Abstract
Although heat-shock preconditioning has been shown to promote cell survival under oxidative stress, the nature of heat-shock response from different cells is variable and complex. Therefore, it remains unclear whether mesenchymal stem cells (MSCs) modified with a single heat-shock protein (Hsp) gene are effective in the repair of a damaged heart. In this study, we genetically engineered rat MSCs with Hsp20 gene (Hsp20-MSCs) and examined cell survival, revascularization, and functional improvement in rat left anterior descending ligation (LAD) model via intracardial injection. We observed that overexpression of Hsp20 protected MSCs against cell death triggered by oxidative stress in vitro. The survival of Hsp20-MSCs was increased by approximately twofold by day 4 after transplantation into the infarcted heart, compared with that of vector-MSCs. Furthermore, Hsp20-MSCs improved cardiac function of infarcted myocardium as compared with vector-MSCs, accompanied by reduction of fibrosis and increase in the vascular density. The mechanisms contributing to the beneficial effects of Hsp20 were associated with enhanced Akt activation and increased secretion of growth factors (VEGF, FGF-2, and IGF-1). The paracrine action of Hsp20-MSCs was further validated in vitro by cocultured adult rat cardiomyocytes with a stress-conditioned medium from Hsp20-MSCs. Taken together, these data support the premise that genetic modification of MSCs before transplantation could be salutary for treating myocardial infarction.
Keywords: Small heat-shock protein 20, Mesenchymal stem cell, Myocardial infarction, Oxidative stress, Paracrine factors, Akt
Introduction
A leading cause of heart failure is myocardial ischemia, which precipitates dysfunction and death of cardiomyocytes. Recently, stem cell therapy, based on mesenchymal stem cells (MSCs) in particular, is quickly emerging as an alternative strategy in myocardial infarction treatment [1, 2]. The promising therapeutic effect(s) of MSCs is dependent on their capacity to survive and engraft in the target tissue [3–5]. However, the transplantation of as many as 6 × 107 of these putative MSCs into infarcted porcine hearts yielded only marginal improvement in cardiac function [6]. Toma et al. further reported that less than 0.44% MSCs survive by day 4 after engraftment in an immunodeficient mouse heart model [7]. Hence, it is imperative to reinforce the stem cells to withstand the rigors of the microenvironment of the infarcted heart incurred from ischemia, inflammatory responses, and pro-apoptotic factors in order to develop an effective therapeutic modality. Several strategies have been proposed to augment the longevity of engrafted cells in the hostile ischemic environment [4]. These include the following: (1) treatment of the host tissue environment to make it more receptive for the donor cells; (2) in vitro manipulation (i.e., preconditioned donor cells); and (3) genetic modulation of the donor cells to make them more robust and resistant to apoptosis. For example, Mangi et al. demonstrated that a direct intramuscular injection of 5 × 106 Akt-engineered MSCs more greatly improved the function of infarcted rat hearts than that of MSCs alone [8].
Recently, our laboratory showed that a small heat-shock protein 20 (Hsp20) interacted with phosphorylated Akt. Consequently, Akt activity was maintained under stress conditions and led to pro-survival effects of cardiomyocytes on oxidative stress [9]. Furthermore, transgenic mice overexpressing Hsp20 in the heart had reduced infarct size and improved recovery of cardiac function after ischemia/reperfusion injury [10]. More importantly, Hsp20 was observed to be the most upregulated in the differentiated human adipose-derived adult stem cells, compared with other Hsp's, suggesting that Hsp20 may play a critical role in stem cell differentiation [11]. In addition, the Hsp20 protein contains the strongest interactive domains with VEGF (116EFHRKYRI123), FGF-2 (110HGFIAREF117), and insulin (73FSVLLDVK81), which overlap with previously identified αB-crystallin chaperone and filament interactive sequences [12]. This suggests that Hsp20 may be important for the folding and processing of these growth factors in vivo and consequently, may prolong their half-life. Taken together, these data led us to a hypothesis that increased levels of Hsp20 in MSCs would be beneficial for cell survival under ischemic conditions via Akt signaling and enhanced secretion of growth factors. Indeed, the present study demonstrates that, compared to green fluorescent protein (GFP)-modified MSCs, Hsp20-engineered MSCs were more resistant to oxidative stress, evidenced by decreased lactic dehydrogenase (LDH) release and reduced cell death. After transplantation into the ischemic rat heart, Hsp20-MSCs enhanced cardiac repair. The mechanisms underlying the salutary effects of Hsp20-MSCs were associated with increased Akt phosphorylation and secretion of growth factors. These observations suggest that Hsp20 may represent a potential therapeutic candidate for treatment of myocardial infarction with gene-based cell therapy.
Materials and Methods
Purification and Adenoviral Transduction of Mesenchymal Stem Cells
MSCs were isolated from the bone marrow of adult Sprague-Dawley (SD) male rats (Harlan Laboratories, Indianapolis, IN, http://www.harlan.com) and expanded as we previously described [13, 14]. All animal procedures were in accordance with the Guidelines for the Care and Use of Laboratory Animals prepared by the National Academy of Sciences and published by the National Institutes of Health (NIH publication no. 85–23, revised 1996) and were approved by the University of Cincinnati Animal Care and Use Committee. The MSCs were cultured with Iscove's modified Dulbecco's medium (IMDM; Gibco, Grand Island, NY, http://www.invitrogen.com) containing 15% fetal bovine serum and antibiotics. Culture medium was changed every 3–4 days; nonadherent hematopoietic cells were removed in this process. Subsequent passages were performed with a 0.25% trypsin solution containing 0.01% EDTA (Sigma-Aldrich, St. Louis, http://www.sigmaaldrich.com) for 10 minutes at 37°C. Passage 3 MSCs were transduced with an adenoviral vector encoding the gene for GFP-MSCs or with a bicistronic vector expressing both genes for GFP and Hsp20 (Hsp20-MSCs). Transduction efficiency was assessed by fluorescence microscopy.
In Vitro Treatment of MSCs with H2O2 and Cell Survival Assay
Prior to H2O2 treatment, MSCs or gene-modified MSCs were incubated with serum-free IMDM for 2 hours. Different doses of H2O2 (0, 100, 200, and 500 μM) were added to the Hsp20-MSCs or GFP-MSCs and incubated for different time periods (0, 1, and 2–5 hours), as indicated. H2O2 has been used by several laboratories as an oxidant that induces cellular damage similar to ischemia or anoxia [15, 16]. The cell viability was determined by the CellTiter 96 AQueous One Solution Cell Proliferation Assay Kit (Promega, Madison, WI, http://www.promega.com), per the manufacturer's instructions. Cell supernatant was analyzed for LDH using an in vitro Toxicology Assay Kit (Sigma) and expressed as units per milliliter (U/ml). Pyruvate Kinase (PK)/LDH enzyme (Sigma, #P-0294) was used as a standard control. Cell apoptosis was examined using In Situ Cell Death Detection kit (TMR red; Roche Diagnostics, Basel, Switzerland, http://www.roche-applied-science.com).
