SCIENTIFIC ABSTRACT
Autism spectrum disorder (ASD) and specific language impairment (SLI) are developmental disorders exhibiting language deficits, but it is unclear whether they arise from similar etiologies. Language impairments have been described in family members of children with ASD and SLI, but few studies have quantified them. In this study, we examined IQ, language, and reading abilities of ASD and SLI children and their first-degree relatives to address whether the language difficulties observed in some children with ASD are familial and to better understand the degree of overlap between these disorders and their broader phenotypes. Participants were 52 autistic children, 36 children with SLI, their siblings, and their parents. The ASD group was divided into those with (ALI, n=32) and without (ALN, n=20) language impairment. Relationships between ASD severity and language performance were also examined in the ASD probands. ALI and SLI probands performed similarly on most measures while ALN probands scored higher. ALN and ALI probands' language scores were not related to ADI-R and ADOS algorithm scores. SLI relatives scored lowest on all measures, and while scores were not in the impaired range, relatives of ALI children scored lower than relatives of ALN children on some measures, though not those showing highest heritability in SLI. Given that ALI relatives performed better than SLI relatives across the language measures, the hypothesis that ALI and SLI families share similar genetic loading for language is not strongly supported.
Keywords: autism spectrum disorder, specific language impairment, parents, siblings, broader phenotype, genetics, language, reading
INTRODUCTION
There is a long-standing debate in the literature about the extent of overlap between the language phenotypes of autism spectrum disorder (ASD) and specific language impairment (SLI) and whether these deficits arise from similar genetic bases (for review, see Williams et al., 2008). Studies have attempted to address this issue by investigating language abilities in first-degree relatives. Atypical language, or a broader language phenotype, has been described in family members of individuals with ASD and SLI, but few studies have quantified these deficits using standardized language measures, particularly those assessing structural aspects of language. Such studies are necessary to test the specificity of the language phenotypes in ASD and SLI as well as identify which aspects may be genetically mediated.
Overlap Between ASD and SLI
Autism and SLI are two developmental disorders that share language as a deficit. In both disorders, concerns are typically raised during the toddler years (Dahlgren & Gillberg, 1989; Tager-Flusberg & Cooper, 1999). Autism and SLI are both considered spectrum disorders (Bishop, 1989; Gillberg & Coleman, 2000; Resnick & Rapin, 1991), and this is supported by the considerable heterogeneity in language abilities observed in affected individuals (Tager-Flusberg et al., 2005; Tomblin & Zhang, 1999). There is also evidence that genes play a significant role in these disorders. Several studies have supported this hypothesis in autism (for reviews, see Bespalova & Buxbaum, 2003; Folstein & Rosen-Sheidley, 2001), and a strong genetic basis of SLI is supported by significant differences in the concordance rates for monozygotic versus dizygotic twins (Bishop et al., 1995; Lewis & Thompson, 1992; Tomblin & Buckwalter, 1998). Segregation analyses provide strong evidence of familial transmission of SLI (Lewis et al., 1993; Tomblin & Zhang, 1999), and several studies have described an increased prevalence of language delay and language-based learning deficits in the parents and siblings of autistic individuals (Bailey et al., 1998; Bolton et al., 1994; Fombonne et al., 1997; Piven et al., 1997a). This relationship may be bi-directional, and siblings of children with SLI may also be at a higher risk of developing autism. A study by Tomblin and colleagues (2003) found that although there were no significant group differences in autism risk to siblings when SLI and control groups were defined categorically, when language was treated as a continuous variable, siblings of children who had poor spoken language skills in kindergarten were at higher risk for autism. Similarly, although Rapin (1996a) described higher rates of autism in siblings of high- and low-functioning children with autism, the rate of autism in siblings of SLI children was higher than that in siblings of non-autistic children with low IQs. Finally, some genetic studies have described overlap in genetic loci implicated in autism and SLI (for ASD review, see Abrahams & Geschwind, 2008; Alarcon et al., 2008; Arking et al., 2008; Bakkaloglu et al., 2008; O'Brien et al., 2003; Vernes et al., 2008; Warburton et al., 2000). Of particular interest is the recent study by Vernes and colleagues (2008) that identified a candidate gene for SLI showing significant associations with non-word repetition performance. This gene, CNTNAP2, has also been implicated in ASD (Alarcon et al., 2008; Arking et al., 2008; Bakkaloglu et al., 2008), especially in individuals who experienced language delay (Alarcon et al., 2008). These findings support the view that there is at least one gene contributing to the common language phenotype observed in these disorders.
Despite these similarities, there are differences between autism and SLI. Autism is defined by qualitative impairments in three realms by the age of three: social interaction, communication, and a restricted repertoire of activities and interests (American Psychiatric Association, 1994). SLI is characterized by delayed onset and slowed acquisition of language as compared to other areas of development (Tager-Flusberg & Cooper, 1999), but according to DSM-IV (American Psychiatric Association, 1994), individuals cannot meet criteria for autism and receive a diagnosis of SLI. There are also differences in the types of language difficulties observed in these disorders. In autism, some individuals express a developmental regression, especially in language, with one study citing rates as high as 33% (Goldberg et al., 2003), while this trajectory is absent in individuals with SLI (Rapin, 1996b). Furthermore, individuals with autism may exhibit other language characteristics, such as echolalia and pronoun reversal, that are not often described in SLI (e.g., Bartak et al., 1975, 1977).
There have been several studies investigating overlap between language phenotypes in autism and SLI. Bartak, Rutter, and Cox (1975, 1977) completed the first studies to directly investigate this relationship. The authors found that although autistic children scored significantly lower on measures of language comprehension, the groups exhibited similar deficits in expressive language and language production. In another study by Kjelgaard and Tager-Flusberg (2001), a large group of children with autism was tested on a variety of standardized language measures, including the Clinical Evaluation of Language Fundamentals – 3rd Edition (CELF-III) and a non-word repetition test. The authors found that, as a group, children with autism performed one standard deviation or more below the mean in total language ability as measured by the CELF-III and on non-word repetition. However, when the group was subdivided based on Total Language Ability on the CELF-III into normal, borderline, or impaired language ability, only the borderline and impaired groups (about 75% of the total sample) scored significantly below the mean on non-word repetition. These children exhibited language profiles of grammar, vocabulary, and phonological processing similar to children with SLI. A follow-up study found that children with autism and language impairment made grammatical tense marking errors that were similar to those of children with SLI (Roberts et al., 2004), further supporting the hypothesis of overlap between these groups. Finally, a recent study by Whitehouse and colleagues (2008) was the first to directly compare children with autism and SLI on non-word repetition performance. The authors found that those children with autism and normal structural language (ALN) scored higher than both the SLI group and the group of autistic children with poor structural language (ALI). Further analyses of group differences in non-word repetition performance and its relation to syllable length in those children who performed poorly on this test (SLI, n=18; ALI, n=8) found similar rates of error on words two or three syllables in length but significantly poorer performance on five-syllable words in the SLI group; however, given the small sample size and number of observations for each syllable length (3 trials each for 2-4 syllables and 4 trials for 5 syllables), the latter analyses require further investigation.
The diagnostic boundaries between autism and SLI have also been questioned. In the early studies by Bartak and colleagues (1975, 1977), about 10% of the original SLI sample displayed some autistic characteristics. When the children from these studies were then followed into middle childhood (Cantwell et al., 1989) and later into adulthood (Howlin et al., 2000; Mawhood et al., 2000), the authors noted that some of the SLI individuals had developed social and behavioral impairments similar to those observed in the autism group. A recent study also noted poorer quality of friendships in adolescents with SLI (Durkin & Conti-Ramsden, 2007). Bishop and Norbury (2002) found that some children with either SLI or pragmatic language impairment, as defined by the Children's Communication Checklist (Bishop, 1998), scored above cutoff on two of the three domains of the Autism Diagnostic Interview – Revised (ADI-R) or met criteria for autistic disorder on this measure. There was, however, also a group of children that failed to exhibit clear autistic symptoms outside of the communication domain. In a similar study by Conti-Ramsden and colleagues (2006a), 14-year-old children with a history of SLI were evaluated on a variety of diagnostic measures, including the ADI-R, the Autism Diagnostic Observation Schedule (ADOS), and the Family History Interview (FHI). The prevalence of ASD in this group was higher than that of the general population, and a number of children exhibited milder autistic behaviors on these measures. More recently, a study by Bishop and colleagues (2008) investigating the hypothesis of diagnostic substitution in autism found that in their sample of 38 individuals who had previously been diagnosed with language disorder in childhood, 13 met criteria for ASD on both the ADI-R and ADOS in adulthood. This study raises the question of whether SLI individuals in previous studies truly developed autistic symptoms later in life or whether they were misdiagnosed in childhood. These results highlight the possible continuity between autism and SLI and the lack of clear boundaries between these heterogeneous disorders.
Language Characteristics of Relatives of Individuals with Autism and Individuals with SLI
Family studies have noted language impairments in first-degree relatives of children with SLI and children with ASD, supporting a genetic basis for these deficits. Using family history questionnaires, several studies have described higher rates of language impairments in parents and siblings of SLI children when compared to relatives of typically developing children (Lahey & Edwards, 1995; Neils & Aram, 1986; Rice et al., 1998; Tallal et al., 1989; Tomblin, 1989; van der Lely & Stollwerck, 1996). As many as 60% of children with SLI have at least one additional family member with language impairments (Lahey & Edwards, 1995), although it is unclear whether the occurrence rates vary depending on the relationship of the family member to the proband (Rice et al., 1995; Tomblin, 1989). Family studies of children with autism have also noted similar features, or a “broader phenotype,” in first-degree relatives, including impairments in language functioning. Studies of twins discordant for autism have reported language difficulties in the non-affected twins (Folstein & Rutter, 1977; Le Couteur et al., 1996). Using family history data, higher rates of communication deficits have been identified in relatives of children with autism when compared to relatives of children with Down syndrome (Bolton et al., 1994; Piven et al., 1997a). These deficits were also greater in biological versus non-biological relatives of children with autism (Szatmari et al., 2000), further supporting the hypothesis that the communication impairments observed in autism and in the broader phenotype have a shared genetic basis.
Studies in family members of children with ASD and SLI have mainly focused on two types of language deficits: those in pragmatic language, or the social use of language, and those in structural language, such as phonology, grammar, and vocabulary difficulties. Pragmatic language deficits are consistently described as part of the ASD broader phenotype. Studies using questionnaires, such as the Autism Spectrum Quotient (Bishop et al., 2004a) or the Children's Communication Checklist (Bishop et al., 2006), have demonstrated clear communication deficits in some parents and siblings of children with ASD when compared to family members of typically developing children. Higher rates of poor narrative performance (Landa et al., 1991) and pragmatic language impairments (Landa et al., 1992; Piven et al., 1997b) have also been identified in parents of children with ASD when compared to parents of typically developing children or children with Down syndrome. More recently, Ruser and colleagues (2007) noted these communication deficits on a modified version of the Pragmatic Rating Scale in both parents of children with autism and parents of children with SLI when compared to parents of children with Down syndrome. Together, these findings suggest that pragmatic deficits are evident in a subset of first-degree relatives of both children with autism and children with SLI and that they may contribute to the broader phenotypes associated with these disorders. Of note, however, one study using the Autism Spectrum Quotient failed to identify impairments in social communication in parents of children with SLI (Whitehouse et al., 2007).
