Abstract
As a means toward understanding the neural bases of schizophrenic thought disturbance, we examined brain activation patterns in response to semantically and superficially encoded words in patients with schizophrenia. Nine male schizophrenic and 9 male control subjects were tested in a visual levels of processing (LOP) task first outside the magnet and then during the fMRI scanning procedures (using a different set of words). During the experiments visual words were presented under two conditions. Under the deep, semantic encoding condition, subjects made semantic judgments as to whether the words were abstract or concrete. Under the shallow, nonsemantic encoding condition, subjects made perceptual judgments of the font size (uppercase/lowercase) of the presented words. After performance of the behavioral task, a recognition test was used to assess the depth of processing effect, defined as better performance for semantically encoded words than for perceptually encoded words. For the scanned version only, the words for both conditions were repeated in order to assess repetition-priming effects. Reaction times were assessed in both testing scenarios. Both groups showed the expected depth of processing effect for recognition, and control subjects showed the expected increased activation of the left inferior prefrontal cortex (LIPC) under semantic encoding relative to perceptual encoding conditions as well as repetition priming for semantic conditions only. In contrast, schizophrenics showed similar patterns of fMRI activation regardless of condition. Most striking in relation to controls, patients showed decreased LIFC activation concurrent with increased left superior temporal gyrus activation for semantic encoding versus shallow encoding. Furthermore, schizophrenia subjects did not show the repetition priming effect, either behaviorally or as a decrease in LIPC activity. In patients with schizophrenia, LIFC underactivation and left superior temporal gyrus overactivation for semantically encoded words may reflect a disease-related disruption of a distributed frontal temporal network that is engaged in the representation and processing of meaning of words, text, and discourse and which may underlie schizophrenic thought disturbance.
Introduction
A hallmark of schizophrenia is an often disabling disturbance of thought. The neuropsychology of schizophrenic thought disorder has yet to be elucidated but it is commonly linked to disease-related deficits in the declarative-episodic memory of verbal material. (Saykin et al., 1991; Nestor et al., 1997). Verbal memory is processed by a distributed network of brain regions, including medial and neocortical temporal regions and perhaps inferior prefrontal cortex. Not surprisingly, many of these very same regions are among the most frequently reported sites of neuropathology in schizophrenia (Shenton et al., 2001; McCarley, 2001). Functional imaging studies of schizophrenia have also demonstrated abnormalities similarly localized within the hippocampus, superior temporal gyrus, and prefrontal cortex (Shergill et al., 2000; Liddle et al., 1992; Weinberger et al., 1992; Heckers et al., 1998; Yurgelun-Todd et al., 1996). It is unclear, however, how these abnormalities relate to the well-known neuropsychological changes in memory seen in chronic schizophrenia as well as how they may contribute to schizophrenic thought disturbance. Nor is it clear how disturbances in specific information processing mechanisms might correspond to both disturbances in thinking and declarative-episodic verbal memory deficits observed in patients with schizophrenia.
Information processing models commonly divide declarative-episodic memory into three stages: encoding, retention or storage, and retrieval. Of particular interest has been the encoding stage, which has been frequently studied using the levels of processing (LOP) framework (Craik and Lockhart, 1972). In the prototypical paradigm, words are studied or encoded under different levels of processing and then subsequently recalled. The pivotal idea is that the greater the depth at which information is encoded, the more likely it will be remembered. Encoding is manipulated by instructing subjects to process material more deeply, as for example to make semantic judgments about to-be-remembered words, such as whether the stimuli represent living or nonliving or abstract or concrete words. This deeper, more elaborate encoding is compared with a shallower, more superficial level of encoding, such as having subjects judge the font (uppercase vs lowercase) of each word presented. Numerous studies have demonstrated the so-called depth of processing effect, as reflected by significantly better recall for words encoded under the deep or semantic condition than for words encoded under shallow conditions where attention is directed to more superficial, perceptual properties of words.
The LOP framework has not, however, been extensively applied to examine the nature of encoding and semantic processing in learning and memory in schizophrenia. The principal advantages of such an approach are several. First, the LOP provides a well-studied paradigm to parse word processing into specific stages and hence should allow the comparison between semantic and more superficial processing stages in schizophrenia. Second, when combined with fMRI, LOP tasks have effectively distinguished between activation due to semantic and nonsemantic processing in healthy subjects (Demb et al., 1995; Gabrieli et al., 1998). FMRI LOP studies of healthy subjects have also demonstrated that semantic encoding becomes more efficient as words are repeated relative to their initial encoding, an effect known as repetition priming, which corresponds to reduced LIFC activation (Gabrieli et al., 1998). Behavioral studies as well as event-related potential studies have demonstrated abnormal semantic priming effects in schizophrenia (Guillem et al., 2001; Minzenberg et al., 2002; Baving et al., 2001; Mathalon et al., 2002; Niznikiewicz et al., 1997), which, in turn, have been related to schizophrenic thought disturbance (Nestor et al., 1998). Finally, the building blocks of human thought are the representations and processing of meaning of words, texts, and discourse. Disturbances in these semantic representations and processes, which may contribute to schizophrenic thought disturbance, can be readily studied using the LOP framework.
We studied patients with schizophrenia and controls matched for age, gender, handedness, and parental socioeconomic status (PSES) using LOP tasks in an fMRI block design (Demb et al., 1995). We posit that semantic processing is represented in part by a distributed neural network that clearly includes LIFC but also extends to superior temporal gyrus as well, as reflected by classic human lesion studies of semantic language disturbances and semantic dementia (e.g., Hart and Gordon, 1990; Hodges et al., 1992). We specifically hypothesize that in relation to age-matched controls, schizophrenic patients will demonstrate abnormal patterns of fMRI activation across LIFC and temporal regions during the LOP semantic encoding condition.
Methods
Subjects
Nine male patients diagnosed with chronic schizophrenia, using DSM-IV criteria based on SCID-P interviews and a review of the medical records, and 9 male control subjects were matched on handedness, PSES, and age. All subjects gave written informed consent prior to participation in the study, and all were compensated for their time.
