Abstract
Objective
Evidence suggests that chronic high levels of behavioral inhibition are a precursor of social anxiety disorder (SAD). This study identified the early risk factors for and developmental pathways to chronic high inhibition among school-age children and its association with SAD by adolescence.
Method
A community sample of 238 children was followed from birth to Grade 9. Mothers, children, and teachers reported on children's behavioral inhibition from Grades 1 to 9. Lifetime history of psychiatric disorders was available for the subset of 60 (25%) children who participated in an intensive laboratory assessment at Grade 9. Four early risk factors were assessed: female gender; exposure to maternal stress during the infancy and preschool periods and at child age 4.5 years; early manifestation of behavioral inhibition, and elevated afternoon salivary cortisol levels.
Results
All four risk factors predicted higher and more chronic inhibition from Grade 1 to Grade 9, and together, defined two developmental pathways. The first pathway in female children was partially mediated by early evidence of behavioral inhibition and elevated cortisol levels at age 4.5 years. The second pathway began with exposure to early maternal stress and was also partially mediated by childhood cortisol levels. By Grade 9, chronic high inhibition was associated with a lifetime history of SAD.
Conclusions
Chronic high levels of behavioral inhibition are associated with SAD by adolescence. The identification of two developmental pathways suggests the potential importance of considering both sets of risk factors in developing preventive interventions for SAD.
Introduction
Behavioral inhibition, an early temperamental characteristic, is a precursor of anxiety disorders, especially social anxiety disorder (SAD) (1-3). In younger children, inhibition is characterized by an increased fear response to both non-social and social novelty; by school-age, it is characterized primarily by social reticence (4). Importantly, children who are chronically inhibited are at greatest risk for the development of anxiety disorders (3, 5). Further, extreme school-age inhibition shares much in common with SAD (6), including fear of meeting/interacting with unfamiliar peers and adults; and recent evidence suggests that it is chronic inhibition that for some individuals evolves into SAD (7).
Considerable research has focused on the chronicity of childhood inhibition. Studies have shown that extreme childhood inhibition is moderately enduring and heritable (4, 8, 9), suggesting that it may represent a marker of genetically-transmitted susceptibility to later chronic inhibition and SAD (10). However, there is also considerable evidence of instability (11), especially in unselected -- as opposed to extreme -- samples of children who are studied over longer intervals that encompass multiple developmental periods (12). Some studies have also found greater chronicity among girls than boys (2, 5, 9, 12), although the findings are very inconsistent (13). Previous findings also suggest that factors other than early inhibition play important roles in the development of chronic inhibition and SAD (14).
One candidate biological substrate is increased activity of the limbic system, which is associated with the generation of fear (4). Imaging studies in monkeys and humans suggest that individual differences in limbic system activity (amygdala and bed nucleus of the stria terminalis) are associated with temperamental inhibition and anxiety-related behaviors (15, 16). Further, inhibition is linked with physiological reactivity across systems regulated by the central nucleus of the amygdala, including the neuroendocrine response system (17). Of particular interest are findings suggesting that increased pituitary-adrenal activity, assessed by salivary cortisol levels, is associated with extreme childhood inhibition (4, 18), although increased cortisol has also been linked with other temperamental styles (19, 20). Further, in the same sample of children as reported on here, our group previously showed that preschoolers with increased afternoon cortisol levels, especially girls, showed higher levels of later inhibition during the transition to primary school (21). However, the role of cortisol in the development of chronic school-age inhibition has not been investigated.
Early environmental factors also play a role in the development of inhibition. Monkeys exposed to early environmental perturbations and negative maternal behaviors tend to develop increased HPA reactivity and stable fear-related behaviors (22, 23). Studies of young children also suggest that maternal behaviors, including being overly solicitous as well as negative behaviors (e.g., low engagement, hostility) associated with maternal depression/distress, might influence the development of increased cortisol levels (24, 25) and early inhibition (26, 27). However, very little is known about the effects on childhood cortisol levels and inhibition of a broader array of family adversities that may create stressful caretaking environments. In the same sample of children as reported on here, our group previously showed that exposure to early maternal stress, assessed across multiple domains, prospectively predicted increased cortisol levels when the children were age 4.5 years (28). However, a cross-sectional study (29) showed no association of psychosocial adversity with inhibition among young children. To our knowledge, there are no prospective studies of such family-wide stressors on the development of chronic school-age inhibition.
This study aimed to identify the early risk factors for, and longitudinal pathways to, chronic high inhibition across Grades 1 to 9, i.e., ages 7 to 15. We also estimated the association of chronic inhibition with lifetime history of SAD for a subset of children at Grade 9. Four potential early risk factors were considered: child gender, exposure to maternal stress during the infancy and preschool periods, and at child age 4.5 years, observations of behavioral inhibition earlier in life, and afternoon cortisol levels. We hypothesized that all four early factors would be associated with the development of chronic high school-age inhibition; that early evidence of behavioral inhibition and cortisol levels would play mediating roles in defining developmental pathways; and that chronic high inhibition would be associated with SAD by adolescence.
