Abstract
Himalayacetus subathuensis is a new pakicetid archaeocete from the Subathu Formation of northern India. The type dentary has a small mandibular canal indicating a lack of auditory specializations seen in more advanced cetaceans, and it has Pakicetus-like molar teeth suggesting that it fed on fish. Himalayacetus is significant because it is the oldest archaeocete known and because it was found in marine strata associated with a marine fauna. Himalayacetus extends the fossil record of whales about 3.5 million years back in geological time, to the middle part of the early Eocene [≈53.5 million years ago (Ma)]. Oxygen in the tooth-enamel phosphate has an isotopic composition intermediate between values reported for freshwater and marine archaeocetes, indicating that Himalayacetus probably spent some time in both environments. When the temporal range of Archaeoceti is calibrated radiometrically, comparison of likelihoods constrains the time of origin of Archaeoceti and hence Cetacea to about 54–55 Ma (beginning of the Eocene), whereas their divergence from extant Artiodactyla may have been as early as 64–65 Ma (beginning of the Cenozoic).
Pakicetus and contemporary archaeocetes have long been the oldest whales known as fossils (1–3). All are from red beds of the lower Kuldana Formation in Pakistan and the upper Subathu Formation in India, which are intercalated in a thicker sequence of Eocene marine sediments. All were deposited in a shallow epicontinental remnant of the Tethys Sea (Neotethys) that formerly separated the Indian subcontinent from the rest of Asia. These red beds yield essentially the same Kuldana and Kalakot freshwater fish and continental vertebrate fauna of early-middle Eocene age (4, 5). When first described, Pakicetus was interpreted as an amphibious initial stage of whale evolution that rested and reproduced on land and entered Tethys opportunistically to feed on fish (1).
We report here a new pakicetid archaeocete from marine strata of the middle Subathu Formation of India. The new pakicetid was found about 100 m lower stratigraphically and 3.5 million years older geologically than the Kuldana–Kalakot-equivalent upper Subathu red bed interval producing Pakicetus elsewhere. This not only extends the fossil record of Cetacea back in time, but also reinforces the idea that whales originated on the margin of Tethys and corroborates interpretation of pakicetids as an initial amphibious stage of cetacean evolution entering Tethys to feed on fish.
SYSTEMATICS
Order Cetacea Brisson 1762 Suborder Archaeoceti Flower 1883 Family Pakicetidae Gingerich & Russell 1990 Himalayacetus subathuensis, new genus and species
Etymology.
Himalaya, Sanskrit, place of snow and cetus, Greek (masculine), whale; subathuensis refers to the Subathu Formation and Subathu type section in the Himalayan foothills yielding the fossil described here.
Holotype.
Left dentary with molar teeth M2–3 (Figs. 1b and 2 a and b), Roorkee University Vertebrate Paleontology Laboratory [RUSB] specimen 2003, collected by A. Sahni, J.-J. Jaeger, V. Courtillot, and E. Buffetaut during a joint Panjab University–Université de Montpellier research project.
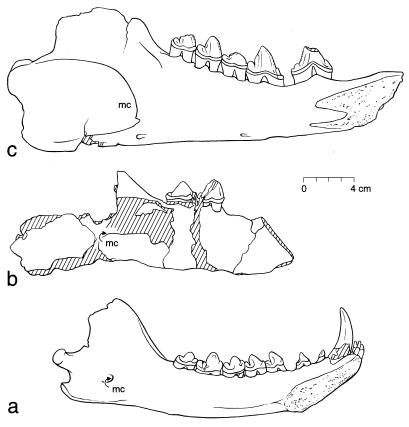
Figure 1.
Himalayacetus subathuensis type specimen compared with an earlier Asian mesonychid and a later Asian archaeocete. All are left dentaries shown in medial view. (a) Sinonyx jiashanensis type (6), late Paleocene, with incisor I1–3, canine C1, premolar P1–4, and molar M1–3 (China; Institute of Vertebrate Palaeontology and Palaeoanthropology IVPP 10760). (b) H. subathuensis type, early Eocene, with M2–3 (India; Roorkee University RUSB 2003). (c) Representative middle Eocene archaeocete with P3–4 and M1–3 (Pakistan; Geological Survey of Pakistan–University of Michigan GSP-UM 3062, drawing reversed from right side). Note similarities in overall size and rounded molar profiles, suggesting similar trophic adaptation. H. subathuensis retains a small mandibular canal (mc) like that of earlier mesonychids, contrasting with the larger mandibular canal of Ambulocetus and all postpakicetid archaeocetes; a small mandibular canal precludes whale-like hearing in water (3).
