Abstract
Peroral infection with Toxoplasma gondii leads to the development of small intestinal inflammation dependent on Th1 cytokines. The role of Th17 cells in ileitis is unknown. We report interleukin (IL)-23–mediated gelatinase A (matrixmetalloproteinase [MMP]-2) up-regulation in the ileum of infected mice. MMP-2 deficiency as well as therapeutic or prophylactic selective gelatinase blockage protected mice from the development of T. gondii–induced immunopathology. Moreover, IL-23–dependent up-regulation of IL-22 was essential for the development of ileitis, whereas IL-17 was down-regulated and dispensable. CD4+ T cells were the main source of IL-22 in the small intestinal lamina propria. Thus, IL-23 regulates small intestinal inflammation via IL-22 but independent of IL-17. Gelatinases may be useful targets for treatment of intestinal inflammation.
Within 8 d after peroral infection with Toxoplasma gondii, susceptible C57BL/6 mice develop massive necrosis in the ileum, leading to death (Liesenfeld et al., 1996). T. gondii–induced ileitis is characterized by a CD4 T cell–dependent overproduction of proinflammatory mediators, including IFN-γ, TNF, and NO (Khan et al., 1997; Mennechet et al., 2002). Activation of CD4+ T cells by IL-12 and IL-18 is critical for the development of small intestinal pathology (Vossenkämper et al., 2004). Recently, we showed that LPS derived from gut flora via Toll-like receptor (TLR)–4 mediates T. gondii–induced immunopathology (Heimesaat et al., 2006). Thus, the immunopathogenesis of T. gondii–induced small intestinal pathology resembles key features of the inflammatory responses in inflammatory bowel disease (IBD) in humans and in models of experimental colitis in rodents (Liesenfeld, 2002). However, most animal models of IBD assessed inflammatory responses in the large intestine, and models of small intestinal pathology are scarce (Kosiewicz et al., 2001; Strober et al., 2002; Olson et al., 2004; Heimesaat et al., 2006).
IL-12 shares the p40 subunit, IL-12Rβ1, and components of the signaling transduction pathways with IL-23 (Parham et al., 2002). There is strong evidence that IL-23, rather than IL-12, is important in the development of colitis (Yen et al., 2006). The association of IL-23R encoding variant Arg381Gln with IBD (Duerr et al., 2006) and the up-regulation of IL-23p19 in colon biopsies from patients with Crohn's disease (Schmidt et al., 2005) underline the importance of IL-23 in intestinal inflammation. Effector mechanisms of IL-23 include the up-regulation of matrixmetalloproteinases (MMPs; Langowski et al., 2006), a large family of endopeptidases that mediate homeostasis of the extracellular matrix. MMPs were significantly up-regulated in experimental models of colitis (Tarlton et al., 2000; Medina et al., 2003) and in colonic tissues of IBD patients (von Lampe et al., 2000).
Studies in mouse models of autoimmune diseases have associated the pathogenic role of IL-23 with the accumulation of CD4+ T cells secreting IL-17, termed Th17 cells (Aggarwal et al., 2003; Cua et al., 2003). Moreover, increased IL-17 expression was reported in the intestinal mucosa of patients with IBD (Fujino et al., 2003; Nielsen et al., 2003; Kleinschek et al., 2009).
In addition to IL-17, Th17 cells also produce IL-22, a member of the IL-10 family (Dumoutier et al., 2000). IL-22, although secreted by certain immune cell populations, does not have any effects on immune cells in vitro or in vivo but regulates functions of some tissue cells (Wolk et al., 2009). Interestingly, IL-22 has been proposed to possess both protective as well as pathogenic roles. In fact, IL-22 mediated psoriasis-like skin alterations (Zheng et al., 2007; Ma et al., 2008; Wolk et al., 2009). In contrast, IL-22 played a protective role in experimental models of colitis (Satoh-Takayama et al., 2008; Sugimoto et al., 2008; Zenewicz et al., 2008; Zheng et al., 2008), in a model of Klebsiella pneumoniae infection in the lung (Aujla et al., 2007), and against liver damage caused by concanavalin A administration (Radaeva et al., 2004; Zenewicz et al., 2007). IL-22 has been reported to be produced by CD4+ T cells (Wolk et al., 2002; Zheng et al., 2007), γδ cells (Zheng et al., 2007), CD11c+ cells (Zheng et al., 2008), and natural killer cells (Cella et al., 2008; Luci et al., 2008; Sanos et al., 2009; Satoh-Takayama et al., 2008; Zheng et al., 2008). The role of IL-22 in small intestinal inflammation remains to be determined.
In the present study, we investigated the role of the IL-23–IL-17 axis in T. gondii–induced small intestinal immunopathology. We show that IL-23 is essential in the development of small intestinal immunopathology by inducing local MMP-2 up-regulation that could be inhibited by prophylactic or therapeutic chemical blockage. Interestingly, IL-23–dependent IL-22 production was markedly up-regulated and essential for the development of ileal inflammation, whereas IL-17 production was down-regulated after T. gondii infection. IL-22 was mostly produced by CD4+ T cells in the small intestinal lamina propria.
RESULTS
IL-23 mediates T. gondii–induced small intestinal immunopathology
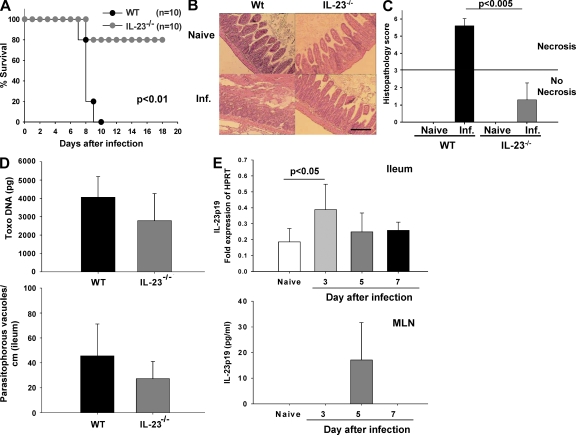
We have previously demonstrated that peroral infection of susceptible C57BL/6 mice with 100 cysts of T. gondii ME49 leads to Th1-type small intestinal necrosis (panileitis; Liesenfeld et al., 1996). To investigate the role of IL-23 in the development of ileal immunopathology, IL-23p19−/− mice were infected orally. All WT mice died within 10 d after infection, whereas 80% of IL-23p19−/− mice survived the acute phase of infection (Fig. 1 A). Severe necrosis of the villi and mucosa was observed in the ilea of WT mice at 8 d after infection, whereas IL-23p19−/− mice showed mild to moderate signs of small intestinal inflammation but no necrosis (Fig. 1 B). Histopathological scores in the ilea of IL-23p19−/− mice were significantly lower compared with WT mice (Fig. 1 C). Neither the amount of T. gondii DNA nor the number of parasitophorous vacuoles containing T.gondii tachyzoites in the ileum differed significantly between both groups at the same time point (Fig. 1 D). Furthermore, no differences were observed in the number of inflammatory foci in the parenchyma and perivascular areas of the liver in WT and IL-23p19−/−–infected mice (unpublished data). There was a significant increase in IL-23p19 mRNA expression in the ileum at day 3 after infection, but levels decreased to those observed in naive mice by day 5 after infection (Fig. 1 E). IL-23 concentrations were below the limit of detection in the ileum at all time points (unpublished data). In mesenteric LNs (MLNs) of naive mice and at day 3 after infection, IL-23 concentrations were below the limit of detection; however, IL-23 concentrations increased markedly on day 5 after infection (Fig. 1 E). Interestingly, CD11b+ but not CD11c+ cells were the main source of IL-23p19 in the small intestinal lamina propria (Fig. S1).
Figure 1.
