Abstract
Proline-rich tyrosine kinase 2 (PYK2) can be activated by angiotensin II (Ang II) and reactive oxygen species. We report that in endothelial cells, Ang II enhances the tyrosine phosphorylation of endothelial NO synthase (eNOS) in an AT1-, H2O2-, and PYK2-dependent manner. Low concentrations (1–100 µmol/liter) of H2O2 stimulated the phosphorylation of eNOS Tyr657 without affecting that of Ser1177, and attenuated basal and agonist-induced NO production. In isolated mouse aortae, 30 µmol/liter H2O2 induced phosphorylation of eNOS on Tyr657 and impaired acetylcholine-induced relaxation. Endothelial overexpression of a dominant-negative PYK2 mutant protected against H2O2-induced endothelial dysfunction. Correspondingly, carotid arteries from eNOS−/− mice overexpressing the nonphosphorylatable eNOS Y657F mutant were also protected against H2O2. In vivo, 3 wk of treatment with Ang II considerably increased levels of Tyr657-phosphorylated eNOS in the aortae of wild-type but not Nox2y/− mice, and this was again associated with a clear impairment in endothelium-dependent vasodilatation in the wild-type but not in the Nox2y/− mice. Collectively, endothelial PYK2 activation by Ang II and H2O2 causes the phosphorylation of eNOS on Tyr657, attenuating NO production and endothelium-dependent vasodilatation. This mechanism may contribute to the endothelial dysfunction observed in cardiovascular diseases associated with increased activity of the renin–angiotensin system and elevated redox stress.
Endothelial dysfunction is recognized as an independent risk factor for the development of cardiovascular diseases, and is characterized by a reduced bioavailability of the antithrombotic and antiatherosclerotic autacoid NO (Davignon and Ganz, 2004). The decrease in NO is directly related to increased vascular oxidative stress, as O2− readily reacts with NO to form peroxynitrite. Perhaps more importantly, the oxidative depletion of tetrahydrobiopterin causes the so-called uncoupling of endothelial NO synthase (eNOS), leading to the production of O2− instead of NO by the enzyme (Schulz et al., 2008). Despite the undisputed role of oxidative stress in the etiology of endothelial dysfunction, large clinical trials with antioxidant therapies have failed to show a beneficial effect on cardiovascular outcome (Thomson et al., 2007). This discrepancy is probably explained at least in part by the formation of other reactive oxygen species from O2− that have more complex roles in intracellular signaling beyond NO scavenging. Several superoxide dismutases convert O2− to the more stable H2O2 that has widespread and more prolonged effects on endothelial cell function (for review see Cai, 2005). H2O2 is in turn eliminated through the actions of catalase and peroxidases. However, it is important to note that the exogenous application of catalase can ameliorate endothelial dysfunction in some models of hypertension (Ulker et al., 2003), whereas catalase aggravates the situation in models characterized by the uncoupling of eNOS (Landmesser et al., 2003). To date, several studies have reported that promoting the conversion of O2− to H2O2 to relieve NO scavenging does not prevent the formation of atherosclerotic lesions and that superoxide dismutase activity actually correlates with lesion size (Tribble et al., 1997; Zanetti et al., 2001). These observations suggest that the impairment of eNOS activity by oxidative stress is more complex than hitherto assumed.
We recently reported that Tyr657 in the reductase domain of eNOS is a critical determinant of enzymatic activity. For example, the phosphorylation of Tyr657 by proline-rich tyrosine kinase 2 (PYK2) decreases eNOS activity, and the mutation of Tyr657 to a phosphomimetic glutamate or aspartate residue completely abolished NO production (Fisslthaler et al., 2008). PYK2 is generally considered to be a redox-sensitive kinase that is activated after stimulation with angiotensin II (Ang II) as well as in other situations associated with elevated oxidative stress (Tai et al., 2002; Yin et al., 2003). As elevated Ang II levels and increased oxidative stress are hallmarks of most cardiovascular diseases and associated with impaired endothelial function, we hypothesized that direct inactivation of eNOS via its tyrosine phosphorylation by PYK2 contributes to the phenomenon of endothelial dysfunction.
RESULTS AND DISCUSSION
Ang II and H2O2 induce activation of endothelial PYK2 and phosphorylation of eNOS on Tyr657, and decrease eNOS activity
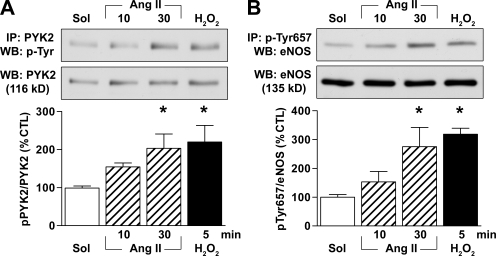
In native porcine aortic endothelial cells, 1 µmol/liter Ang II elicited the time-dependent tyrosine phosphorylation of PYK2 (Fig. 1 A), which correlates with the activation of the kinase (Blaukat et al., 1999). The exogenous application of 500 µmol/liter H2O2 also resulted in PYK2 phosphorylation (Fig. 1 A). Moreover, the activation of PYK2 by Ang II and H2O2 was mirrored by a pronounced increase in the phosphorylation of eNOS on Tyr657 (Fig. 1 B).
