Abstract
Th2 cells drive protective immunity against most parasitic helminths, but few mechanisms have been demonstrated that facilitate pathogen clearance. We show that IL-4 and IL-13 protect against intestinal lumen-dwelling worms primarily by inducing intestinal epithelial cells (IECs) to differentiate into goblet cells that secrete resistin-like molecule (RELM) β. RELM-β is essential for normal spontaneous expulsion and IL-4–induced expulsion of Nippostrongylus brasiliensis and Heligmosomoides polygyrus, which both live in the intestinal lumen, but it does not contribute to immunity against Trichinella spiralis, which lives within IEC. RELM-β is nontoxic for H. polygyrus in vitro but directly inhibits the ability of worms to feed on host tissues during infection. This decreases H. polygyrus adenosine triphosphate content and fecundity. Importantly, RELM-β–driven immunity does not require T or B cells, alternative macrophage activation, or increased gut permeability. Thus, we demonstrate a novel mechanism for host protection at the mucosal interface that explains how stimulation of epithelial cells by IL-4 and IL-13 contributes to protection against parasitic helminthes that dwell in the intestinal lumen.
Gastrointestinal (GI) helminths infect >109 people worldwide (Anthony et al., 2007). Rodent studies demonstrate that IL-4, 5, 9, 13, 25, and 33 promote immunity against worm infections, with IL-4 and IL-13 serving a predominant role in host protection (Fallon et al., 2002, 2006; Humphreys et al., 2008). The IL-4 receptor α chain (IL-4Rα) is essential for both the type I (IL-4Rα/γc) and type II (IL-4Rα/IL-13Rα1) IL-4Rs and is responsible for nearly all known biological effects of IL-4 and IL-13 (Junttila et al., 2008). Indeed, IL-4Rα–deficient mice are highly susceptible to most GI nematode species because of impaired effector functions of bone marrow–derived and non–bone marrow–derived cell lineages (Finkelman et al., 1997; Urban et al., 2001).
The niche inhabited by a particular worm species dictates the mechanism required for expulsion. IL-4/IL-13 stimulation of macrophage arginase production decreases survival of larvae of the hookworm Heligmosomoides polygyrus as they develop in the intestinal wall, but is not involved in IL-4/IL-13 expulsion of adult worms from the intestinal lumen (Anthony et al., 2006). Expulsion of Nippostrongylus brasiliensis adult worms from the intestinal lumen is mast cell, eosinophil, and antibody independent, but it requires IL-4/13–responsive non–bone marrow–derived cells (Urban et al., 2001), even though infected mice that express IL-4Rα only on bone marrow–derived cells develop strong IL-4/IL-13 responses. In contrast, protection against the human roundworm pathogen Trichinella spiralis, which resides in an intestinal epithelial cell (IEC) syncytium, is mast cell dependent and requires IL-4Rα expression by both bone marrow– and non–bone marrow–derived cells (Urban et al., 2000).
The IL-4Rα–expressing cell type required for N. brasiliensis expulsion has been speculated to be smooth muscle (increased contractility) or intestinal epithelium (increased proliferation, permeability, and mucus production), but IL-4/IL-13's effects on smooth muscle cells contribute only slightly to expulsion (Horsnell et al., 2007), and there has been no direct proof that IL-4/IL-13's effects on IEC are required for expulsion. This study demonstrates that IL-4/IL-13 contributes importantly to expulsion of N. brasiliensis and H. polygyrus adults from the intestinal lumen by inducing IEC to differentiate into goblet cells that secrete resistin-like molecule (RELM) β, which interferes with worm nutrition.
RESULTS AND DISCUSSION
Results that IECs constitutively express IL-4Rα (Reinecker and Podolsky, 1995) and can promote Th2 immunity (Zaph et al., 2007) prompted us to investigate whether IEC activation by IL-4/IL-13 promotes GI nematode expulsion. Mice that possess an IEC-specific deletion of IL-4Rα (IL-4Rαflox/−/Villin-Cre) were generated by crossing Villin-Cre transgenic IL-4Rα−/− mice with IL-4Rαflox/flox mice. Villin promoter activity was restricted to small and large intestinal epithelium and was not detected in esophagus, stomach, or lung (Fig. S1 and not depicted).
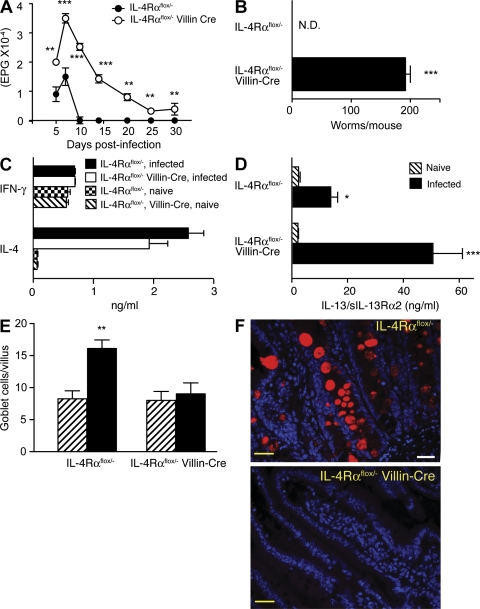
N. brasiliensis larvae migrate from the skin to the lungs (0–3 d after inoculation) and eventually the small intestine (4–9 d). Although most immunocompetent mice, including IL-4Rαflox/− mice, expel worms by day 10, their IL-4Rαflox/−/Villin-Cre+/− littermates fail to completely terminate egg production (Fig. 1 A) or expel adult N. brasiliensis worms (Fig. 1 B) through day 30 but clear parasites by day 45 (not depicted). Thus, IL-4/IL-13 effects on IEC account for most, but not all, of the defect in worm expulsion previously observed in mice deficient in both IL-4 and IL-13 (Fallon et al., 2002). Villin-Cre expression by IL-4Rαflox/− mice did not affect IL-4 or IFN-γ production (Fig. 1 C) but was associated with increased IL-13 (Fig. 1 D), probably as a result of increased worm burden. Despite increased IL-13 levels in IL-4Rαflox/−/Villin-Cre mice, worm-induced goblet cell hyperplasia was abrogated (Fig. 1 E), as was production of RELM-β (Fig. 1 F), a goblet cell–produced molecule that is normally up-regulated by worm infection (Artis et al., 2004). RELM-β expression increased greatly in the small intestine of N. brasiliensis–inoculated mice after larvae migrated to that organ. In contrast, pulmonary RELM-β expression increased only marginally in these mice (Fig. 2 A). Conversely, N. brasiliensis infection induced expression of RELM-α to increase considerably more in the lungs than the intestine (Fig. 2 B).
Figure 1.
