Abstract
Selective degeneration and death of one or more classes of neurons is the defining feature of human neurodegenerative disease. Although traditionally viewed as diseases mainly affecting the most vulnerable neurons, in most instances of inherited disease the causative genes are widely—usually ubiquitously—expressed. Focusing on amyotrophic lateral sclerosis (ALS), especially disease caused by dominant mutations in Cu/Zn superoxide dismutase (SOD1), we review here the evidence that it is the convergence of damage developed within multiple cell types, including within neighboring nonneuronal supporting cells, which is crucial to neuronal dysfunction. Damage to a specific set of key partner cells as well as to vulnerable neurons may account for the selective susceptibility of neuronal subtypes in many human neurodegenerative diseases, including Huntington's disease (HD), Parkinson's disease (PD), prion disease, the spinal cerebellar ataxias (SCAs), and Alzheimer's disease (AD).
Introduction
The great cell biologists of the 19th century, including Rudolph Virchow, the German physician widely known as the father of pathology, and the French physiologist Claude Bernard established the pivotal idea that individual cells function autonomously, while being part of the whole organism. Since then, many pathological conditions including all major neurodegenerative diseases have traditionally been considered mechanistically cell autonomous, meaning that damage within a selective population of affected neurons alone suffices to produce disease.
For most neurodegenerative conditions, injury may originate either from unknown stressors in the case of sporadic disease, or from expression of a mutant gene in the familial forms. With the recognition that essentially all of the genes whose mutation causes the inherited forms of these diseases are widely or ubiquitously expressed, three questions are central for understanding disease mechanism (and for devising therapies). First, what are the mutant-driven toxic mechanisms that mediate disease? Second, is disease actually driven by cell autonomous mechanisms as has previously been widely assumed? Third, what explanation is there for the selectivity in neuronal killing from a widely expressed mutant?
A plethora of damage: eight proposed mechanisms for familial ALS
Amyotrophic lateral sclerosis (ALS) is characterized by selective, premature degeneration and death of motor neurons initiating in mid-adult life. The ensuing progressive paralysis is typically fatal within a handful of years due to respiratory failure. Although the majority of incidences have no apparent hereditary contribution, ∼10% of instances are dominantly inherited. A landmark discovery reported in 1993 initiated the molecular era of ALS research with identification of mutations in the gene encoding for superoxide dismutase 1 (SOD1) as causative in 20% of the inherited cases (Rosen et al., 1993). A major cytoplasmic antioxidant, the ubiquitously expressed SOD1's normal function is to catalytically convert highly reactive superoxide (oxygen with an extra electron) to either hydrogen peroxide or oxygen.
Mice expressing various ALS-related mutants of SOD1 have recapitulated the fatal paralysis seen in human patients, and use of them has been the most important contributor to defining familial ALS disease mechanisms. An important initial realization from these efforts is that disease is caused by one or more acquired toxicity(ies) of the mutant proteins, rather than reduced superoxide dismutase activity. A universal finding is that a proportion of the more than 150 SOD1 mutants (Turner and Talbot, 2008) fails to fold properly, thus implicating accumulation of misfolded SOD1 as a possible toxic contributor in ALS. The misfolded SOD1 forms ubiquitinated cytoplasmic inclusions that occur early in disease and escalate as disease progresses (Bruijn et al., 1997). A sobering reality, however, is that 16 years after the initiating discovery (Rosen et al., 1993) no consensus has yet emerged as to the primary toxicity of mutant SOD1. Instead, a plethora of toxic mechanisms have been proposed to mediate pathogenesis, that is, the course of events that underlie the progressive fatal paralysis from degeneration and death of motor neurons (Fig. 1).
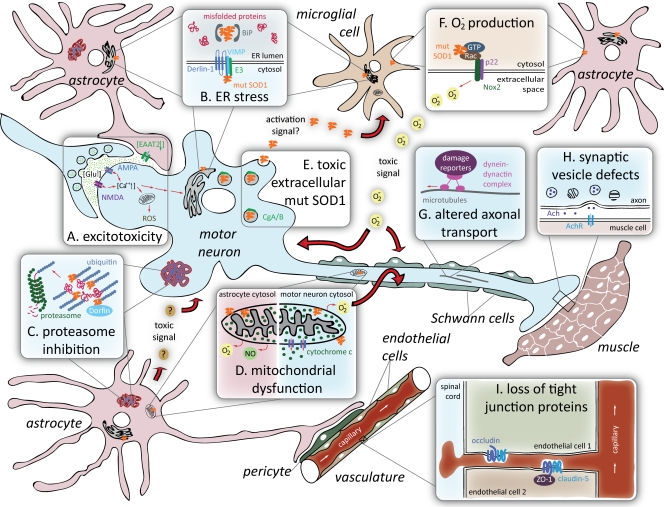
Figure 1.
Proposed mechanisms of toxicity in SOD1-mediated ALS. (A) Excitotoxicity is the hyperactivation of motor neurons resulting from failure to rapidly remove neurotransmitter glutamate from synapses due to deficiency in the glutamate transporter EAAT2 in the neighboring astrocytes. (B) ER stress is induced by abnormal interactions of mutant SOD1 with ER proteins (see text for details). (C) Proteasome inhibition due to “overload” of the proteasome degradation pathway with ubiquitinated misfolded protein aggregates may damage astrocytes and motor neurons. (D) Mitochondrial dysfunction mediated by mutant SOD1 deposition on the mitochondrial membrane provokes release of cytochrome c in motor neurons, whereas in astrocytes it leads to nitroxidative stress. (E) Toxic extracellular mutant SOD1 is secreted from motor neurons and astrocytes (not depicted) after interaction with components of neurosecretory vesicles. (F) Superoxide production from microglia or astrocytes can damage neighboring motor neurons. (G) Altered axonal transport including an increase in retrogradely transported stress-related proteins was reported in mutant SOD1-expressing motor neurons. (H) Synaptic vesicle defects such as stalling and loss from distal synapse in vulnerable motor neurons is an early event in ALS. (I) Loss of tight junction proteins within capillary endothelial cells results in the disruption of the blood–spinal cord barrier and the occurrence of microhemorrhages within the spinal cord well before disease onset.
Excitotoxicity from mishandling of glutamate.
One of the early proposed mechanisms—observed both in SOD1 mutant mouse models and in familial and sporadic ALS patient samples—is glutamate excitotoxicity, the excessive firing of motor neurons derived from failure to rapidly remove synaptic glutamate (Fig. 1 A). Overstimulation by glutamate, the neurotransmitter that triggers motor neurons to fire, can elicit a cascade of toxic events in the postsynaptic motor neuron including repetitive activation of glutamate receptors and the corresponding increase in calcium influx, thus overriding the storage abilities of mitochondria and endoplasmic reticulum (ER). Contributing to this phenomenon is a failure to rapidly clear extracellular glutamate through deficiency in the glutamate transporter EAAT2 in the astrocytic processes that surround synapses of motor neurons (Rothstein et al., 1995; Bruijn et al., 1997; Howland et al., 2002; Yang et al., 2009a,b). Recent evidence has shown that loss of connectivity between upper and lower motor neurons triggers reduced transcription of EAAT2 through reduced expression of a κB motif binding phosphoprotein (KBBP), the mouse homologue of hnRNP K, or human heterogeneous nuclear ribonucleoprotein K (Yang et al., 2009b). Caspase-3 activation has also been linked to production of a truncated form of EAAT2 in SOD1 mutant mouse spinal cords (Boston-Howes et al., 2006).
Mutant SOD1 causes ER stress.
Mutant SOD1 has been argued to trigger ER stress via two different pathways (Fig. 1 B). Mutant SOD1 aggregates were found to accumulate in fractions that are enriched in ER membranes in the affected tissues, a phenomenon that intensifies as disease progresses. These ER-associated SOD1 aggregates bind to the ER-luminal polypeptide chain binding protein (BiP) (Kikuchi et al., 2006), a chaperone that regulates the activation of ER stress transducers such as IRE1, PERK, and ATF6. A later study showed that mutant SOD1 inhibits ER-associated degradation (ERAD), the cell's machinery for eliminating proteins that fail to fold properly inside the ER. The first critical step in the ERAD process is the retrograde transport of misfolded proteins out of the ER lumen into the cytosol, where they are ubiquitinated and subsequently degraded by the proteasome. Multiple ALS-associated mutants of SOD1—including dismutase-active (e.g., SOD1G93A, SOD1A4V) and -inactive (SOD1G85R) mutants but not the wild-type SOD1—interact with derlin-1, a transmembrane ER protein that is instrumental for dislocation of misfolded proteins from the ER to the cytosol. Binding of mutant SOD1 to Derlin-1 inhibits ERAD and thereby generates ER stress (Nishitoh et al., 2008), but only after disease onset, consistent with this being a consequence secondary to some unidentified initiating trigger. Nevertheless, up-regulation of ER-related genes at presymptomatic stages is seen in a subset of vulnerable motor neurons in mice expressing either dismutase-active or -inactive mutants (Saxena et al., 2009). We would note that mutant SOD1 association with the ER may interfere with synthesis of any protein, like EAAT2, whose synthesis and maturation are dependent on passage through the ER.
Mutant SOD1 inhibits the proteasome.
A third proposal for SOD1 mutant toxicity is for misfolded mutant SOD1 inhibition of clearance of damaged proteins by the proteasome, the proteolysis machine for removing abnormally folded proteins from the cytoplasm (Fig. 1 C). In cell culture, mutant SOD1 turns over more rapidly than wild-type and turnover depends on proteasome activity (Hoffman et al., 1996). Intracellular accumulations in familial or sporadic ALS patients are not immunoreactive for proteasome components (Ii et al., 1997; Watanabe et al., 2001) but have been reported to contain Dorfin, a RING finger–type E3 ubiquitin ligase. At least in some instances, Dorfin physically binds and ubiquitinates various SOD1 mutants, thereby enhancing their degradation, but does not affect the stability of wild-type SOD1 (Niwa et al., 2002). A preponderance of biochemical evidence from spinal cords of SOD1 mice has reported decreased activities of the proteasome in lumbar spinal cords of SOD1 mutant mice (Kabashi et al., 2004; Cheroni et al., 2009) or after sustained expression of mutant SOD1 in a culture neuronal line (Urushitani et al., 2002). In this view, a vicious cycle can thus ensue in which protein aggregation not only increases the levels of misfolded mutant SOD1 (Hoffman et al., 1996), but also sequesters essential cellular components (including endogenous SOD1) within the aggregates, causing further damage to the affected cell (Bruijn et al., 1998).
