The human immunodeficiency virus (HIV), the cause of acquired immunodeficiency syndrome (AIDS) has infected an estimated 33 million individuals worldwide{1}. HIV is a member of the lentivirus genus, part of the Retroviridae (retrovirus) family{2}. HIV is associated with immunodeficiency, neoplasia and neurological disease.
The development of an identifiable neurological syndrome in an HIV infected person is the culmination of a chain of events, determined by properties of HIV itself, genetic characteristics of the host, and interactions with the environment (including treatment). HIV-associated neurological syndromes can be classified as primary HIV neurological disease (in which HIV is both necessary and sufficient to cause the illness), secondary or opportunistic neurological disease (in which HIV interacts with other pathogens, resulting in opportunistic infections ((OI)) and tumors), and treatment related neurological disease (such as immune reconstitution inflammatory syndrome or IRIS).
HIV is neuroinvasive (can enter the central nervous system ((CNS)), neurotrophic (can live in neural tissues), and neurovirulent (causes disease of the nervous system){2}. Presumed mechanisms of CNS invasion include the “Trojan horse” mechanism in which HIV-infected monocytes are admitted by the blood-brain barrier and mature into long-lived, persistently infected perivascular macrophages; infection of the choroid plexus; and direct infection of capillary endothelial cells, among others. HIV-infected cells include capillary endothelium, microglia, monocytes, macrophages, astrocytes, and choroid plexus {3}. Neurons and oligodendrocytes are rarely, if ever, infected (although this is still under discussion), and “indirect” mechanisms are postulated to account for most damage {4}. There is a burst of viral replication in primary infection, followed by an aggressive immune response that declines over time, and by a long period of subclinical infection, followed by recrudescence of disease, and death {2}. Persistent infection and inflammation results in blood-brain barrier breakdown, neuronal and axonal injury, neurotoxicity, and clinical symptoms; damage to the immune system, particularly cell-mediated immunity, results in vulnerability to OI.
In addition to its importance as a cause of neurological problems, HIV infection of the CNS constitutes a serious barrier to management and eradication of the virus. The CNS is incompletely permeable to antiretroviral drugs, resulting in subtherapeutic levels of many antiretrovirals {5}; it is part of a protected reservoir {6} (along with the gut and several other organs), where HIV can evade the immune system; and it provides an environment where HIV can replicate, mutate, and re-infect the circulation. HIV stimulates a persistent inflammatory response that may activate pathways leading to other neurodegenerative diseases {7}.
HIV-1-associated syndromes in primary infection
Acute HIV infection is the period from initial infection to complete seroconversion. During this time 40-90% of individuals describe physical symptoms, similar to influenza, or mononucleosis. The most common features include a short period of fever, lymphadenopathy, night sweats, headache, and/or rash {8, 9}. Early CNS infection is usually asymptomatic, but cerebrospinal fluid (CSF) {10} and imaging studies {11} can detect abnormalities even during the “asymptomatic” period that presage later neurological events.
A minority of seroconverters will experience a neurologic event that brings them to medical attention, such as aseptic meningitis, Bell’s palsy {12, 13}, or inflammatory neuropathy. Individuals with symptomatic neurological disease tend to have higher CSF HIV levels than those without. Neurological symptoms may occur before an HIV diagnosis is suspected, e.g., before there are sufficient HIV antibodies to produce a positive HIV enzyme-linked immunosorbent antibody (ELISA, also called an HIV enzyme immunoassay). In such cases, a Western Blot or a polymerase chain reaction (PCR) test for HIV may lead to the diagnosis. Early diagnosis of acute HIV infection is important, as these individuals are at high risk to transmit the virus.
The most common neurologic syndrome associated with primary HIV infection is an acute aseptic (viral) meningitis or meningoencephalitis. The symptoms are similar to other viral meningitides, with fever, headache, stiff neck, and photophobia. Cerebrospinal fluid (CSF) shows a mild lymphocytic pleocytosis, normal or slightly elevated total protein, and normal glucose {14}. HIV may be detectable by antigen or PCR testing {15}. Most individuals will recover with supportive care. A few will have recurrent bouts.
Information on the management of HIV aseptic meningitis is limited to case reports. Initiating treatment with cART, or changing and intensifying the regimen to include more CNS-penetrating drugs, may suppress the symptoms {16}. Others have recurrent meningitis when they stop combined antiretroviral therapy (cART), e.g. during structured treatment interruptions {17}.
HIV Associated Neurocognitive Disorders (HAND)
The most common CNS manifestation of HIV is a chronic neurodegenerative condition characterized by cognitive, central motor and behavioral abnormalities. A variety of names (e.g. AIDS Dementia Complex, HIV Associated Dementia, HIV Associated Cognitive Motor Complex) have been applied to this syndrome. Recently, HIV Associated Neurocognitive Disorder (HAND) {18} has become a widely accepted nosology for classifying individuals with varying levels of HIV-associated neurocognitive deficits. HAND is stratified into 1) asymptomatic neurocognitive impairment (ANI) 2) minor neurocognitive disorder (MND) and 3) HIV Associated Dementia (HAD). ANI is characterized a subclinical decline in cognition. MND is characterized as mild decline in cognition in addition to mild everyday functioning impairment that affects the more difficult activities of daily living). HAD is characterized by significant decline in cognition along with a significant degree of functional impairment that affects routine activities{19}. There is no diagnostic marker or combination of markers for HAND. The diagnosis is made in HIV+ patients with cognitive impairment, after ruling out confounding conditions (CNS OI, neurosyphilis, substance abuse, delirium, toxic-metabolic disorders, psychiatric disease, age related dementias).
Although HAND can affect any neuropsychological domain, the most commonly reported deficits are in attention/concentration, psychomotor speed, memory and learning, information processing, and executive function, while language and visuospatial abilities are relatively unaffected {20}. HAND has been characterized as a “subcortical dementia”{21}, in which deficits in working memory (e.g., “short-term” memory, the ability to remember information over a brief period of time) and executive function (e.g., planning, cognitive flexibility, abstract thinking, rule acquisition, initiating appropriate actions and inhibiting inappropriate actions) tend to occur early on. Deficits in delayed recall are more typical of “cortical” dementias such as Alzheimer’s disease. Like other subcortical dementias, the pattern of episodic memory impairment in HAND is consistent with a mixed encoding and retrieval profile {22}, whereas that of Alzheimer’s Disease consists of rapid forgetting due to inability to encode novel information {23}.
HIV can have profound effects on the pyramidal and extrapyramidal motor systems. Milder manifestations of CNS motor impairment include ataxia, motor slowing, incoordination, and tremor. This may progress to disabling weakness, spasticity, extrapyramidal movement disorders, and paraparesis {24, 25}. Behavioral effects of HAND include apathy, irritability, and psychomotor retardation {26-28}, which can be mistaken for depression. This is difficult to disentangle because of the high rate of major depression and dysthymia in the HIV population, {29}, and because many symptoms queried in depression screening instruments, such as loss of appetite, can be due to HIV. Some AIDS patients develop “manic” symptoms {30}. Again, this must be disentangled from a pre-existing bipolar disorder, or a reaction to drugs. So-called “secondary” or AIDS mania tends to occur in patients with poorly controlled disease, concurrent cognitive deficits, irritability, aggression, and talkativeness, and have hallucinations and paranoia {31}.
Historically, the onset of HAND was associated with low CD4+ counts{32}, other AIDS symptoms{33}, elevated CSF viral load {34}, and elevated CSF markers of immune activation (e.g. beta 2 microglobulin and neopterin) {35}. Much of this dates back to the 1980s when no effective treatment was available and only the most demented patients came to medical attention {36, 37}. At that time, a diagnosis of HAD was considered to be a precursor of death {32}. These aggressive forms of HAND remain prevalent in Third World countries and among individuals with late diagnosis, or who have refused or failed cART. However, such presentations are uncommon in cART-treated individuals, who manifest a milder, more slowly progressing and less lethal “attenuated” form of HAND {38, 39}.These mild presentations typically occur in persons with partial immune reconstitution, higher CD4 + counts, and suppressed viral loads, and are less strongly associated with markers of immune activation {40, 41} {42} {43}. Due to increased awareness of HAND it is also possible that it is identified at an earlier stage.
The traditional neuroimaging approach (in which a diagnosis is made through qualitative image examination) is useful in excluding structural or inflammatory processes, such as abscess, or tumor, that may mimic HAND, but is limited as a diagnostic marker. The HAND brain may appear grossly normal until advanced disease, when atrophy may be noted. White matter changes may appear, typically in the periventricular region. These must be differentiated from other diseases that affect myelin such as progressive multifocal leukoencephalopathy (PML), and unrelated processes such as leukoariosis. Currently, there are few ways to distinguish among these white matter abnormalities except for the pattern and distribution of the lesions, which are not definitive. Advanced, investigative neuroimaging techniques show promise as biomarkers of HAND {44}. Brain mapping indicates that there may be a unique pattern of atrophy in HAND that distinguishes it from other dementias. There are reports of ventricular enlargement, atrophy of the caudate, putamen, nucleus accumbens, and other subcortical regions that may characterize HAND {45, 46} {47}. Another technique that may identify HAND and measure its progression or improvement, is magnetic resonance spectroscopy (MRS){44, 45}. MRS is sensitive to the earliest signs of CNS infection. Lentz et al {48}, reported on subjects followed from early seroconversion and reported initial decline in n-acetylaspartate (NAA), a marker of neuronal viability, in the frontocortical gray matter. NAA levels were found to be decreased in the centrum semiovale white matter of chronically HIV-infected subjects, but not in early infection. This study is still ongoing so further follow up will be needed to map changes over time. Paul et al, {49} reported that impaired neuropsychological performance in HIV is associated with reduced NAA and increased myoinositol (MI, a marker of gliosis) in the basal ganglia and frontal white matter, but not with markers in the parietal region (Figure 1). Flurodeoxyglucose (FDG) positron emission tomography (PET) studies in HIV are few, but show early hypermetabolism in the basal ganglia {50}, followed by hypometabolism in late HAND {51}. This pattern is unlike Alzheimer’s disease and other dementias.
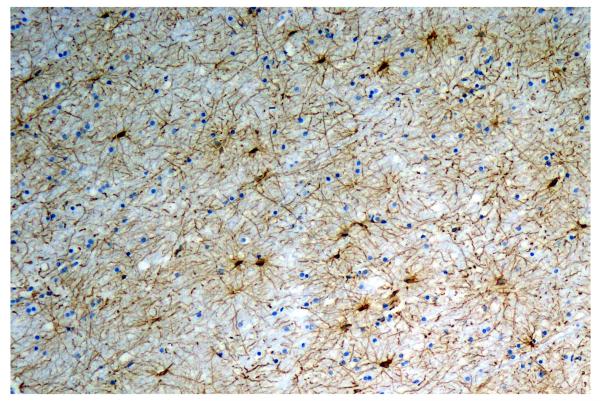
Figure 1.
Marked gliosis of the white matter is a common finding in HIV+ patients at autopsy and may underlie changes seen premortem by magnetic resonance spectroscopy (MRS). Immunoperoxidase stain (brown) for the astrocytic marker, glial fibrillary acidic protein (GFAP).
Neurological and neuropsychological improvement of HAND after treatment with antiretrovirals was established with studies of zidovudine monotherapy in randomized placebo-controlled trials that demonstrated improved neuropsychological test scores versus placebo {52, 53}. Current treatment of HIV is based on the use of cART, that includes two or more antiretroviral agents to suppress HIV, and increase CD4+ counts. The most common initial cART regimens consist of two nucleoside or nucleotide reverse transcriptase inhibitors (NRTIs) combined with either a non-nucleoside reverse transcriptase inhibitor (NNRTI) or a “boosted” protease inhibitor (PI). Ritonavir (in small doses) is a PI most commonly used as a booster; it enhances other PI so they can be given in lower doses. No placebo–controlled trials of cART for HAND have been performed, because it would be unethical to refuse treatment to a symptomatic patient. Based on well-conducted (albeit, not randomized, double blinded, controlled) studies {54}, it appears that cART improves neuropsychological performance {55}. It also appears that cART has reduced the incidence of new cases of severe HAND (HAD), while apparently increasing the number and lifespan of individuals living with milder forms of HAND {38, 56}.
