Abstract
Signaling from the endothelin-A (Ednra) receptor is responsible for initiating multiple signaling pathways within neural crest cells (NCCs). Loss of this initiation is presumably the basis for the craniofacial defects observed in Ednra−/− embryos. However, it is not known whether continued Ednra signaling in NCC derivatives is required for subsequent development of the lower jaw. To address this question, mice containing loxP recombination sequences flanking a portion of the Ednra gene were bred with transgenic mice that express Cre recombinase under control of a Dlx5/6 enhancer element. We find that while Ednra gene inactivation within the mandibular arch of these Ednra conditional knockout embryos is detectable by embryonic day (E) 10.5, mandibular arch-specific gene expression is normal, as is overall mandible development. These results suggest that while Ednra receptor signaling is crucial for early NCC patterning, subsequent Ednra signaling is not essential for mandible bone development.
Keywords: Craniofacial, Neural crest, loxP, Cre recombinase, Knockout mice, Transgenic mice
Introduction
Lower jaw formation is arguably one of the more amazing morphological achievements of embryogenesis. Much of the facial skeleton is formed by cephalic neural crest cells (NCCs), which migrate to the pharyngeal arches from the midbrain/hindbrain region, subsequently giving rise to bone and cartilage (Noden 1983; Lumsden et al. 1991; Serbedzija et al. 1992). NCCs appear to be patterned once in the arches by environmental signals from the surrounding tissues (Le Douarin et al. 1993; Couly et al. 2002; Trainor et al. 2002; Schneider and Helms 2003). However, the ability of NCCs and their derivatives to respond to these signals appears to decrease with time. In E9.0 explanted mandibles, Fgf8-soaked beads can induce widespread Lhx7 expression, though by E11.0, Lhx7 expression is independent of Fgf8 (Tucker et al. 1999). These findings illustrate that NCC patterning signals may only be necessary for the initiation of developmental pathways within NCCs but not during subsequent mesenchymal differentiation.
Endothelin-1 (Edn1), expressed by the surrounding ectoderm, core paraxial mesoderm and pharyngeal pouch endoderm, is also crucial for NCC patterning, acting through the endothelin-A receptor (Ednra) located on NCCs (for review, see Clouthier and Schilling 2004). Targeted inactivation of either Edn1 or Ednra results in severe craniofacial and cardiovascular defects that are attributable to aberrant NCC development (Kurihara et al. 1994; Clouthier et al. 1998; Yanagisawa et al. 1998). These include an apparent homeotic transformation of lower jaw structures to more maxillary-like derivatives (Ozeki et al. 2004; Ruest et al. 2004). While these findings indicate a role for Ednra signaling in initiating early crest cell patterning, it is not clear if continued Ednra signaling is required to maintain patterning mechanisms and whether Ednra signaling is required for later differentiation of the crest derived mesenchyme. To address these questions, we specifically inactivated the Ednra gene in a subset of cells within the mandibular arch using Cre/loxP technology.
Materials and methods
Animals
Ednraflox/flox (Kedzierski et al. 2003) and Dlx5/6-Cre (Ruest et al. 2003) mice were generated as previously described. Ednraflox/flox; Dlx5/6-Cre embryos were generated by crossing Ednraflox/+; Dlx5/6-Cre female mice with Ednraflox/flox male mice. We also generated conditional knockout mice carrying one conventional Ednra mutant allele (Ednra+/−; Clouthier et al. 1998) and one conditional allele (Ednraflox/−).Ednraflox/− female mice were bred with Ednraflox/flox; Dlx5/6-Cre male mice to generate Ednraflox/−; Dlx5/6-Cre embryos. Genotyping was performed by PCR using genomic DNA prepared from tail biopsies or amniotic sacs. Dlx5/6-Cre mouse genotyping was performed as previously described (Ruest et al. 2003). The genotyping of the Ednra conditional allele was performed with the following primers: 5′-ACACAACCATGGTGTCGA-3′ and 5′-CGGTTCTTATCCATCTCATC-3′. These primers flank the 5′ loxP site located between the fifth and sixth exons of the conditionally targeted Ednra gene (Kedzierski et al. 2003), thus producing ~420 bp and ~380 bp bands in Ednraflox/+ animals, a single ~420 bp band in Ednraflox/flox animals and a single ~380 bp band in Ednra+/+mice. Reaction products were visualized on a 1.5% agarose gel. Ednra mutant genotyping was performed as previously described (Clouthier et al. 1998).
To determine whether the conditional Ednra gene had undergone recombination, genomic DNA was extracted from the mandibular pharyngeal arch of E9.5 and E10.5 embryos. To obtain bone DNA, the mandible bone was dissected from 3-month-old Ednraflox/flox; Dlx5/6-Cre females and cleaned of all muscle and tendon tissue. Mandibles were ground in liquid nitrogen and incubated for 48 h in 1 ml of 0.5 M EDTA (pH 8.0)/2% sarkosyl with agitation at room temperature. After addition of TRIS (pH 8.0) and NaCl to 20 mM and 400 mM final concentration, respectively, samples were incubated with proteinase K (10 mg/ml) at 55°C overnight. Following phenol/chloroform extraction, samples were dialyzed in Spectra Float A Lyzer tubes (3500 MWcut-off) for 20 h in TE buffer. After addition of sodium acetate and isopropyl alcohol, DNA was precipitated and washed with 75% ethanol. DNAwas used in a recombinant PCR reaction with the following primers: 5′-ACACAACCATGTTGTCGAGGTCGA-3′ and 5′-GAGAACCTACAACTGGGGACACAAACAC-3′. Recombination of the conditional Ednra allele gives rise to a 1.2 kb band.