IEC IL-4Rα expression is required for expulsion of N. brasiliensis and RELM-β production. (A) Kinetics of fecal egg count in N. brasiliensis–inoculated IL-4Rαflox/− (WT phenotype) and IL-4Rαflox/−/Villin-Cre (selective IEC IL-4Rα deficiency) mice. (B) Intestinal worm burden 30 d after inoculation. Means ± SE of 8–10 mice/group are shown. The experiment was performed three times. (C) IL-4 and IFN-γ secretion measured by IVCCA 10 d after inoculation. (D) Serum levels of IL-13–soluble IL-13Rα2 complexes 10 d after inoculation. The error bars indicate the SE of 8–10 mice per group. ***, P < 0.001. (E) Quantitation of goblet cells per villus 10 d after inoculation. Mean ± SE is shown of 400 villi/group. (F) Immunofluorescence staining for RELM-β (red) and cell nuclei (blue) demonstrates colocalization of RELM-β and goblet cell mucus. 400× magnification. Yellow bars, 10 µm. A representative photo is shown from three independent experiments. **, P < 0.01; *, P < 0.05, compared with WT. N.D., none detected.
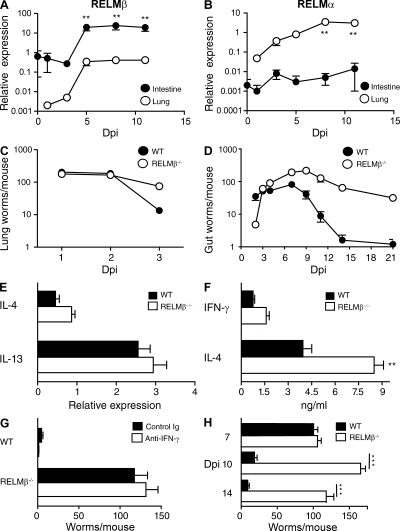
Figure 2.
Intestinal RELM-β expression is important for N. brasiliensis and H. polygyrus expulsion. (A and B) RELM-β (A) and RELM-α (B) mRNA were quantitated by real-time PCR in intestinal and lung tissue after N. brasiliensis infection. Results are representative of two independent experiments with mean ± SE. n = 6–8 mice/group. **, P < 0.01. (C) Lung worm burden in N. brasiliensis–inoculated WT and RELM-β−/−. Mean ± SE is shown of five to six mice per group. Error bars are too small to be seen. The experiment was performed twice. (D) Intestinal worm burden in N. brasiliensis–inoculated WT and RELM-β−/−. Mean ± SE is shown of 8–10 mice/group. The experiment was performed four times. (E) Real-time PCR analysis of IL-4 and IL-13 in gut mRNA 7 d after inoculation. Mean ± SE is shown of five to six mice per group. (F) Serum levels of IFN-γ and IL-4 measured by IVCCA 10 d after inoculation. **, P < 0.01. (G) Intestinal worm burden of WT and RELM-β−/− mice 10 d after inoculation in mice treated with anti–IFN-γ (XMG-6) or isotype control mAb (GL113). (H) Intestinal worm burden 7, 10, and 14 d after second infection of WT or RELM-β−/− with H. polygyrus. Dpi, days post inoculation. The error bars indicate the SE of five to six mice per group. The experiment was performed four times. ***, P < 0.001, compared with WT.
Because RELM-β binds to chemosensory organs of the intestinal worms Trichuris muris and Strongyloides stercoralis and inhibits S. stercoralis chemotaxis in vitro (Artis et al., 2004), and because intact chemotaxis might be required for gut lumen-dwelling worms to locate and feed on host epithelium, we tested whether expulsion of worms that reside in the intestinal lumen is defective in RELM-β−/− mice. Serial counts of worms in the lungs and intestine of N. brasiliensis–inoculated mice (Fig. 2, C and D) demonstrated the following: (a) similar numbers of worm larvae in the lungs for the first 2 d after inoculation; (b) ∼90% of worms in WT mice, but <50% of worms in RELM-β−/−, leave the lungs by day 3; (c) more worms reach the intestine in the WT than the RELM-β−/− by day 2; (d) peak intestinal worm burden is similar to peak lung worm burden for RELM-β−/− but only ∼1/3 of peak lung worm burden for WT; (e) intestinal worms begin to decline between days 6 and 9 for WT versus days 8 and 11 for the RELM-β−/−; (f) the rate of decline is steeper in WT than in RELM-β−/−; and (g) WT mice are worm-free by day 13, whereas RELM-β−/− continue to harbor worms at d 21. Collectively, these observations suggest that RELM-β hastens the exit of worms from the lungs, even though little RELM-β is detectable in that organ, and that more worms eventually reach the intestine in RELM-β−/− than in WT and/or early expulsion from the intestine limits peak intestinal worm number in WT but not RELM-β−/−. Thus, RELM-β limits N. brasiliensis survival through effects on the parasite in the intestine and, most likely, the lung. These effects do not appear to be the result of reduced Th2 cytokine production in RELM-β−/− because there were no differences in intestinal cytokine expression in WT versus RELM-β−/−, and at day 7, IL-4 and IL-13 intestinal messenger RNA (mRNA) transcripts were equivalent between WT and RELM-β−/− (Fig. 2 E). RELM-β−/− produced elevated serum levels of IL-4 and IFN-γ (Fig. 2 F) and similar amounts of IL-13 (not depicted) compared with WT. Increased IFN-γ was not responsible for defective worm expulsion (Fig. 2 G), and infected RELM-β−/− expelled N. brasilensis by day 30 (not depicted).
To evaluate whether RELM-β also promotes expulsion of worms that normally establish a chronic infection of the gut lumen, WT and RELM-β−/− were inoculated with H. polygyrus (Urban et al., 1991). In contrast to N. brasilensis, H. polygyrus has two intestinal niches. Larvae encyst in the intestinal muscularis (days 0–8) and emerge afterward as adults that reside only in the intestinal lumen, where they survive >90 d during primary infection in most mouse strains (Su and Dobson, 1997). During a second infection, however, IL-4Rα–mediated signaling promotes destruction of some encysted H. polygyrus larvae by inducing macrophage production of arginase (Anthony et al., 2006) and then induces expulsion from the gut lumen of the remaining worms that survive beyond day 7 (Anthony et al., 2006). As expected, RELM-β deficiency neither increases H. polygyrus survival during the initial 7 d of a second infection, when encysted larvae are surrounded by arginase-expressing cells (Fig. 2 H and Fig. S2), nor impairs intestinal IL-4 and IL-13 expression at day 10 (Fig. S3 A). However, RELM-β expression was critical for decreasing intestinal worm numbers at days 10 and 14, when adults entered the lumen and began to feed on intestinal mucosa (Fig. 2 H). Thus, IL-4Rα signaling suppresses H. polygyrus during a second infection by two distinct mechanisms: increased macrophage production of arginase promotes killing of gut wall–encysted larvae and IEC differentiation into goblet cells that secrete RELM-β promotes adult worm expulsion.
