Abstract
During thymopoiesis, a unique program of gene expression promotes the development of CD4 regulatory T (T reg) cells. Although Foxp3 maintains a pattern of gene expression necessary for T reg cell function, other transcription factors are emerging as important determinants of T reg cell development. We show that the NF-κB transcription factor c-Rel is highly expressed in thymic T reg cells and that in c-rel−/− mice, thymic T reg cell numbers are markedly reduced as a result of a T cell–intrinsic defect that is manifest during thymocyte development. Although c-Rel is not essential for TGF-β conversion of peripheral CD4+CD25− T cells into CD4+Foxp3+ cells, it is required for optimal homeostatic expansion of peripheral T reg cells. Despite a lower number of peripheral T reg cells in c-rel−/− mice, the residual peripheral c-rel−/− T reg cells express normal levels of Foxp3, display a pattern of cell surface markers and gene expression similar to those of wild-type T reg cells, and effectively suppress effector T cell function in culture and in vivo. Collectively, our results indicate that c-Rel is important for both the thymic development and peripheral homeostatic proliferation of T reg cells.
Regulatory T (T reg) cells represent a class of T lymphocytes that serve to restrict the extent and duration of immune responses, as well as maintain self-tolerance by suppressing autoreactive T cells (Shevach, 2000; Sakaguchi, 2004, 2005; Fontenot and Rudensky, 2005; Ziegler, 2006). Among the different types of T reg cells described to date, CD4+CD25+ cells that express the transcription factor Foxp3 are the most well characterized T cells with immune-suppressive properties (Fontenot and Rudensky, 2005; Sakaguchi, 2005; Ziegler, 2006). Most Foxp3+ CD4 T cells, referred to as CD4 T reg cells, begin to develop in the thymus between 2 and 3 d after birth (Fontenot et al., 2005a). Like conventional CD4+ T cells, thymus-derived CD4+Foxp3+ T cells (also known as natural T reg [nT reg] cells) undergo positive selection through TCR interactions with self-peptides presented by cortical thymic epithelial cells (Bensinger et al., 2001). Although conventional CD4 single-positive (SP) T cells that express TCRs with low to intermediate affinity for self-peptides are selected to undergo maturation, and those with high-affinity TCRs are eliminated by negative selection (Robey and Fowlkes, 1994), T reg cells appear to develop from CD4 SP thymocytes expressing TCRs with a relatively high affinity for self-antigens (Caton et al., 2004; Liston and Rudensky, 2007). In addition to TCR signals, costimulatory molecules and cytokines, which include CD28 (Tang et al., 2003; Sansom and Walker, 2006) and IL-2 (Bayer et al., 2005; Fontenot et al., 2005b), respectively, contribute to the development of nT reg cells. Consistent with multiple signals being required for nT reg cell development, disrupting various intracellular signaling pathways engaged by these receptors impairs nT reg cell differentiation (Liston and Rudensky, 2007). Although the majority of Foxp3+ T reg cells develop in the thymus (Fontenot et al., 2005a), TGF-β is also able to promote the differentiation of mature CD4+CD25−Foxp3− peripheral T cells into CD4+CD25+Foxp3+ cells that possess T cell suppressive properties akin to those of nT reg cells (Chen et al., 2003; Marie et al., 2005).
Although the Foxp3 transcription factor is essential for the T cell–suppressive properties of T reg cells (Fontenot et al., 2003; Hori et al., 2003; Williams and Rudensky, 2007), Foxp3 itself does not appear to be a T reg cell lineage-determining transcription factor (Gavin et al., 2007; Lin et al., 2007; Zheng and Rudensky, 2007). Instead, Foxp3 functions by maintaining a pattern of T reg cell–specific gene expression that is controlled by other transcription factors (Marson et al., 2007; Zheng and Rudensky, 2007; Zheng et al., 2007), which, by inference, must dictate the lineage determination of CD4+Foxp3+ T cells.
The Rel–NF-κB pathway has been implicated in the generation of T reg cells (Siebenlist et al., 2005; Liston and Rudensky, 2007). The transcriptional mediators of this pathway comprise homodimers and heterodimers of five related proteins (c-Rel, RelA, RelB, p50NF-κB1, and p52NF-κB2). In the absence of stimulatory signals, Rel–NF-κB factors are retained in an inactive state within the cytosol by IκB proteins (Hayden and Ghosh, 2008). Signals emanating from the cell surface or intracellular receptors activate a multisubunit IκB kinase (IKK) that, upon phosphorylating IκB, targets it for proteosome-mediated degradation (Scheidereit, 2006), thereby promoting the nuclear translocation of Rel–NF-κB factors. The classical arm of the NF-κB pathway comprises the IKK2 kinase and the transcription factors NF-κB1, RelA, and c-Rel (Gilmore, 2006), whereas the alternate NF-κB pathway requires the IKK1 kinase to activate NF-κB2 and RelB (Gilmore, 2006).
The alternate NF-κB pathway is dispensable for T reg cell development (Siebenlist et al., 2005), whereas inactivation of IKK2 (Schmidt-Supprian et al., 2003) or the combined loss of NF-κB1 and c-Rel (Zheng et al., 2003) markedly reduces thymic and peripheral CD4+CD25+ cell numbers. Despite these links between the classical NF-κB pathway and the generation of T reg cells, the specific roles of individual Rel–NF-kB transcription factors in T reg cell differentiation and function remain to be determined. In this paper, we report that c-Rel is the critical NF-κB transcription factor required to generate normal numbers of thymic and peripheral T reg cells. Consistent with c-Rel being highly expressed in thymic T reg cells, c-rel−/− mice display diminished numbers of thymic CD4+Foxp3+ T cells as a result of a thymocyte-intrinsic survival-independent defect in T reg cell development. Although c-Rel is not essential for TGF-β conversion of CD4+CD25− T cells into CD4+CD25+FoxP3+ cells in culture or in vivo, the reduced capacity of c-rel−/− T reg cells to undergo homeostatic expansion may contribute to the lower numbers of peripheral T reg cells seen in c-rel−/− mice. Despite a significant reduction in number, residual c-rel−/− T reg cells express normal levels of Foxp3 and exhibit a phenotype similar to that of normal T reg cells. Moreover, c-Rel appears to be dispensable for T reg cell immune regulatory function in culture and in vivo. Collectively these findings indicate that c-Rel is a transcription factor that regulates both the thymic development and peripheral homeostatic expansion of T reg cells.
RESULTS
Thymic and peripheral T reg cell numbers are markedly reduced in c-rel−/− mice
Conditional deletion of ikk2 in T lineage cells, which blocks activation of the classical NF-κB pathway, disrupts T reg cell development (Schmidt-Supprian et al., 2003). This finding prompted us to determine the roles of the individual transcriptional mediators of the classical Rel–NF-kB pathway, namely p50NF-κB1, RelA, and c-Rel, in T reg cell development. Initially, thymic CD4+Foxp3+ populations were examined in c-rel−/− and nfkb1−/− mice, as well as in lethally irradiated Ly5.1+ C57BL/6 mice engrafted with embryonic day 13 (E13) rela−/− fetal liver hemopoietic stem cells (Ly5.2+). The use of radiation chimeras is required to examine the role of RelA in T reg cell development as a result of the death of rela−/− embryos at ∼E15 from fetal hepatocyte apoptosis (Beg et al., 1995).
Total thymocyte cellularity and the relative frequencies of CD4−CD8−, CD4+CD8+, CD4+CD8−, and CD4−CD8+ thymocytes in 6–8-wk-old mice are normal in the absence of c-Rel, NF-κB1, or RelA (Grossmann et al., 2000; Pohl et al., 2002). However, the proportion of CD4+CD25+Foxp3+ thymocytes, although normal in nfkb1−/− mice and ∼2.5-fold lower in rela−/− chimeras, are reduced ∼6–7-fold in c-rel−/− mice (Fig. 1 A). c-rel−/− CD4+CD25−Foxp3+ thymocyte numbers are also reduced in similar proportions (Fig. 1 A, i) but, interestingly, this population is slightly elevated in the rela−/− chimeras (Fig. 1 A, ii). Given that c-Rel appeared to be the most important of the Rel–NF-kB transcription factors necessary for the generation of CD4+Foxp3+ thymocytes, subsequent work focused on the role of c-Rel in T reg cell development and function.
Figure 1.
T reg cell populations in Rel–NF-kB–deficient mice. Single cell suspensions from the thymus, spleen, and lymph nodes of Rel–NF-kB–deficient mice stained with antibodies for CD4, CD8, CD25, and Foxp3 were examined by flow cytometry. Representative dot plots from one of six independent experiments are presented with the percentage of cells in the relevant quadrants indicated. (A and B) For every experiment, organs from two mice of each genotype were analyzed separately. (A) Expression profiles of CD4 versus CD8 and Foxp3 versus CD25 (gated on CD4SP cells) for thymocyte populations in wt, nfkb1−/−, and c-rel−/− (i) mice and irradiated recipients of wt or rela−/− E14 fetal liver hemopoietic progenitors (ii). (B) Expression profiles of CD4 versus CD25 and Foxp3 versus CD25 (CD4 gated cells) for CD4 T cell populations in spleen and lymph nodes of wt and c-rel−/− mice. (C) Percentage and numbers of T reg cells in the thymus, spleen, and lymph nodes of individual wt and c-rel−/− mice. Each symbol represents an individual mouse. *, P < 0.05; **, P < 0.02; ***, P < 0.01. The data were compiled from two independent experiments. Horizontal bars represent the mean values of the data.
The peripheral T reg cell population is mainly, but not exclusively, derived from cells generated in the thymus (Fontenot and Rudensky, 2005; Fontenot et al., 2005a; Sakaguchi, 2005; Ziegler, 2006). To ascertain if fewer CD4+Foxp3+ thymocytes in c-rel−/− mice translate into diminished numbers of T reg cells in peripheral lymphoid tissues, CD4+Foxp3+ populations were assessed in the spleen and lymph nodes (Fig. 1 B). Whereas c-rel−/− CD4+CD25− T cell numbers in both lymphoid compartments are normal (Pohl et al., 2002), CD4+CD25+Foxp3+ T cells are reduced ∼3-fold. Loss of c-Rel also leads to a reduction in CD4+CD25−Foxp3+ T cells in these peripheral lymphoid organs. A similar reduction in peripheral CD4+Foxp3+ T cells is also observed in c-rel−/− mice as old as 24 wk of age (unpublished data). These findings, summarized in Fig. 1 C, establish that c-Rel is required to generate normal numbers of thymic and peripheral CD4+Foxp3+ T cells.