Skeleton staining and histology
Skeleton staining was performed as previously described (McLeod 1980). Skeletons were preserved in glycerol or in 25% glycerol/75% ethanol. Skeletons were photographed with an Olympus DP11 digital camera mounted on an Olympus SZX12 stereomicroscope. For histological analysis, E18.5 embryos were fixed in 10% neutral buffered formalin (Sigma), dehydrated in graded ethanols and then embedded in paraffin. Eight-µm sagittal sections were then collected onto Plus-coated slides. Every other section was then counterstained with hematoxylin and eosin (H&E), dehydrated in graded ethanols and then coverslipped using DPX mounting medium (BDH). Sections were examined and photographed on an E600 Nikon microscope fitted with a Spot-RT digital camera. To examine the extent of calcification in the mandible of embryos, contiguous slides to those used for H&E analysis were deparaffinized and rehydrated and then subjected to Van Kossa’s method. Briefly, sections were incubated in 5% silver nitrate for 1 h at room temperature under a 60-W bulb. Slides were then rinsed 3 times in distilled water and incubated in 5% sodium thiosulfate. Sections were rinsed in water and then counterstained with nuclear fast red before dehydrating and mounting as described above. We analyzed more than 20 Ednraflox/flox; Dlx5/6-Cre embryos and ten Ednraflox/−; Dlx5/6-Cre embryos.
In situ hybridization
Whole-mount in situ hybridization analysis was performed as previously described (Clouthier et al. 2000). Embryos were hybridized with digoxigenin (DIG)-labeled cRNA riboprobes against dHAND (Srivastava et al. 1997) and Dlx5 (Depew et al. 1999). Riboprobe labeling was performed using the DIG labeling kit (Roche). Stained embryos were photographed in whole-mount as described above.
Results
Generation of Ednraflox/flox; Dlx5/6-Cre conditional knockout mice
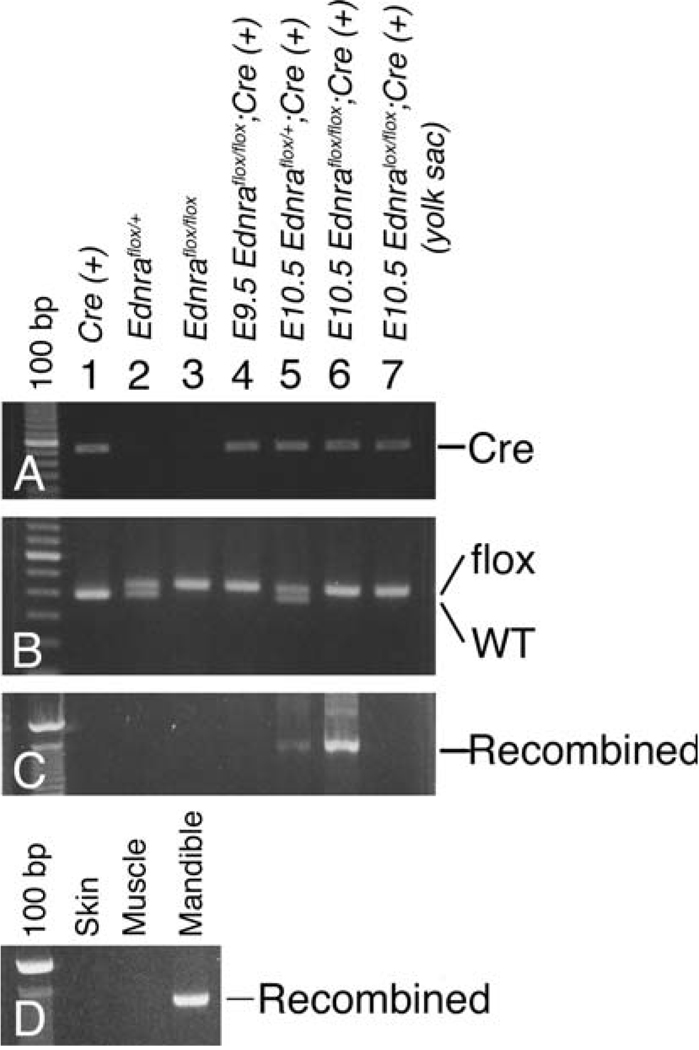
Mice carrying a conditionally targeted Ednra allele (referred to as ETAflox/flox) have been described previously (Kedzierski et al. 2003). Briefly, these mice carry two loxP sites that flank the last three exons of the Ednra gene (hence the term “flox”). We have also previously described the generation and characterization of Dlx5/6-Cre transgenic mice (Ruest et al. 2003). Cre expression in these mice is restricted to the mandibular first arch, with expression first detected at embryonic day (E) 9.5 before being down regulated by E10.5. Ednraflox/flox; Dlx5/6-Cre embryos, generated by crossing Ednraflox/+; Dlx5/6-Cre with Ednraflox/flox mice, were collected at both E9.5 and E10.5 and mandibular arch DNA isolated to verify recombination of the conditional Ednra allele. At E9.5, recombination of the conditional allele was not detected by PCR in either Ednraflox/flox; Dlx5/6-Cre (Fig. 1, lane 4) or ETAflox/+; Dlx5/6-Cre embryos (data not shown). However, by E10.5, recombination of the conditional Ednra allele was observed in embryos carrying both an Edmraflox allele and the Dlx5/6-Cre transgene (lanes 5 and 6). Recombination was not observed in Ednraflox embryos (lanes 2 and 3) or in amniotic sac DNA from Ednraflox/flox; Dlx5/6-Cre (neither Dlx5 nor Dlx6 is expressed in the amniotic sac; Beverdam et al. 2002; Robledo et al. 2002; Ruest et al. 2003). To quantify the extent of recombination, we counted the number of labeled cells in multiple sections through the mandibular arch of E10.5 R26R; Dlx5/6-Cre embryos, finding that 3.2% (±0.5) of cells were labeled (data not shown). While this indicates a low level of recombination, we have shown that Dlx5/6 daughter cells are restricted to the mandibular bone of E16.5 R26R; Dlx5/6-Cre embryos. To determine if this restriction was detectable by recombination PCR, we examined the extent of Ednra gene recombination in the adult mandible bone, comparing it to surrounding tissue. We found that recombination was only present in bone DNA but not in skin or muscle DNA (Fig. 1d).
