Abstract
Engineering solid tissues, including cardiac muscle, requires the inclusion of a microvasculature. Prevascularization in vitro will likely be dependent upon coculturing parenchymal cells with vascular cells, on a matrix that is sufficiently porous to allow microvessel formation. In this study, we examined the behavior and function of endothelial cells on a highly porous elastomeric 3D poly(glycerol sebacate) (PGS) scaffold, to provide a flexible and biocompatible endothelial cell delivery system for developing cardiac engineered tissues with neovascularization potential. Both static and perfusion cell seeding methods were used, and the effects of surface treatment of the scaffold with various extracellular matrix components were examined. Endothelial cell adhesion and phenotype on the PGS scaffold under various flow conditions were also determined. Surface coating with laminin markedly improved the endothelial cell adhesion, survival, and proliferation. The anticoagulant phenotype of adhered endothelial cells was further regulated by the application of flow through regulation of nitric oxide expression. By providing a highly porous scaffolding that contains endothelium with anticoagulant properties, the endothelial cell-seeded PGS scaffold could provide a new basis for subsequent coculture studies with various cell types to develop complex engineered tissue constructs with vascularization capacity.
Keywords: Endothelial cell function, Flow, Vascular tissue engineering, PGS scaffold
INTRODUCTION
In recent years, numerous efforts have been made towards developing functional engineered tissues, which may be a promising tool to repair or replace irreversibly damaged tissues or organs (2,19,29). A successful application of a tissue-engineered construct requires an appropriate scaffold material as well as adequate cell adherence, maximized cell viability, and appropriate cell behavior and function on the scaffold. More importantly, engineering solid tissues requires an inclusion of microvasculature. However, our current inability to vascularize and perfuse thick cell masses has hindered efforts to develop thick and successful living tissue replacements for surgical implantation or in vitro cultivation.
A number of groups have worked for several decades to engineer microvessels in vitro (20,26,43). Recently, enhanced neovascularization of engineered cardiac tissue when implanted into rats was facilitated by endothelial cell coculture (33). Inosculation of engineered capillary-like structures with host vasculature in an endothelialized skin has also been demonstrated (38). Despite the efforts, developing functional vascularized tissues by forming microvasculature within engineered tissues and integrating them with the vascular supply of the host remains a challenge.
Development of microvasculature in vitro will likely be dependent upon coculturing parenchymal cells with vascular cells on a matrix that is sufficiently porous to allow microvessel formation through endothelial adhesion and differentiation. Several biomaterials have been utilized to engineer cardiovascular tissues in vitro. The most widely utilized polymeric scaffold is polyglycolic acid (PGA) (26,30), and naturally occurring hydrogels such as collagen and fibrin gels have also been employed (16,23,46). The main advantage of using polymeric scaffolds is the ability to support the seeding of substrate-dependent cells onto a firm surface, and to provide stable mechanical support, which promotes tissue formation. Polymeric scaffolds can sustain higher mechanical loads than most hydrogels, and therefore have been preferred for many cardiovascular tissue-engineering applications.
A novel biodegradable elastomer, poly(glycerol sebacate) (PGS) can be processed into highly porous scaffolds with mechanical properties suitable for engineering soft tissues (7,11). PGS has been shown to be biocompatible with multiple cell types including neonatal rat cardiomyocytes (30) and Schwann cells (35). It was also shown that the endothelial cells can be cultured on PGS material, though not in a three-dimensional (3D) configuration (8). One of the advantages of PGS scaffolds is that they induce little chronic inflammation when implanted subcutaneously in animal models (35,41). Moreover, highly porous PGS scaffolds can be channeled to mimic a capillary network that can support the vascularization of tissue constructs (30). Thus, PGS may provide an appropriate environment for the cells to adhere and to form microvasculature.
In the current study, we examined a highly porous formulation of PGS for cultivating endothelial cells in a 3D environment. This study was aimed at characterizing the behavior and function of endothelial cells on the PGS scaffold, and defining the tissue-engineering conditions that support endothelial survival and differentiated function. Both static and perfusion seeding methods were studied, and the impact of surface treatments on the PGS scaffold on endothelial behavior, viability, and proliferation were determined. Moreover, endothelial cell adhesion and phenotype on the PGS scaffold under various flow conditions were determined.
We demonstrate that surface treatment of the PGS scaffold improves endothelial cell adhesion, survival, and proliferation in the case of static seeding. Under physiological levels of flow and shear, endothelial cells not only remain adherent, but also express vasodilator and anticoagulant phenotype. Hence, biomimetic physical signals can be used to modulate endothelial cell function in PGS scaffolds in vitro, thereby enhancing their eventual applicability in the cultivation of endothelial- and capillary-containing tissues.
MATERIALS AND METHODS
Cell Culture
Rat aortic endothelial cells (RAECs) were purchased from VEC Technologies (Rensselaer, NY). Cells were cultured in 0.2% gelatin-coated (Sigma, St. Louis, MO) polystyrene tissue culture flasks in MCDB-131 “complete medium” (VEC Technologies) supplemented with 10% FBS, antibiotics, and growth factors. Fresh culture medium was exchanged every 3–4 days. RAECs were passaged when they reached 80% confluency using 0.25% trypsin-EDTA (Gibco BRL). The identity of the cells was confirmed by characteristic cobblestone morphology and acetylated low-density lipoprotein uptake (DiI-Ac-LDL, Biomedical Technologies Inc., Stoughton, MA). RAECs of passage 6–10 were used in this study.
Scaffold Preparation and Coating
Porous PGS biorubber scaffolds were fabricated as previously described in detail (41). Scaffolds were cored into discs (4 mm in diameter, 1 mm in thickness) and sterilized by autoclaving. To facilitate surface wetting, scaffolds were treated with 30-min exchanges in 70%, 50%, and 25% ethanol solution with gentle agitation on an orbital shaker. After ethanol treatment, scaffolds were rinsed with sterile phosphate-buffered saline (PBS, Gibco) for 30 min and twice again with culture medium. Scaffolds were then conditioned in culture medium overnight before cell seeding.
To evaluate the effect of different extracellular matrix proteins on RAECs’ adhesion and function, PGS scaffolds were coated with different substrates prior to cell seeding. Scaffolds were coated with laminin, fibronectin, Matrigel, or collagen, while uncoated PGS scaffolds served as a control. Scaffolds were immersed in Matrigel (2.5 mg/ml in serum-free DMEM, BD Biosciences, Bedford, MA), fibronectin (50 μg/ml in PBS, BD Biosciences), or collagen type I (50 μg/ml in 0.02 N acetic acid, BD Biosciences) at room temperature for 1 h. Scaffolds in laminin solution (10 μg/ml in distilled water, Sigma) were incubated at 37°C for 2 h. Once the scaffolds were coated, they were rinsed with sterile water and culture medium before cell seeding.