Figure 1.
Ang II and H2O2 activate PYK2 and phosphorylate eNOS on Tyr657. (A and B) Porcine aortae were incubated with solvent (Sol), 1 µmol/liter Ang II, or 500 µmol/liter H2O2. The endothelial cells were isolated, and the tyrosine phosphorylation of PYK2 (A) and the phosphorylation of eNOS on Tyr657 (B) were assessed by immunoprecipitation (IP) followed by Western blotting (WB). The graphs summarize data from six samples from three independent experiments. Data are expressed as means ± SEM. *, P < 0.05 versus solvent.
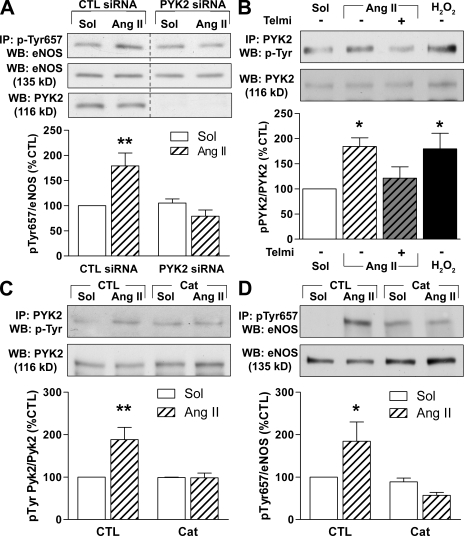
Mouse lung endothelial cells, which maintain responsiveness to Ang II in culture, were used to analyze the pathway leading to eNOS Tyr657 phosphorylation by Ang II. As was the case with the native porcine endothelial cells, 100 nmol/liter Ang II induced the phosphorylation of eNOS on Tyr657, and this was directly dependent on PYK2 activation because Ang II failed to increase eNOS phosphorylation in endothelial cells pretreated with PYK2 siRNA (Fig. 2 A). Furthermore, the activation of PYK2 by Ang II was prevented by 10 µmol/liter telmisartan (Fig. 2 B), demonstrating the involvement of the AT1 receptor. Ang II–induced H2O2 production seems to underlie the activation of PYK2, as the addition of Ang II to mouse lung endothelial cells resulted in the production of H2O2 (Fig. S1), and 150 U/ml polyethylene glycol (PEG)–catalase prevented the Ang II–induced phosphorylation of PYK2 as well as the subsequent phosphorylation of eNOS (Fig. 2, C and D). Collectively, these data indicate that Ang II induces eNOS Tyr657 phosphorylation through an AT1 receptor–, H2O2-, and PYK2-dependent mechanism.
Figure 2.
Ang II phosphorylates eNOS on Tyr657 by activation of AT1, H2O2, and PYK2. (A) Mouse lung endothelial cells were transfected with a scrambled (CTL) siRNA or siRNA directed against PYK2 48 h before incubation with 100 nmol/liter Ang II. Thereafter, eNOS phosphorylated on Tyr657 was immunoprecipitated (IP) and detected by Western blotting (WB). Nonadjacent lanes from the same membrane and exposition are shown. (B) Effect of 100 nmol/liter Ang II for 30 min, Ang II and 10 µmol/liter telmisartan (Telmi), or 30 µmol/liter H2O2 for 5 min on the tyrosine phosphorylation of PYK2. (C and D) Mouse lung homogenates were incubated with 150 U/ml PEG-catalase (Cat) for 1 h before stimulation with 100 nmol/liter Ang II for 20 min. The tyrosine phosphorylation of PYK2 (C) and eNOS (D) were assessed by immunoprecipitation and Western blotting. The graphs summarize data from six samples from three to six independent experiments. Data are expressed as means ± SEM. *, P < 0.05; and **, P < 0.01 versus the corresponding solvent (Sol) control.
