Summary
The elderly population with chronic kidney disease (CKD) is at greater risk for cardiovascular disease than from an independent risk of CKD, as well as from added dyslipidemia of aging and renal dysfunction. Changes in lipid metabolism with more isodense and high-dense, triglyceride-rich particles, low high-density lipoprotein cholesterol, and increased triglyceride levels occur with CKD and aging, which are noted to have significant atherogenic potential. In addition, lipid abnormalities may lead to the progression of CKD. Cardiovascular mortality in the end-stage renal disease population is more than 10 times higher than the general population. Treatment of dyslipidemia in the general population suggests important benefits both in reducing cardiovascular risk and in the prevention of cardiovascular disease. Secondary analyses of elderly subgroups of various large prospective studies with statins suggest treatment benefit with statin use in the elderly. Similarly limited data from secondary analyses of CKD subgroups of larger prospective trials using statins also suggest a possible benefit in cardiovascular outcomes and the progression of kidney disease. However, randomized trials have yet to confirm similar benefits and targets of treatment for dyslipidemia in the elderly with CKD and end-stage renal disease. Treatment in the elderly with CKD should be individualized and outweigh risks of side effects and drug–drug interactions. There is a need for further specific investigation of dyslipidemia of CKD in the aging population in relation to renal disease progression and cardiovascular outcome.
Keywords: Lipid disorder, elderly, chronic kidney disease, end-stage renal disease, dyslipidemia
As the incidence of chronic kidney disease (CKD) increases with an aging population, understanding abnormalities in lipid metabolism become central given the increased risk for poor cardiovascular outcome in this population.1,2 Cardiovascular deaths are 10 to 30 times greater in patients requiring dialysis than similarly aged patients in the general population.3 The majority of patients with CKD also are older, with the US Renal Data System reporting a median age of 64.6 years for incident end-stage renal disease (ESRD), with one-fourth being older than 75 years.4 When comparing cardiovascular risk in patients with CKD with those without CKD in community dwellers of the Framingham study, the mean age of 70 ± 8 years was significantly greater in those without CKD (60 ± 9 y; P < .001).5 An atherogenic lipoprotein phenotype leads to increased triglyceride levels, increased total cholesterol (TC), low high-density lipoprotein cholesterol (HDLc), and often normal to low low-density lipoprotein cholesterol (LDLc), frequently described in patients with CKD and ESRD. The role of how this phenotype and serum lipids enhance atherogenicity in the complex CKD patient frequently plagued with diabetes, hypertension, abnormal mineral metabolism, and a propensity for chronic inflammation is being investigated actively. Although observational and post hoc analyses of clinical studies have suggested that treating with lipid-lowering agents may decrease the progression of CKD,6-10 strict LDLc lowering in the highest-risk ESRD diabetic patient did not change cardiovascular mortality in this group of patients.11 These data obligate a closer examination of the lipoprotein metabolism and serum lipids in elderly CKD patients.
CHARACTERISTICS OF CKD DYSLIPIDEMIA
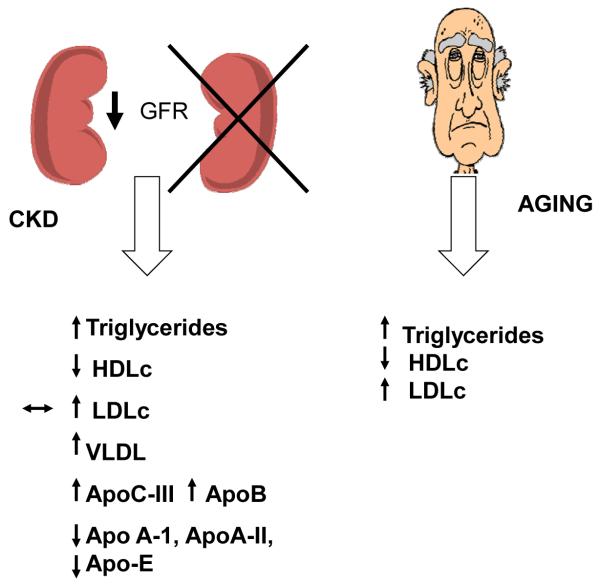
With the progression of renal disease, there is notable change in lipoprotein metabolism and serum lipid levels.12,13 Increased triglyceride levels and low HDLc levels with only mild increase or normal or low LDLc levels are noted in patients with CKD and ESRD. Low levels of apoA-I, apo A-II, and apo-E as well as increases in apoB and apoC-III concentrations often precede abnormalities in serum lipids13-15 (Fig 1). Increases in apoC-III, increased VLDL, and low HDLc levels are found even in patients without hyperlipidemia. Accumulation of smaller, more isodense VLDL remnants results from impaired plasma clearance. As renal function deteriorates, qualitative changes of serum lipids also are seen. VLDL cholesterol content increases whereas VLDL triglyceride content decreases. The opposite occurs with LDL and HDL, with cholesterol concentration decreasing in these particles and triglyceride content increasing, suggesting a redistribution of cholesterol from HDL to VLDL and isodense VLDL, and ineffective removal of triglycerides from LDL and HDL particles.14 Low hepatic triglyceride lipase,16 decreased lipoprotein lipase, increased cholesterol ester transfer protein, in addition to decreased receptor number for these proteins, as well as altered lipoprotein substrates for the receptors in patients with CKD, contribute to the change in lipoprotein metabolism.14,17 In addition, a higher HDL catabolic rate also is seen in dialysis patients, leading to low HDLc levels. Furthermore, decreased lecithin cholesterol acyl transferase in CKD patients leads to cholesterol ester–poor and triglyceride-rich HDL(3) and pre-β-HDL, which become less-effective antioxidative agents.15 These findings were similar in diabetic patients on hemodialysis compared with those patients not on dialysis.18,19
Figure 1.
Lipid abnormalities found with CKD and with aging
Increased lipid peroxidation also may contribute to dyslipidemia of CKD and is exacerbated in the diabetic patient.17 Patients on hemodialysis are noted to have higher markers of oxidative stress,20 including oxidized glutathione and advanced glycation end-products.21-23 Antibody titers to oxidized LDL are increased in patients on hemodialysis compared with similarly aged controls in the general population.24 In addition, low antioxidant levels in hemodialysis patients and higher levels of oxidant stress including advanced glycation end-products and malondialdehyde appears to predict carotid intimamedia thickness.19 Therefore, although quantitative levels of serum lipids may not be increased markedly in CKD, qualitative changes in lipoproteins and serum lipids that occur with progression of renal disease may predispose to increased endothelial abnormalities and subsequent vascular disease.
