Abstract
One of the most rigorously investigated problems in modern neuroscience is to decipher the mechanisms by which experience-induced changes in the central nervous system are translated into behavioral acquisition, consolidation, retention, and subsequent recall of information. Brain-derived neurotrophic factor (BDNF) has recently emerged as one of the most potent molecular mediators of not only central synaptic plasticity, but also behavioral interactions between an organism and its environment. Recent experimental evidence indicates that BDNF modulates synaptic transmission and plasticity by acting across different spatial and temporal domains. BDNF signaling evokes both short- and long-term periods of enhanced synaptic physiology in both pre- and postsynaptic compartments of central synapses. Specifically, BDNF/TrkB signaling converges on the MAP kinase pathway to enhance excitatory synaptic transmission in vivo, as well as hippocampal-dependent learning in behaving animals. Emerging concepts of the intracellular signaling cascades involved in synaptic plasticity induced through environmental interactions resulting in behavioral learning further support the contention that BDNF/TrkB signaling plays a fundamental role in mediating enduring changes in central synaptic structure and function. Here we review recent literature showing the involvement of BDNF/TrkB signaling in hippocampal-dependent learning paradigms, as well as in the types of cellular plasticity proposed to underlie learning and memory.
Activity-induced changes in the central nervous system (CNS) are widely proposed mechanisms that allow organisms to adapt behaviorally, to learn and remember. For more than a century, the most intensely studied aspects of learning and memory have been to determine the cellular and molecular mechanisms of memory acquisition, consolidation, and subsequent recall of information. The first formal ideas regarding the ability of experience to shape and modify brain structures as well as synaptic junctions between nerve cells can be traced back to the later part of the 19th century (Bain 1872; James 1890; Tanzi 1893; Foster and Sherringhton 1897; Cajal 1909). More recently, Donald Hebb proposed a cellular mechanism for associative learning postulating that coincident activity at given synaptic junctions modifies the properties of those synapses, thereby increasing their efficiency (Hebb 1949). This so-called Hebbian principle dominates thinking at present of how use-dependent changes at synapses occur during learning. Investigations into the mechanisms underlying learning have thus primarily focused on the computational unit of the nervous system, the synapse. Synapses are not static; the cellular mechanisms of learning and memory, induced by experience, involve biochemical modification and morphological remodeling of existing synapses, as well as genesis of new synapses, resulting in activity-dependent physiological changes, which collectively define interactions between an organism and its environment. The majority of these investigations have delved into the mechanisms underlying changes in synaptic strength in one of the anatomical regions most relevant to learning and memory, the hippocampus. The study of synaptic plasticity in the hippocampus and its relationship to learning and memory has unveiled a labyrinth of potential mechanisms and molecular signals beyond the scope of the present communication, and has been recently reviewed (Martin et al. 2000; Abel and Lattal 2001).
Not only are the mechanisms of neuronal plasticity during brain development similar to those proposed to occur during learning in the adult nervous system, but biologically similar molecular signals are often conserved to serve similar functions in a diversity of roles, enhancing the evolutionary advantage they confer. Due to their established role during brain development, some of the most attractive molecular candidates for modulating synaptic plasticity during learning and memory processes are members of the family of neurotrophins (NTs). Thus, NTs represent molecular signals initially establishing, then allowing an organism's nervous system to remain in an unbound plastic state. The mammalian NTs, nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3), and neurotrophin 4/5 (NT-4/5), all have been shown to play an essential role in neuronal viability and differentiation, as well as synaptic plasticity in various brain regions relevant to learning and memory (for review, see McAllister et al. 1999). Two types of receptors mediate NT action: high-affinity membrane-bound tyrosine kinase receptors (Trk) and a low-affinity pan-neurotrophin receptor (p75). Specific Trk receptors have preferential affinities for one or more of the NTs: TrkA for NGF, TrkB for BDNF and NT-4/5, and TrkC for NT-3. BDNF is one member providing a target-derived signal for the establishment of neuronal connections during development, as well as continuing to modify these networks long after their establishment.
BDNF is of particular interest in the hippocampus, an area well-known for its role in memory and for its high degree of synaptic plasticity, for this region has the highest neuroanatomical expression of BDNF and its TrkB receptor in the mammalian brain (Murer et al. 2001). Even though BDNF has been repeatedly hypothesized to play a role in learning and memory, evidence of such a role in behaving animals is only beginning to emerge. The extensive literature on the role of NTs in synaptic plasticity, including neurotransmitter release and intracellular signaling cascades, has been reviewed in recent articles (Segal and Greenberg 1996; Schinder and Poo 2000; Thoenen 2000; Poo 2001; Tyler et al. 2002). The aims of the present review are to examine the behavioral evidence supporting the involvement of BDNF-triggered intracellular cascades in hippocampal-mediated learning and memory, as well as to evaluate the evidence showing the involvement of those same signaling cascades in forms of hippocampal excitatory synaptic plasticity thought to underlie mechanistically some forms of learning and memory.
BDNF Signaling Is Necessary for Hippocampal-Dependent Learning
The role of BDNF in activity-dependent forms of synaptic plasticity has been extended by implicating BDNF directly in learning and memory processes; however, the majority of these hypotheses have been based on correlative evidence between the molecular and physiological similarities of BDNF-induced plasticity and the types of plasticity thought to underlie learning and memory. More recently, BDNF/TrkB signaling has been implicated in learning and memory by directly examining its role in a variety of learning paradigms in behaving animals. Despite the fact that BDNF has been shown to play a critical role in a variety of learning paradigms in mice (Bao et al. 1998; Qiao et al. 1998; Aloe et al. 1999; Croll et al. 1999; Horger et al. 1999), monkeys (Tokuyama et al. 2000), zebra finches (Akutagawa and Konishi 1998; Wade 2000), and chicks (Johnston et al. 1999; Johnston and Rose 2001), the behavioral tests used in those studies were not as hippocampal-dependent as spatial learning or contextual fear conditioning. We focus here on behavioral evidence obtained using specific hippocampal-mediated spatial learning and contextual fear conditioning paradigms.
The first behavioral evidence supporting the potential role of BDNF in hippocampal-dependent cognitive performance was the observation of improved spatial memory from rats housed in enriched environments, which was also correlated with an increased BDNF mRNA expression in their hippocampi (Falkenberg et al. 1992). Interestingly, these authors found that there were no differences in basal hippocampal BDNF mRNA expression due to the housing conditions alone (enriched vs. impoverished). However, rats that were housed in the enriched environment and tested repeatedly in the Morris water maze had a 48% higher expression of BDNF mRNA in the hippocampus than did rats that were housed in an enriched environment but did not undergo the Morris water-maze training. It has since been reported that BDNF mRNA levels in the dentate gyrus are increased in rats with good retention at 1, 3, and 6 h posttraining, when compared with poor-retention rats undergoing Morris water-maze training (Ma et al. 1998). BDNF expression in the CA1 region of the hippocampus in rats increases above control values within 30 min of undergoing a contextual conditioning paradigm (Hall et al. 2000). In addition, spatial memory formation is associated with increased BDNF mRNA levels when learning takes place in the eight-arm radial maze, but not when learning is inhibited with a brain nitric oxide synthase inhibitor (7-nitroindazole; Mizuno et al. 2000). Although the increased levels of BDNF mRNA under various experimental manipulations designed to test spatio-cognitive function is good correlative evidence, it does not alone directly show a causal relationship between BDNF and hippocampal-dependent learning.
The behavioral evidence implicating BDNF in spatial learning and memory has revealed a role in the acquisition, consolidation, and subsequent recall of information. Although requiring twice the number of training days to achieve an equal level of performance in the Morris water maze as age-matched wild-type mice, young (6–8-wk), heterozygous BDNF knockout (KO) mice showed no deficits in memory retention (Linnarsson et al. 1997). The potential contribution of the genetic background to the behavioral phenotype has always been a caveat of the knockout approach for the study of the neurobiology of behavior (Gingrich and Hen 2000; Wolfer and Lipp 2000). The studies reviewed here are no exception; they present, however, the critical controls performed in littermates of the same genetic background, and although not absolute, they represent significant comparative observations between siblings of different genotypes. With these caveats in mind, these authors also found learning deficits in aged (10-mo) wild-type and BDNF KO mice compared with their young counterparts, with the age impairments being the greatest in the mutant mice. Consistent with the observations in BDNF KO mice, quenching of endogenous BDNF with function-blocking anti-BDNF antibodies also impairs spatial learning and memory in rats. Compared with controls, rats who received chronic (7-d) intracerebroventricular infusions of anti-BDNF antibody had longer escape latencies, slower rates of decline in escape latencies, and lower performance in probe trials in the Morris water maze, all indicative of impaired spatial learning and memory (Mu et al. 1999). Likewise, disruption of hippocampal BDNF gene expression using BDNF antisense oligonucleotides severely impairs spatial learning and memory in rats. Infusions of BDNF antisense oligonucleotides directly into the dentate gyrus elicited a reduction of BDNF mRNA in the hippocampus. Only when these infusions were performed before memory consolidation took place did they severely impair retention performance in an inhibitory avoidance learning task (Ma et al. 1998). It should be noted that these authors found that the same BDNF antisense oligonucleotide treatment also markedly reduced long-term potentiation (LTP) of excitatory synaptic transmission, the most intensively studied cellular model of hippocampal associative learning (Martin et al. 2000), at perforant path-dentate granule cell synapses. Following a 28-d training period on the eight-arm radial maze task, rats receiving continuous intracerebroventricular BDNF antisense oligonucleotide infusions beginning 4 d prior to and during the subsequent testing period, showed drastic impairments in both reference and working memory compared with control rats (Mizuno et al. 2000). Reference memory refers to trial-independent information retention remaining constant over repeated trials, whereas working memory refers to memory in which the information retained changes across repeated trials, and thereby is trial-dependent.
Mice with experimental alterations in the expression of the BDNF receptor TrkB also show impairments in hippocampal-mediated learning. Mice overexpressing a truncated noncatalytic form of the TrkB receptor, Trkb.T1, showed mild spatial memory deficits in Morris water-maze tasks, although hippocampal slices from these mice expressed normal LTP in the CA1 region (Saarelainen et al. 2000), showing, as briefly discussed below, an apparent dissociation between LTP and memory. Using Cre/loxP recombination and the promoter of α-calcium-calmodulin protein kinase II (α-CaMKII), a conditional trkB knockout mouse (trk-B–CRE) was produced that expresses the knockout transgene only in the forebrain starting at the end of the second postnatal week during development (Minichiello et al. 1999). This conditional knockout approach circumvents the always-present issue of potential developmental defects in constitutive knockout mice, at least to the extent that the period of genetic intervention spans only from approximately the second postnatal week to the time of behavioral testing a few weeks later. Despite this caveat, these authors present clear evidence that trkB–CRE mice failed to successfully learn navigation of the Morris water maze, and their escape latencies never decreased with repeated training and were only partially reduced when the escape platform was visible (Minichiello et al. 1999). Furthermore, trk-B–CRE mice did learn a less stressful eight-arm radial maze task, although with significantly more errors. Following trk-B–CRE testing in a contextual fear conditioning paradigm and a two-way avoidance task, these authors finally concluded that trkB–CRE mutants maintain a degree of task-specific long-term memory; however, they show impaired learning or inappropriate coping responses when they are subjected to complex and/or stressful learning paradigms. These authors also found an interesting dissociation between the impairment of LTP in the heterozygous mice and their lack of any behavioral deficits, because it seems that LTP induction and maintenance do not necessarily translate into behavioral defects in learning and memory in these mice. In fact, several other studies have shown that impairments in hippocampal LTP are not always associated with spatial learning deficits, indicating that spatial learning may not require hippocampal CA1 LTP (Bannerman et al. 1995; Huang et al. 1995; Nosten-Bertrand et al. 1996; Okabe et al. 1998). Alternatively, the precise strength and timing of hippocampal LTP may be the critical point for spatial learning to occur. In addition, some studies reported lack of effects on memory retention of acute or chronic intracerebral administration of BDNF (Fischer et al. 1994; Pelleymounter et al. 1996; Cirulli et al. 2000); however, as stressed by Fischer et al. (1994), the very restricted penetration of BDNF into the brain parenchyma, which is likely to limit or restrict the functional efficacy of the infused protein, could explain their negative results. Based on present behavioral studies, however, there is a general consensus that BDNF participates in hippocampal-dependent learning and memory formation, and, as we shall discuss below, also in synaptic plasticity phenomena considered to be the cellular correlate of associative learning.
