Abstract
The components of the Escherichia coli flagella apparatus are synthesized in a three-level transcriptional cascade activated by the master regulator FlhDC. The cascade coordinates the synthesis rates of a large number of gene products with each other and with nutritional conditions. Recent genome-wide studies have reported that flagellar transcription is altered in cells lacking the transcription regulators DksA or ppGpp, but some or all reported effects could be indirect, and some are contradictory. We report here that the activities of promoters at all three levels of the cascade are much higher in strains lacking dksA, resulting in overproduction of flagellin and hyperflagellated cells. In vitro, DksA/ppGpp inhibits the flhDC promoter and the σ70-dependent fliA promoter transcribing the gene for σ28. However, DksA and ppGpp do not affect the σ28-dependent fliA promoter or the σ28-dependent fliC promoter in vitro, suggesting that the dramatic effects on expression of those genes in vivo are mediated indirectly through direct effects of DksA/ppGpp on FlhDC and σ28 expression. We conclude that DksA/ppGpp inhibits expression of the flagellar cascade during stationary phase and following starvation, thereby coordinating flagella and ribosome assembly and preventing expenditure of scarce energy resources on synthesis of two of the cell’s largest macromolecular complexes.
Introduction
Bacterial flagella are synthesized by systems that are controlled tightly by nutritional and environmental conditions. In Escherichia coli, at least 50 genes, organized in at least 14 operons, contribute to the synthesis and operation of flagella (Macnab, 1992). Synthesis and assembly of flagella are regulated coordinately by a transcriptional cascade composed of 3 levels of gene products (“classes”; for reviews see Chilcott and Hughes, 2000; Chevance and Hughes, 2008).
Class I genes consist of a single operon encoding the proteins FlhD and FlhC that form a multimeric transcriptional activation complex. This “master regulator” stimulates transcription by binding upstream of Class II promoters (Liu and Matsumura, 1994; Wang et al., 2006). Class II genes encode proteins that assemble to form the basal body and hook of the flagellum, as well as the fliA gene which encodes the alternative σ factor σ28, also called σF. σ28 binds to RNA polymerase (RNAP) core enzyme and directs it to Class III promoters (Liu and Matsumura, 1995). Many Class II genes are preceded by a σ28-dependent promoter that likely contributes to expression once σ28 has been synthesized (Liu and Matsumura, 1996; reviewed in Chilcott and Hughes, 2000; Chevance and Hughes, 2008). Class III genes encode the rest of the structural genes of the flagellum, including fliC encoding flagellin, as well as the chemotaxis apparatus.
One of the Class III genes, flgM, encodes an anti-sigma factor that sequesters cellular σ28 until FlgM is exported by the partially-completed flagellum, allowing σ28 to bind to RNAP (reviewed in Chilcott and Hughes, 2000; Chevance and Hughes, 2008). Feedback control by FlgM coordinates expression of flagellar genes with assembly of a functional flagellum, but other mechanisms are needed to control flagellar expression with nutritional and environmental conditions. Many of these act at the level of flhDC transcription. Transcription factors implicated in regulation of the flhDC promoter include CRP (Silverman and Simon, 1974; Soutourina et al., 1999), H-NS (Soutourina et al., 1999), LrhA (Lehnen et al., 2002), OmpR (Shin and Park, 1995), Fur (Stojiljovich et al., 1994), RcsAB (Francez-Charlot et al., 2003), HdfR (Ko and Park, 2000), QseBC (Sperandio et al., 2002), and IHF (Shin and Park, 1995).
Two separate expression microarray studies (Durfee et al, 2008; Traxler et al., 2008) suggested that transcription of flagellar genes also might be inhibited by ppGpp and pppGpp, small molecules initially identified as regulators of ribosome synthesis (for reviews see Paul et al., 2004b; Potrykus and Cashel, 2008; Srivatsan and Wang, 2008). ppGpp and pppGpp (hereafter referred to as ppGpp) are synthesized following nutrient starvation (e.g. amino acid deprivation), redirectling the cell’s resources away from synthesis of the translational machinery and toward other biosynthetic needs as part of a wide-ranging reorganization of the cell’s regulatory networks often referred to as the “stringent response”. ppGpp binds directly to RNAP (Barker et al. 2001; Paul et al., 2005) and controls the expression of a large number of gene products, some of which themselves are transcriptional regulators (Mallik et al., 2006; Sharma and Payne, 2006; Durfee et al., 2008; Traxler et al., 2008). After amino acid starvation, most of the transcripts needed for synthesis and operation of the flagellum decreased temporally in the same order as their appearance in the flagellar cascade (Durfee et al, 2008).
In most cases, regulation of transcription by ppGpp depends on DksA, a small cytoplasmic protein that modifies RNAP and is present at high levels under all conditions that have been tested to date (Paul et al., 2004a; Rutherford et al., 2007). DksA and ppGpp together activate or inhibit transcription depending on the intrinsic kinetic characteristics of the promoter complex (Paul et al., 2004a; Paul et al., 2005; Rutherford et al., 2009; reviewed in Haugen et al., 2008). One study reported that cells lacking dksA displayed increased transcription of flagellar operons, were hyperflagellated, and had increased motility compared to wild-type cells (Aberg et al., 2009). However, another study reported that strains devoid of ppGpp (ΔrelAΔspoT) were nearly amotile and lacked flagella, and that ΔdksA mutants were less motile than wild-type cells, suggesting that DksA/ppGpp positively regulated flagellar expression (Magnusson et al., 2007).
Our long-term interest in the roles of DksA and ppGpp in gene regulation, as well as the apparent contradictions in the literature, encouraged us to investigate the effects of DksA/ppGpp on specific candidate promoters in each of the three flagellar gene classes. We determined whether regulation was direct or indirect and negative or positive. We demonstrate that DksA and ppGpp act directly and negatively on representative Class I and II flagellar promoters and indirectly on Class III promoters, inhibiting flagella production. As a result, the synthesis of ribosomes and flagella, two pathways that consume large amounts of the bacterial cell’s biosynthetic energy, are coordinated by the DksA/ppGpp system, preventing overinvestment of the cell’s resources in these large macromolecular complexes under nutritionally unfavorable conditions.
Results
Transcription of the flhDC operon is inhibited directly by DksA and ppGpp
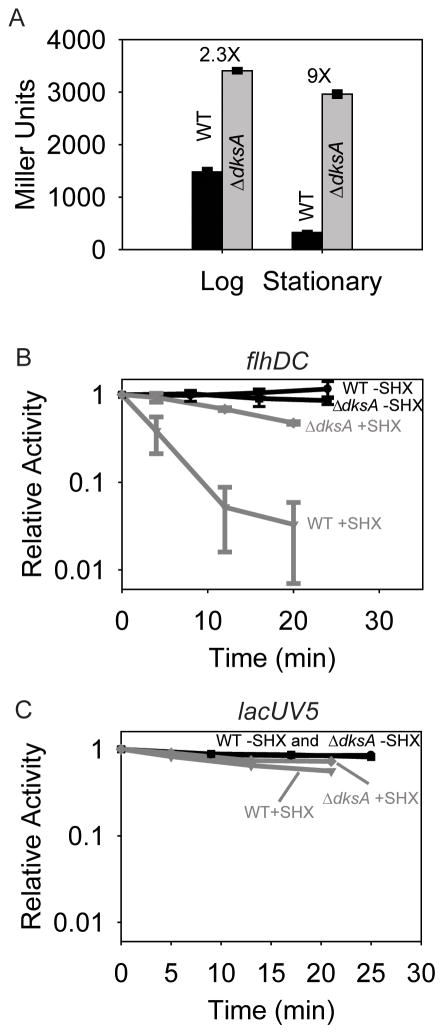
Consistent with some genome-wide expression studies cited above, our own expression microarray studies in mid-log phase in MOPS medium supplemented with glycerol and all 20 amino acids indicated that transcripts from multiple flagellar operons were overexpressed in a ΔdksA strain (data not shown). To examine transcription of several flagellar operons individually and directly, we first examined transcription from flhDC, using an flhDC promoter-lacZ fusion. In defined medium supplemented with glycerol and amino acids, flhDC promoter activity decreased in stationary phase (22–24 hours after inoculation of the culture) relative to log phase (Fig. 1A, compare black bars). However, flhDC promoter activity in the ΔdksA mutant relative to the wild-type strain increased ~2-fold in log phase and ~9-fold in stationary phase. As a consequence, flhDC promoter activity in the ΔdksA mutant decreased little if at all in stationary phase (Fig. 1A, compare grey bars). The regulation of the flhDC promoter with growth phase and the absence of this regulation in the ΔdksA strain are reminiscent of the behavior of rRNA promoters (Paul et al., 2004a).
