Abstract
Although neuroimaging and human lesion studies agree that the medial parietal region plays a critical role in episodic memory, many neuroimaging studies have also implicated lateral parietal cortex, leading some researchers to suggest that the lateral region plays a heretofore underappreciated role in episodic memory. Because there are very few extant lesion data on this matter, we examined memory in six cases of focal lateral parietal damage, using both clinical and experimental measures, in which we distinguished between recollection and familiarity. The patients did not have amnesia, but they did show evidence of disrupted recollection on an anterograde memory task. Although the exact mechanisms remain to be elucidated, lateral parietal damage appears to impair some aspects of episodic memory.
Keywords: recollection, autobiographical memory, working memory, retrosplenial cortex, fMRI
Medial temporal and prefrontal regions of the brain are necessary for episodic memory (i.e., conscious memory for personally-experienced events within a particular spatiotemporal context; for reviews, see Baldo & Shimamura, 2002; Davidson et al., 2006; Moscovitch et al., 2005, 2006; Squire et al., 2004; Tulving, 2002). Recently, however, several independent reviews (Naghavi & Nyberg, 2005; Skinner & Fernandes, 2007; Wagner et al., 2005), following Rugg and colleagues’ observations with event-related potentials (e.g., Rugg & Wilding, 1996), have pointed out that functional neuroimaging studies of episodic memory tend to show significantly greater activation in parietal regions for previously studied items that are correctly recognized as old, compared to unstudied items that are correctly identified as new. Furthermore, some studies have suggested that parietal activations are stronger in cases where one has a vivid, clear recollection (i.e., remembering) of an item and the contextual details surrounding it, as opposed to a more intuitive feeling of familiarity (i.e., knowing that the stimulus has been encountered recently without awareness of the context in which it appeared; Tulving, 1985). These findings have led some researchers to suggest that parietal cortex plays a heretofore underappreciated role in episodic memory. Such an assertion is provocative, however, because memory is not a function that has traditionally been ascribed to the parietal lobe. Classic texts on the functions of parietal cortex have made scant mention of memory (e.g., Critchley, 1953; Luria, 1966), and those on the neuropsychology of memory have said little about the parietal lobe (e.g., Luria, 1976). In the interest of seeking convergence with the functional neuroimaging data, we examined the literature on the effects of parietal lesions on memory, and we report six cases of focal parietal damage, examining memory processes in detail.
Topography and Functions of Parietal Cortex
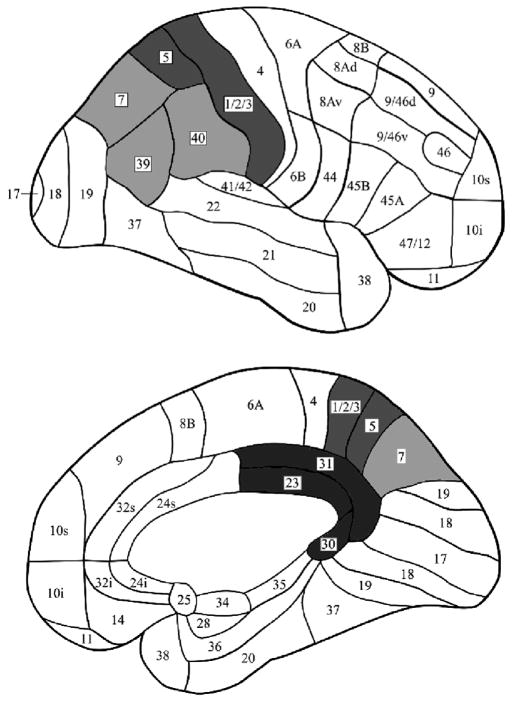
Parietal cortex includes a strip posterior to the central sulcus that is specialized for somatosensory function (Brodmann areas [BAs] 1, 2, 3, and 5). On the medial surface, posterior to this strip lies the precuneus (medial BA 7), which extends posteriorly to the parieto-occipital notch, and is bordered anteriorly and inferiorly by the posterior cingulate gyrus and the retrosplenial region (including BAs 23, 30, and 31). On the lateral surface, posterior to the somatosensory area, are three large zones: the superior parietal lobule, the angular gyrus, and the supramarginal gyrus (roughly corresponding to BAs 7, 39, and 40, respectively; collectively, these zones are commonly referred to as lateral or posterior parietal cortex). For the purposes of our review, we will divide parietal cortex into two broad regions, medial and lateral, and discuss each in turn but focus on the latter. Figure 1 shows the putative distinctions among regions.
Figure 1.
Medial region: Precuneus (and Posterior Cingulate and Retrosplenial areas)
Medial parietal and caudomedial limbic (i.e., posterior cingulate and retrosplenial) activations are ubiquitous in functional imaging studies of episodic and autobiographical memory (among other tasks; for reviews, see Cavanna & Trimble, 2006; Naghavi & Nyberg, 2005; Skinner & Fernandes, 2007; Svoboda et al., 2006; Vincent et al., 2006; Wagner et al., 2005). The neuroimaging data fit well with the human lesion data: Several case studies have reported memory impairment after damage to this area, although the relative contributions of precuneus, posterior cingulate, and retrosplenial cortex are still unclear (for reviews, see Aguirre & D’Esposito, 1999; Maguire, 2001). Patients with damage to this broad area may either show a full-blown amnesia similar to that following medial temporal damage (e.g., Rudge & Warrington, 1991; Valenstein et al., 1987; Von Cramon & Schuri, 1992), or show a more selective topographical disorientation, in which they can recognize familiar landmarks but get lost when asked to go from one place to another (Takahashi et al., 1997).
Given the clear correspondence between the neuroimaging and human lesion data concerning episodic and autobiographical memory, and the strong anatomical connections with medial temporal (Insausti et al., 1987; Insausti & Munoz, 2001; Kobayashi & Amaral, 2003; Morris et al., 1999a; Morris et al., 1999b; Suzuki & Amaral, 1994; Van Hoesen & Pandya, 1975) and dorsolateral prefrontal areas (Goldman-Rakic et al., 1984; Morris et al., 1999a; Petrides & Pandya, 1999; Kobayashi & Amaral, 2003) as well as the anterior and lateroposterior nuclei of the thalamus (Morris et al., 1999a), there is relatively little controversy that the medial parietal and caudomedial limbic areas are involved in episodic and autobiographical memory. For this reason, we will not discuss the medial regions further, and will focus on the lateral parietal region.
Lateral region: Superior Parietal Lobule, Angular Gyrus, and Supramarginal Gyrus
The aforementioned reviews by Naghavi and Nyberg (2005), Skinner and Fernandes (2007), and Wagner et al. (2005; see also Vincent et al., 2006) also showed areas of activation in lateral/posterior parietal regions during episodic memory retrieval, and Svoboda et al. (2006) reported consistent lateral parietal activations in a meta-analysis of autobiographical memory retrieval. This finding is much more provocative than the medial parietal activity because memory is not usually thought to depend on this region of the brain. Traditionally, lateral posterior parietal cortex (including BAs 39, 40, and the posterior part of area 7) is thought to support planning and control of movement, as well as perception of, and attention to, spatial information (for influential models, see Corbetta & Shulman, 2002; Milner & Goodale, 1995; Mishkin, Ungerleider, & Macko, 1983; Nobre et al., 2001; Posner & Peterson, 1990), multisensory integration (Xing & Andersen, 2000) and construction (Critchley, 1953; Luria, 1973). For example, damage to posterior parietal cortex can limit awareness of the outside world, an object, or even of one’s own body, so that only the contralesional half is consciously perceived (i.e., neglect; for theories, see Bisiach & Vallar, 1998; Dankert & Ferber, 2006; Driver & Vuilleumier, 2001; Karnath et al., 2001; Mayer et al., 1999; Rafal, 1997), and can impair the ability to detect multiple objects simultaneously (especially when they are in opposite hemifields; i.e., simultanagnosia; Balint, 1909/1995; Rafal, 2002). Current models have proposed subdivisions of the posterior parietal region along functional lines. For example, Corbetta and Shulman (2002) have suggested that dorsal parietal areas (centered on the intraparietal sulcus) are involved in “top-down” or goal-directed attention, whereas ventral parietal areas (centered on the temporoparietal junction) are involved in “bottom-up” or stimulus-driven detection of behaviorally relevant stimuli.
Nonetheless, based on intrahemispheric connections with medial temporal and frontal regions, it is plausible that lateral posterior parietal cortex could play a role in episodic memory. Lateral posterior parietal cortex has reciprocal connections with entorhinal, parahippocampal, and hippocampal regions of the medial temporal lobe (Blatt et al., 2003; Clower et al., 2001; Insausti & Munoz, 2001; Laveneux et al., 2002; Munoz & Insausti, 2005; Rockland & Van Hoesen, 1999; Suzuki & Amaral, 1994), as well as with the medial parietal region (Kobayashi & Amaral, 2003; Morris et al., 1999b). It is also connected to anterior cingulate and dorsolateral prefrontal cortex, in particular BAs 6, 8, and 46 (Cavada & Goldman-Rakic, 1989; Lewis & Van Essen, 2000; Petrides & Pandya, 1984; Petrides & Pandya, 1999).