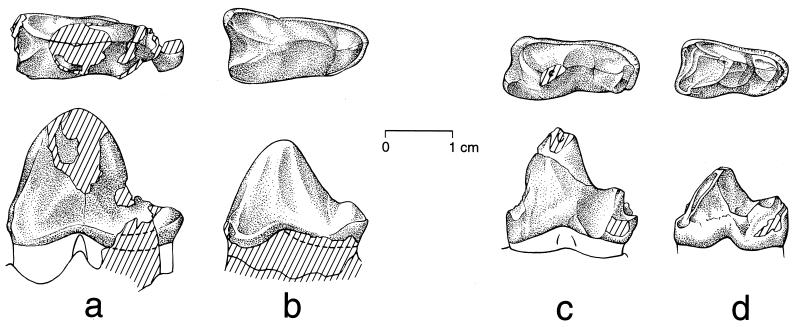
Figure 2.
Teeth of H. subathuensis holotype compared with those of pakicetid Pakicetus attocki and Pakicetus inachus. All teeth are shown in occlusal (Upper) and lateral (Lower) view; anterior is toward the left in all drawings. (a) Crown of left M2 of H. subathuensis, RUSB 2003. (b) Crown of left M3 of H. subathuensis, RUSB 2003. (c) Crown of left M2 of P. attocki (reversed), Howard University–Geological Survey of Pakistan H-GSP 18410 (7). (d) Crown of left M3 of P. inachus, GSP-UM 82 (8). Note the general similarity of molar form seen in Himalayacetus and Pakicetus. The sulcus on the anterior face of the trigonid is found in all archaeocetes. Differences distinguishing Himalayacetus include a larger and more rounded major cusp (protoconid), a smaller and less elevated talonid cusp (hypoconid) on the talonid, and a more anteriorly projecting anteromedial cusp at the base of the trigonid.
Type Locality.
Oyster-rich limestone near the base of the Subathu Formation type section in Kuthar Nala in the Simla Hills of the Lesser Himalaya Range, Himachal Pradesh, India (30° 58′ 54" N, 76° 58′ 36" E; Survey of India 15′ quadrangle 53 B/13).
Age and Distribution.
The type comes from zone IIIc in Mathur’s (9) standard classification of the Subathu Formation (Fig. 3). H. subathuensis is known only from the type locality.
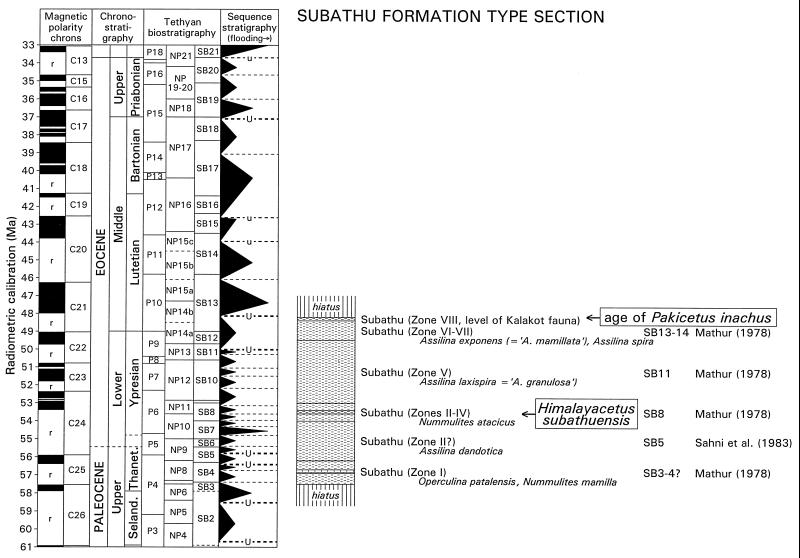
Figure 3.