Figure 1.
The flhDC promoter is inhibited by DksA/ppGpp in vivo.
A. β-galactosidase activities from flhDC promoter-lacZ fusions in log and stationary phases in strains containing (WT; RLG8992) or lacking DksA (ΔdksA; RLG8994). Error bars represent standard deviations (from 6 independent cultures). The numbers above the bars represent the ratio of β-galactosidase activities ΔdksA: WT.
B. Transcription from the same flhDC-lacZ fusions as in Fig. 1A, but measured by primer extension from RNA isolated at varying times after addition of 1 μg/ml (final concentration) serine hydroxamate (SHX) to induce ppGpp synthesis. Error bars represent standard deviations (3 independent experiments).
C. As in panel B, except transcription was measured from a lacUV5-lacZ fusion (in RLG4998 and RLG 8950). A representative experiment is shown.
We next addressed whether DksA/ppGpp affected the activity of the flhDC promoter following amino acid starvation, a condition in which rRNA promoters are inhibited at least 20-fold (Paul et al., 2004a). Promoter activity was measured at different times following addition of serine hydroxamate (SHX), an amino acid analog that prevents charging of seryl-tRNAs and thus induces synthesis of ppGpp. Whereas the amount of transcript made from the flhDC promoter (as measured by primer extension from the flhDC-lacZ fusion; see Materials and Methods) decreased precipitously (~30-fold) in the wild-type strain, it remained relatively constant in the ΔdksA mutant strain, almost as high as in untreated controls (Fig. 1B). In contrast, expression from a control promoter, lacUV5, fused to the same DNA sequences as in the flhDC-lacZ fusion, was affected little or not at all by SHX addition in either the wild-type or ΔdksA strains (Fig. 1C), confirming that effects on flhDC were promoter-specific.
To address whether the effects of DksA/ppGpp on the flhDC promoter were direct, promoter activity was measured in vitro in the presence and absence of DksA and ppGpp. CRP-cAMP activates transcription from this promoter by binding to a site centered at −71.5 relative to the transcription start site (Soutourina et al., 1999). CRP and cAMP were therefore included in the reaction to increase the basal level of transcription and improve detection. Together, DksA and ppGpp inhibited transcription ~7-fold (Fig. 2A), whereas no effect of ppGpp was observed on cAMP-CRP-dependent transcription from the lac promoter (Fig. 2B). The latter result ruled out the possibility that the observed inhibition of transcription from the flhDC promoter by ppGpp/DksA resulted from competition by ppGpp with cAMP binding to CRP.
Figure 2.
The flhDC promoter is inhibited by DksA/ppGpp in vitro. Promoter activity was measured by in vitro transcription in the presence/absence of DksA, ppGpp, or both, from plasmids containing different promoters (as well as the plasmid-derived RNA I promoter). Representative gel images are shown, but the quantitation reflects the average of 3 or more independent experiments (6 or more identical transcription reactions).
A. Single round in vitro transcription from the flhDC promoter (on pRLG8413) in the presence of 50 nM CRP and 200 μM cAMP. Percent activity is relative to the same reaction in the absence of ppGpp and DksA. There is a faint band derived from the vector just above the position of the test band that is activated by DksA/ppGpp (see also Fig. 2C–2D and Fig. 4A–2B).
B. Single round in vitro transcription from the CRP-cAMP-dependent lac promoter (on pRLG3256) in the presence of increasing concentrations of ppGpp. Percent activity is relative to the reaction with CRP (50 nM) and cAMP (200 μM) and without ppGpp (lanes 3–4). RNAP concentration was 10 nM.
C. Single round in vitro transcription from the flhDC promoter (on pRLG8413) in the absence of CRP-cAMP. RNAP concentration was 60 nM.
D. Single round in vitro transcription from the rrnB P1 promoter (on pRLG6555). For direct comparison to the effects of DksA/ppGpp on the cAMP-CRP-activated FlhDC promoter, transcription was performed in the presence of CRP (50 nM) and cAMP (200 μM). CRP-cAMP did not affect rrnB P1 transcription in the presence or absence of DksA/ppGpp (data not shown).
E. Single round in vitro transcription from the lacUV5 promoter (on pRLG3422) in the absence of CRP-cAMP.
F. Effects of DksA/ppGpp on lifetimes of competitor-resistant complexes containing the flhDC promoter (on pRLG8413) or the fis promoter (on pRLG8110). DksA was at 0.25 μM and ppGpp was at 100 μM when indicated. The double-stranded DNA used as competitor (1 μM) contained the full-con promoter sequence (Gaal et al., 2001). A representative experiment is shown. The half-lives of the complexes formed were: fis, 82 minutes, fis+DksA/ppGpp, 22 minutes, flhDC, 48 minutes and flhDC+DksA/ppGpp, 14 minutes. Absolute half-lives varied slightly from experiment to experiment, but the ratio of the half-life without/with DksA/ppGpp was the same in different experiments.
Effects of DksA and ppGpp on the flhDC promoter were also tested in the absence of CRP-cAMP (Fig. 2C). Detection of transcripts under these conditions required increasing the RNAP concentration 6-fold, resulting in transcription from additional sites on the plasmid. The flhDC promoter was still inhibited by DksA/ppGpp in these reactions, indicating that the observed inhibition in Fig. 2A did not result from anti-activation of CRP by ppGpp.
Effects of DksA/ppGpp on the ribosomal RNA promoter rrnB P1 were approximately 2-fold stronger in vitro than on the CRP-activated flhDC promoter (Fig. 2D), but the effects on the flhDC promoter (Figs. 2A and 2C) were much greater than on lacUV5 (Fig. 2E), a promoter that does not require activation by cAMP-CRP, or on the RNA1 promoter (Fig. 2A–E). Taken together, our data demonstrate that DksA/ppGpp directly and specifically inhibits flhDC transcription in vitro.
There is a strict inverse correlation between the half-life of the complex formed between RNAP and rRNA promoters in vitro and inhibition of these promoters in vivo (Haugen et al., 2006). DksA/ppGpp further decreases the lifetimes of these promoters, suggesting that it inhibits transcription, at least in part, by further destabilizing their intrinsically unstable promoter complexes. In order to address the mechanism of DksA/ppGpp action on the flhDC promoter, we measured the lifetime of its competitor-resistant complex ± DksA/ppGpp. DksA/ppGpp reduced the half-life of the competitor-resistant complex formed between RNAP and the flhDC promoter DNA (Fig. 2F). Although (for ease of measurement) solution conditions were chosen that resulted in a relatively long complex half-life, under the same conditions flhDC promoter complexes were shorter-lived than fis promoter complexes (which DksA/ppGpp regulates in vivo; Mallik et al., 2006). Furthermore, DksA/ppGpp had about the same fold-effect on the lifetimes of the fis and flhDC promoters (~3–4 fold; Fig. 2F). These results are consistent with the model that DksA/ppGpp inhibits the flhDC and fis promoters because they have similar intrinsic kinetic properties. However, further studies will be needed to define the mechanism responsible for the effects fo DksA/ppGpp on the flhDC promoter.