In Vitro Experiments with Conditioned Medium
Conditioned medium was generated as follows: 90% confluent GFP- or Hsp20-MSCs were cultured with serum-free IMDM and subjected to 200 μM H2O2 treatment for 5 hours. The medium was then collected and used for in vitro experiments. Adult rat cardiomyocytes (ARCMs) were obtained from Langendorff-per-fused hearts of 4- to 6-week-old female SD rats at 37°C, as described before [17]. Cells were seeded in 12-well plates pre-coated with laminin [10 μg/ml in phosphate-buffered saline (PBS)] and left overnight in standard growth medium (M199; Gibco). One day later, the M199 medium was replaced with serum-free IMDM [normal medium (NM)] or conditioned medium (CM) from either GFP-MSCs or Hsp20-MSCs. The ARCMs were then treated with 50 μM H2O2 for 2 hours. Cell viability assessment was performed with the CellTiter 96 AQueous One Solution Cell Proliferation Assay Kit (Promega), per the manufacturer's instructions. Nuclear fragmentation was determined by Hoechst staining and visualized under a fluorescent microscope, as previously described [17, 18].
Western Blot Analysis, Immunostaining, and Caspase-3 Activity Assay
Protein samples were extracted from MSCs, with the procedures as described in detail elsewhere [9, 19]. Protein samples (10 μg for Hsp20 detection, 150 μg for detection of Akt and its phosphorylation, and Bcl-2 and Bax) were fractionated by 12% SDS-polyacrylamide gel electrophoresis. A primary antibody against Hsp20 (1:5000 dilution; Research Diagnostics, Concord, MA, http://www.researchd.com), rabbit anti-Akt and its phosphorylation (pS473Akt) antibodies, and rabbit anti-Bcl-2 antibody and rabbit anti-Bax antibody (1:500 dilution; Cell Signaling Technology, Beverly, MA, http://www.cellsignal.com) were used; β-actin (1:1000 dilution; Sigma-Aldrich) was served as an internal control. Immunostaining was performed with antibodies to CD34, CD44, CD45, CD71, and CD90 (BD Pharmingen, San Diego, http://www.bdbiosciences.com) as described before [8]. Caspase-3 activity was determined in MSC lysates (200 μg) using Caspase-3/CPP32 Fluorometric Assay kit (BioVision, Inc., Mountain View, CA, http://www.biovision.com).
Measurement of VEGF, FGF-2, and IGF-1 Secretion by ELISA
The levels of VEGF, FGF-2, and IGF-1 released from MSCs into culture medium were directly measured by ELISA kit according to manufacturer's instructions (R&D Systems Inc., Minneapolis, http://www.rndsystems.com). Basal medium was used as a control. The absorbance was measured at 450 and 570 nm.
Myocardial Infarction and MSC Transplantation
A myocardial infarction (MI) model was developed in SD female rats (200–250 g), as previously described [13]. Briefly, isoflurane anesthesia was induced by spontaneous inhalation. The inhalation gas was a mixture of air and oxygen (total oxygen: 40%) and 2.4% isoflurane. A midline cervical skin incision was performed for intubation. The animals were mechanically ventilated with room air supplemented with oxygen (1.5 liters/minute) using a rodent ventilator (Model 683; Harvard Apparatus, South Natick, MA, http://www.harvardapparatus.com). Body temperature was carefully monitored with a probe (Cole-Parmer, Vernon Hill, IL, http://www.coleparmer.com) and was maintained at 37°C throughout the surgical procedure. The heart was exposed by left-side limited thoracotomy and the left anterior descending artery (LAD) was ligated with a 6–0 polyester suture 1 mm from the tip of the normally positioned left auricle. In the sham group, rats underwent a sham operation with loose suture around LAD. Successful infarction was determined by observing a pale discoloration of the left ventricular muscle and an ST elevation on electrocardiograms. Ten minutes after ligation, 1 × 106 GFP-MSCs or Hsp20-MSCs (total of 50 μl) were injected at 3–5 injection sites into anterior and lateral aspects of the viable myocardium bordering the infarction with a 31-gauge needle (BD Bio-sciences, San Diego, http://www.bdbiosciences.com). LAD ligated rats with PBS injection served as controls. Some of the animals were sacrificed for examining cell survival by day 4 after cell implantation. Cardiac function was assessed by echocardiograph, and hearts were harvested and analyzed for various studies by week 4 after cell transplantation.
Real-Time Polymerase Chain Reaction for Donor Cell Survival
Real-time polymerase chain reaction (PCR) was performed for quantification of the Sry-DNA on day 4 after cell transplantation. The rat heart samples from different groups were frozen in liquid nitrogen and powdered. The DNA purification was performed using Genomic DNA Isolation kit (Qiagen, Hilden, Germany, http://www1.qiagen.com) and total RNA was isolated using TRIzol Reagent (Invitrogen, Carlsbad, CA, http://www.invitrogen.com). The concentration of the purified DNA or RNA was determined by spectrophotometry. GFP cDNA was reverse-transcripted using SuperScript III First-Strand Synthesis Super-Mix (Invitrogen). Real-time PCR was performed using iQ SYBR-Green supermix (Bio-Rad, Hercules, CA, http://www.bio-rad.com) in a Bio-Rad iQ5 optical module. Primers for amplification of rat Y-chromosome Sry, GFP, and GAPDH genes are listed below.
Sry: sense primer, 5′-GAG GCA CAA GTT GGC TCA ACA 3′; antisense primer, 5′-CTC CTG CAA AAA GGG CCT TT 3′. GFP: sense, 5′-AAGTTCATCTGCACCACCG-3′; antisense, 5′-TCCTTGAAGAAGATGGTGCG-3′. GAPDH: sense, 5′-TGC AGT GGC AAA GTG GAG-3′; antisense, 5-ACA TAC TCA GCA CCG GCC TC-3′. The cycling conditions were set at 3 minutes at 95°C for initial denaturation, 40 cycles of denaturation at 95°C for 30 seconds, annealing at 59.5°C for 40 seconds, and extension at 72°C for 50 seconds. The data were acquired during the extension step. Melting curves were obtained at the end of the reaction by gradually raising the temperature by 1°C/minute from 59.5°C to 95°C over a time period of 35 minutes.
Echocardiography
Left ventricular (LV) function variables were assessed by trans-thoracic echocardiography, which was performed at 4 weeks after MI using iE33 Ultrasound System (Philips International B.V., Amsterdam, Netherlands, http://www.medical.philips.com) with a 15-MHz probe. After the induction of light general anesthesia, hearts were imaged two dimensionally in long-axis view at the level of the greatest LV diameter. This view was used to position the M-mode cursor perpendicular to the LV anterior and posterior walls. The LV internal end-diastolic diameter (LVIDd) and LV internal end-systolic diameter (LVIDs) were measured from M-mode recordings according to the leading-edge method. LV ejection fraction (LVEF) was calculated as follows: LVEF (%) = [(LVIDd)3 − (LVIDs)3]/(LVIDd)3 × 100. The percentage of LV fractional shortening (FS) was calculated as follows: FS (%) = [(LVIDd − LVIDs)/LVIDd] × 100. All echocardiograph measurements were averaged from at least six separate cardiac cycles.