Misfolded mutant SOD1 damages mitochondria.
A fourth proposal is that misfolded mutant SOD1 damages mitochondria by its deposition onto the cytoplasmic face of the outer membrane (Fig. 1 D) of spinal cord mitochondria (Liu et al., 2004; Vande Velde et al., 2008). Apparent morphological damage to mitochondria is seen presymptomatically within motor neurons of some, but not all, lines of mice that develop SOD1 mutant-mediated ALS. A proportion of both dismutase-active and -inactive mutants, however, has been shown convincingly to be mitochondrially associated (Mattiazzi et al., 2002; Liu et al., 2004; Vijayvergiya et al., 2005; Vande Velde et al., 2008). Mitochondrial association of mutant SOD1 begins presymptomatically (Liu et al., 2004), firmly supporting an important mechanistic contribution to disease initiation.
There is no uniform view of how mitochondrial function is affected. ATP levels have been reported to be diminished in symptomatic (Mattiazzi et al., 2002) and presymptomatic (Browne et al., 2006) mutant spinal cords of one mouse model but to be unchanged in another (Damiano et al., 2006). Mitochondrial calcium buffering capacity was found affected in spinal cords of two different strains of mice and has direct connection to the excitotoxic hypothesis (Damiano et al., 2006). Mitochondrial damage seems not to be neuronally limited, but is also found in spinal cord astrocytes for at least one mutant (Cassina et al., 2008). Still untested is whether association of mutant SOD1 with the mitochondrial outer membrane could trigger changes in other functions vital for mitochondrial homeostasis such as protein import, mitochondrial fission/fusion, ionic balance, or regulation of apoptosis.
Extracellular toxicity from aberrant secretion of mutant SOD1.
A fifth proposed mechanism involves interaction of misfolded mutant SOD1 with components of neurosecretory vesicles, chromogranin A (CgA) and chromogranin B (CgB), which in turn can apparently direct the unexpected cosecretion of mutant SOD1 (Urushitani et al., 2006) by motor neurons or astrocytes (Fig. 1 D). Extracellular mutant SOD1 in turn acts to damage motor neurons through activation of microglia, the innate immunity cells within the spinal cord, so as to ultimately drive neuronal death (Zhao et al., 2009). Moreover, proteasome inhibition was reported to enhance aberrant secretion of mutant SOD1, suggesting a cross talk between these two pathways.
Mutant SOD1 generates extracellular superoxide.
A sixth proposed mechanism of mutant SOD1 is counterintuitive: mutant stimulation of excessive extracellular production of superoxide (O−2) (Harraz et al., 2008). Both wild-type and mutant SOD1 can associate with Rac1, a small GTPase that controls the activation of NADPH oxidase (a multiprotein membrane-associated complex whose catalytic subunit is Nox2). A key normal role of Nox2 in phagocytic cells, including microglia, is to produce highly toxic extracellular superoxide, for example, in order to kill bacteria and other pathogens. Harraz et al. (2008)proposed that association of wild-type SOD1 with Rac1 participates in a tightly regulated mechanism that in reducing conditions activates Nox2. Mutant forms of SOD1 interact with Rac1 with apparent higher affinity, thereby locking Nox2 in its active, superoxide-producing form. Paradoxically, instead of its normal job of removing intracellular superoxide, mutant SOD1 may thus be responsible for driving extracellular production of superoxide (Fig. 1 F). It should be noted that for the proportion of Nox2 imbedded in internal membranes—including newly made Nox2 transiting from the ER to the cell surface—Rac1 binding would stimulate high intracellular superoxide within the Nox2-containing vesicles. Like BiP and Derlin-1, still unexplained is why interaction between Rac1 and mutant SOD1 is not detectable at presymptomatic stages.
Mutant SOD1 causes axonal disorganization and disrupted transport.
A seventh hypothesis for mutant SOD1-dependent toxicity is through interference with axonal cytoskeletal organization and/or inhibition of axonal transport. As the most asymmetric cells in nature, motor neurons have a crucial requirement for axonal transport to deliver the many components synthesized in the cell bodies to axons and synapses. SOD1 mutants have been demonstrated to slow both anterograde (Williamson and Cleveland, 1999) and retrograde (Murakami et al., 2001; Perlson et al., 2009) routes months before neurodegeneration. Indeed, axonal disorganization, especially neurofilament misaccumulation, is a hallmark of both sporadic and inherited forms of ALS. Mutations in neurofilaments are at best causes of a very small proportion of ALS, however (Marszalek et al., 1996). Reduction in retrograde transport by mutation in dynactin, an activator of the retrograde motor cytoplasmic dynein, provokes human motor neuron disease that is substantially less severe than ALS (Puls et al., 2003), whereas mutation in dynein provokes loss of sensory neurons that report position (proprioception), but not motor neurons (Chen et al., 2007; Ilieva et al., 2008). Similar decreases in overall retrograde flow in SOD1 mutant axons is, however, accompanied by an increase in retrogradely transported stress or cell death–related proteins (Fig. 1 G) (Perlson et al., 2009), presumably reflecting increased production of such factors in the distal axons and/or synapses. Likely coupled to errors in delivery early in disease are apparent synaptic vesicle stalling and depletion from distal synapses of vulnerable motor neurons (Pun et al., 2006) (Fig. 1 H). It is conceivable that affinity of misfolded mutant SOD1 for membranes may underlie these affects on synaptic vesicles and their normal synaptic trafficking.
Microhemorrhages of spinal capillaries from mutant SOD1.
A final eighth proposal is that mutant SOD1 damage within cells of the vasculature leads to leakage of toxic products, including iron complexes from hemoglobin, into the spinal cord. Indeed, microhemorrhages within the spinal cord that initiate well before disease onset have been seen in all models of SOD1 mutant-mediated disease in mice (Zhong et al., 2008). This disruption of the blood–spinal cord barrier is accompanied by loss of components of the tight junctions (including ZO-1, occludin, and claudin-5) between endothelial cells that maintain the blood–spinal cord barrier (Fig. 1 I). Indeed, decreased mRNA levels of ZO-1 and occludin have been found in lumbar spinal cords of ALS patients, compared with control samples, suggesting that this mechanism of toxicity is relevant for human disease (Henkel et al., 2009). Although efficient mutant SOD1 gene excision from the endothelial cells that line the capillaries does not affect disease course (Zhong et al., 2009), unresolved is how mutant SOD1 induces the loss of these tight junctions and whether the damage to the capillaries is mediated by mutant SOD1 synthesized within the pericytes or astrocytes that reinforce the initial endothelial cell barrier or SOD1 made outside of the vasculature.
Mutant SOD1 causes damage within multiple cell types
From the confusing diversity of proposed toxic pathways, which is correct? And which cells develop the crucial damage through their own synthesis of ubiquitously expressed mutant SOD1? Initial efforts assumed that disease was cell autonomous, that is, from mutant damage solely within motor neurons. Accordingly, attempts were made to generate disease from selective mutant SOD1 expression only in motor neurons. Although these efforts did not produce disease (Pramatarova et al., 2001; Lino et al., 2002), a later study succeeded with mutant synthesis largely restricted to neurons, using mutant SOD1 expression driven by neuron-specific Thy1.2 promoter (Jaarsma et al., 2008). In this latter paradigm, however, even animals with the highest level of mutant synthesis developed disease only at very late ages and disease progressed slowly without reaching the same degree of paralysis relative to lines expressing the same mutant ubiquitously.
It is now clear that toxicity of mutant SOD1 is not just within motor neurons. Analyses of chimeric mice that were mixtures of normal and SOD1 mutant-expressing cells revealed that high expression levels of mutant SOD1 in most (Clement et al., 2003) or all (Yamanaka et al., 2008a) motor neurons is not sufficient for early onset disease, clearly implicating mutant synthesis by nonmotor neurons in driving disease initiation. Lentiviral reduction of mutant SOD1 synthesis in motor neurons—produced through peripheral injection—led to long-term suppression in the central nervous system of mutant SOD1 synthesis selectively within motor neurons. This transcription-mediated mutant SOD1 suppression slowed disease onset very markedly when applied at a very young age, but was of no benefit at all in slowing the rate of disease progression after onset (Ralph et al., 2005).
Identities of cells beyond motor neurons whose mutant SOD1 synthesis contributes to disease has emerged by cell type–selective excision in mice expressing transgenes flanked by lox sites that permit deletion by action of Cre recombinase. As expected, expressing Cre recombinase under two different promoters largely selective to motor neurons in the central nervous system delayed disease onset from a dismutase-active ALS-linked SOD1 mutant (Fig. 2), but did not alter the rate of progression of disease after onset (Boillée et al., 2006; Yamanaka et al., 2008b). Similar deletion from motor neurons and interneurons (by a Lhx3-driven Cre transgene) primarily delayed disease onset and early disease progression (Wang et al., 2009). Taken together, mutant SOD1 expression in motor neurons determines the initial timing of disease onset and early progression, but very surprisingly does not contribute much to later disease progression.
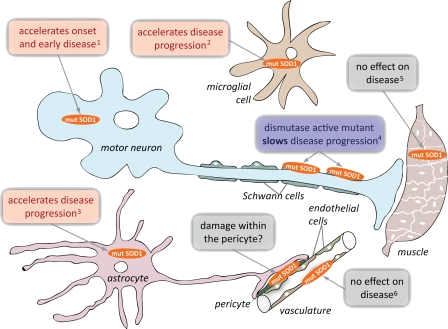
Figure 2.
