Abstract
Repression and activation of the expression of homeotic genes are maintained by proteins encoded by the Polycomb group (PcG) and trithorax group (trxG) genes. Complexes formed by these proteins are targeted by PcG or trxG response elements (PREs/TREs), which share binding sites for several of the same factors. GAGA factor and Zeste bind specifically to PREs/TREs and have been shown to act as both activators and repressors. We have used purified proteins and complexes reconstituted from recombinant subunits to characterize the effects of GAGA and Zeste proteins on PcG function using a defined in vitro system. Zeste directly associates withthe PRC1 core complex (PCC) and enhances the inhibitory activity of this complex on all templates, with a preference for templates withZeste binding sites. GAGA does not stably associate with PCC, but nucleosomal templates bound by GAGA are more efficiently bound and more efficiently inhibited by PCC. Thus Zeste and GAGA factor use distinct means to increase repression mediated by PRC1.
Keywords: Zeste, Polycomb, long-range repression, GAGA factor
Precise spatial and temporal regulation of gene expression during development and throughout the life of the adult is critical in higher eukaryotes. In Drosophila, the identity of body segments is determined by appropriate expression of homeotic selector (HOX) genes. Transiently expressed segmentation gene products and maternal protein contributions establish patterns of HOX gene expression. The active and repressed states of gene expression are maintained by genes of the trithorax group (trxG) and of the Polycomb group (PcG), respectively. When PcG complexes associate with target genes, they form a repressive chromatin structure that is inherited by daughter cells. This cellular memory system allows gene expression patterns to persist throughout the adult life of the organism.
Two distinct PcG complexes have been purified from the fly: Esc/E(z) and PRC1 (Shao et al. 1999; Ng et al. 2000; Saurin et al. 2001; Tie et al. 2001). The Esc/E(z) complex is a methyltransferase with specificity for Lys 27 of the histone H3 tail. HMTase mutations of E(z) result in deregulated silencing of HOX genes in vivo (Muller et al. 2002). The class II PcG complex PRC1 has more than 30 protein subunits, including 5 that have been genetically defined as PcG proteins: Polycomb (Pc), posterior sex combs (PSC), polyhomeotic (Ph), dRING1, and, at substoichiometric levels, sex combs on midlegs (SCM; Shao et al. 1999; Saurin et al. 2001). PRC1 represses transcription in vitro (King et al. 2002). Consistent with the hypothesis that PcG complexes repress genes by affecting chromatin structure, PRC1 inhibits chromatin remodeling of reconstituted nucleosomal arrays by the hSWI/SNF complex in vitro (Shao et al. 1999). A reconstituted recombinant PRC1 core complex (PCC), which contains just the stoichiometric PcG subunits of PRC1 (Pc, PSC, Ph, and dRING1), also inhibits both remodeling and transcription (Francis et al. 2001; King et al. 2002).
PcG effects are mediated by PcG response elements (PREs), large composite regulatory elements that are able to repress transgene reporters in a PcG-dependent manner (Simon et al. 1993; Chan et al. 1994; Christen and Bienz 1994). When introduced at ectopic chromosomal locations, PREs generate new binding sites for PcG proteins and repress the activity of nearby enhancers (Chan et al. 1994). PREs contain binding sites for numerous sequence-specific DNA binding proteins, only one of which is a genetically identified PcG protein, PHO (Brown et al. 1998). Binding sites for early repressors, such as Hunchback and Kruppel, are also found in PREs, as are binding sites for activator proteins (Tillib et al. 1999; Shimell et al. 2000). It has been proposed that the two proteins studied here, Zeste and GAGA factor, contribute to both activation and repression of linked genes (Busturia et al. 2001; Hur et al. 2002; Kosoy et al. 2002).
Zeste is thought to regulate gene expression through effects on chromatin structure, due to its role in transvection (Lewis 1954) and in position effect variegation (Judd 1995). Zeste is a sequence-specific DNA binding protein that binds to the enhancers and promoters of a number of developmentally important genes (Benson and Pirrotta 1988; Pirrotta et al. 1988). Zeste often colocalizes with PcG proteins at repressed loci and has been shown to be involved in maintenance of repression (Hur et al. 2002). Zeste was also found to be a stoichiometric subunit of PRC1 (Shao et al. 1999; Saurin et al. 2001).
