Abstract
The inflammatory response is one of several host alert mechanisms that recruit neutrophils from the circulation to the area of infection. We demonstrate that Bordetella, a bacterial pathogen, exploits an antiinflammatory cytokine, interleukin-10 (IL-10), to evade the host immune system. We identified a Bordetella effector, BopN, that is translocated into the host cell via the type III secretion system, where it induces enhanced production of IL-10. Interestingly, the BopN effector translocates itself into the nucleus and is involved in the down-regulation of mitogen-activated protein kinases. Using pharmacological blockade, we demonstrated that BopN-induced IL-10 production is mediated, at least in part, by its ability to block the extracellular signal-regulated kinase pathway. We also showed that BopN blocks nuclear translocation of nuclear factor κB p65 (NF-κBp65) but, in contrast, promotes nuclear translocation of NF-κBp50. A BopN-deficient strain was unable to induce IL-10 production in mice, resulting in the elimination of bacteria via neutrophil infiltration into the pulmonary alveoli. Furthermore, IL-10–deficient mice effectively eliminated wild-type as well as BopN mutant bacteria. Thus, Bordetella exploits BopN as a stealth strategy to shut off the host inflammatory reaction. These results explain the ability of Bordetella species to avoid induction of the inflammatory response.
Bordetella pertussis is a causative agent of whooping cough (pertussis) in humans (Mattoo and Cherry, 2005). Recent studies suggest that 48.5 million people suffer from pertussis per year, with as many as 295,000 deaths worldwide (Mattoo and Cherry, 2005). Although wide-scale vaccination has been performed in many countries, major concerns exist about pertussis as a reemerging infectious disease (Gzyl et al., 2001; King et al., 2001; He et al., 2003; Raguckas et al., 2007). For this reason, the identification and characterization of new virulence factors as potential protective antigens is critical for the development of more effective and longer-acting vaccines. Possible candidates for such protective antigens include the component proteins of the type III secretion system (T3SS), a protein transport device composed of two distinct portions: (1) a cylindrical basal body that spans the outer and inner membranes of the bacterium and (2) a needle-like structure that protrudes from the bacterial outer membrane and functions as an injector of bacterial proteins (called effectors) into the host cells (Galán and Wolf-Watz, 2006).
Many Gram-negative bacterial pathogens exploit T3SS to deliver effectors into host cells, thereby altering the physiological functions of the infected cells (Finlay and Cossart, 1997). T3SSs are involved in establishing disease processes, and the virulence of pathogens can be greatly reduced in T3SS-deficient strains (Abe et al., 1998). Although B. pertussis infection is highly specific to humans, B. bronchiseptica is a broad host range pathogen that causes kennel cough in dogs, atrophic rhinitis in swine, snuffles in rabbits, and bronchopneumonia in guinea pigs (Goodnow, 1980; Foley et al., 2002). B. bronchiseptica is the evolutionary progenitor of B. pertussis and B. parapertussis, and many virulence factors and effectors delivered by the T3SS are highly conserved among these three strains (Fauconnier et al., 2001). For these reasons, B. bronchiseptica has been used as a model of B. pertussis. In Bordetella, T3SS as well as adherence factors and toxins are positively regulated by a two-component regulatory system composed of BvgA and BvgS (Stibitz et al., 1989). Thus, activation of the BvgA/BvgS system triggers the bacteria to enter the virulent phase. Five type III–secreted proteins—BopB, BopC (also referred to as BteA), BopD, BopN, and Bsp22—have been identified in Bordetella (Yuk et al., 2000; Kuwae et al., 2003, 2006; Nogawa et al., 2004; Panina et al., 2005). We have demonstrated that BopB and BopD make a complex and form translocation pores on the host membrane as a conduit of effectors (Kuwae et al., 2003; Nogawa et al., 2004). Bsp22 polymerizes to form a flexible filamentous structure at the tip of the needle structure and associates with the pore component BopD (Medhekar et al., 2009). The size of the outer diameter and the shape of the Bsp22-mediated filament are similar to those of the enteropathogenic Escherichia coli EspA sheath–like structure that is thought to facilitate the ability of the bacteria to traverse the mucus layer and the glycocalyx of the intestinal epithelium (Sekiya et al., 2001). The Bsp22-derived filament may have a similar function in establishing the T3SS-dependent persistent colonization of the respiratory tract. Finally, the Bordetella BopC/BteA effector can be translocated into the host cells via the T3SS and the BopB/BopD-mediated translocation pore, and induces necrotic cell death in mammalian cell lines (Panina et al., 2005; Kuwae et al., 2006).
In vivo studies using B. bronchiseptica have demonstrated that the T3SS plays a role in the persistent bacterial colonization of the lower respiratory tract by modulating host immune responses; B. bronchiseptica infection alters DC maturation and enhances the production of the antiinflammatory cytokine IL-10 (Skinner et al., 2004; Skinner et al., 2005; Pilione and Harvill, 2006), thereby inhibiting production of proinflammatory cytokines such as IFN-γ. Furthermore, B. bronchiseptica colonization in IL-10−/− mice is significantly reduced compared with that in WT mice (Skinner et al., 2005; Pilione and Harvill, 2006). In contrast, IFN-γ−/− mice exhibit defective clearance of B. bronchiseptica compared with WT mice (Pilione and Harvill, 2006). These results suggest that Bordetella actively enhances the production of the immunosuppressive cytokine IL-10 as a survival strategy, using certain unknown type III effectors.
In this study, we have identified BopN as the effector involved in the up-regulation of IL-10. We report that the IL-10–mediated antiinflammatory response triggered by a bacterial effector is a Bordetella strategy used to escape the host immune system.
RESULTS
The BopN effector is involved in the up-regulation of IL-10
To determine whether the Bordetella type III effectors are involved in the up-regulation of IL-10, we established an in vitro model of infection based on a DC cell line (DC2.4). This model mimics DCs expressed in the lower respiratory tract and allows detection of IL-10 mRNA; the expression of IL-10 is modulated by Bordetella infection in a T3SS-dependent manner.
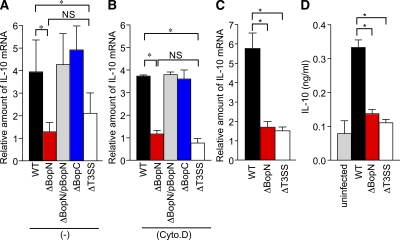
DC2.4 cells were infected with WT B. bronchiseptica or its isogenic derivatives, the BopC mutant (ΔBopC) and the BopN mutant (ΔBopN), for 60 min. The level of IL-10 mRNA was then measured by quantitative real-time PCR (qRT-PCR; Fig. 1 A). A significantly higher level of IL-10 mRNA was produced in cells infected with WT or ΔBopC compared with ΔBopN or a T3SS mutant (ΔT3SS) strain. The up-regulation of IL-10 was restored in cells infected with ΔBopN complemented with a WT bopN clone (ΔBopN/pBopN), indicating that BopN, but not BopC, is involved in IL-10 up-regulation. It is assumed that DC2.4 cells have the ability to uptake bacteria and the resulting phagocytosis event triggers various host-cell signaling pathways. To investigate the involvement of phagocytosis in IL-10 up-regulation, we treated DC2.4 cells with cytochalasin D, a fungal metabolite that inhibits phagocytosis (Fig. 1 B). The level of IL-10 mRNA in DC2.4 cells infected with WT or ΔBopN was not affected by treatment with cytochalasin D. Although the level of IL-10 mRNA in DC2.4 cells infected with ΔT3SS appears to be reduced in the presence of cytochalasin D, no significant difference in the induction of IL-10 was found between ΔBopN and ΔT3SS in the presence or absence of cytochalasin D. Similar results were obtained using Bordetella infection of BM-derived DCs (BMDCs), and the level of IL-10 mRNA was significantly reduced after infection with ΔBopN as well as ΔT3SS relative to WT (Fig. 1 C). We also determined the amount of IL-10 by ELISA (Fig. 1 D), and IL-10 production was significantly reduced after infection with ΔBopN as well as ΔT3SS relative to WT. Collectively, these results suggest that the IL-10 up-regulation depends on BopN function and is independent of phagocytosis.
Figure 1.
BopN is involved in the up-regulation of IL-10. (A and B) DC2.4 cells were cultured in the absence (A) or presence (B) of 1 µM cytochalasin D (Cyto.D) for 30 min, and then infected with B. bronchiseptica WT, BopN effector mutant (ΔBopN), ΔBopN mutant complemented with a WT bopN clone (ΔBopN/pBopN), BopC effector mutant (ΔBopC), or T3SS mutant (ΔT3SS). After 60 min, total RNA was prepared and levels of IL-10 mRNA were assessed by real-time PCR using the comparative cycle threshold method. (C) BMDCs were infected with the indicated strains. After 60 min, total RNA was prepared and amounts of IL-10 mRNAs were assessed by real-time PCR. The values in A–C were normalized to the internal control β-actin and calculated in arbitrary units set to a value of 1 for uninfected cells. (D) BMDCs were infected with the indicated strains. After 60 min, the amount of IL-10 in the culture medium was determined by ELISA. The values are means ± SE from three independent experiments. *, P < 0.05.
BopN is an essential virulence factor
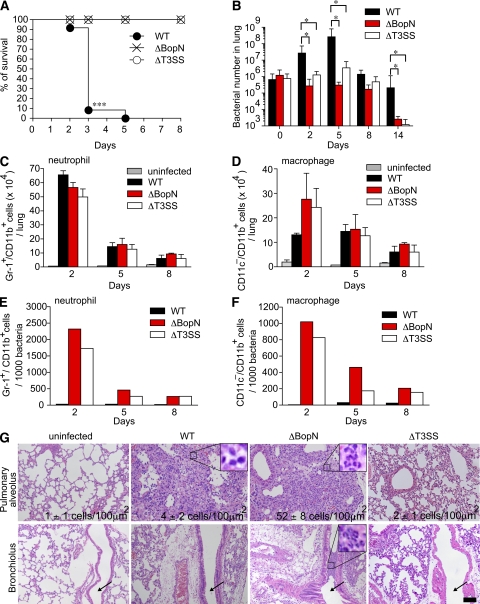
Our in vitro study demonstrated that Bordetella BopN activates the expression of the antiinflammatory cytokine IL-10. This suggested that Bordetella may establish persistent colonization and exert full virulence by perturbation of the inflammatory response. Therefore, we investigated BopN function in vivo. C57BL/6J mice were infected intranasally with WT or ΔBopN B. bronchiseptica (Fig. 2, A and B). Although all mice infected with WT (5 × 106 bacteria) succumbed by day 5 (Fig. 2 A), all mice infected with ΔBopN or ΔT3SS survived, indicating that the BopN effector is an essential virulence factor. Next, we used a lower dose (5 × 105 bacteria) to mimic persistent colonization by Bordetella (Fig. 2 B). Upon infection with WT, the bacterial number in the lung continued to increase for 5 d, and WT bacteria appeared to establish persistent colonization. In contrast, the number of bacteria in the lung did not increase upon infection with either ΔBopN or ΔT3SS and was significantly decreased after 14 d, indicating that BopN is involved in persistent colonization.