The T reg cell deficit in c-rel−/− mice arises in neonates
Mouse CD4+Foxp3+ T cells first arise in the thymus within 2–3 d of birth (Fontenot et al., 2005a), and by day 5 (D5) sufficient T reg cells have been generated to protect mice from developing autoimmune disease (Shevach, 2000; Sakaguchi, 2004, 2005; Fontenot and Rudensky, 2005). To determine if the T reg cell deficit in c-rel−/− mice occurs at the outset of T reg ontogeny or develops as mice age, CD4+CD25+ and CD4+Foxp3+ thymic populations were compared in wt and c-rel−/− D2 and D5 neonates, as well as 28-d-old mice. At D2, the earliest time at which thymic Foxp3+ T cells are detectable (Fontenot et al., 2005a), the percentage of CD4+CD25+ thymocytes (5–6% of CD4 SP cells) was similar in wt and c-rel−/− mice (Fig. 2 A). Although the CD4+Foxp3+ population is very low in D2 mice of both genotypes (Fig. 2 A and Table S1), it was found to be smaller in c-rel−/− mice (∼2.5% vs. <0.02% of CD4+CD25+ thymocytes). By D5, despite an increase in the absolute numbers (Table S1) of CD4+ Foxp3+ thymocytes in both wt and c-rel−/− mice, the proportion of CD4+CD25+Foxp3+ cells in the CD4 SP thymocyte population remains four- to fivefold lower in c-rel−/− mice (Fig. 2 A). Unlike at D2, the difference in T reg cell numbers in these mice at D5 was now also reflected in the relative proportion of CD4+CD25+ thymocytes (∼5 vs. 3% in wt and c-rel−/− mice, respectively; Fig. 2 A). A similar trend was also observed in the CD4+CD25−Foxp3+ thymocyte populations of D2 and D5 neonates (Fig. 2 A). CD4+Foxp3+ (both CD25+ and CD25−) splenic T cells are also reduced in D5 c-rel−/− neonates (Fig. 2 B) and these trends were replicated in the D28 thymic and spleen samples (Fig. 2 B). Together, these data indicate that the deficit of CD4+Foxp3+ T cells in c-rel−/− mice reflects a failure to generate normal numbers of these cells in the thymus during neonatal development.
Figure 2.
Thymic and splenic T reg populations in wt and c-rel−/− neonates. (A and B) Percentages of CD4+CD25+, CD4+CD25+Foxp3+, and CD4+CD25−Foxp3− thymocytes (A) and splenic T cells (B) as a proportion of CD4 SP cells in 2-, 5-, and 28-d-old wt and c-rel−/− mice. Each symbol represents an individual mouse. Data for individual mice were compiled from four independent experiments. Horizontal bars represent the mean values of the data.
The reduction in c-rel−/− T reg cells is caused by a hemopoietic defect
The development of different T cell populations is dependent on thymocyte interactions with other hemopoietic cells, including DCs, as well as nonhemopoietic stroma such as thymic epithelial cells (Takahama, 2006). Rel–NF-kB involvement in the development and function of both hemopoietic (Gerondakis et al., 2006) and epithelial cells (Pasparakis et al., 2002; Gugasyan et al., 2004) prompted the generation of bone marrow chimeras to assess the contributions of the hemopoietic and stromal compartments to the reduction in c-rel−/− T reg cells.
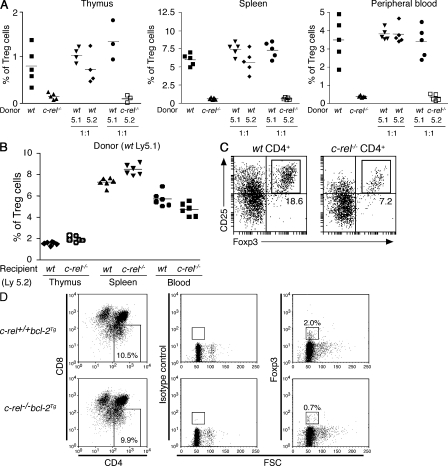
In irradiated wt (Ly5.1) recipients of c-rel−/− bone marrow (Ly5.2), the percentage and absolute number (not depicted) of CD4+CD25+Foxp3+ thymocytes, like those in c-rel−/− mice, were reduced compared with recipients of wt (Ly5.2) bone marrow (Fig. 3 A). In contrast, the CD4+CD25+Foxp3+ thymic population was normal in the reciprocal bone marrow donor/recipient combination where c-rel−/− (Ly5.2) mice were engrafted with Ly5.1+ wt bone marrow (Fig. 3 B). Similar conclusions were made for the T reg cell population in the peripheral lymphoid tissues and blood of the chimeras (Fig. 3, A and B). These experiments indicate that the thymic and peripheral T reg cell deficiency in c-rel−/− mice represents a hemopoietic rather than a stromal defect.
Figure 3.
The c-rel−/− T reg cell deficit results from a thymocyte-intrinsic defect. (A) The c-rel−/− T reg cell deficit arises from a cell-autonomous defect. wt, c-rel−/−, or mixtures of equal numbers of wt (Ly5.1 and Ly5.2) or wt (Ly5.1) and c-rel−/− (Ly5.2) bone marrow cells were engrafted into irradiated recipients (Ly5.2+ mice) and, 8 wk later, T reg cell populations were examined in thymus, spleen, and blood. Each symbol represents an individual mouse. The data are representative of two independent experiments using similar numbers of mice in each experimental group. (B) The T reg cell deficit in c-rel−/− mice is the result of a hemopoietic defect. Irradiated wt and c-rel−/− mice (Ly5.2) were engrafted with wt bone marrow (Ly5.1) and, 8 wk later, donor-derived T reg cell (Ly5.1) numbers were determined in the thymus, spleen, and blood. Each symbol represents an individual mouse. The data are representative of two independent experiments using similar numbers of mice in each experimental group. Horizontal bars represent the mean values of the data. (C) Thymic DC-induced T reg cell differentiation in culture. wt and c-rel−/− CD4+CD25− thymocytes and wt CD8Sirpα+ DC cultured with IL-7 for 5 d were stained with antibodies for CD25 and Foxp3. The data shown is representative of two independent experiments. In both experiments, there were duplicates for cultures using each combination of wt and c-rel−/− thymocyte and DC. (D) Reduced T reg cell differentiation of c-rel−/− CD4+CD8+ thymocytes in culture. Equal numbers of CD4+CD8lo cells generated in culture after anti-CD3 antibody activation of wt and c-rel−/− CD4+CD8+ thymocytes expressing a Bcl-2 transgene (bcl2Tg) were stained with control or Foxp3 specific antibodies. The data shown is representative of three independent experiments. In each experiment, the cultures for cells of each genotype were performed in triplicate.
The defect in c-rel−/− CD4 Foxp3+ thymocyte development is thymocyte intrinsic
Despite bone marrow chimeras establishing that the c-rel−/− T reg cell deficit is the result of a hemopoietic defect, these experiments did not distinguish between defects intrinsic to CD4+ thymocytes and defects in other hemopoietic cells involved in T reg cell differentiation. Two approaches were used to address this question. First, chimeras were generated by introducing an equal mix (1:1) of wt (Ly5.1) and c-rel−/− (Ly5.2) bone marrow cells into irradiated wt (Ly5.2) recipients. The proportion of wt CD4+Foxp3+ cells in the thymus and peripheral lymphoid organs was normal, yet the c-rel−/− CD4+CD25+Foxp3+ and CD4+CD25−Foxp3+ (not depicted) populations were reduced, resembling those in c-rel−/− mice or wt recipients of c-rel−/− bone marrow (Fig. 3 A).
The second approach used cell culture models of thymic T reg cell differentiation. In one model, CD4+CD25−Foxp3− thymocytes were cultured for 5 d with thymic CD8 Sirpa+ DC, which preferentially converts these thymocytes into T reg cells (Proietto et al., 2008). In these cultures, significantly more T reg cells were generated from wt than c-rel−/− CD4+CD25− thymocytes (Fig. 3 C). In another cell culture model, T reg cells were generated by TCR and CD28 costimulation of CD4+CD8+ thymocytes (Tai et al., 2005). This method used wt and c-rel−/− thymocytes expressing a Bcl-2 transgene to prevent the high levels of CD4+CD8+ thymocyte apoptosis that normally accompanies TCR activation (Hettmann et al., 1999). After anti-CD3 plus anti-CD28 antibody activation, cells were cultured for an additional 48 h, after which CD4+CD8lo cells were stained for Foxp3 (Fig. 3 D). Despite TCR and CD28 stimulation generating similar frequencies of wt and c-rel−/− CD4+CD8lo T cells, Foxp3+ cell numbers were ∼3-fold lower in the c-rel−/− CD4+CD8lo population. Together, these experiments establish that c-rel−/− thymocytes have an intrinsic defect in their capacity to differentiate into T reg cells.
The reduction in c-rel−/− thymic T reg cells is not a result of impaired survival
The role of c-Rel in promoting cell survival through the induction of genes encoding Bcl-2 prosurvival proteins (Gerondakis and Strasser, 2003) raised the possibility that a survival defect might account for the reduced number of c-rel−/− thymic or peripheral T reg cells. This was assessed by introducing a bcl-2 transgene (bcl-2Tg) under the control of the hemopoietic-restricted vav promoter (Ogilvy et al., 1999) onto the c-rel−/− background. The bcl-2Tg increased overall thymocyte cellularity in wt and c-rel−/− mice compared with nontransgenic counterparts (not depicted), yet bcl-2Tg expression did not correct the selective reduction in c-rel−/− CD4+CD25+Foxp3+ thymocytes (Fig. 4). Although bcl-2Tg expression led to an increase in the proportion of CD4+CD25−Foxp3+ thymocytes in both wt and c-rel−/− mice when compared with nontransgenic counterparts (compare Fig. 4 and Fig. 1 A), the frequency of this Foxp3+ population remained lower in c-rel−/−bcl-2Tg compared with c-rel+/+bcl-2Tg mice (Fig. 4). This indicated that Bcl2 expression has a differential impact on the CD4+CD25+Foxp3+ and CD4+CD25+Foxp3+ thymocyte populations. An examination of the CD4+Foxp3+ populations in the spleen and lymph nodes of c-rel+/+bcl-2Tg and c-rel−/−bcl-2Tg mice revealed a similar picture to that seen in the thymus, with enforced Bcl-2 expression failing to rescue the diminished c-rel−/− CD25+Foxp3+ population (Fig. 4). Collectively, these data show that the reduced number of CD4+Foxp3+ cells in c-rel−/− mice is not the result of a defect in the Bcl-2 survival pathway.
