Abstract
Spontaneous otoacoustic emissions (SOAEs) were measured longitudinally for durations up to 19.5 years. Initial ages of the subjects ranged from 6 to 41 years. The most compelling finding was a decrease in frequency of all emissions in all subjects, which was approximately linear in %∕year and averaged 0.25%∕year. SOAE levels also tended to decrease with age, a trend that was significant, but not consistent across emissions, either within or across subjects. Levels of individual SOAEs might decrease, increase, or remain relatively constant with age. Several types of frequency∕level instabilities were noted in which some SOAEs within an ear interacted such that their levels were negatively correlated. These instabilities often persisted for many years. SOAEs were also measured in two females over the course of their pregnancies. No changes in SOAE levels or frequencies were seen, that were larger than have been reported in females over a menstrual cycle, suggesting that levels of female gonadal hormones do not have a significant direct effect on SOAE frequencies or levels.
INTRODUCTION
Spontaneous otoacoustic emissions (SOAEs), along with the various stimulus-related OAEs, have been studied extensively since their discovery by Kemp (1979), in part because they provide a noninvasive technique for probing cochlear mechanics. Current models of SOAE generation (Talmadge et al., 1998; Shera, 2003) propose that SOAEs are actively maintained cochlear standing waves, which arise from the coherent reflection of forward traveling waves in the region of maximum basilar-membrane (BM) displacement and the reflection of backward traveling waves at the stapes. In these models, potential SOAE frequencies are related to the total phase change involved in round-trip travel of a wave moving from the stapes to the region of reflection and back. These models explain the quasiperiodicity and characteristic minimal spacing of SOAE frequencies (reviewed in Talmadge et al., 1998) and the influence of middle-ear properties on SOAE frequencies (Shera, 2003).
In the ultra-short term, on the order of 1 min, human SOAEs are apparently frequency-locked based on their extremely low variability in frequency and amplitude. For frequency, half-power bandwidths can be less than 0.1% of SOAE frequency; for amplitude, rms amplitude fluctuations can be less than 6.3% of mean SOAE amplitude (Van Dijk and Wit, 1990). In the short term, on the order of hours to months, SOAE frequencies and amplitudes are more variable. A major source of variability in measurements made over a single recording session are slow drifts of SOAE frequency (up or down) and amplitude (typically increases), which occur over a period of about 30 min after insertion of the microphone in either the measured ear or, in the interim between measurements, in the contralateral ear (Zurek, 1981; Rabinowitz and Widen, 1984; Whitehead, 1988, 1991), apparently as the result of tactually induced efferent effects. The average drifts in frequency are on the order of 0.1%, with maximum shifts on the order of 0.5%; average drifts in amplitude are on the order of 1.5 dB (Whitehead, 1988, 1991).
Repeated measurements over several weeks or months show frequency variability of the same order of magnitude as within-session variability, but much larger amplitude variability. Whitehead (1988) found average maximum frequency shifts of about 0.4% and average maximum amplitude shifts of about 8 dB for 19 SOAEs in one subject measured weekly for 6–8 weeks. Calculations based on the data of Fritze (1983) show a standard deviation of 0.26% for 41 measurements of a single SOAE over 4 months. Some of this weekly or monthly variability is presumably related to small shifts, less than 1%, which follow a diurnal cycle and, in the case of menstruating females, a monthly cycle (Wit, 1985; Wilson, 1986; Bell, 1992; Haggerty et al., 1993; Penner, 1995).
SOAE frequencies and amplitudes are influenced by factors that change middle-ear impedance, including the transmission between the stapes and the cochlea. Elicitation of the middle-ear reflex results in frequency and amplitude shifts, which are mostly increases in frequency of less than 1% and decreases in amplitude of 25 dB or more, with the maximum shifts occurring in SOAEs with frequencies below 3 kHz (Schloth and Zwicker, 1983; Rabinowitz and Widen, 1984; Mott et al., 1989; Harrison and Burns, 1993; Burns et al., 1993b). Increasing or decreasing the static pressure in the ear canal (Kemp, 1979; Wilson and Sutton, 1981; Schloth and Zwicker, 1983; Whitehead, 1988), or increasing or decreasing ambient pressure (Hauser et al., 1993) results in a similar pattern of frequency and amplitude shifts; however, frequency shifts from static pressure changes may be as large as 5% (Kemp, 1979; Wilson and Sutton, 1981). Finally, shifts in posture, which presumably increase middle-ear stiffness via changes in intracochlear pressure, likewise result in a pattern of frequency and amplitude shifts consistent with those elicited by the more direct methods of increasing middle-ear stiffness (Bell, 1992; Whitehead, 1988; De Kleine et al., 2000).
Activation of the cochlear efferent system, for example, with contralateral acoustic stimulation, also can produce small positive shifts in SOAE frequencies, less than 0.3%, and small decreases in amplitude (Mott et al., 1989; Long, 1989). These effects, however, are very difficult to separate from middle-ear reflex effects (Harrison and Burns, 1993).
Finally, there is a small frequency modulation of SOAEs, about 0.1% that correlates with heart rate (Long and Talmadge, 1997). Because most SOAE measurements are analyzed with time-frequency distributions (TFDs) having relatively high-frequency-resolution and low-time-resolution, e.g., spectrally-averaged Fourier transforms, this modulation is usually manifest as low-level sidebands around the SOAE.
The above-cited data are representative of the majority of SOAEs. However, there are many SOAEs that are much less stable both in the short term and in the ultra-short term. In the course of our studies, the author and colleagues have measured a large number of SOAEs whose ultra-short-term frequency variability was more than an order of magnitude greater than that measured by Van Dijk and Wit (1990). Most of these were low-level SOAEs that presumably were perturbed by noise (Talmadge et al., 1993); however, we also have measured a number of high-level frequency-unstable SOAEs. In addition, we have measured a number of SOAEs whose frequencies were stable, but whose amplitude varied in an “on-off” manner over a time frame of tenths-of-seconds to tens-of-seconds (Burns and Keefe, 1992; Burns, 1996).
While the instabilities in individual SOAEs described above are apparently independent of instabilities in other SOAEs in the same ear, another type of SOAE instability that has been documented is the “energy-sharing” of SOAEs where pairs, or groups, of SOAEs co-vary in a highly correlated manner. There are at least three variations of this type of instability. Noncontiguous-linked SOAEs are groups of SOAEs, usually separated by stable SOAEs, whose levels co-vary in an on-off manner; i.e., when one group is present the other is absent (Burns et al., 1984; Whitehead, 1988). The spacing between the frequencies of these SOAEs is consistent with the characteristic minimum spacing of SOAE frequencies–roughly 6%–observed in literature and predicted by the standing-wave models. In some cases the on-off switching from one group of SOAEs to another (i.e., between “states”) can be elicited by changing middle-ear pressure (Whitehead, 1988), or by the presentation of an external tone (Burns et al., 1984). The change from state to state of these unstable SOAEs often results in small, less than 1%, shifts in the frequencies of adjacent stable SOAEs. The time course of spontaneous state shifting can vary from seconds to months.
Another variation of the energy-sharing instability are contiguous-linked SOAEs. In this case the amplitudes of pairs of contiguous SOAEs, whose frequency spacing is again consistent with the characteristic minimum spacing of SOAEs, co-vary inversely. Analyses of this type of instability by short-time Fourier transform (STFT) and other TFDs having high-time-resolution show that this co-variation is typically not an on-off co-variation; rather, both SOAEs are usually present simultaneously, but with one or the other of them at a much lower level (Burns and Pitton, 1993).