Measurement of Fibrosis
Fixed hearts were embedded in paraffin and LV cross sections from apex, mid-LV, and base were stained with Trichrome-Masson. Images of the LV area of each slide were taken by an Olympus BX41 microscope equipped with CCD (MagnaFire; Olympus, Tokyo, http://www.olympus-global.com) camera. Fibrosis area and total LV area of each image were measured using the Image-Pro Plus (Media Cybernetics, Carlsbad, CA, http://www.mediacy.com), and the percentage of the fibrosis was calculated as follows: (fibrosis area/total LV area) × 100.
Determination of Vascular Density
The vascular density in both infarcted and normal myocardial tissue was determined as we previously described [20]. Tissue sections were triple-stained using either CD31 antibody (PECAM-1; Santa Cruz Biotechnology Inc., Santa Cruz, CA, http://www.scbt.com) for examination of angiogenesis and capillary density or α-smooth muscle actin (SMA; Sigma) antibody for evaluation of vascular density, troponin I (Santa Cruz Biotechnology Inc.) for cardiac myocytes, and 4′,6-diamino-2-phenylindole (DAPI; Sigma) for all nuclei. For quantification of positively stained vessels, five sections within the peri-infarcted area of each animal were analyzed by an investigator who was blinded with respect to the cell treatment. Blood vessels were detected at low magnification, ×200.
Statistical Analysis
Results were statistically analyzed with the use of the StatView 5.O software package (Abacus Concepts, Inc., Piscataway, NJ, http://abacus-concepts.com). All values were expressed as mean ± SD and were analyzed by ANOVA for repeated measures, followed by Bonferroni/Dunn test or unpaired t test. p value less than .05 was considered statistically significant.
RESULTS
Increased Hsp20 Expression in Rat MSCs upon Oxidative Stress
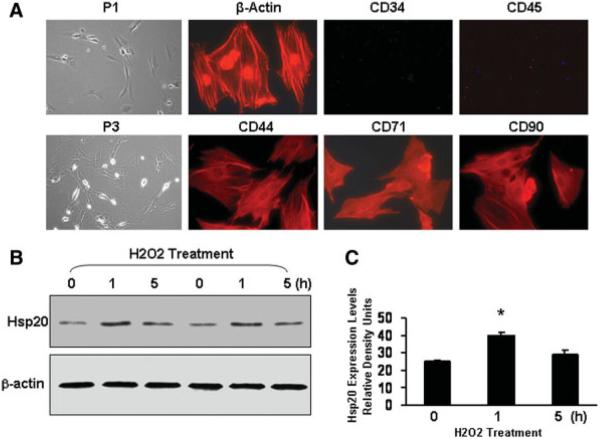
The isolation and expansion of MSCs were performed as in our previous reports [13, 14]. By repeated washing, MSCs can be easily separated from the hematopoietic stem cells because of their preferential attachment to the culture plate. The morphology of rat MSCs from bone marrow displayed a homogenous spindle-shaped population and maintained a similar morphology during the subsequent passages (Fig. 1A). Immunostaining analysis showed that MSCs were devoid of CD34 and CD45 (Fig. 1A), which are markers for hematopoietic cells. In contrast, more than 90% of MSCs expressed CD44, CD71, and CD90 (Fig. 1A). These results are further confirmed by fluorescence-activated cell sorting analysis (data not shown).
Figure 1.
Characterization of mesenchymal stem cells (MSCs) isolated from male rat bone marrow and time course of Hsp20 expression in response to H2O2 treatment (200 μM). (A): Cultured P3 MSCs were immunostained with primary antibodies as indicated. Alexa Fluor 594 goat anti-rabbit or mouse IgG was used as a secondary antibody and also negative controls. Images of phase contrast are at original magnification, ×100; the immunocytochemistry images are at original magnification, ×400. (B): At the indicated intervals, MSCs treated with H2O2 were lysed to assess Hsp20 expression by Western blot analysis. Panel (C) shows the quantitative results from the Western blots. Similar results were observed in three additional, independent experiments. β-Actin was used as internal control. Abbreviations: Hsp, heat-shock protein; P1, passage 1; P3, passage 3.
Recently, we have demonstrated that the expression of Hsp20 was transiently upregulated in cardiomyocytes under doxorubicin treatment and β-agonist stimulation [9, 17]. To further evaluate the expression of Hsp20 in MSCs in response to oxidative stress, we treated MSCs with 200 μM H2O2 for 1 or 5 hours. Western blots and quantitative results (Fig. 1B, 1C) indicated that the levels of Hsp20 were transiently increased by 1.7-fold at 1 hour and then returned to basal at 5 hours. These results suggest that Hsp20 may be instrumental during oxidative stress.
Overexpression of Hsp20 Protects MSCs Against Apoptosis In Vitro
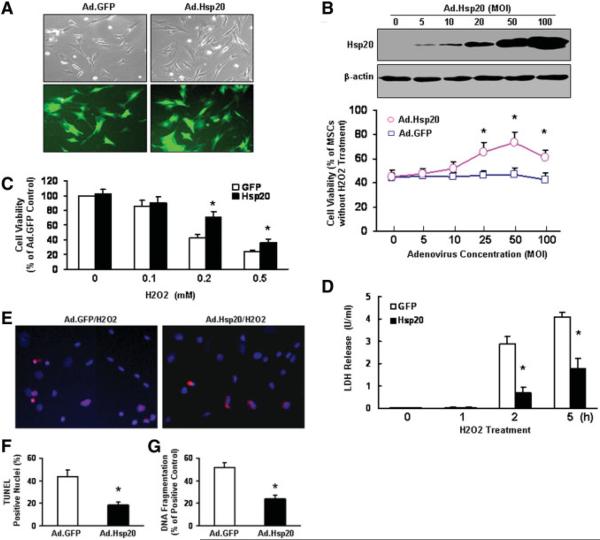
The transfection efficiency of adenoviral vector for genetically modified MSCs was evaluated by green fluorescent protein (GFP) as an internal control after 36–48 hours of gene delivery. A representative GFP expression is shown in Figure 2A. We observed that more than 90% of MSCs were transfected by adenoviral vector having 50 multiplicity of infection (MOI, number of viruses per cell) (Fig. 2A). Furthermore, Hsp20-MSCs and GFP-MSCs had a similar phenotype 3 days after transfection (data not shown). To test the capability of Hsp20-MSCs to protect against cell death in vitro, we infected MSCs with different MOIs of Ad.Hsp20, followed by treatment with 200 μM H2O2 for 2 hours. We observed that a significant increase in cell survival was dependent on the level of Hsp20 overexpression (Fig. 2B). Furthermore, gene-modified MSCs were treated with different concentrations of H2O2 for 2 hours or 200 μM H2O2 for different time points (0, 1, 2, and 5 hours). We found that cell death was greatly reduced in Hsp20-MSCs compared with GFP controls, as measured by (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt (MTS)-based cell incorporation (Fig. 2C) and LDH release (Fig. 2D). However, there was no significant difference in cell death among MSCs, GFP-MSCs, and Hsp20-MSCs when measured under normal conditions. Accordingly, the apoptotic cell number, determined using TUNEL assay (red) (Fig. 2E), was greatly decreased in Hsp20-MSCs (19% ± 2.1%) compared with that of GFP-MSCs (43% ± 5.2%) upon treatment with 200 μM H2O2 for 5 hours (Fig. 2F). These observations were further confirmed by DNA fragmentation assays (Fig. 2G), as measured by the content of cytosolic mono- and oligonucleosomes (180 base pair nucleotides or multiples) by a cell death ELISA kit. Results of the quantitative nucleosome assay showed that levels of mono- and oligonucleosomes were significantly lower in Hsp20-MSCs treated with H2O2 (23% ± 1.9%) relative to treated GFP-MSCs (50% ± 3.1%) (Fig. 2G). Collectively, these findings demonstrate that the Hsp20-modified MSCs could protect against oxidative stress. It is anticipated that Hsp20-engineered MSCs may retain their antiapoptotic properties in the ischemic myocardium.
