Abstract
The progressive rise in the prevalence of allergic diseases since the 1970s is widely attributed to diminished exposure to microbial stimuli, resulting in dysregulated immune functions during early life. Most studies investigating the mechanism behind this phenomenon have focused on postnatal microbial exposure. But emerging evidence suggests that such programming may also occur in the developing fetus as a result of microbial stimulation of the pregnant mother.
Susceptibility to allergic disease: the “hygiene hypothesis”
Current interest in the role of environmental microbial exposure as a determinant of susceptibility to allergic diseases dates back to the hallmark publication by Strachan (1989) introducing the hygiene hypothesis, which inferred from epidemiological evidence that shared infections between siblings during early life protected children against development of allergic disease. On page 2869 of this issue, Conrad et al. (2009) add a new twist to the hygiene hypothesis by showing that allergic risk can also be modulated by microbial exposure before birth. Mice born to dams that were exposed to bacteria during pregnancy were less likely to develop allergic responses than those born to unexposed mothers. And maternal Toll-like receptor (TLR) signals were required for the transmission of protection.
The hygiene hypothesis and immune development
Soon after Strachan's publication, our group identified infancy as the life phase during which risk for primary allergic sensitization is greatest. These studies also uncovered a link between delayed postnatal maturation of adaptive immune function and genetically determined, increased risk for atopy (Holt et al., 1992). Notably, infants from atopic families who were at high risk for developing allergic disease had reduced numbers of immunocompetent CD4+ T cells in the circulation. Moreover clones from high-risk infants produced lower levels of cytokines, particularly Th1 cytokines, resulting in an overall Th2 bias. This suggested that the developmental process underlying the transition from the quiescent and relatively Th2-polarized fetal immune phenotype toward the more active and balanced pattern characteristic of mature adaptive immunity was intrinsically slower in the high-risk population, thus increasing the likelihood of priming Th2-biased immunity against environmental allergens (Holt et al., 1992). Although recent data from prospective studies on birth cohorts still support this general concept (Rowe et al., 2007), it is now clear that T cells are only one component of a complex mosaic of innate and adaptive immune mechanisms that are developmentally compromised in high-risk children (Holt and Sly, 2007).
An obvious question arising from these findings is what drives immune maturation in early life. It is evident from studies on germ-free mice that the primary triggers for postnatal immune maturation is provided by microbial signals that are not available in the intrauterine environment, in particular from commensal bacterial (Holt, 1995) and possibly low-pathogenicity viruses (McIntire et al., 2003) in the gastrointestinal tract. It is now clear that respiratory viral pathogens exacerbate rather than retard diseases such as atopic asthma in children (Sly et al., 2008), reinforcing the emphasis on the gastrointestinal tract as the most likely site for generation of the protective, microbial-induced signals envisaged in the hygiene hypothesis. Indeed, a major international research effort has now developed to characterize the underlying immunological processes, and to identify strains of organisms with “safe” immunomodulatory properties for use as oral therapies in inflammatory diseases, with some (limited) success (Holt and Sly 2007).
Meanwhile, back at the farm…
This gut-centric view has been challenged by findings from landmark human studies relating to asthma and allergy in traditional farming communities. These epidemiological studies defined a unique gradient in allergy and asthma prevalence between children born into traditional farming families and their rural and urban counterparts, with the lowest prevalence occurring in the former. The hallmark characteristic of these farms is barns in which animals (especially dairy cows) are housed over the winter, during which much of the in-barn work is performed by the mother of the family. A series of studies (for review see von Mutius and Radon, 2008) have provided compelling evidence for a link between repeated exposure of the offspring of these farming mothers to microbe-rich barn environments during infancy and reduced susceptibility to subsequent allergy/asthma development, which is associated with persistent changes in various aspects of immune function in the children.
The high microbial burden which appears to mediate protection in these infants is multifaceted and involves both gastrointestinal exposure to components in unpasteurized milk and exposure to high levels of LPS-containing dust (Braun-Fahrländer et al., 2002). Indeed, exposure to LPS in house dust also protects against the subsequent development of allergies in nonfarming settings (Gereda et al., 2000). It is interesting to note that farm exposure during infancy also modulates susceptibility to inflammatory diseases beyond allergy, including ulcerative colitis and Crohn's disease (Bach, 2002; Radon et al., 2007).
