Abstract
In this issue, Gillespie et al. (Gillespie et al. 2009. J. Cell Biol. doi:10.1083/jcb.200907037) demonstrate that the mitogen-activated protein kinase isoform p38-γ plays a crucial role in blocking the premature differentiation of satellite cells, a skeletal muscle stem cell population. p38-γ puts the brakes on skeletal muscle differentiation by promoting the association of the transcription factor MyoD with the histone methyltransferase, KMT1A, which act together in a complex to repress the premature expression of the gene encoding the myogenic transcription factor Myogenin.
Satellite cells are myogenic progenitors that reside between the sarcolemma and basal lamina of the muscle fiber and play a crucial role in postnatal muscle growth and regeneration (for review see Zammit et al., 2006a). During skeletal muscle regeneration, these quiescent muscle precursor cells are “activated” to enter the cell cycle, and give rise to a transient amplifying population of skeletal muscle progenitor cells termed myoblasts. Although quiescent satellite cells express the transcription factor Pax7 (Seale et al., 2000), activated satellite cells express the myogenic transcription factor MyoD (Zammit et al., 2006b). Activated satellite cells face a very important developmental decision to either continue to divide or differentiate into skeletal muscle cells that fuse with existing myofibers. Among the various signaling pathways thought to control this important decision, the p38 mitogen-activated protein kinase (MAPK) family stands out as being crucial. p38 MAPK activity has been known for several years to be necessary to promote skeletal muscle differentiation (Zetser et al., 1999). Indeed, mice lacking p38-α MAPK display delayed myofiber growth and maturation in vivo, and proliferating skeletal muscle cells isolated from such mice display defective differentiation in vitro (Perdiguero et al., 2007). p38-α MAPK activity has been demonstrated to promote skeletal muscle differentiation by (a) enhancing dimerization of myogenic regulatory factors with their E protein binding partners (Lluís et al., 2005), (b) recruiting the SWI-SNF complex to skeletal muscle differentiation genes (Simone et al., 2004), and (c) activating the transcriptional activity of MEF2, which works in a positive feed-forward loop with MyoD to promote differentiation (Penn et al., 2004). In this issue, Gillespie et al. demonstrate that a different p38 MAPK family member, p38-γ, also plays a crucial role in regulating skeletal muscle differentiation. Interestingly (and surprisingly), this study indicates that p38-γ MAPK does not promote skeletal muscle differentiation but instead acts to block the premature induction of this program in activated satellite cells.
Since the discovery of MyoD (Davis et al., 1987), one of a family of muscle regulatory factors that both induce and coordinate the skeletal muscle differentiation program (for review see Tapscott, 2005), it has been apparent that the transcriptional activity of these basic helix-loop-helix (bHLH) transcription factors must be tightly regulated. Although MyoD is expressed in proliferating myoblasts, the skeletal muscle differentiation program is only induced in these cells after mitogen withdrawal. One of the first differentiation-specific transcriptional targets to be induced by MyoD is another muscle regulatory factor, termed Myogenin (Hollenberg et al., 1993), whose expression is essential to execute the differentiation program (Hasty et al., 1993; Nabeshima et al., 1993). The signals and transcriptional regulatory machinery that restrict Myogenin expression to differentiating myocytes has been an area of intense scrutiny during the past decade. Myogenin is a direct transcriptional target of MyoD (Hollenberg et al., 1993), and the myogenin promoter contains both a high-affinity MyoD binding site (a canonical E box) and a low-affinity MyoD binding site (a noncanonical E box), the latter of which is located adjacent to a homeodomain binding site (Berkes et al., 2004). Interaction between MyoD and the homeodomain protein Pbx has been demonstrated to stabilize binding of MyoD to this low-affinity binding site (Berkes et al., 2004). What factors inhibit the inappropriate induction of myogenin in proliferating myoblasts? Early studies by Benezra et al. (1990) documented that expression of Id1—a dominant-negative HLH protein that competes with MyoD for interaction with its obligate DNA-binding partners, the bHLH E proteins—is down-regulated during skeletal muscle differentiation; this observation led to the hypothesis that members of the Id protein family might act to restrict MyoD activity in proliferating myoblasts by blocking interaction of MyoD with DNA (Benezra et al., 1990). However, because chromatin immunoprecipitation assays have more recently demonstrated that MyoD is indeed bound to various transcriptional targets, including Myogenin, in proliferating myoblasts (Mal and Harter, 2003; Blais et al., 2005; Ishibashi et al., 2005; Ohkawa et al., 2006), there must be a non–Id-based mechanism for restricting Myogenin expression to differentiated cells.