Static Cell Seeding
Prior to cell seeding, scaffolds were placed in six-well plates that were coated with 2% bovine serum albumin (BSA) to minimize cell adhesion to the plastic during the initial hour of cell seeding. Both coated and uncoated scaffolds were gently blotted dry and 10 μl of RAEC suspension (50 × 106 cell/ml) was first added to the top surface of the scaffold and maintained in an incubator at 37°C and 5% CO2. For uniform seeding of the cells, scaffolds were taken out of the incubator after 15 min, turned over and another 10 μl of cell suspension was added. Culture medium (10 ml) was carefully added after 1 h and was replaced every 1–2 days. Samples were cultured for 24 h or up to 7 days under static culture conditions at 37°C and 5% CO2.
Perfusion Cell Seeding
RAECs were seeded onto PGS scaffolds using a medium perfusion bioreactor system as described in detail elsewhere (29). Briefly, scaffolds were placed in custom cell-seeding cartridges and RAECs (100 × 106 cells/ml) were seeded at a flow rate of 1.068 ml/min (0.1 mm/s) for 2 h using a syringe pump. After 2 h of seeding, the scaffolds were either cultured statically or subjected to different flow rates to examine the effects of flow on RAEC function. The low flow rate of 0.1 ml/min and high flow rate of 1 ml/min were applied to the cultures for either 72 h or up to 7 days. In order to estimate shear stress within the PGS scaffold, the Darcy permeability (KD) of the PGS scaffold was determined as:
| (Eq. 1) |
where μ is the fluid viscosity (0.8 cP for culture medium at 37°C), Q is the measured volumetric flow rate, L is the thickness of the sample (1 mm), A is the cross-sectional area of the scaffold, and ΔP is the pressure drop across the scaffold construct. The parameters ΔP and Q were measured directly from the PGS scaffold that was housed and perfused within the cartridge.
The average shear stress (τ) within the PGS scaffold to which the RAECs were exposed was estimated by assuming flow around cylindrical polymer surfaces inside the PGS scaffold, based upon the modified Brink-man (5) equation:
| (Eq. 2) |
where B is the Brinkman constant for flow around cylinders (B = 4/π).
Scanning Electron Microscopy
Samples were fixed in 2.5% gluteraldehyde/paraformaldehyde in 100 mM sodium cacodylate solution (Electron microscopy sciences) at pH 7.4 for 2 h (25). After fixation, samples were rinsed in a solution of 100 mM of sodium cacodylate at pH 7.4. Samples were dehydrated with 10-min exchanges in each of 50%, 70%, 80%, 90% ethanol solution, in absolute ethanol for three times, and were then immersed in hexamethyldisilazane (HMDS) for 15 min and air dried at room temperature overnight. The dried samples were coated with gold by a sputter coater for 30 s. Samples were examined using and XL30 ESEM scanning electron microscope (FEI, Hillsboro, OR).
DNA Assay
To quantify the number of cells seeded onto each scaffolds, DNA assay was performed as described (34). Scaffolds were incubated in Proteinase K (0.1 mg/ml in TE buffer, Invitrogen, Carlsbad, CA) overnight at 56°C for DNA extraction. DNA content was measured flourometrically using a picogreen dye (Molecular Probes, Eugene, OR) at a wavelength of 485 nm for excitation and 530 nm for emission.
Cell Viability and Proliferation
For histological evaluation, samples were fixed in 4% paraformaldehyde overnight, dehydrated, embedded in paraffin, and sectioned at 5 μm. To assess cell viability and proliferation, paraffin-embedded sections were deparaffinized and rehydrated according to standard protocol. For cell viability, apoptotic cells were identified by TUNEL staining using a commercially available TdT-FragEL DNA fragmentation detection kit (EMD Biosciences, San Diego, CA) (12). Apoptotic cells were detected using a streptavidin-horseradish peroxidase conjugate and were counterstained with methyl green to visualize the live cells. For cell proliferation, tissue sections were stained using a proliferating cell nuclear antigen (PCNA) staining kit (Zymed Laboratories, San Francisco, CA) (42). PCNA-containing cells were detected using a conjugated biotinylated PCNA monoclonal antibody and streptavidin-peroxidase along with DAB as the chromagen to stain PCNA-positive nuclei a dark brown. The percentage of proliferating or apoptotic cells relative to the total number of cells was obtained from counting positive cells from images taken from three slides per sample.
Western Blots
Construct homogenates were diluted (1:4) in Laemili buffer (Bio-Rad) containing 5% mercaptoethanol and 2% sodium dodecyl sulfate (SDS) and were boiled for 10 min to denature the proteins. The proteins were separated on PDVF gels using 1× Tris-glycine-SDS running buffer (Boston Bioproducts) at a constant voltage of 100 V for 2 h at room temperature followed by electrophoretic transfer. Primary antibodies used for immunoblotting were polyclonal rabbit anti-NOS3 (1:100, Santa Cruz), polyclonal rabbit anti-prostaglandin I synthase (1: 100, Abcam), polyclonal rabbit anti-CD31 (1:100, Santa Cruz), monoclonal mouse anti-thrombomodulin (1:500, Abcam), polyclonal rabbit anti-superoxide dismutase (Abcam, 1:2000), and monoclonal mouse anti-β-actin (Sigma). After multiple washes, blots were incubated with secondary goat anti-rabbit IgG HRP or goat anti-mouse IgG HRP antibodies (1:2000, Santa Cruz), and developed using chemiluminescence (Supersignal, Pierce). Fresh rat aortic tissue homogenate and low passage cultured RAEC lysate were used as positive controls.
Statistical Analysis
Results are presented as mean ± SD. Statistical differences were determined by a two-way ANOVA with a post hoc Tukey’s least significant difference among the different matrices and flow conditions. Statistical significance was accepted for p < 0.05.
RESULTS
Influence of Protein Coating on RAEC Adhesion to PGS Scaffolds
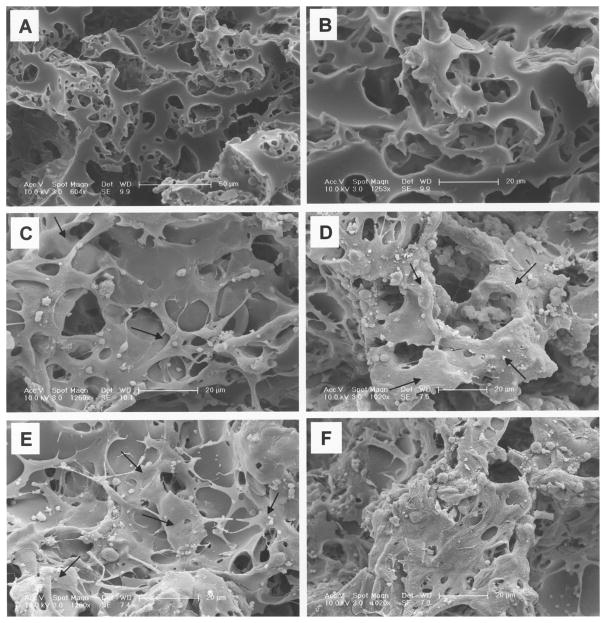
To investigate the effects of surface treatment with extracellular matrix proteins on endothelial cell adhesion and function, PGS scaffolds were coated with laminin, fibronectin, Matrigel, and collagen. Uncoated PGS scaffold served as a control. The uncoated PGS scaffolds exhibited a very open structure with large interconnected pores, as evidenced by scanning electron micrographs (SEM) (Fig. 1A, B). RAECs cultured on uncoated control scaffold for 24 h adhered to PGS to some degree (Fig. 1C), and higher cell density and more cell spreading were observed after 7 days of culture (Fig. 1D). In contrast, cells cultured on the laminin-coated scaffold for 24 h and 7 days were more spread out and extended across scaffold interstices (Fig. 1E, F). Hematoxylin and eosin (H&E) staining also showed higher cell number and more uniform distribution of cells on the laminin-coated PGS compared to other coatings after 24 h of culture, as evident in Fig. 2.