Method
Participants
The data were drawn from a larger longitudinal study, the Wisconsin Study of Families and Work (WSFW) (30). A sample of 570 women over the age of 18 years and living with the baby's biological father were recruited from prenatal clinics in two Midwestern cities during the second trimester of pregnancy. Follow-up assessments were conducted in infancy (1, 4 and 12 months), preschool (age 3.5 and 4.5 years), and at Grades 1, 3, 5, 7 and 9; teachers participated beginning at Grade 1. At Grade 1, 468 (82%) of the families remained in the study. However, because of budgetary constraints, the full Grade 1 assessment (which required home visits) was conducted only on one-half of the first cohort (N = 100) and the complete second cohort (N = 197) of families living within a 4-hour driving distance of the project offices. Of these 297 families and teachers who participated in the full Grade 1 assessment, the present study included the 238 who had complete multi-informant inhibition data through Grade 9 (missing data primarily due to home-schooling and school/teacher refusal). The study was approved by the Institutional Review Board of the University of Wisconsin-Madison. Adult informed consent was obtained at each assessment; beginning at Grade 5, children provided informed assent.
The sample of 238 children consisted of 111 boys and 127 girls, 11% ethnic minorities. At recruitment (1990 – 1991), the mothers ranged in age from 20 to 41 years, median 30; 96% were married. One percent of the mothers had less than a high school degree, 40% were high school graduates, 38% were college graduates, and 21% had education beyond college; educational level of fathers was similar. Annual family income ranged from $7,500 to over $200,000, median $49,000, comparable to the average family income in Wisconsin (31). There were no demographic differences between the 238 participating families and the remainder of the original 570 families, with two exceptions: participating mothers were one year older (M = 29.97 years, SD = 4.20) than non-participating mothers (M = 28.94 years, SD = 4.43), t(568) = -2.80, p = .005; and participating families had higher annual incomes (M = $52,232, SD = $22,797) than non-participating families (M = $48,106, SD = $23,853), F(1,564) = 4.27, p = .039.
Measures
Maternal Stress was assessed in the infancy and preschool periods with maternal reports of five domains: 1) Maternal depression was assessed by the Center for Epidemiological Studies-Depression scale (CES-D) (32); alpha coefficients exceeded .85 for all assessments. 2) Family expressed anger was assessed with the average of three items tapping marital conflict and scores for Anger Expression and the Negative subscale of the Family Expressiveness Questionnaire (33-35); all alphas exceeded .70. 3) Parenting stress was calculated from scores on the Competence and Child Reinforces Parent subscales of the Parenting Stress Inventory (PSI), and the average of three negative items (e.g., “I often feel angry with my child”) from the Childrearing Practices Report (CRPR) (34, 36, 37); all alphas exceeded .65. 4) Role overload was calculated from the Role Restriction subscale of the PSI, and the average of five items from the Role Overload scale (e.g., feeling “pulled apart by conflicting obligations”) (34, 36, 37); all alphas exceeded .75. 5) Financial stress was the average of four items (e.g., “Difficulty making monthly payments”); all alphas exceeded .70. Separately for the infancy and preschool periods, composites were constructed using principal components analysis (PCA), where the first component accounted for at least 50% of the variance and all factor loadings exceeded .50. The two resulting composites were highly correlated (r = .73) and thus, were averaged.
Children's Behavioral Inhibition at age 4.5 years was assessed from videotaped observations of a two-hour home visit conducted by two research assistants. The visit included entrance to the home, saliva collection, cognitive testing, and a series of emotion-eliciting tasks (38), all used to measure the child's reactions to unfamiliar people, objects, and test procedures. After the visit, the research assistants independently reviewed the videotape once for the entire assessment and rated the children on four 5-point items assessing global shyness, initiative with tasks, exploration of unfamiliar objects, and social engagement with the research assistants (all kappas ≥ .80). Each item was averaged across the two research assistants, and the four averaged items were combined into a single composite score using PCA (65% of the variance accounted for; all factor loadings > .71).
Children's Salivary Cortisol Levels at age 4.5 years were assessed with saliva samples (passive drool) collected by parents on three consecutive afternoons. Eighty-six percent of the samples were collected in the target period between 3:00 PM and 7:00 PM, with the remainder collected earlier in the afternoon. Cortisol was assessed with the Pantex RIA (Santa Monica, CA) modified for saliva. The detection limit of the assay (ED80) was .03 ug/dl. The mean inter-assay and intra-assay variation was 7.4% and 3.8%, respectively. Based on previous analyses (21, 28) showing that only antibiotics and cold/allergy medications affected cortisol values in this sample, medication use was coded ‘1’ if the child was currently on these medications, ‘0’ otherwise. To control for time of collection and medication use, the log-transformed cortisol values were residualized for hour of collection and medications.
Children's School-Age Inhibition was assessed from interviews with mothers, teachers, and children during the spring of Grades 1, 3, 5, 7, and 9. Adults completed the MacArthur Health and Behavior Questionnaire (HBQ) (39) Social Inhibition scale (5 items, e.g., “shy asking peers to play”, “shy with unfamiliar adults”); all alpha coefficients > .74. At Grade 1, children were asked a parallel set of items from the Berkeley Puppet Interview Social Scales (BPI-Social) (40) (alpha = .67); at subsequent grades, the BPI was modified to an age-appropriate questionnaire format (all alpha coefficients > .71). Multi-informant scores were computed at each assessment using a PCA-based approach (41) in which the first component measures the core characteristic of interest (here, Inhibition), defined as what the three reports shared in common (i.e., all positive loadings greater than .50), and the second and third components distinguish the sources of error that result from reporters' differing perspectives (i.e., child self-view versus adult view) and contexts (i.e., home versus school). To construct a longitudinal inhibition score reflecting both level and chronicity, the first PCA component scores were used to categorize children at each assessment as being in the lower 25% (score = 1), middle 50% (score = 2), or upper 25% (score = 3) of inhibition; these scores were summed over the five assessments, resulting in a longitudinal score ranging from 5 (lower 25% at all times) to 15 (upper 25% at all times).