Figure 2.
BopN is required for disease process. (A) C57BL/6J mice (n = 27 for each group) were infected intranasally with 5 × 106 B. bronchiseptica WT, ΔbopN, or ΔT3SS, and the survival was monitored for 8 d after infection. ***, P < 0.0001 using the log-rank test. (B) Mice (n = 15 for each group) infected intranasally with the specified strains (5 × 105 bacteria) were sacrificed at the indicated days after infection. Lung specimens were homogenized and plated on BG agar plates. Colonies were counted to determine the number of colonized bacteria per lung. The values are means ± SD from three independent experiments. *, P < 0.05. (C and D) Mice (n = 9 for each group) infected intranasally with the indicated strains (5 × 105 bacteria) were sacrificed, and cells were isolated from the whole lung. The total numbers of neutrophils (C) or macrophages (D) in the lung were determined by FACS analysis. The values are means ± SE from three independent experiments. *, P < 0.05. (E and F) The total number of neutrophils or macrophages in the lung obtained from C or D, respectively, was normalized by the bacterial number in the lung obtained from B. The values are means ± SE from three independent experiments. (G) 2 d after infection (5 × 105 bacteria), lung sections were fixed and stained with H&E. The infiltration of neutrophils and macrophages into the pulmonary alveoli and peribronchiolar edema formation are observed in mice infected with WT, ΔbopN, and ΔT3SS. Arrows indicate bronchioles. The number of neutrophils per 100 µm2 was determined in three randomly obtained images of pulmonary alveoli. The area of the enlarged boxes corresponds to 8 µm2. Bar, 100 µm.
Because neutrophils and macrophages are involved in the elimination of bacteria at an early phase of infection, cells from the lungs of mice infected with Bordetella were isolated and analyzed by FACS with anti–Gr-1, anti-CD11b, and anti-CD11c antibodies (Fig. 2, C and D). The lungs of mice infected with WT, ΔBopN, and ΔT3SS contained a large number of Gr-1+/CD11b+ cells (neutrophils) at 2 d after infection, but the neutrophil number was greatly reduced by 5 and 8 d (Fig. 2 C). In contrast, at 2 d after infection, the total number of CD11c−/CD11b+ cells (macrophages) in WT-infected lungs was lower than the number of macrophages in lungs infected with ΔBopN or ΔT3SS (Fig. 2 D). By 5 and 8 d after infection, the numbers of macrophages in the lungs were approximately equal regardless of the strain of Bordetella. To further investigate the relative neutrophil or macrophage number involved in bacterial elimination, the total numbers of neutrophils (Fig. 2 C) or macrophages (Fig. 2 D) in the lung were normalized by the bacterial number obtained in Fig. 2 B. The relative number of neutrophils or macrophages in the lung was very different between WT versus ΔBopN or ΔT3SS infection (Fig. 2, E and F).
Lung sections were obtained from mice 2 d after infection with B. bronchiseptica WT, ΔbopN, and ΔT3SS (5 × 105 bacteria), and stained with hematoxylin and eosin (H&E; Fig. 2 G). Histological analysis showed edema formation and fibrin deposition in the pulmonary alveoli only in mice infected with the WT strain (Fig. S1). Peribronchiolar edema formation (unpublished data) and infiltration of neutrophils and macrophages into the pulmonary alveoli were observed in mice infected with WT, ΔbopN, and ΔT3SS. However, the degree of neutrophil infiltration into the pulmonary alveoli after ΔBopN infection was significantly higher than the infiltration after WT or ΔT3SS infection (WT, 4 ± 2 cells/100 µm2; ΔbopN, 52 ± 8 cells/100 µm2; ΔT3SS, 2 ± 1 cells/100 µm2; and uninfected, 1 ± 1 cells/100 µm2; Fig. 2 G). A small number of neutrophils was observed in the bronchiolus upon WT infection, whereas a large number of aggregated neutrophils was observed in the bronchiolus upon ΔBopN infection. In contrast, mice infected with ΔT3SS had the appearance of uninfected controls with no histological lesions. These results suggest that BopN counteracts the host inflammatory responses by suppressing neutrophil infiltration into the pulmonary alveoli and bronchiolus, even though the total number of neutrophils in the lung was similar between WT and ΔBopN infection (Fig. 2 C).
Bordetella alters the tracheal microenvironment
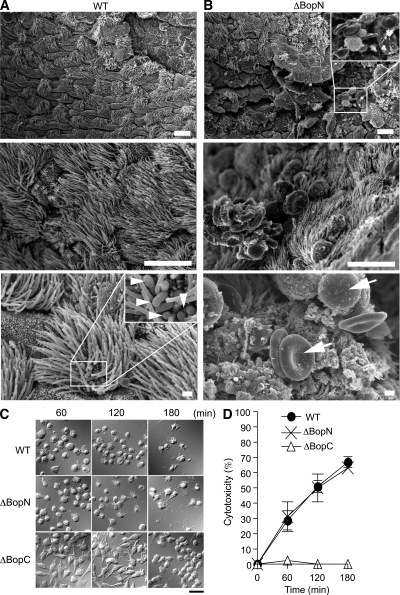
To determine the mechanism by which BopN contributes to bacterial colonization in the trachea, mice infected with WT or ΔBopN B. bronchiseptica were sacrificed 2 d after infection, and tracheal sections were examined by scanning electron microscopy (Fig. 3, A and B). Upon WT B. bronchiseptica infection, bacteria were detected in the trachea (Fig. 3 A, arrowheads) but neither cell-surface damage nor areas of damaged cilia were observed. To our surprise, even though Bordetella virulence was greatly reduced upon loss of BopN function (Fig. 2), we observed cell-surface disruption, an increased frequency of unciliated cells, and infiltration of inflammatory cells and erythrocytes upon ΔBopN infection (Fig. 3 B, arrows). To eliminate the possibility that BopN is involved in the cytotoxic phenotype, DC2.4 cells were infected with Bordetella and examined under a light microscope (Fig. 3 C). In addition, we measured the release of lactate dehydrogenase (LDH) into the extracellular medium from the infected cells (Fig. 3 D). ΔBopC and ΔT3SS (unpublished data) elicited no cytotoxicity in the infected DC2.4 cells. In contrast, infection with WT or ΔBopN produced similar cytotoxic effects, indicating that BopN is not involved in the cytotoxic phenotype. These results are consistent with the hypothesis that BopN functions as an immunosuppressive modulator to down-regulate the host inflammatory responses. Thus, Bordetella has the ability to alter the tracheal microenvironment for its own benefit by exploitation of BopN.
Figure 3.
BopN suppresses inflammatory responses at the bacterial-colonized area. (A and B) Scanning electron micrographs of mouse tracheas infected with WT (A) and ΔBopN Bordetella (B). C57BL/6J mice were infected intranasally with 5 × 106 WT and ΔBopN B. bronchiseptica, and tracheal sections were obtained 2 d after infection. Arrowheads indicate bacteria. Arrows indicate erythrocytes and inflammatory cells. Note that extensive cell-surface disruption, including increased unciliated cells as well as infiltration of inflammatory cells and erythrocytes, is observed in mice infected with ΔBopN but not WT. The areas of the enlarged boxes correspond to 20 µm2 (top) and 4 µm2 (bottom). Bars: (top and middle) 10 µm; (bottom) 1 µm. (C) DC2.4 cells were infected with the indicated strains (m.o.i. = 10) for 60, 120, and 180 min and then analyzed with Nomarski imaging. Both WT and ΔBopN induced significant morphological changes, including rounding and detachment, in the cells. Bar, 20 µm. (D) Time course of the release of LDH into the extracellular medium from DC2.4 cells infected with the indicated strains. The values are the percentages of LDH released from Triton X-100–lysed DC2.4 cells after subtraction of the value measured in uninfected cells. Error bars represent means ± SE from triplicate experiments. The amounts of LDH released after ΔBopN infection were similar to those released after WT infection.
Up-regulation of IL-10 benefits Bordetella
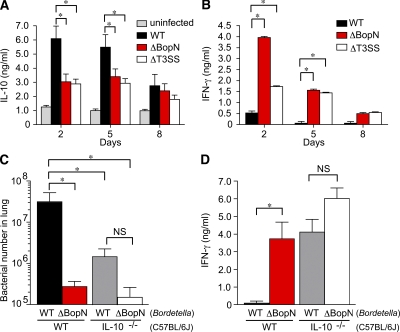
We have clearly demonstrated that BopN is involved in the up-regulation of IL-10 in infected DC2.4 cells (Fig. 1) and that this effector is an essential virulence factor that subverts the host immune system (Fig. 2 and Fig. 3). To further investigate the role of BopN in vivo, mice were infected intranasally with WT B. bronchiseptica or a mutant strain, and the amounts of the cytokine produced were determined by ELISA on homogenized lung specimens (Fig. 4, A and B). The production of the antiinflammatory cytokine IL-10 in the lungs of mice infected with WT was significantly higher than that in the lungs of mice infected with ΔBopN or ΔT3SS on day 2 or 5 (Fig. 4 A). In contrast, the production of the inflammatory cytokine IFN-γ in mice infected with WT was significantly lower than that of mice infected with ΔBopN or ΔT3SS (Fig. 4 B). These results clearly indicate that BopN is involved in the up-regulation of IL-10 production in vivo, thereby leading to the inhibition of IFN-γ production.
Figure 4.