IL-23 mediates T. gondii–induced immunopathology in the gut. (A) Survival of WT and IL-23p19−/− mice after oral infection with 100 cysts of T. gondii. (B) Histopathology of hematoxylin and eosin–stained ileal sections of naive and infected (Inf.) WT and IL-23p19−/− mice 8 d after infection. (C) Histopathology scores of the ileum of naive and infected WT and IL-23p19−/− mice. The horizontal line indicates the border between mild inflammation (<3) and necrosis (>3). (D) T. gondii DNA concentration in ileal biopsies of WT and IL-23p19−/− mice 8 d after infection (top), and the number of T. gondii parasitophorous vacuoles that contained tachyzoites stained with a rabbit anti–T. gondii IgG antibody and counted in the terminal ileum in WT and IL-23p19−/− mice 8 d after infection (bottom). (E) RT-PCR of IL-23p19 mRNA of ileal biopsies of WT mice at different time points after infection (top). Results are expressed as fold changes relative to HPRT mRNA expression. IL-23p19 concentration in supernatants of MLN of WT mice detected by ELISA (bottom). Data (one representative out of three independent experiments is shown) from five mice per group are given as means ± SD, and p-values were determined by the Mann-Whitney U test or by Kaplan-Meier analysis and the log-rank test (A). Bar, 100 µm.
MMP-2 and MMP-9 are up-regulated in the small intestine of T. gondii–infected mice
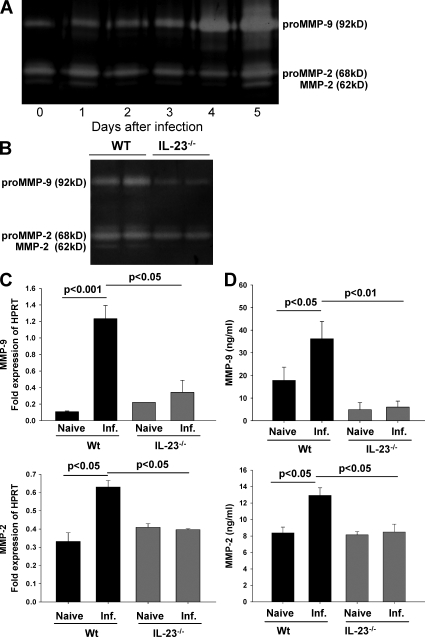
Given that MMPs have been proposed to play an important role in the pathology of IBD (Gao et al., 2005), we determined the enzymatic activity of gelatinases A (MMP-2) and B (MMP-9) in the small intestine of T. gondii–infected WT mice using gelatin zymography. The activity of both MMP-2 and MMP-9 markedly increased after infection and peaked at day 5 after infection (the time point when intestinal pathology starts to develop; Fig. 2 A). An additional band characteristic of active MMP-2 was also observed at the same time point. Because IL-23 was shown to induce the expression of MMP-9, we investigated the enzymatic activity of MMP-9 and MMP-2 in the ileum of WT and IL-23p19−/− mice before and after T. gondii infection. The enzymatic activity of MMP-9 and MMP-2 was lower in IL-23p19−/− compared with WT mice (Fig. 2 B); furthermore, the band of active MMP-2 observed in WT mice after infection was absent in IL-23p19−/− animals at day 8 after infection.
Figure 2.
MMP-2 and MMP-9 are up-regulated in the small intestine of T. gondii–infected mice. (A) MMP-9 and MMP-2 enzymatic activity was measured by gelatin zymography in the ileum of WT-infected mice at different time points after infection. (B) MMP-9 and MMP-2 enzymatic activity measured in the ileum of WT and IL-23p19−/− mice 8 d after infection by gelatin zymography. (C) mRNA of MMP-9 and MMP-2 in ileal biopsies of naive and infected (Inf.) WT and IL-23p19−/− mice 8 d after infection measured by quantitative real-time PCR. Results are expressed as fold changes relative to HPRT mRNA expression. (D) MMP-9 and MMP-2 concentrations in supernatants of ileal biopsies from naive and infected WT and IL-23p19−/− mice detected by ELISA 8 d after infection. Data (one representative out of two independent experiments is shown) of three mice per group are given as means ± SD, and p-values were determined by the Mann-Whitney U test.
The increased enzymatic activity of gelatinases in the ileum of infected WT but not IL-23p19−/− mice was paralleled by increased mRNA and protein levels of either gelatinase at day 8 after infection compared with naive WT mice (Fig. 2, C and D). These results indicate that IL-23 is required for the up-regulation of both MMP-2 and MMP-9 in T. gondii–induced ileitis.
Small intestinal immunopathology is mediated by MMP-2 but not MMP-9
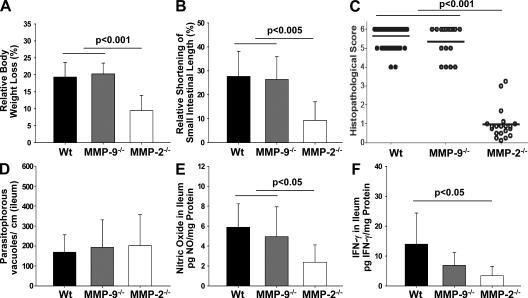
To investigate whether IL-23–dependent up-regulation of MMP-2 and MMP-9 is critical for the development of T. gondii–induced ileitis, we determined the mortality, clinical conditions, and histopathological changes in the ileum of WT, MMP-2−/−, and MMP-9−/− mice after T. gondii infection. On day 8 after infection, MMP-9−/− and WT mice had lost 19.3 ± 4.3% and 20.3 ± 3.1% of their body weight, respectively, whereas MMP-2−/− mice displayed significantly less weight loss (9.4 ± 4.3%; Fig. 3 A). Furthermore, infection resulted in significantly less shortening of the small intestinal length in MMP-2−/− compared with MMP-9−/− and WT mice on day 8 after infection (9.3 ± 7.4% vs. 26.4 ± 8.7 and 27.7 ± 10.1%, respectively (Fig. 3 B). Although WT and MMP-9−/− mice displayed severe necrosis at 8 d after infection, only mild inflammatory changes but no necrosis were observed in the small intestine of MMP-2−/− mice (Fig. 3 C). The number of parasitophorous vacuoles containing T. gondii tachyzoites in the ileum did not differ significantly between the groups at the same time point (Fig. 3 D). Ileal immunopathology in MMP-9−/− and WT mice was accompanied by significantly elevated NO and IFN-γ levels in culture supernatants of ileal biopsies compared with MMP-2−/− mice (Fig. 3, E and F, respectively). All MMP-9−/− as well as WT mice had died by day 9 after infection, whereas 82% of MMP-2−/− mice survived the acute phase of infection and 40% survived for >3 wk (Fig. S2). Interestingly, MMP-2−/− mice that survived the acute phase of infection did not show signs of intestinal inflammation around the time of death (unpublished data), indicating that the deficiency in MMP-2 does not delay but prevents the development of intestinal pathology. Immunohistochemical staining for MMP-2 in the ileum of naive and infected WT and MMP-2−/− mice indicates that cells in the lamina propria produce MMP-2 (Fig. S2). Granulocytes did not seem to secrete MMP-2, because ileal MMP-2 levels did not differ between neutrophil-depleted (anti-Gr1 mAb) and control WT mice (Fig. S3). Collectively, MMP-2 but not MMP-9 appears to be an essential downstream mediator of immunopathology in T. gondii–induced ileitis.
Figure 3.
Small intestinal immunopathology is mediated by MMP-2 but not MMP-9. (A) Relative body weight loss of WT (n = 43), MMP-9−/− (n = 28), and MMP-2−/− (n = 11) mice 8 d after infection. (B) Relative shortening of small intestine of WT (n = 13), MMP-9−/− (n = 6), and MMP-2−/− (n = 11) mice 8 d after infection. (C) Histopathology score of WT (n = 42), MMP-9−/− (n = 17), and MMP-2−/− (n = 11) mice 8 d after infection. Horizontal bars indicate means for each group. (D) T. gondii parasitophorous vacuoles that contained tachyzoites were stained with a rabbit anti–T. gondii IgG antibody and counted in the terminal ileum in WT, MMP-2−/−, and MMP-9−/− mice 8 d after infection. (E) NO levels and (F) IFN-γ concentration in supernatants of ileal biopsies from WT (n = 15), MMP-9−/− (n = 6), and MMP-2−/− (n = 10) mice 8 d after infection. Data are pooled from at least three independent experiments. Mean values, SDs, and significance levels were determined by the Student's t test or by Kaplan-Meier analysis and the log-rank test (A).