Contribution of mutant SOD1 within different cell types in ALS. Despite the apparent selectivity for motor neurons, multiple lines of evidence indicate that nonneuronal cell types contribute to pathogenesis and disease progression in SOD1-mediated neurodegeneration. Mutant SOD1 expression in motor neurons directs the onset and development of early disease, but does not influence its progression. In contrast, mutant SOD1 expression in microglia or astrocytes accelerates disease progression without affecting its onset. Expression of a dismutase-active mutant SOD1 specifically in Schwann cells was found to slow disease progression, but the role of a dismutase-inactive mutant in these cells has not been tested. Mutant SOD1 expression within muscle or endothelial cells does not affect ALS onset or progression, although some reports suggest that muscle might be a direct target of mutant SOD1 toxicity. Lastly, the vasculature is damaged very early in disease, leading to loss of tight junctions between endothelial cells and microhemorrhages, but whether any of this is from mutant SOD1 within pericytes, the terminal astrocyte, or coming from cells outside the vasculature is not established. 1(Ralph et al., 2005; Boillée et al., 2006; Jaarsma et al., 2008), 2(Beers et al., 2006; Boillée et al., 2006; Wang et al., 2009), 3(Yamanaka et al., 2008b), 4(Lobsiger et al., 2009), 5(Holzbaur et al., 2006; Miller et al., 2006; Dobrowolny et al., 2008; Towne et al., 2008), 6(Zhong et al., 2009).
Mutant SOD1 causes microglial damage, which drives rapid ALS progression.
Mutant SOD1 expression in cells other than the motor neurons must therefore be the decisive source(s) that drive disease progression after onset. So which cell types develop damage that contributes to the spread of motor neuron injury? Three independent studies reached a common conclusion: damage within mutant-expressing microglial cells—the macrophages of the central nervous system—is key to rapid disease progression (Fig. 2). Using a CD11b-Cre transgene that yields excision of “floxed” genes only in the myeloid lineage, selective mutant SOD1 gene excision provided the most substantial slowing of disease progression yet seen in a rodent model of inherited ALS. Survival after disease onset was almost tripled (Boillée et al., 2006). Slowed progression after reduced mutant SOD1 synthesis in microglia was subsequently confirmed by an analogous approach in a different mutant transgenic line (Wang et al., 2009). Similarly, bone marrow transplantation to generate complete replacement of mutant SOD1-expressing microglial cells with nontransgenic ones did not affect disease onset, but slowed progression (Beers et al., 2006). Driving disease progression does not seem to require the proliferation of mutant microglia in response to an initial injury, as killing of about half of proliferating microglia did not affect the rate of progression (Gowing et al., 2008).
A controversial point has been whether microglial cells from the periphery enter the brain and spinal cord during disease, and if so, whether they participate in driving or ameliorating pathogenesis. Evidence in favor of both roles has emerged using irradiation and grafting (Kang and Rivest, 2007), but this outcome is likely the result of irradiation-induced disruption of the blood–brain barrier (Mildner et al., 2007). A complex experiment which used parabiosis (thereby avoiding irradiation and bone marrow transplantation) found almost no contribution of peripherally circulating macrophages to microglial proliferation and activation during SOD1 mutant-mediated disease (Ajami et al., 2007).
Astrocytes expressing mutant SOD1 drive disease progression.
Astrocytes are one of the most abundant cell types in the adult nervous system. Closely apposed to motor neurons, they are responsible for supplying nutrients, buffering ions, recycling neurotransmitter precursors, and limiting motor neuron firing through rapid recovery of synaptic glutamate with their glutamate transporters. Although restricting mutant SOD1 expression in astrocytes is not sufficient for disease (Gong et al., 2000), selective reduction of mutant SOD1 in astrocytes (Yamanaka et al., 2008b) slowed disease progression and doubled the length of disease duration after onset (Fig. 2). This was accompanied by delayed microglial activation, demonstrating a functional cross talk between mutant astrocytes and microglia.
Mutant astrocytes also induce changes in their partner motor neurons. In cell culture experiments, normal (but not mutant SOD1-expressing) astrocytes induce the up-regulation of the glutamate receptor subunit GluR2 in neighboring (co-cultured) motor neurons through an unidentified soluble factor(s). The GluR2 subunit produces receptors that are impermeable to Ca2+, thereby protecting the motor neurons from excitotoxic damage. Mutant SOD1 expression in astrocytes abrogated their GluR2-regulating capacity, rendering motor neurons vulnerable to excitotoxicity (Van Damme et al., 2007). Activation of the redox-sensitive nuclear factor erythroid-2–related transcription factor 2 (Nrf2) in astrocytes is thought to coordinate the up-regulation of antioxidant defenses, thereby conferring protection to neighboring neurons. Indeed, astrocyte-selective up-regulation of Nrf2 produced significant delay in disease onset in mouse models overexpressing dismutase-active and -inactive SOD1 mutants (Vargas et al., 2008). Perhaps most importantly, cervical transplantation of lineage-restricted astrocyte precursors delayed progression of mutant SOD1-mediated disease after onset, not only reinforcing the influence of astrocytes on disease progression, but demonstrating the feasibility of cell replacement therapies focused on astrocytes (Lepore et al., 2008).
A cascade of oxidative damage from motor axons to myelinating Schwann cells.
Schwann cells are in intimate contact with the full length of the axons of lower motor neurons. The multiple wraps of myelin provide the electrical insulation essential for rapid signal conduction. (A different cell, the oligodendrocyte, has the job of myelination of upper motor axons.) After axonal damage, Schwann cells also participate—in concert with peripheral macrophages—in clearing debris and in guiding the recovering axon. Selective excision of a dismutase-active mutant SOD1 from a substantial proportion of Schwann cells (70%) (through P0-Cre–mediated excision) yielded a highly unexpected outcome: not only did removal of the mutant gene fail to slow any aspect of disease, it generated a substantial acceleration of the late phase of disease (Lobsiger et al., 2009). Indeed, a retrospective look at disease progression in dismutase-active and -inactive SOD1 mutants confirmed that inactive mutants uniformly generate more rapidly progressing disease, consistent with an ameliorating influence of increased dismutase activity in Schwann cells. These findings implicate an oxidative cascade during disease progression that is triggered within axon-ensheathing Schwann cells and that can be ameliorated by elevated dismutase activity (Fig. 1 D). This provocative possibility now awaits a direct test, which could be posed by elevating dismutase activity selectively within Schwann cells as a means to slow disease progression.
A controversial role of mutant SOD1 damage within muscle.
A mechanistic role for mutant SOD1 in muscle remains controversial. Diminished mutant synthesis by 50–60% in muscle (Miller et al., 2006; Towne et al., 2008) did not affect any aspect of mutant SOD1-mediated disease, findings inconsistent with muscle cells as a direct target of mutant SOD1 toxicity (Fig. 2). Indeed, stimulation of myogenesis to produce chronic muscle hypertrophy (by inhibition of the inhibitory hormone myostatin) provided no benefit in slowing disease (Holzbaur et al., 2006; Miller et al., 2006). In contrast, mutant synthesis selectively within skeletal muscle did provoke damage to muscle (Dobrowolny et al., 2008) that was reminiscent of initial damage seen in SOD1 mutant-mediated ALS. A resolution of the conflicting views should now be undertaken by testing whether increased mutant synthesis in muscle can affect onset or progression in a SOD1 mutant mouse that generates very late disease—as in mice with mutant synthesis restricted to neurons (Jaarsma et al., 2008).
A protective role of T lymphocytes in mutant SOD1-mediated ALS.
T lymphocytes appear to play a protective role in SOD1 mutant-mediated ALS. Preventing T lymphocyte recruitment to the spinal cord of the mutant SOD1 mouse (Beers et al., 2008; Chiu et al., 2008) accelerated disease progression. Lack of T lymphocytes was accompanied by a remarkably blunted microglial proliferation, decrease in mRNA levels for neurotrophic factors (BDNF, GDNF, IGF-1), for EAAT2, for immunomodulatory IL-4, and TGF-β, but increased level of pro-inflammatory mRNA for TNF-α and especially for Nox2 (100-fold!). These results demonstrate that the presence of T lymphocytes, through interactions with microglia and astrocytes and/or directly with motor neurons, modifies the local environment to promote neuroprotection (Beers et al., 2008).
Selectivity in ALS: damage-producing pathways converge in different cells
The collective evidence for SOD1 mutant-mediated ALS is that the disease process is decidedly non-cell autonomous (Fig. 2). Synthesis of mutant SOD1 within motor neurons is a primary determinant of driving disease onset. Mutant synthesis by other cells (the most directly implicated include interneurons and cells that comprise the blood–brain barrier) also contributes in a substantial way to disease initiation. Neighboring glial cells, especially the astrocytes and the microglia, develop mutant damage within them, causing them to accelerate disease progression—very markedly so—whereas mutant synthesis in motor neurons has little influence on progression. Schwann cells are recipients of damage, presumably from the damaged motor axons to which they are partnered, and such damage is at least in part from oxidative species that can be diminished by SOD1 activity within the Schwann cells.
Combining the contributions from different cell types with the diversity in proposed toxic mechanisms (Fig. 1), we propose an explanation for preferential toxicity to motor neurons from ubiquitously expressed mutant SOD1. All of the proposed mechanisms are probably contributors to pathogenesis, but they generate their damage within different cell types. Initiating damage takes place within the motor neurons most susceptible to ALS: here it is hard to imagine that misfolded mutant SOD1 aberrantly aggregated onto mitochondria does not damage their function and/or distribution within the motor neuron and its highly extended axon. Damage to one or more cell types of the blood–brain barrier leads to very early microhemorrhaging within the spinal cord, with release of neurotoxic hemoglobin products, that tips the balance of already damaged motor neurons, thereby driving disease initiation.
ER stress is generated in motor neurons and probably other cell types by misfolded mutant SOD1 bound to either Derlin-1 or BiP. This may affect many proteins that mature in the ER, including those contributing to synaptic vesicles. Not yet established is whether ER stress is also generated by mutant SOD1 within astrocytes, but fully consistent with this is the loss of the mature EAAT2 glutamate transporter, whose reduction is sure to enhance calcium-dependent excitotoxicity within the juxtaposed motor neuron. Damage to both intracellular calcium stores, the ER and the mitochondria, would then accelerate damage within the motor neurons, fueling a feed-forward mechanism through which mutant astrocytes promote disease progression. Misfolded mutant SOD1 within microglia—which become activated and migrate to initial sites of cell injury—triggers unregulated and high production of extracellular superoxide, thereby enflaming the initial injury.
How does this help with understanding the basis of selectivity in neuronal loss? Recognizing the contributions of multiple mechanisms and direct involvement of multiple cell types in disease initiation and progression, the selective vulnerability of motor neurons to toxicity from a ubiquitously expressed mutant can be explained by the accidental convergence of the motor neuron's own inherent functional properties and the combination of mutant damage developed within it and its multiple cell partners.