Current Theories of the Lateral Parietal Region’s Role in Memory
The anatomical connections between the lateral parietal and medial temporal and frontal areas, along with the ubiquity of lateral parietal activations in functional neuroimaging studies of memory, have led researchers to speculate as to what mnemonic functions the lateral parietal region might support. Candidate hypotheses (which are not mutually exclusive) include awareness at retrieval, working memory demands, and retrieval of contextual details. Parietal cortex may support aspects of consciousness and awareness during retrieval, as demonstrated by Bisiach and Luzzatti (1978), who showed that right parietal lesion patients with unilateral neglect omitted left-sided details in describing their memory of their town’s central square. However, when asked to describe the scene from the opposite point of view, the patients could now report the missing details (but omitted the previously reported ones, which now fell in the neglected representational space). This finding suggests that parietal damage can impair conscious retrieval of even well encoded information. A second possibility is that parietal cortex supports some sort of attentional process during memory retrieval. Given that parietal cortex plays a prominent role in attention to the outside world, it is natural to wonder whether it also supports analogous processes in one’s inner world, for example, during memory retrieval (a hypothesis also entertained by Naghavi & Nyberg, 2005; and Wagner et al., 2005).
Parietal activation during retrieval may also reflect rehearsal of retrieved information in working memory. Functional neuroimaging studies have highlighted frontal and parietal activations in working memory, especially in the angular gyrus (BA 40) of the lateral parietal region. Activity in this region is strongly linked to rehearsal of information held in auditory or visual buffers, more so than to the executive components of working memory (for reviews, see Baddeley, 2003; D’Esposito et al., 2006; Martin, 2005; Smith & Jonides, 1998). Lesion studies are generally consistent with this finding (e.g., De Renzi & Nichelli, 1975; Markowitsch et al., 1999; Warrington & Shallice, 1969; Warrington et al., 1971; see also Butters et al., 1970; Samuels et al., 1971). Participants may engage in a greater degree of post-retrieval rehearsal than normal in the scanner due to the unusual demands inherent to the scanning situation.
Finally, parietal activation may reflect the retrieval of contextual information from memory. Wagner et al. (2005), following Rugg and colleagues’ lead (e.g., Rugg & Wilding, 1996) pointed out that in at least some lateral parietal areas, activation tends to be greater when participants are required to retrieve information about the specific contextual details associated with an event, such as remembering which of two actions one performed in relation to a stimulus at encoding (Dobbins, 2003) or in which spatial location a stimulus was studied (Cansino et al., 2002; see also Hayes et al., 2004). This possibility also fits with classic interpretations of the heteromodal role of parietal cortex, in which it posited to act as a crossroads, integrating information from multiple sensory domains (Critchley, 1953).
Previous Lesion Studies
Although it is well accepted that lesions to the medial parietal region can impair memory (reviewed briefly above), it is much less clear that damage to the lateral parietal region can have an impact. Relatively few studies of lateral parietal cortex damage and memory have been reported, and the extant findings are mixed. On the one hand, Warrington and James (1967) reported impaired recognition memory for visual stimuli in right parietal lesion patients, relative to left parietal and right temporal patients. However, interpretation of these findings was hindered by evidence of neglect and overall poor visual perception in the right parietal patients (see also Heilman, 1974; Vuilleumier et al., 2002). On the other hand, Milner (1968) reported intact recognition memory for faces and abstract patterns in unilateral parietal lesion patients (even in those with right hemisphere damage), compared to impaired performance in unilateral temporal lesion patients. More recently, Simons et al. (in press) reported that although lateral parietal regions were significantly active in an fMRI study of action monitoring in healthy people, patients with lateral parietal lesions generally performed well on the very same task.
As outlined above, although there is a theoretical basis for thinking that lateral parietal lesions would affect memory performance, there is as yet little evidence to support this prediction. The previous studies by Milner (1968) and Warrington and James (1967) both used simple recognition memory. Although functional neuroimaging studies suggest a role for the parietal lobe in recognition, it is likely that performance on standard tests of recognition is mediated by other areas (such as the medial temporal lobes) in the presence of parietal damage. In other words, the parietal lobe may be activated by simple item recognition tasks, but may not be required for such tasks. To our knowledge, however, no studies (other than the recent report from Simons et al., in press) have assessed performance of parietal patients on more sophisticated measures of episodic memory that may be reliant on higher order attentional or working memory processes attributed to the parietal lobe. We report two studies in which patients with well-characterized focal damage to lateral parietal cortex were assessed on a variety of such measures: A case series of five patients and a single case study. We collected data on clinical memory tasks as well as on more sophisticated tasks assessing autobiographical memory and the distinction between recollection and familiarity. These are aspects of memory that have received considerable attention in the functional neuroimaging literature on the lateral parietal lobe but not in the lesion literature on this region.
Five Cases of Focal Parietal Lobe Damage
Patients
We selected patients (n = 5) with parietal cortex damage from a larger group of focal lesion patients who had been recruited for neuropsychological research at the Rotman Research Institute at Baycrest. Four patients had left hemisphere damage, and one had right hemisphere damage. All patients were in the stable phase of recovery (at least six months post-morbid) from either stroke or excision of a low-grade tumor. They ranged in age between 44 and 67 years, with between 12 and 16 years of education. Demographic information is shown in Table 1. Patients were scanned with a 1.5T-MR system (General Electric) at the time of testing. With the exception of patient 1050, who received a standard clinical MRI, all patients were scanned with a research MRI protocol including a sagittal T1-weighted 3D volume technique producing 124 1.3mm slices (TR/TE of 35/5 ms, flip angle of 35 degrees, 1.0 NEX, and FOV of 22 cm). Proton density and T2-weighted images with a slice thickness of 3 mm were obtained using an interleaved sequence (TR/TE of 3000/30, 80 ms, 0.5 NEX, and FOV of 22 cm).
Table 1.
Demographic and lesion data
| 1022 | 1040 | 1047 | 1050 | 1051 | |
|---|---|---|---|---|---|
| Sex | M | F | F | M | M |
| Age (years) | 67 | 63 | 64 | 46 | 44 |
| Education (years) | 12 | 13 | 13 | 12 | 16 |
| Etiology | Stroke | Meningioma | Stroke | Meningioma | Stroke |
| Time since injury (months) | 12 | 23 | 10 | 23 | 48 |
| Lesion volume (mm3) | 6517 | 14307 | 9716 | 2601 | 47729 |
Focal lesions in the parietal patients were visualized and defined using Analyze® software (Biomedical Imaging Resource, Mayo Foundation, Rochester, MN, USA). The area of damage was determined by detailed slice-by-slice visual inspection on axial views by a radiologist. In order for a lesion to be traced, it had to appear on more than one slice, with a diameter of at least 3 mm on one of the slices. The boundary of the lesion was manually delineated on each MR T1-weighted axial slice using the Analyze® region of interest (ROI) module. A 3D lesion ROI for each patient was produced by combining all lesion tracings from each slice (see Table 1 for lesion volumes). Lesion localization was determined clinically by a radiologist. Whole-brain volumetric analysis using an updated version of our in-house software (Dade et al., 2004; Kovacevic et al., 2002) corroborated the clinical judgment, showing that the tracings were confined to the parietal lobes.
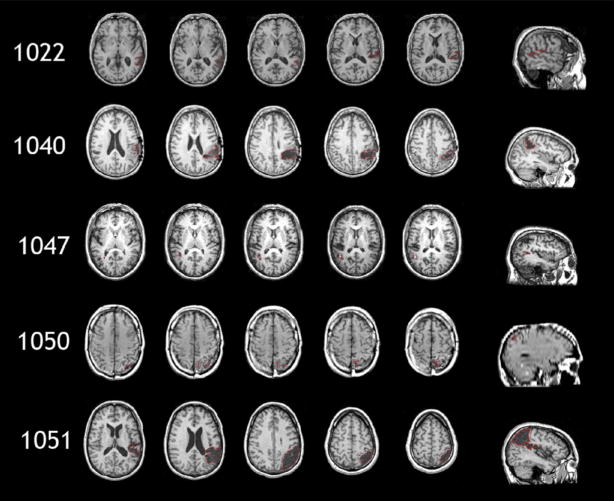
Figure 2 shows the extent of damage (outlined in red) for each patient on the T1-weighted scans. Although the lesions for these patients varied somewhat in terms of their precise location within lateral parietal cortex, none of the patients’ lesions invaded the medial/limbic region. Patient 1022’s lesion was in the left temporo-parietal junction (including the angular gyrus). Patient 1040 showed damage centered on the left inferior parietal zone, with involvement of superior parietal and posterior temporal cortex and white matter deep in these regions. Patient 1047 was the only patient with right sided damage, centered on the temporo-parietal junction (including the angular gyrus). This patient also had minimal damage in the orbitofrontal region, accounting for about 5% of her lesion load. Patient 1050 showed left superior parietal damage. Patient 1051 had left superior and inferior parietal damage, extending to the left posterior temporal lobe and to deep white matter.
Figure 2.
For the purposes of assessing group differences, the patients were compared to a control group (n = 10) of healthy subjects matched to the patients for age (M = 57 years, SD = 9 years) and education (M = 14 years; SD = 2 years). For comparisons of individual patients to controls, we conducted ancillary analyses, in which the three patients in their 60s and the two patients in their 40s were compared to separate age-matched groups (age Ms = 65 and 46 years, SDs = 5 and 6 years, respectively; education Ms = 15 and 16 years, SDs = 2 and 3 years, respectively; Ns = 8 and 14, respectively).
Materials and Method
As part of their assessment, the patients were administered a neuropsychological battery (following standard instructions for administration of each task, over two or three sessions) as part of their assessment, which included measures of vocabulary (Shipley, 1946), executive function (Wisconsin Card Sorting Test [WCST; Stuss et al., 2000], Trail Making Test [TMT; Spreen & Strauss, 1998], and verbal fluency [Spreen & Strauss, 1998]), and verbal learning and memory (Hopkins Verbal Learning Test-Revised; Benedict et al., 1998). Working memory was assessed using a self-ordered pointing task (Petrides & Milner, 1982), which consisted of a booklet containing sheets with arrangements of pictures of items (objects, people, and animals). The position of items on each sheet varied, and subjects were asked to touch all the items in the set, a different one on each sheet. We computed the total number of errors (i.e., selecting an item more than once). Set size increased as the task progressed (i.e., 6, 8, 10, or 12 items per sheet). Neuropsychological results are shown in Table 2.