The CNS penetration of the various components of a cART regimen may vary widely, and there is ongoing discussion regarding the importance of the CNS-penetrability of a cART regimen. Letendre et al., have proposed a CNS-Penetration Effectiveness (CPE) Rank {5} in which cART regimens are ranked by summing up various criteria associated with CNS effectiveness. In a large study, subjects whose regimens scored low on the CPE ranking had higher CSF viral loads{5} In a related study, Marra et al.{57}, confirmed the association of low CPE scores with higher probability of detectable CSF viral load, and poorer neuropsychological performance. An independent group {58} confirmed that higher CPE scores were associated with greater improvements in neuropsychological performance.
In addition to cART there have been attempts to identify non-antiretroviral neuroprotective drugs to avert HAND. A review of clinical studies {59} have been performed on memantine (an N-methyl-D-aspartate antagonist drug), selegiline (a monoamine oxidase-B inhibitor), nimodipine (a calcium channel blocker), and non-approved drugs Peptide T, CPI-1189, and others. None of the drugs studied were effective. Studies of valproic acid (an anticonvulsant) and minocycline (an antibiotic) are in progress.
There are a few studies of psychostimulant drugs to palliate neuropsychiatric symptoms frequently seen in HAND. These included a randomized, placebo-controlled trial of methylphenidate for severe HIV associated fatigue {60}, and a single-blind, placebo-controlled, crossover-design study of methylphenidate for HIV-associated cognitive slowing {61}. Both showed significantly more improvement with methylphenidate than placebo.
HIV-Associated Vacuolar Myelopathy (HIV VM)
Vacuolar myelopathy is the most common spinal cord disease in AIDS, found in up to 30% of Autopsies in the pre-cART era {62}. It is under diagnosed {63} and must be differentiated from other causes of myelopathy such as infection with Human T-cell lymphotropic virus (HTLV) I or II; herpes simplex 1 or 2, varicella zoster, cytomegalovirus, enteroviruses, syphilis and tuberculosis, tumors, and nutritional deficiencies such as B12 {64}. In the past, HIV VM was more common in patients with opportunistic conditions{63}, but this association has not be reviewed in the cART era.
The clinical presentation of HIV VM is slowly progressive leg weakness, which may be asymmetric at first, spasticity, dorsal column (vibration, position) sensory loss, ataxic gait, and urinary frequency and urgency{65}. Erectile dysfunction is an early sign in men. Paresthesias in the legs are common but neuropathic pain rarely rises to the level seen with peripheral neuropathy. There is prominent hyperreflexia, and extensor plantar responses. In advanced cases, patients may become wheelchair-bound and doubly incontinent {65}.The diagnosis should be questioned if symptoms present in an acute fashion, the arms are affected, there is a sensory level, or if there is back pain.
The most important test is a spinal MRI, to rule out abscess or tumor. In many HIV VM cases, the MRI is normal. Some will have high signal hyperintense areas on T2 weighted imaging, primarily in the thoracic region and affecting the posterior columns, that do not enhance with contrast; these areas correlate to vacuolation on histopathology{66}. Cord atrophy has also been reported{67}. A lumbar puncture is important to exclude treatable infections or carcinomatous meningitis. Somatosensory evoked potentials are useful to disentangle cases in which both HIV VM and sensory neuropathy are present {68}.
The precise pathophysiology of HIV VM is unknown. The distribution of pathology, involving the posterior columns and pyramidal tracts, resembles B12 deficiency {69}. The relationship to productive HIV infection within the cord remains controversial {70-73}. Suspected mechanisms include defective methylation due to a deficiency of S-adenosylmethionine {74}, triggered by inflammatory products secreted by activated macrophages and microglia {75} {76}.
There is no specific treatment for HIV VM. A pilot, open label study of L-methionine to address the suspected abnormality of transmethylation mechanisms in HIV VM did not show benefit{77}. There are case reports of improvement with cART{78-80}. However, axonal degeneration is a late feature of HIV VM {81}, and would not be expected to resolve. Patients with HIV VM benefit from physical and occupational therapy, baclofen, tizanidine, dantrolene, and intramuscular botulinum toxin to manage spasticity, pain management, and anticholinergic drugs to improve bladder function{65}.
HIV associated peripheral neuropathies
Many peripheral neuropathic syndromes have been reported in the context of HIV infection. Herein, we will confine our discussion to HIV-associated distal sensory neuropathy, neurotoxic nucleoside neuropathy, and inflammatory demyelinating neuropathy.
HIV associated distal peripheral sensory neuropathy (DSPN) (also called predominantly sensory neuropathy, or distal symmetrical peripheral neuropathy), is the most common neurological problem in AIDS {82}, with incidences ranging from 19-66%, depending upon the age, disease stage, and treatment history of the cohort {83}. The risk factors for HIV DSPN are older age, history of alcohol abuse, and advanced HIV disease (e.g., a low nadir CD4+ count and high plasma HIV viral load){84, 85}, prior use of a neurotoxic antiretroviral drug (e.g., didanosine, stavudine, zalcitabine), and diabetes.
The most universally reported symptoms are paresthesias {86}, that virtually always begin in the feet, as this is a length-dependent neuropathy. Patients complain of burning, of numbness, of hot or cold sensations, and of episodic electric-shock like sensations. Some complain of a sensation that they are walking on sand, or glass. Many cannot bear to wear shoes. The symptoms ascend over time, as far as the thighs, and will also involve the hands in a glove-like fashion. Cramps and fasiculations may develop in the extremities. Most patients do not develop any motor weakness or muscle wasting until late in their course, and this is limited to the distal extremities {87}. The most common physical findings are decreased or absent ankle jerks, diminished vibratory sensation in the legs, and increased threshold to temperature and pinprick (alternatively, some patients develop hyperesthesia) {87}. Some patients will have all the physical findings of neuropathy but do not report pain. They usually have an asymptomatic neuropathy. Others will have hyper-reflexia proximally and hypo-reflexia distally, in which case a mixed myelopathy and neuropathy should be suspected.
The pathogenesis of HIV DSPN is unknown. Related viruses such as feline immunodeficiency virus (FIV) also cause neuropathy in cats {88}. FIV (and HIV-1) infects and activates macrophages{88} and CD8+lymphocytes {89} in the dorsal root ganglion, and these cells can release substances, such as tumor necrosis factor {88}, that are toxic to neurons and oligodendrocytes. The HIV protein gp120 is also neurotoxic, causing hyperesthesia, allodynia, and spinal gliosis{90}. The major neuropathologic features in HIV DSPN include a loss of unmyelinated axons in the distal regions of sensory nerves, followed by Wallerian degeneration of the distal myelinated fibers. Some degree of demyelination and remyelination and has also been reported{91}.
In most cases, an electromyogram (EMG) and nerve conduction studies (NCS) are not necessary to diagnose HIV DSPN {92}. If an electrodiagnostic study is performed, it will demonstrate findings similar to other degenerative, predominantly axonal neuropathies, such as reduced or absent action potentials. A few patients have apparently normal studies. They most likely have a small-fiber neuropathy, and quantitative sensory testing may be helpful if there is a reason to document the clinical diagnosis. It is important to search for processes that can mimic or exacerbate HIV DSPN, including syphilis, diabetes, B12 or folate deficiency, thyroid disease, hepatitis C virus, and any neurotoxic medication.
Treatment of DSPN itself is generally frustrating. Studies of nerve growth factor {93} and the experimental drug prosaptide {94} were unproductive. Randomized, controlled clinical trials of drugs to control neuropathic pain showed positive results for lamotrigine {95}, for an experimental a high-concentration capsaicin dermal patch {96}, cannabinoids {97, 98} and gabapentin {99}. Treatments that were ineffective included amitriptyline {100, 101}, mexilitene,{100}, memantine {102}, Peptide T {103}, and acupuncture {101}.
Nucleoside neuropathy in HIV+ patients (also called antiretroviral toxic neuropathy, or neurotoxic neuropathy), has classically been associated with three NRTI drugs: didanosine (ddI), zalcitabine (ddC), and stavudine (d4T). These drugs were used extensively early in the epidemic, and they are still used in resource-limited settings. Other risk factors include another, prior neuropathy, diabetes, alcoholism, poor nutrition, using higher doses of the offending nucleoside, and use of more than one nucleoside {86, 104}. The clinical and electrophysiological features of neurotoxic neuropathy are very similar to HIV DSPN {105}, but usually begin within six months of starting the offending drug, with a peak around 3 months{106}. It has been proposed that mitochondrial toxicity {107}, competitive inhibition of human mitochondrial DNA polymerase-gamma {108}, downregulation of gene expression for brain derived neurotrophic factor in the dorsal root ganglion{109}, and specific host genetic polymorphisms {110} may predispose to nucleoside neuropathy. The only specific treatment is to remove the offending drug; if this is impossible, it should be maintained at the lowest dose. Many patients take up to three weeks after the drug is discontinued to see any improvement{111}; reduction in symptoms usually occurs by six weeks, but some may take up to six months.
Inflammatory Demyelinating Polyneuropathies (IDP)
The true prevalence of this complication is unknown, but it appears to be relatively rare. There are two major types of HIV inflammatory demyelinating polyneuropathies (HIV IDP). Acute IDP is similar to Guillain- Barre syndrome, and often occurs during or near primary infection {112}. Patients develop the rapid onset of ascending weakness, areflexia, autonomic instability, and some (usually minor) sensory symptoms{113}, but bowel and bladder function is spared. The disease can progress to involve the muscles of respiration. Unlike non-HIV Guillain Barre, there is usually a mild lymphocytic pleocytosis. Since the advent of cART, a few cases have occurred during immune reconstitution {114}.Electrophysiological studies show patchy distribution of abnormalities, including slow or absent nerve conduction, and abnormal F-waves{115}. Treatment consists of supportive care, intravenous gamma globulin, plasma exchange, and possibly cART {116-118}, and is based on case reports and extrapolation from the non-HIV literature {119}.
A chronic IDP (CIDP) may occur in late infection and is often associated with a CD4+ count of under 50 cells/mm3 {120}. Unlike acute HIV IDP, this syndrome progresses slowly and may have a relapsing and remitting nature. It must be differentiated from neuropathies caused by CMV and related viruses. Treatment of HIV CIDP is similar to that of non-HIV related CIDP, with the exception for the need to control HIV infection, and is based on case reports on HIV patients and the non-HIV literature.
Progressive Multifocal Leukoencephalopathy
Progressive multifocal leukoencephalopathy (PML) is a demyelinating disease of the CNS {121}. It is caused by the John Cunningham virus (JCV), a polyoma virus found world-wide, with a seroprevalence of 70-90% {122, 123}. Previously a rare disease, PML became a frequent (up to 5%) complication of AIDS in the 1980s {124}, although the incidence has declined with cART {125}. Recently, PML has received occurred in association with the treatments for multiple sclerosis and rheumatological disorders {126}.
Most cases of AIDS PML occur during severe immunosuppression (under 100 CD4+cells/mm3) although exceptions occur in about 11% of cases {124}. Most present with the subacute onset of altered mental status, accompanied by focal symptoms referable to the location of the one or more PML lesions, such as hemiparesis, hemianopsia, ataxia, vertigo, speech disorders, and seizures {125}. Patients usually do not have headaches, fevers, nausea, vomiting, or papilledema.