Lifetime DSM-IV Diagnoses were obtained with the Kiddie-Schedule for Affective Disorders-Present Lifetime (K-SADS-PL) (42) for the subset (n = 60; 25% of 238) of the children who participated in an intensive laboratory assessment at Grade 9. Recruitment was based on living within close proximity to the laboratory and reporting no exclusionary criteria for functional magnetic resonance imaging (e.g., metal orthodontics); there was no significant difference on the longitudinal inhibition score between these children (M = 10.30, SD = 2.95) and the remaining 178 children (M = 9.87, SD = 2.63), t(236) = -1.06, p = .29. A board-certified child psychiatrist (MJS) with expertise in the K-SADS trained and supervised a child psychiatrist and a clinical child psychologist in its administration. Mothers were interviewed first and children second, with additional interviewing conducted when they disagreed about diagnostic criteria. All K-SADS interviews were reviewed by MJS and the administering clinician, and disagreements were resolved by consensus.
Data Analysis
Data analyses addressed two questions. First, Spearman correlations identified the early risk factors for chronic high inhibition; and a hierarchical multiple regression analysis defined the longitudinal linkages among the risk factors. Child gender and exposure to early maternal stress were included in the first step of the regression analysis. Next, to identify possible mediators and moderators, childhood behavioral inhibition and afternoon cortisol levels at age 4.5 years were entered, together with the four pair-wise interactions of child gender and exposure to maternal stress with each of the two 4.5-year variables; no significant interactions were found. A dichotomous variable was also included to assess any effect of psychotropic medication use at Grade 9 (n = 14; 6%); no significant effect was found. Second, among the subset of children with lifetime psychiatric data, a χ2 analysis ascertained differences in DSM-IV diagnoses according to level and chronicity of school-age inhibition; and t-tests examined differences in risk factors and school-age inhibition for children with and without a lifetime history of SAD.
Results
Descriptive Statistics
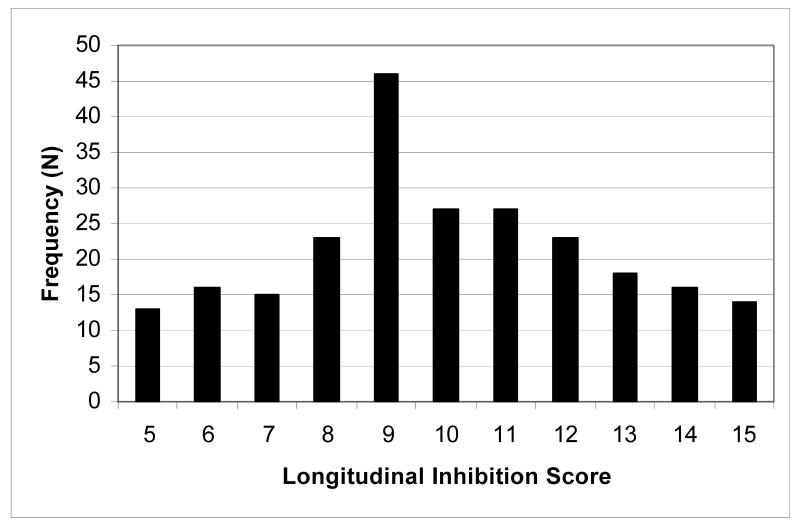
The distribution of the longitudinal inhibition score is shown in Figure 1 (M = 9.98, SD = 2.71). For the χ2 analysis only, these scores were collapsed to form five groups, where chronic inhibition was defined as evidencing the same level (i.e., low, middle, high) for at least four of the five assessments, and never evidencing a non-adjacent level. By this definition, 55% of the children evidenced chronic inhibition, including all children in the “Chronic High” (scores of 15 or 14) and “Chronic Low” (scores of 5 or 6) groups, and three-fourths of those in the “Middle” (scores of 9, 10, or 11) group. None of the children in the “Low-Middle” (scores of 7 or 8) or “Middle-High” (scores of 12 or 13) groups evidenced chronicity.
Figure 1.
Frequency Distribution of Longitudinal Inhibition Score. Scores are summed across the five assessments, where 1 = lower 25%, 2 = middle 50%, and 3 = upper 25% of the distribution of inhibition. N = 238.
Identification of Early Risk Factors and Pathways to Chronic High Inhibition
All four early factors were significantly correlated with the longitudinal inhibition score (Table 1; see supplemental on-line material for correlations with inhibition at each grade). As hypothesized, chronic high inhibition was associated with being female, exposure to higher levels of early maternal stress, and at age 4.5 years, higher levels of behavioral inhibition and afternoon cortisol. Further, each pair of risk factors within a time period (i.e., child gender and exposure to maternal stress beginning at birth, and behavioral inhibition and cortisol levels at age 4.5 years) were uncorrelated; however, there were significant correlations between the earlier and age 4.5 year risk factors, possibly reflecting mediational processes.