Materials
The stimuli were 180 words selected from the University of Western Australia database (www.psy.uwa.edu.au/MRCDataBase/uwa_mrc.htm), which provides parametric ratings of the degree of concreteness of words. Abstract words were defined as having normative ratings ranging from 100 to 300, whereas concrete words had normative ratings of 500–700. Words in two semantic categories [abstract (concreteness rating, 100–300) and concrete (concreteness rating, 500–700)] and two nonsemantic categories (upper- and lowercase font) were matched for number of syllables (one to three syllables) and frequency (Kucera–Francis frequency, 1–100). Words were presented to the subjects in 30-s-long blocks either on a computer screen (behavioral experiment) or with MR compatible visual goggles (Avotec, Inc., Florida, www.avotec.org, for the fMRI experiment) using the Presentation version 0.46 software (Neurobehavioral Systems, www.neurobehavioralsystems.com). Each word for both encoding and recognition tasks was presented in white type (font size 66) printed on a black background and appeared centrally on a screen for 1.5 s, with a 1-s intertrial interval (ITI) before the next word appeared (so the Stimulus Onset Asynchrony (SOA) was equal to 2.5 s). Each block was preceded by a word to instruct the subject as to the judgment (e.g., ABSTRACT or UPPERCASE). Responses were collected using an MRI compatible fiber optic response four-button diamond configuration pad (Current Designs Inc., Philadelphia, PA, http://www.curdes.com). Each subject underwent the same number of procedures performed in the same order. Before the scanning procedures, each session started with a practice session. Subjects were given detailed instructions and then presented with two blocks of 6 randomly selected words and instructions for either a semantic (abstract or concrete) or a nonsemantic (upper- or lowercase) judgment. If more than one error was made, the practice session was repeated. This was followed by the actual behavioral encoding task, in which the same judgments—either a semantic (abstract or concrete) or a nonsemantic (upper- or lowercase)—were made. Finally, a recognition task, in which subjects judged whether the word was new or had been seen in the encoding task was performed. Then subjects were put into the scanner, where only the encoding task using new set of words was performed again. In addition, during the encoding task, but only in the scanner, each block of words was immediately repeated to assess repetition priming. Subjects were required to make the same judgment (i.e., semantic or perceptual) for both the initially presented and repeated conditions. Response time and accuracy were recorded for all tasks.
Behavioral tasks
Encoding
Sixteen 30-s blocks of 12 words were presented on a computer screen. Each block contained 12 words: 3 abstract and uppercase, 3 abstract and lowercase, 3 concrete and uppercase, and 3 concrete and lowercase. Words were placed in a pseudorandom order with the constraint that no more than 3 abstract or concrete and no more than 3 uppercase or lowercase words appeared consecutively. Under the semantic encoding condition, subjects were instructed to press a response button only if they saw a specific semantic category of word (this was counterbalanced across subjects, so half of them were responding only to abstract and half only to concrete words). Under the nonsemantic, perceptual encoding condition, subjects judged the font, responding to only uppercase words (half of the subjects) or to lowercase words (another half of the subjects), using the same response button. Both semantic (abstract and concrete) and nonsemantic (upper- and lowercase) conditions were counterbalanced across subjects.
Recognition
A recognition task consisted of 24 target words (12 from each category) along with 24 foil words (also 12 from each category). Subjects made judgments (by pressing the left or right button) as to whether the word presented on the screen was used before (under the encoding condition) or was new. Unlike under the encoding condition, where the SOA and ITI were constant for all words, here the next word appeared on the computer screen after the subject pressed the button in response to the previous word.
FMRI task
All subjects included in the FMRI task achieved accuracy judgments of 75% or better in the behavioral encoding task. During fMRI scanning, subjects performed the semantic (deep) and perceptual (shallow) encoding tasks. The task was presented in two runs. Within each run, eight 30-s blocks of task were interleaved with eight 30-s rest blocks (blank screen). Instructions to press the button for abstract/concrete or upper/lowercase words were presented visually for 3 s at the end of each rest block. In each block 12 words were presented, 6 from each category (abstract/concrete, lower/uppercase) (see experimental design, Fig. 1). Each word was presented for 1.5 s, with a 1-s intertrial interval. In order to test for repetition priming effects, each block of new words was followed by the block containing the same words but in a different, randomized order. In addition, to examine the relationship between total positive symptoms, hallucinations, delusions, and thought disorder with abnormal brain activation in schizophrenia, the SPM “multiple regression” analysis was used to find relationship between above symptoms (as measured by the scale for the assessment of positive symptoms SAPS) and brain activity observed under three different experimental conditions: deep, shallow, and deep minus shallow.
Fig. 1.
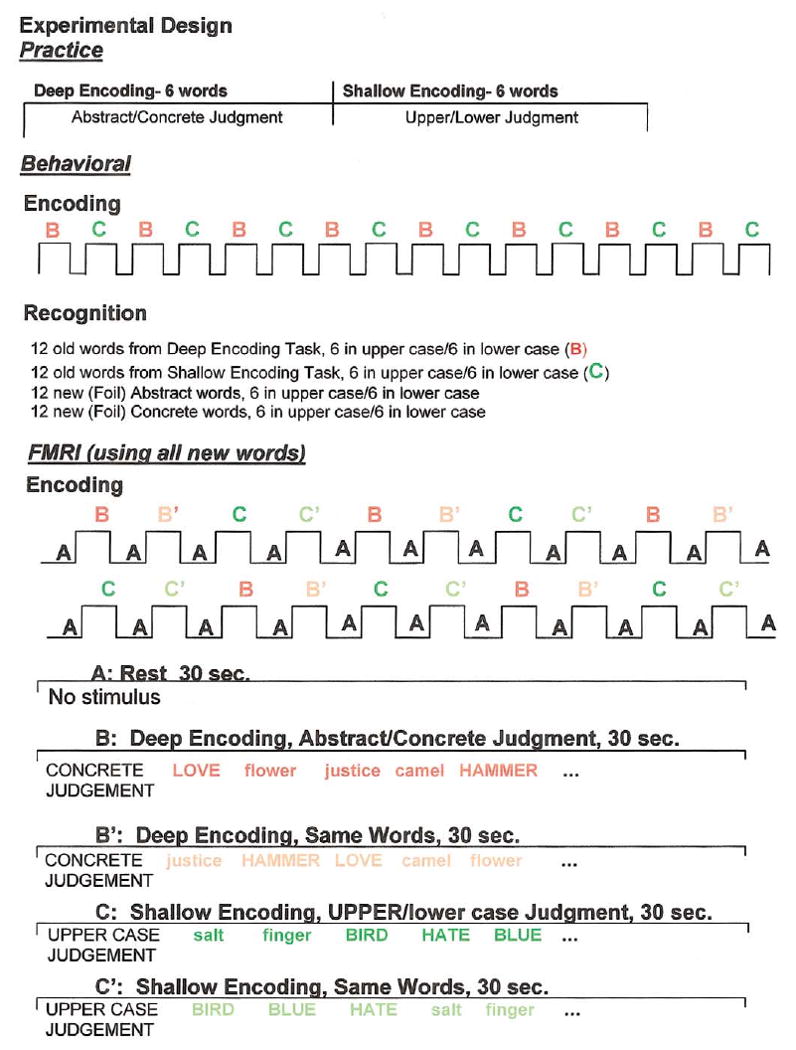
The figure illustrates the design of the experiment.