PERSPECTIVES FROM ANIMAL STUDIES
Animal studies have indicated that the abnormal lipid metabolism in CKD and aging are mediated by altered expression of a number of transcriptional factors and nuclear hormone receptors. In animal models of CKD there is evidence for increased expression of the sterol regulatory element binding protein-1 (SREBP-1) and SREBP-2 in the liver and in the adipose tissue. SREBP-1 and SREBP-2 are master regulators of fatty acid, triglyceride, and cholesterol synthesis and therefore mediate increased serum lipids as well as insulin resistance.25-27 In addition, there also is decreased expression of peroxisome proliferator activated receptor-α, which results in impaired fatty acid oxidation.28 Similar changes also occur in the liver and kidneys of animals with the nephrotic syndrome29-31 and in aging.32-34 Recent studies have indicated that there is decreased expression and activity of the farnesoid X receptor in the liver of aging mice.35 The farnesoid X receptor is a bile acid–activated nuclear hormone receptor that plays an important role in the regulation of bile acid, fatty acid, cholesterol, and glucose metabolism in the liver and in the kidney.36,37 Decreased farnesoid X receptor activity also occurs in the liver and kidney of diabetic animals82 and may mediate the increased expression and activity of SREBP-1 and decreased expression and activity of peroxisome proliferator activated receptor-α.
Caloric restriction is known to prevent age-related alterations in metabolic function and several studies have shown that caloric restriction prevents the age-related increased expression of SREBP-1 and a decrease in peroxisome proliferator activated receptors.38-41 One important mediator of the metabolic effects of caloric restriction is the increased expression of the sirtuin SIRT1, a nicotinamide adenine dinucleotide–dependent deacetylase enzyme. There is now increasing evidence that sirtuin analogues replicate several of the beneficial effects of caloric restriction including adipogenesis, insulin sensitivity and signaling, and lipid metabolism,42-45 and SIRT1 transgenic mice reveal phenotypes resembling caloric restriction.46 Studies in animal models thus continue to be useful in understanding the complex changes in lipid metabolism in aging and CKD.
CKD IN THE ELDERLY
With increasing age, there is also an increase in the prevalence of CKD. The US National Health and Nutrition Examination Survey from 1999 to 2004 estimated that more than a third of adults older than 70 years age have moderate CKD.47 A cross-sectional evaluation of 9,806 older adults in Germany between ages 50 and 74 years who presented for a general health check-up found that 17.4% of subjects had CKD. The prevalence of stages 1 and 2 was 4.6% and 4.7%, respectively, whereas 17.4% of subjects had CKD stage 3 or higher. In this group, nearly 24% of adults with CKD were between the ages of 70 and 74 years.48 When the estimated glomerular filtration rate (eGFR) of 15,536 patients 75 years and older was calculated using serum creatinine and the Modification of Diet in Renal Disease (MDRD) formula from 53 general practices in Great Britain, the prevalence of CKD was noted to be 56.1% for an eGFR of less than 60, 17.7% for an eGFR of less than 45, and 2.7% for an eGFR of less than 30 mL/min.49 Although estimations of GFR in each of these studies may vary, findings that there is a significant prevalence of CKD in the elderly appear consistent. Furthermore, mortality risk appears to increase significantly with decreasing clearance in the elderly.50 When adjusted for covariates including demographics, physical activity, comorbidities, proteinuria, and inflammatory parameters, elderly community subjects followed up for more than 6 years in Europe with a 24-hour creatinine clearance of 60 to 90 mL/min/1.73 m2 had a hazard ratio of 1.70 (95% confidence interval [CI], 1.02-2.83) for mortality, which was even greater in those with clearances less than 60 mL/min/1.73 m2 and hazard ratios of 1.91 (95% CI, 1.11-3.29). The use of the Cockcroft-Gault equation and particularly the MDRD equation to estimate clearances were less prognostic for mortality outcome for clearances between 60 and 90 mL/min (see Pizzarelli et al50).
The question of CKD as an independent risk for poor cardiovascular outcome remains under debate given conflicting outcomes of various observational studies with limited numbers of patients.51 In the larger longitudinal community-based sample (1,120,295 adults) from Kaiser Permanente of Northern California, the prevalence of traditional risk factors was higher in those with a lower GFR. With covariate adjustment of traditional risk factors, proteinuria, serum albumin, and co-existing illness, the relative risk for adverse outcome and all-cause mortality increased with a graded decline of renal function, which remained robust in men, women, older patients, younger patients, as well as in those with and without diabetes.52,53 A progressive rapid decrease in renal function in older community dwellers of the longitudinal Cardiovascular Health Study with a mean follow-up period of 9.9 years was associated with an increased cardiovascular risk and all-cause mortality.54 In this same population, the presence of CKD alone (eGFR < 60 mL/min) in the elderly conferred a 10-year risk for cardiovascular mortality similar to those having a history of pre-existing myocardial infarction or diabetes.55 Thus, declining renal function in the elderly may increase the burden of cardiovascular risk.
DYSLIPIDEMIA WITH INCREASING AGE
Approximately 33% of elderly men and 50% of elderly women have a total cholesterol level greater than 240.56 A greater prevalence of metabolic syndrome including central obesity with insulin resistance, increased blood pressure, and dyslipidemia characterized by increased triglyceride levels, small high dense LDL, and a low concentration of HDL is being noted in older adults.57,58 With a higher prevalence of cardiovascular events in older individuals, dyslipidemia poses a greater attributable risk in this population, although some studies have noted little relationship with coronary heart disease in patients older than 70 years.59 Because protective HDL cholesterol decreases in men and women with aging, the gap for incidence for coronary disease between the sexes narrows. Per US National Health and Nutritional Examination Survey data, approximately 50% of adults older than 65 years are eligible for dietary intervention according to the National Cholesterol Education Program Adult Treatment II guidelines.59,60 Noninvasive testing by ankle-brachial index and carotid intima media thickness as well as echocardiography and electrocardiography in the Cardiovascular Health Study was predictive of cardiac events in the elderly compared with traditional risk markers.59,61 Given a qualitative shift of serum lipoproteins to a more atherogenic phenotype with CKD, the likelihood of an added burden to underlying cardiovascular risk may exist in the elderly with CKD.
DYSLIPIDEMIA AND PROGRESSION OF CKD
Although experimental data support a role for dyslipidemia in the progression of renal disease,62,63 the data in human beings extends primarily from observational studies. High triglyceride levels and low HDL as seen in CKD predicted an increase in the risk of renal dysfunction when participants of the Atherosclerosis Risk in Communities were followed up for approximately 3 years.64 Similarly, a low HDL and high LDL/HDL cholesterol ratio suggested a greater risk for an increase in serum creatinine or an increase in the development of stage 3 CKD (glomerular filtration < 60 mL/min) for individuals followed up over time in the Physicians Health Study and the Framingham Offspring Study.6,65,66 Samuelsson et al8 observed a strong association between plasma triglyceride-rich apoB containing lipoproteins and the rate of decline in GFR. This association also was evident for participants with moderate to severe kidney disease in the MDRD study.7 These data suggest then that lipid lowering may possibly benefit patients with CKD by slowing the rate of progression.