A selective gelatinase inhibitor protects mice against T. gondii–induced immunopathology
Because MMP-2 mediates the development of necrosis in the small intestine after T. gondii infection, we investigated the efficacy of gelatinase-blocking drugs to prevent or treat the development of immunopathology. A nonselective MMP inhibitor (doxycycline) as well as a more “selective” gelatinase inhibitor, RO28-2653, were evaluated. Infected mice treated prophylactically with RO28-2653 survived longer than PBS- or doxycycline-treated animals (Fig. S4 A). Mice treated prophylactically with either doxycycline or RO28-2653 displayed significantly less weight loss as well as less shortening of the small intestine as compared with the PBS control group on day 8 after infection (Fig. S4, B and C). The lengths of small intestines did not differ between individual treatment groups (Fig. S4 C).
Although PBS-treated mice displayed severe ileal necrosis, mice treated prophylactically or therapeutically with doxycycline showed mild signs of inflammation but no necrosis on day 8 after infection (Fig. S4 D). Moreover, therapeutic treatment (initiated 5 d after ileitis induction) with RO28-2653 led to an even more pronounced amelioration of inflammation than doxycycline treatment, and mice only showed minor signs of inflammation (Fig. S4 D). Neither doxycycline nor RO28-2653 affected the numbers of parasites in the small intestinal lamina propria after infection (unpublished data). In addition, significantly lower NO as well as IFN-γ levels were found in the ilea of mice prophylactically treated with either doxycycline or RO28-2653 compared with the PBS-treated group on day 8 after infection (Fig. S4, E and F). Collectively, these results indicate that prophylactic and therapeutic treatment with selective gelatinase inhibitors prevents the development of T. gondii–induced small intestinal necrosis.
Differential regulation of IL-17 and IL-22 after infection with T. gondii
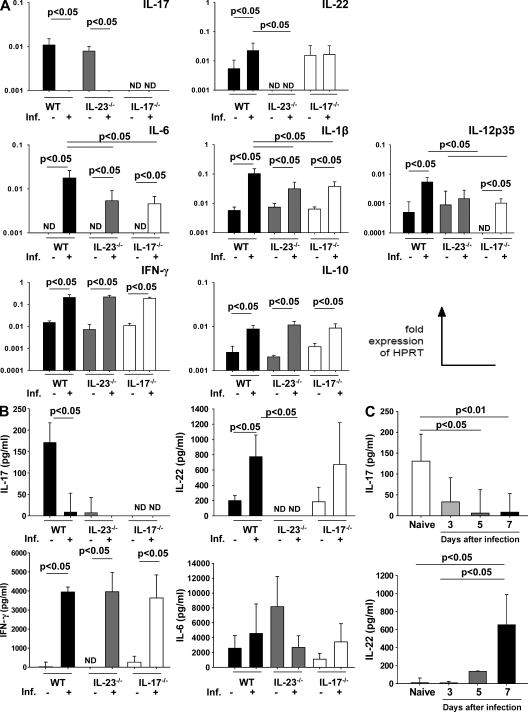
Because IL-23 was required for small intestinal pathology but MMP-2−/− mice still show significant mortality but rather mild pathological changes in the small intestine, we investigated the expression of IL-23–dependent cytokines involved in mucosal inflammatory responses. In naive mice, IL-17 mRNA was similarly expressed in both WT and IL-23p19−/− mice but was absent in IL-17−/− mice (Fig. 4 A). Interestingly, IL-17 was markedly down-regulated in WT and IL-23p19−/− mice 8 d after infection, although no difference was detected between both groups (Fig. 4 A). In contrast, we did not detect IL-22 mRNA in naive and infected IL-23p19−/− mice, but IL-22 mRNA was up-regulated in infected WT mice and detectable at similar levels in naive and infected IL-17−/− mice (Fig. 4 A). Moreover, IL-6 mRNA was not detected in any group of naive mice, whereas infected WT mice displayed higher IL-6 mRNA expression compared with IL-23p19−/−– and IL-17−/−–infected mice (Fig. 4 A). Comparable levels of IL-1β mRNA were found in all naive groups; upon infection, IL-1β transcripts increased significantly in infected WT mice compared with IL-23p19−/−– and IL-17−/−–infected mice (Fig. 4 A). The expression of IL-12p35 mRNA was comparable in naive WT and IL-23p19−/− mice but undetectable in naive IL-17−/− mice. However, IL-12p35 mRNA was significantly up-regulated in infected WT mice as compared with both infected IL-23p19−/− and IL-17−/− mice (Fig. 4 A). Furthermore, levels of IFN-γ and IL-10 mRNA increased similarly in all groups upon infection (Fig. 4 A). In accordance with the mRNA expression data described, protein concentration of IL-17 was markedly down-regulated in infected WT mice and undetectable in infected IL-23p19−/− or IL-17−/− mice. In contrast, upon infection, IL-22 secretion was up-regulated three- to fourfold in WT and IL-17−/− mice but was undetectable in naive and infected IL-23p19−/− mice (Fig. 4 B). IFN-γ was not detectable in naive mice but levels increased after infection. IFN-γ and IL-6 concentrations did not differ significantly in infected WT, IL-23p19−/−, or IL-17−/− mice (Fig. 4 B). Collectively, IFN-γ and IL-22 are up-regulated upon infection with T. gondii, whereas IL-17 is markedly down-regulated.
Figure 4.
Differential regulation of IL-17 and IL-22 after infection with T. gondii. (A) RT-PCR of IL-17, IL-22, IL-6, IL-1β, IL-12p35, IFN-γ, and IL-10 mRNA in the ileum of naive and infected (Inf.) WT, IL-23p19−/−, and IL-17−/− mice 8 d after infection. Results are expressed as fold changes relative to HPRT mRNA expression. (B) IL-17, IL-22, IFN-γ, and IL-6 concentrations (ELISA) in supernatants of ileal biopsies from naive and infected (Inf.) WT, IL-23p19−/−, and IL-17−/− mice 8 d after infection. (C) IL-17 and IL-22 concentrations in supernatants of ileal biopsies from WT mice at different time points after T. gondii infection (ELISA). Data are pooled from at least three independent experiments of five mice per group and are given as means ± SD, and p-values were determined by the Mann-Whitney U test. ND, not detected.
Because IL-17 and IL-22 were differentially expressed in WT-infected mice, we assessed their expression in the ileum at different time points after ileitis induction. IL-17 concentrations decreased gradually during the course of infection with maximum levels in naive mice and minimum levels at day 8 after infection (Fig. 4 C). In contrast, IL-22 was undetectable in WT naive mice, but IL-22 concentrations increased during the course of the ileitis development and peaked at day 8 after infection (Fig. 4 C). These results indicate that IL-22 and IL-17 are inversely regulated during T. gondii–induced ileitis, and IL-22 but not IL-17 is induced by IL-23.
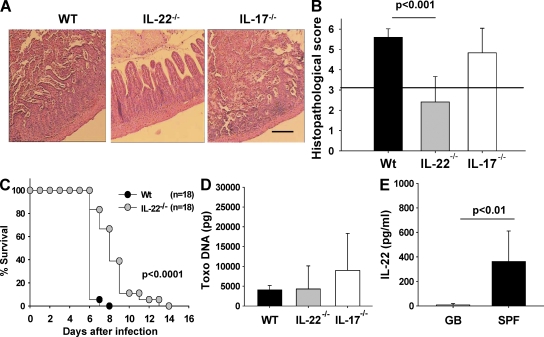
IL-22–deficient mice are resistant to T. gondii–induced immunopathology
Given that the production of IL-22 but not IL-17 was induced by IL-23 and up-regulated in mice developing intestinal immunopathology, we assessed the development of ileal inflammation in WT, IL-22−/−, and IL-17−/− mice after T. gondii infection. Severe necrosis of the mucosa was observed in WT mice, whereas IL-22−/− mice showed only mild signs of inflammation but no necrosis by day 8 after infection (Fig. 5 A). Interestingly, IL-17−/− mice also developed severe necrosis of the mucosa (Fig. 5 A). WT mice displayed a significantly higher ileal histopathological score than IL-22−/− but not IL-17−/− mice (Fig. 5 B). Furthermore, IL-22−/− mice survived significantly longer than WT mice (Fig. 5 C). Interestingly, IL-22−/− mice that survived the acute phase of infection did not show ileal necrosis around the time of death but more frequently displayed bacterial translocation to the spleen and liver than WT mice (unpublished data). The amount of T. gondii DNA in the ileum did not differ between groups of mice 8 d after infection (Fig. 5 D).
