Abstract
We evaluated the expression of epithelial-cell-adhesion-molecule (EpCAM) and the potential of MT201 (adecatumumab), a human monoclonal antibody against EpCAM, in uterine serous papillary carcinoma (USPC). EpCAM expression was evaluated by real-time-PCR and immunohistochemistry (IHC) in a total of 56 USPC fresh-frozen biopsies and paraffin-embedded-tissues. EpCAM surface expression was also evaluated by flow cytometry and IHC in 6 USPC cell lines. Sensitivity to MT201 antibody-dependent-cellular-cytotoxicity (ADCC) and complement-dependent-cytotoxicity (CDC) was tested against a panel of primary USPC cell lines expressing different levels of EpCAM in standard 5-h 51Cr release-assays. EpCAM transcript was significantly overexpressed in fresh-frozen USPC when compared to normal-endometrial-cells (NEC). Median (minimum–maximum) copy number was 943.8 (31.5–1568.3) in tumor samples versus 12.9 (1.0–37.0) in NEC (P < 0.001). By immunohistochemistry, EpCAM expression was found in 96% (26 out of 27) of USPC samples with significantly higher expression compared to normal endometrial cells (P < 0.001). High surface expression of EpCAM was found in 83% (5 out of 6) of the USPC cell lines tested by flow cytometry. EpCAM-positive cell lines were found highly sensitive to MT201-mediated ADCC in vitro, while primary USPC cell lines were resistant to natural killer (NK) cell-dependent cytotoxicity. Human plasma IgG did not significantly inhibit MT201-mediated-cytotoxicity against USPC. EpCAM is highly expressed in uterine serous carcinoma at mRNA and protein levels and primary USPC are highly sensitivity to MT201-mediated cytotoxicity. MT201 might represent a novel therapeutic strategy in patients harboring advanced/recurrent or metastatic USPC refractory to standard treatment modalities.
Keywords: Uterine serous papillary cancer, MT201, Adecatumumab, EpCAM, Endometrial carcinoma, Tumor markers
INTRODUCTION
Cancer of the uterine corpus is the most prevalent gynecologic tumor in women, with an estimated 40,100 cases and 7,470 deaths in the United States in 2008 (1). The majority of cancers of the uterus are early stage, low grade endometrioid tumors (i.e. Type I). These neoplasms are frequently diagnosed in younger women, are associated with a history of hyperestrogenism as the main risk factor, and typically have a favorable prognosis with appropriate therapy. In contrast, Type II endometrial cancers are poorly differentiated tumors, often with serous papillary (USPC) or clear cell histology. Although Type II tumors account for a minority of endometrial cancers, the majority of relapses and deaths occur in this group of patients (2,3).
USPC represents the variant of Type II endometrial carcinoma characterized by the most aggressive biologic behavior (3–12). The microscopic criteria for USPC diagnosis were first outlined by Hendrickson in 1982 (11). Classically, the neoplastic epithelium is characterized by serous differentiation with psammoma bodies present and with predominantly papillary architecture although solid areas can be focally detected (11). Cytologically, pleomorphism, grade III nuclear atypia with prominent nucleoli and vescicular chromatin pattern, and a high mitotic activity are detected. Clinically, USPC has a propensity for early intraabdominal and lymphatic spread even at presentation (3–12). Unlike serous carcinomas of the ovary, USPC is a chemoresistant disease since its onset with responses to combined cisplatinum-based chemotherapy in the order of 20% and of short duration (3–12). The survival rate is dismal, even when USPC is only a minor component of the histologically more common endometrioid adenocarcinoma and widespread metastasis and death may occur even in those cases in which tumor is confined to the endometrium or to an endometrial polyp (3–12). The overall 5-year survival is about 30% for all stages and the recurrence rate after surgery is extremely high (50% to 80%). The poor prognosis of USPC patients mandates the need for a better understanding of the molecular basis of the aggressive biologic behavior of these tumors as well as for the development of novel, target-specific and more effective treatment modalities against this variant of endometrial cancer.
High-throughput genomic analysis represents a new tool for the discovery of novel molecular diagnostic and therapeutic markers. Using this technology, our group has recently evaluated the genetic fingerprints of USPC (13,14). EpCAM (also known as TROP-1, TACSTD1, 17-1A, GA733-2, KSA, KS1/4, 323/A3, CD326), a calcium-independent homophilic cell adhesion molecule of 39–42 kDa, was consistently found as one of the top differentially expressed genes in USPC compared to normal human endometrial cells (NEC) by gene expression profiling (13,14). Of interest, EpCAM antigen is not structurally related to any of the four major families of adhesion molecules such as cadherins, immunoglobulin (Ig) family, selectins, or integrins (15). It is a type I transmembrane glycoprotein consisting of an extracellular domain with two EGF-like repeats and a short cytoplasmic domain of 26 amino acids (15). EpCAM has been reported to be expressed at relatively low levels on basolateral cell surfaces of most human simple epithelia (15). In contrast, high levels of EpCAM expression have been demonstrated in a variety of human epithelial tumors compared to normal human tissue (16). Previous studies have demonstrated that overexpression of EpCAM protein is common in mullerian derived malignancies such as ovarian cancer (17), and more recently, EpCAM overexpression has been shown to represent an independent prognostic marker for reduced survival in breast and ovarian cancer patients (18,19). Very limited information is currently available on EpCAM expression in USPC, a tumor resembling high grade ovarian cancer and characterized by an inborn resistance to chemotherapy and poor prognosis. This information is however important because current medical treatment of chemotherapy-resistant/ recurrent USPC remains dissatisfying, and because a novel human monoclonal antibody against EpCAM has been recently developed (20) and is currently undergoing Phase II studies in breast, prostate and colon cancer patients (21).
In this investigation, we evaluated EpCAM’s potential value as a novel target for USPC therapy by studying its expression at both gene and protein level in patients harboring primary, metastatic and recurrent USPC as well as on fresh uterine serous tumor cell lines established from patients harboring chemotherapy-resistant disease.
MATERIALS AND METHODS
Freshly frozen uterine tissue samples
Study approval was obtained from the Institutional Review Board, and all patients signed an informed consent according to institutional guidelines. Briefly, tumor and normal fresh-frozen tissues were identified, sharp-dissected and snap-frozen in liquid nitrogen within 30 minutes from resection. Tissue fragments from 29 USPC samples (18 Caucasian and 11 African-American) who underwent treatment for International Federation of Gynecology and Obstetrics (FIGO) stage IA to IV serous papillary endometrial adenocarcinoma and 6 normal fresh-frozen endometria (obtained from similar age women undergoing hysterectomy for uterine fibromas or prolapse) were split for histological confirmation and RNA isolation. Samples were embedded in optimal cutting temperature (O.C.T.) medium, microdissected, and the frozen sections were stained with hematoxylin and eosin (H&E) to check epithelial component. All of the neoplastic specimens examined contained at least 70% tumor epithelial cells. Patient characteristics from which tumor and normal samples were obtained are described in Table 1. Of the 29 USPC samples included in the study, 24 were obtained from primary endometrial tissues, 4 from metastatic omental disease and 1 from recurrent disease (i.e., biopsy obtained from a groin lymph node).
Table 1.
