Abstract
The tetrameric prokaryotic potassium channel KcsA is activated by protons acting on the intracellular aspect of the protein and inactivated through conformational changes in the selectivity filter. Inactivation is modulated by a network of interactions within each protomer between the pore helix and residues at the external entrance of the channel. Inactivation is suppressed by the E71A mutation, which perturbs the stability of this network. Here, cell-free protein synthesis followed by protein purification by sodium dodecyl sulfate–polyacrylamide gel electrophoresis was used to produce heterotetramers of KcsA that contain different combinations of wild-type and E71A subunits. Single-channel recordings from these heterotetramers reveal how the network of interactions in individual protomers affects ionic conduction and channel inactivation, suggesting that the latter is a cooperative process.
INTRODUCTION
The potassium channel KcsA from Streptomyces lividans has been used as a model for understanding the structure–function relationships of ion channels (Doyle et al., 1998; MacKinnon, 2004; Gouaux and Mackinnon, 2005). It is a small tetrameric protein with a pore region that resembles that of larger potassium channels (Doyle et al., 1998; MacKinnon, 2004; Gouaux and Mackinnon, 2005). The channel is activated by protons acting on the intracellular aspect of the protein (Cuello et al., 1998; Heginbotham et al., 1999; Thompson et al., 2008) and inactivated through conformational changes in the selectivity filter (Blunck et al., 2006; Cordero-Morales et al., 2006a,b, 2007). Macroscopic current measurements of KcsA channels have shown that after exposure to low pH, the channels are rapidly activated and subsequently enter an inactivated state from which recovery is slow (Gao et al., 2005; Cordero-Morales et al., 2006b; Chakrapani et al., 2007a). As a result, under steady-state conditions, the channel has a low open probability (Cordero-Morales et al., 2006b; Chakrapani et al., 2007b). In single-channel recordings, this activity is characterized by short bursts of activity that are separated by long periods of inactivity (Cordero-Morales et al., 2006b; Chakrapani et al., 2007b). In addition, the steady-state gating of KcsA at low pH is affected by the applied potential (Cuello et al., 1998; Heginbotham et al., 1999; Cordero-Morales et al., 2006a), which influences the rate of entry and exit into the inactivated state as reflected in macroscopic currents (Chakrapani et al., 2007a).
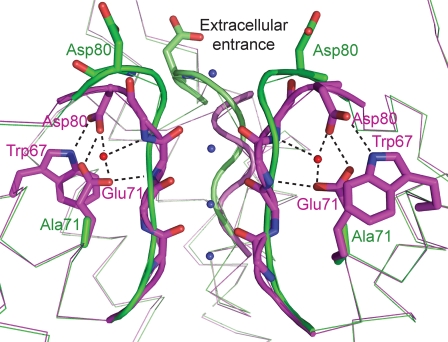
The inactivation of KcsA is modulated by intraprotomeric interactions between Glu71 and Trp67 in the pore helix and Asp80 in the external entrance of the channel (Fig. 1). Therefore, the inactivation of KcsA channels can be described as an allosteric process, where the conformation of the regulatory site (the network of interactions between the pore helix and the external entrance) affects ion permeability through the selectivity filter. The mutation E71A, which disrupts this network, greatly reduces the rate of inactivation as observed in macroscopic currents (Cordero-Morales et al., 2006a,b, 2007 Chakrapani et al., 2007a,b) and produces a remarkably high steady-state open probability in single-channel recordings at low pH (Cordero-Morales et al., 2006a,b, 2007; Chakrapani et al., 2007b). Further, it has been proposed that crystal structures of wild-type (WT) KcsA in the presence of high K+ concentrations, where the selectivity filter contains two ions distributed over the four binding sites (Zhou et al., 2001), represent the permeable (active) configuration of the KcsA selectivity filter (Fig. 1). On the other hand, the structures of WT KcsA at low K+ concentrations (Zhou et al., 2001) and the crystal structure of the M96V mutant (Lockless et al., 2007), where the selectivity filter contains one ion distributed over the two remaining binding sites, are thought to represent the configuration of the selectivity filter during inactivation.
Figure 1.
Superposition of the selectivity filter region of the WT KcsA structure (PDB entry 1K4C; magenta) and the flipped E71A KcsA structure (PDB entry 2ATK; green). There is a network of interactions between the side chains of Asp80, Glu71, and Trp67 in the WT channel. This network is broken in the mutated channel, and the side chains of Asp80 are exposed at the extracellular entrance of the channel. K+ ion binding sites inside the selectivity filter are presented as blue spheres and water molecules as red spheres. One KcsA protomer is omitted for clarity. Both of these structures are believed to represent conductive forms of the selectivity filter exhibiting four K+ ion binding sites with an overall occupancy of two K+ ions. The superposition was created with PyMOL (http://www.pymol.org).
C-type inactivation of eukaryotic voltage-dependent K+ (Kv) channels, which occurs in response to prolonged depolarization, has also been associated with conformational changes in the selectivity filter (Yellen, 1998). C-type inactivation of Kv channels is a cooperative process that involves concerted conformational changes of all four subunits (Ogielska et al., 1995; Panyi et al., 1995). The KcsA inactivation process shares features with C-type inactivation: (a) mutation of Glu71 and Tyr82 in KcsA (Cordero-Morales et al., 2006a,b), and the corresponding residues in Shaker Kv channels, Val438 (Yifrach and MacKinnon, 2002) and Thr449 (López-Barneo et al., 1993; Ogielska et al., 1995), respectively, all affect the inactivation of the channels; (b) the rate of inactivation of KcsA and Shaker Kv channels has a similar dependence on the extracellular potassium ion concentration (López-Barneo et al., 1993; Cordero-Morales et al., 2006b; Chakrapani et al., 2007a). These results, together with the sequence and structural similarities of KcsA and eukaryotic K+ channels in the region of the selectivity filter (Long et al., 2005), suggest that the inactivation processes of the channels are mechanistically similar.
Single-channel recordings are widely used to clarify the mechanisms of action of ion channels. The majority of single-channel activity measurements on ion channels that have been performed in planar lipid bilayers, including those on KcsA channels, have been of proteins overexpressed and purified from bacteria. The ion channels are typically reconstituted into lipid vesicles, which are subsequently fused with bilayers (e.g., Schrempf et al., 1995; Heginbotham et al., 1999). Although this approach has produced valuable data and expanded our understanding of the functional properties of ion channels, time-consuming overexpression and purification steps are involved. It has previously been shown that some β barrel pores, such as staphylococcal α-hemolysin, and the small viral K+ channel Kcv can be expressed in a cell-free system and purified by SDS-PAGE before direct insertion into planar lipid bilayers (Braha et al., 1997; Shim et al., 2007; Mason, 2008). Cell-free protein synthesis has advantages over conventional protein expression techniques, including its rapidity and the capability to synthesize proteins with unnatural or labeled amino acids (Spirin, 2002; Wang et al., 2006). Cell-free synthesis has been used to produce many membrane proteins, including KcsA channels (van Dalen et al., 2000). In addition, it has been shown that KcsA, like the α-hemolysin pore and the Kcv channel, maintains its oligomeric structure during SDS-PAGE (Heginbotham et al., 1997). These results suggested that KcsA tetramers might also be purified directly from polyacrylamide gels in a functional form, which is the approach taken here.
Cell-free protein synthesis followed by direct protein purification by SDS-PAGE was previously exploited to form heteromers of WT and chemically modified mutant subunits of α-hemolysin (Braha et al., 1997). More recently, when this technique was used, the mutant α-hemolysin subunits contained oligoaspartate extensions at their C termini (Howorka et al., 2001). Accordingly, it was possible to separate heteromers with different combinations of WT and mutated subunits based on their differing electrophoretic mobilities. Electrophoresis of KcsA heteromers formed by the coexpression of subunits with different peptide extensions at their C termini was used previously to demonstrate that KcsA forms tetramers (Heginbotham et al., 1997). However, these channels were not purified from the gels and used for functional studies. In the present work, heteromers that contained WT and E71A KcsA protomers with different peptide extensions at their N termini were produced and separated by SDS-PAGE. The extensions were then cleaved by proteolysis, and single-channel recordings were made with heteromers in which the protomers differed from each other only at the desired site of mutation, position 71.