Figure 5.
IL-22–deficient mice are resistant to T. gondii–induced immunopathology. (A) Histopathology of hematoxylin and eosin–stained ileal sections of WT, IL-22−/−, and IL-17−/− mice 8 d after infection. (B) Histopathology scores of the ileum of WT, IL-22−/−, and IL-17−/− mice 8 d after infection. The horizontal line indicates the border between mild inflammation (<3) and necrosis (>3). (C) Survival of WT and IL-22−/− mice after oral infection with 100 cysts of T. gondii. (D) T. gondii DNA concentration in ileum of WT, IL-22−/−, and IL-17−/− mice 8 d after infection. (E) IL-22 concentration in culture supernatants of ileal biopsies from gnotobiotic (GB) and specific pathogen-free (SPF) mice 8 d after infection (ELISA). Data (one representative out of three independent experiments) from three to five mice per group are given as means ± SD, and p-values were determined by the Mann-Whitney U test. Bar, 100 µm.
Lastly, we examined IL-22 levels in the ilea of infected gnotobiotic mice, because we have previously shown that these mice do not develop ileitis after T. gondii infection (Heimesaat et al., 2006). Interestingly, IL-22 was almost undetectable in infected gnotobiotic mice, whereas mice with a normal specific pathogen-free gut flora had significantly higher IL-22 levels in their ilea 8 d after infection (Fig. 5 E). Thus, IL-22 but not IL-17 is a crucial mediator in the development of small intestinal pathology after oral infection with T. gondii.
Source of IL-22 in the lamina propria of the small intestine upon infection
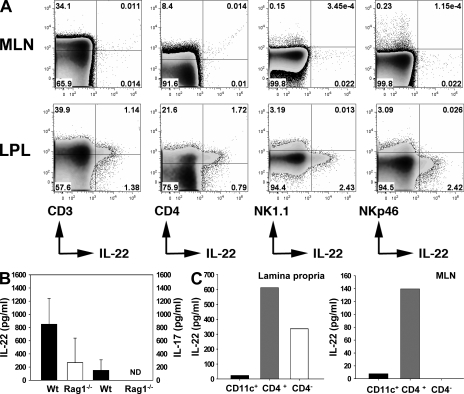
Given the evidence that both T cells and non–T cells can produce IL-22 (Wolk and Sabat, 2006), we investigated the potential source of IL-22 in the ileum of mice after ileitis induction. We performed flow cytometry to identify the source of IL-22 in isolated cells from MLNs and in the small intestinal lamina propria of infected WT mice. Although IL-22–producing cells in MLNs of infected mice were almost undetectable (Fig. 6 A), 2.8% of CD3+ cells produced IL-22 in the small intestinal lamina propria and 45.2% of all IL-22–producing cells were CD3+. IL-22 production was also observed in 7.4% of all CD4+ cells. Interestingly, we were unable to detect IL-22 secretion among NK22 cells because cells that expressed NK1.1 or NKp46 did not secrete IL-22.
Figure 6.
Source of IL-22 in the lamina propria of the small intestine upon induction of ileitis. (A) Flow cytometric analysis of cells isolated from the MLNs and small intestinal lamina propria of infected WT mice 8 d after infection, stained for CD4, CD3, NK1.1, NKp46, and IL-22 (percentages are shown). (B) IL-22 and IL-17 concentration in supernatants of ileal biopsies from WT and RAG1−/− mice 8 d after infection measured by ELISA. (C) IL-22 concentration in supernatants of CD11c+, CD4+, and CD4− sorted cells isolated from the small intestinal lamina propria and MLNs from infected WT mice 8 d after infection. Data shown are representative of three independent experiments. LPL, lamina propria of the small intestine.
To confirm the presence of non–T cells producing IL-22 in the ilea of infected mice, we compared the production of IL-22 in the ileum of infected RAG1−/− (which lack T cells) and WT mice. After ileitis induction, RAG1−/− mice displayed lower IL-22 concentrations in the ileum compared with WT mice, but this difference did not reach statistical significance (Fig. 6 B). IL-17 was not detected in RAG1−/−-infected mice (Fig. 6 B). We obtained similar results by measuring IL-22 levels in culture supernatants of sorted CD4+ and CD4− cells from the MLN and the small intestinal lamina propria cells (Fig. 6 C). Furthermore, supernatants obtained from CD11c+ cells sorted from both MLN and the lamina propria produced very low levels of IL-22 (Fig. 6 C). These results suggest that IL-22 is mostly produced by CD4+ cells in the small intestinal lamina propria, but IL-22 production does not appear to be restricted to T cells.
DISCUSSION
A growing body of evidence from human studies and mouse models of IBD has shown that IL-23 promotes acute as well as chronic intestinal inflammation through the induction of a plethora of proinflammatory mediators. Because the vast majority of these studies have used models of large intestinal inflammation, in the present study, we investigated the role of IL-23 using a well-established mouse model of small intestinal inflammation. In this model, peroral infection with T. gondii induces a hyperinflammatory Th1-type immune response characterized by overproduction of IL-12, IL-18, IFN-γ, TNF, and NO leading to small intestinal immunopathology with massive necrosis (panileitis). Although IL-23 has been shown to induce and/or maintain mucosal inflammatory responses via the induction of Th17 cells, we show in this paper that IL-23 caused small intestinal immunopathology via the up-regulation of MMP-2 and, surprisingly, via the induction of IL-22 but in an IL-17–independent way.
The expression and enzymatic activity of MMP-2 and MMP-9 were enhanced in the small intestine when pathology started to develop. However, only MMP-2–deficient mice were protected against the development of intestinal immunopathology and early death. IL-22 levels in the ileum of WT and MMP-2−/− mice as well as the levels of MMP-2 in the ileum of WT and IL-22−/− mice did not differ between WT and knockout mice, respectively, indicating that IL-22 and MMP-2 mediate T. gondii–induced ileitis through independent pathways.
In accordance with our data, several studies have demonstrated that MMP-2 and MMP-9 are up-regulated during active episodes of IBD in humans as well as in animal models of colitis (Baugh et al., 1998; Castaneda et al., 2005; Yen et al., 2006; Gordon et al., 2008), and that IL-23 is able to induce their expression (Ivanov et al., 2007). Epithelial barrier dysfunction may be involved in the MMP-mediated effects in T. gondii–induced ileitis, because MMP-2−/− mice showed a lower rate of bacterial translocation into the spleen compared with WT mice (unpublished data). Interestingly, granulocytes have been proposed as an important source of MMPs. We found an increased number of granulocytes in the lamina propria after infection (unpublished data). However, neutrophil depletion in WT mice did not prevent the development of ileitis, and MMP concentrations did not differ after depletion of granulocytes. In addition, IL-22 levels in the ileum were similar in granulocyte-depleted and control mice, suggesting that neutrophils are not a source of IL-22 (unpublished data).
We observed that nonselective (doxycycline) and selective (RO28-3653) gelatinase inhibitors ameliorated intestinal pathology when given either prophylactically or therapeutically. Dosages of RO28-2653 used in the present study were similar to those administered in pharmacodynamic studies in rat and mouse models (Kilian et al., 2006; Abramjuk et al., 2007). Although nonselective MMP-blocking agents may cause severe adverse side effects (Bernardo et al., 2002), RO28-2653 did not show major side effects in rat and monkey toxicological studies (unpublished data).
Moreover, RO28-2653 also blocked large intestinal inflammation in a model of dextran sulfate sodium–induced colitis (unpublished data). Thus, selective blockage of gelatinases may be a safe and effective new strategy in the prevention and treatment of intestinal inflammation.
IL-23 has been proposed to induce pathology through the proliferation and maintenance of IL-17–secreting cells (Aggarwal et al., 2003; Bettelli et al., 2007). In contrast, our results demonstrate that the pathogenic role of IL-23 was independent of IL-17 but dependent on IL-22. In agreement with our data, several studies have demonstrated that IL-17 and IL-22 possess distinct roles during immune responses (Cruz et al., 2006; Kreymborg et al., 2007; Schulz et al., 2008; Sugimoto et al., 2008; Wolk et al., 2009). Moreover, our study provides strong evidence that IL-22 and IL-17 are inversely regulated in ileitis.