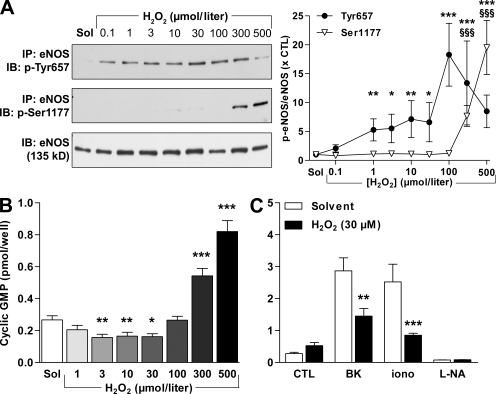
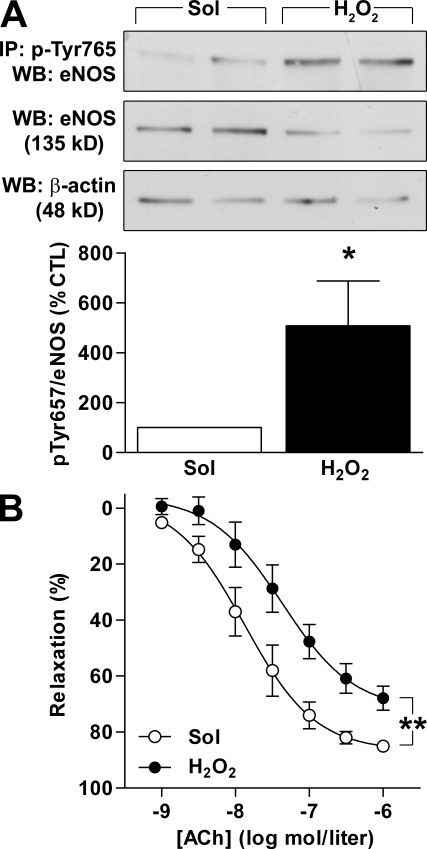
Incubation of cultured human endothelial cells with H2O2 for 5 min also elicited the tyrosine phosphorylation of eNOS. The latter response was detectable at concentrations as low as 100 nmol/liter (Fig. 3 A). At high concentrations (300–500 µmol/liter), however, H2O2 stimulates the influx of Ca2+ into endothelial cells (Edwards et al., 2008), and this was associated with the phosphorylation of eNOS on Ser1177 (Fig. 3 A). This dual effect on eNOS phosphorylation was also reflected by changes in eNOS activity. At low concentrations (e.g., 30 µmol/liter), H2O2 inhibited basal eNOS activity, whereas high concentrations (300–500 µmol/liter) stimulated NO production (Fig. 3 B). The latter phenomenon has also been reported by other authors (Thomas et al., 2002; Dossumbekova et al., 2008). To circumvent this confounding effect in further experiments, we concentrated on determining the responses to a maximum H2O2 concentration of 30 µmol/liter. At this low and potentially physiologically more relevant concentration, H2O2 significantly attenuated the activation of eNOS by bradykinin and ionomycin (Fig. 3 C). Furthermore, incubation of freshly isolated endothelium-intact mouse aortae with 30 µmol/liter H2O2 for 10 min induced the phosphorylation of eNOS on Tyr657 (Fig. 4 A). This concentration of H2O2 elicited only a small nonsignificant (10.7 ± 5.8%; n = 7; P = 0.068) decrease of vascular tone (which was corrected for by using the new plateau as a baseline) and significantly impaired endothelium-dependent relaxation to acetylcholine (ACh; Fig. 4 B). To the best of our knowledge, the present investigation is the first to assess NO production in response to such a large range of H2O2 concentrations and to correlate these changes with the activity as well as the phosphorylation of eNOS on more than one regulatory site.
Figure 3.
H2O2 has a dual effect on eNOS phosphorylation and activity. (A–C) Human endothelial cells were incubated with 0.1–500 µmol/liter H2O2 for 15 min. Thereafter, either (A) eNOS was immunoprecipitated (IP) and its phosphorylation on Tyr657 and Ser1177 were detected by Western blotting (IB), or (B and C) cyclic GMP levels were assessed by radioimmunoassay under basal conditions (B) or after stimulation with 1 µmol/liter bradykinin (BK) for 2 min or 100 nmol/liter ionomycin (iono) for 2 min (C). Some experiments were also performed in the presence of 300 µmol/liter Nωnitro–L-arginine (L-NA) for 60 min. The graphs summarize data from 8–12 experiments. Data are expressed as means ± SEM. *, P < 0.05; **, P < 0.01; and ***, P < 0.001 versus CTL or the corresponding solvent (Sol)-treated group. §§§, P < 0.001 versus solvent for Ser1177 phosphorylation.
Figure 4.
H2O2 induces Tyr657 phosphorylation and impairs endothelium-dependent vasodilatation in isolated mouse aorta. (A) Isolated aortae were incubated in the absence (Sol) or presence of 500 µmol/liter H2O2 for 10 min, and phosphorylation of the immunoprecipitated (IP) eNOS on Tyr657 was detected by Western blotting (WB). (B) ACh-induced vasodilatation was assessed in the absence (Sol) or presence of 30 µmol/liter H2O2 in aortic ring segments in the continuous presence of 10 µmol/liter diclofenac. The graphs summarize the results obtained in 4–10 different experiments. Data are expressed as means ± SEM. *, P < 0.05; and **, P < 0.01 versus CTL.
