Abstract
The pre- and postnatal environment may represent a window of opportunity for allergy and asthma prevention, and the hygiene hypothesis implies that microbial agents may play an important role in this regard. Using the cowshed-derived bacterium Acinetobacter lwoffii F78 together with a mouse model of experimental allergic airway inflammation, this study investigated the hygiene hypothesis, maternal (prenatal) microbial exposure, and the involvement of Toll-like receptor (TLR) signaling in prenatal protection from asthma. Maternal intranasal exposure to A. lwoffii F78 protected against the development of experimental asthma in the progeny. Maternally, A. lwoffii F78 exposure resulted in a transient increase in lung and serum proinflammatory cytokine production and up-regulation of lung TLR messenger RNA. Conversely, suppression of TLRs was observed in placental tissue. To investigate further, the functional relevance of maternal TLR signaling was tested in TLR2/3/4/7/9−/− knockout mice. The asthma-preventive effect was completely abolished in heterozygous offspring from A. lwoffii F78–treated TLR2/3/4/7/9−/− homozygous mother mice. Furthermore, the mild local and systemic inflammatory response was also absent in these A. lwoffii F78–exposed mothers. These data establish a direct relationship between maternal bacterial exposures, functional maternal TLR signaling, and asthma protection in the progeny.
Allergic bronchial asthma has become a major public health burden in industrialized countries, and the incidence of this chronic inflammatory disease has been steadily growing over the last decades. Although many susceptibility genes have been identified (Moffatt et al., 2007; Vercelli, 2008), genetics alone cannot explain the velocity of these changes. Additional factors must have contributed to the growing incidence of asthma. In agreement with the hygiene hypothesis, epidemiological studies indicate that lack of early childhood exposure to microbial agents may contribute to increased susceptibility to the development of allergic diseases (Strachan, 2000). Moreover, environments characterized by a diverse and concentrated microbial milieu, such as traditional farming sites, may protect from allergic diseases (Riedler et al., 2001; Majkowska-Wojciechowska et al., 2007; Midodzi et al., 2007; von Mutius and Radon, 2008). In this regard, analysis of cowshed dust samples has identified the Gram-negative, nonpathogenic bacterium Acinetobacter lwoffii F78 as a potential allergo-protective agent (Korthals et al., 2008). Although experimental approaches using a mouse model of acute allergic airway inflammation suggest a strong allergy protective potential of this bacterial strain (Debarry et al., 2007), nothing is known about the effects of prenatal exposure to A. lwoffii F78 and possible downstream mechanisms affecting allergic inflammatory responses in the progeny.
Respiratory allergies, including bronchial asthma, are chronic inflammatory diseases that are based on a complex deregulated interaction of both innate and adaptive immune responses (Hammad and Lambrecht, 2008; Holgate and Polosa, 2008). Regarding the maturation of the adaptive immune system, it is now well established that the development of functionally active T cell subsets starts already prenatally (Warner et al., 2000), and it has been proposed that immunoprogramming by environmental influences may occur at this early developmental stage. Indeed, studies have demonstrated that many factors affecting the initiation and course of respiratory allergies appear to act within a narrow window of opportunity, either prenatally and/or early in life (Ege et al., 2006; Rowe et al., 2007). It is still unresolved, however, how protective signals are transferred from the mother to the developing fetus.
Regarding mechanisms by which A. lwoffii F78 may exert its protective effects, consideration must be given to innate immune processes that participate in initial microbial recognition. Innate immunity functions as a first line of defense that uses, in addition to physical barriers, a pattern recognition receptor (PRR) system that can identify a broad spectrum of microbial components. Toll-like receptors (TLRs) are a major class of PRRs expressed by several cell types, including epithelium and immune cells, that play a vital role in the initiation of the immune response (Gon, 2008; Kawai and Akira, 2009).
Combining prenatal exposure to the cowshed bacterium A. lwoffii F78 with a mouse model of allergic airway inflammation, we show in this report that prenatal exposure to farming-related microbes protects from the development of allergic phenotypes in the next generation. The process by which this protection is achieved involves a low level, local, and systemic maternal innate immune response, and the transference of protective immunity from the mother to the fetus is fully dependent on the action of the maternal TLR2, 3, 4, 7, and/or 9.
RESULTS AND DISCUSSION
Prenatal A. lwoffii F78 application protects against experimental asthma pathology in adult offspring
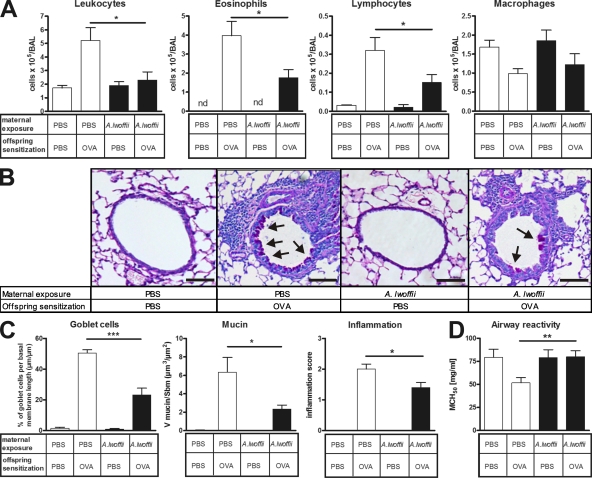
As shown in Fig. 1, the development of experimental asthma was largely prevented in OVA-sensitized and -challenged offspring from A. lwoffii F78–exposed mothers. This was exhibited by a significantly lower influx of eosinophils and lymphocytes into the airway lumen as compared with OVA-sensitized and -challenged offspring from sham-exposed control mothers (Fig. 1 A). In accordance, histological analyses of tissue inflammation revealed a significantly reduced degree of airway inflammation with fewer mucus-producing goblet cells (Fig. 1, B and C). Lung provocation tests, in which airway hyperreactivity is defined as a lower concentration of β-methacholine (MCh) required to induce a 50% decrease in lung function compared with a negative control, demonstrated that prenatal treatment with A. lwoffii F78 resulted in normalization of airway reactivity (Fig. 1 D).