Figure 1.
Scanning electron micrograph (SEM) of poly(glycerol-sebacate) (PGS) elastomer scaffold (A, B) (original magnification at 604× and 1253×, respectively). SEM of RAECs seeded on (C) uncoated control PGS scaffold after 24 h of culture and (D) after 7 days. Arrows indicate spread out cells. (E) RAECs cultured on laminin-coated PGS scaffold after 24 h of culture and (F) after 7 days. Higher numbers of attached cells are observed with longer culture and laminin coating.
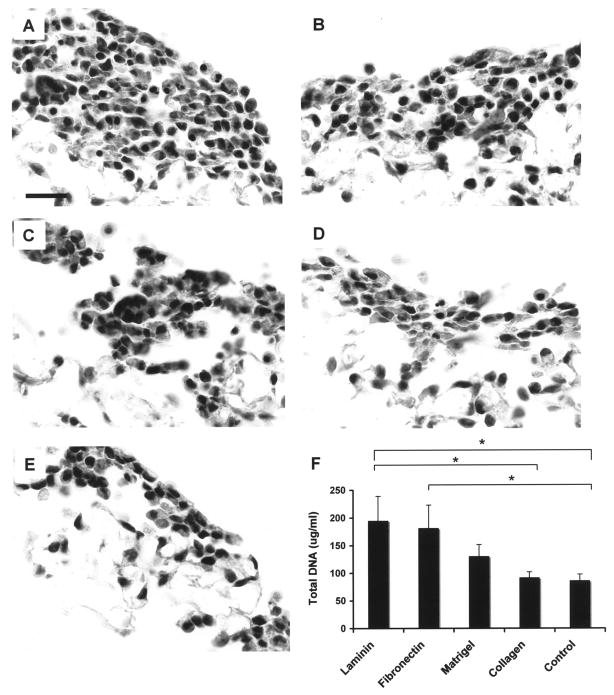
Figure 2.
Representative micrographs of hematoxylin and eosin (H&E) staining and DNA assay of uncoated/coated RAEC seeded after 24 h of culture. (A) Laminin coated, (B) fibronectin coated, (C) Matrigel coated, (D) collagen coated, (E) uncoated control. Scale bars: 20 μm (A–E). (F) DNA assay indicates that more cells adhered to laminin- and fibronectin-coated scaffolds compared to uncoated scaffold. *p < 0.05 (n = 4, Ave. ± SD).
After 24 h of culture, the average DNA content was significantly higher in the laminin-coated and fibronectin-coated scaffolds compared to the uncoated control scaffolds (194.90 ± 44.13, 181.47 ± 41.53, and 86.18 ± 11.57 μg/ml, respectively, p < 0.05 vs. uncoated control) indicating a higher number of attached cells in the laminin- and fibronectin-coated scaffolds (Fig. 2F). This result demonstrates that the laminin and fibronectin significantly improved endothelial adherence to the PGS scaffold after only 1 day.
Effects of Extracellular Matrix Treatment on RAEC Proliferation and Apoptosis
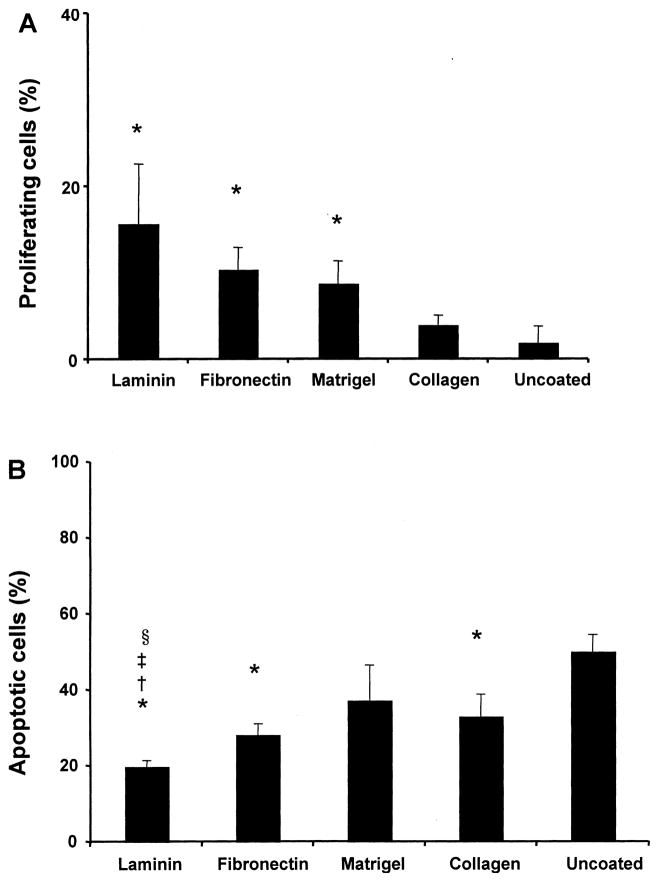
The impact of the surface coating on the proliferation and survival of statically seeded RAEC was examined using PCNA and TUNEL staining on paraffin-embedded sections of uncoated and coated scaffolds. PCNA-positive cells were counted as a percentage of the total number of cells after 24 h of culture. PCNA staining revealed significantly more positive nuclei in laminin-coated scaffolds than in uncoated (15.72 ± 6.9% vs. 1.97 ± 1.9%, respectively, p = 0.03) (Fig. 3A), indicating that laminin-coated scaffolds supported more RAEC proliferation than uncoated. Compared to the uncoated control scaffolds, fibronectin and Matrigel coating were also effective in promoting RAEC proliferation in the PGS scaffold.
Figure 3.
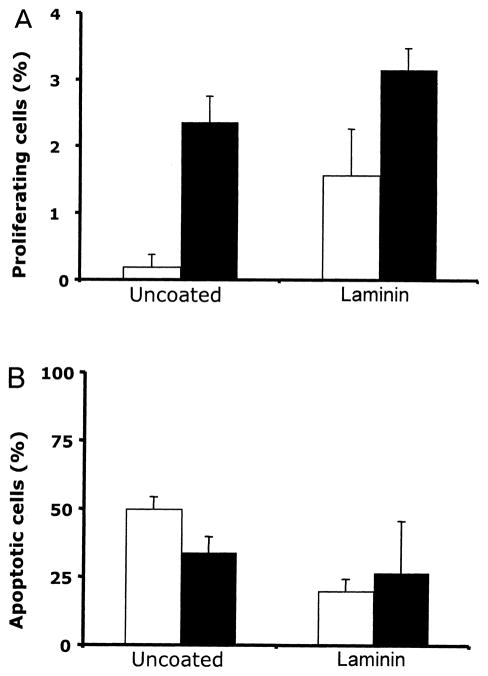
Proliferation and apoptosis of statically seeded RAEC. (A) The percentage of proliferating RAEC after 24 h of culture on coated/uncoated scaffolds. Surface coating of PGS scaffold with laminin, fibronectin, and Matrigel resulted in significantly more proliferative endothelial cells (*p < 0.05 vs. uncoated scaffold, n = 3 slides counted, Ave. ± SD). (B) The percentage of apoptotic cells expressed as the proportion of TUNEL-positive cell nuclei to the total number of nuclei (*p < 0.01 vs. uncoated, ‡p < 0.05 vs. fibronectin, †p < 0.05 vs. Matrigel, §p < 0.05 vs. collagen, n = 3 slides counted, Ave. ± SD).