By this approach, we reveal how regulatory sites in the KcsA channel affect inactivation. These regulatory sites are the networks of interaction within individual protomers between the pore helix and the external entrance of the channel. Based on our results, we propose an inactivation mechanism that features cooperativity between the four subunits. In addition, this study shows that the E71A mutation causes the formation of a ring of charges at the extracellular entrance of the channel that increases inward ionic conduction.
MATERIALS AND METHODS
Unless otherwise stated, chemicals and oligonucleotides were purchased from Sigma-Aldrich. Enzymes and prestained gel markers were purchased from New England Biolabs, Inc. and lipids from Avanti Polar Lipids, Inc.
Genetic constructs
Constructs were assembled in the pT7-SC1 expression vector (Cheley et al., 1997) and verified by sequencing of the entire genes. The KcsA gene encoding the point mutant E71A was generated by PCR mutagenesis and in vivo recombination as described previously (Jones, 1995; Howorka and Bayley, 1998). The same technique was used to generate the genes for KcsA with N-terminal polypeptide extensions by using overlapping primers to form the coding sequences for the extensions. Two extensions were generated: “long,” with the amino acid sequence MHHHHHHNNNNNNNNRLEQKLISEEDLL…, and “short,” with the sequence MHHHHHHL.... In both cases, the sequences were followed by a tobacco etch virus (TEV) protease recognition site (ENLYFQG) and eight asparagine residues.
Cell-free coupled transcription and translation
Polypeptides were generated by coupled in vitro transcription and translation (IVTT) by using an Escherichia coli T7-S30 extract system for circular DNA (Promega). The complete 1-mM amino acid mixture minus cysteine and the complete 1-mM amino acid mixture minus methionine were mixed in equal volumes to obtain the working amino acid solution required to generate high concentrations of the proteins. The amino acids (10 µl) were mixed with premix solution (40 µl), 4 µl [35S]methionine (1,175 Ci mmol−1, 10 mCi ml−1; MP Biomedicals) and 30 µl T7 S30 extract supplemented with 20 µg ml−1 (final concentration) rifampicin (Cheley et al., 1997). To form heteromers, different ratios of the plasmid DNA encoding WT KcsA with the long polypeptide extension and E71A-KcsA with the short polypeptide extension were mixed (total volume 16 µl, from 400–ng µl−1 stock solutions of each DNA). Synthesis was performed for 60 min at 37°C to generate 100 µl IVTT polypeptide solution. Laemmli sample buffer (2×, 100 µl; Bio-Rad Laboratories) was then added and the sample was loaded, without heating, into two 5-mm wells of a 15-cm-long, 1.5-mm-thick 10% SDS polyacrylamide gel. Both the stacking and the separation gels were supplemented with 10 mM KCl. The gel was run overnight at 45 V, and then for a few more hours at 100 V to achieve the maximal separation between the different heteromers. The unfixed gel was vacuum dried without heating onto 3MM chromatography paper (GE Healthcare) and visualized by exposure to film (BioMax MR-1; Kodak). Each of the heteromer-containing bands was cut from the gel by using the autoradiogram as a guide. The excised pieces were rehydrated with buffer (200–300 µl) containing 10 mM HEPES, pH 7.0, and 10 mM KCl for 1 h. After removing the paper, each gel strip was thoroughly crushed in the solution, and the protein was separated from the gel by centrifugation through a 0.2-µm cellulose acetate filter (Rainin). The protein was stored frozen at −80°C.
Proteolysis with TEV protease
The polypeptide extensions of the heteromers were removed by proteolysis with TEV protease. Before proteolysis, the storage buffer of the protein was exchanged for 50 mM Tris HCl, pH 8.0, 0.5 mM EDTA, 0.02% (wt/vol) n-dodecyl-β-d-maltoside, and 10 mM KCl by dilution and concentration by centrifugation, three times at 14,000 g for 18 min, with a filter (YM-10 Microcon; Millipore). 1 mM (final concentration) dithiothreitol and AcTEV protease (1 µl, 10 U µl−1; Invitrogen) were added to a protein sample (50 µl) (Parks et al., 1994; Suh-Lailam and Hevel, 2009). The sample was incubated overnight at 25°C and stored at −80°C. This material was used directly for electrical recordings.
Single-channel electrical recording and analysis
Single-channel recordings were performed by using the folded planar lipid bilayer method (Montal and Mueller, 1972). The apparatus consisted of a 0.025-mm-thick Teflon septum glued between two Delrin chambers. The septum contained an aperture of 100-µm diameter, which was treated with 10% (vol/vol) hexadecane in pentane. Each chamber was filled with 1 ml of filter-sterilized buffered salt solution. Unless otherwise stated, the salt solutions were 200 mM KCl and 10 mM HEPES, pH 7.0, in the cis chamber and 200 mM KCl and 10 mM succinic acid, pH 4.0, in the trans chamber (solutions were titrated with KOH or HCl).
1-Palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine (POPE) and 1-palmitoyl-2-oleoyl-sn-glycero-3-[phospho-rac-(1-glycerol)] (POPG) (8 µl; 7.5 and 2.5 mg ml−1, respectively, in pentane) or 1,2-diphytanoyl-sn-glycero-3-phosphocholine (DPhPC; 8 µl; 10 mg ml−1 in pentane) were added to the surface of each chamber, and the pentane was allowed to evaporate to leave behind a lipid monolayer. A lipid bilayer was formed by lowering and raising the liquid levels in the chambers below and above the aperture.
The electrical current was detected with two Ag/AgCl electrodes, amplified with a patch clamp amplifier (Axopatch 200B; MDS Analytical Technologies), filtered with a low-pass Bessel filter (80 dB per decade) with a corner frequency of 5 kHz, and digitized with an A/D converter (DigiData 1320; MDS Analytical Technologies) at a sampling frequency of 50 kHz. In all experiments, the cis chamber was at ground. A positive current represents the movement of cations from trans to cis. The bilayer recording platform, chambers, amplifying headstage, and micromanipulator were enclosed in a Faraday cage to shield against ambient electrical noise. KcsA channels were directly inserted into the bilayer from the cis (ground) chamber either by adding the protein to the bath solution and stirring or by using an agarose probe, as described previously (Holden and Bayley, 2005).
Traces of 2-min duration (beginning in the open state) were further filtered with a digital Gaussian filter at 1 kHz in Clampfit 10 (MDS Analytical Technologies). Amplitude histograms were fitted to Gaussian functions, and the quoted unitary conductance values were obtained from the peak (mean) values of the fits. Open probabilities were determined with the Single-Channel Search module from Clampfit. When the activity of more than one channel was observed in a trace, the single-channel open probability was determined by dividing the total open probability (NPo) by the total number of active channels (N). NPo is calculated by Clampfit:
where n is the number of channels in level n (1, 2, … N), tn is the total amount of time spent in level n during the entire trace, and T is the total recording time. A value for N was obtained from the highest current level observed during a recording.
RESULTS
In vitro expression of functional KcsA channels
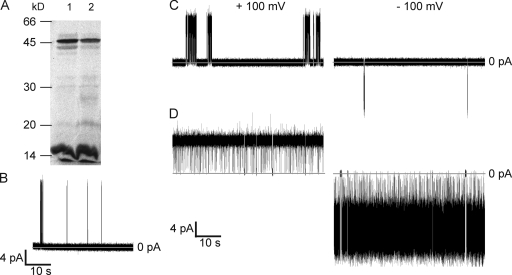
KcsA was expressed and labeled with [35S]methionine in a cell-free IVTT system. Analysis by SDS-PAGE revealed two major bands with Mr values of ∼15 and ∼45 kD (Fig. 2 A). The molecular mass of KcsA, predicted from the polypeptide sequence, is 18 kD for the monomer and hence 72 kD for the tetramer. Tetrameric KcsA has been shown previously to migrate in SDS polyacrylamide gels with Mr < 72 kD (Heginbotham et al., 1997), and we surmised that the ∼45-kD band is the tetramer.