In fact, post hoc subgroup analysis of some prospective trials using 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors (statins) to treat hyperlipidemia suggest a slowing in decline of renal function and proteinuria, although this was not observed uniformly for all statin trials. Pooled data of 18,569 participants from 3 randomized double-blind controlled trials comparing pravastatin 40 mg/d and placebo, of whom 3,402 had an eGFR of 30 to 59.9 mL/min/1.73 m2, showed that pravastatin caused an absolute reduction in the rate of loss of eGFR of 0.22 mL/min/1.73 m2 by MDRD (95% CI, 0.07-0.37).10 Meta-analysis of several randomized controlled trials also showed that 1.2 mL/min slower decline in GFR occurred in statin-treated patients. A reduction in proteinuria also was observed.67,68 This may be owing to the beneficial effect of statins on vessel stiffening and endothelial function from its anti-inflammatory effect. However, the beneficial effect to decreasing urine protein with statin treatment has not been uniform.69 Furthermore, renal function remained unchanged in those patients randomized to fluvastatin therapy for the prevention of cardiac events and declined when pooled analysis of 10,000 hyperlipidemic patients using rosuvastatin (5-40 mg) was performed. Long-term use of rosuvastatin, however, suggested either no change or an increase in GFR.70 With the paucity of randomized trials specifically addressing the effect of hyperlipidemia on renal disease progression and the presence of only conflicting observational data, definitive conclusions cannot be drawn. The use of statins in the elderly for renoprotection alone remains premature at this time.
LIPID LOWERING AND CARDIOVASCULAR OUTCOMES IN THE ELDERLY
Secondary prevention trials using LDLc-lowering therapies have shown a benefit of lipid lowering on both cardiac events and all-cause mortality, with limited data available in older subjects. However, evidence from subgroup analysis of older patients from lipid-lowering trials suggests a similar benefit in cardiovascular outcomes in the elderly compared with that seen in younger subjects (Table 1).
Table 1.
Lipid Lowering With Statins on Cardiovascular Outcomes in the Elderly
| Clinical Trial |
Elderly in Subgroup, n |
Subgroup age, y |
Medication used/dosage |
Outcome | Follow-up period |
|---|---|---|---|---|---|
| LIPID | 3,514 | 65-75 | Pravastatin 40 mg versus placebo |
Statin decreased the risk of all CVD events and all-cause mortality |
6 y |
| PROSPER | 5,804 | 70-82 | Pravastatin 40 mg versus placebo |
Statin decreased LDLc by 34% and decreased CHD and nonfatal MI |
3 y |
| CARE | 1,283 | 65-75 | Pravastatin 40 mg versus placebo |
Clinically significant risk reduction for cardiovascular and cerebrovascular events |
5 y |
| 4S | 1,021 | ≥65 | Simvastatin 20-40 mg |
Simvastatin decreased relative risk for CHD by 43% and also reduced all-cause and CHD mortality |
5.4 y |
| SAGE | 893 | 65-85 | Atorvastatin 80 mg versus pravastatin 40 mg |
Decrease in ischemia duration in both groups, high-dose group improved cardiovascular events and all-cause mortality |
12 mo |
| CARDS | 1,129 | 65-75 | Atorvastatin 10 mg |
Relative risk reduction of 38% in first major cardiovascular event |
3.9 y |
| TNT | 3,809 | ≥65 | Atorvastatin 10 versus 80 mg |
80-mg group showed a decrease in absolute risk of major cardiovascular events of 2.3% and relative risk of 19% |
4.9 y |
Abbreviations: LIPID, Long-Term Intervention with Pravastatin in Ischemic Disease; CVD, cardiovascular disease; PROSPER, Prospective Study of Pravastatin in the Elderly at Risk; MI, myocardial infarction; CARE, Cholesterol and Recurrent Events; 4S, Scandinavian Simvastatin Survival Study; SAGE, Study Assessing Goals in the Elderly; CARDS, Collaborative Atorvastatin Diabetes Study; TNT, Treating to New Targets.
Several trials evaluating the use of pravastatin compared with placebo have noted a reduced cardiovascular risk in the elderly. In the Long-Term Intervention with Pravastatin in Ischemic Disease study, 3,514 patients aged 65 to 75 years with previous myocardial infarction or unstable angina were randomized to either treatment with pravastatin 40 mg/d or placebo. Pravastatin use reduced the risk for all cardiovascular disease events, with similar relative effects observed in both older and younger patients. Because older patients have greater risk than younger patients for cardiovascular events, the absolute benefit of treatment was significantly more important in older patients.71 Another randomized trial compared pravastatin 40 mg with placebo in a slightly larger group of 5,804 older men and women, ages 70 to 82 years, with a known history or risk of vascular disease. The Prospective Study of Pravastatin in the Elderly at Risk evaluated a composite of coronary death, nonfatal myocardial infarction, and fatal or nonfatal stroke over an average of 3 years of follow-up evaluation. Pravastatin lowered the LDL cholesterol by 34%, with lower numbers of coronary heart disease death and nonfatal myocardial infarction. There was no observed difference in all-cause mortality or risk of stroke. However, a more frequent diagnosis for new cancer was reported for those treated with pravastatin. A subsequent meta-analysis of trials using any statin revealed no increase in overall cancer risk with statins. Based on these findings, a strategy of managing vascular risk in the middle-aged population was extended to the elderly.72 A subset analysis of the Cholesterol and Recurrent Events trial included 1,283 patients aged 65 to 75 years with myocardial infarction, a total plasma cholesterol level of 208 mg/dL, and a LDL of 138 mg/dL, given pravastatin 40 mg/d or placebo, suggested clinically important risk reduction in the risk for major cardiac events and stroke with pravastatin use. It was estimated that for every 1,000 older patients treated, 225 cardiovascular hospitalizations would be prevented compared with 121 hospitalizations in 1,000 younger patients.73