Transcription of the fliA gene is inhibited directly by DksA and ppGpp
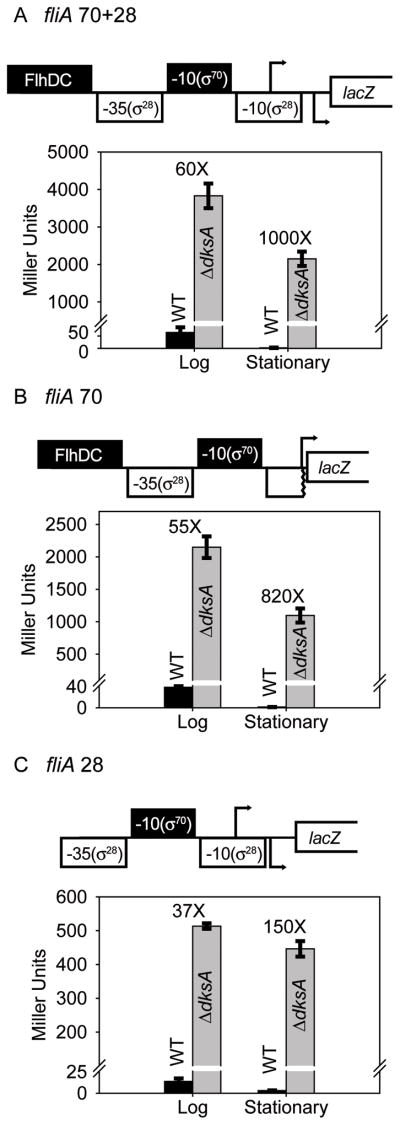
We next examined the effects of DksA/ppGpp on expression from a Class II flagellar gene, fliA. There are actually two fliA promoters, one of which, referred to here as fliA 70, is σ70 and FlhDC-dependent. The second fliA promoter, ~11–12 bp downstream of fliA 70 and referred to here as fliA 28, is σ28-dependent and not activated by FlhDC (Fig. 3A–C). We constructed promoter-lacZ fusions containing each of the promoters separately as well as the two together (see Table 1 for sequence endpoints and each panel in Fig. 3 for schematics). The activities of each of the promoter constructs decreased upon transition to stationary phase in the wild-type strain but not in the ΔdksA strain, in qualitative agreement with the results described above for the flhDC promoter. The absence of dksA resulted in 37- to 60-fold higher promoter activities than in the wild-type strain in log phase and as much as ~1000-fold higher promoter activities in stationary phase (Fig. 3A–C).
Figure 3.
Class II and III fliA promoters are inhibited by DksA/ppGpp in vivo. A–C. Schematic representation of fliA promoters fused to lacZ are presented above each graph. The -10 element of the fliA 70 promoter used for recognition by Eσ70 and a FlhDC binding site is filled in black. The -10 and -35 elements of the σ28-dependent promoter (fliA 28) are not shaded. Endpoints of the promoter fragments used for constructing the lacZ fusions are provided in Table 1. fliA 70+28 contains both promoters. fliA 70 is missing part of the fliA 28 -10 element and the fliA 28 transcription start site; fliA 28 lacks sequences upstream of -26 relative to the fliA 70 transcription start site.
A. fliA 70+28 promoter. β-galactosidase activities in log and stationary phases measured in wild-type and ΔdksA strains. Error bars represent measurements from 6 independent cultures. The numbers above the bars represent the ratio of β-galactosidase activities ΔdksA: WT.
B. fliA 70 promoter. See panel A.
C. fliA 28 promoter. See panel A.
Table 1.
Strains and plasmids used in this work.
| Strain | Relevant Genotype | Promoter-lacZ fusion | Promoter Endpoints | Source |
|---|---|---|---|---|
| VH1000 | RLG3499=MG1655 pyrE+ lacZ− lacI− | Gaal et al., 1997 | ||
| RLG4998 | VH1000 | lacUV5 | −59/+36 | Barker et al., 2001 |
| RLG8950 | RLG4998 dksA::tet | lacUV5 | −59/+36 | This work |
| RLG8992 | VH1000 | flhDC | −95/+50 | This work |
| RLG8994 | RLG8992 dksA::tet | flhDC | −95/+50 | This work |
| RLG8430 | VH1000 | fliA 70+28 | −103/+15* | This work |
| RLG8431 | VH1000 | fliA 70 | −103/+2 | This work |
| RLG8432 | VH1000 | fliA 28 | −37/+5 | This work |
| RLG8433 | VH1000 | fliC | −125/+50 | This work |
| RLG8434 | RLG8430 dksA::tet | fliA 70+28 | −103/+15* | This work |
| RLG8435 | RLG8431 dksA::tet | fliA 70 | −103/+2 | This work |
| RLG8436 | RLG8432 dksA::tet | fliA 28 | −37/+5 | This work |
| RLG8437 | RLG8433 dksA::tet | fliC | −125/+50 | This work |
| RLG8984 | MG1655 strR fliC::kan | P.S. Cohen |
| Plasmid | Description | Promoter Endpoints | Source |
|---|---|---|---|
| pRLG770 | Transcription vector | Ross et al., 1990 | |
| pRLG8413 | pRLG770 containing flhDC promoter | −95/+50 | This work |
| pRLG8423 | pRLG770 containing fliA 70 promoter | −103/+2 | This work |
| pRLG8424 | pRLG770 containing fliA 28 promoter | −37/+5 | This work |
| pRLG8425 | pRLG770 containing fliC promoter | −125/+50 | This work |
| pRLG3422 | pRLG770 containing lacUV5 promoter | −46/+1 | Gaal et al., 1997 |
| pRLG6555 | pRLG770 containing rrnB P1 promoter | −66/+9 | Barker and Gourse, 2001 |
| pRLG8110 | pRLG770 containing fis promoter | −166/+83 | Mallik et al., 2006 |
| pRLG3256 | pRLG770 containing lac promoter with CRP binding site | −80/+57 | W. Ross and R.L.G., unpublished |
Promoter endpoints are numbered relative to the transcription start site. Fragments marked with an asterisk are numbered with respect to the transcription start site for the promoter recognized by the σ70 RNAP holoenzyme (fliA 70). All promoter-lacZ fusions are on phage lambda prophage on the bacterial chromosome. Plasmids were used as templates for in vitro transcription.
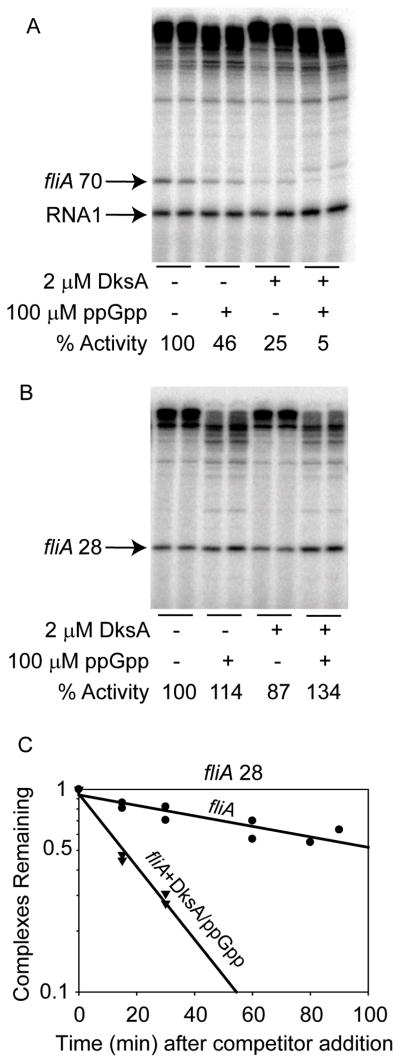
We also examined Class II transcription in vitro (Fig. 4). The fliA 70 promoter was inhibited directly by DksA and/or ppGpp (Fig. 4A), but the fliA 28 promoter was not (Fig. 4B). Because DksA/ppGpp reduced the lifetime of the fliA 28 promoter complex (Fig. 4C), the lack of an effect of DksA/ppGpp on transcriptional output likely results from the intrinsic kinetic characteristics of this promoter rather than from an inability of DksA/ppGpp to bind to the σ28 RNAP holoenzyme (see Discussion). The effect of DksA/ppGpp on the lifetime of the fliA 70 promoter complex was not examined, because transcription from this promoter was completely dependent on FlhDC (which affected complex half-life measurements by our methods; data not shown). Taken together, our data suggest that DksA/ppGpp regulates some Class II flagellar promoters directly and others indirectly by inhibiting FlhDC production.
