Abstract
Division plane specification in animal cells has long been presumed to involve direct contact between microtubules of the anaphase mitotic spindle and the cell cortex. In this issue, von Dassow et al. (von Dassow et al. 2009. J. Cell. Biol. doi:10.1083/jcb.200907090) challenge this assumption by showing that spindle microtubules can effectively position the division plane at a distance from the cell cortex.
Cell division, or cytokinesis, is accomplished via constriction of an equatorially localized contractile ring composed of filamentous actin and myosin II (Rappaport, 1996). Accurate division plane specification is essential to properly partition the cytoplasm and permit each daughter cell to receive a single copy of the genome. To ensure this accuracy, microtubules of the mitotic spindle signal to the cell cortex upon anaphase onset and promote assembly of the contractile ring between the separating chromosomes. The precise mechanism by which microtubules position the contractile ring, however, remains elusive.
Early models on the nature of the spindle-derived signal proposed that astral rays (later found to be microtubules) position the division plane by either locally promoting contractility at the cell equator or inhibiting contractility at the cell poles (Rappaport, 1996). Recent evidence, though, suggests that distinct microtubule populations within a single cell provide multiple signals to promote accurate division (Canman et al., 2003; Bringmann and Hyman, 2005; Chen et al., 2008; Foe and von Dassow, 2008; von Dassow, 2009).
The anaphase mitotic spindle contains several subtypes of microtubules, each of which is likely to contribute to division plane specification. Although kinetochore microtubules drive chromosome segregation during anaphase, nonkinetochore microtubules extend and maintain close proximity with the assembling central spindle (Mastronarde et al., 1993). Central spindle microtubules are highly stable (Salmon et al., 1976) and organize into an antiparallel bundled array between the separating chromosomes (Mastronarde et al., 1993). Preventing central spindle assembly usually results in a complete failure in cytokinesis, and prevents division plane specification in many cell types (Glotzer, 2005). Astral microtubules, however, are highly dynamic and grow out circumferentially from the centrosomes toward the cell cortex. Increasing evidence suggests that the astral microtubule signal inhibits contractility (see below; Canman et al., 2000; Kurz et al., 2002; Lewellyn et al., 2009).
Regardless of the mechanism of division plane specification via microtubules, nearly all current models depend on direct contact between microtubules of the mitotic spindle and the cell cortex. Most of these models were based on observations that at the time of division plane specification, astral microtubules contact the cell cortex in nearly all systems studied. Nonkinetochore and/or central spindle microtubules have also been proposed to deliver critical contractile signals to the cell equator (Murata-Hori and Wang, 2002; Canman et al., 2003; Somers and Saint, 2003; Verbrugghe and White, 2004; Lewellyn et al., 2009; Vale et al., 2009). Yet in many cell types (especially early embryos), central spindle microtubules are at some distance from the cell cortex during division plane specification. Despite this, signal delivery for both astral and central spindle microtubules was proposed to occur via direct transport along microtubules to the cell cortex. The study of von Dassow et al. in this issue, however, indicates that accurate division plane specification does not require any close microtubule/cortical contact and may occur via a diffusion-based mechanism (see also Salmon and Wolniak, 1990).
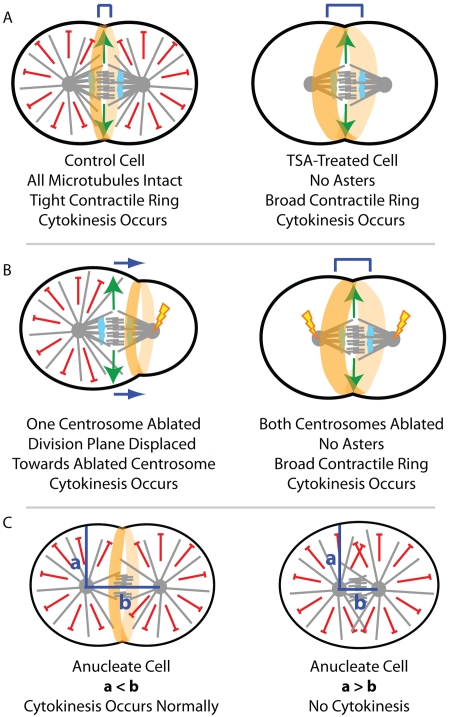
By treating echinoderm and Xenopus embryos with controlled levels of trichostatin A (TSA), which destabilizes acetylated dynamic microtubules via inhibition of the tubulin deacetylase HDAC6 (Matsuyama et al., 2002), von Dassow et al. (2009) were able to preferentially prevent astral microtubule growth while leaving central spindle microtubules intact. TSA treatment did not block anaphase onset or central spindle assembly, but resulted in the complete disruption of all direct microtubule contact with the cell cortex. Nevertheless, TSA-treated cells were able to undergo cytokinesis successfully (Fig. 1 A). The lack of astral microtubules in TSA-treated cells was also recapitulated by double centrosome ablation, and again the cells were able to undergo cytokinesis (Fig. 1 B). In both experiments, cytokinesis occurred in a timely manner, but the contractile ring was broader than in control cells. Together, these data suggest that spindle microtubules are sufficient to provide a diffusible stimulatory signal capable of defining the cell division plane without any direct contact with the cell cortex (von Dassow et al., 2009).