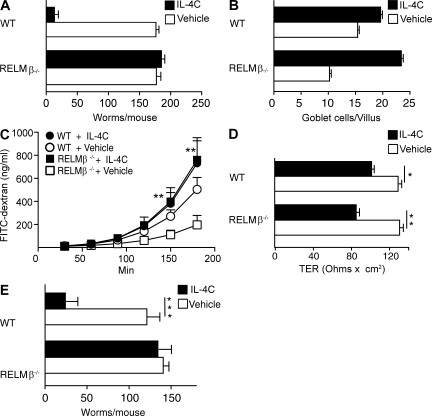
Although immunity against N. brasiliensis and H. polygyrus is CD4+ T cell dependent (Voehringer et al., 2006), the CD4+ T cell requirement is restricted to IL-4/IL-13 production, inasmuch as treatment with a long-acting form of IL-4 (IL-4C) induces worm expulsion by CD4+ T cell–depleted mice (Urban et al., 1995). IL-4C–induced T cell–independent worm expulsion is RELM-β dependent; IL-4C induced N. brasiliensis expulsion in anti-CD4 mAb-treated WT but not RELM-β−/− (Fig. 3 A). In contrast, IL-4C has similar effects on intestinal physiology in WT and RELM-β−/−: increased goblet cell number (Fig. 3 B), increased permeability (Fig. 3 C), decreased trans-epithelial resistance (Fig. 3 D), and increased luminal fluid (not depicted). IL-4C treatment of mice that harbored H. polygyrus adults also markedly increased goblet cell number (not depicted), permeability, (Fig. S3 B), and intestinal fluid (not depicted) and eliminated most worms from WT, but not RELM-β−/−, during a 1° infection (Fig. 3 E). Collectively, our results show that RELM-β induction by IL-4Rα signaling in IEC is essential for normal expulsion of at least two intestinal lumen-dwelling helminth parasites, although differences in N. brasiliensis expulsion kinetics between RELM-β−/− and IL-4Rαflox/−/Villin-Cre and between IL-4Rαflox/−/Villin-Cre and IL-4Rα−/− mice indicate that RELM-β production is not the sole mechanism by which IL-4Rα–activated IECs expel GI nematodes and that IEC activation is not the sole mechanism by which IL-4/IL-13 promote intestinal worm expulsion.
Figure 3.
RELM-β contribution to IL-4–mediated N. brasiliensis and H. polygyrus expulsion. (A) Worm counts 9 d after N. brasiliensis inoculation of WT or RELM-β−/− treated with anti-CD4 mAb and vehicle or IL-4C. The experiment was performed three times. The error bars indicate the SE of five to six mice per group. (B) Number of goblet cells per villus 10 d after inoculation in the experiment shown in A. Data show mean ± SE of 400 villi/group. (C) FITC-dextran permeability of muscle-free jejunum segments from mice in the experiment shown in A. (D) Transepithelial resistance of muscle-free segments of jejunum from mice in the experiment shown in A. *, P < 0.05 compared with WT or WT vehicle-treated group; **, P < 0.01. The error bars in C and D indicate the SE of five to six mice per group. (E) Intestinal worm burdens 14 d after primary infection with H. polygyrus in WT or RELM-β−/−. Mice were treated with vehicle or IL-4C on days 8, 10, and 12. ***, P < 0.001. Means ± SE are shown of 8–10 mice/group. The experiment was performed three times.
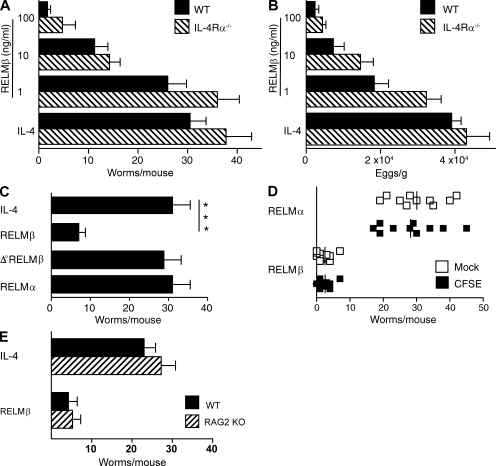
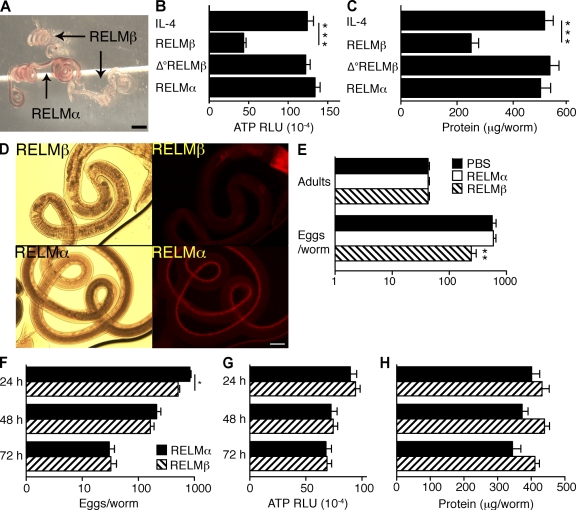
RELM-β, which acts on mouse macrophages to promote IFN-γ–driven intestinal inflammation (Nair et al., 2008) in addition to its direct effect on worm chemotaxis, might expel worms through effects on the parasite or the host. To distinguish between these possibilities, H. polygyrus adult worms were isolated from chronically infected mice, exposed to PBS, rRELM-β, or rRELM-α, a closely related molecule which limits Th2 responses in worm-infected mice (Nair et al., 2009; Pesce et al., 2009), washed, reinoculated into naive WT and IL-4Rα–deficient hosts, and evaluated for survival and fecundity. In vitro treatment with rRELM-β caused a dose-dependent decrease in in vivo worm survival (Fig. 4 A) and fecundity (Fig. 4 B) within 2 d, although rRELM-β effects were noted as early as 16 h after inoculation (not depicted). In contrast, in vitro treatment of worms with IL-4, RELM-α (which differs from RELM-β by absence of a cysteine residue; Patel et al., 2004), or heat-denatured RELM-β had no detectable effect (Fig. 4 C). It is unlikely that worm-associated RELM-β promotes worm expulsion by indirect actions on the host, because only RELM-β–treated worms had decreased survival when mice were inoculated with equal numbers of RELM-α- and RELM-β–treated worms that were differentiated by labeling with CFSE (Fig. 4 D). In addition, RELM-β effects did not require T or B cells because RELM-β–-treated worms had decreased survival in RAG-2–deficient mice (Fig. 4 E). Finally, it is unlikely that RELM-β protects against H. polygyrus before larval encystment because treatment of infective larvae before inoculation had no effect on larval encystment, adult worm number, or egg production (unpublished data).
Figure 4.