Fig. 1.
Analysis of the Ednra gene recombination in Ednraflox/flox; Dlx5/6-Cre embryos. Genomic PCR analysis of DNA extracted from adult mouse tails (lanes 1–3), embryonic mandibular pharyngeal arches (lanes 4–6) or embryonic yolk sac (lane 7). The genotype of each animal is listed above the lane number. a CrePCR amplification generates a band ~500 bp. b loxP PCR reveals a 375 bp band, representing the wild type (WT) allele, a 425 bp band, representing the mutant allele (flox), or both. c Recombinant PCR detects the recombined Ednraflox allele and generates a 1200 bp band. d Recombinant PCR of DNA extracted from the skin, muscle and bone of the lower jaw of adult Ednraflox/flox; Dlx5/6-Cre mice
Lower jaw development in Ednraflox/flox; Dlx5/6-Cre embryos
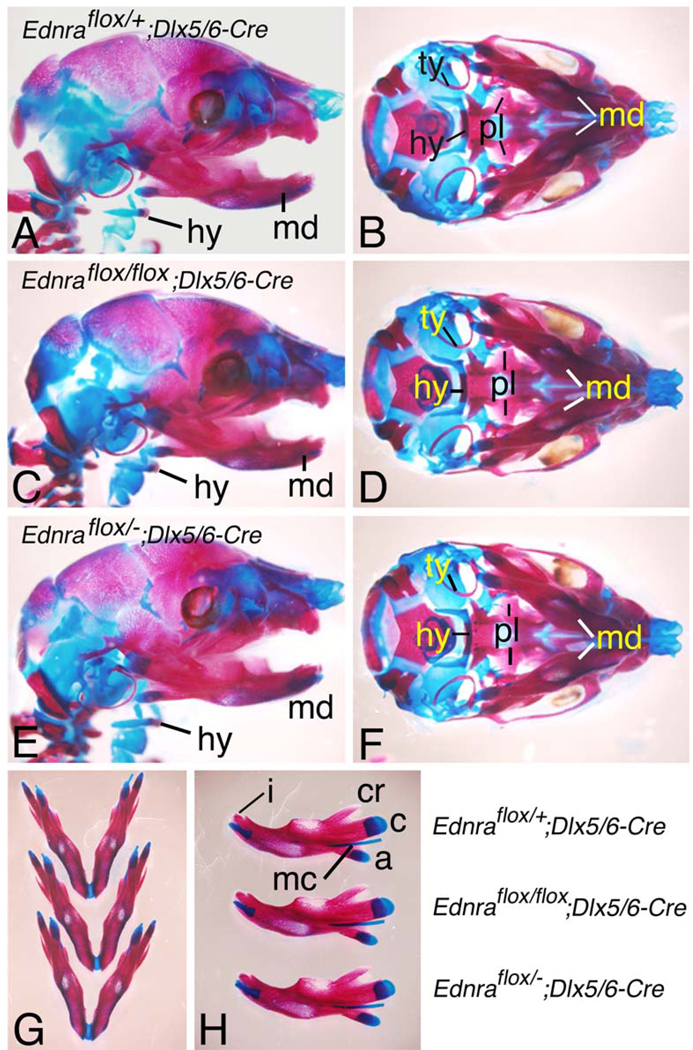
The mandible of Ednra−/−embryos has significant defects in NCC-derived structures of the first mandibular arch (Clouthier et al. 1998; Ruest et al. 2004). To examine whether similar defects were present in Ednraflox/flox; Dlx5/6-Cre embryos, we examined the skulls of E18.5 embryos. We focused our attention on the mandible bone, since β-galactosidase-labeled cells in Dlx5/6-Cre; R26R embryos, representing Dlx5/6 daughter cells, are found almost exclusively in the mandible bone of E16.5 embryos (Ruest et al. 2003). In Ednraflox/flox; Dlx5/6-Cre embryos (Fig. 2c,d), no obvious differences were apparent in the mandible bone when compared with either Ednraflox/+; Dlx5/6-Cre (Fig. 2a,b) or Ednraflox/flox (data not shown) control littermates. The absence of defects in Ednraflox/flox; Dlx5/6-Cre embryos could reflect insufficient recombination of the Ednra conditional allele, resulting in genetic mosaicism (Nagy 2000; Kwan 2002). To address this issue, we also examined lower jaw structures in Ednraflox/−; Dlx5/6-Cre embryos. Since one Ednra allele of Ednraflox/− animals already contains a traditional mutation, recombination would only have to occur once to result in an Ednra mutant genotype. However, defects were also not present in Ednraflox/−; Dlx5/6-Cre embryos (Fig. 2e, f).