BopN induces IL-10 production in vivo. (A and B) Detection of IL-10 (A) or IFN-γ (B) production in Bordetella-infected mice is shown. C57BL/6J mice (n = 9 for each group) were infected intranasally with 5 × 105 B. bronchiseptica. Homogenized lung specimens were prepared from mice infected with the indicated strains at 2, 5, or 8 d after infection, and the amounts of cytokines in the specimens were determined by ELISA. (C) C57BL/6J (WT) or IL-10−/− mice (n = 3 for each group) on a C57BL/6J background were infected intranasally with the indicated strains (5 × 105 bacteria). 2 d after infection, lung specimens were homogenized and plated on BG agar plates to detect the number of colonizing bacteria. (D) IFN-γ production in the lungs of Bordetella-infected mice (n = 3 for each group). Homogenized lung specimens were prepared and the amounts of cytokines were determined by ELISA. The values are means ± SE from three independent experiments. *, P < 0.05.
To further investigate whether the major biological target of BopN in the pathophysiology of Bordetella infection is the up-regulation of IL-10, WT and IL-10−/− mice were infected intranasally with WT or ΔBopN B. bronchiseptica. As expected (Skinner et al., 2005), WT Bordetella colonization in IL-10−/− mice was significantly decreased compared with that in C57BL/6J mice 2 d after infection (Fig. 4 C). Importantly, although the colonization by WT Bordetella was significantly higher than that of ΔBopN in C57BL/6J mice, no significant difference was found between WT and ΔBopN colonization in IL-10−/− mice (Fig. 4 C). In addition, although the production of IFN-γ in the lungs of C57BL/6J mice infected with WT Bordetella was significantly lower than that of mice infected with ΔBopN, no significant difference was found comparing WT and ΔBopN infection in IL-10−/− mice; large amounts of IFN-γ were produced in the lungs of IL-10−/− mice upon infection with either WT or ΔBopN (Fig. 4 D). This genetic evidence indicates that the major in vivo target of BopN is indeed the regulation of IL-10 production and that BopN exploits IL-10 to establish persistent colonization during Bordetella infection.
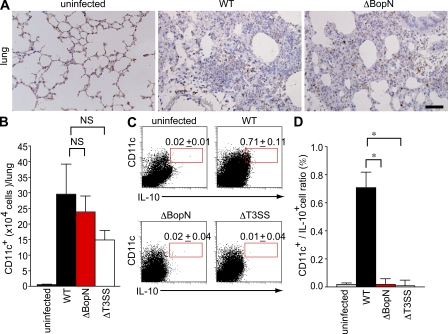
CD11c+ cells are recruited into pulmonary alveoli during Bordetella infection
IL-10 production by DCs and macrophages is essential for the control of excessive inflammatory responses. Our study demonstrated that the disease process of Bordetella infection involves the function of BopN in IL-10 up-regulation. To further confirm whether Bordetella exploits IL-10–producing cells during infection, we performed immunostaining on lung sections obtained from mice infected with WT or ΔBopN Bordetella. Immunostaining for CD11c, one of the main surface antigens present in DCs or macrophages (Fig. 5 A), showed extensive recruitment of CD11c+ cells into the pulmonary alveoli of WT Bordetella-infected lung tissues. A similar observation was obtained using ΔBopN-infected tissue, suggesting that the absence of BopN does not affect CD11c+ cell recruitment to the lung. We also determined the number of CD11c+ cells in the lung by FACS analysis (Fig. 5 B). Again, the total number of CD11c+ cells in the lung of mice infected with WT was similar to that in the lung of mice infected with ΔBopN (Fig. 5 B). However, the percentage of IL-10+ CD11c+ cells in the lung upon WT infection was significantly higher than that in the lung upon ΔBopN infection (Fig. 5, C and D). Collectively, these results indicate that Bordetella allows recruitment of CD11c+ cells during infection, thus leading to the up-regulation of IL-10 production by BopN.
Figure 5.
CD11c+ cells migrate into the lung and produce IL-10 during Bordetella infection. (A) C57BL/6J mice were infected intranasally with 5 × 105 WT or ΔBopN B. bronchiseptica. 2 d after infection, lung sections were immunostained with anti-CD11c monoclonal antibodies (brown). Note that extensive recruitment of CD11c+ cells into the pulmonary alveoli was observed in mice infected with WT and ΔBopN. Bar, 50 µm. (B) 2 d after infection, the total numbers of CD11c+ cells in the lungs of mice (n = 3 for each group) infected with the indicated strains were determined by FACS analysis. (C) The lung cells from mice (n = 3 for each group) infected with Bordetella were pooled, stained with FITC-conjugated anti–IL-10 and PE-conjugated anti-CD11c, and determined by FACS analysis (percentages are shown). (D) The histogram shows the results obtained in C expressed as a ratio of IL-10+/CD11c+ cells. The values in B and D are means ± SE from three independent experiments. *, P < 0.05.
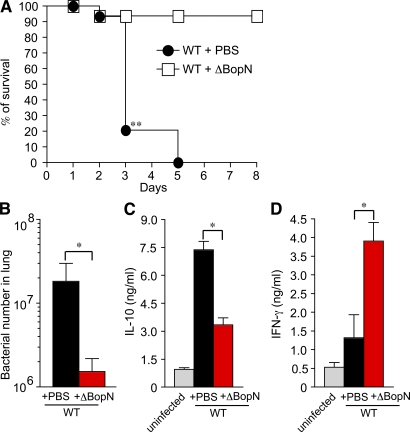
Preinfection with ΔBopN allows mice to survive a lethal dose of Bordetella
Our in vitro and in vivo data led us to hypothesize that if mice were pretreated with ΔBopN, which allows strong host inflammatory responses, they might be able to survive a subsequent lethal dose of Bordetella WT bacteria. Hence, we performed a successive infection study (Fig. 6). C57BL/6J mice were infected intranasally with ΔBopN (5 × 105 bacteria) or PBS (mock preinfection) 24 h before WT Bordetella infection (9 × 105 bacteria). As shown in Fig. 6 A, although all mice with mock preinfection were killed by a lethal dose of WT bacteria, 24 out of 27 mice preinfected with ΔBopN survived the WT infection. The in vivo successive infection study clearly demonstrates that preinduction of host inflammatory responses by an avirulent ΔBopN strain contributes to survival of the mice upon a lethal dose of WT Bordetella. Indeed, the number of bacteria in the lung was greatly decreased by the ΔBopN preinfection (Fig. 6 B). Furthermore, the up-regulation of IL-10 production by WT Bordetella was disturbed by preinfection with ΔBopN (Fig. 6 C), and the production of IFN-γ in mice preinfected with ΔBopN was significantly higher than that of mice undergoing mock preinfection (Fig. 6 D). Collectively, these results confirm that the up-regulation of IL-10 by the BopN effector, resulting in the suppression of IFN-γ signaling, is a significant stealth strategy by which Bordetella evades the host immune system.
Figure 6.
In vivo successive infection study. (A) C57BL/6J mice (n = 27 for WT + PBS and WT + ΔBopN) were infected intranasally with 5 × 105 ΔBopN or PBS (mock infection) 24 h before infection with a lethal dose of WT Bordetella (9 × 105), and the survival was monitored for 8 d. **, P < 0.005 using the log-rank test. (B) 2 d after the WT lethal-dose infection, the lung specimens of mice (n = 3 for WT + PBS preinfection and WT + ΔBopN preinfection) were homogenized and plated on BG agar plates to detect the number of colonized bacteria. (C and D) 2 d after the WT lethal-dose infection, homogenized lung specimens were prepared from mice (n = 3 for WT + PBS and WT + ΔBopN) infected with the indicated strains, and the amount of IL-10 (C) or IFN-γ (D) in the specimen was determined by ELISA. The values in B–D are means ± SD from three independent experiments. *, P < 0.05.
BopN regulates the mitogen-activated protein kinase (MAPK) signaling pathways
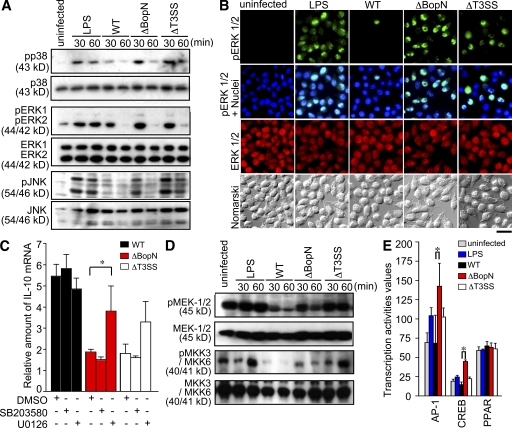
Infection with B. bronchiseptica triggers the down-regulation (dephosphorylation) of MAPKs (Reissinger et al., 2005) in a T3SS-dependent manner. To examine whether the BopN effector is involved in MAPK signaling alterations, we assessed MAPK signaling in DC2.4 cells infected with Bordetella by immunoblot analysis using phosphospecific anti-p38, anti–extracellular signal-regulated kinase (ERK) 1 and –ERK2, or anti–c-Jun N-terminal kinase (JNK) antibodies (Fig. 7 A). Band intensities of the phosphorylated and total MAPK products were quantified using the ImageJ application (Fig. S2 A). At 30 min after infection, the amounts of phospho-p38 in DC2.4 cells infected with WT were significantly lower than those in DC2.4 cells infected with ΔBopN or ΔT3SS. Similarly, the level of phosphorylated (active) ERK2 and JNK in DC2.4 cells infected with WT was lower than that in ΔBopN or ΔT3SS (Fig. 7 A and Fig. S2 A). To further confirm the down-regulation of MAPK signaling by BopN, we assessed the subcellular localization of ERK after Bordetella infection and compared the localization of total ERK with that of active phosphorylated ERK (Fig. 7 B). The specificity of phospho-ERK antibodies was also confirmed by pretreatment of cells with a specific MEK1/2 inhibitor, U0126 (Fig. S2 B). In addition, we determined the percentages of cells showing phosphorylated ERK (Fig. S2 C). The number of cells showing phosphorylated ERK was significantly decreased in WT infection relative to ΔBopN infection. These results clearly indicate that BopN is involved in the down-regulation of MAPKs. To examine the relationship between the down-regulation of MAPKs and IL-10 production, MAPK inhibitors were used (Fig. 7 C). Inhibition of the p38 pathway with a specific inhibitor, SB203580, did not greatly affect the level of IL-10 mRNA expression. However, the level of IL-10 mRNA was significantly increased in DC2.4 cells infected with ΔBopN when DCs were treated with the specific MEK1/2 inhibitor U0126 (Fig. 7 C). We assessed the activity of the MEK1/2 and MKK3/MKK6 signaling by immunoblotting (Fig. 7 D). The amounts of phospho-MEK1/2 and phospho-MKK3/MKK6 in DC2.4 cells infected with WT were lower than those in DC2.4 cells infected with ΔBopN or ΔT3SS (Fig. 7 D).