The PRE sequences to which Zeste and PcG proteins are localized frequently contain GAGA factor consensus binding sites as well. GAGA factor is encoded by the trithorax-like gene, Trl, and has been characterized as a trxG protein (Farkas et al. 1994). However, Trl null mutations result in suppression of silencing of the iab-7 PRE (Hagstrom et al. 1997), and in expression of silenced reporter transgene under the control of a fragment of the Ubx PRE (Horard et al. 2000), indicating that GAGA also has a repressive activity. Pc and GAGA colocalize at PREs of repressed genes and cofractionate, suggesting the existence of a GAGA-containing PcG complex, distinct from PRC1 (Strutt et al. 1997; Horard et al. 2000). Mutation of the GAGA consensus sites in PREs abrogates PcG binding to these elements, strongly suggesting a role in targeting of PcG complexes (Horard et al. 2000).
One hypothesis for how Zeste and GAGA factor might be involved in PcG-mediated repression is that these proteins increase the local concentration of regulatory complexes by direct interactions (“targeting”), akin to gene-specific activator proteins. We have used purified proteins to characterize the ability of Zeste and GAGA protein to interact with PRC1 subunits. Surprisingly, we find that Zeste does not act analogously to many gene-specific activators, but instead forms a stable complex with the PcG proteins of PRC1. Inclusion of Zeste in the complex results in greater repressive activity on templates that both contain and lack Zeste binding sites. In contrast, GAGA does not form a stable complex with the same PcG proteins. However, when prebound to nucleosomal arrays, GAGA recruits PCC to the arrays, making it more effective in inhibiting the chromatin remodeling activity of SWI/SNF. Thus, both of these proteins can contribute to targeting and regulating the activity of PcG complexes, though they use different means to do so.
Results and Discussion
Zeste copurifies with PcG proteins of PRC1; GAGA factor does not
To characterize the role of GAGA and Zeste in PcG repression, we first asked whether either of these proteins could form a stable complex with the PRC1 functional core complex, PCC (Francis et al. 2001). We coinfected Sf9 cells with recombinant baculoviruses expressing PSC, PC, dRING1, and a Flag-PH. Immunopurification from nuclear extracts yielded PCC, as expected (Fig. 1A, lane 1). When HA-tagged Zeste was coinfected with the four PcG proteins, a complex containing all five proteins was purified (hereafter referred to as PCC-Z; Zeste was estimated by staining intensity to associate at stoichiometric levels [data not shown], as seen with PRC1 [Saurin et al. 2001]). Coinfection with HA-GAGA expressing virus yielded PCC only (Fig. 1A, lanes 2,3). Western blot analysis of flow through and purified fractions further demonstrated that Zeste associated with PCC but GAGA did not (Fig. 1B).
Figure 1.
Zeste but not GAGA forms a complex with PcG proteins of PCC. (A) Colloidal Coomassie-stained 8% SDS-PAGE of protein complexes purified by affinity chromatography of nuclear extracts from Sf9 cells coinfected with recombinant baculovirus expressing Flag-PH, Psc, Pc, and dRING1 (lane 1) and either HA-GAGA (lane 2) or HA-Zeste (lane 3). (B) Western blot analysis of complexes purified in the presence of HA-GAGA or HA-Zeste. (C) Silver-stained 8% SDS-PAGE gel of proteins purified by anti-HA affinity chromatography of Sf9 cells infected with recombinant baculovirus expressing HA-GAGA or HA-GAGAΔPOZ.
To characterize the effects of GAGA protein on PCC activity, we purified intact GAGA and a POZ domain mutant form of GAGA (GAGAΔPOZ, see following) using the appropriate HA-tagged viral construct (Fig. 1C). The function of these proteins is described following.
Zeste increases repressive activity
Both PRC1 and PCC inhibit the activity of the ATP-dependent chromatin remodeling complex SWI/SNF in vitro (Shao et al. 1999; Francis et al. 2001). To determine whether association of Zeste with PCC components alters the efficiency of repression, we compared the abilities of PCC-Z and PCC to inhibit SWI/SNF activity in an assay for nucleosome remodeling. We used a template made up of 10 5S nucleosome positioning sequences flanking a central region large enough for two additional nucleosomes (G5E4, Fig. 2A) and assembled it into a nucleosomal array by salt dialysis (Steger et al. 1997). SWI/SNF remodeling increases access of XbaI to its cutting site (e.g., Fig. 2B, lanes 1,2; Polach and Widom 1995). As has been observed previously, PRC1 inhibited SWI/SNF activity more efficiently than did PCC (Fig. 2B, lanes 3-5,8-10). PCCZ inhibited remodeling with efficiency similar to that of PRC1 and with greater efficiency than PCC (Fig. 2B, lanes 11-13, 2C). We conclude that the addition of a sequence-specific DNA binding protein, Zeste, increases activity of the core components to a level comparable to the native PRC1.