ALS as the tip of the iceberg: non-cell autonomy in neurodegenerative disease
Although our understanding of non-cell autonomy is most advanced for cases of SOD1-mediated familial ALS, it is likely that this is going to be a unifying theme in many other neurodegenerative diseases as almost all of the causative proteins are widely or ubiquitously expressed. For ALS, within the last 18 months, dominant mutations in two other widely expressed genes, TDP-43 (Sreedharan et al., 2008; Van Deerlin et al., 2008) and FUS/TLS (Kwiatkowski et al., 2009; Vance et al., 2009), have been reported. Both of these new ALS-causing genes encode proteins intimately associated with RNA processing and are at least partially depleted from nuclei in mutant-expressing cells. It remains to be determined if the underlying disease mechanism is from loss of nuclear function, gain of one or more toxic properties, or both. Moreover, TDP-43 misaccumulation in motor neurons and astrocytes (Neumann et al., 2006; Cairns et al., 2007) is seen in most instances of sporadic or familial ALS, so noncell-autonomous disease seems likely to be a universal feature of ALS. The first report of a prion promoter-driven mutant TDP-43 mouse line has just been published (Wegorzewska et al., 2009) and will assuredly be followed by further work assessing if the neurological phenotype described is mutant specific.
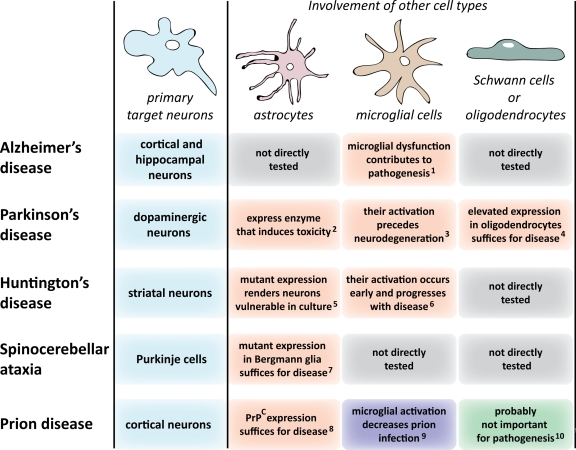
Parkinson's disease and multiple system atrophy.
A hallmark of the second most common age-related neurodegenerative disease—Parkinson's disease (PD)—is the loss of dopaminergic neurons in a brain region called the substantia nigra (for review see Dauer and Przedborski, 2003). Intraneuronal accumulations of α-synuclein, a highly abundant presynaptic protein, are a defining pathology of sporadic and many inherited instances of PD. Although most incidences of disease are sporadic, multiple genetic causes are known (for review see Hardy et al., 2009), including dominant mutations in α-synuclein or increased synthesis of normal α-synuclein. Although increased intraneuronal synthesis of α-synuclein can damage those neurons (Masliah et al., 2000), elevated expression of α-synuclein selectively within the axon-ensheathing oligodendrocytes can also induce neurodegeneration of the associated neurons (Yazawa et al., 2005), firmly suggesting a non–cell autonomous component to pathogenesis. Indeed, in multiple system atrophy (MSA), whose clinical presentation includes Parkinsonism, ataxia, and autonomic failure, α-synuclein–containing inclusions are actually more prominent in oligodendrocytes (for review see Dauer and Przedborski, 2003).
Additional evidence for a non–cell autonomous mechanism has come from chemically induced PD. MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) can induce a Parkinsonian syndrome in humans and rodents almost indistinguishable from PD (Dauer and Przedborski, 2003). The conversion of MPTP to the damaging MPP+ depends on monoamine oxidase B, an enzyme expressed predominantly by astrocytes (Nakamura et al., 1990; Ekblom et al., 1993) and serotoninergic neurons, but not by the affected dopaminergic neurons (Kitahama et al., 1991; Luque et al., 1995; Jahng et al., 1997). Myeloperoxidase, an oxidant-producing enzyme, is expressed by the neighboring astrocytes in brains of both PD patients and MPTP-induced animal models, and its genetic knockdown increases resistance to MPTP toxicity (Choi et al., 2005). Microglial activation precedes neurodegeneration (at least in animal studies) and genetic reduction of the inducible nitric oxide synthase expressed by microglial cells or inhibition of microglial activation (by the antibiotic minocycline) ameliorates dopaminergic neurodegeneration (Liberatore et al., 1999; Wu et al., 2002). T lymphocytes were shown to have invaded the brain in both postmortem human PD specimens and in the MPTP-treated mice during the course of neurodegeneration (Brochard et al., 2009). Moreover, MPTP-induced dopaminergic cell death was markedly attenuated in the absence of mature T lymphocytes (Brochard et al., 2009). Thus, in this chemically induced PD model, converging damage within astrocytes, microglia, and invading T cells, as well as the target neurons, creates an oxidative milieu detrimental to the neurons and which is probably further exacerbated by neuronally restricted oxidative pathways (Teismann et al., 2003; Hunot et al., 2004).
Huntington's disease.
Huntington's disease (HD) is a dominant, fatal, progressive disease characterized by prominent, age-dependent degeneration and death of striatal medium spiny neurons. The lesion that underlies nearly all instances of HD is CAG expansion within the widely expressed huntingtin gene. Many more cells beyond the striatum are affected, especially cortical pyramidal neurons, which send their axons to synapse on striatal neurons. Indeed, one third of total brain mass is lost by end-stage disease (Zoghbi and Orr, 2000).
Like the preceding diseases covered in this review, HD disease mechanism is non-cell autonomous and based upon pathological cell–cell interactions. Progressive motor deficits and striato-cortical neuropathology in mice have been observed when mutant huntingtin expression was activated (with nervous system–specific nestin-Cre) (Gu et al., 2007) in multiple neuronal and glial cell types, including striatal medium spiny neurons, cortical interneurons, and cortical pyramidal neurons. Conversely, when Cre synthesis was restricted to cortical pyramidal neurons (Gu et al., 2005) or striatal medium spiny neurons (Gu et al., 2007), no motor deficits or cortical neuropathology were observed despite mutant huntingtin aggregation.
Substantial evidence also has revealed that mutant huntingtin damage within microglia and astrocytes are likely pathogenic contributors, including progressive reactive microgliosis (Sapp et al., 2001). The inflammatory modulator minocycline delays disease in mice generated by widespread expression of mutant huntingtin exon 1 (R6/2), accompanied by decreased accumulation of microglial-derived iNOS activity (Chen et al., 2000). Mutant huntingtin accumulates in astroglial nuclei of diseased brains, accompanied by decreased levels of the EAAT2 glutamate transporter and transporter activity in HD mouse models (Shin et al., 2005), and mutant astrocytes increase neuronal vulnerability to excitotoxicity in cell culture (Shin et al., 2005).
Spinocerebellar ataxias.
Spinocerebellar ataxias (SCAs), characterized by cerebellar degeneration, lead to progressive motor incoordination (for review see Taroni and DiDonato, 2004). The most affected cells are the large, cerebellar Purkinje neurons. Intimate nonneuronal neighbors to these neurons are the Bergmann glia, the cerebellum's specialized astrocytes that use long finger-like processes to enwrap the huge dendritic trees of Purkinje cells. Mutations in at least 25 genes cause ataxias, 6 of which (SCA1, 2, 3, 6, 7, and 17) represent dominant, polyglutamine repeat expansions (polyQ). Each of these mutant gene products is widely expressed. The Purkinje neurons are killed even when they do not make the mutant ataxin (Garden et al., 2002) or, even more provocatively, when mutant ataxin is expressed only within the Bergmann glia (Custer et al., 2006), demonstrating a non–cell autonomous disease mechanism.
Prion diseases.
Transmissible spongiform encephalopathies, including the bovine spongiform encephalopathy (BSE; i.e., mad cow disease), originate not just sporadically or through inherited mutations but notably also by an infectious agent, the prion (for review see Aguzzi and Calella, 2009). Prions consist of an aggregated form of a ubiquitously expressed cellular prion protein (PrPC) (Prusiner, 1982; Basler et al., 1986). PrPC expression is necessary both for toxicity and prion replication because mice devoid of PrPC (Prnpo/o) are resistant to infection and disease (Büeler et al., 1993). A cell's susceptibility to prion toxicity depends on the localization of PrPC on the plasma membrane (Chesebro et al., 2005). Although clinical symptoms of prion diseases stem from neuronal injury, neighboring glial cells seem to play a decisive role in prion pathogenesis. Non-cell autonomy in disease mechanism has been shown in at least two contexts. First, for infection, as in the recent outbreak of bovine spongiform encephalopathy in Britain or the current epidemic of prion-mediated wasting disease in American deer (Tamgüney et al., 2009), an initial noncell-autonomous contribution is after ingestion of prions. The route of prion movement from the digestive system to the brain, a process called neuroinvasion, has been shown to generally use prion amplification in the spleen and lymph nodes in order to deliver an effective dose to the nervous system (for review see Nuvolone et al., 2009). Second, astrocytes show morphological abnormalities early in disease (Eklund et al., 1967), and in some cases they are the first places of prion aggregation (Diedrich et al., 1991). Neuronal synthesis of PrPC can be critical, as demonstrated by reversal of prion disease through excision of neuronal PrPC in mice with established prion infection (Mallucci et al., 2003). Nevertheless, mice expressing PrPC at higher than normal levels but exclusively in astrocytes develop prion disease despite lack of neuronal PrPC (Raeber et al., 1997). In this paradigm, accumulated prion aggregates lead to reactive changes in astrocytes without damaging them, but trigger toxic events in nearby PrPC-negative neurons (Jeffrey et al., 2004). Microglia cells may play the opposite role in prion diseases, possibly containing prion infections, because microglial ablation markedly increased prion titers in a slice culture system (Falsig et al., 2008).
Alzheimer's disease and tauopathies.