A definitive diagnosis is established by biopsy or autopsy. However, a diagnosis of probable PML can be made with a supportive clinical history along with correlative radiological and laboratory findings. The most common neuroimaging findings {127} are one or more space-occupying white matter lesions that are non-enhancing, hyperintense on T2 and hypointense on T1, and spare the cortical U-fibers. Having said that, some cases do enhance, a feature associated with a better prognosis {128}. There is no definitive MRI marker of PML. Cerebrospinal fluid examination (CSF) is invaluable in ruling out other infections, but otherwise is nonspecific, with mild pleocytosis, elevated total protein, and normal glucose. A positive JCV PCR is considered diagnostic in a case with typical clinical and imaging features; however, the sensitivity and specificity of this test is under discussion, as JCV has been detected in the CSF of immune suppressed persons without PML {129} and may not be detected in cART-treated patients with tissue-diagnosed PML or those with low JCV viral loads {130}. In a severe case such as shown in Figure 2 gross examination at autopsy may show multifocal areas of tan-gay discoloration and softening. The gray-white junction is a favored site. The larger lesions may be necrotizing at the center with loss of both myelin and axons and replacement by confluent lipid-laden macrophages. At the periphery there are scattered virally infected oligodendroglial cells with enlarged nuclei that contain deep amphophilic (purple) viral inclusions. PML lesions also contain grossly enlarged, pleomorphic “pseudoneoplastic” astrocytes. If necessary the diagnosis can be confirmed with immunohistochemistry or in situ hybridization for JCV.
Figure 2.
This coronal section through the formalin-fixed brain of an HIV+ person who succumbed to progressive multifocal leukoencephalopathy (PML) shows the characteristic multifocal areas of white matter discoloration.
There is no specific treatment for PML with or without AIDS. Multiple agents have been tried without success including topotecan {131}, cytarabine {132, 133}, and cidofovir {134}. However, cART has improved the course of AIDS PML, decreasing the mortality rate, improving the neuroimaging features, improving survival, and decreasing CSF JCV viral load {135, 136} {125}. Patients who survive AIDS PML are likely to have serious residual neurocognitive deficits. Levine et al (2008) reported that eight patients with past PML differed as a group from AIDS patients without history of CNS-OI with regards to information processing and motor functioning{137}. Further, while the PML group was less severely impaired overall than those with history of AIDS and toxoplasmosis, their deficits in information processing and motor functioning were the most severe of all groups examined.
Cytomegalovirus (CMV)
Cytomegalovirus (CMV), a member of the Herpesvirus family, can infect the brain, spinal cord, meninges, retina, the dorsal root ganglion of peripheral nerves, and many visceral organs {138}. Approximately 60% of the population show evidence of exposure to CMV {139} but the prevalence is higher in homosexual men {140}. Cytomegalovirus (CMV) establishes a life-long, latent infection without clinical disease in immunocompetent individuals after initial infection, and may remain latent for years, or reactivate under conditions such as HIV or other immunodeficient states. CMV and HIV are known to transactivate each other in vitro {141}.
Typically, CMV of the nervous system presents in individuals with CD4+ counts under 50 CD4+ cells/mm3, CMV viremia, and one or more systemic site(s) of infection {138}. Neurological CMV disease can present as encephalitis, ventriculitis, myelitis, radiculoganglionitis and peripheral polyneuropathy, or various combinations thereof {138, 142}. Presenting signs and symptoms are extremely variable depending upon the area affected; CMV encephalitis and ventriculitis may present with fever, lethargy, confusion, or coma, seizures, and cranial nerve palsies, ataxia and hemiparesis, or even coma; some patients present with dementia, which may or may not be due to a concurrent HIV encephalitis {143, 144}. CMV infection of the spinal cord may cause either a transverse myelitis or a myeloradiculitis characterized by flaccid paraparesis associated with back pain, incontinence, areflexia, paresthesias and sensory loss, and ascending weakness{145, 146}.
The CSF CMV PCR is considered the gold standard for identifying and quantifying CNS CMV and for following the response to therapy {147}. The literature also refers to a CSF profile that consists of a polymorphonuclear pleocytosis, {148}, but this is not a consistent finding{145}.
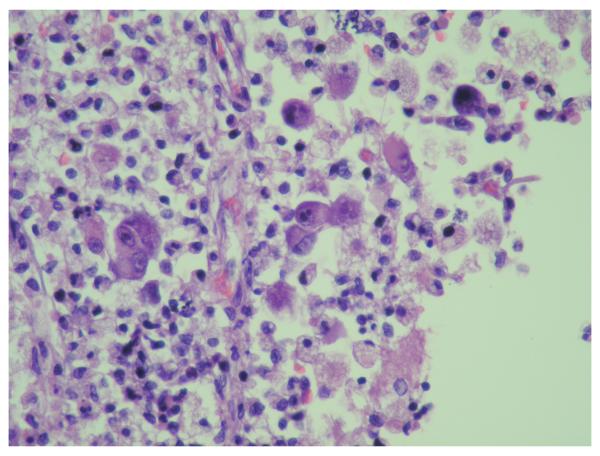
Unlike HIV, CMV can directly infect astrocytes, neurons, oligodendroglia, endothelial, ependyma, and meningeal cells {149, 150}, and can directly kill, neural cells, e.g., by inducing apoptosis {151, 152}. The most common pathological finding is a microglial nodule encephalitis, but other findings include ventriculoencephalitis (a focal or diffuse destruction of the ependymal lining and necrosis of periventricular tissue); focal necrosis; and isolated cytomegalic cells{153}.(Figure.3)
Figure 3.
Cytomegalovirus (CMV) infection of cells may result in the morphological changes shown here ie, cytomegaly and both intranuclear and intracytoplasmic viral inclusions. Immunohistochemistry for CMV (not shown) may detect additional infected cells that appear normal. Hematoxylin and Eosin stain.
Randomized, placebo-controlled trial data regarding treatment of CNS CMV is lacking. Based on data extrapolated from case reports and clinical trials of other organ systems, it is recommended that treatment be initiated immediately with intravenous ganciclovir, at an induction dose of 5mg/kg twice daily{54}. Intravenous foscarnet 90 mg/kg twice a day, can be used in lieu of ganciclovir{54} but has greater renal toxicity. Cidofovir can be used if these regimens fail {54}. Based on information extrapolated from other CMV infections, patients should continue chronic suppressive therapy, until CD4+ cell counts rise above 100 cells/mm3 {154}. Patients who have not started cART should do so, and those with suboptimal cART should have that adjusted {155}.
Cryptococal meningitis (CM)
Cryptococcus neoformans is an encapsulated yeast found throughout the world. It is spread through inhalation of spores, which can be found in dust and bird droppings. The initial infection is usually a self-limited pneumonitis. In most individuals the immune system clears the disease, but some the organism remains in a latent state within granulomas {156}, from which it can disseminate to multiple organs, particularly in immunosuppressed patients. In AIDS, the most common presentation is a subacute meningoencephalitis, usually in a patient with under 100 CD4+ cells/mm3. Cryptococcus has an affinity for the CNS, possibly related to its consumption of catecholamines {157}.
Common presenting symptoms of CM include malaise, headache, and fever. As the disease progresses, patients may develop seizures and signs of increased intracranial pressure (nausea, vomiting, visual loss, diplopia, coma){156}. A diagnosis of CM can be made by visualizing the yeast in CSF, using India ink; or by detecting cryptococcal antigen in the CSF using the latex agglutination test {54}. If lumbar puncture is contraindicated, a presumptive diagnosis can be made with a serum antigen test. AIDS patients may not have a CSF cellular pleocytosis, abnormal protein, or low CSF glucose{158}. Neuroimaging may be normal, but abnormalities such as masses (cryptococcomas), dilated perivascular spaces, or pseudocysts, are associated with higher blood and CSF antigen titers {159}.
Immediate treatment is essential to prevent loss of brain and loss of life, as this is a lethal disease and even with optimal treatment, the mortality rate is still 15% {160}. The recommended initial standard treatment is amphotericin B, at a dose of 0.7 mg/kg daily, combined with flucytosine, at a dose of 100 mg/kg daily in four divided doses, for at least 2 weeks for those with normal renal function {155}. Primary treatment with fluconazole has failed {161}. In addition to antifungal therapy and cART, it is important to manage increased intracranial pressure, as this may lead to permanent neurologic deficits, blindness, and death {162}. The CSF can be removed by repeated lumbar puncture, or a lumbar drain or shunt may be necessary{54}. After at least a 2-week period of successful induction therapy, defined as significant clinical improvement and a negative repeat CSF culture, amphotericin B and flucytosine may be discontinued and follow-up therapy initiated with fluconazole 400 mg daily {155}. This should continue for at least eight weeks. Discontinuation of secondary prophylaxis can be considered in patients with sterile CSF, clinical improvement, and an increase in CD4+ cell count to at least 200 cells/mm3.
With treatment, most HIV+ individuals will survive CM. Long-term outcomes in neurocognitive functioning have only recently been examined. In an exploratory study, Levine et. al., examined neurocognitive functioning in a cohort of fifteen individuals with history of AIDS and CM, compared to 61 individuals with AIDS, but without history of CNS disease. Those with a history of CM continued to demonstrate deficits in verbal fluency and motor functioning relative to HIV infected controls without CM.
Toxoplasmosis encephalitis (TE)
Toxoplasma gondii is a ubiquitous intracellular protozoan pathogen of both humans and animals. From 15% to 85% of the world’s adult human population is infected with Toxoplasma gondii depending on geographical location. The definitive host is the cat, but the parasite can be carried by all mammals. Infection can be acquired transplacentally, or by ingesting contaminated water, undercooked meat, soil, or cat feces. Once in the gut, the parasite disseminates to the brain, muscles, and eyes, and invades cells, where it forms intracellular cysts. Most primary infections are asymptomatic or there may be flu-like symptoms. The parasite may remain latent for years; cases of AIDS-associated Toxoplasmosis encephalitis (TE) almost always result from reactivation, usually when the CD4+ count has declined below 200 cells/mm3; higher risk is present when the CD4+ is under 50 cells/mm3 {155, 163}.
Fever, headache, focal neurological deficit, cognitive dysfunction, seizures, and altered mental status are the most common presenting symptoms of TE {164, 165}. Because these are highly inflammatory and necrotic lesions with mass effect, elevated intracranial pressure is often a serious problem. The typical neuroimaging presentation includes multiple (in seventy percent of cases), contrast-enhancing lesions, frequently surrounded by edema {166}. Most lesions are supratentorial, and located at the gray-white matter junction or in the basal ganglia. MRI typically shows several T2 weighted hyperintense lesions with enhancement on postcontrast T1 images{166}. Some lesions are hemmorhagic.{167}. The most important differential diagnosis in AIDS patients is primary central nervous system lymphoma (PCNSL). Some investigators advocate the use of thallium-201 brain single-photon emission CT {168} or positron emission tomography{169} (PET) to differentiate between PCNSL (which has a high rate of uptake) and TE (which does not). However, most physicians still require a tissue diagnosis before treating a patient for PCNSL because of the lack of specificity of these techniques. Cerebrospinal fluid (CSF) frequently is not sampled in TE, because the mass lesions may make lumbar puncture unsafe. It is useful in excluding other pathogens. Almost all AIDS patients with TE are have toxoplasma immunoglobulin G (IgG) antibodies in blood. While a definitive diagnosis requires brain biopsy, a response to empiric toxoplasmosis treatment is also considered to be diagnostic {54}; failure to respond is an indication for biopsy{155}. The response to anti-parasitic therapy may be confounded, however, if corticosteroids are required to reduce brain inflammation, intracranial pressure, and prevent herniation.
The treatment of choice for toxoplasmosis encephalitis is a combination of pyrimethamine (200 mg oral loading dose followed by 50 mg by mouth per day, plus sulfadiazine 1 gram by mouth, four times a day (to treat the parasite) and leucovorin (folinic acid) 10 mg by mouth per day, to reduce toxicity caused by pyrimethamine {54, 155}. An alternative regimen is pyrimethamine 200 mg by mouth loading dose followed by 50 mg by mouth per day, plus clindamycin 600 mg by mouth four times a day plus leucovorin 10 mg per day. Acute therapy should be continued for at least six weeks, provided that the patient is improving, and longer in cases with extensive disease. Secondary prophylaxis should be continued until the lesions are resolved, symptoms have improved, cART has raised the CD4+ cell count to at least 200 cells/mm3, and viral load is suppressed{170}.