Figure 7.
Molecular characterization of the function of BopN in MAPK pathways. (A) Immunoblot analysis of MAPK activities in the lysates of DC2.4 cells. DC2.4 cells were stimulated with 10 µg/ml LPS or infected with the indicated Bordetella strains for the specified time periods. The lysates were analyzed by Western blotting with antibodies against phospho-p38 (top), phospho-ERK1/2 (third from top), and phospho-JNK (fifth from top). The membranes were stripped and reblotted with antibodies against p38 (second panel), ERK1/2 (fourth from top), and JNK (bottom). (B) Immunofluorescence microscopy of DC2.4 cells stimulated with LPS for 30 min or infected for 30 min with the indicated strains. DC2.4 cells were fixed and stained with anti–phospho-ERK1/2 (green), anti-ERK1/2 (red), and DAPI (blue). Data are representative of three independent experiments. Bar, 20 µm. (C) 10 µM of MEK1/2 inhibitor U0126, 50 µM of p38 kinase inhibitor SB203580, and vehicle control (DMSO) were added to DC2.4 culture medium for 1 h before infection (m.o.i. = 100) with the indicated strains. Total RNA was prepared from the treated cells, and the amounts of IL-10 mRNA produced were assessed by qRT-PCR. (D) DC2.4 cells were infected with the indicated strains (m.o.i. = 100) for the specified time periods, and the amounts of phospho-MEK1/2 and phospho-MKK3/MKK6 were analyzed using immunoblotting. Three independent experiments were performed and a representative immunoblot is shown. (E) DC2.4 cells were stimulated with 10 µg/ml LPS or infected with the indicated strains for 30 min, and nuclear fractions were prepared and analyzed using a Multiplex transcription factor profiling kit. Differences in transcription factor activation were determined by Bio-Plex. The values in C and E are means ± SE from three independent experiments. *, P < 0.05.
To characterize the transcription factors affected by BopN, nuclear extracts were prepared from DC2.4 cells infected with WT, ΔbopN, and ΔT3SS Bordetella, and transcription factor activities were analyzed using a Multiplex transcription factor profiling kit. The level of AP-1 and CREB activation in DC2.4 cells was significantly reduced after infection with WT relative to ΔBopN infection (Fig. 7 E). These observations were consistent with the fact that MAPK signaling is down-regulated by the function of BopN, because both AP-1 and CREB transcription factors are downstream of MAPKs. Collectively, BopN down-regulates both MAPK kinase and MAPK signaling pathways.
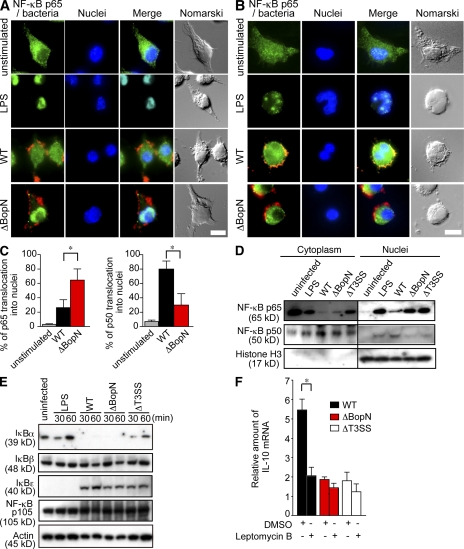
BopN regulates the NF-κB signaling pathways
It has also been reported that NF-κB pathways are modulated during B. bronchiseptica infection (Yuk et al., 2000). Therefore, the translocation of NF-κB into the nucleus was investigated using DC2.4 cells infected with Bordetella. DC2.4 cells were infected with WT or ΔBopN for 20 min and then fixed and permeabilized. NF-κB translocation was analyzed by immunofluorescence microscopy using anti–NF-κBp65 or anti–NF-κBp50 antibodies (Fig. 8, A and B). As expected (Yuk et al., 2000), the translocation of NF-κBp65 into nuclei was inhibited by WT B. bronchiseptica infection (Fig. 8 A). In contrast, the nuclear translocation of NF-κBp65 was intact in the absence of BopN (Fig. 8 A, ΔBopN). The difference in NF-κBp65 translocation into nuclei between WT and ΔBopN infection was statistically significant (Fig. 8 C). Conversely, the nuclear translocation of NF-κBp50 observed after WT infection was significantly blocked in the absence of BopN (Fig. 8, B and C). To further confirm the subcellular distribution of NF-κB, DC2.4 cells infected with Bordetella for 30 min were separated into nuclear and cytosolic fractions. The resulting fractions were subjected to immunoblot analysis using anti–NF-κBp65 and anti–NF-κBp50 antibodies (Fig. 8 D). NF-κBp65 translocation into nuclei was greatly inhibited by WT Bordetella infection. In contrast, NF-κBp65 was detected in the nuclear fraction of DC2.4 cells infected with ΔBopN or ΔT3SS. Conversely, the nuclear translocation of NF-κBp50 observed after WT infection was largely blocked in the absence of BopN (Fig. 8 D). Thus, BopN participates in altering the nuclear compartmentalization of NF-κB p65 and p50 subunits.
Figure 8.
Molecular characterization of the mechanism by which BopN affects NF-κB pathways. (A and B) Immunofluorescence microscopy of DC2.4 cells 30 min after stimulation with 10 µg/ml LPS or infection with WT or ΔBopN Bordetella (m.o.i. = 100). The cells were stained with anti–B. bronchiseptica antibodies (red), anti–NF-κBp65 (A, green), or NF-κBp50 (B, green) antibodies, and DAPI for nuclei (blue). Bars, 10 µm. (C) DC2.4 cells infected with WT or ΔBopN Bordetella were randomly picked from the immunofluorescence micrographs (A and B), and the percentages of cells showing nuclear translocation of NF-κBp65 (left) or NF-κBp50 (right) were determined. Percentages were based on a count of 50 cells, and the values are means ± SD from three independent experiments. *, P < 0.05. (D) DC2.4 cells were stimulated by 10 µg/ml LPS or infected with the indicated strains for 30 min, and then separated into nuclear and cytosolic fractions. The resulting fractions were subjected to immunoblot analyses using anti–NF-κBp65 and anti–NF-κBp50 antibodies. Histone H3 antibodies were used as a nuclear control. The majority of both p65 and p50 was localized in the cytoplasm (A, B, and D) of unstimulated cells. (E) Immunoblot analysis of IκBα, IκBβ, IκBϵ, or NF-κBp105 in the lysates of DC2.4 cells. DC2.4 cells were stimulated with 10 µg/ml LPS or infected with the indicated strains, and total cell lysates were analyzed using immunoblotting with anti-IκBα, anti-IκBβ, anti-IκBϵ, or anti–NF-κBp105 antibodies. (F) 20 nM leptomycin B, an inhibitor of nuclear export, or vehicle control (DMSO) was added to DC2.4 culture medium for 24 h before infection with the indicated strains. Total RNA was prepared from DC2.4 cells, and amounts of IL-10 mRNA were assessed by qRT-PCR. The values are means ± SE from three independent experiments. *, P < 0.05.
Inhibition of inhibitor of NF-κB (IκB) degradation by a bacterial effector is one bacterial strategy to prevent NF-κB translocation into nuclei (see Discussion). To determine if BopN is involved in the inhibition of IκB degradation, DC2.4 cells were stimulated with 10 µg/ml LPS or infected with WT, ΔBopN, and ΔT3SS B. bronchiseptica, and the cell lysates were analyzed using immunoblotting with anti-IκBα, anti-IκBβ, anti-IκBϵ, and anti–NF-κBp105 antibodies (Fig. 8 E). Degradation of IκBα was detected in DC2.4 cells infected with WT and ΔBopN B. bronchiseptica. In contrast, degradation of IκBβ, IκBϵ, and NF-κBp105 was not detected during Bordetella infection. Thus, IκBα degradation is triggered by Bordetella infection, but this event is independent of BopN function.
To investigate whether the nuclear export of proteins, such as NF-κB, is involved in Bordetella infection–induced IL-10 production, DC2.4 cells were treated with leptomycin B, an inhibitor of nuclear export, and then infected with WT or ΔBopN (Fig. 8 F). The enhanced production of IL-10 in DC2.4 cells infected with WT Bordetella was significantly reduced in the presence of leptomycin B. Immunofluorescence microscopy analyses showed that NF-κBp65 could be detected in the nuclei of DC2.4 cells infected with WT Bordetella in the presence of leptomycin B (unpublished data). Conversely, the amounts of nuclear NF-κBp50 in DC2.4 cells infected with WT Bordetella were somewhat reduced in the presence of leptomycin B. In contrast to WT infection, localization of neither NF-κB p65 nor p50 in DC2.4 cells infected with ΔBopN was affected by leptomycin B (unpublished data). Thus, altered NF-κB translocation may contribute to the effects of BopN on the up-regulation of IL-10.
BopN alone modulates nuclear NF-κB subunit compartmentalization and down-regulates MAPK signaling
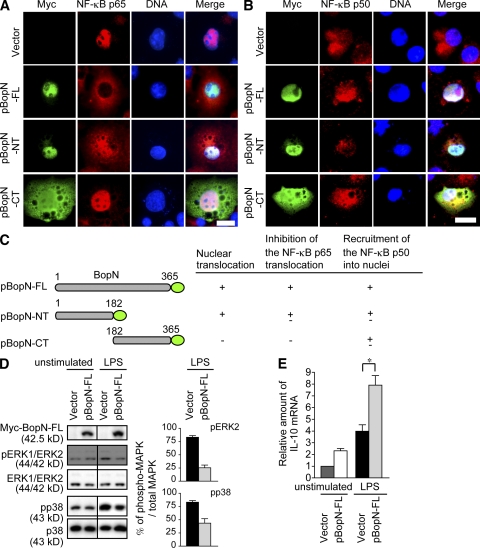
To analyze the precise function of BopN in the process of NF-κB nuclear translocation, we constructed expression vectors for the production of BopN fused with a Myc-tag, BopN full length (pBopN-FL; aa 1–365), BopN N-terminal half (pBopN-NT; aa 1–182), and BopN C-terminal half (pBopN-CT; aa 182–365), and the resulting constructs were introduced into Cos7 cells (Fig. 9, A–C). Interestingly, the N-terminal half (aa 1–182) of BopN was sufficient for the translocation of BopN into the nucleus, although the C-terminal half was required to completely block NF-κBp65 nuclear translocation. In contrast to NF-κBp65, most of the NF-κBp50 was localized in the cytosol of control Cos7 cells (vector alone), but translocation of NF-κBp50 into nuclei was facilitated by introduction of pBopN-FL (Fig. 9, A–C). Similar observations were obtained upon introduction of pBopN-FL into a macrophage cell line, RAW264.7, and nuclear translocation of BopN was detected in the presence or absence of LPS (Fig. S3). Collectively, our results show that BopN has the ability to translocate itself into the nucleus and promotes nuclear translocation of NF-κBp50.
