Abstract
This study investigated the mechanisms responsible for the disrupted homeostasis of reduced glutathione (GSH) in aging muscles with stress (14 days of hind-limb unloading [HU]). Adult and old rats were randomized into four groups: weight bearing and 3, 7, and 14 days of HU. Soleus muscles were harvested to investigate the activity or content of enzymes involved in GSH metabolism (utilization and synthesis). The activities of glutathione S transferase, glutathione reductase, γ-glutamyl transpeptidase, and glutamate cysteine ligase (GCL) were determined. The protein content of the two subunits of GCL, catalytic subunit (GCLC) and modifier subunit (GCLM), were evaluated. The major results, failure to maintain the accelerated GCLC production and GCL activity, are associated with the GSH depletion in aging muscles with 14 days of HU. The results suggest that the regulation of GCL, especially the catalytic subunit, with stress may be compromised in aging muscles.
Keywords: GCL, GCLC, Hind-limb unloading
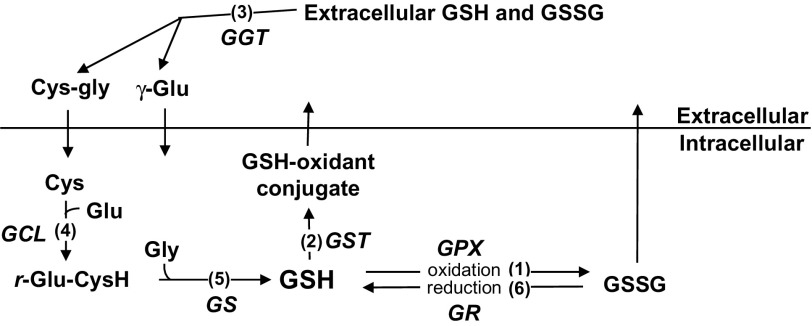
THE reduced form of glutathione (GSH), a tripeptide composed of glutamate, cysteine, and glycine, is a crucial antioxidant that protects cells by being a cofactor in redox reactions as well as by direct conjugation with oxidants (Figure 1). In redox reactions, GSH donates electrons to hydrogen peroxides that are reduced to water by the enzyme glutathione peroxidase (GPX). In the reaction of direct conjugations, GSH conjugates with a variety of oxidants as well as with protein cysteine residues (S-glutathionylation). The reaction of GSH conjugation with oxidants is catalyzed by the enzyme glutathione S transferase (GST). These GSH-dependent detoxifying reactions protect cells from oxidative damage, but consequently, they reduce intracellular GSH levels.
Figure 1.
Metabolism of glutathione (reduced glutathione, GSH). GSH, a tripeptide composed of glutamate (Glu), cysteine (Cys), and glycine (Gly), is a crucial antioxidant in the cells. (1) GSH is used as a cofactor in the redox reaction catalyzed by glutathione peroxidase (GPX). In this redox reaction, hydrogen peroxide and other peroxides are reduced, whereas GSH is oxidized to glutathione disulfide (GSSG). (2) The detoxifying function of GSH is also achieved by direct conjugation with oxidants. This direct conjugation reaction is catalyzed by glutathione S transferase (GST). (3) GSSG and GSH–oxidant conjugates are transported out of cells where they are degraded by γ-glutamyl transferase (GGT). This degradation ensures substrate available for GSH biosynthesis intracellularly. The intracellular synthesis of GSH is by two consecutive enzymatic reactions catalyzed by glutamate cysteine ligase (GCL; 4) and glutathione synthetase (GS; 5). (6) GSH is also replenished by the reduction of GSSG catalyzed by glutathione reductase (GR).
The replenishment of GSH is achieved by recycling and biosynthesis (Figure 1). The recycling of GSH is catalyzed by glutathione reductase (GR) with NADPH as the cofactor. The biosynthesis of GSH is regulated by the substrate availability and the synthesis rate. Substrate availability is mainly determined by γ-glutamyl transferase (GGT), which initiates the extracellular degradation of GSH conjugates and GSH into γ-glutamyl amino acids and cysteine–glycine peptide. This reaction ensures substrate available for GSH biosynthesis intracellularly. The intracellular synthesis of GSH is by two consecutive ATP-dependent enzymatic reactions. In the first reaction, glutamate is coupled with cysteine to form γ-glutamylcysteine (γ-GC) catalyzed by glutamate cysteine ligase (GCL), the rate-limiting enzyme of GSH biosynthesis. In the second reaction, γ-GC is coupled with glycine to form GSH catalyzed by glutathione synthetase.
The homeostasis of GSH is altered with various cellular stresses, including oxidative stress. Aging, a process of chronic oxidative stress, has been shown to affect GSH levels in a tissue-specific manner (1). In skeletal muscles, GSH levels are increased with aging in type I (oxidative) muscles (2,3). The increased GSH level in aged muscles is likely a compensatory response for the chronic oxidative stress that helps maintain the redox balance of muscle cells.
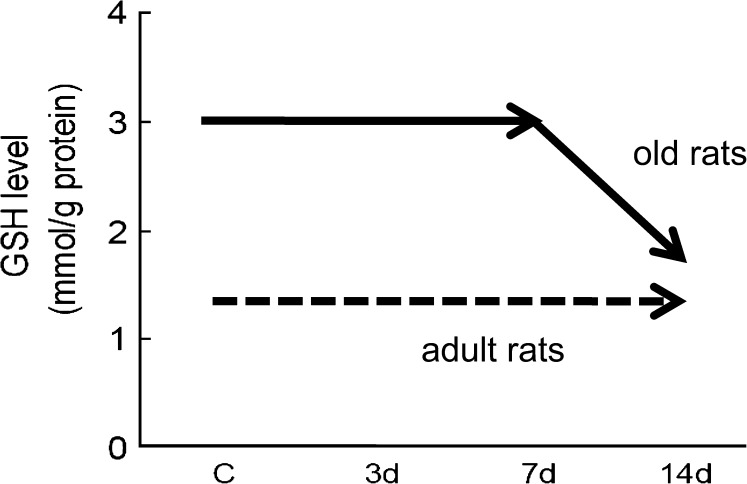
Importantly, the ability of aged organisms to adapt to additional stressors appears to be compromised. Our previous study found that in adult animals, the GSH levels in muscles are stable with the additional stress of muscle disuse, which has been shown to induce oxidative stress of the affected muscles (3,4). In contrast, GSH levels in aging muscles with the same stress dropped dramatically (3) (Figure 2). These findings prompted the focused examination of GSH metabolism in the current study. Thus, the aim of this study was to determine the enzymes that are associated with the impaired GSH homeostasis in aging muscles with additional stress.
Figure 2.