Figure 2.
PCC-Z is more active than PCC and as active as PRC1 in inhibiting SWI/SNF remodeling of a nucleosomal array. (A) Schematic representation of the G5E4 nucleosomal array used in the restriction enzyme accessibility assay. (B) Products generated from XbaI enzyme digestion of the G5E4 array as a representation of increased or decreased accessibility because of changes in local chromatin structure. Lanes labeled “-SWI/SNF” are controls for basal levels of enzyme accessibility. Lanes labeled “+SWI/SNF” are controls for the effect of SWI/SNF in the absence of PcG. PcG at concentrations of 0.1 nM, 0.2 nM, and 0.5 nM were incubated in a reaction containing 0.8 nM nucleosomes for 30 min at 30°C, followed by addition of 2 nM SWI/SNF and restriction enzyme. The percent uncut is indicated below the lanes. (C) Graphic representation of B. The data represents results from at least three experiments and the error bars represent standard deviations. The percent inhibition is calculated with the following equation: (% Uncut with hSWI/SNF and PcG - % Uncut with hSWI/SNF)/(% Uncut without hSWI/SNF - % Uncut with hSWI/SNF).
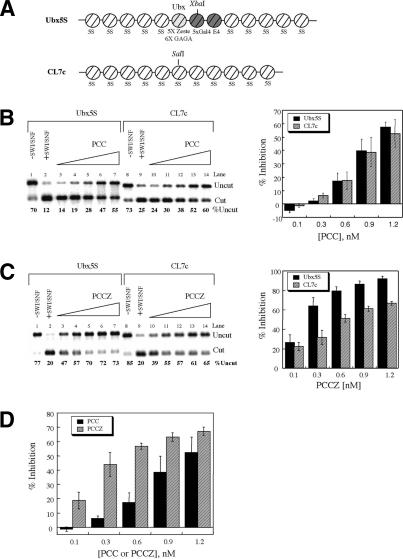
The central region of the G5E4 template contains several Zeste binding sites. To determine the effect of Zeste binding sites on the activity of the PCC-Z complex, we compared activity on a template devoid of consensus Zeste-binding sites (CL7c) with a template into which we inserted a portion of the Ubx promoter with known high-affinity Zeste binding sites (Ubx5s; Fig. 3A; Benson and Pirrotta 1988). Competition filter binding studies demonstrated, as anticipated, that PCC-Z bound nucleosomal array templates that contained Zeste binding sites 10-fold better than templates that did not (data not shown). Thus, we infer that the Zeste protein complexed in PCC-Z is able to recognize its binding site. We then compared the ability of the two complexes to inhibit chromatin remodeling on nucleosomal arrays with or without Zeste binding sites (Fig. 3). The results are represented by the percent inhibition of restriction enzyme cleavage, which is used to normalize for any differences in the levels of assembly between array preparations. PCC inhibited chromatin remodeling by SWI/SNF to the same extent on both arrays, whereas PCC-Z more efficiently inhibited remodeling of the array containing Zeste binding sites (Fig. 3B,C). It is important to note, however, that PCC-Z enhanced inhibition even on templates with no consensus Zeste binding site (Fig. 3D). Zeste might have a direct effect on the properties of the core subunits of PRC1, influencing their activity independent of targeting them to specific DNA sequences. In addition, nonspecific DNA binding abilities of Zeste may make a significant contribution to PRC1 activity.
Figure 3.
PCC-Z more effectively inhibits chromatin remodeling on arrays with and without Zeste binding sites. (A) Schematic representation of the nucleosomal arrays used in this assay, Ubx5S and CL7c (see text for description). (B,C) Products of digestion with either XbaI (Ubx5s, black bars) or SalI (CL7c, hatched bars) after incubation with increasing amounts of PCC (B) or PCC-Z (C) and SWI/SNF. Controls are as in Figure 2B. Representative gels are shown. Data are represented as percent inhibition and represent data from at least three experiments. (D) Data from B and C plotted together to highlight the enhanced repressive activity of PCC-Z (hatched bars) as compared with PCC (black bars) on CL7c arrays, which do not have zeste binding sites.
The ability of Zeste to interact stably with PCC subunits and alter activity of the resultant complex differs from what is normally seen with sequence-specific eukaryotic DNA binding proteins. Aspects of this behavior are reminiscent of proteins such as the bacterial sigma factors, in that both Zeste and sigma factors can bind to core subunits of the regulated complex, and both proteins alter the function of the resultant complex on specific and on nonspecific templates (Hinkle et al. 1972). However, Zeste increases function of PCC subunits regardless of whether they contain a Zeste binding site, and sigma reduces binding of RNA polymerase core subunits to nonspecific templates and enhances binding to promoter-containing templates (Dombroski et al. 1992).