The impairment of NO-mediated vasodilatation by H2O2 is PYK2 dependent
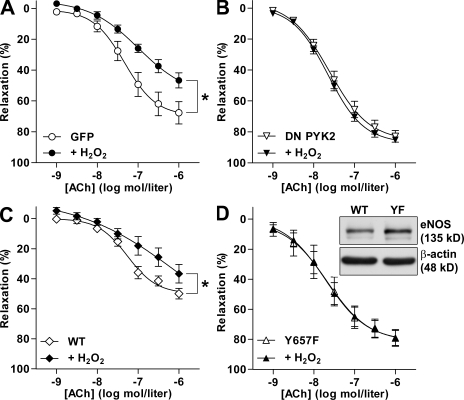
It is clear that H2O2 can potentially affect the eNOS/cyclic GMP signaling cascade and subsequent vasodilatation at several different levels. In addition to stimulating the phosphorylation of eNOS, H2O2 can alter the activity of the soluble guanylyl cyclase (Meurer et al., 2005), as well as of several other tyrosine kinases and tyrosine phosphatases. To relate the decreased endothelium-dependent relaxation to the activation of PYK2, responses to ACh were compared in carotid arteries from wild-type mice in which the endothelial layer was infected with adenoviruses to overexpress either GFP or a dominant-negative (DN) PYK2. Although endothelial infection followed by organ culture is a simple method and allows the transduction of many proteins of interest, including “rescue” experiments in tissues from knockout mice, it is not free of complications. Indeed, we observed that the maintenance of carotid arteries in culture for 48 h led to a significant increase in the endogenous production of H2O2 that was not affected by the expression of GFP or DN PYK2 (Fig. S2 top). Correspondingly, responses to ACh in solvent-treated arteries expressing GFP were moderately impaired (maximal response = 68 ± 7%; Fig. 5 A; in comparison, freshly isolated wild-type arteries typically relax to ∼90%; Fig. 4 B). However, the exogenous application of 30 µmol/liter H2O2 further impaired endothelium-dependent relaxations (Fig. 5 A). Overexpression of the DN PYK2 mutant significantly improved ACh-stimulated responses in solvent-treated arteries (maximal response = 83 ± 4%) and completely protected the arteries against the effect of exogenous H2O2 (Fig. 5 B). Such findings indicate that PYK2 activation may underlie both the basal reduction in vascular responsiveness as well as the acute impairment of endothelial function elicited by the exogenous application of H2O2.
Figure 5.
H2O2-induced endothelial dysfunction involves PYK2 and eNOS phosphorylation on Tyr657. (A–D) ACh-induced vasodilatation was assessed in the absence or presence of 30 µmol/liter H2O2 in carotid arteries from wild-type (A and B) or eNOS−/− (C and D) mice infected with adenoviruses to express GFP (A), a DN PYK2 mutant (B), wild-type eNOS (WT; C), or the Y657F eNOS mutant (YF; D). The inset shows expression of adenovirally delivered wild-type and Y657F eNOS. The graphs summarize the results obtained in six to eight different experiments. Data are expressed as means ± SEM. *, P < 0.05 versus the appropriate solvent control.
To demonstrate a causal relation between the phosphorylation of eNOS on Tyr657 and impaired endothelial function, experiments were repeated in carotid arteries from eNOS−/− mice overexpressing either the wild-type eNOS or a nonphosphorylatable Y657F (tyrosine replaced by phenylalanine) eNOS mutant. Consistent with the endogenous H2O2 production in eNOS−/− carotid arteries after organ culture (Fig. S2 bottom), vessels transduced with wild-type eNOS relaxed to a maximum of only 50 ± 4%. The application of H2O2, however, further decreased endothelium-dependent relaxation in response to ACh (Fig. 5 C). In carotid arteries expressing similar levels of the Y657F eNOS mutant, relaxations to ACh were significantly improved under basal conditions (maximum relaxation = 79 ± 6%) and completely unaffected by exogenous H2O2 (Fig. 5 D). As organ culture enhanced endogenous H2O2 production in vessels from wild-type and eNOS−/− mice alike, it seems that the oxidant is generated by vascular sources other than eNOS. Whatever the mechanism responsible, we observed that DN PYK2 was able to protect vessels against H2O2-induced endothelial dysfunction as well as against the attenuated responsiveness to ACh that was associated with organ culture itself. As mutation of Tyr657 to the nonphosphorylatable phenylalanine conferred protection, it seems that phosphorylation rather than the uncoupling of eNOS accounts for the effects observed.
In addition to its effects on PYK2, H2O2 is reported to inactivate the protein tyrosine phosphatase SHP-2 (Tang et al., 2005). SHP-2 is of potential relevance to the current study because it is reported to deactivate PYK2 (Chauhan et al., 2000), and we have previously demonstrated that eNOS and SHP-2 can be coprecipitated from cultured endothelial cells (Dixit et al., 2005). However, as the reported IC50 value for the H2O2-induced inactivation of SHP-2 is ∼75 µmol/liter, it seems unlikely that SHP-2 inactivation is the main mechanism by which H2O2 influences eNOS activity. At this stage, we cannot exclude the involvement of an additional phosphatase because H2O2 can result in the oxidation of the critical cysteine residues and, thus, inactivate a series of tyrosine phosphatases (Thomas et al., 2008). However, as the expression of DN PYK2 was sufficient to abrogate the H2O2-induced endothelial dysfunction, we speculate that kinase activation is the predominant mechanism underlying the effects described.