Persistent neuropsychological deficits are evident in many survivors of TE. Examining the long term neurocognitive outcomes of individuals who survived AIDS CNS-OIs, Levine et al (2008) found that those with past TE performed worse on all but one neuropsychological domain than those with history of other AIDS CNS OI, including PML and CM {137}.
Primary CNS LYMPHOMA (PCNSL) in AIDS
Primary CNS lymphoma (PCNSL) arises in and is confined to, the CNS. It is second most common mass lesion in AIDS. The major risk factor is a CD4+ count under 100 cells/mm3. These tumors are promoted by immunosuppression, chronic antigenic stimulation, and cytokine overproduction. In the setting of immunosuppression, PCNSL is almost always associated with Epstein-Barr Virus (EBV) {171}, a ubiquitous herpes virus with a seroprevalence of 90%. Most EBV infections are asymptomatic, or present as acute mononucleosis. The EBV can remain latent in B cells, and can immortalize the cell. In AIDS, immune surveillance fails and the immortalized EBV-infected B cells are no longer held in check {172}. Thus the risk of PCNSL is greatly increased. Prior to cART, PCNSL occurred in 5% of those with neurological symptoms {173, 174}. The use of cART has resulted in lower incidence of PCNSL and improved survival {175, 176}.
The presenting symptoms of PCNSL include lethargy, confusion, impaired memory, headache, seizures, or focal weakness. Many patients develop cranial neuropathies and/or ocular involvement. Increased intracranial pressure and herniation can result in papilledema, and coma if untreated.
The usual neuroimaging findings on CT or MRI are one or sometimes multiple, contrast-enhancing lesions surrounded by edema, with mass effect. On MRI, they are hyperintense on T1 imaging and often show a periventricular distribution These lesions typically have a high uptake of radioactive tracers on thallium 201 SPECT {168} or fluorodeoxyglucose PET {169}, as opposed to TE. If it is safe to perform a lumbar puncture, the CSF may be helpful. EBV can be detected and quantified by PCR in the CSF{177} and plasma {178} of these patients, and may be a biomarker of PCNSL. However, diagnosis ultimately depends on a tissue diagnosis.
AIDS PCNSL are almost always high-grade, diffuse B-cell lymphomas, often of immunoblastic subtype. Compared with similar tumors seen in immunocompetent individuals, they are more likely to be multifocal and necrotic. Biopsy can be problematic{179}, especially a needle biopsy, because of the small sample, the possibility of extensive necrosis of the tumor, and the angiocentric nature. Administration of corticosteroids to reduce cerebral edema prior to biopsy can result in lysis of most neoplastic lymphocytes, resulting in a non-diagnostic biopsy{179}.
The tumor is treated with cranial irradiation (usual adult dose is fractionated 4000-5000 cGy), and by instituting or optimizing cART. Chemotherapy, if used, typically includes methotrexate, and there are also some positive results using antiviral therapies (e.g., ganciclovir) that decrease EBV viral load {180}, but there are no large controlled trials that establish optimal therapy.
Unfortunately, many treatments for AIDS PCNSL can result in residual cognitive impairment, particularly when both whole brain radiation and methotrexate-based chemotherapy are used {181}. The literature on this topic almost exclusively involves non-HIV cases. Harder et al. {182} reported on the neurocognitive status and quality of life among 19 non-HIV patients treated for PCNSL. All patients were in remission after combined whole-brain radiation and methotrexate-based chemotherapy. Neurocognitive and quality of life scores were compared to demographically-matched controls who had systemic malignancies and had undergone chemotherapy or non-brain radiotherapy. The authors found neurocognitive impairment in twelve patients with PCNSL, with four showing severe cognitive deficits. Only two control subjects were cognitively impaired according to their criteria. Only 42% of the PCNSL patients returned to work, as compared to 81% of controls.
Immune reconstitution inflammatory syndrome (IRIS)
The immune reconstitution inflammatory syndromes (IRIS) is a serious problem complicating the treatment of AIDS{183}. It refers to a group of syndromes characterized by paradoxical clinical worsening that usually occurs within the first four to eight weeks after starting cART {155}. The reconstituted immune system generates an inflammatory response, resulting in either a worsening of a known, underlying infection, or the unmasking of a subclinical, indolent infection. This exaggerated “dysregulated” inflammatory response is characterized by massive infiltration of CD8+ cells. Neuroimaging features include development of, or increase in, contrast enhancement, and unusual patterns of contrast enhancement {184}. Intracranial pressure may rise {185}, requiring the use of corticosteroids. Among the most common CNS infections reported to be involved in IRIS are HIV encephalitis {186-188}, TE {187, 189}, CM {185}, and PML {184} {155}. Risk factors for IRIS include taking cART for the first time, active or subclinical OI, CD4+ counts under 50 cells/mm3, high CD8+ cells, anemia, and a rapid decline in HIV viral load {190, 191}. There are relatively few biopsy or autopsy studies of IRIS, in part because most patients survive the syndrome. Some studies have reported both active lesions containing the pathogen (HIV-associated multinucleated giant cells, JCV, Toxoplasma parasites, etc.), and “sterile” lesions with inflammatory infiltrates. The treatment of CNS IRIS with corticosteroids has been advocated and remains controversial, as there are no formal studies, but should be considered if increased intracranial pressure is present.
The continuing evolution of the HIV epidemic has spurred an intense interest into a hitherto neglected area of medicine, neuroinfectious diseases and their consequences. This work has broad applications for the study of CNS tumors, dementias, neuropathies, and CNS disease in other immunosuppressed individuals.
Acknowledgments
This work was supported by Grants No. NS38841, U01MH083500, and R03DA026099, from the National Institutes of Health
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.UNAIDS . Report on the global AIDS epidemic 2008. UNAIDS; Geneva: 2007. [Google Scholar]
- 2.Patrick MK, Johnston JB, Power C. Lentiviral neuropathogenesis: comparative neuroinvasion, neurotropism, neurovirulence, and host neurosusceptibility. J Virol. 2002;76:7923–7931. doi: 10.1128/JVI.76.16.7923-7931.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wiley CA, Schrier RD, Nelson JA, et al. Cellular localization of human immunodeficiency virus infection within the brains of acquired immune deficiency syndrome patients. Proceedings of the National Academy of Sciences of the United States of America. 1986;83:7089–7093. doi: 10.1073/pnas.83.18.7089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaul M, Lipton SA. Mechanisms of neuronal injury and death in HIV-1 associated dementia. Curr HIV Res. 2006;4:307–318. doi: 10.2174/157016206777709384. [DOI] [PubMed] [Google Scholar]
- 5.Letendre S, Marquie-Beck J, Capparelli E, et al. Validation of the CNS Penetration-Effectiveness rank for quantifying antiretroviral penetration into the central nervous system. Arch Neurol. 2008;65:65–70. doi: 10.1001/archneurol.2007.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clements JE, Li M, Gama L, et al. The central nervous system is a viral reservoir in simian immunodeficiency virus--infected macaques on combined antiretroviral therapy: a model for human immunodeficiency virus patients on highly active antiretroviral therapy. Journal of neurovirology. 2005;11:180–189. doi: 10.1080/13550280590922748-1. [DOI] [PubMed] [Google Scholar]
- 7.Brew BJ, Crowe SM, Landay A, et al. Neurodegeneration and ageing in the HAART era. J Neuroimmune Pharmacol. 2009;4:163–174. doi: 10.1007/s11481-008-9143-1. [DOI] [PubMed] [Google Scholar]
- 8.Huang ST, Lee HC, Liu KH, et al. Acute human immunodeficiency virus infection. Journal of microbiology, immunology, and infection = Wei mian yu gan ran za zhi. 2005;38:65–68. [PubMed] [Google Scholar]
- 9.Fox R, Eldred LJ, Fuchs EJ, et al. Clinical manifestations of acute infection with human immunodeficiency virus in a cohort of gay men. AIDS (London, England) 1987;1:35–38. [PubMed] [Google Scholar]
- 10.Resnick L, Berger JR, Shapshak P, et al. Early penetration of the blood-brain-barrier by HIV. Neurology. 1988;38:9–14. doi: 10.1212/wnl.38.1.9. [DOI] [PubMed] [Google Scholar]
- 11.Tarasow E, Wiercinska-Drapalo A, Kubas B, et al. Cerebral MR spectroscopy in neurologically asymptomatic HIV-infected patients. Acta Radiol. 2003;44:206–212. doi: 10.1080/j.1600-0455.2003.00028.x. [DOI] [PubMed] [Google Scholar]
- 12.Krasner CG, Cohen SH. Bilateral Bell’s palsy and aseptic meningitis in a patient with acute human immunodeficiency virus seroconversion. West J Med. 1993;159:604–605. [PMC free article] [PubMed] [Google Scholar]
- 13.Serrano P, Hernandez N, Arroyo JA, et al. Bilateral Bell palsy and acute HIV type 1 infection: report of 2 cases and review. Clin Infect Dis. 2007;44:e57–61. doi: 10.1086/511876. [DOI] [PubMed] [Google Scholar]
- 14.Hollander H, Stringari S. Human immunodeficiency virus-associated meningitis. Clinical course and correlations. Am J Med. 1987;83:813–816. doi: 10.1016/0002-9343(87)90635-8. [DOI] [PubMed] [Google Scholar]
- 15.Hollander H, Levy JA. Neurologic abnormalities and recovery of human immunodeficiency virus from cerebrospinal fluid. Ann Intern Med. 1987;106:692–695. doi: 10.7326/0003-4819-106-5-692. [DOI] [PubMed] [Google Scholar]
- 16.Wendel KA, McArthur JC. Acute meningoencephalitis in chronic human immunodeficiency virus (HIV) infection: putative central nervous system escape of HIV replication. Clin Infect Dis. 2003;37:1107–1111. doi: 10.1086/378300. [DOI] [PubMed] [Google Scholar]
- 17.Worthington MG, Ross JJ. Aseptic meningitis and acute HIV syndrome after interruption of antiretroviral therapy: implications for structured treatment interruptions. AIDS (London, England) 2003;17:2145–2146. doi: 10.1097/00002030-200309260-00026. [DOI] [PubMed] [Google Scholar]