Figure 2.
Effect of increased Hsp20 expression on MSCs survival after H2O2 treatment. (A): There was no morphological change between the empty vector Ad.GFP- and Ad.Hsp20-infected MSCs, and more than 90% of MSCs were infected by recombinant adenovirus vector after 36 hours, as indicated by GFP fluorescence. (B): The cytoprotective effect of Hsp20 was observed in MSCs infected with different concentrations of Ad.Hsp20, which was dependent on the levels of Hsp20 overexpression. (C): Hsp20-MSCs showed significantly higher cell viability than GFP-MSCs after treatment with H2O2 at 0.2 or 0.5 mM for 2 hours. (D): Hsp20-MSCs were resistant to H2O2-induced cell death, as evidenced by decreased LDH release, compared with GFP-MSCs. (E–G): Hsp20-MSCs reduced H2O2-induced apoptosis, as measured by TUNEL staining (red) (E,F) and DNA fragmentation assay by a cell-death-detection ELISA kit (G). Similar results were observed in three additional, independent experiments. *, p < .05 versus H2O2-treated GFP-MSCs. Abbreviations: GFP, green fluorescent protein; Hsp, heat-shock protein; LDH, lactate dehydrogenase; MOI, multiplicity of infection; MSCs, mesenchymal stem cells.
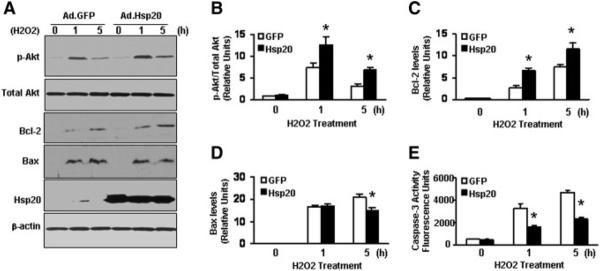
Hsp20-Modified MSCs Upregulated Akt Phosphorylation Under Oxidative Stress
Recently, we showed that Hsp20 interacted with phosphorylated Akt and maintained Akt activity in cardiomyocytes upon doxorubicin treatment [9]. To elucidate the mechanism of enhanced survival in Hsp20-MSCs under oxidative stress, we first assessed the expression levels of Akt phosphorylation (S473-Akt) in H2O2-treated Hsp20-MSCs and GFP-MSCs. Western blots (Fig. 3A) showed that the endogenous Akt was dramatically activated by 7.3 ± 1.2-fold in GFP-MSCs at 1 hour after H2O2 treatment, which was further enhanced in Hsp20-MSCs (12 ± 1.9-fold) related to nontreatment controls. Furthermore, the phosphorylated Akt was decreased to near basal levels in GFP-MSCs, but remained significantly elevated in Hsp20-MSCs after a 5-hour treatment with H2O2 (3.1 ± 0.5-fold vs. 6.8 ± 0.7-fold, respectively; Fig. 3B). In addition, although both Bcl-2 and Bax levels were markedly increased in MSCs upon H2O2 treatment, the degree of Bcl-2 increase was significantly higher in Hsp20-MSCs than that of GFP-MSCs (Fig. 3C), and the increase of Bax level was attenuated in Hsp20-MSCs by a 5-hour treatment with H2O2, related to GFP controls (Fig. 3D). Accordingly, caspase-3 activity was reduced by 45%–50% in H2O2-treated Hsp20-MSCs compared with that of GFP controls (Fig. 3E). Taken together, these data indicate that protective effects of Hsp20 against cell death are associated with activation of Akt and increased ratio of Bcl-2/Bax; consequently, caspase-3 activation was inhibited.
Figure 3.
Effects of Hsp20 on the expression of Akt-caspase-3 signaling cascades in H2O2-treated MSCs. (A,B): There were no differences in the phosphorylation levels of Akt at the S473 site between Hsp20-MSCs and GFP-MSCs under basal conditions. However, upon H2O2 treatment for 1 hour, the levels of p-S473-Akt were significantly increased in both Hsp20-MSCs and GFP-MSCs, with higher levels increased in Hsp20-MSCs than in GFP-MSCs. This significant increase in Akt phosphorylation was also observed in Hsp20-MSCs after H2O2 treatment for 5 hours. (A,C): Bcl-2 was upregulated in MSCs upon H2O2 treatment. The degree of increase in Bcl-2 levels was significantly higher in Hsp20-MSCs than in GFP-MSCs at both 1 and 5 hours of H2O2 treatment. (A,D): Addition of H2O2 induced Bax expression in MSCs. The level of Bax expression was significantly reduced in Hsp20-MSCs than in GFP-MSCs at 5 hours of H2O2 treatment. (E): Caspase-3 activity was markedly decreased in Hsp20-MSCs after H2O2 treatment, compared to that in H2O2-treated GFP-MSCs. Similar results were observed in three additional, independent experiments. *, p < .05 versus H2O2-treated GFP-MSCs. β-Actin was used as internal control. Abbreviations: GFP, green fluorescent protein; Hsp, heat-shock protein; MSCs, mesenchymal stem cells.
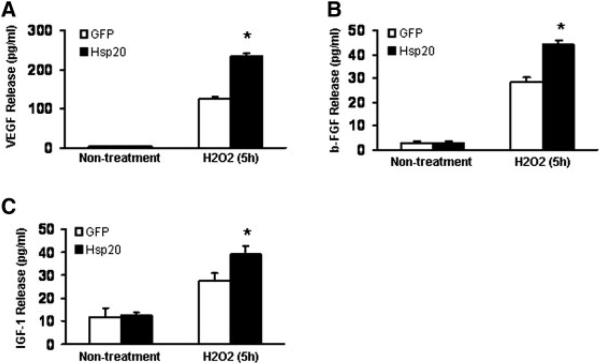
Hsp20-MSCs Enhanced Release of Growth Factors VEGF, FGF-2, and IGF-1 Under Oxidative Stress
To identify potential paracrine mechanisms responsible for the therapeutic effect of Hsp20-MSCs, we examined the secretion of major growth factors (VEGF, FGF-2, and IGF-1). GFP-MSCs and Hsp20-MSCs were cultured under oxidative stress for up to 5 hours, and secretion of growth factors was quantitated by Quantikine rat VEGF, FGF-basic, and IGF-1 immunoassay. A more than 80% increase in the secretion of VEGF was detected in Hsp20-MSCs compared with that in GFP-MSCs when the cells were treated with H2O2 for 5 hours (Fig. 4A). Similarly, there was an approximately 55% increase of FGF-2 and an approximately 39% increase of IGF-1 in Hsp20-MSCs related to that in GFP-MSCs upon H2O2 treatment for 5 hours (Fig. 4B, 4C). However, no significant difference was observed between the groups when the cells were cultured under either normal conditions or H2O2 treatment for 1 and 2 hours (data not shown). These data suggest that the high-level secretion of growth factors from Hsp20-MSCs may provide cardioprotective and proangiogenic effects.