Figure 4.
Only the fliA 70 promoter recognized by σ70 RNAP is inhibited directly by DksA/ppGpp in vitro.
A. Single round in vitro transcription from the fliA 70 promoter in the presence of 75 nM FlhDC and Eσ70. Quantitation reflects averages from 6 identical transcription reactions. There is a faint band derived from the vector just above the position of the test transcript that is activated by DksA/ppGpp (see also Fig. 2A, 2C, 2D and Fig. 4B).
B. Single round in vitro transcription from the fliA 28 promoter with Eσ28 RNAP. The RNA I promoter is not recognized by this RNAP. Quantitation reflects averages from 6 identical transcription reactions.
C. Effects of DksA/ppGpp on half-life of the fliA 28 complex. DksA was present at 2 μM where indicated. Heparin was 10 μg/ml. Curves reflect the averages of duplicate experiments. The absolute half-lives of the complexes formed were: fliA, 109 minutes and fliA+DksA/ppGpp, 15 minutes.
DksA/ppGpp strongly regulates transcription of fliC, but inhibition appears to be indirect
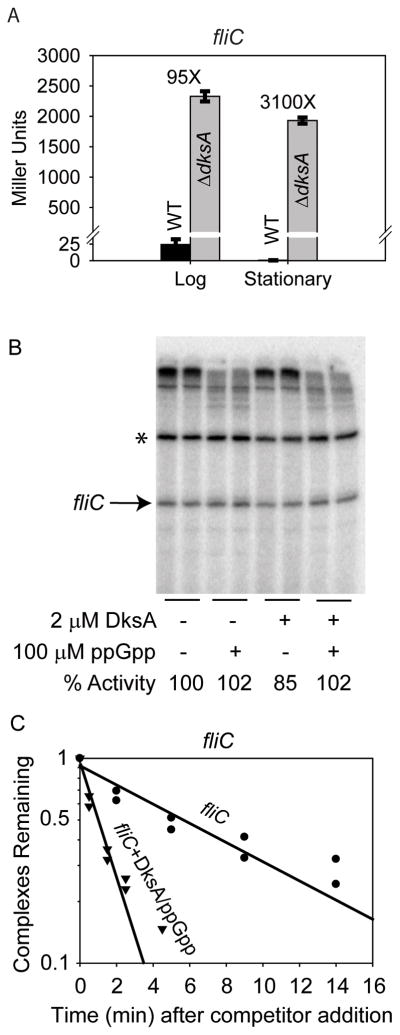
We next tested effects of DksA/ppGpp on the Class III flagellin promoter, fliC, recognized by σ28 RNAP. fliC-lacZ activity decreased in stationary phase in the wild-type strain, but not in the ΔdksA strain (Fig. 5A). As with the Class II promoters, the magnitude of the effect in the ΔdksA strain was very large, both in log and stationary phase (~95-fold and ~3000-fold increases, respectively). DksA and/or ppGpp did not inhibit transcription from the fliC promoter in vitro (Fig. 5B), suggesting that the effects observed in the ΔdksA strain were indirect from overexpression of σ28 (although we have not tested this hypothesis). DksA/ppGpp shortened the lifetime of the RNAP-promoter complex formed at this promoter (Fig. 5C), indicating that DksA/ppGpp binds to Eσ28 holoenzyme, but suggesting that, like fliA 28, the fliC promoter does not have the requisite kinetic properties for DksA/ppGpp to inhibit transcriptional output (see above and Discussion). Taken together from the limited number of flagellar promoters examined, our data suggest that effects of DksA/ppGpp on Class I and II Eσ70-dependent promoters might be mediated directly by DksA/ppGpp, whereas Class II and III Eσ28-dependent promoters might be regulated by DksA and ppGpp indirectly through their direct effects on production of FlhDC and σ28.
Figure 5.
DksA inhibits the fliC promoter in vivo but not in vitro.
A. β-galactosidase activity from the fliC promoter-lacZ fusion in log and stationary phases in WT and ΔdksA strains. Error bars reflect results from 6 independent cultures.
B. Single round in vitro transcription from the fliC promoter with EσF RNAP. The RNAI transcript is not transcribed by this RNAP. The identity of the prominent band (marked by an asterisk) above the fliC transcript band is unclear, but it probably derives from readthrough of the first of the two terminators in the plasmid vector and termination at the second terminator. Quantitation reflects the average from 6 reactions.
C. Effects of DksA/ppGpp on lifetime of the fliC promoter complex. DksA (2 μM) and ppGpp (100 μM) were included where indicated. Heparin was at 10 μg/ml. Curves are from duplicate experiments. The absolute half-lives of the complexes formed were: fliC, 6.4 minutes and fliC+DksA/ppGpp, 1.1 minutes.
Flagellin and flagella are greatly increased in cells lacking DksA
Two approaches were taken to investigate the significance of the increase in flagellar transcription in the ΔdksA mutant, i.e. whether it led to an increase in the amount of flagellin and in the number of flagella in vivo. Flagellin was measured by western blotting. As shown in Fig. 6A, we did not detect flagellin in log or stationary phase in wild-type cells under these growth conditions (or in cells lacking fliC, data not shown), but cells lacking dksA produced high levels of flagellin in both growth phases. In order to determine whether this overproduction of flagellin resulted in increased numbers of flagella, stationary phase cells were negatively-stained with Nano-W and examined by transmission electron microscopy (see Materials and Methods). Consistent with the levels of flagellin produced under these conditions, no obvious flagella were observed on the surface of the wild-type cells (Fig. 6B), but there were multiple flagella on the ΔdksA mutant cells (Fig. 6C). We also observed the characteristic morphological properties reported previously for stationary phase and ΔdksA mutant cells (see figure legend). We conclude that the observed increase in flagellar promoters and overproduction of flagellin in the ΔdksA mutant lead to hyperflagellation.
Figure 6.
Strains lacking DksA overproduce flagellin and are hyperflagellated.
A. Western blot from wild-type, ΔdksA, and fliC::kan cell lysates (strains RLG8992, RLG8994, and RLG8984, respectively) using anti-flagellin antibody. Protein size standards run in an adjacent lane indicated that the band was the appropriate size for flagellin (51kD; data not shown). Flagellin was not detectable in the wild-type strain under these growth conditions.
B. Transmission electron microscopy of wild-type stationary-phase cells negatively stained with NANO-W (Nanoprobes Inc). The cells shown in each panel (B–C) are representative, and the populations in each culture were uniform. The bar is a 1 μm size marker. Small size is typical of cells in stationary phase.
C. ΔdksA. Arrows point to flagella. ΔdksA cells display cell-division defects and thus are elongated (Paul et. al., 2004).
Discussion
DksA and ppGpp directly inhibit expression of the flagellar cascade
We found that DksA and ppGpp work together to inhibit the synthesis of flagella, at least in part by directly inhibiting two key σ70-dependent promoters, one for the master regulator FlhDC and one for the flagellar sigma factor σ28. We suggest that direct inhibition of FlhDC and σ28 synthesis by DksA/ppGpp in turn results in inhibition of Class II and III promoters, linking expression of all the components of the flagellar machinery with expression of the translation apparatus. In retrospect, given the high amounts of energy expended by the cell in synthesizing these large and abundant macromolecular machines, it is not surprising that their regulation would be linked.
We emphasize that until the basis for the resistance of the σ28-dependent promoters to DksA/ppGpp has been determined, we cannot rule out that some other σ28-dependent promoters that we have not yet tested could be inhibited directly. In any case, it appears that the direct effects of DksA/ppGpp on the flhDC and fliA 70 promoters may be sufficient for tight control of the entire flagellar cascade.