Figure 4.
Bcl-2 transgene expression fails to rescue the T reg cell deficit in c-rel−/− mice. CD4+CD8− thymocytes and CD4+CD25+ spleen and lymph node cells from c-rel+/+bcl2Tg and c-rel−/−bcl2Tg mice were stained with antibodies for CD25 and Foxp3. Representative dot plots from an individual mouse of either genotype are presented with the percentage of cells in the relevant quadrants indicated. Two mice of each genotype were analyzed in each experiment and the data are from one of three independent experiments.
c-Rel is up-regulated in thymic T reg cells
The reduction in c-rel−/− thymic T reg cells resulting from a thymocyte intrinsic defect raised the issue of c-Rel expression during thymopoiesis and how this might relate to T reg cell development. This was determined by Western blot analysis of c-Rel expression in total cell lysates from CD4+CD8+, CD4−CD8+, CD4+CD25−, and CD4+CD25+ thymocytes (Fig. 5 A). Whereas c-Rel is barely detectable in CD4+CD8+ cells and its expression is only marginally higher in CD4−CD8+ and CD4+CD25− thymocytes, c-Rel levels, like those of Foxp3, are dramatically elevated in the CD4+CD25+ population. RelA levels in contrast do not differ between CD4+CD25− and CD4+CD25+ thymocytes (Fig. 5 A). Consistent with the protein expression data, real-time PCR confirmed that c-rel, but not rela, messenger RNA (mRNA) is specifically up-regulated in CD4+CD25+ thymocytes (Fig. 5 B). nfkb1 mRNA levels are also somewhat elevated in CD4+CD25+ thymocytes compared with CD4+C25− cells. These findings, coupled with the phenotype in c-rel−/− mice, indicated that high levels of c-Rel may be necessary for the maintenance of the T reg cell population. Surprisingly, Western blotting of cytosolic and nuclear fractions from wt CD4+CD25+ thymocytes revealed that c-Rel could only be detected in the cytosol (Fig. 5 C), indicating that constitutive c-Rel activation does not appear to be essential for the maintenance of thymic T reg cells. The expected pattern of c-Rel expression in the cytoplasm and nucleus of resting and activated B cells (Fig. S1) validated the accuracy of the fractionation process used to examine T reg cells.
Figure 5.
c-Rel is highly expressed in thymic T reg cells. (A) Western blot analysis of c-Rel, RelA, and Foxp3 expression in different thymocyte populations. Western blot analysis of total cell lysates was used to determine c-Rel and Foxp3 expression in purified wt populations of CD4−CD8+, CD4+CD8+, CD4+CD25−, and CD4+CD25+ thymocytes and RelA expression in wt CD4+CD25− and CD4+CD25+ thymocyte populations. ERK or actin expression served as loading controls. The data are representative of two independent experiments using different lysate samples prepared from thymocyte populations purified from the pooled thymi of three mice for each experiment. (B) c-rel, rela, and nfkb1 mRNA levels in thymocyte populations. The levels of c-rel, rela, and nfkb1 mRNA expressed in purified CD4+CD25+ and CD4+CD25− thymocyte populations were determined by real-time PCR. The data represents duplicate PCRs performed on individual samples from each of four independent sets of cells. Error bars represent SEM. (C) c-Rel is localized in the cytoplasm of thymic T reg cells. Nuclear and cytoplasmic fractions from purified CD4+CD25+ thymocytes were subjected to Western blotting analysis using antibodies for c-Rel, ERK, and histone H3. The data shown is representative of two independent experiments using different preparations of nuclear and cytoplasmic lysates.
c-Rel is required for the homeostatic proliferation of peripheral T reg cells
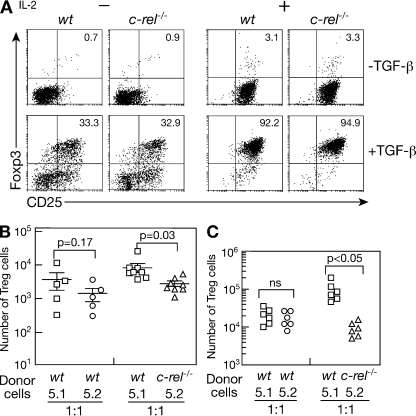
Although thymus-derived CD4+Foxp3+ T cells are the major source of T reg cells (Fontenot and Rudensky, 2005; Fontenot et al., 2005a; Sakaguchi, 2005; Ziegler, 2006), the TGF-β–induced differentiation of CD4+CD25− T cells into T reg cells (Chen et al., 2003; Marie et al., 2005), coupled with T reg cell proliferation, collectively determines the size of the peripheral T reg cell population. To determine if c-Rel is necessary for TGF-β–dependent conversion of CD4+CD25− T cells into T reg cells, wt and c-rel−/− CD4+CD25− T cell cultures were initially subjected to TCR activation in the absence or presence of TGF-β. Given that IL-2 enhances the generation of TGF-β–induced T reg cells in culture by promoting T cell proliferation (Kretschmer et al., 2005) and TCR-mediated CD4+CD25− T cell proliferation requires the c-Rel dependent expression of IL-2 (Köntgen et al., 1995; Rao et al., 2003), the influence of IL-2 on TGF-β–induced c-rel−/− T reg cell differentiation was also determined (Fig. 6 A).
Figure 6.
The roles of c-Rel in T reg cell conversion and homeostatic expansion. (A) TGF-β induced differentiation of c-rel−/− T reg cells in culture. wt and c-rel−/− splenic CD4+CD25− T cells incubated with anti-CD3 antibodies and irradiated T cell–depleted allophycocyanin (APC) in the absence or presence of TGF-β (with or without IL-2) for 4 d were stained with antibodies specific for CD25 and Foxp3. Representative dot plots from one of four independent experiments are presented with the percentage of cells in the relevant quadrants indicated. In each experiment, there were triplicate cultures for each set of stimulation conditions. (B) T reg cell conversion in vivo. Mesenteric lymph node (MLN) cells isolated from rag-1−/− mice injected 18 d earlier with either a 1:1 mix of purified splenic CD4+CD25− wt (Ly5.1+ and Ly5.2+) or wt (Ly5.1+) and c-rel−/− (Ly5.2+) T cells were stained with antibodies specific for CD4, CD8, CD45.1 (Ly5.1), and Foxp3. The data compares the absolute numbers of wt (Ly5.1+ and Ly5.2+, squares and circles, respectively) and c-rel−/− (Ly5.2+, triangles) CD4+Foxp3+ cells detected in the mesenteric lymph nodes of individual mice receiving each mixture of cells. Horizontal bars represent the mean values of the data. Error bars represent SD. The data were analyzed using a paired Student's t test and is representative of two independent experiments, with a minimum of five mice in each recipient group. (C). c-Rel is necessary for optimal homeostatic expansion of T reg cells in lymphopenic mice. MLN cells isolated from rag-1−/− mice injected between 4 and 8 wk earlier with either a 1:1 mix of purified wt (Ly5.1+, squares; Ly5.2+, circles) or wt (Ly5.1+, squares) and c-rel−/− (Ly5.2+, triangles) splenic CD4+CD25+ T cells were stained with antibodies specific for CD4, CD8, CD45.1 (Ly5.1), and Foxp3. The data shows the number of MLN T reg cells of each donor type in recipient mice (six per group) from one experiment. The data shown is representative of three independent experiments with five or six mice in each recipient group.
Without TGF-β, ∼1% of activated wt and c-rel−/− cells were Foxp3+. In the absence of IL-2, this increased to ∼33% for cells of both genotypes in the presence of TGF-β (Fig. 6 A). After the addition of IL-2 during TGF-β–induced T reg cell differentiation, the proportion of cultured wt and c-rel−/− CD4+Foxp3+ T cells increased to between 93 and 95% (Fig. 6 A). Although the frequency of TGF-β–generated wt and c-rel−/− Foxp3+ cells was similar, absolute numbers of c-rel−/− Foxp3+ cells recovered in culture were two- to threefold lower than for wt Foxp3+ cells, irrespective of whether IL-2 was present (Fig. S2). This reduced number of c-rel−/−Foxp3+ cells reflects a decrease in c-rel−/− CD4+ T cells recovered after TGF-β stimulation (Fig. S2) that coincides with diminished TCR-mediated c-rel−/− T cell proliferation (Fig. S3).
The conversion of c-rel−/− CD4+CD25− T cells into CD4+Foxp3+ cells in vivo was also examined (Curotto de Lafaille et al., 2004; Almeida et al., 2006). The relative efficiency with which a T reg cell population develops from c-rel−/− CD4+CD25− cells was determined by injecting a 1:1 ratio of wt (Ly5.1) and c-rel−/− (Ly5.2) CD4+CD25− T cells into rag-1−/− mice. 18 d later, the number (Fig. 6 B) and frequency (Fig. S4) of CD4+Foxp3+ cells from each donor population in the lymph nodes of individual mice was compared using paired data analysis. Although naive c-rel−/− CD4+CD25− T cells can convert to T reg cells in vivo, the absolute number and percentage of c-rel−/− T reg cells was reduced when compared with the wt population.
To determine if the impaired T reg cell proliferation observed in culture also occurs in vivo, we examined the homeostatic expansion of c-rel−/− T reg cells under competitive conditions in a lymphopenic host. A 1:1 mix of wt (Ly5.1+ and Ly5.2+) or wt (Ly5.1+) and c-rel−/− (Ly5.2+) CD4+CD25+ T cells was introduced into rag-1−/− mice, and between 4 and 12 wk later the number of T reg cells in lymph nodes from the different donors was determined. In contrast with control mice, which retain a similar ratio of wt Ly5.1+ and Ly5.2+ T reg cells to the starting populations after homeostatic expansion, the number of c-rel−/− T reg cells was always significantly less compared with cotransferred wt T reg cells (Fig. 6 C). This indicates that the capacity of c-rel−/− T reg cells to undergo homeostatic expansion is reduced.