Studies assessing structural language abilities in parents and siblings of individuals with SLI have described clinically impaired performance in some relatives on standardized measures with rates ranging from 21% to 63% (Conti-Ramsden et al., 2006b; Plante et al., 1996; Tomblin & Buckwalter, 1998). Similar deficits have also been found in phonological processing, including poor performance on a non-word repetition task (Barry et al., 2007; Bishop et al., 1999; Bishop et al., 1996). Studies evaluating structural language in relatives of autistic individuals, however, have produced mixed results. Folstein and colleagues (1999) found higher rates of early language difficulties and poorer performance on a nonsense word reading task in parents, but not siblings, of children with autism versus relatives of children with Down syndrome. Amongst the relatives of children with autism, parents and siblings with a history of language impairment performed more poorly than family members without a positive history on tests of verbal intelligence, reading, spelling, and nonsense word reading. Similarly, Bishop and colleagues (2006) described abnormalities in structural language in some siblings of children with autism, suggesting that the broader phenotype of autism may overlap with SLI. Another study described poorer phonological processing, reading, writing, and vocabulary abilities in brothers, but not mothers, fathers, or sisters, of autistic females when compared to relatives of individuals with Down Syndrome (Plumet et al., 1995). Other studies, though, have suggested that structural language deficits may not be part of the broader autism phenotype. Pilowsky and colleagues (2003) investigated language abilities of siblings of children with autism, children with SLI, and children with mental retardation and found no differences between the groups on a variety of language abilities, including verbal intelligence, receptive and expressive language, and reading, writing, and spelling performance. Another study failed to identify phonological processing deficits in first-degree relatives of children with autism on non-word repetition and nonsense word reading tests (Bishop et al., 2004b). Similarly, a recent comparison of parents of children with autism, children with SLI, and typically developing children on various language measures found no evidence of overlap between the broader phenotypes of the autism and SLI groups (Whitehouse et al., 2007). This absence of overlap remained even after the parents of children with autism were divided based on the proband's performance on a non-word repetition task, although the sample of parents of children with autism and language impairments was small (n=9). To explain the existence of linguistic deficits in autism but not in the first-degree relatives, the authors hypothesized that these deficits are not heritable but rather a consequence of the ASD phenotype and its effect on language development.
Given that only a subset of children with ASD exhibit language profiles that overlap with those of children with SLI (Kjelgaard & Tager-Flusberg, 2001; Tager-Flusberg & Joseph, 2003), these studies (Bishop et al., 2004b; Pilowsky et al., 2003; Whitehouse et al., 2007) may have found no overlap between the broader phenotypes of these disorders because they combined samples of relatives of autistic children with and without language impairment. One of these studies attempted to investigate this in parents of autistic children but had small sample sizes, characterized language impairment in the proband using only one measure, and only included relatives of higher-functioning children (Whitehouse et al., 2007). In addition, the majority of studies on the broader phenotype in autism investigated either parents or siblings. Only one study included probands, siblings, and parents, but it was limited to comparisons of families with children with autism to those with typically developing children and only focused on deficits in phonological processing (Bishop et al., 2004b).
In the current study, we included families of autistic children without language impairment (ALN), families of autistic children with language impairment (ALI), and families of children with SLI. We studied the proband, both parents, and the sibling closest in age to the proband in each family. All probands were thoroughly assessed to confirm diagnoses, and SLI probands who exhibited autistic symptoms (i.e. met diagnostic cutoffs for social impairments or the presence of stereotyped or repetitive behaviors) were excluded from the sample. Language impairment was defined with tests that detect clinically significant language impairments in older children (Bishop et al., 1996; Conti-Ramsden, 2003; Conti-Ramsden et al., 2001; for review, see Coady & Evans, 2008), demonstrate heritability in SLI (Barry et al., 2007; Bishop et al., 1999; Bishop et al., 1996), and are highly sensitive in identifying language impairment in children with autism (Kjelgaard & Tager-Flusberg, 2001). To address whether the linguistic deficits observed in ASD are secondary to the ASD phenotype, as suggested by Whitehouse and colleagues (2007), we investigated the relationship between performance on language and non-word repetition tests and scores on the algorithm domains of the ADI-R and ADOS. Group comparisons were also made on a wide variety of measures, including assessments of intelligence, receptive and expressive language, phonological processing, lexical comprehension, and reading ability, in probands, siblings, mothers, and fathers from these families. We hypothesized that ALN probands and family members would perform better than ALI and SLI families on these measures and that ALI and SLI probands and family members would perform similarly. Such findings would provide strong support for the view that the language impairments in ALI and SLI are based on the same etiology. Impaired performance in relatives of ALI children versus relatives of ALN children would suggest that the language difficulties observed in these children are familial, whereas comparable performance would suggest that these language difficulties are not part of the broader ASD phenotype.
METHODS
Participants
Two groups of families were recruited for this study: families with children with autism spectrum disorders (ASD; N=52) and families with a child with specific language impairment (SLI; N=36). The ASD families were then divided into two groups: families with a child with ASD without language impairment (ALN; N=20) and families with a child with ASD and language impairment (ALI; N=32). We studied the probands, the sibling closest in age to the proband, and both parents (Table 1). All probands had verbal IQ scores higher than 50 as measured by the Wechsler Intelligence Scale for Children (WISC-III) (Wechsler, 1991), and probands and siblings were between the ages of 6 and 16 years. All first-degree relatives were screened using a structured family and personal history interview to exclude individuals meeting criteria for ASD. Participants in the study were required to speak standard English as their first language.
Table 1.
Participant Characteristics
| PROBAND DIAGNOSIS | ||||||
|---|---|---|---|---|---|---|
| ALN | ALI | SLI | Results | F or χ2 | p | |
| PROBANDS (ALN = 20, ALI = 32, SLI=36) | ||||||
| Chronological age (years) | 10.3 ± 2.6 | 10.4 ± 2.6 | 11.6 ± 1.6 | No differences | F 2,85 = 3.13 | 0.05 |
| Parental Education Sum* | 12.5 ± 1.0 | 10.5 ± 1.2 | 9.4 ± 1.4 | ALN > ALI > SLI | Overall: χ2 = 59.78 | < 0.0001 |
| ALN vs. ALI: χ2 = 25.22 | 0.0003 | |||||
| ALN vs. SLI: χ2 = 34.32 | < 0.0001 | |||||
| ALI vs. SLI: χ2 = 16.14 | 0.0065 | |||||
| Gender | 18 M / 2 F | 28 M / 4 F | 17 M / 19 F | SLI > ALI = ALN (proportion of females) | Overall: χ2 = 17.83 | 0.0001 |
| ALN vs. ALI: χ2 = 0.08 | 0.78 | |||||
| ALN vs. SLI: χ2 = 10.04 | 0.0015 | |||||
| ALI vs. SLI: χ2 = 12.28 | 0.0005 | |||||
| SIBLINGS (ALN = 19, ALI = 31, SLI = 36) | ||||||
| Chronological age (years) | 10.7 ± 3.3 | 9.6 ± 2.2 | 11.8 ± 2.3 | SLI > ALI, ALN = ALI, ALN = SLI | F 2,83 = 6.92 | 0.0017 |
| Gender | 10 M / 9 F | 16 M / 15 F | 15 M / 21 F | No differences | χ2 = 0.90 | 0.64 |
| MOTHERS (ALN = 20, ALI = 31, SLI = 35) | ||||||
| Chronological age (years) | 42.4 ± 4.1 | 40.1 ± 3.8 | 38.8 ± 5.3 | ALN > SLI, ALN = ALI, ALI = SLI | F 2,83 = 3.96 | 0.023 |
| Education* | 6.0 ± 0.8 | 5.4 ± 0.8 | 4.8 ± 0.8 | ALN = ALI > SLI | Overall: χ2 = 29.13 | 0.0001 |
| ALN vs. ALI: χ2 = 7.58 | 0.11 | |||||
| ALN vs. SLI: χ2 = 20.28 | 0.0004 | |||||
| ALI vs. SLI: χ2 = 9.89 | 0.042 | |||||
| FATHERS (ALN = 19, ALI = 31, SLI = 35) | ||||||
| Chronological age (years) | 43.7 ± 5.1 | 42.0 ± 4.2 | 39.7 ± 5.4 | ALN > SLI, ALN = ALI, ALI = SLI | F 2,82 = 4.22 | 0.018 |
| Education* | 6.5 ± 0.5 | 5.2 ± 1.0 | 4.6 ± 1.0 | ALN > ALI = SLI | Overall: χ2 = 45.15 | < 0.0001 |
| ALN vs. ALI: χ2 = 22.23 | 0.0002 | |||||
| ALN vs. SLI: χ2 = 35.92 | < 0.0001 | |||||
| ALI vs. SLI: χ2 = 6.99 | 0.14 | |||||
Education based on the 7-point Hollingshead scale. Higher values indicate higher education levels.
Two sites participated in this study: Tufts-New England Medical Center (Tufts-NEMC) and the University of Iowa. SLI families from the Iowa site were recruited from a longitudinal cohort (see Tomblin et al., 2000) that had been sampled from a cross-sectional population sample of kindergarten children (Tomblin et al., 1997). To avoid bias toward ascertaining SLI families who were concerned that their child may have symptoms of autism, SLI families at the Boston site were recruited through classes and services specifically for children with language impairment or language-based learning disorders. Recruitment of the ASD families was carried out through services for children with autism and Asperger syndrome at both the Iowa and Boston sites. After recruitment, as part of the consent process, the families were notified that the purpose of the study was to investigate inherited contributions to both autism and SLI, and they understood that once enrolled in the study the children would be assessed both for autism and SLI.
Table 1 summarizes the characteristics and group differences for all groups. Group comparisons for age were tested using oneway ANOVAs, and post-hoc comparisons were made using Tukey-Kramer HSD tests. Comparisons for gender distribution and parental education, as measured using the Hollingshead scale (Hollingshead, 1965), were tested using Pearson chi-square tests.
Diagnosis of Autism in the Proband
All the ASD probands met criteria for ASD on the basis of clinical impression, and diagnoses were confirmed using the Autism Diagnostic Interview – Revised (ADI-R) (Lord et al., 1994) and the Autistic Diagnostic Observation Schedule – Generic (ADOS-G) (Lord et al., 2000). Thirty-seven probands met criteria for autism on both the ADI-R and the ADOS-G. Seven probands met criteria for autism on the ADI-R and criteria for ASD on the ADOS-G. Four probands met criteria for autism on the ADI-R but did not meet criteria for ASD on the ADOS-G. Four probands met criteria for ASD on the ADOS-G and met criteria for autism on either social or communication on the ADI-R and scored within two points on the other domain. Based on these scores, all of the probands met criteria for either autism or ASD as defined by the Collaborative Programs of Excellence in Autism (CPEA, http://www.autismresearchnetwork.org) (Lainhart et al., 2006).
Probands were screened for the following exclusionary criteria: known genetic disorders (e.g., Fragile X, Rett's syndrome, tuberous sclerosis, neurofibromatosis, cerebral palsy, phenylketonuria), deafness, frank neurological damage, and major physical abnormalities.
SLI probands were screened for autistic symptoms using the ADI-R and ADOS-G. None of the SLI probands included in the study met criteria for autism or ASD on the social or repetitive behavior domains of either measure. Ranges (and medians) for these domains in the included SLI sample were 0-9 (and 2) for ADI reciprocal social interaction, 0-2 (and 0) for ADI repetitive behaviors and stereotyped patterns, and 0-3 (and 0) for ADOS social interaction. Fourteen children with SLI that were originally recruited for this study were excluded from the SLI group. Six met criteria for either ASD or autism on the ADOS but did not meet criteria for autism on the ADI-R. Three met criteria for autism or ASD on the social domain of both the ADI-R and the ADOS, four only met criteria for autism on the social domain of the ADI-R, and seven only met criteria for ASD on the social domain of the ADOS. These diagnostic findings are further addressed in another manuscript (Leyfer et al., 2008). SLI probands were not excluded for meeting criteria for autism or ASD on the communication domain of the ADI-R or ADOS.