The third variation of this energy-sharing, the bimodal energy-sharing SOAE, is a SOAE that shifts between two frequencies whose spacing is usually smaller than the characteristic minimum spacing between adjacent SOAEs (Whitehead, 1988; Burns and Keefe, 1992). Analyses of these SOAEs with TFDs having high-time-resolution show that the switching between frequencies is in an on-off manner and is very rapid, often occurring over times that are shorter than the time-resolution of the TFDs (Burns and Keefe, 1992; Burns and Pitton, 1993). Presumably this instability reflects a single SOAE that has a bimodal frequency distribution. For this instability the time course of the shifting varies from tenths-of-seconds to months.
There are relatively few data on the long-term (over years) frequency and amplitude stability of SOAEs. Kohler and Fritze (1992) reported on measurements of 13 SOAEs in 7 ears over a single measurement interval of from 3.5 to 7.2 years. All SOAEs showed a decrease in frequency that averaged 0.14%∕year across all emissions.1Burns et al. (1993a) measured 54 emissions from 12 subjects for periods up to over 9 years and also noted a decline in frequency of all emissions, which averaged about 0.2%∕year. Although there is an obvious trend in these data for a long-term decrease in SOAE frequency with age, the small magnitude of this apparent shift, relative to the magnitude of the short-term shifts in SOAE frequency from other factors noted above, requires that longitudinal measurements of SOAE frequencies be carried out over a very long period.
The author and three of his children are “super emitters” who had participated in the study of Burns et al. (1993a) on SOAE stability.2 Having easy additional access to these ears provided the author with a core group of subjects well suited for a continuation and expansion of the earlier study. In the end, repeated measurements were available for some subjects for over 19 years.
METHODS
Long-term longitudinal measurements
The subjects for the long-term longitudinal study were 6 children (initial age range 6–12 years) and 12 adults (initial age range 21–41 years). Details concerning length of measurement period, number of measurements, initial age, etc., are given in Table 1. Subjects 3, 4, 5, and 6 are the author’s children and subject 18 is the author. The other adult subjects had been measured initially while graduate students in the Speech and Hearing Sciences Department at the University of Washington and had either remained in the Seattle area or had made the mistake of revisiting the department. At the time of the initial measurements, all subjects had normal tympanograms and thresholds within normal limits at all audiometric frequencies, with the exception of the 41-year-old author who had a permanent threshold shift of greater than 40 dB at 8000 Hz in both ears. The minimum measurement period for inclusion in the study was 4 years.
Table 1.
Individual subject information and average SOAE changes. The numbers in parentheses are standard deviations.
| Subject | Initial age | Sex | No. of SOAEs | Years measured | Measurements | Average frequency shift (%∕year) | Average level shift (dB∕year) |
|---|---|---|---|---|---|---|---|
| 1 | 6 | F | 3 | 4.0 | 4 | −0.27 (0.07) | −0.6 (0.5) |
| 2 | 6 | F | 19 | 4.1 | 5 | −0.26 (0.09) | 0.4 (2.3) |
| 3 | 7 | F | 6 | 19.3 | 16 | −0.19 (0.04) | −0.7 (0.3) |
| 4 | 9 | F | 11 | 16.3 | 15 | −0.23 (0.03) | −0.5 (5.8) |
| 5 | 9 | M | 2 | 13.5 | 11 | −0.19 | −0.5 |
| 6 | 12 | F | 9 | 19.5 | 15 | −0.35 (0.06) | −0.6 (5.0) |
| 7 | 23 | F | 3 | 14.1 | 3 | −0.19 (0.07) | 0.0 (0.4) |
| 8 | 27 | F | 4 | 7.1 | 3 | −0.29 (0.07) | −0.6 (1.4) |
| 9 | 28 | F | 2 | 13.8 | 3 | −0.16 | −0.9 |
| 10 | 30 | F | 6 | 6.4 | 8 | −0.13 (0.07) | −1.1 (9.3) |
| 11 | 30 | F | 2 | 5.1 | 2 | −0.27 | −0.1 |
| 12 | 31 | F | 10 | 5.0 | 2 | −0.21 (0.06) | −1.5 (1.2) |
| 13 | 32 | F | 2 | 5.0 | 2 | −0.13 | −0.3 |
| 14 | 36 | F | 3 | 5.1 | 2 | −0.41 (0.01) | −0.2 (0.6) |
| 15 | 36 | M | 1 | 4.1 | 2 | −0.17 | −2.1 |
| 16 | 37 | M | 1 | 6.8 | 2 | −0.41 | −0.8 |
| 17 | 38 | M | 1 | 15.0 | 5 | −0.21 | −0.5 |
| 18 | 41 | M | 11 | 19.3 | 47 | −0.28 (0.07) | −0.3 (0.5) |
SOAE measurements for the subjects studied the longest, those measured over periods of greater than 16 years, commenced in mid-1983 to early 1984. Measurements from 1983 to April 1986 were made with a custom-built insert microphone that utilized a Knowles model 1842 microphone and a Grason–Stadler oto-admittance meter earpiece fitted with an appropriately sized rubber eartip. From April 1986 to April 1988, measurements were obtained with an Etymotic model ER-10 insert microphone using foam eartips. From April 1988 until the end of the study in 2003 measurements were made with an Etymotic model ER-10B microphone, which also used the eartips of an oto-admittance meter. All microphones were calibrated in a Zwislocki coupler mounted in a KEMAR mannequin. Measurements were obtained with the subjects sitting quietly in a sound-treated chamber.3 Tympanograms were measured at the initial session and at any session where SOAE measurements indicated that there might be a middle-ear problem (e.g., no SOAEs, or very low levels, in an ear which had previously had SOAEs), but were not routinely measured at every session.
Microphone signals were high-pass filtered (c∕o 300 Hz, roll-off 6 dB∕octave). From 1983 until 1991, the signals were analyzed online with a Wavetek-Rockland 5820A spectrum analyzer. Each measurement was typically based on 64 spectral averages. The frequency region from 0 to 10 kHz was examined. Frequency measurements were made with an analysis binwidth of 1.25 Hz; however, an “improved accuracy” option of the analyzer, utilizing weighted averaging of the analysis bins adjacent to that containing the maximum energy, gave a nominal frequency-measurement resolution of 0.125 Hz. Level measurements were made with an analysis binwidth of 12.5 Hz to account, to some degree, for the wider apparent bandwidth of the less-frequency-stable SOAEs.
From 1991 until the end of the study, microphone signals were digitally recorded (44.1 kHz sampling rate) and analyzed offline. Typically, recordings of 20-s duration were analyzed via discrete Fourier transform (DFT) with spectral averaging with a 2:1 overlap factor. Frequency measurements were based on spectra obtained by applying a 262144-sample Hanning window, which gave an analysis binwidth of 0.17 Hz. Level measurements were obtained using a 4096-sample Hanning window, which gave an analysis binwidth of 10.8 Hz..
For the pre-1991 (online) measurements the existence criteria for SOAEs were based on measurement repeatability and comparison with calibration data. For the offline measurements SOAEs are clearly discriminable from electrical pure-tone artifacts based on their bandwidths in the high-frequency-resolution mode; that is, even the most frequency-stable SOAEs have a wider half-power bandwidth than a pure-tone electrical signal, and SOAEs also have a pronounced broadening near the noise floor (Long and Talmadge, 1997).
The SOAEs followed were selected to some extent. For example, super-emitter subjects can have many borderline, low-level, highly frequency-unstable SOAEs, which often disappear from session to session, and whose frequency is difficult to characterize. These weak SOAEs were not followed longitudinally; however, some initially frequency-stable SOAEs that later became unstable were followed as possible. Additionally, in some cases, SOAEs that later became stable were not present in the initial measurement sessions, but appeared in later years. Some of the latter SOAEs, in particular, those which comprised one member of an energy-sharing pair, were followed but are not included in the averaged data.