Figure 1.
Prenatal A. lwoffii F78 exposure significantly prevented the extent of the asthmatic phenotype in the offspring. White bars represent offspring exposed prenatally to control PBS, and black bars represent offspring exposed prenatally to A. lwoffii F78. (A) Differential leukocyte numbers in the BAL of offspring. (B) Representative lung tissue cross sections stained with PAS to visualize mucus-producing goblet cells (arrows) and airway inflammation in offspring. Bars, 100 µm. (C) Quantification of mucus-producing goblet cells, mucus volume, and inflammation in offspring airways. (D) Offspring airway responsiveness to MCh. Results represent one out of two independently performed experiments with similar outcomes (n ≥ 8 per group). Means ± SEM are shown. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
An examination of OVA-specific antibody titers in OVA-sensitized and -challenged offspring revealed a trend toward decreased Th2 antibody titers in mice prenatally treated with A. lwoffii F78 (OVA-specific IgE = 155 ± 60.54 ng/ml vs. 72.64 ± 9.202 ng/ml, and OVA-specific IgG1 = 7,076 ± 920.7 ng/ml vs. 5,088 ± 924.6 ng/ml; prenatal PBS control vs. prenatal A. lwoffii F78, respectively), but these differences did not reach statistical significance. Several possible reasons may explain this finding. Offspring were sensitized using the adjuvant aluminum hydroxide, which is known to elicit a very strong Th2 response (Brewer et al., 1999) and specifically affect antibody titers (Conrad et al., 2009). Although prenatal A. lwoffii F78 treatment appears to positively influence antibody titers, its effect may not be strong enough to override the effects of the adjuvant used. Additionally, it is also possible that the protection produced by prenatal A. lwoffii F78 treatment occurs in a B cell–independent manner. If this was the case, it would indicate that treatment does not prevent sensitization per se but rather initiates mechanisms that prevent or dampen the elicitation of the inflammatory response to allergen challenge.
A. lwoffii F78 is not the only type of bacteria that can engender protective effects through prenatal treatment. Previous experiments revealed that prenatal treatment with both Lactobacillus rhamnosus GG (Blümer et al., 2007) and the Gram-negative cell-wall component LPS (Blümer et al., 2005) also had a protective effect on the development of experimental asthma in the next generation. The strongest effect, however, was observed using A. lwoffii F78, because treatment with A. lwoffii F78 prevented the development of airway hyperresponsiveness together with a prevention of allergic airway inflammation. Therefore, we selected A. lwoffii F78 to further explore underlying mechanisms in terms of innate and adaptive immune responses. Because Gram-positive and -negative microbes differ significantly in terms of usage of PRRs, it is very likely that the mechanism of asthma protection depends on the type of microbe or microbial compounds used. In the current study, we have elucidated a novel mechanism for asthma protection by a Gram-negative bacterium. Further studies are necessary to investigate cellular and molecular pathways underlying asthma protection in other model situations.
Using A. lwoffii F78, a Gram-negative bacterium isolated from the stable dusts of traditional farms, and a mouse model of experimental asthma, our data demonstrate a direct cause–effect relationship between maternal (prenatal) exposure to farm-related bacteria and protection from allergic airway inflammation in the progeny. Because only mother mice were directly exposed to A. lwoffii F78, the reduced asthma phenotype observed in the progeny must have resulted from a transfer of protective effects from the mother to the fetus.
Generation of a quintuple TLR2/3/4/7/9−/− knockout mouse
After demonstrating the protective effects of prenatal A. lwoffii F78 treatment, subsequent investigations sought to compare the action of A. lwoffii F78 in a system where its recognition is abolished (or greatly reduced) in the mother. Because the recognition of A. lwoffii F78 is likely very complex and it is conceivable that the TLR family plays a large role in maternal A. lwoffii F78 recognition, we designed an experimental system in which the recognition of a broad spectrum of pathogen-associated molecular patterns was selectively impaired in the mother. Because MyD88−/− or MYD88−/−/TRIF−/− mice lack not only TLR activity but are also deficient in IL-1 cytokine family member signaling, which plays a role in the regulation of inflammatory responses, we considered these mice unsuitable for our experimental approach. Instead, TLR2−/−, TLR3−/−, TLR4−/−, TLR7−/−, and TLR9−/− quintuple knockout mice (TLR2/3/4/7/9−/−) were created by intercrossing respective single TLR knockout mice. The TLR2/3/4/7/9−/− mouse was best suited to the experimental design because, as a result of heterodimer formation, knockout of TLR2 also functionally eliminates TLR1 and 6 function. This leaves only TLR5, which is unlikely to be involved in A. lwoffii F78 recognition. TLR2/3/4/7/9−/− mice were healthy, bred normally under regular specific pathogen-free conditions, and were fully responsive toward challenge with cytokines and unrelated pathogen-associated molecular patterns.
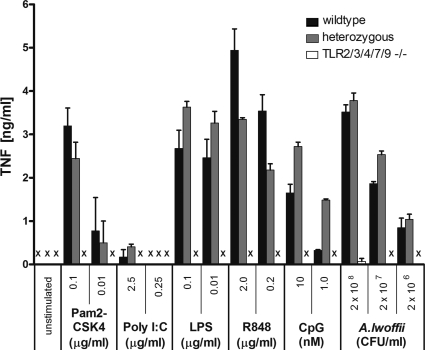
To demonstrate the principal functionality of the TLR signaling system in TLR2/3/4/7/9−/− knockout mice, BM-derived macrophages (BMDMs) from homozygous, heterozygous, and wild-type animals were tested in vitro for responsiveness to TLR agonists and A. lwoffii F78 specifically (Fig. 2). As expected, BMDMs from TLR2/3/4/7/9−/− animals generated no response to TLR agonists or A. lwoffii F78, whereas BMDMs from heterozygous animals reacted similarly to the different stimuli as wild-type mice.