Table 2.
Neuropsychological and experimental test data
| Test | 1022 | 1040 | 1047 | 1050 | 1051 | Controls |
t | Sig.* | |
|---|---|---|---|---|---|---|---|---|---|
| Mean | S.D. | ||||||||
| Shipley vocabulary | 27 | 38 | 32 | 29 | 32 | 34.5 | 3.9 | −1.33 | 0.21 |
| HVLT-R | |||||||||
| Recall | 19 | 30 | 25 | 24 | 19 | 25.4 | 3.7 | −0.91 | 0.38 |
| Retention | 7 | 11 | 8 | 10 | 8 | 8.7 | 1.9 | 0.10 | 0.92 |
| Recognition | 10 | 12 | 12 | 11 | 12 | 11.2 | 0.6 | 0.50 | 0.62 |
| Trail Making Test (s) | |||||||||
| Part A | 66 | 51 | 51 | 26 | 22 | 27.7 | 9.3 | 1.76 | 0.14 |
| Part B | 408 | 90 | 73 | 80 | 109 | 92.9 | 68 | 1.10 | 0.29 |
| Verbal Fluency (Letter) | 31 | 42 | 42 | 38 | 17 | 43 | 13.5 | −1.30 | 0.22 |
| WCST | |||||||||
| Categories | 3 | 3 | 5 | 7 | 9 | 6.9 | 3.5 | −0.83 | 0.42 |
| PPC | 43 | 35 | 40 | 18 | 18 | 19.4 | 10.4 | 1.90 | 0.08 |
| PPR | 16 | 16 | 11 | 3 | 4 | 6.2 | 6.1 | 1.12 | 0.28 |
| Self-ordered pointing errors | 35 | 4 | 12 | 12 | 10 | 10.3 | 6.6 | 0.92 | 0.38 |
| Remember/Know Source Memory | |||||||||
| Cued recall | 0.17 | 0.71 | 0.17 | 0.54 | 0.46 | 0.40 | 0.21 | 0.11 | 0.91 |
| Recognition | 0.79 | 1.00 | 0.79 | 0.96 | 0.75 | 0.96 | 0.04 | −2.58 | 0.02 |
| Source memory | 0.38 | 0.75 | 0.63 | 0.54 | 0.50 | 0.62 | 0.14 | −0.73 | 0.48 |
| Remember | 0.50 | 0.75 | 0.25 | 0.25 | 0.50 | 0.85 | 0.11 | −4.94 | 0.0003 |
| Remember/Know Remote Autobiographical Memory | |||||||||
| Remember | 0.82 | 0.55 | 0.75 | 0.85 | 0.66 | 0.24 | −0.42 | 0.68 | |
| No memory | 0 | 0.25 | 0.13 | 0.05 | 0.23 | 0.17 | −1.71 | 0.11 | |
| Never | 0.12 | 0.20 | 0.04 | 0.20 | 0.07 | 0.07 | 1.01 | 0.33 | |
| Autobiographical Interview | |||||||||
| Internal | 56.00 | 69.00 | 46.40 | 56.40 | 48.40 | 60.66 | 16.32 | −0.69 | 0.51 |
| External | 58.40 | 97.40 | 30.40 | 33.40 | 28.40 | 28.42 | 22.95 | 1.54 | 0.15 |
| Internal to total ratio | 0.50 | 0.43 | 0.58 | 0.63 | 0.63 | 0.70 | 0.09 | −2.95 | 0.01 |
Note. Raw scores, with individual scores below z = −1.96 bolded to denote impaired performance (see text for details). HVLT-R: Hopkins Verbal Learning Test—Revised, WCST: Wisconsin Card Sorting Test, PPC: perseverations of previous criterion, PPR: perseverations of previous response (see Stuss et al., 2000, for details). Patient 1022 did not complete the Autobiographical Remember/Know task.
Alpha = 0.05, two tailed (statistically significant results in bold).
In addition, the patients completed several experimental tasks, three of which are discussed here. The Remember/Know Source Memory task assessed recall, recognition, source memory, and “Remember/Know” judgments (Söderlund et al., in press). Patients studied 72 pairings of words with clever definitions (e.g., a talkative featherbrain -- parakeet; taken from Tulving & Watkins, 1977) and made a rating of cleverness for each pairing to encourage deep encoding. Half of the word-definition pairings were presented visually on a computer screen, and the other half were presented auditorily over loudspeakers (in separate blocks). After 30 minutes, the examiner read aloud the definitions as cues for participants to supply the defined word (e.g., “A talkative featherbrain - ?”; cued recall). Participants were informed that some definitions would be ‘old’ ones that were presented earlier, while other definitions would be ‘new’ items not encountered before. Participants were then asked if they recognized the item from the encoding list (recognition). If a participant failed to recall a word in the cued recall part, he/she was informed of the correct response and asked whether the item had been presented earlier or not. For each definition recognized as old, participants were asked whether they heard the item on the speakers or read the item on the computer screen (source recall). They were next asked to make a decision about their subjective experience of remembering the item (remember/know; Tulving, 1985; Gardiner, 1988). Proportions of hits were assessed for cued recall, recognition, and source, as well as the proportion of “remember” responses. All proportions were corrected for false alarms (i.e., when a participant qualified a new item as old).
We also administered a test of remote autobiographical memory that incorporated the remember/know procedure. Participants were asked to recall 25 typical autobiographical events (e.g., first job interview; giving a gift). For each event, the participant would choose among four responses: He or she remembered (in the same sense as in the previous task; Tulving, 1985) a single instance of the event occurring at any point during his or her lifespan, merely knew the event had taken place, thought the event had taken place but had absolutely no memory of it, or thought that the event had never taken place.
Finally, we administered the Autobiographical Interview (Levine et al., 2002). Briefly, participants were asked to recall five unique events (which took place at a specific time and in a specific place) from across their lifetimes (one each from childhood, teenage years, early adulthood, middle adulthood, and within the last year; for further details on method, see Levine et al., 2002). Only minimal, general cues (e.g., Can you tell me anything more about that?) were provided at this stage (free recall). This was followed by a semi-structured interview, in which the examiner asked a series of specific probe questions (e.g., In what part of the room did this event take place? What sounds do you remember?) to elicit further details about each event (the specific probe condition). The transcripts of each patient’s interview were scored by raters who had been extensively trained with high inter-rater reliability (intra-class correlation coefficient > 90%) already established according to standard procedures used in our laboratory (Levine et al., 2002). Comparison subjects’ and patients’ memories were pooled and assigned to scorers at random. Scorers were blind to subject group. Scoring involved counting bits of information in each report (following a standardized procedure) and separating them into two kinds: Internal details (details that were related to the event, e.g., perceptual details, thoughts and emotions, time, and place) that reflected episodic recall, and external details (extraneous information not directly related to the event, semantic information, and metacognitive or editorial statements) that reflected non-episodic or semantic recall (for further information on this distinction, see Levine et al., 2002). Data presented are collapsed across the five life periods.
Results and Discussion
Table 2 shows results on the standard neuropsychological measures (top half) and the experimental measures (bottom half). For each measure, we show each patient’s score (with those beyond the normal range for their age-appropriate control groups, that is z < −1.96, in bold), the mean and standard deviation for an overall control group of 10 healthy comparison subjects matched for age and education, and results of a t-test comparing the patient group to the overall group of 10 controls.
Overall, the patients were within the normal range on vocabulary, and generally they were not impaired (although most scored below average) on the executive measures. On the verbal memory task (HVLT-R), overall the patients scored below average, although only one fell into the impaired range (1051 on recall). Working memory (assessed using self-ordered pointing) was intact in all the patients except for 1022, who made a larger number of errors than normal.
On the experimental Remember/Know Source Memory task, all were within the normal range on cued recall. The patients were impaired on the recognition component of the task (with patient 1051 scoring outside of the normal range). However, parameter estimation for this measure is affected by restricted range in controls, who were at ceiling. When asked to report in which modality the word-definition pairings had been studied (i.e., source memory), no patient was significantly impaired. This may relate to the degree of perceptual separation between auditory and visual stimuli, as compared to the more subtle source manipulations that are used in other tasks. The largest effect was noted for “remember” responses, where four of five patients were severely impaired, and the fifth (1040) scored below average. This finding suggests that parietal cortex may support processes that are essential for the conscious experience of memory (i.e., recollection).1
Results from one of the two autobiographical memory tasks, however, were inconsistent with this view. Despite the fact that the Remember/Know Source Memory and Remember/Know Remote Autobiographical tasks used similar instructions for distinguishing between “remembering” and “knowing”, on the Remember/Know Remote Autobiographical task all but one of the patients’ “remember” scores were better than normal, and the patient who was below average (1047) was not significantly so. There are several possible reasons for the discrepancy between the two tasks: For example, the source memory task examined the anterograde domain, whereas the remote memory task chiefly examined the retrograde domain. Also, the events examined in the Remember/Know Source Memory task (word-definition pairings) were relatively similar to one another and context-poor, whereas those in the Remember/Know Remote Autobiographical task were unique, richly detailed, and vivid real-life experiences. Furthermore, participants were allowed to select events from across the lifespan, allowing for a relatively large pool of events from which to draw. Many of these events may be ones that the patients revisited in memory often, so that they may have had the impression that they were recollected vividly, when in fact they may contain fewer details than normal about the event itself. This interpretation is supported by their performance on a subsequent test in which details unique to autobiographical events were measured directly, rather than relying on the individual’s subjective impression (see below).