Table 1.
Spearman Correlations Among Risk Factors and Longitudinal Inhibition Score
| Exposure to maternal stress, birth to age 4.5 years | Early behavioral inhibition, age 4.5 years | Afternoon basal cortisol levels, age 4.5 years | Longitudinal inhibition score, Grades 1, 3, 5, 7, 9 | |
|---|---|---|---|---|
| Child gender (0 = boys; 1 = girls) | .017 | .127 * | .139 * | .265 ** |
| Exposure to maternal stress, birth to age 4.5 years | -- | -.084 | .137 * | .225 ** |
| Early behavioral inhibition, age 4.5 years | -- | .041 | .224 ** | |
| Afternoon basal cortisol levels, age 4.5 years | -- | .209 ** |
N = 238
p < .05;
p < .01
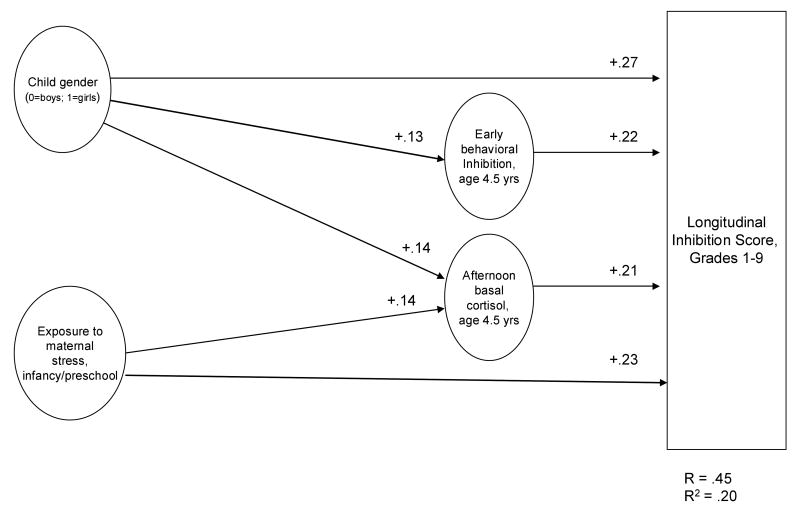
The hierarchical multiple regression analysis identified two mediational chains (Figure 2). The first chain began with the association of child gender with longitudinal inhibition (β = 1.34, p < .001), which at a trend level (Sobel's test, p = .069) was partially mediated (i.e., the effect was reduced but remained significant; β = 1.13, p = .001) by early behavioral inhibition and cortisol levels at age 4.5 years. The second chain began with exposure to early maternal stress (β = .64, p = .001), which was partially mediated (β = .61, p = .001; Sobel's test, p = .048) by children's cortisol levels at age 4.5 years.
Figure 2.
Longitudinal Model of Inhibition, Grade 1 to Grade 9. Coefficients are bivariate Spearman correlations (see Table 1). All correlations are significant, p < .05. N = 238.
Association of Chronic High Inhibition with Social Anxiety Disorder
Among the 60 children with available lifetime DSM-IV data, there were significant differences in diagnostic status of the five longitudinal inhibition chronicity groups, χ2(16, 60) = 31.36, p = .01 (Table 2). Whereas 80% of children in the “Chronic High” group received at least one lifetime psychiatric diagnosis, all in the internalizing (anxiety and/or mood) domain, the percentage decreased across the groups to only 20% in the “Chronic Low” group, all in the externalizing domain. Most importantly, 50% of children in the “Chronic High” group received a lifetime SAD diagnosis compared with 27% in the “Middle-High” group, 5% in the “Middle” group, and none in the “Low-Middle” and “Chronic Low” groups. Further, among the three higher chronicity groups, there were few differences in other anxiety (predominantly Specific Phobias) or mood disorders.
Table 2.
Lifetime Diagnoses by Longitudinal Inhibition Groupsa for Subset of Participants
| DSM-IV Lifetime Diagnosis | Chronic Low n (%) |
Low-Middle n (%) |
Middle n (%) |
Middle-High n (%) |
Chronic High n (%) |
|---|---|---|---|---|---|
| Social Anxiety Disorder (SAD) | 0 | 0 | 1 (5) | 4 (27) | 5 (50) |
| Other Anxiety Disorder w/o SADb | 0 | 2 (40) | 1 (5) | 3 (20) | 2 (20) |
| Mood Disorder w/o any Anxiety Disorder | 0 | 0 | 2 (10) | 1 (7) | 1 (10) |
| Externalizing Disorder Only | 2 (20) | 0 | 5 (25) | 0 | 0 |
| No Lifetime Diagnosis | 8 (80) | 3 (60) | 11 (55) | 7 (47) | 2 (20) |
| TOTAL | 10 (100) | 5 (100) | 20 (100) | 15 (100) | 10 (100) |
Longitudinal inhibition scores defining groups: Chronic Low = 5, 6; Low-Middle = 7, 8; Middle = 9, 10, 11; Middle-High = 12, 13; Chronic High = 14, 15.