Figure 2.
Expression of functional KcsA channels by coupled IVTT. (A) Assembly of KcsA channels during IVTT. Products of IVTT were separated in a 12.5% SDS polyacrylamide gel supplemented with 10 mM KCl. Lane 1, WT KcsA; lane 2, E71A KcsA. The proteins were labeled with [35S]methionine and visualized by exposure of the dried gel to x-ray film. (B–D) Representative single-channel recordings of KcsA purified by SDS-PAGE. (B) WT KcsA in POPE/POPG (3:1) bilayers at +150 mV. (C) WT KcsA in DPhPC bilayers at ±100 mV. (D) E71A KcsA in DPhPC bilayers at ±100 mV. The measurements were made in 200 mM KCl, buffered with 10 mM HEPES, pH 7.0, in the cis chamber and 200 mM KCl, buffered with 10 mM succinic acid, pH 4.0, in the trans chamber. The traces were digitally filtered at 1 kHz for display.
For single-channel recording, the KcsA tetramers formed by IVTT were isolated from an SDS polyacrylamide gel by elution into 10 mM HEPES, pH 7.0. The protein was added to the cis chamber (ground) of a bilayer apparatus, and the solution was stirred until single-channel activity was seen. Alternatively, the protein was introduced directly into the bilayer by using an agarose probe (Holden and Bayley, 2005). During this study, folded lipid bilayers were used (Montal and Mueller, 1972), composed initially of either DPhPC or a 3:1 ratio of POPE and POPG.
Previous studies have suggested a requirement for a negatively charged lipid for channel activity (Heginbotham et al., 1998; Valiyaveetil et al., 2002). Typically then, for single-channel recordings, KcsA is reconstituted into vesicles and fused with bilayers of 3:1 POPE/POPG (Heginbotham et al., 1999; LeMasurier et al., 2001; Marius et al., 2008) or soybean asolectin lipids (Cortes et al., 2001). However, we found that KcsA functions in a lipid bilayer composed solely of DPhPC with similar activity, in terms of channel gating and ion conductance, compared with that observed in POPE/POPG bilayers (Fig. 2, B and C). As the S30 cell extract contains a high level of E. coli lipids (van Dalen et al., 2002; Conlan, 2003), it is possible that the tetramer formed by IVTT contains tightly bound PG, which might be retained throughout the purification process and thereby allow the channel to function in a DPhPC bilayer. All subsequent experiments were performed with DPhPC bilayers.
To be active in reconstituted systems, the intracellular domain of KcsA should be at pH <5 (Cuello et al., 1998). Therefore, the chamber solutions were buffered with 10 mM HEPES, pH 7.0, in the cis chamber, and with 10 mM succinic acid, pH 4.0, in the trans chamber. This ensured that the active channels were of known orientation with respect to the bilayer (Heginbotham et al., 1999). The cis chamber therefore represented the extracellular side of a cell and the trans chamber the intracellular side. The channels displayed the well-documented activity of KcsA: burst-like behavior, with a higher open probability at positive potentials compared with negative potentials (Fig. 2 C) (Cordero-Morales et al., 2006b; Chakrapani et al., 2007b). The openings at negative potentials were significantly noisier than at positive potentials (not depicted) and the I-V curve displayed partial outward rectification (Fig. 3). WT KcsA had a conductance of 108 ± 8 pS at +100 mV and 50 ± 10 pS at −100 mV in 200 mM KCl. These values are in reasonable agreement with previously reported values of 56 pS at +100 mV and 31 pS at −100 mV recorded in 100 mM KCl (Heginbotham et al., 1999). This anticipated single-channel behavior demonstrates that it is possible to examine the functional properties of a K+ channel expressed in vitro and purified directly from a gel, thereby eliminating the time-consuming labor of traditional protocols.
Figure 3.
The addition of a peptide extension to the N terminus of KcsA does not affect its activity. I-V curves of WT (squares) and E71A (triangles) KcsA without (filled symbols) or with the G8N extension at the N terminus (open symbols). Each data point represents the mean ± SD from at least three separate bilayers. All the measurements were done in 200 mM KCl, buffered with 10 mM HEPES, pH 7.0, in the cis chamber and 200 mM KCl, buffered with 10 mM succinic acid, pH 4.0, in the trans chamber.
Formation of heteromeric channels
It had been shown that KcsA heteromers with different combinations of subunits, containing short and long peptide extensions, can be separated by SDS-PAGE (Heginbotham et al., 1997). Therefore, we attempted to form heteromeric KcsA channels by co-translation of two different constructs. We added DNAs encoding WT and E71A KcsA polypeptides, which contained, respectively, long and short peptide extensions at the N terminus (see Materials and methods), to an IVTT mix. When the products were examined by SDS-PAGE, the E71A tetramer was not observed, and it was supposed that it either did not form or dissociated into monomers in SDS. Cations known to permeate or strongly block K+ channels (such as K+, Rb+, and Cs+) confer stability to the WT tetramer (Krishnan et al., 2005). Indeed, the addition of 10 mM KCl to the SDS polyacrylamide gels increased the stability of the E71A tetramer sufficiently for purification for single-channel recording. Therefore, 10 mM KCl was routinely added to gels and to the storage solutions of the purified protein.
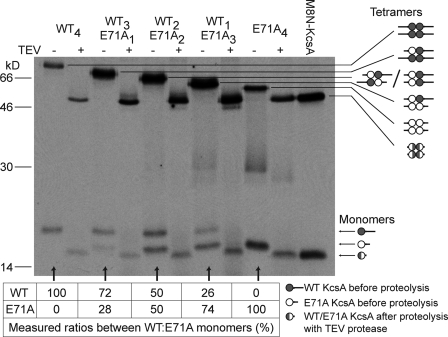
When the WT/E71A tetramers were analyzed by SDS-PAGE, a ladder of five evenly spaced bands was evident (Fig. 4). The different heteromeric combinations had been separated according to their molecular masses, conferred by the N-terminal extensions. However, by using this method it was not possible to distinguish between the two potential WT2E71A2 tetramers, where the mutant protomers are in either symmetric or asymmetric configurations (Fig. 4). The tetramers with different ratios of WT and E71A subunits were isolated from the gel, and the protein was eluted into 10 mM KCl and 10 mM HEPES, pH 7.0. Most of the purified protein retained its tetrameric structure, as shown by the rerunning of samples in SDS polyacrylamide gels (Fig. 5). The ratios of the two subunits in the dissociated tetramers were as anticipated based on the expected “parent” tetramer structures (Fig. 5).
Figure 4.
Expression of heteromeric KcsA channels by coupled IVTT. The samples were separated in a 12.5% SDS polyacrylamide gel supplemented with 10 mM KCl. The proteins were labeled with [35S]methionine and visualized by exposure of the dried gel to x-ray film. Lanes 1–5: protein products from plasmid DNAs encoding WT-long and E71A-short in the ratios 4:0, 1:3, 2:2, 3:1, and 0:4. A schematic representation of the distribution of subunits in the heterotetramers is shown (right).
Figure 5.
Electrophoretically purified KcsA heteromers. The five WT/E71A heteromers were purified by SDS-PAGE as described in Materials and methods and rerun in a 12.5% SDS polyacrylamide gel supplemented with 10 mM KCl, with or without treatment with TEV protease. The WT subunits had the long extension and the E71A subunits the short extension. Without treatment with the protease, the relative mobilities of the heteromers take on a staircase appearance because of the different total masses of the extensions. After proteolysis with TEV, all of the heteromers have the same mobility as a KcsA tetramer with an eight-Asn extension at the N terminus (right lane, M8N-KcsA; M, Met). The proteins were labeled with [35S]methionine and visualized by exposure of the dried gel to x-ray film. A schematic representation of the distribution of subunits in the heterotetramers is shown (right). The table at the bottom of the figure presents the ratios of WT KcsA and E71A KcsA subunits from spontaneously dissociated tetramers (not treated with TEV protease) on the same gel. The ratios were calculated with Quantity One software (Bio-Rad Laboratories).