The lacZ fusions and in vitro transcription templates utilized here were designed to test effects of DksA/ppGpp on transcription initiation from representative Class I, II, and III promoters, independent of potential effects on other steps in gene expression. However, direct or indirect effects of DksA/ppGpp, e.g. on mRNA or protein stability or on translation, could contribute to the magnitude of the effects on synthesis of the flagellar components. Furthermore, although we did not observe direct effects of DksA/ppGpp on the two σ28-dependent promoters tested, in theory others could be inhibited directly. Finally, as many as six flhDC promoters have been reported in Salmonella typhimurium, so it is possible that flhDC promoter control by DksA/ppGpp could be more complicated in that bacterium (Yanagihara et al., 1999).
Multiple signals balance motility with conservation of nutrients
Inhibitory effects of DksA/ppGpp on σ70-dependent Class I and II promoters were amplified dramatically on the promoters genetically “downstream”, even in the presence of cAMP-CRP, which has been shown to activate flhDC transcription. Therefore, we suggest that DksA/ppGpp plays a major role in preventing expression of this pathway even when other regulatory inputs, such as cAMP-CRP, are operational.
Cells grown in carbon sources of declining nutritional quality (as judged by cell growth rate) often display increasing levels of motility (Liu et al., 2005), because cAMP concentration usually increases as the growth rate decreases. Thus, an increase in CRP activity, with the accompanying activation of the flhDC promoter, can account for graded increases in motility in carbon sources of declining nutritional quality (Buettner et al., 1973, Bettenbrock et al., 2007). However, the inverse correlation between growth rate and motility breaks down in cells growing in very poor carbon sources (e.g. proline; Liu et al., 2005) or when cells are in stationary phase (Amsler et al., 1993). Our data suggest that DksA/ppGpp functions as an independent signal that supercedes other signals and shuts down transcription of the flagellar cascade in very poor carbon sources and in stationary phase.
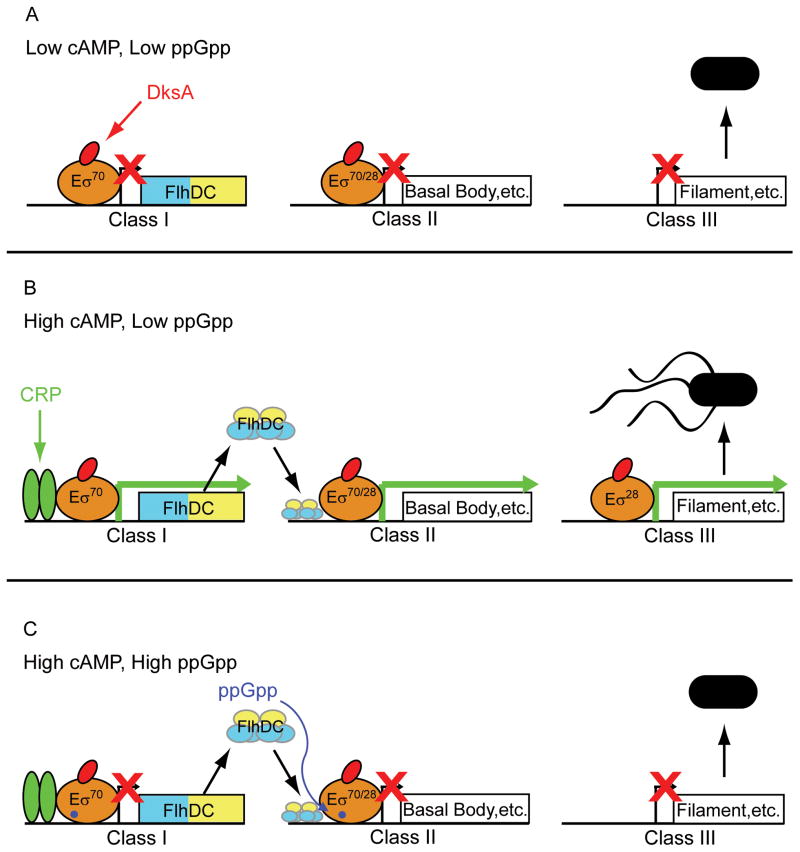
Fig. 7 represents flagellar output in three different situations. Fig. 7A illustrates the situation when cAMP and ppGpp concentrations are both low (which occurs when cells are growing in a relatively rich medium in log phase). The result is that flagella production is inhibited. Fig. 7B illustrates the situation when cAMP is high and ppGpp is low (which occurs in a nutritionally poor medium in log phase). In this case, flagella are synthesized. Fig. 7C illustrates the situation when cAMP and ppGpp are both high (which occurs when cells are starved for an essential nutrient and/or in stationary phase). In this case, flagella synthesis is again inhibited. Bettenbrock and colleagues (2007) speculated previously that the DksA/ppGpp system might be responsible for overriding activation of CRP-cAMP-dependent promoters at very low growth rates.
Figure 7.
CRP-cAMP and DksA/ppGpp contributions to the flagellar cascade. Other inputs to the system are left out for clarity. The dual promoters (σ70- and σ28-dependent) in the Class II promoter are represented by a single arrow. Gene products produced by each flagellar gene class are indicated. FlhDC is represented as a heterohexamer binding upstream of a generic Class II Eσ70-dependent promoter. RNAP is represented as a tan oval. DksA is represented as a red oval. ppGpp is represented as a purple dot bound to RNAP. The cAMP-CRP dimer is represented as green ovals. Flagellated motile cells and unflagellated nonmotile are indicated in black. A green arrow indicated that the promoter is active. A red “X” indicates that the promoter is inactive.
A. When cAMP and ppGpp levels are low (e.g. in a rich medium in log phase) flagellar expression and motility is weak, because FlhDC expression is not high enough to activate the fliA promoter and produce σ28.
B. When cAMP levels are high and ppGpp levels are low (e.g. in a nutritionally relatively poor medium in log phase), flagella levels and motility are high, because FlhDC expression is high enough to activate the fliA promoter and produce σ28, whereas the ppGpp concentration is too low to strongly inhibit transcription.
C. When both cAMP and ppGpp levels are high (e.g. in a nutritionally very poor medium, after starvation, or in stationary phase), CRP can activate the flagellar cascade, but DksA/ppGpp overrides this activation by inhibiting transcription from the flhDC and fliA 70 promoters.
It has been proposed that when σS-dependent transcription is high, cyclic-di-GMP reduces flagellar function in some stress, starvation, or stationary phase conditions, triggering a switch from motility to curli-mediated adhesion (which is σS-dependent; Pratt and Silhavy, 1998; Pesavento et al., 2008). Hengge and colleagues suggested that ppGpp might also participate in the switch from motility to curli-mediated adhesion (Pesavento et al., 2008). Such a model is appealing: not only does DksA/ppGpp inhibit flagellar expression directly, as shown here, but ppGpp also activates σS-dependent transcription under some conditions (Bougdour and Gottesman, 2007) which could facilitate the curli switch. Thus, cyclic-di-GMP-dependent regulation would complement the regulation by DksA/ppGpp described here.
Overproduction of flagella leads to changes in motility
Conflicting reports have appeared recently about the effects of DksA and ppGpp on motility (reviewed in Pesavento and Hengge, 2009). Magnusson and colleagues (2007) reported that cells lacking the dksA gene were less motile than wild-type cells, whereas Aberg and colleagues (2009) reported that ΔdksA mutants were more motile than wild-type cells. Estimates of bacterial cell motility as measured by the diameter of growth in soft agar are subject to effects of nutritional conditions, agar concentration, cell morphology, etc. Because cells lacking dksA grow slower than wild-type cells (especially in a defined medium) and exhibit defects in cell division, in our experience it is difficult to draw conclusions about the motility of ΔdksA mutants using this assay. Our conclusions (that flagellar promoters are transcribed at a higher level in ΔdksA mutants, that ΔdksA mutants overexpress flagellar components, and that DksA inhibits the flhDC and fliA promoters in vitro) were based on assays that directly report on the appearance of flagella, flagellar proteins, and promoter activity and are in agreement with the interpretation of Aberg and colleagues (2009). However, the magnitude of the effects reported here is much larger. This difference likely reflects the fact that DksA works synergistically with ppGpp, and DksA/ppGpp effects are much larger in a minimal medium where ppGpp concentrations are much higher than in a rich medium (Murray et al., 2003; Paul et al., 2004a).