Perhaps a more unexpected finding was that exposure of mothers to the barn environment during pregnancy was also a major factor in modifying immune function and reducing risk of allergic disease in their offspring (Ege et al., 2008; von Mutius and Radon, 2008). Newborns of farm-exposed mothers displayed enhanced levels of regulatory T (T reg) cell activity at birth, which was associated with reduced Th2 cytokine production after cord blood stimulation (Schaub et al., 2009). Stimulated cord blood cells from these infants also showed increased expression of TLR2 and CD14, suggesting innate immune cell activation. In a parallel study, 5-13-yr-old children of farm-exposed mothers displayed reduced rates of sensitization and concomitantly increased TLR2, TLR4, and CD14 gene expression in PBMCs (Ege et al., 2006). It should be emphasized that the effects in the children were unrelated to the farm exposure levels at the time of their PBMC collection, and instead were related exclusively to the mothers' exposures during pregnancy.
Transplacental immune programming
These findings raise the intriguing possibility that appropriate stimulation of the maternal immune system during pregnancy may generate signals capable of “educating” the fetal immune system to pave the way for efficient functional maturation after birth. Is this plausible? There is some evidence that supports prenatal programming, including data from birth cohort studies showing that neonatal patterns of cytokine production are significant predictors of subsequent risk for allergy and asthma (Macaubas et al., 2003). The general concept of signaling between the maternal and fetal immune systems is not new and encompasses fundamental protective mechanisms ranging from transplacental transfer of protective antibodies to allostimulation of tolerogenic T reg cells in the fetus by migrating maternal cells (Mold et al., 2008). However, in the human allergy/asthma field, the major focus has been on the converse scenario: maternal transmission of disease risk (Lim and Kobzik, 2009). The mechanisms involved in transmission of risk from mother-to-fetus are incompletely understood. Based on limited data from animal models, it has been speculated that transplacental transfer of Th2-trophic cytokines and/or cells from atopic dams may be involved (Matson et al., 2007).
Conversely, a variety of experimental mouse models (for review see Lim and Kobzik, 2009; Matson et al., 2007) have demonstrated that stimulation of a strong Th1 environment during pregnancy can markedly reduce the susceptibility of the offspring to experimental asthma. This includes exposure to aerosolized LPS via the respiratory mucosa, which mimics some aspects of the human farm exposures discussed above. These data collectively suggest that maternal signaling to the fetal immune system exists on several levels and is likely to vary qualitatively depending on the stimuli present in the maternal environment.
Maternal farm exposure and asthma: a mouse model
Conrad et al. (2009) describe a mouse model designed to specifically examine the implications of maternal farm exposure. The group used a bacterial strain, Acinetobacter lwoffi, a common component of barn dust that has the potential capacity to protect against atopy. The progeny of mice given repeated intranasal treatments with A. lwoffi during pregnancy developed strong resistance to experimental atopic asthma development in response to subsequent sensitization and aerosol challenge with ovalbumin. Intriguingly, the resistance to eosinophilia, goblet cell hyperplasia, and airway hyperresponsiveness after challenge was independent of changes in specific IgE, which were not significantly reduced by the treatment. This finding is reminiscent of successful immunotherapy in humans in which reductions in symptoms typically precede decreases in IgE titers.
The protective effect was dependent on intact maternal TLR signaling, as offspring of mice lacking multiple TLRs (TLR2, 3, 4, 7, and 9) generated allergic responses comparable to those seen in the offspring of nonexposed mothers. Exposure to A. lwoffi triggered a mild-to-moderate local inflammatory response in the lungs of wild-type animals, which was accompanied by the up-regulation of mRNA specific for TLR1, 2, 3, 5, 7, and 9, and the production of TNF, IL-12, and IL-6 in the BAL and serum. It was particularly notable that the lung response was also accompanied by down-regulation of TLR gene expression in the placenta, which the authors suggest occurs predominantly on the maternal side, with concomitantly reduced expression of a broad range of cytokine genes (Conrad et al., 2009). This TLR down-regulation could be partially reproduced in primary placental cultures treated with IL-6.
These findings suggest that controlled microbial stimulation at the maternal mucosal surface (in this case, the respiratory tract) during pregnancy, at a level that elicits mild, subclinical inflammation, can generate diffusible signals that function as the immunological equivalent of a lullaby in placental tissue, dampening baseline innate immune activity and attenuating local cytokine production. The association between these effects and the subsequent resistance to allergic disease in offspring suggests that these changes at the fetomaternal interface had positive effects on functional maturation of the fetal immune system. It would be of interest to analyze the nature of these changes in follow-up studies and to determine whether exposure of the offspring to the same microbial stimuli after birth (as is the norm with the farm children) further amplifies their acquired resistance to allergy and asthma. It is noteworthy that Conrad et al. (2009) ruled out direct transfer of bacterial endotoxin as a significant factor in the antenatal programming process. It is also important to emphasize that although A. lwoffii is a potent mediator of the transplacental effects observed, there is no reason to believe that this organism is unique in this regard, and indeed the authors report qualitatively similar effects with other microbial agents.