For further work, the peptide extensions at the N termini of the KcsA subunits in the tetramers were removed by proteolysis with TEV protease to form subunits that differed from each other only at the mutated position 71 (Fig. 5). After the proteolysis step, all the subunits in a tetramer (WT or E71A) retain a short extension at the N terminus comprising one Gly residue followed by eight Asn residues (G8N). Interestingly, in the absence of the G8N linker between the N terminus of the protomers and the TEV recognition site, protease cleavage was very inefficient (not depicted). It has been determined by site-directed spin labeling (Cortes et al., 2001) and nuclear magnetic resonance spectroscopy (Baker et al., 2007) that the N terminus of the KcsA channel forms an α helix. It is possible that this well-ordered secondary structure prevents the binding of the protease or inhibits protease activity when it is adjacent to the recognition site. After the proteolysis step, the KcsA tetramers with different ratios of WT and E71A subunits all formed active channels in planar lipid bilayers (Fig. 6). Comparisons of the properties of WT and E71A KcsA channels with or without the G8N extension demonstrated that the presence of the extension did not affect the activity of channels in terms of their unitary conductance values (Fig. 3) and gating activities (compare Figs. 2 and 6).
Figure 6.
Representative single-channel recordings of WT/E71A heteromers purified by SDS-PAGE. All measurements were done at ±100 mV in 200 mM KCl, buffered with 10 mM HEPES, pH 7.0, in the cis chamber and 200 mM KCl, buffered with 10 mM succinic acid, pH 4.0, in the trans chamber. The traces were digitally filtered at 1 kHz for display. All-points histograms of the traces are shown.
The effect of the E71A mutation on the KcsA inward conductance
The I-V curve of WT KcsA in 200 mM KCl reflects significant outward rectification (Fig. 3). By comparison, under the same conditions, E71A homotetramers show mild inward rectification. Although the mutation has little effect on the magnitude of the current in the outward direction, it has a major effect on the inward current, e.g., at −100 mV the conductance of WT channels is 50 ± 10 pS (n = 4), and the conductance of E71A channels is about three times higher, 148 ± 5 pS (n = 4). Similar results were previously published (Thompson et al., 2008). The E71V homotetramer in planar lipid bilayers showed a similar phenomenon, with an even larger inward conductance (Choi and Heginbotham, 2004). The E71A homotetramers exhibited significant noise in single-channel recordings (Fig. 2). The origin of the noise is unclear, but it is of a similar magnitude in currents recorded from WT tetramers.
Comparison of the crystal structures of WT (Zhou et al., 2001) and E71A (Cordero-Morales et al., 2006b) KcsA channels suggests a possible explanation for the differences in the conductance values. In the WT KcsA crystal structure, the side chain of Asp80 faces into the protein core (Zhou et al., 2001) (Fig. 1). In one of the two structures of E71A (called “flipped”), the Asp80 side chain moves 8 Å and is situated in the extracellular entrance of the channel, exposed to the bulk solvent (Fig. 1) (Cordero-Morales et al., 2006b). The authors suggest that eliminating the carboxyl–carboxylate interaction between Asp80 and Glu71 increases the flexibility of Asp80 side chain and allows the observed movement (Cordero-Morales et al., 2006b). Based on the conformational change observed in the flipped structure, the exposed carboxyl side chains of Asp80 creates an external ring of negative charge (Fig. 1). This ring would increase the inward conductance of the channel by decreasing the height of the energy barrier for ion entry through the external entrance into the selectivity filter.
The effect of charged rings on conduction has been studied in various ion channels, including BK potassium channels (Qian et al., 2002; Brelidze et al., 2003; Carvacho et al., 2008), acetylcholine receptors (Imoto et al., 1988), and mutated KcsA (Nimigean et al., 2003). For all of these channels, it was demonstrated that the presence of negatively charged amino acids in one of the entrances into the channel increases the rate of transport of cations from this entrance to the other side of the membrane.
To further explore the effect of a negatively charged ring at the extracellular entrance of E71A KcsA, we determined the effect of pH on the conductance of the channel. If the source of the increase of inward conductance in E71A KcsA channels is a ring of charge at the extracellular entrance formed by the exposed carboxyl side chains of Asp80, it is predicted that a reduction in the pH on the extracellular side of the channel would cause a decrease in the inward current as a result of protonation of the Asp side chains. When the pH in the cis chamber (the extracellular side of the channel) is decreased from pH 7.0 to 3.0, only the inward conductance of E71A KcsA is reduced, whereas for WT KcsA, both the inward and the outward conductances are unaffected (Fig. 7). Therefore, the results support the idea that the rise in the inward conductance of the mutated channel is caused, at least in part, by the exposure of the Asp80 carboxyl side chains at the extracellular entrance of the channel, as shown in the flipped E71A KcsA crystal structure. On the other hand, the WT KcsA inward conductance was not changed at low extracellular pH because the carboxyl side chain of Asp80 is buried inside the protein, as shown in the WT crystal structure (Fig. 1), and does not form a ring of charge at the extracellular entrance.
Figure 7.
Currents through WT4 and E71A4 at pH 7.0 and 3.0 in the cis chamber. I-V curves of WT4 (squares) and E71A4 (triangles) at pH 7.0 (filled symbols) and 3.0 (open symbols) in the cis chamber. Each data point represents the mean ± SD from at least three separate bilayers. All the measurements were done in 200 mM KCl, buffered with 10 mM succinic acid, pH 4.0, in the trans chamber and with 200 mM KCl, buffered with 10 mM HEPES, pH 7.0, or with 10 mM succinic acid, pH 3.0, in the cis chamber.
Measurements on the WT/E71A heteromers gave a further opportunity to determine the effect of Ala substitution at position 71 on the channel conductance (Fig. 8 A). The I-V curves of the WT/E71A heteromers show that as the number of E71A subunits in the tetramers is increased, the inward current increases (Fig. 8 B). These results suggest that each of the E71A subunits in the tetramer makes a cumulative contribution to the elevation of the inward current.
Figure 8.
(A) I-V relationships for homotetramers and heterotetramers of KcsA. Diamonds, WT4; squares, WT3E71A1; triangles, WT2E71A2; circles, WT1E71A3; crosses, E71A4. Each data point represents the mean ± SD from at least four separate bilayers. (B) Unitary conductance values of WT/E71A heteromers at −100 mV. Each data point represents the mean ± SD from at least 11 separate bilayers. The measurements were done in 200 mM KCl, buffered with 10 mM HEPES, pH 7.0, in the cis chamber and 200 mM KCl, buffered with 10 mM succinic acid, pH 4.0, in the trans chamber.
Inactivation of KcsA
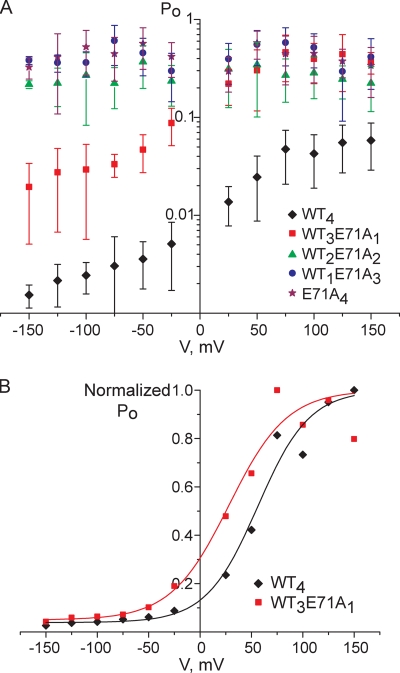
Proton-dependent activation of KcsA is followed by an inactivation process similar to C-type inactivation. As a result, at low pH, homotetrameric WT KcsA channels are characterized by short bursts of activity that are separated by long periods of inactivity (Cordero-Morales et al., 2006b; Chakrapani et al., 2007b). Under our conditions, WT4 KcsA channels behave as described in the earlier reports (Fig. 6). WT4 channels indeed have a low open probability, Po < 0.1, and as the bilayer is depolarized, the open probability of the channel increases (Fig. 9 A and Table I) (Cordero-Morales et al., 2006a; Chakrapani et al., 2007b). The inactivation gate is located in the selectivity filter of the channel, and inactivation is suppressed by the E71A mutation (Cordero-Morales et al., 2006b). In contrast with WT4, previous work has shown that the open probability of E71A4 at low pH is close to one, Po = 1 (Cordero-Morales et al., 2006b; Chakrapani et al., 2007b; Thompson et al., 2008). In the experiments presented here, E71A4 channels exhibited variable and lower open probabilities compared with those reported previously (e.g., Po = 0.58 ± 0.18 at +50 mV; n = 12) (Fig. 9 A and Table I). The basis of the differences between this study and previous work is not clear. Nevertheless, we do find that the open probability of E71A4 is much higher than that of WT4 (Fig. 9 A and Table I) and is practically voltage independent (Cordero-Morales et al., 2007).