Environmental Interactions Modulate BDNF Expression in the Hippocampus
One of the basic premises of synaptic plasticity's involvement in learning and memory consolidation is that it is an activity-dependent process. Any candidate proposed to play a role in learning by modulating synaptic plasticity should therefore also be regulated in an activity-dependent manner. Again, BDNF is a strong candidate, because its expression is regulated by neuronal activity, as evidenced by increased BDNF mRNA levels in the hippocampus of rats following the induction of seizure activity (Zafra et al. 1990; Ballarin et al. 1991; Ernfors et al. 1991; Isackson et al. 1991). Subsequently, BDNF mRNA levels were shown to increase sixfold in the granule cells of the dentate gyrus 30 min after the induction of epileptiform activity (Ernfors et al. 1991). Despite the fact that mRNA levels alone do not always represent protein levels, increases in the amount of BDNF protein have also been found in the hippocampus following seizure activity (Nawa et al. 1995; Smith et al. 1997; Yan et al. 1997). Whereas both BDNF mRNA and protein levels increase in the hippocampus following epileptiform activity, investigations have also shown similar results under a variety of more physiological experimental conditions. Stimulation patterns capable of inducing LTP also increase BDNF mRNA in the hippocampus (Patterson et al. 1992; Castren et al. 1993). Interestingly, it has been found that bioelectrical activity in general regulates BDNF mRNA levels. In cultured neurons, BDNF mRNA levels were found to increase following depolarization with either high-potassium solutions or with the excitatory amino acid, glutamate (Zafra et al. 1991; Lindholm et al. 1994; Berninger et al. 1995). Conversely, inhibition of electrical activity with either γ-aminobutyric acid (GABA) or with GABAA receptor agonists decreases BDNF expression (Zafra et al. 1991; Lindholm et al. 1994; Berninger et al. 1995). Furthermore, BDNF expression is modulated in the hippocampus by a variety of neurotransmitter and neuromodulatory systems, all of which have been implicated in hippocampal-dependent learning. Hippocampal BDNF expression is regulated by cholinergic (da Penha Berzaghi et al. 1993; Lapchak et al. 1993; Knipper et al. 1994; French et al. 1999), serotonergic (Nibuya et al. 1995; Vaidya et al. 1997, 1999), and nitric oxide (Xiong et al. 1999) manipulations, as well as by both gluco- and mineralocorticoids (Schaaf et al. 1998; Hansson et al. 2000).
In addition to these in vitro manipulations, and more relevant to learning and memory, BDNF expression is also regulated in vivo by various environmental interactions. Increased physical activity increases, and decreased physical activity decreases BDNF mRNA and protein levels in the rat hippocampus (Neeper et al. 1995, 1996; Oliff et al. 1998; Widenfalk et al. 1999; Russo-Neustadt et al. 2000). In the hippocampus of rats presented with enriched environments, BDNF levels are also increased (Falkenberg et al. 1992; Ickes et al. 2000). It has been established that both exercise and exposure to enriched environments improve performance in a variety of hippocampal-dependent learning paradigms. In fact, learning itself has been shown to markedly increase the levels of BDNF in the hippocampus (Kesslak et al. 1998; Hall et al. 2000). The mechanisms of activity-regulated BDNF expression have only recently begun to be illuminated through investigations into the structure and regulatory mechanisms of the BDNF gene itself.
The BDNF gene, a slave to activity, is intricately designed, allowing distinctive responses to a wide variety of environmental stimuli throughout development. The BDNF gene is comprised of four 5′ exons, each preceded by its own promoter region, and one 3′ exon from which the biologically active protein is ultimately transcribed (Metsis et al. 1993; Timmusk et al. 1993). This structure permits alternate splicing of four different BDNF mRNAs that are independently transcribed, based on neuroanatomical localization and the type of neuronal activity triggering the process; in addition, each one of the four splice variants has two distinct polyadenylation sites, giving rise to eight different mRNA species. Each one of the independently regulated 5′ exons is capable of being spliced to the 3′ coding region, thereby providing a mechanism for differential anatomical and functional BDNF expression within the CNS (Timmusk et al. 1993). Considering that all these mRNA species are translated into the same BDNF protein, various physiological stimuli may selectively activate different promoters, providing a complexity reflecting different stability, trafficking, and targeting within neurons. Although different mechanisms exist for each one, to some degree all exons are regulated in an activity-dependent fashion (for review, see Murer et al. 2001; Tao et al. 2002). Understanding the precise mechanisms of activity-dependent transcription of the BDNF gene, including its spatio-temporal pattern of activation, will provide insights into the integration between environmental stimuli and the associated plasticity mediated by BDNF/TrkB signaling. Although it is not yet clear exactly how activity-dependent expression of BDNF plays a role in plasticity, it seems as though “plasticity is built into the BDNF gene” (Black 1999).
Activity-Dependent BDNF Release Allows Spatio-Temporally Restricted BDNF Signaling
It is generally accepted that those cellular processes proposed to underlie learning and memory, such as LTP in the hippocampus, involve both pre- and postsynaptic changes at existing synapses. In this context, bidirectional BDNF release and signaling at synapses would allow modifications affecting both pre- and postsynaptic compartments, making it an ideal candidate for mediating learning-induced changes by fine tuning the strength of synaptic connections. In fact, BDNF has been shown to have effects on hippocampal physiology by acting pre-, post-, and perisynaptically (for review, see Poo 2001). Several fundamental issues regarding the cellular localization, release, and transport of BDNF have been raised, including the role of neuronal activity in the release of BDNF. Furthermore, does BDNF undergo retrograde and/or anterograde transport and/or signaling to mediate its effects on hippocampal synaptic plasticity, and ultimately learning and memory?
BDNF immunoreactivity has been found in the nucleus and surrounding cytoplasm, dendritic shafts and spines, axons, and axon terminals of hippocampal pyramidal neurons (for review, see Murer et al. 2001). Consistent with its ligand localization and signaling at synapses, TrkB immuno-reactivity was detected in neuronal cell bodies, along axons and dendritic shafts, as well as at excitatory synapses in both the axon terminals and dendritic spines of hippocampal neurons (Drake et al. 1999; Tyler and Pozzo-Miller 2001). Additionally, both BDNF and its TrkB receptor are often coexpressed in the same neuron (Kokaia et al. 1993; Miranda et al. 1993). These findings support the notion that BDNF exerts its actions by activating TrkB receptors, which trigger signal transduction pathways in both pre- and postsynaptic compartments, acting in both autocrine and paracrine fashions. Classically, NTs were described as target-derived factors, which signal and are transported in a retrograde fashion; however, recently emerging evidence from the CNS also indicates anterograde transport and signaling, from somata to axonal and dendritic processes.
Most experimental evidence indicates that hippocampal neurons release BDNF via the regulated pathway in an activity-dependent manner. BDNF immunoreactivity has been localized to large dense-core vesicles in hippocampal neurons (Fawcett et al. 1997; Smith et al. 1997; Haubensak et al. 1998; Moller et al. 1998). Recently, activity-dependent release of BDNF was shown using optical imaging of a BDNF–green fluorescence protein (GFP) fusion protein in transfected hippocampal neurons maintained in primary culture (Hartmann et al. 2001; Kojima et al. 2001). The localization within synaptic compartments, combined with recent evidence of anterograde transport, supports the hypothesis that BDNF may act directly at synapses to modulate synaptic transmission and plasticity. BDNF mRNA has been found in the granule layer of the dentate gyrus, and BDNF immunoreactivity in mossy fibers indicates anterograde transport of BDNF protein from dentate granule neurons (Conner et al. 1997; Yan et al. 1997). Perhaps the strongest evidence of anterograde transport of BDNF was provided by nuclear injections of BDNF–GFP cDNA, followed by subsequent visualization of the BDNF–GFP fusion protein in individual living neurons. Fluorescent puncta representing BDNF–GFP not only moved in the anterograde direction, from nucleus to axon terminals, but also underwent activity-dependent transfer from pre- to postsynaptic neurons across the synapse (Kohara et al. 2001). The bidirectional signaling of BDNF at synaptic junctions is in good agreement with physiological evidence indicating that BDNF enhances synaptic transmission by acting both pre- and postsynaptically. Furthermore, the properties of BDNF transport (retro- and anterograde), release (activity-dependent regulated secretion), and signaling (paracrine and autocrine actions at pre- and postsynaptic compartments) seem to make it an ideally suited molecule in facilitating coincidence detection, thereby modulating the long-term changes in synaptic strength thought to underlie learning and memory consolidation.
BDNF/TrkB Signaling Mediates Morphological Plasticity in Hippocampal Neurons
In addition to modifying the strength of established neuronal connections, several neurotrophins also play a fundamental role in the establishment of proper neuronal networks during development. In fact, in the peripheral nervous system and in some regions of the central nervous system, NTs are implicated in guiding and directing the growth of developing axons, as well as regulating their innervation density (for review, see Murer et al. 2001). With respect to learning-induced structural changes, it was proposed more than a century ago that changes in the structure and/or number of synapses may underlie memory formation following learning (Tanzi 1893; Cajal 1909). Indeed, it has been subsequently shown that increases in synaptic density follow the acquisition of new behaviors as a consequence of learning (Bailey and Kandel 1993). Furthermore, spatial training in a complex environment not only improved performance in hippocampal-dependent spatial tasks, but also increased spine density in basal dendrites of hippocampal CA1 pyramidal neurons (Moser et al. 1994, 1997). With an intriguing similarity, the induction of LTP in the hippocampus not only increases BDNF mRNA expression (Patterson et al. 1992; Castren et al. 1993; Dragunow et al. 1993), but also increases spine density (Trommald et al. 1996), the appearance of new spines (Engert and Bonhoeffer 1999; Maletic-Savatic et al. 1999), and/or the formation of multiple spine synapses (Toni et al. 1999). Interestingly, NTs themselves are capable of exerting morphological effects on axons (Gallo and Letourneau 1998) and dendrites (McAllister et al. 1995) similar to those following the induction of hippocampal LTP. Similarly, exposure to enriched environments not only increases BDNF mRNA expression and improves spatial learning, but it has been shown to have profound effects on spine morphology (Greenough and Volkmar 1973). Considering this evidence, it is possible that BDNF signaling meditates the morphological changes following hippocampal LTP induction, as well as those induced through environmental interactions. It is thought that all these changes mediate neuronal information processing, and thereby play a role in learning and memory.
Consistent with the functional convergence of intracellular signals among BDNF signaling, synaptic plasticity, and learning and memory, BDNF/TrkB signaling has also been shown to enhance dendritic growth and branching (McAllister et al. 1995), as well as spine dynamics (Horch et al. 1999) in pyramidal neurons from the visual cortex. In this context, BDNF has the capacity to translate neuronal activity into structural changes in the formation of ocular dominance columns in the visual cortex (Cabelli et al. 1995; for review, see McAllister et al. 1999). In addition, BDNF increases dendritic spine density in cerebellar Purkinje neurons in culture (Shimada et al. 1998). The axons of entorhinal and commissural fibers from TrkB−/− knockout mice had fewer axon collaterals and decreased density of axonal varicosities in both the hippocampus proper (CA1 and CA3 regions), as well as in the dentate gyrus (Martinezet al. 1998). These mice also show decreased synaptic innervation in both the stratum radiatum and the stratum lacunosum-moleculare of the hippocampus proper, as well as in the molecular layer of the dentate gyrus (Martinezet al. 1998). Furthermore, BDNF increases the number of dendritic spines on apical dendrites of CA1 pyramidal neurons (Fig. 1), as well as the number of CA3–CA1 excitatory synapses in hippocampal slice cultures (Tyler and Pozzo-Miller 2001).