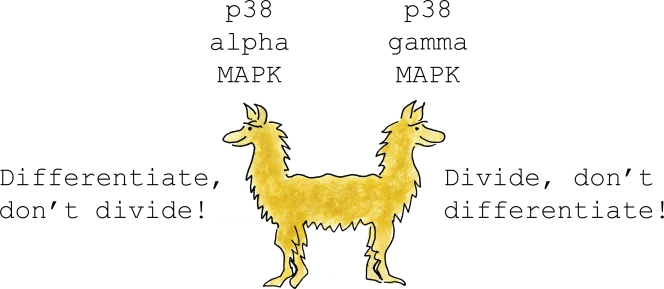
An initial clue to resolve this issue was the identification of the histone modification mark H3K9me3 over the myogenin promoter specifically in proliferating myoblasts (Mal and Harter, 2003). This histone modification is associated with facultative heterochromatin and is “read” by the transcriptional corepressor HP1, which in turn can interact with other heterochromatin-associated proteins (for review see Lomberk et al., 2006). Importantly, Mal demonstrated that MyoD could be isolated in complex with the histone methyl-transferase KMT1A (also known as Suv39h1), the “writer” of H3K9me3 modification, specifically in proliferating myoblasts but not in differentiated myocytes (Mal, 2006). This important observation suggested that differential interaction of MyoD with KMT1A in proliferating myoblasts versus differentiated myocytes may act to restrict transcriptional activation of target genes by MyoD to the latter. What signals modulate interaction of MyoD with KMT1A differentially in myoblasts versus differentiated myocytes? Gillespie et al. (2009) demonstrate that p38-γ MAPK phosphorylates the carboxy terminus of MyoD on serines 199 and 200, leading to increased interaction of MyoD with KMT1A. Consistent with these observations, this study also demonstrates that satellite cell–derived myoblasts isolated from mice lacking p38-γ MAPK display decreased KMT1A association with chromatin encompassing the myogenin promoter, and consequently display both accelerated expression of Myogenin and decreased proliferation in vitro. Most importantly, satellite cells in p38-γ–null mice display increased expression of Myogenin, loss of Pax7 expression, and markedly defective skeletal muscle differentiation in vivo (Gillespie et al., 2009). Surprisingly, another group did not observe either regeneration defects in p38-γ–null mice or altered differentiation of myoblasts derived from these animals (Ruiz-Bonilla et al., 2008), which suggests that mouse strain–specific genetic modifiers may alter the response of myoblasts to p38-γ deficiency. The consistent set of observations of Gillespie et al. (2009) make a compelling argument that p38-γ MAPK plays a crucial role in blocking the premature induction of Myogenin and consequent differentiation of activated satellite cells. In striking contrast, satellite cells in p38-α–null mice display increased expression of Pax7, augmented proliferation, and defective differentiation (Perdiguero et al., 2007), which is the inverse phenotype of p38-γ deficiency. Thus, the present study by Gillespie et al. (2009), together with other prior studies, highlights the surprisingly distinct role for differing members of the p38 MAPK family in either promoting or inhibiting the differentiation of activated satellite cells. It seems likely that the balance between p38-α and p38-γ MAPK activity in activated satellite cells serves as an accelerator or brake, respectively, to drive the process of skeletal muscle differentiation. Together, they resemble the conflicting interests of the two-headed pushmi-pullyu, who struggles to go in two opposite directions simultaneously (Fig. 1; Lofting, 1920). Although the activity of the pro-differentiation signal p38-α MAPK is known to be regulated during skeletal muscle differentiation by the cell surface Ig superfamily member Cdo (Takaesu et al., 2006), the signals that specifically regulate p38-γ MAPK activity in proliferating myoblasts or differentiated myocytes are unknown. Lastly, it seems likely that p38 MAPK family members play an additional important role during satellite cell activation, as treatment of quiescent satellite cells with SB203580, a p38-α/β MAPK inhibitor, blocks both the proliferation and induction of MyoD in such cells (Jones et al., 2005), and thus suggests that this kinase family also plays an important role in regulating entry of quiescent satellite cells to become a transient amplifying population.