RELM-β directly inhibits fecundity and survival of adult H. polygyrus. (A) Numbers of adult worms recovered 2 d after inoculation of WT and IL-4Rα–deficient (IL-4Rα−/−) BALB/c mice with H. polygyrus adult worms treated in vitro with rIL-4 or 1–100 ng/ml of rRELM-β. The experiment was performed three times. (B) Fecal egg counts from the experiment shown in A. The experiment was performed three times. (C) Intestinal worm burden 2 d after inoculation of IL-4Rα−/− with H. polygyrus adults treated in vitro with rRELM-α, rRELM-β, heat-treated (Δ°) rRELM-β, or rIL-4. The experiment was performed three times. (D) Intestinal worm burden of WT mice 2 d after oral challenge with equal numbers of rRELM-α– or rRELM-β–pretreated H. polygyrus adults. Worms were labeled with CFSE or vehicle (mock) to distinguish RELM-β– from RELM-α–pretreated worms. The experiment was performed three times, with selective CFSE labeling of RELM-β–treated worms in some experiments and RELM-α–treated worms in other experiments. The vertical bars indicate the mean of five to six mice per group. ***, P < 0.001. (E) Intestinal worm burden 2 d after inoculation of WT or RAG-2–deficient mice with untreated or RELM-β–treated H. polygyrus adult worms. The experiment was performed two times. The error bars indicate the SE of five to six mice per group.
Because rRELM-β interferes with chemotaxis in vitro (Artis et al., 2004), it might promote expulsion of adult worms by blocking their ability to sense their source of nutrition. Indeed, rRELM-β–pretreated adults recovered from host intestines appeared pale, compared with the red color of RELM-α–treated worms (a consequence of erythrocyte ingestion; Fig. 5 A) and had decreased ATP and protein content compared with worms pretreated with control proteins (Fig. 5, B and C). Furthermore, RELM-β pretreatment of H. polygyrus adults inhibited their in vivo ingestion of rhodamine B–labeled host IEC (Fig. 5 D; Bansemir and Sukhdeo, 2001).
Figure 5.
RELM-β impairs feeding and reduces ATP and protein content in H. polygyrus adult worms. (A) Representative photomicrographs of H. polygyrus adults recovered from WT mice 2 d after oral gavage with adult worms pretreated with rRELM-α or rRELM-β. 4× magnification. The experiment was performed four times. Bar, 2 mm. (B) ATP levels of individual adult worms recovered from WT mice 2 d after oral inoculation. Worms were treated with rIL-4, rRELM-β, Δ°rRELM-β, or rRELM-α before inoculation of mice. Data are expressed as relative luminescence units (RLU). Error bars indicate means for 15–17 worms/group. The experiment was performed three times. (C) Protein content of adult worms treated as in Fig. 4 A. (D) H. polygyrus adults were treated with rRELM-α or rRELM-β and inoculated into naive WT. Mice were injected i.v. 2 d later with rhodamine B and worms were recovered from mouse intestines 1 h afterward. 100× magnification. The experiment was performed three times. Bar, 100 µm. (E) Number of viable H. polygyrus adult worms and eggs produced per worm after 16 h in vitro culture in medium supplemented with saline and 1 µg/ml of rRELM-α or rRELM-β. The experiment was performed three times. (F) Fecundity of H. polygyrus adult worms cultured for 24, 48, or 72 h with rRELM-α or rRELM-β. Worms were moved to fresh culture wells every 24 h to allow determination of egg production for each 24-h period. (G) ATP levels of adult H. polygyrus worms cultured for 24, 48, or 72 h with rRELM-α or rRELM-β. (H) Protein content of adult H. polygyrus worms cultured for 24, 48, or 72 h with rRELM-α or rRELM-β. The experiments were performed three times. The error bars indicate the SE of 15–17 worms per group. *, P < 0.05; **, P < 0.01; ***, P < 0.001, compared with IL-4–treated worms.
These observations were also compatible with the possibility that direct RELM-β toxicity to H. polygyrus causes multiple secondary abnormalities, including inability to ingest food. To test this possibility, H. polygyrus adults were cultured in vitro with rRELM-α or rRELM-β for 3 d. rRELM-β modestly, but significantly, suppressed initial egg production (Fig. 5, E and F) but had no effect on worm survival (100% under both conditions; Fig. 5 E), ATP or protein content (Fig. 5, G and H), motility, or ability to coil (which is thought to contribute to adherence to the host intestine; not depicted). Thus, RELM-β appears to protect against H. polygyrus in vivo by inhibiting its ability to locate, adhere to, or ingest host tissues rather than through a general toxic effect. Our studies, however, leave open additional possibilities. For example, RELM-β could sensitize worms to another factor present in the gut lumen that interferes with food ingestion, chemotaxis, or motility.
If RELM-β promotes expulsion of intestinal luminal adult worms by inhibiting their ability to locate their food source, it should not affect worm parasites that live entirely or partially within host intestinal epithelium, such as Trichinella spiralis or Trichuris muris (Gagliardo et al., 2002). These worms would not need to locate IEC and would likely be at least partially protected from RELM-β secreted into the gut lumen. Indeed, RELM-β−/− has recently been shown to have normal protective immunity against T. muris (Nair et al., 2008) and we find no defect in expulsion of T. spiralis in RELM-β−/− (Fig. S4), although protective immunity against both worms is IL-4Rα dependent (Else et al., 1994; Urban et al., 2001). Combined, these observations provide evidence that IL-4/IL-13 induction of IEC RELM-β production is selectively important for expulsion of worms that live in the intestinal lumen.
The role of IEC in host protection is complex and includes a critical inducer function (production of the Th2-promoting cytokine TSLP in mice infected with Trichuris muris; Zaph et al., 2007) as well as the effector function shown here. The critical role of the mucus-associated protein RELM-β is consistent with a mucus-trapping hypothesis, whereby IL-4/IL-13–dependent goblet cell hyperplasia drives worm expulsion through production of a protective layer of mucus-containing bioactive molecules (especially RELM-β) that encapsulates worms and prevents their ability to feed. Such a mechanism most likely works in concert with other effects of IL-4R signaling, including increased smooth muscle responsiveness (Zhao et al., 2003, 2008), establishment of a mucus barrier (Kuperman et al., 2002; Ramalingam et al., 2008), CD8+ T cell proliferation (Morris et al., 2009), chemokine secretion (Fulkerson et al., 2004), and adhesion molecule expression (Hickey et al., 1999), that together protect hosts against different worm parasites.
Taken in the context of previous observations, our studies demonstrate the remarkable versatility of functions mediated by IL-4R signaling in host protection against nematode parasites (Finkelman et al., 1997). Signaling through this receptor appears to protect against T. spiralis by stimulating isotype switching to IgE, promoting mast cell development, and increasing sensitivity to mast cell–produced mediators (Strait et al., 2003), to protect against lethal inflammation caused by Schistosoma mansoni by causing macrophages to produce arginase (Herbert et al., 2004), to protect against N. brasiliensis by inducing IEC to differentiate into goblet cells that produce RELM-β, to protect against H. polygyrus first through an arginase-dependent mechanism directed against larvae encysted in the gut wall (Anthony et al., 2006) and then through a RELM-β–dependent mechanism directed against luminal adults, and to protect against Trichuris muris by promoting the proliferation and shedding of the IEC in which these worms embed (Cliffe et al., 2005). Further still, these mechanisms are distinct from IL-4Rα–mediated RELM-α production that down-modulates worm-driven lung inflammation (Nair et al., 2009;Pesce et al., 2009).