Figure 1.
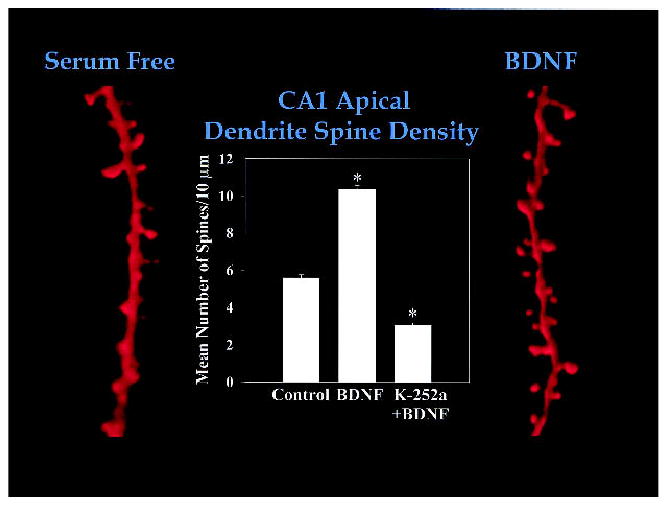
BDNF increases dendritic spine density in CA1 pyramidal neurons of hippocampal slices. Representative apical dendritic segments from CA1 pyramidal neurons from a serum-free (control; left) and a BDNF-treated hippocampal slice culture (250 ng/mL, 5–7 div; right). CA1 pyramidal neurons were filled with Alexa-594 during whole-cell recording, and later imaged by laser-scanning confocal microscopy. (Middle) Histograms of the number of dendritic spines per 10 μm of apical dendrite. The tyrosine kinase inhibitor K-252a blocked the BDNF-induced increase in dendritic spine density. Adapted from Tyler and Pozzo-Miller (2001).
BDNF induces enduring structural changes in a manner consistent with those morphological modifications classically and mechanistically thought to underlie learning and memory. Because most observations of BDNF-induced structural changes have been made using embryonic neurons in primary culture or cultured brain slices from young animals still undergoing developmental plasticity, it remains to be established if BDNF/TrkB signaling induces similar morphological changes in the mature CNS. Furthermore, several interesting issues need to be addressed, such as whether BDNF/TrkB signaling modifies dendritic spine morphology. Considering that fundamental biophysical properties of the dendritic spine, such as Ca2+ compartmentalization, depend on the morphology of the spine head and neck (Yuste and Bonhoeffer 2001), it will be of paramount importance to establish if the activity-dependent release of BDNF promotes the sculpting of dendritic spines in hippocampal pyramidal neurons (W.J. Tyler and L.D. Pozzo-Miller, in prep.). Taken together, these observations support the hypothesis that BDNF/TrkB signaling mediates the structural changes in hippocampal neuron spine density triggered by LTP-inducing stimulation paradigms in vitro, as well as during learning and environmental interactions in behaving animals.
The Effects of BDNF/TrkB Signaling Modulate LTP and NMDA Receptor Function: A Role in Cellular Models of Learning and Memory
Induction of LTP increases BDNF mRNA levels in hippocampal and dentate neurons (Patterson et al. 1992; Castren et al. 1993; Dragunow et al. 1993; Bramham et al. 1996). Furthermore, LTP induction is impaired at CA3–CA1 synapses of hippocampal slices from BDNF knockout mice, despite normal basal synaptic transmission (Korte et al. 1995; Patterson et al. 1996; Pozzo-Miller et al. 1999). These deficits are not a developmental defect because they can be rescued following either exogenous application of recombinant BDNF (Patterson et al. 1996; Pozzo-Miller et al. 1999) or adenovirus-mediated transfection of the BDNF gene (Korte et al. 1996) in hippocampal slices in vitro from BDNF-KO mice. Supporting the hypothesis that BDNF plays a role in hippocampal synaptic plasticity, it was further shown that LTP is attenuated following both theta burst and tetanic stimulation in slices pretreated with function-blocking anti-BDNF antibodies or the fusion protein TrkB–IgG, a molecular scavenger of endogenous BDNF (Figurov et al. 1996; Kang et al. 1997). Indicative of a permissive role for BDNF in hippocampal LTP, Figurov and colleagues (1996) found that BDNF allows the induction of LTP in hippocampal slices from neonatal rats (P12–P13), normally incapable of expressing LTP, likely because of the lack of endogenous BDNF expression at this age. In addition to its permissive role in the induction phase of LTP, BDNF has also been shown to play a role in the maintenance phase of LTP (Korte et al. 1998). Furthermore, infusion of BDNF into the dentate gyrus induced a slowly developing, long-lasting potentiation of excitatory synaptic transmission at medial perforant path-granule cell synapses in anesthetized rats (Messaoudi et al. 1998). Interestingly, the properties, time course (Fig. 3A), and transcription dependency of this in vivo BDNF-induced LTP (Messaoudi et al. 1998, 2002; Ying et al. 2002) were similar to those described for mRNA and protein synthesis-dependent late phase LTP (L-LTP) in the hippocampus (Nguyen et al. 1994).
Figure 3.
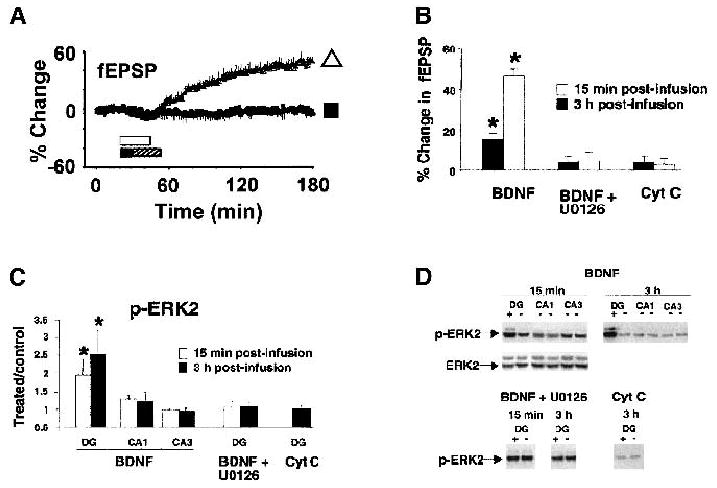
In vivo BDNF infusions into the dentate gyrus induce LTP and are coupled to an increase in ERK activation. (A) Time-course plots of medial perforant path-evoked EPSPs during BDNF infusion (white triangle; 2 μg/2 μL). BDNF was infused 300 μm above the medial perforant-granule cell synapses, during the period indicated by the white bar (n = 16). An MEK inhibitor (U0126; 30 μm) pre- and coinfused with BDNF blocked the BDNF-LTP. The period of MEK inhibitor (black bar) and BDNF plus MEK inhibitor (hatched bar) application are indicated. Values are means ±SEM expressed in percentage of baseline. (B) Bar graph of the EPSP slope changes obtained at 15 min and 3 h after infusion; asterisks indicate p < 0.05 significant difference from baseline. Cytochrome C, which has a similar molecular weight and charge as BDNF, was used as protein control. Infusion of cytochrome C (2 μgin 2 μL, 25 min) had no significant effect on the fEPSP slope. (C) Group mean ±SEM changes in p-ERK2 immunoreactivity levels based on densitometric analysis. Optical density values are expressed as a ratio between the treated and untreated (control) side for each hippocampal subfield. BDNF infusion increased activation of ERK2 at 15 min (n = 8) and 3 h (n = 7) in the infused dentate gyrus. BDNF infused into the dentate gyrus had no effect on ERK2 phosphorylation in hippocampal regions CA1 or CA3. Delivery of U0126 abolished the increase in ERK2 phosphorylation at both 15 min and 3 h (n = 4) at both time points. Cytochrome C infusion had no effect on p-ERK2 immunoreactivity levels. (D) Representative p-ERK immunoblots for the group data shown in C. Total ERK2 protein levels were unchanged following BDNF infusion. Adapted from Ying et al. (2002).
Several observations have shown a role for intact BDNF/TrkB signaling in making the transition from the early phase (E-LTP) to L-LTP. A 15-min pretreatment of hippocampal slices with the scavenger TrkB–IgG before induction of LTP resulted in a decaying potentiation in the CA1 region, which returned to baseline within 150 min, with no effects on basal synaptic transmission (Figurov et al. 1996; Kang et al. 1997; Korte et al. 1998). BDNF knockout mice show virtually no L-LTP (Korte et al. 1998); however, in a small number of cases, E-LTP can be induced in these mice (Korte et al. 1998; Pozzo-Miller et al. 1999). Korte et al. (1998) propose that BDNF/TrkB signaling may differentially regulate E-LTP and L-LTP, and/or TrkB ligands may not be necessary for all forms of observed E-LTP.
Coincident timing of pre- and postsynaptic activity is known to be a critical requirement for induction of long-term changes in synaptic strength (Bi and Poo 2001). Whereas there is extensive evidence of the modulatory effects of BDNF/TrkB signaling on presynaptic plasticity (for review, see Tyler et al. 2002), BDNF has also been shown to have intriguing postsynaptic effects. Changes in postsynaptic responsiveness to presynaptic glutamate release may include enhanced glutamate receptor function, as well as modulation of voltage-gated ion channels. Furthermore, enhanced postsynaptic responsiveness is thought to provide a mechanism for facilitating the temporal specificity requirements of coincidence detection, thereby resulting in greater changes in synaptic strength even when presynaptic glutamate release has not been enhanced (Bi and Poo 2001). In addition to the profound enhancement of presynaptic neurotransmitter release induced by BDNF, the evidence supporting the postsynaptic effects of BDNF/TrkB signaling alone generates possibilities for a role of BDNF-induced plasticity in learning and memory.
In the developing hippocampus, BDNF enhances synaptic transmission postsynaptically by selectively modulating the NMDA subtype of glutamate receptors. Levine et al. (1995) first showed that BDNF rapidly increases spontaneous synaptic transmission in primary hippocampal neurons in vitro, mediated by postsynaptic protein tyrosine phosphorylation. Introduction of K-252a, a general Trk receptor tyrosine kinase inhibitor, into the postsynaptic neuron had no effects on the frequency of spontaneous excitatory postsynaptic currents (sEPSC), but it prevented the BDNF-mediated increase in sEPSC amplitude (Levine et al. 1995). Furthermore, intracellular addition of okadaic acid, a protein phosphatase inhibitor, enhanced the effect of BDNF on sEPSC amplitude, strengthening the hypothesis that BDNF/TrkB signaling triggers protein phosphorylation of glutamate receptors (Levine et al. 1995). Interestingly, it has been previously shown that phosphorylation of tyrosine residues of NMDA receptor subunits enhances NMDA receptor function (Wang and Salter 1994). Consistent with this observation, TrkB activation has been shown to rapidly phosphorylate the NMDA receptor subunits NR1 and NR2B in isolated postsynaptic densities (Suen et al. 1997; Lin et al. 1998). With respect to NMDA receptor subunit involvement in learning and memory, restricted genetic deletion of the NR1 subunit in the CA1 region of the hippocampus using the conditional Cre/loxP recombination approach produces impaired NMDA-dependent LTP, and spatial memory (Tsien et al. 1996a,b). It was subsequently reported that BDNF increases NMDA-mediated currents, as well as the NMDA receptor component of evoked EPSCs in cultured hippocampal neurons (Sakai et al. 1997; Levine et al. 1998; Song et al. 1998; Crozier et al. 1999). Specifically, single-channel analysis indicated that BDNF rapidly increased the single-channel open probability (Po) of NMDA receptors, but did not affect the mean open time or unitary conductance in outside-out patch (Jarvis et al. 1997) or cell-attached (Levine et al. 1998) recordings in cultured hippocampal neurons. The actions of BDNF on NMDA receptor function in the hippocampus have direct implications concerning its ability to facilitate dendritic spine Ca2+ influx, and thus intracellular signaling mechanisms relevant to cellular models of learning and memory, such as LTP.