Chronic treatment with Ang II stimulates the phosphorylation of eNOS on Tyr657 and endothelial dysfunction
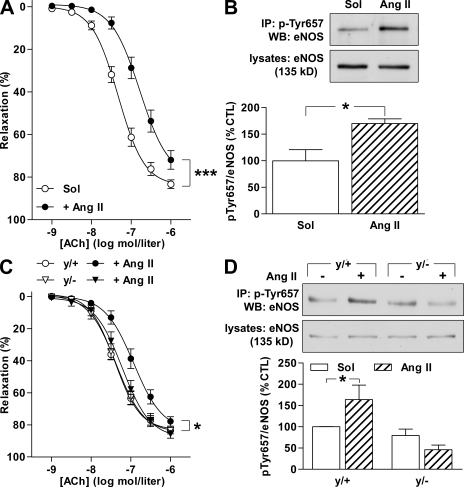
Our results indicated that the phosphorylation of eNOS on Tyr657 is acutely related to a decrease in endothelial function, but the pathophysiological consequences of endothelial dysfunction (e.g., atherosclerosis) generally develop over prolonged periods. To determine the physiological relevance of the mechanism described, we assessed eNOS phosphorylation in aortae from mice treated with Ang II over 3 wk, a procedure previously shown to induce hypertension and vascular oxidative stress as well as endothelial dysfunction (Jung et al., 2005). In segments of aortae isolated from wild-type mice treated with 1 mg/kg/day Ang II for 3 wk, a clear impairment in endothelial function was observed (Fig. 6 A). Although eNOS expression was unaltered by the Ang II treatment, eNOS Tyr657 phosphorylation was significantly higher in the Ang II–treated group (Fig. 6 B).
Figure 6.
Chronic Ang II infusion induces Tyr657 phosphorylation of eNOS in wild-type but not in Nox2y/− mice. (A–D) Wild-type mice (A and B), and Nox2y/− mice (y/−) or the corresponding wild-type littermates (y/+; C and D) were treated with vehicle (Sol) or 1 mg/kg/day Ang II for 3 wk, after which endothelium-dependent relaxation in response to ACh (A and C) and eNOS Tyr657 phosphorylation (B and D) were assessed in aortic segments. The graphs summarize data from eight mice per group stratified over two (A and B) or four (C and D) independent experiments; immunoprecipitations (IP) were performed on lysates from two pooled aortae. Data are expressed as means ± SEM. *, P < 0.05; and ***, P < 0.001 versus the corresponding vehicle (Sol) control. WB, Western blotting.
Many of the consequences of Ang II exposure are related to the formation of reactive oxygen species, such as H2O2, as a consequence of the activation of NADPH oxidases (for review see Garrido and Griendling, 2009). In endothelial cells, Ang II activates mainly the Nox2 (gp91phox) and Nox4 subunits, and the genetic disruption of Nox2 prevents the endothelial dysfunction observed in a high-renin model of hypertension (Jung et al., 2004). We therefore assessed eNOS phosphorylation in mice lacking the Nox2 subunit. Because the gene encoding Nox2 is located on the X chromosome, we compared male Nox2-deficient (Nox2y/−) mice to their wild-type (Nox2y/+) counterparts. We found that the treatment of Nox2y/− mice with Ang II did not result in the attenuation of endothelium-dependent responses to ACh (Fig. 6 C) and did not increase eNOS phosphorylation on Tyr657 (Fig. 6 D).
Endothelium-derived NO is not only essential for controlling vascular tone but is also a critical determinant for vascular gene expression, the recruitment of circulating cells, and the progression of vascular diseases such as atherosclerosis and restenosis (Davignon and Ganz, 2004). Correspondingly, eNOS gene transfer is considered an attractive therapeutic option to improve endothelial function. However, although the exogenous expression of eNOS successfully prevented smooth muscle cell migration in vitro (Largiadèr et al., 2008), a similar approach in human atherosclerotic lesions failed because the heterologously expressed eNOS was inactive (Tanner et al., 2007). In the latter study, the supplementation of samples with essential cofactors, including tetrahydrobiopterin and l-arginine, failed to stimulate NO production, indicating that mechanisms other than enzyme uncoupling determine NO production in atherosclerotic lesions. It is tempting to speculate that the inactivation of eNOS as a consequence of its phosphorylation by PYK2 may play an important role; certainly in the setting of atherosclerosis, PYK2 may be sufficiently activated to influence eNOS. Therefore, it follows that the expression of the nonphosphorylatable eNOS Y657F mutant could represent a more successful therapeutic approach.
MATERIALS AND METHODS
Materials.