Figure 9.
Voltage dependences of the open probabilities of WT/E71A heteromers. (A) Plots of open probability (Po) versus voltage. Diamonds, WT4; squares, WT3E71A1; triangles, WT2E71A2; circles, WT1E71A3; stars, E71A4. Each data point represents the mean ± SD from at least three separate bilayers. The measurements were done in 200 mM KCl, buffered with 10 mM HEPES, pH 7.0, in the cis chamber and 200 mM KCl, buffered with 10 mM succinic acid, pH 4.0, in the trans chamber. (B) Normalized values of the data shown in A for WT4 and WT3E71A1. Values were normalized to the maximum Po value, which was set at Po = 1. Diamonds, WT4; squares, WT3E71A1. The continuous curves are the best fits to Boltzmann functions for WT4 (black) and WT3E71A1 (red).
Table I.
Open probabilities of WT/E71A heteromers
| WT4 | WT3E71A1 | WT2E71A2 | WT1E71A3 | E71A4 | ||||||
| V, mV | Po | n | Po | n | Po | n | Po | n | Po | n |
| −50 | 0.0036 ± 0.0018 | 11 | 0.047 ± 0.020 | 5 | 0.31 ± 0.17 | 9 | 0.46 ± 0.19 | 10 | 0.57 ± 0.26 | 9 |
| 50 | 0.025 ± 0.016 | 11 | 0.30 ± 0.19 | 7 | 0.27 ± 0.18 | 9 | 0.56 ± 0.22 | 11 | 0.58 ± 0.18 | 12 |
All measurements were made in 200 mM KCl, buffered with 10 mM HEPES, pH 7.0, in the cis chamber and 200 mM KCl, buffered with 10 mM succinic acid, pH 4.0, in the trans chamber. Values are mean ± SD.
Measurements of the single-channel activity of the WT/E71A heteromers gave a closer view of the mechanism behind the transition of the selectivity filter between active and inactive states. The open probability of WT3E71A1 channels, where one WT subunit is substituted with one E71A subunit, was higher than that of WT4 channels at all the potentials examined (Fig. 9 A and Table I). However, like that of WT4 channels, the open probability of WT3E71A1 channels was voltage dependent, and it increased as the membrane was depolarized. At high depolarizing potentials (above +50 mV), the open probability of WT3E71A1 channels approached the same level as that of the E71A4 channels (Fig. 9 A and Table I). Because of the high variability in the open probability values, it was impossible to determine the exact values of V1/2 and the gating charge (z) for WT4 and WT3E71A1. However, the plots of normalized open probability as a function of membrane potential of both channels can be fit to Boltzmann distributions with similar slopes (i.e., similar gating charges), in which the V1/2 of WT3E71A1 is shifted to a more negative potential (Fig. 9 B). These results suggest that the presence of even one E71A subunit destabilizes the inactive state of the selectivity filter in the tetrameric channel.
Heteromers with additional E71A subunits (i.e., WT2E71A2 and WT1E71A3 channels) had open probability values of the same order of magnitude as E71A4 channels (Fig. 9 A and Table I). Although it is difficult to make a precise comparison of the open probability values of WT2E71A2, WT1E71A3, and E71A4 tetramers because of their high variability, it is clear that in all of these channels, the open probability is no longer voltage dependent (Fig. 9 A and Table I). These results suggest that the presence of two or more E71A subunits in a KcsA tetramer provides maximal suppression of the transition of the selectivity filter into the inactivated state.
DISCUSSION
The potassium channel KcsA has been used extensively as a model for the understanding of structure–function relationships in ion channels. Here, it has been demonstrated that functional KcsA can be expressed in vitro by cell-free coupled IVTT by using an E. coli extract. The tetrameric proteins formed in the IVTT mix are stable in SDS and therefore can be purified by SDS-PAGE. The extracted protein inserts spontaneously into planar lipid bilayers. By using single-channel recording, it was shown that the activity of KcsA extracted from gels is similar to the activity of KcsA purified by conventional means.
Cell-free expression and purification of heteromeric KcsA channels
Cell-free protein expression and purification by SDS-PAGE eliminates the time-consuming steps of traditional membrane protein purification protocols. In addition, in vitro synthesis provides numerous useful options, including the labeling of amino acids and the incorporation of unnatural amino acids (Spirin, 2002; Wang et al., 2006). Here, we have exploited one such application, namely, the production of heteromers of an oligomeric protein. Natural KcsA is a homotetramer. Therefore, it is difficult to analyze the contributions to protein function of individual subunits and the interactions between them. To this end, heterooligomers formed from WT and mutated subunits can be used. Two major techniques have been used previously for the production of heteromeric ion channels. The first is in vivo coexpression of WT and mutated polypeptides. This technique, combined with data-fitting analyses of macroscopic currents, was useful in determining the subunit stoichiometry of channels such as Shaker Kv (MacKinnon, 1991). The second technique uses tandemly fused polypeptides connected by flexible linkers. The genes are built from WT and mutant genes fused tail to head. This technique has been used in analyses of subunit cooperativity in processes including the activation of Kv channels (Zandany et al., 2008). One of the limitations of the first approach is the stochastic (or otherwise) sorting of the WT and mutant subunits in the heteromers. The second method addresses this problem. However, the fact that the subunits are fused tail to head can affect their ability to function normally or to insert correctly into membranes (McCormack et al., 1992; Sack et al., 2008).
In the study presented here, an IVTT system was used to produce heteromers of WT and mutant KcsA subunits with peptide extensions at their N termini. These extensions allowed heteromers with different ratios of WT and mutant subunits to be separated on the basis of size by electrophoresis. In this way, our technique overcomes the limitations of the other approaches; heteromers are produced in which the subunits are not covalently connected and the stoichiometry of the subunits is known. In recent years, this method has been used extensively to form heteroheptamers of α-hemolysin pores (Bayley and Jayasinghe, 2004). In the study presented here, the peptide extensions were removed by proteolysis, so that the subunits in the tetramers differed only by the desired mutation at position 71. This was an important step because the extensions were up to about one quarter of the mass of each monomer and situated close to the channel entrance. They might therefore have influenced channel activity. Both KcsA and α-hemolysin form stable oligomers in SDS polyacrylamide gels and therefore can be purified by electrophoresis. “Native” gel electrophoresis might be suitable for the separation and purification of oligomeric proteins that are not stable to SDS-PAGE.
The E71A mutation affects KcsA channel stability and ion conduction
E71A, the mutation used throughout this work, was shown to affect the stability of KcsA channels and their gating and conductance properties. The source of these effects is likely to be the elimination of the carboxyl–carboxylate interaction between Asp80 and Glu71 (Cordero-Morales et al., 2006b). As a result, the side chain of Asp80 becomes more flexible, which disrupts its interaction with Trp67. Consequently, Trp67 is able to switch from one rotameric state to another (Cordero-Morales et al., 2006b). In WT channels, the side chain of Trp67 is turned toward the adjacent protomer and lies in close proximity to several residues, including Arg89, which has been implicated in the stability of the tetramers (Irizarry et al., 2002). In E71A4, one of the rotamers of Trp67 is orientated away from the contact plane with the adjacent protomer (whereas the other is located as found in the WT channel). Therefore, the flexibility of Trp67 in E71A channels weakens the stability of the tetramer. Inorganic cations known to permeate or strongly block K+ channels confer stability to the KcsA channel (Krishnan et al., 2005), and indeed we found that the addition of 10 mM KCl to SDS polyacrylamide gels increased the stability of the E71A homotetramer.