Imaging was performed using a 1.5-T whole body MRI Echospeed system (General Electric Medical Systems, Milwaukee, WI). First a sagittal anatomical localizer image was acquired, and then the 174 EPI BOLD scans (24 oblique coronal slices, 6 mm thick, TR, 3 s; TE, 40 ms; flip angle, 90°) were acquired perpendicular to the long axis of the hippocampus. The total length of each of the two runs was 8 min and 42 s. The first 4 scans of each block were discarded, and the rest were subjected to statistical analysis.
Data were analyzed using Statistical Parametric Mapping (SPM99). FMRI data were coregistered to the first scan of the first session (in order to correct for head movement), normalized to the Montreal Neurology Institute (MNI) template using a nonlinear, 12-parameter affine transformation registration, and smoothed with a 6-mm FWHM Gaussian filter. Activation maps, including contrasts of tested conditions (semantic words vs rest, nonsemantic words vs rest, semantic vs nonsemantic words) were constructed separately for each subject, and random effect analyses were performed using the general linear model and random effect t tests. Since the multiple voxelwise comparison correction is quite stringent and prone to false negatives (Ashburner and Friston, 2000), we used an alternative approach that takes into account spatial clustering, testing the probability of chance occurrence of the observed spatial extent of contiguous voxels (Friston et al., 1995). First, the SPM map, after Z transformation, was thresholded at a higher P value (P < 0.001). Local maxima of Z value were reported as separate regions if they were more than 6 mm apart within a cluster (half-width of the smoothing Gausian kernel). Those regions with P < 0.05 (corrected for spatial extent) were considered significantly different between groups (Friston et al., 1995; Wright et al., 1999). The extent threshold was used so that only these activations were displayed on the figures (nonsignificant clusters were not displayed). For correlational analysis, lower threshold, of P < 0.01, and a priori selected region (left STG) were used for statistical analysis.
Results
There were no group differences in age or handedness (Table 1). Groups differed in education and socioeconomic status, likely the effect of the disorder, but did not differ in parental socioeconomic status.
Table 1.
Sample characteristics
| Schizophrenic subjects (n = 9) | Control subjects (n = 9) | |
|---|---|---|
| Age | 39.7 ± 8.1 | 43.2 ± 5.0 |
| Education | 11.7 ± 2.1a | 16.1 ± 2.3 |
| Socioeconomic status (SES) | 4.3 ± 0.7b | 2.0 ± 1.1 |
| Parental SES | 2.3 ± 1.3 | 2.9 ± 1.3 |
| Handedness | 0.82 ± 0.2 | 0.71 ± 0.2 |
| Score of Mini Mental status | 28.8 ± 1.2 | 29.4 ± 0.7 |
| Verbal IQ | 84.2 ± 14.0c | 109.0 ± 9.5 |
| Age of onset | 22.1 ± 2.9 | — |
| Chlorpromazine equivalent of neuroleptic dose | 591.7 ± 329.4 | — |
t(16) = 4.4; P < 0.001.
t(16) = 5.6; P < 0.001.
t(16) = 4.6; P < 0.001.
Behavioral results
There were no statistical differences between groups in task accuracy during encoding of either the behavioral (t(16) = 0.7, P = 0.49) or functional (t(16) = 1.4, P = 0.18) experiments. All subjects reached 75% accuracy in encoding judgments and proceeded to the fMRI experiment. We excluded one control subject because of equipment failure (the computer did not record the subject's responses). Recognition accuracy did not differ between groups (t(16) = 1.31, P = 0.21, for deeply or t(16) = 1.3, P = 0.22, for shallowly encoded words). Subjects in both groups recognized more words seen under the deep encoding condition than the shallow encoding condition (controls, t(16) = 3.32, P = 0.016; schizophrenics, t(16) = 2.57, P = 0.04). Reaction times for new versus previously viewed words were compared; schizophrenics did not show a reduction in reaction times under either of the encoding conditions (despite more words remembered when deeply encoded) (deep, t(16) = 0.93, P = 0.38; shallow, t(16) = 0.15; P = 0.17). Control subjects, on the other hand, did show repetition priming effects (decreased reaction time for both deeply and shallowly encoded words); (deep, t(16) = 2.4; P = 0.044; shallow, t(16) = 3.15, P = 0.014).
FMRI results
Control subjects
For the semantic (deep) encoding condition, control subjects showed activation in several brain regions, including left (x = −52, y = 26, z = 16; z = 3.62) and right (42, 22, −14; z = 3.78) inferior frontal gyri, right superior frontal gyrus (46, 8, 44; z = 4.65), anterior cingulate gyri bilaterally (0, 18, 44; z = 3.93), and occipital lobes bilaterally (−22, −94, −4, z = 4.65; and 36, −84, −6, z = 3.85). For the nonsemantic (shallow) encoding condition, control subjects showed activation in the cingulate gyri (−4, 10, 50; z = 4.44) and occipital lobes (−26, −88, −14, z = 4.62; and 30, −86, −8; z = 3.97), but not in the frontal lobes. The inferior frontal gyrus (bilaterally) showed increased activity under the semantic compared to the nonsemantic encoding condition (left, −48, 46, −8, z = 4.64; and right, 40, 22, −18; z = 4.86; see Fig. 2). In addition, the LIPC showed priming-related decreases of activity for repeated words under the semantic (−50, 38, −14; z = 4.33; see Fig. 3), but not the nonsemantic condition. (Reaction times acquired subsequently were also decreased for repeated words under the semantic condition.)
Fig. 2.