Diagnosis of Language Impairment
Diagnosis of language impairment (ALI and SLI groups) was made if the proband had a positive history of language delay and/or deficits and met at least one of the following criteria: 1) a standard score lower than one standard deviation below the mean (standard score < 85) for Total Language Ability on the (CELF-III; Semel et al., 1995) or 2) a standard score lower than one standard deviation below the mean (standard score < 7) on the Non-Word Repetition subtest of the Comprehensive Test of Phonological Processing (CTOPP; Wagner et al., 1999). Deficits on these tests contribute to the defining phenotype of SLI (Tager-Flusberg & Cooper, 1999) and have been identified as good clinical markers for this disorder (Conti-Ramsden, 2003; Conti-Ramsden et al., 2001; for review, see Coady & Evans, 2008). In the ALI proband group, 8 met criteria for language impairment on both tests, 5 met criteria only on non-word repetition, and 16 met criteria only on total language ability. 3 ALI probands met criteria on non-word repetition but did not have total language ability scores. In the SLI proband group, 16 met criteria on both tests, 3 met criteria only on non-word repetition, and 17 met criteria only on total language ability. Of note, 6/19 ALN probands and 25/32 ALI probands exhibited delays in language acquisition as defined by late onset of first words or phrases on the ADI-R (data was not available for one ALN proband).
Procedures
1. IQ Tests
Four subtests of the Wechsler Intelligence Scales were administered to participants to assess intellectual ability (Wechsler, 1991, 1997). The WISC-III was administered to probands and siblings, and the WAIS-III was administered to parents. Z-scores for verbal IQ were calculated using an algorithm based on Vocabulary and Similarities standard scores ((Similarities + Vocabulary − 20)/5.564), and z-scores for performance IQ were calculated using an algorithm based on Picture Arrangement and Block Design standard scores ((Picture Arrangement + Block Design − 20)/5.144). Z-scores for full-scale IQ were calculated using an algorithm based on the standard scores from the four subtests mentioned above ((Similarities + Vocabulary + Picture Arrangement + Block Design − 40)/9.469). The three z-scores (verbal, performance, and full-scale IQ) were converted to standard scores using the following formula: 100 + 15(z-score) (Ruser et al., 2007).
2. Language Assessment
The Clinical Evaluation of Language Fundamentals – 3rd Edition (CELF-III; Semel et al., 1995) was administered to probands and siblings. The CELF-III is a measure designed to evaluate semantics, morphology, syntax, and memory for language. Z-scores for receptive language were calculated using an algorithm based on standard scores from Concepts and Directions (CD, all ages), Sentence Structure (SS, ages 6-8), and Word Classes (WC, ages 9-16). Receptive language z-scores were (SS + CD − 20)/5.109 for children ages 6-8 and (WC + CD − 20)/5.265 for children ages 9-16. Z-scores for expressive language were calculated using an algorithm based on standard scores from Recalling Sentences (RS, all ages), Word Structure (WS, ages 6-8), and Formulated Sentences (FS, ages 9-16). Expressive language z-scores were (RS + WS − 20)/5.213 for children ages 6-8 and (RS + FS − 20)/5.126 for children ages 9-16. Z-scores for total language ability were calculated using an algorithm based on standard scores from the four age-appropriate subtests described above. Total language z-scores were (SS +CD + RS + WS − 40)/9.468 for children ages 6-8 and (WC + CD + RS + FS − 40)/9.353 for children ages 9-16. The three z-scores (receptive, expressive, and total language) were converted to standard scores using the following formula: 100 + 15(z-score). Norms are available for the subtests and composite scores for individuals aged 6 to 21 years.
The Comprehensive Test of Phonological Processing (CTOPP; Wagner et al., 1999) was administered to all participants. The CTOPP assesses phonological processing in three realms: phonological awareness, phonological memory, and rapid naming. Phonological awareness is a measure of one's ability to recognize and use the sound structure of oral language. Phonological memory is an assessment of one's ability to code information and store it in working memory. Rapid naming examines one's ability to efficiently retrieve phonological information from long-term memory. There are two versions of the test, one for children aged 5-6 and the other for individuals aged 7 to adulthood. The former contains seven core subtests while the latter only contains six. All of the core subtests were administered, and the composites were calculated as documented in the examiner's manual. Norms are available for the subtests and composite scores.
In addition to the composite scores, the non-word repetition subtest of the CTOPP was used as a language measure. This subtest is designed to evaluate one's ability to repeat non-words ranging in length from three to fifteen sounds and is a good measure of phonological memory. Non-words are composed of random phonemes and follow rules of standard English phonology and stress patterns; however, these non-words are intentionally designed to be dissimilar to existing English words to discourage the use of other strategies besides phonological memory.
The Peabody Picture Vocabulary Test – 3rd Edition (PPVT-III; Dunn & Dunn, 1997) was administered to all participants. The PPVT-III measures lexical comprehension by asking subjects to select one of four pictures based on the word stated by the examiner. Norms are available for ages 2;6 through adulthood.
The Woodcock-Johnson Psycho-Educational Battery – Revised (WJ-R; Woodcock & Johnson, 1990) was administered to all participants. The WJ-R is a comprehensive battery used to measure a wide range of reading abilities. Participants were administered three standard subtests (Letter-Word Identification, Passage Comprehension, and Dictation) and one supplemental subtest (Word Attack). Two composite scores were also calculated as documented in the examiner's manual: Basic Reading, which is based on the performance on Letter-Word Identification and Word Attack, and Broad Reading, which is based on Letter-Word Identification and Passage Comprehension. Norms are available for ages 2;0 through adulthood for the subtests and composite scores.
Testing was usually conducted in one day with ample opportunity for breaks but if necessary was conducted over two sessions. All data were analyzed with JMP 7.0 (SAS Institute Inc., 2007).
Data Analysis
Between group differences in the proband groups were assessed using oneway ANOVAs for all measures. Between group differences in siblings for CELF-III receptive, expressive, and total language ability scores were also assessed using oneway ANOVAs. Between group differences in family members for IQ, PPVT, CTOPP, and WJ-R were assessed using a mixed effects model with score as the dependent variable, group as the between-subjects factor (family identifier nested within it as a random effect), and relationship to proband and relationship to proband-by-group as fixed effects. Post-hoc comparisons for all analyses were made using Tukey-Kramer HSD with adjustments for multiple comparisons within the model.
Proportion of First-Degree Relatives Performing in the Normal and Language-Impaired Range
Family members were categorized into language-normal and language-impaired based on the same criteria described above for an ALN or ALI diagnosis on the CELF-III (mothers, fathers, and siblings) and non-word repetition (siblings only). Between-group differences in the number of first-degree relatives performing in the language-impaired and language-normal ranges were examined using Pearson Chi-Square analyses. To examine whether the rate of impaired performance on these measures differed between brothers and sisters of children with ASD or SLI, we used a Pearson Chi-Square analysis comparing the number of brothers and sisters categorized as language-normal and language-impaired for each diagnostic group.
Relationship of Language Abilities to ASD Severity
Relationships between total language ability (CELF-III) and non-word repetition (CTOPP) with algorithm scores on the ADI-R and ADOS were examined using pairwise correlations across the ALN and ALI groups. The algorithm domains included verbal communication, reciprocal social interaction, repetitive behaviors and stereotyped patterns, and a total sum (verbal communication + social interaction + repetitive behaviors and stereotyped patterns) on the ADI-R and communication, reciprocal social interaction, and a total sum (communication + social interaction + imagination/creativity + stereotyped behaviors and repetitive interests) on the ADOS. Between-group differences on the total sums for both the ADI-R and ADOS were also examined using two-tailed Student's t-tests. Only those children who were administered a module 3 ADOS (ALN 16/20, ALI 25/32) were included in the ADOS analyses to control for differences in the number of algorithm items.
RESULTS
Group comparison data are summarized in Table 2 (IQ), Table 3 (CELF-III and PPVT), Table 4 (CTOPP), and Table 5 (WJ-R).
Table 2.
Performance on WISC-III (probands and siblings) and WAIS-III (parents)
| PROBAND DIAGNOSIS | ||||||||
|---|---|---|---|---|---|---|---|---|
| ALN | ALI | SLI | Results (Probands and Family) | F | p | η p2 | ||
| FULL-SCALE IQ | ||||||||
| PROBANDS | 113.0 ± 16.5 | 86.5 ± 19.2 | 85.4 ± 12.8 | ALN > ALI = SLI | F2,84 = 20.71 | < 0.0001 | 0.33 | |
| SIBLINGS | 120.7 ± 13.9 | 114.5 ± 12.5 | 93.4 ± 15.5 |
|
Group: F2,85 = 36.51 | < 0.0001 | 0.61 | |
| MOTHERS | 117.6 ± 11.4 | 109.3 ± 12.9 | 96.7 ± 13.5 | ALN > ALI > SLI | Relation: F 2,164 = 0.67 | 0.51 | 0.01 | |
| FATHERS | 119.8 ± 11.0 | 109.5 ± 13.3 | 96.7 ± 12.7 | Group*Relation: F 4,164 = 2.10 | 0.08 | 0.05 | ||
| VERBAL IQ | ||||||||
| PROBANDS | 113.5 ± 15.5 | 85.1 ± 20.4 | 84.6 ± 12.2 | ALN > ALI = SLI | F2,84 = 22.86 | < 0.0001 | 0.35 | |
| SIBLINGS | 117.4 ± 11.5 | 112.8 ± 12.0 | 93.8 ± 16.2 |
|
Group: F2,85 = 42.22 | < 0.0001 | 0.64 | |
| MOTHERS | 119.8 ± 10.3 | 110.4 ± 12.3 | 93.9 ± 13.1 | ALN > ALI > SLI | Relation: F 2,164 = 0.02 | 0.98 | 0.0004 | |
| FATHERS | 120.3 ± 9.9 | 108.3 ± 13.7 | 96.0 ± 12.1 | Group*Relation: F 4,164 = 1.28 | 0.28 | 0.03 | ||
| PERFORMANCE IQ | ||||||||
| PROBANDS | 109.4 ± 20.4 | 91.3 ± 20.9 | 89.9 ± 14.6 | ALN > ALI = SLI | F2,84 = 7.78 | 0.0008 | 0.16 | |
| SIBLINGS | 119.3 ± 15.8 | 112.8 ± 16.8 | 94.7 ± 16.2 |
|
Group: F2,85 = 17.98 | < 0.0001 | 0.35 | |
| MOTHERS | 110.9 ± 14.0 | 105.8 ± 13.3 | 100.5 ± 13.0 | ALN = ALI > SLI | Relation: F 2,164 = 1.45 | 0.24 | 0.02 | |
| FATHERS | 114.4 ± 13.1 | 108.4 ± 13.7 | 98.3 ± 14.0 | Group*Relation: F 4,164 = 3.17 | 0.015 | 0.07 | ||
Brackets indicate analyses using a mixed effects model with score as the dependent variable, group as the between-subjects factor (family identifier nested within it as a random effect), and relationship to proband and relationship to proband-by-group as fixed effects.
Table 3.