Phenotypic properties of c-rel−/− CD4+ T reg cells
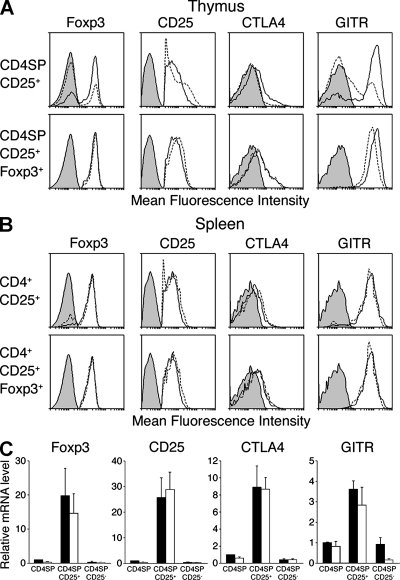
Although c-Rel is necessary for generating normal numbers of thymic and peripheral T reg cells, it was unclear what impact the absence of c-Rel had on the phenotype of the remaining T reg cells. This issue was addressed by comparing the mRNA and protein expression of various characteristic T reg cell markers in wt and c-rel−/− CD4+CD25+ thymocytes and splenic T cells (Fig. 7). Real-time PCR and intracellular staining established that the levels of Foxp3 expression are equivalent in wt and c-rel−/− CD4+CD25+ thymocytes and splenic T cells (Fig. 7, A and C). This indicates that c-Rel does not control Foxp3 expression. Although CD25 and CTLA4 protein expression is normal in c-rel−/− thymic and splenic T reg cells, glucocorticoid-induced TNF receptor–related (GITR) protein levels are marginally lower on c-rel−/− CD4+CD25+ thymocytes but not c-rel−/− CD4+CD25+ splenic T cells (Fig. 7, A and B). These data are also borne out by mRNA analyses (Fig. 7 C and not depicted).
Figure 7.
Foxp3, CD25, CTLA4, and GITR expression in thymic and splenic CD4+CD25+ and CD4+CD25+Foxp3+ T cells. (A and B) wt and c-rel−/− CD4+CD25+ thymocytes (A) and splenocytes (B) were stained with antibodies for Foxp3, CD25, CTLA4, or GITR. Representative profiles from one of five independent experiments are shown for wt (line) and c-rel−/− (dashed line) cells stained with control (filled) and test (open) antibodies. In each experiment, a single mouse of each genotype was used. (C) Relative mRNA levels for foxp3, cd25, ctla4, and gitr were determined by RT-PCR from purified populations of wt (closed) and c-rel−/− (open) CD4SP, CD4+CD25+, and CD4+CD25− thymocytes. The data represents the mean value from duplicate PCRs performed on individual samples from each of three independent sets of cells.
Genes expressed in CD4+ T cells, including those relevant to CD4+Foxp3+ T reg cell function (Marson et al., 2007; Zheng et al., 2007) and genes known to be c-Rel targets (Bunting et al., 2007), were also examined by real-time PCR (Table I). As expected, a group of these genes were enriched in the thymic or splenic CD4+CD25+ population compared with the CD4+CD25− population (Table I). Several of these genes, including Il10, 41BB, CREM, MAP3K8, Irf4, Bcl2l1, Ox40, and Ebi3, showed a decrease in expression (generally ∼2–3-fold) in c-rel−/− CD4+CD25+ thymocytes compared with WT CD4+CD25− thymocytes (Table I). Expression of a smaller number of the genes examined, namely CD127, CD40L, and Bcl2, was decreased in the thymic CD4+CD25+ population compared with CD4+CD25− cells (Table I), and, interestingly, expression of several of these genes were increased in the absence of c-Rel (Table I). In contrast, loss of c-Rel did not significantly influence gene expression patterns in splenic CD4+CD25+ T cells (unpublished data). Therefore, we conclude that c-Rel appears to have a role in modulating the gene expression profile of thymic CD4+CD25+ cells.
Table I.
Comparison of mRNA levels in CD4SPCD25+ and CD4SPCD25− thymocytes in WT and c-rel−/− mice
| Gene name | Fold enrichment in CD4SPCD25+ compared with CD4SPCD25− thymocytes | Fold change in CD4SPCD25+ thymocytes between WT and c-rel−/− mice |
| I110 | 63 | 0.23 |
| Ebi3 | 31.25 | 0.38 |
| Tnfrsf9 (41BB) | 26.6 | 0.46 |
| Nt5e (CD73) | 7.9 | 0.55 |
| Tnfrsf4 (Ox40) | 6.9 | 0.53 |
| Map3k8 | 3.2 | 0.17 |
| Crem | 2.8 | 0.09 |
| Bc1211 | 2.75 | 0.54 |
| Irf4 | 2.25 | 0.33 |
| 117r (CD127) | 0.31 | 1.8 |
| Cd401g | 0.28 | 3.1 |
| Bc12 | 0.22 | 3.0 |
Values given for fold enrichment and fold change are means of at least two independent biological replicates with duplicate PCRs performed for each experiment.
c-Rel is not required for T reg cell function in culture
To evaluate the immune suppressive properties of c-rel−/− T reg cells, we first determined their capacity to inhibit CD4+ effector T cell proliferation in culture. Initially, suppression assays were performed using spleen and lymph node CD4+CD25+ T cells isolated from wt and c-rel−/− mice. In these assays, c-rel−/− T reg cells were found to be slightly less effective at suppressing T cell proliferation than wt T reg cells (Fig. 8 A). Given that the frequency of Foxp3+ cells in the c-rel−/− CD4+CD25+ T cell population was slightly less compared with wt CD4+CD25+ T cells (Fig. 7 B and Fig. S5), to determine if the reduced suppressive capacity of c-rel−/− CD4+CD25+ splenic T cells simply reflected fewer numbers of Foxp3+ T reg cells in this population, we also examined the suppressive properties of c-rel−/− T reg cells generated in culture. First, wt and c-rel−/− CD4+CD25− T cells were transduced with retroviruses coexpressing gfp and murine foxp3. Greater than 96% of wt and c-rel−/− T cells infected with the GFP-Foxp3 virus expressed Foxp3, and Western blotting confirmed that its levels were equivalent in both groups of cells (Fig. S5). Whereas T cells infected with a GFP-only control virus failed to suppress CD4+CD25− T cell proliferation (not depicted), both wt and c-rel−/− GFP+Foxp3+ T cells were equally effective at inhibiting T cell proliferation (Fig. 8 B). wt and c-rel−/− CD4+CD25+ T cells generated by stimulating activated CD4+CD25− T cells with TGF-β plus IL-2 (both ∼97–98% Foxp3+; Fig. S5) were also found to be equally effective at suppressing T cell proliferation (Fig. 8 C). Collectively, these data indicate that in culture c-Rel is dispensable for the immune suppressive properties of T reg cells.
Figure 8.
c-rel−/− T reg cells inhibit T cell proliferation in culture and suppress CD4 T cell-dependent colitis. Varying numbers of wt and c-rel−/− T reg cells (suppressors) from various sources (see A–C) incubated in culture with a fixed number of wt splenic CD4+CD25− T cells (effectors), expressed as a ratio, were activated with anti-CD3 antibodies and irradiated T cell–depleted APC for 72 h. Proliferation was monitored by 3H-thymidine incorporation. The data shown in A–C is the SEM for four independent experiments using each different source of T reg cells. Every data point was performed in triplicate for each experiment. (A) Splenic T reg cells from wt and c-rel−/− mice. (B) Foxp3+ cells generated by transducing wt and c-rel−/− CD4+CD25− splenic T cells with GFP-Foxp3 retroviruses. (C) Foxp3+ cells generated from TGF-β + IL-2–treated wt and c-rel−/− CD4+CD25− splenic T cells. rag-1−/− mice injected with CD4+CD45RBhi T cells (4 × 105) in the absence or presence of wt or c-rel−/− CD4+CD25+ T cells (105, >87% Foxp3+) were monitored regularly for up to 10 wk. During this period, mice displaying signs of illness or weight loss were sacrificed. After 10 wk, the remaining mice were weighed and sacrificed. The colons were removed from all mice and processed for histology. (D) Representative hematoxylin and eosin–stained colon sections from rag-1−/− mice receiving CD4+CD45RBhi T cells alone or with wt or c-rel−/− CD4+CD25+ T cells (n = 12 mice for each cohort). Bars, 875 µM. (E) Histology scores for rag-1−/− mice receiving CD4+CD45RBhi T cells alone or with wt or c-rel−/− CD4+CD25+ T cells based on a modification of Berg et al. (1996). *, P < 0.02; **, P < 0.05.
c-rel−/− T reg cells inhibit T cell–dependent colitis
To determine if c-rel−/− T reg cells can inhibit T cell–mediated autoimmune disease, we used a model of T cell–dependent colitis induced in Rag-deficient mice after the adoptive transfer of CD4+CD25−CD45RBhi T cells that can be prevented by cotransferring T reg cells (Asseman et al., 1999). CD4+CD25−CD45RBhi T cells (4 × 105) were injected into rag-1−/− mice alone or with 105 wt or c-rel−/− CD4+CD25+ T cells. Mice were monitored for signs of illness (weight loss and diarrhea) over a 10-wk period. All sick mice and any remaining healthy mice were euthanized and histological analysis of colon sections performed. Whereas all mice injected with only CD4+CD25−CD45RBhi T cells developed colitis, no histological evidence of colitis was observed in animals that had also received wt or c-rel−/− splenic T reg cells (Fig. 8, D and E). This indicates that c-Rel is dispensable for the immune suppressive function of T reg cells in this model of autoimmune disease.
DISCUSSION
Little is currently known about the roles of the classical NF-κB pathway in the development or function of T reg cells. In this paper, we show that among the transcription factors operating downstream of this pathway, c-Rel is the most important for T reg cell development. T cell–intrinsic expression of c-Rel, although necessary for generating normal numbers of thymic T reg cells, is not essential for TGF-β–mediated T reg cell differentiation. Despite a need for c-Rel during nT reg cell development and peripheral T reg cell homeostasis, c-rel−/− T reg cells effectively suppress CD4+ effector T cell proliferation in culture and prevent the induction of colitis in rag1−/− mice engrafted with CD4+CD45RBhi T cells. These findings support a model in which the development of T reg cells in the thymus and maintenance of the T reg cell phenotype have distinct molecular requirements, with c-Rel belonging to that class of transcription factors whose function is important in regulating thymic T reg cell differentiation.
Previously, nonredundant roles for c-Rel in the T cell lineage were thought to be restricted to mature T cells and involve functions such as proliferation, survival, and cytokine production (Gerondakis et al., 2006). In this paper, we establish that c-Rel is required during thymic T reg cell development, with the absence of c-Rel leading to a marked reduction in both CD4+CD25+Foxp3+ and CD4+CD25−Foxp3+ thymocytes. The importance of c-Rel being expressed in thymic T reg cells during their development, as opposed to thymic stromal cells or other hemopoietic cells such as DC, was confirmed using mixed bone marrow chimeras and cell culture models of T reg cell differentiation. Consistent with this conclusion, c-Rel expression is markedly elevated in thymic T reg cells compared with conventional CD4 or CD8SP thymocytes.