Fig. 2.
Analysis of jaw development in E18.5 Ednraflox/flox; Dlx5/6-Cre and Ednraflox/−; Dlx5/6-Cre conditional knockout embryos. Lateral (a,c,e), ventral (b,d,f,g) and intralateral (h) views of Ednraflox/+; Dlx5/6-Cre (control; a,b), Ednraflox/flox; Dlx5/6-Cre (c,d) and Ednraflox/−; Dlx5/6-Cre (e,f) conditional knockout embryos stained with alizarin red and alcian blue. a,f Regardless of the genotype, defects are not observed in any skeletal structures, including the mandible, Meckel’s cartilage, malleus and incisors. g,h In comparison to a control mandible, Ednraflox/flox; Dlx5/6-Cre and Ednraflox/−; Dlx5/6-Cre conditional knockout mandibles do not display any morphological differences in their shape, length or processes. a Articular process; c condylar process; cr coronoid process; i incisor; md mandible; mc Meckel’s cartilage; hy hyoid; ty tympanic ring
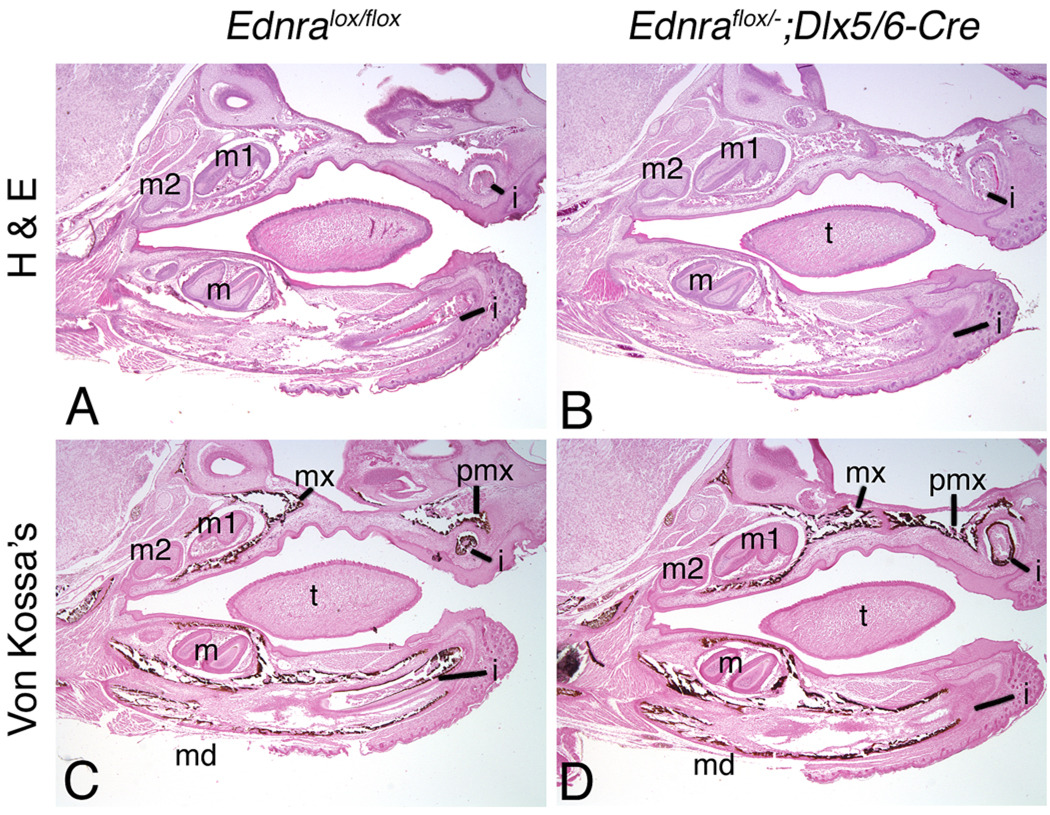
Histological analysis of E18.5 embryos also did not reveal any structural differences in the mandible bone between Ednraflox/flox (control; Fig. 3a), Ednraflox/flox; Dlx5/6-Cre (data not shown) and Ednraflox/−; Dlx5/6-Cre embryos (Fig. 3b). The size of the dental papilla and the extent of early dentin matrix formation around the teeth also appeared normal and suggested that odontogenesis was unaffected in Ednraflox/−; Dlx5/6-Cre embryos.We also examined calcification of the mandibular bone at E18.5 using Van Kossa’s method, which results in a black deposit of reduced silver in the presence of calcium. Calcified matrices along the mandible bone appeared similar between Ednraflox/flox (control; Fig. 3c) and Ednraflox/−; Dlx5/6-Cre (Fig. 3d) embryos. The absence of defects was reflected in adult Ednraflox/flox; Dlx5/6-Cre mice, which are viable and fertile at least to 15 months, indicating normal dentition, growth, musculature, innervation and tendon connections.