Low-Middle = 1 subject with Specific Phobia, 1 with GAD; Middle = 1 with PTSD; Middle-High = 3 with Specific Phobia; Chronic High = 1 with GAD, 1 with PTSD.
χ2 (16, 60) = 31.36, p = .01.
Because of the overlapping time interval over which inhibition (and risk factor) ratings and lifetime SAD diagnoses were obtained, additional analyses were conducted to substantiate the prospective nature of this association. Comparisons of adolescents with and without a lifetime SAD diagnosis showed that there were no significant group differences for the early risk factors or Grade 1 inhibition, but beginning at Grade 3, SAD-diagnosed adolescents had significantly higher inhibition scores (Table 3). This pattern corresponds closely with the interview descriptions of the SAD-diagnosed adolescents as being “always” shy since very early childhood, with the defining clinical features of significant distress and impairment manifesting later in elementary or middle school when the demands of social relationships, including performance situations, increased.
Table 3.
Comparisons of Adolescents With and Without A Lifetime Diagnosis of Social Anxiety Disorder
| Social Anxiety Disorder Diagnosis | Statistics | ||
|---|---|---|---|
| Positive (n=10)a Mean (SD) |
Negative (n=50) Mean (SD) |
t (p) | |
| Exposure to maternal stress, birth to age 4.5 years | 0.628 (0.825) | 0.011 (1.019) | -1.798 (.08) |
| Afternoon basal cortisol levels, age 4.5 years | -0.116 (0.144) | 0.031 (0.359) | 1.269 (.21) |
| Early behavioral inhibition, age 4.5 years | 0.012 (1.130) | -0.050 (1.081) | -0.163 (.88) |
| Inhibition: b | |||
| Grade 1 | 0.613 (1.135) | -0.040 (1.123) | -1.675 (.10) |
| Grade 3 | 1.371 (1.309) | -0.115 (1.010) | -4.029 (<.01) |
| Grade 5 | 1.334 (0.887) | -0.115 (0.974) | -4.131 (<.01) |
| Grade 7 | 1.183 (0.811) | -0.101 (0.955) | -3.964 (<.01) |
| Grade 9 | 1.135 (0.756) | -0.148 (0.934) | -4.068 (<.01) |
n = 8 current/ongoing; n = 2 past only (1 manifest Grades 6 to 8; 1 manifest Grades 3 to 7).
First PCA component scores (see Measures).
Discussion
This study aimed to identify the early risk factors for, and developmental pathways to, chronic high levels of school-age inhibition and its association with SAD in adolescence. As hypothesized, all four early factors significantly predicted chronic high inhibition. Although behavioral inhibition and cortisol levels at 4.5 years were unrelated, each played a mediating role in pathways to later chronic high levels of inhibition. Further, chronic high levels of inhibition were significantly associated with a lifetime history of SAD by Grade 9.
The results extend those of previous studies of the chronicity of childhood inhibition, focusing on early behavioral inhibition as a risk factor for the development of chronic high school-age inhibition and its association with SAD by adolescence. Although the correlation of early behavioral inhibition with later chronic high inhibition (.224) was lower than two-point stability estimates found in some studies of children selected for extreme inhibition (43), it was consistent with previous studies of unselected children assessed over longer intervals and when measures of inhibition differ across developmental periods (i.e., observations of early inhibition and informant reports of school-age inhibition) (12). Importantly, the specific association of chronic high levels of school-age inhibition with SAD by Grade 9 was consistent with previous findings showing that it is the chronicity of high childhood inhibition that is most likely to evolve into SAD (7).
Two developmental pathways were identified. The “gender pathway” suggests that girls are at greater risk for developing chronic high inhibition, in part, because they evidence greater inhibition and afternoon cortisol levels as preschoolers. It has been suggested that these gender differences may reflect differences in cultural expectations and socialization patterns such that some degree of inhibition and increased stress responsivity is considered “gender appropriate” in girls whereas in boys, it is less likely to be reinforced and may even be discouraged (12). However, it should be noted that these mediating effects were only statistically marginal, and previous findings of the association of gender with chronic inhibition are very inconsistent (2, 5, 12, 13).
The “stress pathway” highlighted that children exposed to greater maternal stress beginning at birth are at greater risk for developing chronic high inhibition, in part, because they evidence increased afternoon cortisol levels as preschoolers. Our group previously reported that both early stress exposure and preschool cortisol levels predicted children's overall mental health symptoms in Grade 1 (28). Further, previous research has established the associations of numerous interrelated components of maternal stress, including those assessed here, with children's internalizing and externalizing problems (44-46). Thus, this may be a pathway to more generalized mental health problems and not as specific to chronic high inhibition as the pathway including early behavioral inhibition.
There are several limitations to this study. First, the sample was relatively homogenous, reflecting primarily Caucasian, middle-class families living in the Midwest. Second, we did not have diagnostic information for the full sample of children. Third, the longitudinal model explained one-fifth of the variance in chronic high inhibition, suggesting that there are other important risk factors that we did not assess, including family psychiatric history, child cognitive factors, other child affective and regulatory traits, specific parenting behaviors, and non-shared environmental factors (e.g., adverse life events) (47). Finally, because we defined chronic high inhibition by the number of assessments at which children were in the upper 25%, we did not take advantage of analytic techniques that would allow for missing data longitudinally as well as examination of other patterns of change in inhibition over time.