Transcriptional repression is increased by Zeste association
Both PRC1 and PCC are able to repress transcription of a chromatin template in vitro (King et al. 2002). We asked whether adding the Zeste subunit to PCC would affect its ability to repress transcription (Fig. 4A). Both PCC and PCC-Z were able to repress transcription of a chromatin template (Fig. 4B), but PCC-Z repressed transcription more efficiently than did PCC. The concentration of PCC required was on average fivefold higher than the concentration of PCC-Z needed to repress transcription to a similar degree. PCC-Z was typically able to fully repress transcription at a molar ratio to template of -1:1, similar to the molar ratio needed for PRC1 to fully repress transcription (King et al. 2002). We conclude that the presence of the Zeste subunit allows PCC-Z to repress transcription more efficiently than PCC, and that PCC-Z is comparable in efficiency to native PRC1.
Figure 4.
PCC-Z inhibits transcription more efficiently than PCC. (A) Transcription reactions were performed as shown. (B) PCC or PCCZ was incubated with the Ubx5S array. PCC-Z and PCC were added at 63 pM, 125 pM, 250 pM, 0.5 nM, and 1 nM. Subsequently, Gal4-VP16, HeLa nuclear extract, and nucleoside triphosphates were added to the reaction as indicated. Transcripts were visualized by primer extension, resolved on sequencing gels, and quantitated by PhosphorImager. Quantitation is given as a fraction of control (no PCC or no PCC-Z) reactions.
GAGA factor enhances the inhibitory activity of PCC
These experiments show that Zeste, which binds tightly to PRC1 components and is part of a bona fide embryonic PRC1, is capable of enhancing the activity of PCC. The GAGA protein does not form a stable complex with PCC components and does not copurify with embryonic PRC1, but does associate with PRC1 components in immunoprecipitation experiments from embryonic chromatin (Horard et al. 2000). We therefore asked if PCC is recruited to templates bound by GAGA factor.
Both PCC and PRC1 must be incubated with target templates prior to the addition of SWI/SNF to inhibit remodeling (Fig. 5A; Shao et al. 1999). We reasoned that if PCC were recruited by GAGA factor the complex would not require preincubation with these templates to compete with SWI/SNF. To test this, we used the restriction enzyme accessibility assay on the Ubx5S template prebound with GAGA. This template contains GAGA sites from the Ubx promoter (Tillib et al. 1999). To these GAGA-bound arrays, PCC, SWI/SNF, and XbaI were added simultaneously. Inhibition of SWI/SNF activity by PCC was enhanced on templates prebound by GAGA (Fig. 5, cf. A, “no preincubation”, and B, “+GAGA”; quantified in panel C). Although PCC was more active when prebound to the template (Fig. 5A), repression of the GAGA-bound template was more efficient still (Fig. 5A, cf. “+GAGA” and “preincubation”). Addition of GAGA at the same time as PCC did not increase PCC activity and GAGA alone had no effect on chromatin remodeling by SWI/SNF (data not shown).
Figure 5.
GAGA-bound nucleosomal templates do not require preincubation with PCC for inhibitory effects against SWI/SNF chromatin remodeling. (A) Effects of preincubating nucleosomal arrays with PCC alone. Arrays were incubated at 30°C with either PCC (0.05 nM to 1.5 nM PCC/0.8 nM nucleosomes) or buffer alone for 30 min prior to addition of SWI/SNF and XbaI, and digestion products run on a 0.8% agarose gel. Typical results are shown. (B) Experimental protocol for effects of GAGA. The Ubx5s nucleosomal array (0.8 nM nucleosomes) was incubated with either 1 nM GAGA, 1 nM GAGAΔPOZ, or buffer for 15 min. PCC, SWI/SNF, and XbaI were added simultaneously, and the reaction was allowed to proceed for 60 min. Resulting digestion products were run on 0.8% agarose gel. Results from a typical experiment are shown. (B,C) The effect of PCC on SWI/SNF remodeling in the absence of GAGA (black bars), with 1 nM GAGA (hatched bars), and with 1 nM GAGAΔPOZ (open bars) are plotted as percent inhibition. (D) GAGA bound arrays are bound preferentially by PCC. Immobilized nucleosomal arrays were incubated with GAGA or buffer alone for 15 min at 30°C. Free protein was washed away. PCC or buffer and 10×, 100×, or 1000× free nucleosomal arrays were added and reactions were incubated for 30 min at 30°C. Free material was washed away. Immobilized templates and the material associated with them (Bound) and wash material (Unbound) were loaded onto 8% SDS-PAGE and analyzed by Western blot using antibodies against the Flag tag (Flag-PH) or against the HA tag (HA-GAGA).