On the Autobiographical Interview, because the overall pattern of results was quite similar for the free recall and specific probe conditions, only data from the specific probe condition are reported in Table 2. Overall, the patients were above average on production of external details (i.e., semantic information about the events, or extraneous information not related to the events), but below average on production of internal details (i.e., episodic information related to the event, such as perceptual details, experienced thoughts and emotions, and so on). Examination of performance across time periods did not reveal a clear temporal gradient for this effect, nor did we see clear evidence in support of greater effects on time periods pre- or post-lesion. It should be noted, however, that we may have had insufficient power to detect such effects given the selection of only one memory per time period. We also calculated a ratio of internal-to-total details as an index of specificity of autobiographical recall, regardless of the total verbal output. All patients scored below average on this ratio, one significantly so (case 1040). Taken together, the internal and external detail scores suggest that the parietal patients were weak when recalling episodic aspects of autobiographical memory, despite relatively good memory for semantic elements.
Although autobiographical memory has rarely been examined in parietal lesion patients, Hunkin et al. (1995) reported a relevant case who claimed to have no episodic memories of his life before the age of 19, when he had suffered a closed head injury. MRI revealed bilateral occipital and parietal lesions (but no medial temporal damage), and formal memory testing yielded results consistent with the patient’s complaint. In patients with frontotemporal lobar degeneration, episodic autobiographical memory as assessed by the Autobiographical Interview was related to left inferior parietal parenchymal volume (along with bilateral temporal and left posterior cingulate/retrosplenial volumes; McKinnon et al., in press). Consistent with these patient reports, several functional neuroimaging studies of autobiographical recollection have revealed significant lateral parietal activity (e.g., Addis et al., 2004; Gilboa et al., 2004; Greenberg et al., 2005; Levine et al., 2004; for a review, see Svoboda et al., 2006), in addition to medial parietal, retrosplenial, and posterior cingulate activity. For example, Levine et al. (2004) played back audiotapes of descriptions of real-life experiences to volunteers who had recorded them over several weeks. Hearing episodic information from these reports in the scanner was associated with a greater degree of right lateral parietal activity than when hearing recordings of personal semantic information made simultaneously to the episodic recordings. Curiously, lateral parietal activation in autobiographical memory tends to be centered around the temporoparietal junction (reviewed in Svoboda et al., 2006), which is inferior to the intraparietal region Wagner et al. (2005) focused on in their review of laboratory recognition memory tasks.
Additional Case
At the time that we were examining the main group of patients, one of us (E. C.) conducted a search of her records and found an additional patient with lateral parietal damage. Although the materials used were different from those administered to the previous patients, our goal in examining this additional patient was the same as for the previous group: To determine how lateral parietal damage might affect memory.
Patient SM
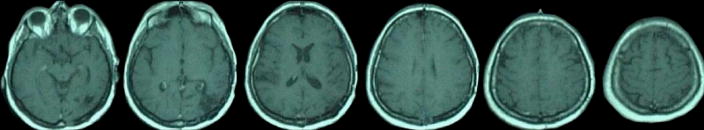
Patient SM was a 45 year old woman with 8 years of education. She had a lesion in left posterior parietal cortex (shown on T1-weighted magnetic resonance imaging [MRI] with gadolinium contrast for detection of residual tumour 6 months post-lesion in Figure 3), following surgery to remove a brain tumor four years earlier. She received a battery of clinical and experimental memory tests.
Figure 3.
Materials and Method
All testing was conducted in the patient’s native language of Italian, using Italian adaptations of the following measures (all clinical materials and methods from Spinnler & Tognoni, 1987) to evaluate global function (Mini-Mental State Exam [Folstein et al., 1975] and Standard Raven’s Matrices), language (Verbal Judgment Task, which requires people to judge whether sentences contain an absurdity or not, and to explain the meaning of common proverbs), neglect (the Bell cancellation task, in which people cross out as many bells as possible, which are intermixed among pictures of other objects on a sheet of A4 paper; Gauthier et al., 1989), executive function (Tower of London, Wisconsin Card Sorting, and verbal fluency tests), and memory (digit span, the Italian version of the Wechsler Memory Scale, the Buschke-Fuld Test, and a prose-passage recall task). The Buschke-Fuld Test (1974) is a standardized selective-reminding list learning task involving free recall; we used the Consistent Long Term Retrieval score from it (CLTR, i.e. the number of words recalled without further reminding until the last trial). On the prose-passage recall task, participants were read a short story and were required to recall immediately as many details about it as they could. Following the recall task, the passage was read again to them, and after a filled 10 minute interval they were asked to recall the passage again. A recall score accounting for both immediate and delayed performance was calculated based on Spinnler & Tognoni (1987).
SM was also administered a DRM recognition paradigm (Deese, 1959; Roediger & McDermott, 1995). Participants were presented with 8 lists of 15 words semantically related to a central theme (i.e. the critical lure; e.g., snore, pillow, and night, related to central theme sleep). These were Italian translations of the lists of semantic associates from Stadler, Roediger & McDermott (1999) and had already been employed in previous research (Ciaramelli, Ghetti, Fratterelli, & Ladavas, 2006). Recognition memory was tested immediately after the presentation of all the lists, using 24 studied and 24 unstudied words. Of the unstudied words, 8 were semantically related to the studied words (i.e., were critical lures) and 16 were not (i.e., target controls and lure controls). During the recognition test participants were also asked to label endorsed words according to the Remember/Know distinction (Tulving, 1985). In the DRM paradigm, hit rates and false-alarm rates to critical lures are corrected for baseline false-alarm rates to target controls and lure controls, respectively, resulting in a measure of true recognition (i.e. “corrected true recognition;” Schacter et al., 1996) and a measure of illusory recognition of words consistent with the gist of the studied lists (i.e. “corrected false recognition”). On this task, amnesic patients typically show lower levels of both corrected true and corrected false recognition compared to normal controls (Schacter et al., 1996; Melo et al., 1999; Ciaramelli et al., 2006), arguably due to difficulty remembering the gist of the studied lists. Patient SM was compared to six healthy controls matched for age, sex, and education.
Results and Discussion
Neuropsychological test results are shown in Table 3. Although SM had a mild contralesional hemianopia, she did not show neglect (i.e., she crossed out an equivalent number of bells in the left and right hemifields on the Bell cancellation test; Gauthier et al., 1989) or language problems. General intellectual skills were also intact, as assessed by performance on the Mini Mental State Exam and the Verbal Judgment Task (Spinnler & Tognoni, 1987). On the Wechsler Memory Scale, her overall score was close to normal (i.e., her General Memory index score was 90, where the normal mean and standard deviation are 100 and 15, respectively). However, she showed a severe deficit on the Paired Associates subtest of the WMS involving semantically unrelated words. Also, she had significant difficulty recalling a list of unrelated words (on an Italian version of the Buschke-Fuld selective reminding test; see Spinnler & Tognoni, 1987 for normative data) and recalling the Logical Memory story from the WMS, although her recall of a prose passage was borderline (Spinnler & Tognoni, 1987). Despite these problems with verbal memory, she performed relatively well on the visuospatial memory task on the WMS (visual reproduction), consistent with the fact that her damage was restricted to the left hemisphere. On the working memory measures from the WMS, she scored within the normal range on both forward and backward digit span.
Table 3.
Neuropsychological data for patient SM
| MMSE (raw) | 30 |
| Verbal judgement Task | 10 |
| Weschler Memory Scale | |
| General memory index | 90 |
| Subtests (raw) | |
| Information | 6 |
| Orientation | 5 |
| Mental control | 6 |
| Logical memory | 5.5 |
| Digit span forward | 5 |
| Digit span backward | 4 |
| Visual reproduction | 9 |
| Paired associates (easy) | 7.5 |
| Paired associates (hard) | 0 |
| Buschke–Fuld Test | |
| Consistent long term retrieval | 3 |
| Prose Recall Test | 6 |
| Tower of London Test | |
| Total Move Score | 10 |
| Rule Violation Score | 10 |
| WCST | |
| Number of categories (raw) | 6 |
| Perseverative responses | 10 |
| Verbal Fluency (Letter) | 10 |
| Digit Span | 10 |
| Bell Cancellation Test (raw) | |
| Left Bell (threshold = 15) | 16 |
| Right Bell (threshold = 15) | 16 |
Note. Scaled scores (unless noted otherwise), with higher scores indicating better performance. Scaled scores <5 (or equivalent for raw or index scores) are bolded to denote impaired performance.
On the experimental DRM recognition memory task (see Table 4), patient SM showed lower levels of both corrected true and corrected false recognition, a pattern indicating impaired memory for the gist of the studied lists, similar to results of previous studies of amnesic patients (Schacter et al., 1996; Melo et al., 1999; Ciaramelli et al., 2006). Interestingly, she showed reduced corrected true and false recognition compared to normal controls for Remember but not for Know responses, which suggests impaired recollection.
Table 4.