To examine the viability of RAECs that were statically seeded onto PGS scaffolds, cells were cultured on coated or uncoated scaffolds for 24 h. Tissue sections were labeled using TUNEL staining, and TUNEL-positive cells as a percentage of total were counted. Laminin-coated scaffolds exhibited not only a higher number of overall seeded endothelial cells, but also a higher number of live cells compared to the uncoated scaffolds. The percentage of apoptotic cells was significantly lower in laminin-coated scaffolds compared to the un-coated scaffolds (20 ± 1.5% vs. 50 ± 4.5%, p < 0.05), and all of the other coated scaffolds (Fig. 3B). In addition, the apoptotic activity of cells on fibronectin-coated and collagen-coated scaffolds was lower than the un-coated group, suggesting that the surface treatment of the scaffold can preserve RAEC viability (p < 0.05). Although laminin-coated scaffolds improved the endothelial cell viability, the percentage of apoptotic cells remained fairly high, at 20% after 24 h.
To test whether toxic components were leaching from the PGS scaffolds and impairing RAEC viability, cell-free scaffolds were incubated in culture medium for 7 days at 37°C to produce scaffold-conditioned medium. RAECs were cultured in the conditioned medium for 5 days, and cell viability and growth were examined. There were no differences in cell morphology or cell number between RAEC cultured in control medium versus scaffold-conditioned medium, implying that no cytotoxic components leached from PGS scaffolds (data not shown). Thus, the observed high levels of TUNEL staining were more likely due to limitations of mass transfer inside the bulk of the scaffold, as opposed to toxicity of the scaffold material per se.
Effects of Perfusion Cell Seeding on RAEC Adhesion to PGS Scaffolds
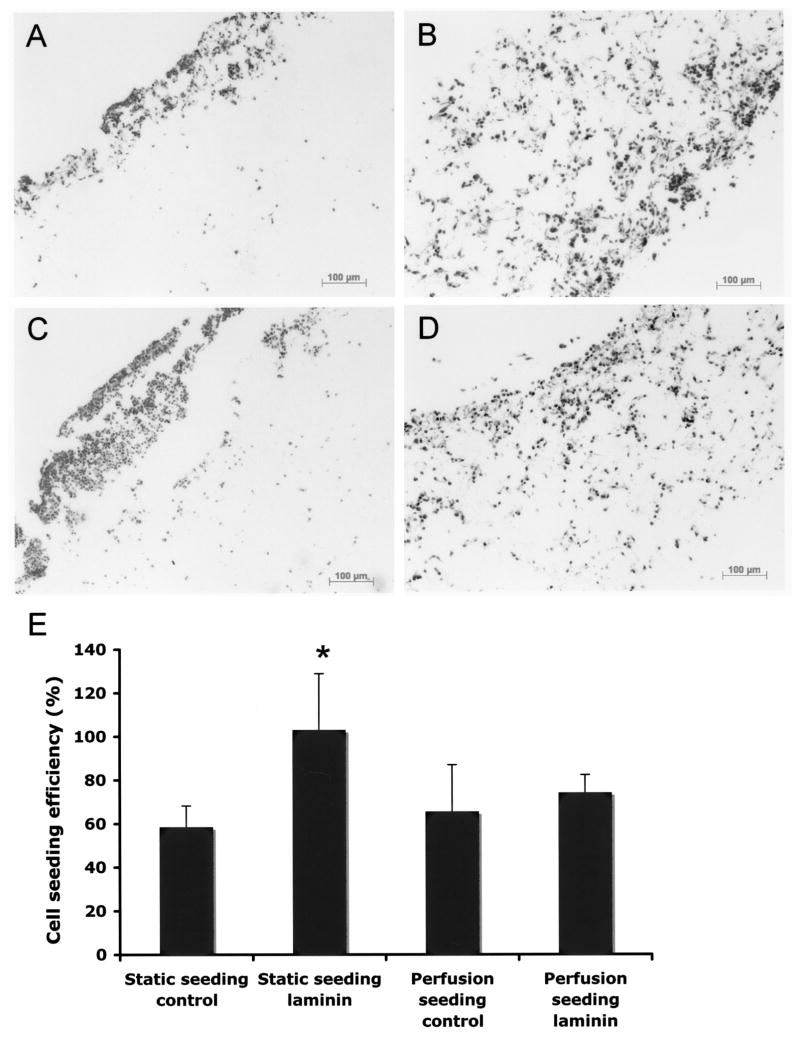
To compare the effects of static versus perfusion cell seeding, a perfusion bioreactor was used to allow medium flow through the scaffold (29). In contrast to the static seeding, where the majority of the cells were found on the outer surface of the uncoated scaffolds (Fig. 4A), much improved uniformity of the cells throughout the construct was observed after 2 h of perfusion seeding on uncoated scaffolds (Fig. 4B). Perfusion seeding improved the cell uniformity throughout the scaffold in the laminin-coated scaffolds as well, as shown with H&E staining (Fig. 4C, D). Cell seeding efficiency, calculated based upon DNA assay, revealed that laminin had a significant effect on adhered cell density under static conditions as described previously. However, there was no difference in cell seeding efficiency between laminin-coated and uncoated scaffolds with perfusion seeding, indicating that the impact of perfusion seeding was at least as great as the impact of surface coating of PGS scaffolds (p > 0.1) (Fig. 4E). In addition, the total number of cells was similar in either statically seeded or perfusion seeded for both uncoated and laminin-coated scaffolds, indicating that perfusion seeding resulted in a more spatially uniform distribution of cells within the scaffold rather than seeding a higher number of cells.
Figure 4.
Representative micrographs of H&E staining after 2 h of static versus perfusion seeding. Statically seeded uncoated and laminin-coated scaffolds are shown in (A) and (C), respectively. Perfusion seeded scaffold demonstrate uniform cell distribution throughout the thickness in both uncoated (B) and laminin-coated scaffolds (D).
