Abstract
Mycobacterium tuberculosis is a leading killer worldwide, yet the adjuvancy of its cell wall has proven to be a valuable therapeutic tool for vaccination and immunotherapy. Much research effort has focused on the mycobacterial glycolipid trehalose-6,6’-dimycolate (TDM), a potent immunostimulant that is also known as cord factor. Now, the identification of the monocyte-inducible C-type lectin (Mincle) as an essential receptor for TDM provides new insight into the formation of the characteristic granulomas in tuberculosis and an avenue for rational adjuvant design.
A dirty little secret
Mycobacterium tuberculosis is a devastating pathogen that kills >1.5 million people each year, but controlled use of its cell wall activates macrophages in ways that can be harnessed for therapy. For example, M. bovis Bacille Calmette-Guérin (BCG) is one of the most widely used antitumor adjuvant therapies in humans. Injection of BCG into the bladder mediates regression of transitional cell carcinomas by stimulating a vigorous local immune response, bathing tumors in cytokines and activated immune cells (Brandau and Suttmann, 2007). Similarly, Freund’s adjuvant, an emulsion of mycobacterial cell wall components in paraffin oil, is mixed with antigens to optimize memory T and B cell responses in mice (Freund et al., 1948). In 1989, Charles Janeway Jr. dubbed the widespread use of Freund’s adjuvant “the immunologist’s dirty little secret” (Janeway, 1989). This practice was considered a secret because the co-stimulatory properties of adjuvants were overshadowed by the focus on antigen interactions with T and B cell receptors. 20 yr later, the role of innate receptors in licensing adaptive responses is no longer a secret, but Freund’s adjuvant remains dirty. Freund’s and BCG adjuvants contain hundreds of natural molecules, yet the particular components that mediate therapeutic effects have not been identified.
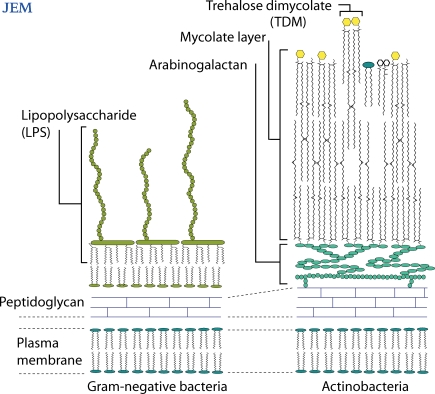
Janeway advocated systematically sorting through the components of empirically discovered adjuvants to identify the individual stimulatory molecules and their cellular receptors, especially those that lead to interleukin-1 (IL-1) secretion (Janeway, 1989). For Gram-negative bacteria, lipopolysaccharide (LPS) is the most potent cell wall component that leads to innate immune activation and sepsis (Fig. 1). Discovery of the mechanism by which LPS activates CD14, Toll-like receptor 4 (TLR4), and myeloid differentiation factor 88 (MyD88) represents a major advance in understanding Gram-negative bacterial sepsis, a cytokine-mediated disease. On page 2879 of this issue, Ishikawa et al. (2009) identify a ligand-receptor interaction that plays a role in the characteristic immune process of mycobacterial infection: granuloma formation. Until recently, Mincle was an orphan receptor for which ligands were unknown. Now, this lectin is shown to bind and mediate responses to an immunodominant glycolipid in the mycobacterial cell wall, TDM (Fig. 1). Here, we discuss Mincle as a target for rational adjuvant design and review TLRs and C-type lectins as two major innate pathways for host response during M. tuberculosis infection.
Figure 1.
Actinobacteria contain a unique outer layer composed of mycolate lipids. One of those lipids, TDM, is an immunostimulant whose potency compares to Gram-negative bacterial LPS, but the nature of the inflammation triggered by these two molecules differ.
Cord factor controls inflammation, not cording
In search of a bacterial factor that mediates a characteristic growth pattern known as cording, Hubert Bloch isolated a glycolipid from the tubercle bacillus (Bloch, 1950), the structure of which was solved as TDM (Fig. 1; Noll et al., 1956). Although widely known as cord factor, this glycolipid is no longer believed to be the cause of bacterial cording. Instead, TDM has remained the focus of sustained research for five decades because of its ability to potently activate the innate immune system of mammals (Hunter et al., 2006). M. tuberculosis is encased in an outer membrane formed of very long chain α-branched, β-hydroxy fatty acids (Fig. 1). These unusual lipids are known as mycolic acids because the lipid type is found only in mycobacteria and related actinobacteria. Mycolate lipids like TDM are foreign molecules and can be considered Actinomycetales-specific biomarkers. Therefore, identifying the cellular receptors for TDM might begin to explain why mycobacteria, in contrast to many other types of bacteria, generate prominent granulomas during natural infection.