Results of experimental DRM recognition memory task for patient SM
| Old responses |
R responses |
K responses |
||||
|---|---|---|---|---|---|---|
| SM | Controls | SM | Controls | SM | Controls | |
| Targets | 0.62 | 0.85 | 0.25 | 0.47 | 0.37 | 0.38 |
| Target controls | 0.16 | 0.01 | 0.00 | 0.00 | 0.16 | 0.01 |
| Corrected true recognition | 0.46 | 0.84 | 0.25 | 0.47 | 0.21 | 0.37 |
| Critical lures | 0.75 | 0.77 | 0.12 | 0.52 | 0.62 | 0.25 |
| Lure controls | 0.25 | 0.00 | 0.00 | 0.00 | 0.25 | 0.00 |
| Corrected false recognition | 0.50 | 0.77 | 0.12 | 0.52 | 0.37 | 0.25 |
Note. Percentage of old, remember (R), and know (K) responses by participant and item type in the DRM paradigm, with scores below z = −1.96 bolded to denote impaired performance. Based on standard high-threshold procedures (e.g., Melo et al., 1999), we calculated corrected true recognition scores by subtracting the proportion of “old” responses to target-controls (i.e., items from non-studied lists) from the proportion of old responses to targets, and corrected false recognition scores by subtracting the proportion of “old” responses to lure-controls (i.e., critical lures of non-studied lists) from the proportion of old responses to critical lures.
During her assessment, patient SM complained spontaneously of memory problems in real life. She reported that she often felt that she did not know where her memories had come from, and was not confident in her own memories. We observed this phenomenon in the laboratory: While performing the experimental yes-no recognition memory task, after a hit she would ask “Am I right? Really? I am?” The experimental and anecdotal evidence from this patient (who had damage restricted to posterior parietal cortex) converge to suggest that she had a deficit in recollection and associated subjective states of remembering.
General Discussion
Although medial temporal and prefrontal systems are essential for episodic memory (for reviews, see Baldo & Shimamura, 2002; Davidson et al., 2006; Moscovitch et al., 2005, 2006; Squire et al., 2004; Tulving, 2002), recent functional neuroimaging studies have reported significant activation in other regions, including parietal cortex. As outlined in the introduction, both neuroimaging and human lesion studies indicate that the medial parietal region plays a critical role in episodic memory. However, based on independent reviews of the functional neuroimaging literature, Naghavi and Nyberg (2005), Skinner and Fernandes (2007), and Wagner et al. (2005; see also Vincent et al., 2006) suggested that lateral posterior parietal cortex may also be important for episodic memory. We evaluated this hypothesis by examining data from patients with focal damage to this region, and found that, although the patients could not be described as amnestic, they appeared to have disordered conscious recollection.
As noted in the introduction, there are several ways in which parietal damage might affect memory. Current hypotheses about lateral parietal effects on memory include awareness at retrieval, working memory demands, and memory for contextual details. Parietal cortex may support aspects of consciousness and awareness during retrieval. An intriguing example from our study that fits with this explanation is patient SM. She experienced a lack of confidence in her memories and said that her memories lacked experiential richness and robustness. Four of the five patients in our main study also demonstrated significantly reduced recollection as expressed by “remember” responses on an anterograde memory test. Furthermore, patients’ richness of recollection as assessed by the Autobiographical Interview was reduced. These data fit with the functional neuroimaging literature. That is, in fMRI studies in which participants are asked to report their subjective experience for each item they recognize (e.g., using the remember/know paradigm), parietal activations are often stronger for items that are accompanied by a vivid feeling of “remembering” than for those yielding a more impoverished feeling of “knowing” (Henson et al., 1999; Eldridge et al., 2000; M. E. Wheeler & Buckner, 2004; for a review, see Skinner & Fernandes, 2007).2 This finding is compatible with Naghavi and Nyberg’s (2005) explanation for parietal activations during memory retrieval: They noted that similar frontal and parietal regions are active across a variety of tasks – attention, imagery, working memory, and episodic memory retrieval – which reflect conscious awareness. Other neuroimaging data, however, suggest that this explanation might be too simple. For instance, Giovanello, Kensinger, and Schacter (2005) reported that whether one sees differential parietal activation for remembering versus knowing can depend on other factors, such as list length. Also, several studies have shown a dissociation within the parietal cortex, with some subregions more active during recollection but others more active during familiarity (e.g., Daselaar et al., 2006; Wagner et al., 2005; Yonelinas et al., 2005).
Another possibility is that parietal cortex supports attentional processing during memory retrieval (Naghavi & Nyberg, 2005; Wagner et al., 2005). Such attentional processing may not merely reflect the search of the memory store. If this were the case, then one might expect in fMRI to see equivalent parietal activation between trials in which one successfully recognizes an old item, versus those in which one rejects a new item; however, parietal activation is often stronger for the former than the latter (Wagner et al., 2005). Rather, parietal activation may reflect post-retrieval attentional capture by the old item, which supports one’s subjective sense of accurate retrieval. A related notion is that parietal activation during retrieval reflects rehearsal of retrieved information in working memory. Although working memory was normal in many of the patients with impaired recollection, this was assessed by the self-ordered pointing test (Petrides & Milner, 1982), which, strictly speaking, is a test of monitoring within working memory. This test has been validated in patients with mid-dorsolateral prefrontal and medial temporal lesions, but not as a measure of parietal function. A severe working memory deficit was clinically noted in one patient, 1051 (as evidenced by impaired performance on auditory and visual span tasks and on Trial 1 of verbal and design learning tasks), who was uniquely impaired on verbal learning and recognition tests, although he performed normally on the self-ordered pointing test; this patient had the largest lateral parietal lesion of the group, extending to deep white matter.
The last possibility that we mentioned in the introduction was that parietal activation reflects the retrieval of contextual information from memory, a notion raised by Wagner et al. (2005) and explored extensively by Rugg and colleagues (e.g., Rugg & Wilding, 1996; Uncapher et al., 2006; Vilberg & Rugg, in press), which fits with traditional models of parietal involvement in cross-modal perception (Critchley, 1953). This explanation gets mixed support from our data. On the one hand, some of our parietal lesion patients showed poor recollection of episodic details on the Autobiographical Interview. Furthermore, using the same Autobiographical Interview in patients with frontotemporal lobar degeneration, we found that the pattern of reduced internal and increased external details was related to the integrity of the left inferior parietal region (McKinnon et al., in press), replicating the present findings in an independent sample. On the other hand, the patients in the present study were all relatively good at remembering the modality (seen versus heard) in which they had studied word-definition pairings. Furthermore, M. L. Smith (personal communication, January 20, 2006) reported that she and Brenda Milner tested patients with extensive parietal lesions on a spatial memory task in which participants were required to remember the positions of different objects in an array (i.e., Smith & Milner, 1989). Parietal lesion patients had no trouble remembering the spatial context in which each of the different objects had been seen, although patients with medial temporal damage were reliably impaired. Finally, Simons et al. (in press) reported that patients with lateral parietal lesions were not significantly impaired when asked to recollect whether they had made a judgment involving either semantic category membership or pleasantness for each of series of faces and words at encoding (one of their patients was impaired but this was attributed to concomitant frontal lobe damage).
Conclusions and Future Work
We sought to examine the assertion that the lateral parietal region plays a previously-underappreciated role in episodic memory. Although functional neuroimaging data support this assertion, there are very few patient lesion data on this matter. Consistent with clinical impressions and previous studies, we found that patients with focal lateral parietal damage did not have amnesia, yet the patients did show evidence of disrupted recollection. This may be due to attentional or working memory effects at retrieval, or effects on retrieval of contextual information. At present there is insufficient evidence to indicate which of these processes are involved. These data, although preliminary, warrant further research on the ways in which the parietal region might support memory.
Future work will have to take into account several outstanding questions. First, to what degree are the effects of parietal damage on memory similar to, or different from, damage to other regions? In the present study, we found that parietal patients did not show a severe memory impairment like that which follows bilateral medial temporal lobe damage. However, it would be useful in future work to contrast the effects of parietal damage with damage to other regions that appear to be involved in memory, and are connected to the lateral parietal region, such as prefrontal cortex. Second, do different sub-regions of the lateral parietal area have specialized functions (as suggested by Wagner et al., 2005)? Note that this question is currently under investigation with respect to the prefrontal cortex: For example, some sub-regions of prefrontal cortex may be more important for recollection compared to familiarity (e.g., Duarte et al., 2005; Eldridge et al., 2000; Henson et al., 1999; M. E. Wheeler & Buckner, 2004; M. A. Wheeler & Stuss, 2003; for a review, see Skinner & Fernandes, 2007), but the exact brain-behaviour pattern one finds may depend on exactly what kind of paradigm one uses (cf. Ciaramelli & Ghetti, 2007; Duarte et al., 2006; Levine et al., 1999). In the present study, we examined only a small group of patients, making it difficult to say for certain whether different subregions of the lateral parietal zone play different roles in memory. Third, do the left and right hemispheres make different contributions, and if so, do they differ along material- or process-specific lines? In the current study, all but one of our patients had left hemisphere damage, and the tasks that we used were mostly verbal. Considering the possibly greater role of right compared to left parietal cortex in visual attention, there may be some hemispheric specialization in memory processes. Although a larger-scale patient study could help answer this question, it would also be useful to have a comprehensive meta-analysis of single-trial fMRI studies of episodic memory. Such a meta-analysis could contrast activation patterns seen, for example, with verbal compared to non-verbal stimuli, or during the encoding compared to the retrieval phase, in order to help guide lesion research. The reviews of parietal activation by Naghavi and Nyberg (2005), Skinner and Fernandes (2007), and Wagner et al. (2005) were each relatively selective, and none considered potential hemispheric differences in depth.