Despite these limitations, the findings highlight early risk factors comprising two developmental pathways to chronic high school-age inhibition and the specific association with a lifetime history of SAD by adolescence. The results suggest that a trajectory of high inhibition may begin with the difficulties experienced by girls and/or children exposed to early maternal stress who are more likely to be temperamentally inhibited and/or to evidence increased cortisol levels as they face the new social challenges associated with the transition to school and the progressive shift from family to peer relationships. Across the school years, the increasingly complex demands of social relationships may contribute to heightened levels of distress and functional impairment, demarcating the shift from chronic high inhibition to the clinically significant symptoms of SAD. If confirmed, such a developmental model has implications for both prevention and intervention strategies aimed at reducing the risk of SAD. Prevention strategies prior to the school transition might target the modifiable risk factor of maternal stress; and intervention strategies after the school transition might target social competencies of extremely inhibited children to reduce the risk of chronic high levels of inhibition evolving into SAD.
Supplementary Material
Table 1a. Spearman Correlations of Early Risk Factors with School-Age Inhibition Scores at Each Assessment
Acknowledgments
Funding was provided by National Institute of Mental Health grants RO1-MH44340, P50-MH52354, and P50-MH69315; the John D. and Catherine T. MacArthur Foundation Research Network on Psychopathology and Development (David J. Kupfer, Chair); and the University of Wisconsin HealthEmotions Research Institute.
Footnotes
Drs. Essex, Klein, Slattery and Goldsmith report no competing interests. Dr. Kalin is on the Advisory Boards or serves as Consultant to AstraZeneca, Bristol-Myers-Squibb, CeNeRx Biopharma, Corcept Therap, Cyberonics, Elsevier, Eli Lilly and Company, Forest Laboratories, General Electric Corp (GE Healthcare), GlaxoSmithKline, Jazz Pharmaceutical, Neuronetics, Novartis, Otsuka American Pharmaceuticals, Takeda International, and Wyeth Pharmaceutical. He is founder and owner of Promoter Neurosciences, LLC and owns stock or equity options in Corcept and CeNeRx.
References
- 1.Hirshfeld-Becker DR, Biederman J, Henin A, Faraone SV, Davis S, Harrington K, Rosenbaum JF. Behavioral inhibition in preschool children at risk is a specific predictor of middle childhood social anxiety: A five-year follow-up. Journal of Developmental and Behavioral Pediatrics. 2007;28:225–233. doi: 10.1097/01.DBP.0000268559.34463.d0. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz CE, Snidman N, Kagan J. Adolescent social anxiety as an outcome of inhibited temperament in childhood. Journal of the American Academy of Child & Adolescent Psychiatry. 1999;38(8):1008–1015. doi: 10.1097/00004583-199908000-00017. [DOI] [PubMed] [Google Scholar]
- 3.Prior M, Smart D, Sanson A, Oberklaid F. Does shy-inhibited temperament in childhood lead to anxiety problems in adolescence? Journal of the American Academy of Child & Adolescent Psychiatry. 2000;39(4):461–468. doi: 10.1097/00004583-200004000-00015. [DOI] [PubMed] [Google Scholar]
- 4.Kagan J, Reznick JS, Snidman N. Biological bases of childhood shyness. Science. 1988;240:167–171. doi: 10.1126/science.3353713. [DOI] [PubMed] [Google Scholar]
- 5.Hirschfeld DR, Rosenbaum JF, Biederman J, Bolduc EA, Faraone SV, Snidman N, Reznick JS, Kagan J. Stable behavioral inhibition and its association with anxiety disorder. Journal of the American Academy of Child & Adolescent Psychiatry. 1992;31(1):103–111. doi: 10.1097/00004583-199201000-00016. [DOI] [PubMed] [Google Scholar]
- 6.Heiser NA, Turner SM, Beidel DC. Shyness: Relationship to social phobia and psychiatric disorders. Behaviour Research & Therapy. 2003;41:209–221. doi: 10.1016/s0005-7967(02)00003-7. [DOI] [PubMed] [Google Scholar]
- 7.Chronis-Tuscano A, Degnan KA, Pine DS, Perez-Edgar K, Henderson HA, Diaz Y, Raggi VL, Fox NA. Stable early maternal report of behavioral inhibition predicts lifetime social anxiety disorder in adolescence. Journal of the American Acadamy of Child and Adolescent Psychiatry. 2009;48(9):1–8. doi: 10.1097/CHI.0b013e3181ae09df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pfeifer M, Goldsmith HH, Davidson RJ, Rickman M. Continuity and change in inhibited and uninhibited children. Child Development. 2002;73(5):1474–1485. doi: 10.1111/1467-8624.00484. [DOI] [PubMed] [Google Scholar]
- 9.Robinson JL, Kagan J, Reznick JS, Corley R. The heritability of inhibited and unhibited behavior: A twin study. Developmental Psychology. 1992;28:1030–1037. [Google Scholar]