To further characterize this effect, we examined a GAGA factor deletion mutant (GAGAΔPOZ; Fig. 1C) that is less efficient in binding to multiple GAGA sites and is defective in oligomerization (Katsani et al. 1999). GAGAΔPOZ was unable to increase PCC activity when used at identical concentrations to the intact GAGA protein (Fig. 5B, quantified in panel C). Molar concentrations of GAGAΔPOZ up to six times higher than those used for full-length GAGA had no effect on PCC activity. GAGAΔPOZ bound to nucleosomal templates in an immobilized template binding assay (see following; data not shown). Although we have not examined whether this effect of GAGA on PCC requires GAGA sites on the template (all templates characterized for the functional assay protocol used earlier contain GAGA sites within the 5S repeat sequence, which is required for nucleosome positioning), these experiments demonstrate that this recruitment effect requires that the GAGA factor have an intact BTB/POZ domain. This suggests that the multimerization activity of GAGA might contribute significantly to recruitment of PCC, because the BTB/POZ domain is also required for GAGA factor to multimerize and bind two DNA molecules simultaneously (Mahmoudi et al. 2002).
PCC is recruited to nucleosomal arrays by GAGA
To demonstrate directly that GAGA enhances template recognition by PCC, we developed a recruitment assay. The Ubx5S DNA was biotinylated, assembled into chromatin, and immobilized on streptavidin-coated magnetic beads. The nucleosomal arrays were incubated with either buffer only or GAGA for 15 min at 30°C prior to addition of PCC. Following a 20-min binding period, array-bound beads and all material bound to them were separated magnetically from unbound protein. Western blot analysis demonstrated that PCC (Fig. 5D, left panel) and GAGA (Fig. 5D, right panel) components bound to the nucleosomal array-bead complex and that PCC association was increased on arrays bound by GAGA (Fig. 5D, cf. lanes 1 and 5). GAGA and PCC bound only minimally to unconjugated beads (data not shown). Increasing amounts of competitor nucleosomal array resulted in a loss of PCC association with the array-bead complex, but did not affect PCC association with the GAGA array-bead complex (Fig. 5D, cf. lanes 1-4 and 5-8). These results demonstrate further that PCC has a higher affinity for nucleosomal templates bound by the GAGA factor. Prebinding the GAGAΔPOZ protein did not lead to increased recruitment of PCC (data not shown).
These experiments show that a template prebound by GAGA factor is more efficiently bound and repressed by PCC than an unbound template. GAGA factor might recruit or stabilize PCC binding by directly interacting with its subunits, or GAGA might alter the template in a manner that favors PCC binding. It has been shown previously that GAGA factor oligomers are able to bind multiple templates simultaneously, bringing them together. Binding by GAGA factor might create a network of templates that is more efficiently bound and recognized by PCC than an individual template might be.
Mechanisms that regulate and target PRC1 repression
In the defined in vitro system used here, both Zeste and GAGA factor can enhance the activity of a PRC1 core complex to repress remodeling of a nucleosomal template. Zeste binds directly to these PcG proteins to generally increase their repressive function, and prebinding GAGA factor to the template recruits the PRC1 core to that template. Previous genetic and mechanistic studies have suggested that regulation of PRC1 repression is a complicated process involving targeting by sequence-specific DNA binding proteins, covalent modification of histone tails, and perhaps targeting by siRNAs. The differences in function of GAGA and Zeste suggest that their role in PcG repression is more complex than previously suspected. One hypothesis for how sequence-specific factors establish PcG repression is that they create a binding surface with greater affinity for PRC1. Although our experiments with GAGA factor are consistent with this hypothesis, our experiments with Zeste suggest that additional mechanisms contribute to targeting by sequence-specific factors. Zeste binds tightly to the core components of PRC1 and enhances their activity even when templates do not contain targeting sequences. This might be important in facilitating the ability of PRC1 repression to spread away from PRE elements, and thus may facilitate the repression of large domains by PRC1.
Although originally identified as an activator, Zeste can also function in vivo as a repressor. For example, in zeste mutant flies, a transgene containing the Ubx promoter modified to contain only Zeste binding sites is derepressed in the anterior and posterior segments of the embryo (Hur et al. 2002). Two distinct substitution mutants of zeste express proteins that repress rather than activate the white gene but retain activator function required for transvection, suggesting that Zeste has both inherent activation and repression activities that can be separated (Rosen et al. 1998). It is possible that, when incorporated into PRC1, Zeste is configured so as to only display surfaces responsible for repression.