Performance on CELF-III and PPVT
| PROBAND DIAGNOSIS | ||||||||
|---|---|---|---|---|---|---|---|---|
| ALN | ALI | SLI | Results (Probands and Family) | F | p | η p2 | ||
| CELF-III | ||||||||
| TOTAL LANGUAGE * | ||||||||
| PROBANDS | 107.2 ± 15.0 | 72.0 ± 16.2 | 73.3 ± 9.5 | ALN > ALI = SLI | F 2,81 = 48.79 | < 0.0001 | 0.55 | |
| SIBLINGS | 115.6 ± 10.8 | 103.9 ± 13.5 | 89.3 ± 16.6 | ALN > ALI > SLI | F 2,81 = 21.86 | < 0.0001 | 0.35 | |
| RECEPTIVE LANGUAGE | ||||||||
| PROBANDS | 106.9 ± 15.1 | 76.6 ± 18.0 | 76.9 ± 9.0 | ALN > ALI = SLI | F 2,82 = 35.06 | < 0.0001 | 0.46 | |
| SIBLINGS | 116.4 ± 10.5 | 104.3 ± 13.3 | 92.0 ± 15.9 | ALN > ALI > SLI | F 2,81 = 19.69 | < 0.0001 | 0.33 | |
| EXPRESSIVE LANGUAGE | ||||||||
| PROBANDS | 105.0 ± 16.0 | 72.5 ± 12.8 | 74.9 ± 10.0 | ALN > ALI = SLI | F 2,83 = 46.25 | < 0.0001 | 0.53 | |
| SIBLINGS | 111.8 ± 13.1 | 102.8 ± 13.7 | 88.8 ± 15.8 | ALN = ALI > SLI | F 2,81 = 17.13 | < 0.0001 | 0.30 | |
| PPVT | ||||||||
| PROBANDS | 111.1 ± 11.9 | 87.5 ± 17.4 | 91.5 ± 9.9 | ALN > ALI = SLI | F 2,84 = 20.30 | < 0.0001 | 0.33 | |
| SIBLINGS | 118.1 ± 12.0 | 113.5 ± 12.2 | 99.7 ± 12.0 |
|
Group: F 2,84 = 26.01 | < 0.0001 | 0.46 | |
| MOTHERS | 111.1 ± 13.6 | 105.6 ± 12.5 | 95.7 ± 10.0 | ALN = ALI > SLI | Relation: F 2,164 = 11.04 | < 0.0001 | 0.12 | |
| FATHERS | 112.4 ± 8.7 | 105.0 ± 9.8 | 97.8 ± 9.3 | Group*Relation: F 4,164 = 1.11 | 0.35 | 0.03 | ||
Brackets indicate analyses using a mixed effects model with score as the dependent variable, group as the between-subjects factor (family identifier nested within it as a random effect), and relationship to proband and relationship to proband-by-group as fixed effects.
This measure was used to diagnosis language impairment in ALI and SLI probands
Table 4.
Performance on CTOPP
| PROBAND DIAGNOSIS | ||||||||
|---|---|---|---|---|---|---|---|---|
| ALN | ALI | SLI | Results (Probands and Family) | F | p | η p2 | ||
| PHONOLOGICAL AWARENESS | ||||||||
| PROBANDS | 107.4 ± 10.1 | 87.9 ±17.6 | 82.3 ± 11.0 | ALN > ALI = SLI | F 2,84 = 21.59 | < 0.0001 | 0.34 | |
| SIBLINGS | 109.7 ± 12.1 | 101.3 ± 9.1 | 93.0 ± 15.5 |
|
Group: F 2,82 = 11.57 | < 0.0001 | 0.22 | |
| MOTHERS | 105.5 ± 13.6 | 100.5 ± 12.4 | 93.4 ± 16.2 | ALN = ALI > SLI | Relation: F 2,162 = 10.39 | < 0.0001 | 0.11 | |
| FATHERS | 99.5 ± 14.4 | 92.7 ± 16.8 | 86.5 ± 15.2 | Group*Relation: F 4,162 = 0.28 | 0.89 | 0.01 | ||
| PHONOLOGICAL MEMORY | ||||||||
| PROBANDS | 103.0 ± 8.7 | 82.1 ± 14.5 | 81.2 ± 10.3 | ALN > ALI = SLI | F 2,84 = 24.72 | < 0.0001 | 0.37 | |
| SIBLINGS | 106.2 ± 15.0 | 97.9 ± 10.4 | 90.9 ± 12.1 |
|
Group: F 2,81 = 18.13 | < 0.0001 | 0.31 | |
| MOTHERS | 105.1 ± 7.3 | 97.5 ± 11.6 | 88.6 ± 13.3 | ALN = ALI > SLI | Relation: F 2,160 = 0.61 | 0.54 | 0.004 | |
| FATHERS | 100.6 ± 12.3 | 99.5 ± 12.8 | 88.4 ± 12.7 | Group*Relation: F 4,160 = 1.00 | 0.41 | 0.03 | ||
| RAPID NAMING | ||||||||
| PROBANDS | 96.8 ± 19.0 | 81.8 ± 18.8 | 87.1 ± 19.7 | ALN > SLI, ALN = ALI, ALI = SLI | F 2,79 = 3.44 | 0.04 | 0.08 | |
| SIBLINGS | 104.3 ± 14.2 | 102.9 ± 11.7 | 92.3 ± 15.4 |
|
Group: F 2,80 = 5.74 | 0.0047 | 0.09 | |
| MOTHERS | 100.6 ± 18.0 | 99.1 ± 14.5 | 93.4 ± 13.2 | ALN = ALI > SLI | Relation: F 2,160 = 0.34 | 0.71 | 0.002 | |
| FATHERS | 100.5 ± 17.7 | 100.8 ± 17.4 | 94.5 ± 16.8 | Group*Relation: F 4,160 = 0.47 | 0.76 | 0.01 | ||
| NONWORD REPETITION * | ||||||||
| PROBANDS | 9.9 ± 1.6 | 7.0 ± 2.4 | 6.3 ± 1.6 | ALN > ALI = SLI | F 2,84 = 22.46 | < 0.0001 | 0.35 | |
| SIBLINGS | 10.1 ± 2.5 | 9.4 ± 2.0 | 7.4 ± 2.0 |
|
Group: F 2,80 = 20.00 | < 0.0001 | 0.30 | |
| MOTHERS | 8.7 ± 1.6 | 7.8 ± 1.8 | 6.1 ± 2.2 | ALN = ALI > SLI | Relation: F 2,160 = 17.09 | < 0.0001 | 0.16 | |
| FATHERS | 8.3 ± 2.2 | 7.6 ± 2.6 | 6.3 ± 1.7 | Group*Relation: F 4,160 = 0.39 | 0.82 | 0.01 | ||
Brackets indicate analyses using a mixed effects model with score as the dependent variable, group as the between-subjects factor (family identifier nested within it as a random effect), and relationship to proband and relationship to proband-by-group as fixed effects.
This measure was used to diagnosis language impairment in ALI and SLI probands
Table 5.
Performance on WJ-R
| PROBAND DIAGNOSIS | ||||||||
|---|---|---|---|---|---|---|---|---|
| ALN | ALI | SLI | Results (Probands and Family) | F | p | η p2 | ||
| BROAD READING COMPOSITE | ||||||||
| PROBANDS | 106.6 ± 15.0 | 91.6 ± 21.7 | 87.1 ± 9.9 | ALN > ALI = SLI | F 2,84 = 9.20 | 0.0002 | 0.18 | |
| SIBLINGS | 122.5 ± 15.1 | 112.2 ± 13.9 | 98.8 ± 16.6 |
|
Group: F 2,83 = 37.71 | < 0.0001 | 0.52 | |
| MOTHERS | 120.5 ± 16.2 | 113.2 ± 14.3 | 95.1 ± 14.0 | ALN > ALI > SLI | Relation: F 2,161 = 1.31 | 0.27 | 0.01 | |
| FATHERS | 120.8 ± 11.1 | 109.1 ± 16.7 | 93.1 ± 13.8 | Group*Relation: F 4,161 = 0.49 | 0.74 | 0.01 | ||
| READING SKILL COMPOSITE | ||||||||
| PROBANDS | 110.0 ± 17.3 | 93.8 ± 21.5 | 85.5 ± 12.3 | ALN > ALI = SLI | F 2,84 = 12.57 | < 0.0001 | 0.23 | |
| SIBLINGS | 119.9 ± 15.0 | 110.1 ± 14.1 | 95.6 ± 17.6 |

|
Group: F 2,83 = 34.55 | < 0.0001 | 0.51 | |
| MOTHERS | 121.1 ± 15.9 | 114.6 ± 15.7 | 95.6 ± 14.6 | ALN > ALI > SLI | Relation: F 2,162 = 0.88 | 0.42 | 0.01 | |
| FATHERS | 119.9 ± 11.6 | 109.5 ± 16.5 | 93.3 ± 15.5 | Group*Relation: F 4,162 = 0.35 | 0.84 | 0.01 | ||
| DICTATION | ||||||||
| PROBANDS | 97.3 ± 13.0 | 84.8 ± 25.1 | 81.4 ± 7.5 | ALN > ALI = SLI | F 2,84 = 5.57 | 0.005 | 0.12 | |
| SIBLINGS | 107.1 ± 13.0 | 99.1 ± 10.1 | 88.6 ± 13.1 |
|
Group: F 2,83 = 28.35 | < 0.0001 | 0.45 | |
| MOTHERS | 103.3 ± 11.7 | 99.4 ± 10.0 | 88.5 ± 11.5 | ALN > ALI > SLI | Relation: F 2,162 = 7.06 | 0.001 | 0.08 | |
| FATHERS | 101.5 ± 10.0 | 93.6 ± 12.8 | 82.8 ± 11.7 | Group*Relation: F 4,162 = 0.43 | 0.78 | 0.01 | ||
| PASSAGE COMPREHENSION | ||||||||
| PROBANDS | 107.1 ± 16.3 | 92.3 ± 21.2 | 90.3 ± 9.0 | ALN > ALI = SLI | F 2,84 = 7.38 | 0.001 | 0.15 | |
| SIBLINGS | 122.7 ± 13.1 | 110.5 ± 14.6 | 101.1 ± 14.7 |
|
Group: F 2,83 = 28.37 | < 0.0001 | 0.42 | |
| MOTHERS | 118.8 ± 16.1 | 111.0 ± 14.9 | 95.3 ± 15.7 | ALN > ALI > SLI | Relation: F 2,162 = 3.22 | 0.04 | 0.04 | |
| FATHERS | 116.8 ± 12.3 | 107.3 ± 17.8 | 93.7 ± 16.1 | Group*Relation: F 4,162 = 0.55 | 0.70 | 0.02 | ||
| WORD ATTACK | ||||||||
| PROBANDS | 112.8 ± 21.3 | 95.6 ± 19.9 | 84.4 ± 16.2 | ALN > ALI > SLI | F 2,84 = 14.31 | < 0.0001 | 0.25 | |
| SIBLINGS | 117.8 ± 14.5 | 105.2 ± 13.1 | 92.9 ± 18.3 |
|
Group: F 2,83 = 24.13 | < 0.0001 | 0.43 | |
| MOTHERS | 119.3 ± 17.7 | 114.0 ± 16.5 | 96.4 ± 16.2 | ALN > ALI > SLI | Relation: F 2,162 = 2.90 | 0.06 | 0.03 | |
| FATHERS | 114.6 ± 11.4 | 107.3 ± 16.6 | 94.5 ± 16.9 | Group*Relation: F 4,162 = 0.86 | 0.49 | 0.02 | ||
Brackets indicate analyses using a mixed effects model with score as the dependent variable, group as the between-subjects factor (family identifier nested within it as a random effect), and relationship to proband and relationship to proband-by-group as fixed effects.
Broad Reading = Letter-Word Identification and Passage Comprehension, Basic Reading = Letter-Word Identification and Word Attack
Group Comparisons - Probands
As expected based on our definition of the proband groups, ALI and SLI probands had comparable receptive, expressive, and total language scores on the CELF-III and non-word repetition scores, and both groups scored lower than ALN probands. ALI and SLI probands also performed similarly on verbal, performance, and full-scale IQ, lexical comprehension, phonological awareness and memory, broad reading, reading skill, dictation, and passage comprehension with scores in both groups lower than that of the ALN group. ALN probands scored similarly to ALI probands on rapid naming but better than SLI probands. There was no difference in rapid naming performance between ALI and SLI probands. Word attack scores were highest in the ALN probands and lowest in the SLI probands.