It has also been reported that flagellar promoters respond oppositely to DksA and ppGpp under some conditions in vivo (Magnusson et al., 2007). Because both ppGpp and DksA functioned as inhibitors in our purified transcription reactions, such opposing effects must be indirect, and/or mediated by factors not present in our in vitro system, and/or they could occur on step(s) other than transcription initiation.
Mechanism of Action of DksA/ppGpp
DksA/ppGpp decreases the lifetime of complexes formed between RNAP and promoter DNA at all promoters that have been tested to date, but transcription is inhibited only from a subset of these promoters. We have proposed previously that promoters that form intrinsically short-lived competitor-resistant complexes with RNAP are particularly sensitive to inhibition by DksA/ppGpp. However, the promoter sequence determinants that result in short-lived promoter complexes are complex. For example, mutations in the -10, -35, or extended -10 elements, between the transcription start site and the -10 element, and between the -10 and -35 elements all can have large effects on the intrinsic half-life of a promoter complex (Haugen et al., 2006). The determinants of the stability of the flhDC promoter complex have not been investigated in depth, but we note that its intrinsic lifetime is comparable to that formed by the fis promoter which is inhibited by DksA/ppGpp in vivo and in vitro (Mallik et al., 2006).
Transcription from the two σ28-dependent promoters investigated here was not inhibited directly by DksA/ppGpp. Further study will be required to understand the mechanism by which the fliC promoter resists direct inhibition by DksA/ppGpp. DksA and ppGpp together affected promoter-σ28 RNAP lifetime (Fig. 4C and Fig. 5C), implying they bind to the complex. However, we did not test these factors individually, and we have not yet demonstrated that effects of DksA/ppGpp on σ28 RNAP-promoter complexes are mechanistically similar to those on σ70 holoenzyme-promoter complexes or that the σ28 RNAP-promoter complex half-life measured in these assays is even relevant for regulation of these promoters. Examination of effects of DksA and ppGpp on σ28 RNAP-promoter complexes in vitro is proceeding as part of a more extensive study on effects of these factors on promoter complexes containing alternative holoenzymes.
Materials and Methods
Strains, plasmids, and proteins
Construction of promoter-lacZ fusions on phage λ and isolation of strains lysogenic for these prophage have been described elsewhere (Simons et al., 1987; Rao et al., 1994). All promoter-lacZ fusions are on phage lambda prophage on the bacterial chromosome (“system II”; Rao et al., 1994). Plasmids used for in vitro transcription were constructed by insertion of the promoter fragment of interest into the EcoRI and HindIII sites of plasmid pRLG770 (Ross et al., 1990) and were verified by sequencing. The endpoints of the promoter fragments used for these constructions are provided in Table 1. Construction of ΔdksA strains was performed by transduction of the dksA::tet insertion-deletion from strain RLG8124 with P1vir (Rutherford et al., 2009). The fliC::kan strain was constructed as described (Gauger et al., 2007), except the gene was inactivated with a gene for kanamycin-resistance instead of one for chloramphenicol-resistance, using the same primers except pKD4 as a template instead of pKD3 (Datsenko and Wanner, 2000). RNAP and σ70 were purified by standard methods (Rutherford et al., 2009 and references therein). CRP was purified according as described (Ghosaini et al., 1988). His-tagged DksA was purified as described (Paul et al., 2004a). Purified σ28 was a generous gift from Richard Burgess (UW-Madison). cAMP was purchased from Sigma, and ppGpp was purchased from Trilink, Inc. FlhDC was overexpressed and purified by affinity chromatography (HiTrap heparin, GE Healthcare; Liu and Matsumura, 1994) and eluted in 0.5 M NaCl in 50 mM Tris (pH 7.9), dialyzed against a solution of 50% glycerol, 0.5M NaCl, 50 mM Tris (pH 7.9), and stored at −20° C.
β-galactosidase assays
Cells were grown in MOPS medium (Neidhardt et al., 1974) supplemented with glycerol (0.4%), all 20 amino acids (80 μg/ml each except 40 μg/ml for tryptophan and tyrosine), and thiamine (10 μg/ml) at 37°C for at least 3 generations before sampling. At an OD600 of ~0.3, 1 ml samples were removed to tubes on ice containing 4 ml of Z-buffer (Miller et al., 1972), incubated on ice for at least 10 min, lysed by sonication, and β-galactosidase activity was measured (Barker et al., 2001). The remainder of the culture was allowed to continue growth for a total of 22–24 hours after inoculation, after which β-galactosidase activity was measured in the same way. Background (β-galactosidase activity from a promoterless lacZ fusion) was less than 1 Miller Unit under these conditions (Rao et al., 1994).
RNA extraction and Primer Extension
RNA levels were quantified from promoter-lacZ fusions after cells were treated with serine hydroxamate (SHX; 1 μg/ml final concentration) (Sigma) to initiate amino acid starvation (Paul et al., 2004a). RNA extraction by boiling lysis followed by primer extension for measuring transcription in vivo has been described in detail (Ross and Gourse, 2009). Briefly, RNA samples were resuspended in a solution consisting of 50–100 μl 10 mM Tris pH 8.0 and 300 ng of a primer annealing to the leader region of the lacZ transcript (5′-GTTTTCCCAGTCACGAC-3′). The primer was end-labeled with 32P-γ-ATP using polynucleotide kinase (Promega). Extension with reverse transcriptase was allowed to proceed for 30 min at 48° C, the reaction was terminated with a formamide stop solution, aliquots were electrophoresed on a 6% polyacrylamide-7 M urea denaturing gel, the gel was dried, and the results were visualized and quantified by phosphorimaging. The amount of extension product was normalized to a recovery marker RNA added at the cell lysis stage.
In vitro transcription
Promoter fragments of interest were ligated into the EcoRI and HindIII sites of pRLG770 to generate supercoiled plasmid templates for in vitro transcription (Ross et al., 1990). Single-round transcription reactions were preformed essentially as described (Paul et al., 2004a) except that all reactions contained 60 mM NaCl, and heparin (10 μg/ml) was used as the competitor to prevent reinitiation. The concentrations of the following (when present) are in parentheses: DksA (2 μM), ppGpp (100 μM; TriLink, Inc.), FlhDC (75 nM), CRP (50 nM), cAMP (200 μM), ATP, GTP, and CTP (200 μM), UTP (10 μM), and ~ 1 μCi of 32P-αUTP. RNAP was at 10 nM except in Fig. 2C, when it was at 60 nM. For the fliA promoter constructs, transcription occurred only with the RNAP containing the appropriate σ factor (data not shown).
Promoter Complex Decay Assay
Half-lives were determined using a transcription-based assay (Barker et al., 2001). Supercoiled plasmid DNA (1 nM) was preincubated with DksA (0.25 μM or 2 μM as indicated in the legends) and ppGpp (100 μM) in transcription buffer (Paul et al., 2004a) for 10 min. After competitor addition, 10 μl aliquots were removed to a tube containing NTPs (final concentrations as described above) at various times and incubated for 10 min before the addition of formamide stop solution, electrophoresis on 6% polyacrylamide gels, and analysis by phosphorimaging. The results were plotted, and the half-lives were calculated using Sigmaplot (Jandel Scientific). Only test bands at least ~2-fold above background were used for the analysis.