It is widely believed that PcG activity is targeted and maintained throughout the course of development by multiple systems. For instance, ESC/E(z) can methylate H3 K27, and PC can bind to this modification, suggesting that a methylation mark might also play a key role in targeting PRC1 and/or in regulating the spread of PRC1 activity. The combined effects of factors such as GAGA that target PRC1 activity, factors such as Zeste that augment PRC1 activity, and other systems such as those for covalent modification of histones might be necessary for faithful maintenance of PRC1 association with a template. It is likely that further mechanisms, such as RNAi, also contribute (Pal-Bhadra et al. 2002).
These multiple mechanisms might be additive or synergistic. Additionally, redundancy between them would provide a failsafe scheme for maintenance of repression. For instance, if methylation at H3 K27 and increased function by Zeste each were sufficient to establish repression by PRC1, then repression could be established even if one or the other were to fail. Consistent with this hypothesis of redundant function, we have performed experiments in which both Zeste and GAGA were present and to date have not seen any significant additive or synergistic effects on PCC function (data not shown). The establishment of defined in vitro systems, such as used here, will aid in unraveling the connections between the different mechanisms that contribute to regulation of PcG function.
Materials and methods
Protein purification
PCC was prepared as described (Francis et al. 2001). In brief, Sf9 cells were coinfected with baculovirus expressing Flag-PH, Psc, Pc, and dRING1. Nuclear extracts were prepared and complex was affinity purified using M2 anti-Flag beads (Sigma). Beads were washed in up to 2M KCl and proteins were eluted with Flag peptide in BC300 (20 mM HEPES at pH 8.0, 0.2 mM EDTA, 20% glycerol, 300 mM KCl, 0.2 mM PMSF, 0.5 mM DTT, protease inhibitors) with 0.05% NP40 and 10 μM ZnCl2. For Zeste or GAGA associations with PCC, baculovirus expressing HA-Zeste or HA-GAGA were coinfected with PCC components. HA-GAGA and GAGAΔPOZ were expressed in Sf9 cells and purified on anti-HA affinity matrix (Roche).
Restriction enzyme accessibility assay
Nucleosomal arrays (NAs) were assembled by salt dialysis confirmed by EcoRI digestion between nucleosomes as described (Francis et al. 2001). The G5E4 and CL7c arrays have been described (Polach and Widom 1995; Ikeda et al. 1999). Ubx5S was constructed by inserting a 175-bp fragment of the Ubx promoter into PstI of G5E4. The restriction enzyme accessibility assay was performed as described (Francis et al. 2001) with the following variations: no HeLa polynucleosomes were added, 0.3 U/μL of SalI for CL7c and XbaI for G5E4 and Ubx5S were used. In Figures 2 and 3, PcG proteins were incubated with NAs for 30 min prior to addition of SWI/SNF and restriction enzyme. In Figure 5A, NAs are incubated with GAGA or GAGAΔPOZ for 15 min prior to the simultaneous addition of PCC, hSWI/SNF, and restriction enzyme. Digestion proceeded for 60 min and stopped with 2 μL DSB (50 mM Tris, 100 mM EDTA, 1% SDS, 25% glycerol, BPB, and XC) and 1 μL 10 mg/mL proteinase K and incubated at 55°C for 60 min. Samples were resolved on agarose gels and exposed to PhosphorImager screens for quantitation (Molecular Dynamics).
In vitro transcription
In vitro transcription reactions were performed as described (King et al. 2002), omitting the hSWI/SNF remodeling.
Recruitment assay
The Ubx5S DNA was biotinylated and reconstituted by salt dialysis, and NA integrity was assayed. Approximately 300 ng of NA was immobilized onto 50 μg of streptavidin-coated magnetic beads (Dynal M-280) at 4°C in BB (10 mM HEPES at pH 8.0, 0.1 mM EDTA, 10% glycerol, 2 mM MgCl2, 0.25 mM DTT, 300 mM KCl, 100 μg/mL BSA). Bead-NA complexes were washed two times in BB, blocked once in BB + 5 mg/mL PVP and 60 mg/mL casein, followed by two more washes in BB and resuspended in 50 μL BB. Binding reactions with the immobilized NAs were carried out under the same conditions of the restriction enzyme assay for 15 min (GAGA) and 30 min (PCC) at 30°C. Following the binding reaction, “unbound” material was removed by a wash in BB. After one more wash, immobilized beads and the “bound” proteins were resuspended in BB + 1× SDS-PAGE loading buffer. Samples were resolved by 8% SDS-PAGE and proteins were visualized by Western blot using antibodies against Flag (PH) or HA (GAGA).