Our results also show that the inward conductance of E71A KcsA channels is much higher than that of WT channels and is reduced at low pH. In the crystal structure of the E71A homotetramers (in the flipped conformation; Cordero-Morales et al., 2006b), Asp80 residues are exposed at the extracellular entrance. Therefore, our findings suggest that the exposed side chains create a ring of negative charge that decreases the height of the energy barrier for ion entry into the selectivity filter and therefore increases the inward current. At low pH, the Asp80 side chains become protonated, and their effect on the electrostatic potential at the entrance is reduced. By using the WT-E71A heteromers, it was shown that the addition of each E71A subunit to the tetrameric channel makes a cumulative contribution to the elevation of the inward conductance. In the flipped crystal structure of E71A tetramers, the distance between the carboxyl groups of the Asp80 side chains is ∼9 Å. Because the Debye length in 200 mM KCl is ∼7 Å, the independence of the contributions of each carboxyl group makes sense. Therefore, the stability of the intraprotomeric network of interactions between the pore helix and the external entrance of the channel is a major factor in the outward rectification of the WT KcsA channel.
The inactivation of KcsA as an allosteric process
Based on electrophysiological measurements of mutated channels and molecular dynamics simulations, it has been proposed that an intraprotomeric network of interactions between Glu71 and Trp67 in the pore helix and Asp80 in the external entrance of KcsA (Fig. 1) promotes a distortion of the filter into a nonconductive configuration, leading to inactivation (Cordero-Morales et al., 2006b). In addition, because the open probability of the channel is increased at high positive applied potentials, it has been suggested that membrane depolarization relieves the inactivation of the channel by pulling Glu71 toward the intracellular side of the bilayer, thereby disrupting the network (Cordero-Morales et al., 2006a). The open probability of E71A4 is much higher than that of WT4, and it is voltage independent. Based on these results, it was proposed that the E71A mutation essentially abolishes inactivation because the presence of Ala in position 71 cannot support the network of interactions (Cordero-Morales et al., 2006b, 2007).
As mentioned earlier, our measurements of the unitary conductance of WT/E71A heteromers suggest that the identity of the residue at position 71 (Glu or Ala) in a protomer determines whether Asp80 in the same subunit is buried in the protein or exposed at the external entrance. Therefore, the network of interactions that is described above only exists in WT subunits and not in E71A subunits, whether they are present in homotetramers or heterotetramers. From our examination of inactivation, we surmise that the tetrameric selectivity filter can be in only one of two configurations: active (conductive) or inactive (nonconductive). For example, we did not observe intermediate current steps between the closed and the open levels (although we cannot reject the possibility that very short-lived states exist that were not acquired in our measurements). In other words, the transition of the four selectivity filter sequences (TVGYG) between their active and inactive conformations is concerted. Therefore, we can describe the inactivation of KcsA as an allosteric process, where the permeability of the selectivity filter (the active site) is affected by the state of the four independent networks of interactions between the pore helices and the external entrance (together: the regulatory sites). Each WT subunit in a tetramer contains a functional regulatory site that stabilizes the inactive conformation of the selectivity filter. In an E71A protomer, this regulatory site is defective.
Our results show that under strongly depolarizing potentials (above +50 mV), the open probabilities of WT/E71A heteromers with one or more E71A subunits are similar to the open probability of E71A homotetramers (Fig. 9 A and Table I). Therefore, we suggest that under these conditions, the transition of the selectivity filter sequences into the inactive state in all E71A-containing heteromers is defective. On the other hand, channels with four WT subunits have a much lower open probability in this voltage range, reflected by their greater tendency to inactivate. The network of interactions that stabilizes the inactivated state is relatively weak under a strong depolarizing potential, which pulls Glu71 toward the intracellular side of the bilayer, disrupting the network of interactions between Glu71, Trp67, and Asp80. We propose that, under these conditions, the concerted transition of the selectivity filter sequences of all four subunits into their inactive conformation will happen only when all of the subunits are stabilized in that conformation by the regulatory network (Fig. 10).
Figure 10.
A model for inactivation of KcsA channels as an allosteric process. Upon inactivation, there is a concerted transition of all four selectivity filter sequences (TVGYG) from the active conformation (green symbols) to the inactive conformation (red symbols). The conformation of the selectivity filter (the “active site” by analogy with allosteric enzymes) is regulated by networks of interactions between the pore helix and the external entrance of the channel that are formed independently within each subunit of the tetramer (the “regulatory sites” by analogy with allosteric enzymes). The selectivity filter sequence of each WT KcsA protomer is stabilized in its inactive conformation (striped symbols). On the other hand, the regulatory sites of E71A protomers are defective and do not support the stabilization of the inactive conformation of the selectivity filter sequences (open symbols). Under highly depolarizing potentials (top panel), the network of interactions is weakened (Cordero-Morales et al., 2006a). Under these conditions, the concerted transition of all four selectivity filter sequences into inactive conformations happens only when all four are stabilized by their regulatory site. When the membrane potential is negative (bottom panel), the network of interactions in the regulatory site becomes stronger. As a result, the concerted transition of the four selectivity filter sequences into inactive conformations occurs even when one of the sequences is not stabilized in the inactive conformation by its regulatory site. Pale red symbols correspond to unfavorable states of the KcsA channel.
A decrease in the membrane potential does not affect the open probability of channels that contain two or more E71A subunits. In contrast, the open probabilities of E71A1WT3 and WT4 are reduced as the membrane potential becomes more negative (Fig. 9, A and B, and Table I). It has been suggested that at more negative potentials, the network of interactions in WT subunits that stabilizes inactivation becomes stronger (Cordero-Morales et al., 2006a). We propose that, under these conditions, the concerted transition of the selectivity filter sequences of all the subunits of a tetramer into the inactive conformation occurs even when the regulatory network is broken in one of the subunits (Fig. 10). However, because the open probability of channels with one E71A protomer is higher than WT4 across the entire measured voltage range (Fig. 9 A), we also propose that at hyperpolarized potentials (when the network of interactions is relatively strong), the destabilization of the regulatory network in one protomer still exerts a negative effect on the concerted transition of the selectivity filter sequences into the inactive conformation.
Conclusion
These studies of WT/E71A KcsA heteromers have offered a glimpse into the mechanism of inactivation of the KcsA channel. It was previously shown that the C-type inactivation of Kv channels is a cooperative process that involves a concerted conformational change in all four protomers (Ogielska et al., 1995; Panyi et al., 1995). Superimposition of the KcsA crystal structure (PDB entry 1K4C) with the crystal structures of the Kv1.2 channel from the Shaker family (PDB entries 2A79 and 2R9R) and the KvAP channel (PDB entry 1ORQ) suggests that in these channels there also exist networks of interactions between the pore helix and the external entrance of the channel. In addition, mutation of the residue corresponding to Glu71 of KcsA in the Shaker Kv (Val438) (Yifrach and MacKinnon, 2002) and Kv2.1 (Ile369) channels (De Biasi et al., 1993) affects the C-type inactivation of these channels. Therefore, we can speculate that C-type inactivation of voltage-gated channels, like KcsA, is also regulated by an allosteric site. In contrast with the KcsA channel, it has been shown that C-type inactivation in Shaker Kv channels is voltage independent, at least in the range of −25 to +50 mV (Hoshi et al., 1994). As proposed earlier (Chakrapani et al., 2007a), the absence of a charged residue in “position 71” of these channels might be the basis for the difference in the voltage dependency. Further, it was shown that when the conserved Val in position 370 (equivalent to position 71 in KcsA) of the Shaker channel Kv1.2 was mutated to Glu, the inactivation of channel was accelerated (Cordero-Morales et al., 2007).