Figure 1.
What's an activated satellite cell to do? Although p38-α MAPK activity simultaneously promotes the differentiation of activated satellite cells and inhibits their proliferation, p38-γ MAPK activity works in an opposing fashion to block differentiation of these cells, and instead promotes their proliferation. Thus, the conflicting signals of p38-α versus p38-γ MAPK activity in activated satellite cells resemble the conflicting interests of the two headed pushmi-pullyu, who struggles to go in two opposite directions simultaneously (Lofting, 1920).
References
- Benezra R., Davis R.L., Lockshon D., Turner D.L., Weintraub H. 1990. The protein Id: a negative regulator of helix-loop-helix DNA binding proteins. Cell. 61:49–59 10.1016/0092-8674(90)90214-Y [DOI] [PubMed] [Google Scholar]
- Berkes C.A., Bergstrom D.A., Penn B.H., Seaver K.J., Knoepfler P.S., Tapscott S.J. 2004. Pbx marks genes for activation by MyoD indicating a role for a homeodomain protein in establishing myogenic potential. Mol. Cell. 14:465–477 10.1016/S1097-2765(04)00260-6 [DOI] [PubMed] [Google Scholar]
- Blais A., Tsikitis M., Acosta-Alvear D., Sharan R., Kluger Y., Dynlacht B.D. 2005. An initial blueprint for myogenic differentiation. Genes Dev. 19:553–569 10.1101/gad.1281105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R.L., Weintraub H., Lassar A.B. 1987. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell. 51:987–1000 10.1016/0092-8674(87)90585-X [DOI] [PubMed] [Google Scholar]
- Gillespie M.A., Le Grand F., Scimè A., Kuang S., von Maltzahn J., Seale V., Cuenda A., Ranish J.A., Rudnicki M.A. 2009. p38-γ–dependent gene silencing restricts entry into the myogenic differentiation program. J. Cell Biol. 187:991–1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasty P., Bradley A., Morris J.H., Edmondson D.G., Venuti J.M., Olson E.N., Klein W.H. 1993. Muscle deficiency and neonatal death in mice with a targeted mutation in the myogenin gene. Nature. 364:501–506 10.1038/364501a0 [DOI] [PubMed] [Google Scholar]
- Hollenberg S.M., Cheng P.F., Weintraub H. 1993. Use of a conditional MyoD transcription factor in studies of MyoD trans-activation and muscle determination. Proc. Natl. Acad. Sci. USA. 90:8028–8032 10.1073/pnas.90.17.8028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi J., Perry R.L., Asakura A., Rudnicki M.A. 2005. MyoD induces myogenic differentiation through cooperation of its NH2- and COOH-terminal regions. J. Cell Biol. 171:471–482 10.1083/jcb.200502101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones N.C., Tyner K.J., Nibarger L., Stanley H.M., Cornelison D.D., Fedorov Y.V., Olwin B.B. 2005. The p38α/β MAPK functions as a molecular switch to activate the quiescent satellite cell. J. Cell Biol. 169:105–116 10.1083/jcb.200408066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lluís F., Ballestar E., Suelves M., Esteller M., Muñoz-Cánoves P. 2005. E47 phosphorylation by p38 MAPK promotes MyoD/E47 association and muscle-specific gene transcription. EMBO J. 24:974–984 10.1038/sj.emboj.7600528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lofting H. 1920. The Story of Doctor Dolittle: Being the History of His Peculiar Life at Home and Astonishing Adventures in Foreign Parts. Frederick A. Stokes, New York: 180 pp [Google Scholar]