For Alzheimer's disease (AD), the high frequency of instances of this well-known progressive dementia makes it the disease with the highest burden on society. Genetics has uncovered gene products central to pathogenesis, including α-secretase, β-secretase, γ-secretase, and amyloid precursor protein (APP). All are widely or ubiquitously expressed. An aberrant processing product of APP (Aβ) is thought by most investigators to be the pathogenic species. There are clear hints that pathogenesis may be noncell autonomous. Mutations in presenilin 1 (PS1), the catalytic part of the γ-secretase complex, cause familial AD. Co-culture of wild-type neural progenitor cells with microglia expressing PS1 mutations or with conditioned media from those microglia—but not normal microglia—impairs proliferation of the neural cells (Choi et al., 2008). Although neuroinflammation is increasingly recognized as a pathological component of AD, provocative recent evidence has supported microglial dysfunction—rather than activation—as a probable, primary event in sporadic AD (Streit et al., 2009). In this comprehensive histopathological study, analysis of 19 human AD specimens from patients with a broad range of AD pathology showed that degenerating neuronal structures positive for tau are invariably colocalized with severely dystrophic, fragmented microglial cells. A microglial contribution to pathogenesis is also supported by recent genome-wide association studies in which two of three genes linked to AD (clusterin [also known as ApoJ] and complement component [3b/4b] receptor 1) are involved in neuroinflammatory responses of microglia and astrocytes (Harold et al., 2009; Lambert et al., 2009).
Finally, the group of disorders that share misaccumulation of tau—the axonal microtubule-associated protein—are collectively referred to as tauopathies. Included here are a proportion of frontal temporal dementia caused by mutation in tau, progressive supranuclear palsy, and corticobasal degeneration. Intracellular tau accumulations are not restricted to the vulnerable neurons, but are found also in astrocytes and oligodendrocytes (Forman et al., 2005), suggestive of damage to multiple cell types as contributors to pathogenesis.
Selective neuronal vulnerability in neurodegenerative disease: the neighborhood matters
Disease mechanism in each of the major neurodegenerative diseases we have discussed is almost assuredly non-cell autonomous. We propose that neuronal selectivity in each disorder can be most persuasively explained not by unique vulnerability to damage developed solely within the neurons whose loss is characteristic of each disorder, but by the coincidental convergence of multiple disease-causing mutant gene products provoking damage within the vulnerable neuron and multiple neighboring cell types (Fig. 3). This is a very positive insight for the prospects of effective therapies, as they could be envisioned by targeting any of the mechanisms and cell types involved.
Figure 3.
Non–cell autonomous pathogenesis in neurodegenerative diseases. This figure summarizes current evidence suggesting the contribution of apparently unaffected cell types in pathogenic mechanisms of neurodegenerative diseases, other than ALS. 1(Choi et al., 2008; Streit et al., 2009), 2(Nakamura et al., 1990; Ekblom et al., 1993), 3(Liberatore et al., 1999), 4(Yazawa et al., 2005), 5(Shin et al., 2005), 6(Sapp et al., 2001), 7(Custer et al., 2006), 8(Raeber et al., 1997; Jeffrey et al., 2004), 9(Falsig et al., 2008), 10(Prinz et al., 2004).
Acknowledgments
Thanks to the Cleveland lab, especially to Sandrine Da Cruz and Philippe Parone for comments on the manuscript.
H. Ilieva receives salary support from a Career Development Award from the Muscular Dystrophy Association. M. Polymenidou is the recipient of a Human Frontier Science Program Long Term Fellowship. D.W. Cleveland receives salary support from the Ludwig Institute for Cancer Research.
Footnotes
Abbreviations used in this paper:
- AD
- Alzheimer's disease
- ALS
- amyotrophic lateral sclerosis
- HD
- Huntington's disease
- PD
- Parkinson's disease
- SCA
- spinal cerebellar ataxia
- SOD1
- superoxide dismutase
References
- Aguzzi A., Calella A.M. 2009. Prions: protein aggregation and infectious diseases. Physiol. Rev. 89:1105–1152 10.1152/physrev.00006.2009 [DOI] [PubMed] [Google Scholar]
- Ajami B., Bennett J.L., Krieger C., Tetzlaff W., Rossi F.M. 2007. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat. Neurosci. 10:1538–1543 10.1038/nn2014 [DOI] [PubMed] [Google Scholar]
- Basler K., Oesch B., Scott M., Westaway D., Wälchli M., Groth D.F., McKinley M.P., Prusiner S.B., Weissmann C. 1986. Scrapie and cellular PrP isoforms are encoded by the same chromosomal gene. Cell. 46:417–428 10.1016/0092-8674(86)90662-8 [DOI] [PubMed] [Google Scholar]
- Beers D.R., Henkel J.S., Xiao Q., Zhao W., Wang J., Yen A.A., Siklos L., McKercher S.R., Appel S.H. 2006. Wild-type microglia extend survival in PU.1 knockout mice with familial amyotrophic lateral sclerosis. Proc. Natl. Acad. Sci. USA. 103:16021–16026 10.1073/pnas.0607423103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beers D.R., Henkel J.S., Zhao W., Wang J., Appel S.H. 2008. CD4+ T cells support glial neuroprotection, slow disease progression, and modify glial morphology in an animal model of inherited ALS. Proc. Natl. Acad. Sci. USA. 105:15558–15563 10.1073/pnas.0807419105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boillée S., Yamanaka K., Lobsiger C.S., Copeland N.G., Jenkins N.A., Kassiotis G., Kollias G., Cleveland D.W. 2006. Onset and progression in inherited ALS determined by motor neurons and microglia. Science. 312:1389–1392 10.1126/science.1123511 [DOI] [PubMed] [Google Scholar]
- Boston-Howes W., Gibb S.L., Williams E.O., Pasinelli P., Brown R.H., Jr., Trotti D. 2006. Caspase-3 cleaves and inactivates the glutamate transporter EAAT2. J. Biol. Chem. 281:14076–14084 10.1074/jbc.M600653200 [DOI] [PubMed] [Google Scholar]
- Brochard V., Combadière B., Prigent A., Laouar Y., Perrin A., Beray-Berthat V., Bonduelle O., Alvarez-Fischer D., Callebert J., Launay J.M., et al. 2009. Infiltration of CD4+ lymphocytes into the brain contributes to neurodegeneration in a mouse model of Parkinson disease. J. Clin. Invest. 119:182–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne S.E., Yang L., DiMauro J.P., Fuller S.W., Licata S.C., Beal M.F. 2006. Bioenergetic abnormalities in discrete cerebral motor pathways presage spinal cord pathology in the G93A SOD1 mouse model of ALS. Neurobiol. Dis. 22:599–610 10.1016/j.nbd.2006.01.001 [DOI] [PubMed] [Google Scholar]
- Bruijn L.I., Becher M.W., Lee M.K., Anderson K.L., Jenkins N.A., Copeland N.G., Sisodia S.S., Rothstein J.D., Borchelt D.R., Price D.L., Cleveland D.W. 1997. ALS-linked SOD1 mutant G85R mediates damage to astrocytes and promotes rapidly progressive disease with SOD1-containing inclusions. Neuron. 18:327–338 10.1016/S0896-6273(00)80272-X [DOI] [PubMed] [Google Scholar]
- Bruijn L.I., Houseweart M.K., Kato S., Anderson K.L., Anderson S.D., Ohama E., Reaume A.G., Scott R.W., Cleveland D.W. 1998. Aggregation and motor neuron toxicity of an ALS-linked SOD1 mutant independent from wild-type SOD1. Science. 281:1851–1854 10.1126/science.281.5384.1851 [DOI] [PubMed] [Google Scholar]
- Büeler H., Aguzzi A., Sailer A., Greiner R.A., Autenried P., Aguet M., Weissmann C. 1993. Mice devoid of PrP are resistant to scrapie. Cell. 73:1339–1347 10.1016/0092-8674(93)90360-3 [DOI] [PubMed] [Google Scholar]
- Cairns N.J., Neumann M., Bigio E.H., Holm I.E., Troost D., Hatanpaa K.J., Foong C., White C.L., III, Schneider J.A., Kretzschmar H.A., et al. 2007. TDP-43 in familial and sporadic frontotemporal lobar degeneration with ubiquitin inclusions. Am. J. Pathol. 171:227–240 10.2353/ajpath.2007.070182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassina P., Cassina A., Pehar M., Castellanos R., Gandelman M., de León A., Robinson K.M., Mason R.P., Beckman J.S., Barbeito L., Radi R. 2008. Mitochondrial dysfunction in SOD1G93A-bearing astrocytes promotes motor neuron degeneration: prevention by mitochondrial-targeted antioxidants. J. Neurosci. 28:4115–4122 10.1523/JNEUROSCI.5308-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M., Ona V.O., Li M., Ferrante R.J., Fink K.B., Zhu S., Bian J., Guo L., Farrell L.A., Hersch S.M., et al. 2000. Minocycline inhibits caspase-1 and caspase-3 expression and delays mortality in a transgenic mouse model of Huntington disease. Nat. Med. 6:797–801 10.1038/80538 [DOI] [PubMed] [Google Scholar]
- Chen X.J., Levedakou E.N., Millen K.J., Wollmann R.L., Soliven B., Popko B. 2007. Proprioceptive sensory neuropathy in mice with a mutation in the cytoplasmic Dynein heavy chain 1 gene. J. Neurosci. 27:14515–14524 10.1523/JNEUROSCI.4338-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheroni C., Marino M., Tortarolo M., Veglianese P., De Biasi S., Fontana E., Zuccarello L.V., Maynard C.J., Dantuma N.P., Bendotti C. 2009. Functional alterations of the ubiquitin-proteasome system in motor neurons of a mouse model of familial amyotrophic lateral sclerosis. Hum. Mol. Genet. 18:82–96 10.1093/hmg/ddn319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesebro B., Trifilo M., Race R., Meade-White K., Teng C., LaCasse R., Raymond L., Favara C., Baron G., Priola S., et al. 2005. Anchorless prion protein results in infectious amyloid disease without clinical scrapie. Science. 308:1435–1439 10.1126/science.1110837 [DOI] [PubMed] [Google Scholar]