Figure 9.
BopN alters the nuclear compartmentalization of NF-κB. (A and B) A mammalian expression vector, pcDNA3.1/His(-)A, encoding the full-length BopN-FL (aa 1–365) or truncated versions of BopN (-NT, aa 1–182; -CT, aa 182–365) with a Myc-tag fused at its C terminus was introduced into Cos7 cells. 24 h after transfection, cells were stimulated with 10 µg/ml LPS for 10 min, and then fixed and stained with anti-Myc antibodies to detect BopN (green), anti–NF-κBp65 (A, red), or NF-κBp50 (B, red), and DAPI for nuclei (blue). Bars, 20 µm. (C) Schematic diagrams of BopN fusion constructs with a Myc-tag (green) used in translocation assays (A and B). aa numbers of full-length and truncated BopN are shown. (D) The pBopN-FL was introduced into RAW264.7 cells. After LPS stimulation for 30 min, BopN-Myc fusion protein and phospho-MAPKs were detected by immunoblot analysis (left). Band intensity of phospho-ERK2 and phospho-p38 were quantified by the ImageJ application (right). The percentages of phospho-MAPK/total MAPK were determined from three independent experiments. (E) The level of IL-10 mRNA in RAW264.7 cells transfected with pBopN-FL was measured by qRT-PCR. The values presented for D and E are means ± SE from three independent experiments. *, P < 0.05.
To further investigate whether BopN is directly involved in the down-regulation of MAPK signaling, pBopN-FL was introduced into RAW264.7 cells and MAPK activity was analyzed by immunoblotting (Fig. 9 D). The amounts of phospho-ERK2 and phospho-p38 in RAW264.7 were increased by LPS stimulation. However, the activation (phosphorylation) of both MAPKs was reduced by the introduction of pBopN-FL into RAW264.7 cells. Interestingly, the level of IL-10 mRNA was significantly increased by introduction of pBopN-FL into RAW264.7 cells (Fig. 9 E). These results clearly indicate that BopN alone has an ability to induce IL-10 production by down-regulation of the MAPK signaling pathways. To eliminate the possibility that the enhanced production of IL-10 down-regulates MAPKs, IL-10−/−–BMDCs were infected with WT or ΔBopN Bordetella and alterations in MAPKs were detected with immunoblotting (Fig. S4). During Bordetella infection, the levels of active or inactive MAPKs in IL-10−/−–BMDCs were similar to those in WT-BMDCs, and the down-regulation of MAPK signaling by BopN also occurred in the absence of IL-10. These results suggest that altered MAPK activation can be attributed to BopN function and not to the enhanced production of IL-10.
DISCUSSION
BopN has long been thought to be a regulator for the Bordetella T3SS, because BopN shows weak homology (19%) to Yersinia YopN, which functions as a regulator to block secretion of Yop effectors (Forsberg et al., 1991). Secretion of Yop effectors via the T3SS is induced by contact between the bacterium and the host cell. In vitro, Yop secretion is dependent on calcium, being blocked in the presence and induced in the absence of calcium (Michiels et al., 1990). The block of Yop secretion in the presence of calcium requires a functional YopN; YopN deficiency results in constitutive secretion of Yop proteins in the presence or absence of calcium and before contact with the host cell (Forsberg et al., 1991). In this study, we demonstrated that BopN is an effector protein but not a regulator of other Bop proteins, and that the BopN effector translocates itself into the nuclei of host cells and triggers the up-regulation of IL-10. This alters the microenvironment and allows Bordatella to escape from the host immune system. We also have confirmed that the Bordetella BopN-deficient strain does not affect secretion of other type III–secreted proteins during in vitro growth (unpublished data).
In B. bronchiseptica, the gene encoding BopN is located within the Bsc T3SS locus, and BopN in B. bronchiseptica shows extensive identity (99%) to the BopN of B. pertussis Tohama I and B. parapertussis 12822, suggesting that the function of BopN is probably equivalent throughout the Bordetella species. To our knowledge, two effectors, BopC and BopN (this study), have been identified and characterized in B. bronchiseptica. The BopC effector induces necrotic cell death in infected cultured cells (Panina et al., 2005; Kuwae et al., 2006). However, as shown in Fig. 3 A, no histopathological lesions, including disruption of ciliated cells, were detected in mice infected with WT B. bronchiseptica (BopC-positive phenotype), suggesting that the respiratory tract is not a BopC target.
To evade the host immune system, certain bacterial pathogens have evolved strategies to subvert activation of host signaling pathways. For example, Shigella delivers OspF into the host cell via T3SS. This effector is translocated into the nucleus and dephosphorylates MAPKs, thereby preventing phosphorylation of histone H3 at Ser10 (Arbibe et al., 2007). Thus, epigenetic modification by the OspF effector results in inhibition of NF-κB chromatin access leading to the inactivation of IL-8 and other genes essential for innate immune responses (Thomson et al., 1999; Saccani et al., 2002; Arbibe et al., 2007). Recently, OspF was characterized as a phosphothreonine lyase, which removes phosphate groups from the phosphothreonine residues of MAPKs (Li et al., 2007). However, BopN has no sequence similarity to OspF and does not contain His-Cys-X5-Arg-Ser/Thr, a catalytic active site for specific phosphatases (Zhang, 2002). The precise mechanism by which MAPK is inactivated is unclear, but this signaling pathway is a target of BopN function (Fig. 7 and Fig. 9).
We have shown that Bordatella evades the host immune system and establishes persistent colonization via induction of IL-10. In general, ERK activation is required for host cells to produce high IL-10. However, our results clearly indicate that blockade of the ERK pathway by the specific MEK inhibitor U0126 allowed enhanced production of IL-10 in DC2.4 cells infected with ΔBopN (Fig. 7 C). In contrast, pharmacological blockade of p38 activation does not affect IL-10 induction. Although the functional significance of altered JNK activation is still unclear, these results suggest that BopN regulation of IL-10 is mediated, at least in part, by its ability to modulate ERK activation.
The transcription factor NF-κB, which consists of homo- and heterodimers of the p50, p52, p65 (also called RelA), RelB, and c-Rel subunits, plays a central role in regulating host immune and inflammatory responses (Baldwin, 1996; Ghosh et al., 1998; Karin and Ben-Neriah, 2000). In unstimulated cells, NF-κB is associated with members of the IκB protein family in the cytoplasm as an inactive form. When a bacterial pathogen enters the host, various stimuli are detected by Toll-like receptors (TLRs) and nucleotide-binding oligomerization domain protein–like receptors. These signaling pathways trigger activation of the IκB kinase to phosphorylate IκBs, leading to ubiquitination of phospho-IκBs and their degradation via the ubiquitin–proteasome pathway, thus permitting nuclear translocation of NF-κB. (Sun and Ley, 2008). Nuclear NF-κB activates the expression of immunoregulatory genes, including IFN-γ, as well as several other proinflammatory cytokines. Expression of these genes is required for the triggering of inflammatory responses that eliminate bacterial pathogens by innate immunity. The subversion of the NF-κB signaling pathway is a bacterial mechanism by which to evade the host immune system. For example, Shigella OspG effector associates with ubiquitin-conjugating enzymes that ubiquitinate phospho-IκBα, thus blocking NF-κB translocation into nuclei (Kim et al., 2005). We showed that IκBα degradation is not affected by BopN function (Fig. 8 E). Furthermore, although OspG must be located in the host cytosol to exert its effects on degradation of phospho-IκBα, our data clearly showed that the BopN effector has the ability to independently translocate itself into the nucleus and contains a nuclear localization signal located in the N-terminal half (aa 1–182; Fig. 9). It has been reported that Bordetella inhibits the nuclear translocation of NF-κBp65 in a T3SS-dependent manner (Yuk et al., 2000). In this study, we showed that BopN blocks nuclear translocation of NF-κBp65 and promotes nuclear translocation of NF-κBp50. The translocation of NF-κBp50 into the nucleus is involved in the up-regulation of IL-10, as previously described (Driessler et al., 2004; Cao et al., 2006). As shown in Fig. 8 F, leptomycin B inhibits IL-10 production, suggesting that the relocation of NF-κB components contributes to the effects of BopN on IL-10 production.
In Yersinia, LcrV is located at the tip of the T3SS needle structure (Mueller et al., 2005) and is involved in effector translocation. LcrV reportedly enhances production of IL-10 via association with TLR2 (Sing et al., 2005). However, TLR2−/− mice were not protected against subcutaneous plague infection (Pouliot et al., 2007). Recently, Depaolo et al. (2008) demonstrated that LcrV-mediated TLR6 is involved in the up-regulation of IL-10 via activation of the JNK pathway. They further confirmed that IL-10−/− and TLR6−/− mice are significantly protected from plague infection. Thus, both Bordetella and Yersinia species induce the up-regulation of IL-10, but they exploit different host-cell signaling pathways using different virulence factors.
We demonstrated that BopN has the ability to alter both MAPKs and the nuclear translocation of NF-κB, resulting in activation or inactivation of various transcriptional factors. Furthermore, we confirmed that NF-κB activation in DC2.4 cells infected with ΔBopN was similar to that of DC2.4 cells infected with WT (Fig. S5). To our surprise, the level of CRE-ATF, Myc-Max, and SP-1 activation in DC2.4 cells was significantly increased after infection with WT relative to ΔBopN, even though, in general, activation of these transcription factors is dependent on MAPK signaling. SP-1 plays an important role in IL-10 transcription (Chanteux et al., 2007). Thus, Bordetella appears to exploit a transcription factor, SP-1, for the up-regulation of IL-10 during infection, although the BopN-mediated signaling pathway remains to be fully elucidated.