- Lomberk G., Wallrath L., Urrutia R. 2006. The Heterochromatin Protein 1 family. Genome Biol. 7:228 10.1186/gb-2006-7-7-228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mal A.K. 2006. Histone methyltransferase Suv39h1 represses MyoD-stimulated myogenic differentiation. EMBO J. 25:3323–3334 10.1038/sj.emboj.7601229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mal A., Harter M.L. 2003. MyoD is functionally linked to the silencing of a muscle-specific regulatory gene prior to skeletal myogenesis. Proc. Natl. Acad. Sci. USA. 100:1735–1739 10.1073/pnas.0437843100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabeshima Y., Hanaoka K., Hayasaka M., Esumi E., Li S., Nonaka I., Nabeshima Y. 1993. Myogenin gene disruption results in perinatal lethality because of severe muscle defect. Nature. 364:532–535 10.1038/364532a0 [DOI] [PubMed] [Google Scholar]
- Ohkawa Y., Marfella C.G., Imbalzano A.N. 2006. Skeletal muscle specification by myogenin and Mef2D via the SWI/SNF ATPase Brg1. EMBO J. 25:490–501 10.1038/sj.emboj.7600943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn B.H., Bergstrom D.A., Dilworth F.J., Bengal E., Tapscott S.J. 2004. A MyoD-generated feed-forward circuit temporally patterns gene expression during skeletal muscle differentiation. Genes Dev. 18:2348–2353 10.1101/gad.1234304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perdiguero E., Ruiz-Bonilla V., Gresh L., Hui L., Ballestar E., Sousa-Victor P., Baeza-Raja B., Jardí M., Bosch-Comas A., Esteller M., et al. 2007. Genetic analysis of p38 MAP kinases in myogenesis: fundamental role of p38alpha in abrogating myoblast proliferation. EMBO J. 26:1245–1256 10.1038/sj.emboj.7601587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Bonilla V., Perdiguero E., Gresh L., Serrano A.L., Zamora M., Sousa-Victor P., Jardí M., Wagner E.F., Muñoz-Cánoves P. 2008. Efficient adult skeletal muscle regeneration in mice deficient in p38beta, p38gamma and p38delta MAP kinases. Cell Cycle. 7:2208–2214 [DOI] [PubMed] [Google Scholar]
- Seale P., Sabourin L.A., Girgis-Gabardo A., Mansouri A., Gruss P., Rudnicki M.A. 2000. Pax7 is required for the specification of myogenic satellite cells. Cell. 102:777–786 10.1016/S0092-8674(00)00066-0 [DOI] [PubMed] [Google Scholar]
- Simone C., Forcales S.V., Hill D.A., Imbalzano A.N., Latella L., Puri P.L. 2004. p38 pathway targets SWI-SNF chromatin-remodeling complex to muscle-specific loci. Nat. Genet. 36:738–743 10.1038/ng1378 [DOI] [PubMed] [Google Scholar]
- Takaesu G., Kang J.S., Bae G.U., Yi M.J., Lee C.M., Reddy E.P., Krauss R.S. 2006. Activation of p38α/β MAPK in myogenesis via binding of the scaffold protein JLP to the cell surface protein Cdo. J. Cell Biol. 175:383–388 10.1083/jcb.200608031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapscott S.J. 2005. The circuitry of a master switch: Myod and the regulation of skeletal muscle gene transcription. Development. 132:2685–2695 10.1242/dev.01874 [DOI] [PubMed] [Google Scholar]
- Zammit P.S., Partridge T.A., Yablonka-Reuveni Z. 2006a. The skeletal muscle satellite cell: the stem cell that came in from the cold. J. Histochem. Cytochem. 54:1177–1191 10.1369/jhc.6R6995.2006 [DOI] [PubMed] [Google Scholar]
- Zammit P.S., Relaix F., Nagata Y., Ruiz A.P., Collins C.A., Partridge T.A., Beauchamp J.R. 2006b. Pax7 and myogenic progression in skeletal muscle satellite cells. J. Cell Sci. 119:1824–1832 10.1242/jcs.02908 [DOI] [PubMed] [Google Scholar]
- Zetser A., Gredinger E., Bengal E. 1999. p38 mitogen-activated protein kinase pathway promotes skeletal muscle differentiation. Participation of the Mef2c transcription factor. J. Biol. Chem. 274:5193–5200 10.1074/jbc.274.8.5193 [DOI] [PubMed] [Google Scholar]