- 18.Antinori A, Arendt G, Becker JT, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Woods SP, Moore DJ, Weber E, et al. Cognitive neuropsychology of HIV-associated neurocognitive disorders. Neuropsychology review. 2009;19:152–168. doi: 10.1007/s11065-009-9102-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reger M, Welsh R, Razani J, et al. A meta-analysis of the neuropsychological sequelae of HIV infection. J Int Neuropsychol Soc. 2002;8:410–424. doi: 10.1017/s1355617702813212. [DOI] [PubMed] [Google Scholar]
- 21.Tross S, Price RW, Navia B, et al. Neuropsychological characterization of the AIDS dementia complex: a preliminary report. AIDS (London, England) 1988;2:81–88. doi: 10.1097/00002030-198804000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Delis DC, Peavy G, Heaton R, et al. Do patients with HIV-associated minor cognitive/ motor disorder exhibit a ‘subcortical’ memory profile? Evidence using the California verbal learning test. Assessment. 1995;2:151–165. [Google Scholar]
- 23.Pillon B, Deweer B, Michon A, et al. Are explicit memory disorders of progressive supranuclear palsy related to damage to striatofrontal circuits? Comparison with Alzheimer’s, Parkinson’s, and Huntington’s diseases. Neurology. 1994;44:1264–1270. doi: 10.1212/wnl.44.7.1264. [DOI] [PubMed] [Google Scholar]
- 24.Navia BA, Cho ES, Petito CK, et al. The AIDS dementia complex: II. Neuropathology. Ann Neurol. 1986;19:525–535. doi: 10.1002/ana.410190603. [DOI] [PubMed] [Google Scholar]
- 25.Tisch S, Brew B. Parkinsonism in hiv-infected patients on highly active antiretroviral therapy. Neurology. 2009;73:401–403. doi: 10.1212/WNL.0b013e3181b04b0d. [DOI] [PubMed] [Google Scholar]
- 26.Castellon SA, Hinkin CH, Myers HF. Neuropsychiatric disturbance is associated with executive dysfunction in HIV-1 infection. J Int Neuropsychol Soc. 2000;6:336–347. doi: 10.1017/s1355617700633088. [DOI] [PubMed] [Google Scholar]
- 27.Cole MA, Castellon SA, Perkins AC, et al. Relationship between psychiatric status and frontal-subcortical systems in HIV-infected individuals. J Int Neuropsychol Soc. 2007;13:549–554. doi: 10.1017/S135561770707066X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mateos JL Ayuso, Singh AN, Catalan J. Drug treatment of HIV associated neuropsychiatric syndromes. Neurologia (Barcelona, Spain) 2000;15:164–171. [PubMed] [Google Scholar]
- 29.Bing EG, Burnam MA, Longshore D, et al. Psychiatric disorders and drug use among human immunodeficiency virus-infected adults in the United States. Archives of general psychiatry. 2001;58:721–728. doi: 10.1001/archpsyc.58.8.721. [DOI] [PubMed] [Google Scholar]
- 30.Lyketsos CG, Schwartz J, Fishman M, et al. AIDS mania. The Journal of neuropsychiatry and clinical neurosciences. 1997;9:277–279. doi: 10.1176/jnp.9.2.277. [DOI] [PubMed] [Google Scholar]
- 31.Nakimuli-Mpungu E, Musisi S, Mpungu SK, et al. Primary mania versus HIV-related secondary mania in Uganda. The American journal of psychiatry. 2006;163:1349–1354. doi: 10.1176/ajp.2006.163.8.1349. quiz 1480. [DOI] [PubMed] [Google Scholar]
- 32.Portegies P, Enting RH, de Gans J, et al. Presentation and course of AIDS dementia complex: 10 years of follow-up in Amsterdam, The Netherlands. AIDS (London, England) 1993;7:669–675. [PubMed] [Google Scholar]
- 33.McArthur JC, Hoover DR, Bacellar H, et al. Dementia in AIDS patients: incidence and risk factors. Multicenter AIDS Cohort Study. Neurology. 1993;43:2245–2252. doi: 10.1212/wnl.43.11.2245. [DOI] [PubMed] [Google Scholar]
- 34.Ellis RJ, Moore DJ, Childers ME, et al. Progression to neuropsychological impairment in human immunodeficiency virus infection predicted by elevated cerebrospinal fluid levels of human immunodeficiency virus RNA. Arch Neurol. 2002;59:923–928. doi: 10.1001/archneur.59.6.923. [DOI] [PubMed] [Google Scholar]
- 35.Stern Y, McDermott MP, Albert S, et al. Factors associated with incident human immunodeficiency virus-dementia. Arch Neurol. 2001;58:473–479. doi: 10.1001/archneur.58.3.473. [DOI] [PubMed] [Google Scholar]
- 36.Navia BA, Jordan BD, Price RW. The AIDS dementia complex: I. Clinical features. Ann Neurol. 1986;19:517–524. doi: 10.1002/ana.410190602. [DOI] [PubMed] [Google Scholar]
- 37.Snider WD, Simpson DM, Nielsen S, et al. Neurological complications of acquired immune deficiency syndrome: analysis of 50 patients. Annals of neurology. 1983;14:403–418. doi: 10.1002/ana.410140404. [DOI] [PubMed] [Google Scholar]
- 38.McArthur JC. HIV dementia: an evolving disease. Journal of neuroimmunology. 2004;157:3–10. doi: 10.1016/j.jneuroim.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 39.Brew BJ. Evidence for a change in AIDS dementia complex in the era of highly active antiretroviral therapy and the possibility of new forms of AIDS dementia complex. Aids. 2004;18(Suppl 1):S75–78. [PubMed] [Google Scholar]
- 40.Ernst T, Chang L. Effect of aging on brain metabolism in antiretroviral-naive HIV patients. Aids. 2004;18(Suppl 1):S61–67. [PubMed] [Google Scholar]
- 41.Dore GJ, Correll PK, Li Y, et al. Changes to AIDS dementia complex in the era of highly active antiretroviral therapy. AIDS (London, England) 1999;13:1249–1253. doi: 10.1097/00002030-199907090-00015. [DOI] [PubMed] [Google Scholar]
- 42.Sevigny JJ, Albert SM, McDermott MP, et al. Evaluation of HIV RNA and markers of immune activation as predictors of HIV-associated dementia. Neurology. 2004;63:2084–2090. doi: 10.1212/01.wnl.0000145763.68284.15. [DOI] [PubMed] [Google Scholar]
- 43.Cysique LA, Brew BJ, Halman M, et al. Undetectable cerebrospinal fluid HIV RNA and beta-2 microglobulin do not indicate inactive AIDS dementia complex in highly active antiretroviral therapy-treated patients. Journal of acquired immune deficiency syndromes (1999) 2005;39:426–429. doi: 10.1097/01.qai.0000165799.59322.f5. [DOI] [PubMed] [Google Scholar]
- 44.Descamps M, Hyare H, Stebbing J, et al. Magnetic resonance imaging and spectroscopy of the brain in HIV disease. Journal of HIV therapy. 2008;13:55–58. [PubMed] [Google Scholar]
- 45.Paul RH, Ernst T, Brickman AM, et al. Relative sensitivity of magnetic resonance spectroscopy and quantitative magnetic resonance imaging to cognitive function among nondemented individuals infected with HIV. J Int Neuropsychol Soc. 2008;14:725–733. doi: 10.1017/S1355617708080910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paul RH, Brickman AM, Navia B, et al. Apathy is associated with volume of the nucleus accumbens in patients infected with HIV. J Neuropsychiatry Clin Neurosci. 2005;17:167–171. doi: 10.1176/appi.neuropsych.17.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chiang MC, Dutton RA, Hayashi KM, et al. 3D pattern of brain atrophy in HIV/AIDS visualized using tensor-based morphometry. NeuroImage. 2007;34:44–60. doi: 10.1016/j.neuroimage.2006.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lentz MR, Kim WK, Lee V, et al. Changes in MRS neuronal markers and T cell phenotypes observed during early HIV infection. Neurology. 2009;72:1465–1472. doi: 10.1212/WNL.0b013e3181a2e90a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Paul RH, Yiannoutsos CT, Miller EN, et al. Proton MRS and neuropsychological correlates in AIDS dementia complex: evidence of subcortical specificity. J Neuropsychiatry Clin Neurosci. 2007;19:283–292. doi: 10.1176/jnp.2007.19.3.283. [DOI] [PubMed] [Google Scholar]
- 50.Rottenberg DA, Sidtis JJ, Strother SC, et al. Abnormal cerebral glucose metabolism in HIV-1 seropositive subjects with and without dementia. J Nucl Med. 1996;37:1133–1141. [PubMed] [Google Scholar]
- 51.Rottenberg DA, Moeller JR, Strother SC, et al. The metabolic pathology of the AIDS dementia complex. Ann Neurol. 1987;22:700–706. doi: 10.1002/ana.410220605. [DOI] [PubMed] [Google Scholar]
- 52.Schmitt FA, Bigley JW, McKinnis R, et al. Neuropsychological outcome of zidovudine (AZT) treatment of patients with AIDS and AIDS-related complex. The New England journal of medicine. 1988;319:1573–1578. doi: 10.1056/NEJM198812153192404. [DOI] [PubMed] [Google Scholar]
- 53.Sidtis JJ, Gatsonis C, Price RW, et al. Zidovudine treatment of the AIDS dementia complex: results of a placebo-controlled trial. AIDS Clinical Trials Group. Annals of neurology. 1993;33:343–349. doi: 10.1002/ana.410330403. [DOI] [PubMed] [Google Scholar]
- 54.Portegies P, Solod L, Cinque P, et al. Guidelines for the diagnosis and management of neurological complications of HIV infection. Eur J Neurol. 2004;11:297–304. doi: 10.1111/j.1468-1331.2004.00856.x. [DOI] [PubMed] [Google Scholar]
- 55.Sacktor NC, Lyles RH, Skolasky RL, et al. Combination antiretroviral therapy improves psychomotor speed performance in HIV-seropositive homosexual men. Multicenter AIDS Cohort Study (MACS) Neurology. 1999;52:1640–1647. doi: 10.1212/wnl.52.8.1640. [DOI] [PubMed] [Google Scholar]
- 56.Brodt HR, Kamps BS, Gute P, et al. Changing incidence of AIDS-defining illnesses in the era of antiretroviral combination therapy. AIDS (London, England) 1997;11:1731–1738. doi: 10.1097/00002030-199714000-00010. [DOI] [PubMed] [Google Scholar]
- 57.Marra CM, Zhao Y, Clifford DB, et al. Impact of combination antiretroviral therapy on cerebrospinal fluid HIV RNA and neurocognitive performance. AIDS (London, England) 2009;23:1359–1366. doi: 10.1097/QAD.0b013e32832c4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tozzi V, Balestra P, Salvatori MF, et al. Changes in cognition during antiretroviral therapy: comparison of 2 different ranking systems to measure antiretroviral drug efficacy on HIV-associated neurocognitive disorders. Journal of acquired immune deficiency syndromes (1999) 2009;52:56–63. doi: 10.1097/qai.0b013e3181af83d6. [DOI] [PubMed] [Google Scholar]
- 59.Uthman OA, Abdulmalik JO. Adjunctive therapies for AIDS dementia complex. Cochrane Database Syst Rev. 2008:CD006496. doi: 10.1002/14651858.CD006496.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Breitbart W, Rosenfeld B, Kaim M, et al. A randomized, double-blind, placebo-controlled trial of psychostimulants for the treatment of fatigue in ambulatory patients with human immunodeficiency virus disease. Arch Intern Med. 2001;161:411–420. doi: 10.1001/archinte.161.3.411. [DOI] [PubMed] [Google Scholar]
- 61.Hinkin CH, Castellon SA, Hardy DJ, et al. Methylphenidate improves HIV-1-associated cognitive slowing. J Neuropsychiatry Clin Neurosci. 2001;13:248–254. doi: 10.1176/jnp.13.2.248. [DOI] [PubMed] [Google Scholar]
- 62.Petito CK. Review of central nervous system pathology in human immunodeficiency virus infection. Annals of neurology. 1988;23(Suppl):S54–57. doi: 10.1002/ana.410230715. [DOI] [PubMed] [Google Scholar]