In placenta veritas?
Homeostatic regulation of the immunological milieu of the placenta has traditionally been far removed from the normal purview of allergy/asthma researchers. However, the growing realization that the long-term programming of immunological and respiratory phenotypes that underpin these diseases is initiated during infancy (Holt et al., 2005) is fuelling interest in this area. The new findings reported by Conrad et al. will undoubtedly add to this interest.
It is a widely accepted precept that maintenance of the placental microenvironment in a “noninflamed” steady state represents the ideal for healthy development of the fetus and that efficient local surveillance for microbial pathogens is central to fetal survival (Mor et al., 2005). How this balance is achieved remains largely unknown, but the cellular players in the game of fetal survival are essentially the same as those responsible for maintenance of immunological homeostasis in the airway mucosa, where they help to determine susceptibility versus resistance to allergy and asthma. In the maternal decidua, interactions between dendritic cells (DCs) and macrophages play a central role in maintaining tolerance to fetal antigens (Blois et al., 2007). The equivalent myeloid populations in the airway mucosa normally maintain tolerance to incoming aeroallergens (Holt et al., 2008), and it may thus be reasonable to extrapolate somewhat between the two organ systems.
In this regard, DC populations in the airway mucosa are highly dynamic even in the steady-state, continuously shuttling antigen between the mucosal surface and the draining lymph nodes. These cells respond rapidly to inhaled microbial ligands, further amplifying local immune surveillance by increasing recruitment of bone marrow–derived immature DCs and, later, monocytes (Holt et al., 2008). This mechanism provides the essential local link between the innate and adaptive arms of the immune response and appears to be an essential component of host defense.
Unless fine control of this process is maintained, however, the host response to microbial exposure can itself become pathological. An archetypal example of this is the demonstration that cytokine signals generated in the inflamed airways during virus-induced asthma exacerbations can induce proinflammatory functions in immature DCs and monocytes before their release from the bone marrow (Subrata et al., 2009). Moreover, inflammation-associated signaling from the periphery to the bone marrow can also program regulatory phenotypes in migratory myeloid cell populations (Gordon, 2003). Once released into the circulation, these myeloid cells are free to migrate into any tissue (including potentially the decidua) to subsequently modulate local immunological processes. It is thus possible that the effects observed in the mouse placenta by intranasal exposure to A. lwoffi may occur in part via this indirect route rather than exclusively by the direct passage of cytokines into decidual tissue itself (Fig. 1). It should be noted, however, that lymphangiogenesis in human decidual tissues is much more robust than in the mouse, and this is likely to influence migration and turnover of DCs and other myeloid cells (Collins et al., 2009). This difference may result in species differences in sensitivity to external stimuli such as those described here (Conrad et al., 2009), and this possibility needs to be addressed in follow-up studies. Despite this caveat, these findings have established a potentially important set of principles relating to maternal environmental exposure during pregnancy and subsequent immune functions in offspring that, if substantiated, will be applicable to a much broader range of disease than simply asthma and allergy.
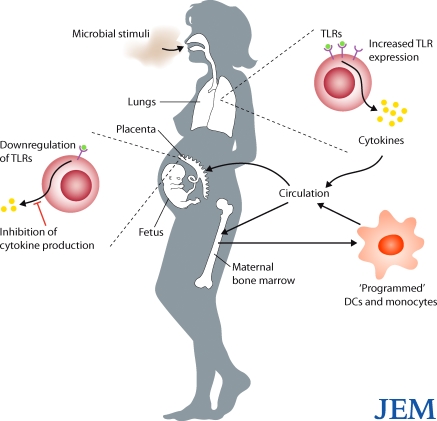
Figure 1.
Proposed mechanisms by which maternal exposure to bacteria protects against allergies in offspring. Aerosol exposure to microbe-containing dust particles induces mild-to-moderate inflammation in the lungs, including increased expression of TLRs and production of cytokines. Cytokines might then enter the bloodstream and be delivered directly to the placental tissues, where they depress TLR expression, cytokine production, and influence resident myeloid cell functions. Circulating cytokines might also enter the maternal bone marrow, where they stimulate and “program” myeloid precursor cells. Programmed DCs and monocytes could then enter the circulation, and some of these cells could traffic to the decidua, where they could replace resident myeloid populations and influence the local inflammatory milieu.