Characteristics of Patients
| Pathology and Tissue Type (number) |
Age in Years | Race | Stage | |||||
|---|---|---|---|---|---|---|---|---|
| Mean | (SD) | AA* | C** | I | II | III | IV | |
| None | ||||||||
| Fresh Frozen NEC (6) | 57 | (7) | 3 | 3 | ||||
| Formalin Fixed NEC (5) | 63 | (9) | 2 | 3 | ||||
| Primary/Metastatic/Recurrent USPC | ||||||||
| Fresh Frozen USPC (29) | 68 | (7) | 11 | 18 | 6 | 2 | 12 | 9 |
| Formalin Fixed USPC (27) | 69 | (5) | 10 | 17 | 8 | 3 | 9 | 7 |
| USPC cell lines | ||||||||
| Primary USPC (6) | 66 | (11) | 4 | 2 | 3 | 3 | ||
AA: African-American.
C: Caucasian. Note that the Primary/Metastatic/Recurrent USPC tested as fresh frozen samples in RT-PCR experiments differ from the formalin fixed samples used in IHC experiments.
Quantitative real-time PCR
RNA isolation from a total of 35 fresh frozen samples (29 USPC and 6 normal endometria) and the 6 primary USPC cell lines used in the cytotoxicity experiments were performed using TRIzol Reagent (Invitrogen) according to the manufacturer's instructions. Quantitative PCR was done with a 7500 Real Time PCR System using the manufacturer's recommended protocol (Applied Biosystems, Foster City, CA) to evaluate expression of EpCAM in all samples. Each reaction was run in duplicate. Briefly, 5 µg of total RNA from each sample was reverse transcribed using SuperScript III first-strand cDNA synthesis (Invitrogen). Five microliters of reverse transcribed RNA samples (from 500 µL of total volume) were amplified by using the TaqMan Universal PCR Master Mix (Applied Biosystems) to produce PCR products specific for EpCAM. The primers and probe for EpCAM (TACSTD1) were obtained from Applied Biosystems (Hs00158980_m1). The comparative threshold cycle (CT) method (Applied Biosystems) was used to determine gene expression in each sample relative to the value observed in the lowest nonmalignant endometrial epithelial cell sample, using glyceraldehyde-3-phosphate dehydrogenase (Assay ID Hs99999905_m1) RNA as internal controls.
EpCAM immunostaining of formalin-fixed tumor tissues
A total of 27 USPC specimens (22 primary and 5 metastatic obtained from uterine serous tumors with single cell differentiation, i.e., pure USPC), and 5 normal endometrium control tissues obtained from similar age women were evaluated by standard immunohistochemical staining on formalin-fixed tumor tissue for EpCAM surface expression. Patient characteristics from which tumor and normal samples were obtained are described in Table 1. Study blocks were selected after histopathologic review by a surgical pathologist. The most representative block was selected for each specimen. Briefly, immunohistochemical stains were performed on 4-µm-thick sections of formalin-fixed, paraffin-embedded tissue. After pretreatment with 10 mM citrate buffer at pH 6.0 using a steamer, they were incubated with mouse anti-EpCAM antibodies ESA/EpCAM/Ab-3 (clone323/A3) cat # MS-181 (NeoMarkers Inc. Fremont, CA). Antigen-bound primary antibody was detected using standard avidin-biotin immunoperoxidase complex (Dako Corp., Carpinteria, CA). Cases with less than 10% membranous staining in tumor cells were considered negative for EpCAM expression. The intensity of membranous immunoreactivity for EpCAM in tumor cells was subjectively scored as follow: (a) 0, negative; (b) 1+, weak membrane staining; (c) 2+, medium staining; and (d) 3+, strong membrane staining. Appropriate negative and positive controls were performed with each case.
Establishment of uterine serous cancer cell lines and NEC primary cell lines
A total of six uterine serous papillary carcinoma and three normal endometrial short term cell cultures (i.e., tissues obtained from women undergoing hysterectomy for uterine fibromas or prolapse) were established after sterile processing of samples from surgical biopsies as previously described (13,14). Patient characteristics from which tumor cell lines were obtained are described in Table 1. All USPC patients from which the cell lines were established experienced clinical progression of the disease during chemotherapy. Three out of six of these in vivo chemotherapy resistant tumors were confirmed to be highly resistant to multiple chemotherapeutic agents when measured for percentage cell inhibition (PCI) by in vitro Extreme Drug Resistance (EDR) assay (Oncotech Inc. Irvine, CA) (22, data not shown). Briefly, tissue was mechanically minced to portions no larger than 1–3 mm3 in an enzyme solution made of 0.14% collagenase type I (Sigma) and 0.01% DNAse (Sigma, 2000 KU/mg) in RPMI 1640, and incubated in the same solution in a magnetic stirring apparatus for 1 hour at room temperature. Enzymatically dissociated cells were then washed twice in RPMI-1640 with 10% fetal bovine serum (FBS) and maintained in RPMI supplemented with 10% FBS, 200 µg/ml penicillin and 200 µg/ml streptomycin at 37°C, 5% CO2 in 75-cm2 tissue culture flasks or Petri dishes (Corning, NY). 48–72 hrs after seeding on plasticware, non-adherent cells and contaminant inflammatory cells were gently removed from the culture by multiple washing with phosphate-buffered saline (PBS). The epithelial purity of the NEC and USPC cell lines was evaluated by immunocytochemical staining with antibody against pan-cytokeratin as previously described (13,14). Only cell cultures composed of at least 99% epithelial cells were retained for flow cytometry experiments
EpCAM immunohistochemistry of cell blocks obtained from primary USPC cell lines cultured in vitro
Cell cultures from six primary USPC cell lines were trypsinized and cells were suspended in Cytorich fixative (Richard Allen Scientific, Kalamazoo, MI), then centrifuged for 5 min at 2650 rpm. The supernatant was pipetted without disturbing the cell button. Four drops of human plasma and four drops of thromboplastin (Simplastin® Excel, Biomerieux, Durham, NC) were added to resuspend the cell button. The specimens were set aside until a clot formed (generally 5 minutes). The clot was then placed in a meshbag, fixed in 10% buffered formalin and processed as per routine histological technique. EpCAM immunohistochemical stains were performed on 5 µm sections of the paraffin-embedded cell blocks. After pretreatment with 10 mM citrate buffer at pH 6.0 using a steamer, the slides were incubated with anti ESA/EpCAM MAb (Clone MOC-31) (Neomarkers/Thermo Scientific, Fremont, CA). The DAKO EnVision™ kit was used for secondary detection and the reaction was visualized by DAB chromogen (DAKO, Carpinteria, CA). The reactions were scored (0 to 3+) as described above. Appropriate positive and negative controls were used with each case.
Flow cytometry
Adecatumumab (i.e., human recombinant IgG1 antibody MT201, kindly provided by Micromet AG, Munich, Germany) was used for our flow cytometry and ADCC studies. Clinical grade MT201 was produced by the manufacturer in CHO cells and formulated in phosphate-buffered saline at 10 mg/mL. Briefly, six freshly established uterine serous tumor cell lines obtained from the above described patients who experienced progression on chemotherapy were stained by MT201. A FITC-conjugated goat anti-human F(ab1)2 immunoglobulin was used as a secondary reagent (BioSource International, Camarillo, CA). Analysis was conducted with a FACScalibur instrument using cell Quest software (Becton Dickinson).