Figure 4.
Increased secretion of (A) VEGF, (B) FGF-2, and (C) IGF-1 in Hsp20-MSCs at 5 hours of incubation with H2O2 (0.2 mM), compared with GFP-MSCs. *, p < .05. Abbreviations: GFP, green fluores-cent protein; Hsp, heat-shock protein; MSCs, mesenchymal stem cells.
Hsp20-MSCs Conditioned Medium Protects Adult Rat Cardiomyocytes Against Oxidative Stress
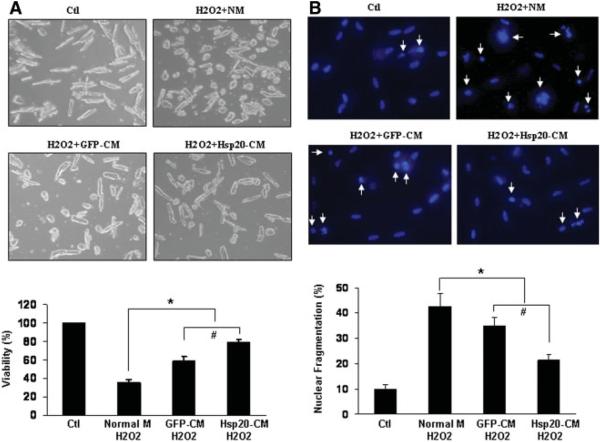
To further evaluate the effects of conditioned medium from cultured MSCs in vitro on adult rat cardiomyocytes (ARCMs) subjected to oxidative stress, we collected the serum-free conditioned medium IMDM from MSCs after 5 hours of treatment with/without H2O2. Initially, the ARCM standard growth medium was replaced with IMDM normal medium or oxidatic-conditioned medium from GFP-MSCs or Hsp20-MSCs; the ARCMs were subsequently treated with 50 μM H2O2 for 2 hours. Cell viability was assessed by MTS incorporation. After exposure to H2O2 in the presence of normal medium, the cell viability was considerably reduced by approximately 60% related to that of nontreated controls (Fig. 5A). Although the cytoprotective effect was observed in the presence of both GFP-MSCs and Hsp20-MSCs oxidative-stressed medium, the greater degree of protection was conferred by the Hsp20-MSC conditioned medium, with an increase by approximately 20% in the number of viable ARCMs as compared with that by the conditioned medium from H2O2-stressed GFP-MSCs (Fig. 5A). Furthermore, the relative number of apoptotic ARCMs exposed to H2O2 for 2 hours was quantified by nuclear condensation after staining with Hoechst 33342 (Fig. 5B). In the presence of Hsp20-MSC oxidative-stressed medium, the number of apoptotic nuclei was reduced to 21 ± 1.9% as compared with that of the normal medium (43 ± 5.1%, p < .05) or the GFP-MSC H2O2-conditioned medium (35 ± 3.2%, p < .05), respectively (Fig. 5B). Thus, these results indicate that a paracrine cytoprotective mechanism is involved with growth factors secreted by the MSCs under ischemic conditions.
Figure 5.
Effect of the mesenchymal stem cell conditioned medium on viability and apoptosis of adult rat cardiomyocytes (ARCMs) exposed to H2O2. (A): Representative microphotographs of ARCMs grown in control NM or different CM under H2O2 (50 μM) treatment for 2 hours. Cell viability was determined by MTS analysis, and the results of three independent measurements were summarized in the bar graph. *, p < .05 versus control; #, p < .05 versus GFP conditioned medium. (B): Representative images of cardiomyocytes stained with Hoechst 33342 to detect apoptotic nuclei. Apoptotic nuclei are pyknotic (arrows), whereas nonapoptotic nuclei are longitudinal. Pyknotic nuclei were counted and expressed as the percentage of total nuclei. Bars represent mean ± SD from three independent experiments with more than 200 nuclei counted per group. *, p < .05 versus control; #, p < .05 versus GFP conditioned medium. Abbreviations: CM, conditioned medium; GFP, green fluores-cent protein; Hsp, heat-shock protein; NM, normal medium.
Hsp20 Enhances Implanted MSCs Survival in the Infarcted Heart
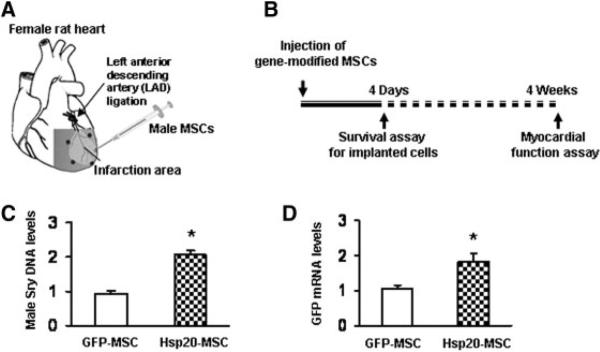
To further validate the protective properties of the Hsp20-MSCs in vivo, we transplanted 1 × 106 Hsp20-MSCs or GFP-MSCs into the female viable left ventricular (LV) myocardium bordering infarction (Fig. 6A). PBS was injected into control animals. The survival of implanted cells was determined by real-time PCR for GFP mRNA and rat Y-chromosome Sry DNA. The results showed that the degree of cell survival in the Hsp20-MSC group was greater than that in the GFP-MSCs following cell transplantation, with approximately twofold enhancement of cell survival by day 4 (n = 4, p < .5) (Fig. 6B–6D). These data indicate that manipulation of MSCs with Hsp20 gene enhances cellular survival after transplantation.
Figure 6.
Effects of Hsp20 overexpression on transplanted MSC survival in vivo. (A): Diagram of in vivo gene-modified MSC therapy for myocardial infarction. After left anterior descending artery ligation, phosphate-buffered saline, male GFP-MSCs, or male Hsp20-MSCs were injected into 3–5 spots in the border zone surrounding the infarcted zone of the female rat heart. (B–D): Four days after injection, real-time polymerase chain reaction for Sry gene and GFP mRNA showed a significant 2.1-fold and 1.83-fold increase, respectively, in donor cell survival in the Hsp20-MSC group as compared with that in the GFP-MSC group. *, p < .05, n = 4 hearts). The GAPDH gene or mRNA was used as internal control. Abbreviations: GFP, green fluorescent protein; Hsp, heat-shock protein; MSCs, mesenchymal stem cells.