- Chiu I.M., Chen A., Zheng Y., Kosaras B., Tsiftsoglou S.A., Vartanian T.K., Brown R.H., Jr., Carroll M.C. 2008. T lymphocytes potentiate endogenous neuroprotective inflammation in a mouse model of ALS. Proc. Natl. Acad. Sci. USA. 105:17913–17918 10.1073/pnas.0804610105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi D.K., Pennathur S., Perier C., Tieu K., Teismann P., Wu D.C., Jackson-Lewis V., Vila M., Vonsattel J.P., Heinecke J.W., Przedborski S. 2005. Ablation of the inflammatory enzyme myeloperoxidase mitigates features of Parkinson's disease in mice. J. Neurosci. 25:6594–6600 10.1523/JNEUROSCI.0970-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S.H., Veeraraghavalu K., Lazarov O., Marler S., Ransohoff R.M., Ramirez J.M., Sisodia S.S. 2008. Non-cell-autonomous effects of presenilin 1 variants on enrichment-mediated hippocampal progenitor cell proliferation and differentiation. Neuron. 59:568–580 10.1016/j.neuron.2008.07.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement A.M., Nguyen M.D., Roberts E.A., Garcia M.L., Boillée S., Rule M., McMahon A.P., Doucette W., Siwek D., Ferrante R.J., et al. 2003. Wild-type nonneuronal cells extend survival of SOD1 mutant motor neurons in ALS mice. Science. 302:113–117 10.1126/science.1086071 [DOI] [PubMed] [Google Scholar]
- Custer S.K., Garden G.A., Gill N., Rueb U., Libby R.T., Schultz C., Guyenet S.J., Deller T., Westrum L.E., Sopher B.L., La Spada A.R. 2006. Bergmann glia expression of polyglutamine-expanded ataxin-7 produces neurodegeneration by impairing glutamate transport. Nat. Neurosci. 9:1302–1311 10.1038/nn1750 [DOI] [PubMed] [Google Scholar]
- Damiano M., Starkov A.A., Petri S., Kipiani K., Kiaei M., Mattiazzi M., Flint Beal M., Manfredi G. 2006. Neural mitochondrial Ca2+ capacity impairment precedes the onset of motor symptoms in G93A Cu/Zn-superoxide dismutase mutant mice. J. Neurochem. 96:1349–1361 10.1111/j.1471-4159.2006.03619.x [DOI] [PubMed] [Google Scholar]
- Dauer W., Przedborski S. 2003. Parkinson's disease: mechanisms and models. Neuron. 39:889–909 10.1016/S0896-6273(03)00568-3 [DOI] [PubMed] [Google Scholar]
- Diedrich J.F., Bendheim P.E., Kim Y.S., Carp R.I., Haase A.T. 1991. Scrapie-associated prion protein accumulates in astrocytes during scrapie infection. Proc. Natl. Acad. Sci. USA. 88:375–379 10.1073/pnas.88.2.375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrowolny G., Aucello M., Rizzuto E., Beccafico S., Mammucari C., Boncompagni S., Bonconpagni S., Belia S., Wannenes F., Nicoletti C., et al. 2008. Skeletal muscle is a primary target of SOD1G93A-mediated toxicity. Cell Metab. 8:425–436 10.1016/j.cmet.2008.09.002 [DOI] [PubMed] [Google Scholar]
- Ekblom J., Jossan S.S., Bergström M., Oreland L., Walum E., Aquilonius S.M. 1993. Monoamine oxidase-B in astrocytes. Glia. 8:122–132 10.1002/glia.440080208 [DOI] [PubMed] [Google Scholar]
- Eklund C.M., Kennedy R.C., Hadlow W.J. 1967. Pathogenesis of scrapie virus infection in the mouse. J. Infect. Dis. 117:15–22 [DOI] [PubMed] [Google Scholar]
- Falsig J., Julius C., Margalith I., Schwarz P., Heppner F.L., Aguzzi A. 2008. A versatile prion replication assay in organotypic brain slices. Nat. Neurosci. 11:109–117 10.1038/nn2028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman M.S., Lal D., Zhang B., Dabir D.V., Swanson E., Lee V.M., Trojanowski J.Q. 2005. Transgenic mouse model of tau pathology in astrocytes leading to nervous system degeneration. J. Neurosci. 25:3539–3550 10.1523/JNEUROSCI.0081-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garden G.A., Libby R.T., Fu Y.H., Kinoshita Y., Huang J., Possin D.E., Smith A.C., Martinez R.A., Fine G.C., Grote S.K., et al. 2002. Polyglutamine-expanded ataxin-7 promotes non-cell-autonomous purkinje cell degeneration and displays proteolytic cleavage in ataxic transgenic mice. J. Neurosci. 22:4897–4905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Y.H., Parsadanian A.S., Andreeva A., Snider W.D., Elliott J.L. 2000. Restricted expression of G86R Cu/Zn superoxide dismutase in astrocytes results in astrocytosis but does not cause motoneuron degeneration. J. Neurosci. 20:660–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowing G., Philips T., Van Wijmeersch B., Audet J.N., Dewil M., Van Den Bosch L., Billiau A.D., Robberecht W., Julien J.P. 2008. Ablation of proliferating microglia does not affect motor neuron degeneration in amyotrophic lateral sclerosis caused by mutant superoxide dismutase. J. Neurosci. 28:10234–10244 10.1523/JNEUROSCI.3494-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X., Li C., Wei W., Lo V., Gong S., Li S.H., Iwasato T., Itohara S., Li X.J., Mody I., et al. 2005. Pathological cell-cell interactions elicited by a neuropathogenic form of mutant Huntingtin contribute to cortical pathogenesis in HD mice. Neuron. 46:433–444 10.1016/j.neuron.2005.03.025 [DOI] [PubMed] [Google Scholar]
- Gu X., André V.M., Cepeda C., Li S.H., Li X.J., Levine M.S., Yang X.W. 2007. Pathological cell-cell interactions are necessary for striatal pathogenesis in a conditional mouse model of Huntington's disease. Mol. Neurodegener. 2:8 10.1186/1750-1326-2-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy J., Lewis P., Revesz T., Lees A., Paisan-Ruiz C. 2009. The genetics of Parkinson's syndromes: a critical review. Curr. Opin. Genet. Dev. 19:254–265 10.1016/j.gde.2009.03.008 [DOI] [PubMed] [Google Scholar]
- Harold D., Abraham R., Hollingworth P., Sims R., Gerrish A., Hamshere M.L., Pahwa J.S., Moskvina V., Dowzell K., Williams A., et al. 2009. Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer's disease. Nat. Genet. 41:1088–1093 10.1038/ng.440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harraz M.M., Marden J.J., Zhou W., Zhang Y., Williams A., Sharov V.S., Nelson K., Luo M., Paulson H., Schöneich C., Engelhardt J.F. 2008. SOD1 mutations disrupt redox-sensitive Rac regulation of NADPH oxidase in a familial ALS model. J. Clin. Invest. 118:659–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkel J.S., Beers D.R., Wen S., Bowser R., Appel S.H. 2009. Decreased mRNA expression of tight junction proteins in lumbar spinal cords of patients with ALS. Neurology. 72:1614–1616 10.1212/WNL.0b013e3181a41228 [DOI] [PubMed] [Google Scholar]
- Hoffman E.K., Wilcox H.M., Scott R.W., Siman R. 1996. Proteasome inhibition enhances the stability of mouse Cu/Zn superoxide dismutase with mutations linked to familial amyotrophic lateral sclerosis. J. Neurol. Sci. 139:15–20 10.1016/S0022-510X(96)00031-7 [DOI] [PubMed] [Google Scholar]
- Holzbaur E.L., Howland D.S., Weber N., Wallace K., She Y., Kwak S., Tchistiakova L.A., Murphy E., Hinson J., Karim R., et al. 2006. Myostatin inhibition slows muscle atrophy in rodent models of amyotrophic lateral sclerosis. Neurobiol. Dis. 23:697–707 10.1016/j.nbd.2006.05.009 [DOI] [PubMed] [Google Scholar]
- Howland D.S., Liu J., She Y., Goad B., Maragakis N.J., Kim B., Erickson J., Kulik J., DeVito L., Psaltis G., et al. 2002. Focal loss of the glutamate transporter EAAT2 in a transgenic rat model of SOD1 mutant-mediated amyotrophic lateral sclerosis (ALS). Proc. Natl. Acad. Sci. USA. 99:1604–1609 10.1073/pnas.032539299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunot S., Vila M., Teismann P., Davis R.J., Hirsch E.C., Przedborski S., Rakic P., Flavell R.A. 2004. JNK-mediated induction of cyclooxygenase 2 is required for neurodegeneration in a mouse model of Parkinson's disease. Proc. Natl. Acad. Sci. USA. 101:665–670 10.1073/pnas.0307453101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ii K., Ito H., Tanaka K., Hirano A. 1997. Immunocytochemical co-localization of the proteasome in ubiquitinated structures in neurodegenerative diseases and the elderly. J. Neuropathol. Exp. Neurol. 56:125–131 10.1097/00005072-199702000-00002 [DOI] [PubMed] [Google Scholar]
- Ilieva H.S., Yamanaka K., Malkmus S., Kakinohana O., Yaksh T., Marsala M., Cleveland D.W. 2008. Mutant dynein (Loa) triggers proprioceptive axon loss that extends survival only in the SOD1 ALS model with highest motor neuron death. Proc. Natl. Acad. Sci. USA. 105:12599–12604 10.1073/pnas.0805422105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaarsma D., Teuling E., Haasdijk E.D., De Zeeuw C.I., Hoogenraad C.C. 2008. Neuron-specific expression of mutant superoxide dismutase is sufficient to induce amyotrophic lateral sclerosis in transgenic mice. J. Neurosci. 28:2075–2088 10.1523/JNEUROSCI.5258-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahng J.W., Houpt T.A., Wessel T.C., Chen K., Shih J.C., Joh T.H. 1997. Localization of monoamine oxidase A and B mRNA in the rat brain by in situ hybridization. Synapse. 25:30–36 [DOI] [PubMed] [Google Scholar]
- Jeffrey M., Goodsir C.M., Race R.E., Chesebro B. 2004. Scrapie-specific neuronal lesions are independent of neuronal PrP expression. Ann. Neurol. 55:781–792 10.1002/ana.20093 [DOI] [PubMed] [Google Scholar]