Figure 2.
Heterozygous TLR2/3/4/7/9+/− mice respond similarly to TLR agonists as wild-type mice. Macrophages prepared from BM cells of wild-type, heterozygous, and homozygous TLR2/3/4/7/9−/− mice were in vitro stimulated with specific TLR agonists or A. lwoffii F78, as indicated. Pam2CSK4, Poly I:C, LPS, R848, and CpG specifically stimulate TLR2, 3, 4, 7, and 9, respectively. Results are from one experiment performed in duplicate (n = 2 per group). Means ± SEM are shown. X, not detectable.
Application of A. lwoffii F78 induces maternal local and systemic innate immune responses
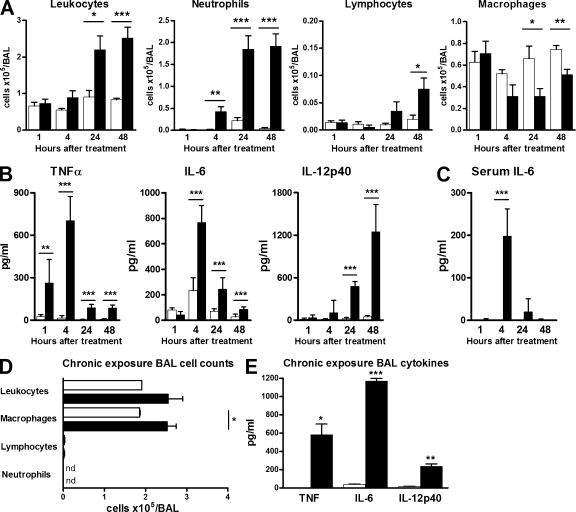
As a first step in investigating the mechanism by which maternal A. lwoffii F78 treatment confers protection against experimental asthma in progeny, a kinetic was performed to determine the immediate local effects of intranasal A. lwoffii F78 administration. Acutely (analysis at 1, 4, 24, or 48 h after application of a single dose) and chronically (treated three times per week for five consecutive weeks) exposed mice equivalent in age to the breeding mothers were examined. After a single intranasal A. lwoffii F78 application, a fast and transient inflammatory response was observed within the airways. This response was primarily characterized by an increase of inflammatory cytokines in the bronchoalveolar lavage (BAL) fluid that peaked at different time points: TNF after 1 h, IL-6 after 4 h, and IL-12p40 after 24 h (Fig. 3 B). Additionally, an influx of neutrophils and lymphocytes into the BAL fluid was observed, with the maximum after 24 and 48 h, respectively (Fig. 3 A). In serum, IL-6 levels peaked after 4 h (Fig. 3 C).
Figure 3.
Single or chronic A. lwoffii F78 exposure induces local and systemic innate immune responses. All animals used were age equivalent to breeding mothers. White bars represent PBS control exposure, and black bars represent A. lwoffii F78 exposure. (A–C) Single-exposure kinetic. Results represent one out of two independently performed experiments with similar outcomes (n ≥ 7 per group). (A) Differential leukocyte numbers in the BAL. (B) Local concentration of proinflammatory cytokines in BAL fluid. (C) Serum IL-6 concentration. (D and E) Chronic exposure three times per week for 5 wk. Results represent one out of two independently performed experiments with similar outcomes (n = 4 per group). (D) Differential leukocyte numbers in BAL. (E) Local concentration of proinflammatory cytokines in BAL fluid after chronic exposure to A. lwoffii F78. Means ± SEM are shown. *, P < 0.05; **, P < 0.01; ***, P < 0.001. nd, not detectable.
In contrast to the single-dose response, the maternal airways showed partial adaptation to chronic A. lwoffii F78 exposure, as neutrophils and lymphocytes were no longer detectable after 5 wk of treatment (Fig. 3 D). However, TNF, IL-6, and IL-12p40 levels still remained increased in BAL fluids (Fig. 3 E). These data indicate that chronic exposure leads to a weak but persistent innate immune response possibly induced by TLR-mediated activation of airway epithelial cells or locally residing innate immune cells such as macrophages (Medzhitov, 2007). Notably, acute and chronic exposure to A. lwoffii F78 was well tolerated by treated animals. After intranasal microbial application, mice exhibited normal behavior and, throughout the study, did not show signs of weight loss or lethargy. As demonstrated previously, chronic treatment with the nonpathogenic A. lwoffii F78 did not result in infection, as histological lung sections from chronically treated mice showed no signs of inflammation and the lung tissues were cleared of the bacteria (Debarry et al., 2007).
To analyze the immune response generated in mother mice by intranasal A. lwoffii F78 treatment in the absence of major microbial sensors such as TLRs, TLR2/3/4/7/9−/− mice were treated a single time with A. lwoffii F78. Analysis of BAL cell counts, as well as BAL and serum cytokines, revealed that in the absence of specific TLRs, animals mount none of the responses observed in wild-type mice, suggesting that the initiation of innate immune responses to A. lwoffii F78 is fully dependent on TLR function (unpublished data).