Threshold fine structure measurements
The correlation between SOAE frequencies and minima in the microstructure of the auditory threshold is well documented and is addressed in the current models of SOAE generation (e.g., Schloth, 1983; Long and Tubis, 1988; Talmadge et al., 1998). Specifically, minima in threshold fine structure correspond to maxima in stimulus-frequency otoacoustic emission (SFOAE) fine structure, which in turn correspond to frequencies at which SOAEs may be present. That is, SOAEs always correspond to a threshold minimum, but not all threshold minima have a SOAE associated with them. For subject 18, who was followed over a period of 19.5 years, threshold microstructure was measured in both ears at the time of initial measurement (1983), 11 (1994), and 19 years (2003). Thresholds were obtained for pure tones at frequencies separated by 10 Hz intervals using a computerized Bekesey-tracking procedure. 500-ms duration tones were presented at the rate of 1∕s. Consecutive tones increased or decreased in level by a fixed decibel increment. The direction of level change was controlled by the subject. Thresholds were based on the last six of ten turn-arounds.
Longitudinal measurements during pregnancy
One explanation posited for the monthly variation of SOAE frequency in females is based on a direct effect of the hormones estrogen and∕or progesterone on the generation mechanism of SOAEs (Haggerty et al., 1993; Penner, 1995). Because the levels of these hormones vary much more dramatically over the course of a pregnancy than during a normal menstrual cycle, variation in SOAE frequencies would presumably also vary more dramatically during pregnancy. SOAEs in two females were measured every few weeks over the course of their pregnancies. Subject P1 was followed from 28 week prepartum to 48 week postpartum. Subject P2 was followed from 38 week prepartum to 58 week postpartum. For subject P1, tympanograms were also measured at each session. These two subjects were not part of the main study.
RESULTS
Longitudinal frequency shifts
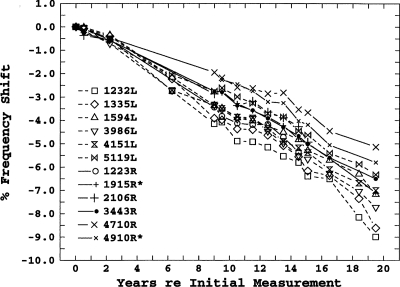
96 SOAEs from the 18 subjects were measured. All 96 SOAEs showed decreases in frequency over their respective measurement periods. Figure 1 shows the relative changes in SOAE frequencies over a period of 19.5 years for subject 6, who was 12 years old when first measured. The initial frequency and the ear of each SOAE are given in the figure legend. These results are representative in that all SOAEs showed a gradual decrease in frequency over time; these decreases were approximately linear in percent-frequency-shift-per-year, and there was a tendency for lower-frequency SOAEs to show a greater shift.
Figure 1.
Longitudinal frequency shifts for 11 SOAEs in subject 6. The * indicates the member of a contiguous-linked SOAE pair not present in the initial years of the study.
For the 77 SOAEs that were measured at least three times, the plots of frequency shift by time were fitted by linear regression. Of these, the 45 SOAEs that were measured for periods of at least 12 years all had coefficients of determination greater than 0.85. The slopes of the linear fits for all SOAEs are shown in Table 1 as an average slope for each subject. For subjects whose SOAEs were measured only twice, the slopes were calculated from the two measurements. The average slope across all SOAEs was −0.25%∕year. The range of slopes for all SOAEs was from −0.033%∕year to −0.539%∕year; the range for SOAEs measured for at least 12 years was from −0.132%∕year to −0.440%∕year. Lower initial frequencies tended to show larger negative slopes.
A linear regression of frequency-shift-slope on initial frequency, initial level, initial age, duration of measurement, and ear showed a small but significant effect of initial frequency (β=0.015%∕year kHz; r=0.242, p=0.017). No other predictors were significant.
Some other aspects of the results shown in Fig. 1 are noteworthy. This subject was one of the subjects with noncontiguous-linked unstable SOAEs studied by Burns et al. (1984), and the initial longitudinal measurements were taken at about the same time as the measurements analyzed in that paper. Those data were later reanalyzed by Keefe et al. (1990) who concluded that the SOAEs that comprised the noncontiguous-linked SOAEs could be characterized as high harmonics of a common low fundamental. The SOAEs with frequencies of 1232, 1335, and 1594 Hz shown in Fig. 1 are three of those SOAEs. If they remained harmonics of a common fundamental they would, of course, be expected to show exactly the same percentage frequency shifts over time. This was the case for the first 2 years and for the first 6 years for two of the SOAEs, but after that they all showed significantly different frequency shifts. The instability, a spontaneous “state switching” between two groups of SOAEs, was not seen in any measurements following the 2-year measurements, and state switching could not be induced with the presentation of external tones, as it had been in the original study. It also should be noted that this ear had both the largest average SOAE shift (−0.385%∕year) of any ear studied, as well as the largest shift for an individual SOAE measured more than 12 years (−0.440%∕year).
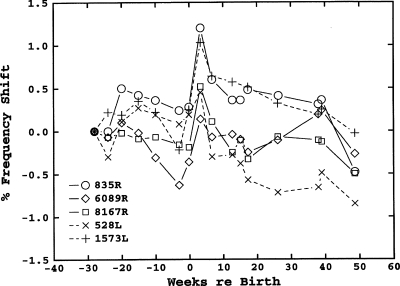
The frequency shifts for the right ear of subject 6 show the same form as the left-ear data, but the average frequency shift across SOAEs (−0.2%∕year) is smaller. Although frequency shifts in the two ears were not significantly different across subjects, in this subject the difference is highly significant (t=7.12, p<0.001), despite a similar range of SOAE frequencies. The two SOAE frequencies, which are followed by *s, 1915, and 4910, are examples of the contiguous-linked instability described in the Introduction. These SOAEs both appeared at about the eighth year of measurement (age 19): Their frequency shifts at that point were arbitrarily plotted as the same as their energy-sharing neighbors, 2106 and 4710, respectively. Both the 4710 and 4910 SOAEs were simultaneously present in measurements until the end of the study, whereas the 2106 SOAE was eventually replaced by the 1915 SOAE at about 15 years (age 27). The two emissions comprising each pair show similar frequency-shift slopes, and, in fact, the 4710 and 4910 frequency shifts show a similar fine structure in these plots.
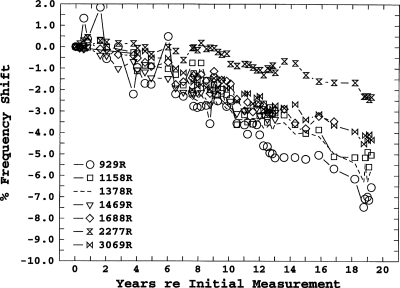
Figure 2 shows the frequency shifts for the right ear of subject 18. Several aspects of these data are of interest. First, this ear exhibits two of the instabilities we have characterized as bimodal: SOAEs that are separated in frequency by less than the characteristic minimum SOAE spacing and whose amplitudes co-vary in an on-off manner. The irregular fine structure of the shifts in the 929-Hz SOAE illustrates this phenomenon. The initial SOAE frequencies for this subject (at age 41) are based on an average of 57 measurements per SOAE taken over a period of 1 month. Most of the frequency measurements showed an approximately normal distribution, with an average standard deviation across SOAEs of 0.18%. The 929-Hz SOAE, however, showed a bimodal distribution with modes at 929 and 941 Hz. The 929-Hz mode was chosen as the reference because that was the predominate mode, but the obvious irregularity of the fine structure of the frequency-shift plot during the first 10 years reflects the fact that for some of the longitudinal measurements, the SOAE was in the higher-frequency mode.
Figure 2.
Longitudinal frequency shifts for six SOAEs in the right ear of subject 18. The dashed line indicates the member of a bimodal-SOAE pair not present in the initial years of the study.