References
- Bach J.F. 2002. The effect of infections on susceptibility to autoimmune and allergic diseases. N. Engl. J. Med. 347:911–920 10.1056/NEJMra020100 [DOI] [PubMed] [Google Scholar]
- Blois S.M., Kammerer U., Alba Soto C., Tometten M.C., Shaikly V., Barrientos G., Jurd R., Rukavina D., Thomson A.W., Klapp B.F., et al. 2007. Dendritic cells: key to fetal tolerance? Biol. Reprod. 77:590–598 10.1095/biolreprod.107.060632 [DOI] [PubMed] [Google Scholar]
- Braun-Fahrländer C., Riedler J., Herz U., Eder W., Waser M., Grize L., Maisch S., Carr D., Gerlach F., Bufe A., et al. ; Allergy and Endotoxin Study Team 2002. Environmental exposure to endotoxin and its relation to asthma in school-age children. N. Engl. J. Med. 347:869–877 10.1056/NEJMoa020057 [DOI] [PubMed] [Google Scholar]
- Collins M.K., Tay C.S., Erlebacher A. 2009. Dendritic cell entrapment within the pregnant uterus inhibits immune surveillance of the maternal/fetal interface in mice. J. Clin. Invest. 119:2062–2073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad M.L., Fersti R., Teich R., Brand S., Blumer N., Yildirim A.O., Patrascan C.C., Hanuszkiewicz A., Akira S., Wagner H., et al. 2009. Maternal TLR signaling is required for prenatal asthma protection by the non-pathogenic microbe Acinetobacter lwoffii F78. J. Exp. Med. 2869–2877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ege M.J., Bieli C., Frei R., van Strien R.T., Riedler J., Ublagger E., Schram-Bijkerk D., Brunekreef B., van Hage M., Scheynius A., et al. ; Parsifal Study team 2006. Prenatal farm exposure is related to the expression of receptors of the innate immunity and to atopic sensitization in school-age children. J. Allergy Clin. Immunol. 117:817–823 10.1016/j.jaci.2005.12.1307 [DOI] [PubMed] [Google Scholar]
- Ege M.J., Herzum I., Buchele G., Krauss-Etschmann S., Lauener R.P., Roponen M., Hyvarinen A., Vuitton D.A., Riedler J., Brunekreef B., et al. 2008. Prenatal exposure to a farm environment modifies atopic sensitization at birth. J. Allergy Clin. Immunol. 122:407–412 [DOI] [PubMed] [Google Scholar]
- Gereda J.E., Leung D.Y.M., Thatayatikom A., Streib J.E., Price M.R., Klinnert M.D., Liu A.H. 2000. Relation between house-dust endotoxin exposure, type 1 T-cell development, and allergen sensitisation in infants at high risk of asthma. Lancet. 355:1680–1683 10.1016/S0140-6736(00)02239-X [DOI] [PubMed] [Google Scholar]
- Gordon S. 2003. Alternative activation of macrophages. Nat. Rev. Immunol. 3:23–35 10.1038/nri978 [DOI] [PubMed] [Google Scholar]
- Holt P.G. 1995. Environmental factors and primary T-cell sensitisation to inhalant allergens in infancy: reappraisal of the role of infections and air pollution. Pediatr. Allergy Immunol. 6:1–10 10.1111/j.1399-3038.1995.tb00250.x [DOI] [PubMed] [Google Scholar]
- Holt P.G., Sly P.D. 2007. Prevention of allergic respiratory disease in infants: current aspects and future perspectives. Curr. Opin. Allergy Clin. Immunol. 7:547–555 10.1097/ACI.0b013e3282f14a17 [DOI] [PubMed] [Google Scholar]
- Holt P.G., Clough J.B., Holt B.J., Baron-Hay M.J., Rose A.H., Robinson B.W.S., Thomas W.R. 1992. Genetic ‘risk’ for atopy is associated with delayed postnatal maturation of T-cell competence. Clin. Exp. Allergy. 22:1093–1099 10.1111/j.1365-2222.1992.tb00135.x [DOI] [PubMed] [Google Scholar]
- Holt P.G., Upham J.W., Sly P.D. 2005. Contemporaneous maturation of immunologic and respiratory functions during early childhood: implications for development of asthma prevention strategies. J. Allergy Clin. Immunol. 116:16–24, quiz :25 10.1016/j.jaci.2005.04.017 [DOI] [PubMed] [Google Scholar]