Current models of thymic T reg cell differentiation favor a multistep process controlled by TCR, costimulatory, and cytokine signals, each of which is required during specific stages of development (Liston and Rudensky, 2007). Given that TCR and CD28 signaling are both crucial for T reg cell development (Liston and Rudensky, 2007), with c-Rel functioning downstream of both receptors in conventional CD4+ T cells (Venkataraman et al., 1995; Kahn-Perlès et al., 1997), c-Rel could conceivably control thymic T reg cell differentiation at a single or multiple points. Although our data demonstrates that a proportion of CD25+Foxp3− CD4SP thymocytes, a population enriched for T reg cell precursors (Lio and Hsieh, 2008), appears relatively normal at the onset of T reg cell development in c-rel−/− neonates, we cannot rule out that the generation of the CD25+Foxp3− T reg cell precursor population in c-rel−/− mice is not affected. Alternatively, given that Foxp3+ T reg thymocytes are markedly diminished in 5-d-old c-rel−/− neonates, c-Rel may promote the development of T reg cells arising from a CD25+Foxp3− CD4SP precursor or control the expansion of T reg cells once differentiation is complete. Although the differentiation of T reg cells from a CD4+CD25+Foxp3− precursor is IL-2 dependent (Bayer et al., 2005; Fontenot et al., 2005b) and c-Rel regulates il2 transcription in conventional CD4 T cells (Köntgen et al., 1995; Rao et al., 2003), the inability of mixed bone marrow chimeras to reverse the thymic c-rel−/− T reg cell deficit indicates that it is not the result of a failure to express IL-2 or other soluble factors. Furthermore, the inability of a Bcl-2 transgene to reverse the c-rel−/− T reg cell deficit rules out a role for c-Rel in controlling thymic T reg cell survival during or after differentiation, a function served by c-Rel in several other cell types (Gerondakis and Strasser, 2003; Gerondakis et al., 2006). Recent studies (Barnes et al., 2009; Molinero et al., 2009) have demonstrated that mice lacking functional CARMA1, a component of the CMB (CARMA1–Bcl10–Malt1) TCR adaptor complex required for NF-κB activation (Lin and Wang, 2004), also have markedly reduced numbers of thymic T reg cells. Although CARMA1 is required for both TCR-dependent NF-κB and JNK2 activation (Barnes et al., 2009), the finding that the T reg cell deficit in CARMA mutant mice, like the c-rel−/− mice, arises from a T cell–intrinsic defect that is independent of Bcl-2 prosurvival signals (Barnes et al., 2009; Molinero et al., 2009), provides strong support for a model in which the TCR-dependent activation of c-Rel via CARMA1 is important in T reg cell development.
The intriguing finding that c-Rel is cytosolic rather than nuclear in thymic T reg cells lends support to the notion that c-Rel is required for the differentiation rather than the maintenance of T reg cells. However, sustained expression of c-Rel in thymic and splenic (unpublished data) T reg cells points to the likelihood that c-Rel serves additional functions in these cells in response to other signals. It remains to be determined what types of stimuli, which could include antigen, costimulatory, and cytokine signals, promote the nuclear expression of c-Rel in T reg cells once differentiation in the thymus is complete.
c-Rel is not required for TGF-β–dependent differentiation of CD4+CD25− T cells into Foxp3+ T reg cells in culture, nor is it essential for T reg cell conversion in vivo. Although the size of the T reg cell population that arises in a lymphopenic environment from c-rel−/− CD4+CD25− T cells is reduced when compared with wt cells, it remains unclear whether this reflects a genuine defect in the efficiency with which conversion occurs in vivo or whether it is simply the result of the impaired proliferation of the converted T reg cells, which is controlled by c-Rel. So although a failure to produce normal T reg cell numbers in the thymus almost certainly accounts for some of the peripheral T reg cell deficit in c-rel−/− mice, the reduced capacity of c-rel−/− T reg cells to undergo normal homeostatic expansion is also likely to contribute to the overall reduction in peripheral c-rel−/− T reg cell numbers.
Despite being reduced in number, the remaining thymic c-rel−/− T reg cells, as assessed by key T reg cell marker expression, appear relatively normal. Foxp3 mRNA and protein levels are unchanged, as is the expression of characteristic T reg cell markers such as CTLA4, CD25, CD37, and CD73. Similar findings were made for splenic and lymph node T reg cells from c-rel−/− mice, plus c-rel−/− T reg cells generated in culture either by TGF-β treatment or enforced Foxp3 expression (unpublished data). Nevertheless, the expression of several genes was found to be lower or higher (∼2–3-fold in most instances) in c-rel−/− CD4+CD25+ thymocytes but not in peripheral T reg cells, which is consistent with the main role of c-Rel in the generation of thymic Foxp3+ cells. These differences point to c-Rel serving as a modulator of transcription in thymic T reg cells, which is consistent with its role in other cell types, including CD4+CD25− effector T cells (Bunting et al., 2007). It remains to be determined whether these differences in gene expression identified to date contribute to the reduction in c-rel−/− thymic T reg cell numbers.
Residual c-rel−/− T reg cells can inhibit CD4 effector T cell proliferation in culture and, importantly, prevent colitis in rag1−/− mice engrafted with CD4+CD45RBhi T cells. This indicates that the immune modulatory function of the remaining c-rel−/− T reg cells appears to be relatively normal. The remaining T reg cells in c-rel−/− mice also appear to be sufficient in number and activity to control antigen-dependent T cell effector responses. T reg depletion in vivo by anti-CD25 antibody administration increases the frequency of influenza virus-specific CD8 cytotoxic T cells in mice during the primary immune response (Haeryfar et al., 2005). Nevertheless, the number of influenza virus–specific CD8 effector T cells induced in the spleen and lungs of influenza virus infected c-rel−/− mice is normal (Harling-McNabb et al., 1999). Similarly, although the OVA-induced proliferation of OT-I TCR transgenic T cells is enhanced after T reg depletion (Salem et al., 2007; Fig. S6 A), the expansion of antigen-stimulated OT-I CD8 T cells is equivalent in wt and c-rel−/− mice (Fig. S6 B; Mintern et al., 2002). Although these findings indicate that there are sufficient T reg cells that function relatively normally in c-rel−/− mice to prevent the development of autoimmune disease and limit effector T cell activity during certain immune responses, it remains a possibility that defects in other c-rel−/− cells, such as effector T cells or DC, also contribute to the failure of autoimmune disease to develop in these mice.
Collectively, the data presented in this paper indicates that c-Rel belongs to a class of transcription factors required for thymic T reg cell differentiation and peripheral homeostatic proliferation but not the immune function of mature T reg cells. However, this needs to be confirmed by examining the function of c-rel−/− T reg cells across the gamut of immune responses controlled by T reg cells.
MATERIALS AND METHODS
Mice.
C57BL/6 mice (Ly5.1 and Ly5.2) were used as wt controls. c-rel−/− (Köntgen et al., 1995), nfkb1−/−(Sha et al., 1995), rela+/−(Beg et al., 1995), rag-1−/−, vav-bcl-2 transgenic (bcl-2Tg; Ogilvy et al., 1999), and c-rel−/−bcl-2Tg (Grumont et al., 2002) mice are all on a C57BL/6 background (Ly5.2, N: 9–12 backcrossed generations; rela+/−, 35 backcrossed generations). OT-I (C57BL/6) mice were on a Ly5.1 background. With the exception of E14 rela+/+, rela+/−, and rela−/− embryos used for fetal liver hemopoietic cell engraftment of irradiated recipients and neonates used for ontogeny studies, all other experimental mice were used between 7 and 10 wk of age. Genotypes of embryos from rela+/− intercrosses were determined by PCR. All animal protocols were approved by the Research Directorate of Melbourne Health, the Animal Ethics Committee of the Australian National University, and the University of Queensland Animal Ethics Committee.
Reagents.
The following antibodies were used: biotin, PE, APC, or Cy5-conjugated anti-CD4 (GK1.5), anti–mouse biotin-conjugated anti-CD3ϵ (145-2C11), FITC-conjugated anti-CD25 (PC61.1), PE-conjugated anti-GITR (DTA-1), PE-conjugated anti–CTLA-4 (UC10-4B9), and PE- or APC-conjugated anti–mouse Foxp3 (FJK-16S). Streptavidin-conjugated PE-Cy5 and PE-conjugated CD45RB (C363.16A) were purchased from eBioscience. Streptavidin-peridinin chlorophyll-a protein (SA-PerCP) was obtained from BD. PE-conjugated CD25 (7D4) anti-biotin microbeads were obtained from Miltenyi Biotec. TGF-β and recombinant murine IL-2 were purchased from R&D Systems and PeproTech, respectively. Dulbecco's Modified Eagle Medium supplemented with 10% FBS was used for cell culture.
Isolation of CD4+CD25+ and CD4+CD25− T cells.
CD25+ and CD25− CD4SP thymocytes were purified by first using an enrichment step that involved depleting anti–CD8-biotin–labeled CD8+ cells with anti-biotin microbeads (Miltenyi Biotec), after which the unbound flow-through fraction stained with anti–CD4-PE, anti–CD8-biotin plus SA-PE-Cy5, and anti–CD25-FITC was sorted for CD4+CD25+ and CD4+CD25− thymocytes using a MoFlo (Dako). T reg cells in spleen and lymph nodes were isolated from wt and c-rel−/− mice by preenriching cell suspensions stained with biotinylated anti-CD4 using anti-biotin magnetic beads (Miltenyi Biotec), followed by staining with anti–CD4-PE and anti–CD25-FITC and sorting for CD4+CD25+ and CD4+CD25− T cells. The purity of sorted CD4+CD25+ T cells was typically >96%.
Flow cytometric analysis.
T cells in single cell suspensions of thymus, spleen, and lymph nodes prepared from wt and c-rel−/− neonates (D2 and D5) or adult mice were stained with anti–CD4-APC and anti–CD25-FITC antibodies. Thymocytes were also stained with biotinylated anti-CD8 antibodies and SA-PerCP. Intracellular Foxp3 stains were then performed using PE-conjugated anti-Foxp3 antibodies (eBioscience). In some experiments, intracellular staining with anti–CTLA-4–PE antibodies was performed after surface staining cells for CD4, CD25, CD8, GITR, and Foxp3 (APC-conjugated Foxp3 antibodies). Stained cells were analyzed using a FACSCalibur (BD) or LSR (BD), and the data was analyzed using CellQuest Pro software (BD).
Generation and analysis of radiation bone marrow chimeras.