- 63.Dal Pan GJ, Glass JD, McArthur JC. Clinicopathologic correlations of HIV-1-associated vacuolar myelopathy: an autopsy-based case-control study. Neurology. 1994;44:2159–2164. doi: 10.1212/wnl.44.11.2159. [DOI] [PubMed] [Google Scholar]
- 64.Berger JR, Sabet A. Infectious myelopathies. Seminars in neurology. 2002;22:133–142. doi: 10.1055/s-2002-36536. [DOI] [PubMed] [Google Scholar]
- 65.Di Rocco A, Simpson DM. AIDS-associated vacuolar myelopathy. AIDS patient care and STDs. 1998;12:457–461. doi: 10.1089/apc.1998.12.457. [DOI] [PubMed] [Google Scholar]
- 66.Sartoretti-Schefer S, Blattler T, Wichmann W. Spinal MRI in vacuolar myelopathy, and correlation with histopathological findings. Neuroradiology. 1997;39:865–869. doi: 10.1007/s002340050523. [DOI] [PubMed] [Google Scholar]
- 67.Chong J, Di Rocco A, Tagliati M, et al. MR findings in AIDS-associated myelopathy. Ajnr. 1999;20:1412–1416. [PMC free article] [PubMed] [Google Scholar]
- 68.Tagliati M, Di Rocco A, Danisi F, et al. The role of somatosensory evoked potentials in the diagnosis of AIDS-associated myelopathy. Neurology. 2000;54:1477–1482. doi: 10.1212/wnl.54.7.1477. [DOI] [PubMed] [Google Scholar]
- 69.Petito CK, Navia BA, Cho ES, et al. Vacuolar myelopathy pathologically resembling subacute combined degeneration in patients with the acquired immunodeficiency syndrome. The New England journal of medicine. 1985;312:874–879. doi: 10.1056/NEJM198504043121402. [DOI] [PubMed] [Google Scholar]
- 70.Petito CK, Vecchio D, Chen YT. HIV antigen and DNA in AIDS spinal cords correlate with macrophage infiltration but not with vacuolar myelopathy. J Neuropathol Exp Neurol. 1994;53:86–94. doi: 10.1097/00005072-199401000-00011. [DOI] [PubMed] [Google Scholar]
- 71.Rosenblum M, Scheck AC, Cronin K, et al. Dissociation of AIDS-related vacuolar myelopathy and productive HIV-1 infection of the spinal cord. Neurology. 1989;39:892–896. doi: 10.1212/wnl.39.7.892. [DOI] [PubMed] [Google Scholar]
- 72.Eilbott DJ, Peress N, Burger H, et al. Human immunodeficiency virus type 1 in spinal cords of acquired immunodeficiency syndrome patients with myelopathy: expression and replication in macrophages. Proc Natl Acad Sci U S A. 1989;86:3337–3341. doi: 10.1073/pnas.86.9.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Geraci A, Di Rocco A, Liu M, et al. AIDS myelopathy is not associated with elevated HIV viral load in cerebrospinal fluid. Neurology. 2000;55:440–442. doi: 10.1212/wnl.55.3.440. [DOI] [PubMed] [Google Scholar]
- 74.Di Rocco A, Bottiglieri T, Werner P, et al. Abnormal cobalamin-dependent transmethylation in AIDS-associated myelopathy. Neurology. 2002;58:730–735. doi: 10.1212/wnl.58.5.730. [DOI] [PubMed] [Google Scholar]
- 75.Tan SV, Guiloff RJ. Hypothesis on the pathogenesis of vacuolar myelopathy, dementia, and peripheral neuropathy in AIDS. Journal of neurology, neurosurgery, and psychiatry. 1998;65:23–28. doi: 10.1136/jnnp.65.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tan SV, Guiloff RJ, Henderson DC, et al. AIDS-associated vacuolar myelopathy and tumor necrosis factor-alpha (TNF alpha) Journal of the neurological sciences. 1996;138:134–144. doi: 10.1016/0022-510x(95)00354-5. [DOI] [PubMed] [Google Scholar]
- 77.Di Rocco A, Werner P, Bottiglieri T, et al. Treatment of AIDS-associated myelopathy with L-methionine: a placebo-controlled study. Neurology. 2004;63:1270–1275. doi: 10.1212/01.wnl.0000140469.18782.05. [DOI] [PubMed] [Google Scholar]
- 78.Bizaare M, Dawood H, Moodley A. Vacuolar myelopathy: a case report of functional, clinical, and radiological improvement after highly active antiretroviral therapy. Int J Infect Dis. 2008;12:442–444. doi: 10.1016/j.ijid.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 79.Di Rocco A, Tagliati M. Remission of HIV myelopathy after highly active antiretroviral therapy. Neurology. 2000;55:456. doi: 10.1212/wnl.55.3.456. [DOI] [PubMed] [Google Scholar]
- 80.Staudinger R, Henry K. Remission of HIV myelopathy after highly active antiretroviral therapy. Neurology. 2000;54:267–268. doi: 10.1212/wnl.54.1.267. [DOI] [PubMed] [Google Scholar]
- 81.Rottnek M, Di Rocco A, Laudier D, et al. Axonal damage is a late component of vacuolar myelopathy. Neurology. 2002;58:479–481. doi: 10.1212/wnl.58.3.479. [DOI] [PubMed] [Google Scholar]
- 82.Verma S, Estanislao L, Simpson D. HIV-associated neuropathic pain: epidemiology, pathophysiology and management. CNS Drugs. 2005;19:325–334. doi: 10.2165/00023210-200519040-00005. [DOI] [PubMed] [Google Scholar]
- 83.Letendre SL, Ellis RJ, Everall I, et al. Neurologic complications of HIV disease and their treatment. Top HIV Med. 2009;17:46–56. [PMC free article] [PubMed] [Google Scholar]
- 84.Lopez OL, Becker JT, Dew MA, et al. Risk modifiers for peripheral sensory neuropathy in HIV infection/AIDS. Eur J Neurol. 2004;11:97–102. doi: 10.1046/j.1351-5101.2003.00713.x. [DOI] [PubMed] [Google Scholar]
- 85.Lichtenstein KA, Armon C, Baron A, et al. Modification of the incidence of drug-associated symmetrical peripheral neuropathy by host and disease factors in the HIV outpatient study cohort. Clin Infect Dis. 2005;40:148–157. doi: 10.1086/426076. [DOI] [PubMed] [Google Scholar]
- 86.Simpson DM. Selected peripheral neuropathies associated with human immunodeficiency virus infection and antiretroviral therapy. Journal of neurovirology. 2002;8(Suppl 2):33–41. doi: 10.1080/13550280290167939. [DOI] [PubMed] [Google Scholar]
- 87.Cornblath DR, McArthur JC. Predominantly sensory neuropathy in patients with AIDS and AIDS-related complex. Neurology. 1988;38:794–796. doi: 10.1212/wnl.38.5.794. [DOI] [PubMed] [Google Scholar]
- 88.Kennedy JM, Hoke A, Zhu Y, et al. Peripheral neuropathy in lentivirus infection: evidence of inflammation and axonal injury. AIDS (London, England) 2004;18:1241–1250. doi: 10.1097/00002030-200406180-00002. [DOI] [PubMed] [Google Scholar]
- 89.Zhu Y, Antony J, Liu S, et al. CD8+ lymphocyte-mediated injury of dorsal root ganglion neurons during lentivirus infection: CD154-dependent cell contact neurotoxicity. J Neurosci. 2006;26:3396–3403. doi: 10.1523/JNEUROSCI.4767-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Herzberg U, Sagen J. Peripheral nerve exposure to HIV viral envelope protein gp120 induces neuropathic pain and spinal gliosis. Journal of neuroimmunology. 2001;116:29–39. doi: 10.1016/s0165-5728(01)00288-0. [DOI] [PubMed] [Google Scholar]
- 91.de la Monte SM, Gabuzda DH, Ho DD, et al. Peripheral neuropathy in the acquired immunodeficiency syndrome. Annals of neurology. 1988;23:485–492. doi: 10.1002/ana.410230510. [DOI] [PubMed] [Google Scholar]
- 92.Evans SR, Clifford DB, Kitch DW, et al. Simplification of the research diagnosis of HIV-associated sensory neuropathy. HIV clinical trials. 2008;9:434–439. doi: 10.1310/hct0906-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.McArthur JC, Yiannoutsos C, Simpson DM, et al. A phase II trial of nerve growth factor for sensory neuropathy associated with HIV infection. AIDS Clinical Trials Group Team 291. Neurology. 2000;54:1080–1088. doi: 10.1212/wnl.54.5.1080. [DOI] [PubMed] [Google Scholar]
- 94.Evans SR, Simpson DM, Kitch DW, et al. A randomized trial evaluating Prosaptide for HIV-associated sensory neuropathies: use of an electronic diary to record neuropathic pain. PLoS One. 2007;2:e551. doi: 10.1371/journal.pone.0000551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Simpson DM, McArthur JC, Olney R, et al. Lamotrigine for HIV-associated painful sensory neuropathies: a placebo-controlled trial. Neurology. 2003;60:1508–1514. doi: 10.1212/01.wnl.0000063304.88470.d9. [DOI] [PubMed] [Google Scholar]
- 96.Simpson DM, Brown S, Tobias J. Controlled trial of high-concentration capsaicin patch for treatment of painful HIV neuropathy. Neurology. 2008;70:2305–2313. doi: 10.1212/01.wnl.0000314647.35825.9c. [DOI] [PubMed] [Google Scholar]
- 97.Abrams DI, Jay CA, Shade SB, et al. Cannabis in painful HIV-associated sensory neuropathy: a randomized placebo-controlled trial. Neurology. 2007;68:515–521. doi: 10.1212/01.wnl.0000253187.66183.9c. [DOI] [PubMed] [Google Scholar]
- 98.Ellis RJ, Toperoff W, Vaida F, et al. Smoked medicinal cannabis for neuropathic pain in HIV: a randomized, crossover clinical trial. Neuropsychopharmacology. 2009;34:672–680. doi: 10.1038/npp.2008.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hahn K, Arendt G, Braun JS, et al. A placebo-controlled trial of gabapentin for painful HIV-associated sensory neuropathies. Journal of neurology. 2004;251:1260–1266. doi: 10.1007/s00415-004-0529-6. [DOI] [PubMed] [Google Scholar]
- 100.Kieburtz K, Simpson D, Yiannoutsos C, et al. A randomized trial of amitriptyline and mexiletine for painful neuropathy in HIV infection. AIDS Clinical Trial Group 242 Protocol Team. Neurology. 1998;51:1682–1688. doi: 10.1212/wnl.51.6.1682. [DOI] [PubMed] [Google Scholar]
- 101.Shlay JC, Chaloner K, Max MB, et al. Acupuncture and amitriptyline for pain due to HIV-related peripheral neuropathy: a randomized controlled trial. Terry Beirn Community Programs for Clinical Research on AIDS. Jama. 1998;280:1590–1595. doi: 10.1001/jama.280.18.1590. [DOI] [PubMed] [Google Scholar]
- 102.Schifitto G, Yiannoutsos CT, Simpson DM, et al. A placebo-controlled study of memantine for the treatment of human immunodeficiency virus-associated sensory neuropathy. Journal of neurovirology. 2006;12:328–331. doi: 10.1080/13550280600873835. [DOI] [PubMed] [Google Scholar]
- 103.Simpson DM, Dorfman D, Olney RK, et al. Peptide T in the treatment of painful distal neuropathy associated with AIDS: results of a placebo-controlled trial. The Peptide T Neuropathy Study Group. Neurology. 1996;47:1254–1259. doi: 10.1212/wnl.47.5.1254. [DOI] [PubMed] [Google Scholar]
- 104.Moore RD, Wong WM, Keruly JC, et al. Incidence of neuropathy in HIV-infected patients on monotherapy versus those on combination therapy with didanosine, stavudine and hydroxyurea. AIDS (London, England) 2000;14:273–278. doi: 10.1097/00002030-200002180-00009. [DOI] [PubMed] [Google Scholar]
- 105.Verma A. Epidemiology and clinical features of HIV-1 associated neuropathies. J Peripher Nerv Syst. 2001;6:8–13. doi: 10.1046/j.1529-8027.2001.006001008.x. [DOI] [PubMed] [Google Scholar]
- 106.Arenas-Pinto A, Bhaskaran K, Dunn D, et al. The risk of developing peripheral neuropathy induced by nucleoside reverse transcriptase inhibitors decreases over time: evidence from the Delta trial. Antiviral therapy. 2008;13:289–295. [PubMed] [Google Scholar]