Although IL-22 was up-regulated, IL-17 production was turned off in the ileum of infected mice. High concentrations of IFN-γ in the small intestine of mice might have contributed to the down-regulation of IL-17 production, as previously shown in models of adjuvant-induced arthritis (Kim et al., 2008) and during mycobacterial infection (Cruz et al., 2006).
Importantly, we found that IL-23–induced up-regulation of IL-22 was essential for the development of small intestinal immunopathology. IL-22−/− mice did not develop small intestinal necrosis although they harbored the same number of parasites as both WT and IL-17−/− mice. These data support a new pathogenic rather than protective role of IL-22 in the small intestine.
Although IL-22 has been reported to promote psoriasis-like skin alterations (Wolk et al., 2004; Wolk et al., 2006; Zheng et al., 2007; Ma et al., 2008; Wolk et al., 2009), an increasing number of studies have reported a rather protective role of IL-22, especially in hepatitis and colitis experimental models (Zenewicz et al., 2007; Cella et al., 2008; Satoh-Takayama et al., 2008; Sugimoto et al., 2008; Zenewicz et al., 2008). In regard to intestinal inflammation, IL-22 protected mice from colitis in a CD45RBhi transfer model (Zenewicz et al., 2008), and IL-22 deficiency rendered mice susceptible to Citrobacter rodentium– and dextran sulfate sodium–induced colitis (Satoh-Takayama et al., 2008; Zheng et al., 2008). Furthermore, IL-22 induces the expression of antimicrobial peptides in epithelial cells (Liang et al., 2006; Aujla et al., 2007; Zheng et al., 2008).
These contrasting features of IL-22 raise important questions about how the same protein could behave in opposite ways. First, the role of IL-22 in inflammation could be tissue specific. IL-22 might exert pathogenic functions in keratinocytes and epithelial cells of the small intestine while playing a protective role in the large intestinal and lung epithelium, as well as in hepatocytes. Second, IL-22 may exert different functions dependent on its amount and duration in tissues. In the present study, increasing levels of IL-22 in the ileum were found during ileitis, and they peaked at day 8 after infection when intestinal pathology was full blown and mice began to succumb to infection. Third, the diversity of experimental models used (pathogen and chemical induced) may contribute to the contrasting roles of IL-22. At this point, we can only speculate on the potential pathogenic effector mechanisms mediated by IL-22 in the small intestine. Neutralization of IL-22 has recently been reported to block CXCL-8 expression by intestinal epithelial cells after stimulation with T memory cells (Kleinschek et al., 2009). Lastly, pathogenic IL-22–producing cells might constitute a subpopulation of cells, different from Th17 and NK22 cells. IL-22 production is higher in Th1 cells than in Th17 cells (Volpe et al., 2008), and a unique IL-22–producing population of NKp46+RORγt+ natural killer cells, termed NK22 cells, was identified in the dermis, lamina propria, and other mucosa-associated lymphoid tissues (Cella et al., 2008; Luci et al., 2008; Sanos et al., 2009; Satoh-Takayama et al., 2008; Zenewicz et al., 2008). Furthermore, a human Th cell population that secretes IL-22 but not IL-17 nor IFN-γ has been recently reported (Duhen et al., 2009; Trifari et al., 2009). In the present study, we used a well-characterized IL-22 mAb (Zheng et al., 2007) to identify the source of IL-22 after ileitis induction. CD4+ T cells were the predominant source of IL-22. IL-17 was down-regulated and dispensable for the development of intestinal necrosis. Interestingly, flow cytometry pointed toward non-CD4, non-CD3 T cells as an additional source of IL-22 in the ilea of infected mice. This was confirmed by our finding of IL-22 production in the ileum of RAG1−/− mice, which do not have T cells, and in supernatants of CD4− cells sorted from the lamina propria of infected mice. However, flow cytometry did not associate IL-22 production with NK cells, as recently described (Cella et al., 2008; Luci et al., 2008; Sanos et al., 2009; Satoh-Takayama et al., 2008; Zenewicz et al., 2008). We cannot formally rule out the presence of NK22 cells, because the mAb used to detect IL-22–producing cells may have failed to detect IL-22 secretion by non–T cells because of a short half-life of IL-22 or a low sensitivity of this mAb. Therefore, we assume that CD4+ T cells are the main producers of IL-22 but other non–T cells contribute to IL-22 production. In agreement with a previous study, IL-22 production was also dependent on the presence of the normal gut flora, because gnotobiotic mice displayed significantly lower IL-22 concentrations (Satoh-Takayama et al., 2008).
In conclusion, IL-22 induced by IL-23 but not IL-17 is a key mediator of immunopathology in the small intestine. IL-23 also induced the local up-regulation of MMP-2 that was a crucial downstream effector molecule for the development of small intestinal inflammation that could be effectively inhibited by chemical blockage of gelatinases.
MATERIALS AND METHODS
Mice.
Female WT, IL-23p19−/−, IL-17A−/−, RAG1−/−, MMP-2−/−, MMP-9−/− (all on a C57BL/6 background), and NMRI mice were 8–12 wk of age and bred and maintained in the Forschungsinstitut für Experimentelle Medizin (Charité Medical School, Berlin, Germany). Clinical conditions and body weights were determined daily, and all experiments were conducted according to the German animal protection laws. Animal protocols were approved by the Landesamt für Gesundheit und Soziales.
Parasites and infection.
Cysts of the T. gondii ME49 strain were obtained from brains of NMRI mice that had been infected i.p. with 10 cysts for 2–3 mo. For peroral infection, mice were infected with 100 cysts in a volume of 0.3 ml in PBS (pH 7.4) by gavage, as described previously (Heimesaat et al., 2006).
Sampling procedures, determination of small intestinal length, histological scores, and parasite load.
Mice were sacrificed with Halothan (Eurim-Pharm) 8 d after infection. The relative shortening of the small intestine was calculated by dividing the difference of the mean length of the small intestine from naive control mice minus the length from infected mice at day 8 after infection and then multiplied by 100 over the mean length of naive mice small intestines. Histological scores and parasite loads were determined in fixed and paraffin-embedded tissue sections taken from the terminal ileum, as previously described (Heimesaat et al., 2006).
Detection of T. gondii.
Approximately 1 cm of ileum tissue was homogenized in a rotor–stator in 500 µl of lysis buffer containing 100 mM Tris-HCl [pH 8], 200 mM NaCl, 5 mM EDTA, 0.2% SDS, and 200 µg/ml proteinase K. The amplification mixture consisted of 2 µl of 10× reaction mix (LightCycler FastStart Master Hybridization Probes; Roche), 2 mM MgCl2, 1 µM of each oligonucleotide primer (TOX-9, 5′-AGGAGAGATATCAGGACTGTAG-3′; TOX-10, 5′-GCGTCGTCTCGTCTAGATCG-3′), 0.2 µM of each oligonucleotide probe (TOX-HP-1, 5′-GAGTCGGAGAGGGAGAAGATGTT-FAM-3′; TOX-HP-2, 5′-RED-640-CCGGCTTGGCTGCTTTTCCTG-PH-3′), and 500 ng of template DNA in a final volume of 20 µl. The amplification was performed using one cycle at 95°C for 10 min, 50 cycles at 95°C for 10 s/52°C for 20 s/72°C for 30 s, and one cycle at 40°C for 30 s with a single fluorescence detection point at the end of the cycle. A standard curve was performed using 500–5 pg T. gondii DNA of GFP tachyzoites. Fluorescence was analyzed by LightCycler Data Analysis software (version 3.5; Roche). T. gondii parasitophorous vacuoles that contained tachyzoites were stained with a rabbit anti–T. gondii IgG antibody and counted in the terminal ileum, as previously described (Vossenkämper et al., 2004).
Gelatin zymography.
The activity of MMP-2 and MMP-9 was measured by zymography under nonreducing conditions. In brief, ileum samples of infected and noninfected mice were snap frozen in liquid nitrogen and homogenized in lysis buffer (50 mM Hepes [pH 7.5], 150 mM NaCl, 1 mM MgCl2, 1 mM CaCl2, and 1% Triton X-100). MMPs were enriched by binding for 1 h at 4°C to gelatin-agarose beads (Sigma-Aldrich). Bound MMPs were eluted in 80 µl of 1× nonreducing SDS loading buffer. Samples were subjected to SDS-PAGE in 8% polyacrylamide gels containing 0.1% gelatin. After staining with Coomassie brilliant blue and destaining, gels were scanned and visualized using an image analyzer (LAS-1000; Fujifilm). A mixture of human MMP-2/9 was used as a standard.