Fig. 3.
Analysis of general histology and calcification in E18.5 Ednraflox/−;Dlx5/6-Cre conditional knockout mandibles. Sagittal paraffin sections through the head of E18.5 Ednraflox/flox (used as control; a,c) and Ednraflox/−;Dlx5/6-Cre (b,d) embryos stained with either hematoxylin and eosin (H&E; a,b) or Van Kossa’s method counterstained with nuclear fast red (c,d). a,b Histological analysis of H&E-stained sections illustrate that differences are not observed in either the mandibular/alveolar bone structure or incisors of control (a) and Ednraflox/−;Dlx5/6-Cre (b) embryos. c,d Analysis of calcification by Van Kossa’s method, which produces a precipitate of reduced silver metal in the presence of calcium, reveals no differences between control (c) and Ednraflox/−;Dlx5/6-Cre (d) embryos. Silver metal deposits were also observed along the incisors of Ednraflox/−; Dlx5/6-Cre embryos, though this is not apparent in the section shown in d. i Incisor; m; molar; m1 first molar; m2 second molar; md mandible; mx maxilla; pmx pre-maxilla; t tongue
Normal gene expression within the mandibular arch of Ednraflox/flox; Dlx5/6-Cre embryos
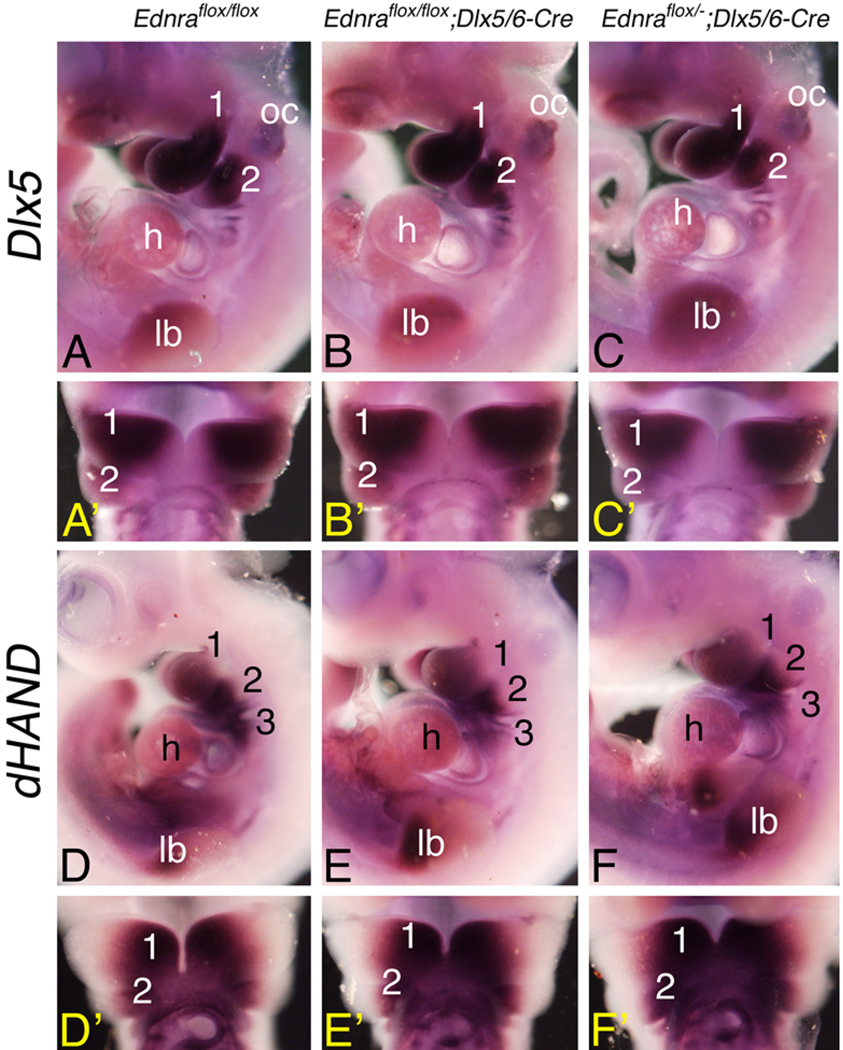
Ednra signaling is required for normal signaling of at least eight transcription factors involved in neural crest cell development, including the bHLH transcription factor dHAND/HAND2 and the Distal-less homeobox family member Dlx5 (Clouthier et al. 1998,Clouthier et al. 2000; Thomas et al. 1998; Ivey et al. 2003;Park et al. 2004; Ruest et al. 2004). Since defects were not observed in either Ednraflox/flox; Dlx5/6-Cre or Ednraflox/−; Dlx5/6-Cre embryos, we examined whether this reflected normal gene expression within the mandibular arch. In both Ednraflox/flox; Dlx5/6-Cre and Ednraflox/−; Dlx5/6-Cre embryos, expression of both Dlx5 (Fig. 4a–c) and dHAND (Fig. 4d–f) was normal in the pharyngeal arch mesenchyme. This suggests that inactivation of the conditional Ednra gene is occurring after activation of the signaling cascade(s) involving both Dlx5 and dHAND.
Fig. 4.