TDM strongly contributes to inflammation seen in mycobacterial infection. For example, injection of pure TDM in mice stimulates the formation of lung granulomas (Bekierkunst et al., 1969) and induces a generalized wasting that mimics certain features of consumption in tuberculosis patients (Kato, 1968; Ryll et al., 2001). Although mycobacteria contain several classes of immunostimulants, TDM has been demonstrated to be the most potent stimulator of IL-1, TNF, and granuloma formation among the BCG cell wall glycolipids (Geisel et al., 2005). After early studies showed that natural TDMs enhanced antibody production by B cells (Bekierkunst et al., 1971), synthetic TDMs with simplified structures are being developed as therapeutic adjuvants (Davidsen et al., 2005). These studies provide a strong rationale to understand which receptors on macrophages and dendritic cells mediate the response.
Vetting candidate receptors
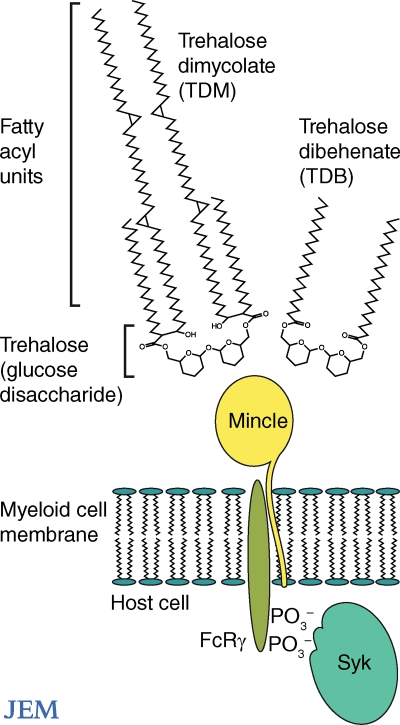
The most extensively studied innate receptors for mycobacteria are TLRs and C-type lectins. Both cell surface receptor families lack intracellular signaling motifs and depend on adaptor proteins for signaling. TLRs signal through the adaptor MyD88. C-type lectins signal through adaptors DAP10, DAP12, or Fc receptor γ chain (FcRγ), all of which bind phosphorylated Syk proteins with immunoreceptor tyrosine activation motifs (ITAMs; Fig. 2). Before the studies of Mincle reported here, it was suspected that either TLRs (Bowdish et al., 2009) or C-type lectins (Werninghaus et al., 2009) might be responsible for TDM-induced activation of macrophages. Either receptor family represents a plausible candidate based on their general patterns of ligand specificity. The TLR2-1 heterodimer is a lipid binding receptor that uses two hydrophobic pockets to sequester the hydrophobic triacylation units on the N terminus of bacterial proteins (Jin et al., 2007). The finding that TLR2, in combination with other receptors, influences macrophage activation by TDM raised the possibility that TLR2 might in some way capture the mycolate lipid units (Bowdish et al., 2009). In contrast, C-type lectins are carbohydrate-binding proteins and would be predicted to bind TDM’s trehalose disaccharide (Fig. 2).
Figure 2.
The mycobacterial lipid TDM and its synthetic analogue TDB exist as symmetric trehalose glycolipid structures. Their receptor, Mincle, couples with Syk and the FcRγ chain.
The case for C-type lectins as TDM receptors was supported by a study earlier this year showing that TDM signaling involves Syk and FcRγ (Werninghaus et al., 2009). Deletion of Syk or any of its downstream signaling proteins (Card9, Bcl10, or Malt1) essentially eliminated measurable responses to TDM by mouse macrophages. Additionally, testing of Syk-associated proteins showed that the FcRγ, but not DAP12, was necessary for recognition of TDM (Fig. 2). Looking further upstream for cell surface receptors, it was determined that one of the FcRγ-associated proteins, Dectin-1, was not the putative receptor. The authors predicted that the TDM responses would involve another FcRγ-associated receptor such as Dectin-2, dendritic cell activating receptor, or Mincle (Werninghaus et al., 2009).