Figure 1.
Testing models of division plane specification by targeting distinct microtubule populations. By selectively eliminating astral microtubules with either controlled TSA-treatment (A) or by double centrosome ablation (B), von Dassow et al. (2009) provide strong evidence that microtubule contact with the cell cortex is not essential for successful cytokinesis. When a single centrosome was ablated, the division plane was displaced away from the ablated aster (B); this suggests that astral microtubules provide an inhibitory signal. Further, anucleate cells would only complete cytokinesis if the intracentrosomal distance exceeded the distance from the centrosomes to the cell cortex (C).
The authors noticed that cytokinesis occurred selectively at a position with reduced microtubule density in control cells; therefore, they explored the role of astral microtubules in division plane positioning. By selectively ablating one centrosome just before anaphase onset, von Dassow et al. (2009) were also able to provide strong support for an inhibitory role of astral microtubules in division plane specification. When a single centrosome was ablated, the division plane was displaced away from the remaining astral microtubules and toward the ablated centrosome (Fig. 1 B). Further evidence for an inhibitory role of astral microtubules in cytokinesis came from close examination of the intracentrosomal distance in anucleate cells that were able to undergo cytokinesis relative to those that were not. Cells were only able to undergo cytokinesis when the intracentrosomal distance exceeded the distance from the centrosomes to the cell cortex (Fig. 1 C), which suggests that cytokinesis requires an aster-free zone. The authors propose a mechanism in these anucleate cells whereby global activation of contractility drives division plane specification refined by a zone of astral separation (von Dassow et al., 2009). However, one possibility is that a central spindle still forms in these anucleate cells and thus provides the same diffusion-based signal that promotes division in cells without asters. Indeed, antiparallel arrays of bundled microtubules that resemble the central spindle are known to form between asters without intervening chromosomes in other systems (Savoian et al., 1999).
To summarize, the results described by von Dassow et al. (2009) support a model in which central spindle microtubules provide a diffusible stimulatory signal to promote the assembly of a broad contractile ring, which is then refined by astral microtubules into a tight contractile ring. It is tempting to speculate on the molecular nature of this diffusible signal and mechanism of the astral refinement during cytokinesis. Signaling via the small GTPase Rho is required for cytokinesis and is dependent on spindle microtubules (Bement et al., 2005; Piekny et al., 2005). von Dassow et al. (2009) showed that in TSA-treated cells lacking astral microtubules, the equatorial zone of active Rho GTPase is broader relative to control cells. Rho activation is promoted (at least in part) via the central spindle–localized GTP exchange factor, ECT2 (Glotzer, 2005). In parallel, the GTPase-activating protein (GAP) CYK4/MgcRacGAP also associates with the central spindle, where it acts to both limit the zone of Rho activity (Miller and Bement, 2009) and to promote the inactivation of another small GTPase, Rac (D'Avino et al., 2004; Yoshizaki et al., 2004; Canman et al., 2008). Perhaps in parallel to central spindle mediated activation of Rho signaling, local inactivation of the inhibitory Rac signal via CYK4 GAP activity would further specify the division plane, even at a distance (Fig. 2). When the dynamic asters are present, they could then additionally amplify Rac signaling at the cell poles via a similar mechanism to what occurs during cell motility (Wittmann and Waterman-Storer, 2001). This local feedback loop would reinforce the positive signal coming from the central spindle via Rho activation and could help delimit active Rho at the cell equator (Fig. 2). Certainly, understanding how Rho activation can be propagated to the cell cortex via diffusion in such an accurate manner will be a major future challenge.