Effects of Perfusion Seeding on RAEC Survival and Proliferation
PCNA and TUNEL stainings were performed to assess the number of proliferating and apoptotic cells following perfusion seeding. RAECs were perfusion seeded for 2 h, followed by 24 h of static culture, to match statically seeded scaffolds. Our results show that perfusion seeding enhanced RAEC proliferation and survival in the uncoated scaffolds (Fig. 5A). A nearly 10-fold increase in the number of proliferating cells from 1.9 ± 1.85% to 23.66 ± 3.87% (p < 0.05) was observed in perfusion-seeded uncoated scaffolds compared to statically seeded uncoated scaffolds. The percentage of proliferating cells tended to be higher in laminin-coated scaffolds with perfusion seeding compared to statically seeded scaffolds (31.55 ± 3.21% and 15.7 ± 6.92%, respectively), although the difference was not statistically significant (p > 0.1) (Fig. 5A). The percentage of apoptotic cells in uncoated and statically seeded scaffolds decreased significantly from 50.03 ± 4.51% to 35.31 ± 6.58% by perfusion seeding (p < 0.05) (Fig. 5B). The percentage of apoptotic cells in statically seeded laminin-coated scaffolds was not statistically different from the perfusion-seeded laminin-coated scaffolds (20.02 ± 4.51% to 28.42 ± 20.37%, respectively). Based on PCNA and TUNEL staining, no difference in cell proliferation and survival between laminin-coated and uncoated scaffolds with perfusion seeding was observed, similar to the cell seeding efficiency described above. This result again indicates that the perfusion seeding had a significant impact on uncoated scaffolds on cell proliferation and survival, and not on laminin-coated scaffolds. This in turn implies that cell viability and proliferation were enhanced by perfusion, likely due to the more uniform monolayer distribution of seeded cells, while laminin coating conferred survival even in statically seeded constructs where cells were piled on top of one another rather than in a monolayer.
Figure 5.
Impact of perfusion on RAEC proliferation and survival. (A) The percentage of proliferating RAEC on uncoated and laminin-coated scaffolds with static (white bars) or perfusion seeding (black bars). Increase in the number of proliferating cells was observed with the perfusion seeding on both uncoated and laminin-coated scaffolds after 24 h of culture (n = 3). (B) The percentage of apoptotic cells decreased significantly on uncoated scaffolds with perfusion seeding (n = 3), although no difference was observed with laminin-coated scaffolds.
Effects of Flow on RAEC Function and Phenotype
To characterize the impact of bulk flow and shear stress on RAEC function in PGS, samples were perfusion seeded for 2 h and then cultured under either low flow at 0.1 ml/min, or high flow at 1 ml/min, for 72 h. Static culture for 72 h served as a control.
In order to estimate shear stress within the scaffolds, Darcy’s permeability for PGS was first measured from Equation 1 under a range of pressures and flow rates. The measured values were fitted to obtain permeability at flow rates of 0.1 and 1 ml/min. The extrapolated permeability values for low flow (0.1 ml/min) and high flow (1 ml/min) were 1.9 × 10−8 and 3.9 × 10−8 cm2, respectively. The average shear stress for low flow (0.1 ml/min) and high flow (1 ml/min), calculated using the permeability values and Equation 2, resulted in a shear stress of 0.8 and 5.5 dynes/cm2, for low and high flows, respectively.
Figure 6 shows representative H&E micrographs of RAECs after 72 h in the absence of flow (Fig. 6A) or under low flow (Fig. 6B) or high flow (Fig. 6C). Cells remained well adhered and spread out in the PGS scaffolds under all three conditions, and cell density was not grossly affected by either high or low shear after 72 h.
Figure 6.
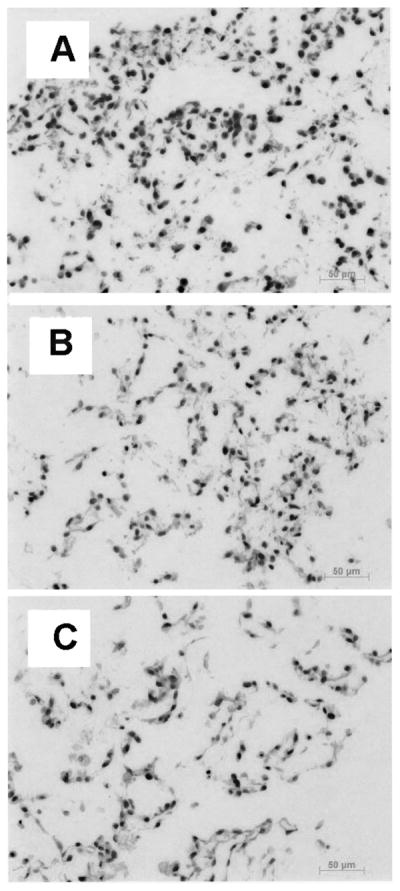
Representative micrographs of H&E staining after 72 h in the absence of flow (A), under low flow (B), or high flow (C). Cells remained well adhered and spread out in the PGS scaffolds even at the high flow rate.
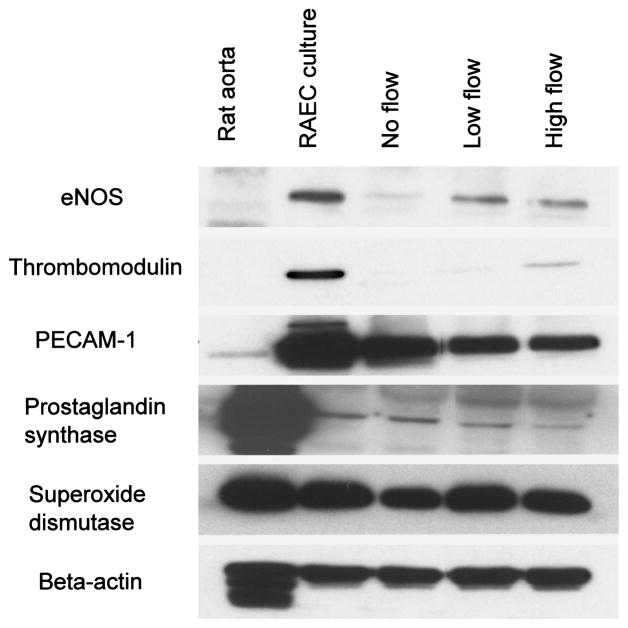
In order to assess the impact of shear on RAEC phenotype in the scaffolds, immunoblotting was performed. Western blot revealed that RAEC express endothelial nitric oxide synthase (eNOS), thrombomodulin, PECAM- 1, prostaglandin synthase, and superoxide dismutase under static culture conditions in flasks (Fig. 7). The expression of eNOS, which is important in vascular homeostasis, vessel remodeling, and angiogenesis, was upregulated by both low and high flow compared to no flow in the PGS scaffolds. This is consistent with the animal studies showing upregulation of eNOS expression by shear stress (24). In a quiescent state, eNOS is negatively regulated by several proteins (10), but when a stimulus such as shear stress is present, eNOS activity is enhanced. The expression of thrombomodulin, which is an antithrombogenic protein, also increased in a flow-dependent manner. This is also consistent with previous studies showing upregulation of thrombomodulin by fluid shear stress (36). However, no significant changes were observed in PECAM-1, prostaglandin I synthase, and superoxide dismutase expression with flow. In addition, these latter three molecules were not different in their expression between static flask culture and PGS culture, while eNOS and thrombomodulin both were decreased in RAEC on PGS scaffolds compared to flasks under static conditions.