The reduced glutathione (GSH) level was maintained in adult rats with an additional stress (hind-limb unloading [HU]); however, the level dropped significantly in aging muscles with the same stress. c = weight bearing; 3d, 7d, and 14d = HU for 3, 7, and 14 days. [Figure is drawn from the data in Chen and colleagues (3).]
METHODS
Animals and Stress Intervention
Eighty male Fischer 344 rats, aged 13 months (the age at which more than 90% of this rat strain have survived, n = 40) and 26 months (the age at which 25% have survived, n = 40), were purchased from the Minneapolis Veterans Administration Aged Rodent Colony that was maintained by the University of Minnesota. Rats were randomized into four groups: normal weight bearing (c) and hind-limb unloading (HU) for 3, 7, and 14 days. Muscle disuse by HU was used as a form of stress for skeletal muscles because previous studies have shown that HU altered antioxidant capacities (3,5,6) and increased protein oxidation and ubiquitination (7–9).
The HU intervention was achieved as described previously (3,9). Briefly, the tail of the rat was attached to a swivel mounted at the top of the cage. The height of the suspension was adjusted to prevent the hind limbs from touching the floor. All the animals were housed in a research animal facility and were checked daily for any abnormal response to suspension. The rats were anesthetized with pentobarbital sodium (35 mg/kg body weight) after the intervention. Soleus muscles were harvested, weighed, and immediately frozen in liquid nitrogen. The frozen soleus muscles were stored in a −80°C freezer until later analysis. The protocol of this study was approved by the University of Minnesota Institutional Animal Care and Use Committee.
Enzyme Activity Assays
GR, GGT, and GST activities.—
Frozen soleus muscles were homogenized in buffer containing 20 mM 3-(4-morpholino)propane sulfonic acid (MOPS), 62 mM sucrose, and 0.1 mM ethylenediaminetetraacetic acid (EDTA) (pH 7.2). The supernatant collected after centrifugation at 12,000g for 25 minutes was used for the analysis of enzyme activities. Protein concentration in the supernatant was measured by the Bradford method (10).
GR activity was determined as previously described (11). Briefly, tissue homogenates were added to assay medium containing 100 mM thiourea, 10 mM NADPH, 30 mM glutathione disulfide (GSSG), 200 mM MOPS, 620 mM sucrose, and 1 mM EDTA (pH 7.2) to initiate the reaction. GR activity was determined spectrophotometrically by measuring the disappearance of NADPH at a wavelength of 340 nm at 30°C for 5 minutes. Values were expressed in micromoles per minute per milligram protein.
GGT activity was measured as previously described (12). Briefly, homogenates were incubated in an assay medium containing 100 mM Tris–HCL (pH 8.0), 5 mM γ-glutamyl-p-nitroanilide, and 100 mM glycylglycine for 30 minutes at 37°C. The enzymatic reaction was stopped by precipitating proteins with 12N acetic acid followed by centrifugation at 8,160g for 3 minutes. GGT activity was then determined spectrophotometrically by measuring the absorbance of the supernatant fraction at 410 nm at 37°C (12). Values were expressed in nanomoles per milligram protein.
GST activity was determined as previously described (12). Briefly, homogenates were mixed in a medium containing 100 mM KPO4 (pH 6.5) and 20 mM GSH, and the reaction was initiated by adding 1-chloro-2,4-dinitrobenzene (CDNB). GST activity was then determined spectrophotometrically by measuring the formation of CDNB–GSH conjugates at 340 nm at 30°C for 5 minutes. Values were expressed in micromoles per minute per milligram protein.
GCL Activity Assay.—
GCL activity was determined by a fluorescence assay developed by White and colleagues (13) and Wu and colleagues (14) with our optimization of the reaction time specifically for muscle tissues. In detail, frozen soleus muscles were homogenized in 20 mM Tris, 1 mM EDTA, 250 mM sucrose, 20 mM sodium borate, and 2 mM serine (TES/SB buffer). Homogenates were centrifuged at 10,000g at 4°C for 10 minutes. The supernatants were collected and then centrifuged again at 15,000g at 4°C for 20 minutes. Protein concentrations in the supernatants were determined using bicinchoninic acid (BCA) Protein Assay Kit (Pierce, Rockford, IL) with bovine serum albumin as the standard.
In the GCL activity assay, 30 μL of homogenate was added to 30 μL of GCL reaction cocktail (400 mM Tris, 40 mM ATP, 40 mM L-glutamic acid, 2 mM EDTA, 20 mM sodium borate, 2 mM serine, and 40 mM MgCl2) and incubated at 37°C for 5 minutes. After 5 minutes of incubation, 30 μL of 30 mM cysteine (dissolved in TES/SB buffer) was added and the mixture was incubated for 13 minutes at 37°C. The enzymatic reaction in the mixture was stopped by precipitating proteins with 200 mM 5-sulfosalicylic acid (SSA). After placing on ice for 20 minutes, the mixture was centrifuged at 2,000g at 4°C for 10 minutes.
Following centrifugation, 20 μL of supernatant that contained γ-GC product was added to a 96-well plate designed for fluorescence detection. For each assay, 20 μL of γ-GC standards containing 30 μL of GCL reaction cocktail, 30 μL of 200 mM SSA, 30 μL of H2O, and 30 μL of γ-GC standard solution (0, 20, 40, 60, 80, 100, 120, and 140 μM of γ-GC in TES/SB buffer) were added to the same 96-well plate to generate the standard curve. Next, 180 μL of 2,3-naphthalenedicarboxyaldehyde (NDA) was added into each well. The plate was incubated in the dark at room temperature for 30 minutes. After the incubation, the formation of NDA–γ-GC was measured (472 nm excitation/528 nm emission) using a fluorescent plate reader (Applied Biosystems, Foster City, CA). The production of γ-GC in each sample was calculated with standard curve. Values were expressed in nanomoles per minute per milligram protein.
Protein Content of Catalytic Subunit of Glutamate Cysteine Ligase and Modifier Subunit of Glutamate Cysteine Ligase
Protein content of catalytic subunit of glutamate cysteine ligase (GCLC) and modifier subunit of glutamate cysteine ligase (GCLM) were determined by Western blot. Briefly, soleus muscles were homogenized in DNase buffer containing 20 mM Tris (pH 6.8), 1 mM CaCl2, 5 mM MgCl2, and 150 units/mL DNase I (Roche Diagnostics, Indianapolis, IN). The homogenate was then placed on ice for 40 minutes. Next, urea buffer containing 6 M urea, 2% sodium dodecyl sulfate (SDS), and 20 mM Tris (pH 6.8) was added into the homogenate followed by homogenization. The homogenate was centrifuged at 600g at 4°C for 15 minutes, and the supernatant was collected. Protein concentrations in the supernatants were determined using the BCA Protein Assay Kit.