Phagocytosed B. pertussis bacteria are easily killed by neutrophils (Lenz et al., 2000) and IFN-γ production is required for Bordetella clearance from the lung (Pilione and Harvill, 2006). Our in vivo successive infection study directly showed that IFN-γ production is involved in Bordetella clearance and that mice can survive a lethal dose of WT Bordetella when the up-regulation of IL-10, as a Bordetella strategy, was perturbed by preinfection with the avirulent ΔBopN strain (Fig. 6). Recently, it was reported that the T3SS of B. pertussis is also involved in persistent colonization in mice and that, indeed, BopN was detected as a protein secreted via T3SS (Fennelly et al., 2008). Another study showed that B. pertussis can induce IL-10 production in BMDCs (McGuirk et al., 2002). Thus, the up-regulation of IL-10 by the BopN effector is likely a stealth strategy by which Bordetella establishes an immunosuppressive environment such that the resulting microenvironment is advantageous for persistent colonization.
MATERIALS AND METHODS
Bacterial strains and plasmids.
E. coli DH10B, MC1061, and SM10λpir were used as hosts for the construction of various plasmids. The WT strain used in this study was B. bronchiseptica S798 (Kuwae et al., 2003). The type III secretion mutant (ΔT3SS) and BopC mutant (ΔBopC) were derived from S798 WT (Kuwae et al., 2003, 2006). The inoculum for liquid culture of Bordetella strains was prepared from fresh colonies grown on Bordet-Gengou (BG) agar, and these bacteria were cultured in Stainer-Scholte liquid medium at a starting A600 of 0.2 with vigorous shaking at 37°C for 18 h, as previously described (Cotter and Miller, 1994, 1997; Martínez de Tejada et al., 1996). The B. bronchiseptica strain BopN mutant (ΔBopN) was created as follows. pDONR201 (Invitrogen) and pABB-CRS2 (Sekiya et al., 2001) were used as the cloning and positive suicide vectors, respectively. A 2.4-kbp DNA fragment containing the bopN gene was amplified by PCR with the primers B1-bopN (5′-AAAAAGCAGGCTCCGCCTGGGCCTCGGCCTCT-3′) and B2-bopN (5′-AGAAAGCTGGGTGCCAGGGCCAGCAGGGACC-3′) using B. bronchiseptica S798 genomic DNA as the template. The resulting PCR product was cloned into pDONR201 to obtain pDONR-bopN using the adaptor PCR method in the Gateway cloning system (Invitrogen). Inverse PCR was performed with the primers R1-bopN (5′-GGAATTCATTGGGGGCGGCATCGATAC-3′) and R2-bopN (5′-GGAATTCAACGCGATAGCAATGGAGAA-3′) using circular pDONR-bopN as the template. The underlined portions indicate the EcoRI sites. The resulting PCR products were digested with EcoRI and self-ligated to obtain pDONR-ΔbopN, which contained a 1,044-bp deletion within the open reading frame of bopN. pDONR-ΔbopN was mixed with pABB-CRS2 to obtain pABB-ΔbopN using the Gateway cloning system. pABB-ΔbopN was introduced into E. coli SM10λpir and was transconjugated into the B. bronchiseptica S798 (streptomycin resistant), as previously described (Donnenberg and Kaper, 1991). The resulting mutant strain was designated as B. bronchiseptica ΔBopN. For the complementation of the bopN defect in ΔBopN, pBopN was constructed as follows. A 1.2-kbp fragment encoding bopN was amplified by PCR with the primers B1-bopN–comp (5′-AAAAAGCAGGCTGCGACACTCACTGCACCAGT-3′) and B2-bopN–comp (5′-AGAAAGCTGGGTATCCTGGCCGAACTGATGCA-3′) using B. bronchiseptica S798 genomic DNA as the template. The resulting fragment was cloned into pDONR201 and designated as pDONR-bopN–comp. To control the transcription of the bopN gene by the fha promoter and rrnB terminator in Bordetella, pDONR-fhaP (Kuwae et al., 2006), pDONR-bopN–comp, pDONR-rrnB (Kuwae et al., 2006), and pRK415 R4-R3-F (Kuwae et al., 2006) were mixed and treated with LR Clonase Plus (Invitrogen) to clone the fha promoter, bopN, and rrnB terminator into pRK415 R4-R3-F using the MultiSite Gateway system (Invitrogen), and the resulting plasmid was designated as pBopN. To express the BopN-Myc fusion protein in mammalian cells, pBopN-FL was constructed as follows. The bopN gene was amplified by PCR with the primers 5′-GGAATTCGCCACCATGGCTCGTATCGATGCCG-3′ (forward) and 5′-CGGGATCCTGCGTTCTCCATTGCTATCG-3′ (reverse) using B. bronchiseptica S798 genomic DNA as the template. To obtain the plasmid encoding truncated versions of the BopN-NT or BopN-CT, PCR was performed with the primer sets 5′-GGAATTCGCCACCATGGCTCGTATCGATGCCG-3′ (forward) and 5′-CGGGATCCCGCTTGCGCGGCCAGTTGC-3′ (reverse) or 5′-GGAATTCGCCACCATGGGTGTCACGCAGCAATACC-3′ (forward) and 5′-CGGGATCCTGCGTTCTCCATTGCTATCG-3′ (reverse). The underlined portions, GAATTC and GGATCC, indicate the EcoRI and BamHI sites, respectively. The EcoRI-BamHI fragment of the resulting PCR product was cloned into the EcoRI and BamHI sites of pcDNA3.1/His(-)A (Invitrogen) to obtain pBopN-FL, pBopN-NT, and pBopN-CT, respectively.
Mice.
5–6-wk-old C57BL/6J mice were purchased from Nihon SLC. IL-10−/− mice on a C57BL/6J background were obtained from the Jackson Laboratory.
Cells and transfection.
The DC line DC2.4 was provided by K.M. Rock (University of Massachusetts, Worcester, MA) and grown in RPMI 1640 (Invitrogen) containing 10% FCS (Sigma-Aldrich), 55 µM 2-mercaptoethanol, 100 U/ml penicillin, and 100 µg/ml streptomycin at 37°C under an atmosphere of 5% CO2. Cos7 cells (American Type Culture Collection) were maintained in Dulbecco's modified Eagle's medium (Sigma-Aldrich) with 10% FCS. The mouse macrophage cell line RAW264.7 (American Type Culture Collection) was maintained in RPMI 1640 medium with 10% FCS. Cos7 and RAW264.7 cells were transfected with 2.5 µg/ml pcDNA3.1/His(-)A, pBopN-FL, pBopN-NT, and pBopN-CT using Lipofectamine LTX (Invitrogen) according to the manufacturer's protocol. After transfection, the cells were incubated at 37°C for 24 h and fixed in 4% paraformaldehyde.
Inhibitors.
For the MAPK inhibition experiments, the MEK1/2 inhibitor U0126 (EMD) or the p38 kinase inhibitor SB203580 (EMD) was added to medium at 10 and 50 µM, respectively, for 1 h before LPS stimulation or Bordetella infection. To block nuclear export, leptomycin B (EMD) was added to medium at 20 nM for 24 h before the Bordetella infection.
Preparation of DCs from BM.
BM cells prepared from the tibias and femurs of mice were cultured at 1.5 × 106 cells/ml in RPMI 1640 medium with 10% FCS in the presence of 10 ng/ml GM-CSF (Fitzgerald Industries). BM cells were maintained for 3 d, and then one half of the medium was replaced by fresh medium. BM cells were further maintained for 3 d, and then DCs were purified using anti-CD11c microbeads with an autoMACS separation system (Miltenyi Biotec).
LDH assay.
DC2.4 cells were seeded in 24-well plates at 2.5 × 105 cells/well and incubated for 20 h. The precultured bacteria described in Bacterial strains and plasmids were added to the cells at a multiplicity of infection (m.o.i.) of 10 and centrifuged for 5 min. After incubation at 37°C under an atmosphere of 5% CO2 for the time periods indicated in the figures, the amounts of LDH were measured spectrophotometrically using a CytoTox 96 Non-Radioactive Cytotoxicity Assay kit (Promega).
In vivo infection of mice.
After arrival, all mice were housed for 1 wk before experiments. Mice were infected intranasally with 50 µl (5 × 106 or 5 × 105 CFU) of a B. bronchiseptica overnight culture started from an individually isolated colony on BG agar plates. To determine the amount of bacterial colonization in the lung, the whole lung was homogenized in 10 ml of cold PBS using a digital homogenizer (Potter-Elvehjem; As One, Inc.). The resulting homogenates were serially diluted with cold PBS and plated on BG agar plates, and colonies were then counted to calculate the number of CFUs per mouse. For histological analysis, lung tissues were fixed in 4% paraformaldehyde in PBS and stained with H&E. For detection of DCs or macrophages that had migrated into the lung, lung tissues were fixed and immunostained with anti-CD11c monoclonal antibodies (Endogen). For scanning electron microscopy observation, trachea sections were fixed in 2.5% glutaraldehyde in PBS. Three 5–6-wk-old mice were used for each time point. All animal experiments were conducted according to protocols approved by the Experimental Animal Center of the Kitasato University.
Flow cytometry.
Lung cells were incubated in 100 µl DMEM at 4°C for 1 h and stained with PE-Cy7–conjugated anti–Gr-1 (clone RB6-8c5; eBioscience), FITC-conjugated anti-CD11b (clone M1/70; BD), and PE-conjugated anti-CD11c (clone HL3; BD). For ex vivo intracellular cytokine staining, lung cells were cultured at 37°C for 4.5 h in PBS supplemented with 2% FCS and GolgiStop (BD). Cells were stained with PE-conjugated anti-CD11c antibodies, and then fixed and permeabilized with Cytofix/Cytoperm solution (BD). After fixation and permeabilization, cells were stained with FITC-conjugated anti–IL-10 (clone JES5-16E3; BD). Flow cytometric analysis (FACS) was performed with a multi–flow cytometry system (EPICS Elite; Beckman Coulter) using the EXPO 32 Elite software package (Beckman Coulter).
Fluorescence staining.