Figure 7.
The effects of flow on RAEC protein expression after 72 h of culture. Western analysis revealed low levels of eNOS and thromobomodulin expression with no flow, and upregulation in response to both low and high flow. No change was observed in PECAM-1, prostacyclin, and superoxide dismutase expression with flow. β-Actin served as a loading control.
DISCUSSION
The objective of this study was to examine the biocompatibility of porous PGS scaffolds, and the function of RAEC as affected by various extracellular matrix proteins and by shear stress. By characterizing RAEC adhesion, survival, proliferation, and phenotype under both static and flow conditions, our study demonstrates that the RAEC-seeded 3D PGS scaffold provides a biocompatible and flexible endothelial cell delivery system for developing engineered tissues with neovascularization potential.
PGS scaffolds were initially coated with various extracellular matrices to determine biocompatibility and optimal endothelial cell-seeding conditions. Among several extracellular matrix proteins, laminin coating proved to be the most effective for promoting RAEC adhesion, survival, and proliferation. Previous studies have shown that coating surfaces with extracellular matrix components such as laminin, fibronectin, and gelatin can impact the interaction between a biomaterial and cells resulting in enhanced cell attachment, proliferation, differentiation, and migration (3,21,31). Barcells et al. showed improved bovine aortic endothelial cell adhesion on polystyrene discs with protein coating, and reported that the enhancement of cell adhesion was independent of the type of coating proteins (3). Sales et al. showed that precoating of PGS scaffold with various matrix proteins resulted in increased endothelial progenitor cell adhesion, growth, and phenotype (31) most effectively with fibronectin. Overall, our studies show that precoating with laminin resulted in the highest density of attached cells, and not only the highest fraction of proliferative cells but also lowest fraction of apoptotic cells compared to the uncoated scaffolds, suggesting that the laminin coating is the most effective among the other tested matrices. Precoating the scaffold with fibronectin and Matrigel also enhanced cell adhesion and proliferation compared to the uncoated control scaffold. While there was no significant difference in RAEC adhesion to collagen- coated scaffolds compared to the uncoated control scaffolds, collagen coating improved cell survival compared to the uncoated control.
It has been reported that endothelial cell proliferation and survival are influenced by interactions between integrins on the basal surface and the underlying extracellular matrix proteins (1,13,18). In particular, activation of the mitogen-activated protein kinase (MAPK) signal transduction pathway in endothelial cells is critical for proliferation and angiogenesis (32,39). However, the specific extracellular matrix component involved in supporting these processes is less understood. The results from our study suggest that higher survival and proliferation of RAECs observed with laminin and fibronectin coating may be due to interactions of integrin receptors such as α2β1 and α5β3 via the MAPK signaling pathway (27,40).
With the static seeding method, a majority of the cells were found on the outer surface within 100 μm from the edge, rather than in the center. Previously it has been shown that perfusion yields high seeded cardiac cell populations as well as improved viability of cardiac tissue constructs in long-term culture (29,30). Thus, we extended our study using a custom flow bioreactor for medium perfusion seeding. With the perfusion seeding, uniform cell distribution throughout the thickness of the scaffold was achieved and cells spread in a monolayer appearance, although the total number of seeded cells was similar to statically seeded constructs.
In contrast to the static seeding condition where precoating with laminin had a significant effect on cell density, the difference in the cell density between uncoated and laminin-coated scaffold became insignificant with the perfusion seeding. This suggests that the perfusion cell seeding minimized the effects of laminin coating in the adherence of RAEC to the PGA scaffolds. Endothelial cells are substrate dependent, and although optimal matrix stiffness varies among different cell types, cells on soft matrices are minimally adhesive and prone to apoptosis in general (14,44). In the static seeding condition, cells were mostly stacked on top of each other, and hence experiencing a softer substrate of cells underneath. In contrast, perfusion seeding resulted in a monolayer distribution of cells, wherein most cells were on a stiffer matrix (~60 KPa modulus), which endothelial cells prefer for survival. Therefore, it is possible that the monolayer distribution of endothelial cells caused by perfusion seeding promoted similar cell adhesion and survival to endothelial cells on the laminin-coated scaffold, where laminin provided antiapoptotic, integrin-mediated signals for endothelial cell survival. It is likely that the high levels of apoptosis that were observed in the static seeding experiment were due to the overdense seeding and nutrient limitations. A cell density of 100 × 106 cell/ml was chosen to determine the feasibility of high cell loading, and for subsequent cocultures with parenchymal cells, which require higher cell density (7, 28,29).
In vivo, endothelium is exposed to shear stress ranging from 1–20 dyn/cm2 (4,6) and expresses high levels of native anticoagulants such as thrombomodulin, and vasodilators such as prostacyclin (9) and nitric oxide synthase (eNOS) (17) and low levels of proinflammatory or prothrombogenic molecules. However, in static in vitro cultures, endothelial cell phenotype is altered, and cells become more proliferative and the endogenous anticoagulant properties of native endothelium are attenuated. Thrombomodulin, which is the most important endogenous anticoagulant in the pulmonary and cardiac vasculatures, is substantially down regulated in vitro.
Our study shows that the application of flow to the cells seeded onto 3D PGS scaffold can directly influence endothelial cell function, especially their anticoagulant phenotype. By applying flow to the RAEC-seeded PGS scaffold, we observed an upregulation of eNOS and thrombomodulin in response to shear stress compared to statically cultured RAEC. This increase in expression of eNOS and thrombomodulin that was mediated by flow was somewhat eclipsed by an overall decrease in expression of these molecules in cells seeded on PGS, compared to cells grown on tissue culture plastic. While the cause of the decrease in eNOS and thrombomodulin on PGS remains unclear, it is likely not due to toxicity, because other EC markers were preserved and toxicity studies of this material were negative. Hence, it may be the 3D topography of the PGS scaffold that induced changes in the expression of eNOS and thrombomodulin under static conditions. Further experiments are required to test this possibility.
The expression of PECAM-1, which is the endothelial adhesion protein, remained unchanged with flow, suggesting that the adhesion of endothelial cells in the scaffolds was not affected by the applied shear stress. This is consistent with previous reports wherein PECAM-1 remains relatively constant under a number of experimental conditions (45). In addition, the expression of prostaglandin synthase and superoxide dismutase remained unchanged with flow. This is at variance with some previous reports, which suggest an upregulation of prostacyclin (15,22) and superoxide dismutase (37) by shear stress. Because calculations of the applied shear stress were based upon simplifying assumptions, the true applied shear may be have been different inside the PGS scaffold, and the magnitude of shear may not have been sufficient to generate changes in expression of these latter molecules.
This study demonstrates the feasibility of using 3D biodegradable PGS scaffold for culturing RAECs. The PGS scaffold proved to be stable and nontoxic. A perfusion cell-seeding system not only allowed uniform cell distribution within the scaffolds, but also provided dynamic culture conditions wherein endothelial cell function in response to flow could be examined. Endothelial cell adhesion, proliferation, and survival were affected both by flow and by adhesion molecules. Application of physiological shear stress improved the anticoagulant phenotype of cultured RAEC without decreasing cell density, rendering them more suitable for eventual inclusion into microvascular structures. This 3D culture system is well suited for a wide range of vascular tissue-engineering applications, including cell delivery systems or cocultures with other cells. The porous PGS scaffolding therefore provides a tool for in vitro study of multicellular processes such as tissue vascularization.