ADCC measurement
A standard 5-h chromium (51Cr) release assay was performed to measure the cytotoxic reactivity of Ficoll-Hypaque separated peripheral blood lymphocytes (PBL) obtained from several healthy donors against all 6 USPC target cell lines. The release of 51Cr from pre-loaded target cells was measured as evidence of tumor cell lysis, after exposure of tumor cells to varying concentrations of MT201 (ranging from 0.5 µg/ml to 100 µg/ml). Controls included the incubation of target cells alone, with PBL, or mAb separately. The chimeric anti-CD20 IgG1 mAb rituximab (Rituxan, Genentech, CA) was used as antibody isotype control for MT201 in all bioassays. ADCC was calculated as the percentage of killing of target cells observed with mAb plus effector cells, compared to the 51Cr release from target cells incubated in the absence of mAb or effector cells.
IL-2 enhancement of ADCC
To investigate the effect of IL-2 on MT201-mediated ADCC, effector PBLs were incubated for 5 hours at 37°C at a final concentration of IL-2 (Aldesleukin; Chiron Therapeutics, Emeryville, CA) ranging from 50 to 100 IU/ml in 96-well microtiter plates. Target cells were primary USPC cell lines exposed to MT201 (concentrations ranging from 0.5 µg/ml to 100 µg/ml), whereas controls included the incubation of target cells alone or with PBLs in the presence or absence of IL-2 or mAb, respectively. Rituximab was used as a control mAb. ADCC was calculated as the percentage of killing of target cells observed with mAb plus effector PBLs, as compared with target cells incubated alone. Each experiment was performed with PBLs obtained from at least two normal donors, with results from a representative donor presented.
Test for complement-mediated target cell lysis, and for inhibition by γ-immunoglobulin
A standard 5-h chromium (51Cr) release assay identical to those used for ADCC assays, except that human plasma (as a source of complement) diluted 1:2 was added in place of the effector cells, was used to test for complement-mediated target cell lysis. To test for the possible inhibition of ADCC against USPC cell lines by physiological human plasma concentrations of γ-immunoglobulin, heat inactivated (56 °C for 30 min) human plasma was diluted 1:2 before being added in the presence or absence of effector PBL. In some experiments, non-heat-inactivated human plasma (diluted 1:2) was added in the presence of effector PBL. Controls included the incubation of target cells alone or with either lymphocytes or mAb separately. Rituximab was used as isotype control mAb.
Statistical analysis
For q-RT-PCR data, the right-skewing was removed by taking copy-number ratios relative to the lowest-expressing NEC sample (“relative copy numbers”), log2-transforming them to ΔCTs, and comparing the results via unequal-variance t-test for the USPC-versus-NEC difference. Group means with 95% confidence limits (CIs) were calculated by computing them on the ΔCTs and then reverse-transforming the results to obtain means (95% CIs) of relative copy numbers. Differences in EpCAM expression by flow cytometry were analyzed by the unpaired t test, and a P value < 0.05 among samples was considered to be significant. The Wilcoxon rank-sum (WRS) test was used to compare USPC types to normal endometrium for differences in IHC staining intensities. Sample-type differences were expressed as odds ratios accompanied by 95% confidence limits. Kruskal-Wallis test and chi-square analysis was used to evaluate differences in MT201-induced ADCC levels in primary tumor cell lines. Statistical analysis was performed using SPSS version 15 (SPSS, Chicago, IL). A P-value of <0.05 was considered statistically significant.
RESULTS
EpCAM Transcript Levels in Uterine Serous Carcinomas
USPCs are rare tumors which may present in either pure forms, or admixed with endometrioid or clear cell carcinoma (i.e., mixed USPC). To minimize the risk of contamination of USPC RNA with that of normal cells or tumor cells with different histology, we extracted RNA to be evaluated for EpCAM expression by qRT-PCR from primary USPC with single type differentiation (i.e., pure USPC). A total of 35 specimens including 6 flash-frozen normal endometria tissues, 24 primary, 4 metastatic and 1 recurrent USPC were evaluated for EpCAM expression by qRT-PCR assays. Figure 1a shows expression details for EpCAM in USPC tested versus NEC. No significant difference in EpCAM expression was found when primary tumors were compared to metastatic/recurrent USPC. In contrast, EpCAM transcript was significantly overexpressed in fresh frozen USPC when compared to fresh-frozen normal endometrial cells (NEC). The mean (minimum-maximum) copy number in tumor samples was 515.4 (31.5–1568.3) versus 8.1 (1.0–25.5) in NEC (P <0.001). The -fold change in mean relative copy numbers was 64.3 (Figure 1a; P=0.0002).