Equal amounts (20 μg for GCLC and 50 μg for GCLM, determined by linear responses of respective antibody) of protein were loaded onto 12% SDS–polyacrylamide gels and separated by electrophoresis using Mini-Vertical Gel Electrophoresis Units (Amersham Biosciences, Cleveland, OH). Ten microliters of GCLC-positive control (Thermo Fisher Scientific, Fremont, CA) was loaded in each gel for Western blot probed with GCLC antibody. Proteins resolved on the gels were transferred to polyvinylidene difluoride (PVDF) membranes using a Mini Trans-Blot Electrophoretic Transfer Cells (Bio-Rad, Hercules, CA) at 110 V for 3 hours. An internal control (a muscle sample) was loaded and transferred on each blot. The band intensity of all samples was normalized to the intensity of this internal control, thus permitting the comparison of samples across multiple blots.
The protein-bound PVDF membranes were incubated overnight at 4°C with the polyclonal GCLC antibody (Thermo Fisher Scientific; 1:1,000) or monoclonal GCLM antibody (Santa Cruz Biotechnology, Santa Cruz, CA; 1:1,000). Blots probed with GCLC antibody were incubated with goat anti-rabbit alkaline phosphatase–conjugated secondary antibody (Bio-Rad; 1:3.000) at room temperature for 1 hour. Substrate BCIP-NBT (5-bromo-4-chloro-3′-iodolyl phosphate p-toluidine-nitroblue tetrazolium chloride) was used for colorimetric visualization of the immunoreactions on membranes. The immunoblots were imaged using a GS-800 Calibrated Densitometer (Bio-Rad). Blots probed with GCLM antibody were incubated with goat anti-mouse horseradish peroxidase (HRP)–conjugated secondary antibody (Bio-Rad; 1:3,000) at room temperature for 1 hour. Chemiluminescent Substrate Kits (SuperSignal West Pico; Fisher Scientific, Pittsburgh, PA) were used to detect HRP-labeled probes on the blots. The immunoblots were imaged using a ChemiDoc Imaging System (Bio-Rad). The intensity of immunoreactions on the blots was quantified using Quantity One software (Bio-Rad) (9).
Statistics
Data are presented as mean ± SEM. Two-way analysis of variance (ANOVA) and Tukey–Kramer multiple comparison post hoc test were used to determine whether the age of the animals influences the responses of GSH metabolism–related enzymes in muscles under stress. If a significant interaction was detected between age and stress, the effects of age and stress were examined separately by one-way ANOVA with Tukey–Kramer multiple comparison post hoc test. Differences were considered significant when p < .05.
RESULTS
GSH Utilization
Activities of GST.—
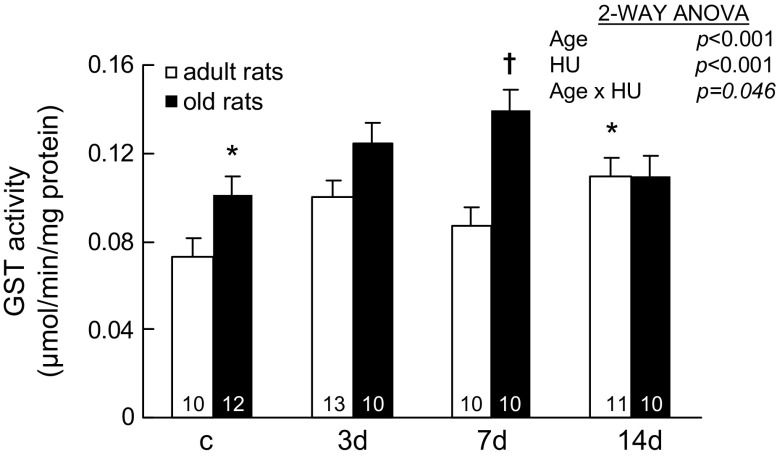
GST catalyzes the reaction of glutathione conjugation with endogenous compounds and xenobiotics. GST activities changed with HU, and the changes were age dependent (Age × HU, p = .046; Figure 3). In adult rats, GST activities in muscles with 14 days of HU were 0.109 ± 0.009 μmol/min/mg protein, which were significantly higher than the values seen in the muscles of weight-bearing control rats. In old rats, GST activities in muscles with 7 days of HU were higher than values seen in the muscles of weight-bearing control rats (0.139 ± 0.009 and 0.101 ± 0.008 μmol/min/mg protein, respectively). However, GST activities in muscles of old rats with 14 days of HU (0.110 ± 0.009 μmol/min/mg protein) were not different from the values seen in the muscles of old control rats.
Figure 3.
Activity of glutathione S transferase (GST) from rats with weight bearing (c) and 3, 7, and 14 days (d) of hind-limb unloading (HU). The number of animals in each group is noted. Values are mean ± SEM. The effect of HU was dependent on age (p = .046). *Significantly different from adult weight-bearing rats. †Significantly different from old weight-bearing rats. ANOVA = analysis of variance.
Aging also affected GST activities. GST activities in normal weight-bearing muscles of old rats were 38% higher than the values seen in the muscles of adult rats.
GSH Replenishment
Activities of GR.—
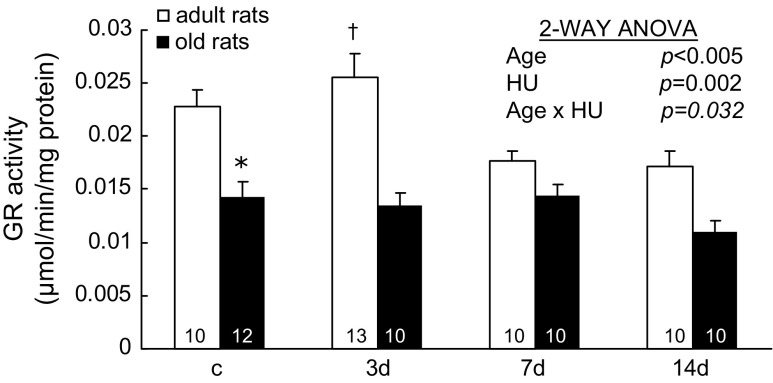
GR catalyzes the reduction reaction of glutathione disulfide (the oxidized form of glutathione) to glutathione (1). GR activities changed with HU age specifically (Age × HU, p = .032; Figure 4). In adult rats, GR activity in muscles with 3 days of HU was 0.026 ± 0.002 μmol/min/mg protein, which was 44% and 53% higher than the values seen in the muscles with 7 days and 14 days of HU (0.018 ± 0.001 and 0.017 ± 0.001 μmol/min/mg protein, respectively). In old rats, GR activities did not change significantly with HU.
Figure 4.
Activity of glutathione reductase (GR) from rats with weight bearing (c) and 3, 7, and 14 days (d) of hind-limb unloading (HU). The number of animals in each group is noted. Values are mean ± SEM. The effect of HU was dependent on age (p = .032). *Significantly different from adult weight-bearing rats. †Significantly different from adult 7- and 14-day rats. ANOVA = analysis of variance.