Application of A. lwoffii F78 alters maternal TLR expression patterns
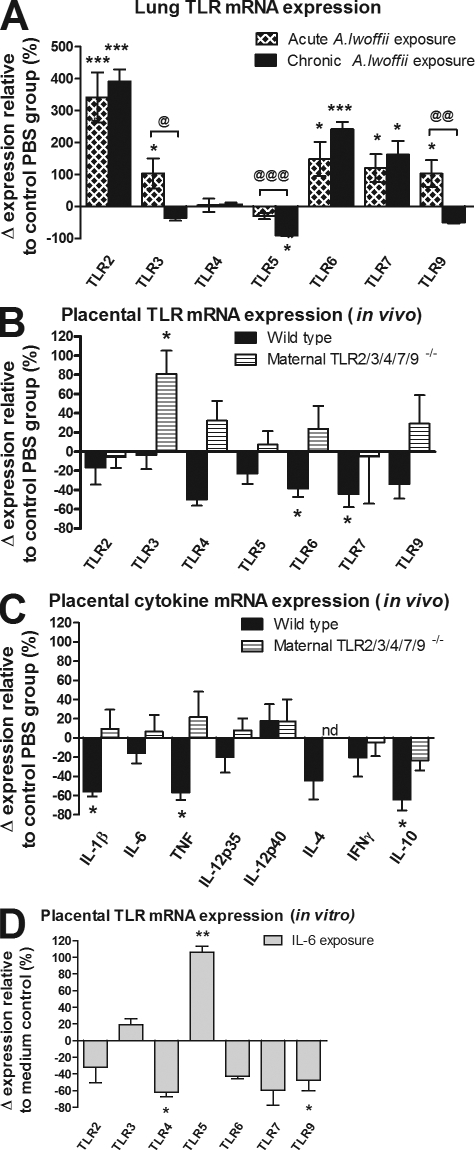
Maternal treatment with A. lwoffii F78 resulted in modulation of lung TLR mRNA expression. Examination of lung-tissue TLR mRNAs after acute A. lwoffii F78 application revealed an up-regulation of TLR2, 3, 6, 7, and 9 expression. (Fig. 4 A). Although changes in TLR mRNA expression occurred at varying time points for different receptors, all aforementioned TLRs were up-regulated at the 4-h time point. Further examination of maternal lung TLR expression after chronic exposure to A. lwoffii F78 revealed similar TLR expression patterns, with the exception of TLR3 and 9, which appear to have attenuated expression after chronic exposure (Fig. 4 A). Interestingly, TLR4 mRNA expression remained unchanged throughout the entire observation period.
Figure 4.
Maternal treatment with A. lwoffii F78 modulates expression of immune-relevant genes in lung and placental tissue. Bars represent the relative change in mRNA expression in the treated compared with the control group. All mRNA expression levels were calculated by setting the mRNA expression of the control group as 100% and individually calculating the Δ expression for each mRNA sample. The calculated change in mRNA expression for each noncontrol individual was then plotted, and the mean and SEM calculated. (A) TLR mRNA expression in the wild-type maternal lung 4 h after intranasal A. lwoffii F78 exposure for both a single acute exposure and a 5-wk chronic exposure. (B) TLR mRNA expression in placentas from both wild-type and TLR2/3/4/7/9−/− mice on day 18 of gestation. (C) Cytokine mRNA expression in placentas from both wild-type and TLR2/3/4/7/9−/− mice on day 18 of gestation. (D) TLR mRNA expression from wild-type primary placental cell culture incubated with IL-6. In vivo results are from two independently performed experiments with similar outcomes (n ≥ 5 animals per group with two placentas per animal). In vitro results are from one experiment performed in triplicate (n = 3 per group). Means ± SEM are shown. *, P < 0.05; **, P < 0.01; and ***, P < 0.001 for the treated group compared with control. @, P < 0.05; @@, P < 0.01; and @@@, P < 0.001 for acute versus chronic treatment. nd, not detectable.
Up-regulation of TLR mRNA in the maternal lung was accompanied by TLR down-regulation in the placenta. Examination of placentas from A. lwoffii F78–treated mother mice showed a significant down-regulation of TLR6 and 7 mRNA (compared with sham-treated controls), accompanied by a nonsignificant trend toward lower mRNA levels in all other TLRs tested (Fig. 4 B). Furthermore, the down-regulation of placental TLR mRNA expression was paralleled by a significant suppression of the placental proinflammatory mediators IL-1β and TNF (Fig. 4 C). A similar effect was observed in terms of T cell effector responses on the level of IL-4 (Th2), IFN-γ (Th1), and IL-10 (T reg) mRNA levels.
To further study the effects of maternal TLRs on placental mRNA expression, TLR and cytokine mRNA from the placentas of sham- or A. lwoffii F78–treated TLR2/3/4/7/9−/− homozygous knockout mother mice carrying TLR2/3/4/7/9+/− heterozygous offspring were examined. These experiments revealed that in the absence of maternal TLRs, fetal placentas exhibit a different TLR mRNA expression pattern than that of the whole placenta (Fig. 4 B). These results indicate that the TLR mRNA down-regulation seen in wild-type A. lwoffii F78–treated animals may occur mainly on the maternal side of the placenta. Quantitative real-time PCR analysis of placental cytokine mRNAs revealed that in the absence of maternal TLRs, there is no longer a difference in placental cytokine production between PBS- and A. lwoffii F78–treated groups (Fig. 4 C). This further supports the hypothesis that maternal TLRs are required for the transfer of protective antiallergic effects to the fetus.
To investigate how signals generated by intranasal A. lwoffii F78 treatment might be transferred from the maternal lung to the placenta (feto–maternal interface), subsequent experiments were designed to examine the effect of serum IL-6 (generated by A. lwoffii F78 exposure) on placental tissue. In vitro primary placental culture revealed that incubation with IL-6 resulted in an alteration of placental TLR mRNA expression; TLR4 and 9 were significantly down-regulated upon exposure to IL-6, with TLR2, 6, and 7 following a similar trend (Fig. 4 D). The local transient proinflammatory response to A. lwoffii F78 in the maternal airways was accompanied by counterregulatory activities in the placental tissue, indicating (a) a systemic distribution of signals such as IL-6 after local bacterial exposure and (b) the generation of a tissue-specific response in the placenta.