Acknowledgments
We thank Peter Verrijzer for generously supplying GAGA and Zeste expression vectors. We are grateful to all members of the Kingston Lab, in particular Marc Lavigne and Nicole Francis, for helpful suggestions and ideas.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Article and publication are at http://www.genesdev.org/cgi/doi/10.1101/gad.1143303.
References
- Benson M. and Pirrotta, V. 1988. The Drosophila zeste protein binds cooperatively to sites in many gene regulatory regions: Implications for transvection and gene regulation. EMBO J. 7: 3907-3915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J.L., Mucci, D., Whiteley, M., Dirksen, M.L., and Kassis, J.A. 1998. The Drosophila Polycomb group gene pleiohomeotic encodes a DNA binding protein with homology to the transcription factor YY1. Mol. Cell 1: 1057-1064. [DOI] [PubMed] [Google Scholar]
- Busturia A., Lloyd, A., Bejarano, F., Zavortink, M., Xin, H., and Sakonju, S. 2001. The MCP silencer of the Drosophila Abd-B gene requires both Pleiohomeotic and GAGA factor for the maintenance of repression. Development 128: 2163-2173. [DOI] [PubMed] [Google Scholar]
- Chan C.S., Rastelli, L., and Pirrotta, V. 1994. A Polycomb response element in the Ubx gene that determines an epigenetically inherited state of repression. EMBO J. 13: 2553-2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christen B. and Bienz, M. 1994. Imaginal disc silencers from Ultrabithorax: Evidence for Polycomb response elements. Mech. Dev. 48: 255-266. [DOI] [PubMed] [Google Scholar]
- Dombroski A.J., Walter, W.A., Record Jr., M.T., Siegele, D.A., and Gross, C.A. 1992. Polypeptides containing highly conserved regions of transcription initiation factor sigma 70 exhibit specificity of binding to promoter DNA. Cell 70: 501-512. [DOI] [PubMed] [Google Scholar]
- Farkas G., Gausz, J., Galloni, M., Reuter, G., Gyurkovics, H., and Karch, F. 1994. The Trithorax-like gene encodes the Drosophila GAGA factor. Nature 371: 806-808. [DOI] [PubMed] [Google Scholar]
- Francis N.J., Saurin, A.J., Shao, Z., and Kingston, R.E. 2001. Reconstitution of a functional core polycomb repressive complex. Mol. Cell 8: 545-556. [DOI] [PubMed] [Google Scholar]
- Hagstrom K., Muller, M., and Schedl, P. 1997. A Polycomb and GAGA dependent silencer adjoins the Fab-7 boundary in the Drosophila bithorax complex. Genetics 146: 1365-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkle D.C., Ring, J., and Chamberlin, M.J. 1972. Studies of the binding of Escherichia coli RNA polymerase to DNA. 3. Tight binding of RNA polymerase holoenzyme to single-strand breaks in T7 DNA. J. Mol. Biol. 70: 197-207. [DOI] [PubMed] [Google Scholar]
- Horard B., Tatout, C., Poux, S., and Pirrotta, V. 2000. Structure of a polycomb response element and in vitro binding of polycomb group complexes containing GAGA factor. Mol. Cell. Biol. 20: 3187-3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hur M.W., Laney, J.D., Jeon, S.H., Ali, J., and Biggin, M.D. 2002. Zeste maintains repression of Ubx transgenes: Support for a new model of Polycomb repression. Development 129: 1339-1343. [DOI] [PubMed] [Google Scholar]
- Ikeda K., Steger, D.J., Eberharter, A., and Workman, A.L. 1999. Activation domain-specific and general transcription stimulation by native histone acetyltransferase complexes. Mol. Cell. Biol. 19: 855-863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judd B.H. 1995. Mutations of zeste that mediate transvection are recessive enhancers of position-effect variegation in Drosophila melanogaster. Genetics 141: 245-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsani K.R., Hajibagheri, M.A., and Verrijzer, C.P. 1999. Co-operative DNA binding by GAGA transcription factor requires the conserved BTB/POZ domain and reorganizes promoter topology. EMBO J. 18: 698-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King I.F., Francis, N.J., and Kingston, R.E. 2002. Native and recombinant polycomb group complexes establish a selective block to template accessibility to repress transcription in vitro. Mol. Cell. Biol. 22: 7919-7928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosoy A., Pagans, S., Espinas, M.L., Azorin, F., and Bernues, J. 2002. GAGA factor down-regulates its own promoter. J. Biol. Chem. 277: 42280-42288. [DOI] [PubMed] [Google Scholar]