Group Comparisons – Family Members
Performance was highest in ALN relatives and lowest in SLI relatives (i.e. ALN > ALI > SLI) on the following measures: verbal and full-scale IQ, receptive and total language ability, and all measures of reading ability. ALN and ALI relatives performed similarly but significantly higher than SLI relatives (i.e. ALN = ALI > SLI) on the following measures: performance IQ, expressive language, lexical comprehension, and all measures of phonological processing. For performance IQ, a significant group by relationship interaction was obtained. Scores for ALN and ALI siblings were similar to one another but significantly higher than the scores of the SLI siblings. ALN fathers scored higher than SLI fathers but there were no significant group differences between ALN and ALI fathers or ALI and SLI fathers, and no group differences for mothers of all three groups. There were significant main effects of relationship for non-word repetition, phonological awareness, dictation, and passage comprehension. For non-word repetition, mothers and fathers scored similarly but poorer than siblings across groups. For phonological awareness and dictation, siblings and mothers scored similarly but higher than fathers across groups. For passage comprehension, siblings scored higher than fathers while there were no differences between siblings and mothers or mothers and fathers on this measure across groups.
Proportion of First-Degree Relatives Performing in the Normal and Language-Impaired Range
Table 6 displays the number and percentage of first-degree relatives that performed in the normal or language-impaired range on total language ability or non-word repetition.
Table 6.
Proportion of First-Degree Relatives Performing in the Normal and Language-Impaired Range on CELF-III or Non-word Repetition
| PROBAND DIAGNOSIS | ||||||
|---|---|---|---|---|---|---|
| ALN | ALI | SLI | Results | χ 2 | p | |
| SIBLINGS | ||||||
| LN | 17 (8M/9F) (89%) | 26 (12M/14F) (84%) | 21 (8M/13F) (58%) | SLI > ALI = ALN | Overall: χ2 = 8.61 | 0.014 |
| LI | 2 (2M/0F) (11%) | 5 (4M/1F) (16%) | 15 (7M/8F) (42%) | ALN vs. ALI: χ2 = 0.31 | 0.58 | |
| ALN vs. SLI: χ2 = 5.65 | 0.018 | |||||
| ALI vs. SLI: χ2 = 5.19 | 0.022 | |||||
| By Gender (SLI): χ 2 = 0.26 | 0.61 | |||||
| By Gender (ASD): χ2 = 3.71 | 0.054 | |||||
| MOTHERS | ||||||
| LN | 19 (95%) | 22 (71%) | 14 (40%) | SLI > ALI > ALN | Overall: χ2 = 17.74 | 0.0001 |
| LI | 1 (5%) | 9 (29%) | 21 (60%) | ALN vs. ALI: χ2 = 4.45 | 0.035 | |
| ALN vs. SLI: χ2 = 16.04 | < 0.0001 | |||||
| ALI vs. SLI: χ2 = 6.36 | 0.011 | |||||
| FATHERS | ||||||
| LN | 15 (79%) | 20 (65%) | 16 (46%) | SLI > ALN, ALN = ALI, ALI = SLI | Overall: χ2 = 6.08 | 0.048 |
| LI | 4 (21%) | 11 (35%) | 19 (54%) | ALN vs. ALI: χ2 = 1.17 | 0.28 | |
| ALN vs. SLI: χ2 = 5.56 | 0.018 | |||||
| ALI vs. SLI: χ2 = 2.34 | 0.13 | |||||
Number (and percentage) of first-degree relatives that performed in the language-impaired (LI) and normal (LN) range on non-word repetition (mothers, fathers, and siblings) and total language ability (siblings only).
There were significant group differences in the proportion of siblings, mothers, and fathers performing in the language-impaired range on non-word repetition (mothers, fathers, and siblings) and CELF-III total language ability (siblings only). For siblings, this proportion was highest in the SLI group but was similar between ALN and ALI siblings. For mothers, this proportion was highest in the SLI group and lowest in the ALN group. For fathers, this proportion was significantly higher in the SLI group versus the ALN group; however, there was no group difference between ALN and ALI fathers or between ALI and SLI fathers.
Relationship of Language Abilities to ASD Severity
Figure 1 displays scatterplots of ADOS and ADI-R algorithm scores in the ALN and ALI probands. There were no group differences between ALN and ALI probands for the sum scores for the ADI-R (ALN Mean=47.37, S.D.=8.80; ALI Mean=46.72, S.D.=7.25; t(49)=0.29, p=0.78) or the ADOS (ALN Mean=14.75, S.D.=4.92; ALI Mean=14.84, S.D.=4.92; t(39)=0.06, p=0.95). There were also no significant correlations between total language ability on the CELF-III and the algorithm domain scores of the ADI-R or the ADOS across the ALN and ALI groups. There were no significant correlations between non-word repetition and the algorithm domains of the ADI-R. Only the social domain score on the ADOS was correlated with non-word repetition (r=0.36, p=0.02); however, this relationship did not maintain significance after a Bonferroni correction for multiple comparisons.
Figure 1.
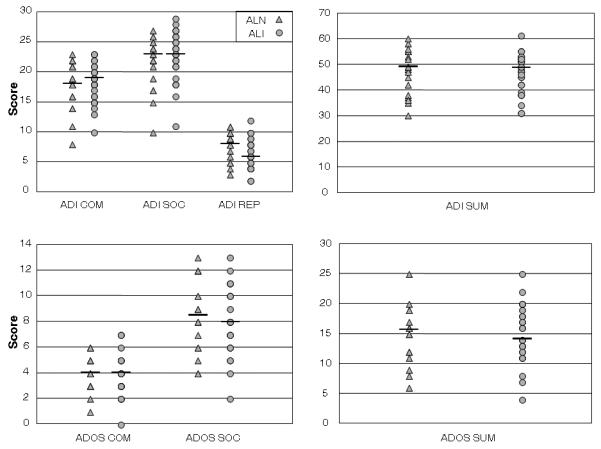
Scatterplots of algorithm scores for verbal communication (ADI COM), reciprocal social interaction (ADI SOC), repetitive behaviors and stereotyped patterns (ADI REP), and total sum (ADI SUM) on the ADI-R and communication (ADOS COM), reciprocal social interaction (ADOS SOC), and total sum (ADOS SUM) on the ADOS in ALN and ALI probands. Horizontal lines indicate medians for each group on each algorithm score.
DISCUSSION
Probands
ALN children scored significantly higher than both ALI and SLI children on the majority of the assessments, while no differences were observed between ALI and SLI probands except for higher performance by ALI probands on word attack on the WJ-R. Group differences were expected on the CELF-III and non-word repetition given how our samples were defined, but these differences extended beyond basic language abilities. ALI and SLI children also had significantly lower non-verbal IQ, phonological processing, lexical comprehension, and reading abilities than ALN children. Poor reading ability has been documented in children with SLI (Catts et al., 2008; Catts et al., 2002; Flax et al., 2003; McArthur et al., 2000), but it is interesting that ALI children also demonstrated these deficits. A study by Nation and colleagues (2006) described significant heterogeneity in reading abilities in children with autism with scores ranging from floor to ceiling, so perhaps those children performing in the lower range in their study fit the ALI profile. Our findings are also consistent with previous studies that have noted overlap in language phenotypes of children with autism and children with SLI (Bartak et al., 1975, 1977; Kjelgaard & Tager-Flusberg, 2001; Roberts et al., 2004; Whitehouse et al., 2008). Importantly, there were similarities between the ALI and SLI groups in our sample despite excluding SLI children who met criteria for ASD on any domain of the ADI-R or ADOS besides communication, thus minimizing the likelihood of misdiagnosis in the SLI group.
It has been hypothesized that the language deficits observed in ALI are a consequence of the ASD phenotype (Whitehouse et al., 2007). In our study, however, there were no group differences between the sum scores of ALN and ALI probands on either the ADI-R or ADOS, and there were no significant correlations between any language measures and ASD severity. These findings indicate that the language difficulties observed in the ALI group are not secondary to the severity of the ASD phenotype. Primary etiological candidates for the language impairments in ASD have been suggested based on genetic and neuroimaging research. Several genetic loci have been implicated in genetic studies that have subset their ASD samples on the basis of language delay or impairment (Abrahams & Geschwind, 2008; Alarcon et al., 2008; Arking et al., 2008; Bakkaloglu et al., 2008; O'Brien et al., 2003; Vernes et al., 2008; Warburton et al., 2000). Neuroimaging studies have described structural abnormalities in perisylvian cortices, especially in children with autism and language impairment (De Fossé et al., 2004; Herbert et al., 2002). Similar abnormalities have also been reported in children with SLI (De Fossé et al., 2004; Gauger et al., 1997; Herbert et al., 2005; C. M. Leonard et al., 2002; Plante et al., 1991) and their first-degree relatives (Jackson & Plante, 1996; Plante, 1991), suggesting that these anomalies are heritable neurobiological markers for language impairment.
Neuroanatomical studies comparing ASD and SLI in regions beyond the perisylvian cortices have yielded less consistent findings. Herbert and colleagues (2005) described asymmetry patterns in other cortical regions that were present in both autistic and SLI children but not typically developing children; however, these findings have not been replicated in other studies of SLI children. While some studies found increased total brain volume, perhaps due to disproportionately greater white matter volumes, in both ASD (Aylward et al., 2002; Carper et al., 2002; Courchesne et al., 2001; Filipek et al., 1992; Hardan et al., 2001; Piven et al., 1996; Piven et al., 1995; Sparks et al., 2002) and SLI (Filipek et al., 1992; Herbert et al., 2003; Woodhouse et al., 1996), other studies have found decreased total brain volume in SLI (Jernigan et al., 1991; C. Leonard et al., 2006; C. M. Leonard et al., 2002). These findings suggest that there may be more limited shared etiology between ASD and SLI than has been argued by some researchers.
First-Degree Relatives
SLI family members performed the worst of the three groups on all measures, and over half scored in the language-impaired range on the CELF-III or non-word repetition, supporting other studies that have shown high heritability of language deficits in SLI. When compared to the proportion of ALN and ALI family members performing in the language-impaired range on these measures, this difference was most pronounced among siblings and mothers. There was no difference, however, in the proportion of brothers versus sisters from SLI families performing in the language-impaired range. Poor performance on non-word repetition tasks has previously been described in parents of children with SLI in comparison to parents of both typically developing (Barry et al., 2007) and autistic children (Whitehouse et al., 2007). In our study, on average, non-word repetition performance was borderline or impaired in the relatives of SLI children, and as a group SLI relatives scored lower than the relatives of the ALI and ALN children. In contrast to Pilowsky and colleagues (2003), we found that siblings of SLI children performed significantly lower on the CELF-III compared to both ALN and ALI siblings. These conflicting results may be attributable to differences in the inclusion criteria for the SLI probands between studies. SLI children in Pilowsky and colleagues' study only had to have normal intelligence and score more than one standard deviation below the mean on the CELF-III, while the SLI children in our study also had to have a documented positive history of language delay or deficits. Furthermore, all of the children in Pilowsky and colleagues' study were native Hebrew speakers, and there is some evidence that the characteristic SLI phenotype varies depending on the language (Dromi et al., 1999; L. B. Leonard et al., 2000; Owen et al., 2001; Stokes et al., 2006; Thordardottir & Namazi, 2007).
In addition to poor performance on primary language measures, SLI family members also scored lower than ALN and ALI family members on measures of verbal IQ, phonological processing, and reading ability. Whitehouse and colleagues (2007) also found relatively poor performance on language measures in parents of SLI children when compared to parents of autistic children. Similarly, Flax and colleagues (2003) described elevated rates of reading impairments in first-degree relatives of SLI children as well as a high co-occurrence of reading impairment with language impairment. Overall, these findings suggest that the heritable SLI phenotype is not restricted to basic language ability but also encompasses reading deficits.
The pattern of language and reading performance of the relatives of ALN and ALI probands was not consistent. On some measures, there were no significant differences between the ALN and ALI relatives. These included expressive language, lexical comprehension, and all measures of phonological processing, including non-word repetition. On other measures, including verbal IQ, total and receptive language, and all reading measures, the siblings or parents of the ALI probands scored lower than the relatives of the ALN probands. However, it is unclear whether these group differences indicate familial aggregation of susceptibility to those language and reading impairments or if these group differences are attributable to above normal performance in the ALN relatives and their higher education levels (ALN parents on average completed college whereas ALI parents on average only partially completed college).