Absence of endotoxin in amniotic fluid
It has been previously demonstrated that some environmental components that are able to pass the feto–maternal interface, via the amniotic membrane and swallowing of amniotic fluid, may directly activate the developing fetal immune system (Harju et al., 2005). It is also known that the presence of bacterial compounds, such as endotoxin in the placental compartment, may result in the abortion of the fetus (Entrican, 2002). To rule out the possibility of microbial component transfer between mother and fetus, maternal fluids were tested for the presence of endotoxin derived from A. lwoffii F78 exposure. In both maternal blood plasma and amniotic fluid, no differences were found in endotoxin levels between control and A. lwoffii F78–treated mothers (unpublished data).
In addition to measuring endotoxin levels in the maternal fluids, an experiment was designed to measure endotoxin-induced inflammatory cytokine expression in fetal tissue from TLR2/3/4/7/9−/− mothers mated to wild-type males. In this particular case, the mother will not respond to A. lwoffii F78, but if bacterial components such as endotoxin are made locally available to the heterozygous fetus, an inflammatory response should be detected in the fetal tissue. Comparison of inflammatory cytokine mRNA (TNF, IL-1β, and IL-6) in the fetal tissue from sham- or A. lwoffii F78–treated mothers revealed no differences in mRNA expression (unpublished data). These results provide strong evidence that the fetus is not exposed to A. lwoffii F78–derived endotoxin from maternal treatment. Finally, observation of the experimental asthma phenotype in heterozygous offspring from A. lwoffii F78–treated TLR2/3/4/7/9−/− mothers (discussed in detail in the next section) demonstrates that even if small quantities of endotoxin are made available to the developing fetus, it has no measurable phenotypic effect.
Protective effects of prenatal A. lwoffii F78 exposure require functional maternal TLR expression
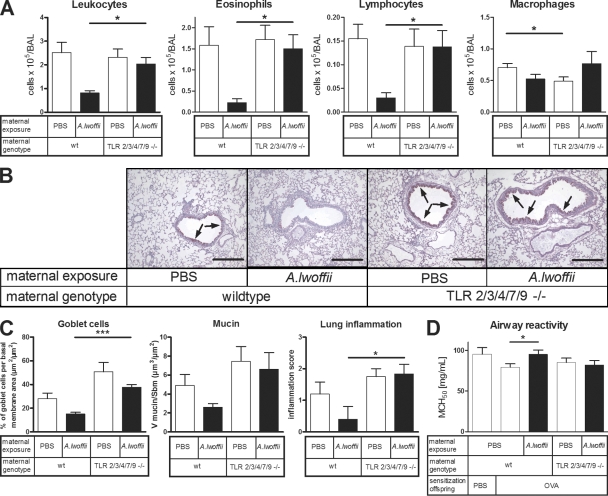
To analyze the requirement of maternal TLR signaling for prenatal allergo-protection, female TLR2/3/4/7/9−/− mother mice, treated with A. lwoffii F78 or control PBS, were mated with wild-type males to generate heterozygous offspring that have a fully functional TLR response (Fig. 2). At 4 wk of age, experimental asthma was induced in these offspring. Functional knockout of the five TLRs in the mothers resulted in a loss of the A. lwoffii F78 asthma-protective effect in the offspring, as demonstrated by fully developed airway inflammation, goblet cell metaplasia, and mucus production in these animals (Figs. 5, A–C). Mice of the C57BL/6 background by themselves develop less hyperreactivity of the airways (Whitehead et al., 2003). Nonetheless, a significant improvement in lung function could be detected in sensitized offspring from A. lwoffii F78–exposed wild-type mothers, whereas heterozygous offspring of TLR-dysfunctional mothers did not show normalization after prenatal bacterial treatment (Fig. 5 D).
Figure 5.
Asthma-protective effects of prenatal A. lwoffii F78 treatment are dependent on maternal TLRs. White bars represent offspring exposed prenatally to control PBS, and black bars represent offspring exposed prenatally to A. lwoffii F78. All offspring were sensitized and challenged with OVA. (A) Differential leukocyte numbers in the BAL of offspring. (B) Representative lung tissue cross sections stained with PAS to visualize mucus-producing goblet cells (arrows) and airway inflammation in offspring. Bars, 200 µm. (C) Quantification of mucus-producing goblet cells, mucus volume, and inflammation in offspring. (D) Offspring airway responsiveness to MCh. Results represent two individually performed experiments (WT mother–PBS, n = 13 OVA-treated offspring; WT mother–A. lwoffii F78, n = 11 OVA-treated offspring; TLR KO mother–PBS, n = 10 OVA-treated offspring; and TLR KO mother–PBS A. lwoffii F78, n = 16 OVA-treated offspring). Means ± SEM are shown. *, P < 0.05; ***, P < 0.001.
Conclusion
Our data provide strong evidence in favor of the hygiene hypothesis and demonstrate how prenatal asthma-protective effects from maternal microbial treatment are transferred from the mother to the progeny. For the first time, a causal relationship between prenatal exposure to stable-derived bacteria, functional maternal TLR signaling, and protection against experimental asthma is described. A. lwoffii F78 acts via the up-regulation of maternal lung TLR expression in conjunction with initiation of a transient acute local Th1-like inflammatory response. This local response is followed by systemic distribution of inflammatory cytokines and a down-regulation of TLR and cytokine expression in the placenta. Furthermore, as offspring from A. lwoffii F78–treated TLR2/3/4/7/9−/− mothers are no longer protected from experimental asthma, this strongly indicates that maternal TLR signaling plays a pivotal role in the transfer of protective effects. Collectively, the results of our study elucidate a novel mechanism for prenatal asthma protection and may incite novel clinical approaches for allergy and asthma prevention.
MATERIALS AND METHODS
Animals.
6–8-wk-old female BALB/c and C57BL/6 mice were obtained from Harlan Winkelmann. Mice were kept under specific pathogen-free housing conditions and were supplied with water and an OVA-free diet ad libitum. All animal experiments were performed in accordance with German and international guidelines and were approved by local authorities (Regierungspräsidium Gießen).