Evidence that signaling through a single cytokine receptor induces multiple effector mechanisms, each of which protects against different species of worms, is consistent with the existence of host receptors for helminth-derived molecules that recognize this class of pathogens but that are unable to distinguish between worm species. Instead, IL-4/IL-13 production elicits a large set of effector mechanisms that produce comprehensive immunity.
MATERIALS AND METHODS
Mice.
IL-4Rαflox/− and IL-4Rαflox/−/Villin-Cre mice were generated by crossing and backcrossing Villin-Cre transgenic mice (The Jackson Laboratory) to IL-4Rα−/− mice to produce IL-4Rα−/−/Villin-Cre mice and then crossing these mice to IL-4Rαflox/flox mice. Because these mice have a mixed genetic background, IL-4Rαflox/−/Villin-Cre−/− mice were used as littermate controls for IL-4Rαflox/−/Villin-Cre+/− mice. RELM-β− mice (Hogan et al., 2006) were bred onto a C57BL/6 background for nine generations before use in our studies. WT C57BL/6 mice were bred at the Cincinnati Veterans Affairs Medical Center (VAMC) and used as controls. WT BALB/c and IL-4Rα−/− mice were purchased from Taconic. Mice were age, sex, and background strain matched in each experiment. Procedures were performed in accordance with the guide for care and use of laboratory animals and approved by the Cincinnati VAMC Institutional Animal Care and Use Committee.
Immunological reagents.
rmIL-4, rmRELM-α, and rmRELM-β were purchased from PeproTech. Hybridomas that secrete neutralizing rat IgG1 anti–mouse IFN-γ mAb (XMG-6; Mosmann and Fong, 1989) and an isotype-matched control mAb (GL113) were obtained originally from DNAX. The hybridoma that secretes GK1.5 (rat IgG2b anti-CD4; Miller and Jenkins, 1985) was obtained from the American Type Culture Collection. Hybridomas were grown as ascites in Pristane-primed athymic nude mice and mAbs were purified by ammonium sulfate precipitation and DE-52 cation exchange chromatography. FITC-Dextran (molecular mass 4.4 kD) and horseradish peroxidase (HRP; molecular mass 40 kD) were purchased from Sigma-Aldrich.
Long-acting IL-4 (IL-4C).
L-4/anti–IL-4 mAb complexes (IL-4C) were prepared by mixing rmIL-4 with the rat IgG1 anti-IL-4 mAb 11B11 (Finkelman et al., 1993; Shea-Donohue et al., 2001) at a 2:1 molar (1:5 weight) ratio (Urban et al., 1995). Mice were injected i.p with IL-4C containing 5 µg of IL-4 and 25 µg of 11B11. IL-4C slowly dissociates, releasing biologically active IL-4 for 3–5 d. Because IL-4Cs contain only one molecule of IgG mAb and 11B11 blocks IL-4R binding of IL-4, IL-4Cs do not activate complement, interact more strongly with Fc receptors than monomeric IgG of the same rat IgG2b isotype or bind to IL-4R. IL-4C does not promote worm expulsion or cell differentiation in IL-4Rα–deficient mice.
Cytokine assays.
Relative rates of in vivo IL-4 and IFN-γ secretion were determined by the in vivo cytokine capture assay (IVCCA; Finkelman and Morris, 1999). Injected biotin-labeled anti-cytokine mAbs in this assay form complexes with the secreted cytokines they specifically bind that have a much longer in vivo half-life than free cytokines. Consequently, the complexes accumulate in vivo and can be measured by ELISA, using wells coated with mAbs that bind to an epitope on the cytokine that is not blocked by the injected mAb. Bound biotin-mAb–cytokine complexes are detected with HRP-streptavidin, followed by a luminogenic substrate. To measure IL-4 and IFN-γ secretion, mice were injected i.v. with 10 µg biotin-BVD4-1D11 (anti–IL-4 mAb) or 10 µg biotin-R46-A2 (anti-IFN-γ), and serum was collected 6 h later and analyzed with microtiter plate wells coated with BVD6-24G2.3 (anti–IL-4) or AN-18 (anti–IFN-γ), respectively. All of these mAbs were purchased from BD.
Because secreted IL-13 is rapidly bound by soluble IL-13Rα2 in serum and the complex has a long in vivo half-life, relative IL-13 secretion was evaluated by measuring serum levels of the IL-13–soluble IL-13Rα2 complex. ELISA wells were coated with an anti–IL-13 mAb (C531; Centocor, Inc.) and the bound complex was detected with biotin-labeled affinity-purified goat anti–IL-13Rα2 antibody followed by HRP-SA and luminogenic substrate (Khodoun et al., 2007).
Real-time PCR.
RNA was obtained from intestinal tissue and DNase I treated. Complementary DNA was generated using SuperScript II reverse transcription (Invitrogen). Real-time PCR was performed on a Gene Amp 7500 instrument (Applied Biosystems) with the SYBR green detection reagent. Relative expression was calculated with the 1/ΔΔct method using vimentin as the housekeeping gene (Livak and Schmittgen, 2001). Primer sequences used were the following: vimentin, forward 5′-TGACCGGCTTGTATGCTATC-3′ and reverse 5′-CAGTGTGAGCCAGGATATAG-3′; RELM-α, forward 5′-AGATGGGCCTCCTGCCCTGCTGGG-3′ and reverse 5′-ACCTGGTGACGGGCGACGACGGTT-3′; RELM-β, forward 5′-ATGGGTGTCACTGGATGTGCTT-3′ and reverse 5′-AGCACTGGCAGTGGCAAGTA-3′; IL-4, forward 5′-GTCATCCTGCTCTTCTTTCT-3′ and reverse 5′-GCTCACTCTCTGTGGTGTT-3′; and IL-13, forward 5′-TCTGTGTCTCTCCCTCTGA-3′ and reverse 5′-ATCCTCTGGGTCCTGTAGA-3′.
Parasites.
Mice were given a primary inoculation s.c. with 500 N. brasiliensis third-stage larvae (L3). Maintenance and recovery of mouse-adapted N. brasiliensis has been described previously (Urban et al., 2001). T. spiralis L1 were recovered by 1% pepsin/1% HCl digests of muscle from mice infected with this parasite for 90–120 d. Naive mice were inoculated by oral gavage with 200 L3 in 0.2 ml PBS/0.2% agarose (BD). H. polygyrus–infective ensheathed infective–stage larvae (L3) were propagated and stored at 4°C until use (Urban et al., 1995). For primary inoculation, mice received 200 L3 by oral gavage. For secondary inoculation, mice were first treated with the anthelminthic pyrantel pamoate 14 d after primary inoculation, allowed to rest for 28 d, and then rechallenged by oral gavage with 200 L3. Worm burdens were analyzed 7, 8, 9, 10, and 14 d after the second inoculation. Worm burdens were assessed by opening mouse intestines longitudinally and incubating them in PBS at 37°C for 3 h in a modified Baermann apparatus in which tissues were placed in a sieve atop a 250-ml beaker. Parasites that collected at the bottom were counted. For fecal egg counts, feces were collected, weighed, and incubated in saturated NaCl solution and eggs were counted using McMaster slides.