Figure 2.
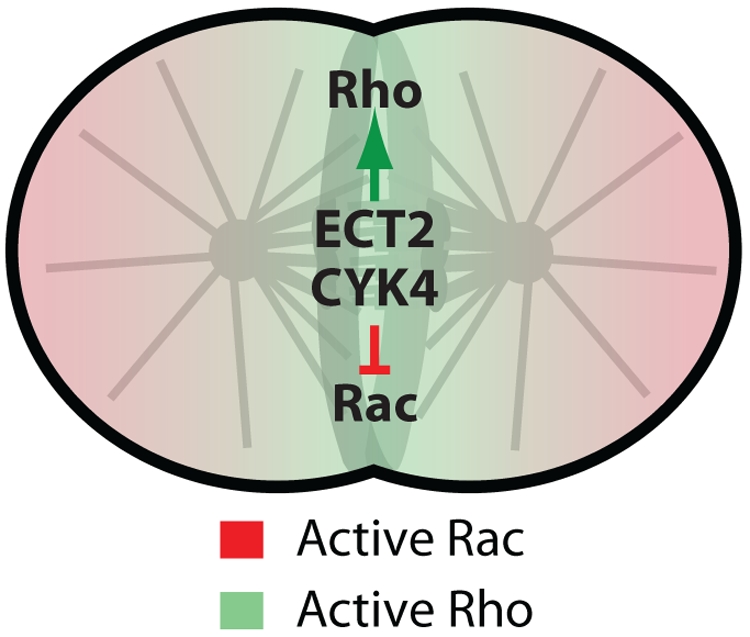
Model for central spindle–mediated signaling via Rho family small GTPases. Central spindle–localized guanine nucleotide exchange factor ECT2 leads to Rho activation at the cell equator. At the same time, central spindle–localized CYK4 (a Rho family GAP) would also locally inactivate the inhibitory Rac signal. Further refinement of the zone of active Rho by astral microtubule–activated Rac could then sharpen the Rho zone into a tight contractile ring.
References
- Bement W.M., Benink H.A., von Dassow G. 2005. A microtubule-dependent zone of active RhoA during cleavage plane specification. J. Cell Biol. 170:91–101 10.1083/jcb.200501131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bringmann H., Hyman A.A. 2005. A cytokinesis furrow is positioned by two consecutive signals. Nature. 436:731–734 10.1038/nature03823 [DOI] [PubMed] [Google Scholar]
- Canman J.C., Hoffman D.B., Salmon E.D. 2000. The role of pre- and post-anaphase microtubules in the cytokinesis phase of the cell cycle. Curr. Biol. 10:611–614 10.1016/S0960-9822(00)00490-5 [DOI] [PubMed] [Google Scholar]
- Canman J.C., Cameron L.A., Maddox P.S., Straight A., Tirnauer J.S., Mitchison T.J., Fang G., Kapoor T.M., Salmon E.D. 2003. Determining the position of the cell division plane. Nature. 424:1074–1078 10.1038/nature01860 [DOI] [PubMed] [Google Scholar]
- Canman J.C., Lewellyn L., Laband K., Smerdon S.J., Desai A., Bowerman B., Oegema K. 2008. Inhibition of Rac by the GAP activity of centralspindlin is essential for cytokinesis. Science. 322:1543–1546 10.1126/science.1163086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W., Foss M., Tseng K.F., Zhang D. 2008. Redundant mechanisms recruit actin into the contractile ring in silkworm spermatocytes. PLoS Biol. 6:e209 10.1371/journal.pbio.0060209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Avino P.P., Savoian M.S., Glover D.M. 2004. Mutations in sticky lead to defective organization of the contractile ring during cytokinesis and are enhanced by Rho and suppressed by Rac. J. Cell Biol. 166:61–71 10.1083/jcb.200402157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foe V.E., von Dassow G. 2008. Stable and dynamic microtubules coordinately shape the myosin activation zone during cytokinetic furrow formation. J. Cell Biol. 183:457–470 10.1083/jcb.200807128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glotzer M. 2005. The molecular requirements for cytokinesis. Science. 307:1735–1739 10.1126/science.1096896 [DOI] [PubMed] [Google Scholar]
- Kurz T., Pintard L., Willis J.H., Hamill D.R., Gönczy P., Peter M., Bowerman B. 2002. Cytoskeletal regulation by the Nedd8 ubiquitin-like protein modification pathway. Science. 295:1294–1298 10.1126/science.1067765 [DOI] [PubMed] [Google Scholar]