Another example of this instability is the 1378 and 1469 Hz SOAE pair. For the first 6 years only the (nominal) 1469 Hz SOAE was present.4 For the period from 7 to 13 years, sometimes only one or the other was present, and sometimes both were present during a single measurement, from 14 to 19 years only the (nominal) 1378 Hz SOAE was present. Further analyses of the recordings from measurement sessions where both SOAEs were present, using TFDs having higher-time-resolution than our standard DFT analysis, showed that the two SOAEs were not present simultaneously. Rather, there was an on-off switching between the two frequencies, usually with an on-time on the order of 1 s. The subject heard this switching as a “trill-like” tinnitus, a rapid alteration between two pitches a flat semitone apart, that has been described in another paper (Burns and Keefe, 1992). This tinnitus allowed an estimate of the effective level of the SOAEs by loudness matching (Burns, 1996).5 Although it is virtually impossible to see in Fig. 2 because of the scales used, the slopes and fine structure of the frequency shifts of these two SOAEs are nearly identical.
Finally, there is the anomalous behavior of the 2277 Hz SOAE. This SOAE did not show any significant frequency shift for the first 9 years, the longest shift-free period of any SOAE measured. It also showed the shallowest slope (−0.132%∕year) of any of the emissions measured for at least 12 years.
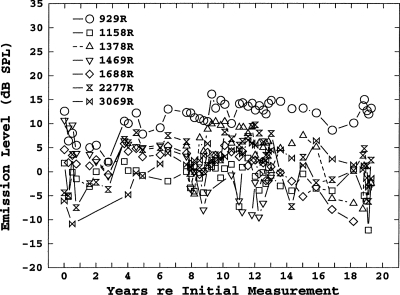
Longitudinal level shifts
Longitudinal shifts in level were much more variable than shifts in frequency. A representative example is shown in Fig. 3, the level shifts for the right ear of subject 18. Although, overall, there was a general decrease in SOAE levels, individual SOAEs might show a decrease, an increase, or no change. However, among the 45 SOAEs measured for longer than 12 years, 39 SOAEs showed a decrease in level and only 6 showed either an increase in level or no change. As noted, it was also common for individual SOAEs to disappear, or for new ones to appear. This was particularly true in subjects with numerous SOAEs in one ear.
Figure 3.
Longitudinal level shifts for 11 SOAEs in subject 18.
Simple curves could not be fitted to most of the plots of shifts in level. Therefore the estimates of level-shifts-per-year shown in Table 1 were simply calculated from the differences in levels between the initial and final levels. These average changes are somewhat biased by the fact that they include the large decreases in levels for SOAEs that eventually disappeared, but do not include SOAEs that were not initially present but appeared later in the longitudinal measurements. Generally, SOAEs having higher initial levels showed greater level shifts.
A linear regression of level-shift-slope on initial frequency, initial level, initial age, and duration of measurement showed a highly significant effect of initial level (β=−0.063 dB∕year dB SPL; r=−0.401, p=0.00). No other predictors were significant.
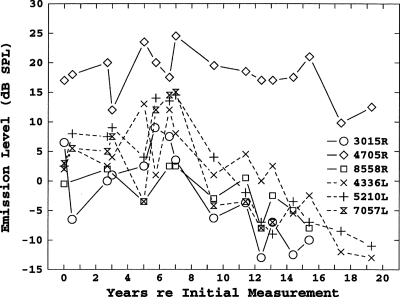
The results shown in Fig. 3 were representative of the vast majority of subjects with multiple SOAEs. Only one subject with more than four SOAEs showed consistent decreases in the levels of all SOAEs. The results for this subject are shown in Fig. 4. After the seventh measurement year, which corresponds to age 14, all her SOAEs, with the possible exception of the strongest, showed consistent decreases in level, and three eventually disappeared completely.
Figure 4.
Longitudinal level shifts for six SOAEs in subject 3.
Threshold fine structure
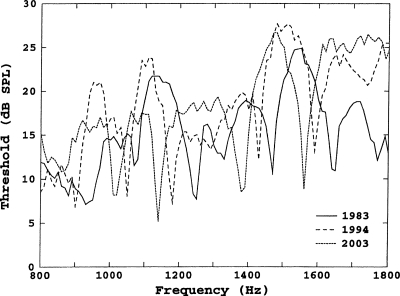
Threshold fine structure measurements for the left ear of subject 18, taken at the time of initial measurements (1983, age 41), at 11 years (1994), and at final measurements (2003), are shown in Fig. 5. Minima in the fine structure shifted down in frequency by an amount consistent with the shifts in SOAE frequencies. For example, the minima at 1640∕50 Hz (1983), 1590 Hz (1994), and 1560 Hz (2003), correspond to a SOAE whose frequencies were 1642 Hz in 1983, 1595 Hz in 1994, and 1565 Hz in 2003. As noted in the Introduction, a SOAE is not always associated with a minimum. The minima at 1470 Hz in 1983 and 1430 Hz in 1994 are associated with a SOAE that was at 1465 Hz in 1983 and 1431 Hz in 1994. This SOAE was no longer present after 1995; however, the minimum at 1380∕1390 Hz in 2003 presumably corresponds to the same SFOAE maximum, which no longer had a measurable SOAE. Threshold fine structure measurements obtained in the right ear of this subject also were consistent with his SOAE shifts.
Figure 5.
Behavior threshold fine structure for subject 18, over the range from 800 to 1800 Hz, measured in the initial (1983), 11th (1994), and final (2003) years of study.
SOAE changes during pregnancy
Figure 6 shows the frequency shifts in the SOAEs of subject P1 for the period from 28 week prepartum to 48 week postpartum. The frequency shifts in subject P2 were roughly similar. The only consistent change in SOAE frequencies across the two subjects was an increase in frequency for all SOAEs from the last prepartum measurement to the first postpartum measurement, which averaged about 0.6% in both subjects. There was no consistent pattern in SOAE level changes over the course of pregnancy in either subject.
Figure 6.
Longitudinal frequency shifts for five SOAEs in subject P1 during 28 weeks of her pregnancy and for 48 weeks after giving birth.
DISCUSSION
Long-term frequency shifts
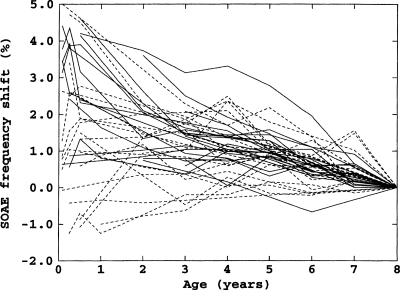
Perhaps the most compelling result reported here was the ongoing decrease in SOAE frequencies of about 0.25%∕year. This rate of decrease was independent of subject age, i.e., it was essentially the same for subjects with initial ages of 6 years as for subjects with final ages of 60 years. Evidence from a separate longitudinal study of SOAEs in children from ages 1 month to 8 years suggests that this decrease starts shortly after birth (Burns, 1999). Figure 7 shows the frequency shifts of 41 SOAEs from 18 children.6 Although there is clearly much more variability in the children’s data, especially in the first several years, from the age of 6 months most SOAEs decreased in frequency and the average rate of decrease was roughly the same as that of the subjects in the present study.
Figure 7.
Longitudinal frequency shifts for 21 SOAEs from 9 female subjects (solid lines) and 20 SOAEs from 9 male subjects (dashed lines) from ages 1 month to 8 years.
Interestingly, in preterm infants the opposite occurs. Brienesse et al. (1997) found that SOAE frequencies increased in preterm infants. The rate of this increase declined from over 1%∕week at 30 week conceptional age to 0%∕week at 45–50 week conceptional age. Thus, the overall picture of SOAE frequencies over a lifespan is a rapid increase in frequency in the months just prior to term birth, which changes to a slow decrease starting in the months just after term birth.