The mono-/polyclonal eNOS antibodies were from Santa Cruz Biotechnology, Inc.; the specific phospho-Tyr657 eNOS antibody was generated as previously described (Fisslthaler et al., 2008) by Eurogentec; and the anti-PYK2 antibody was from BD. Tyrosine-phosphorylated proteins were detected with a mixture of four antibodies: P-Tyr-100 (Cell Signaling Technology), Py20 (Santa Cruz Biotechnology, Inc.), Py20 (BD), and clone 4G10 (Millipore). All other substances were obtained from Sigma-Aldrich.
Cell culture.
The use of human umbilical vein endothelial cells was approved by the ethics committee of the Johann Wolfgang Goethe University medical faculty. Human umbilical vein endothelial cells and mouse lung endothelial cells were isolated and cultured as previously described (Fleming et al., 2005). In some experiments, cyclic GMP production was assessed with a specific radioimmunoassay (GE Healthcare), as previously described (Fisslthaler et al., 2008). To silence PYK2 gene expression, mouse lung endothelial cells were transfected with a mixture of siRNA duplexes (5′-AUCUGAGGCAGGCUGUUCCUCUUCU-3′, 5′-UUUCGUUCCAGGUAGUGUCCCAGCU-3′, and 5′-UUCUCCAGCACUCCGAUGACAUCCU-3′; Invitrogen) or with control oligonucleotides of medium GC content (Eurogentec).
Porcine aortae collected at the local slaughterhouse were cut open lengthwise, fixed in stainless steel frames containing Hepes-Tyrode solution, and incubated at 37°C for a total of 1 h. 1 µmol/liter Ang II was added 10 or 30 min before, and 500 µmol/liter H2O2 was added 5 min before the end of the incubation period. Endothelial cells were recovered by scraping and centrifugation for 4 min at 4,000 rpm and 4°C.
Freshly isolated mouse lungs were cut into ∼1-mm3 pieces, suspended in modified Tyrode’s solution containing 100 µmol/liter l-arginine, and equilibrated for 2 h in a normal CO2 incubator. 150 U/ml PEG-catalase was added as indicated in the figures for 1 h before stimulation with 100 nmol/liter Ang II for 15 min.
Immunoprecipitation and immunoblotting.
Cells, isolated aortae, and lungs were lysed in Triton X-100 buffer. eNOS, tyrosine-phosphorylated proteins, or PYK2 were immunopreciptated with the appropriate antibodies. Detergent-soluble proteins or immunoprecipitates were heated with SDS-PAGE sample buffer and separated by SDS-PAGE, and specific proteins were detected by immunoblotting as previously described (Fleming et al., 2005).
Animal experiments.
Animal experiments were approved by the Regierungspräsidium Darmstadt (F18/14). 6–9-wk-old male C57/b6 mice were obtained from Charles River. Male C57/b6 eNOS−/− mice (provided by A. Gödecke, Heinrich-Heine-Universität, Düsseldorf, Germany) and Nox2y/− mice (The Jackson Laboratory) were bred at the animal facilities at Frankfurt University. In a subgroup of 16 wild-type, 16 Nox2y/−, and 16 Nox2y/+ mice, osmotic mini-pumps (ALZET) delivering either vehicle (0.9% NaCl; 8 animals per group) or [5val]–Ang II (1 mg/kg/day for 3 wk; 8 animals per group) were implanted subcutaneously under isoflurane anesthesia.
Adenoviruses encoding GFP, wild-type eNOS, Y657F eNOS, or DN PYK2 were generated and delivered to the endothelial layer of carotid arteries from wild-type or eNOS−/− mice, as described previously (Dixit et al., 2005; Fisslthaler et al., 2008). In brief, mice were euthanized with isoflurane, and the aortic arches and carotid arteries were prepared free of adhering tissue but left in situ. Each vessel was filled with MCDB 131 containing 2% FCS (FBS GOLD; PAA) and viral particles (1.5 × 108 PFU/ml). The vessel was slightly pressurized, ligated at both ends, and incubated in medium containing 2% FCS for 4 h. After the initial incubation, the ligatures were removed and the vessel was maintained in tissue culture for an additional 40–44 h.
3-mm aortic or carotid artery rings were connected to isometric force transducers at a resting force of 1 or 0.5 g, respectively, for standard organ chamber experiments. Relaxations to cumulatively increasing concentrations of ACh were recorded in vessels preconstricted to 80% of the maximal KCl (80 mmol/liter)-induced contraction using phenylephrine. All experiments were performed in the presence of 10 µmol/liter diclofenac, and relaxations are denoted in the figures as the percentage of the maximal relaxation obtained by 10 µmol/liter sodium nitroprusside.
Statistical analysis.
Data are expressed as means ± SEM. Statistical evaluation was performed on the absolute values or on log-transformed data with the Student’s t test for unpaired data, one-way analysis of variance (ANOVA) followed by a Bonferroni t test, or ANOVA for repeated measures where appropriate. P < 0.05 was considered statistically significant.
Online supplemental material.