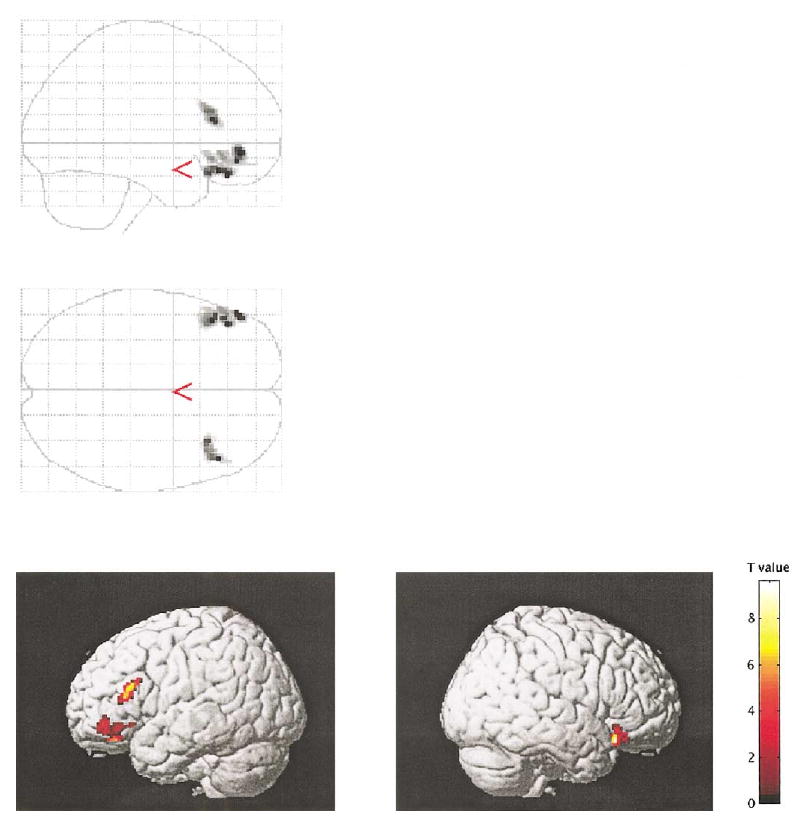
The figure illustrates the increased activation in semantic relative to nonsemantic encoding in control subjects. Only significant clusters (P < 0.05 corrected for cluster size) are displayed.
Fig. 3.
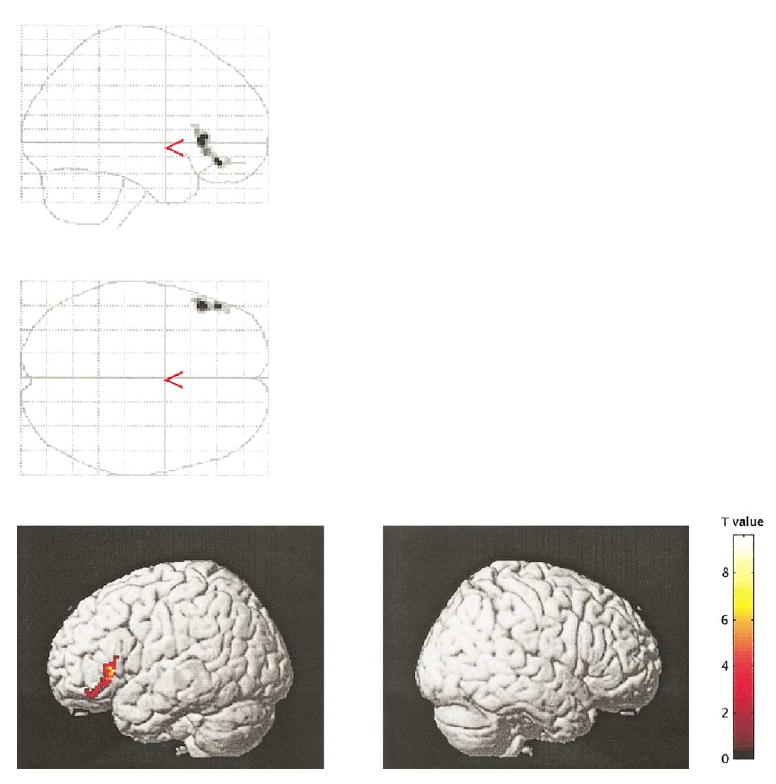
The figure demonstrates the repetition priming effect (priming-related decrease in activity). Only significant clusters (P < 0.05 corrected for cluster size) are displayed.
Schizophrenic subjects
For the semantic encoding condition, schizophrenic subjects showed activation in the left inferior frontal gyrus (−52, 14, −12; z = 3.82), left (−46, 12, 32; z = 4.64) and right (56, 18, 28; z = 4.71) middle frontal gyrus, left posterior superior temporal gyrus (−62, −16, 22; z = 5.43), left parietal lobe (−26, −68, 48; z = 3.60), cingulate gyri (0, 18, 48; z = 3.91), and occipital lobes bilaterally (−40, −70, −28, z = 4.94; and 30, −66, −28; z = 5.06). For the nonsemantic encoding condition, schizophrenic patients showed activation in the same areas as they did under the semantic condition, with no statistically significant differences between activation under the semantic and nonsemantic conditions. In addition, the LIPC did not show decreased activity with repetition priming for either semantically or nonsemantically encoded words. Subsequent statistical analyses with regard to semantic versus nonsemantic condition differences indicated that schizophrenics, when compared with control subjects, showed decreased activation in the left inferior frontal gyrus (46, 48, −10; z = 4.07) (Fig. 4) and increased activation in the left superior temporal gyrus (−54, −22, 8; z = 4.52) (Fig. 4).
Fig. 4.
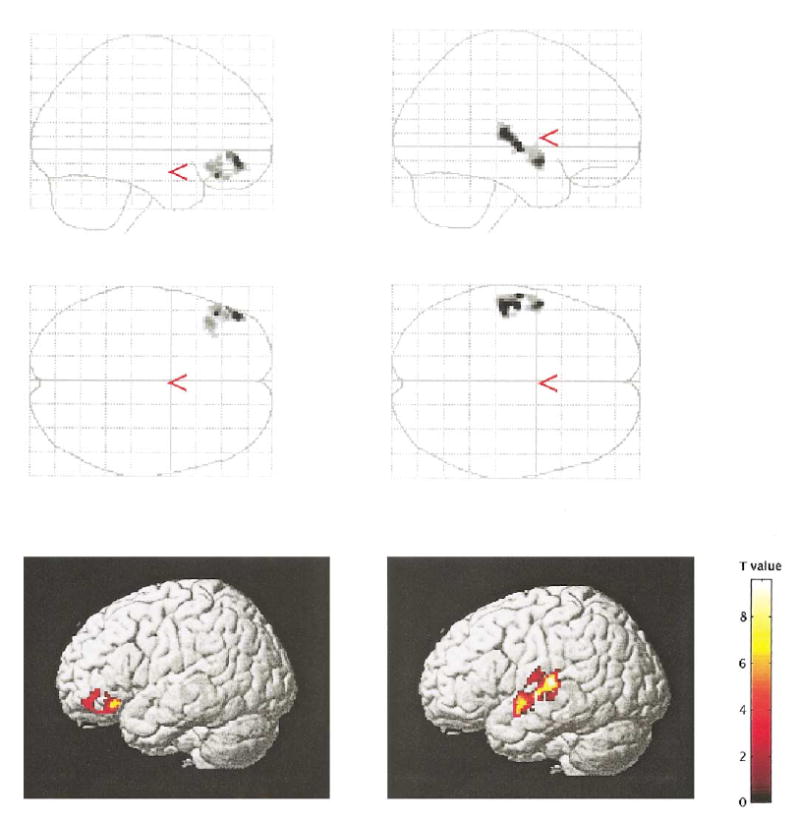
Semantic versus nonsemantic encoding: left side, regions characterized by increased activation in control subjects relative to schizophrenia subjects; right side, regions characterized by increased activation in schizophrenia subjects relative to control subjects. Only significant clusters (P < 0.05 corrected for cluster size) are displayed.