Acknowledgments
We thank Dr. Jin Gao for processing the scaffolds, Robert Maidhof and Dr. Anna Marsano for their help with developing the perfusion seeding methods, and Sevanne Halajian for measuring the permeability of the scaffolds. Financial support from the National Institutes of Health (R01-HL076485) is gratefully acknowledged.
References
- 1.Albelda SM, Daise M, Levine EM, Buck CA. Identification and characterization of cell-substratum adhesion receptors on cultured human endothelial cells. J Clin Invest. 1989;83:1992–2002. doi: 10.1172/JCI114109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Au P, Tam J, Fukumura D, Jain RK. Bone marrow-derived mesenchymal stem cells facilitate engineering of long-lasting functional vasculature. Blood. 2008;111:4551–4558. doi: 10.1182/blood-2007-10-118273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balcells M, Edelman ER. Effect of pre-adsorbed proteins on attachment, proliferation, and function of endothelial cells. J Cell Physiol. 2002;191:155–161. doi: 10.1002/jcp.10087. [DOI] [PubMed] [Google Scholar]
- 4.Benson TJ, Nerem RM, Pedley TJ. Assessment of wall shear stress in arteries, applied to the coronary circulation. Cardiovasc Res. 1980;14:568–576. doi: 10.1093/cvr/14.10.568. [DOI] [PubMed] [Google Scholar]
- 5.Brinkman HC. Problems of fluid flow through swarms of particles and through macromolecules in solution. Research. 1949;2:190–194. [PubMed] [Google Scholar]
- 6.Dewey CF, Jr, Bussolari SR, Gimbrone MA, Jr, Davies PF. The dynamic response of vascular endothelial cells to fluid shear stress. J Biomech Eng. 1981;103:177–185. doi: 10.1115/1.3138276. [DOI] [PubMed] [Google Scholar]
- 7.Engelmayr GC, Jr, Cheng M, Bettinger CJ, Borenstein JT, Langer R, Freed LE. Accordion-like honeycombs for tissue engineering of cardiac anisotropy. Nat Mater. 2008;7:1003–1010. doi: 10.1038/nmat2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fidkowski C, Kaazempur-Mofrad MR, Borenstein J, Vacanti JP, Langer R, Wang Y. Endothelialized microvasculature based on a biodegradable elastomer. Tissue Eng. 2005;11:302–309. doi: 10.1089/ten.2005.11.302. [DOI] [PubMed] [Google Scholar]
- 9.Frangos JA, Eskin SG, McIntire LV, Ives CL. Flow effects on prostacyclin production by cultured human endothelial cells. Science. 1985;227:1477–1479. doi: 10.1126/science.3883488. [DOI] [PubMed] [Google Scholar]
- 10.Fulton D, Gratton JP, Sessa WC. Post-translational control of endothelial nitric oxide synthase: Why isn’t calcium/calmodulin enough? J Pharmacol Exp Ther. 2001;299:818–824. [PubMed] [Google Scholar]
- 11.Gao J, Crapo PM, Wang Y. Macroporous elastomeric scaffolds with extensive micropores for soft tissue engineering. Tissue Eng. 2006;12:917–925. doi: 10.1089/ten.2006.12.917. [DOI] [PubMed] [Google Scholar]
- 12.Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grant DS, Kleinman HK. Regulation of capillary formation by laminin and other components of the extracellular matrix. Exs. 1997;79:317–333. doi: 10.1007/978-3-0348-9006-9_13. [DOI] [PubMed] [Google Scholar]
- 14.Guo WH, Frey MT, Burnham NA, Wang YL. Substrate rigidity regulates the formation and maintenance of tissues. Biophys J. 2006;90:2213–2220. doi: 10.1529/biophysj.105.070144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hanada T, Hashimoto M, Nosaka S, Sasaki T, Nakayama K, Masumura S, Yamauchi M, Tamura K. Shear stress enhances prostacyclin release from endocardial endothelial cells. Life Sci. 2000;66:215–220. doi: 10.1016/s0024-3205(99)00583-4. [DOI] [PubMed] [Google Scholar]
- 16.Isenberg BC, Williams C, Tranquillo RT. Endothelialization and flow conditioning of fibrin-based media-equivalents. Ann Biomed Eng. 2006;34:971–985. doi: 10.1007/s10439-006-9101-0. [DOI] [PubMed] [Google Scholar]
- 17.Kuchan MJ, Frangos JA. Role of calcium and calmodulin in flow-induced nitric oxide production in endothelial cells. Am J Physiol. 1994;266:C628–636. doi: 10.1152/ajpcell.1994.266.3.C628. [DOI] [PubMed] [Google Scholar]
- 18.Languino LR, Gehlsen KR, Wayner E, Carter WG, Engvall E, Ruoslahti E. Endothelial cells use alpha 2 beta 1 integrin as a laminin receptor. J Cell Biol. 1989;109:2455–2462. doi: 10.1083/jcb.109.5.2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee E, Kim D, Azeloglu EU, Costa KD. Engineered cardiac organoid chambers: Toward a functional biological model ventricle. Tissue Eng. 2008;14:215–225. doi: 10.1089/tea.2007.0351. [DOI] [PubMed] [Google Scholar]
- 20.L’Heureux N, McAllister TN, de la Fuente LM. Tissue-engineered blood vessel for adult arterial revascularization. N Engl J Med. 2007;357:1451–1453. doi: 10.1056/NEJMc071536. [DOI] [PubMed] [Google Scholar]
- 21.Madri JA, Pratt BM, Yannariello-Brown J. Matrix-driven cell size change modulates aortic endothelial cell proliferation and sheet migration. Am J Pathol. 1988;132:18–27. [PMC free article] [PubMed] [Google Scholar]
- 22.McCormick SM, Whitson PA, Wu KK, McIntire LV. Shear stress differentially regulates PGHS-1 and PGHS-2 protein levels in human endothelial cells. Ann Biomed Eng. 2000;28:824–833. doi: 10.1114/1.1289472. [DOI] [PubMed] [Google Scholar]
- 23.Mol A, van Lieshout MI, Damde Veen CG, Neuenschwander S, Hoerstrup SP, Baaijens FP, Bouten CV. Fibrin as a cell carrier in cardiovascular tissue engineering applications. Biomaterials. 2005;26:3113–3121. doi: 10.1016/j.biomaterials.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 24.Nadaud S, Philippe M, Arnal JF, Michel JB, Soubrier F. Sustained increase in aortic endothelial nitric oxide synthase expression in vivo in a model of chronic high blood flow. Circ Res. 1996;79:857–863. doi: 10.1161/01.res.79.4.857. [DOI] [PubMed] [Google Scholar]