- 107.Lee H, Hanes J, Johnson KA. Toxicity of nucleoside analogues used to treat AIDS and the selectivity of the mitochondrial DNA polymerase. Biochemistry. 2003;42:14711–14719. doi: 10.1021/bi035596s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Peltier AC, Russell JW. Recent advances in drug-induced neuropathies. Current opinion in neurology. 2002;15:633–638. doi: 10.1097/00019052-200210000-00015. [DOI] [PubMed] [Google Scholar]
- 109.Zhu Y, Antony JM, Martinez JA, et al. Didanosine causes sensory neuropathy in an HIV/AIDS animal model: impaired mitochondrial and neurotrophic factor gene expression. Brain. 2007;130:2011–2023. doi: 10.1093/brain/awm148. [DOI] [PubMed] [Google Scholar]
- 110.Canter JA, Haas DW, Kallianpur AR, et al. The mitochondrial pharmacogenomics of haplogroup T: MTND2*LHON4917G and antiretroviral therapy-associated peripheral neuropathy. The pharmacogenomics journal. 2008;8:71–77. doi: 10.1038/sj.tpj.6500470. [DOI] [PubMed] [Google Scholar]
- 111.Berger AR, Arezzo JC, Schaumburg HH, et al. 2′,3′-dideoxycytidine (ddC) toxic neuropathy: a study of 52 patients. Neurology. 1993;43:358–362. doi: 10.1212/wnl.43.2.358. [DOI] [PubMed] [Google Scholar]
- 112.Hagberg L, Malmvall BE, Svennerholm L, et al. Guillain-Barre syndrome as an early manifestation of HIV central nervous system infection. Scandinavian journal of infectious diseases. 1986;18:591–592. doi: 10.3109/00365548609021668. [DOI] [PubMed] [Google Scholar]
- 113.de Castro G, Bastos PG, Martinez R, et al. Episodes of Guillain-Barre syndrome associated with the acute phase of HIV-1 infection and with recurrence of viremia. Arquivos de neuro-psiquiatria. 2006;64:606–608. doi: 10.1590/s0004-282x2006000400016. [DOI] [PubMed] [Google Scholar]
- 114.Teo EC, Azwra A, Jones RL, et al. Guillain--Barre syndrome following immune reconstitution after antiretroviral therapy for primary HIV infection. Journal of HIV therapy. 2007;12:62–63. [PubMed] [Google Scholar]
- 115.McLeod JG. Electrophysiological studies in the Guillain-Barre syndrome. Annals of neurology. 1981;9(Suppl):20–27. doi: 10.1002/ana.410090705. [DOI] [PubMed] [Google Scholar]
- 116.Cornblath DR. Treatment of the neuromuscular complications of human immunodeficiency virus infection. Annals of neurology. 1988;23(Suppl):S88–91. doi: 10.1002/ana.410230723. [DOI] [PubMed] [Google Scholar]
- 117.Bani-Sadr F, Neuville S, Crassard I, et al. Acute Guillain-Barre syndrome during the chronic phase of HIV infection and dramatic improvement under highly active antiretroviral therapy. AIDS (London, England) 2002;16:1562. doi: 10.1097/00002030-200207260-00017. [DOI] [PubMed] [Google Scholar]
- 118.Sloan DJ, Nicolson A, Miller AR, et al. Human immunodeficiency virus seroconversion presenting with acute inflammatory demyelinating polyneuropathy: a case report. J Med Case Reports. 2008;2:370. doi: 10.1186/1752-1947-2-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gorshtein A, Levy Y. Intravenous immunoglobulin in therapy of peripheral neuropathy. Clinical reviews in allergy & immunology. 2005;29:271–279. doi: 10.1385/CRIAI:29:3:271. [DOI] [PubMed] [Google Scholar]
- 120.Wulff EA, Wang AK, Simpson DM. HIV-associated peripheral neuropathy: epidemiology, pathophysiology and treatment. Drugs. 2000;59:1251–1260. doi: 10.2165/00003495-200059060-00005. [DOI] [PubMed] [Google Scholar]
- 121.Astrom KE, Mancall EL, Richardson EP., Jr. Progressive multifocal leuko-encephalopathy; a hitherto unrecognized complication of chronic lymphatic leukaemia and Hodgkin’s disease. Brain. 1958;81:93–111. doi: 10.1093/brain/81.1.93. [DOI] [PubMed] [Google Scholar]
- 122.Padgett BL, Walker DL. Prevalence of antibodies in human sera against JC virus, an isolate from a case of progressive multifocal leukoencephalopathy. The Journal of infectious diseases. 1973;127:467–470. doi: 10.1093/infdis/127.4.467. [DOI] [PubMed] [Google Scholar]
- 123.Weber T, Trebst C, Frye S, et al. Analysis of the systemic and intrathecal humoral immune response in progressive multifocal leukoencephalopathy. The Journal of infectious diseases. 1997;176:250–254. doi: 10.1086/514032. [DOI] [PubMed] [Google Scholar]
- 124.Berger JR, Pall L, Lanska D, et al. Progressive multifocal leukoencephalopathy in patients with HIV infection. Journal of neurovirology. 1998;4:59–68. doi: 10.3109/13550289809113482. [DOI] [PubMed] [Google Scholar]
- 125.Engsig FN, Hansen AB, Omland LH, et al. Incidence, clinical presentation, and outcome of progressive multifocal leukoencephalopathy in HIV-infected patients during the highly active antiretroviral therapy era: a nationwide cohort study. The Journal of infectious diseases. 2009;199:77–83. doi: 10.1086/595299. [DOI] [PubMed] [Google Scholar]
- 126.Yousry TA, Major EO, Ryschkewitsch C, et al. Evaluation of patients treated with natalizumab for progressive multifocal leukoencephalopathy. The New England journal of medicine. 2006;354:924–933. doi: 10.1056/NEJMoa054693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Whiteman ML, Post MJ, Berger JR, et al. Progressive multifocal leukoencephalopathy in 47 HIV-seropositive patients: neuroimaging with clinical and pathologic correlation. Radiology. 1993;187:233–240. doi: 10.1148/radiology.187.1.8451420. [DOI] [PubMed] [Google Scholar]
- 128.Du Pasquier RA, Koralnik IJ. Inflammatory reaction in progressive multifocal leukoencephalopathy: harmful or beneficial? Journal of neurovirology. 2003;9(Suppl 1):25–31. doi: 10.1080/13550280390195315. [DOI] [PubMed] [Google Scholar]
- 129.Hammarin AL, Bogdanovic G, Svedhem V, et al. Analysis of PCR as a tool for detection of JC virus DNA in cerebrospinal fluid for diagnosis of progressive multifocal leukoencephalopathy. Journal of clinical microbiology. 1996;34:2929–2932. doi: 10.1128/jcm.34.12.2929-2932.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang Y, Kirby JE, Qian Q. Effective use of JC virus PCR for diagnosis of progressive multifocal leukoencephalopathy. Journal of medical microbiology. 2009;58:253–255. doi: 10.1099/jmm.0.004432-0. [DOI] [PubMed] [Google Scholar]
- 131.Royal W, 3rd, Dupont B, McGuire D, et al. Topotecan in the treatment of acquired immunodeficiency syndrome-related progressive multifocal leukoencephalopathy. Journal of neurovirology. 2003;9:411–419. doi: 10.1080/13550280390201740. [DOI] [PubMed] [Google Scholar]
- 132.Enting RH, Portegies P. Cytarabine and highly active antiretroviral therapy in HIV-related progressive multifocal leukoencephalopathy. Journal of neurology. 2000;247:134–138. doi: 10.1007/pl00007794. [DOI] [PubMed] [Google Scholar]
- 133.Hall CD, Dafni U, Simpson D, et al. Failure of cytarabine in progressive multifocal leukoencephalopathy associated with human immunodeficiency virus infection. AIDS Clinical Trials Group 243 Team. The New England journal of medicine. 1998;338:1345–1351. doi: 10.1056/NEJM199805073381903. [DOI] [PubMed] [Google Scholar]
- 134.Marra CM, Rajicic N, Barker DE, et al. A pilot study of cidofovir for progressive multifocal leukoencephalopathy in AIDS. AIDS (London, England) 2002;16:1791–1797. doi: 10.1097/00002030-200209060-00012. [DOI] [PubMed] [Google Scholar]
- 135.Clifford DB, Yiannoutsos C, Glicksman M, et al. HAART improves prognosis in HIV-associated progressive multifocal leukoencephalopathy. Neurology. 1999;52:623–625. doi: 10.1212/wnl.52.3.623. [DOI] [PubMed] [Google Scholar]
- 136.Giudici B, Vaz B, Bossolasco S, et al. Highly active antiretroviral therapy and progressive multifocal leukoencephalopathy: effects on cerebrospinal fluid markers of JC virus replication and immune response. Clin Infect Dis. 2000;30:95–99. doi: 10.1086/313598. [DOI] [PubMed] [Google Scholar]
- 137.Levine AJ, Hinkin CH, Ando K, et al. An exploratory study of long-term neurocognitive outcomes following recovery from opportunistic brain infections in HIV+ adults. J Clin Exp Neuropsychol. 2008:1–8. doi: 10.1080/13803390701819036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Griffiths P. Cytomegalovirus infection of the central nervous system. Herpes. 2004;11(Suppl 2):95A–104A. [PubMed] [Google Scholar]
- 139.Staras SA, Dollard SC, Radford KW, et al. Seroprevalence of cytomegalovirus infection in the United States, 1988-1994. Clin Infect Dis. 2006;43:1143–1151. doi: 10.1086/508173. [DOI] [PubMed] [Google Scholar]
- 140.Embil JA, Pereira LH, MacNeil JP, et al. Levels of cytomegalovirus seropositivity in homosexual and heterosexual men. Sex Transm Dis. 1988;15:85–87. doi: 10.1097/00007435-198804000-00003. [DOI] [PubMed] [Google Scholar]
- 141.Skolnik PR, Kosloff BR, Hirsch MS. Bidirectional interactions between human immunodeficiency virus type 1 and cytomegalovirus. J Infect Dis. 1988;157:508–514. doi: 10.1093/infdis/157.3.508. [DOI] [PubMed] [Google Scholar]
- 142.McCutchan JA. Clinical impact of cytomegalovirus infections of the nervous system in patients with AIDS. Clin Infect Dis. 1995;21(Suppl 2):S196–201. doi: 10.1093/clinids/21.supplement_2.s196. [DOI] [PubMed] [Google Scholar]
- 143.Arribas JR, Storch GA, Clifford DB, et al. Cytomegalovirus encephalitis. Ann Intern Med. 1996;125:577–587. doi: 10.7326/0003-4819-125-7-199610010-00008. [DOI] [PubMed] [Google Scholar]
- 144.Fiala M, Singer EJ, Graves MC, et al. AIDS dementia complex complicated by cytomegalovirus encephalopathy. J Neurol. 1993;240:223–231. doi: 10.1007/BF00818709. [DOI] [PubMed] [Google Scholar]
- 145.Miller RF, Fox JD, Thomas P, et al. Acute lumbosacral polyradiculopathy due to cytomegalovirus in advanced HIV disease: CSF findings in 17 patients. Journal of neurology, neurosurgery, and psychiatry. 1996;61:456–460. doi: 10.1136/jnnp.61.5.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.So YT, Olney RK. Acute lumbosacral polyradiculopathy in acquired immunodeficiency syndrome: experience in 23 patients. Ann Neurol. 1994;35:53–58. doi: 10.1002/ana.410350109. [DOI] [PubMed] [Google Scholar]
- 147.Cinque P, Bossolasco S, Bestetti A, et al. Molecular studies of cerebrospinal fluid in human immunodeficiency virus type 1-associated opportunistic central nervous system diseases--an update. J Neurovirol. 2002;8(Suppl 2):122–128. doi: 10.1080/13550280290167957. [DOI] [PubMed] [Google Scholar]
- 148.de Gans J, Tiessens G, Portegies P, et al. Predominance of polymorphonuclear leukocytes in cerebrospinal fluid of AIDS patients with cytomegalovirus polyradiculomyelitis. J Acquir Immune Defic Syndr. 1990;3:1155–1158. [PubMed] [Google Scholar]