Bone marrow cells (2 × 107) isolated from the femurs of wt or c-rel−/− mice were injected into 8-wk-old lethally irradiated (2 × 550 Rad) recipients. The different combinations of bone marrow chimeras were wt or c-rel−/− (both Ly5.2) bone marrow transferred into wt (Ly5.1) mice; wt (Ly5.1) or c-rel−/− (Ly5.2) bone marrow transferred into c-rel−/− (Ly5.2) mice; and a 1:1 mix (107 cells each) of wt (Ly5.1) and c-rel−/− (Ly5.2) bone marrow injected into wt (Ly5.1) mice. Reconstitution of the hemopoietic compartment with donor stem cells was assessed 6 wk after transplantation by examining peripheral blood collected from the retroorbital vein. T reg cells from the thymus and spleen of successfully engrafted mice were analyzed 8 wk after transplantation.
rela−/− hemopoietic cell chimeras.
Control (rela+/+ or rela+/−) and rela−/− thymocytes and T cells (all Ly5.2+) were generated by engrafting irradiated (2 × 550 Rad) wt (Ly5.1) mice with 106 E13 fetal liver hemopoietic progenitors as previously described (Grossmann et al., 1999)
Isolation of thymic DC.
Pooled thymuses were used for DC extraction as described elsewhere (O'Keeffe et al., 2005). In brief, single cell thymic suspensions prepared by mechanical processing and collagenase digestion were treated with EDTA. Light density cells were then collected by centrifugation and non-DC lineage cells depleted using magnetic beads coupled to anti–rat-IgG after coating them with rat anti–mouse anti-CD3, anti-CD90, anti-TER119, anti-RB68C5, anti-CD19, anti-CD11b, and anti-F4/80. For conventional DC isolation, enriched preparations were stained with anti-CD11c-FITC, anti-CD8-PE, anti-CD45RA-APC, and anti–Sirpα-biotin plus SA-PE-Cy7. Conventional DCs with high forward scatter and bright staining for CD11c were then separated into CD8+Sirpα− and CD8−Sirpα+ populations. The purity of the sorted DC was >98%.
Generation of thymic T reg cells in culture.
T reg cell differentiation in culture was performed using two methods. The first approach involved coculturing different combinations of purified wt thymic CD8−Sirpα+ DC (104 cells) with CD4+CD25− (2 × 104) thymocytes from wt or c-rel−/− mice in the presence of IL-7 for 5 d (Proietto et al., 2008). T reg cells were identified by staining with anti–CD4-Cy5, anti–CD25-FITC, and Foxp3-PE antibodies. The second culture method (Tai et al., 2005) involves activating wt or c-rel−/− CD4+CD8+ thymocytes (106) isolated from c-rel+/+bcl-2Tg and c-rel−/−bcl-2Tg mice with 10 µg/ml of plate-bound anti-CD3 and 10 µg/ml of anti-CD28 antibodies for 24 h, followed by an additional 48-h in culture without activation, after which CD4+CD8lo cells were stained for Foxp3 as described in this section.
Quantitative RT-PCR.
Total RNA was extracted from sorted thymic and splenic CD4+, CD4+CD25+, and CD4+CD25− T cells using TRI reagent (Sigma-Aldrich) according to the manufacturer's specifications. Total RNA was DNase treated in 20 mM Tris, pH 8.3, 50 mM KCl, and 2 mM MgCl2 using 1 U RNase-free DNase I (Roche) per 1 µg of total RNA at 37°C for 30 min, followed by heat inactivation at 75°C for 5 min. 0.2–1 µg DNase-treated total RNA was reverse transcribed using the first-strand complementary DNA (cDNA) synthesis system (OriGene) according to the manufacturer's instructions. Quantitative real-time PCR was performed using an ABI PRISM 7500 or 7900HT sequence detection system (Applied Biosystems). Reactions were performed in MicroAmp Optical 96-well or 384-well reaction plates (Applied Biosystems) in a final volume of 20 or 10 µl, respectively, containing SYBR Green Master Mix (Applied Biosystems), 300 nM of forward and reverse primers, and 1/10-diluted cDNA, according to the manufacturer's guidelines (Applied Biosystems). To normalize for experimental variation in cDNA synthesis and RNA input, PCRs for ubiquitin-conjugating enzyme (UBC) E2D 2 were conducted in parallel. The primer pairs used are listed in Table S2.
Western blotting.
Equal amounts of total cellular protein from FACS-purified populations of thymocytes (CD4+CD8+, CD8+CD4−, CD4+CD25−, and CD4+CD25+) and splenic T cells (CD4+CD25− and CD4+CD25+) were loaded on 10% Novex gels (Invitrogen) and then subjected to electrophoresis and Western blotting, performed as previously described (Banerjee et al., 2006) using RelA and c-Rel–specific antisera (Grumont and Gerondakis, 1994) or anti-Foxp3 antibodies. Blots were stripped and reprobed with anti–β-actin antibodies. Western blots on nuclear and cytoplasmic extracts, prepared as previously described (Grumont and Gerondakis, 1994), were conducted as described earlier in this section and probed with antibodies to c-Rel, after which the blots were stripped and reprobed with anti-ERK (Santa Cruz Biotechnology, Inc.) or histone H3–specific antibodies (Santa Cruz Biotechnology, Inc.).
TGF-β–induced T reg cell differentiation in culture.
Purified wt or c-rel−/− CD4+CD25− splenic T cells (2 × 105) were cultured with 1 µg/ml of plate bound anti-CD3 antibody plus 2 × 106 irradiated T cell–depleted splenocytes in the presence or absence of 2 ng/ml of recombinant human TGF-β (R&D Systems) for 4 d. Cells were then harvested and T reg cells quantified by staining for Foxp3 expression. The effect of exogenous IL-2 on TGF-β–dependent T reg cell conversion was determined by adding 50 U/ml of recombinant murine IL-2 (PeproTech) on D0 and D2 of the 4-d incubation. To assess the proliferation of TGF-β–converted cells, 5 × 104 CD4+CD25− T cells were cultured with anti-CD3 antibodies, 5 × 105 irradiated T cell–depleted splenocytes, and IL-2 in the presence or absence of TGF-β for 3 d and cultures pulsed with 1 µCi of [3H] thymidine for the final 8 h of culture. Incorporated radioactivity was measured by scintillation counting.
T reg cell conversion in vivo.
CD4+CD25− splenic T cells (>96% purity) isolated by flow cytometry from wt (C57BL/6, Ly5.1+, and Ly5.2+) and c-rel−/− (Ly5.2+) mice were mixed at a 1:1 ratio, and 5 × 104 cells from mixtures of either wt (Ly5.1+ and Ly5.2+) or wt (Ly5.1+) and c-rel−/− (Ly5.2+) CD4+CD25− T cells were injected intravenously into rag-1−/− mice. 18 d later, cell suspensions from the spleen and mesenteric lymph nodes of individual mice were stained with anti–CD4-APC, anti–CD8-PerCP, anti–CD45.1-PE, and either anti–CD25-FITC or anti–FoxP3-FITC. Stained cells were analyzed using a FACSCalibur and the data analyzed using Flow Jo software (Tree Star, Inc.).
T reg cell homeostatic proliferation.
CD4+CD25+ splenic T cells (>90% Foxp3+) purified by flow cytometry from wt (C57BL/6, Ly5.1+ and Ly5.2+) and c-rel−/− (Ly5.2+) mice were mixed in equal numbers (1:1 ratio) and 5 × 104 cells from mixtures either wt (Ly5.1+ and Ly5.2+) or wt (Ly5.1+) and c-rel−/−(Ly5.2+) CD4+CD25+ T cells were injected intravenously into rag-1−/− mice. Between 4 and 8 wk later, cell suspensions from the spleens and mesenteric lymph nodes of individual mice were stained with anti–CD4-PerCP, anti–CD8-PE, anti–CD45.1-APC, and anti–FoxP3-FITC antibodies. Stained cells were analyzed using a FACSCalibur and the data analyzed using Flow Jo software.
Infecting T cells with Foxp3-expressing retroviruses.
A full-length cDNA clone encoding mouse Foxp3 (provided by S. Barry, Child Health Research Institute, Adelaide, Australia) was inserted into the pMY-EGFP retroviral vector (Grumont et al., 2004), and stocks of control (MY-EGFP) or MY-EGFP/Foxp3 virus were generated by transfecting Phoenix cells. Purified CD4+CD25− T cells activated in culture with anti-CD3 antibodies plus IL-2 (50 U/ml) were infected with MY-EGFP or MY-EGFP/Foxp3 virus using a spin infection procedure (Grumont et al., 2004). After two rounds of infection, ∼20–30% of cultured cells were GFP positive.
In vitro suppression assay.
Purified wt CD4+CD25− T cells (2.5 × 104) together with varying ratios of wt or c-rel−/− splenic CD4+CD25+ T cells were activated with 1 µg/ml anti-CD3 mAb (145-2C11) in the presence of 105 irradiated (3,000 Rad) T cell–depleted wt splenocytes in 96-well round bottom plates for 72 h. These assays were also performed using either TGF-β–converted T reg cells or CD4+CD25+ T cells generated by infecting wt or c-rel−/− CD4+CD25− T cells with a retrovirus expression Foxp3. All cultures were pulsed with 1 µCi of 3H-thymidine for the final 8 h of incubation and then analyzed by scintillation counting.
In vivo suppression of experimentally induced colitis.
The induction of experimental colitis was performed as previously described (Asseman et al., 1999). In brief, 4 × 105 wt CD4+CD45RBhi cells were delivered by intravenous injection (200 µl) into rag-1−/− mice with or without 105 CD4+CD25+ splenic T cells isolated from wt or c-rel−/− mice. All animals were sacrificed 10 wk after the transfer of cells, with those mice that lost >10% of their initial body weight or were ill sacrificed beforehand. Colons were fixed with 10% formalin solution and stained with hematoxylin and eosin. Histological scoring was done in a blind fashion according to the colitis severity index (epithelial hyperplasia, 0–3; cellular infiltrate, 0–3; cryptic abscess, 0–1) as previously described (Berg et al., 1996).
Online supplemental material.