Cardiac Function and Fibrosis
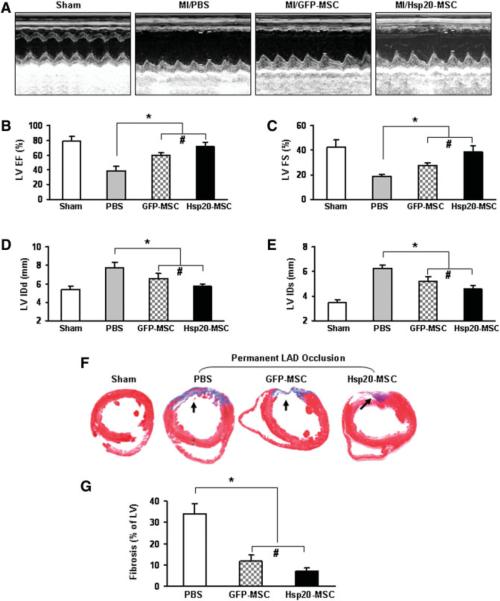
M-mode echocardiography measurements for left ventricular function demonstrated that LVIDs, LVIDd, LVEF, and LV fractional shortening (LVFS) were improved significantly more in the Hsp20-MSC group (LVIDs: 4.3 ± 0.4 mm; LVIDd: 5.5 ± 0.2 mm; LVEF: 71 ± 6.1%; and LVFS: 39 ± 4.9%) than in the GFP-MSC group (LVIDs: 5.1 ± 0.3 mm; LVIDd: 6.4 ± 0.6 mm; LVEF: 59 ± 3.6%; and LVFS: 27 ± 2.4%; n = 8, p < .05) at 4 weeks after MI (Fig. 7A–7E). Compared with PBS-treated hearts, both Hsp20-MSCs and the GFP-MSC group had significant improvements in cardiac function (Fig. 7A–7E). Furthermore, the percentage of fibrosis in the left ventricle wall was 40% lower in Hsp20-MSC treated hearts than in GFP-MSC treated hearts (11.8 ± 2.9% of GFP-MSC group vs. 7.0 ± 1.6% of Hsp20-MSC group; n = 8, p < .05), and both groups showed significant reduction in the percentage of fibrosis compared with that of PBS controls (39% ± 4.3%) (Fig. 7F, 7G).
Figure 7.
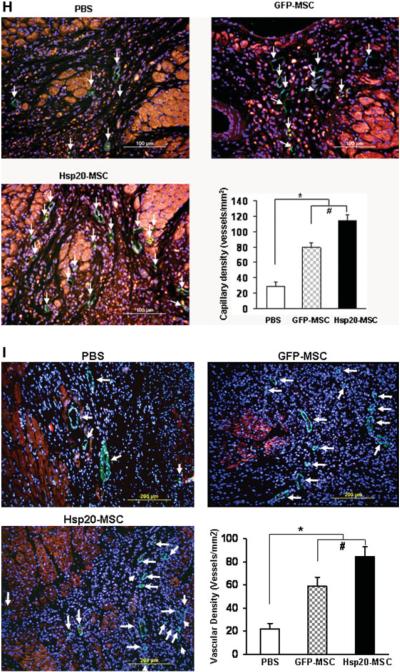
Effects of Hsp20-modified MSCs on cardiac remodeling and myocardial function in sham or MI hearts 4 weeks after therapeutic intervention. (A): Representative M-mode echocardiograms are shown for sham, PBS-treated, GFP-MSCs, and Hsp20-MSCs groups. Quantitative data are shown in (B) ejection fraction (EF), (C) fractional shortening (FS), (D) LVIDd, and (E) LVIDs from sham or transplanted hearts. (F) Masson's trichrome staining showed fibrosis (arrows) and (G) quantitative analysis in recipient hearts. Blood vessels were stained for (H) CD-31 (capillary density) or (I) α-smooth muscle actin (SMA) in hearts of the PBS group, GFP-MSCs, and Hsp20-MSCs and their quantitative analysis. Original magnification: ×200. Data are means ± SD; n = 8 hearts per group. *, p < .05 versus the PBS group; #, p < .05 versus GFP-MSC group. Abbreviations: red, cardiac troponin I; green, CD31 (H) and SMA (I) (arrows); blue, 4′,6-diamino-2-phenylindole; GFP, green fluorescent protein; Hsp, heat-shock protein; LAD, left anterior descending artery; LV, left ventricular; LVEF, LV ejection fraction; LVFS, LV fractional shortening; LVIDd, LV internal end-diastolic diameter; LVIDs, LV internal end-systolic diameter; MI, myocardial infarction; MSCs, mesenchymal stem cells; PBS, phosphate-buffered saline.
Blood Vessel Density
To evaluate the effects of implanted Hsp20-MSCs on formation of new blood vessels in vivo, CD31 (PECAM-1), a marker for endothelial cells, was used to determine the capillary density at the border zone of MI. We observed that both GFP-MSC treated and Hsp20-MSC treated groups revealed a significant increase in the capillary density, compared with that of PBS-treated hearts (Fig. 7H). However, the angiogenesis in Hsp20-MSC implanted hearts was further enhanced by 37%, compared to that of GFP-MSC controls (Fig. 7H). Similarly, vascular density, determined by immunostaining with SMA (a marker for vascular smooth muscle cells), was significantly increased in both GFP-MSC (59.2 ± 7.6 vessels per mm2) and Hsp20-MSC (84.5 ± 8.4 vessels per mm2) groups as compared with that of PBS-treated hearts with MI (21.9 ± 4.8 vessels per mm2) (Fig. 7I). Meanwhile, Hsp20-MSC groups showed a 43% higher vascular density as compared to that of GFP-MSC treated hearts (n = 8, p < .05) (Fig. 7I). These results suggest that Hsp20 enhances the paracrine effects of MSCs on infarcted hearts.
Discussion
Heat-shock treatment has been shown to enhance survival of implanted neonatal cardiomyocytes, skeletal myoblasts, and hESC-derived cardiomyocytes [3, 21, 22]. Heat shock can be typically achieved by heating cells to 43°C for 30–60 minutes 1 day before transplantation, and this yields upregulation of many heat-shock proteins (Hsp's) [3, 21]. Previous studies have shown that several Hsp's including Hsp70, Hsp60, and Hsp20 limit cell death by inhibiting pro-apoptotic signaling, acting as molecular chaperones for damaged proteins and opposing oxidative stress [23–27]. Although we have demonstrated that cardiac-specific overexpression of Hsp20 protected hearts against ischemia/reperfusion-induced injury [10], effects of direct injection of Hsp20 protein to ischemic hearts remain elusive. Furthermore, it is unclear whether Hsp modified-MSCs exert a beneficial effect on the infarcted heart post-engraftment. In this study, we identified the key roles of Hsp20 in protecting grafted MSC survival under hypoxia/ischemia in vitro and in vivo. Overexpression of Hsp20 reduced MSC death under hypoxic conditions. In vivo transplantation of Hsp20-MSCs enhanced the survival rate of MSCs in the LAD ligation model, which may lead to the attenuation of postinfarction LV remodeling and functional improvement. These observations suggest that genetic modification of MSCs with Hsp20 could be of significant value in improving the efficacy of stem cell therapy following a broad range of cardiac diseases. Our current study further supports the previous findings from Mangi et al. [8], Tang et al. [28], and Li et al. [29], who showed that the therapeutic efficacy of MSCs was closely related to in situ survival of cells implanted in the infarcted heart.