Although lesion studies are essential for determining whether a particular brain region is critical for a particular cognitive process, the lesion method is far from perfect. Interpretation of lesion data is complicated by many factors, including diaschisis (when damage to one area has effects on other areas that are connected to it), post-injury reorganization, and degeneracy (where a lesion causes a shift from one brain region or mental process to another, with no apparent effect on behavior). Thus, a particularly fruitful technique may be to combine functional neuroimaging and lesion approaches where patients’ lesions are well-defined, and memory performance is studied in depth (Price & Friston, 2002). Another potentially useful strategy is to induce temporary functional lesions using transcranial magnetic stimulation, although the behavioral effects of this method tend to be quite small (for an initial study, see Rossi et al., 2006). Because every method in cognitive neuroscience has its own strengths and weaknesses, using a combination of them is crucial to developing a more sophisticated picture of how episodic memory is supported by the brain.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Addis DR, Moscovitch M, Crawley AP, McAndrews MP. Recollective qualities modulate hippocampal activation during autobiographical memory retrieval. Hippocampus. 2004;14:752–762. doi: 10.1002/hipo.10215. [DOI] [PubMed] [Google Scholar]
- Aguirre GK, D’Esposito M. Topographical disorientation: A synthesis and taxonomy. Brain. 1999;122:1613–1628. doi: 10.1093/brain/122.9.1613. [DOI] [PubMed] [Google Scholar]
- Baddeley A. Working memory: Looking back and looking forward. Nature Reviews Neuroscience. 2003;4:829–839. doi: 10.1038/nrn1201. [DOI] [PubMed] [Google Scholar]
- Baldo JV, Shimamura AP. Frontal lobes and memory. In: Baddeley AD, Kopelman MD, Wilson BA, editors. The Handbook of Memory Disorders. 2. West Sussex: Wiley; 2002. [Google Scholar]
- Balint R. Psychic paralysis of gaze, optic ataxia, and spatial disorder of attention (reprinted from Monatsschrift fur psychiatrie und neurologie, vol 25, pg 51–81, 1909) Cognitive Neuropsychology. 1995;12:265–281. [Google Scholar]
- Benedict RHB, Schretlen D, Groninger L, Brandt J. Hopkins Verbal Learning Test--Revised: Normative data and analysis of inter-form and test-retest reliability. Clinical Neuropsychologist. 1998;12:43–55. [Google Scholar]
- Bisiach E, Luzzatti C. Unilateral neglect of representational space. Cortex. 1978;14:129–133. doi: 10.1016/s0010-9452(78)80016-1. [DOI] [PubMed] [Google Scholar]
- Bisiach E, Vallar G. Hemineglect in humans. In: Boller F, Grafman J, editors. Handbook of Neuropsychology. Vol. 1. Amsterdam: Elsevier; 1988. pp. 195–222. [Google Scholar]
- Blatt GJ, Pandya DN, Rosene DL. Parcellation of cortical afferents to three distinct sectors in the parahippocampal gyrus of the rhesus monkey: An anatomical and neurophysiological study. Journal of Comparative Neurology. 2003;466:161–179. doi: 10.1002/cne.10866. [DOI] [PubMed] [Google Scholar]
- Buschke H, Fuld PA. Evaluating storage, retention, and retrieval in disordered memory and learning. Neurology. 1974;24:1019–1025. doi: 10.1212/wnl.24.11.1019. [DOI] [PubMed] [Google Scholar]
- Butters N, Samuels I, Goodglass H, Brody B. Short-term visual and auditory memory disorders after parietal and frontal lobe damage. Cortex. 1970;6:440–459. doi: 10.1016/s0010-9452(70)80008-9. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L. Imaging cognition II: An empirical review of 275 PET and fMRI studies. Journal of Cognitive Neuroscience. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- Cansino S, Maquet P, Dolan RJ, Rugg MD. Brain activity underlying encoding and retrieval of source memory. Cerebral Cortex. 2002;12:1048–1056. doi: 10.1093/cercor/12.10.1048. [DOI] [PubMed] [Google Scholar]
- Cavada C, Goldman-Rakic PS. Posterior parietal cortex in rhesus-monkey. 2. evidence for segregated corticocortical networks linking sensory and limbic areas with the frontal-lobe. Journal of Comparative Neurology. 1989;287:422–445. doi: 10.1002/cne.902870403. [DOI] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: A review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Ciaramelli E, Ghetti S. What are confabulators’ memories made of? A study of subjective and objective measures of recollection in confabulation. Neuropsychologia. 2007;45:1489–1500. doi: 10.1016/j.neuropsychologia.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Ciaramelli E, Ghetti S, Frattarelli M, Ladavas E. When true memory availability promotes false memory: Evidence from confabulating patients. Neuropsychologia. 2006;44:1866–77. doi: 10.1016/j.neuropsychologia.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Clower DM, West RA, Lynch JC, Strick PL. The inferior parietal lobule is the target of output from the superior colliculus, hippocampus, and cerebellum. Journal of Neuroscience. 2001;21:6283–6291. doi: 10.1523/JNEUROSCI.21-16-06283.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Craik FIM. Effects of dividing attention on encoding and retrieval processes. In: Roediger HL III, Nairne JS, Neath I, Surprenant AM, editors. The nature of remembering: Essays in honor of Robert G. Crowder. Washington, DC: American Psychological Association; 2001. pp. 55–68. [Google Scholar]
- Critchley M. The parietal lobes. Oxford, UK: Williams and Wilkins; 1953. [Google Scholar]
- Dade LA, Gao FQ, Kovacevic N, Roy P, Rockel C, O’Toole CM, Lobaugh NJ, Feinstein A, Levine B, Black SE. Semiautomatic brain region extraction: a method of parcellating brain regions from structural magnetic resonance images. Neuroimage. 2004;22:1492–1502. doi: 10.1016/j.neuroimage.2004.03.023. [DOI] [PubMed] [Google Scholar]
- Danckert J, Ferber S. Revisiting unilateral neglect. Neuropsychologia. 2006;44:987–1006. doi: 10.1016/j.neuropsychologia.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Fleck MS, Cabeza R. Triple dissociation in the medial temporal lobes: Recollection, familiarity, and novelty. Journal of Neurophysiology. 2006;96:1902–1911. doi: 10.1152/jn.01029.2005. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Prince SE, Cabeza R. When less means more: Deactivations during encoding that predict subsequent memory. NeuroImage. 2004;23:921–927. doi: 10.1016/j.neuroimage.2004.07.031. [DOI] [PubMed] [Google Scholar]
- Davidson PSR, Troyer AK, Moscovitch M. Frontal lobe contributions to recognition and recall: Linking basic research with clinical evaluation and remediation. Journal of the International Neuropsychological Society. 2006;12:210–223. doi: 10.1017/S1355617706060334. [DOI] [PubMed] [Google Scholar]
- Deese J. On the prediction of occurrence of particular verbal intrusions in immediate recall. Journal of Experimental Psychology. 1959;58:17–22. doi: 10.1037/h0046671. [DOI] [PubMed] [Google Scholar]
- De Renzi E, Nichelli P. Verbal and non-verbal short-term memory impairment following hemispheric damage. Cortex. 1975;11:341–354. doi: 10.1016/s0010-9452(75)80026-8. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Cooney JW, Gazzaley A, Gibbs SEB, Postle BR. Is the prefrontal cortex necessary for delay task performance? evidence from lesion and fMRI data. Journal of the International Neuropsychological Society. 2006;12:248–260. doi: 10.1017/S1355617706060322. [DOI] [PubMed] [Google Scholar]
- Dimond SJ. Brain circuits for consciousness. Brain Behavior and Evolution. 1976;13:376–395. doi: 10.1159/000123823. [DOI] [PubMed] [Google Scholar]
- Dobbins IG, Rice HJ, Wagner AD, Schacter DL. Memory orientation and success: Separable neurocognitive components underlying episodic recognition. Neuropsychologia. 2003;41:318–333. doi: 10.1016/s0028-3932(02)00164-1. [DOI] [PubMed] [Google Scholar]
- Driver J, Vuilleumier P. Perceptual awareness and its loss in unilateral neglect and extinction. Cognition. 2001;79:39–88. doi: 10.1016/s0010-0277(00)00124-4. [DOI] [PubMed] [Google Scholar]
- Duarte A, Ranganath C, Knight RT. Effects of unilateral prefrontal lesions on familiarity, recollection, and source memory. Journal of Neuroscience. 2005;25:8333–8337. doi: 10.1523/JNEUROSCI.1392-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge LL, Knowlton BT, Furmanski CS, Bookheimer SY, Engel SA. Remembering episodes: A selective role for the hippocampus during retrieval. Nature Neuroscience. 2000;3:1149–1152. doi: 10.1038/80671. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, Mchugh PR. Mini-mental state - practical method for grading cognitive state of patients for clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gardiner JM. Functional-aspects of recollective experience. Memory & Cognition. 1988;16:309–313. doi: 10.3758/bf03197041. [DOI] [PubMed] [Google Scholar]
- Gauthier L, Dehaut F, Joanette Y. The bells test - a quantitative and qualitative test for visual neglect. International Journal of Clinical Neuropsychology. 1989;11:49–54. [Google Scholar]
- Gilboa A, Winocur G, Grady CL, Hevenor SJ, Moscovitch M. Remembering our past: Functional neuroanatomy of recollection of recent and very remote personal events. Cerebral Cortex. 2004;14:1214–1225. doi: 10.1093/cercor/bhh082. [DOI] [PubMed] [Google Scholar]