TLR-knockout mice.
Wild-type and TLR2−/− (Spiller et al., 2007), TLR3−/− (Honda et al., 2003), TLR4−/− (Hoshino et al., 1999), TLR7−/− (Hemmi et al., 2002), and TLR9−/− (Hemmi et al., 2000) mice were backcrossed toward the genetic C57BL/6 background at least six times, and were subsequently intercrossed to generate TLR2−/−/TLR3−/−/TLR4−/−/TLR7−/−/TLR9−/− (TLR2/3/4/7/9−/−) mice. TLR2/3/4/7/9−/− mice were healthy under regular housing conditions and displayed no obvious abnormalities.
Prenatal A. lwoffii F78 exposure.
According to protocols previously optimized for microbial concentration and treatment timing (Blümer et al., 2005, 2007), female mice of the BALB/c, C57BL/6, and C57BL6 TLR2/3/4/7/9−/− backgrounds received intranasal applications of 108 CFU of freeze-dried A. lwoffii F78 reconstituted in a volume of 50 µl PBS 11, 9, 6, 4, and 2 d before mating, as well as every other day three times per week during gestation. Age-matched control animals were sham treated with PBS.
Sensitization, challenge, and treatment of offspring.
Offspring from A. lwoffii F78– or control-treated mothers were sensitized to OVA at the age of 25 and 39 d by intraperitoneal injection of 10 µg OVA (grade VI; Sigma-Aldrich) emulsified in 1.5 mg Al(OH)3 (Thermo Fisher Scientific) in a total volume of 200 µl. The sham group received PBS with Al(OH)3. At days 44, 45, and 46 after birth, animals were exposed to aerosolized OVA (1% wt/vol diluted in PBS) for 20 min. Lung function was analyzed 24 h (day 47) after the last aerosol challenge, and another 24 h later (day 48), mice were terminally anesthetized with ketamine plus rompun for further analyses (described in the following paragraphs).
Assessment of offspring airway reactivity.
24 h after the last challenge, lung function analysis was performed in conscious animals using noninvasive head-out body plethysmography. The midexpiratory airflow (EF50) of bronchial responsiveness to MCh was measured as described previously (Neuhaus-Steinmetz et al., 2000). Airway hyperreactivity is represented in the figures by a lower MCh concentration that induces a 50% reduction in baseline EF50 (MCh50).
BAL and differential cell counts.
Offspring from A. lwoffii F78– or control-treated mother mice were sacrificed 48 h after the last challenge. Using a tracheal cannula, BAL was performed one time using 1 ml PBS containing 1× protease inhibitor cocktail (Roche). Total leukocytes were counted with an automated cell counter (Casy TT; Schaerfe Systems), and cell-free supernatant was stored at −20°C for cytokine measurement. Cytospin preparations were stained with Diff-Quick (Merz & Dade AG), and standard morphological criteria were used to identify cell types. 100 cells were counted per cytospin.
Measurement of offspring serum antibodies.
Blood samples were taken from the axillary vessels, and serum levels of anti-OVA IgE and OVA IgG1 were measured by ELISA as previously described (Blümer et al., 2007).
Measurement of cytokines.
Cell-free supernatants from BAL fluids, cell-culture supernatants, and blood serum were analyzed for IL-5, IL-6, IL-10, IL-12p40, IFN-γ, (Opteia Kit; BD), and TNF (Invitrogen) using ELISA technology as described by the manufacturers. Serum samples were diluted 1:5 before measurement. Detection limits were 10 pg/ml for IL-4, IL-5, and TNF, 5 pg/ml for IFN-γ, and 4 pg/ml for IL-6 and IL12-p40, respectively.
Histology and quantitative morphological analysis of mucin in offspring airways.
Directly after BAL, lungs were fixed with 10% formalin via the trachea, removed, and stored in 10% formalin. Lung tissues were embedded into paraffin, and 3-µm sections were stained with periodic acid-Schiff (PAS). Goblet cell metaplasia and the volume of epithelial mucin (V mucin) per surface area of airway epithelial basal membrane (Sbm; as shown in the figures) were determined by means of point and intersection counting using the computer-assisted stereology tool box (CAST-Grid 2.0; Olympus; Sel et al., 2008). The inflammatory score was quantified as follows: 0, normal; 1, few inflammatory cells; 2, one to two cell layer ring of inflammatory cells; and 3, three or more cell layer ring of inflammatory cells.
Quantitative analysis of mRNA expression in maternal lung, placental, and fetal tissue.
Total RNA was extracted from the lung tissue of wild-type or TLR2/3/4/7/9−/− mice intranasally exposed to A. lwoffii F78 or control PBS either acutely (once) or chronically (treatment three times per week for 5 wk), as well as from placental and fetal torso tissue from gestation day 18 of chronically exposed mice. Acute and chronically exposed mice were age matched to the breeding mothers. Total RNA was then reverse transcribed (Omniscript RT Kit; QIAGEN) using oligo (dT) primers. A RotorGene (Corbett) and the QuantiTect SYBR Green PCR Kit (QIAGEN) were used for quantitative real-time PCR of TLRs, IL-1β, IL-6, TNF, IL-12p35, IL-12p40, IL-4, IFN-γ, IL-10, and L32 (housekeeping control). Quantitative real-time PCR data were analyzed by using the previously described 2−(ΔΔCt) method (Livak and Schmittgen, 2001). All mRNA expression levels were calculated by setting the mRNA expression of the control group as 100% and individually calculating the Δ expression for each mRNA sample. The calculated change in mRNA expression for each noncontrol individual was then plotted, and the mean and SEM calculated.
Cell purification and in vitro stimulation.