Figure 1.
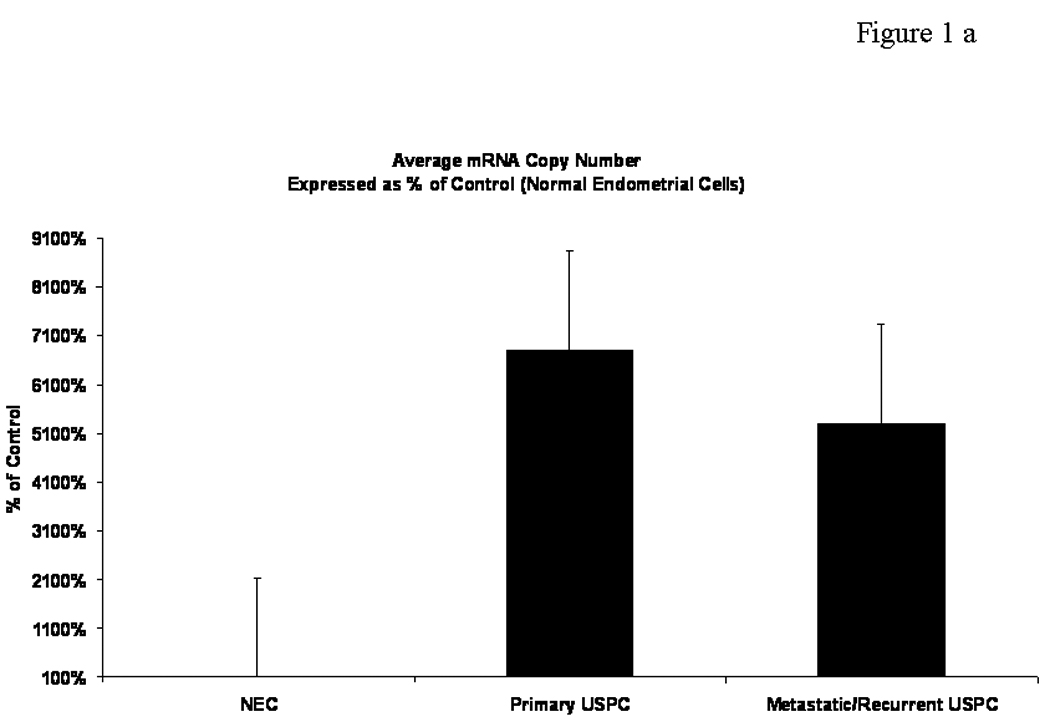
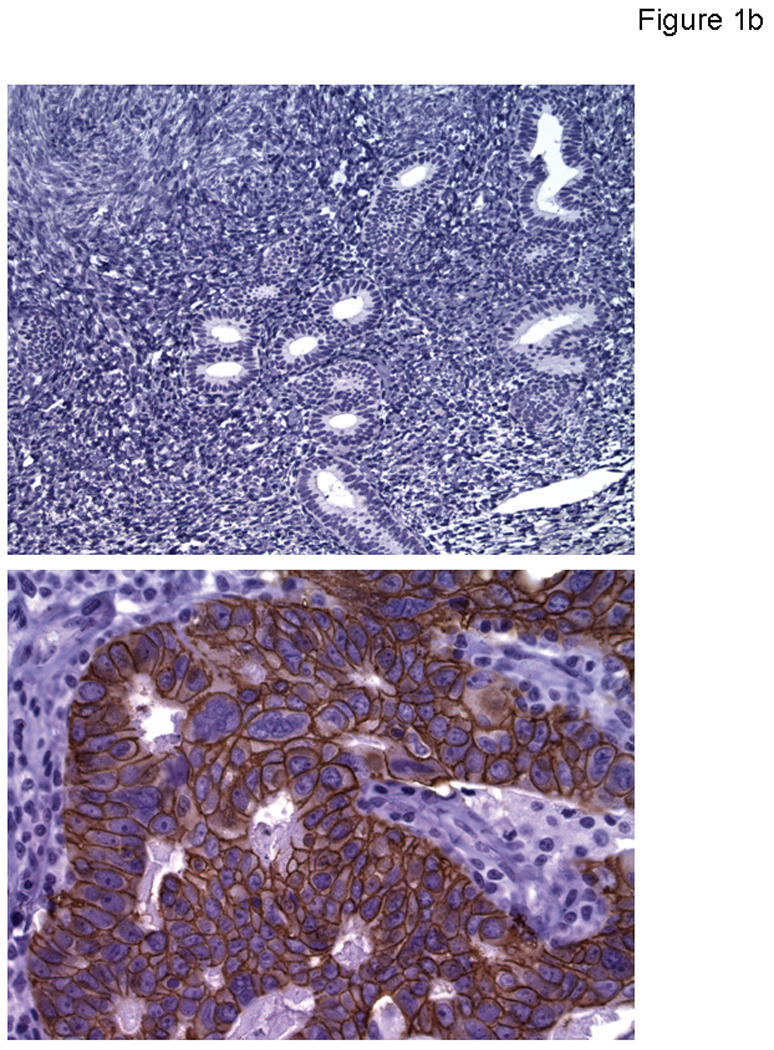
Figure 1a. EpCAM mRNA expression levels in primary and metastatic/recurrent USPC compared to normal endometrial tissues (NEC). EpCAM transcript was found to have significantly higher expression in primary and metastatic/recurrent carcinoma when compared pairwise to fresh-frozen normal endometrial tissues (P values <0.0001)
Figure 1b. Representative image of normal endometrium showing negative EpCAM immunohistochemical reaction (Upper Panel, 200 x), and a representative primary USPC showing strong (3+) EpCAM expression by immunohistochemistry (Lower Panel, 400 x).
EpCAM Expression by Immunohistochemistry on Primary/Metastatic USPC versus Normal Endometrial Tissue
To determine whether the high expression of EpCAM mRNA detected by q-RT-PCR assays in primary/metastatic/recurrent USPC also resulted in high expression of the protein on the surface of tumor cells, we performed immunohistochemical analysis on formalin-fixed, paraffin-embedded tumor tissue from a separate set of 27 USPCs. As representatively shown in Figure 1b, the intensity of EpCAM staining was significantly higher among the tumor specimens compared to normal endometrial controls (WRS P value = <0.0001). With a single exception, all tumors tested by IHC showed membranous positivity for EpCAM (i.e., 26 out of 27 samples). In this regard, only 4 out of the 27 specimens were found to have a low positivity (1+) for EpCAM protein, while the remaining specimens available for IHC testing showed moderate (i.e., 2+ : 7 samples) or strong (i.e., 3 + : 15 samples) EpCAM positivity (Figure 1b).
EpCAM Surface Expression by Flow Cytometry in Primary USPC Cancer Cell Lines
A total of 6 freshly established USPC cell lines derived in our laboratory from USPC patients were tested for EpCAM expression by FACS analysis. Table 2 shows EpCAM surface expression levels by flow cytometry, qRT-PCR and IHC in all primary USPC cell lines. Five out of six (83%) of the primary tumor cell lines were found to express high surface levels of EpCAM (i.e., 100% positive cells; mean fluorescence intensity [MFI] range from 43 to 148) (Table 2). As controls, NEC primary cultures were used. NEC showed negative to negligible levels of EpCAM expression by flow cytometry (Table 2). EpCAM surface expression results from flow cytometric analysis were found to be in good agreement with EpCAM expression results found by qRT-PCR and by IHC in all six primary USPC cell lines.
Table 2.
EpCAM mRNA and protein expression in UPSC cell lines
| Cell Line | IHC | RT-PCR | Flow cytometry | |
|---|---|---|---|---|
| Cells (%) | MFI | |||
| Control | - | 1 | - | - |
| USPC ARK-1 | 1+ | 1072 | 100 | 43 |
| USPC ARK-2 | 2+ | 8732 | 100 | 148 |
| USPC ARK-3 | 3+ | 9584 | 100 | 110 |
| USPC ARK-4 | 0 | 1.5 | 30 | 31 |
| USPC ARK-5 | 3+ | 8888 | 100 | 116 |
| USPC ARK-6 | 3+ | 5691 | 100 | 86 |
IHC, Immunohistochemistry; RT-PCR, Real-time Polymerase Chain Reaction; MFI, Mean Fluorescence Intensity
USPC Cell Lines Are Highly Resistant to NK Cell Activity but Sensitive to MT201-mediated ADCC
Next, we evaluated primary USPC cell lines for their sensitivity to natural killer cell-mediated cytotoxicity when challenged with PBL collected from multiple healthy donors in a standard 5-h 51Cr release assay. We initially performed dose titration experiments using different doses of MT201 (i.e., from 0.5 µg/ml to 100 µg/ml) and a constant effector (PBL) to target cell (USPC) ratios (1:25). As shown for two representative USPC cell lines (Figure 2, upper and middle panel), we found the lysis of primary USPC cell lines to plateau at 5 to 10 µg/ml of MT201 in multiple experiments. On the basis of these results we used 5 µg/ml of MT201 in the majority of our following cytotoxicity experiments. Importantly, all USPC cell lines were consistently found to be resistant to NK cell-mediated lysis when combined with PBL in the absence of the antibody at effector : target (E/T) cell ratios varying from 25 : 1 to 50 : 1 (mean killing = 2.8 %) (Table 3). Similarly, USPC cell lines incubated with the isotype control antibody rituximab were not significantly lysed (mean lysis = 3.0 %) (Table 3). In strong contrast, all EpCAM-positive cell lines (5 out of 6 USPC) were found to be highly sensitive to PBL when incubated with MT201 (range of lysis from 23% to 62%; mean 33%) (Table 3 and Figure 2). These experiments were repeated a minimum of 3 times for each USPC cell line.
Figure 2.