The question engendered by the present data is the source of the apparently life-long decrease in SOAE frequencies, which begins at about 6 months of age. As noted, experimental manipulations that increase middle-ear stiffness result in increases in SOAE frequencies, in some cases by as much as 5% (e.g., Kemp, 1979). A relationship between SOAE frequency and middle-ear characteristics is predicted by the standing-wave model because changes in middle-ear stiffness alter the output impedance of the cochlea and thus alter the phase of reflected reverse-traveling waves at the stapes. Shera (2003) specifically addressed the frequency shifts associated with changes in middle-ear stiffness [see Eq. 19 and Fig. 5 in Shera, 2003]: Increases in middle-ear stiffness result in increases in SOAE frequencies, and decreases in middle-ear stiffness result in decreases in SOAE frequencies. Therefore, the slow decrease in SOAE frequencies with age observed in our subjects might be the result of a slow decrease in middle–ear stiffness from ages 6 to 60.
However, there are a number of arguments against this explanation. First, the SOAE frequency shifts produced by changes in middle-ear stiffness are strongly dependent on SOAE frequency. For example, a 100% increase in stiffness results in about a 1.5% increase in frequency for a 1000-Hz SOAE, but only about a 0.25% increase for a 4000-Hz SOAE (Shera, 2003), a sixfold effect. While there was a significant effect of SOAE initial frequency in our results, it was small; the difference in the percentage frequency shift between the lowest and highest SOAE frequencies in a given subject was at most a factor of 2, and was usually less.
Second, the magnitude of the frequency shifts in our subjects could only result from changes in middle-ear stiffness that also would result in low-frequency threshold shifts. For example, shifts in SOAE frequency greater than 2% are seen only for ear-canal pressure changes of greater than ±200 kPa (Kemp, 1979; Wilson and Sutton, 1981). Our subjects showed total frequency shifts of up to 9% with no apparent effects on hearing thresholds.7
Third, the rate of frequency shifts in our subjects was relatively uniform over ages ranges (6–12) where metrics of middle-ear function still show maturation effects (e.g., Okabe et al., 1988). Frequency-shift rate was also uniform through adulthood, where no changes in middle-ear function have been reported, and continued to be uniform in the oldest subject who was age 60 at the end of the study. Feeney and Sanford (2004) reported aging effects on middle-ear function in a subject group including 60 year olds. These non-uniform changes in middle-ear function from ages 6 to 60 suggest it is unlikely that changes in middle-ear stiffness could account for the uniform frequency shifts over this age range.
The major determinate of SOAE frequency is the place-frequency map of the BM, which is, in turn, primarily a function of the exponentially varying stiffness of the BM from base to apex. A continual decrease in BM stiffness with age could account for the decline in SOAE frequency with age. According to current models (e.g., Talmadge et al., 1998) there are two components to BM stiffness, the passive stiffness and an “active” component provided by the so-called cochlear amplifier. The passive component is mainly determined by the transverse fiber bands of the BM (e.g., Olson and Mountain, 1994). There are no reports in literature from which to assess any possible morphological changes in these bands in the mammalian BM over age ranges equivalent to those covered in our study, nor are there any reports on measurements of passive BM stiffness over this age range.
There are indirect measurements in humans, which could be interpreted as reflecting a change in BM stiffness with age. Ramotowski and Kimberley (1998) measured human BM traveling-wave delay in 91 subjects from 22 to 78 years of age and found a significant increase in delay as a function of age. This increase in delay, which is independent of hearing threshold differences, and is the opposite of what would be expected from an increase in tuning bandwidth with age, could be the result of a decrease in BM traveling-wave velocity due to a decrease in BM stiffness. Among the many caveats in comparing these data to our frequency-shift data are that the rate of increase in traveling-wave delay appears to become larger with age, which is not the case in the SOAE frequency-shift data, and there are no data on the age range from infancy to young adult. Also note that if these data do reflect decrease in BM stiffness, it could be due to changes in either the passive or the active component.
Because a component of stiffness is provided by the cochlear amplifier, loss of efficiency of the amplifier would presumably result in a reduction in stiffness and a concomitant decrease in SOAE frequencies. All types of otoacoustic emissions (OAEs) are manifestations of the existence of the cochlear amplifier, and their levels are assumed to be an indirect reflection of cochlear-amplifier efficiency. Therefore, studies of OAE levels with age should provide evidence regarding a possible decrease in BM stiffness due to a decrease in efficiency of the cochlear amplifier with age. However, the conductive mechanism also must be taken into account. For example, the well-documented high levels of OAEs in infants relative to adults are probably entirely attributable to immaturities in the ear canal and middle ear (Abdala and Keefe, 2006; Keefe and Abdala, 2007). A study comparing SOAE power (Burns and Keefe, 1997), a measurement which accounts for differences in probe impedance and placement as well as ear-canal size, showed no differences in the average power of SOAEs between 8-year-old children and adults.8 The results of the present study showed a general decrease in SOAE levels with age, but not a consistent and uniform decrease in all SOAE levels as was the case for SOAE frequency. Studies on evoked OAE levels as a function of age in adults show little consensus in the results (e.g., Cilento et al., 2003). Some studies show a decrease in OAE levels with age, which are independent of changes in hearing threshold, and others do not. In most cases where threshold-independent decreases in OAE levels are seen, high frequencies show significantly greater decreases than low frequencies (Dorn et al., 1998).
Finally, the place-frequency map also could be shifted to lower frequencies by a uniform addition of BM mass (per unit length). For example, Long and Talmadge (1997) concluded, on the basis of modeling results, that the most likely explanation for the small modulations in the frequency of SOAEs that are correlated with heartbeat is the small increase in mass of the BM that occurs with the increase in blood flow with each heartbeat. As with BM stiffness, there are no measurements of the morphology of mammalian BMs over the age range equivalent to that in our study that might provide correlative evidence for a continuous increase in BM mass with age.
Long-term level shifts
In contrast with the totally consistent findings on frequency shifts with age, the findings for level shifts were much less consistent, both within and across subjects. Although there was an overall trend for SOAE levels to decrease with age, in most subjects individual SOAE levels might decrease, increase, or stay the same. A portion of this inconsistency is obviously related to the inherent variability of SOAE level measurements, both within and across sessions, relative to frequency measurements. SOAE levels are much more sensitive to external- and middle-ear acoustics: Probe calibration, probe placement, cerumen in the ear canal, changes in ear-canal size, and changes in middle-ear function all can have significant affects on SOAE levels. Large changes in the frequency stability of SOAEs also would affect their level measurements, given the constant binwidth of the measurements (12.5 Hz, 1983–1990; 10.8 Hz, 1991–2003).
Another factor is the interdependency often seen in the levels of SOAEs in ears with numerous SOAEs. As discussed above, often the decrease in level (or disappearance) of a particular SOAE is accompanied by the increase in level (or appearance) of a neighboring SOAE. Because we did not include SOAEs that appeared after the initial measurement session in the data analysis, this “energy conservation” aspect of SOAE levels was not totally taken into account. The significant correlation between initial level and level shift—higher initial level SOAEs showed greater level shifts—presumably reflects the obvious: Higher level SOAEs have more level to lose before disappearing below the noise floor.
Although in most subjects there was no evidence of an overall decline in SOAE levels, a few subjects did show a consistent decline in level with age. An example of this was shown in Fig. 4. This subject was first measured at age 6. Her SOAE levels were stable up to age 14, but all SOAEs in both ears showed a decrease in level from age 14 until the final measurement at age 26. The subject’s thresholds and tympanograms were normal at age 26, and her transient-evoked OAE levels were in the high end of the normal range. The frequency shifts of her SOAEs were consistent with those of the other subjects, and showed no obvious changes in slope between the periods, which corresponded to stable and decreasing SOAE levels. In short, there was nothing exceptional about this subject other than the uniform decrease in SOAE levels.
The obvious explanation for a decrease in SOAE levels with age would be a decrease in the efficiency of the cochlear amplifier with age. As discussed in Sec. 4A, the evidence for such a decrease is equivocal.