It is important to note that overall, there were no differences between the ALN and ALI relatives on either expressive language or phonological processing, two measures exhibiting high heritability in SLI. This is consistent with previous studies concluding that phonological deficits are not clearly part of the heritable language phenotype in ASD (Bishop et al., 2004b; Whitehouse et al., 2007). While at the group level there were no differences in non-word repetition, the proportion of mothers of ALI probands who scored in the impaired range on this measure was significantly higher compared to the mothers of ALN probands. Based on our definition of language impairment for this analysis, one would expect approximately 16% of mothers to be categorized as language impaired, but 29% of ALI mothers in our sample scored within this range. There was also a trend towards a greater proportion of brothers versus sisters of ASD children scoring in the language-impaired range on the CELF-III or non-word repetition, which is consistent with findings of higher rates of learning and speech issues in brothers versus sisters of individuals with ASD (Interactive Autism Network, 2007; Plumet et al., 1995). These findings suggest that there may be some genetic component to the language impairments in ASD that is transmitted through the maternal line to their male children (cf. Ruser et al., 2007); however, replications of these findings on independent samples are needed before such conclusions are warranted.
Contrary to our predictions, relatives of ALI probands scored higher than relatives of SLI probands on all the language and reading measures. Moreover, language impairments are more prevalent in SLI families than ALI families. As many as 60% of children with SLI have at least one additional family member with language impairments (Lahey & Edwards, 1995), whereas the rate of speech and language deficits is approximately 20-25% in ASD families (Bartak et al., 1975; Piven et al., 1997a). These percentages are consistent with those of first-degree relatives performing in the impaired range on non-word repetition or total language ability in our study. Given these differences between ASD and SLI, the language deficits in these populations cannot be linked to the identical genetic etiology (Williams et al., 2008). It remains to be seen, however, whether there is some limited partial overlap between these populations as suggested by the association between the CNTNAP2 gene, a component of the FOXP2 pathway, with both ASD and SLI (Alarcon et al., 2008; Vernes et al., 2008). Further analyses of this pathway and its relationship to language abilities in ASD and SLI families are warranted to better understand its role in the ALI and SLI language phenotype.
It may also be that, in comparison to relatives of SLI probands, ALI relatives are more likely to outgrow early language impairments or be better able to compensate for them. Because our sample only included siblings ages 6 and older, we were unable to see if at a younger age siblings of ALI children exhibit greater evidence of language impairment. This hypothesis is supported by a longitudinal study by Gamliel and colleagues (2007) reporting that a number of siblings of ASD children who scored poorly on language measures at age 14 months had improved functioning at age 54 months. It has also been suggested that the language impairments observed in ALI children diminish with age (Williams et al., 2008); however, our data do not support this hypothesis as there were no differences on any of our language measures between the ALI and SLI groups of children. Further investigations of the developmental trajectories of language and reading abilities in ALI and SLI probands as well as identification of the strengths and weaknesses of affected individuals and their relatives are necessary to better understand the relationship between autism and SLI. Moreover, longitudinal studies of language development in siblings of ALI and SLI children would provide insight into whether there is greater overlap in the broader phenotypes of these disorders at an early age and what risk factors predict language performance later in life. Finally, neuroimaging studies of first-degree relatives of children with ASD and SLI are also needed to examine whether the similarities in abnormal brain structure between these disorders are heritable and are related to language impairment. Understanding the overlap of and differences between these complex disorders has important implications for diagnosis and treatment in clinical practice.
ACKNOWLEDGEMENTS
This research was supported by grants from NINDS (RO1 NS 38668; F30 NS 055511), a grant from NIDCD (U19 DC 03610), which is part of the NICHD/NIDCD funded Collaborative Programs of Excellence in Autism, and a pre-doctoral fellowship from the National Alliance for Autism Research. We are grateful to Brian Winklosky, Deborah Arin, Beth Rosen-Sheidley, Emily Presseau, and Carey Wagner at Tufts-New England Medical Center and Marlea O'Brien and Marcia St. Clair at the University of Iowa for collecting the data reported in this paper. We would also like to thank Dr. Howard Cabral at Boston University School of Public Health for his statistical guidance. We offer special thanks to the children and families who participated in this study.
Supporting Grants:
NINDS; R01 NS 38668 and F30 NS 055511
NIDCD; U19 DC 03610
National Alliance for Autism Research
LITERATURE CITED
- Abrahams BS, Geschwind DH. Advances in autism genetics: On the threshold of a new neurobiology. Nature Reviews Genetics. 2008;9(5):341–355. doi: 10.1038/nrg2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alarcon M, Abrahams BS, Stone JL, Duvall JA, Perederiy JV, Bomar JM, et al. Linkage, association, and gene-expression analyses identify cntnap2 as an autism-susceptibility gene. American Journal of Human Genetics. 2008;82(1):150–159. doi: 10.1016/j.ajhg.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th ed. Author; Washington, DC: 1994. [Google Scholar]
- Arking DE, Cutler DJ, Brune CW, Teslovich TM, West K, Ikeda M, et al. A common genetic variant in the neurexin superfamily member cntnap2 increases familial risk of autism. American Journal of Human Genetics. 2008;82(1):160–164. doi: 10.1016/j.ajhg.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aylward EH, Minshew NJ, Field K, Sparks BF, Singh N. Effects of age on brain volume and head circumference in autism. Neurology. 2002;59(2):175–183. doi: 10.1212/wnl.59.2.175. [DOI] [PubMed] [Google Scholar]
- Bailey A, Palferman S, Heavey L, Le Couteur A. Autism: The phenotype in relatives. Journal of Autism and Developmental Disorders. 1998;28(5):369–392. doi: 10.1023/a:1026048320785. [DOI] [PubMed] [Google Scholar]
- Bakkaloglu B, O'Roak BJ, Louvi A, Gupta AR, Abelson JF, Morgan TM, et al. Molecular cytogenetic analysis and resequencing of contactin associated protein-like 2 in autism spectrum disorders. American Journal of Human Genetics. 2008;82(1):165–173. doi: 10.1016/j.ajhg.2007.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry JG, Yasin I, Bishop DV. Heritable risk factors associated with language impairments. Genes, Brain and Behavior. 2007;6(1):66–76. doi: 10.1111/j.1601-183X.2006.00232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartak L, Rutter M, Cox A. A comparative study of infantile autism and specific development receptive language disorder. I. The children. British Journal of Psychiatry. 1975;126:127–145. doi: 10.1192/bjp.126.2.127. [DOI] [PubMed] [Google Scholar]
- Bartak L, Rutter M, Cox A. A comparative study of infantile autism and specific developmental receptive language disorders. Iii. Discriminant function analysis. Journal of Autism and Child Schizophrenia. 1977;7(4):383–396. doi: 10.1007/BF01540396. [DOI] [PubMed] [Google Scholar]
- Bespalova IN, Buxbaum JD. Disease susceptibility genes for autism. Annals of Medicine. 2003;35(4):274–281. doi: 10.1080/07853890310005966. [DOI] [PubMed] [Google Scholar]
- Bishop DV. Autism, asperger's syndrome and semantic-pragmatic disorder: Where are the boundaries? British Journal of Disorders of Communication. 1989;24(2):107–121. doi: 10.3109/13682828909011951. [DOI] [PubMed] [Google Scholar]
- Bishop DV. Development of the children's communication checklist (ccc): A method for assessing qualitative aspects of communicative impairment in children. Journal of Child Psychology and Psychiatry. 1998;39(6):879–891. [PubMed] [Google Scholar]
- Bishop DV, Bishop SJ, Bright P, James C, Delaney T, Tallal P. Different origin of auditory and phonological processing problems in children with language impairment: Evidence from a twin study. Journal of Speech, Language, and Hearing Research. 1999;42(1):155–168. doi: 10.1044/jslhr.4201.155. [DOI] [PubMed] [Google Scholar]
- Bishop DV, Maybery M, Maley A, Wong D, Hill W, Hallmayer J. Using self-report to identify the broad phenotype in parents of children with autistic spectrum disorders: A study using the autism-spectrum quotient. Journal of Child Psychology and Psychiatry. 2004a;45(8):1431–1436. doi: 10.1111/j.1469-7610.2004.00849.x. [DOI] [PubMed] [Google Scholar]
- Bishop DV, Maybery M, Wong D, Maley A, Hallmayer J. Characteristics of the broader phenotype in autism: A study of siblings using the children's communication checklist-2. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics. 2006;141(2):117–122. doi: 10.1002/ajmg.b.30267. [DOI] [PubMed] [Google Scholar]
- Bishop DV, Maybery M, Wong D, Maley A, Hill W, Hallmayer J. Are phonological processing deficits part of the broad autism phenotype? American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics. 2004b;128(1):54–60. doi: 10.1002/ajmg.b.30039. [DOI] [PubMed] [Google Scholar]
- Bishop DV, Norbury CF. Exploring the borderlands of autistic disorder and specific language impairment: A study using standardised diagnostic instruments. Journal of Child Psychology and Psychiatry. 2002;43(7):917–929. doi: 10.1111/1469-7610.00114. [DOI] [PubMed] [Google Scholar]
- Bishop DV, North T, Donlan C. Genetic basis of specific language impairment: Evidence from a twin study. Developmental Medicine and Child Neurology. 1995;37(1):56–71. doi: 10.1111/j.1469-8749.1995.tb11932.x. [DOI] [PubMed] [Google Scholar]
- Bishop DV, North T, Donlan C. Nonword repetition as a behavioural marker for inherited language impairment: Evidence from a twin study. Journal of Child Psychology and Psychiatry. 1996;37(4):391–403. doi: 10.1111/j.1469-7610.1996.tb01420.x. [DOI] [PubMed] [Google Scholar]
- Bishop DV, Whitehouse AJ, Watt HJ, Line EA. Autism and diagnostic substitution: Evidence from a study of adults with a history of developmental language disorder. Developmental Medicine and Child Neurology. 2008;50(5):341–345. doi: 10.1111/j.1469-8749.2008.02057.x. [DOI] [PubMed] [Google Scholar]
- Bolton P, Macdonald H, Pickles A, Rios P, Goode S, Crowson M, et al. A case-control family history study of autism. Journal of Child Psychology and Psychiatry. 1994;35(5):877–900. doi: 10.1111/j.1469-7610.1994.tb02300.x. [DOI] [PubMed] [Google Scholar]
- Cantwell DP, Baker L, Rutter M, Mawhood L. Infantile autism and developmental receptive dysphasia: A comparative follow-up into middle childhood. Journal of Autism and Developmental Disorders. 1989;19(1):19–31. doi: 10.1007/BF02212715. [DOI] [PubMed] [Google Scholar]