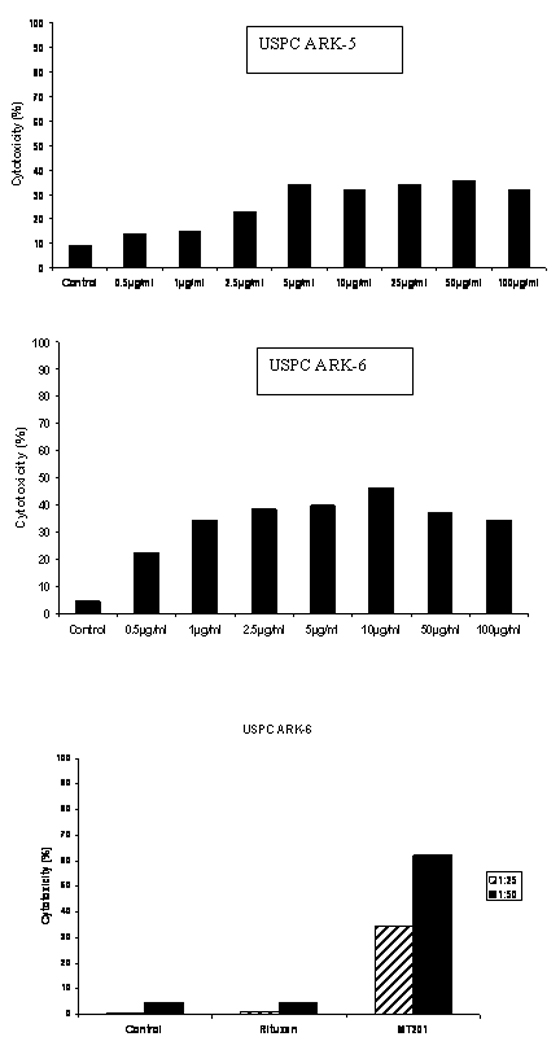
Dose titration cytotoxicity experiments using MT201 against USPC ARK-5 (upper panel) and USPC ARK-6 (middle panel) cell lines at 25 : 1 effector-target cell ratio. ADCC was found to plateau at a dose of 5 µg/ml to 10 µg/ml in multiple experiments. Lower Panel: example of ADCC in USPC ARK-6 cell line at different effector to target cell ratios in presence or absence of MT201 in 5-h chromium release cytotoxicity assay. High levels of ADCC MT201 induced cytotoxicity was evident against USPC ARK-6 cells while negligible cytotoxicity was detectable in the absence of MT201 or in the presence of rituximab.
Table 3.
MT201-dependent cytotoxicity in USPC cell lines
| Control | Rituximab | MT201 | P Value* | |
|---|---|---|---|---|
| USPC ARK-1 | 9.6% | 12.6% | 23.4% | |
| USPC ARK-2 | 0.5% | 0.04% | 62.8% | |
| USPC ARK-3 | 0.7% | 2.0% | 36.1% | |
| USPC ARK-4 | 0.6% | 0.4% | 0.2% | |
| USPC ARK-5 | 4.7% | 3.8% | 48.8% | |
| USPC ARK-6 | 4.7% | 4.6% | 62.2% | |
| Average** ± SD | 2.8% ± 3.0 | 3.0% ± 3.5 | 33% ± 18.0 | <0.0001 |
Cytotoxicty results in Ep-CAM-positive cell lines versus Controls by Kruskall-Wallis test and chi-square analysis.
Average ± SD of 22 cytotoxicity experiments.
Effect of Complement and Physiological Concentrations of IgG on MT201-mediated ADCC against USPC
To evaluate primary USPC cell lines for their sensitivity to complement-mediated cytotoxicity, and to evaluate possible inhibition of ADCC by physiological serum concentrations of IgG, USPC cell lines were challenged by adding human plasma diluted 1 : 2 (with or without heat inactivation) in the presence or absence of the effector cells and MT201 to our standard 5-h 51Cr release assays. As representatively shown in Figure 3a, addition of untreated plasma with or without MT201 was not able to induce significant cytotoxicity against USPC ARK-2 cell line. These data illustrate the lack of significant cytotoxicity mediated by complement proteins in the absence of effector cells. Addition of physiological concentrations of serum IgG (i.e., heat-inactivated plasma diluted 1 : 2) to PBL in the presence of MT201 consistently reduced the degree of ADCC achieved in the presence of MT201 (Figure 3a). In contrast, addition of untreated plasma (diluted 1 : 2) to PBL in the presence of MT201 consistently increased MT201-mediated cytotoxicity against USPC (p < 0.03) (Figure 3a).
Figure 3.
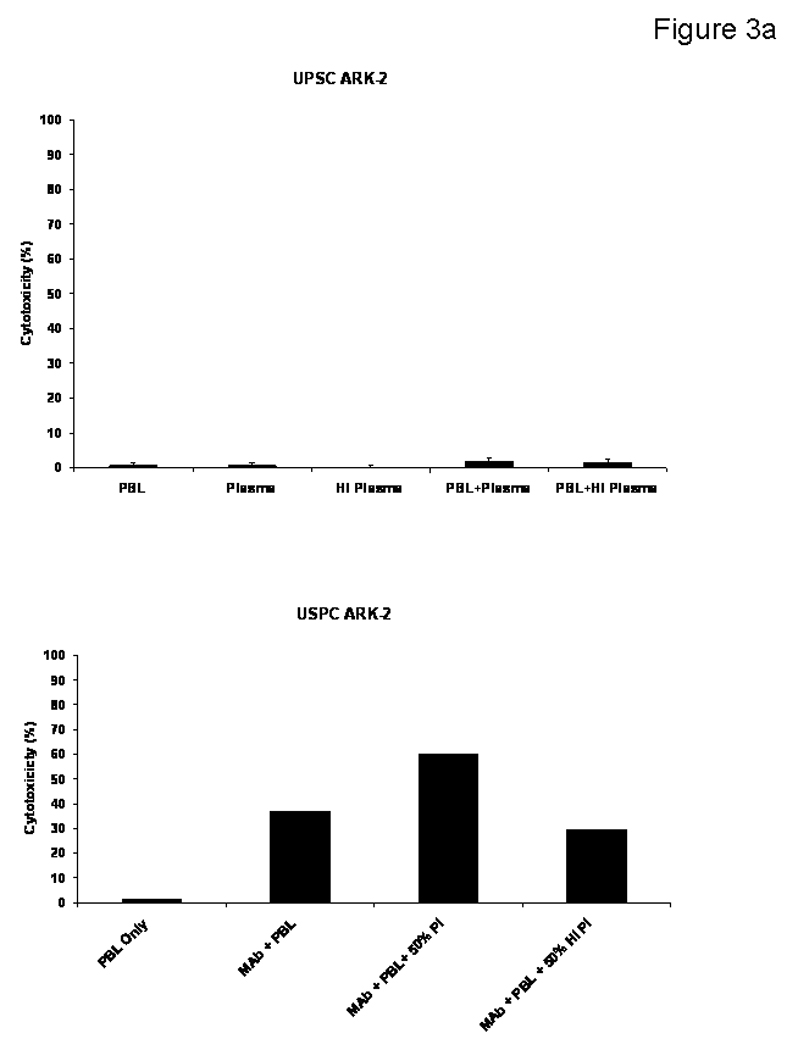
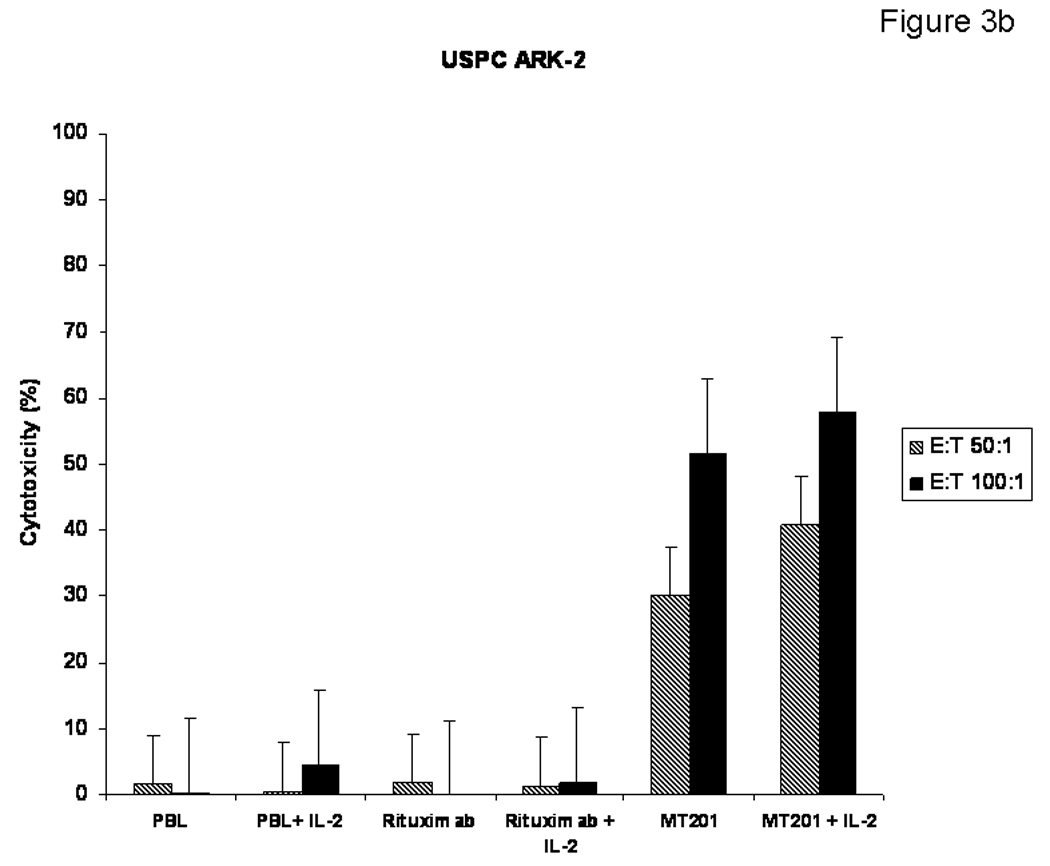
Figure 3a. USPC cell lines were challenged by adding human plasma diluted 1:2 (with or without heat inactivation) in the presence or absence of the effector cells and MT201 to standard 5-h 51Cr release assays. The addition of PBLs with or without plasma (untreated or heat-inactivated) was not able to induce significant cytotoxicity against USPC ARK-2 cell line (Upper Panel). Addition of physiological concentrations of IgG (i.e., heat-inactivated plasma diluted 1 : 2) to PBL in the presence of MT201 did not significantly alter the degree of ADCC achieved in the presence of MT201 (lower panel). In contrast, addition of untreated plasma (diluted 1:2) to PBL in the presence of MT201 consistently increased MT201-mediated cytotoxicity against USPC (p < 0.03) (lower panel).
Figure 3b. The effect of low doses of interleukin-2 (IL-2) in combination with MT201 (5 µg/ml) on ADCC against USPC cell lines. PBL from healthy donors were incubated for 5 to 72 h in the presence of 100 IU/ml of IL-2. MT201-mediated ADCC was significantly increased in the presence of low doses of IL-2. No significant increase in cytotoxicity was detected after 5-h IL-2 treatment in the absence of MT201 or in the presence of rituximab isotype control mAb.
IL-2 Enhancement of ADCC against USPC