Fig. S1 shows c-Rel expression in nuclear and cytoplasmic fractions of resting and activated B cells. Fig. S2 shows cultured CD4+ and T reg cell numbers after TGF-β (with or without IL-2 treatment). Fig. S3 shows that TCR-activated c-rel−/− T cells demonstrate diminished proliferation after TGF-β treatment. Fig. S4 shows T reg cell conversion in vivo. Fig. S5 shows Foxp3 expression in populations of purified peripheral nT reg cells and converted CD4+CD25− T cells. Fig. S6 shows T reg cell control of antigen-specific effector T cell proliferation in c-rel−/− mice. Table S1 shows the numbers of CD4+CD25+, CD4+CD25+Foxp3+, and CD4+CD25−Foxp3+ cells in the thymus and spleen of 2-, 5-, and 28-d-old wt and c-rel−/− mice. Table S2 shows sequences of the primers used for determining quantitative real-time PCR analysis of gene expression in wt and c-rel−/− CD4+CD25− and CD4+CD25+ thymocytes and splenic T cells. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20091411/DC1.
Acknowledgments
The authors thank J. Adams for the Bcl-2 transgenic mice, Lina Ma for expert technical assistance, and Drs. Kristine Hardy and Stephen Daley for useful discussions.
This work was supported by a Program grant from the Leukemia and Lymphoma Society (SCOR grant 7015) and grants from the National Health and Medical Research Council of Australia (Program 257502 and Project 543141).
The authors have no conflicting financial interests.
Footnotes
Abbreviations used:
- APC
- allophycocyanin
- cDNA
- complementary DNA
- GITR
- glucocorticoid-induced TNF receptor related
- IKK
- IκB kinase
- mRNA
- messenger RNA
- nT reg
- natural T reg
- SP
- single positive
- T reg
- regulatory T
- UBC
- ubiquitin-conjugating enzyme
References
- Almeida A.R., Zaragoza B., Freitas A.A. 2006. Competition controls the rate of transition between the peripheral pools of CD4+CD25− and CD4+CD25+ T cells. Int. Immunol. 18:1607–1613 doi: 10.1093/intimm/dxl093 [DOI] [PubMed] [Google Scholar]
- Asseman C., Mauze S., Leach M.W., Coffman R.L., Powrie F. 1999. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J. Exp. Med. 190:995–1004 doi: 10.1084/jem.190.7.995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee A., Gugasyan R., McMahon M., Gerondakis S. 2006. Diverse Toll-like receptors utilize Tpl2 to activate extracellular signal-regulated kinase (ERK) in hemopoietic cells. Proc. Natl. Acad. Sci. USA. 103:3274–3279 doi: 10.1073/pnas.0511113103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes M.J., Krebs P., Harris N., Eidenschenk C., Gonzalez-Quintial R., Arnold C.N., Crozat K., Sovath S., Moresco E.M., Theofilopoulos A.N., et al. 2009. Commitment to the regulatory T cell lineage requires CARMA1 in the thymus but not in the periphery. PLoS Biol. 7:e51 doi: 10.1371/journal.pbio.1000051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer A.L., Yu A., Adeegbe D., Malek T.R. 2005. Essential role for interleukin-2 for CD4+CD25+ T regulatory cell development during the neonatal period. J. Exp. Med. 201:769–777 doi: 10.1084/jem.20041179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beg A.A., Sha W.C., Bronson R.T., Ghosh S., Baltimore D. 1995. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-kappa B. Nature. 376:167–170 doi: 10.1038/376167a0 [DOI] [PubMed] [Google Scholar]
- Bensinger S.J., Bandeira A., Jordan M.S., Caton A.J., Laufer T.M. 2001. Major histocompatibility complex class II–positive cortical epithelium mediates the selection of CD4+25+ immunoregulatory T cells. J. Exp. Med. 194:427–438 doi: 10.1084/jem.194.4.427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg D.J., Davidson N., Kühn R., Müller W., Menon S., Holland G., Thompson-Snipes L., Leach M.W., Rennick D. 1996. Enterocolitis and colon cancer in interleukin-10-deficient mice are associated with aberrant cytokine production and CD4(+) TH1-like responses. J. Clin. Invest. 98:1010–1020 doi: 10.1172/JCI118861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunting K., Rao S., Hardy K., Woltring D., Denyer G.S., Wang J., Gerondakis S., Shannon M.F. 2007. Genome-wide analysis of gene expression in T cells to identify targets of the NF-kappa B transcription factor c-Rel. J. Immunol. 178:7097–7109 [DOI] [PubMed] [Google Scholar]
- Caton A.J., Cozzo C., Larkin J., III, Lerman M.A., Boesteanu A., Jordan M.S. 2004. CD4(+) CD25(+) regulatory T cell selection. Ann. N. Y. Acad. Sci. 1029:101–114 doi: 10.1196/annals.1309.028 [DOI] [PubMed] [Google Scholar]
- Chen W., Jin W., Hardegen N., Lei K.J., Li L., Marinos N., McGrady G., Wahl S.M. 2003. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-β induction of transcription factor Foxp3. J. Exp. Med. 198:1875–1886 doi: 10.1084/jem.20030152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curotto de Lafaille M.A., Lino A.C., Kutchukhidze N., Lafaille J.J. 2004. CD25- T cells generate CD25+Foxp3+ regulatory T cells by peripheral expansion. J. Immunol. 173:7259–7268 [DOI] [PubMed] [Google Scholar]
- Fontenot J.D., Rudensky A.Y. 2005. A well adapted regulatory contrivance: regulatory T cell development and the forkhead family transcription factor Foxp3. Nat. Immunol. 6:331–337 doi: 10.1038/ni1179 [DOI] [PubMed] [Google Scholar]
- Fontenot J.D., Gavin M.A., Rudensky A.Y. 2003. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 4:330–336 doi: 10.1038/ni904 [DOI] [PubMed] [Google Scholar]
- Fontenot J.D., Dooley J.L., Farr A.G., Rudensky A.Y. 2005a. Developmental regulation of Foxp3 expression during ontogeny. J. Exp. Med. 202:901–906 doi: 10.1084/jem.20050784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontenot J.D., Rasmussen J.P., Gavin M.A., Rudensky A.Y. 2005b. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat. Immunol. 6:1142–1151 doi: 10.1038/ni1263 [DOI] [PubMed] [Google Scholar]
- Gavin M.A., Rasmussen J.P., Fontenot J.D., Vasta V., Manganiello V.C., Beavo J.A., Rudensky A.Y. 2007. Foxp3-dependent programme of regulatory T-cell differentiation. Nature. 445:771–775 doi: 10.1038/nature05543 [DOI] [PubMed] [Google Scholar]
- Gerondakis S., Strasser A. 2003. The role of Rel/NF-kappaB transcription factors in B lymphocyte survival. Semin. Immunol. 15:159–166 doi: 10.1016/S1044-5323(03)00036-8 [DOI] [PubMed] [Google Scholar]
- Gerondakis S., Grumont R., Gugasyan R., Wong L., Isomura I., Ho W., Banerjee A. 2006. Unravelling the complexities of the NF-kappaB signalling pathway using mouse knockout and transgenic models. Oncogene. 25:6781–6799 doi: 10.1038/sj.onc.1209944 [DOI] [PubMed] [Google Scholar]
- Gilmore T.D. 2006. Introduction to NF-kappaB: players, pathways, perspectives. Oncogene. 25:6680–6684 doi: 10.1038/sj.onc.1209954 [DOI] [PubMed] [Google Scholar]
- Grossmann M., Metcalf D., Merryfull J., Beg A.A., Baltimore D., Gerondakis S. 1999. The combined absence of the transcription factors Rel and RelA leads to multiple hemopoietic cell defects. Proc. Natl. Acad. Sci. USA. 96:11848–11853 doi: 10.1073/pnas.96.21.11848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossmann M., O'Reilly L.A., Gugasyan R., Strasser A., Adams J.M., Gerondakis S. 2000. The anti-apoptotic activities of Rel and RelA required during B-cell maturation involve the regulation of Bcl-2 expression. EMBO J. 19:6351–6360 doi: 10.1093/emboj/19.23.6351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grumont R.J., Gerondakis S. 1994. The subunit composition of NF-kappa B complexes changes during B-cell development. Cell Growth Differ. 5:1321–1331 [PubMed] [Google Scholar]
- Grumont R.J., Strasser A., Gerondakis S. 2002. B cell growth is controlled by phosphatidylinositol 3-kinase-dependent induction of Rel/NF-kappaB regulated c-myc transcription. Mol. Cell. 10:1283–1294 doi: 10.1016/S1097-2765(02)00779-7 [DOI] [PubMed] [Google Scholar]
- Grumont R., Lock P., Mollinari M., Shannon F.M., Moore A., Gerondakis S. 2004. The mitogen-induced increase in T cell size involves PKC and NFAT activation of Rel/NF-kappaB-dependent c-myc expression. Immunity. 21:19–30 doi: 10.1016/j.immuni.2004.06.004 [DOI] [PubMed] [Google Scholar]
- Gugasyan R., Voss A., Varigos G., Thomas T., Grumont R.J., Kaur P., Grigoriadis G., Gerondakis S. 2004. The transcription factors c-rel and RelA control epidermal development and homeostasis in embryonic and adult skin via distinct mechanisms. Mol. Cell. Biol. 24:5733–5745 doi: 10.1128/MCB.24.13.5733-5745.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeryfar S.M.M., DiPaolo R.J., Tscharke D.C., Bennink J.R., Yewdell J.W. 2005. Regulatory T cells suppress CD8+ T cell responses induced by direct priming and cross-priming and moderate immunodominance disparities. J. Immunol. 174:3344–3351 [DOI] [PubMed] [Google Scholar]
- Harling-McNabb L., Deliyannis G., Jackson D.C., Gerondakis S., Grigoriadis G., Brown L.E. 1999. Mice lacking the transcription factor subunit Rel can clear an influenza infection and have functional anti-viral cytotoxic T cells but do not develop an optimal antibody response. Int. Immunol. 11:1431–1439 doi: 10.1093/intimm/11.9.1431 [DOI] [PubMed] [Google Scholar]
- Hayden M.S., Ghosh S. 2008. Shared principles in NF-kappaB signaling. Cell. 132:344–362 doi: 10.1016/j.cell.2008.01.020 [DOI] [PubMed] [Google Scholar]