Analysis of gene expression in E10.5 conditional knockout embryos. Whole-mount in situ hybridization analysis of Dlx5 (a–c) and dHAND (d–f) expression in E10.5 Ednraflox/flox (control; a,d), Ednraflox/flox; Dlx5/6-Cre (b,e) and Ednraflox/−; Dlx5/6-Cre (c,f) embryos, presented in lateral (a–f) and ventral (a′–f′) views. (a–f) No differences in Dlx5 and dHAND expression are observed in the mandibular (1) and second (2) pharyngeal arches between the different genotypes. oc Otic capsule; h heart; lb limb bud; 3 third pharyngeal arch
Discussion
Our previous analysis of Dlx5/6-Cre; R26R embryos demonstrated that Cre expression in Dlx5/6-Cre embryos occurs between E9.5 and E10.5, with Dlx5/6 daughter cells restricted to the mandible bone of E18.5 embryos (Ruest et al. 2003). However, when crossed into the conditional Ednraflox/flox background, neither Ednraflox/flox; Dlx5/6-Cre nor Ednraflox/−; Dlx5/6-Cre embryos develop defects in lower jaw structures. The Ednraflox line has been used previously to inactivate Ednra expression specifically in myocardial cells of the adult heart, with Edn1 binding assays illustrating loss of Ednra receptor function (Kedzierski et al. 2003). While the size of E9.5 and E10.5 pharyngeal arches make binding assays unfeasible, our PCR analysis shows that the conditional Ednra allele is recombined by E10.5 in Ednraflox/flox; Dlx5/6-Cre embryos. While we do not observe recombination at E9.5, the time period that Cre transgene expression first appears, this absence may simply reflect a very limited recombination of the conditional Ednra allele at that time. However, even at E10.5, recombination is only observed in 3.2% of the arch mesenchyme cells, which could suggest that inefficient recombination leads to an absence of mandibular defects. Yet, our results with Ednraflox/−; Dlx5/6-Cre embryos argue against inefficient recombination of the conditional Ednra allele. Rather, we believe that we are targeting a small group of cells that will later participate in mandible bone formation. A lack of phenotype is probably due to the timing of recombination rather than the extent of recombination, as we have shown using chimera analysis that the Ednra mutation acts in a cell autonomous manner (Clouthier et al. 2003). This argues against rescue of mutant cells by neighboring cells within the arches. This is also supported by a lack of change in gene expression patterns. Taken together, these aspects indicate, though do not prove, that an absence of phenotype is more probably due to the timing of gene recombination rather than an absence of recombination.
Ednra signaling is crucial for NCC development in mouse (Clouthier et al. 1998), rat (Spence et al. 1999), zebrafish (Miller et al. 2000) and chick (Kempf et al. 1998). We have clarified the function of Ednra receptor signaling in this study, illustrating that it does not appear to be required for mandible bone development after NCC patterning. However, it is still possible that Ednra receptor function is required within the adult mandibular bone. Ednra receptors are located on osteoblasts (Stern et al. 1995; Suzuki et al. 1997), including those in craniofacial bone (Kitano et al. 1998). Further, recent evidence suggests that Ednra signaling may play a role in the bone formation observed during osteoblastic bone metastases (Yin et al. 2003). These findings may indicate that Ednra receptor signaling is required for adult bone remodeling. It is plausible that such a mechanism could be mediated by Dlx5, as Dlx5 expression is observed in broken bones of adult mice (Miyama et al. 1999). It will be interesting to examine whether the absence of the Ednra gene in Ednraflox/flox; Dlx5/6-Cre or Ednraflox/−; Dlx5/6-Cre mice affects mandible fracture repair.
Acknowledgements
The authors would like to thank Shelley Dixon and Tinisha Taylor for technical assistance. M.Y. is an investigator of the Howard Hughes Medical Institute. R.M.K is a trainee in the Medical Scientist Training Program at the University of Texas Southwestern Medical Center at Dallas. D.E.C. is a recipient of a Career Development Award from the NIDCR/NIH.
This work was supported in part by grants from the National Institutes of Health and the American Heart Association to D.E.C.
Contributor Information
Louis-Bruno Ruest, Department of Molecular, Cellular and Craniofacial Biology and the Birth Defects Center, University of Louisville, Louisville, KY, 40292, USA.
Rafal Kedzierski, Department of Molecular Genetics, University of Texas Southwestern Medical Center, Dallas, TX, 75390, USA.
Masashi Yanagisawa, Department of Molecular Genetics, University of Texas Southwestern Medical Center, Dallas, TX, 75390, USA; Howard Hughes Medical Institute, University of Texas Southwestern Medical Center, Dallas, TX, 75390, USA.