GR activities also changed with aging. GR activities in normal weight-bearing soleus muscles of old rats were 37% lower compared with the values of adult rats.
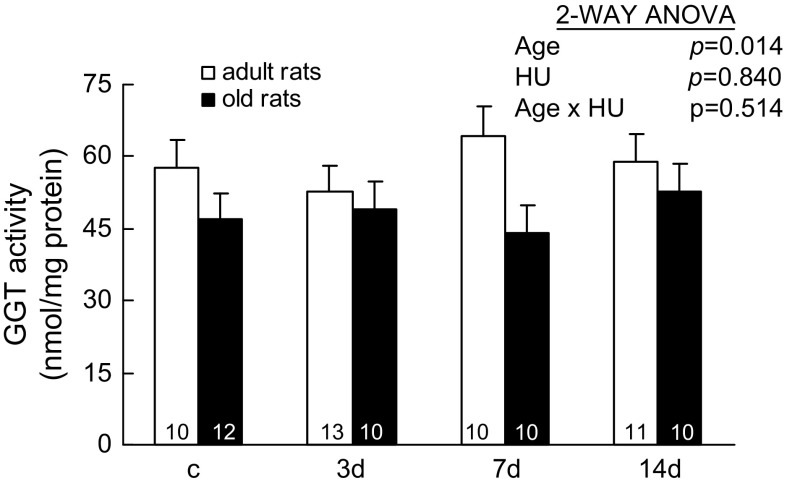
Activities of GGT.—
GGT, a membrane-bound enzyme, initiates the extracellular catabolism of GSH and GSH conjugates, which makes amino acid substrates available for GSH synthesis intracellularly (15). GGT activities in muscles did not change with HU; however, they changed with aging (two-way ANOVA, p = .014; Figure 5). The activities of GGT were 18% lower in soleus muscles of old rats compared with the values seen in the adult rats.
Figure 5.
Activity of γ-glutamyl transferase (GGT) from rats with weight bearing (c) and 3, 7, and 14 days (d) of hind-limb unloading (HU). GGT activity is determined by assaying after 30 minutes of incubation. The number of animals in each group is noted. Values are mean ± SEM. The effect of HU was independent of age. Adult rats had higher GGT activities than those of old rats. ANOVA = analysis of variance.
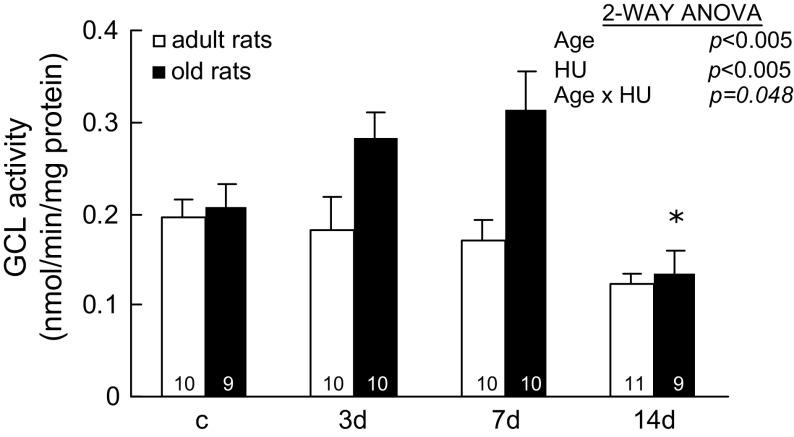
Activities of GCL.—
GCL is the rate-limiting enzyme of GSH synthesis (15). GCL activities change age specifically with HU (Age × HU, p = .048; Figure 6). In adult rats, GCL activities did not change significantly with HU. In old rats, GCL activities in muscles with 14 days of HU were 0.13 ± 0.02 nmol/min/mg protein, which were significantly lower than the activities of muscles with 3 and 7 days of HU (0.28 ± 0.03 and 0.31 ± 0.04 nmol/min/mg protein, respectively).
Figure 6.
Activity of glutamate cysteine ligase (GCL) from rats with weight bearing (c) and 3, 7, and 14 days (d) of hind-limb unloading (HU). The number of animals in each group is noted. Values are mean ± SEM. The effect of HU was dependent on age (p = .048). *Significantly different from old 3- and 7-day rats. ANOVA = analysis of variance.
Protein Content of GCL Subunits
In order to understand why GCL activity in soleus muscles of old rats decreased from 7 days of HU to 14 days of HU, the protein content of the two subunits of GCL were determined.
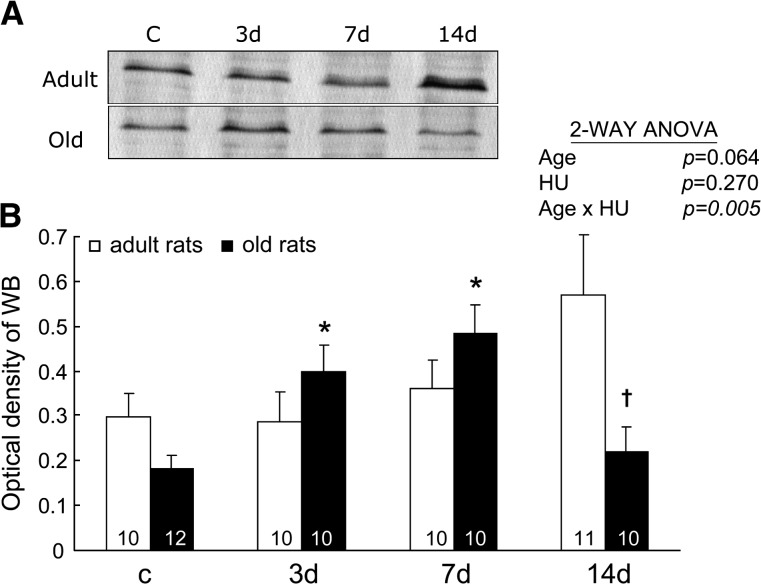
The content of GCLC.—
The GCLC produces the catalytic function of GCL (16). Figure 7 shows representative immunoblots (A) and densitometric analysis (B) of GCLC from soleus muscles of rats. The relative GCLC protein expressions changed with HU, and the changes were age related (Age × HU, p = .005). In adult rats, the relative GCLC contents tended to increase with HU, although this change did not reach significance (one-way ANOVA, p < .1). In old rats, the relative GCLC contents in muscles with 3 and 7 days of HU were 2.2 and 2.7 times higher, respectively, than the values seen in muscles with weight bearing. However, the GCLC contents in muscles of old rats with 14 days of HU returned to control levels.
Figure 7.