Long-term stability of frequency∕level instabilities
The disappearance or appearance of individual SOAEs, both in the short term and the long term, is consistent with the standing-wave model because of the stochastic nature of the impedance irregularities that are the basis of the reflected waves, and the spatial variations in the nonlinear amplification necessary to maintain the waves (Talmadge et al., 1998; Shera, 2003). That is, the model predicts the nominal frequencies at which SOAEs can occur, but whether a SOAE will be present at one of these frequencies at any particular time, and its level, depends on factors that can vary both in the short and long term. However, the energy-sharing instabilities noted in Sec. 3A, i.e., noncontiguous-linked SOAEs, contiguous-linked SOAEs, and bimodal SOAEs, presumably reflect somewhat more complicated dynamics, perhaps interacting standing waves between tonotopic reflection sites. The fact that some of these instabilities are themselves quite stable over years suggests that at least a portion of the basis for these instabilities may be robust irregularities in BM morphology, for example, extra rows of outer hair cells at a particular location (Lonsbury-Martin et al., 1988).
Threshold fine structure
The fact that the threshold fine structure of subject 18 shifted in frequency along with the shifts in his SOAE frequencies is completely consistent with both the empirical and modeling results (Talmadge et al., 1998) on the relationship between SOAEs and threshold fine structure.
SOAE changes during pregnancy
McFadden (2008, 2009) presented strong evidence that prepartum exposure to male gonadal hormones directly affects the cochlear processes that produce SOAEs; for example, the exposure to high levels of male hormones in the womb “masculinizes” the ears of female opposite-sex-dizygotic twins such that they tend to have fewer and weaker SOAEs. There also are a number of auditory measurements, both physiological and psychophysical, which fluctuate during the menstrual cycle [summarized by McFadden (1998)]. It is therefore not unreasonable to suggest, as do Haggerty et al. (1993) and Penner (1995), that the monthly variations in frequency of female SOAEs could be directly related to fluctuating levels of the female gonadal hormones estrogen and progesterone over the menstrual cycle.9
The results of our measurements on two subjects during pregnancy do not lend much support to this idea, however. The only consistent frequency shift in the SOAEs in the two subjects was a small (about 0.6%) increase in frequency between the last prepartum measurement and the first postpartum measurement. This is within the range of shifts seen over the menstrual cycle for females; whereas estrogen and progesterone levels in pregnancy, and, in particular, from late postpartum to just prepartum, vary by almost two orders of magnitude more than they do during a menstrual cycle (Tulchincky et al., 1972). In addition, both during a normal menstrual cycle and during pregnancy there are other changes occurring such as fluctuating levels of interstitial fluids that could affect middle-ear function (e.g., Cox, 1980), which as noted above, profoundly affects SOAE frequencies. In the one pregnancy subject where tympanograms were obtained at each measurement session, the pre-postpartum increase in frequency correlated with a decrease in peak compliance of about 0.1 ml. Also, as McFadden (1998) noted, most of the auditory measures that fluctuate over the menstrual cycle are either definitely, or most likely, mediated at post-cochlear levels of the auditory system. It thus seems more likely that the small frequency shifts seen in SOAEs in females over the menstrual cycle are related to middle ear-effects rather than levels of female hormones per se.
Relevance of SOAE frequency shifts to pitch coding
The following second-order correlation is presented for consideration by those readers interested in the apparently never-ending saga of whether pure-tone pitch is coded primarily by place or temporal information. The average SOAE frequency shift of 0.25%∕year leads to a semitone shift in frequency after 24 years. Most possessors of absolute pitch (AP) report that their AP shifts by a semitone about every 20 years (Ward, 1999). The direction of pitch shift is, assuming place coding of pitch, consistent with a decrease in the frequency corresponding to a particular place along the BM as the result of, for example, a decrease in stiffness of the BM. The existence of a drug, which reversibly shifts AP (Chaloupka et al., 1994), suggests an obvious experiment, using subjects who possess both AP and SOAEs, which would lead either to a first-order correlation between SOAE frequency shifts and shifts in AP, or would illustrate the irrelevance of these shifts to pitch coding.
CONCLUSIONS
SOAEs uniformly decrease in frequency at about 0.25%∕year from shortly after birth to at least age 60. SOAEs also decrease in level with age, but unlike the decrease in frequency, decreases in level are not uniform or consistent either within or across subjects. Female gonadal hormones probably do not have a significant effect on SOAE frequencies.
ACKNOWLEDGMENTS
This research was supported by long-since-expired NIH Grant No. P01 DC00520. The author would like to thank Doug Keefe, Lynne Werner, and a particularly thorough anonymous reviewer for helpful comments on previous versions of this manuscript.
Footnotes
Kohler and Fritze (1992) gave the average yearly shift as 1.4%. However, a perusal of their data shows that this was an error and the actual value is 0.14%.
Super-emitters are arbitrarily defined as possessing 12 or more SOAEs in at least one ear; possessing at least one SOAE greater than 10 dB SPL; and, at some point, possessing one of the linked instabilities discussed in the Introduction.
Some investigators prefer to base SOAE frequency measurements on the value obtained after the slow drift, which often follows placement of the microphone in the ear, has dissipated. However, this entails having the subjects sit quietly in the booth for up to 30 min, which is obviously not practical for young subjects, and therefore we did not do so. In any case, it is not clear that waiting for this apparently efferent-based shift, which is highly variable across subjects, would lead to less-variable repeated measurements across sessions.
SOAEs in this paper are denoted by their nominal frequencies, i.e., their frequencies when they were first measured. By the time the SOAE at 1378 Hz appeared, in the sixth year of measurements, the 1469 Hz SOAE had declined in frequency to 1440 Hz. At this point, then, the separation between these SOAEs (about 4%) was smaller than the characteristic minimum spacing of about 6% in this frequency range, although still within the range of variability of characteristic minimum spacing (Shera, 2003). In this sense this SOAE pair was atypical for a bimodal instability, where the usual spacing is on the order of 1%–2%.
Stable SOAEs are usually inaudible to their possessor or are very faint, even when measured at relatively high levels in the ear canal. Presumably this is because SOAEs are subject to the same type of adaptation that is measured psychophysically for low-level, pure-tone stimuli, i.e., “loudness adaptation” and “tone decay” (e.g., Scharf, 1983). SOAES that are unstable in level and∕or frequency are, to varying degrees, released from this adaptation. For bimodal SOAEs, which apparently switch frequencies and are either “on” or “off” at a particular frequency, and for temporal conditions where the “on-times” of these SOAEs are long enough that loudness integration is complete (about 200 ms) and short enough so there is no loudness adaptation (about 500 ms), the equivalent level of the SOAE can be determined by comparing their level in the ear canal, obtained from STFT analyses, with the levels of tones matched in loudness to the SOAEs. For the 1378∕1469 pair, and another bimodal pair in the same subject, the SOAEs were matched in loudness by tones whose levels in the ear canal were from 15 to 20 dB higher than the levels of the SOAEs in the ear canal. The value of 30 dB given in Burns, 1996 illustrates the danger of the practice of submitting abstracts based on preliminary data.
Note that, because some of the SOAEs in infants were not seen in the earliest measurement sessions due to noisy measurement conditions, the reference for frequency shifts in this figure is the final (8-year-old) measurement rather than the initial measurement as in our other figures.
The subjects who showed the largest cumulative frequency shift were those studied the longest, the author and two of his children (subjects 18, 3, and 6). Thresholds measured in these subjects at the end of the study were within normal limits with the exception of the high-frequency (8 kHz) loss in the author, which had also been present at time of initial measurements.