DC2.4 cells were seeded in a 6-well plate at 5 × 105 cells/well and incubated for 20 h, and then infected with Bordetella strains at an m.o.i. of 10. After 60 min, DCs were fixed for 15 min with 4% paraformaldehyde in PBS and subjected to immunofluorescence staining with anti–NF-κBp65 and anti–NF-κBp50 antibodies (Santa Cruz Biotechnology, Inc.). As a secondary antibody, Alexa Fluor 488 goat anti–rabbit IgG (Invitrogen) was used. Nuclei were stained with DAPI (Invitrogen). Bacteria were visualized with rabbit anti–B. bronchiseptica serum (Denka Seiken) followed by Alexa Fluor 594–conjugated secondary antibody. Numbers of NF-κBp65 translocated into nuclei were scored by examining 50 cells per coverslip under a fluorescence microscope. Phosphorylated or total ERKs were stained with anti–phospho-ERK or anti-ERK antibody (Cell Signaling Technology), and Alexa Fluor 488 goat anti–mouse IgG or Alexa Fluor 594 goat anti–rabbit IgG (Invitrogen) were used as secondary antibodies, respectively. BopN-Myc fusion proteins were stained with anti-Myc antibody (Santa Cruz Biotechnology, Inc.) and Alexa Fluor 488 goat anti–mouse IgG (Invitrogen).
mRNA analysis by real-time PCR.
DC2.4 cells were seeded in 24-well plates at 2.5 × 105 cells/well, incubated for 20 h, and infected with Bordetella strains at an m.o.i. of 10. After incubation for the times indicated in the figures, total RNA was isolated using an RNeasy Mini Kit (QIAGEN), and 5 µg RNA from each sample was reverse transcribed using oligo (dT) primers and Omniscript RT (QIAGEN). The resulting 5 µl cDNA was amplified by SYBR Premix Ex Taq (Takara Bio Inc.) using mouse IL-10 primers and β-actin primers in a LightCycler apparatus (Roche). Primer pairs specific for IL-10 (forward, 5′-AGCTGGACAACATACTGCTA-3′; reverse, 5′-TGGGCCATGCTTCTCTG-3′) and β-actin (forward, 5′-GTGGGCCGCTCTAGCCACCAA-3′; reverse, 5′-TCTTTGATGTCACGCACGATTTC-3′) were used. The specificity was checked by analyzing the melting curves, and results were calculated using the comparative cycle threshold method, in which the amount of target mRNA is normalized to the internal control β-actin and calculated in arbitrary units set to a value of 1 for uninfected cells.
Cytokine detection.
Lung specimens were homogenized as described in In vivo infection of mice, and concentrations of IL-10 or IFN-γ in the homogenates were determined by an ELISA development kit (DuoSet; R&D Systems). The absorbance of each well was determined using a plate reader (Biotrak II; GE Healthcare).
Immunoblotting.
DC2.4 cells infected with B. bronchiseptica were washed with PBS and solubilized with SDS sample buffer. The resulting samples were sonicated and boiled for 5 min, separated by SDS-PAGE on a 10% gel, and transferred to polyvinylidene fluoride membranes (Millipore). Proteins were analyzed by immunoblotting with anti–phospho–p38 MAPK (Thr180/Tyr182), anti–phospho–p44/p42 MAPK (Thr202/Tyr204; E10), anti–phospho–stress-activated protein kinase (SAPK)/JNK (Thr183/Tyr185), anti–phospho-MEK1/2 (Ser217/221), and anti–phospho-MKK3/MKK6 (Ser189/207) antibodies (Cell Signaling Technology). Anti-IκBα, anti-IκBβ, anti-IκBϵ, and anti–NF-κBp105 antibodies (Cell Signaling Technology) were used to detect IκBα, IκBβ, IκBϵ, and NF-κBp105, respectively. To ensure equal protein loading, the membrane was stripped and reprobed with anti–p38 MAPK (Santa Cruz Biotechnology, Inc.), anti–p44/p42 MAPK, anti-SAPK/JNK, anti-MKK3, anti-MEK1/2 (47E6), or anti–β-actin antibodies (Cell Signaling Technology). The detection of specific protein signals was performed using a detection kit (ECL; GE Healthcare). Nuclear and cytosolic fractions were prepared using a subcellular proteome extraction kit (ProteoExtract; EMD). Lysates of DC2.4 cells infected with B. bronchiseptica were separated into nuclear and cytosolic fractions, and the resulting fractions were analyzed by immunoblotting with anti–NF-κBp65 and anti–NF-κBp50 (Santa Cruz Biotechnology, Inc.), and anti–histone H3 (Cell Signaling Technology) antibodies. Protein expression levels were determined by densitometry analysis of immunoblots using ImageJ (version 1.42; National Institutes of Health).
Transcription factor profiling.
Nuclear fractions were prepared from DC2.4 cells infected with B. bronchiseptica using the ProteoExtract subcellular proteome extraction kit and analyzed using a transcription factor profiling kit (Multiplex; Marligen). Positive binding of each transcription factor to the DNA probe was detected using Bio-Plex (Bio-Rad Laboratories).
Statistics.
Statistical analyses were performed using the Mann-Whitney U test or the unpaired Student's t test, with P < 0.05 considered statistically significant. Survival curves were generated by the Kaplan-Meier method, and statistical analyses were performed using the log-rank test.
Online supplemental material.
Fig. S1 shows H&E staining of lung sections of mice infected with B. bronchiseptica. Fig. S2 shows densitometry analyses of immunoblots, immunofluorescence micrographs of MAPK activities, and confirmation of the specificity of anti-ERK antibodies by immunoblotting. Fig. S3 shows immunofluorescence micrographs to identify localization of NF-κBp65 and NF-κBp50 in a macrophage cell line transfected with a bopN clone. Fig. S4 shows immunoblot analysis of phospho-MAPKs in WT- and IL-10−/−–derived BMDCs infected with B. bronchiseptica. Fig. S5 shows transcription factor profiling during the Bordetella infection. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20090494/DC1.
Acknowledgments
Dr. C. Sasakawa has generously reviewed the paper and gave us critical comments.
This work was supported in part by the Ministry of Education, Culture, Sports, Science, and Technology of Japan through Grants-in-Aid for Scientific Research (B, 18390136 and 21390133) and for Scientific Research on Priority Areas (19041066 and 21022045). Support was also received in the form of operating grants from a Kitasato University Research Grant for Young Researchers (2008).
S. Koyasu is a consultant for Medical and Biological Laboratories, Co. Ltd. The authors otherwise have no financial conflicts of interest.
Footnotes
Abbreviations used:
- BG
- Bordet-Gengou
- BMDC
- BM-derived DC
- ERK
- extracellular signal-regulated kinase
- IκB
- inhibitor of NF-κB
- JNK
- c-Jun N-terminal kinase
- LDH
- lactate dehydrogenase
- MAPK
- mitogen-activated protein kinase
- m.o.i.
- multiplicity of infection
- qRT-PCR
- quantitative real-time PCR
- T3SS
- type III secretion system
- TLR
- Toll-like receptor
References
- Abe A., Heczko U., Hegele R.G., Brett Finlay B. 1998. Two enteropathogenic Escherichia coli type III secreted proteins, EspA and EspB, are virulence factors. J. Exp. Med. 188:1907–1916 10.1084/jem.188.10.1907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbibe L., Kim D.W., Batsche E., Pedron T., Mateescu B., Muchardt C., Parsot C., Sansonetti P.J. 2007. An injected bacterial effector targets chromatin access for transcription factor NF-kappaB to alter transcription of host genes involved in immune responses. Nat. Immunol. 8:47–56 10.1038/ni1423 [DOI] [PubMed] [Google Scholar]
- Baldwin A.S., Jr. 1996. The NF-κB and IκB proteins: new discoveries and insights. Annu. Rev. Immunol. 14:649–683 10.1146/annurev.immunol.14.1.649 [DOI] [PubMed] [Google Scholar]
- Cao S., Zhang X., Edwards J.P., Mosser D.M. 2006. NF-kappaB1 (p50) homodimers differentially regulate pro- and anti-inflammatory cytokines in macrophages. J. Biol. Chem. 281:26041–26050 10.1074/jbc.M602222200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanteux H., Guisset A.C., Pilette C., Sibille Y. 2007. LPS induces IL-10 production by human alveolar macrophages via MAPKinases- and Sp1-dependent mechanisms. Respir. Res. 8:71 10.1186/1465-9921-8-71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter P.A., Miller J.F. 1994. BvgAS-mediated signal transduction: analysis of phase-locked regulatory mutants of Bordetella bronchiseptica in a rabbit model. Infect. Immun. 62:3381–3390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter P.A., Miller J.F. 1997. A mutation in the Bordetella bronchiseptica bvgS gene results in reduced virulence and increased resistance to starvation, and identifies a new class of Bvg-regulated antigens. Mol. Microbiol. 24:671–685 10.1046/j.1365-2958.1997.3821741.x [DOI] [PubMed] [Google Scholar]
- Depaolo R.W., Tang F., Kim I., Han M., Levin N., Ciletti N., Lin A., Anderson D., Schneewind O., Jabri B. 2008. Toll-like receptor 6 drives differentiation of tolerogenic dendritic cells and contributes to LcrV-mediated plague pathogenesis. Cell Host Microbe. 4:350–361 10.1016/j.chom.2008.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnenberg M.S., Kaper J.B. 1991. Construction of an eae deletion mutant of enteropathogenic Escherichia coli by using a positive-selection suicide vector. Infect. Immun. 59:4310–4317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driessler F., Venstrom K., Sabat R., Asadullah K., Schottelius A.J. 2004. Molecular mechanisms of interleukin-10-mediated inhibition of NF-kappaB activity: a role for p50. Clin. Exp. Immunol. 135:64–73 10.1111/j.1365-2249.2004.02342.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauconnier A., Veithen A., Gueirard P., Antoine R., Wacheul L., Locht C., Bollen A., Godfroid E. 2001. Characterization of the type III secretion locus of Bordetella pertussis. Int. J. Med. Microbiol. 290:693–705 [DOI] [PubMed] [Google Scholar]