The relative content of the catalytic subunit of glutamate cysteine ligase. Representative immunoblots (A) and densitometric analysis (B) are shown from rats with weight bearing (c) and 3, 7, and 14 days (d) of hind-limb unloading (HU). The number of animals in each group is noted. Values are mean ± SEM. The effect of HU was dependent on age (p = .005). *Significantly different from old weight-bearing rats. †Significantly different from old 7-day rats. ANOVA = analysis of variance.
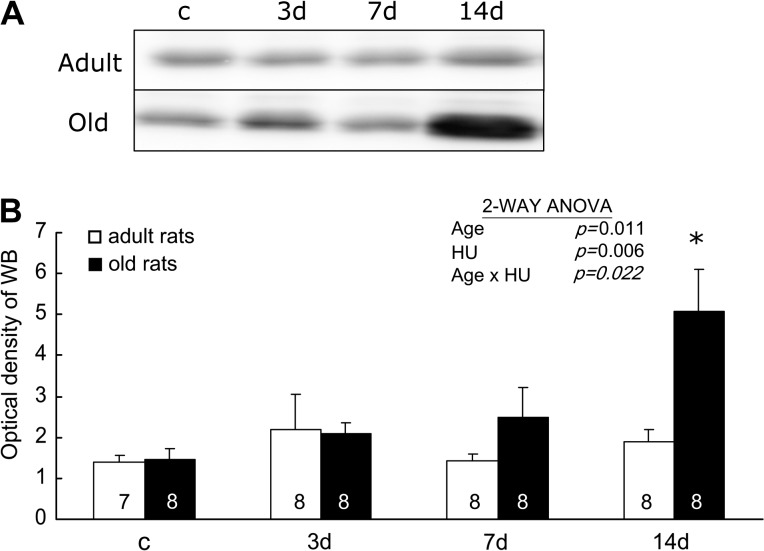
The content of GCLM.—
The modifier subunit of GCL lowers the Km (increases the affinity) for glutamate and ATP and increases the concentration of GSH required for GCL inhibition (Ki) (16). Figure 8 shows representative immunoblots (A) and summary of the densitometric analysis (B) of GCLM from soleus muscles of rats. The changes of the relative contents of GCLM with HU were age specific (Age × HU, p = .022). In adult rats, the relative GCLM contents in muscles did not change with HU. In old rats, the relative GCLM contents in muscles with 14 days of HU were greater than the values seen in the muscles with weight bearing and 3 and 7 days of HU (3.48-, 2.42-, and 2.03-folds higher, respectively).
Figure 8.
The relative content of the modifier subunit of glutamate cysteine ligase. Representative immunoblots (A) and densitometric analysis (B) are shown from rats with weight bearing (c) and 3, 7, and 14 days (d) of hind-limb unloading (HU). The number of animals in each group is noted. Values are mean ± SEM. The effect of HU was dependent on age (p = .022). *Significantly different from old weight-bearing, 3- and 7-day rats. ANOVA = analysis of variance.
DISCUSSION
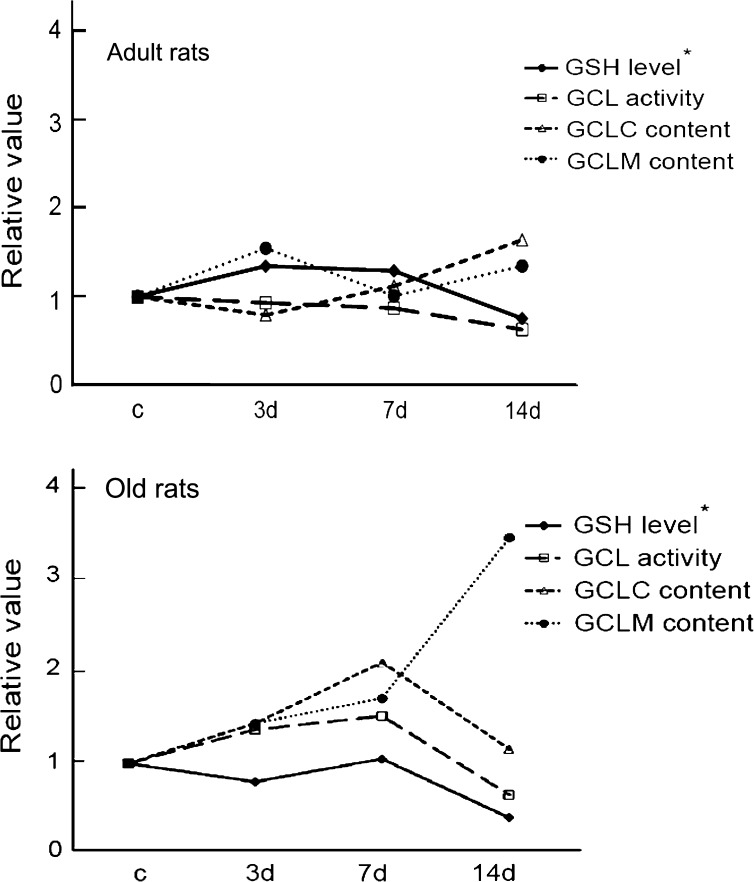
With aging, organisms display a reduction in their ability to adapt to stress (17–19). Our previous study showed that aged muscles have an impaired ability to maintain the GSH level with extended stress (14 days of HU; Figure 9) (3). The aim of this study was to determine the mechanism behind the age-related differences in muscle GSH levels in response to stress. The major finding of this study is that the decreased activity of GCL, the rate-limiting enzyme of GSH biosynthesis, explains at least part of the GSH depletion in muscles of old rats with stress (Figure 9). GCL activity in aging muscles declines under prolonged stress, and this decline is closely related to a reduction in the expression of the GCLC. The dramatic changes in components responsible for GSH metabolism observed in aging muscles are in sharp contrast to the attenuated response to HU observed in muscles from adult rats.
Figure 9.
Summary of the major findings. The changes of glutamate cysteine ligase (GCL) activity and protein content for the catalytic subunit of glutamate cysteine ligase (GCLC) and modifier subunit of glutamate cysteine ligase (GCLM) in adult (top) and old (bottom) rats are plotted to demonstrate the relationship of these variables with the changes of reduced glutathione (GSH) content. Values are relative to control for each age. c = weight bearing; 3d, 7d, and 14d = hind-limb unloading for 3, 7, and 14 days. *Drawn from the data in Chen and colleagues (3).