Fig. S1 shows the Ang II–induced production of H2O2 in cultured endothelial cells. Fig. S2 shows the endogenous production of H2O2 in carotid arteries kept in tissue culture. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20090449/DC1.
Acknowledgments
The authors are indebted to I. Winter, K. Bruch, and M. Piepenbrock for expert technical assistance.
This study was supported by EICOSANOX, an integrated project under the European Commission Framework 6 Program (contract LSHM-CT-2004-005033); by the Deutsche Forschungsgemeinschaft (Exzellenzcluster 147 “Cardio-Pulmonary Systems”); and by a young investigator grant from the Johann Wolfgang Goethe University (to A.E. Loot).
The authors have no conflicting financial interests.
Footnotes
Abbreviations used:
- ACh
- acetylcholine
- Ang II
- angiotensin II
- DN
- dominant negative
- eNOS
- endothelial NO synthase
- PEG
- polyethylene glycol
- PYK2
- proline-rich tyrosine kinase 2
References
- Blaukat A., Ivankovic-Dikic I., Grönroos E., Dolfi F., Tokiwa G., Vuori K., Dikic I. 1999. Adaptor proteins Grb2 and Crk couple Pyk2 with activation of specific mitogen-activated protein kinase cascades. J. Biol. Chem. 274:14893–14901 10.1074/jbc.274.21.14893 [DOI] [PubMed] [Google Scholar]
- Cai H. 2005. Hydrogen peroxide regulation of endothelial function: origins, mechanisms, and consequences. Cardiovasc. Res. 68:26–36 10.1016/j.cardiores.2005.06.021 [DOI] [PubMed] [Google Scholar]
- Chauhan D., Pandey P., Hideshima T., Treon S., Raje N., Davies F.E., Shima Y., Tai Y.T., Rosen S., Avraham S., et al. 2000. SHP2 mediates the protective effect of interleukin-6 against dexamethasone-induced apoptosis in multiple myeloma cells. J. Biol. Chem. 275:27845–27850 [DOI] [PubMed] [Google Scholar]
- Davignon J., Ganz P. 2004. Role of endothelial dysfunction in atherosclerosis. Circulation. 109(Suppl. 1):III27–III32 [DOI] [PubMed] [Google Scholar]
- Dixit M., Loot A.E., Mohamed A., Fisslthaler B., Boulanger C.M., Ceacareanu B., Hassid A., Busse R., Fleming I. 2005. Gab1, SHP2, and protein kinase A are crucial for the activation of the endothelial NO synthase by fluid shear stress. Circ. Res. 97:1236–1244 10.1161/01.RES.0000195611.59811.ab [DOI] [PubMed] [Google Scholar]
- Dossumbekova A., Berdyshev E.V., Gorshkova I., Shao Z., Li C., Long P., Joshi A., Natarajan V., Vanden Hoek T.L. 2008. Akt activates NOS3 and separately restores barrier integrity in H2O2-stressed human cardiac microvascular endothelium. Am. J. Physiol. Heart Circ. Physiol. 295:H2417–H2426 10.1152/ajpheart.00501.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards D.H., Li Y., Griffith T.M. 2008. Hydrogen peroxide potentiates the EDHF phenomenon by promoting endothelial Ca2+ mobilization. Arterioscler. Thromb. Vasc. Biol. 28:1774–1781 10.1161/ATVBAHA.108.172692 [DOI] [PubMed] [Google Scholar]
- Fisslthaler B., Loot A.E., Mohamed A., Busse R., Fleming I. 2008. Inhibition of endothelial nitric oxide synthase activity by proline-rich tyrosine kinase 2 in response to fluid shear stress and insulin. Circ. Res. 102:1520–1528 10.1161/CIRCRESAHA.108.172072 [DOI] [PubMed] [Google Scholar]
- Fleming I., Fisslthaler B., Dixit M., Busse R. 2005. Role of PECAM-1 in the shear-stress-induced activation of Akt and the endothelial nitric oxide synthase (eNOS) in endothelial cells. J. Cell Sci. 118:4103–4111 10.1242/jcs.02541 [DOI] [PubMed] [Google Scholar]
- Garrido A.M., Griendling K.K. 2009. NADPH oxidases and angiotensin II receptor signaling. Mol. Cell. Endocrinol. 302:148–158 10.1016/j.mce.2008.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung O., Schreiber J.G., Geiger H., Pedrazzini T., Busse R., Brandes R.P. 2004. gp91phox-containing NADPH oxidase mediates endothelial dysfunction in renovascular hypertension. Circulation. 109:1795–1801 10.1161/01.CIR.0000124223.00113.A4 [DOI] [PubMed] [Google Scholar]