Real-time PCR.
RNA was isolated from organs using the RNeasy Mini Kit (QIAGEN). mRNA was reverse transcribed and analyzed in triplicate assays by TaqMan PCR using a sequence detection system (ABI Prism 7700; Applied Biosystems), as described previously (Wolk et al., 2002). For detection of mouse IFN-γ, IL-12, IL-10, IL-6, IL-1β, IL-17A, IL-22, MMP-2, MMP-9, and IL-23p19, assays including double-fluorescent probes in combination with assays for the mouse housekeeping gene hypoxanthine phosphoribosyltransferase (HPRT) were purchased from Applied Biosystems or developed by us (IL-22). Expression levels were calculated relative to the HPRT expression.
Cytokine and MMP ELISA.
Concentrations of IL-6, IL-10, TNF, IFN-γ (BD), IL-22, IL-17, MMP-9, and MMP-2 (R&D Systems) were determined in supernatants of organ cultures according to the manufacturers' protocols.
Treatment with doxycycline and RO28-2653.
C57BL/6 mice were treated perorally by gavage twice daily with either doxycycline (50 mg/kg body weight/day; Sigma-Aldrich) or RO28-2653 (75 mg/kg body weight/day; Roche) in 0.3 ml PBS. One group of mice was treated starting 5 d before T. gondii infection until 8 d after infection (prophylactic regimen), and another group from 5 d (the time point when inflammatory changes in the gut mucosa start to develop) until 8 d after infection (therapeutic regimen). PBS-treated mice served as negative controls.
Determination of NO concentrations.
NO concentrations in supernatants were determined by Griess reaction, as previously described (Heimesaat et al., 2006).
Lamina propria and MLN cell isolation, sorting, and flow cytometry.
The small intestine, dissected from its mesentery and Peyer's patches, was cut into 2-cm pieces and incubated in 5 mM EDTA, 1 mM dithiothreitol (Sigma-Aldrich), and RPMI 1640. Thereafter, pieces were incubated in RPMI 1640 containing 500 µg/ml liberase CI (Roche) and 0.05% DNase. MLNs were removed and subsequently minced through a 70-µm filter. For intracellular staining, cells were stimulated for 6 h with 50 ng/ml 12-O-tetradecanoylphorbol-13-acetate (Sigma-Aldrich), 750 ng/ml ionomycin (Sigma-Aldrich), and GolgiStop (BD) at 37°C. Stainings and cell sorting with anti-CD4 (L3T4 clone, allophycocyanin [APC]-Cy7 conjugated), anti-CD8 (53-6.7 clone, Pacific blue conjugated), anti-CD11c (HL3 clone, PerCP conjugated), anti-NK1.1 (PK136 clone, PE-Cy7 conjugated), anti-γδ (GL3 clone, FITC conjugated; BD), anti-NKp46 (PE conjugated; R&D Systems), and anti–IL-22 (APC conjugated; Genentech) were performed. Cells were analyzed with flow cytometers (FACSCalibur or LSR II; BD).
Depletion of neutrophils.
Neutrophils were depleted using an anti-Gr1 mAb (RB6-8C5) at days 3 and 5 after infection with T. gondii. Each mouse was injected i.p. with 0.15 mg/ml anti-Gr1. Control animals were left untreated. Neutrophil depletion was confirmed by flow cytometric analyses in all experimental animals.
Generation of gnotobiotic mice.
To remove the commensal gut flora, C57BL/6 mice were treated by adding 1 g/liter ampicillin (ratiopharm), 500 mg/liter vancomycin (Cell Pharm), 200 mg/liter ciprofloxacin (Bayer Vital), 250 mg/liter imipenem (MSD), and 1 g/liter metronidazole (Fresenius) to the drinking water ad libitum for 6–8 wk, as described previously (Heimesaat et al., 2006).
Statistical analyses.
For statistical analyses, the Mann-Whitney U test, the Student's t test, or the log-rank test (for Kaplan-Meier analysis of survival) was performed, as indicated in the figures. P ≤ 0.05 was considered significant.
Online supplemental material.
Fig. S1 shows IL-23p19 mRNA from sorted lamina propria and MLN CD11b+ and CD11c+ cells. Fig. S2 shows survival of WT, MMP-2−/−, and MMP-9−/− mice after T. gondii infection. Fig. S3 A shows immunohistochemistry staining of MMP-2 in ileal biopsies of WT and MMP-2−/− mice, and Fig. S3 B shows MMP-2 concentration in the ileum of WT mice after neutrophil depletion. Fig. S4 (A–D) shows survival, body weight loss, small intestinal length shortening, and histopathology scores, respectively, of mice treated with PBS, doxycycline, or RO28-2653. Fig. S4 (E and F) show NO and IFN-γ concentrations, respectively, in the ileum from mice treated with PBS, doxycycline, or RO28-2653. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20090900/DC1.
Acknowledgments
We would like to thank G. Reifenberger, M. Wattrodt, D. Trautmann, U.B. Göbel, N. Kassner, S. Rutz, G. Alber, and the staff of the animal facility of the Charité Medical School for their technical expertise and/or discussion.
This study was supported by grants from the German Research Foundation to O. Liesenfeld (SFB633, B6), S. Bereswill (SFB633, A7), and C. Loddenkemper (SFB633, Z1).
H.-W. Krell is employed by Roche Diagnostics. The authors have no further conflicting financial interests.
Footnotes
Abbreviations used:
- HPRT
- hypoxanthine phosphoribosyltransferase
- IBD
- inflammatory bowel disease
- MLN
- mesenteric LN
- MMP
- matrixmetalloproteinase
References
- Abramjuk C., Lein M., Rothaug W., Krell H.W., Loening S.A., Jung K. 2007. Enhanced inhibitory effect of the matrix metalloproteinase inhibitor Ro 28-2653 in combination with estramustine and etoposide on the prostate carcinoma in the rat Dunning orthotopic tumor model. Cancer Chemother. Pharmacol. 59:275–282 10.1007/s00280-006-0269-7 [DOI] [PubMed] [Google Scholar]
- Aggarwal S., Ghilardi N., Xie M.H., de Sauvage F.J., Gurney A.L. 2003. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J. Biol. Chem. 278:1910–1914 10.1074/jbc.M207577200 [DOI] [PubMed] [Google Scholar]
- Aujla S.J., Dubin P.J., Kolls J.K. 2007. Interleukin-17 in pulmonary host defense. Exp. Lung Res. 33:507–518 10.1080/01902140701756604 [DOI] [PubMed] [Google Scholar]
- Baugh M.D., Evans G.S., Hollander A.P., Davies D.R., Perry M.J., Lobo A.J., Taylor C.J. 1998. Expression of matrix metalloproteases in inflammatory bowel disease. Ann. NY Acad. Sci. 859:249–253 10.1111/j.1749-6632.1998.tb11139.x [DOI] [PubMed] [Google Scholar]
- Bernardo M.M., Brown S., Li Z.H., Fridman R., Mobashery S. 2002. Design, synthesis, and characterization of potent, slow-binding inhibitors that are selective for gelatinases. J. Biol. Chem. 277:11201–11207 10.1074/jbc.M111021200 [DOI] [PubMed] [Google Scholar]
- Bettelli E., Oukka M., Kuchroo V.K. 2007. T(H)-17 cells in the circle of immunity and autoimmunity. Nat. Immunol. 8:345–350 10.1038/ni0407-345 [DOI] [PubMed] [Google Scholar]
- Castaneda F.E., Walia B., Vijay-Kumar M., Patel N.R., Roser S., Kolachala V.L., Rojas M., Wang L., Oprea G., Garg P., et al. 2005. Targeted deletion of metalloproteinase 9 attenuates experimental colitis in mice: central role of epithelial-derived MMP. Gastroenterology. 129:1991–2008 10.1053/j.gastro.2005.09.017 [DOI] [PubMed] [Google Scholar]