Sorting through cell walls
With this information, it might have been possible to discover a receptor for TDM by analyzing molecules in the Syk–FcRγ pathway, but the work reported here instead pursued the identification of Mincle ligands using reporter constructs. After demonstrating that Mincle binds to FcRγ in its transmembrane segment (Yamasaki et al., 2008), Mincle and FcRγ were transfected together with an ITAM-dependent expression system into lymphoid cells to generate a reporter line. Whereas early studies of Mincle suggested that it is activated by Candida albicans (Bugarcic et al., 2008; Wells et al., 2008) or fungal saprophytes of the Malassezia genus (Yamasaki et al., 2009), Ishikawa et al. (2009) linked Mincle to M. tuberculosis, a pathogen of worldwide importance. Following up on this important observation, the authors used the reporter to identify the particular cell wall structures that activate Mincle.
First, a targeted approach was used based on the annotation of Mincle’s carbohydrate recognition domain (CRD; Balch et al., 2002) as a mannose-binding motif (Fig. 2). To examine the contribution of mannose residues for Mincle activation, lipids from mycobacteria lacking mannosyl transferases and mannolipids were tested one by one for Mincle activity. The targeted experiments, however, yielded no results. Next, the authors undertook a systematic approach whereby all chromatographically separated cell wall components were exposed to the reporter line without regard to whether their structures contained mannose. This approach led to successful identification of TDM as the Mincle ligand. Thus, mycobacterial recognition by Mincle does not depend upon mannose-containing glycolipids. Although the final answer awaits further quantitative structure–function studies, the mannose-binding motif in Mincle likely contacts some aspect of the glucose disaccharide, trehalose, in TDM (Fig. 2). The apparent contradiction is resolved by understanding that proteins with mannose motifs also bind structurally related sugars like glucose.
This unbiased discovery approach is less dependent on assumptions, yet it is simple and effective. Although transcriptional profiling has transformed our ability to investigate responses to defined stimuli, it requires physically arraying libraries on chips and detection of individual transcripts. In contrast, all microbe-derived small molecules can be rapidly extracted and the eluent loaded onto a matrix to provide a stream of compounds that are individually fed into a biological testing system. This global analysis of bacterial extracts provides information about the number, relative potency, and structural relatedness of all bioactive compounds in an organism (Moody et al., 2004; Geisel et al., 2005).
Is Mincle the major pathway?
In mice, Mincle deletion essentially abolishes many aspects of the in vivo response to purified TDM, including granulomatous lung inflammation (Ishikawa et al., 2009). Thus, Mincle plays an essential role in response to TDM. However, mycobacteria produce several classes of immunostimulants, and only further investigation will determine whether blocking Mincle–TDM interactions also alters the course of mycobacterial infection. M. tuberculosis produces several compounds that act in parallel via TLR2, TLR9, mannose receptor, Dectin-1, NOD2, and other receptors. Illustrating the redundant nature of innate response to this pathogen, wild-type and TLR-deficient mice show only modest differences in outcomes of experimental infection (Su et al., 2005), despite the remarkably strong host responses to mycobacterial TLR-2 ligands when they are administered as individual stimuli. M. tuberculosis is a readily communicable pathogen that has infected one third of all humans, so it is unsurprising that effective immunity does not rely on a single receptor. However, in the current study, Ishikawa et al. (2009) found that dual deletion of Myd88 and Mincle pathways reduces key aspects of the overall immune response, including TNF production. Thus, the discovery of the role of the Mincle–FcRγ–Syk pathway in TB infection now provides a tool to tease out the separate and interrelated roles of C-type lectins and TLRs as two dominant pathways in host response to mycobacteria.
Mincle opens new adjuvant avenues
Microbial products are typically screened for bioactivity at micromolar doses, but LPS, TDM, and other natural molecules are stimulatory at lower, nanomolar concentrations. Because even trace contaminates in natural products can lead to misidentification of bioactive compounds, chemical synthesis is typically necessary to validate ligands for sensitive innate receptors. The report by Ishikawa et al. (2009) is convincing because Mincle could be activated by a synthetic analogue, trehalose dibehenate (TDB). This result also provides insight into potential molecular mechanisms of activation. Recognition of TDB by Mincle proves that activation does not require the long, branched, complex structures found in natural mycolic acids. Instead, Mincle binding appears to be specific for the ester linkage of a fatty acid to trehalose (Fig. 2). Humans express a functional Mincle orthologue (Arce et al., 2004), so synthetic and simplified trehalose glycolipids (Davidsen et al., 2005) might be developed as adjuvants for human use. Both TDM and TDB are composed of two glucose mycolate units, which are mirror images of each other (Fig. 2). Each of the glucose mycolate units is a potential Mincle epitope. Because TDM analogues that lack elements of the second unit are not strongly stimulatory, a simple, but untested model is that TDM serves as a divalent activator, which cross-links two Mincle receptors. Further testing of this model is of clinical import because it predicts that TDB-like molecules with mono- or multivalent epitopes might be developed as blockers or superagonists of Mincle. It would be tremendously satisfying to see the immunologist’s dirty little secret cleaned up to exploit a potentially important pathway of macrophage and dendritic cell activation.