There are at least two mechanisms contributing to the resistance of Hsp20-MSCs against hypoxia/ischemia stress. We have previously shown that overexpression of Hsp20 in cardiomyocytes maintained Akt/caspase-3 signaling cascade, which resulted in attenuation of doxorubicin-triggered cardiomyocyte death [9]. In the present study, we verified this protective mechanism in MSCs. We treated MSCs with H2O2 to induce the oxidative damage that occurs in the myocardium after ischemic injury. Cell death induced by H2O2 was reduced in Hsp20-MSCs compared with that in GFP-MSCs. Furthermore, Western blot analysis clearly showed that Akt remained in its phosphorylated form in Hsp20-MSCs longer than in GFP-MSCs. It is well documented that activation of Akt plays a critical role in cell survival [30]. Overexpression of Akt improved neonatal cardiomyocyte graft survival, and several groups showed that Akt enhanced mesenchymal stem cell survival after transplantation in both rat and porcine myocardial infarction models [3, 8, 31, 32]. Moreover, Bcl-2, an anti-apoptotic protein, has been shown to be regulated by Akt [33] and enhances survival of transplanted stem cells in the injured heart [29]. Hence, our in vitro results suggest that adenoviral transfer of Hsp20 may contribute to the protection of an ischemic myocardium, possibly through an enhanced Akt signaling pathway.
Another cytoprotective mechanism of Hsp20 may involve the paracrine effects by secretion of growth factors. The present study clearly demonstrated the increased secretion of VEGF, FGF-2, and IGF-1 from Hsp20-MSCs in response to the hypoxic conditions. These paracrine factors from stem cells have been shown to protect cocultured adult cardiomyocytes against hypoxic/ischemic stress [34–36]. Accordingly, we observed that adult rat cardiomyocytes cultured with conditioned medium from Hsp20-MSCs survived better than those cultured with conditioned medium from GFP-MSCs. Therefore, transplantation of Hsp20-MSCs could provide adequate magnitude and duration of VEGF, FGF-2, and IGF-1 release in the ischemic myocardium, which would provide cardioprotective effects and induce functional collateral vessels, leading to the salvaging of ischemic myocardium and decrease the infarcted area. Indeed, recent studies from Dzau's group and others have demonstrated that MSCs produce and secrete a broad variety of adhesive molecules, chemokines, and growth factors such as SDF-1, VEGF, FGF-2, IGF-1, and HGF acting in a paracrine fashion, which attenuated pathological cardiac remodeling and promoted neovascularization [29, 34–37]. Hence, it is impossible to exclude other paracrine factors (except these growth factors examined in the present study) contributing to the protective effects of Hsp20-MSCs on infarcted hearts. Future studies using gene/protein arrays will be helpful to identify other important paracrine players generated in Hsp20-MSCs.
Although it has been shown that implanted MSCs trans-differentiate into cardiomyocytes via cell fusion dependent or independent mechanisms [28, 29, 32], the number of newly generated cardiac cells is too low to tell the difference between the Hsp20-MSC group and controls. Therefore, our study did not precisely define whether Hsp20-MSCs turned into cardiomyocytes. In summary, this study for the first time demonstrates that modified MSCs with a single heat-shock protein gene, Hsp20, reduced cell death under hypoxic conditions via maintenance of Akt activation and enhanced paracrine action. Transplantation of Hsp20-modified MSCs ameliorates LV remodeling and improves LV function. Thus, Hsp20 may be an important survival factor and new therapeutic candidate for treatment of ischemic heart disease with gene-based cell therapy. Further work on the long-term persistence, function, and phenotype of Hsp20-modified MSCs needs to be done for evaluation of Hsp20's role in myocardial regeneration.
Acknowledgments
This study was supported by NIH grants HL-087861 and HL087861-03S1 (Dr. G. C. Fan), HL-089824 and HL-081859 (Dr. Y. Wang), and HL-083236 (Dr. M. Xu).
Footnotes
Disclosure of Potential Conflicts of Interest The authors indicate no potential conflicts of interest.
References
- 1.Passier R, van Laake LW, Mummery CL. Stem-cell-based therapy and lessons from the heart. Nature. 2008;453:322–329. doi: 10.1038/nature07040. [DOI] [PubMed] [Google Scholar]
- 2.Sussman MA, Murry CE. Bones of contention: Marrow-derived cells in myocardial regeneration. J Mol Cell Cardiol. 2008;44:950–953. doi: 10.1016/j.yjmcc.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang M, Methot D, Poppa V, et al. Cardiomyocyte grafting for cardiac repair: Graft cell death and anti-death strategies. J Mol Cell Cardiol. 2001;33:907–921. doi: 10.1006/jmcc.2001.1367. [DOI] [PubMed] [Google Scholar]
- 4.Haider HKh, Ashraf M. Strategies to promote donor cell survival: Combining preconditioning approach with stem cell transplantation. J Mol Cell Cardiol. 2008;45:554–566. doi: 10.1016/j.yjmcc.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robey TE, Saiget MK, Reinecke H, et al. Systems approaches to preventing transplanted cell death in cardiac repair. J Mol Cell Cardiol. 2008;45:567–581. doi: 10.1016/j.yjmcc.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shake JG, Gruber PJ, Baumgartner WA, et al. Mesenchymal stem cell implantation in a swine myocardial infarct model: engraftment and functional effects. Ann Thorac Surg. 2002;73:1919–1925. doi: 10.1016/s0003-4975(02)03517-8. [DOI] [PubMed] [Google Scholar]
- 7.Toma C, Pittenger MF, Cahill KS, et al. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002;105:93–98. doi: 10.1161/hc0102.101442. [DOI] [PubMed] [Google Scholar]
- 8.Mangi AA, Noiseux N, Kong D, et al. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med. 2003;9:1195–1201. doi: 10.1038/nm912. [DOI] [PubMed] [Google Scholar]
- 9.Fan GC, Zhou X, Wang X, et al. Heat shock protein 20 interacting with phosphorylated Akt reduces doxorubicin-triggered oxidative stress and cardiotoxicity. Circ Res. 2008;103:1270–1279. doi: 10.1161/CIRCRESAHA.108.182832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fan GC, Ren X, Qian J, et al. Novel cardioprotective role of a small heat-shock protein, Hsp20, against ischemia/reperfusion injury. Circulation. 2005;111:1792–1799. doi: 10.1161/01.CIR.0000160851.41872.C6. [DOI] [PubMed] [Google Scholar]