- Cella M., Fuchs A., Vermi W., Facchetti F., Otero K., Lennerz J.K., Doherty J.M., Mills J.C., Colonna M. 2008. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature. 457:722–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz A., Khader S.A., Torrado E., Fraga A., Pearl J.E., Pedrosa J., Cooper A.M., Castro A.G. 2006. Cutting edge: IFN-gamma regulates the induction and expansion of IL-17-producing CD4 T cells during mycobacterial infection. J. Immunol. 177:1416–1420 [DOI] [PubMed] [Google Scholar]
- Cua D.J., Sherlock J., Chen Y., Murphy C.A., Joyce B., Seymour B., Lucian L., To W., Kwan S., Churakova T., et al. 2003. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 421:744–748 10.1038/nature01355 [DOI] [PubMed] [Google Scholar]
- Duerr R.H., Taylor K.D., Brant S.R., Rioux J.D., Silverberg M.S., Daly M.J., Steinhart A.H., Abraham C., Regueiro M., Griffiths A., et al. 2006. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science. 314:1461–1463 10.1126/science.1135245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duhen T., Geiger R., Jarrossay D., Lanzavecchia A., Sallusto F. 2009. Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nat. Immunol. 10:857–863 10.1038/ni.1767 [DOI] [PubMed] [Google Scholar]
- Dumoutier L., Louahed J., Renauld J.C. 2000. Cloning and characterization of IL-10-related T cell-derived inducible factor (IL-TIF), a novel cytokine structurally related to IL-10 and inducible by IL-9. J. Immunol. 164:1814–1819 [DOI] [PubMed] [Google Scholar]
- Fujino S., Andoh A., Bamba S., Ogawa A., Hata K., Araki Y., Bamba T., Fujiyama Y. 2003. Increased expression of interleukin 17 in inflammatory bowel disease. Gut. 52:65–70 10.1136/gut.52.1.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Q., Meijer M.J., Kubben F.J., Sier C.F., Kruidenier L., van Duijn W., van den Berg M., van Hogezand R.A., Lamers C.B., Verspaget H.W. 2005. Expression of matrix metalloproteinases-2 and -9 in intestinal tissue of patients with inflammatory bowel diseases. Dig. Liver Dis. 37:584–592 10.1016/j.dld.2005.02.011 [DOI] [PubMed] [Google Scholar]
- Gordon J.N., Pickard K.M., Di Sabatino A., Prothero J.D., Pender S.L., Goggin P.M., MacDonald T.T. 2008. Matrix metalloproteinase-3 production by gut IgG plasma cells in chronic inflammatory bowel disease. Inflamm. Bowel Dis. 14:195–203 10.1002/ibd.20302 [DOI] [PubMed] [Google Scholar]
- Heimesaat M.M., Bereswill S., Fischer A., Fuchs D., Struck D., Niebergall J., Jahn H.K., Dunay I.R., Moter A., Gescher D.M., et al. 2006. Gram-negative bacteria aggravate murine small intestinal Th1-type immunopathology following oral infection with Toxoplasma gondii. J. Immunol. 177:8785–8795 [DOI] [PubMed] [Google Scholar]
- Ivanov S., Bozinovski S., Bossios A., Valadi H., Vlahos R., Malmhäll C., Sjöstrand M., Kolls J.K., Anderson G.P., Lindén A. 2007. Functional relevance of the IL-23-IL-17 axis in lungs in vivo. Am. J. Respir. Cell Mol. Biol. 36:442–451 10.1165/rcmb.2006-0020OC [DOI] [PubMed] [Google Scholar]
- Khan I.A., Schwartzman J.D., Matsuura T., Kasper L.H. 1997. A dichotomous role for nitric oxide during acute Toxoplasma gondii infection in mice. Proc. Natl. Acad. Sci. USA. 94:13955–13960 10.1073/pnas.94.25.13955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilian M., Gregor J.I., Heukamp I., Hanel M., Ahlgrimm M., Schimke I., Kristiansen G., Ommer A., Walz M.K., Jacobi C.A., Wenger F.A. 2006. Matrix metalloproteinase inhibitor RO 28-2653 decreases liver metastasis by reduction of MMP-2 and MMP-9 concentration in BOP-induced ductal pancreatic cancer in Syrian hamsters: inhibition of matrix metalloproteinases in pancreatic cancer. Prostaglandins Leukot. Essent. Fatty Acids. 75:429–434 10.1016/j.plefa.2006.08.004 [DOI] [PubMed] [Google Scholar]
- Kim E.Y., Chi H.H., Bouziane M., Gaur A., Moudgil K.D. 2008. Regulation of autoimmune arthritis by the pro-inflammatory cytokine interferon-gamma. Clin. Immunol. 127:98–106 10.1016/j.clim.2008.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinschek M.A., Boniface K., Sadekova S., Grein J., Murphy E.E., Turner S.P., Raskin L., Desai B., Faubion W.A., de Waal Malefyt R., et al. 2009. Circulating and gut-resident human Th17 cells express CD161 and promote intestinal inflammation. J. Exp. Med. 206:525–534 10.1084/jem.20081712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosiewicz M.M., Nast C.C., Krishnan A., Rivera-Nieves J., Moskaluk C.A., Matsumoto S., Kozaiwa K., Cominelli F. 2001. Th1-type responses mediate spontaneous ileitis in a novel murine model of Crohn's disease. J. Clin. Invest. 107:695–702 10.1172/JCI10956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreymborg K., Etzensperger R., Dumoutier L., Haak S., Rebollo A., Buch T., Heppner F.L., Renauld J.C., Becher B. 2007. IL-22 is expressed by Th17 cells in an IL-23-dependent fashion, but not required for the development of autoimmune encephalomyelitis. J. Immunol. 179:8098–8104 [DOI] [PubMed] [Google Scholar]
- Langowski J.L., Zhang X., Wu L., Mattson J.D., Chen T., Smith K., Basham B., McClanahan T., Kastelein R.A., Oft M. 2006. IL-23 promotes tumour incidence and growth. Nature. 442:461–465 10.1038/nature04808 [DOI] [PubMed] [Google Scholar]
- Liang S.C., Tan X.Y., Luxenberg D.P., Karim R., Dunussi-Joannopoulos K., Collins M., Fouser L.A. 2006. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J. Exp. Med. 203:2271–2279 10.1084/jem.20061308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesenfeld O. 2002. Oral infection of C57BL/6 mice with Toxoplasma gondii: a new model of inflammatory bowel disease? J. Infect. Dis. 185(Suppl. 1):S96–S101 10.1086/338006 [DOI] [PubMed] [Google Scholar]
- Liesenfeld O., Kosek J., Remington J.S., Suzuki Y. 1996. Association of CD4+ T cell–dependent, interferon-γ–mediated necrosis of the small intestine with genetic susceptibility of mice to peroral infection with Toxoplasma gondii. J. Exp. Med. 184:597–607 10.1084/jem.184.2.597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luci C., Reynders A., Ivanov I.I., Cognet C., Chiche L., Chasson L., Hardwigsen J., Anguiano E., Banchereau J., Chaussabel D., et al. 2008. Influence of the transcription factor RORgammat on the development of NKp46(+) cell populations in gut and skin. Nat. Immunol. 10:75–82 [DOI] [PubMed] [Google Scholar]
- Ma H.L., Liang S., Li J., Napierata L., Brown T., Benoit S., Senices M., Gill D., Dunussi-Joannopoulos K., Collins M., et al. 2008. IL-22 is required for Th17 cell-mediated pathology in a mouse model of psoriasis-like skin inflammation. J. Clin. Invest. 118:597–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina C., Videla S., Radomski A., Radomski M.W., Antolín M., Guarner F., Vilaseca J., Salas A., Malagelada J.R. 2003. Increased activity and expression of matrix metalloproteinase-9 in a rat model of distal colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 284:G116–G122 [DOI] [PubMed] [Google Scholar]
- Mennechet F.J., Kasper L.H., Rachinel N., Li W., Vandewalle A., Buzoni-Gatel D. 2002. Lamina propria CD4+ T lymphocytes synergize with murine intestinal epithelial cells to enhance proinflammatory response against an intracellular pathogen. J. Immunol. 168:2988–2996 [DOI] [PubMed] [Google Scholar]