- Giovanello KS, Kensinger EA, Schacter DL. Left parietal contributions to episodic retrieval: Effects of retrieval demand. Society for Neuroscience Abstracts 2005 [Google Scholar]
- Greenberg DL, Rice HJ, Cooper JJ, Cabeza R, Rubin DC, LaBar KS. Co-activation of the amygdala, hippocampus and inferior frontal gyrus during autobiographical memory retrieval. Neuropsychologia. 2005;43:659–674. doi: 10.1016/j.neuropsychologia.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Hayes SM, Ryan L, Schnyer DM, Nadel L. An fMRI Study of Episodic Memory: Retrieval of Object, Spatial, and Temporal Information. Behavioral Neuroscience. 2004;118:885–896. doi: 10.1037/0735-7044.118.5.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilman KM, Watson RT, Schulman HM. Unilateral memory defect. Journal of Neurology Neurosurgery and Psychiatry. 1974;37:790–793. doi: 10.1136/jnnp.37.7.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson RNA, Rugg MD, Shallice T, Josephs O, Dolan RJ. Recollection and familiarity in recognition memory: An event-related functional magnetic resonance imaging study. Journal of Neuroscience. 1999;19:3962–3972. doi: 10.1523/JNEUROSCI.19-10-03962.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insausti R, Amaral DG, Cowan WM. The entorhinal cortex of the monkey. II. Cortical afferents. Journal of Comparative Neurology. 1987;264:356–395. doi: 10.1002/cne.902640306. [DOI] [PubMed] [Google Scholar]
- Insausti R, Munoz M. Cortical projections of the non-entorhinal hippocampal formation in the cynomolgus monkey (macaca fascicularis) European Journal of Neuroscience. 2001;14:435–451. doi: 10.1046/j.0953-816x.2001.01662.x. [DOI] [PubMed] [Google Scholar]
- Karnath HO, Ferber S, Himmelbach M. Spatial awareness is a function of the temporal not the posterior parietal lobe. Nature. 2001;411:950–953. doi: 10.1038/35082075. [DOI] [PubMed] [Google Scholar]
- Kirchhoff BA, Wagner AD, Maril A, Stern CE. Prefrontal-temporal circuitry for episodic encoding and subsequent memory. Journal of Neuroscience. 2000;20:6173–6180. doi: 10.1523/JNEUROSCI.20-16-06173.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y, Amaral DG. Macaque monkey retrosplenial cortex: II. cortical afferents. Journal of Comparative Neurology. 2003;466:48–79. doi: 10.1002/cne.10883. [DOI] [PubMed] [Google Scholar]
- Kovacevic N, Lobaugh NJ, Bronskill MJ, Levine B, Feinstein A, Black SE. A robust method for extraction and automatic segmentation of brain images. Neuroimage. 2002;17:1087–1100. doi: 10.1006/nimg.2002.1221. [DOI] [PubMed] [Google Scholar]
- Lavenex P, Suzuki WA, Amaral DG. Perirhinal and parahippocampal cortices of the macaque monkey: Projections to the neocortex. Journal of Comparative Neurology. 2002;447:394–420. doi: 10.1002/cne.10243. [DOI] [PubMed] [Google Scholar]
- Levine B, Svoboda E, Hay JF, Winocur G. Aging and autobiographical memory: Dissociating episodic from semantic retrieval. Psychology and Aging. 2002;17:677–689. [PubMed] [Google Scholar]
- Levine B, Freedman M, Dawson D, Black SE, Stuss DT. Ventral frontal contribution to self-regulation: Convergence of episodic memory and inhibition. Neurocase. 1999;5:263–275. [Google Scholar]
- Levine B, Turner GR, Tisserand D, Hevenor SJ, Graham SJ, McIntosh AR. The functional neuroanatomy of episodic and semantic autobiographical remembering: A prospective functional MRI study. Journal of Cognitive Neuroscience. 2004;16:1633–1646. doi: 10.1162/0898929042568587. [DOI] [PubMed] [Google Scholar]
- Lewis JW, Van Essen DC. Corticocortical connections of visual, sensorimotor, and multimodal processing areas in the parietal lobe of the macaque monkey. Journal of Comparative Neurology. 2000;428:112–137. doi: 10.1002/1096-9861(20001204)428:1<112::aid-cne8>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Luria AR. Higher Cortical Functions in Man. Oxford, UK: Basic Books; 1966. [Google Scholar]
- Luria AR. The Neuropsychology of Memory. Oxford, UK: V. H. Winston & Sons; 1976. [Google Scholar]
- Maguire EA. The retrosplenial contribution to human navigation: A review of lesion and neuroimaging findings. Scandinavian Journal of Psychology. 2001;42:225–238. doi: 10.1111/1467-9450.00233. [DOI] [PubMed] [Google Scholar]
- Markowitsch HJ, Kalbe E, Kessler J, von Stockhausen HM, Ghaemi M, Heiss WD. Short-term memory deficit after focal parietal damage. Journal of Clinical and Experimental Neuropsychology. 1999;21:784–797. doi: 10.1076/jcen.21.6.784.853. [DOI] [PubMed] [Google Scholar]
- Martin RC. Components of short-term memory and their relation to language processing - Evidence from neuropsychology and neuroimaging. Current Directions in Psychological Science. 2005;14:204–208. [Google Scholar]
- Mayer E, Martory MD, Pegna AJ, Landis T, Delavelle J, Annoni JM. A pure case of gerstmann syndrome with a subangular lesion. Brain. 1999;122:1107–1120. doi: 10.1093/brain/122.6.1107. [DOI] [PubMed] [Google Scholar]
- McKinnon MC, Nica EI, Sengdy P, Kovacevic N, Moscovitch M, Freedman M, Miller BL, Black SE, Levine B. Autobiographical memory and patterns of brain atrophy in frontotemporal lobar degeneration. Journal of Cognitive Neuroscience. doi: 10.1162/jocn.2008.20126. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo B, Winocur G, Moscovitch M. False recall and false recognition: An examination of the effects of selective and combined lesions to the medial temporal lobe diencephalon and frontal lobe structures. Cognitive Neuropsychology. 1999;16:343–359. [Google Scholar]
- Milner B. Visual recognition and recall after right temporal-lobe excision in man. Neuropsychologia. 1968;6:191–209. doi: 10.1016/j.yebeh.2003.08.027. [DOI] [PubMed] [Google Scholar]
- Milner AD, Goodale MA. The visual brain in action. Oxford, UK: Oxford University Press; 1995. [Google Scholar]
- Mishkin M, Ungerleider LG, Macko KA. Object vision and spatial vision - 2 cortical pathways. Trends in Neurosciences. 1983;6:414–417. [Google Scholar]
- Morcom AM, Good CD, Frackowiak RSJ, Rugg MD. Age effects on the neural correlates of successful memory encoding. Brain. 2003;126:213–229. doi: 10.1093/brain/awg020. [DOI] [PubMed] [Google Scholar]
- Morris R, Petrides M, Pandya DN. Architecture and connections of retrosplenial area 30 in the rhesus monkey (macaca mulatta) European Journal of Neuroscience. 1999a;11:2506–2518. doi: 10.1046/j.1460-9568.1999.00672.x. [DOI] [PubMed] [Google Scholar]
- Morris R, Pandya DN, Petrides M. Fiber system linking the mid-dorsolateral frontal cortex with the retrosplenial/presubicular region in the rhesus monkey. Journal of Comparative Neurology. 1999b;407:183–192. doi: 10.1002/(sici)1096-9861(19990503)407:2<183::aid-cne3>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Moscovitch M. Memory and working-with-memory - a component process model based on modules and central systems. Journal of Cognitive Neuroscience. 1992;4:257–267. doi: 10.1162/jocn.1992.4.3.257. [DOI] [PubMed] [Google Scholar]
- Moscovitch M, Nadel L, Winocur G, Gilboa A, Rosenbaum RS. The cognitive neuroscience of remote episodic, semantic and spatial memory. Current Opinion in Neurobiology. 2006;16:179–190. doi: 10.1016/j.conb.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Moscovitch M, Rosenbaum RS, Gilboa A, Addis DR, Westmacott R, Grady C, McAndrews MP, Levine B, Black S, Winocur G, Nadel L. Functional neuroanatomy of remote episodic, semantic and spatial memory: A unified account based on multiple trace theory. Journal of Anatomy. 2005;207:35–66. doi: 10.1111/j.1469-7580.2005.00421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz M, Insausti R. Cortical efferents of the entorhinal cortex and the adjacent parahippocampal region in the monkey (macaca fascicularis) European Journal of Neuroscience. 2005;22:1368–1388. doi: 10.1111/j.1460-9568.2005.04299.x. [DOI] [PubMed] [Google Scholar]
- Naghavi HR, Nyberg L. Common fronto-parietal activity in attention, memory, and consciousness: Shared demands on integration? Consciousness and Cognition. 2005;14:390–425. doi: 10.1016/j.concog.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Petrides M, Milner B. Deficits on subject-ordered tasks after frontal-lobe and temporal-lobe lesions in man. Neuropsychologia. 1982;20:249–262. doi: 10.1016/0028-3932(82)90100-2. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Dorsolateral prefrontal cortex: Comparative cytoarchitectonic analysis in the human and the macaque brain and corticocortical connection patterns. European Journal of Neuroscience. 1999;11:1011–1036. doi: 10.1046/j.1460-9568.1999.00518.x. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Projections to the frontal-cortex from the posterior parietal region in the rhesus-monkey. Journal of Comparative Neurology. 1984;228:105–116. doi: 10.1002/cne.902280110. [DOI] [PubMed] [Google Scholar]
- Picton TW, Stuss DT, Shallice T, Alexander MP, Gillingham S. Keeping time: Effects of focal frontal lesions. Neuropsychologia. 2006;44:1195–1209. doi: 10.1016/j.neuropsychologia.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Posner MI, Petersen SE. The attention system of the human brain. Annual Review of Neuroscience. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Price CJ, Friston KJ. Degeneracy and cognitive anatomy. Trends in Cognitive Science. 2002;6:416–421. doi: 10.1016/s1364-6613(02)01976-9. [DOI] [PubMed] [Google Scholar]