To investigate the effect of low doses of interleukin-2 (IL-2) in combination with MT201 (5 µg/ml) on ADCC against USPC cell lines, PBL from healthy donors were incubated for 5 to 72 h in the presence of 100 IU/ml of IL-2. As representatively shown in Figure 3b, MT201-mediated ADCC was significantly increased in the presence of low doses of IL-2 (P=0.04). Administration of 100 IU/ml of IL-2 to the effector PBL at the start of the assay increased the cytotoxic activity against USPC cell lines compared to the use of MT201 alone, while no significant increase in cytotoxicity was detected after 5 h of IL-2 treatment in the absence of MT201 and/or in the presence of the isotype control mAb rituximab (Figure 3b).
DISCUSSION
Our research group has recently analyzed the genetic expression profile of uterine serous carcinoma by oligonucleotide microarrays (13,14). Among the most up-regulated genes in USPC compared to NEC tissues, we consistently identified the gene encoding EpCAM. EpCAM is known as a surface glycoprotein whose expression is highest during embryogenesis and in association with neoplastic changes in many carcinomas of different origin (15,16). Lowest expression of EpCAM is generally found in adult mature tissues, where it is commonly only detectable on the basolateral surface of epithelia (15,16). Due to its restricted accessibility in highly structured epithelium, EpCAM is considered not accessible to intravenously administered anti-EpCAM mAb, as demonstrated by comparing antibody accessibility to human EpCAM-expressing syngeneic tumors and normal epithelial tissues in transgenic mice (23). Hence, despite EpCAM’s wide-spread expression in several human normal tissues, the potentially high density and accessibility of EpCAM on metastatic/chemotherapy-resistant USPC may suggest EpCAM as a promising target for antibody-based therapies of uterine serous tumors refractory to standard treatment modalities.
In this study, we have analyzed EpCAM gene and protein expression in a cohort of USPC tissues. In addition, we have immunohistochemically investigated EpCAM expression and localization in normal endometrium, and compared such expression to primary/metastatic/recurrent uterine serous tumors. Our findings demonstrate that (i) EpCAM mRNA and protein are significantly up-regulated in primary/metastatic/recurrent USPC compared to normal endomtrial tissues; (ii) normal endometrial cells express low EpCAM transcript and showed a negative immunostaining for the protein; (iii) five out of six freshly established tumor cell lines derived from patients harboring advanced stages USPC and experiencing progression of disease on chemotherapy expressed high levels of EpCAM on their cell surfaces as measured by IHC and flow cytometry, and (iv) primary USPC cell lines overexpressing EpCAM are highly susceptible to ADCC mediated by MT201, a human mAb recently developed for targeting EpCAM-expressing cancers.
Our new results may have important implication for the treatment of USPC patients harboring tumors resistant to chemotherapy. In high grade ovarian serous carcinoma, a tumor resembling USPC, several lines of evidence seem to support an association between increased expression of EpCAM and tumor progression (19). Recent studies have revealed a more versatile function for EpCAM that is not limited only to cell adhesion but includes diverse processes such as signaling, cell migration, proliferation and differentiation (24). EpCAM was shown to be a signaling molecule activated through regulated intramembrane proteolysis (25). Once released, its short cytoplasmic tail EpICD can associate with components of the wnt signaling pathway, and after nuclear transport, a large nuclear complex containing EpICD induce transcription of c-myc and cyclins. In our study, high EpCAM levels were found by flow cytometry in five out of six of the freshly established USPC cell lines tested for EpCAM surface expression. Taken together, these observations are in agreement with the possibility that aberrant expression of EpCAM may account for the enhanced invasive behavior and the increased cell survival of chemotherapy-resistant uterine serous cancer cells.