Other statins such as simvastatin use in the Scandinavian Simvastatin Survival Study, and atorvastatin use in the Study Assessing Goals in the Elderly, Collaborative Atorvastatin Diabetes Study, and Treating to New Targets study also suggested favorable risk for statin use in the elderly to decrease cardiovascular events and mortality. The Scandinavian Simvastatin Survival Study trial provided a post hoc assessment of the efficacy and safety of simvastatin and included 1,021 patients age 65 years or older who had angina or a prior myocardial infarction. Mean changes in serum lipid levels were similar in the older compared with the younger group. Relative risk for coronary heart disease (CHD) mortality in patients older than age 65 years decreased by 43% in the simvastatin group. The study showed significant risk reduction for both all-cause and CHD mortality in patients older than 65 years of age.74 That older men and women with coronary artery disease benefit from intensive statin therapy has been suggested by the Study Assessing Goals in the Elderly trial. This trial randomized 893 patients with coronary artery disease who were 65 to 85 years of age to atorvastatin 80 mg/d or pravastatin 40 mg/d. The primary end point, absolute change from baseline in total duration of ischemia at 12 months, was reduced significantly in both groups. However, the intensive therapy group had a trend toward fewer major acute cardiovascular events (hazard ratio, 0.71; 95% CI, 0.46-1.09) and a significantly greater reduction in all-cause mortality (hazard ratio, 0.33; 95% CI, 0.13-0.83).75 Further evidence showing benefit of lipid lowering with statin therapy in elderly subjects comes from post hoc analysis of the Collaborative Atorvastatin Diabetes Study. The efficacy and safety of atorvastatin was compared among 1,229 patients aged 65 to 75 years at randomization with 1,709 younger patients. Atorvastatin 10 mg/d resulted in a 38% reduction in the relative risk of the first major cardiovascular events in older patients and a 37% reduction in younger patients.76 To assess the efficacy and safety of high-dose atorvastatin in patients age 65 years and older a secondary analysis of 3,809 of 10,001 patients with coronary heart disease and a LDL level less than 130 mg/dL in the Treating to New Targets study was performed. Patients were assigned randomly to atorvastatin, 10 or 80 mg/d. In patients 65 years of age or older, absolute risk was reduced by 2.3% and relative risk was reduced by 19% for major cardiovascular events in favor of the high-dose atorvastatin group.77
Thus, evidence from major clinical trials that studied the effect of statins in patients with CHD show a clear beneficial effect in younger individuals, and subsequent subgroup analysis of elderly patients also showed reductions in all-cause mortality, in major CHD events, and in the number of revascularization procedures. Intensive statin therapy in patient groups at risk for myocardial infarction also reduces the risk of death, risk of myocardial infarction and unstable angina, and the need for revascularization procedures in elderly patients. Furthermore, evaluation of intensive lipid lowering has confirmed the benefits of statins in the elderly population.78,79 However, the benefits of treatment need to be weighed carefully against risks in the elderly. Elderly patients often take multiple medications and are at significant risk of drug–drug interactions. Several available statin medications, with the exception of atorvastatin and fluvastatin, are metabolized by cytochrome P450 3A4 and therefore can interact with commonly used medications such as amiodarone, macrolide antibiotics, calcium channel blockers, fibric acid derivatives, and cyclosporine. These interactions can result in an increased frequency of statin-related hepatotoxicity and myopathy. Therefore, dose adjustment is necessary with creatinine clearances of less than 30 mL/min for statins other than atorvastatin and fluvastatin. In these cases hydrophilic statins such as pravastatin may be preferred to lipophilic statins.80 Dietary modifications may be helpful for the treatment of hypertriglyceridemia in the elderly with CKD. Special consideration, however, should be given to the patients who are at increased risk for malnutrition, including patients with dementia and physical inabilities, to avoid exacerbating malnutrition.
LIPID LOWERING IN CKD AND ESRD
Although changes in lipoprotein metabolism and serum lipids occur with increasing renal dysfunction, data suggesting a benefit of lipid-lowering agents, principally statins, among patients with CKD, remain limited (Table 2). A subgroup analysis of 1,329 CKD patients in the Heart Protection Study, including patients with a creatinine level from 1.3 to 2.3 mg/dL over 5 years’ duration, showed a relative risk reduction of 28% (95% CI, 0.75-0.85; P = .05) with simvastatin use of 40 mg/d.81 The multicenter, placebo-controlled Assessment of Lescol in Renal Transplant trial enrolled 2,102 patients and examined the effect of fluvastatin 40 to 80 mg/d versus placebo in renal transplant recipients receiving immunosuppressive therapy with cyclosporine who had mild to moderate increases in LDL cholesterol levels. Patients had a mean serum creatinine level of 1.6 mg/dL and the study duration was 5 years. By using cardiac death and nonfatal myocardial infarction as the primary end point, this study showed a statistically significant risk reduction of 35% with fluvastatin use.82 Again, a subset analysis of the Cholesterol and Recurrent Events trial was performed on 1,711 participants with CKD. After a median follow-up period of 58.9 months, the incidence of the primary end point (death from coronary disease or symptomatic nonfatal myocardial infarction) was lower in participants receiving pravastatin 40 mg than in those receiving placebo.83 When 864 patients with microalbuminuria were randomized to either fosinopril 20 mg/d, pravastatin 40 mg/d, or matching placebo over 4 years in the Prevention of Renal and Vascular End Stage Disease Intervention Trial, a 13% nonsignificant reduction in cardiovascular mortality and hospitalization for cardiovascular morbidity was observed.69 Fibric acid derivatives are effective in lowering triglyceride levels in patients with severe hypertriglyceridemia, however, these medications are associated with an increased risk of rhabdomyolysis in patients with renal insufficiency.84 Thus, they need to be monitored carefully because the risk may exceed the potential benefit.
Table 2.
Lipid Lowering on Cardiovascular Outcomes in CKD/ESRD
| Clinical Trial |
CKD in Subgroup, n |
Serum Creatinine Level, mg/dL |
Medication Used/Dosage |
Outcome | Follow- Up Period, y |
|---|---|---|---|---|---|
| HPS | 1,329 | 1.5-2.3 | Simvastatin 40 mg |
Cardiovascular relative risk reduction of 28% |
5 |
| ALERT | 2,102 | Mean, 1.6 | Fluvastatin 40-80 mg versus placebo |
35% risk reduction in cardiac death and nonfatal MI with fluvastatin |
5 |
| CARE | 1,711 | Mean, 1.0-1.4 | Pravastatin 40 mg versus placebo |
Decreased incidence of CHD nonfatal MI |
Median, 4.9 |
| PREVEND IT |
864 | Mean, 0.8-1.2 + microalbuminuria |
Fosinopril 20 mg/pravastatin 40 mg versus placebo |
13% reduction— not significant in cardiovascular mortality and hospitalization |
4 |
| 4D | 1,255 patients in trial |
ESRD | Atorvastatin 20 mg versus placebo |
No change in cardiovascular mortality |
Median, 4 |
Abbreviations: HPS, xxxxx; ALERT, Assessment of Lescol in Renal Transplant; MI, myocardial infarction; CARE, Cholesterol and Recurrent Events; PREVEND IT, Prevention of Renal and Vascular End Stage Disease Intervention Trial; 4D, Duetche Diabetes Dialysis Study of Atorvastatin in Patients with Type 2 Diabetes Mellitus Undergoing Hemodialysis.