In conclusion, comparison of the single-channel activity of homomeric and heteromeric channels formed from WT and E71A KcsA subunits shows that the stability of the intraprotomeric network of interactions between the pore helix and the external entrance of the channel makes a major contribution to both the outward rectification of the channel and to its transition into an inactive state. The effect of the stability of the network on the ion conductance is graded: the more protomers with a stabilized network, the greater the reduction in the inward current. On the other hand, channel inactivation, which is a concerted process, occurs only when, depending on the membrane polarization, the network of interactions is stabilized in three or four protomers.
Acknowledgments
This work was supported by a grant from the Medical Research Council. H. Bayley is the holder of a Royal Society Wolfson Research Merit Award.
Christopher Miller served as editor.
Footnotes
Abbreviations used in this paper:
- DPhPC
- 1,2-diphytanoyl-sn-glycero-3-phosphocholine
- IVTT
- in vitro transcription and translation
- POPE
- 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine
- POPG
- 1-palmitoyl-2-oleoyl-sn-glycero-3-[phospho-rac-(1-glycerol)]
- TEV
- tobacco etch virus
- WT
- wild-type
References
- Baker K.A., Tzitzilonis C., Kwiatkowski W., Choe S., Riek R. 2007. Conformational dynamics of the KcsA potassium channel governs gating properties. Nat. Struct. Mol. Biol. 14:1089–1095 10.1038/nsmb1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayley H., Jayasinghe L. 2004. Functional engineered channels and pores (Review). Mol. Membr. Biol. 21:209–220 10.1080/09687680410001716853 [DOI] [PubMed] [Google Scholar]
- Blunck R., Cordero-Morales J.F., Cuello L.G., Perozo E., Bezanilla F. 2006. Detection of the opening of the bundle crossing in KcsA with fluorescence lifetime spectroscopy reveals the existence of two gates for ion conduction. J. Gen. Physiol. 128:569–581 10.1085/jgp.200609638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braha O., Walker B., Cheley S., Kasianowicz J.J., Song L., Gouaux J.E., Bayley H. 1997. Designed protein pores as components for biosensors. Chem. Biol. 4:497–505 10.1016/S1074-5521(97)90321-5 [DOI] [PubMed] [Google Scholar]
- Brelidze T.I., Niu X., Magleby K.L. 2003. A ring of eight conserved negatively charged amino acids doubles the conductance of BK channels and prevents inward rectification. Proc. Natl. Acad. Sci. USA. 100:9017–9022 10.1073/pnas.1532257100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvacho I., Gonzalez W., Torres Y.P., Brauchi S., Alvarez O., Gonzalez-Nilo F.D., Latorre R. 2008. Intrinsic electrostatic potential in the BK channel pore: role in determining single channel conductance and block. J. Gen. Physiol. 131:147–161 10.1085/jgp.200709862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrapani S., Cordero-Morales J.F., Perozo E. 2007a. A quantitative description of KcsA gating I: macroscopic currents. J. Gen. Physiol. 130:465–478 10.1085/jgp.200709843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrapani S., Cordero-Morales J.F., Perozo E. 2007b. A quantitative description of KcsA gating II: single-channel currents. J. Gen. Physiol. 130:479–496 10.1085/jgp.200709844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheley S., Malghani M.S., Song L., Hobaugh M., Gouaux J.E., Yang J., Bayley H. 1997. Spontaneous oligomerization of a staphylococcal alpha-hemolysin conformationally constrained by removal of residues that form the transmembrane beta-barrel. Protein Eng. 10:1433–1443 10.1093/protein/10.12.1433 [DOI] [PubMed] [Google Scholar]
- Choi H., Heginbotham L. 2004. Functional influence of the pore helix glutamate in the KcsA K+ channel. Biophys. J. 86:2137–2144 10.1016/S0006-3495(04)74273-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlan S.P. 2003. Biochemical and biophysical characterization of a monomeric porin, OmpG. PhD thesis. Texas A&M University, College Station, TX: 180 pp [Google Scholar]
- Cordero-Morales J.F., Cuello L.G., Perozo E. 2006a. Voltage-dependent gating at the KcsA selectivity filter. Nat. Struct. Mol. Biol. 13:319–322 10.1038/nsmb1070 [DOI] [PubMed] [Google Scholar]
- Cordero-Morales J.F., Cuello L.G., Zhao Y., Jogini V., Cortes D.M., Roux B., Perozo E. 2006b. Molecular determinants of gating at the potassium-channel selectivity filter. Nat. Struct. Mol. Biol. 13:311–318 10.1038/nsmb1069 [DOI] [PubMed] [Google Scholar]
- Cordero-Morales J.F., Jogini V., Lewis A., Vásquez V., Cortes D.M., Roux B., Perozo E. 2007. Molecular driving forces determining potassium channel slow inactivation. Nat. Struct. Mol. Biol. 14:1062–1069 10.1038/nsmb1309 [DOI] [PubMed] [Google Scholar]
- Cortes D.M., Cuello L.G., Perozo E. 2001. Molecular architecture of full-length KcsA: role of cytoplasmic domains in ion permeation and activation gating. J. Gen. Physiol. 117:165–180 10.1085/jgp.117.2.165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuello L.G., Romero J.G., Cortes D.M., Perozo E. 1998. pH-dependent gating in the Streptomyces lividans K+ channel. Biochemistry. 37:3229–3236 10.1021/bi972997x [DOI] [PubMed] [Google Scholar]
- De Biasi M., Hartmann H.A., Drewe J.A., Taglialatela M., Brown A.M., Kirsch G.E. 1993. Inactivation determined by a single site in K+ pores. Pflugers Arch. 422:354–363 10.1007/BF00374291 [DOI] [PubMed] [Google Scholar]
- Doyle D.A., Morais Cabral J., Pfuetzner R.A., Kuo A., Gulbis J.M., Cohen S.L., Chait B.T., MacKinnon R. 1998. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science. 280:69–77 10.1126/science.280.5360.69 [DOI] [PubMed] [Google Scholar]
- Gao L., Mi X., Paajanen V., Wang K., Fan Z. 2005. Activation-coupled inactivation in the bacterial potassium channel KcsA. Proc. Natl. Acad. Sci. USA. 102:17630–17635 10.1073/pnas.0505158102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouaux E., Mackinnon R. 2005. Principles of selective ion transport in channels and pumps. Science. 310:1461–1465 10.1126/science.1113666 [DOI] [PubMed] [Google Scholar]
- Heginbotham L., Odessey E., Miller C. 1997. Tetrameric stoichiometry of a prokaryotic K+ channel. Biochemistry. 36:10335–10342 10.1021/bi970988i [DOI] [PubMed] [Google Scholar]
- Heginbotham L., Kolmakova-Partensky L., Miller C. 1998. Functional reconstitution of a prokaryotic K+ channel. J. Gen. Physiol. 111:741–749 10.1085/jgp.111.6.741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heginbotham L., LeMasurier M., Kolmakova-Partensky L., Miller C. 1999. Single streptomyces lividans K+ channels: functional asymmetries and sidedness of proton activation. J. Gen. Physiol. 114:551–560 10.1085/jgp.114.4.551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden M.A., Bayley H. 2005. Direct introduction of single protein channels and pores into lipid bilayers. J. Am. Chem. Soc. 127:6502–6503 10.1021/ja042470p [DOI] [PubMed] [Google Scholar]
- Hoshi T., Zagotta W.N., Aldrich R.W. 1994. Shaker potassium channel gating. I: transitions near the open state. J. Gen. Physiol. 103:249–278 10.1085/jgp.103.2.249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howorka S., Bayley H. 1998. Improved protocol for high-throughput cysteine scanning mutagenesis. Biotechniques. 25:764–766, 768, 770 passim [DOI] [PubMed] [Google Scholar]
- Howorka S., Cheley S., Bayley H. 2001. Sequence-specific detection of individual DNA strands using engineered nanopores. Nat. Biotechnol. 19:636–639 10.1038/90236 [DOI] [PubMed] [Google Scholar]