- Jung O., Brandes R.P., Kim I.H., Schweda F., Schmidt R., Hammock B.D., Busse R., Fleming I. 2005. Soluble epoxide hydrolase is a main effector of angiotensin II-induced hypertension. Hypertension. 45:759–765 10.1161/01.HYP.0000153792.29478.1d [DOI] [PubMed] [Google Scholar]
- Landmesser U., Dikalov S., Price S.R., McCann L., Fukai T., Holland S.M., Mitch W.E., Harrison D.G. 2003. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J. Clin. Invest. 111:1201–1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Largiadèr T., Eto M., Payeli S.K., Greutert H., Viswambharan H., Lachat M., Zünd G., Yang Z., Tanner F.C., Lüscher T.F. 2008. Endothelial nitric oxide synthase gene transfer inhibits human smooth muscle cell migration via inhibition of Rho A. J. Cardiovasc. Pharmacol. 52:369–374 10.1097/FJC.0b013e31818953d0 [DOI] [PubMed] [Google Scholar]
- Meurer S., Pioch S., Gross S., Müller-Esterl W. 2005. Reactive oxygen species induce tyrosine phosphorylation of and Src kinase recruitment to NO-sensitive guanylyl cyclase. J. Biol. Chem. 280:33149–33156 10.1074/jbc.M507565200 [DOI] [PubMed] [Google Scholar]
- Schulz E., Jansen T., Wenzel P., Daiber A., Münzel T. 2008. Nitric oxide, tetrahydrobiopterin, oxidative stress, and endothelial dysfunction in hypertension. Antioxid. Redox Signal. 10:1115–1126 10.1089/ars.2007.1989 [DOI] [PubMed] [Google Scholar]
- Tai L.K., Okuda M., Abe J., Yan C., Berk B.C. 2002. Fluid shear stress activates proline-rich tyrosine kinase via reactive oxygen species-dependent pathway. Arterioscler. Thromb. Vasc. Biol. 22:1790–1796 10.1161/01.ATV.0000034475.40227.40 [DOI] [PubMed] [Google Scholar]
- Tang H., Hao Q., Rutherford S.A., Low B., Zhao Z.J. 2005. Inactivation of SRC family tyrosine kinases by reactive oxygen species in vivo. J. Biol. Chem. 280:23918–23925 10.1074/jbc.M503498200 [DOI] [PubMed] [Google Scholar]
- Tanner F.C., van der Loo B., Shaw S., Greutert H., Bachschmid M.M., Berrozpe M., Rozenberg I., Blau N., Siebenmann R., Schmidli J., et al. 2007. Inactivity of nitric oxide synthase gene in the atherosclerotic human carotid artery. Basic Res. Cardiol. 102:308–317 10.1007/s00395-007-0650-7 [DOI] [PubMed] [Google Scholar]
- Thomas S.R., Chen K., Keaney J.F., Jr 2002. Hydrogen peroxide activates endothelial nitric-oxide synthase through coordinated phosphorylation and dephosphorylation via a phosphoinositide 3-kinase-dependent signaling pathway. J. Biol. Chem. 277:6017–6024 10.1074/jbc.M109107200 [DOI] [PubMed] [Google Scholar]
- Thomas S.R., Witting P.K., Drummond G.R. 2008. Redox control of endothelial function and dysfunction: molecular mechanisms and therapeutic opportunities. Antioxid. Redox Signal. 10:1713–1765 10.1089/ars.2008.2027 [DOI] [PubMed] [Google Scholar]
- Thomson M.J., Puntmann V., Kaski J.C. 2007. Atherosclerosis and oxidant stress: the end of the road for antioxidant vitamin treatment? Cardiovasc. Drugs Ther. 21:195–210 10.1007/s10557-007-6027-1 [DOI] [PubMed] [Google Scholar]
- Tribble D.L., Gong E.L., Leeuwenburgh C., Heinecke J.W., Carlson E.L., Verstuyft J.G., Epstein C.J. 1997. Fatty streak formation in fat-fed mice expressing human copper-zinc superoxide dismutase. Arterioscler. Thromb. Vasc. Biol. 17:1734–1740 [DOI] [PubMed] [Google Scholar]
- Ulker S., McMaster D., McKeown P.P., Bayraktutan U. 2003. Impaired activities of antioxidant enzymes elicit endothelial dysfunction in spontaneous hypertensive rats despite enhanced vascular nitric oxide generation. Cardiovasc. Res. 59:488–500 10.1016/S0008-6363(03)00424-3 [DOI] [PubMed] [Google Scholar]
- Yin G., Yan C., Berk B.C. 2003. Angiotensin II signaling pathways mediated by tyrosine kinases. Int. J. Biochem. Cell Biol. 35:780–783 10.1016/S1357-2725(02)00300-X [DOI] [PubMed] [Google Scholar]
- Zanetti M., Sato J., Jost C.J., Gloviczki P., Katusic Z.S., O’Brien T. 2001. Gene transfer of manganese superoxide dismutase reverses vascular dysfunction in the absence but not in the presence of atherosclerotic plaque. Hum. Gene Ther. 12:1407–1416 10.1089/104303401750298562 [DOI] [PubMed] [Google Scholar]