Although improved cardiovascular outcomes have been suggested in CKD, similar findings of treatment benefit have not been evident in patients with ESRD. Degoulet et al85 reported an increased mortality rate in dialysis patients with low cholesterol. This paradox of low cholesterol, confirmed by others, which in this disease population serves as a marker of malnutrition, questions the benefits of lipid lowering in patients with underlying hemodialysis.86-88 The randomized placebo-controlled Duetche Diabetes Dialysis Study of Atorvastatin in Patients with Type 2 Diabetes Mellitus Undergoing Hemodialysis found no significant benefit in cardiovascular mortality with atorvastatin in 1,255 patients undergoing hemodialysis11 and appears to confirm the futility of lipid lowering in patients with ESRD. Current ongoing trials, A Study to Evaluate the Use of Rosuvastatin in Subjects on Regular Hemodialysis: an Assessment of Survival and Cardiovascular Events, a prospective international trial to assess the effects of rosuvastatin on cardiovascular morbidity and mortality in ESRD patients on chronic hemodialysis, and the Study of Heart and Renal Protection, which uses combined simvastatin and ezetimibe therapy, will address the effect of statins on the outcomes in predialysis and dialysis patients.89-91 These trials may clarify further the putative renoprotective effect of statin treatment in patients with CKD and analyze the morbidity and mortality from cardiovascular causes in hemodialysis patients.
SUMMARY
Metabolic changes that occur with progressive renal failure and aging predispose patients to lipid abnormalities with increased atherogenic potential. Changes in expression of transcriptional and nuclear hormone receptors may be contributing. Suggestions of further CKD progression and increased cardiovascular risk from abnormal lipid metabolism causes concern for preventive treatment. At present, secondary analysis of large treatment trials with various statin agents suggest cardiovascular risk reduction in the elderly and those with earlier stages of CKD. However, strict LDL lowering in ESRD patients did not improve mortality. Therefore, treatment options for the elderly with CKD, particularly with advanced CKD/ESRD, should continue to be weighed carefully against risks for each patient. Continued evaluation of lipid abnormalities in this high-risk population may help clarify standards of evaluation and treatment.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Foley RN, Murray AM, Li S, et al. Chronic kidney disease and the risk for cardiovascular disease, renal replacement, and death in the United States Medicare population, 1998 to 1999. J Am Soc Nephrol. 2005;16:489–95. doi: 10.1681/ASN.2004030203. [DOI] [PubMed] [Google Scholar]
- 2.Coresh J, Astor BC, Greene T, Eknoyan G, Levey AS. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis. 2003;41:1–12. doi: 10.1053/ajkd.2003.50007. [DOI] [PubMed] [Google Scholar]
- 3.Parfrey PS, Foley RN, Harnett JD, Kent GM, Murray D, Barre PE. Outcome and risk factors of ischemic heart disease in chronic uremia. Kidney Int. 1996;49:1428–34. doi: 10.1038/ki.1996.201. [DOI] [PubMed] [Google Scholar]
- 4.United States Renal Data System Annual Data Report; Bethesda, MD: US Department of Public Health and Human Services, Public Health Service, National Institutes of Health. 2006. [Google Scholar]
- 5.Parikh NI, Hwang SJ, Larson MG, Meigs JB, Levy D, Fox CS. Cardiovascular disease risk factors in chronic kidney disease: overall burden and rates of treatment and control. Arch Intern Med. 2006;166:1884–91. doi: 10.1001/archinte.166.17.1884. [DOI] [PubMed] [Google Scholar]
- 6.Cases A, Coll E. Dyslipidemia and the progression of renal disease in chronic renal failure patients. Kidney Int Suppl. 2005:S87–93. doi: 10.1111/j.1523-1755.2005.09916.x. [DOI] [PubMed] [Google Scholar]
- 7.Hunsicker LG, Adler S, Caggiula A, et al. Predictors of the progression of renal disease in the Modification of Diet in Renal Disease Study. Kidney Int. 1997;51:1908–19. doi: 10.1038/ki.1997.260. [DOI] [PubMed] [Google Scholar]
- 8.Samuelsson O, Mulec H, Knight-Gibson C, et al. Lipoprotein abnormalities are associated with increased rate of progression of human chronic renal insufficiency. Nephrol Dial Transplant. 1997;12:1908–15. doi: 10.1093/ndt/12.9.1908. [DOI] [PubMed] [Google Scholar]
- 9.Fried LF, Orchard TJ, Kasiske BL. Effect of lipid reduction on the progression of renal disease: a meta-analysis. Kidney Int. 2001;59:260–9. doi: 10.1046/j.1523-1755.2001.00487.x. [DOI] [PubMed] [Google Scholar]
- 10.Tonelli M, Isles C, Craven T, et al. Effect of pravastatin on rate of kidney function loss in people with or at risk for coronary disease. Circulation. 2005;112:171–8. doi: 10.1161/CIRCULATIONAHA.104.517565. [DOI] [PubMed] [Google Scholar]
- 11.Wanner C, Krane V, Marz W, et al. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med. 2005;353:238–48. doi: 10.1056/NEJMoa043545. [DOI] [PubMed] [Google Scholar]
- 12.Tsimihodimos V, Dounousi E, Siamopoulos KC. Dyslipidemia in chronic kidney disease: an approach to pathogenesis and treatment. Am J Nephrol. 2008;28:958–73. doi: 10.1159/000144024. [DOI] [PubMed] [Google Scholar]
- 13.Harper CR, Jacobson TA. Managing dyslipidemia in chronic kidney disease. J Am Coll Cardiol. 2008;51:2375–84. doi: 10.1016/j.jacc.2008.03.025. [DOI] [PubMed] [Google Scholar]
- 14.Vaziri ND. Dyslipidemia of chronic renal failure: the nature, mechanisms, and potential consequences. Am J Physiol Renal Physiol. 2006;290:F262–72. doi: 10.1152/ajprenal.00099.2005. [DOI] [PubMed] [Google Scholar]
- 15.Kaysen GA. Lipid and lipoprotein metabolism in chronic kidney disease. J Ren Nutr. 2009;19:73–7. doi: 10.1053/j.jrn.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 16.Mordasini R, Frey F, Flury W, Klose G, Greten H. Selective deficiency of hepatic triglyceride lipase in uremic patients. N Engl J Med. 1977;297:1362–6. doi: 10.1056/NEJM197712222972502. [DOI] [PubMed] [Google Scholar]