- 10.Smoller JW, Yamaki LH, Fagerness JA, Biederman J, Racette SR, Laird NM, Kagan J, Snidman N, Faraone SV, Hirshfeld-Becker D, Tsuang MT, Slaugenhaupt SA, Rosenbaum JF, Sklar PB. The corticotropin-releasing hormone gene and behavioral inhibition in children at risk for panic disorder. Biological Psychiatry. 2005;57(12):1485–1492. doi: 10.1016/j.biopsych.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 11.Degnan KA, Fox NA. Behavioral inhibition and anxiety disorders: Multiple levels of a resilience process. Development and Psychopathology. 2007;19:729–746. doi: 10.1017/S0954579407000363. [DOI] [PubMed] [Google Scholar]
- 12.Kerr M, Lambert WW, S H, Kackenberg-Larsson I. Stability of inhibition in a Swedish longitudinal sample. Child Development. 1994;65:138–146. [Google Scholar]
- 13.Henderson HA, Fox NA, Rubin KH. Temperamental contributions to social behavior: The moderating roles of frontal EEG asymmetry and gender. Journal of the American Acadamy of Child & Adolescent Psychiatry. 2001;40:68. doi: 10.1097/00004583-200101000-00018. [DOI] [PubMed] [Google Scholar]
- 14.Hirshfeld-Becker DR, Micco JA, Simoes NA, Henin A. High risk studies and developmental antecedents of anxiety disorders. American Journal of Medical Genetics. 2008;148(2):99–117. doi: 10.1002/ajmg.c.30170. [DOI] [PubMed] [Google Scholar]
- 15.Kalin NH, Shelton SE, Fox AS, Oakes TR, Davidson RJ. Brain regions associated with the expression and contextual regulation of anxiety in primates. Biological Psychiatry. 2005;58:796–804. doi: 10.1016/j.biopsych.2005.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwartz CE, Wright CI, Shin LM, Kagan J, Rauch SL. Inhibited and uninhibited infants “grown up”: Adult amygdalar response to novelty. Science. 2003;300:1952–3. doi: 10.1126/science.1083703. [DOI] [PubMed] [Google Scholar]
- 17.Fox NA, Henderson HA, Marshall PJ, Nichols KE, Ghera MM. Behavioral inhibition: Linking biology and behavior within a developmental framework. Annual Review of Psychology. 2005;56:235–262. doi: 10.1146/annurev.psych.55.090902.141532. [DOI] [PubMed] [Google Scholar]
- 18.Schmidt LA, Fox NA, Rubin KH, Sternberg EM, Gold PW, Smith CC, Schulkin J. Behavioral and neuroendocrine responses in shy children. Developmental Psychobiology. 1997;30(2):127–140. doi: 10.1002/(sici)1098-2302(199703)30:2<127::aid-dev4>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 19.Gunnar MR, Tout K, deHaan M, Pierce S, Stansbury K. Temperament, social competence, and adrenocortical activity in preschoolers. Developmental Psychobiology. 1997;31(1):65–85. doi: 10.1002/(sici)1098-2302(199707)31:1<65::aid-dev6>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 20.Schmidt LA, Santesso DL, Schulkin J, Segalowitz SJ. Shyness is a necessary but not sufficient condition for high salivary cortisol in typically developing 10 year-old children. Personality and Individual Differences. 2007;43:1541–1551. [Google Scholar]
- 21.Smider NA, Essex MJ, Kalin NH, Buss KA, Klein MH, Davidson RJ, Goldsmith HH. Salivary cortisol as a predictor of socioemotional adjustment during kindergarten: A prospective study. Child Development. 2002;73(1):75–92. doi: 10.1111/1467-8624.00393. [DOI] [PubMed] [Google Scholar]
- 22.Coplan JD, Andrews MW, Rosenblum LA, Owens MJ, Friedman S, Gorman JM, Nemeroff CB. Persistent elevations of cerebrospinal fluid concentrations of corticotropin-releasing factor in adult nonhuman primates exposed to early-life stressors: Implications for the pathophysiology of mood and anxiety disorders. Proceeds of the National Academy of Science USA. 1996;93:1619–1623. doi: 10.1073/pnas.93.4.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanchez MM, Ladd CO, Plotsky PM. Early adverse experience as a developmental risk factor for later psychopathology: Evidence from rodent and primate models. Development and Psychopathology. 2001;13:419–449. doi: 10.1017/s0954579401003029. [DOI] [PubMed] [Google Scholar]
- 24.Blair C, Granger DA, Kivlighan KT, Mills-Koonce R, Willoughby M, Greenberg MT, Hibel LC, Fortunato CK. Family Life Project Investigators: Maternal and child contributions to cortisol response to emotional arousal in young children from low-income, rural communities. Developmental Psychology. 2008;44(4):1095–1109. doi: 10.1037/0012-1649.44.4.1095. [DOI] [PubMed] [Google Scholar]
- 25.Hessl D, Dawson G, Frey K, Panagiotides H, Self H, Yamada E, Osterling J. A longitudinal study of children of depressed mothers: Psychobiological findings related to stress. In: Hann DM, Huffman LC, Lederhendler KK, Minecke D, Bethesda MD, editors. Advancing research on developmental plasticity: Integrating the behavioral sciences and the neurosciences of mental health. National Institutes of Mental Health; 1998. [Google Scholar]
- 26.Degnan KA, Henderson HA, Fox NA, Rubin KH. Predicting social wariness in middle childhood: The moderating roles of childcare history, maternal personality and maternal behavior. Social Development. 2008;17(3):471–487. doi: 10.1111/j.1467-9507.2007.00437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rubin KH, Burgess KB, Hastings PD. Stability and social-behavioral consequences of toddlers' inhibited temperament and parenting behaviors. Child Development. 2002;73(2):483–495. doi: 10.1111/1467-8624.00419. [DOI] [PubMed] [Google Scholar]