In vitro worm culture.
A modified Baermann apparatus was used to allow recovery of H. polygyrus adult worms from the intestinal lumen of donor mice 14–20 d after oral inoculation with 200 H. polygyrus L3. Worms were washed several times with PBS containing penicillin/streptomycin/gentamicin, counted, and incubated at 37°C 5% CO2 in RPMI for up to 3 d. Worms were incubated with rmRELM-β, rmRELM-α, or rmIL-4 (PeproTech). Media and recombinant proteins were replenished every 24 h over the course of the experiment.
Oral worm inoculation.
H. polygyrus adult worms were recovered from the intestinal lumen of donor mice 14–20 d after oral inoculation with 200 H. polygyrus L3. Recipient mice were injected twice i.p. at 16-h intervals (1 mg/dose) with a proton pump inhibitor (Protonix; Pfizer) before oral challenge with 100 H. polygyrus adult worms. Worms were pretreated with 100 ng/ml rmRELM-β, heat inactivated rmRELM-β (65°C for 30 min), rmRELM-α, or recombinant mouse IL-4 (PeproTech) for 2 h at room temperature and washed by centrifugation with PBS before inoculation by oral gavage. Adult worms and fecal eggs were counted 2 d later. Worms were collected no more than 30 min after tissues were placed in a Baermann apparatus in experiments that evaluated adult worm fluorescence staining and ATP content.
Detection of host epithelial cell ingestion in vivo by H. polygyrus.
Mice harboring H. polygyrus adults were injected i.v. with rhodamine B (1 mg/ml in saline) 1 h before sacrifice (Bansemir and Sukhdeo, 2001). Worms were photographed with a fluorescence microscope (Nikon) using an SPT Diagnostics imaging system and Simple PCI C-Imaging systems software.
Intestinal permeability determination in vitro.
1-cm segments of mucosa were mounted in U2500 Dual Channel Ussing chambers that exposed 0.30 cm2 of tissue to 10 ml Krebs buffer. Agar-salt bridges and electrodes were used to measure the potential difference. After a 15-min equilibrium period, basal short-circuit current (Isc), representing the net ion flux at baseline, and tissue resistance were determined. Every 50 s, the tissues were short-circuited at 1 V (EC 800 Epithelial Cell Voltage Clamp; Warner Instruments) and the Isc was monitored continuously. In addition, every 50 s the clamp voltage was adjusted to 3 mV for 5 s to allow calculation of tissue resistance using Ohm's law. After a second 15-min period, concentration-dependent changes in Isc (ΔIsc) were determined for the cumulative addition of methacholine to the serosal side of the stripped mucosa. In some experiments, 2.2 mg/ml FITC-dextran was added to the mucosal reservoir after equilibrium period and baseline potential difference and resistance had been established. Medium (0.25/10 ml) was removed from the serosal reservoir and replaced with fresh medium every 20 min over a period of 180 min for measurement of FITC-dextran concentration. To measure HRP concentration, 120 µl of sample was added to 800 µl of phosphate buffer containing 0.003% H2O2 and 80 µg/ml o-dianisidine (Sigma-Aldrich), and peroxidase activity was determined from the rate of increase in optical density at 460 nm during a 1.5-min period. The luminal-to-serosal flux was calculated using a standard formula and expressed as nanograms per milliliter. FITC-dextran concentration was determined from a standard curve of FITC-dextran concentration versus fluorescence using a FLX800 96-well microplate fluorescence reader (excitation, 490 nm; emission, 530 nm).
Intestinal permeability determination in vivo.
Mice were anesthetized by inhalation of Metophen (Schering-Plough Animal Health), after which 20 ml/kg body weight of phosphate-buffered saline, pH 7.4, containing 22 mg/ml FITC-dextran and 1 mg/ml HRP, was administered by gavage. A blood sample (∼150 µl) in a capillary tube was obtained by orbital retrobulbar puncture 4 h after administration of the markers. The blood samples were centrifuged (3,000 rpm at 4°C) for 20 min. 50 µl of plasma was mixed with an equal volume of phosphate-buffered saline, pH 7.4, and HRP activity and FITC-dextran levels were quantitated as described in the beginning of this section.
Microscopy/histology.
Histologically processed sections of jejunum tissue (5 µm) were stained with the periodic acid Schiff reagent and goblet cell number was determined from coded slides. Formalin-fixed paraffin-embedded jejunum was used for immunofluorescent detection of RELM-β+ goblet cells (Texas red) and cell nuclei (DAPI). A microscope (Eclipse E600; Nikon) fitted with a 40× objective oil-immersion lens (Plan Apo; Nikon) was used for image acquisition at ambient temperature (25°C). Photographs were obtained with a SPOT Diagnostics RT slider digital color camera using a SPOT Diagnostics imaging system (SPOT version 3.5 for windows software; Diagnostic Instruments, Inc.). Photographs of RELM-α– versus RELM-β–treated worms were taken at 4× using a National digital stereomicroscope with Motic images Plus 2.0 software (Motic China group).
ATP assay.
The ATPLite kit (Perkin Elmer) was used to measure the ATP content of individual H. polygyrus adult worms (Ishiwata and Watanabe, 2007).
Statistical analysis.
The statistical significance of differences between two groups was determined using a Students' t test or the Mann-Whitney U test. P-values of <0.05 were considered significant. All analyses were performed with Prism 4.0 software (GraphPad Software, Inc.). Data are expressed as means ± SEM.
Online supplemental material.
Fig. S1 demonstrates that villin promoter activity is restricted to small and large intestinal epithelium. Fig. S2 demonstrates that arginase I is highly expressed in tissue cysts that surround H. polygyrus larvae in RELM-β−/− mice. Fig. S3 shows IL-4 and IL-13 intestinal mRNA transcripts 10 d after inoculation of WT and RELM-β−/− mice infected with H. polygyrus, and an equivalent increase of intestinal permeability between WT and RELM-β−/− mice infected with H. polygyrus. Fig. S4 demonstrates that WT and RELM-β−/− mice exhibit similar kinetics of worm expulsion and mast cell degranulation after infection with T. spiralis. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20091268/DC1.
Acknowledgments
We thank Crystal Potter and Amanda Roloson for technical assistance.
This work was supported by the U.S. Department of Veterans Affairs and National Institutes of Health grants RO1GM083204, RO1 GM49758, and RO1 AI052099.
The authors declare no competing financial interests.