- Kabashi E., Agar J.N., Taylor D.M., Minotti S., Durham H.D. 2004. Focal dysfunction of the proteasome: a pathogenic factor in a mouse model of amyotrophic lateral sclerosis. J. Neurochem. 89:1325–1335 10.1111/j.1471-4159.2004.02453.x [DOI] [PubMed] [Google Scholar]
- Kang J., Rivest S. 2007. MyD88-deficient bone marrow cells accelerate onset and reduce survival in a mouse model of amyotrophic lateral sclerosis. J. Cell Biol. 179:1219–1230 10.1083/jcb.200705046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi H., Almer G., Yamashita S., Guégan C., Nagai M., Xu Z., Sosunov A.A., McKhann G.M., II, Przedborski S. 2006. Spinal cord endoplasmic reticulum stress associated with a microsomal accumulation of mutant superoxide dismutase-1 in an ALS model. Proc. Natl. Acad. Sci. USA. 103:6025–6030 10.1073/pnas.0509227103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitahama K., Denney R.M., Maeda T., Jouvet M. 1991. Distribution of type B monoamine oxidase immunoreactivity in the cat brain with reference to enzyme histochemistry. Neuroscience. 44:185–204 10.1016/0306-4522(91)90260-U [DOI] [PubMed] [Google Scholar]
- Kwiatkowski T.J., Jr., Bosco D.A., Leclerc A.L., Tamrazian E., Vanderburg C.R., Russ C., Davis A., Gilchrist J., Kasarskis E.J., Munsat T., et al. 2009. Mutations in the FUS/TLS gene on chromosome 16 cause familial amyotrophic lateral sclerosis. Science. 323:1205–1208 10.1126/science.1166066 [DOI] [PubMed] [Google Scholar]
- Lambert J.C., Heath S., Even G., Campion D., Sleegers K., Hiltunen M., Combarros O., Zelenika D., Bullido M.J., Tavernier B., et al. ; European Alzheimer's Disease Initiative Investigators 2009. Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer's disease. Nat. Genet. 41:1094–1099 10.1038/ng.439 [DOI] [PubMed] [Google Scholar]
- Lepore A.C., Rauck B., Dejea C., Pardo A.C., Rao M.S., Rothstein J.D., Maragakis N.J. 2008. Focal transplantation-based astrocyte replacement is neuroprotective in a model of motor neuron disease. Nat. Neurosci. 11:1294–1301 10.1038/nn.2210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberatore G.T., Jackson-Lewis V., Vukosavic S., Mandir A.S., Vila M., McAuliffe W.G., Dawson V.L., Dawson T.M., Przedborski S. 1999. Inducible nitric oxide synthase stimulates dopaminergic neurodegeneration in the MPTP model of Parkinson disease. Nat. Med. 5:1403–1409 10.1038/70978 [DOI] [PubMed] [Google Scholar]
- Lino M.M., Schneider C., Caroni P. 2002. Accumulation of SOD1 mutants in postnatal motoneurons does not cause motoneuron pathology or motoneuron disease. J. Neurosci. 22:4825–4832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Lillo C., Jonsson P.A., Vande Velde C., Ward C.M., Miller T.M., Subramaniam J.R., Rothstein J.D., Marklund S., Andersen P.M., et al. 2004. Toxicity of familial ALS-linked SOD1 mutants from selective recruitment to spinal mitochondria. Neuron. 43:5–17 10.1016/j.neuron.2004.06.016 [DOI] [PubMed] [Google Scholar]
- Lobsiger C.S., Boillee S., McAlonis-Downes M., Khan A.M., Feltri M.L., Yamanaka K., Cleveland D.W. 2009. Schwann cells expressing dismutase active mutant SOD1 unexpectedly slow disease progression in ALS mice. Proc. Natl. Acad. Sci. USA. 106:4465–4470 10.1073/pnas.0813339106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luque J.M., Kwan S.W., Abell C.W., Da Prada M., Richards J.G. 1995. Cellular expression of mRNAs encoding monoamine oxidases A and B in the rat central nervous system. J. Comp. Neurol. 363:665–680 10.1002/cne.903630410 [DOI] [PubMed] [Google Scholar]
- Mallucci G., Dickinson A., Linehan J., Klöhn P.C., Brandner S., Collinge J. 2003. Depleting neuronal PrP in prion infection prevents disease and reverses spongiosis. Science. 302:871–874 [DOI] [PubMed] [Google Scholar]
- Marszalek J.R., Williamson T.L., Lee M.K., Xu Z., Hoffman P.N., Becher M.W., Crawford T.O., Cleveland D.W. 1996. Neurofilament subunit NF-H modulates axonal diameter by selectively slowing neurofilament transport. J. Cell Biol. 135:711–724 10.1083/jcb.135.3.711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masliah E., Rockenstein E., Veinbergs I., Mallory M., Hashimoto M., Takeda A., Sagara Y., Sisk A., Mucke L. 2000. Dopaminergic loss and inclusion body formation in alpha-synuclein mice: implications for neurodegenerative disorders. Science. 287:1265–1269 10.1126/science.287.5456.1265 [DOI] [PubMed] [Google Scholar]
- Mattiazzi M., D'Aurelio M., Gajewski C.D., Martushova K., Kiaei M., Beal M.F., Manfredi G. 2002. Mutated human SOD1 causes dysfunction of oxidative phosphorylation in mitochondria of transgenic mice. J. Biol. Chem. 277:29626–29633 10.1074/jbc.M203065200 [DOI] [PubMed] [Google Scholar]
- Mildner A., Schmidt H., Nitsche M., Merkler D., Hanisch U.K., Mack M., Heikenwalder M., Brück W., Priller J., Prinz M. 2007. Microglia in the adult brain arise from Ly-6ChiCCR2+ monocytes only under defined host conditions. Nat. Neurosci. 10:1544–1553 10.1038/nn2015 [DOI] [PubMed] [Google Scholar]
- Miller T.M., Kim S.H., Yamanaka K., Hester M., Umapathi P., Arnson H., Rizo L., Mendell J.R., Gage F.H., Cleveland D.W., Kaspar B.K. 2006. Gene transfer demonstrates that muscle is not a primary target for non-cell-autonomous toxicity in familial amyotrophic lateral sclerosis. Proc. Natl. Acad. Sci. USA. 103:19546–19551 10.1073/pnas.0609411103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami T., Nagano I., Hayashi T., Manabe Y., Shoji M., Setoguchi Y., Abe K. 2001. Impaired retrograde axonal transport of adenovirus-mediated E. coli LacZ gene in the mice carrying mutant SOD1 gene. Neurosci. Lett. 308:149–152 10.1016/S0304-3940(01)02036-5 [DOI] [PubMed] [Google Scholar]
- Nakamura S., Kawamata T., Akiguchi I., Kameyama M., Nakamura N., Kimura H. 1990. Expression of monoamine oxidase B activity in astrocytes of senile plaques. Acta Neuropathol. 80:419–425 10.1007/BF00307697 [DOI] [PubMed] [Google Scholar]
- Neumann M., Sampathu D.M., Kwong L.K., Truax A.C., Micsenyi M.C., Chou T.T., Bruce J., Schuck T., Grossman M., Clark C.M., et al. 2006. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 314:130–133 10.1126/science.1134108 [DOI] [PubMed] [Google Scholar]
- Nishitoh H., Kadowaki H., Nagai A., Maruyama T., Yokota T., Fukutomi H., Noguchi T., Matsuzawa A., Takeda K., Ichijo H. 2008. ALS-linked mutant SOD1 induces ER stress- and ASK1-dependent motor neuron death by targeting Derlin-1. Genes Dev. 22:1451–1464 10.1101/gad.1640108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa J., Ishigaki S., Hishikawa N., Yamamoto M., Doyu M., Murata S., Tanaka K., Taniguchi N., Sobue G. 2002. Dorfin ubiquitylates mutant SOD1 and prevents mutant SOD1-mediated neurotoxicity. J. Biol. Chem. 277:36793–36798 10.1074/jbc.M206559200 [DOI] [PubMed] [Google Scholar]
- Nuvolone M., Aguzzi A., Heikenwalder M. 2009. Cells and prions: a license to replicate. FEBS Lett. 583:2674–2684 10.1016/j.febslet.2009.06.014 [DOI] [PubMed] [Google Scholar]
- Perlson E., Jeong G.B., Ross J.L., Dixit R., Wallace K.E., Kalb R.G., Holzbaur E.L. 2009. A switch in retrograde signaling from survival to stress in rapid-onset neurodegeneration. J. Neurosci. 29:9903–9917 10.1523/JNEUROSCI.0813-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pramatarova A., Laganière J., Roussel J., Brisebois K., Rouleau G.A. 2001. Neuron-specific expression of mutant superoxide dismutase 1 in transgenic mice does not lead to motor impairment. J. Neurosci. 21:3369–3374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinz M., Montrasio F., Furukawa H., van der Haar M.E., Schwarz P., Rülicke T., Giger O.T., Häusler K.G., Perez D., Glatzel M., Aguzzi A. 2004. Intrinsic resistance of oligodendrocytes to prion infection. J. Neurosci. 24:5974–5981 10.1523/JNEUROSCI.0122-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusiner S.B. 1982. Novel proteinaceous infectious particles cause scrapie. Science. 216:136–144 10.1126/science.6801762 [DOI] [PubMed] [Google Scholar]
- Puls I., Jonnakuty C., LaMonte B.H., Holzbaur E.L., Tokito M., Mann E., Floeter M.K., Bidus K., Drayna D., Oh S.J., et al. 2003. Mutant dynactin in motor neuron disease. Nat. Genet. 33:455–456 10.1038/ng1123 [DOI] [PubMed] [Google Scholar]
- Pun S., Santos A.F., Saxena S., Xu L., Caroni P. 2006. Selective vulnerability and pruning of phasic motoneuron axons in motoneuron disease alleviated by CNTF. Nat. Neurosci. 9:408–419 10.1038/nn1653 [DOI] [PubMed] [Google Scholar]
- Raeber A.J., Race R.E., Brandner S., Priola S.A., Sailer A., Bessen R.A., Mucke L., Manson J., Aguzzi A., Oldstone M.B., et al. 1997. Astrocyte-specific expression of hamster prion protein (PrP) renders PrP knockout mice susceptible to hamster scrapie. EMBO J. 16:6057–6065 10.1093/emboj/16.20.6057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph G.S., Radcliffe P.A., Day D.M., Carthy J.M., Leroux M.A., Lee D.C., Wong L.F., Bilsland L.G., Greensmith L., Kingsman S.M., et al. 2005. Silencing mutant SOD1 using RNAi protects against neurodegeneration and extends survival in an ALS model. Nat. Med. 11:429–433 10.1038/nm1205 [DOI] [PubMed] [Google Scholar]
- Rosen D.R., Siddique T., Patterson D., Figlewicz D.A., Sapp P., Hentati A., Donaldson D., Goto J., O'Regan J.P., Deng H.X., et al. 1993. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 362:59–62 10.1038/362059a0 [DOI] [PubMed] [Google Scholar]