Comparison of control and schizophrenic subjects
Additionally, to examine further the differences between controls and schizophrenics, we looked at the differences of activation separately for semantic and nonsemantic conditions across the groups. Direct comparison of the control group versus schizophrenics under the semantic condition revealed that schizophrenic subjects were characterized by statistically significant decreases in the activation in both inferior frontal areas (right, 52, 28, −2, z = 4.85; and left, −52, 28, 6; z = 4.13) (Fig. 5), but increased activation in the cingulate region (0, 10, 36; z = 3.80) as well as a cluster extending from the left STG to the left inferior parietal lobes (−54, −6, 18; z = 4.98) (Fig. 5).
Fig. 5.
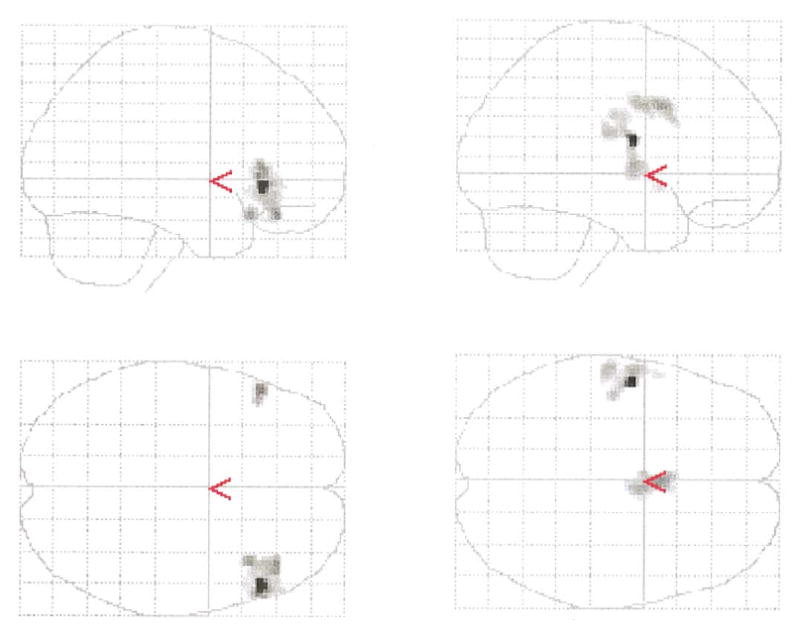
Semantic encoding condition: left side, regions characterized by increased activation in control subjects relative to schizophrenia subjects; right side, regions demonstrating increased activation in schizophrenia subjects relative to control subjects.
The correlation analysis revealed a significant relationship between increased activation within the left superior temporal region and positive symptoms under the shallow encoding condition in schizophrenia (52, 22, 12; z = 3.76) (Fig. 6). There were no relationships between the other tested clinical symptoms and studied regions for the three conditions.
Fig. 6.
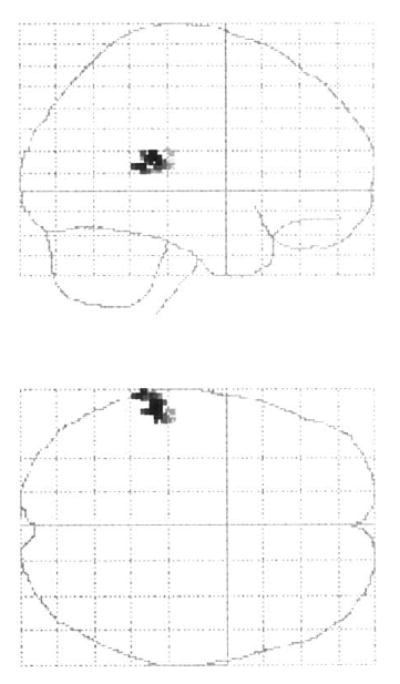
Voxels displaying a significant correlation between increased activation under the shallow encoding condition and positive symptoms in schizophrenic subjects (P < 0.01).
Discussion
The principal finding indicated that, in relation to control subjects, schizophrenic patients showed different patterns of fMRI activation for semantically encoded words. When asked to make semantic judgments of words, schizophrenic patients showed significantly reduced LIFC activation but significantly increased left superior temporal gyrus activation. Schizophrenic patients thus failed to show the increase in LIFC activation under the semantic versus nonsematic encoding condition demonstrated by control subjects. In fact, in the patients, LIFC as well as parietal, temporal, and cingulate areas were equally active under both deep (semantic) and shallow (perceptual) encoding conditions.
Several other studies have demonstrated that patients with schizophrenia show a similar abnormal pattern of brain activation across frontal and temporal lobes in response to verbal tasks. Although these studies most often have emphasized a disease-related LIFC reduction in activation in response to verbal task demands, overactivation in temporal and or parietal regions have also been reported in schizophrenia in many of the studies (Gur, 1978; Hazlett et al., 2000; Fletcher, 1998; Frith et al., 1995; Yurgelun-Todd et al., 1996; O'Leary et al., 1996). Left hemisphere dysfunction in schizophrenia has been hypothesized and documented for decades (see Gur, 1978, for a review). Gur (1978) showed in a series of behavioral studies that left hemisphere function was abnormal in the initial processing of verbal information in schizophrenia and that the abnormality was likely due to hyperactivation of the left hemisphere. More recent neuroimaging studies have confirmed earlier hypotheses and have shown temporal lobe hyperactivation during performance of a number of disparate tasks. Hazlett et al. (2000) found that patients with schizophrenia showed increased temporal lobe activation when using a serial ordering memory strategy. Similarly, Fletcher (1998) reported a relative failure of superior temporal and inferior parietal deactivation for patients with schizophrenia while performing a verbal memory task. This failure of deactivation was independent of task performance, suggesting to the authors that it might reflect a core feature of schizophrenia. They postulated that hyperactivation of temporal regions might have been related to an overelaboration of verbal information that is unconstrained in patients with schizophrenia. Whether this hyperactivation of semantic networks is intrinsic to the temporal lobe or whether it reflects a failure of LIFC-mediated cognitive control is unclear.