- Fennelly N.K., Sisti F., Higgins S.C., Ross P.J., van der Heide H., Mooi F.R., Boyd A., Mills K.H. 2008. Bordetella pertussis expresses a functional type III secretion system that subverts protective innate and adaptive immune responses. Infect. Immun. 76:1257–1266 10.1128/IAI.00836-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay B.B., Cossart P. 1997. Exploitation of mammalian host cell functions by bacterial pathogens. Science. 276:718–725 10.1126/science.276.5313.718 [DOI] [PubMed] [Google Scholar]
- Foley J.E., Rand C., Bannasch M.J., Norris C.R., Milan J. 2002. Molecular epidemiology of feline bordetellosis in two animal shelters in California, USA. Prev. Vet. Med. 54:141–156 10.1016/S0167-5877(02)00022-3 [DOI] [PubMed] [Google Scholar]
- Forsberg A., Viitanen A.M., Skurnik M., Wolf-Watz H. 1991. The surface-located YopN protein is involved in calcium signal transduction in Yersinia pseudotuberculosis. Mol. Microbiol. 5:977–986 10.1111/j.1365-2958.1991.tb00773.x [DOI] [PubMed] [Google Scholar]
- Galán J.E., Wolf-Watz H. 2006. Protein delivery into eukaryotic cells by type III secretion machines. Nature. 444:567–573 10.1038/nature05272 [DOI] [PubMed] [Google Scholar]
- Ghosh S., May M.J., Kopp E.B. 1998. NF-κB and Rel proteins: evolutionarily conserved mediators of immune responses. Annu. Rev. Immunol. 16:225–260 10.1146/annurev.immunol.16.1.225 [DOI] [PubMed] [Google Scholar]
- Goodnow R.A. 1980. Biology of Bordetella bronchiseptica. Microbiol. Rev. 44:722–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gzyl A., Augustynowicz E., van Loo I., Slusarczyk J. 2001. Temporal nucleotide changes in pertactin and pertussis toxin genes in Bordetella pertussis strains isolated from clinical cases in Poland. Vaccine. 20:299–303 10.1016/S0264-410X(01)00356-5 [DOI] [PubMed] [Google Scholar]
- He Q., Mäkinen J., Berbers G., Mooi F.R., Viljanen M.K., Arvilommi H., Mertsola J. 2003. Bordetella pertussis protein pertactin induces type-specific antibodies: one possible explanation for the emergence of antigenic variants? J. Infect. Dis. 187:1200–1205 10.1086/368412 [DOI] [PubMed] [Google Scholar]
- Karin M., Ben-Neriah Y. 2000. Phosphorylation meets ubiquitination: the control of NF-[κ]B activity. Annu. Rev. Immunol. 18:621–663 10.1146/annurev.immunol.18.1.621 [DOI] [PubMed] [Google Scholar]
- Kim D.W., Lenzen G., Page A.L., Legrain P., Sansonetti P.J., Parsot C. 2005. The Shigella flexneri effector OspG interferes with innate immune responses by targeting ubiquitin-conjugating enzymes. Proc. Natl. Acad. Sci. USA. 102:14046–14051 10.1073/pnas.0504466102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King A.J., Berbers G., van Oirschot H.F., Hoogerhout P., Knipping K., Mooi F.R. 2001. Role of the polymorphic region 1 of the Bordetella pertussis protein pertactin in immunity. Microbiology. 147:2885–2895 [DOI] [PubMed] [Google Scholar]
- Kuwae A., Ohishi M., Watanabe M., Nagai M., Abe A. 2003. BopB is a type III secreted protein in Bordetella bronchiseptica and is required for cytotoxicity against cultured mammalian cells. Cell. Microbiol. 5:973–983 10.1046/j.1462-5822.2003.00341.x [DOI] [PubMed] [Google Scholar]
- Kuwae A., Matsuzawa T., Ishikawa N., Abe H., Nonaka T., Fukuda H., Imajoh-Ohmi S., Abe A. 2006. BopC is a novel type III effector secreted by Bordetella bronchiseptica and has a critical role in type III-dependent necrotic cell death. J. Biol. Chem. 281:6589–6600 10.1074/jbc.M512711200 [DOI] [PubMed] [Google Scholar]
- Lenz D.H., Weingart C.L., Weiss A.A. 2000. Phagocytosed Bordetella pertussis fails to survive in human neutrophils. Infect. Immun. 68:956–959 10.1128/IAI.68.2.956-959.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Xu H., Zhou Y., Zhang J., Long C., Li S., Chen S., Zhou J.M., Shao F. 2007. The phosphothreonine lyase activity of a bacterial type III effector family. Science. 315:1000–1003 10.1126/science.1138960 [DOI] [PubMed] [Google Scholar]
- Martínez de Tejada G., Miller J.F., Cotter P.A. 1996. Comparative analysis of the virulence control systems of Bordetella pertussis and Bordetella bronchiseptica. Mol. Microbiol. 22:895–908 10.1046/j.1365-2958.1996.01538.x [DOI] [PubMed] [Google Scholar]
- Mattoo S., Cherry J.D. 2005. Molecular pathogenesis, epidemiology, and clinical manifestations of respiratory infections due to Bordetella pertussis and other Bordetella subspecies. Clin. Microbiol. Rev. 18:326–382 10.1128/CMR.18.2.326-382.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuirk P., McCann C., Mills K.H. 2002. Pathogen-specific T regulatory 1 cells induced in the respiratory tract by a bacterial molecule that stimulates interleukin 10 production by dendritic cells: a novel strategy for evasion of protective T helper type 1 responses by Bordetella pertussis. J. Exp. Med. 195:221–231 10.1084/jem.20011288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medhekar B., Shrivastava R., Mattoo S., Gingery M., Miller J.F. 2009. Bordetella Bsp22 forms a filamentous type III secretion system tip complex and is immunoprotective in vitro and in vivo. Mol. Microbiol. 71:492–504 10.1111/j.1365-2958.2008.06543.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michiels T., Wattiau P., Brasseur R., Ruysschaert J.M., Cornelis G. 1990. Secretion of Yop proteins by Yersiniae. Infect. Immun. 58:2840–2849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller C.A., Broz P., Müller S.A., Ringler P., Erne-Brand F., Sorg I., Kuhn M., Engel A., Cornelis G.R. 2005. The V-antigen of Yersinia forms a distinct structure at the tip of injectisome needles. Science. 310:674–676 10.1126/science.1118476 [DOI] [PubMed] [Google Scholar]
- Nogawa H., Kuwae A., Matsuzawa T., Abe A. 2004. The type III secreted protein BopD in Bordetella bronchiseptica is complexed with BopB for pore formation on the host plasma membrane. J. Bacteriol. 186:3806–3813 10.1128/JB.186.12.3806-3813.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panina E.M., Mattoo S., Griffith N., Kozak N.A., Yuk M.H., Miller J.F. 2005. A genome-wide screen identifies a Bordetella type III secretion effector and candidate effectors in other species. Mol. Microbiol. 58:267–279 10.1111/j.1365-2958.2005.04823.x [DOI] [PubMed] [Google Scholar]
- Pilione M.R., Harvill E.T. 2006. The Bordetella bronchiseptica type III secretion system inhibits gamma interferon production that is required for efficient antibody-mediated bacterial clearance. Infect. Immun. 74:1043–1049 10.1128/IAI.74.2.1043-1049.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouliot K., Pan N., Wang S., Lu S., Lien E., Goguen J.D. 2007. Evaluation of the role of LcrV-Toll-like receptor 2-mediated immunomodulation in the virulence of Yersinia pestis. Infect. Immun. 75:3571–3580 10.1128/IAI.01644-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raguckas S.E., VandenBussche H.L., Jacobs C., Klepser M.E. 2007. Pertussis resurgence: diagnosis, treatment, prevention, and beyond. Pharmacotherapy. 27:41–52 10.1592/phco.27.1.41 [DOI] [PubMed] [Google Scholar]
- Reissinger A., Skinner J.A., Yuk M.H. 2005. Downregulation of mitogen-activated protein kinases by the Bordetella bronchiseptica type III secretion system leads to attenuated nonclassical macrophage activation. Infect. Immun. 73:308–316 10.1128/IAI.73.1.308-316.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccani S., Pantano S., Natoli G. 2002. p38-dependent marking of inflammatory genes for increased NF-κB recruitment. Nat. Immunol. 3:69–75 10.1038/ni748 [DOI] [PubMed] [Google Scholar]
- Sekiya K., Ohishi M., Ogino T., Tamano K., Sasakawa C., Abe A. 2001. Supermolecular structure of the enteropathogenic Escherichia coli type III secretion system and its direct interaction with the EspA-sheath-like structure. Proc. Natl. Acad. Sci. USA. 98:11638–11643 10.1073/pnas.191378598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sing A., Reithmeier-Rost D., Granfors K., Hill J., Roggenkamp A., Heesemann J. 2005. A hypervariable N-terminal region of Yersinia LcrV determines Toll-like receptor 2-mediated IL-10 induction and mouse virulence. Proc. Natl. Acad. Sci. USA. 102:16049–16054 10.1073/pnas.0504728102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner J.A., Reissinger A., Shen H., Yuk M.H. 2004. Bordetella type III secretion and adenylate cyclase toxin synergize to drive dendritic cells into a semimature state. J. Immunol. 173:1934–1940 [DOI] [PubMed] [Google Scholar]
- Skinner J.A., Pilione M.R., Shen H., Harvill E.T., Yuk M.H. 2005. Bordetella type III secretion modulates dendritic cell migration resulting in immunosuppression and bacterial persistence. J. Immunol. 175:4647–4652 [DOI] [PubMed] [Google Scholar]
- Stibitz S., Aaronson W., Monack D., Falkow S. 1989. Phase variation in Bordetella pertussis by frameshift mutation in a gene for a novel two-component system. Nature. 338:266–269 [DOI] [PubMed] [Google Scholar]
- Sun S.C., Ley S.C. 2008. New insights into NF-kappaB regulation and function. Trends Immunol. 29:469–478 10.1016/j.it.2008.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson S., Clayton A.L., Hazzalin C.A., Rose S., Barratt M.J., Mahadevan L.C. 1999. The nucleosomal response associated with immediate-early gene induction is mediated via alternative MAP kinase cascades: MSK1 as a potential histone H3/HMG-14 kinase. EMBO J. 18:4779–4793 10.1093/emboj/18.17.4779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuk M.H., Harvill E.T., Cotter P.A., Miller J.F. 2000. Modulation of host immune responses, induction of apoptosis and inhibition of NF-kappaB activation by the Bordetella type III secretion system. Mol. Microbiol. 35:991–1004 10.1046/j.1365-2958.2000.01785.x [DOI] [PubMed] [Google Scholar]
- Zhang Z.Y. 2002. Protein tyrosine phosphatases: structure and function, substrate specificity, and inhibitor development. Annu. Rev. Pharmacol. Toxicol. 42:209–234 10.1146/annurev.pharmtox.42.083001.144616 [DOI] [PubMed] [Google Scholar]