- Lewellyn L., Dumont J., Desai A., Oegema K. 2009. Analyzing the effects of delaying aster separation on furrow formation during cytokinesis in the C. elegans embryo. Mol. Biol. Cell. In press 10.1091/mbc.E09-01-0089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastronarde D.N., McDonald K.L., Ding R., McIntosh J.R. 1993. Interpolar spindle microtubules in PTK cells. J. Cell Biol. 123:1475–1489 10.1083/jcb.123.6.1475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama A., Shimazu T., Sumida Y., Saito A., Yoshimatsu Y., Seigneurin-Berny D., Osada H., Komatsu Y., Nishino N., Khochbin S., et al. 2002. In vivo destabilization of dynamic microtubules by HDAC6-mediated deacetylation. EMBO J. 21:6820–6831 10.1093/emboj/cdf682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A.L., Bement W.M. 2009. Regulation of cytokinesis by Rho GTPase flux. Nat. Cell Biol. 11:71–77 10.1038/ncb1814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata-Hori M., Wang Y.L. 2002. Both midzone and astral microtubules are involved in the delivery of cytokinesis signals: insights from the mobility of aurora B. J. Cell Biol. 159:45–53 10.1083/jcb.200207014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piekny A., Werner M., Glotzer M. 2005. Cytokinesis: welcome to the Rho zone. Trends Cell Biol. 15:651–658 10.1016/j.tcb.2005.10.006 [DOI] [PubMed] [Google Scholar]
- Rappaport R. 1996. Cytokinesis in Animal Cells. Cambridge University Press, Cambridge, England, UK: 386 pp [Google Scholar]
- Salmon E.D., Wolniak S.M. 1990. Role of microtubules in stimulating cytokinesis in animal cells. Ann. N. Y. Acad. Sci. 582:88–98 10.1111/j.1749-6632.1990.tb21670.x [DOI] [PubMed] [Google Scholar]
- Salmon E.D., Goode D., Maugel T.K., Bonar D.B. 1976. Pressure-induced depolymerization of spindle microtubules. III. Differential stability in HeLa cells. J. Cell Biol. 69:443–454 10.1083/jcb.69.2.443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savoian M.S., Earnshaw W.C., Khodjakov A., Rieder C.L. 1999. Cleavage furrows formed between centrosomes lacking an intervening spindle and chromosomes contain microtubule bundles, INCENP, and CHO1 but not CENP-E. Mol. Biol. Cell. 10:297–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers W.G., Saint R. 2003. A RhoGEF and Rho family GTPase-activating protein complex links the contractile ring to cortical microtubules at the onset of cytokinesis. Dev. Cell. 4:29–39 10.1016/S1534-5807(02)00402-1 [DOI] [PubMed] [Google Scholar]
- Vale R.D., Spudich J.A., Griffis E.R. 2009. Dynamics of myosin, microtubules, and Kinesin-6 at the cortex during cytokinesis in Drosophila S2 cells. J. Cell Biol. 186:727–738 10.1083/jcb.200902083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbrugghe K.J., White J.G. 2004. SPD-1 is required for the formation of the spindle midzone but is not essential for the completion of cytokinesis in C. elegans embryos. Curr. Biol. 14:1755–1760 10.1016/j.cub.2004.09.055 [DOI] [PubMed] [Google Scholar]
- von Dassow G. 2009. Concurrent cues for cytokinetic furrow induction in animal cells. Trends Cell Biol. 19:165–173 10.1016/j.tcb.2009.01.008 [DOI] [PubMed] [Google Scholar]
- von Dassow G., Verbrugghe K.J.C., Miller A.L., Sider J.R., Bement W.M. 2009. Action at a distance during cytokinesis. J. Cell Biol. 187:831–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann T., Waterman-Storer C.M. 2001. Cell motility: can Rho GTPases and microtubules point the way? J. Cell Sci. 114:3795–3803 [DOI] [PubMed] [Google Scholar]
- Yoshizaki H., Ohba Y., Parrini M.C., Dulyaninova N.G., Bresnick A.R., Mochizuki N., Matsuda M. 2004. Cell type-specific regulation of RhoA activity during cytokinesis. J. Biol. Chem. 279:44756–44762 10.1074/jbc.M402292200 [DOI] [PubMed] [Google Scholar]