There is also evidence from imaging studies of both neurological and healthy subjects for the involvement of temporal/parietal regions in semantic processing. These include lesion studies of patients with aphasia (Hart and Gordon, 1990), semantic dementia (Hodges et al., 1992), and category-specific semantic impairments along with normal neuroimaging studies of category-specific representations. Taken together, these findings have demonstrated that semantic representations are stored and/or processed in a distributed manner in left temporal, posterior superior temporal, and inferior parietal regions (Binder, 1997; Hart and Gordon, 1990; Goodglass and Baker, 1976; Hodges et al., 1992). Overactivation of these regions may thus reflect neurobiological evidence of a disturbance in the functioning of widely distributed semantic networks, which would be consistent with independent lines of evidence from behavioral and neuroimaging studies of patients with schizophrenia.
Along with increased temporal lobe activation, schizophrenic patients also showed reduced LIFC activation for the semantic encoding of words. Neuroanatomic studies show that the LIPC in monkeys receives the largest input from posterior or temporal lobe regions that are thought to process higher order visual representations (Petrides and Pandya, 2002). A theory that is consistent with this organizational structure is that the ventrolateral prefrontal cortex is the site where information is initially received, held in working memory, and organized from posterior association areas (Petrides, 1994; D'Esposito and Postle, 2002). The dorsolateral prefrontal region (primarily the middle frontal gyrus) is then recruited when the information must be manipulated and possibly also for some maintenance purposes (D'Esposito and Postle, 2002).
Several studies have demonstrated reduced prefrontal activation for semantic tasks or tasks involving verbal material in patients with schizophrenia. For example, Ragland and colleagues (2001) showed reduced LIPC activation in patients for both word encoding and word recognition. Ragland et al. suggested that patients with schizophrenia process words on a more superficial level and do not spontaneously use semantic information to guide encoding and retrieval, a result that has been reported by others as well (e.g., Brebion et al., 1997). They also suggested that reduced LIFC activation might reflect impaired executive or strategic processes, as opposed to a selective impairment of lexical–semantic processing (see also Curtis et al., 1999). Other lines of evidence have also pointed to prefrontal cortex as especially important in exerting cognitive control and implementing extramnemonic executive control processes (D'Esposito et al., 2000; Miller and Cohen, 2001).
In healthy controls, the fMRI activation patterns observed using LOP tasks showed a remarkable consistency of results, consisting mainly of a greater left inferior prefrontal activation under deep encoding conditions that was associated with better memory at recall. Although it is generally agreed that there are two anatomically based subdivisions of LIPC, the precise functional role of these regions is still a matter of active debate. The more anterior portion of LIPC (BA 47/45) may be responsible for maintaining semantic information, while the more posterior/superior portion (BA 44/6) may be related to general word retrieval and/or phonological processing (Swick, 1998; Wagner et al., 1997; Thompson-Schill et al., 1997). Several alternatives for the function of anterior LIPC have been proposed, including controlled semantic retrieval (i.e., Wagner et al., 2001), semantic working memory (Petersen et al., 1988; Kapur et al., 1994; Demb et al., 1995; Buckner and Koutstaal, 1998; Gabrieli et al., 1998), and the selection of task-relevant representations among distractors (Thompson-Schill et al., 1997). For more comprehensive reviews of prefrontal function see D'Esposito et al. (2000), Miller and Cohen (2001), and Fletcher and Henson (2001). The prefrontal cortex may function in working memory by reactivating, or keeping active, those primary and association cortices in other cortical areas that are responsible for information storage. In line with these theoretical points of view, LIFC underactivation for semantically encoded words may reflect a disease-related failure to implement executive control over lexical material (see Ragland et al., 2001; Brebion et al., 1997). By contrast, the overactivation of the left superior temporal gyrus might reflect hyperactivation of semantic representations in schizophrenia. This would be consistent with both behavioral priming and N400 studies of subjects with schizophrenia (e.g., Niznikiewicz et al., 1997). Such semantically induced hyperactivation may reflect aberrant processes, such as excessively fast decay or excessively dominant, prepotent associates that prevail regardless of context. Moreover, not only might these regions become hyperactive to verbal stimuli, but we found that this aberrant hyperactivation may also be associated with positive clinical symptoms. Additional abnormalities may stem from reduced LIFC activation, resulting in a failure of modulation or executive control over posterior regions (Nestor et al., 1998).
In addition, since schizophrenics demonstrated more differential activation of the cingulate gyrus under deep versus shallow conditions than controls, increased effort as a possible source of the differentiation should be at least taken into account. Although studies demonstrate reduced anterior cingulate (AC) activity in patients during attention demanding tasks (Carter et al., 1997; Pantelis and Maruff, 2002; Rubia et al., 2001), studies investigating resting states in patients show increased activation in AC as well as STG, MTG, hippocampus, and the parahippocampal gyrus (Shergill et al., 2000; Silbersweig et al., 1995; Liddle et al., 1992). This suggests that the AC may be included in the complex circuitry commonly associated with schizophrenia pathophysiology and hyperactivation. Furthermore, given the role of the AC in intentional selection, it is possible that during simple tasks requiring minor recruitment of attention, such as the judgment tasks used in the present study, patients may need to overcompensate and provide an increased effort compared to normal control subjects.
Control subjects showed the expected repetition priming fMRI effect of decreased LIPC for repeated semantically encoded words. Schizophrenic subjects failed to show repetition priming related to decreased activity for repeated semantically encoded words. Demb et al. (1995) interpreted decreased LIPC activation as evidence that repeated semantic processing requires less neuronal activity relative to initial processing for healthy subjects. By reason of their failure to show repetition priming, patients with schizophrenia may require more neuronal activity in response to making semantic judgments during encoding. However, whether this activity-dependent change is mediated primary by prefrontal structures is not entirely clear, as similar repetition priming effects have been demonstrated in subjects with lesions of the left prefrontal cortex (Swick, 1998).