- Nielsen O.H., Kirman I., Rüdiger N., Hendel J., Vainer B. 2003. Upregulation of interleukin-12 and -17 in active inflammatory bowel disease. Scand. J. Gastroenterol. 38:180–185 10.1080/00365520310000672 [DOI] [PubMed] [Google Scholar]
- Olson T.S., Bamias G., Naganuma M., Rivera-Nieves J., Burcin T.L., Ross W., Morris M.A., Pizarro T.T., Ernst P.B., Cominelli F., Ley K. 2004. Expanded B cell population blocks regulatory T cells and exacerbates ileitis in a murine model of Crohn disease. J. Clin. Invest. 114:389–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parham C., Chirica M., Timans J., Vaisberg E., Travis M., Cheung J., Pflanz S., Zhang R., Singh K.P., Vega F., et al. 2002. A receptor for the heterodimeric cytokine IL-23 is composed of IL-12Rbeta1 and a novel cytokine receptor subunit, IL-23R. J. Immunol. 168:5699–5708 [DOI] [PubMed] [Google Scholar]
- Radaeva S., Sun R., Pan H.N., Hong F., Gao B. 2004. Interleukin 22 (IL-22) plays a protective role in T cell-mediated murine hepatitis: IL-22 is a survival factor for hepatocytes via STAT3 activation. Hepatology. 39:1332–1342 10.1002/hep.20184 [DOI] [PubMed] [Google Scholar]
- Sanos S.L., Bui V.L., Mortha A., Oberle K., Heners C., Johner C., Diefenbach A. 2009. RORgammat and commensal microflora are required for the differentiation of mucosal interleukin 22-producing NKp46+ cells. Nat. Immunol. 10:83–91 10.1038/ni.1684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh-Takayama N., Vosshenrich C.A., Lesjean-Pottier S., Sawa S., Lochner M., Rattis F., Mention J.J., Thiam K., Cerf-Bensussan N., Mandelboim O., et al. 2008. Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity. 29:958–970 10.1016/j.immuni.2008.11.001 [DOI] [PubMed] [Google Scholar]
- Schmidt C., Giese T., Ludwig B., Mueller-Molaian I., Marth T., Zeuzem S., Meuer S.C., Stallmach A. 2005. Expression of interleukin-12-related cytokine transcripts in inflammatory bowel disease: elevated interleukin-23p19 and interleukin-27p28 in Crohn's disease but not in ulcerative colitis. Inflamm. Bowel Dis. 11:16–23 10.1097/00054725-200501000-00003 [DOI] [PubMed] [Google Scholar]
- Schulz S.M., Köhler G., Schütze N., Knauer J., Straubinger R.K., Chackerian A.A., Witte E., Wolk K., Sabat R., Iwakura Y., et al. 2008. Protective immunity to systemic infection with attenuated Salmonella enterica serovar enteritidis in the absence of IL-12 is associated with IL-23-dependent IL-22, but not IL-17. J. Immunol. 181:7891–7901 [DOI] [PubMed] [Google Scholar]
- Strober W., Fuss I.J., Blumberg R.S. 2002. The immunology of mucosal models of inflammation. Annu. Rev. Immunol. 20:495–549 10.1146/annurev.immunol.20.100301.064816 [DOI] [PubMed] [Google Scholar]
- Sugimoto K., Ogawa A., Mizoguchi E., Shimomura Y., Andoh A., Bhan A.K., Blumberg R.S., Xavier R.J., Mizoguchi A. 2008. IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J. Clin. Invest. 118:534–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarlton J.F., Whiting C.V., Tunmore D., Bregenholt S., Reimann J., Claesson M.H., Bland P.W. 2000. The role of up-regulated serine proteases and matrix metalloproteinases in the pathogenesis of a murine model of colitis. Am. J. Pathol. 157:1927–1935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifari S., Kaplan C.D., Tran E.H., Crellin N.K., Spits H. 2009. Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from T(H)-17, T(H)1 and T(H)2 cells. Nat. Immunol. 10:864–871 10.1038/ni.1770 [DOI] [PubMed] [Google Scholar]
- Volpe E., Servant N., Zollinger R., Bogiatzi S.I., Hupé P., Barillot E., Soumelis V. 2008. A critical function for transforming growth factor-beta, interleukin 23 and proinflammatory cytokines in driving and modulating human T(H)-17 responses. Nat. Immunol. 9:650–657 10.1038/ni.1613 [DOI] [PubMed] [Google Scholar]
- von Lampe B., Barthel B., Coupland S.E., Riecken E.O., Rosewicz S. 2000. Differential expression of matrix metalloproteinases and their tissue inhibitors in colon mucosa of patients with inflammatory bowel disease. Gut. 47:63–73 10.1136/gut.47.1.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vossenkämper A., Struck D., Alvarado-Esquivel C., Went T., Takeda K., Akira S., Pfeffer K., Alber G., Lochner M., Förster I., Liesenfeld O. 2004. Both IL-12 and IL-18 contribute to small intestinal Th1-type immunopathology following oral infection with Toxoplasma gondii, but IL-12 is dominant over IL-18 in parasite control. Eur. J. Immunol. 34:3197–3207 10.1002/eji.200424993 [DOI] [PubMed] [Google Scholar]
- Wolk K., Sabat R. 2006. Interleukin-22: a novel T- and NK-cell derived cytokine that regulates the biology of tissue cells. Cytokine Growth Factor Rev. 17:367–380 10.1016/j.cytogfr.2006.09.001 [DOI] [PubMed] [Google Scholar]
- Wolk K., Kunz S., Asadullah K., Sabat R. 2002. Cutting edge: immune cells as sources and targets of the IL-10 family members? J. Immunol. 168:5397–5402 [DOI] [PubMed] [Google Scholar]
- Wolk K., Kunz S., Witte E., Friedrich M., Asadullah K., Sabat R. 2004. IL-22 increases the innate immunity of tissues. Immunity. 21:241–254 10.1016/j.immuni.2004.07.007 [DOI] [PubMed] [Google Scholar]
- Wolk K., Witte E., Wallace E., Döcke W.D., Kunz S., Asadullah K., Volk H.D., Sterry W., Sabat R. 2006. IL-22 regulates the expression of genes responsible for antimicrobial defense, cellular differentiation, and mobility in keratinocytes: a potential role in psoriasis. Eur. J. Immunol. 36:1309–1323 10.1002/eji.200535503 [DOI] [PubMed] [Google Scholar]
- Wolk K., Haugen H.S., Xu W., Witte E., Waggie K., Anderson M., Vom Baur E., Witte K., Warszawska K., Philipp S., et al. 2009. IL-22 and IL-20 are key mediators of the epidermal alterations in psoriasis while IL-17 and IFN-gamma are not. J. Mol. Med. 87:523–536 10.1007/s00109-009-0457-0 [DOI] [PubMed] [Google Scholar]
- Yen D., Cheung J., Scheerens H., Poulet F., McClanahan T., McKenzie B., Kleinschek M.A., Owyang A., Mattson J., Blumenschein W., et al. 2006. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J. Clin. Invest. 116:1310–1316 10.1172/JCI21404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenewicz L.A., Yancopoulos G.D., Valenzuela D.M., Murphy A.J., Karow M., Flavell R.A. 2007. Interleukin-22 but not interleukin-17 provides protection to hepatocytes during acute liver inflammation. Immunity. 27:647–659 10.1016/j.immuni.2007.07.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenewicz L.A., Yancopoulos G.D., Valenzuela D.M., Murphy A.J., Stevens S., Flavell R.A. 2008. Innate and adaptive interleukin-22 protects mice from inflammatory bowel disease. Immunity. 29:947–957 10.1016/j.immuni.2008.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y., Danilenko D.M., Valdez P., Kasman I., Eastham-Anderson J., Wu J., Ouyang W. 2007. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 445:648–651 10.1038/nature05505 [DOI] [PubMed] [Google Scholar]
- Zheng Y., Valdez P.A., Danilenko D.M., Hu Y., Sa S.M., Gong Q., Abbas A.R., Modrusan Z., Ghilardi N., de Sauvage F.J., Ouyang W. 2008. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat. Med. 14:282–289 10.1038/nm1720 [DOI] [PubMed] [Google Scholar]