- Carper RA, Moses P, Tigue ZD, Courchesne E. Cerebral lobes in autism: Early hyperplasia and abnormal age effects. Neuroimage. 2002;16(4):1038–1051. doi: 10.1006/nimg.2002.1099. [DOI] [PubMed] [Google Scholar]
- Catts HW, Bridges MS, Little TD, Tomblin JB. Reading achievement growth in children with language impairments. Journal of Speech, Language, and Hearing Research. 2008;51(6):1569–1579. doi: 10.1044/1092-4388(2008/07-0259). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catts HW, Fey ME, Tomblin JB, Zhang X. A longitudinal investigation of reading outcomes in children with language impairments. Journal of Speech, Language, and Hearing Research. 2002;45(6):1142–1157. doi: 10.1044/1092-4388(2002/093). [DOI] [PubMed] [Google Scholar]
- Coady JA, Evans JL. Uses and interpretations of non-word repetition tasks in children with and without specific language impairments (sli) International Journal of Language & Communication Disorders. 2008;43(1):1–40. doi: 10.1080/13682820601116485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti-Ramsden G. Processing and linguistic markers in young children with specific language impairment (sli) Journal of Speech, Language, and Hearing Research. 2003;46(5):1029–1037. doi: 10.1044/1092-4388(2003/082). [DOI] [PubMed] [Google Scholar]
- Conti-Ramsden G, Botting N, Faragher B. Psycholinguistic markers for specific language impairment (sli) Journal of Child Psychology and Psychiatry. 2001;42(6):741–748. doi: 10.1111/1469-7610.00770. [DOI] [PubMed] [Google Scholar]
- Conti-Ramsden G, Simkin Z, Botting N. The prevalence of autistic spectrum disorders in adolescents with a history of specific language impairment (sli) Journal of Child Psychology and Psychiatry. 2006a;47(6):621–628. doi: 10.1111/j.1469-7610.2005.01584.x. [DOI] [PubMed] [Google Scholar]
- Conti-Ramsden G, Simkin Z, Pickles A. Estimating familial loading in sli: A comparison of direct assessment versus parental interview. Journal of Speech, Language, and Hearing Research. 2006b;49(1):88–101. doi: 10.1044/1092-4388(2006/007). [DOI] [PubMed] [Google Scholar]
- Courchesne E, Karns CM, Davis HR, Ziccardi R, Carper RA, Tigue ZD, et al. Unusual brain growth patterns in early life in patients with autistic disorder: An mri study. Neurology. 2001;57(2):245–254. doi: 10.1212/wnl.57.2.245. [DOI] [PubMed] [Google Scholar]
- Dahlgren SO, Gillberg C. Symptoms in the first two years of life. A preliminary population study of infantile autism. European Archives of Psychiatry and Neurological Sciences. 1989;238(3):169–174. doi: 10.1007/BF00451006. [DOI] [PubMed] [Google Scholar]
- De Fossé L, Hodge SM, Makris N, Kennedy DN, Caviness VS, Jr., McGrath L, et al. Language-association cortex asymmetry in autism and specific language impairment. Annals of Neurology. 2004;56(6):757–766. doi: 10.1002/ana.20275. [DOI] [PubMed] [Google Scholar]
- Dromi E, Leonard LB, Adam G, Zadunaisky-Ehrlich S. Verb agreement morphology in hebrew-speaking children with specific language impairment. Journal of Speech, Language, and Hearing Research. 1999;42(6):1414–1431. doi: 10.1044/jslhr.4206.1414. [DOI] [PubMed] [Google Scholar]
- Dunn LM, Dunn LM. Peabody picture vocabulary test. 3rd ed. American Guidance Service; Circle Pines, MN: 1997. [Google Scholar]
- Durkin K, Conti-Ramsden G. Language, social behavior, and the quality of friendships in adolescents with and without a history of specific language impairment. Child Development. 2007;78(5):1441–1457. doi: 10.1111/j.1467-8624.2007.01076.x. [DOI] [PubMed] [Google Scholar]
- Filipek PA, Richelme C, Kennedy DN, Rademacher J, Pitcher DA, Zidel S, et al. Morphometric analysis of the brain in developmental language disorders and autism [abstract] Annals of Neurology. 1992;32(3):475. [Google Scholar]
- Flax JF, Realpe-Bonilla T, Hirsch LS, Brzustowicz LM, Bartlett CW, Tallal P. Specific language impairment in families: Evidence for co-occurrence with reading impairments. Journal of Speech, Language, and Hearing Research. 2003;46(3):530–543. doi: 10.1044/1092-4388(2003/043). [DOI] [PubMed] [Google Scholar]
- Folstein SE, Rosen-Sheidley B. Genetics of autism: Complex aetiology for a heterogeneous disorder. Nature Reviews Genetics. 2001;2(12):943–955. doi: 10.1038/35103559. [DOI] [PubMed] [Google Scholar]
- Folstein SE, Rutter M. Infantile autism: A genetic study of 21 twin pairs. Journal of Child Psychology and Psychiatry. 1977;18(4):297–321. doi: 10.1111/j.1469-7610.1977.tb00443.x. [DOI] [PubMed] [Google Scholar]
- Folstein SE, Santangelo SL, Gilman SE, Piven J, Landa R, Lainhart J, et al. Predictors of cognitive test patterns in autism families. Journal of Child Psychology and Psychiatry. 1999;40(7):1117–1128. [PubMed] [Google Scholar]
- Fombonne E, Bolton P, Prior J, Jordan H, Rutter M. A family study of autism: Cognitive patterns and levels in parents and siblings. Journal of Child Psychology and Psychiatry. 1997;38(6):667–683. doi: 10.1111/j.1469-7610.1997.tb01694.x. [DOI] [PubMed] [Google Scholar]
- Gamliel I, Yirmiya N, Sigman M. The development of young siblings of children with autism from 4 to 54 months. Journal of Autism and Developmental Disorders. 2007;37(1):171–183. doi: 10.1007/s10803-006-0341-5. [DOI] [PubMed] [Google Scholar]
- Gauger LM, Lombardino LJ, Leonard CM. Brain morphology in children with specific language impairment. Journal of Speech, Language, and Hearing Research. 1997;40(6):1272–1284. doi: 10.1044/jslhr.4006.1272. [DOI] [PubMed] [Google Scholar]
- Gillberg C, Coleman M. The biology of the autistic syndromes. 3rd ed. Mac Keith Press; London: 2000. [Google Scholar]
- Goldberg WA, Osann K, Filipek PA, Laulhere T, Jarvis K, Modahl C, et al. Language and other regression: Assessment and timing. Journal of Autism and Developmental Disorders. 2003;33(6):607–616. doi: 10.1023/b:jadd.0000005998.47370.ef. [DOI] [PubMed] [Google Scholar]
- Hardan AY, Minshew NJ, Mallikarjuhn M, Keshavan MS. Brain volume in autism. Journal of Child Neurology. 2001;16(6):421–424. doi: 10.1177/088307380101600607. [DOI] [PubMed] [Google Scholar]
- Herbert MR, Harris GJ, Adrien KT, Ziegler DA, Makris N, Kennedy DN, et al. Abnormal asymmetry in language association cortex in autism. Annals of Neurology. 2002;52(5):588–596. doi: 10.1002/ana.10349. [DOI] [PubMed] [Google Scholar]
- Herbert MR, Ziegler DA, Deutsch CK, O'Brien LM, Kennedy DN, Filipek PA, et al. Brain asymmetries in autism and developmental language disorder: A nested whole-brain analysis. Brain. 2005;128(Pt 1):213–226. doi: 10.1093/brain/awh330. [DOI] [PubMed] [Google Scholar]
- Herbert MR, Ziegler DA, Makris N, Bakardjiev A, Hodgson J, Adrien KT, et al. Larger brain and white matter volumes in children with developmental language disorder. Developmental Science. 2003;6(4):F11–F22. [Google Scholar]
- Hollingshead A. Two factor index of social position. Yale University Press; New Haven, CT: 1965. [Google Scholar]
- Howlin P, Mawhood L, Rutter M. Autism and developmental receptive language disorder--a follow-up comparison in early adult life. Ii: Social, behavioural, and psychiatric outcomes. Journal of Child Psychology and Psychiatry. 2000;41(5):561–578. doi: 10.1111/1469-7610.00643. [DOI] [PubMed] [Google Scholar]
- Interactive Autism Network Ian research report. 2007 2007 may; from http://www.iancommunity.org/cs/ian_research_reports/ian_research_report_may_2007.
- Jackson T, Plante E. Gyral morphology in the posterior sylvian region in families affected by developmental language disorder. Neuropsychology Review. 1996;6(2):81–94. doi: 10.1007/BF01875369. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Hesselink JR, Sowell E, Tallal PA. Cerebral structure on magnetic resonance imaging in language- and learning-impaired children. Archives of Neurology. 1991;48(5):539–545. doi: 10.1001/archneur.1991.00530170103028. [DOI] [PubMed] [Google Scholar]
- Kjelgaard M, Tager-Flusberg H. An investigation of language impairment in autism: Implications for genetic subgroups. Language and Cognitive Processes. 2001;16(2/3):287–308. doi: 10.1080/01690960042000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahey M, Edwards J. Specific language impairment: Preliminary investigation of factors associated with family history and with patterns of language performance. Journal of Speech and Hearing Research. 1995;38(3):643–657. doi: 10.1044/jshr.3803.643. [DOI] [PubMed] [Google Scholar]
- Lainhart JE, Bigler ED, Bocian M, Coon H, Dinh E, Dawson G, et al. Head circumference and height in autism: A study by the collaborative program of excellence in autism. American Journal of Medical Genetics Part A. 2006;140(21):2257–2274. doi: 10.1002/ajmg.a.31465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landa R, Folstein SE, Isaacs C. Spontaneous narrative-discourse performance of parents of autistic individuals. Journal of Speech and Hearing Research. 1991;34(6):1339–1345. doi: 10.1044/jshr.3406.1339. [DOI] [PubMed] [Google Scholar]
- Landa R, Piven J, Wzorek MM, Gayle JO, Chase GA, Folstein SE. Social language use in parents of autistic individuals. Psychological Medicine. 1992;22(1):245–254. doi: 10.1017/s0033291700032918. [DOI] [PubMed] [Google Scholar]
- Le Couteur A, Bailey A, Goode S, Pickles A, Robertson S, Gottesman I, et al. A broader phenotype of autism: The clinical spectrum in twins. Journal of Child Psychology and Psychiatry. 1996;37(7):785–801. doi: 10.1111/j.1469-7610.1996.tb01475.x. [DOI] [PubMed] [Google Scholar]
- Leonard C, Eckert M, Given B, Virginia B, Eden G. Individual differences in anatomy predict reading and oral language impairments in children. Brain. 2006;129(Pt 12):3329–3342. doi: 10.1093/brain/awl262. [DOI] [PubMed] [Google Scholar]
- Leonard CM, Lombardino LJ, Walsh K, Eckert MA, Mockler JL, Rowe LA, et al. Anatomical risk factors that distinguish dyslexia from sli predict reading skill in normal children. Journal Of Communication Disorders. 2002;35(6):501–531. doi: 10.1016/s0021-9924(02)00120-x. [DOI] [PubMed] [Google Scholar]
- Leonard LB, Dromi E, Adam G, Zadunaisky-Ehrlich S. Tense and finiteness in the speech of children with specific language impairment acquiring hebrew. International Journal of Language & Communication Disorders. 2000;35(3):319–335. doi: 10.1080/136828200410609. [DOI] [PubMed] [Google Scholar]
- Lewis BA, Cox NJ, Byard PJ. Segregation analysis of speech and language disorders. Behavior Genetics. 1993;23(3):291–297. doi: 10.1007/BF01082469. [DOI] [PubMed] [Google Scholar]
- Lewis BA, Thompson LA. A study of developmental speech and language disorders in twins. Journal of Speech and Hearing Research. 1992;35(5):1086–1094. doi: 10.1044/jshr.3505.1086. [DOI] [PubMed] [Google Scholar]
- Leyfer OT, Tager-Flusberg H, Dowd M, Tomblin JB, Folstein SE. Overlap between autism and specific language impairment: Comparison of autism diagnostic interview and autism diagnostic observation schedule scores. Autism Research. 2008;1(5):284–296. doi: 10.1002/aur.43. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr., Leventhal BL, DiLavore PC, et al. The autism diagnostic observation schedule-generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30(3):205–223. [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism diagnostic interview-revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Mawhood L, Howlin P, Rutter M. Autism and developmental receptive language disorder--a comparative follow-up in early adult life. I: Cognitive and language outcomes. Journal of Child Psychology and Psychiatry. 2000;41(5):547–559. doi: 10.1111/1469-7610.00642. [DOI] [PubMed] [Google Scholar]