- 17.Koniger M, Quaschning T, Wanner C, Schollmeyer P, Kramer-Guth A. Abnormalities in lipoprotein metabolism in hemodialysis patients. Kidney Int Suppl. 1999;71:S248–50. doi: 10.1046/j.1523-1755.1999.07166.x. [DOI] [PubMed] [Google Scholar]
- 18.Gonzalez AI, Schreier L, Elbert A, et al. Lipoprotein alterations in hemodialysis: differences between diabetic and nondiabetic patients. Metabolism. 2003;52:116–21. doi: 10.1053/meta.2003.50018. [DOI] [PubMed] [Google Scholar]
- 19.Dursun B, Dursun E, Capraz I, Ozben T, Apaydin A, Suleymanlar G. Are uremia, diabetes, and atherosclerosis linked with impaired antioxidant mechanisms? J Investig Med. 2008;56:545–52. doi: 10.2310/JIM.0b013e3181641ce3. [DOI] [PubMed] [Google Scholar]
- 20.Ramos R, Martinez-Castelao A. Lipoperoxidation and hemodialysis. Metabolism. 2008;57:1369–74. doi: 10.1016/j.metabol.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 21.Annuk M, Fellstrom B, Akerblom O, Zilmer K, Vihalemm T, Zilmer M. Oxidative stress markers in pre-uremic patients. Clin Nephrol. 2001;56:308–14. [PubMed] [Google Scholar]
- 22.Mimic-Oka J, Simic T, Djukanovic L, Reljic Z, Davicevic Z. Alteration in plasma antioxidant capacity in various degrees of chronic renal failure. Clin Nephrol. 1999;51:233–41. [PubMed] [Google Scholar]
- 23.Ferretti G, Bacchetti T, Masciangelo S, Pallotta G. Lipid peroxidation in hemodialysis patients: effect of vitamin C supplementation. Clin Biochem. 2008;41:381–6. doi: 10.1016/j.clinbiochem.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 24.Maggi E, Bellazzi R, Falaschi F, et al. Enhanced LDL oxidation in uremic patients: an additional mechanism for accelerated atherosclerosis? Kidney Int. 1994;45:876–83. doi: 10.1038/ki.1994.115. [DOI] [PubMed] [Google Scholar]
- 25.Korczynska J, Stelmanska E, Nogalska A, et al. Upregulation of lipogenic enzymes genes expression in white adipose tissue of rats with chronic renal failure is associated with higher level of sterol regulatory element binding protein-1. Metabolism. 2004;53:1060–5. doi: 10.1016/j.metabol.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 26.Chmielewski M, Sucajtys-Szulc E, Kossowska E, Swierczynski J, Rutkowski B, Boguslawski W. Increased gene expression of liver SREBP-2 in experimental chronic renal failure. Atherosclerosis. 2007;191:326–32. doi: 10.1016/j.atherosclerosis.2006.05.044. [DOI] [PubMed] [Google Scholar]
- 27.Chmielewski M, Sucajtys E, Swierczynski J, Rutkowski B, Boguslawski W. Contribution of increased HMG-CoA reductase gene expression to hypercholesterolemia in experimental chronic renal failure. Mol Cell Biochem. 2003;246:187–91. [PubMed] [Google Scholar]
- 28.Mori Y, Hirano T, Nagashima M, Shiraishi Y, Fukui T, Adachi M. Decreased peroxisome proliferator-activated receptor alpha gene expression is associated with dyslipidemia in a rat model of chronic renal failure. Metabolism. 2007;56:1714–8. doi: 10.1016/j.metabol.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 29.Kim HJ, Vaziri ND. Sterol regulatory element-binding proteins, liver X receptor, ABCA1 transporter, CD36, scavenger receptors A1 and B1 in nephrotic kidney. Am J Nephrol. 2009;29:607–14. doi: 10.1159/000193631. [DOI] [PubMed] [Google Scholar]
- 30.Zhou Y, Zhang X, Chen L, et al. Expression profiling of hepatic genes associated with lipid metabolism in nephrotic rats. Am J Physiol Renal Physiol. 2008;295:F662–71. doi: 10.1152/ajprenal.00046.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim CH, Kim HJ, Mitsuhashi M, Vaziri ND. Hepatic tissue sterol regulatory element binding protein 2 and low-density lipoprotein receptor in nephrotic syndrome. Metabolism. 2007;56:1377–82. doi: 10.1016/j.metabol.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 32.Jiang T, Liebman SE, Lucia MS, Li J, Levi M. Role of altered renal lipid metabolism and the sterol regulatory element binding proteins in the pathogenesis of age-related renal disease. Kidney Int. 2005;68:2608–20. doi: 10.1111/j.1523-1755.2005.00733.x. [DOI] [PubMed] [Google Scholar]
- 33.Nogalska A, Sucajtys-Szulc E, Swierczynski J. Leptin decreases lipogenic enzyme gene expression through modification of SREBP-1c gene expression in white adipose tissue of aging rats. Metabolism. 2005;54:1041–7. doi: 10.1016/j.metabol.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 34.Pallottini V, Martini C, Cavallini G, et al. Modified HMG-CoA reductase and LDLr regulation is deeply involved in age-related hypercholesterolemia. J Cell Biochem. 2006;98:1044–53. doi: 10.1002/jcb.20951. [DOI] [PubMed] [Google Scholar]
- 35.Vila L, Roglans N, Alegret M, et al. Hypertriglyceridemia and hepatic steatosis in senescence-accelerated mouse associate to changes in lipid-related gene expression. J Gerontol A Biol Sci Med Sci. 2007;62:1219–27. doi: 10.1093/gerona/62.11.1219. [DOI] [PubMed] [Google Scholar]
- 36.Jiang T, Wang XX, Scherzer P, et al. Farnesoid X receptor modulates renal lipid metabolism, fibrosis, and diabetic nephropathy. Diabetes. 2007;56:2485–93. doi: 10.2337/db06-1642. [DOI] [PubMed] [Google Scholar]
- 37.Lefebvre P, Cariou B, Lien F, Kuipers F, Staels B. Role of bile acids and bile acid receptors in metabolic regulation. Physiol Rev. 2009;89:147–91. doi: 10.1152/physrev.00010.2008. [DOI] [PubMed] [Google Scholar]
- 38.Piper MD, Bartke A. Diet and aging. Cell Metab. 2008;8:99–104. doi: 10.1016/j.cmet.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 39.Martini C, Pallottini V, Cavallini G, Donati A, Bergamini E, Trentalance A. Caloric restrictions affect some factors involved in age-related hypercholesterolemia. J Cell Biochem. 2007;101:235–43. doi: 10.1002/jcb.21158. [DOI] [PubMed] [Google Scholar]