Neurotrophins have also been shown to modulate voltage-gated ion channels relevant to neuronal excitability (Lesser et al. 1997). The most intriguing evidence of neurotrophin modulation of neuron excitability by activating ionic conductances is the observation that rapid and local BDNF application evokes a fast membrane depolarization capable of eliciting action potentials in hippocampal pyramidal, dentate granule, and cerebellar Purkinje neurons (Kafitzet al. 1999). This membrane depolarization is mediated by TrkB-receptor-dependent activation of a TTX-insensitive, persistent Na+ current (Kafitz et al. 1999). Furthermore, this BDNF-mediated fast membrane depolarization cooperates with paired synaptic release of glutamate to lower the threshold of LTP induction by removing the Mg2+ block of NMDA receptors and enhancing Ca2+ influx into dendritic spines through NMDA channels (Kovalchuk et al. 2002). These novel observations further extend the possibilities for BDNF-induced modulation of neuronal excitability, synaptic transmission, and plasticity acting across different time domains depending on the spatio-temporal pattern of the signaling cascades triggered by TrkB receptor activation. Owing to their voltage sensitivity, NMDA receptors are considered molecular coincidence detectors underlying Hebbian learning principles (Bi and Poo 2001). The modulation of these molecular coincidence detectors by BDNF/TrkB signaling illustrates a potentially fundamental role for BDNF in learning and memory processes.
BDNF/TrkB Signaling and the Convergence of Intracellular Cascades Relevant to Learning and Synaptic Plasticity
Activation of the full-length catalytic form of Trk receptors involves ligand-initiated receptor dimerization, and autophosphorylation of specific tyrosine residues in their intracellular domain (for reviews, see Segal and Greenberg 1996; Huang and Reichardt 2001). The autophosphorylated tyrosine residues of Trk receptors provide interaction sites for adapter proteins, leading to the activation of several other enzymes. Ultimately, the propagation of these cascades leads to the activation of several central intracellular signaling pathways. Some of the proteins activated by autophosphorylated Trk receptors are phospholipase C-γ (PLC-γ; Obermeier et al. 1994; Stephens et al. 1994); the adapter proteins Shc, rAPS, and SH2-B (Obermeier et al. 1994; Stephens et al. 1994; Qian et al. 1998); phosphatidylinositol 3-kinase (PI3K; Kaplan and Stephens 1994; Stephens et al. 1994); and the tyrosine kinase Fyn (Iwasaki et al. 1998). These activated proteins also lead to Ras-dependent activation of the mitogen-activated protein (MAP) kinase, ERK (extracellular signal-regulated kinase; Kaplan and Stephens 1994; Sweatt 2001). Activated PLC-γ hydrolyzes phosphotidylinositol 4,5-bisphosphate to produce inositoltriphosphate (IP3), which in turn triggers Ca2+ release from internal stores, and diacylglycerol (DAG), which activates protein kinase C (PKC; Kaplan and Stephens 1994). The intracellular Ca2+ signal subsequently activates several forms of calcium/calmodulin-dependent kinases (CaMK; Finkbeiner et al. 1997). Through these signaling cascades, BDNF has been shown to induce phosphorylation and activation of the cyclic AMP response element-binding protein (CREB), which triggers gene transcription, through both CaMK-regulated (Finkbeiner et al. 1997) and MAPK-dependent pathways (Finkbeiner et al. 1997; Ying et al. 2002).
The link between BDNF/TrkB intracellular signaling, synaptic plasticity, and hippocampal-dependent learning is strengthened by the observations that similar molecules play fundamental roles in these processes (Fig. 2). Genetic manipulations indeed illustrate the overlap of signaling pathways induced by BDNF/TrkB and those previously shown to be relevant to synaptic plasticity, and learning and memory. Mice with genetic deletions or mutations of PKC (Abeliovich et al. 1993), α-CaMKII (Silva et al. 1992), CREB (Bourtchuladze et al. 1994), and Fyn (Grant et al. 1992) have all been shown to have deficits in spatial learning and/or retention of spatial information. Again, these findings do not directly illustrate a direct role for BDNF in hippocampal-dependent learning and memory, but rather lend support to this hypothesis, because BDNF/TrkB signaling positively modulates those same molecules that, when altered, impair hippocampal-dependent learning.
Figure 2.
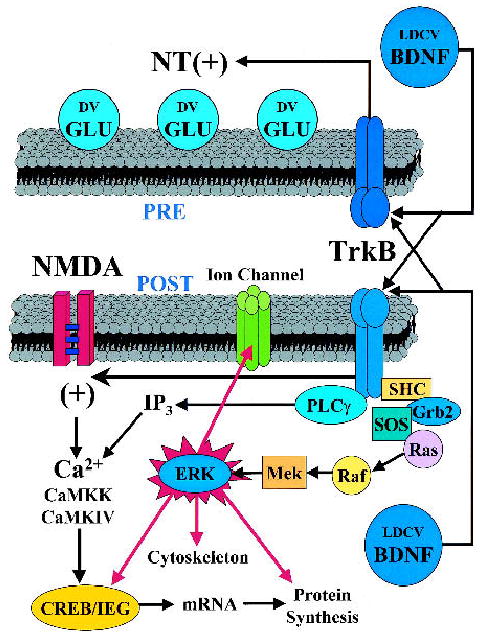
BDNF/TrkB signaling convergence on the MAP kinase pathway is implicated in both synaptic plasticity and learning and memory. The figure summarizes the evidence that BDNF is released from, and acts on, both pre- and postsynaptic compartments. BDNF has been repeatedly shown to enhance the release of the neurotransmitter glutamate from presynaptic nerve terminals. Postsynaptically, BDNF has been shown to phosphorylate some subunits of NMDA receptors. Following the binding of BDNF to the TrkB receptor, the signaling cascade converges on the MAP kinase pathway through the activation of ERK. Phosphorylated ERK then activates one or more of several targets such as CREB, other immediate early genes (IEG), cytoskeletal elements, protein synthesis, and/or voltage- and ligand-gated ion channels. The MAP kinase pathway has been shown to have a clear role in learning and memory (for review, see Sweatt 2001). (LDCV) Large dense core vesicle; (BDNF) brain-derived neurotrophic factor; (DV) docked vesicle; (Glu) glutamate; (NT) neurotrophin; (Pre) presynaptic terminal; (Post) postsynaptic spine; (NMDA) N-methyl-D-aspartate; (TrkB) tyrosine kinase B receptor; (IP3) inositoltriphosphate; (CaMKK) calcium calmodulin kinase kinase; (CaMKIV) calcium calmodulin kinase 4; (CREB) cyclic AMP response element-binding protein; (IEG) immediate early gene; (ERK) extracellular signal-regulated kinase; (MAPK) mitogen-activated protein kinase; (MEK) MAPK kinase; (PLC-γ) phospholipase C-γ; (mRNA) messenger RNA; (Raf) proto-oncogenic serine/threonine protein kinase; (Ras) rat sarcoma proto-oncogenic G-protein; (Grb2) growth factor receptor-bindingprotein 2; (SH-2) src homology domain 2; (SHC) SH-2-containingprotein; (SOS) nucleotide exchange factor son of sevenless.
The signaling pathway underlying the BDNF-induced synaptic potentiation in the dentate gyrus as first described by Messaoudi et al. (1998) has recently been investigated (for review, see Bramham et al. 2002). Ying et al. (2002) found that BDNF microinfusion into the dentate gyrus in vivo leads to a slowly developing and long-lasting potentiation of excitatory synaptic transmission at medial perforant path-granule cell synapses, hence coined BDNF-LTP, with a concomitant and rapid increase in phosphorylated ERK (p-ERK; Fig. 3). Furthermore, local infusion of specific MAP kinase kinase (MEK) inhibitors (PD98059 and U0126) during BDNF administration blocked both in vivo BDNF-LTP, as well as the increase in p-ERK. These observations show that the induction of in vivo BDNF-LTP is dependent on MEK-ERK signaling, consistent with the findings that both LTP induction and spatial learning induce enhanced phosphorylation of TrkB and ERK in the dentate gyrus (Gooney et al. 2002). Furthermore, ERK signaling is required for activation of CREB and up-regulation of the immediate early gene activity-regulated cytoskeleton-associated protein (Arc; Ying et al. 2002). Arc translation, in turn, is pivotally involved in the formation of L-LTP and hippocampal-dependent long-term memory (Guzowski et al. 2000). Because of the involvement of the MAPK pathway in memory formation and synaptic plasticity (for review, see Sweatt 2001), and combined with the findings previously discussed regarding BDNF-LTP, this pathway seems a likely target for investigations examining the effect of BDNF in behavioral learning paradigms. In fact, recent work by Alonso et al. (2002) shows the requirement of BDNF in the formation of both short- and long-term memory involving ERK-dependent and -independent mechanisms.
Alonso et al. (2002) show that BDNF signaling via ERK1/2 is required in a hippocampal-dependent one-trial avoidance learning paradigm in rats. Bilateral hippocampal CA1 infusions of a function-blocking anti-BDNF antibody prior to avoidance training impaired short-term memory retention scores. Further support for the involvement of BDNF in the one-trial avoidance task is provided by the observation that the infusion of exogenous BDNF into the CA1 region facilitates short-term memory retention scores (Fig. 4A; Alonso et al. 2002). In additional experiments, not only did BDNF infusion elicit a rapid increase in p-ERK, but the infusion of anti-BDNF antibody decreased p-ERK (Alonso et al. 2002). In the same study, BDNF was also shown to play a role in the formation of long-term memory (Fig. 4B). As shown in Figure 4, these authors found differences in the BDNF and anti-BDNF antibody effects between short-term (Fig. 4A) and long-term (Fig. 4B) memory test session performances. These results are consistent with previous work by Izquierdo et al. (1998) supporting the hypothesis that short- and long-term memory formation essentially involve separate mechanisms. Indeed, Alonso et al. (2002) found a similar dissociation, because an amnesic effect was induced by anti-BDNF antibody infusion 15 min prior to training, coupled with the absence of a BDNF effect at the same time point (Fig. 4B). These data indicate that, at least at that time point, the limiting factor to produce a memory enhancement seems not to be the amount of endogenous BDNF, but rather a downstream event triggered by BDNF signaling. As previously mentioned, the requirement of endogenous BDNF in the CA1 region involves different signaling cascades during different memory consolidation process. Consistent with recent findings obtained regarding contextual conditioning (Hall et al. 2000), Alonso et al. (2002) also showed that one-trial inhibitory avoidance training resulted in a rapid and transient induction of BDNF mRNA expression in the hippocampus. This induction of BDNF expression was not accompanied by rapid changes in the levels of BDNF protein. Several studies have shown that changes in BDNF mRNA and protein are not always correlated (Nanda and Mack 2000; Pollock et al. 2001), including the increase in BDNF mRNA expression after LTP induction in the rat hippocampus (Dragunow et al. 1993), which is not necessarily accompanied by changes in BDNF protein levels (Walton et al. 1999). The lack of change in BDNF protein levels may reflect an increased turnover rate of BDNF protein, or may represent the lack of translation of the newly synthesized BDNF mRNA, indicating that the BDNF gene may be regulated at the level of translation as well as of transcription. Alternatively, experience-dependent changes in BDNF protein trafficking may account for this discrepancy (Pollock et al. 2001). However, evidence showing that BDNF protein levels do not necessarily increase in parallel with BDNF mRNA levels indicates that the latter must be interpreted with caution when attempting to discern its function. Taken together with the physiological in vivo BDNF-LTP reported by Ying et al. (2002), these results strongly indicate that BDNF signaling is converging on the MAP kinase pathway to induce both behavioral and physiological long-lasting and enduring changes consistent with the role of BDNF in spatial learning and memory. Albeit the evidence from Ying et al. (2002) comes from the dentate gyrus region, whereas Alonso et al. (2002) provide evidence from the CA1 region of the hippocampus proper, these studies provide definitive evidence that BDNF indeed plays a fundamental role in hippocampal-dependent learning and memory.