Acknowledgments
D.B. Moody is supported by the Harvard Initiative for Global Health, the Burroughs Wellcome Fund for Translational Research, and the National Institute of Allergy and Infectious Disease (R01049313 071155, AI). I. Matsunaga is supported by the Shimizu Foundation and a Grant in Aid from the Japan Society for Promotion of Science (19510216).
References
- Arce I., Martínez-Muñoz L., Roda-Navarro P., Fernández-Ruiz E. 2004. The human C-type lectin CLECSF8 is a novel monocyte/macrophage endocytic receptor. Eur. J. Immunol. 34:210–220 10.1002/eji.200324230 [DOI] [PubMed] [Google Scholar]
- Balch S.G., Greaves D.R., Gordon S., McKnight A.J. 2002. Organization of the mouse macrophage C-type lectin (Mcl) gene and identification of a subgroup of related lectin molecules. Eur. J. Immunogenet. 29:61–64 10.1046/j.1365-2370.2002.00266.x [DOI] [PubMed] [Google Scholar]
- Bekierkunst A., Levij I.S., Yarkoni E., Vilkas E., Adam A., Lederer E. 1969. Granuloma formation induced in mice by chemically defined mycobacterial fractions. J. Bacteriol. 100:95–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekierkunst A., Yarkoni E., Flechner I., Morecki S., Vilkas E., Lederer E. 1971. Immune response to sheep red blood cells in mice pretreated with mycobacterial fractions. Infect. Immun. 4:256–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch H. 1950. Studies on the virulence of tubercle bacilli; isolation and biological properties of a constituent of virulent organisms. J. Exp. Med. 91:197–218 10.1084/jem.91.2.197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowdish D.M., Sakamoto K., Kim M.J., Kroos M., Mukhopadhyay S., Leifer C.A., Tryggvason K., Gordon S., Russell D.G. 2009. MARCO, TLR2, and CD14 are required for macrophage cytokine responses to mycobacterial trehalose dimycolate and Mycobacterium tuberculosis. PLoS Pathog. 5:e1000474 10.1371/journal.ppat.1000474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandau S., Suttmann H. 2007. Thirty years of BCG immunotherapy for non-muscle invasive bladder cancer: a success story with room for improvement. Biomed. Pharmacother. 61:299–305 10.1016/j.biopha.2007.05.004 [DOI] [PubMed] [Google Scholar]
- Bugarcic A., Hitchens K., Beckhouse A.G., Wells C.A., Ashman R.B., Blanchard H. 2008. Human and mouse macrophage-inducible C-type lectin (Mincle) bind Candida albicans. Glycobiology. 18:679–685 10.1093/glycob/cwn046 [DOI] [PubMed] [Google Scholar]
- Davidsen J., Rosenkrands I., Christensen D., Vangala A., Kirby D., Perrie Y., Agger E.M., Andersen P. 2005. Characterization of cationic liposomes based on dimethyldioctadecylammonium and synthetic cord factor from M. tuberculosis (trehalose 6,6′-dibehenate)-a novel adjuvant inducing both strong CMI and antibody responses. Biochim. Biophys. Acta. 1718:22–31 10.1016/j.bbamem.2005.10.011 [DOI] [PubMed] [Google Scholar]
- Freund J., Thomson K.J., et al. 1948. Antibody formation and sensitization with the aid of adjuvants. J. Immunol. 60:383–398 [PubMed] [Google Scholar]
- Geisel R.E., Sakamoto K., Russell D.G., Rhoades E.R. 2005. In vivo activity of released cell wall lipids of Mycobacterium bovis bacillus Calmette-Guérin is due principally to trehalose mycolates. J. Immunol. 174:5007–5015 [DOI] [PubMed] [Google Scholar]