- 40.Zhu M, Miura J, Lu LX, et al. Circulating adiponectin levels increase in rats on caloric restriction: the potential for insulin sensitization. Exp Gerontol. 2004;39:1049–59. doi: 10.1016/j.exger.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 41.Zhu M, de Cabo R, Lane MA, Ingram DK. Caloric restriction modulates early events in insulin signaling in liver and skeletal muscle of rat. Ann N Y Acad Sci. 2004;1019:448–52. doi: 10.1196/annals.1297.082. [DOI] [PubMed] [Google Scholar]
- 42.Feige JN, Lagouge M, Canto C, et al. Specific SIRT1 activation mimics low energy levels and protects against diet-induced metabolic disorders by enhancing fat oxidation. Cell Metab. 2008;8:347–58. doi: 10.1016/j.cmet.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 43.Banks AS, Kon N, Knight C, et al. SirT1 gain of function increases energy efficiency and prevents diabetes in mice. Cell Metab. 2008;8:333–41. doi: 10.1016/j.cmet.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brooks CL, Gu W. How does SIRT1 affect metabolism, senescence and cancer? Nat Rev Cancer. 2009;9:123–8. doi: 10.1038/nrc2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lavu S, Boss O, Elliott PJ, Lambert PD. Sirtuins—novel therapeutic targets to treat age-associated diseases. Nat Rev Drug Discov. 2008;7:841–53. doi: 10.1038/nrd2665. [DOI] [PubMed] [Google Scholar]
- 46.Bordone L, Cohen D, Robinson A, et al. SIRT1 transgenic mice show phenotypes resembling calorie restriction. Aging Cell. 2007;6:759–67. doi: 10.1111/j.1474-9726.2007.00335.x. [DOI] [PubMed] [Google Scholar]
- 47.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–47. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 48.Zhang QL, Koenig W, Raum E, Stegmaier C, Brenner H, Rothenbacher D. Epidemiology of chronic kidney disease: results from a population of older adults in Germany. Prev Med. 2008 doi: 10.1016/j.ypmed.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 49.Roderick PJ, Atkins RJ, Smeeth L, et al. Detecting chronic kidney disease in older people; what are the implications? Age Ageing. 2008;37:179–86. doi: 10.1093/ageing/afm180. [DOI] [PubMed] [Google Scholar]
- 50.Pizzarelli F, Lauretani F, Bandinelli S, et al. Predictivity of survival according to different equations for estimating renal function in community-dwelling elderly subjects. Nephrol Dial Transplant. 2008 doi: 10.1093/ndt/gfn594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Culleton BF, Hemmelgarn BR. Is chronic kidney disease a cardiovascular disease risk factor? Semin Dial. 2003;16:95–100. doi: 10.1046/j.1525-139x.2003.16024.x. [DOI] [PubMed] [Google Scholar]
- 52.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 53.Go AS, Lo JC. Epidemiology of non-dialysis-requiring chronic kidney disease and cardiovascular disease. Curr Opin Nephrol Hypertens. 2006;15:296–302. doi: 10.1097/01.mnh.0000222698.30207.aa. [DOI] [PubMed] [Google Scholar]
- 54.Rifkin DE, Shlipak MG, Katz R, et al. Rapid kidney function decline and mortality risk in older adults. Arch Intern Med. 2008;168:2212–8. doi: 10.1001/archinte.168.20.2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rashidi A, Sehgal AR, Rahman M, O’Connor AS. The case for chronic kidney disease, diabetes mellitus, and myocardial infarction being equivalent risk factors for cardiovascular mortality in patients older than 65 years. Am J Cardiol. 2008;102:1668–73. doi: 10.1016/j.amjcard.2008.07.060. [DOI] [PubMed] [Google Scholar]
- 56.Abrams J, Vela BS, Coultas DB, Samaan SA, Malhotra D, Roche RJ. Coronary risk factors and their modification: lipids, smoking, hypertension, estrogen, and the elderly. Curr Probl Cardiol. 1995;20:533–610. [PubMed] [Google Scholar]
- 57.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–9. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 58.Windler E, Schoffauer M, Zyriax BC. The significance of low HDL-cholesterol levels in an ageing society at increased risk for cardiovascular disease. Diab Vasc Dis Res. 2007;4:136–42. doi: 10.3132/dvdr.2007.032. [DOI] [PubMed] [Google Scholar]
- 59.Wenger NK. Dyslipidemia as a risk factor at elderly age. Am J Geriatr Cardiol. 2004;13:4–9. [PubMed] [Google Scholar]
- 60.Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults. Summary of the second report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel II) JAMA. 1993;269:3015–23. [PubMed] [Google Scholar]
- 61.Kuller LH, Shemanski L, Psaty BM, et al. Subclinical disease as an independent risk factor for cardiovascular disease. Circulation. 1995;92:720–6. doi: 10.1161/01.cir.92.4.720. [DOI] [PubMed] [Google Scholar]
- 62.Keane WF, Kasiske BL, O’Donnell MP. Lipids and progressive glomerulosclerosis. A model analogous to atherosclerosis. Am J Nephrol. 1988;8:261–71. doi: 10.1159/000167599. [DOI] [PubMed] [Google Scholar]
- 63.Joles JA, Kunter U, Janssen U, et al. Early mechanisms of renal injury in hypercholesterolemic or hypertriglyceridemic rats. J Am Soc Nephrol. 2000;11:669–83. doi: 10.1681/ASN.V114669. [DOI] [PubMed] [Google Scholar]
- 64.Muntner P, Coresh J, Smith JC, Eckfeldt J, Klag MJ. Plasma lipids and risk of developing renal dysfunction: the atherosclerosis risk in communities study. Kidney Int. 2000;58:293–301. doi: 10.1046/j.1523-1755.2000.00165.x. [DOI] [PubMed] [Google Scholar]
- 65.Schaeffner ES, Kurth T, Curhan GC, et al. Cholesterol and the risk of renal dysfunction in apparently healthy men. J Am Soc Nephrol. 2003;14:2084–91. doi: 10.1681/ASN.V1482084. [DOI] [PubMed] [Google Scholar]
- 66.Fox CS, Larson MG, Leip EP, Culleton B, Wilson PW, Levy D. Predictors of new-onset kidney disease in a community-based population. JAMA. 2004;291:844–50. doi: 10.1001/jama.291.7.844. [DOI] [PubMed] [Google Scholar]
- 67.Sandhu S, Wiebe N, Fried LF, Tonelli M. Statins for improving renal outcomes: a meta-analysis. J Am Soc Nephrol. 2006;17:2006–16. doi: 10.1681/ASN.2006010012. [DOI] [PubMed] [Google Scholar]
- 68.Levi M. Do statins have a beneficial effect on the kidney? Nat Clin Pract Nephrol. 2006;2:666–7. doi: 10.1038/ncpneph0336. [DOI] [PubMed] [Google Scholar]