Figure 4.
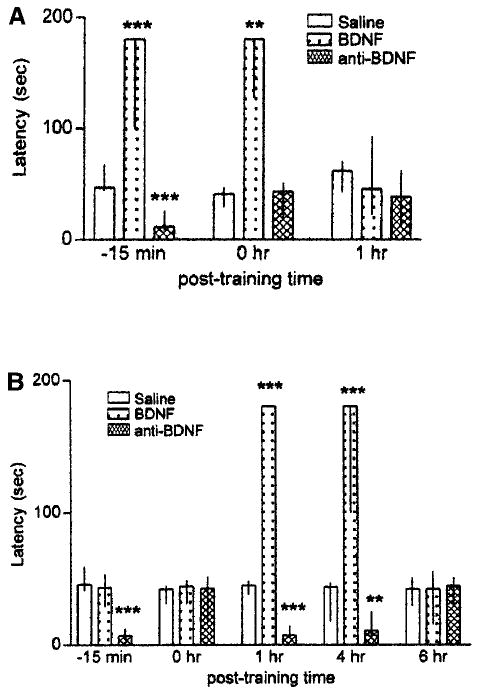
Hippocampal BDNF is required for the formation of both short- and long-term memory. (A) Median (interquartile range) latency to step down from the platform of the inhibitory avoidance box in the test session 1.5 h after training. Histograms represent latency scores for animals receiving bilateral CA1 infusions of saline, BDNF (0.25 μg/side), or a function-blocking anti-BDNF antibody (0.5 μg/side), 15 min prior to, immediately before, or 1 h following avoidance training. (B) Median (interquartile range) latency to step down from the platform of the inhibitory avoidance box in the test session 24 h after training. Histograms represent latency scores for animals receiving bilateral CA1 infusions of saline, BDNF (0.25 μg/side), or anti-BDNF antibody (0.5 μg/side), 15 min prior to, immediately before, or 1, 4, or 6 h following avoidance training. Adapted from Alonso et al. (2002).
Conclusions
BDNF/TrkB signaling is conserved during ontogeny, mediating both developmental and adult synaptic plasticity. Classically, BDNF provides a target-derived factor promoting the survival and differentiation of different populations of neurons in the developing nervous system. In the adult nervous system, BDNF induces several forms of neuronal plasticity in the hippocampus, thus making it an ideal candidate to mediate learning and memory. Although the behavioral evidence implicating BDNF in hippocampal-mediated learning is convincing, it has yet to be determined how endogenous BDNF signaling is translated into hippocampal synaptic plasticity during the learning process. The time course, spatial compartmentalization, and signaling specificity of the intracellular cascades triggered by the BDNF/TrkB complex, as well as its subsequent actions on its molecular targets following an animal's exposure to environmental stimuli, will need to be thoroughly investigated before we can fully understand how this signaling pathway plays a role in synaptic plasticity and learning in the intact nervous system. Continued investigations and generation of novel approaches will advance our basic understanding of the molecular biology and physiology of BDNF-induced plasticity. This progress will also provide important contributions to our understanding of fundamental issues in neuroscience such as the role of hippocampal synapses in governing spatial learning by acquiring, processing, integrating, storing, recalling, and synthesizing information within the central nervous system.
Acknowledgments
The authors thank Jorge Medina (Universidad de Buenos Aires, Argentina) and Alcino Silva (UCLA) for comments on the manuscript. This work was supported by NIH grants RO1-NS40593, MRRC P30-HD38985, and PO1-HD38760, as well as by AMGEN for the generous supply of BDNF (LDP-M).
References
- Abel T, Lattal KM. Molecular mechanisms of memory acquisition, consolidation and retrieval. Curr Opin Neurobiol. 2001;11:180–187. doi: 10.1016/s0959-4388(00)00194-x. [DOI] [PubMed] [Google Scholar]
- Abeliovich A, Paylor R, Chen C, Kim JJ, Wehner JM, Tonegawa S. PKC γ mutant mice exhibit mild deficits in spatial and contextual learning. Cell. 1993;75:1263–1271. doi: 10.1016/0092-8674(93)90614-v. [DOI] [PubMed] [Google Scholar]
- Akutagawa E, Konishi M. Transient expression and transport of brain-derived neurotrophic factor in the male zebra finch's song system during vocal development. Proc Natl Acad Sci. 1998;95:11429–11434. doi: 10.1073/pnas.95.19.11429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloe L, Properzi F, Probert L, Akassoglou K, Kassiotis G, Micera A, Fiore M. Learning abilities, NGF and BDNF brain levels in two lines of TNF-α transgenic mice, one characterized by neurological disorders, the other phenotypically normal. Brain Res. 1999;840:125–137. doi: 10.1016/s0006-8993(99)01748-5. [DOI] [PubMed] [Google Scholar]
- Alonso M, Vianna MRM, Depino AM, Souza TMe, Pereira P, Szapiro G, Viola H, Pitossi F, Izquierdo I, Medina JH. BDNF-triggered events in the rat hippocampus are required for both short and long-term memory formation. Hippocampus. 2002;12:551–560. doi: 10.1002/hipo.10035. [DOI] [PubMed] [Google Scholar]
- Bailey CH, Kandel ER. Structural changes accompanying memory storage. Annu Rev Physiol. 1993;55:397–426. doi: 10.1146/annurev.ph.55.030193.002145. [DOI] [PubMed] [Google Scholar]
- Bain A. Mind and body: The theories of their relation. Henry S. King; London: 1872. [Google Scholar]
- Ballarin M, Ernfors P, Lindefors N, Persson H. Hippocampal damage and kainic acid injection induce a rapid increase in mRNA for BDNF and NGF in the rat brain. Exp Neurol. 1991;114:35–43. doi: 10.1016/0014-4886(91)90082-n. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Good MA, Butcher SP, Ramsay M, Morris RG. Distinct components of spatial learning revealed by prior training and NMDA receptor blockade. Nature. 1995;378:182–186. doi: 10.1038/378182a0. [DOI] [PubMed] [Google Scholar]
- Bao S, Chen L, Qiao X, Knusel B, Thompson RF. Impaired eye-blink conditioning in waggler, a mutant mouse with cerebellar BDNF deficiency. Learn Mem. 1998;5:355–364. [PMC free article] [PubMed] [Google Scholar]
- Berninger B, Marty S, Zafra F, da Penha Berzaghi M, Thoenen H, Lindholm D. GABAergic stimulation switches from enhancing to repressing BDNF expression in rat hippocampal neurons during maturation in vitro. Development. 1995;121:2327–2335. doi: 10.1242/dev.121.8.2327. [DOI] [PubMed] [Google Scholar]
- Bi G, Poo M. Synaptic modification by correlated activity: Hebb's postulate revisited. Annu Rev Neurosci. 2001;24:139–166. doi: 10.1146/annurev.neuro.24.1.139. [DOI] [PubMed] [Google Scholar]
- Black IB. Trophic regulation of synaptic plasticity. J Neurobiol. 1999;41:108–118. [PubMed] [Google Scholar]
- Bourtchuladze R, Frenguelli B, Blendy J, Cioffi D, Schutz G, Silva AJ. Deficient long-term memory in mice with a targeted mutation of the cAMP-responsive element-binding protein. Cell. 1994;79:59–68. doi: 10.1016/0092-8674(94)90400-6. [DOI] [PubMed] [Google Scholar]
- Bramham CR, Southard T, Sarvey JM, Herkenham M, Brady LS. Unilateral LTP triggers bilateral increases in hippocampal neurotrophin and trk receptor mRNA expression in behaving rats: Evidence for interhemispheric communication. J Comp Neurol. 1996;368:371–382. doi: 10.1002/(SICI)1096-9861(19960506)368:3<371::AID-CNE4>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Bramham CR, Messaoudi E, Bårdsen K. Neurotrophins and synaptic plasticity in the adult hippocampus. In: Lester DS, et al., editors. Site-selective neurotoxicity. Taylor and Francis; London, UK: 2002. pp. 61–77. [Google Scholar]
- Cabelli RJ, Hohn A, Shatz CJ. Inhibition of ocular dominance column formation by infusion of NT-4/5 or BDNF. Science. 1995;267:1662–1666. doi: 10.1126/science.7886458. [DOI] [PubMed] [Google Scholar]
- Cajal S Ramón y. Histologie du Système Nerveux de l'Homme et des Vertébrés. Maloine, Paris: 1909. Reprinted in 1952 by Consejo Superior de Investigaciones Científicas, Instituto Ramón y Cajal, Madrid. [Google Scholar]
- Castren E, Pitkanen M, Sirvio J, Parsadanian A, Lindholm D, Thoenen H, Riekkinen PJ. The induction of LTP increases BDNF and NGF mRNA but decreases NT-3 mRNA in the dentate gyrus. Neuroreport. 1993;4:895–898. doi: 10.1097/00001756-199307000-00014. [DOI] [PubMed] [Google Scholar]
- Cirulli F, Berry A, Alleva E. Intracerebroventricular administration of brain-derived neurotrophic factor in adult rats affects analgesia and spontaneous behavior but not memory retention in a Morris water maze task. Neurosci Lett. 2000;287:207–210. doi: 10.1016/s0304-3940(00)01173-3. [DOI] [PubMed] [Google Scholar]
- Conner JM, Lauterborn JC, Yan Q, Gall CM, Varon S. Distribution of brain-derived neurotrophic factor (BDNF) protein and mRNA in the normal adult rat CNS: Evidence for anterograde axonal transport. J Neurosci. 1997;17:2295–2313. doi: 10.1523/JNEUROSCI.17-07-02295.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croll SD, Suri C, Compton DL, Simmons MV, Yancopoulos GD, Lindsay RM, Wiegand SJ, Rudge JS, Scharfman HE. Brain-derived neurotrophic factor transgenic mice exhibit passive avoidance deficits, increased seizure severity and in vitro hyperexcitability in the hippocampus and entorhinal cortex. Neuroscience. 1999;93:1491–1506. doi: 10.1016/s0306-4522(99)00296-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crozier RA, Black IB, Plummer MR. Blockade of NR2B-containing NMDA receptors prevents BDNF enhancement of glutamatergic transmission in hippocampal neurons. Learn Mem. 1999;6:257–266. [PMC free article] [PubMed] [Google Scholar]
- da Penha Berzaghi M, Cooper J, Castren E, Zafra F, Sofroniew M, Thoenen H, Lindholm D. Cholinergic regulation of brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF) but not neurotrophin-3 (NT-3) mRNA levels in the developing rat hippocampus. J Neurosci. 1993;13:3818–3826. doi: 10.1523/JNEUROSCI.13-09-03818.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragunow M, Beilharz E, Mason B, Lawlor P, Abraham W, Gluckman P. Brain-derived neurotrophic factor expression after long-term potentiation. Neurosci Lett. 1993;160:232–236. doi: 10.1016/0304-3940(93)90420-p. [DOI] [PubMed] [Google Scholar]
- Drake CT, Milner TA, Patterson SL. Ultrastructural localization of full-length trkB immunoreactivity in rat hippocampus suggests multiple roles in modulating activity-dependent synaptic plasticity. J Neurosci. 1999;19:8009–8026. doi: 10.1523/JNEUROSCI.19-18-08009.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engert F, Bonhoeffer T. Dendritic spine changes associated with hippocampal long-term synaptic plasticity. Nature. 1999;399:66–70. doi: 10.1038/19978. [DOI] [PubMed] [Google Scholar]
- Ernfors P, Bengzon J, Kokaia Z, Persson H, Lindvall O. Increased levels of messenger RNAs for neurotrophic factors in the brain during kindling epileptogenesis. Neuron. 1991;7:165–176. doi: 10.1016/0896-6273(91)90084-d. [DOI] [PubMed] [Google Scholar]