Western Blotting
Cells were grown in the MOPS-glycerol medium described above to an OD600 of 0.4 (log phase) or overnight (stationary phase, 22–24 hours after inoculation). Samples (1 ml) were added to tubes containing 111 μl 100% TCA, vortexed, incubated on ice for at least 5 min, and microcentrifuged at 14K RPM for 15 min at 4°C. After supernatant removal, the pellet was suspended in 50–100 μl of SDS resuspension buffer (2% SDS, 20 mM sodium phosphate pH 7.5, 10 mM EDTA, 0.1 mM PMSF) and heated at 95°C. After protein concentration determination, 20 μg aliquots were electrophoresed on 4–12% Bis-Tris gels (Invitrogen), transferred to PVDF membranes using a semi-dry transfer apparatus, probed with anti-flagellin antibodies (a generous gift from Doug Weibel and Matt Copeland, UW-Madison) and HRP-conjugated goat anti-rabbit IgG antibodies (Santa Cruz Biotechnologies), and imaged using ECL+ (Amersham) and a Typhoon phosphorimager-scanner.
Electron Microscopy
Cells were grown overnight in the MOPS-glycerol medium described above and stained with methylamine tungstate (NANO-W; Nanoprobes, Inc). Transmission electron microscopy was performed by the UW-Madison Medical School Electron Microscope Facility. The strains used were RLG8992 (WT), RLG8994 (ΔdksA) and RLG8984 (ΔfliC).
Acknowledgments
We thank members of our laboratory for helpful comments, M. Copeland, S. Parkinson, and J. Armitage for antibodies to FliC, P. Matsumura and A. Campos for the flhDC overexpression vector, M. Leatham for the fliC::kan strain, R. Burgess for purified σ28 protein, B. August and the UW Medical School Electron Microscope Facility for the TEM images, D. Jin for providing information before publication, and K. Hughes, P. Matsumura, and W. Ross for examining the manuscript. Work in our laboratory is supported by a grant from the National Institutes of Health (R37 GM37048).
References
- Aberg A, Fernández-Vázquez J, Cabrer-Panes JD, Sánchez A, Balsalobre C. Similar and divergent effects of ppGpp and DksA deficiencies on transcription in Escherichia coli. J Bacteriol. 2009;191:3226–3236. doi: 10.1128/JB.01410-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amsler CD, Cho M, Matsumura P. Multiple factors underlying the maximum motility of Escherichia coli as cultures enter post-exponential growth. J Bacteriol. 1993;175:6238–9244. doi: 10.1128/jb.175.19.6238-6244.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnosti DN, Chamberlin MJ. Secondary σ factor controls transcription of flagellar and chemotaxis genes in Escherichia coli. Proc Natl Acad Sci USA. 1989;86:830–834. doi: 10.1073/pnas.86.3.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker MM, Gaal T, Josaitis CA, Gourse RL. Mechanism of regulation of transcription initiation by ppGpp. I. Effects of ppGpp on transcription initiation in vivo and in vitro. J Mol Biol. 2001;305:673–688. doi: 10.1006/jmbi.2000.4327. [DOI] [PubMed] [Google Scholar]
- Barker MM, Gourse RL. Regulation of rRNA transcription correlates with nucleoside triphosphate sensing. J Bacteriol. 2001;183:6315–6323. doi: 10.1128/JB.183.21.6315-6323.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettenbrock K, Sauter T, Jahreis K, Kremling A, Lengeler JW, Gilles ED. Correlation between growth rates, EIIACrr phosphorylation, and intracellular cyclic AMP levels in Escherichia coli K-12. J Bacteriol. 2007;189:6891–6900. doi: 10.1128/JB.00819-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bougdour A, Gottesman S. ppGpp regulation of RpoS degradation via anti-adaptor protein IraP. Proc Natl Acad Sci USA. 2007;104:12896–12901. doi: 10.1073/pnas.0705561104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buettner MJ, Spitz E, Rickenberg HV. Cyclic adenosine 3′,5′-monophosphate in Escherichia coli. J Bacteriol. 1973;114:1068–1073. doi: 10.1128/jb.114.3.1068-1073.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevance FF, Hughes KT. Coordinating assembly of a bacterial macromolecular machine. Nat Rev Microbiol. 2008;6:455–65. doi: 10.1038/nrmicro1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilcott GS, Hughes KT. Coupling of flagellar gene expression to flagellar assembly in Salmonella enterica serovar Typhimurium and Escherichia coli. Microbiol Mol Biol Rev. 2000;64:694–708. doi: 10.1128/mmbr.64.4.694-708.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli using PCR products. Proc Natl Acad Sci USA. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durfee T, Hansen AM, Zhi H, Blattner FR, Jin DJ. Transcription profiling of the stringent response in Escherichia coli. J Bacteriol. 2008;190:1084–1096. doi: 10.1128/JB.01092-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francez-Charlot A, Laugel B, Van Gemert A, Dubarry N, Wiorowski F, Castanie-Cornet MP, et al. RcsCDB his-asp phosphorelay system negatively regulates the flhDC operon in Escherichia coli. Mol Microbiol. 2003;49:823–832. doi: 10.1046/j.1365-2958.2003.03601.x. [DOI] [PubMed] [Google Scholar]
- Gaal T, Bartlett MS, Ross W, Turnbough CL, Jr, Gourse RL. Transcription regulation by initiating NTP concentration: rRNA synthesis in bacteria. Science. 1997;278:2092–2097. doi: 10.1126/science.278.5346.2092. [DOI] [PubMed] [Google Scholar]
- Gauger EJ, Leatham MP, Mercado-Lubo R, Laux DC, Conway T, Cohen PS. Role of motility and the flhDC operon in Escherichia coli MG1655 colonization of the mouse intenstine. Infect Immun. 2007;75:3315–3324. doi: 10.1128/IAI.00052-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosaini LR, Brown AM, Sturtevant JM. Scanning calorimetric study of the thermal unfolding of catabolite activator protein from Escherichia coli in the absence and presence of cyclic mononucleotides. Biochemistry. 1988;27:5257–5261. doi: 10.1021/bi00414a046. [DOI] [PubMed] [Google Scholar]
- Haugen SP, Berkmen MB, Ross W, Gaal T, Ward C, Gourse RL. rRNA promoter regulation by nonoptimal binding of sigma region 1.2: An additional recognition element for RNA polymerase. Cell. 2006;125:1069–1082. doi: 10.1016/j.cell.2006.04.034. [DOI] [PubMed] [Google Scholar]
- Haugen SP, Ross W, Gourse RL. Advances in bacterial promoter recognition and its control by factors that do not bind DNA. Nat Rev Microbiol. 2008;6:507–519. doi: 10.1038/nrmicro1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko M, Park C. H-NS-dependent regulation of flagellar synthesis is mediated by a LysR family protein. J Bacteriol. 2000;182:4670–4672. doi: 10.1128/jb.182.16.4670-4672.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehnen D, Blumer C, Polen T, Wackwitz B, Wendisch VF, Unden G. LrhA as a new transcriptional key regulator of flagella, motility and chemotaxis genes in Escherichia coli. Mol Microbiol. 2002;45:521–532. doi: 10.1046/j.1365-2958.2002.03032.x. [DOI] [PubMed] [Google Scholar]
- Liu M, Durfee T, Cabrera JE, Zhao K, Jin DJ, Balttner FR. Global transcriptional programs reveal a carbon source foraging strategy by Escherichia coli. J Biol Chem. 2005;280:15921–15927. doi: 10.1074/jbc.M414050200. [DOI] [PubMed] [Google Scholar]
- Liu X, Matsumura P. The FlhD/FlhC complex, a transcriptional activator of the Escherichia coli flagella class II operons. J Bacteriol. 1994;176:7345–7351. doi: 10.1128/jb.176.23.7345-7351.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Matsumura P. An alternative sigma factor controls transcription of flagellar class-III operons in Escherichia coli: gene sequence, overproduction, purification and characterization. Gene. 1995;164:81–84. doi: 10.1016/0378-1119(95)00480-t. [DOI] [PubMed] [Google Scholar]