Our observations suggest that targeting cancer cells with high surface expression of EpCAM, may be a novel, potentially effective option to treat residual/resistant USPC disease after standard adjuvant chemotherapy. Consistent with this view, we have tested in this study the ability of MT201, a recently developed human anti-EpCAM antibody (20) currently in phase II clinical trials in breast and colon cancer patients (21), for its ability to kill in vitro multiple primary USPC cell lines expressing different levels of EpCAM. In this regard, with the exception of USPC-ARK-4, all primary USPC cell lines available to this study (i.e., USPC-ARK-1 to USPC-ARK-6) were found to express significant levels of EpCAM by flow cytometry. Importantly, with no exception, the primary USPC cell lines studied were found to be highly resistant to lysis by natural killer cells. These data suggest that in addition to their high resistance to chemotherapy, radiation treatment and hormonal therapy (3–12), USPC cells are also intrinsically highly resistant to natural killer activity. Furthermore, complement-mediated tumor cell lysis (in the absence of effector cells) was not observed, which may be due to the presence of membrane-associated complement regulatory proteins such as CD35 (complement receptor 1), CD55 (decay accelerating factor), or CD46 (membrane cofactor protein) on USPC, as previously reported for other human tumors resistant to complement-dependent cytotoxicity (26). In agreement with our qRT-PCR and protein expression results, all primary USPC cell lines overexpressing EpCAM were found to be highly susceptible to ADCC when incubated with effector cells in the presence of MT201. These data, therefore, demonstrate that although these tumor cells are per se extremely resistant to any standard cytotoxic therapy in the clinic, they remain highly sensitive to lysis by NK cells when these are engaged by EpCAM-specific antibody MT201.
In vivo,ADCC activity is known to be dependent upon the availability of the effector cells to interact with the antibody at the target site in the presence of high concentrations of irrelevant human IgG. In this study, we show that ADCC against USPC was not significantly inhibited by high concentrations (up to 50%) of human plasma. In fact, a consistent increase in cytotoxicity was detected in the presence of effector cells and non-heat inactivated human plasma. These data, therefore, suggest that in the presence of effector PBL, human plasma may augment MT201-mediated cytotoxicity against USPC. Moreover, these results indicate that the binding of MT201 to the Fc receptor on mononuclear effector cells is likely enabled in the in vivo situation.
Treatment of cancer patients with combinations of mAbs and cytokines does not amount to a mere addition to the benefit of each treatment modality alone, but has been demonstrated to have synergistic potential (27,28). Recently, low doses of rIL-2 have been given by continuous infusion or subcutaneously, with remarkable immunologic results coupled with negligible toxicity (29,30). This point is noteworthy because, both in experimental models and in patients, modulation of both the number and function of NK cells has been previously associated with tumor progression (31,32) and, in addition, substantially suppressed ADCC responses have been reported in several cancer patients (33). Importantly, however, cytotoxicity levels in patients who demonstrate suppressed ADCC can be increased in vitro to levels similar to those of normal donors by prior exposure of effector cells to IL-2 (34). Consistent with this view, a significant increase in ADCC against USPC was detected after exposure of effector cells to low doses of IL-2 in vitro for a brief time (i.e., for 5 h). Longer time periods of incubation (up to 3 days) with IL-2 under the same conditions showed similar results (data not shown). These data suggest that the administration of low (i.e., non toxic) doses of IL-2 in vivo, giving rise to a lytic effector cell that is markedly enhanced in its function by the addition of an antibody bridge, may significantly increase the efficacy of MT201 therapy in USPC patients. Furthermore, on the basis of the high resistance of USPC to standard cytotoxic anti-cancer therapy, these combined therapies might be particularly important in the treatment of USPC patients.
In conclusion, this is the first report on EpCAM protein expression and MT201 therapeutic activity in USPC, the most aggressive and chemotherapy resistant variant of endometrial cancer. Our study has demonstrated that EpCAM expression is highly and consistently expressed at mRNA and protein level in the majority of primary, metastatic and recurrent USPC as well as on primary cell lines established from patients harboring chemotherapy resistant tumors. The high density and the membranous localization of EpCAM on USPC cells, combined with its negative expression in mesothelial type cells in the abdominal cavity (data not shown), suggests that this protein could represent an accessible tumor target antigen for both intravenous (i.v.) and intraperitoneal (i.p.) antibody-based therapies. Consistent with this view, the first clinical i.p. application of a well-tolerated bispecific, trifunctional anti-EpCAM antibody has recently demonstrated effective tumor cell destruction, substantial decreased ascites accumulation, and reduced necessity of paracentesis in advanced ovarian carcinoma patients harboring malignant tumors refractory to salvage chemotherapy (35). The future design and implementation of clinical trials in this regard will ultimately determine the validity of this novel therapeutic approach in patients harboring USPC.
Acknowledgments
Supported in part by grants from the Angelo Nocivelli, the Berlucchi and the Camillo Golgi Foundation, Brescia, Italy, NIH R01 CA122728-01A2 to AS, and grants 501/A3/3 and 0027557 from the Italian Institute of Health (ISS) to AS. This investigation was also supported by NIH Research Grant CA-16359 from the National Cancer Institute.
Abbreviations
- USPC
uterine serous papillary carcinoma
- EpCAM
Epithelial Cell Adhesion Molecule
- RT-PCR
reverse transcriptase-polymerase chain reaction
- SEM
standard error of the mean
- NEC
normal endometrial controls
- FBS
fetal bovine serum
- q-RT-PCR
quantitative real time-PCR
- CT
comparative threshold cycle.
Footnotes
Conflict of interest: None
REFERENCES
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. Vol. 58. CA: a Cancer Journal for Clinicians; 2008. pp. 71–96. [DOI] [PubMed] [Google Scholar]
- 2.Bohkman JV. Two pathogenetic types of endometrial carcinoma. Gynecol Oncol. 1983;15:10–17. doi: 10.1016/0090-8258(83)90111-7. [DOI] [PubMed] [Google Scholar]
- 3.Hamilton CA, Cheung MK, Osann K, et al. Uterine papillary serous and clear cell carcinomas predict for poorer survival compared to grade 3 endometrioid corpus cancers. Brit J Cancer. 2006;94(5):642–646. doi: 10.1038/sj.bjc.6603012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sherman ME, Bitterman P, Rosenshein NB, et al. Uterine serous carcinoma. A morphologically diverse neoplasm with unifying clinicopathological features. Am J Surg Pathol. 1992;16:600–610. doi: 10.1097/00000478-199206000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Carcangiu ML, Chambers JT. Uterine papillary serous carcinoma: a study on 108 cases with emphasis on prognostic significance of associated endometrioid carcinoma, absence of invasion, and concomitant ovarian cancer. Gynecol Oncol. 1992;47:298–305. doi: 10.1016/0090-8258(92)90130-b. [DOI] [PubMed] [Google Scholar]