- Falkenberg T, Mohammed AK, Henriksson B, Persson H, Winblad B, Lindefors N. Increased expression of brain-derived neurotrophic factor mRNA in rat hippocampus is associated with improved spatial memory and enriched environment. Neurosci Lett. 1992;138:153–156. doi: 10.1016/0304-3940(92)90494-r. [DOI] [PubMed] [Google Scholar]
- Fawcett JP, Aloyz R, McLean JH, Pareek S, Miller FD, McPherson PS, Murphy RA. Detection of brain-derived neurotrophic factor in a vesicular fraction of brain synaptosomes. J Biol Chem. 1997;272:8837–8840. doi: 10.1074/jbc.272.14.8837. [DOI] [PubMed] [Google Scholar]
- Figurov A, Pozzo-Miller LD, Olafsson P, Wang T, Lu B. Regulation of synaptic responses to high-frequency stimulation and LTP by neurotrophins in the hippocampus. Nature. 1996;381:706–709. doi: 10.1038/381706a0. [DOI] [PubMed] [Google Scholar]
- Finkbeiner S, Tavazoie SF, Maloratsky A, Jacobs KM, Harris KM, Greenberg ME. CREB: A major mediator of neuronal neurotrophin responses. Neuron. 1997;19:1031–1047. doi: 10.1016/s0896-6273(00)80395-5. [DOI] [PubMed] [Google Scholar]
- Fischer W, Strevaag A, Wiegand SJ, Lindsay RM, Björklund A. Reversal of spatial memory impairments in aged rats by nerve growth factor and neurotrophins 3 and 4/5 but not by brain-derived neurotrophic factor. Proc Natl Acad Sci. 1994;91:8607–8611. doi: 10.1073/pnas.91.18.8607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster M, Sherringhton CS. A text book of physiology. Part III. The central nervous system. Macmillan; London: 1897. [Google Scholar]
- French SJ, Humby T, Horner CH, Sofroniew MV, Rattray M. Hippocampal neurotrophin and trk receptor mRNA levels are altered by local administration of nicotine, carbachol and pilocarpine. Brain Res Mol Brain Res. 1999;67:124–136. doi: 10.1016/s0169-328x(99)00048-0. [DOI] [PubMed] [Google Scholar]
- Gallo G, Letourneau PC. Localized sources of neurotrophins initiate axon collateral sprouting. J Neurosci. 1998;18:5403–5414. doi: 10.1523/JNEUROSCI.18-14-05403.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingrich JA, Hen R. The broken mouse: The role of development, plasticity and environment in the interpretation of phenotypic changes in knockout mice. Curr Opin Neurobiol. 2000;10:146–152. doi: 10.1016/s0959-4388(99)00061-6. [DOI] [PubMed] [Google Scholar]
- Gooney M, Shaw K, Kelly A, O'Mara SM, Lynch MA. Long-term potentiation and spatial learning are associated with increased phosphorylation of TrkB and extracellular signal-regulated kinase (ERK) in the dentate gyrus: Evidence for a role for brain-derived neurotrophic factor. Behav Neurosci. 2002;116:455–463. doi: 10.1037//0735-7044.116.3.455. [DOI] [PubMed] [Google Scholar]
- Grant SG, O'Dell TJ, Karl KA, Stein PL, Soriano P, Kandel ER. Impaired long-term potentiation, spatial learning, and hippocampal development in fyn mutant mice. Science. 1992;258:1903–1910. doi: 10.1126/science.1361685. [DOI] [PubMed] [Google Scholar]
- Greenough WT, Volkmar FR. Pattern of dendritic branching in occipital cortex of rats reared in complex environments. Exp Neurol. 1973;40:491–504. doi: 10.1016/0014-4886(73)90090-3. [DOI] [PubMed] [Google Scholar]
- Guzowski JF, Lyford GL, Stevenson GD, Houston FP, McGaugh JL, Worley PF, Barnes CA. Inhibition of activity-dependent Arc protein expression in the rat hippocampus impairs the maintenance of long-term potentiation and the consolidation of long-term memory. J Neurosci. 2000;20:3993–4001. doi: 10.1523/JNEUROSCI.20-11-03993.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J, Thomas KL, Everitt BJ. Rapid and selective induction of BDNF expression in the hippocampus during contextual learning. Nat Neurosci. 2000;3:533–535. doi: 10.1038/75698. [DOI] [PubMed] [Google Scholar]
- Hansson AC, Cintra A, Belluardo N, Sommer W, Bhatnagar M, Bader M, Ganten D, Fuxe K. Gluco- and mineralocorticoid receptor-mediated regulation of neurotrophic factor gene expression in the dorsal hippocampus and the neocortex of the rat. Eur J Neurosci. 2000;12:2918–2934. doi: 10.1046/j.1460-9568.2000.00185.x. [DOI] [PubMed] [Google Scholar]
- Hartmann M, Heumann R, Lessman L. Synaptic secretion of BDNF after high-frequency stimulation of glutamatergic synapses. EMBO J. 2001;20:5887–5897. doi: 10.1093/emboj/20.21.5887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haubensak W, Narz F, Heumann R, Lessmann V. BDNF–GFP containing secretory granules are localized in the vicinity of synaptic junctions of cultured cortical neurons. J Cell Sci. 1998;111:1483–1493. doi: 10.1242/jcs.111.11.1483. [DOI] [PubMed] [Google Scholar]
- Hebb DO. The organization of behavior. Wiley; New York: 1949. [DOI] [PubMed] [Google Scholar]
- Horch HW, Kruttgen A, Portbury SD, Katz LC. Destabilization of cortical dendrites and spines by BDNF. Neuron. 1999;23:353–364. doi: 10.1016/s0896-6273(00)80785-0. [DOI] [PubMed] [Google Scholar]
- Horger BA, Iyasere CA, Berhow MT, Messer CJ, Nestler EJ, Taylor JR. Enhancement of locomotor activity and conditioned reward to cocaine by brain-derived neurotrophic factor. J Neurosci. 1999;19:4110–4122. doi: 10.1523/JNEUROSCI.19-10-04110.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Neurotrophins: Roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YY, Kandel ER, Varshavsky L, Brandon EP, Qi M, Idzerda RL, McKnight GS, Bourtchuladze R. A genetic test of the effects of mutations in PKA on mossy fiber LTP and its relation to spatial and contextual learning. Cell. 1995;83:1211–1222. doi: 10.1016/0092-8674(95)90146-9. [DOI] [PubMed] [Google Scholar]
- Ickes BR, Pham TM, Sanders LA, Albeck DS, Mohammed AH, Granholm AC. Long-term environmental enrichment leads to regional increases in neurotrophin levels in rat brain. Exp Neurol. 2000;164:45–52. doi: 10.1006/exnr.2000.7415. [DOI] [PubMed] [Google Scholar]
- Isackson PJ, Huntsman MM, Murray KD, Gall CM. BDNF mRNA expression is increased in adult rat forebrain after limbic seizures: Temporal patterns of induction distinct from NGF. Neuron. 1991;6:937–948. doi: 10.1016/0896-6273(91)90234-q. [DOI] [PubMed] [Google Scholar]
- Iwasaki Y, Gay B, Wada K, Koizumi S. Association of the Src family tyrosine kinase Fyn with TrkB. J Neurochem. 1998;71:106–111. doi: 10.1046/j.1471-4159.1998.71010106.x. [DOI] [PubMed] [Google Scholar]
- Izquierdo I, Barros DM, Izquierdo L, Mello e Souza T, Souza M, Medina JH. Mechanisms for memory types differ. Nature. 1998;393:635–636. doi: 10.1038/31371. [DOI] [PubMed] [Google Scholar]
- James W. Principles of psychology. Henry Holt; New York: 1890. [Google Scholar]
- Jarvis CR, Xiong ZG, Plant JR, Churchill D, Lu WY, MacVicar BA, MacDonald JF. Neurotrophin modulation of NMDA receptors in cultured murine and isolated rat neurons. J Neurophysiol. 1997;78:2363–2371. doi: 10.1152/jn.1997.78.5.2363. [DOI] [PubMed] [Google Scholar]
- Johnston AN, Clements MP, Rose SP. Role of brain-derived neurotrophic factor and presynaptic proteins in passive avoidance learning in day-old domestic chicks. Neuroscience. 1999;88:1033–1042. doi: 10.1016/s0306-4522(98)00362-5. [DOI] [PubMed] [Google Scholar]
- Kafitz KW, Rose CR, Thoenen H, Konnerth A. Neurotrophin-evoked rapid excitation through TrkB receptors. Nature. 1999;401:918–921. doi: 10.1038/44847. [DOI] [PubMed] [Google Scholar]
- Kang H, Welcher AA, Shelton D, Schuman EM. Neurotrophins and time: Different roles for TrkB signaling in hippocampal long-term potentiation. Neuron. 1997;19:653–664. doi: 10.1016/s0896-6273(00)80378-5. [DOI] [PubMed] [Google Scholar]
- Kaplan DR, Stephens RM. Neurotrophin signal transduction by the Trk receptor. J Neurobiol. 1994;25:1404–1417. doi: 10.1002/neu.480251108. [DOI] [PubMed] [Google Scholar]
- Kesslak JP, So V, Choi J, Cotman CW, Gomez-Pinilla F. Learning upregulates brain-derived neurotrophic factor messenger ribonucleic acid: A mechanism to facilitate encoding and circuit maintenance? Behav Neurosci. 1998;112:1012–1019. doi: 10.1037//0735-7044.112.4.1012. [DOI] [PubMed] [Google Scholar]
- Knipper M, da Penha Berzaghi M, Blochl A, Breer H, Thoenen H, Lindholm D. Positive feedback between acetylcholine and the neurotrophins nerve growth factor and brain-derived neurotrophic factor in the rat hippocampus. Eur J Neurosci. 1994;6:668–671. doi: 10.1111/j.1460-9568.1994.tb00312.x. [DOI] [PubMed] [Google Scholar]
- Kohara K, Kitamura A, Morishima M, Tsumoto T. Activity-dependent transfer of brain-derived neurotrophic factor to postsynaptic neurons. Science. 2001;291:2419–2423. doi: 10.1126/science.1057415. [DOI] [PubMed] [Google Scholar]
- Kojima M, Takei N, Numakawa T, Ishikawa Y, Suzuki S, Matsumoto T, Katoh-Semba R, Nawa H, Hatanaka H. Biological characterization and optical imaging of brain-derived neurotrophic factor-green fluorescent protein suggest an activity-dependent local release of brain-derived neurotrophic factor in neurites of cultured hippocampal neurons. J Neurosci Res. 2001;64:1–10. doi: 10.1002/jnr.1080. [DOI] [PubMed] [Google Scholar]
- Kokaia Z, Bengzon J, Metsis M, Kokaia M, Persson H, Lindvall O. Coexpression of neurotrophins and their receptors in neurons of the central nervous system. Proc Natl Acad Sci. 1993;90:6711–6715. doi: 10.1073/pnas.90.14.6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korte M, Carroll P, Wolf E, Brem G, Thoenen H, Bonhoeffer T. Hippocampal long-term potentiation is impaired in mice lacking brain-derived neurotrophic factor. Proc Natl Acad Sci. 1995;92:8856–8860. doi: 10.1073/pnas.92.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korte M, Griesbeck O, Gravel C, Carroll P, Staiger V, Thoenen H, Bonhoeffer T. Virus-mediated gene transfer into hippocampal CA1 region restores long-term potentiation in brain-derived neurotrophic factor mutant mice. Proc Natl Acad Sci. 1996;93:12547–12552. doi: 10.1073/pnas.93.22.12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korte M, Kang H, Bonhoeffer T, Schuman E. A role for BDNF in the late-phase of hippocampal long-term potentiation. Neuropharmacology. 1998;37:553–559. doi: 10.1016/s0028-3908(98)00035-5. [DOI] [PubMed] [Google Scholar]
- Kovalchuk Y, Hanse E, Kafitz KW, Konnerth A. Postsynaptic induction of BDNF-mediated long-term potentiation. Science. 2002;295:1729–1734. doi: 10.1126/science.1067766. [DOI] [PubMed] [Google Scholar]