- Imoto K., Busch C., Sakmann B., Mishina M., Konno T., Nakai J., Bujo H., Mori Y., Fukuda K., Numa S. 1988. Rings of negatively charged amino acids determine the acetylcholine receptor channel conductance. Nature. 335:645–648 10.1038/335645a0 [DOI] [PubMed] [Google Scholar]
- Irizarry S.N., Kutluay E., Drews G., Hart S.J., Heginbotham L. 2002. Opening the KcsA K+ channel: tryptophan scanning and complementation analysis lead to mutants with altered gating. Biochemistry. 41:13653–13662 10.1021/bi026393r [DOI] [PubMed] [Google Scholar]
- Jones D.H. 1995. PCR mutagenesis and recombination in vivo. PCR Primer: A Laboratory Manual Dieffenbach C.W., Dveksler G.S., Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY: 591–601 [Google Scholar]
- Krishnan M.N., Bingham J.P., Lee S.H., Trombley P., Moczydlowski E. 2005. Functional role and affinity of inorganic cations in stabilizing the tetrameric structure of the KcsA K+ channel. J. Gen. Physiol. 126:271–283 10.1085/jgp.200509323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeMasurier M., Heginbotham L., Miller C. 2001. KcsA: it's a potassium channel. J. Gen. Physiol. 118:303–314 10.1085/jgp.118.3.303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockless S.W., Zhou M., MacKinnon R. 2007. Structural and thermodynamic properties of selective ion binding in a K+ channel. PLoS Biol. 5:e121 10.1371/journal.pbio.0050121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long S.B., Campbell E.B., Mackinnon R. 2005. Crystal structure of a mammalian voltage-dependent Shaker family K+ channel. Science. 309:897–903 10.1126/science.1116269 [DOI] [PubMed] [Google Scholar]
- López-Barneo J., Hoshi T., Heinemann S.H., Aldrich R.W. 1993. Effects of external cations and mutations in the pore region on C-type inactivation of Shaker potassium channels. Receptors Channels. 1:61–71 [PubMed] [Google Scholar]
- MacKinnon R. 1991. Determination of the subunit stoichiometry of a voltage-activated potassium channel. Nature. 350:232–235 10.1038/350232a0 [DOI] [PubMed] [Google Scholar]
- MacKinnon R. 2004. Potassium channels and the atomic basis of selective ion conduction (Nobel Lecture). Angew. Chem. Int. Ed. Engl. 43:4265–4277 10.1002/anie.200400662 [DOI] [PubMed] [Google Scholar]
- Marius P., Zagnoni M., Sandison M.E., East J.M., Morgan H., Lee A.G. 2008. Binding of anionic lipids to at least three nonannular sites on the potassium channel KcsA is required for channel opening. Biophys. J. 94:1689–1698 10.1529/biophysj.107.117507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason A.E. 2008. Single-channel characterisation of potassium channels with high temperature studies. PhD thesis. University of Oxford, England, UK: 200 pp [Google Scholar]
- McCormack K., Lin L., Iverson L.E., Tanouye M.A., Sigworth F.J. 1992. Tandem linkage of Shaker K+ channel subunits does not ensure the stoichiometry of expressed channels. Biophys. J. 63:1406–1411 10.1016/S0006-3495(92)81703-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montal M., Mueller P. 1972. Formation of bimolecular membranes from lipid monolayers and a study of their electrical properties. Proc. Natl. Acad. Sci. USA. 69:3561–3566 10.1073/pnas.69.12.3561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimigean C.M., Chappie J.S., Miller C. 2003. Electrostatic tuning of ion conductance in potassium channels. Biochemistry. 42:9263–9268 10.1021/bi0348720 [DOI] [PubMed] [Google Scholar]
- Ogielska E.M., Zagotta W.N., Hoshi T., Heinemann S.H., Haab J., Aldrich R.W. 1995. Cooperative subunit interactions in C-type inactivation of K channels. Biophys. J. 69:2449–2457 10.1016/S0006-3495(95)80114-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panyi G., Sheng Z., Deutsch C. 1995. C-type inactivation of a voltage-gated K+ channel occurs by a cooperative mechanism. Biophys. J. 69:896–903 10.1016/S0006-3495(95)79963-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks T.D., Leuther K.K., Howard E.D., Johnston S.A., Dougherty W.G. 1994. Release of proteins and peptides from fusion proteins using a recombinant plant virus proteinase. Anal. Biochem. 216:413–417 10.1006/abio.1994.1060 [DOI] [PubMed] [Google Scholar]
- Qian X., Nimigean C.M., Niu X., Moss B.L., Magleby K.L. 2002. Slo1 tail domains, but not the Ca2+ bowl, are required for the β1 subunit to increase the apparent Ca2+ sensitivity of BK channels. J. Gen. Physiol. 120:829–843 10.1085/jgp.20028692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack J.T., Shamotienko O., Dolly J.O. 2008. How to validate a heteromeric ion channel drug target: assessing proper expression of concatenated subunits. J. Gen. Physiol. 131:415–420 10.1085/jgp.200709939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrempf H., Schmidt O., Kümmerlen R., Hinnah S., Müller D., Betzler M., Steinkamp T., Wagner R. 1995. A prokaryotic potassium ion channel with two predicted transmembrane segments from Streptomyces lividans. EMBO J. 14:5170–5178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim J.W., Yang M., Gu L.-Q. 2007. In vitro synthesis, tetramerization and single channel characterization of virus-encoded potassium channel Kcv. FEBS Lett. 581:1027–1034 10.1016/j.febslet.2007.02.005 [DOI] [PubMed] [Google Scholar]
- Spirin A.S. 2002. Cell-free protein synthesis. Cell-Free Translation Systems. Spirin A.S., editor Springer, New York: 3–20 [Google Scholar]
- Suh-Lailam B.B., Hevel J.M. 2009. Efficient cleavage of problematic tobacco etch virus (TEV)-protein arginine methyltransferase constructs. Anal. Biochem. 387:130–132 10.1016/j.ab.2008.12.031 [DOI] [PubMed] [Google Scholar]
- Thompson A.N., Posson D.J., Parsa P.V., Nimigean C.M. 2008. Molecular mechanism of pH sensing in KcsA potassium channels. Proc. Natl. Acad. Sci. USA. 105:6900–6905 10.1073/pnas.0800873105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valiyaveetil F.I., Zhou Y., MacKinnon R. 2002. Lipids in the structure, folding, and function of the KcsA K+ channel. Biochemistry. 41:10771–10777 10.1021/bi026215y [DOI] [PubMed] [Google Scholar]
- van Dalen A., Schrempf H., Killian J.A., de Kruijff B. 2000. Efficient membrane assembly of the KcsA potassium channel in Escherichia coli requires the protonmotive force. EMBO Rep. 1:340–346 10.1093/embo-reports/kvd067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dalen A., Hegger S., Killian J.A., de Kruijff B. 2002. Influence of lipids on membrane assembly and stability of the potassium channel KcsA. FEBS Lett. 525:33–38 10.1016/S0014-5793(02)03061-2 [DOI] [PubMed] [Google Scholar]
- Wang L., Xie J., Schultz P.G. 2006. Expanding the genetic code. Annu. Rev. Biophys. Biomol. Struct. 35:225–249 10.1146/annurev.biophys.35.101105.121507 [DOI] [PubMed] [Google Scholar]
- Yellen G. 1998. The moving parts of voltage-gated ion channels. Q. Rev. Biophys. 31:239–295 10.1017/S0033583598003448 [DOI] [PubMed] [Google Scholar]
- Yifrach O., MacKinnon R. 2002. Energetics of pore opening in a voltage-gated K(+) channel. Cell. 111:231–239 10.1016/S0092-8674(02)01013-9 [DOI] [PubMed] [Google Scholar]
- Zandany N., Ovadia M., Orr I., Yifrach O. 2008. Direct analysis of cooperativity in multisubunit allosteric proteins. Proc. Natl. Acad. Sci. USA. 105:11697–11702 10.1073/pnas.0804104105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Morais-Cabral J.H., Kaufman A., MacKinnon R. 2001. Chemistry of ion coordination and hydration revealed by a K+ channel-Fab complex at 2.0 Å resolution. Nature. 414:43–48 10.1038/35102009 [DOI] [PubMed] [Google Scholar]