- Lewis E.B. 1954. The theory and application of a new method of detecting chromosomal rearrangements in Drosophila melanogaster. Am. Nat. 88: 225-239. [Google Scholar]
- Mahmoudi T., Katsani, K.R., and Verrijzer, C.P. 2002. GAGA can mediate enhancer function in trans by linking two separate DNA molecules. EMBO J. 21: 1775-1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller J., Hart, C.M., Francis, N.J., Vargas, M.L., Sengupta, A., Wild, B., Miller, E.L., O'Connor, M.B., Kingston, R.E., and Simon, J.A. 2002. Histone methyltransferase activity of a Drosophila Polycomb group repressor complex. Cell 111: 197-208. [DOI] [PubMed] [Google Scholar]
- Ng J., Hart, C.M., Morgan, K., and Simon, J.A. 2000. A Drosophila ESC-E(Z) protein complex is distinct from other polycomb group complexes and contains covalently modified ESC. Mol. Cell. Biol. 20: 3069-3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal-Bhadra M., Bhadra, U., and Birchler, J.A. 2002. RNAi related mechanisms affect both transcriptional and posttranscriptional transgene silencing in Drosophila. Mol. Cell 9: 315-327. [DOI] [PubMed] [Google Scholar]
- Pirrotta V., Bickel, S., and Mariani, C. 1988. Developmental expression of the Drosophila zeste gene and localization of zeste protein on polytene chromosomes. Genes & Dev. 2: 1839-1850. [DOI] [PubMed] [Google Scholar]
- Polach K.J. and Widom, J. 1995. Mechanism of protein access to specific DNA sequences in chromatin: A dynamic equilibrium model for gene regulation. J. Mol. Biol. 254: 130-149. [DOI] [PubMed] [Google Scholar]
- Rosen C., Dorsett, D., and Jack, J. 1998. A proline-rich region in the Zeste protein essential for transvection and white repression by Zeste. Genetics 148: 1865-1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saurin A.J., Shao, Z., Erdjument-Bromage, H., Tempst, P., and Kingston, R.E. 2001. A Drosophila Polycomb group complex includes Zeste and dTAFII proteins. Nature 412: 655-660. [DOI] [PubMed] [Google Scholar]
- Shao Z., Raible, F., Mollaaghababa, R., Guyon, J.R., Wu, C.T., Bender, W., and Kingston, R.E. 1999. Stabilization of chromatin structure by PRC1, a Polycomb complex. Cell 98: 37-46. [DOI] [PubMed] [Google Scholar]
- Shimell M.J., Peterson, A.J., Burr, J., Simon, J.A., and O'Connor, M.B. 2000. Functional analysis of repressor binding sites in the iab-2 regulatory region of the abdominal-A homeotic gene. Dev. Biol. 218: 38-52. [DOI] [PubMed] [Google Scholar]
- Simon J., Chiang, A., Bender, W., Shimell, M.J., and O'Connor, M. 1993. Elements of the Drosophila bithorax complex that mediate repression by Polycomb group products. Dev. Biol. 158: 131-144. [DOI] [PubMed] [Google Scholar]
- Steger D.J., Owen-Hughes, T., John, S., and Workman, J.L. 1997. Analysis of transcription factor-mediated remodeling of nucleosomal arrays in a purified system. Methods 12: 276-285. [DOI] [PubMed] [Google Scholar]
- Strutt H., Cavalli, G., and Paro, R. 1997. Co-localization of Polycomb protein and GAGA factor on regulatory elements responsible for the maintenance of homeotic gene expression. EMBO J. 16: 3621-3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tie F., Furuyama, T., Prasad-Sinha, J., Jane, E., and Harte, P.J. 2001. The Drosophila Polycomb Group proteins ESC and E(Z) are present in a complex containing the histone-binding protein p55 and the histone deacetylase RPD3. Development 128: 275-286. [DOI] [PubMed] [Google Scholar]
- Tillib S., Petruk, S., Sedkov, Y., Kuzin, A., Fujioka, M., Goto, T., and Mazo, A. 1999. Trithorax- and Polycomb-group response elements within an Ultrabithorax transcription maintenance unit consist of closely situated but separable sequences. Mol. Cell. Biol. 19: 5189-5202. [DOI] [PMC free article] [PubMed] [Google Scholar]