- Lapchak PA, Araujo DM, Hefti F. Cholinergic regulation of hippocampal brain-derived neurotrophic factor mRNA expression: Evidence from lesion and chronic cholinergic drug treatment studies. Neuroscience. 1993;52:575–585. doi: 10.1016/0306-4522(93)90407-7. [DOI] [PubMed] [Google Scholar]
- Lesser SS, Sherwood NT, Lo DC. Neurotrophins differentially regulate voltage-gated ion channels. Mol Cell Neurosci. 1997;10:173–183. doi: 10.1006/mcne.1997.0656. [DOI] [PubMed] [Google Scholar]
- Levine ES, Dreyfus CF, Black IB, Plummer MR. Brain-derived neurotrophic factor rapidly enhances synaptic transmission in hippocampal neurons via postsynaptic tyrosine kinase receptors. Proc Natl Acad Sci. 1995;92:8074–8077. doi: 10.1073/pnas.92.17.8074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine ES, Crozier RA, Black IB, Plummer MR. Brain-derived neurotrophic factor modulates hippocampal synaptic transmission by increasing N-methyl-D-aspartic acid receptor activity. Proc Natl Acad Sci. 1998;95:10235–10239. doi: 10.1073/pnas.95.17.10235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SY, Wu K, Levine ES, Mount HT, Suen PC, Black IB. BDNF acutely increases tyrosine phosphorylation of the NMDA receptor subunit 2B in cortical and hippocampal postsynaptic densities. Brain Res Mol Brain Res. 1998;55:20–27. doi: 10.1016/s0169-328x(97)00349-5. [DOI] [PubMed] [Google Scholar]
- Lindholm D, da Penha Berzaghi M, Cooper J, Thoenen H, Castren E. Brain-derived neurotrophic factor and neurotrophin-4 increase neurotrophin-3 expression in the rat hippocampus. Intl J Dev Neurosci. 1994;12:745–751. doi: 10.1016/0736-5748(94)90054-x. [DOI] [PubMed] [Google Scholar]
- Linnarsson S, Bjorklund A, Ernfors P. Learning deficit in BDNF mutant mice. Eur J Neurosci. 1997;9:2581–2587. doi: 10.1111/j.1460-9568.1997.tb01687.x. [DOI] [PubMed] [Google Scholar]
- Ma YL, Wang HL, Wu HC, Wei CL, Lee EH. Brain-derived neurotrophic factor antisense oligonucleotide impairs memory retention and inhibits long-term potentiation in rats. Neuroscience. 1998;82:957–967. doi: 10.1016/s0306-4522(97)00325-4. [DOI] [PubMed] [Google Scholar]
- Maletic-Savatic M, Malinow R, Svoboda K. Rapid dendritic morphogenesis in CA1 hippocampal dendrites induced by synaptic activity. Science. 1999;283:1923–1927. doi: 10.1126/science.283.5409.1923. [DOI] [PubMed] [Google Scholar]
- Martin SJ, Grimwood PD, Morris RG. Synaptic plasticity and memory: An evaluation of the hypothesis. Annu Rev Neurosci. 2000;23:649–711. doi: 10.1146/annurev.neuro.23.1.649. [DOI] [PubMed] [Google Scholar]
- Martinez A, Alcantara S, Borrell V, Del Rio JA, Blasi J, Otal R, Campos N, Boronat A, Barbacid M, Silos-Santiago I, et al. TrkB and TrkC signaling are required for maturation and synaptogenesis of hippocampal connections. J Neurosci. 1998;18:7336–7350. doi: 10.1523/JNEUROSCI.18-18-07336.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister AK, Lo DC, Katz LC. Neurotrophins regulate dendritic growth in developing visual cortex. Neuron. 1995;15:791–803. doi: 10.1016/0896-6273(95)90171-x. [DOI] [PubMed] [Google Scholar]
- McAllister AK, Katz LC, Lo DC. Neurotrophins and synaptic plasticity. Annu Rev Neurosci. 1999;22:295–318. doi: 10.1146/annurev.neuro.22.1.295. [DOI] [PubMed] [Google Scholar]
- Messaoudi E, Bardsen K, Srebro B, Bramham CR. Acute intrahippocampal infusion of BDNF induces lasting potentiation of synaptic transmission in the rat dentate gyrus. J Neurophysiol. 1998;79:496–499. doi: 10.1152/jn.1998.79.1.496. [DOI] [PubMed] [Google Scholar]
- Messaoudi E, Ying SW, Kanhema T, Croll SD, Bramham CR. BDNF triggers transcription-dependent, late phase LTP in vivo. J Neurosci. 2002;22:7453–7461. doi: 10.1523/JNEUROSCI.22-17-07453.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metsis M, Timmusk T, Arenas E, Persson H. Differential usage of multiple brain-derived neurotrophic factor promoters in the rat brain following neuronal activation. Proc Natl Acad Sci. 1993;90:8802–8806. doi: 10.1073/pnas.90.19.8802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minichiello L, Korte M, Wolfer D, Kuhn R, Unsicker K, Cestari V, Rossi-Arnaud C, Lipp HP, Bonhoeffer T, Klein R. Essential role for TrkB receptors in hippocampus-mediated learning. Neuron. 1999;24:401–414. doi: 10.1016/s0896-6273(00)80853-3. [DOI] [PubMed] [Google Scholar]
- Miranda RC, Sohrabji F, Toran-Allerand CD. Neuronal colocalization of mRNAs for neurotrophins and their receptors in the developing central nervous system suggests a potential for autocrine interactions. Proc Natl Acad Sci. 1993;90:6439–6443. doi: 10.1073/pnas.90.14.6439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno M, Yamada K, Olariu A, Nawa H, Nabeshima T. Involvement of brain-derived neurotrophic factor in spatial memory formation and maintenance in a radial arm maze test in rats. J Neurosci. 2000;20:7116–7121. doi: 10.1523/JNEUROSCI.20-18-07116.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller JC, Kruttgen A, Heymach JV, Jr, Ghori N, Shooter EM. Subcellular localization of epitope-tagged neurotrophins in neuroendocrine cells. J Neurosci Res. 1998;51:463–472. doi: 10.1002/(SICI)1097-4547(19980215)51:4<463::AID-JNR6>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Moser MB, Trommald M, Andersen P. An increase in dendritic spine density on hippocampal CA1 pyramidal cells following spatial learning in adult rats suggests the formation of new synapses. Proc Natl Acad Sci. 1994;91:12673–12675. doi: 10.1073/pnas.91.26.12673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser MB, Trommald M, Egeland T, Andersen P. Spatial training in a complex environment and isolation alter the spine distribution differently in rat CA1 pyramidal cells. J Comp Neurol. 1997;380:373–381. doi: 10.1002/(sici)1096-9861(19970414)380:3<373::aid-cne6>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Mu JS, Li WP, Yao ZB, Zhou XF. Deprivation of endogenous brain-derived neurotrophic factor results in impairment of spatial learning and memory in adult rats. Brain Res. 1999;835:259–265. doi: 10.1016/s0006-8993(99)01592-9. [DOI] [PubMed] [Google Scholar]
- Murer MG, Yan Q, Raisman-Vozari R. Brain-derived neurotrophic factor in the control human brain, and in Alzheimer's disease and Parkinson's disease. Prog Neurobiol. 2001;63:71–124. doi: 10.1016/s0301-0082(00)00014-9. [DOI] [PubMed] [Google Scholar]
- Nanda SA, Mack KJ. Seizure and sensory stimulation result in different patterns of brain derived neurotrophic factor protein expression in the barrel cortex and hippocampus. Mol Brain Res. 2000;78:1–14. doi: 10.1016/s0169-328x(00)00054-1. [DOI] [PubMed] [Google Scholar]
- Nawa H, Carnahan J, Gall C. BDNF protein measured by a novel enzyme immunoassay in normal brain and after seizure: Partial disagreement with mRNA levels. Eur J Neurosci. 1995;7:1527–1535. doi: 10.1111/j.1460-9568.1995.tb01148.x. [DOI] [PubMed] [Google Scholar]
- Neeper SA, Gomez-Pinilla F, Choi J, Cotman CW. Exercise and brain neurotrophins. Nature. 1995;373:109. doi: 10.1038/373109a0. [DOI] [PubMed] [Google Scholar]
- Neeper SA, Gomez-Pinilla F, Choi J, Cotman CW. Physical activity increases mRNA for brain-derived neurotrophic factor and nerve growth factor in rat brain. Brain Res. 1996;726:49–56. [PubMed] [Google Scholar]
- Nguyen PV, Abel T, Kandel ER. Requirement of a critical period of transcription for induction of a late phase of LTP. Science. 1994;265:1104–1107. doi: 10.1126/science.8066450. [DOI] [PubMed] [Google Scholar]
- Nibuya M, Morinobu S, Duman RS. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J Neurosci. 1995;15:7539–7547. doi: 10.1523/JNEUROSCI.15-11-07539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosten-Bertrand M, Errington ML, Murphy KP, Tokugawa Y, Barboni E, Kozlova E, Michalovich D, Morris RG, Silver J, Stewart CL, et al. Normal spatial learning despite regional inhibition of LTP in mice lacking Thy-1. Nature. 1996;379:826–829. doi: 10.1038/379826a0. [DOI] [PubMed] [Google Scholar]
- Obermeier A, Bradshaw RA, Seedorf K, Choidas A, Schlessinger J, Ullrich A. Neuronal differentiation signals are controlled by nerve growth factor receptor/Trk binding sites for SHC and PLC γ. EMBOJ. 1994;13:1585–1590. doi: 10.1002/j.1460-2075.1994.tb06421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabe S, Collin C, Auerbach J, Meiri N, Bergzon J, Kennedy M, Segal M, McKay R. Hippocampal synaptic plasticity in mice overexpressing an embryonic subunit of the NMDA receptor. J Neurosci. 1998;18:4177–4188. doi: 10.1523/JNEUROSCI.18-11-04177.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliff HS, Berchtold NC, Isackson P, Cotman CW. Exercise-induced regulation of brain-derived neurotrophic factor (BDNF) transcripts in the rat hippocampus. Brain Res Mol Brain Res. 1998;61:147–153. doi: 10.1016/s0169-328x(98)00222-8. [DOI] [PubMed] [Google Scholar]
- Patterson SL, Grover LM, Schwartzkroin PA, Bothwell M. Neurotrophin expression in rat hippocampal slices: A stimulus paradigm inducing LTP in CA1 evokes increases in BDNF and NT-3 mRNAs. Neuron. 1992;9:1081–1088. doi: 10.1016/0896-6273(92)90067-n. [DOI] [PubMed] [Google Scholar]
- Patterson SL, Abel T, Deuel TA, Martin KC, Rose JC, Kandel ER. Recombinant BDNF rescues deficits in basal synaptic transmission and hippocampal LTP in BDNF knockout mice. Neuron. 1996;16:1137–1145. doi: 10.1016/s0896-6273(00)80140-3. [DOI] [PubMed] [Google Scholar]
- Pelleymounter MA, Cullen MJ, Baker MB, Gollub M, Wellman C. The effects of intrahippocampal BDNF and NGF on spatial learning in aged Long Evans rats. Mol Chem Neuropathol. 1996;29:211–226. doi: 10.1007/BF02815003. [DOI] [PubMed] [Google Scholar]
- Pollock GS, Vernon E, Forbes ME, Yan Q, Ma YT, Hsieh T, Robichon R, Frost DO, Johnson JE. Effects of early visual experience and diurnal rhythms on BDNF mRNA and protein levels in the visual system, hippocampus, and cerebellum. J Neurosci. 2001;21:3923–3931. doi: 10.1523/JNEUROSCI.21-11-03923.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poo MM. Neurotrophins as synaptic modulators. Nat Rev Neurosci. 2001;2:24–32. doi: 10.1038/35049004. [DOI] [PubMed] [Google Scholar]