- 149.van Den Pol AN, Mocarski E, Saederup N, et al. Cytomegalovirus cell tropism, replication, and gene transfer in brain. J Neurosci. 1999;19:10948–10965. doi: 10.1523/JNEUROSCI.19-24-10948.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wiley CA, Schrier RD, Denaro FJ, et al. Localization of cytomegalovirus proteins and genome during fulminant central nervous system infection in an AIDS patient. J Neuropathol Exp Neurol. 1986;45:127–139. doi: 10.1097/00005072-198603000-00003. [DOI] [PubMed] [Google Scholar]
- 151.Chiou SH, Liu JH, Chen SS, et al. Apoptosis of human retina and retinal pigment cells induced by human cytomegalovirus infection. Ophthalmic research. 2002;34:77–82. doi: 10.1159/000048332. [DOI] [PubMed] [Google Scholar]
- 152.Odeberg J, Wolmer N, Falci S, et al. Human cytomegalovirus inhibits neuronal differentiation and induces apoptosis in human neural precursor cells. J Virol. 2006;80:8929–8939. doi: 10.1128/JVI.00676-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Morgello S, Cho ES, Nielsen S, et al. Cytomegalovirus encephalitis in patients with acquired immunodeficiency syndrome: an autopsy study of 30 cases and a review of the literature. Hum Pathol. 1987;18:289–297. doi: 10.1016/s0046-8177(87)80012-6. [DOI] [PubMed] [Google Scholar]
- 154.Jabs DA, Bolton SG, Dunn JP, et al. Discontinuing anticytomegalovirus therapy in patients with immune reconstitution after combination antiretroviral therapy. Am J Ophthalmol. 1998;126:817–822. doi: 10.1016/s0002-9394(98)00285-2. [DOI] [PubMed] [Google Scholar]
- 155.Kaplan JE, Benson C, Holmes KH, et al. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Recomm Rep. 2009;58:1–207. quiz CE201-204. [PubMed] [Google Scholar]
- 156.Jarvis JN, Harrison TS. HIV-associated cryptococcal meningitis. Aids. 2007;21:2119–2129. doi: 10.1097/QAD.0b013e3282a4a64d. [DOI] [PubMed] [Google Scholar]
- 157.Polacheck I, Platt Y, Aronovitch J. Catecholamines and virulence of Cryptococcus neoformans. Infect Immun. 1990;58:2919–2922. doi: 10.1128/iai.58.9.2919-2922.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Moosa MY, Coovadia YM. Cryptococcal meningitis in Durban, South Africa: a comparison of clinical features, laboratory findings, and outcome for human immunodeficiency virus (HIV)-positive and HIV-negative patients. Clin Infect Dis. 1997;24:131–134. doi: 10.1093/clinids/24.2.131. [DOI] [PubMed] [Google Scholar]
- 159.Charlier C, Dromer F, Leveque C, et al. Cryptococcal neuroradiological lesions correlate with severity during cryptococcal meningoencephalitis in HIV-positive patients in the HAART era. PLoS One. 2008;3:e1950. doi: 10.1371/journal.pone.0001950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Lortholary O, Poizat G, Zeller V, et al. Long-term outcome of AIDS-associated cryptococcosis in the era of combination antiretroviral therapy. Aids. 2006;20:2183–2191. doi: 10.1097/01.aids.0000252060.80704.68. [DOI] [PubMed] [Google Scholar]
- 161.Larsen RA, Leal MA, Chan LS. Fluconazole compared with amphotericin B plus flucytosine for cryptococcal meningitis in AIDS. A randomized trial. Ann Intern Med. 1990;113:183–187. doi: 10.7326/0003-4819-113-3-183. [DOI] [PubMed] [Google Scholar]
- 162.Graybill JR, Sobel J, Saag M, et al. Diagnosis and management of increased intracranial pressure in patients with AIDS and cryptococcal meningitis. The NIAID Mycoses Study Group and AIDS Cooperative Treatment Groups. Clin Infect Dis. 2000;30:47–54. doi: 10.1086/313603. [DOI] [PubMed] [Google Scholar]
- 163.Nascimento LV, Stollar F, Tavares LB, et al. Risk factors for toxoplasmic encephalitis in HIV-infected patients: a case-control study in Brazil. Ann Trop Med Parasitol. 2001;95:587–593. doi: 10.1080/00034980120073931. [DOI] [PubMed] [Google Scholar]
- 164.Ho YC, Sun HY, Chen MY, et al. Clinical presentation and outcome of toxoplasmic encephalitis in patients with human immunodeficiency virus type 1 infection. J Microbiol Immunol Infect. 2008;41:386–392. [PubMed] [Google Scholar]
- 165.Luft BJ, Remington JS. Toxoplasmic encephalitis in AIDS. Clin Infect Dis. 1992;15:211–222. doi: 10.1093/clinids/15.2.211. [DOI] [PubMed] [Google Scholar]
- 166.Bousson V, Brunereau L, Meyohas M, et al. Brain imaging in AIDS. J Radiol. 1999;80:99–107. [PubMed] [Google Scholar]
- 167.Bhagavati S, Choi J. Frequent hemorrhagic lesions in cerebral toxoplasmosis in AIDS patients. J Neuroimaging. 2009;19:169–173. doi: 10.1111/j.1552-6569.2008.00274.x. [DOI] [PubMed] [Google Scholar]
- 168.Ruiz A, Ganz WI, Post MJ, et al. Use of thallium-201 brain SPECT to differentiate cerebral lymphoma from toxoplasma encephalitis in AIDS patients. AJNR Am J Neuroradiol. 1994;15:1885–1894. [PMC free article] [PubMed] [Google Scholar]
- 169.Pierce MA, Johnson MD, Maciunas RJ, et al. Evaluating contrast-enhancing brain lesions in patients with AIDS by using positron emission tomography. Ann Intern Med. 1995;123:594–598. doi: 10.7326/0003-4819-123-8-199510150-00005. [DOI] [PubMed] [Google Scholar]
- 170.Miro JM, Lopez JC, Podzamczer D, et al. Discontinuation of primary and secondary Toxoplasma gondii prophylaxis is safe in HIV-infected patients after immunological restoration with highly active antiretroviral therapy: results of an open, randomized, multicenter clinical trial. Clin Infect Dis. 2006;43:79–89. doi: 10.1086/504872. [DOI] [PubMed] [Google Scholar]
- 171.MacMahon EM, Glass JD, Hayward SD, et al. Epstein-Barr virus in AIDS-related primary central nervous system lymphoma. Lancet. 1991;338:969–973. doi: 10.1016/0140-6736(91)91837-k. [DOI] [PubMed] [Google Scholar]
- 172.Birx DL, Redfield RR, Tosato G. Defective regulation of Epstein-Barr virus infection in patients with acquired immunodeficiency syndrome (AIDS) or AIDS-related disorders. N Engl J Med. 1986;314:874–879. doi: 10.1056/NEJM198604033141403. [DOI] [PubMed] [Google Scholar]
- 173.Petito CK, Cho ES, Lemann W, et al. Neuropathology of acquired immunodeficiency syndrome (AIDS): an autopsy review. J Neuropathol Exp Neurol. 1986;45:635–646. doi: 10.1097/00005072-198611000-00003. [DOI] [PubMed] [Google Scholar]
- 174.Levy RM, Bredesen DE, Rosenblum ML. Neurological manifestations of the acquired immunodeficiency syndrome (AIDS): experience at UCSF and review of the literature. J Neurosurg. 1985;62:475–495. doi: 10.3171/jns.1985.62.4.0475. [DOI] [PubMed] [Google Scholar]
- 175.Wolf T, Brodt HR, Fichtlscherer S, et al. Changing incidence and prognostic factors of survival in AIDS-related non-Hodgkin’s lymphoma in the era of highly active antiretroviral therapy (HAART) Leuk Lymphoma. 2005;46:207–215. doi: 10.1080/10428190400015733. [DOI] [PubMed] [Google Scholar]
- 176.Skiest DJ, Crosby C. Survival is prolonged by highly active antiretroviral therapy in AIDS patients with primary central nervous system lymphoma. Aids. 2003;17:1787–1793. doi: 10.1097/00002030-200308150-00007. [DOI] [PubMed] [Google Scholar]
- 177.Bossolasco S, Cinque P, Ponzoni M, et al. Epstein-Barr virus DNA load in cerebrospinal fluid and plasma of patients with AIDS-related lymphoma. J Neurovirol. 2002;8:432–438. doi: 10.1080/13550280260422730. [DOI] [PubMed] [Google Scholar]
- 178.Fan H, Kim SC, Chima CO, et al. Epstein-Barr viral load as a marker of lymphoma in AIDS patients. J Med Virol. 2005;75:59–69. doi: 10.1002/jmv.20238. [DOI] [PubMed] [Google Scholar]
- 179.Gerstner E, Batchelor T. Primary CNS lymphoma. Expert Rev Anticancer Ther. 2007;7:689–700. doi: 10.1586/14737140.7.5.689. [DOI] [PubMed] [Google Scholar]
- 180.Bossolasco S, Falk KI, Ponzoni M, et al. Ganciclovir is associated with low or undetectable Epstein-Barr virus DNA load in cerebrospinal fluid of patients with HIV-related primary central nervous system lymphoma. Clin Infect Dis. 2006;42:e21–25. doi: 10.1086/499956. [DOI] [PubMed] [Google Scholar]
- 181.Correa DD, DeAngelis LM, Shi W, et al. Cognitive functions in survivors of primary central nervous system lymphoma. Neurology. 2004;62:548–555. doi: 10.1212/01.wnl.0000109673.75316.d8. [DOI] [PubMed] [Google Scholar]
- 182.Harder H, Holtel H, Bromberg JE, et al. Cognitive status and quality of life after treatment for primary CNS lymphoma. Neurology. 2004;62:544–547. doi: 10.1212/wnl.62.4.544. [DOI] [PubMed] [Google Scholar]
- 183.Riedel DJ, Pardo CA, McArthur J, et al. Therapy Insight: CNS manifestations of HIV-associated immune reconstitution inflammatory syndrome. Nat Clin Pract Neurol. 2006;2:557–565. doi: 10.1038/ncpneuro0303. [DOI] [PubMed] [Google Scholar]
- 184.Vendrely A, Bienvenu B, Gasnault J, et al. Fulminant inflammatory leukoencephalopathy associated with HAART-induced immune restoration in AIDS-related progressive multifocal leukoencephalopathy. Acta Neuropathol. 2005;109:449–455. doi: 10.1007/s00401-005-0983-y. [DOI] [PubMed] [Google Scholar]
- 185.York J, Bodi I, Reeves I, et al. Raised intracranial pressure complicating cryptococcal meningitis: immune reconstitution inflammatory syndrome or recurrent cryptococcal disease? J Infect. 2005;51:165–171. doi: 10.1016/j.jinf.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 186.Miller RF, Isaacson PG, Hall-Craggs M, et al. Cerebral CD8+ lymphocytosis in HIV-1 infected patients with immune restoration induced by HAART. Acta Neuropathol. 2004;108:17–23. doi: 10.1007/s00401-004-0852-0. [DOI] [PubMed] [Google Scholar]
- 187.Venkataramana A, Pardo CA, McArthur JC, et al. Immune reconstitution inflammatory syndrome in the CNS of HIV-infected patients. Neurology. 2006;67:383–388. doi: 10.1212/01.wnl.0000227922.22293.93. [DOI] [PubMed] [Google Scholar]
- 188.Langford TD, Letendre SL, Marcotte TD, et al. Severe, demyelinating leukoencephalopathy in AIDS patients on antiretroviral therapy. Aids. 2002;16:1019–1029. doi: 10.1097/00002030-200205030-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Pfeffer G, Prout A, Hooge J, et al. Biopsy-proven immune reconstitution syndrome in a patient with AIDS and cerebral toxoplasmosis. Neurology. 2009;73:321–322. doi: 10.1212/WNL.0b013e3181af788e. [DOI] [PubMed] [Google Scholar]
- 190.Shelburne SA, Visnegarwala F, Darcourt J, et al. Incidence and risk factors for immune reconstitution inflammatory syndrome during highly active antiretroviral therapy. Aids. 2005;19:399–406. doi: 10.1097/01.aids.0000161769.06158.8a. [DOI] [PubMed] [Google Scholar]
- 191.Robertson J, Meier M, Wall J, et al. Immune reconstitution syndrome in HIV: validating a case definition and identifying clinical predictors in persons initiating antiretroviral therapy. Clin Infect Dis. 2006;42:1639–1646. doi: 10.1086/503903. [DOI] [PubMed] [Google Scholar]