To summarize, persons with schizophrenia showed abnormal fMRI patterns of underactivation of LIFC and overactivation of left superior temporal gyrus for semantically encoded words. They did so despite demonstrating the depth of processing effect of better recognition for words encoded semantically than for words that were perceptually or superficially encoded. Schizophrenic patients also showed evidence of a reduced repetition priming effect, which likely reflects a more general failure to modulate brain activity in response to task demands, whether semantic or perceptual. A distributed network of frontal and temporal regions supports human thought, defined in part by a basic ability to associate representations with arbitrary symbols, from which the nature of word meanings or semantics is derived. Schizophrenia is classically described as a disorder of thought. The current findings therefore provide fMRI evidence that schizophrenia may disrupt the coordinated activity of such a distributed brain network that is engaged in the representation and processing of meaning of words, text, and discourse from which human thought emerges.
Acknowledgments
The authors thank Marie Fairbanks for her administrative assistance. Additionally, we gratefully acknowledge the support of the National Institute of Health (R01 MH 40799 to R.W.M. and K02 to M.E.S.), the Department of Veterans Affairs Merit Awards (R.W.M., M.E.S., P.G.N.), and the National Center for Research Resources (11747 to R.K.).
References
- Adams J, Faux SF, et al. ERP abnormalities during semantic processing in schizophrenia. Schizophr Res. 1993;10(3):247–257. doi: 10.1016/0920-9964(93)90059-r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry—the methods. NeuroImage. 2000;11(6 Pt 1):805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Baving L, Wagner M, et al. Increased semantic and repetition priming in schizophrenic patients. J Abnorm Psychol. 2001;110(1):67–75. doi: 10.1037//0021-843x.110.1.67. [DOI] [PubMed] [Google Scholar]
- Binder JR. Neuroanatomy of language processing studied with functional MRI. Clin Neurosci. 1997;4(2):87–94. [PubMed] [Google Scholar]
- Brebion G, Amador X, et al. Mechanisms underlying memory impairment in schizophrenia. Psychol Med. 1997;27(2):383–93. doi: 10.1017/s0033291796004448. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Koutstaal W. Functional neuroimaging studies of encoding, priming, and explicit memory retrieval. Proc Natl Acad Sci USA. 1998;95(3):891–898. doi: 10.1073/pnas.95.3.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Mintun M, et al. Anterior cingulate gyrus dysfunction and selective attention deficits in schizophrenia: [15O]H2O PET study during single-trial Stroop task performance. Am J Psychiatry. 1997;154(12):1670–1675. doi: 10.1176/ajp.154.12.1670. [DOI] [PubMed] [Google Scholar]
- Craik FI, Lockhart RS. Levels of processing: a framework for memory research. J Verbal Learning Verbal Behav. 1972;11:617–684. [Google Scholar]
- Curtis VA, Bullmore ET, et al. Attenuated frontal activation in schizophrenia may be task dependent. Schizophr Res. 1999;37(1):35–44. doi: 10.1016/s0920-9964(98)00141-8. [DOI] [PubMed] [Google Scholar]
- Demb JB, Desmond JE, et al. Semantic encoding and retrieval in the left inferior prefrontal cortex: a functional MRI study of task difficulty and process specificity. J Neurosci. 1995;15(9):5870–5878. doi: 10.1523/JNEUROSCI.15-09-05870.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Esposito M, Postle BR, et al. Prefrontal cortical contributions to working memory: evidence from event-related fMRI studies. Exp Brain Res. 2000;133(1):3–11. doi: 10.1007/s002210000395. [DOI] [PubMed] [Google Scholar]
- Fletcher P. The missing link: a failure of fronto-hippocampal integration in schizophrenia. Nat Neurosci. 1998;1(4):266–267. doi: 10.1038/1078. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Henson RN. Frontal lobes and human memory: insights from functional neuroimaging. Brain. 2001;124(Pt 5):849–881. doi: 10.1093/brain/124.5.849. [DOI] [PubMed] [Google Scholar]
- Friston K, Holmes A, et al. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp. 1995;2:189–210. [Google Scholar]
- Frith CD, Friston KJ, et al. Regional brain activity in chronic schizophrenic patients during the performance of a verbal fluency task. Br J Psychiatry. 1995;167(3):343–349. doi: 10.1192/bjp.167.3.343. [DOI] [PubMed] [Google Scholar]
- Gabrieli JD, Poldrack RA, et al. The role of left prefrontal cortex in language and memory. Proc Natl Acad Sci USA. 1998;95(3):906–913. doi: 10.1073/pnas.95.3.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodglass H, Baker E. Semantic field, naming, and auditory comprehension in aphasia. Brain Language. 1976;3(3):359–374. doi: 10.1016/0093-934x(76)90032-8. [DOI] [PubMed] [Google Scholar]
- Guillem F, Bicu M, et al. Memory impairment in schizophrenia: a study using event-related potentials in implicit and explicit tasks. Psychiatry Res. 2001;104(2):157–173. doi: 10.1016/s0165-1781(01)00305-5. [DOI] [PubMed] [Google Scholar]
- Gur RE. Left hemisphere dysfunction and left hemisphere overactivation in schizophrenia. J Abnorm Psychol. 1978;87:226–238. doi: 10.1037//0021-843x.87.2.226. [DOI] [PubMed] [Google Scholar]
- Hart J, Jr, Gordon B. Delineation of single-word semantic comprehension deficits in aphasia, with anatomical correlation. Ann Neurol. 1990;27(3):226–231. doi: 10.1002/ana.410270303. [DOI] [PubMed] [Google Scholar]
- Hazlett EA, Buchsbaum MS, et al. Hypofrontality in unmedicated schizophrenia patients studied with PET during performance of a serial verbal learning task. Schizophr Res. 2000;43(1):33–46. doi: 10.1016/s0920-9964(99)00178-4. [DOI] [PubMed] [Google Scholar]
- Heckers S, Rauch SL, et al. Impaired recruitment of the hippocampus during conscious recollection in schizophrenia. Nat Neurosci. 1998;1(4):318–323. doi: 10.1038/1137. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Patterson K, et al. Semantic dementia. Progressive fluent aphasia with temporal lobe atrophy. Brain. 1992;115(Pt 6):1783–1806. doi: 10.1093/brain/115.6.1783. [DOI] [PubMed] [Google Scholar]