- Hunter R.L., Olsen M.R., Jagannath C., Actor J.K. 2006. Multiple roles of cord factor in the pathogenesis of primary, secondary, and cavitary tuberculosis, including a revised description of the pathology of secondary disease. Ann. Clin. Lab. Sci. 36:371–386 [PubMed] [Google Scholar]
- Ishikawa E., Ishikawa T., Morita Y.S., Toyonaga K., Yamada H., Takeuchi O., Kinoshita T., Akira S., Yoshikai Y., Yamasaki S. 2009. Direct recognition of the mycobacterial glycolipid, trehalose dimycolate, by C-type lectin Mincle. J. Exp. Med. 206:2879–2888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janeway C.A., Jr. 1989. Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb. Symp. Quant. Biol. 54:1–13 [DOI] [PubMed] [Google Scholar]
- Jin M.S., Kim S.E., Heo J.Y., Lee M.E., Kim H.M., Paik S.G., Lee H., Lee J.O. 2007. Crystal structure of the TLR1-TLR2 heterodimer induced by binding of a tri-acylated lipopeptide. Cell. 130:1071–1082 10.1016/j.cell.2007.09.008 [DOI] [PubMed] [Google Scholar]
- Kato M. 1968. Studies of a biochemical lesion in experimental tuberculosis in mice. 8. Effect of derivatives and chemical analogues of cord factor on structure and function of mouse liver mitochondria. Am. Rev. Respir. Dis. 98:668–676 [DOI] [PubMed] [Google Scholar]
- Moody D.B., Young D.C., Cheng T.Y., Rosat J.P., Roura-Mir C., O’Connor P.B., Zajonc D.M., Walz A., Miller M.J., Levery S.B., et al. 2004. T cell activation by lipopeptide antigens. Science. 303:527–531 10.1126/science.1089353 [DOI] [PubMed] [Google Scholar]
- Noll H., Bloch H., Asselineau J., Lederer E. 1956. The chemical structure of the cord factor of Mycobacterium tuberculosis. Biochim. Biophys. Acta. 20:299–309 10.1016/0006-3002(56)90289-X [DOI] [PubMed] [Google Scholar]
- Ryll R., Kumazawa Y., Yano I. 2001. Immunological properties of trehalose dimycolate (cord factor) and other mycolic acid-containing glycolipids—a review. Microbiol. Immunol. 45:801–811 [DOI] [PubMed] [Google Scholar]
- Su S.B., Silver P.B., Grajewski R.S., Agarwal R.K., Tang J., Chan C.C., Caspi R.R. 2005. Essential role of the MyD88 pathway, but nonessential roles of TLRs 2, 4, and 9, in the adjuvant effect promoting Th1-mediated autoimmunity. J. Immunol. 175:6303–6310 [DOI] [PubMed] [Google Scholar]
- Wells C.A., Salvage-Jones J.A., Li X., Hitchens K., Butcher S., Murray R.Z., Beckhouse A.G., Lo Y.L., Manzanero S., Cobbold C., et al. 2008. The macrophage-inducible C-type lectin, mincle, is an essential component of the innate immune response to Candida albicans. J. Immunol. 180:7404–7413 [DOI] [PubMed] [Google Scholar]
- Werninghaus K., Babiak A., Gross O., Hölscher C., Dietrich H., Agger E.M., Mages J., Mocsai A., Schoenen H., Finger K., et al. 2009. Adjuvanticity of a synthetic cord factor analogue for subunit Mycobacterium tuberculosis vaccination requires FcRgamma-Syk-Card9-dependent innate immune activation. J. Exp. Med. 206:89–97 10.1084/jem.20081445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki S., Ishikawa E., Sakuma M., Hara H., Ogata K., Saito T. 2008. Mincle is an ITAM-coupled activating receptor that senses damaged cells. Nat. Immunol. 9:1179–1188 10.1038/ni.1651 [DOI] [PubMed] [Google Scholar]
- Yamasaki S., Matsumoto M., Takeuchi O., Matsuzawa T., Ishikawa E., Sakuma M., Tateno H., Uno J., Hirabayashi J., Mikami Y., et al. 2009. C-type lectin Mincle is an activating receptor for pathogenic fungus, Malassezia. Proc. Natl. Acad. Sci. USA. 106:1897–1902 10.1073/pnas.0805177106 [DOI] [PMC free article] [PubMed] [Google Scholar]