- Rothstein J.D., Van Kammen M., Levey A.I., Martin L.J., Kuncl R.W. 1995. Selective loss of glial glutamate transporter GLT-1 in amyotrophic lateral sclerosis. Ann. Neurol. 38:73–84 10.1002/ana.410380114 [DOI] [PubMed] [Google Scholar]
- Sapp E., Kegel K.B., Aronin N., Hashikawa T., Uchiyama Y., Tohyama K., Bhide P.G., Vonsattel J.P., DiFiglia M. 2001. Early and progressive accumulation of reactive microglia in the Huntington disease brain. J. Neuropathol. Exp. Neurol. 60:161–172 [DOI] [PubMed] [Google Scholar]
- Saxena S., Cabuy E., Caroni P. 2009. A role for motoneuron subtype-selective ER stress in disease manifestations of FALS mice. Nat. Neurosci. 12:627–636 10.1038/nn.2297 [DOI] [PubMed] [Google Scholar]
- Shin J.Y., Fang Z.H., Yu Z.X., Wang C.E., Li S.H., Li X.J. 2005. Expression of mutant huntingtin in glial cells contributes to neuronal excitotoxicity. J. Cell Biol. 171:1001–1012 10.1083/jcb.200508072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreedharan J., Blair I.P., Tripathi V.B., Hu X., Vance C., Rogelj B., Ackerley S., Durnall J.C., Williams K.L., Buratti E., et al. 2008. TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science. 319:1668–1672 10.1126/science.1154584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit W.J., Braak H., Xue Q.S., Bechmann I. 2009. Dystrophic (senescent) rather than activated microglial cells are associated with tau pathology and likely precede neurodegeneration in Alzheimer's disease. Acta Neuropathol. 118:475–485 10.1007/s00401-009-0556-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamgüney G., Miller M.W., Wolfe L.L., Sirochman T.M., Glidden D.V., Palmer C., Lemus A., DeArmond S.J., Prusiner S.B. 2009. Asymptomatic deer excrete infectious prions in faeces. Nature. 461:529–532 10.1038/nature08289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taroni F., DiDonato S. 2004. Pathways to motor incoordination: the inherited ataxias. Nat. Rev. Neurosci. 5:641–655 10.1038/nrn1474 [DOI] [PubMed] [Google Scholar]
- Teismann P., Tieu K., Choi D.K., Wu D.C., Naini A., Hunot S., Vila M., Jackson-Lewis V., Przedborski S. 2003. Cyclooxygenase-2 is instrumental in Parkinson's disease neurodegeneration. Proc. Natl. Acad. Sci. USA. 100:5473–5478 10.1073/pnas.0837397100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towne C., Raoul C., Schneider B.L., Aebischer P. 2008. Systemic AAV6 delivery mediating RNA interference against SOD1: neuromuscular transduction does not alter disease progression in fALS mice. Mol. Ther. 16:1018–1025 10.1038/mt.2008.73 [DOI] [PubMed] [Google Scholar]
- Turner B.J., Talbot K. 2008. Transgenics, toxicity and therapeutics in rodent models of mutant SOD1-mediated familial ALS. Prog. Neurobiol. 85:94–134 10.1016/j.pneurobio.2008.01.001 [DOI] [PubMed] [Google Scholar]
- Urushitani M., Kurisu J., Tsukita K., Takahashi R. 2002. Proteasomal inhibition by misfolded mutant superoxide dismutase 1 induces selective motor neuron death in familial amyotrophic lateral sclerosis. J. Neurochem. 83:1030–1042 10.1046/j.1471-4159.2002.01211.x [DOI] [PubMed] [Google Scholar]
- Urushitani M., Sik A., Sakurai T., Nukina N., Takahashi R., Julien J.P. 2006. Chromogranin-mediated secretion of mutant superoxide dismutase proteins linked to amyotrophic lateral sclerosis. Nat. Neurosci. 9:108–118 10.1038/nn1603 [DOI] [PubMed] [Google Scholar]
- Van Damme P., Bogaert E., Dewil M., Hersmus N., Kiraly D., Scheveneels W., Bockx I., Braeken D., Verpoorten N., Verhoeven K., et al. 2007. Astrocytes regulate GluR2 expression in motor neurons and their vulnerability to excitotoxicity. Proc. Natl. Acad. Sci. USA. 104:14825–14830 10.1073/pnas.0705046104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Deerlin V.M., Leverenz J.B., Bekris L.M., Bird T.D., Yuan W., Elman L.B., Clay D., Wood E.M., Chen-Plotkin A.S., Martinez-Lage M., et al. 2008. TARDBP mutations in amyotrophic lateral sclerosis with TDP-43 neuropathology: a genetic and histopathological analysis. Lancet Neurol. 7:409–416 10.1016/S1474-4422(08)70071-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance C., Rogelj B., Hortobágyi T., De Vos K.J., Nishimura A.L., Sreedharan J., Hu X., Smith B., Ruddy D., Wright P., et al. 2009. Mutations in FUS, an RNA processing protein, cause familial amyotrophic lateral sclerosis type 6. Science. 323:1208–1211 10.1126/science.1165942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vande Velde C., Miller T.M., Cashman N.R., Cleveland D.W. 2008. Selective association of misfolded ALS-linked mutant SOD1 with the cytoplasmic face of mitochondria. Proc. Natl. Acad. Sci. USA. 105:4022–4027 10.1073/pnas.0712209105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas M.R., Johnson D.A., Sirkis D.W., Messing A., Johnson J.A. 2008. Nrf2 activation in astrocytes protects against neurodegeneration in mouse models of familial amyotrophic lateral sclerosis. J. Neurosci. 28:13574–13581 10.1523/JNEUROSCI.4099-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayvergiya C., Beal M.F., Buck J., Manfredi G. 2005. Mutant superoxide dismutase 1 forms aggregates in the brain mitochondrial matrix of amyotrophic lateral sclerosis mice. J. Neurosci. 25:2463–2470 10.1523/JNEUROSCI.4385-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Sharma K., Grisotti G., Roos R.P. 2009. The effect of mutant SOD1 dismutase activity on non-cell autonomous degeneration in familial amyotrophic lateral sclerosis. Neurobiol. Dis. 35:234–240 10.1016/j.nbd.2009.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M., Dykes-Hoberg M., Culotta V.C., Price D.L., Wong P.C., Rothstein J.D. 2001. Histological evidence of protein aggregation in mutant SOD1 transgenic mice and in amyotrophic lateral sclerosis neural tissues. Neurobiol. Dis. 8:933–941 10.1006/nbdi.2001.0443 [DOI] [PubMed] [Google Scholar]
- Wegorzewska I., Bell S., Cairns N.J., Miller T.M., Baloh R.H. 2009. TDP-43 mutant transgenic mice develop features of ALS and frontotemporal lobar degeneration. Proc. Natl. Acad. Sci. USA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson T.L., Cleveland D.W. 1999. Slowing of axonal transport is a very early event in the toxicity of ALS-linked SOD1 mutants to motor neurons. Nat. Neurosci. 2:50–56 10.1038/4553 [DOI] [PubMed] [Google Scholar]
- Wu D.C., Jackson-Lewis V., Vila M., Tieu K., Teismann P., Vadseth C., Choi D.K., Ischiropoulos H., Przedborski S. 2002. Blockade of microglial activation is neuroprotective in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson disease. J. Neurosci. 22:1763–1771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka K., Boillee S., Roberts E.A., Garcia M.L., McAlonis-Downes M., Mikse O.R., Cleveland D.W., Goldstein L.S. 2008a. Mutant SOD1 in cell types other than motor neurons and oligodendrocytes accelerates onset of disease in ALS mice. Proc. Natl. Acad. Sci. USA. 105:7594–7599 10.1073/pnas.0802556105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka K., Chun S.J., Boillee S., Fujimori-Tonou N., Yamashita H., Gutmann D.H., Takahashi R., Misawa H., Cleveland D.W. 2008b. Astrocytes as determinants of disease progression in inherited amyotrophic lateral sclerosis. Nat. Neurosci. 11:251–253 10.1038/nn2047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Gozen O., Vidensky S., Robinson M.B., Rothstein J.D. 2009a. Epigenetic regulation of neuron-dependent induction of astroglial synaptic protein GLT1. Glia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Gozen O., Watkins A., Lorenzini I., Lepore A., Gao Y., Vidensky S., Brennan J., Poulsen D., Won Park J., et al. 2009b. Presynaptic regulation of astroglial excitatory neurotransmitter transporter GLT1. Neuron. 61:880–894 10.1016/j.neuron.2009.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazawa I., Giasson B.I., Sasaki R., Zhang B., Joyce S., Uryu K., Trojanowski J.Q., Lee V.M. 2005. Mouse model of multiple system atrophy alpha-synuclein expression in oligodendrocytes causes glial and neuronal degeneration. Neuron. 45:847–859 10.1016/j.neuron.2005.01.032 [DOI] [PubMed] [Google Scholar]
- Zhao W., Beers D.R., Henkel J.S., Zhang W., Urushitani M., Julien J.P., Appel S.H. 2009. Extracellular mutant SOD1 induces microglial-mediated motoneuron injury. Glia. 58:231–243 10.1002/glia.20919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Z., Deane R., Ali Z., Parisi M., Shapovalov Y., O'Banion M.K., Stojanovic K., Sagare A., Boillee S., Cleveland D.W., Zlokovic B.V. 2008. ALS-causing SOD1 mutants generate vascular changes prior to motor neuron degeneration. Nat. Neurosci. 11:420–422 10.1038/nn2073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Z., Ilieva H., Hallagan L., Bell R., Singh I., Paquette N., Thiyagarajan M., Deane R., Fernandez J.A., Lane S., et al. 2009. Activated protein C therapy slows ALS-like disease in mice by transcriptionally inhibiting SOD1 in motor neurons and microglia cells. J. Clin. Invest. 119:3437–3449 10.1172/JCI38476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoghbi H.Y., Orr H.T. 2000. Glutamine repeats and neurodegeneration. Annu. Rev. Neurosci. 23:217–247 10.1146/annurev.neuro.23.1.217 [DOI] [PubMed] [Google Scholar]