David E. Clouthier, Department of Molecular, Cellular and Craniofacial Biology and the Birth Defects Center, University of Louisville, Louisville, KY, 40292, USA, clouthier@louisville.edu, Tel.: +1-502-8522452, Fax: +1-502-8524702
References
- Beverdam A, Merlo GR, Paleari L, Mantero S, Genova F, Barbieri O, Janvier P, Levi G. Jaw transformation with gain of symmetry after Dlx5/Dlx6 inactivation: mirror of the past. Genesis. 2002;34:221–227. doi: 10.1002/gene.10156. [DOI] [PubMed] [Google Scholar]
- Clouthier DE, Schilling TF. Understanding endothelin-1 function during craniofacial development in the mouse and zebrafish. Birth Defects Res (Part C) 2004;72:190–199. doi: 10.1002/bdrc.20007. [DOI] [PubMed] [Google Scholar]
- Clouthier DE, Hosoda K, Richardson JA, Williams SC, Yanagisawa H, Kuwaki T, Kumada M, Hammer RE, Yanagisawa M. Cranial and cardiac neural crest defects in endothelin-A receptor-deficient mice. Development. 1998;125:813–824. doi: 10.1242/dev.125.5.813. [DOI] [PubMed] [Google Scholar]
- Clouthier DE, Williams SC, Yanagisawa H, Wieduwilt M, Richardson JA, Yanagisawa M. Signaling pathways crucial for craniofacial development revealed by endothelin-A receptor-deficient mice. Dev Biol. 2000;217:10–24. doi: 10.1006/dbio.1999.9527. [DOI] [PubMed] [Google Scholar]
- Clouthier DE, Williams SC, Hammer RE, Richardson JA, Yanagisawa H. Cell-autonomous and non-autonomous actions of endothelin-A receptor signaling in craniofacial and cardiovascular development. Dev Biol. 2003;261:506–519. doi: 10.1016/s0012-1606(03)00128-3. [DOI] [PubMed] [Google Scholar]
- Couly GF, Creazzo TL, Bennaceur S, Vincent C, Le Douarin NM. Interactions between Hox-negative cephalic neural crest cells and the foregut endoderm in patterning the facial skeleton in the vertebrate head. Development. 2002;129:1061–1073. doi: 10.1242/dev.129.4.1061. [DOI] [PubMed] [Google Scholar]
- Depew MJ, Liu JK, Long JE, Presley R, Meneses JJ, Pedersen RA, Rubenstein JL. Dlx5 regulates regional development of the branchial arches and sensory capsules. Development. 1999;126:3831–3846. doi: 10.1242/dev.126.17.3831. [DOI] [PubMed] [Google Scholar]
- Ivey K, Tyson B, Ukidwe P, McFadden DG, Levi G, Olson EN, Srivastava D, Wilkie TM. Gαq and Gα11 proteins mediate endothelin-1 signaling in neural crest-derived pharyngeal arch mesenchyme. Dev Biol. 2003;255:230–237. doi: 10.1016/s0012-1606(02)00097-0. [DOI] [PubMed] [Google Scholar]
- Kedzierski RM, Grayburn PA, Kisanuki YY, Williams CS, Hammer RE, Richardson JA, Schneider MD, Yanagisawa M. Cardiomyocyte-specific endothelin a receptor knockout mice have normal cardiac function and an unaltered hypertrophic response to angiotensin II and isoproterenol. Mol Cell Biol. 2003;23:8226–8232. doi: 10.1128/MCB.23.22.8226-8232.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempf H, Linares C, Corvol P, Gasc JM. Pharmacological inactivation of the endothelin type A receptor in the early chick embryo: a model of mispatterning of the branchial arch derivatives. Development. 1998;125:4931–4941. doi: 10.1242/dev.125.24.4931. [DOI] [PubMed] [Google Scholar]
- Kitano Y, Kurihara H, Kurihara Y, Maemura K, Ryo Y, Yazaki Y, Harii K. Gene expression of bone matrix proteins and endothelin receptors in endothelin-1-deficient mice revealed by in situ hybridization. J Bone Miner Res. 1998;13:237–244. doi: 10.1359/jbmr.1998.13.2.237. [DOI] [PubMed] [Google Scholar]
- Kurihara Y, Kurihara H, Suzuki H, Kodama T, Maemura K, Nagai R, Oda H, Kuwaki T, Cao W-H, Kamada N, et al. Elevated blood pressure and craniofacial abnormalities in mice deficient in endothelin-1. Nature. 1994;368:703–710. doi: 10.1038/368703a0. [DOI] [PubMed] [Google Scholar]
- Kwan K-M. Conditional alleles in mice: practical considerations for tissue-specific knockouts. Genesis. 2002;32:49–62. doi: 10.1002/gene.10068. [DOI] [PubMed] [Google Scholar]
- Le Douarin NM, Ziller C, Couly GF. Patterning of neural crest derivatives in the avian embryo: in vivo and in vitro studies. Dev Biol. 1993;159:24–49. doi: 10.1006/dbio.1993.1219. [DOI] [PubMed] [Google Scholar]
- Lumsden A, Sprawson N, Graham A. Segmental origin and migration of neural crest cells in the hindbrain region of the chick embryo. Development. 1991;113:1281–1291. doi: 10.1242/dev.113.4.1281. [DOI] [PubMed] [Google Scholar]
- McLeod MJ. Differential staining of cartilage and bone in whole mouse fetuses by alcian blue and alizarin red S. Teratology. 1980;22:299–301. doi: 10.1002/tera.1420220306. [DOI] [PubMed] [Google Scholar]
- Miller CT, Schilling TF, Lee K-H, Parker J, Kimmel CB. sucker encodes a zebrafish endothelin-1 required for ventral pharyngeal arch development. Development. 2000;127:3815–3838. doi: 10.1242/dev.127.17.3815. [DOI] [PubMed] [Google Scholar]