- 11.DeLany JP, Floyd ZE, Zvonic S, et al. Proteomic analysis of primary cultures of human adipose-derived stem cells: Modulation by adipo-genesis. Mol Cell Proteomics. 2005;4:731–740. doi: 10.1074/mcp.M400198-MCP200. [DOI] [PubMed] [Google Scholar]
- 12.Ghosh JG, Shenoy AK, Jr,, Clark JI. Interactions between important regulatory proteins and human alphaB crystallin. Biochemistry. 2007;46:6308–6317. doi: 10.1021/bi700149h. [DOI] [PubMed] [Google Scholar]
- 13.Zhang D, Fan GC, Zhou X, et al. Over-expression of CXCR4 on mesenchymal stem cells augments myoangiogenesis in the infarcted myocardium. J Mol Cell Cardiol. 2008;44:281–292. doi: 10.1016/j.yjmcc.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dai Y, Ashraf M, Zuo S, et al. Mobilized bone marrow progenitor cells serve as donors of cytoprotective genes for cardiac repair. J Mol Cell Cardiol. 2008;44:607–617. doi: 10.1016/j.yjmcc.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miyawaki H, Wang Y, Ashraf M. Oxidant stress with hydrogen peroxide attenuates calcium paradox injury: Role of protein kinase C and ATP-sensitive potassium channel. Cardiovasc Res. 1998;37:691–699. doi: 10.1016/s0008-6363(97)00249-6. [DOI] [PubMed] [Google Scholar]
- 16.Pasha Z, Wang Y, Sheikh R, et al. Preconditioning enhances cell survival and differentiation of stem cells during transplantation in infarcted myocardium. Cardiovasc Res. 2008;77:134–142. doi: 10.1093/cvr/cvm025. [DOI] [PubMed] [Google Scholar]
- 17.Fan GC, Chu G, Mitton B, et al. Small heat-shock protein Hsp20 phosphorylation inhibits beta-agonist-induced cardiac apoptosis. Circ Res. 2004;94:1474–1482. doi: 10.1161/01.RES.0000129179.66631.00. [DOI] [PubMed] [Google Scholar]
- 18.Fan GC, Yuan Q, Song G, et al. Small heat-shock protein Hsp20 attenuates beta-agonist-mediated cardiac remodeling through apoptosis signal-regulating kinase 1. Circ Res. 2006;99:1233–1242. doi: 10.1161/01.RES.0000251074.19348.af. [DOI] [PubMed] [Google Scholar]
- 19.Ren XP, Wu J, Wang X, et al. MicroRNA-320 is involved in the regulation of cardiac ischemia/reperfusion injury by targeting heat-shock protein 20. Circulation. 2009;119:2357–2366. doi: 10.1161/CIRCULATIONAHA.108.814145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao T, Zhang D, Millard RW, et al. Stem cell homing and angiomyo-genesis in transplanted hearts are enhanced by combined intramyocardial SDF-1{alpha} delivery and endogenous cytokine signaling. Am J Physiol Heart Circ Physiol. 2009;296:H976–H986. doi: 10.1152/ajpheart.01134.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suzuki K, Smolenski RT, Jayakumar J, et al. Heat shock treatment enhances graft cell survival in skeletal myoblast transplantation to the heart. Circulation. 2000;102:III216–III221. doi: 10.1161/01.cir.102.suppl_3.iii-216. [DOI] [PubMed] [Google Scholar]
- 22.Laflamme MA, Chen KY, Naumova AV, et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007;25:1015–1024. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- 23.Trost SU, Omens JH, Karlon WJ, et al. Protection against myocardial dysfunction after a brief ischemic period in transgenic mice expressing inducible heat shock protein 70. J Clin Invest. 1998;101:855–862. doi: 10.1172/JCI265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okubo S, Wildner O, Shah MR, et al. Gene transfer of heat-shock protein 70 reduces infarct size in vivo after ischemia/reperfusion in the rabbit heart. Circulation. 2001;103:877–881. doi: 10.1161/01.cir.103.6.877. [DOI] [PubMed] [Google Scholar]
- 25.Jayakumar J, Suzuki K, Sammut IA, et al. Heat shock protein 70 gene transfection protects mitochondrial and ventricular function against ischemia-reperfusion injury. Circulation. 2001;104:I303–I307. doi: 10.1161/hc37t1.094932. [DOI] [PubMed] [Google Scholar]
- 26.Lin KM, Lin B, Lian IY, et al. Combined and individual mitochondrial HSP60 and HSP10 expression in cardiac myocytes protects mitochondrial function and prevents apoptotic cell deaths induced by simulated ischemia-reoxygenation. Circulation. 2001;103:1787–1792. doi: 10.1161/01.cir.103.13.1787. [DOI] [PubMed] [Google Scholar]
- 27.Fan GC, Chu G, Kranias EG. Hsp20 and its cardioprotection. Trends Cardiovasc Med. 2005;15:138–141. doi: 10.1016/j.tcm.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 28.Tang YL, Tang Y, Zhang YC, et al. Improved graft mesenchymal stem cell survival in ischemic heart with a hypoxia-regulated heme oxygenase-1 vector. J Am Coll Cardiol. 2005;46:1339–1350. doi: 10.1016/j.jacc.2005.05.079. [DOI] [PubMed] [Google Scholar]
- 29.Li W, Ma N, Ong LL, et al. Bcl-2 engineered MSCs inhibited apoptosis and improved heart function. Stem Cells. 2007;25:2118–2127. doi: 10.1634/stemcells.2006-0771. [DOI] [PubMed] [Google Scholar]
- 30.Miyamoto S, Rubio M, Sussman MA. Nuclear and mitochondrial signalling Akts in cardiomyocytes. Cardiovasc Res. 2009;82:272–285. doi: 10.1093/cvr/cvp087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lim SY, Kim YS, Ahn Y, et al. The effects of mesenchymal stem cells transduced with Akt in a porcine myocardial infarction model. Cardiovasc Res. 2006;70:530–542. doi: 10.1016/j.cardiores.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 32.Noiseux N, Gnecchi M, Lopez-Ilasaca M, et al. Mesenchymal stem cells overexpressing Akt dramatically repair infarcted myocardium and improve cardiac function despite infrequent cellular fusion or differentiation. Mol Ther. 2006;14:840–850. doi: 10.1016/j.ymthe.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 33.Belkhiri A, Dar AA, Zaika A, et al. t-Darpp promotes cancer cell survival by up-regulation of Bcl2 through Akt-dependent mechanism. Cancer Res. 2008;68:395–403. doi: 10.1158/0008-5472.CAN-07-1580. [DOI] [PubMed] [Google Scholar]
- 34.Gnecchi M, He H, Noiseux N, et al. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB J. 2006;20:661–669. doi: 10.1096/fj.05-5211com. [DOI] [PubMed] [Google Scholar]
- 35.Gnecchi M, Zhang Z, Ni A, et al. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204–1219. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Uemura R, Xu M, Ahmad N, et al. Bone marrow stem cells prevent left ventricular remodeling of ischemic heart through paracrine signaling. Circ Res. 2006;98:1414–1421. doi: 10.1161/01.RES.0000225952.61196.39. [DOI] [PubMed] [Google Scholar]
- 37.Mirotsou M, Zhang Z, Deb A. Secreted frizzled related protein 2 (Sfrp2) is the key Akt-mesenchymal stem cell-released paracrine factor mediating myocardial survival and repair. Proc Natl Acad Sci U S A. 2007;104:1643–1648. doi: 10.1073/pnas.0610024104. [DOI] [PMC free article] [PubMed] [Google Scholar]