- Holt P.G., Strickland D.H., Wikström M.E., Jahnsen F.L. 2008. Regulation of immunological homeostasis in the respiratory tract. Nat. Rev. Immunol. 8:142–152 10.1038/nri2236 [DOI] [PubMed] [Google Scholar]
- Lim R.H., Kobzik L. 2009. Maternal transmission of asthma risk. Am. J. Reprod. Immunol. 61:1–10 10.1111/j.1600-0897.2008.00671.x [DOI] [PubMed] [Google Scholar]
- Macaubas C., de Klerk N.H., Holt B.J., Wee C., Kendall G., Firth M., Sly P.D., Holt P.G. 2003. Association between antenatal cytokine production and the development of atopy and asthma at age 6 years. Lancet. 362:1192–1197 10.1016/S0140-6736(03)14542-4 [DOI] [PubMed] [Google Scholar]
- Matson A.P., Zhu L., Lingenheld E.G., Schramm C.M., Clark R.B., Selander D.M., Thrall R.S., Breen E., Puddington L. 2007. Maternal transmission of resistance to development of allergic airway disease. J. Immunol. 179:1282–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntire J.J., Umetsu S.E., Macaubas C., Hoyte E.G., Cinnioglu C., Cavalli-Sforza L.L., Barsh G.S., Hallmayer J.F., Underhill P.A., Risch N.J., et al. 2003. Immunology: hepatitis A virus link to atopic disease. Nature. 425:576 10.1038/425576a [DOI] [PubMed] [Google Scholar]
- Mold J.E., Michaëlsson J., Burt T.D., Muench M.O., Beckerman K.P., Busch M.P., Lee T.H., Nixon D.F., McCune J.M. 2008. Maternal alloantigens promote the development of tolerogenic fetal regulatory T cells in utero. Science. 322:1562–1565 10.1126/science.1164511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mor G., Romero R., Aldo P.B., Abrahams V.M. 2005. Is the trophoblast an immune regulator? The role of Toll-like receptors during pregnancy. Crit. Rev. Immunol. 25:375–388 10.1615/CritRevImmunol.v25.i5.30 [DOI] [PubMed] [Google Scholar]
- Radon K., Windstetter D., Poluda A.L., Mueller B., von Mutius E., Koletzko S.; Chronische Autoimmunerkrankungen und Kontakt zu Tieren (Chronic Autoimmune Disease and Animal Contact) Study Group 2007. Contact with farm animals in early life and juvenile inflammatory bowel disease: a case-control study. Pediatrics. 120:354–361 10.1542/peds.2006-3624 [DOI] [PubMed] [Google Scholar]
- Rowe J., Kusel M., Holt B.J., Suriyaarachchi D., Serralha M., Hollams E., Yerkovich S.T., Subrata L.S., Ladyman C., Sadowska A., et al. 2007. Prenatal versus postnatal sensitization to environmental allergens in a high-risk birth cohort. J. Allergy Clin. Immunol. 119:1164–1173 10.1016/j.jaci.2007.02.016 [DOI] [PubMed] [Google Scholar]
- Schaub B., Liu J., Hoppler S., Schleich I., Huehn J., Olek S., Wieczorek G., Illi S., von Mutius E. 2009. Maternal farm exposure modulates neonatal immune mechanisms through regulatory T cells. J. Allergy Clin. Immunol. 123:774–782 [DOI] [PubMed] [Google Scholar]
- Sly P.D., Boner A.L., Björksten B., Bush A., Custovic A., Eigenmann P.A., Gern J.E., Gerritsen J., Hamelmann E., Helms P.J., et al. 2008. Early identification of atopy in the prediction of persistent asthma in children. Lancet. 372:1100–1106 10.1016/S0140-6736(08)61451-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strachan D.P. 1989. Hay fever, hygiene, and household size. BMJ. 299:1259–1260 10.1136/bmj.299.6710.1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subrata L.S., Bizzintino J., Mamessier E., Bosco A., McKenna K.L., Wikström M.E., Goldblatt J., Sly P.D., Hales B.J., Thomas W.R., et al. 2009. Interactions between innate antiviral and atopic immunoinflammatory pathways precipitate and sustain asthma exacerbations in children. J. Immunol. 183:2793–2800 10.4049/jimmunol.0900695 [DOI] [PubMed] [Google Scholar]
- von Mutius E., Radon K. 2008. Living on a farm: impact on asthma induction and clinical course. Immunol. Allergy Clin. North Am. 28:631–647) 10.1016/j.iac.2008.03.010 [DOI] [PubMed] [Google Scholar]