- Rafal RD. Hemispatial neglect: Neuropsychological approaches and issues. In: Feinberg TE, Farah MJ, editors. Behavioral neurology and neuropsychology. New York: McGraw-Hill; 1997. pp. 319–335. [Google Scholar]
- Rafal RD. Balint’s syndrome. In: D’Esposito M, editor. Neurological foundations of cognitive neuroscience. Cambridge, MA: MIT Press; 2002. [Google Scholar]
- Rockland KS, Van Hoesen GW. Some temporal and parietal cortical connections converge in CA1 of the primate hippocampus. Cerebral Cortex. 1999;9:232–237. doi: 10.1093/cercor/9.3.232. [DOI] [PubMed] [Google Scholar]
- Roediger HL, Mcdermott KB. Creating false memories - remembering words not presented in lists. Journal of Experimental Psychology-Learning Memory and Cognition. 1995;21:803–814. [Google Scholar]
- Rossi S, Pasqualetti P, Zito G, Vecchio F, Cappa SF, Miniussi C, Babiloni C, Rossini PM. Prefrontal and parietal cortex in human episodic memory: an interference study by repetitive transcranial magnetic stimulation. European Journal Of Neuroscience. 2006;23:793–800. doi: 10.1111/j.1460-9568.2006.04600.x. [DOI] [PubMed] [Google Scholar]
- Rudge P, Warrington EK. Selective impairment of memory and visual-perception in splenial tumors. Brain. 1991;114:349–360. doi: 10.1093/brain/114.1.349. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Wilding EL. An event-related potential study of recognition memory with and without retrieval of source. Brain. 1996;119:889–905. doi: 10.1093/brain/119.3.889. [DOI] [PubMed] [Google Scholar]
- Samuels I, Butters N, Goodglas H. Visual memory deficits following cortical and limbic lesions - effect of field of presentation. Physiology & Behavior. 1971;6:447–452. doi: 10.1016/0031-9384(71)90181-8. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Verfaellie M, Pradere D. Neuropsychology of memory illusions: False recall and recognition in amnesic patients. Journal of Memory and Language. 1996;35:319–334. [Google Scholar]
- Shipley WC. Institute of Living Scale. Los Angeles: Western Psychological Services; 1946. [Google Scholar]
- Simons JS, Peers PV, Hwang DY, Ally BA, Fletcher PC, Budson AE. Is the parietal lobe necessary for recollection in humans. Neuropsychologia. doi: 10.1016/j.neuropsychologia.2007.07.024. in press. [DOI] [PubMed] [Google Scholar]
- Skinner EI, Fernandes MA. Neural correlates of recollection and familiarity: A review of neuroimaging and patient data. Neuropsychologia. 2007;45:2163–2179. doi: 10.1016/j.neuropsychologia.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Smith EE, Jonides J. Neuroimaging analyses of human working memory. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:12061–12068. doi: 10.1073/pnas.95.20.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ML, Milner B. Right hippocampal impairment in the recall of spatial location: encoding deficit or rapid forgetting? Neuropsychologia. 1989;27:71–81. doi: 10.1016/0028-3932(89)90091-2. [DOI] [PubMed] [Google Scholar]
- Söderlund H, Black SE, Miller BL, Freedman M, Levine B. Episodic memory and regional atrophy in frontotemporal lobar degeneration. Neuropsychologia. doi: 10.1016/j.neuropsychologia.2007.08.003. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinnler H, Tognoni G. Standardizzazione e taratura italiana di test neuropsicologici (Italian norms for a battery of neuropsychological tests) The Italian Journal of Neurological Science. 1987;6(Suppl 8):1–120. [PubMed] [Google Scholar]
- Spreen O, Srauss E. A compendium of neuropsychological tests: administration, norms, and commentary. 2. New York: Oxford University Press; 1998. [Google Scholar]
- Squire LR, Stark CE, Clark RE. The medial temporal lobe. Annual Review of Neuroscience. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- Stadler MA, Roediger HL, McDermott KB. Norms for word lists that create false memories. Memory & Cognition. 1999;27:494–500. doi: 10.3758/bf03211543. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Levine B, Alexander MP, Hong J, Palumbo C, Hamer L, et al. Wisconsin card sorting test performance in patients with focal frontal and posterior brain damage: Effects of lesion location and test structure on separable cognitive processes. Neuropsychologia. 2000;38:388–402. doi: 10.1016/s0028-3932(99)00093-7. [DOI] [PubMed] [Google Scholar]
- Suzuki WA, Amaral DG. Perirhinal and parahippocampal cortices of the macaque monkey - cortical afferents. Journal of Comparative Neurology. 1994;350:497–533. doi: 10.1002/cne.903500402. [DOI] [PubMed] [Google Scholar]
- Svoboda E, McKinnon MC, Levine B. The functional neuroanatomy of autobiographical memory: A meta-analysis. Neuropsychologia. 2006;44:2189–2208. doi: 10.1016/j.neuropsychologia.2006.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N, Kawamura M, Shiota J, Kasahata N, Hirayama K. Pure topographic disorientation due to right retrosplenial lesion. Neurology. 1997;49:464–469. doi: 10.1212/wnl.49.2.464. [DOI] [PubMed] [Google Scholar]
- Tulving E. Memory and consciousness. Canadian Psychology-Psychologie Canadienne. 1985;26:1–12. [Google Scholar]
- Tulving E. Episodic memory: from mind to brain. Annual Review of Psychology. 2002;53:1–25. doi: 10.1146/annurev.psych.53.100901.135114. [DOI] [PubMed] [Google Scholar]
- Tulving E, Watkins OC. Recognition failure of words with a single meaning. Memory & Cognition. 1977;5:513–522. doi: 10.3758/BF03197394. [DOI] [PubMed] [Google Scholar]
- Uncapher MR, Otten LJ, Rugg MD. Episodic encoding is more than the sum of its parts: an fMRI investigation of multifeatural encoding. Neuron. 2006;52:547–556. doi: 10.1016/j.neuron.2006.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenstein E, Bowers D, Verfaellie M, Heilman KM, Day A, Watson RT. Retrosplenial amnesia. Brain. 1987;110:1631–1646. doi: 10.1093/brain/110.6.1631. [DOI] [PubMed] [Google Scholar]
- Van Hoesen GW, Pandya DN. Some connections of entorhinal (area 28) and perirhinal (area 35) cortices of rhesus-monkey. I. Temporal-lobe afferents. Brain Research. 1975;95:1–24. doi: 10.1016/0006-8993(75)90204-8. [DOI] [PubMed] [Google Scholar]
- Vilberg KL, Rugg MD. Dissociation of the neural correlates of recollection and familiarity: Further evidence from fMRI. Neuropsychologia in press. [Google Scholar]
- Vincent JL, Snyder AZ, Fox MD, Shannon BJ, Andrews JR, Raichle ME, Buckner RL. Coherent spontaneous activity identifies a hippocampal-parietal memory network. Journal of Neurophysiology. 2006;96:3517–3531. doi: 10.1152/jn.00048.2006. [DOI] [PubMed] [Google Scholar]
- Von Cramon DY, Schuri U. The septohippocampal pathways and their relevance to human-memory - a case-report. Cortex. 1992;28:411–422. doi: 10.1016/s0010-9452(13)80151-7. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Schwartz S, Clarke K, Husain M, Driver J. Testing memory for unseen visual stimuli in patients with extinction and spatial neglect. Journal of Cognitive Neuroscience. 2002;14:875–886. doi: 10.1162/089892902760191108. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Schacter DL, Rotte M, Koutstaal W, Maril A, Dale AM, et al. Building memories: Remembering and forgetting of verbal experiences as predicted by brain activity. Science. 1998;281:1188–1191. doi: 10.1126/science.281.5380.1188. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Shannon BJ, Kahn I, Buckner RL. Parietal lobe contributions to episodic memory retrieval. Trends in Cognitive Sciences. 2005;9:445–453. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Warrington EK, Logue V, Pratt RTC. Anatomical localisation of selective impairment of auditory verbal short-term memory. Neuropsychologia. 1971;9:377–387. doi: 10.1016/0028-3932(71)90002-9. [DOI] [PubMed] [Google Scholar]
- Warrington EK, Shallice T. Selective impairment of auditory verbal short-term memory. Brain. 1969;92:885–896. doi: 10.1093/brain/92.4.885. [DOI] [PubMed] [Google Scholar]
- Wheeler MA, Stuss DT. Remembering and knowing in patients with frontal lobe injuries. Cortex. 2003;39:827–846. doi: 10.1016/s0010-9452(08)70866-9. [DOI] [PubMed] [Google Scholar]
- Wheeler ME, Buckner RL. Functional-anatomic correlates of remembering and knowing. NeuroImage. 2004;21:1337–1349. doi: 10.1016/j.neuroimage.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Xing J, Andersen RA. Models of the posterior parietal cortex which perform multimodal integration and represent space in several coordinate frames. Journal of Cognitive Neuroscience. 2000;12:601–614. doi: 10.1162/089892900562363. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP. The Nature of Recollection and Familiarity: A review of 30 Years of Research. Journal of Memory and Language. 2002;46:441–517. [Google Scholar]
- Yonelinas AP, Kroll NEA, Dobbins I, Lazzara M, Knight RT. Recollection and familiarity deficits in amnesia: Convergence of remember-know, process dissociation, and receiver operating characteristic data. Neuropsychology. 1998;12:323–339. doi: 10.1037//0894-4105.12.3.323. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP, Otten LJ, Shaw KN, Rugg MD. Separating the brain regions involved in recollection and familiarity in recognition memory. Journal of Neuroscience. 2005;25:3002–3008. doi: 10.1523/JNEUROSCI.5295-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]