- 25.Niklason LE, Abbott W, Gao J, Klagges B, Hirschi KK, Ulubayram K, Conroy N, Jones R, Vasanawala A, Sanzgiri S, Langer R. Morphologic and mechanical characteristics of engineered bovine arteries. J Vasc Surg. 2001;33:628–638. doi: 10.1067/mva.2001.111747. [DOI] [PubMed] [Google Scholar]
- 26.Niklason LE, Gao J, Abbott WM, Hirschi KK, Houser S, Marini R, Langer R. Functional arteries grown in vitro. Science. 1999;284:489–493. doi: 10.1126/science.284.5413.489. [DOI] [PubMed] [Google Scholar]
- 27.Qin H, Ishiwata T, Wang R, Kudo M, Yokoyama M, Naito Z, Asano G. Effects of extracellular matrix on phenotype modulation and MAPK transduction of rat aortic smooth muscle cells in vitro. Exp Mol Pathol. 2000;69:79–90. doi: 10.1006/exmp.2000.2321. [DOI] [PubMed] [Google Scholar]
- 28.Radisic M, Euloth M, Yang L, Langer R, Freed LE, Vunjak-Novakovic G. High-density seeding of myocyte cells for cardiac tissue engineering. Biotechnol Bioeng. 2003;82:403–414. doi: 10.1002/bit.10594. [DOI] [PubMed] [Google Scholar]
- 29.Radisic M, Marsano A, Maidhof R, Wang Y, Vunjak-Novakovic G. Cardiac tissue engineering using perfusion bioreactor systems. Nat Protoc. 2008;3:719–738. doi: 10.1038/nprot.2008.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Radisic M, Park H, Chen F, Salazar-Lazzaro JE, Wang Y, Dennis R, Langer R, Freed LE, Vunjak-Novakovic G. Biomimetic approach to cardiac tissue engineering: Oxygen carriers and channeled scaffolds. Tissue Eng. 2006;12:2077–2091. doi: 10.1089/ten.2006.12.2077. [DOI] [PubMed] [Google Scholar]
- 31.Sales VL, Engelmayr GC, Jr, Johnson JA, Jr, Gao J, Wang Y, Sacks MS, Mayer JE., Jr Protein precoating of elastomeric tissue-engineering scaffolds increased cellularity, enhanced extracellular matrix protein production, and differentially regulated the phenotypes of circulating endothelial progenitor cells. Circulation. 2007;116:I55–63. doi: 10.1161/CIRCULATIONAHA.106.6806637. [DOI] [PubMed] [Google Scholar]
- 32.Seger R, Krebs EG. The MAPK signaling cascade. FASEB J. 1995;9:726–735. [PubMed] [Google Scholar]
- 33.Sekine H, Shimizu T, Hobo K, Sekiya S, Yang J, Yamato M, Kurosawa H, Kobayashi E, Okano T. Endothelial cell coculture within tissue-engineered cardiomyocyte sheets enhances neovascularization and improves cardiac function of ischemic hearts. Circulation. 2008;118:S145–152. doi: 10.1161/CIRCULATIONAHA.107.757286. [DOI] [PubMed] [Google Scholar]
- 34.Singer VL, Jones LJ, Yue ST, Haugland RP. Characterization of PicoGreen reagent and development of a fluorescence-based solution assay for double-stranded DNA quantitation. Anal Biochem. 1997;249:228–238. doi: 10.1006/abio.1997.2177. [DOI] [PubMed] [Google Scholar]
- 35.Sundback CA, Shyu JY, Wang Y, Faquin WC, Langer RS, Vacanti JP, Hadlock TA. Biocompatibility analysis of poly(glycerol sebacate) as a nerve guide material. Biomaterials. 2005;26:5454–5464. doi: 10.1016/j.biomaterials.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 36.Takada Y, Shinkai F, Kondo S, Yamamoto S, Tsuboi H, Korenaga R, Ando J. Fluid shear stress increases the expression of thrombomodulin by cultured human endothelial cells. Biochem Biophys Res Commun. 1994;205:1345–1352. doi: 10.1006/bbrc.1994.2813. [DOI] [PubMed] [Google Scholar]
- 37.Topper JN, Cai J, Falb D, Gimbrone MA., Jr Identification of vascular endothelial genes differentially responsive to fluid mechanical stimuli: Cyclooxygenase-2, manganese superoxide dismutase, and endothelial cell nitric oxide synthase are selectively up-regulated by steady laminar shear stress. Proc Natl Acad Sci USA. 1996;93:10417–10422. doi: 10.1073/pnas.93.19.10417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tremblay PL, Hudon V, Berthod F, Germain L, Auger FA. Inosculation of tissue-engineered capillaries with the host’s vasculature in a reconstructed skin transplanted on mice. Am J Transplant. 2005;5:1002–1010. doi: 10.1111/j.1600-6143.2005.00790.x. [DOI] [PubMed] [Google Scholar]
- 39.Vinals F, Pouyssegur J. Confluence of vascular endothelial cells induces cell cycle exit by inhibiting p42/p44 mitogen-activated protein kinase activity. Mol Cell Biol. 1999;19:2763–2772. doi: 10.1128/mcb.19.4.2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang J, Milner R. Fibronectin promotes brain capillary endothelial cell survival and proliferation through alpha5-beta1 and alphavbeta3 integrins via MAP kinase signalling. J Neurochem. 2006;96:148–159. doi: 10.1111/j.1471-4159.2005.03521.x. [DOI] [PubMed] [Google Scholar]
- 41.Wang Y, Ameer GA, Sheppard BJ, Langer R. A tough biodegradable elastomer. Nat Biotechnol. 2002;20:602–606. doi: 10.1038/nbt0602-602. [DOI] [PubMed] [Google Scholar]
- 42.Waseem NH, Lane DP. Monoclonal antibody analysis of the proliferating cell nuclear antigen (PCNA). Structural conservation and the detection of a nucleolar form. J Cell Sci. 1990;96(Pt 1):121–129. doi: 10.1242/jcs.96.1.121. [DOI] [PubMed] [Google Scholar]
- 43.Weinberg CB, Bell E. A blood vessel model constructed from collagen and cultured vascular cells. Science. 1986;231:397–400. doi: 10.1126/science.2934816. [DOI] [PubMed] [Google Scholar]
- 44.Wells RG. The role of matrix stiffness in regulating cell behavior. Hepatology. 2008;47:1394–1400. doi: 10.1002/hep.22193. [DOI] [PubMed] [Google Scholar]
- 45.Wong D, Dorovini-Zis K. Platelet/endothelial cell adhesion molecule-1 (PECAM-1) expression by human brain microvessel endothelial cells in primary culture. Brain Res. 1996;731:217–220. doi: 10.1016/0006-8993(96)00673-7. [DOI] [PubMed] [Google Scholar]
- 46.Zimmermann WH, Melnychenko I, Wasmeier G, Didie M, Naito H, Nixdorff U, Hess A, Budinsky L, Brune K, Michaelis B, Dhein S, Schwoerer A, Ehmke H, Eschenhagen T. Engineered heart tissue grafts improve systolic and diastolic function in infarcted rat hearts. Nat Med. 2006;12:452–458. doi: 10.1038/nm1394. [DOI] [PubMed] [Google Scholar]