Day 18 placentas were isolated from BALB/c mice, and a single-cell suspension of the whole placenta was prepared by meshing through a 100-µm filter. Because mice have hemochorial placentation in which both the maternal and fetal sides of the placenta are exposed to maternal blood in vivo, all placental cell types were used for the cell culture. Primary placental cells were cultured at 5 × 106 cells per well in triplicate with 10 ng/ml IL-6 for 4 h. mRNA was isolated, cDNA was constructed, and quantitative real-time PCR was performed for TLR2, 3, 4, 5, 6, 7, and 9.
BMDMs from wild-type, heterozygous TLR2/3/4/7/9+/−, and homozygous TLR2/3/4/7/9−/− knockout mice were stimulated as indicated for 8 h (Fig. 2), after which supernatants were tested using ELISA. TLR ligands used were dipalmitoylated hexpapeptide for TLR2 (Pam2CSK4; EMC Microcollections), polyinosinic–polycytidylic acid for TLR3 (Poly I:C; Sigma-Aldrich), LPS for TLR4 (ultra-pure Salmonella minnesota Re595; Sigma-Aldrich), resiquimod for TLR7 (R848; InvivoGen), and oligodeoxynucleotide CpG for TLR9 (TIB MOLBIOL).
Statistical analysis.
All numerical data were expressed as means ± SEM. Assuming normal distribution, data were analyzed for significance using the Student's unpaired t test. Calculations were performed with Prism software (version 4.01; GraphPad Software, Inc.).
Acknowledgments
We thank T. Ruppersberg, A. Rühl, and A. Spies-Naumann for excellent technical assistance.
This work was supported by the Deutsche Forschungsgemeinschaft (SFB/TR22) and Stiftung P.E. Kempkes, Marburg (10/07).
The authors have no conflicting financial interests.
Footnotes
Abbreviations used:
- BAL
- bronchoalveolar lavage
- BMDM
- BM-derived macrophage
- MCh
- β-methacholine
- PAS
- periodic acid-Schiff
- PRR
- pattern recognition receptor
- TLR
- Toll-like receptor
References
- Blümer N., Herz U., Wegmann M., Renz H. 2005. Prenatal lipopolysaccharide-exposure prevents allergic sensitization and airway inflammation, but not airway responsiveness in a murine model of experimental asthma. Clin. Exp. Allergy. 35:397–402 10.1111/j.1365-2222.2005.02184.x [DOI] [PubMed] [Google Scholar]
- Blümer N., Sel S., Virna S., Patrascan C.C., Zimmermann S., Herz U., Renz H., Garn H. 2007. Perinatal maternal application of Lactobacillus rhamnosus GG suppresses allergic airway inflammation in mouse offspring. Clin. Exp. Allergy. 37:348–357 10.1111/j.1365-2222.2007.02671.x [DOI] [PubMed] [Google Scholar]
- Brewer J.M., Conacher M., Hunter C.A., Mohrs M., Brombacher F., Alexander J. 1999. Aluminium hydroxide adjuvant initiates strong antigen-specific Th2 responses in the absence of IL-4- or IL-13-mediated signaling. J. Immunol. 163:6448–6454 [PubMed] [Google Scholar]
- Conrad M.L., Yildirim A.O., Sonar S.S., Kiliç A., Sudowe S., Lunow M., Teich R., Renz H., Garn H. 2009. Comparison of adjuvant and adjuvant-free murine experimental asthma models. Clin. Exp. Allergy. 39:1246–1254 10.1111/j.1365-2222.2009.03260.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debarry J., Garn H., Hanuszkiewicz A., Dickgreber N., Blümer N., von Mutius E., Bufe A., Gatermann S., Renz H., Holst O., Heine H. 2007. Acinetobacter lwoffii and Lactococcus lactis strains isolated from farm cowsheds possess strong allergy-protective properties. J. Allergy Clin. Immunol. 119:1514–1521 10.1016/j.jaci.2007.03.023 [DOI] [PubMed] [Google Scholar]
- Ege M.J., Bieli C., Frei R., van Strien R.T., Riedler J., Ublagger E., Schram-Bijkerk D., Brunekreef B., van Hage M., Scheynius A., et al. ; Parsifal Study team 2006. Prenatal farm exposure is related to the expression of receptors of the innate immunity and to atopic sensitization in school-age children. J. Allergy Clin. Immunol. 117:817–823 10.1016/j.jaci.2005.12.1307 [DOI] [PubMed] [Google Scholar]
- Entrican G. 2002. Immune regulation during pregnancy and host-pathogen interactions in infectious abortion. J. Comp. Pathol. 126:79–94 10.1053/jcpa.2001.0539 [DOI] [PubMed] [Google Scholar]
- Gon Y. 2008. Toll-like receptors and airway inflammation. Allergol. Int. 57:33–37 10.2332/allergolint.R-07-157 [DOI] [PubMed] [Google Scholar]
- Hammad H., Lambrecht B.N. 2008. Dendritic cells and epithelial cells: linking innate and adaptive immunity in asthma. Nat. Rev. Immunol. 8:193–204 10.1038/nri2275 [DOI] [PubMed] [Google Scholar]
- Harju K., Ojaniemi M., Rounioja S., Glumoff V., Paananen R., Vuolteenaho R., Hallman M. 2005. Expression of toll-like receptor 4 and endotoxin responsiveness in mice during perinatal period. Pediatr. Res. 57:644–648 10.1203/01.PDR.0000156212.03459.A9 [DOI] [PubMed] [Google Scholar]
- Hemmi H., Takeuchi O., Kawai T., Kaisho T., Sato S., Sanjo H., Matsumoto M., Hoshino K., Wagner H., Takeda K., Akira S. 2000. A Toll-like receptor recognizes bacterial DNA. Nature. 408:740–745 10.1038/35047123 [DOI] [PubMed] [Google Scholar]