GSH Metabolism Is Altered With Aging
The alterations of GSH metabolism with aging (a condition of chronic oxidative stress) are tissue specific (2,20–26). While brain and liver are the most studied tissues, few studies investigate the age-related changes of GSH metabolism in skeletal muscles. Identifying the age-related changes of GSH homeostasis is important because GSH regulates the redox balance of the cells. Previously, we have shown that GSH content and GPX, an enzyme that uses GSH as a substrate in redox reactions, are upregulated with aging (3). In the current study, we investigate GST, another key enzyme that facilitates GSH usage in the detoxification reaction, and found that GST activity increased in soleus muscles (composed of type I fibers) with aging (Figure 3). This age-related increase in GST activity was also observed in muscles before (2,21). Taken together, these results suggest that the GSH-related detoxifying machinery of skeletal muscles is upregulated with aging. The mechanisms underlying the age-related upregulation of the GSH and the GSH-dependent detoxifying enzymes are unknown; however, the upregulation is likely a compensatory adaptation responding to the chronic oxidative stress developed during the aging process (17,27).
On the other hand, the activities of the enzymes that replenish GSH (GGT, GR, and GCL) are not upregulated in soleus muscles with aging. In fact, the current study shows that aging is accompanied by lower GR and GGT activities (Figures 4 and 5, respectively) and has no effect on GCL activity (Figure 6) in soleus muscles. The results are consistent with the finding of Leeuwenburgh and colleagues (2). The reduced GR and GGT activities suggest that either the efficiency or the expression levels of these enzymes may be lower with aging. The non-changed GCL activity is most likely due to the chronic feedback inhibition from the elevated levels of GSH (16).
In addition to the possible mechanisms discussed earlier, other factors (eg, composition of the muscles) may contribute in part to the age-related differences in the GSH metabolism. For example, aging muscles have been reported to have less capillarization and altered fiber type composition (28). Because whole-muscle homogenates were used in this study, the influence of the age-related changes of muscle and extracellular matrix composition on GSH metabolism cannot be excluded.
GSH Metabolism Is Impaired in Aging Muscles Under Stress
Although the antioxidant system shows a compensatory adaptation with aging, the ability of aged organisms to positively respond to an additional stress appears to be compromised. Our previous study, focusing on the influence of age for skeletal muscle antioxidants to adapt to stress, revealed that GSH levels in muscles under stress (muscle disuse) are maintained in adult rats but not in old rats. Specifically, the GSH level in muscles of old rats did not change initially with stress (muscle disuse for 3 and 7 days); however, it dropped to 40% of the control values with prolonged stress (muscle disuse for 14 days). The main focus of the current study was to identify the mechanism underlying the dramatic decrease of GSH levels in the muscles of old rats with 14 days of HU.
GSH replenishment.—
One possible mechanism for the decreased GSH levels in the aging muscles with stress (3) is a decrease in GSH replenishment. The results of the present study suggest that the decline of GCL activity contributes to the decrease of GSH levels (Figure 9). GCL activity in muscles of old rats increased initially with stress, but the elevated activity was not maintained with prolonged stress (ie, activity level dropped with muscle disuse at 14 days). Moreover, the pattern of GCL activities (change in activity levels for a 14-day period of stress) is very similar to the kinetics of GSH content in muscles of old rats with additional stress (3).
GCL activity is regulated by its two subunits, the catalytic subunit (GCLC) and the modifier subunit (GCLM). GCLC (73 kDa) contains the active site for the ATP-dependent bond formation between glutamate and cysteine and possesses all the catalytic activity of GCL (29). Studies have shown that GCLC alone is necessary and sufficient for γ-GC formation (30,31). GCLM (31 kDa), although having no catalytic activity, enhances enzyme activity by lowering the Km (increasing the affinity) of GCLC to glutamate and ATP and increasing the concentration of GSH required to inhibit GCL activity (Ki). Yang and colleagues (31) demonstrated that Gclm homozygous knockout mice had lower GSH levels, increased Km (reduced affinity) of GCLC to glutamate and were more sensitive to oxidative stress compared with wild-type littermates. These results demonstrate the important contribution GCLM makes in regulating GSH levels.
In order to understand why GCL activity decreased in the muscles of old rats under prolonged stress, we investigated the protein content of the two subunits of GCL. We found that in adult animals, GCLC protein content did not change significantly during the 14 days of HU (Figure 7). In contrast, GCLC protein expression in old animals increased initially with stress (3 and 7 days of HU) and then dramatically decreased with prolonged stress (14 days of HU). Because GCLC possesses catalytic function of GCL, the failure to maintain the accelerated production of GCLC at 14 days of HU contributes to the decline of GCL activity in muscles of old rats with prolonged stress.
Interestingly, the increase of GCLM protein content with stress occurs later (14 days) than that of GCLC protein content in aging muscles (3 and 7 days), suggesting an uncoupling response of the two subunits of GCL to HU (Figure 9). This uncoupling of GCLC and GCLM is also reported in aging brain and liver with increased oxidative stress (26,32). The mechanism of the uncoupled response in muscle is likely because GCLC and GCLM are encoded by genes on different chromosomes that can be regulated differently.
The increase of GCLM protein expression in aging muscles with prolonged stress (14 days of HU) found in the current study is likely a compensatory adaptation to rescue GCL activity because as noted earlier, GCLM increases the catalytic function of GCLC. Experiments where the GCLC content is maintained showed that addition of GCLM protein increases GCL activity, and conversely, reduced GCLM content decreases GCL activity (26,31,33). However, we found that the increased GCLM content does not increase GCL activity. This finding is likely due to the significantly lower content of GCLC at 14 days of HU (Figure 9). Collectively, these results suggest that the influence of GCLM on the changes of GCL activity with stress is likely dependent on the changes of GCLC content.
Possible mechanism of the reduced GCLC expression with prolonged stress.—
Many mechanisms such as oxidative stress and cysteine deprivation (low protein diet) have been proposed to increase GCLC protein content (15,33). However, little is known about the mechanism underlying the decreased GCLC protein expression. One potential factor related to the decreased GCLC expression is transforming growth factor-β1 (TGF-β1). Studies show that TGF-β1 downregulates GCLC expression at the transcription level and causes a decline of GCL activity in a dose- and time-dependent manner (34,35). TGF-β1 protein expression has been determined in skeletal muscles where it increased in muscles with 7 days of disuse (36). Importantly, the same study also showed that the timing of the activation of GCLC gene activator and suppressor in disused muscles is different. The activation of TGF-β1 was later than that of tumor necrosis factor-α (TNF-α), a cytokine that induces GCLC transcription, which increased at 3 days of muscle disuse (36). Thus, it is possible that the increased TNF-α is related to the initial increase of GCLC protein expression in aging muscles with stress, and the later increased TGF-β1 regulates the decrease of GCLC protein expression in aging muscles with prolonged stress. Further investigation is needed to test this hypothesis.
Two other enzymes that are related to GSH replenishment, GR and GGT, do not change in aging muscles under stress. This finding of the enzyme-specific responses with stress is similar to that of Lapenna and colleagues (37) where severe ischemia–reperfusion of myocardium affected GCL activity but not GR and GGT activities. The underlying mechanism of the enzyme-specific responses is probably due to the different signal pathways induced by oxidative stress that affect the regulation of GCL, GR, and GGT.