- 6.Goff BA, Kato D, Schmidt RA, et al. Uterine papillary serous carcinoma: pattern of metastatic spread. Gynecol Oncol. 1994;54:264–268. doi: 10.1006/gyno.1994.1208. [DOI] [PubMed] [Google Scholar]
- 7.Carcangiu ML, Chambers JT. Early pathologic stage clear cell carcinoma and uterine papillary serous carcinoma of the endometrium, comparison of clinicopathological features and survival. Int J Gynecol Pathol. 1995;14:30–38. doi: 10.1097/00004347-199501000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Levenback C, Burke TW, Silva E, et al. Uterine papillary serous carcinoma (USPC) treated with cisplatin, doxorubicin, and cyclophosphamide (PAC) Gynecol Oncol. 1992;46:317–321. doi: 10.1016/0090-8258(92)90224-7. [DOI] [PubMed] [Google Scholar]
- 9.Nicklin JL, Copeland LJ. Endometrial papillary serous carcinoma: pattern of spread and treatment. Clin Obstet Gynecol. 1996;39:686–695. doi: 10.1097/00003081-199609000-00016. [DOI] [PubMed] [Google Scholar]
- 10.Trope C, Kristensen GB, Abeler VM. Clear-cell and papillary serous cancer: treatment options. Best Pract & Res in Clin Obstet & Gynaecol. 2001;15(3):433–446. doi: 10.1053/beog.2000.0187. [DOI] [PubMed] [Google Scholar]
- 11.Hendrickson M, Ross J, Eifel P, Martinez A, Kempson R. Uterine papillary serous carcinoma: a highly malignant form of endometrial adenocarcinoma. Am J Surg Pathol. 1982;6(2):93–108. doi: 10.1097/00000478-198203000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Chan JK, Loizzi V, Youssef M, et al. Significance of comprehensive surgical staging in noninvasive papillary serous carcinoma of the endometrium. Gynecol Oncol. 2003;90(1):181–185. doi: 10.1016/s0090-8258(03)00195-1. [DOI] [PubMed] [Google Scholar]
- 13.Santin AD, Zhan F, Bellone S, et al. Discrimination between uterine serous papillary carcinomas and ovarian serous papillary tumors by gene expression profiling. Brit J Cancer. 2004;90:1814–1824. doi: 10.1038/sj.bjc.6601791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Santin AD, Zhan F, Cane' S, et al. Gene expression fingerprint of uterine serous papillary carcinoma: identification of novel molecular markers for uterine serous cancer diagnosis and therapy. Brit J Cancer. 2005;92(8):1561–1573. doi: 10.1038/sj.bjc.6602480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Balzar M, Winter MJ, de Boer CJ, Litvinov SV. The biology of the 17-1A antigen (Ep-CAM) J Mol Med. 1999;77(10):699–712. doi: 10.1007/s001099900038. [DOI] [PubMed] [Google Scholar]
- 16.Spurr NK, Durbin H, Sheer D, Parkar M, Bobrow L, Bodmer WF. Characterization and chromosomal assignment of a human cell surface antigen defined by the monoclonal antibody AUAI. Int J Cancer. 1986;38(5):631–636. doi: 10.1002/ijc.2910380503. [DOI] [PubMed] [Google Scholar]
- 17.Kim JH, Herlyn D, Wong KK, et al. Identification of epithelial cell adhesion molecule autoantibody in patients with ovarian cancer. Clin Cancer Res. 2003;9(13):4782–4791. [PubMed] [Google Scholar]
- 18.Gastl G, Spizzo G, Obrist P, Dunser M, Mikuz G. EpCAM overexpression in breast cancer as a predictor of survival. Lancet. 2000;356(9246):1981–1982. doi: 10.1016/S0140-6736(00)03312-2. [DOI] [PubMed] [Google Scholar]
- 19.Spizzo G, Went P, Dirnhofer S, et al. Overexpression of epithelial cell adhesion molecule (EpCAM) is an independent prognostic marker for reduced survival of patients with epithelial ovarian cancer. Gynecol Oncol. 2006;103(2):483–488. doi: 10.1016/j.ygyno.2006.03.035. [DOI] [PubMed] [Google Scholar]
- 20.Naundorf S, Preithner S, Mayer P, et al. In vitro and in vivo activity of MT201, a fully human monoclonal antibody for pancarcinoma treatment. Int J Cancer. 2002;100(1):101–110. doi: 10.1002/ijc.10443. [DOI] [PubMed] [Google Scholar]
- 21.Kirman I, Whelan RL. Drug evaluation: adecatumumab, an engineered human anti-EpCAM antibody. Curr Opin Mol Ther. 2007;9(2):190–196. [PubMed] [Google Scholar]
- 22.Holloway RW, Mehta RS, Finkler NJ, et al. Association between in vitro platinum resistance in the EDR assay and clinical outcomes for ovarian cancer patients. Gynecol Oncol. 2002;87:8–16. doi: 10.1006/gyno.2002.6797. [DOI] [PubMed] [Google Scholar]
- 23.McLaughlin PM, Harmsen MC, Dokter WH, et al. The epithelial glycoprotein 2 (EGP-2) promoter-driven epithelial-specific expression of EGP-2 in transgenic mice: a new model to study carcinoma-directed immunotherapy. Cancer Res. 2001;61(10):4105–4111. [PubMed] [Google Scholar]
- 24.Trzpis M, McLaughlin PM, de Leij LM, Harmsen MC. Epithelial cell adhesion molecule: more than a carcinoma marker and adhesion molecule. Am J Path. 2007;171(2):386–395. doi: 10.2353/ajpath.2007.070152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maetzel D, Denzel S, Mack B. Nuclear signalling by tumour-associated antigen EpCAM. Nature Cell Biology. 2009;11(2):162–171. doi: 10.1038/ncb1824. [DOI] [PubMed] [Google Scholar]
- 26.Devine D. The regulation of complement on cell surfaces. Transfus Med Rev. 1991;5:123–131. doi: 10.1016/s0887-7963(91)70199-5. [DOI] [PubMed] [Google Scholar]
- 27.Caron PC, Lai LT, Scheinberg DA. Interleukin-2 enhancement of cytotoxicity by humanized monoclonal antibody M195 (anti-CD33) in myelogenous leukemia. Clin. Cancer Res. 1995;1:63–70. [PubMed] [Google Scholar]
- 28.Hooijberg E, Sein JJ, van der Berk PCM, et al. Eradication of large human B cell tumors in nude mice with unconjugated CD20 monoclonal antibodies and Interleukin 2. Cancer Res. 1995;55:2627–2634. [PubMed] [Google Scholar]
- 29.Stein RC, Malkovska V, Morgan S, et al. The clinical effects of prolonged treatment of patients with advanced cancer with low-dose subcutaneous interleukin 2. Br J Cancer. 1991;63:275–282. doi: 10.1038/bjc.1991.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Caligiuri MA, Murray C, Robertson MJ, et al. Selective modulation of human natural killer cells in vivo following prolonged infusion of low-doses recombinant interleuikin 2. J Clin Invest. 1993;91:123–128. doi: 10.1172/JCI116161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Introna M, Mantovani A. Natural killer cells in human solid tumors. Cancer Metastasis Rev. 1983;2:337–340. doi: 10.1007/BF00048566. [DOI] [PubMed] [Google Scholar]
- 32.Choe B, Frost P, Morrison N, Rose N. Natural killer cell activity of prostatic cancer patients. Cancer Invest. 1987;5:285–288. [PubMed] [Google Scholar]
- 33.Ortaldo JR, Woodhouse CS, Morgan AC, Jr, Herberman RB, Cheresh DA, Reisfeld RA. Analysis of effector cells in human antibody-dependent cellular cytotoxicity with murine monoclonal antibodies. J Immunol. 1987;138:3566–3572. [PubMed] [Google Scholar]
- 34.Honsik CJ, Jung G, Reisfeld RA. Lymphokine-activated killer cells targeted by monoclonal antibodies to the disialogangliosides GD2 and GD3 specifically lyse humaan tumor cells of neuroectodermal origin. Proc Natl Acad Sci USA. 1986;83:7893–7897. doi: 10.1073/pnas.83.20.7893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burges A, Wimberger P, Kumper C, et al. Effective relief of malignant ascites in patients with advanced ovarian cancer by a trifunctional anti-EpCAM x anti-CD3 antibody: a phase I/II study. Clin Cancer Res. 2007;13(13):3899–3905. doi: 10.1158/1078-0432.CCR-06-2769. [DOI] [PubMed] [Google Scholar]