- McArthur GM, Hogben JH, Edwards VT, Heath SM, Mengler ED. On the “specifics” of specific reading disability and specific language impairment. Journal of Child Psychology and Psychiatry. 2000;41(7):869–874. [PubMed] [Google Scholar]
- Nation K, Clarke P, Wright B, Williams C. Patterns of reading ability in children with autism spectrum disorder. Journal of Autism and Developmental Disorders. 2006;36(7):911–919. doi: 10.1007/s10803-006-0130-1. [DOI] [PubMed] [Google Scholar]
- Neils J, Aram DM. Family history of children with developmental language disorders. Perceptual and Motor Skills. 1986;63(2 Pt 1):655–658. doi: 10.2466/pms.1986.63.2.655. [DOI] [PubMed] [Google Scholar]
- O'Brien EK, Zhang X, Nishimura C, Tomblin JB, Murray JC. Association of specific language impairment (sli) to the region of 7q31. American Journal of Human Genetics. 2003;72(6):1536–1543. doi: 10.1086/375403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen AJ, Dromi E, Leonard LB. The phonology-morphology interface in the speech of hebrew-speaking children with specific language impairment. Journal Of Communication Disorders. 2001;34(4):323–337. doi: 10.1016/s0021-9924(01)00053-3. [DOI] [PubMed] [Google Scholar]
- Pilowsky T, Yirmiya N, Shalev RS, Gross-Tsur V. Language abilities of siblings of children with autism. Journal of Child Psychology and Psychiatry. 2003;44(6):914–925. doi: 10.1111/1469-7610.00175. [DOI] [PubMed] [Google Scholar]
- Piven J, Arndt S, Bailey J, Andreasen N. Regional brain enlargement in autism: A magnetic resonance imaging study. Journal of the American Academy of Child and Adolescent Psychiatry. 1996;35(4):530–536. doi: 10.1097/00004583-199604000-00020. [DOI] [PubMed] [Google Scholar]
- Piven J, Arndt S, Bailey J, Havercamp S, Andreasen NC, Palmer P. An mri study of brain size in autism. American Journal of Psychiatry. 1995;152(8):1145–1149. doi: 10.1176/ajp.152.8.1145. [DOI] [PubMed] [Google Scholar]
- Piven J, Palmer P, Jacobi D, Childress D, Arndt S. Broader autism phenotype: Evidence from a family history study of multiple-incidence autism families. American Journal of Psychiatry. 1997a;154(2):185–190. doi: 10.1176/ajp.154.2.185. [DOI] [PubMed] [Google Scholar]
- Piven J, Palmer P, Landa R, Santangelo S, Jacobi D, Childress D. Personality and language characteristics in parents from multiple-incidence autism families. American Journal of Medical Genetics. 1997b;74(4):398–411. [PubMed] [Google Scholar]
- Plante E. Mri findings in the parents and siblings of specifically language-impaired boys. Brain and Language. 1991;41(1):67–80. doi: 10.1016/0093-934x(91)90111-d. [DOI] [PubMed] [Google Scholar]
- Plante E, Shenkman K, Clark MM. Classification of adults for family studies of developmental language disorders. Journal of Speech and Hearing Research. 1996;39(3):661–667. doi: 10.1044/jshr.3903.661. [DOI] [PubMed] [Google Scholar]
- Plante E, Swisher L, Vance R, Rapcsak S. Mri findings in boys with specific language impairment. Brain and Language. 1991;41(1):52–66. doi: 10.1016/0093-934x(91)90110-m. [DOI] [PubMed] [Google Scholar]
- Plumet MH, Goldblum MC, Leboyer M. Verbal skills in relatives of autistic females. Cortex. 1995;31(4):723–733. doi: 10.1016/s0010-9452(13)80023-8. [DOI] [PubMed] [Google Scholar]
- Rapin I. Historical data. In: Rapin I, editor. Preschool children with inadequate communication. MacKeith; London: 1996a. pp. 58–97. [Google Scholar]
- Rapin I. Practitioner review: Developmental language disorders: A clinical update. Journal of Child Psychology and Psychiatry. 1996b;37(6):643–655. doi: 10.1111/j.1469-7610.1996.tb01456.x. [DOI] [PubMed] [Google Scholar]
- Resnick TJ, Rapin I. Language disorders in childhood. Psychiatric Annals. 1991;21(2):709–716. [Google Scholar]
- Rice ML, Haney KR, Wexler K. Family histories of children with sli who show extended optional infinitives. Journal of Speech, Language, and Hearing Research. 1998;41(2):419–432. doi: 10.1044/jslhr.4102.419. [DOI] [PubMed] [Google Scholar]
- Rice ML, Wexler K, Cleave PL. Specific language impairment as a period of extended optional infinitive. Journal of Speech and Hearing Research. 1995;38(4):850–863. doi: 10.1044/jshr.3804.850. [DOI] [PubMed] [Google Scholar]
- Roberts JA, Rice ML, Tager-Flusberg H. Tense marking in children with autism. Applied Psycholinguistics. 2004;25(3):429–448. [Google Scholar]
- Ruser TF, Arin D, Dowd M, Putnam S, Winklosky B, Rosen-Sheidley B, et al. Communicative competence in parents of children with autism and parents of children with specific language impairment. Journal of Autism and Developmental Disorders. 2007;37(7):1323–1336. doi: 10.1007/s10803-006-0274-z. [DOI] [PubMed] [Google Scholar]
- SAS Institute Inc . Jmp (Version 7.0) Cary, NC: 2007. [Google Scholar]
- Semel E, Wiig EH, Secord WA. Clinical evaluation of language fundamentals. 3rd ed. The Psychological Corporation; San Antonio, TX: 1995. [Google Scholar]
- Sparks BF, Friedman SD, Shaw DW, Aylward EH, Echelard D, Artru AA, et al. Brain structural abnormalities in young children with autism spectrum disorder. Neurology. 2002;59(2):184–192. doi: 10.1212/wnl.59.2.184. [DOI] [PubMed] [Google Scholar]
- Stokes SF, Wong AM, Fletcher P, Leonard LB. Nonword repetition and sentence repetition as clinical markers of specific language impairment: The case of cantonese. Journal of Speech, Language, and Hearing Research. 2006;49(2):219–236. doi: 10.1044/1092-4388(2006/019). [DOI] [PubMed] [Google Scholar]
- Szatmari P, MacLean JE, Jones MB, Bryson SE, Zwaigenbaum L, Bartolucci G, et al. The familial aggregation of the lesser variant in biological and nonbiological relatives of pdd probands: A family history study. Journal of Child Psychology and Psychiatry. 2000;41(5):579–586. doi: 10.1111/1469-7610.00644. [DOI] [PubMed] [Google Scholar]
- Tager-Flusberg H, Cooper J. Present and future possibilities for defining a phenotype for specific language impairment. Journal of Speech, Language, and Hearing Research. 1999;42(5):1275–1278. doi: 10.1044/jslhr.4205.1275. [DOI] [PubMed] [Google Scholar]
- Tager-Flusberg H, Joseph RM. Identifying neurocognitive phenotypes in autism. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 2003;358(1430):303–314. doi: 10.1098/rstb.2002.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tager-Flusberg H, Paul R, Lord C. Language and communication in autism. In: Volkmar FR, Paul R, Klin A, Cohen D, editors. Handbook of autism and pervasive developmental disorders. 3rd ed. John Wiley & Sons; Hoboken, NJ: 2005. pp. 335–364. [Google Scholar]
- Tallal P, Ross R, Curtiss S. Familial aggregation in specific language impairment. Journal of Speech and Hearing Disorders. 1989;54(2):167–173. doi: 10.1044/jshd.5402.167. [DOI] [PubMed] [Google Scholar]
- Thordardottir ET, Namazi M. Specific language impairment in french-speaking children: Beyond grammatical morphology. Journal of Speech, Language, and Hearing Research. 2007;50(3):698–715. doi: 10.1044/1092-4388(2007/049). [DOI] [PubMed] [Google Scholar]
- Tomblin JB. Familial concentration of developmental language impairment. Journal of Speech and Hearing Disorders. 1989;54(2):287–295. doi: 10.1044/jshd.5402.287. [DOI] [PubMed] [Google Scholar]
- Tomblin JB, Buckwalter PR. Heritability of poor language achievement among twins. Journal of Speech, Language, and Hearing Research. 1998;41(1):188–199. doi: 10.1044/jslhr.4101.188. [DOI] [PubMed] [Google Scholar]
- Tomblin JB, Hafeman LL, O'Brien M. Autism and autism risk in siblings of children with specific language impairment. International Journal of Language & Communication Disorders. 2003;38(3):235–250. doi: 10.1080/1368282031000086363. [DOI] [PubMed] [Google Scholar]
- Tomblin JB, Records NL, Buckwalter P, Zhang X, Smith E, O'Brien M. Prevalence of specific language impairment in kindergarten children. Journal of Speech, Language, and Hearing Research. 1997;40(6):1245–1260. doi: 10.1044/jslhr.4006.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomblin JB, Zhang X. Language patterns and etiology in children with specific language impairment. In: Tager-Flusberg H, editor. Neurodevelopmental disorders. MIT Press; Cambridge, MA: 1999. pp. 361–382. [Google Scholar]
- Tomblin JB, Zhang X, Buckwalter P, Catts H. The association of reading disability, behavioral disorders, and language impairment among second-grade children. Journal of Child Psychology and Psychiatry. 2000;41(4):473–482. [PubMed] [Google Scholar]
- van der Lely HK, Stollwerck L. A grammatical specific language impairment in children: An autosomal dominant inheritance? Brain and Language. 1996;52(3):484–504. doi: 10.1006/brln.1996.0026. [DOI] [PubMed] [Google Scholar]
- Vernes SC, Newbury DF, Abrahams BS, Winchester L, Nicod J, Groszer M, et al. A functional genetic link between distinct developmental language disorders. New England Journal of Medicine. 2008;359(22):2337–2345. doi: 10.1056/NEJMoa0802828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner RK, Torgesen JK, Rashotte CA. Comprehensive test of phonological processing. PRO-ED, Inc; Austin, TX: 1999. [Google Scholar]
- Warburton P, Baird G, Chen W, Morris K, Jacobs BW, Hodgson S, et al. Support for linkage of autism and specific language impairment to 7q3 from two chromosome rearrangements involving band 7q31. American Journal of Medical Genetics. 2000;96(2):228–234. doi: 10.1002/(sici)1096-8628(20000403)96:2<228::aid-ajmg20>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler intelligence scale for children. 3rd ed. The Psychological Corporation; San Antonio, TX: 1991. [Google Scholar]
- Wechsler D. Wechsler adult intelligence scale. 3rd ed. The Psychological Corporation; San Antonio, TX: 1997. [Google Scholar]
- Whitehouse AJ, Barry JG, Bishop DV. The broader language phenotype of autism: A comparison with specific language impairment. Journal of Child Psychology and Psychiatry. 2007;48(8):822–830. doi: 10.1111/j.1469-7610.2007.01765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehouse AJ, Barry JG, Bishop DV. Further defining the language impairment of autism: Is there a specific language impairment subtype? Journal Of Communication Disorders. 2008;41(4):319–336. doi: 10.1016/j.jcomdis.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Williams D, Botting N, Boucher J. Language in autism and specific language impairment: Where are the links? Psychological Bulletin. 2008;134(6):944–963. doi: 10.1037/a0013743. [DOI] [PubMed] [Google Scholar]
- Woodcock RW, Johnson MB. Woodcock-johnson psycho-educational battery – revised. Riverside; Itasca, IL: 1990. [Google Scholar]
- Woodhouse W, Bailey A, Rutter M, Bolton P, Baird G, Le Couteur A. Head circumference in autism and other pervasive developmental disorders. Journal of Child Psychology and Psychiatry. 1996;37(6):665–671. doi: 10.1111/j.1469-7610.1996.tb01458.x. [DOI] [PubMed] [Google Scholar]