- Pozzo-Miller LD, Gottschalk W, Zhang L, McDermott K, Du J, Gopalakrishnan R, Oho C, Sheng ZH, Lu B. Impairments in high-frequency transmission, synaptic vesicle docking, and synaptic protein distribution in the hippocampus of BDNF knockout mice. J Neurosci. 1999;19:4972–4983. doi: 10.1523/JNEUROSCI.19-12-04972.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian X, Riccio A, Zhang Y, Ginty DD. Identification and characterization of novel substrates of Trk receptors in developing neurons. Neuron. 1998;21:1017–1029. doi: 10.1016/s0896-6273(00)80620-0. [DOI] [PubMed] [Google Scholar]
- Qiao X, Chen L, Gao H, Bao S, Hefti F, Thompson RF, Knusel B. Cerebellar brain-derived neurotrophic factor-TrkB defect associated with impairment of eyeblink conditioning in Stargazer mutant mice. J Neurosci. 1998;18:6990–6999. doi: 10.1523/JNEUROSCI.18-17-06990.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo-Neustadt AA, Beard RC, Huang YM, Cotman CW. Physical activity and antidepressant treatment potentiate the expression of specific brain-derived neurotrophic factor transcripts in the rat hippocampus. Neuroscience. 2000;101:305–312. doi: 10.1016/s0306-4522(00)00349-3. [DOI] [PubMed] [Google Scholar]
- Saarelainen T, Pussinen R, Koponen E, Alhonen L, Wong G, Sirvio J, Castren E. Transgenic mice overexpressing truncated trkB neurotrophin receptors in neurons have impaired long-term spatial memory but normal hippocampal LTP. Synapse. 2000;38:102–104. doi: 10.1002/1098-2396(200010)38:1<102::AID-SYN11>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Sakai N, Yamada M, Numakawa T, Ogura A, Hatanaka H. BDNF potentiates spontaneous Ca2+ oscillations in cultured hippocampal neurons. Brain Res. 1997;778:318–328. doi: 10.1016/s0006-8993(97)01052-4. [DOI] [PubMed] [Google Scholar]
- Schaaf MJ, de Jong J, de Kloet ER, Vreugdenhil E. Downregulation of BDNF mRNA and protein in the rat hippocampus by corticosterone. Brain Res. 1998;813:112–120. doi: 10.1016/s0006-8993(98)01010-5. [DOI] [PubMed] [Google Scholar]
- Schinder AF, Poo M. The neurotrophin hypothesis for synaptic plasticity. Trends Neurosci. 2000;23:639–645. doi: 10.1016/s0166-2236(00)01672-6. [DOI] [PubMed] [Google Scholar]
- Segal RA, Greenberg ME. Intracellular signaling pathways activated by neurotrophic factors. Annu Rev Neurosci. 1996;19:463–489. doi: 10.1146/annurev.ne.19.030196.002335. [DOI] [PubMed] [Google Scholar]
- Shimada A, Mason CA, Morrison ME. TrkB signaling modulates spine density and morphology independent of dendrite structure in cultured neonatal Purkinje cells. J Neurosci. 1998;18:8559–8570. doi: 10.1523/JNEUROSCI.18-21-08559.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva AJ, Paylor R, Wehner JM, Tonegawa S. Impaired spatial learning in α-calcium-calmodulin kinase II mutant mice. Science. 1992;257:206–211. doi: 10.1126/science.1321493. [DOI] [PubMed] [Google Scholar]
- Smith MA, Zhang LX, Lyons WE, Mamounas LA. Anterograde transport of endogenous brain-derived neurotrophic factor in hippocampal mossy fibers. Neuroreport. 1997;8:1829–1834. doi: 10.1097/00001756-199705260-00008. [DOI] [PubMed] [Google Scholar]
- Song DK, Choe B, Bae JH, Park WK, Han IS, Ho WK, Earm YE. Brain-derived neurotrophic factor rapidly potentiates synaptic transmission through NMDA, but suppresses it through non-NMDA receptors in rat hippocampal neuron. Brain Res. 1998;799:176–179. doi: 10.1016/s0006-8993(98)00474-0. [DOI] [PubMed] [Google Scholar]
- Stephens RM, Loeb DM, Copeland TD, Pawson T, Greene LA, Kaplan DR. Trk receptors use redundant signal transduction pathways involving SHC and PLC-γ 1 to mediate NGF responses. Neuron. 1994;12:691–705. doi: 10.1016/0896-6273(94)90223-2. [DOI] [PubMed] [Google Scholar]
- Suen PC, Wu K, Levine ES, Mount HT, Xu JL, Lin SY, Black IB. Brain-derived neurotrophic factor rapidly enhances phosphorylation of the postsynaptic N-methyl-D-aspartate receptor subunit 1. Proc Natl Acad Sci. 1997;94:8191–8195. doi: 10.1073/pnas.94.15.8191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweatt JD. The neuronal MAP kinase cascade: A biochemical signal integration system subserving synaptic plasticity and memory. J Neurochem. 2001;76:1–10. doi: 10.1046/j.1471-4159.2001.00054.x. [DOI] [PubMed] [Google Scholar]
- Tanzi E. I fatti e le induzioni nell'odierna isologia del sistema nervosa. Revista Sperimentale di Freniatria e di Medicina Legale. 1893;19:419–472. [Google Scholar]
- Tao X, West AE, Chen WG, Corfas G, Greenberg ME. A calcium-responsive transcription factor, CaRF, that regulates neuronal activity-dependent expression of BDNF. Neuron. 2002;33:383–395. doi: 10.1016/s0896-6273(01)00561-x. [DOI] [PubMed] [Google Scholar]
- Thoenen H. Neurotrophins and activity-dependent plasticity. Prog Brain Res. 2000;128:183–191. doi: 10.1016/S0079-6123(00)28016-3. [DOI] [PubMed] [Google Scholar]
- Timmusk T, Palm K, Metsis M, Reintam T, Paalme V, Saarma M, Persson H. Multiple promoters direct tissue-specific expression of the rat BDNF gene. Neuron. 1993;10:475–489. doi: 10.1016/0896-6273(93)90335-o. [DOI] [PubMed] [Google Scholar]
- Tokuyama W, Okuno H, Hashimoto T, Xin Li Y, Miyashita Y. BDNF upregulation during declarative memory formation in monkey inferior temporal cortex. Nat Neurosci. 2000;3:1134–1142. doi: 10.1038/80655. [DOI] [PubMed] [Google Scholar]
- Toni N, Buchs PA, Nikonenko I, Bron CR, Muller D. LTP promotes formation of multiple spine synapses between a single axon terminal and a dendrite. Nature. 1999;402:421–425. doi: 10.1038/46574. [DOI] [PubMed] [Google Scholar]
- Trommald M, Hulleberg G, Andersen P. Long-term potentiation is associated with new excitatory spine synapses on rat dentate granule cells. Learn Mem. 1996;3:218–228. doi: 10.1101/lm.3.2-3.218. [DOI] [PubMed] [Google Scholar]
- Tsien JZ, Chen DF, Gerber D, Tom C, Mercer EH, Anderson DJ, Mayford M, Kandel ER, Tonegawa S. Subregion- and cell type-restricted gene knockout in mouse brain. Cell. 1996a;87:1317–1326. doi: 10.1016/s0092-8674(00)81826-7. [DOI] [PubMed] [Google Scholar]
- Tsien JZ, Huerta PT, Tonegawa S. The essential role of hippocampal CA1 NMDA receptor-dependent synaptic plasticity in spatial memory. Cell. 1996b;87:1327–1338. doi: 10.1016/s0092-8674(00)81827-9. [DOI] [PubMed] [Google Scholar]
- Tyler WJ, Pozzo-Miller LD. BDNF enhances quantal neurotransmitter release and increases the number of docked vesicles at the active zones of hippocampal excitatory synapses. J Neurosci. 2001;21:4249–4258. doi: 10.1523/JNEUROSCI.21-12-04249.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler WJ, Perrett S, Pozzo-Miller LD. The role of neurotrophins in neurotransmitter release. Neuroscientist. 2002 doi: 10.1177/1073858402238511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya VA, Marek GJ, Aghajanian GK, Duman RS. 5-HT2A receptor-mediated regulation of brain-derived neurotrophic factor mRNA in the hippocampus and the neocortex. J Neurosci. 1997;17:2785–2795. doi: 10.1523/JNEUROSCI.17-08-02785.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya VA, Terwilliger RM, Duman RS. Role of 5-HT2A receptors in the stress-induced down-regulation of brain-derived neurotrophic factor expression in rat hippocampus. Neurosci Lett. 1999;262:1–4. doi: 10.1016/s0304-3940(99)00006-3. [DOI] [PubMed] [Google Scholar]
- Wade J. TrkB-like immunoreactivity in the song system of developing zebra finches. J Chem Neuroanat. 2000;19:33–39. doi: 10.1016/s0891-0618(00)00051-x. [DOI] [PubMed] [Google Scholar]
- Walton M, Henderson C, Mason-Parker S, Lawlor P, Abraham WC, Bilkey D, Dragunow M. Immediate early gene transcription and synaptic modulation. J Neurosci Res. 1999;58:96–106. [PubMed] [Google Scholar]
- Wang YT, Salter MW. Regulation of NMDA receptors by tyrosine kinases and phosphatases. Nature. 1994;369:233–235. doi: 10.1038/369233a0. [DOI] [PubMed] [Google Scholar]
- Widenfalk J, Olson L, Thoren P. Deprived of habitual running, rats downregulate BDNF and TrkB messages in the brain. Neurosci Res. 1999;34:125–132. doi: 10.1016/s0168-0102(99)00051-6. [DOI] [PubMed] [Google Scholar]
- Wolfer DP, Lipp HP. Dissecting the behaviour of transgenic mice: Is it the mutation, the genetic background, or the environment? Exp Physiol. 2000;85:627–634. [PubMed] [Google Scholar]
- Xiong H, Yamada K, Han D, Nabeshima T, Enikolopov G, Carnahan J, Nawa H. Mutual regulation between the intercellular messengers nitric oxide and brain-derived neurotrophic factor in rodent neocortical neurons. Eur J Neurosci. 1999;11:1567–1576. doi: 10.1046/j.1460-9568.1999.00567.x. [DOI] [PubMed] [Google Scholar]
- Yan Q, Rosenfeld RD, Matheson CR, Hawkins N, Lopez OT, Bennett L, Welcher AA. Expression of brain-derived neurotrophic factor protein in the adult rat central nervous system. Neuroscience. 1997;78:431–448. doi: 10.1016/s0306-4522(96)00613-6. [DOI] [PubMed] [Google Scholar]
- Ying SW, Futter M, Rosenblum K, Webber MJ, Hunt SP, Bliss TV, Bramham CR. Brain-derived neurotrophic factor induces long-term potentiation in intact adult hippocampus: Requirement for ERK activation coupled to CREB and upregulation of Arc synthesis. J Neurosci. 2002;22:1532–1540. doi: 10.1523/JNEUROSCI.22-05-01532.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuste R, Bonhoeffer T. Morphological changes in dendritic spines associated with long-term synaptic plasticity. Annu Rev Neurosci. 2001;24:1071–1089. doi: 10.1146/annurev.neuro.24.1.1071. [DOI] [PubMed] [Google Scholar]
- Zafra F, Hengerer B, Leibrock J, Thoenen H, Lindholm D. Activity dependent regulation of BDNF and NGF mRNAs in the rat hippocampus is mediated by non-NMDA glutamate receptors. EMBO J. 1990;9:3545–3550. doi: 10.1002/j.1460-2075.1990.tb07564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zafra F, Castren E, Thoenen H, Lindholm D. Interplay between glutamate and γ-aminobutyric acid transmitter systems in the physiological regulation of brain-derived neurotrophic factor and nerve growth factor synthesis in hippocampal neurons. Proc Natl Acad Sci. 1991;88:10037–10041. doi: 10.1073/pnas.88.22.10037. [DOI] [PMC free article] [PubMed] [Google Scholar]