- 69.Asselbergs FW, Diercks GF, Hillege HL, et al. Effects of fosinopril and pravastatin on cardiovascular events in subjects with microalbuminuria. Circulation. 2004;110:2809–16. doi: 10.1161/01.CIR.0000146378.65439.7A. [DOI] [PubMed] [Google Scholar]
- 70.Vidt DG, Cressman MD, Harris S, Pears JS, Hutchinson HG. Rosuvastatin-induced arrest in progression of renal disease. Cardiology. 2004;102:52–60. doi: 10.1159/000077704. [DOI] [PubMed] [Google Scholar]
- 71.Hunt D, Young P, Simes J, et al. Benefits of pravastatin on cardiovascular events and mortality in older patients with coronary heart disease are equal to or exceed those seen in younger patients: results from the LIPID trial. Ann Intern Med. 2001;134:931–40. doi: 10.7326/0003-4819-134-10-200105150-00007. [DOI] [PubMed] [Google Scholar]
- 72.Shepherd J, Blauw GJ, Murphy MB, et al. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. 2002;360:1623–30. doi: 10.1016/s0140-6736(02)11600-x. [DOI] [PubMed] [Google Scholar]
- 73.Lewis SJ, Moye LA, Sacks FM, et al. Effect of pravastatin on cardiovascular events in older patients with myocardial infarction and cholesterol levels in the average range. Results of the Cholesterol and Recurrent Events (CARE) trial. Ann Intern Med. 1998;129:681–9. doi: 10.7326/0003-4819-129-9-199811010-00002. [DOI] [PubMed] [Google Scholar]
- 74.Miettinen TA, Pyorala K, Olsson AG, et al. Cholesterol-lowering therapy in women and elderly patients with myocardial infarction or angina pectoris: findings from the Scandinavian Simvastatin Survival Study (4S) Circulation. 1997;96:4211–8. doi: 10.1161/01.cir.96.12.4211. [DOI] [PubMed] [Google Scholar]
- 75.Deedwania P, Stone PH, Bairey Merz CN, et al. Effects of intensive versus moderate lipid-lowering therapy on myocardial ischemia in older patients with coronary heart disease: results of the Study Assessing Goals in the Elderly (SAGE) Circulation. 2007;115:700–7. doi: 10.1161/CIRCULATIONAHA.106.654756. [DOI] [PubMed] [Google Scholar]
- 76.DeMicco DLD, Betteridge DJ, Colhoun HM, Durrington PN, Hitman GA, Neil HA, et al. Effect of atorvastatin in patients 65 years of age with type 2 diabetes: subgroup analysis from the collaborative atorvastatin diabetes study (CARDS) Circulation. 2005;112:U907–U. [Google Scholar]
- 77.Wenger NK, Lewis SJ, Herrington DM, Bittner V, Welty FK. Outcomes of using high- or low-dose atorvastatin in patients 65 years of age or older with stable coronary heart disease. Ann Intern Med. 2007;147:1–9. doi: 10.7326/0003-4819-147-1-200707030-00002. [DOI] [PubMed] [Google Scholar]
- 78.Maroo BP, Lavie CJ, Milani RV. Efficacy and sSafety of intensive statin therapy in the elderly. Am J Geriatr Cardiol. 2008;17:92–100. [PubMed] [Google Scholar]
- 79.Maroo BP, Lavie CJ, Milani RV. Secondary prevention of coronary heart disease in elderly patients following myocardial infarction—are all HMG-CoA reductase inhibitors alike? Drugs Aging. 2008;25:649–64. doi: 10.2165/00002512-200825080-00003. [DOI] [PubMed] [Google Scholar]
- 80.Chong PH, Seeger JD, Franklin C. Clinically relevant differences between the statins: implications for therapeutic selection. Am J Med. 2001;111:390–400. doi: 10.1016/s0002-9343(01)00870-1. [DOI] [PubMed] [Google Scholar]
- 81.Szolkiewicz M, Sucajtys E, Chmielewski M, et al. Increased rate of cholesterologenesis—a possible cause of hypercholesterolemia in experimental chronic renal failure in rats. Horm Metab Res. 2002;34:234–7. doi: 10.1055/s-2002-32135. [DOI] [PubMed] [Google Scholar]
- 82.Jardine AG, Holdaas H, Fellstrom B, et al. Fluvastatin prevents cardiac death and myocardial infarction in renal transplant recipients: post-hoc subgroup analyses of the ALERT study. Am J Transplant. 2004;4:988–95. doi: 10.1111/j.1600-6143.2004.00445.x. [DOI] [PubMed] [Google Scholar]
- 83.Tonelli M, Moye L, Sacks FM, Kiberd B, Curhan G. Pravastatin for secondary prevention of cardiovascular events in persons with mild chronic renal insufficiency. Ann Intern Med. 2003;138:98–104. doi: 10.7326/0003-4819-138-2-200301210-00010. [DOI] [PubMed] [Google Scholar]
- 84.Pierides AM, Alvarez-Ude F, Kerr DN. Clofibrate-induced muscle damage in patients with chronic renal failure. Lancet. 1975;2:1279–82. doi: 10.1016/s0140-6736(75)90613-3. [DOI] [PubMed] [Google Scholar]
- 85.Degoulet P, Legrain M, Reach I, et al. Mortality risk factors in patients treated by chronic hemodialysis. Report of the Diaphane collaborative study. Nephron. 1982;31:103–10. doi: 10.1159/000182627. [DOI] [PubMed] [Google Scholar]
- 86.Lowrie EG, Lew NL. Death risk in hemodialysis patients: the predictive value of commonly measured variables and an evaluation of death rate differences between facilities. Am J Kidney Dis. 1990;15:458–82. doi: 10.1016/s0272-6386(12)70364-5. [DOI] [PubMed] [Google Scholar]
- 87.Iseki K, Yamazato M, Tozawa M, Takishita S. Hypocholesterolemia is a significant predictor of death in a cohort of chronic hemodialysis patients. Kidney Int. 2002;61:1887–93. doi: 10.1046/j.1523-1755.2002.00324.x. [DOI] [PubMed] [Google Scholar]
- 88.Kilpatrick RD, McAllister CJ, Kovesdy CP, Derose SF, Kopple JD, Kalantar-Zadeh K. Association between serum lipids and survival in hemodialysis patients and impact of race. J Am Soc Nephrol. 2007;18:293–303. doi: 10.1681/ASN.2006070795. [DOI] [PubMed] [Google Scholar]
- 89.Fellstrom B, Zannad F, Schmieder R, et al. Effect of rosuvastatin on outcomes in chronic haemodialysis patients—design and rationale of the AURORA study. Curr Control Trials Cardiovasc Med. 2005;6:9. doi: 10.1186/1468-6708-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fellstrom B, Holdaas H, Jardine AG, et al. Effect of rosuvastatin on outcomes in chronic haemodialysis patients: baseline data from the AURORA study. Kidney Blood Press Res. 2007;30:314–22. doi: 10.1159/000106803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Baigent C, Landry M. Study of Heart and Renal Protection (SHARP) Kidney Int Suppl. 2003:S207–10. doi: 10.1046/j.1523-1755.63.s84.4.x. [DOI] [PubMed] [Google Scholar]