For SOAEs measured in the ears of both adults and children, the power absorbed by the Etymotic ER-10C probe from the majority of SOAEs ranged from 0.1 to 10.0 aW, with the power absorbed from the highest-level SOAEs as large as 400 aW. The power was estimated from the SOAE SPL recorded by the probe microphone, the measured source impedance of the ER-10C probe, and the results calculated using an acoustic transmission-line model of the ear canal between the tympanic membrane and the probe. The model assumed that the ear canal was adequately represented by a finite cylindrical tube with rigid and loss-free walls; these assumptions are thought to be valid for older children and adults. The model was specified in terms of ear-canal cross-sectional area and length. The ear-canal volume was estimated using a tympanometric measurement, assuming the same insertion depth for the tympanometry probe and the ER-10C probe. Any small differences in insertion depth would produce only small errors in the calculated power because of the absence of standing-wave effects in the ear canal in the frequency range of the SOAE measurements. The ear-canal cross-sectional area was estimated acoustically based on reflectance measurements (Keefe and Abdala, 2007), and the ear-canal length was calculated as the ratio of volume to area.
Haggerty et al. (1993) also suggested the possibility that the pineal hormone melatonin, the levels of which correlate with the circadian rhythm, might control the menstrual-cycle variations in SOAE frequency. However, more recent results (Parry et al., 2006) indicate that melatonin levels are stable over the menstrual cycle in normal subjects.
References
- Abdala, C., and Keefe, D. H. (2006). “Effects of middle-ear immaturity on distortion product otoacoustic emission suppression tuning in infant ears,” J. Acoust. Soc. Am. 10.1121/1.2359237 120, 3832–3842. [DOI] [PubMed] [Google Scholar]
- Bell, A. (1992). “Circadian and menstrual rhythms in frequency variations of spontaneous otoacoustic emissions from human ears,” Hear. Res. 10.1016/0378-5955(92)90012-C 58, 91–100. [DOI] [PubMed] [Google Scholar]
- Brienesse, P., Anteunis, L. J. C., Maertzdorf, W., Blanco, C. E., and Manni, J. J. (1997). “Frequency shift of individual spontaneous otoacoustic emissions in preterm infants,” Pediatr. Res. 42, 478–483. [DOI] [PubMed] [Google Scholar]
- Burns, E. M. (1996). “Equivalent levels of SOAEs estimated from loudness matches to unstable SOAEs,” in Abstracts of the 19th Midwinter Research Meeting of the ARO, edited by Popelka R. (Association of Research in Otolaryngology, Des Moines, IA: ), p. 182.
- Burns, E. M. (1999). “Longitudinal measurements of SOAEs in children revisited, for the last time, really,” in Abstracts of the 22nd Midwinter Research Meeting of the ARO, edited by Popelka R. (Association of Research in Otolaryngology, Des Moines, IA: ), p. 92.
- Burns, E. M., Campbell, S. L., Arehart, K. H., and Keefe, D. H. (1993a). “Long-term stability of spontaneous otoacoustic emissions,” in Abstracts of the 16th Midwinter Research Meeting of the ARO, edited by Lim D. (Association of Research in Otolaryngology, Des Moines, IA: ), p. 98.
- Burns, E. M., Harrison, W. A., Bulen, J. C., and Keefe, D. H. (1993b). “Voluntary contraction of middle ear muscles: Effects on input impedance, energy reflectance and spontaneous otoacoustic emissions,” Hear. Res. 10.1016/0378-5955(93)90239-W 67, 117–127. [DOI] [PubMed] [Google Scholar]
- Burns, E. M., and Keefe, D. H. (1992). “Intermittent tinnitus resulting from unstable otoacoustic emissions,” in Tinnitus 91: Proceedings of the Fourth International Tinnitus Seminar, edited by Aran J. M. and Dauman R. (Kugler, Amsterdam: ).
- Burns, E. M., and Keefe, D. H. (1997). “SOAEs and power transfer in the middle and external ears of children and adults,” in Abstracts of the 20th Midwinter Research Meeting of the ARO, edited by Popelka R. (Association of Research in Otolaryngology, Des Moines, IA: ), p. 168.
- Burns, E. M., and Pitton, J. W. (1993). “Time-frequency analyses of coherent frequency fluctuations among spontaneous otoacoustic emissions,” J. Acoust. Soc. Am. 93, 2314 (Abstract). [Google Scholar]
- Burns, E. M., Strickland, E. A., Tubis, A., and Jones, K. L. (1984). “Interactions among spontaneous otoacoustic emissions. I. Distortion products and linked emissions,” Hear. Res. 10.1016/0378-5955(84)90116-3 16, 271–278. [DOI] [PubMed] [Google Scholar]
- Chaloupka, V., Mitchell, S., and Muirhead, R. (1994). “Observation of a reversible, medication-induced change in pitch perception,” J. Acoust. Soc. Am. 10.1121/1.411437 96, 145–149. [DOI] [PubMed] [Google Scholar]
- Cilento, B. W., Norton, S. J., and Gates, G. A. (2003). “The effects of aging and hearing loss on distortion product otoacoustic emissions,” Otolaryngol.-Head Neck Surg. 10.1016/S0194-5998(03)00637-5 129, 382–389. [DOI] [PubMed] [Google Scholar]
- Cox, J. R. (1980). “Hormonal influence on auditory function,” Hear. Res. 1, 219–222. [DOI] [PubMed] [Google Scholar]
- De Kleine, E., van Dijk, P., and Avan, P. (2000). “The behavior of spontaneous otoacoustic emissions during and after postural changes,” J. Acoust. Soc. Am. 10.1121/1.429403 107, 3308–3316. [DOI] [PubMed] [Google Scholar]
- Dorn, P. A., Piskorski, P., Keefe, D. H., Neely, S. T., and Gorga, M. P. (1998). “On the existence of an age/threshold/frequency interaction in distortion product otoacoustic emissions,” J. Acoust. Soc. Am. 10.1121/1.423339 104, 964–971. [DOI] [PubMed] [Google Scholar]
- Feeney, P. M., and Sanford, C. A. (2004). “Age effects in the human middle ear: Wideband acoustical measures,” J. Acoust. Soc. Am. 10.1121/1.1808221 116, 3546–3558. [DOI] [PubMed] [Google Scholar]
- Fritze, W. (1983). “Registration of spontaneous cochlear emissions by means of Fourier transformation,” Eur. Arch. Otorhinolaryngol. 238, 189–196. [DOI] [PubMed] [Google Scholar]
- Haggerty, H. S., Lusted, H. S., and Morton, S. C. (1993). “Statistical quantification of 24-hour and monthly variabilities of spontaneous otoacoustic emission frequency in humans,” Hear. Res. 10.1016/0378-5955(93)90050-B 70, 31–49. [DOI] [PubMed] [Google Scholar]
- Harrison, W. A., and Burns, E. M. (1993). “Effects of contralateral acoustic stimulation on spontaneous otoacoustic emissions,” J. Acoust. Soc. Am. 10.1121/1.407349 94, 2649–2658. [DOI] [PubMed] [Google Scholar]
- Hauser, R., Probst, R., and Harris, F. P. (1993). “Effects of atmospheric pressure variation on spontaneous, transiently evoked, and distortion product otoacoustic emissions in normal human ears,” Hear. Res. 10.1016/0378-5955(93)90101-6 69, 133–145. [DOI] [PubMed] [Google Scholar]
- Keefe, D. H., and Abdala, C. (2007). “Theory of forward and reverse middle-ear transmission applied to otoacoustic emissions in infant and adult ears,” J. Acoust. Soc. Am. 10.1121/1.2427128 121, 978–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe, D. H., Burns, E. M., Ling, R., and Laden, B. (1990). “Chaotic dynamics of otoacoustic emissions,” in Mechanics and Biophysics of Hearing, edited by Dallos P., Geisler C., Mathews J., Ruggero M., and Steele C. (Springer-Verlag, Berlin: ), pp. 194–201. [Google Scholar]
- Kemp, D. T. (1979). “The evoked cochlear mechanical response and the auditory microstructure—Evidence for a new element in cochlear mechanics,” Scand. Audiol. Suppl. 9, 35–47. [PubMed] [Google Scholar]
- Kohler, W., and Fritze, W. (1992). “A long-term observation of spontaneous oto-acoustic emissions (SOAEs),” Scand. Audiol. 21, 55–58. [DOI] [PubMed] [Google Scholar]
- Long, G. L., and Talmadge, C. L. (1997). “Spontaneous otoacoustic emission frequency is modulated by heartbeat,” J. Acoust. Soc. Am. 102, 2831–2848. [DOI] [PubMed] [Google Scholar]
- Long, G. R. (1989). “Modification of the frequency and level of otoacoustic emissions by contralateral stimulation, in a subject with no acoustic reflexes in one ear,” in Abstracts of the 12th Midwinter Research Meeting of the ARO, edited Lim D. (Association of Research in Otolaryngology, Columbus, OH: ), p. 228.