Footnotes
Abbreviations used:
- GI
- gastrointestinal
- HRP
- horseradish peroxidase
- IEC
- intestinal epithelial cell
- IVCCA
- in vivo cytokine capture assay
- mRNA
- messenger RNA
- RELM
- resistin-like molecule
References
- Anthony R.M., Urban J.F., Jr., Alem F., Hamed H.A., Rozo C.T., Boucher J.L., Van Rooijen N., Gause W.C. 2006. Memory T(H)2 cells induce alternatively activated macrophages to mediate protection against nematode parasites. Nat. Med. 12:955–960 10.1038/nm1451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony R.M., Rutitzky L.I., Urban J.F., Jr., Stadecker M.J., Gause W.C. 2007. Protective immune mechanisms in helminth infection. Nat. Rev. Immunol. 7:975–987 10.1038/nri2199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artis D., Wang M.L., Keilbaugh S.A., He W., Brenes M., Swain G.P., Knight P.A., Donaldson D.D., Lazar M.A., Miller H.R., et al. 2004. RELMbeta/FIZZ2 is a goblet cell-specific immune-effector molecule in the gastrointestinal tract. Proc. Natl. Acad. Sci. USA. 101:13596–13600 10.1073/pnas.0404034101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansemir A.D., Sukhdeo M.V. 2001. Intestinal distribution of worms and host ingesta in Nippostrongylus brasiliensis. J. Parasitol. 87:1470–1472 [DOI] [PubMed] [Google Scholar]
- Cliffe L.J., Humphreys N.E., Lane T.E., Potten C.S., Booth C., Grencis R.K. 2005. Accelerated intestinal epithelial cell turnover: a new mechanism of parasite expulsion. Science. 308:1463–1465 10.1126/science.1108661 [DOI] [PubMed] [Google Scholar]
- Else K.J., Finkelman F.D., Maliszewski C.R., Grencis R.K. 1994. Cytokine-mediated regulation of chronic intestinal helminth infection. J. Exp. Med. 179:347–351 10.1084/jem.179.1.347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon P.G., Jolin H.E., Smith P., Emson C.L., Townsend M.J., Fallon R., Smith P., McKenzie A.N. 2002. IL-4 induces characteristic Th2 responses even in the combined absence of IL-5, IL-9, and IL-13. Immunity. 17:7–17 10.1016/S1074-7613(02)00332-1 [DOI] [PubMed] [Google Scholar]
- Fallon P.G., Ballantyne S.J., Mangan N.E., Barlow J.L., Dasvarma A., Hewett D.R., McIlgorm A., Jolin H.E., McKenzie A.N. 2006. Identification of an interleukin (IL)-25–dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion. J. Exp. Med. 203:1105–1116 10.1084/jem.20051615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelman F.D., Morris S.C. 1999. Development of an assay to measure in vivo cytokine production in the mouse. Int. Immunol. 11:1811–1818 10.1093/intimm/11.11.1811 [DOI] [PubMed] [Google Scholar]
- Finkelman F.D., Madden K.B., Morris S.C., Holmes J.M., Boiani N., Katona I.M., Maliszewski C.R. 1993. Anti-cytokine antibodies as carrier proteins. Prolongation of in vivo effects of exogenous cytokines by injection of cytokine-anti-cytokine antibody complexes. J. Immunol. 151:1235–1244 [PubMed] [Google Scholar]
- Finkelman F.D., Shea-Donohue T., Goldhill J., Sullivan C.A., Morris S.C., Madden K.B., Gause W.C., Urban J.F., Jr 1997. Cytokine regulation of host defense against parasitic gastrointestinal nematodes: lessons from studies with rodent models. Annu. Rev. Immunol. 15:505–533 10.1146/annurev.immunol.15.1.505 [DOI] [PubMed] [Google Scholar]
- Fulkerson P.C., Zimmermann N., Hassman L.M., Finkelman F.D., Rothenberg M.E. 2004. Pulmonary chemokine expression is coordinately regulated by STAT1, STAT6, and IFN-γ. J. Immunol. 173:7565–7574 [DOI] [PubMed] [Google Scholar]
- Gagliardo L.F., McVay C.S., Appleton J.A. 2002. Molting, ecdysis, and reproduction of Trichinella spiralis are supported in vitro by intestinal epithelial cells. Infect. Immun. 70:1853–1859 10.1128/IAI.70.4.1853-1859.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert D.R., Hölscher C., Mohrs M., Arendse B., Schwegmann A., Radwanska M., Leeto M., Kirsch R., Hall P., Mossmann H., et al. 2004. Alternative macrophage activation is essential for survival during schistosomiasis and downmodulates T helper 1 responses and immunopathology. Immunity. 20:623–635 10.1016/S1074-7613(04)00107-4 [DOI] [PubMed] [Google Scholar]
- Hickey M.J., Granger D.N., Kubes P. 1999. Molecular mechanisms underlying IL-4-induced leukocyte recruitment in vivo: a critical role for the α 4 integrin. J. Immunol. 163:3441–3448 [PubMed] [Google Scholar]
- Hogan S.P., Seidu L., Blanchard C., Groschwitz K., Mishra A., Karow M.L., Ahrens R., Artis D., Murphy A.J., Valenzuela D.M., et al. 2006. Resistin-like molecule β regulates innate colonic function: barrier integrity and inflammation susceptibility. J. Allergy Clin. Immunol. 118:257–268 10.1016/j.jaci.2006.04.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsnell W.G., Cutler A.J., Hoving J.C., Hoving C.J., Mearns H., Myburgh E., Arendse B., Finkelman F.D., Owens G.K., Erle D., Brombacher F. 2007. Delayed goblet cell hyperplasia, acetylcholine receptor expression, and worm expulsion in SMC-specific IL-4Ralpha-deficient mice. PLoS Pathog. 3:e1 10.1371/journal.ppat.0030001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys N.E., Xu D., Hepworth M.R., Liew F.Y., Grencis R.K. 2008. IL-33, a potent inducer of adaptive immunity to intestinal nematodes. J. Immunol. 180:2443–2449 [DOI] [PubMed] [Google Scholar]
- Ishiwata K., Watanabe N. 2007. Nippostrongylus brasiliensis: reversibility of reduced-energy status associated with the course of expulsion from the small intestine in rats. Exp. Parasitol. 117:80–86 10.1016/j.exppara.2007.03.019 [DOI] [PubMed] [Google Scholar]
- Junttila I.S., Mizukami K., Dickensheets H., Meier-Schellersheim M., Yamane H., Donnelly R.P., Paul W.E. 2008. Tuning sensitivity to IL-4 and IL-13: differential expression of IL-4Rα, IL-13Rα1, and γc regulates relative cytokine sensitivity. J. Exp. Med. 205:2595–2608 10.1084/jem.20080452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodoun M., Lewis C.C., Lewis C., Yang J.Q., Orekov T., Potter C., Wynn T., Mentink-Kane M., Hershey G.K., Wills-Karp M., Finkelman F.D. 2007. Differences in expression, affinity, and function of soluble (s)IL-4Ralpha and sIL-13Ralpha2 suggest opposite effects on allergic responses. J. Immunol. 179:6429–6438 [DOI] [PubMed] [Google Scholar]