- Miyama K, Yamada G, Yamamoto TS, Takagi C, Miyado K, Sakai M. A BMP-inducible gene, Dlx5, regulates osteoblast differentiation and mesoderm induction. Dev Biol. 1999;208:123–133. doi: 10.1006/dbio.1998.9197. [DOI] [PubMed] [Google Scholar]
- Nagy A. Cre recombinase: the universal reagent for genome tailoring. Genesis. 2000;26:99–109. [PubMed] [Google Scholar]
- Noden DM. The role of the neural crest in patterning of avian cranial skeletal, connective, and muscle tissues. Dev Biol. 1983;96:144–165. doi: 10.1016/0012-1606(83)90318-4. [DOI] [PubMed] [Google Scholar]
- Ozeki H, Kurihara Y, Tonami K, Watatani K, Kurihara H. Endothelin-1 regulates the dorsoventral branchial arch patterning in mice. Mech Dev. 2004;121:387–395. doi: 10.1016/j.mod.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Park BK, Sperber SM, Choudhury A, Ghanem N, Hatch GT, Sharpe PT, Thomas BL, Ekker M. Intergenic enhancers with distinct activities regulate Dlx gene expression in the mesenchyme of the branchial arches. Dev Biol. 2004;268:532–545. doi: 10.1016/j.ydbio.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Robledo RF, Rajan L, Li X, Lufkin T. The Dlx5 and Dlx6 homeobox genes are essential for craniofacial, axial, and appendicular skeletal development. Genes Dev. 2002;16:1089–1101. doi: 10.1101/gad.988402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruest L-B, Hammer RE, Yanagisawa M, Clouthier DE. Dlx5/6-enhancer directed expression of Cre recombinase in the pharyngeal arches and brain. Genesis. 2003;37:188–194. doi: 10.1002/gene.10247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruest L-B, Xiang X, Lim KC, Levi G, Clouthier DE. Endothelin-A receptor-dependent and independent signaling pathways in establishing mandibular identity. Development. 2004;131:4413–4423. doi: 10.1242/dev.01291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider RA, Helms JA. The cellular and molecular origins of beak morphology. Science. 2003;299:565–568. doi: 10.1126/science.1077827. [DOI] [PubMed] [Google Scholar]
- Serbedzija GN, Bronner-Fraser M, Fraser SE. Vital dye analysis of cranial neural crest cell migration in the mouse embryo. Development. 1992;116:297–307. doi: 10.1242/dev.116.2.297. [DOI] [PubMed] [Google Scholar]
- Spence S, Anderson C, Cukierski M, Patrick D. Teratogenic effects of the endothelin receptor antagonist L-753,037 in the rat. Reprod Toxicol. 1999;13:15–29. doi: 10.1016/s0890-6238(98)00064-1. [DOI] [PubMed] [Google Scholar]
- Srivastava D, Thomas T, Lin Q, Kirby ML, Brown D, Olson EN. Regulation of cardiac mesodermal and neural crest development by the bHLH transcription factor, dHAND. Nat Genet. 1997;16:154–160. doi: 10.1038/ng0697-154. [DOI] [PubMed] [Google Scholar]
- Stern PH, Tatrai A, Semler DE, Lee SK, Lakatos P, Strieleman PJ, Tarjan G, Sander JL. Endothelin receptors, second messengers, and actions in bone. J Nutr. 1995;125:2028S–2032S. doi: 10.1093/jn/125.suppl_7.2028S. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Shinoda J, Watanabe-Tomita Y, Ozaki N, Oiso Y, Kozawa O. ETA receptor mediates the signaling of endothelin-1 in osteoblast-like cells. Bone. 1997;21:143–146. doi: 10.1016/s8756-3282(97)00096-3. [DOI] [PubMed] [Google Scholar]
- Thomas T, Kurihara H, Yamagishi H, Kurihara Y, Yazaki Y, Olson EN, Srivastava D. A signaling cascade involving endothelin-1, dHAND and Msx1 regulates development of neural-crest-derived branchial arch mesenchyme. Development. 1998;125:3005–3014. doi: 10.1242/dev.125.16.3005. [DOI] [PubMed] [Google Scholar]
- Trainor PA, Ariza-McNaughton L, Krumlauf R. Role of the isthmus and FGFs in resolving the paradox of neural crest plasticity and prepatterning. Science. 2002;295:1288–1291. doi: 10.1126/science.1064540. [DOI] [PubMed] [Google Scholar]
- Tucker SA, Yamada G, Grigoriou M, Pachnis V, Sharpe PT. Fgf-8 determines rostral-caudal polarity in the first branchial arch. Development. 1999;126:51–61. doi: 10.1242/dev.126.1.51. [DOI] [PubMed] [Google Scholar]
- Yanagisawa H, Hammer RE, Richardson JA, Williams SC, Clouthier DE, Yanagisawa M. Role of endothelin-1/endothelin-A receptor-mediated signaling pathway in the aortic arch patterning in mice. J Clin Invest. 1998;102:22–33. doi: 10.1172/JCI2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin JJ, Mohammad KS, Kokonen SM, Harris S, Wu-Wong JR, Wessale JL, Padley RJ, Garrett IR, Chirgwin JM, Guise TA. A causal role for endothelin-1 in the pathogenesis of osteoblastic bone metastases. Proc Natl Acad Sci USA. 2003;100:10954–10959. doi: 10.1073/pnas.1830978100. [DOI] [PMC free article] [PubMed] [Google Scholar]