- Long, G. R., and Tubis, A. (1988). “Investigations into the nature of the association between threshold microstructure and otoacoustic emissions,” Hear. Res. 10.1016/0378-5955(88)90055-X 36, 125–138. [DOI] [PubMed] [Google Scholar]
- Lonsbury-Martin, B. L., Martin, G. K., Probst, R., and Coats, A. C. (1988). “Spontaneous otoacoustic emissions in the nonhuman primate. II. Cochlear anatomy,” Hear. Res. 10.1016/0378-5955(88)90021-4 33, 69–94. [DOI] [PubMed] [Google Scholar]
- McFadden, D. (1998). “Sex differences in the auditory system,” Dev. Neuropsychol. 14, 261–298. [Google Scholar]
- McFadden, D. (2008). “What do sex, twins, spotted hyenas, ADHD, and sexual orientation have in common?,” Perspect. Psychol. Sci. 3, 309–323. [DOI] [PubMed] [Google Scholar]
- McFadden, D. (2009). “Masculinization of the mammalian cochlea,” Hear. Res. [DOI] [PMC free article] [PubMed]
- Mott, J. B., Norton, S. J., Neely, S. T., and Warr, W. B. (1989). “Changes in otoacoustic emissions produced by acoustic stimulation of the contralateral ear,” Hear. Res. 10.1016/0378-5955(89)90068-3 38, 229–242. [DOI] [PubMed] [Google Scholar]
- Okabe, K., Tanaka, S., Hamada, H., Miura, T., and Funai, H. (1988). “Acoustic impedance measurement of normal ears of children,” J. Acoust. Soc. Jpn. 9, 287–294. [Google Scholar]
- Olson, E. S., and Mountain, D. C. (1994). “Mapping the cochlear partition’s stiffness to its cellular architecture,” J. Acoust. Soc. Am. 10.1121/1.408331 95, 395–400. [DOI] [PubMed] [Google Scholar]
- Parry, B., Martinez, L. F., Maurer, E., López, A., Sorenson, D., and Meliska, C. (2006). “Sleep rhythms and women’s mood. Part I: Menstrual cycle, pregnancy and postpartum,” Sleep Med. Rev. 10, 129–144. [DOI] [PubMed] [Google Scholar]
- Penner, M. J. (1995). “Frequency variation of spontaneous otoacoustic emissions during a naturally occurring menstrual cycle, amenorrhea, and oral contraception: A brief report,” Ear Hear. 16, 428–432. [DOI] [PubMed] [Google Scholar]
- Rabinowitz, W. M., and Widen, G. P. (1984). “Interaction of spontaneous oto-acoustic emissions and external sounds,” J. Acoust. Soc. Am. 10.1121/1.391618 76, 1713–1720. [DOI] [PubMed] [Google Scholar]
- Ramotowski, D., and Kimberley, B. (1998). “Age and the human cochlear traveling wave delay,” Ear Hear. 10.1097/00003446-199804000-00003 19, 111–119. [DOI] [PubMed] [Google Scholar]
- Scharf, B. (1983). “Loudness adaptation,” in Hearing Research and Theory, edited by Tobias J. V. and Schubert E. D. (Academic, San Diego, CA: ), Vol. 2, pp. 1–56. [Google Scholar]
- Schloth, E. (1983). “Spectral composition of spontaneous oto-acoustic emissions and fine structure of the threshold in quiet,” Acustica 53, 250–256. [Google Scholar]
- Schloth, E., and Zwicker, E. (1983). “Mechanical and acoustical influences on spontaneous otoacoustic emissions,” Hear. Res. 10.1016/0378-5955(83)90063-1 11, 285–293. [DOI] [PubMed] [Google Scholar]
- Shera, C. A. (2003). “Mammalian spontaneous otoacoustic emissions are amplitude-stabilized cochlear standing waves,” J. Acoust. Soc. Am. 10.1121/1.1575750 114, 244–262. [DOI] [PubMed] [Google Scholar]
- Talmadge, C. L., Long, G. R., Murphy, W. J., and Tubis, A. (1993). “New off-line method for detecting spontaneous otoacoustic emissions in human subjects,” Hear. Res. 10.1016/0378-5955(93)90032-V 71, 170–182. [DOI] [PubMed] [Google Scholar]
- Talmadge, C. L., Tubis, A., Long, G. R., and Piskorski, P. (1998). “Modeling otoacoustic emission and hearing threshold fine structures,” J. Acoust. Soc. Am. 10.1121/1.424364 104, 1517–1543. [DOI] [PubMed] [Google Scholar]
- Tulchincky, D., Hobel, C. J., Yeager, E., and Marshall, J. R. (1972). “Plasma estrone, estraiol, estroil, progesterone, and 17-hydroxprogesterone in human pregnancy,” Am. J. Obstet. Gynecol. 112, 1095–1100. [DOI] [PubMed] [Google Scholar]
- Van Dijk, P., and Wit, H. P. (1990). “Amplitude and frequency fluctuations of spontaneous otoacoustic emissions,” J. Acoust. Soc. Am. 10.1121/1.400199 88, 1779–1793. [DOI] [PubMed] [Google Scholar]
- Ward, W. D. (1999). “Absolute pitch,” in The Psychology of Music, edited by Deutsch D. (Academic, San Diego, CA: ), pp. 265–298. [Google Scholar]
- Whitehead, M. L. (1988). “Some properties of otoacoustic emissions in vertebrate ears, and their relationship to other hearing mechanisms,” Ph.D. thesis, University of Keele (Keele, UK). [Google Scholar]
- Whitehead, M. L. (1991). “Slow variations of the amplitude and frequency of spontaneous otoacoustic emissions,” Hear. Res. 10.1016/0378-5955(91)90060-M 53, 269–280. [DOI] [PubMed] [Google Scholar]
- Wilson, J. P. (1986). “The influence of temperature on frequency-tuning mechanisms,” in Peripheral Auditory Mechanisms, edited by Allen J. B., Hubbard A., St. Neely S., and Tubis A. (Springer-Verlag, Berlin: ), pp. 229–236. [Google Scholar]
- Wilson, J. P., and Sutton, G. J. (1981). “Acoustic correlates of tonal tinnitus,” in Tinnitus, edited by Evered D. and Lawrenson G. (Pitman Medical, London: ), pp. 82–107. [DOI] [PubMed] [Google Scholar]
- Wit, H. P. (1985). “Diurnal cycle for spontaneous oto-acoustic emission frequency,” Hear. Res. 18, 197–199. [DOI] [PubMed] [Google Scholar]
- Zurek, P. M. (1981). “Spontaneous narrowband acoustic signals emitted by human ears,” J. Acoust. Soc. Am. 10.1121/1.385481 69, 514–523. [DOI] [PubMed] [Google Scholar]