- Kuperman D.A., Huang X., Koth L.L., Chang G.H., Dolganov G.M., Zhu Z., Elias J.A., Sheppard D., Erle D.J. 2002. Direct effects of interleukin-13 on epithelial cells cause airway hyperreactivity and mucus overproduction in asthma. Nat. Med. 8:885–889 [DOI] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 25:402–408 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- Miller S.D., Jenkins M.K. 1985. In vivo effects of GK1.5 (anti-L3T4a) monoclonal antibody on induction and expression of delayed-type hypersensitivity. Cell. Immunol. 92:414–426 10.1016/0008-8749(85)90022-X [DOI] [PubMed] [Google Scholar]
- Morris S.C., Heidorn S.M., Herbert D.R., Perkins C., Hildeman D.A., Khodoun M.V., Finkelman F.D., Finkelman F.D. 2009. Endogenously produced IL-4 nonredundantly stimulates CD8+ T cell proliferation. J. Immunol. 182:1429–1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T.R., Fong T.A. 1989. Specific assays for cytokine production by T cells. J. Immunol. Methods. 116:151–158 10.1016/0022-1759(89)90198-1 [DOI] [PubMed] [Google Scholar]
- Nair M.G., Guild K.J., Du Y., Zaph C., Yancopoulos G.D., Valenzuela D.M., Murphy A., Stevens S., Karow M., Artis D. 2008. Goblet cell-derived resistin-like molecule β augments CD4+ T cell production of IFN-γ and infection-induced intestinal inflammation. J. Immunol. 181:4709–4715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair M.G., Du Y., Perrigoue J.G., Zaph C., Taylor J.J., Goldschmidt M., Swain G.P., Yancopoulos G.D., Valenzuela D.M., Murphy A., et al. 2009. Alternatively activated macrophage-derived RELM-α is a negative regulator of type 2 inflammation in the lung. J. Exp. Med. 206:937–952 10.1084/jem.20082048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S.D., Rajala M.W., Rossetti L., Scherer P.E., Shapiro L. 2004. Disulfide-dependent multimeric assembly of resistin family hormones. Science. 304:1154–1158 10.1126/science.1093466 [DOI] [PubMed] [Google Scholar]
- Pesce J.T., Ramalingam T.R., Wilson M.S., Mentink-Kane M.M., Thompson R.W., Cheever A.W., Urban J.F., Jr., Wynn T.A. 2009. Retnla (relmalpha/fizz1) suppresses helminth-induced Th2-type immunity. PLoS Pathog. 5:e1000393 10.1371/journal.ppat.1000393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramalingam T.R., Pesce J.T., Sheikh F., Cheever A.W., Mentink-Kane M.M., Wilson M.S., Stevens S., Valenzuela D.M., Murphy A.J., Yancopoulos G.D., et al. 2008. Unique functions of the type II interleukin 4 receptor identified in mice lacking the interleukin 13 receptor α1 chain. Nat. Immunol. 9:25–33 10.1038/ni1544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinecker H.C., Podolsky D.K. 1995. Human intestinal epithelial cells express functional cytokine receptors sharing the common γ c chain of the interleukin 2 receptor. Proc. Natl. Acad. Sci. USA. 92:8353–8357 10.1073/pnas.92.18.8353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea-Donohue T., Sullivan C., Finkelman F.D., Madden K.B., Morris S.C., Goldhill J., Piñeiro-Carrero V., Urban J.F., Jr 2001. The role of IL-4 in Heligmosomoides polygyrus-induced alterations in murine intestinal epithelial cell function. J. Immunol. 167:2234–2239 [DOI] [PubMed] [Google Scholar]
- Strait R.T., Morris S.C., Smiley K., Urban J.F., Jr., Finkelman F.D. 2003. IL-4 exacerbates anaphylaxis. J. Immunol. 170:3835–3842 [DOI] [PubMed] [Google Scholar]
- Su Z., Dobson C. 1997. Genetic and immunological adaptation of Heligmosomoides polygyrus in mice. Int. J. Parasitol. 27:653–663 10.1016/S0020-7519(97)00004-0 [DOI] [PubMed] [Google Scholar]
- Urban J.F., Jr., Katona I.M., Paul W.E., Finkelman F.D. 1991. Interleukin 4 is important in protective immunity to a gastrointestinal nematode infection in mice. Proc. Natl. Acad. Sci. USA. 88:5513–5517 10.1073/pnas.88.13.5513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban J.F., Jr., Maliszewski C.R., Madden K.B., Katona I.M., Finkelman F.D. 1995. IL-4 treatment can cure established gastrointestinal nematode infections in immunocompetent and immunodeficient mice. J. Immunol. 154:4675–4684 [PubMed] [Google Scholar]
- Urban J.F., Jr., Schopf L., Morris S.C., Orekhova T., Madden K.B., Betts C.J., Gamble H.R., Byrd C., Donaldson D., Else K., Finkelman F.D. 2000. Stat6 signaling promotes protective immunity against Trichinella spiralis through a mast cell- and T cell-dependent mechanism. J. Immunol. 164:2046–2052 [DOI] [PubMed] [Google Scholar]
- Urban J.F., Jr., Noben-Trauth N., Schopf L., Madden K.B., Finkelman F.D. 2001. Cutting edge: IL-4 receptor expression by non-bone marrow-derived cells is required to expel gastrointestinal nematode parasites. J. Immunol. 167:6078–6081 [DOI] [PubMed] [Google Scholar]
- Voehringer D., Reese T.A., Huang X., Shinkai K., Locksley R.M. 2006. Type 2 immunity is controlled by IL-4/IL-13 expression in hematopoietic non-eosinophil cells of the innate immune system. J. Exp. Med. 203:1435–1446 10.1084/jem.20052448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaph C., Troy A.E., Taylor B.C., Berman-Booty L.D., Guild K.J., Du Y., Yost E.A., Gruber A.D., May M.J., Greten F.R., et al. 2007. Epithelial-cell-intrinsic IKK-β expression regulates intestinal immune homeostasis. Nature. 446:552–556 10.1038/nature05590 [DOI] [PubMed] [Google Scholar]
- Zhao A., McDermott J., Urban J.F., Jr., Gause W., Madden K.B., Yeung K.A., Morris S.C., Finkelman F.D., Shea-Donohue T. 2003. Dependence of IL-4, IL-13, and nematode-induced alterations in murine small intestinal smooth muscle contractility on Stat6 and enteric nerves. J. Immunol. 171:948–954 [DOI] [PubMed] [Google Scholar]
- Zhao A., Urban J.F., Jr., Anthony R.M., Sun R., Stiltz J., van Rooijen N., Wynn T.A., Gause W.C., Shea-Donohue T. 2008. Th2 cytokine-induced alterations in intestinal smooth muscle function depend on alternatively activated macrophages. Gastroenterology. 135:217–225.e1 10.1053/j.gastro.2008.03.077 [DOI] [PMC free article] [PubMed] [Google Scholar]