GSH utilization.—
Increased GSH utilization is another possible mechanism that depletes GSH level in aging muscles with prolonged stress. In the current study, we found that the increased GST activity (increased GSH utilization) was accompanied by the unaltered GSH levels. Additionally, the decreased GST activity (decreased GSH utilization) is accompanied by decreased GSH levels instead of increased GSH level. This finding of the coordinate reductions of GST activity and GSH level indicates that the decrease of GST activity is the reflection of the decreased content of GSH, which is the substrate of GST. Consequences of the reduced GSH level and GST activity are likely increasing the susceptibility of cells to oxidative damage.
In addition to oxidative stress in muscles with HU, other general physiological stress responses may contribute potentially to our finding. HU has been shown to induce general physiological stress responses, such as adrenal hypertrophy, thymus involution, greater glucocorticoid levels, and decreased food intake and body weight (38). The general physiological stress responses with HU may be age dependent. For example, Stump and colleagues (39) reported that the decrease of body weight with 14 days of HU was significant in old rats but not in adult rats. The changes in general physiological stress responses with HU can potentially affect the GSH metabolisms in muscles. Further investigation is needed to test this hypothesis.
CONCLUSIONS
The decrease in GCL activities is at least partially responsible for the decline of GSH levels in aging muscles with disuse. This decreased GCL activity is associated with the reduction of GCLC protein expression. The impairment in GSH homeostasis may render aging muscle more susceptible to the ongoing oxidative damage from stress. The greater susceptibility to oxidants in the stressed muscles of old rats than adult rats is likely associated with the age-related changes of muscle function in the disused muscles.
FUNDING
This project was partially funded by AG-17768 and AG-21626 (L.V.T.) from the National Institutes of Health. C.C. was funded by the Doctoral Dissertation Fellowship from the Graduate School at the University of Minnesota.
Acknowledgments
We thank Janice Shoeman and Sheng Zhong for technical assistance.
References
- 1.Maher P. The effects of stress and aging on glutathione metabolism. Ageing Res Rev. 2005;4(2):288–314. doi: 10.1016/j.arr.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 2.Leeuwenburgh C, Fiebig R, Chandwaney R, Ji LL. Aging and exercise training in skeletal muscle: responses of glutathione and antioxidant enzyme systems. Am J Physiol. 1994;267(2 Pt 2):R439–R445. doi: 10.1152/ajpregu.1994.267.2.R439. [DOI] [PubMed] [Google Scholar]
- 3.Chen CN, Brown-Borg HM, Rakoczy SG, Thompson LV. Muscle disuse: adaptation of antioxidant systems is age dependent. J Gerontol A Biol Sci Med Sci. 2008;63(5):461–466. doi: 10.1093/gerona/63.5.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Powers SK, Kavazis AN, McClung JM. Oxidative stress and disuse muscle atrophy. J Appl Physiol. 2007;102(6):2389–2397. doi: 10.1152/japplphysiol.01202.2006. [DOI] [PubMed] [Google Scholar]
- 5.Lawler JM, Song W, Demaree SR. Hindlimb unloading increases oxidative stress and disrupts antioxidant capacity in skeletal muscle. Free Radic Biol Med. 2003;35(1):9–16. doi: 10.1016/s0891-5849(03)00186-2. [DOI] [PubMed] [Google Scholar]
- 6.Ikemoto M, Okamura Y, Kano M, et al. A relative high dose of vitamin E does not attenuate unweighting-induced oxidative stress and ubiquitination in rat skeletal muscle. J Physiol Anthropol Appl Hum Sci. 2002;21(5):257–263. doi: 10.2114/jpa.21.257. [DOI] [PubMed] [Google Scholar]
- 7.Vermaelen M, Marini JF, Chopard A, Benyamin Y, Mercier J, Astier C. Ubiquitin targeting of rat muscle proteins during short periods of unloading. Acta Physiol Scand. 2005;185(1):33–40. doi: 10.1111/j.1365-201X.2005.01446.x. [DOI] [PubMed] [Google Scholar]
- 8.Koesterer TJ, Dodd SL, Powers S. Increased antioxidant capacity does not attenuate muscle atrophy caused by unweighting. J Appl Physiol. 2002;93(6):1959–1965. doi: 10.1152/japplphysiol.00511.2002. [DOI] [PubMed] [Google Scholar]
- 9.Chen CN, Ferrington DA, Thompson LV. Carbonic anhydrase III and four-and-a-half LIM protein 1 are preferentially oxidized with muscle unloading. J Appl Physiol. 2008;105(5):1554–1561. doi: 10.1152/japplphysiol.90680.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 11.Brown-Borg HM, Bode AM, Bartke A. Antioxidative mechanisms and plasma growth hormone levels: potential relationship in the aging process. Endocrine. 1999;11(1):41–48. doi: 10.1385/ENDO:11:1:41. [DOI] [PubMed] [Google Scholar]
- 12.Brown-Borg HM, Rakoczy SG. Glutathione metabolism in long-living Ames dwarf mice. Exp Gerontol. 2005;40(1–2):115–120. doi: 10.1016/j.exger.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 13.White CC, Viernes H, Krejsa CM, Botta D, Kavanagh TJ. Fluorescence-based microtiter plate assay for glutamate-cysteine ligase activity. Anal Biochem. 2003;318(2):175–180. doi: 10.1016/s0003-2697(03)00143-x. [DOI] [PubMed] [Google Scholar]
- 14.Wu H, White CC, Isanhart JP, McBride TJ, Kavanagh TJ, Hooper MJ. Optimization and application of glutamate cysteine ligase measurement in wildlife species. Ecotoxicol Environ Saf. 2009;72(2):572–578. doi: 10.1016/j.ecoenv.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 15.Lu SC. Regulation of glutathione synthesis. Mol Aspects Med. 2008;30(1–2):42–59. doi: 10.1016/j.mam.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Franklin CC, Backos DS, Mohar I, White CC, Forman HJ, Kavanagh TJ. Structure, function, and post-translational regulation of the catalytic and modifier subunits of glutamate cysteine ligase. Mol Aspects Med. 2009;30(1–2):86–98. doi: 10.1016/j.mam.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lambertucci RH, Levada-Pires AC, Rossoni LV, Curi R, Pithon-Curi TC. Effects of aerobic exercise training on antioxidant enzyme activities and mRNA levels in soleus muscle from young and aged rats. Mech Ageing Dev. 2007;128(3):267–275. doi: 10.1016/j.mad.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 18.Siu PM, Pistilli EE, Murlasits Z, Alway SE. Hindlimb unloading increases muscle content of cytosolic but not nuclear Id2 and p53 proteins in young adult and aged rats. J Appl Physiol. 2006;100(3):907–916. doi: 10.1152/japplphysiol.01012.2005. [DOI] [PubMed] [Google Scholar]