- Hemmi H., Kaisho T., Takeuchi O., Sato S., Sanjo H., Hoshino K., Horiuchi T., Tomizawa H., Takeda K., Akira S. 2002. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat. Immunol. 3:196–200 10.1038/ni758 [DOI] [PubMed] [Google Scholar]
- Holgate S.T., Polosa R. 2008. Treatment strategies for allergy and asthma. Nat. Rev. Immunol. 8:218–230 10.1038/nri2262 [DOI] [PubMed] [Google Scholar]
- Honda K., Sakaguchi S., Nakajima C., Watanabe A., Yanai H., Matsumoto M., Ohteki T., Kaisho T., Takaoka A., Akira S., et al. 2003. Selective contribution of IFN-alpha/beta signaling to the maturation of dendritic cells induced by double-stranded RNA or viral infection. Proc. Natl. Acad. Sci. USA. 100:10872–10877 10.1073/pnas.1934678100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino K., Takeuchi O., Kawai T., Sanjo H., Ogawa T., Takeda Y., Takeda K., Akira S. 1999. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J. Immunol. 162:3749–3752 [PubMed] [Google Scholar]
- Kawai T., Akira S. 2009. The roles of TLRs, RLRs and NLRs in pathogen recognition. Int. Immunol. 21:317–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korthals M., Ege M.J., Tebbe C.C., von Mutius E., Bauer J. 2008. Application of PCR-SSCP for molecular epidemiological studies on the exposure of farm children to bacteria in environmental dust. J. Microbiol. Methods. 73:49–56 10.1016/j.mimet.2008.01.010 [DOI] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 25:402–408 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- Majkowska-Wojciechowska B., Pełka J., Korzon L., Kozłowska A., Kaczała M., Jarzebska M., Gwardys T., Kowalski M.L. 2007. Prevalence of allergy, patterns of allergic sensitization and allergy risk factors in rural and urban children. Allergy. 62:1044–1050 10.1111/j.1398-9995.2007.01457.x [DOI] [PubMed] [Google Scholar]
- Medzhitov R. 2007. Recognition of microorganisms and activation of the immune response. Nature. 449:819–826 10.1038/nature06246 [DOI] [PubMed] [Google Scholar]
- Midodzi W.K., Rowe B.H., Majaesic C.M., Senthilselvan A. 2007. Reduced risk of physician-diagnosed asthma among children dwelling in a farming environment. Respirology. 12:692–699 10.1111/j.1440-1843.2007.01134.x [DOI] [PubMed] [Google Scholar]
- Moffatt M.F., Kabesch M., Liang L., Dixon A.L., Strachan D., Heath S., Depner M., von Berg A., Bufe A., Rietschel E., et al. 2007. Genetic variants regulating ORMDL3 expression contribute to the risk of childhood asthma. Nature. 448:470–473 10.1038/nature06014 [DOI] [PubMed] [Google Scholar]
- Neuhaus-Steinmetz U., Glaab T., Daser A., Braun A., Lommatzsch M., Herz U., Kips J., Alarie Y., Renz H. 2000. Sequential development of airway hyperresponsiveness and acute airway obstruction in a mouse model of allergic inflammation. Int. Arch. Allergy Immunol. 121:57–67 10.1159/000024298 [DOI] [PubMed] [Google Scholar]
- Riedler J., Braun-Fahrländer C., Eder W., Schreuer M., Waser M., Maisch S., Carr D., Schierl R., Nowak D., von Mutius E.; ALEX Study Team 2001. Exposure to farming in early life and development of asthma and allergy: a cross-sectional survey. Lancet. 358:1129–1133 10.1016/S0140-6736(01)06252-3 [DOI] [PubMed] [Google Scholar]
- Rowe J., Kusel M., Holt B.J., Suriyaarachchi D., Serralha M., Hollams E., Yerkovich S.T., Subrata L.S., Ladyman C., Sadowska A., et al. 2007. Prenatal versus postnatal sensitization to environmental allergens in a high-risk birth cohort. J. Allergy Clin. Immunol. 119:1164–1173 10.1016/j.jaci.2007.02.016 [DOI] [PubMed] [Google Scholar]
- Sel S., Wegmann M., Dicke T., Sel S., Henke W., Yildirim A.O., Renz H., Garn H. 2008. Effective prevention and therapy of experimental allergic asthma using a GATA-3-specific DNAzyme. J. Allergy Clin. Immunol. 121:910–916.e5 [DOI] [PubMed] [Google Scholar]
- Spiller S., Dreher S., Meng G., Grabiec A., Thomas W., Hartung T., Pfeffer K., Hochrein H., Brade H., Bessler W., et al. 2007. Cellular recognition of trimyristoylated peptide or enterobacterial lipopolysaccharide via both TLR2 and TLR4. J. Biol. Chem. 282:13190–13198 10.1074/jbc.M610340200 [DOI] [PubMed] [Google Scholar]
- Strachan D.P. 2000. Family size, infection and atopy: the first decade of the “hygiene hypothesis”. Thorax 55(Suppl. 1):S2–S10 10.1136/thorax.55.suppl_1.S2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercelli D. 2008. Discovering susceptibility genes for asthma and allergy. Nat. Rev. Immunol. 8:169–182 10.1038/nri2257 [DOI] [PubMed] [Google Scholar]
- von Mutius E., Radon K. 2008. Living on a farm: impact on asthma induction and clinical course. Immunol. Allergy Clin. North Am. 28:631–647 10.1016/j.iac.2008.03.010 [DOI] [PubMed] [Google Scholar]
- Warner J.A., Jones C.A., Jones A.C., Warner J.O. 2000. Prenatal origins of allergic disease. J. Allergy Clin. Immunol. 105:S493–S498 10.1016/S0091-6749(00)90049-6 [DOI] [PubMed] [Google Scholar]
- Whitehead G.S., Walker J.K., Berman K.G., Foster W.M., Schwartz D.A. 2003. Allergen-induced airway disease is mouse strain dependent. Am. J. Physiol. Lung Cell. Mol. Physiol. 285:L32–L42 [DOI] [PubMed] [Google Scholar]