- Hettmann T., DiDonato J., Karin M., Leiden J.M. 1999. An essential role for nuclear factor κB in promoting double positive thymocyte apoptosis. J. Exp. Med. 189:145–158 doi: 10.1084/jem.189.1.145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori S., Nomura T., Sakaguchi S. 2003. Control of regulatory T cell development by the transcription factor Foxp3. Science. 299:1057–1061 doi: 10.1126/science.1079490 [DOI] [PubMed] [Google Scholar]
- Kahn-Perlès B., Lipcey C., Lécine P., Olive D., Imbert J. 1997. Temporal and subunit-specific modulations of the Rel/NF-kappaB transcription factors through CD28 costimulation. J. Biol. Chem. 272:21774–21783 doi: 10.1074/jbc.272.35.21774 [DOI] [PubMed] [Google Scholar]
- Köntgen F., Grumont R.J., Strasser A., Metcalf D., Li R., Tarlinton D., Gerondakis S. 1995. Mice lacking the c-rel proto-oncogene exhibit defects in lymphocyte proliferation, humoral immunity, and interleukin-2 expression. Genes Dev. 9:1965–1977 doi: 10.1101/gad.9.16.1965 [DOI] [PubMed] [Google Scholar]
- Kretschmer K., Apostolou I., Hawiger D., Khazaie K., Nussenzweig M.C., von Boehmer H. 2005. Inducing and expanding regulatory T cell populations by foreign antigen. Nat. Immunol. 6:1219–1227 doi: 10.1038/ni1265 [DOI] [PubMed] [Google Scholar]
- Lin X., Wang D. 2004. The roles of CARMA1, Bcl10, and MALT1 in antigen receptor signaling. Semin. Immunol. 16:429–435 doi: 10.1016/j.smim.2004.08.022 [DOI] [PubMed] [Google Scholar]
- Lin W., Haribhai D., Relland L.M., Truong N., Carlson M.R., Williams C.B., Chatila T.A. 2007. Regulatory T cell development in the absence of functional Foxp3. Nat. Immunol. 8:359–368 doi: 10.1038/ni1445 [DOI] [PubMed] [Google Scholar]
- Lio C.-W.J., Hsieh C.-S. 2008. A two-step process for thymic regulatory T cell development. Immunity. 28:100–111 doi: 10.1016/j.immuni.2007.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston A., Rudensky A.Y. 2007. Thymic development and peripheral homeostasis of regulatory T cells. Curr. Opin. Immunol. 19:176–185 doi: 10.1016/j.coi.2007.02.005 [DOI] [PubMed] [Google Scholar]
- Marie J.C., Letterio J.J., Gavin M., Rudensky A.Y. 2005. TGF-β1 maintains suppressor function and Foxp3 expression in CD4+CD25+ regulatory T cells. J. Exp. Med. 201:1061–1067 doi: 10.1084/jem.20042276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marson A., Kretschmer K., Frampton G.M., Jacobsen E.S., Polansky J.K., MacIsaac K.D., Levine S.S., Fraenkel E., von Boehmer H., Young R.A. 2007. Foxp3 occupancy and regulation of key target genes during T-cell stimulation. Nature. 445:931–935 doi: 10.1038/nature05478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintern J.D., Belz G., Gerondakis S., Carbone F.R., Heath W.R. 2002. The cross-priming APC requires a Rel-dependent signal to induce CTL. J. Immunol. 168:3283–3287 [DOI] [PubMed] [Google Scholar]
- Molinero L.L., Yang J., Gajewski T., Abraham C., Farrar M.A., Alegre M.-L. 2009. CARMA1 controls an early checkpoint in the thymic development of FoxP3+ regulatory T cells. J. Immunol. 182:6736–6743 doi: 10.4049/jimmunol.0900498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keeffe M., Grumont R.J., Hochrein H., Fuchsberger M., Gugasyan R., Vremec D., Shortman K., Gerondakis S. 2005. Distinct roles for the NF-kappaB1 and c-Rel transcription factors in the differentiation and survival of plasmacytoid and conventional dendritic cells activated by TLR-9 signals. Blood. 106:3457–3464 doi: 10.1182/blood-2004-12-4965 [DOI] [PubMed] [Google Scholar]
- Ogilvy S., Metcalf D., Print C.G., Bath M.L., Harris A.W., Adams J.M. 1999. Constitutive Bcl-2 expression throughout the hematopoietic compartment affects multiple lineages and enhances progenitor cell survival. Proc. Natl. Acad. Sci. USA. 96:14943–14948 doi: 10.1073/pnas.96.26.14943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasparakis M., Courtois G., Hafner M., Schmidt-Supprian M., Nenci A., Toksoy A., Krampert M., Goebeler M., Gillitzer R., Israel A., et al. 2002. TNF-mediated inflammatory skin disease in mice with epidermis-specific deletion of IKK2. Nature. 417:861–866 doi: 10.1038/nature00820 [DOI] [PubMed] [Google Scholar]
- Pohl T., Gugasyan R., Grumont R.J., Strasser A., Metcalf D., Tarlinton D., Sha W., Baltimore D., Gerondakis S. 2002. The combined absence of NF-kappa B1 and c-Rel reveals that overlapping roles for these transcription factors in the B cell lineage are restricted to the activation and function of mature cells. Proc. Natl. Acad. Sci. USA. 99:4514–4519 doi: 10.1073/pnas.072071599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proietto A.I., van Dommelen S., Zhou P., Rizzitelli A., D'Amico A., Steptoe R.J., Naik S.H., Lahoud M.H., Liu Y., Zheng P., et al. 2008. Dendritic cells in the thymus contribute to T-regulatory cell induction. Proc. Natl. Acad. Sci. USA. 105:19869–19874 doi: 10.1073/pnas.0810268105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao S., Gerondakis S., Woltring D., Shannon M.F. 2003. c-Rel is required for chromatin remodeling across the IL-2 gene promoter. J. Immunol. 170:3724–3731 [DOI] [PubMed] [Google Scholar]
- Robey E., Fowlkes B.J. 1994. Selective events in T cell development. Annu. Rev. Immunol. 12:675–705 doi: 10.1146/annurev.iy.12.040194.003331 [DOI] [PubMed] [Google Scholar]
- Sakaguchi S. 2004. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu. Rev. Immunol. 22:531–562 doi: 10.1146/annurev.immunol.21.120601.141122 [DOI] [PubMed] [Google Scholar]
- Sakaguchi S. 2005. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat. Immunol. 6:345–352 doi: 10.1038/ni1178 [DOI] [PubMed] [Google Scholar]
- Salem M.L., Kadima A.N., El-Naggar S.A., Rubinstein M.P., Chen Y., Gillanders W.E., Cole D.J. 2007. Defining the ability of cyclophosphamide preconditioning to enhance the antigen-specific CD8+ T-cell response to peptide vaccination: creation of a beneficial host microenvironment involving type I IFNs and myeloid cells. J. Immunother. 30:40–53 doi: 10.1097/01.cji.0000211311.28739.e3 [DOI] [PubMed] [Google Scholar]
- Sansom D.M., Walker L.S. 2006. The role of CD28 and cytotoxic T-lymphocyte antigen-4 (CTLA-4) in regulatory T-cell biology. Immunol. Rev. 212:131–148 doi: 10.1111/j.0105-2896.2006.00419.x [DOI] [PubMed] [Google Scholar]
- Scheidereit C. 2006. IkappaB kinase complexes: gateways to NF-kappaB activation and transcription. Oncogene. 25:6685–6705 doi: 10.1038/sj.onc.1209934 [DOI] [PubMed] [Google Scholar]
- Schmidt-Supprian M., Courtois G., Tian J., Coyle A.J., Israël A., Rajewsky K., Pasparakis M. 2003. Mature T cells depend on signaling through the IKK complex. Immunity. 19:377–389 doi: 10.1016/S1074-7613(03)00237-1 [DOI] [PubMed] [Google Scholar]
- Sha W.C., Liou H.-C., Tuomanen E.I., Baltimore D. 1995. Targeted disruption of the p50 subunit of NF-kappa B leads to multifocal defects in immune responses. Cell. 80:321–330 doi: 10.1016/0092-8674(95)90415-8 [DOI] [PubMed] [Google Scholar]
- Shevach E.M. 2000. Regulatory T cells in autoimmunity. Annu. Rev. Immunol. 18:423–449 doi: 10.1146/annurev.immunol.18.1.423 [DOI] [PubMed] [Google Scholar]
- Siebenlist U., Brown K., Claudio E. 2005. Control of lymphocyte development by nuclear factor-kappaB. Nat. Rev. Immunol. 5:435–445 doi: 10.1038/nri1629 [DOI] [PubMed] [Google Scholar]
- Tai X., Cowan M., Feigenbaum L., Singer A. 2005. CD28 costimulation of developing thymocytes induces Foxp3 expression and regulatory T cell differentiation independently of interleukin 2. Nat. Immunol. 6:152–162 doi: 10.1038/ni1160 [DOI] [PubMed] [Google Scholar]
- Takahama Y. 2006. Journey through the thymus: stromal guides for T-cell development and selection. Nat. Rev. Immunol. 6:127–135 doi: 10.1038/nri1781 [DOI] [PubMed] [Google Scholar]
- Tang Q., Henriksen K.J., Boden E.K., Tooley A.J., Ye J., Subudhi S.K., Zheng X.X., Strom T.B., Bluestone J.A. 2003. Cutting edge: CD28 controls peripheral homeostasis of CD4+CD25+ regulatory T cells. J. Immunol. 171:3348–3352 [DOI] [PubMed] [Google Scholar]
- Venkataraman L., Burakoff S.J., Sen R. 1995. FK506 inhibits antigen receptor–mediated induction of c-rel in B and T lymphoid cells. J. Exp. Med. 181:1091–1099 doi: 10.1084/jem.181.3.1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams L.M., Rudensky A.Y. 2007. Maintenance of the Foxp3-dependent developmental program in mature regulatory T cells requires continued expression of Foxp3. Nat. Immunol. 8:277–284 doi: 10.1038/ni1437 [DOI] [PubMed] [Google Scholar]
- Zheng Y., Rudensky A.Y. 2007. Foxp3 in control of the regulatory T cell lineage. Nat. Immunol. 8:457–462 doi: 10.1038/ni1455 [DOI] [PubMed] [Google Scholar]
- Zheng Y., Vig M., Lyons J., Van Parijs L., Beg A.A. 2003. Combined deficiency of p50 and cRel in CD4+ T cells reveals an essential requirement for nuclear factor κB in regulating mature T cell survival and in vivo function. J. Exp. Med. 197:861–874 doi: 10.1084/jem.20021610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y., Josefowicz S.Z., Kas A., Chu T.T., Gavin M.A., Rudensky A.Y. 2007. Genome-wide analysis of Foxp3 target genes in developing and mature regulatory T cells. Nature. 445:936–940 doi: 10.1038/nature05563 [DOI] [PubMed] [Google Scholar]
- Ziegler S.F. 2006. FOXP3: of mice and men. Annu. Rev. Immunol. 24:209–226 doi: 10.1146/annurev.immunol.24.021605.090547 [DOI] [PubMed] [Google Scholar]