- 28.Essex MJ, Klein MH, Cho E, Kalin NH. Maternal stress beginning in infancy may sensitize children to later stress exposure: Effects on cortisol and behavior. Biological Psychiatry. 2002;52(8):776–784. doi: 10.1016/s0006-3223(02)01553-6. [DOI] [PubMed] [Google Scholar]
- 29.Hirschfeld-Becker DR, Biederman J, Faraone SV, Segool BA, Buchwald BA, Rosenbaum JF. Lack of association between behavioral inhibition and psychosocial adversity factors in children at risk for anxiety disorders. American Journal of Psychiatry. 2004;161(3):547–555. doi: 10.1176/appi.ajp.161.3.547. [DOI] [PubMed] [Google Scholar]
- 30.Hyde JS, Klein MH, Essex MJ, Clark R. Maternity leave and women's mental health. Psychology of Women Quarterly. 1995;19:257–285. [Google Scholar]
- 31.Housing and Household Economic Statistics Division. U.S. Census Bureau: 2008. Median income for 4-person families, by state. http://www.census.gov/hhes/www/income/4person.html. [Google Scholar]
- 32.Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1(3):385–401. [Google Scholar]
- 33.Halberstadt AG. Family socialization of emotional expression and nonverbal communication styles and skills. Journal of Personality and Social Psychology. 1986;51(4):827–836. [Google Scholar]
- 34.Barnett RC, Marshall NL. Preliminary manual for the Role-Quality Scales. Wellesley College: Center for Research on Women; 1989. [Google Scholar]
- 35.Spielberger CD, Krasner SS, Solomon EP, Janisse MP. The experience, expression, and control of anger. New York: Springer Verlag/Publishers; 1988. pp. 89–108. [Google Scholar]
- 36.Abidin RR. Parenting Stress Index. Charlottesville, VA: Pediatric Psychology Press; 1986. [Google Scholar]
- 37.Block JH. The Child-Rearing Practices Report (CRPR): A set of Q items for the description of parental socialization attitudes and values. Berkeley, CA: Institute of Human Development; 1965. [Google Scholar]
- 38.Goldsmith HH, Reilly J, Lemery KS, Longley S, Prescott A. Technical report. Department of Psychology, University of Wisconsin-Madison; 1993. Preliminary manual for the Preschool Laboratory Temperament Assessment Battery (Version 1.0) [Google Scholar]
- 39.Essex MJ, Boyce WT, Goldstein LH, Armstrong JM, Kraemer HC, Kupfer DJ. The confluence of mental, physical, social, and academic difficulties in middle childhood: II. Developing the MacArthur Health and Behavior Questionnaire. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41(5):588–603. doi: 10.1097/00004583-200205000-00017. [DOI] [PubMed] [Google Scholar]
- 40.Ablow JC, Measelle JR, MacArthur Working Group on Outcome Assessment . Manual for the Berkeley Puppet Interview: Symptomatology, social, and academic modules (BPI 1.0) Pittsburgh, PA: MacArthur Foundation Research Network on Psychopathology and Development, University of Pittsburgh; 2003. [Google Scholar]
- 41.Kraemer HC, Measelle JR, Ablow JC, Essex MJ, Boyce WT, Kupfer DJ. A new approach to integrating data from multiple informants in psychiatric assessment and research: Mixing and matching contexts and perspectives. American Journal of Psychiatry. 2003;160:1566–1577. doi: 10.1176/appi.ajp.160.9.1566. [DOI] [PubMed] [Google Scholar]
- 42.Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson DE, Ryan ND. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): Initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36(7):980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 43.Hirschfeld-Becker DR, Biederman J, Rosenbaum JF. Behavioral inhibition. In: Morris TL, March JS, editors. Anxiety disorders in children and adolescents. New York: The Guilford Press; 2004. [Google Scholar]
- 44.Essex MJ, Kraemer HC, Armstrong JM, Boyce WT, Goldsmith HH, Klein MH, Woodward H, Kupfer DJ. Exploring risk factors for the emergence of children's mental health problems. Archives of General Psychiatry. 2006;63:1246–1256. doi: 10.1001/archpsyc.63.11.1246. [DOI] [PubMed] [Google Scholar]
- 45.Nomura Y, Wickramaratne PJ, Warner V, Mufson L, Weissman MM. Family discord, parental depression and psychopathology in offspring: Ten-year follow-up. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41(4):402–409. doi: 10.1097/00004583-200204000-00012. [DOI] [PubMed] [Google Scholar]
- 46.Repetti RL, Taylor SE, Seeman TE. Risky families: Family social environments and the mental and physical health of offspring. Psychological Bulletin. 2002;128(2):330–366. [PubMed] [Google Scholar]
- 47.Rapee RM, Spence SH. The etiology of social phobia: Empirical evidence and an initial model. Clinical Psychology Review. 2004;24:737–768. doi: 10.1016/j.cpr.2004.06.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table 1a. Spearman Correlations of Early Risk Factors with School-Age Inhibition Scores at Each Assessment