- Kapur S, Rose R, et al. The role of the left prefrontal cortex in verbal processing: semantic processing or willed action. Neuroreport. 1994;5(16):2193–2196. doi: 10.1097/00001756-199410270-00051. [DOI] [PubMed] [Google Scholar]
- Liddle PF, Friston KJ, et al. Cerebral blood flow and mental processes in schizophrenia. J R Soc Med. 1992;85(4):224–227. doi: 10.1177/014107689208500415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathalon DH, Faustman WO, et al. N400 and automatic semantic processing abnormalities in patients with schizophrenia. Arch Gen Psychiatry. 2002;59(7):641–648. doi: 10.1001/archpsyc.59.7.641. [DOI] [PubMed] [Google Scholar]
- McCarley RW. Structural magnetic resonance imaging studies in schizophrenia. In: Davis KL, Charney D, Coyle J, Nemeroff C, editors. Neuropsychopharmacology: the fifth generation of progress. Lippincott, Williams, & Wilkins; Baltimore: 2001. [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Minzenberg MJ, Ober BA, et al. Semantic priming in schizophrenia: a review and synthesis. J Int Neuropsychol Soc. 2002;8(5):699–720. doi: 10.1017/s1355617702801357. [DOI] [PubMed] [Google Scholar]
- Nestor PG, Kimble MO, et al. Aberrant semantic activation in schizophrenia: a neurophysiological study. Am J Psychiatry. 1997;154(5):640–646. doi: 10.1176/ajp.154.5.640. [DOI] [PubMed] [Google Scholar]
- Nestor PG, Shenton ME, et al. A neuropsychological analysis of schizophrenic thought disorder. Schizophr Res. 1998;29(3):217–225. doi: 10.1016/s0920-9964(97)00101-1. [DOI] [PubMed] [Google Scholar]
- Niznikiewicz MA, O'Donnell BF, et al. ERP assessment of visual and auditory language processing in schizophrenia. J Abnorm Psychol. 1997;106(1):85–94. doi: 10.1037//0021-843x.106.1.85. [DOI] [PubMed] [Google Scholar]
- O'Leary DS, Andreasen NC, et al. Auditory attentional deficits in patients with schizophrenia. A positron emission tomography study. Arch Gen Psychiatry. 1996;53(7):633–41. doi: 10.1001/archpsyc.1996.01830070083013. [DOI] [PubMed] [Google Scholar]
- Pantelis C, Maruff P. The cognitive neuropsychiatric approach to investigating the neurobiology of schizophrenia and other disorders. J Psychosom Res. 2002;53(2):655–664. doi: 10.1016/s0022-3999(02)00434-8. [DOI] [PubMed] [Google Scholar]
- Petersen SE, Fox PT, et al. Positron emission tomographic studies of the cortical anatomy of single-word processing. Nature. 1988;331(6157):585–589. doi: 10.1038/331585a0. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Comparative cytoarchitectonic analysis of the human and the macaque ventrolateral prefrontal cortex and corticocortical connection patterns in the monkey. Eur J Neurosci. 2002;16(2):291–310. doi: 10.1046/j.1460-9568.2001.02090.x. [DOI] [PubMed] [Google Scholar]
- Ragland JD, Gur RC, et al. Effect of schizophrenia on frontotemporal activity during word encoding and recognition: a PET cerebral blood flow study. Am J Psychiatry. 2001;158(7):1114–1125. doi: 10.1176/appi.ajp.158.7.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K, Russell T, et al. An fMRI study of reduced left prefrontal activation in schizophrenia during normal inhibitory function. Schizophr Res. 2001;52(1–2):47–55. doi: 10.1016/s0920-9964(00)00173-0. [DOI] [PubMed] [Google Scholar]
- Saykin AJ, Gur RC, et al. Neuropsychological function in schizophrenia: selective impairment in memory and learning. Arch Gen Psychiatry. 1991;48(7):618–624. doi: 10.1001/archpsyc.1991.01810310036007. [DOI] [PubMed] [Google Scholar]
- Shenton ME, Dickey CC, et al. A review of MRI findings in schizophrenia. Schizophr Res. 2001;49(1–2):1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shergill SS, Brammer MJ, et al. Mapping auditory hallucinations in schizophrenia using functional magnetic resonance imaging. Arch Gen Psychiatry. 2000;57(11):1033–1038. doi: 10.1001/archpsyc.57.11.1033. [DOI] [PubMed] [Google Scholar]
- Silbersweig DA, Stern E, et al. A functional neuroanatomy of hallucinations in schizophrenia. Nature. 1995;378(6553):176–179. doi: 10.1038/378176a0. [DOI] [PubMed] [Google Scholar]
- Swick D. Effects of prefrontal lesions on lexical processing and repetition priming: an ERP study. Brain Res Cogn Brain Res. 1998;7(2):143–57. doi: 10.1016/s0926-6410(98)00019-6. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, Esposito MD, et al. Role of left inferior prefrontal cortex in retrieval of semantic knowledge: a reevaluation. Proc Natl Acad Sci USA. 1997;94(26):14792–14797. doi: 10.1073/pnas.94.26.14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AD, Gabrieli JD, et al. Dissociations between familiarity processes in explicit recognition and implicit perceptual memory. J Exp Psychol Learn Mem Cogn. 1997;23(2):305–323. doi: 10.1037//0278-7393.23.2.305. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Pare-Blagoev EJ, et al. Recovering meaning: left prefrontal cortex guides controlled semantic retrieval. Neuron. 2001;31(2):329–338. doi: 10.1016/s0896-6273(01)00359-2. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, Berman KF, et al. Evidence of dysfunction of a prefrontal-limbic network in schizophrenia: a magnetic resonance imaging and regional cerebral blood flow study of discordant monozygotic twins. Am J Psychiatry. 1992;149(7):890–897. doi: 10.1176/ajp.149.7.890. [DOI] [PubMed] [Google Scholar]
- Wright IC, Ellison ZR, et al. Mapping of grey matter changes in schizophrenia. Schizophr Res. 1999;35(1):1–14. doi: 10.1016/s0920-9964(98)00094-2. [DOI] [PubMed] [Google Scholar]
- Yurgelun-Todd DA, Waternaux CM, et al. Functional magnetic resonance imaging of schizophrenic patients and comparison subjects during word production. Am J Psychiatry. 1996;153(2):200–205. doi: 10.1176/ajp.153.2.200. [DOI] [PubMed] [Google Scholar]