- 19.Kayani AC, Morton JP, McArdle A. The exercise-induced stress response in skeletal muscle: failure during aging. Appl Physiol Nutr Metab. 2008;33(5):1033–1041. doi: 10.1139/H08-089. [DOI] [PubMed] [Google Scholar]
- 20.Hollander J, Bejma J, Ookawara T, Ohno H, Ji LL. Superoxide dismutase gene expression in skeletal muscle: fiber-specific effect of age. Mech Ageing Dev. 2000;116(1):33–45. doi: 10.1016/s0047-6374(00)00130-5. [DOI] [PubMed] [Google Scholar]
- 21.Ji LL, Dillon D, Wu E. Alteration of antioxidant enzymes with aging in rat skeletal muscle and liver. Am J Physiol. 1990;258(4 Pt 2):R918–R923. doi: 10.1152/ajpregu.1990.258.4.R918. [DOI] [PubMed] [Google Scholar]
- 22.Kim HG, Hong SM, Kim SJ, et al. Age-related changes in the activity of antioxidant and redox enzymes in rats. Mol Cells. 2003;16(3):278–284. [PubMed] [Google Scholar]
- 23.Liu R, Choi J. Age-associated decline in gamma-glutamylcysteine synthetase gene expression in rats. Free Radic Biol Med. 2000;28(4):566–574. doi: 10.1016/s0891-5849(99)00269-5. [DOI] [PubMed] [Google Scholar]
- 24.Mosoni L, Breuille D, Buffiere C, Obled C, Mirand PP. Age-related changes in glutathione availability and skeletal muscle carbonyl content in healthy rats. Exp Gerontol. 2004;39(2):203–210. doi: 10.1016/j.exger.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 25.Ohrloff C, Hockwin O, Olson R, Dickman S. Glutathione peroxidase, glutathione reductase and superoxide dismutase in the aging lens. Curr Eye Res. 1984;3(1):109–115. doi: 10.3109/02713688408997191. [DOI] [PubMed] [Google Scholar]
- 26.Zhu Y, Carvey PM, Ling Z. Age-related changes in glutathione and glutathione-related enzymes in rat brain. Brain Res. 2006;1090(1):35–44. doi: 10.1016/j.brainres.2006.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Franco AA, Odom RS, Rando TA. Regulation of antioxidant enzyme gene expression in response to oxidative stress and during differentiation of mouse skeletal muscle. Free Radic Biol Med. 1999;27(9-10):1122–1132. doi: 10.1016/s0891-5849(99)00166-5. [DOI] [PubMed] [Google Scholar]
- 28.Rogers MA, Evans WJ. Changes in skeletal muscle with aging: effects of exercise training. Exerc Sport Sci Rev. 1993;21:65–102. [PubMed] [Google Scholar]
- 29.Botta D, White CC, Vliet-Gregg P, et al. Modulating GSH synthesis using glutamate cysteine ligase transgenic and gene-targeted mice. Drug Metab Rev. 2008;40(3):465–477. doi: 10.1080/03602530802186587. [DOI] [PubMed] [Google Scholar]
- 30.Dalton TP, Dieter MZ, Yang Y, Shertzer HG, Nebert DW. Knockout of the mouse glutamate cysteine ligase catalytic subunit (Gclc) gene: embryonic lethal when homozygous, and proposed model for moderate glutathione deficiency when heterozygous. Biochem Biophys Res Commun. 2000;279(2):324–329. doi: 10.1006/bbrc.2000.3930. [DOI] [PubMed] [Google Scholar]
- 31.Yang Y, Dieter MZ, Chen Y, Shertzer HG, Nebert DW, Dalton TP. Initial characterization of the glutamate-cysteine ligase modifier subunit Gclm(−/−) knockout mouse. Novel model system for a severely compromised oxidative stress response. J Biol Chem. 2002;277(51):49446–49452. doi: 10.1074/jbc.M209372200. [DOI] [PubMed] [Google Scholar]
- 32.Lu SC, Huang ZZ, Yang JM, Tsukamoto H. Effect of ethanol and high-fat feeding on hepatic gamma-glutamylcysteine synthetase subunit expression in the rat. Hepatology. 1999;30(1):209–214. doi: 10.1002/hep.510300134. [DOI] [PubMed] [Google Scholar]
- 33.Lee JI, Kang J, Stipanuk MH. Differential regulation of glutamate-cysteine ligase subunit expression and increased holoenzyme formation in response to cysteine deprivation. Biochem J. 2006;393(Pt 1):181–190. doi: 10.1042/BJ20051111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arsalane K, Dubois CM, Muanza T, et al. Transforming growth factor-beta1 is a potent inhibitor of glutathione synthesis in the lung epithelial cell line A549: transcriptional effect on the GSH rate-limiting enzyme gamma-glutamylcysteine synthetase. Am J Respir Cell Mol Biol. 1997;17(5):599–607. doi: 10.1165/ajrcmb.17.5.2833. [DOI] [PubMed] [Google Scholar]
- 35.Jardine H, MacNee W, Donaldson K, Rahman I. Molecular mechanism of transforming growth factor (TGF)-beta1-induced glutathione depletion in alveolar epithelial cells. Involvement of AP-1/ARE and Fra-1. J Biol Chem. 2002;277(24):21158–21166. doi: 10.1074/jbc.M112145200. [DOI] [PubMed] [Google Scholar]
- 36.Hirose T, Nakazato K, Song H, Ishii N. TGF-beta1 and TNF-alpha are involved in the transcription of type I collagen alpha2 gene in soleus muscle atrophied by mechanical unloading. J Appl Physiol. 2008;104(1):170–177. doi: 10.1152/japplphysiol.00463.2006. [DOI] [PubMed] [Google Scholar]
- 37.Lapenna D, de Gioia S, Ciofani G, et al. Impaired glutathione biosynthesis in the ischemic-reperfused rabbit myocardium. FEBS Lett. 1996;391(1–2):76–78. doi: 10.1016/0014-5793(96)00705-3. [DOI] [PubMed] [Google Scholar]
- 38.Thomason DB, Booth FW. Atrophy of the soleus muscle by hindlimb unweighting. J Appl Physiol. 1990;68(1):1–12. doi: 10.1152/jappl.1990.68.1.1. [DOI] [PubMed] [Google Scholar]
- 39.Stump CS, Tipton CM, Henriksen EJ. Muscle adaptations to hindlimb suspension in mature and old Fischer 344 rats. J Appl Physiol. 1997;82(6):1875–1881. doi: 10.1152/jappl.1997.82.6.1875. [DOI] [PubMed] [Google Scholar]