- Liu X, Mastumura P. Differential regulation of multiple overlapping promoters in flagellar class II operons in Escherichia coli. Mol Microbiol. 1996;21:613–620. doi: 10.1111/j.1365-2958.1996.tb02569.x. [DOI] [PubMed] [Google Scholar]
- Macnab RM. Genetics and biogenesis of bacterial flagella. Annu Rev Genet. 1992;26:131–158. doi: 10.1146/annurev.ge.26.120192.001023. [DOI] [PubMed] [Google Scholar]
- Magnusson LU, Gummesson B, Joksimović P, Farewell A, Nyström T. Identical, independent, and opposing roles of ppGpp and DksA in Escherichia coli. J Bacteriol. 2007;189:5193–5202. doi: 10.1128/JB.00330-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallik P, Paul BJ, Rutherford ST, Gourse RL, Osuna R. DksA is required for growth phase-dependent regulation, growth rate-dependent control, and stringent control of fis expression in Escherichia coli. J Bacteriol. 2006;188:5775–5782. doi: 10.1128/JB.00276-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JH. Experiments in Molecular Genetics. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1972. [Google Scholar]
- Murray HD, Schneider DA, Gourse RL. Control of rRNA expression by small molecules is dynamic and nonredundant. Mol Cell. 2003;12:125–34. doi: 10.1016/s1097-2765(03)00266-1. [DOI] [PubMed] [Google Scholar]
- Neidhardt FC, Bloch PL, Smith DF. Culture medium for enterobacteria. J Bacteriol. 1974;119:736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnishi K, Kutsukake K, Suzuki H, Lino T. A novel transcriptional regulation mechanism in the flagellar regulon of Salmonella typhimurium: an antisigma factor inhibits the activity of the flagellum-specific sigma factor, sigma F. Mol Microbiol. 1992;6:3149–3157. doi: 10.1111/j.1365-2958.1992.tb01771.x. [DOI] [PubMed] [Google Scholar]
- Paul BJ, Barker MM, Ross W, Schneider DA, Webb C, Foster JW, Gourse RL. DksA: A critical component of the transcription initiation machinery that potentiates the regulation of rRNA promoters by ppGpp and the initiating NTP. Cell. 2004a;118:311–322. doi: 10.1016/j.cell.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Paul BJ, Ross W, Gaal T, Gourse RL. rRNA transcription in Escherichia coli. Annu Rev Genet. 2004b;38:749–770. doi: 10.1146/annurev.genet.38.072902.091347. [DOI] [PubMed] [Google Scholar]
- Paul BJ, Berkmen MB, Gourse RL. DksA potentiates direct activation of amino acid promoters by ppGpp. Proc Natl Acad Sci USA. 2005;102:7823–7828. doi: 10.1073/pnas.0501170102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesavento C, Becker G, Sommerfeldt N, Possling A, Tschowri N, Mehlis A, Hengge R. Inverse regulatory coordination of motility and curli-mediated adhesion in Escherichia coli. Genes Dev. 2008;22:2434–2446. doi: 10.1101/gad.475808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesavento C, Hengge R. Bacterial nucleotide-based second messengers. Curr Opin Microbiol. 2009;12:170–6. doi: 10.1016/j.mib.2009.01.007. [DOI] [PubMed] [Google Scholar]
- Potrykus K, Cashel M. (p)ppGpp: still magical? Annu Rev Microbiol. 2008;62:35–51. doi: 10.1146/annurev.micro.62.081307.162903. [DOI] [PubMed] [Google Scholar]
- Pratt LA, Silhavy TJ. Crl stimulates RpoS activity during stationary phase. Mol Microbiol. 1998;29:1225–1236. doi: 10.1046/j.1365-2958.1998.01007.x. [DOI] [PubMed] [Google Scholar]
- Rao L, Ross W, Appleman JA, Gaal T, Leirmo S, Schlax PJ, et al. Factor independent activation of rrnB P1: An “extended” promoter with an upstream element that dramatically increases promoter strength. J Mol Biol. 1994;235:1421–1435. doi: 10.1006/jmbi.1994.1098. [DOI] [PubMed] [Google Scholar]
- Ross W, Thompson JF, Newlands JT, Gourse RL. E. coli Fis protein activates ribosomal RNA transcription in vitro and in vivo. EMBO J. 1990;9:3733–3742. doi: 10.1002/j.1460-2075.1990.tb07586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross W, Gourse RL. Analysis of RNA polymerase-promoter complex formation. Methods. 2009;47:13–24. doi: 10.1016/j.ymeth.2008.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford ST, Lemke JJ, Vrentas CE, Gaal T, Ross W, Gourse RL. Effects of DksA, GreA, and GreB on transcription initiation: Insights into the mechanisms of factors that bind in the secondary channel of RNA polymerase. J Mol Biol. 2006;366:1243–1257. doi: 10.1016/j.jmb.2006.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford ST, Villers CL, Lee JH, Ross W, Gourse RL. Allosteric control of Escherichia coli rRNA promoter complexes by DksA. Genes Dev. 2009;23:236–248. doi: 10.1101/gad.1745409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma AK, Payne SM. Induction of expression of hfq by DksA is essential for Shigella flexneri virulence. Mol Microbiol. 2006;62:469–479. doi: 10.1111/j.1365-2958.2006.05376.x. [DOI] [PubMed] [Google Scholar]
- Shin S, Park C. Modulation of flagellar expression in Escherichia coli by acetyl phosphate and the osmoregulator OmpR. J Bacteriol. 1995;177:4696–4702. doi: 10.1128/jb.177.16.4696-4702.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman M, Simon M. Characterization of Escherichia coli flagellar mutants that are insensitive to catabolite repression. J Bacteriol. 1974;120:1196–1203. doi: 10.1128/jb.120.3.1196-1203.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons RW, Houman F, Kleckner N. Improved single and multicopy lac-based cloning vectors for protein and operon fusions. Gene. 1987;53:85–96. doi: 10.1016/0378-1119(87)90095-3. [DOI] [PubMed] [Google Scholar]
- Soutourina O, Kolb A, Krin E, Laurent-Winter C, Rimsky S, Danchin A, Bertin P. Multiple control of flagellum biosynthsis in Escherichia coli: Role of H-NS protein and the cycle AMP-catabolite activator protein complex in transcription of the flhDC master operon. J Bacteriol. 1999;181:7500–7508. doi: 10.1128/jb.181.24.7500-7508.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperandio V, Torres AG, Kaper JB. Quorum sensing Escherichia coli regulators B and C (QseBC): A novel two-component regulatory system involved in the regulation of flagella and motility by quorum sensing in E. coli. Mol Microbiol. 2002;43:809–821. doi: 10.1046/j.1365-2958.2002.02803.x. [DOI] [PubMed] [Google Scholar]
- Srivatsan A, Wang JD. Control of bacterial transcription, translation and replication by (p)ppGpp. Curr Opin Microbiol. 2008;11:100–105. doi: 10.1016/j.mib.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Stojiljkovich I, Baumler AJ, Hantke K. Fur regulon in Gram-negative bacteria: identification and characterization of new iron-regulated Escherichia coli genes by a Fur titration assay. J Mol Biol. 1994;236:531–545. doi: 10.1006/jmbi.1994.1163. [DOI] [PubMed] [Google Scholar]
- Traxler MF, Summers SM, Nguyen HT, Zacharia VM, Hightower GA, Smith JT, Conway T. The global, ppGpp-mediated stringent response to amino acid starvation in Escherichia coli. Mol Microbiol. 2008;68:1128–1148. doi: 10.1111/j.1365-2958.2008.06229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Fleming RT, Westbrook EM, Matsumura R, McKay DB. Structure of the Escherichia coli FlhDC complex, a prokaryotic heteromeric regulator of transcription. J Mol Bio. 2006;355:798–808. doi: 10.1016/j.jmb.2005.11.020. [DOI] [PubMed] [Google Scholar]
- Yanagihara S, Iyoda S, Ohnishi K, Iino T, Kutsukake K. Structure and transcriptional control of the flagellar master operon of Salmonella typhimurium. Genes Genet Syst. 1999;74:105–1111. doi: 10.1266/ggs.74.105. [DOI] [PubMed] [Google Scholar]