Correlation chart relating Subathu Formation stratigraphy to the Eocene time scale and showing evidence constraining the geological age of H. subathuensis. Time scale, planktonic zonations (P and NP), and radiometric calibration are from Berggren et al. (10) as correlated with sea-level sequence stratigraphy by Hardenbol et al. (11). Shallow-benthic foraminiferal zonation (SB) is from Serra-Kiel et al. (12). Note low sea stands and the positions of major unconformities (U) just before and after the Ypresian–Lutetian early-to-middle Eocene boundary. The Kuldana-Kalakot faunal zone that yields Pakicetus in Kashmir and Pakistan (box with arrow) overlies beds with Assilina exponens and Assilina spira of shallow benthic zones SB13–14 here (9, 12) and beds with Orbitolites complanatus of shallow benthic zone SB12 elsewhere (12, 13), indicating that they are early middle Eocene in age. The Subathu zone IIIc marine fauna with H. subathuensis (box with arrow) is associated with Nummulites atacicus of shallow benthic zone SB8 (9, 12), indicating that Himalayacetus is middle early Eocene in age.
Diagnosis.
Himalayacetus has the general molar form characteristic of Archaeoceti. It is clearly a pakicetid because it lacks the enlarged mandibular canal seen in all of the more advanced archaeocetes (Fig. 1). Himalayacetus is similar in size to later Pakicetus (7, 8) but differs in having lower molars with a larger and more rounded major cusp (protoconid) on the trigonid and a smaller and less elevated minor cusp (hypoconid) on the talonid (Fig. 2). Himalayacetus differs from Pakicetus, Gandakasia (14), and Ambulocetus (15) in having the anteromedial cusp at the base of the trigonid projecting more anteriorly than the anterolateral cusp.
DESCRIPTION AND COMPARISON
H. subathuensis is represented by a partial left dentary with alveoli for M1, the slightly damaged crown of M2, and the complete crown of M3. The base of a shallow mandibular canal is preserved relatively high on the medial side of the dentary (Fig. 1b). The top of the canal is not preserved, but the base is so high and the width is so narrow that it cannot have been enlarged like that of Ambulocetus (15) or later archaeocetes.
The crown of M1 is missing, but alveoli preserved on the occlusal surface of the dentary indicate that it was approximately 29 mm long. The trigonid of M2 has a high, narrow, rounded protoconid, partly broken, with a prominent anterior paracristid running down to the base of the crown, where it joined a once substantial paraconid. A weaker anterior crest is also present, forming a raised anterobuccal edge and bordering the shallowly depressed anterior fovea. The anterobuccal crest joins the remnant of a basal cusp that appears to have been less prominent than the paraconid. There is no trace of a metaconid. A posterior cristid runs down the back of the trigonid to a low hypoconid cusp on the talonid. The medial part is damaged, but the talonid was clearly narrow. The crown, as a whole, has a very weak buccal cingulid and a slightly stronger lingual cingulid. M2 measures 26.3 mm long and 10.1 mm wide at the base of the crown. The trigonid was at least 21.0 mm high at the apex of the protoconid, and the talonid was about 7.0 mm high.
The crown of M3 (Fig. 2b) is similar to that of M2 but is better preserved, with the only substantial differences being of proportion. M3 measures 22.1 mm long and 10.6 mm wide at the base of the crown. The trigonid is 17.5 mm high at the apex of the protoconid, and the talonid is 7.4 mm high.
Comparing what is known of all three molars, it appears that there was a modest Pakicetus-like size gradient, with molar size decreasing posteriorly. Molar morphology is generally similar to that of other known early and early-middle Eocene archaeocetes. Ichthyolestes pinfoldi (14) is too small to be confused with Himalayacetus. The lower molars of Gandakasia potens (14) and Ambulocetus natans (15) are larger. The closest resemblance is to a species of Pakicetus (16, 17) but, as stated above, molars of Himalayacetus have larger and more rounded protoconids, lower and narrower talonids, and a more anteriorly positioned anteromedial cusp at the base of the trigonid (Fig. 2).
AGE OF HIMALAYACETUS
Himalayacetus is significant in being the oldest cetacean known to date, predating Pakicetus and its contemporaries by some 3.5 million years. Pakicetus was thought to be late early Eocene in age when it was first described (1), because the red beds in which it is found were interpreted as representing a low sea stand (18). At that time a single low sea stand was recognized in the early-to-middle Eocene transition (19, 20). It was near the end of the early Eocene; Pakicetus-bearing red beds were thought to represent this low sea stand, and Pakicetus was thus thought to be late early Eocene in age.
Two low sea stands are now recognized in the early-to-middle Eocene transition (11), one late in the early Eocene and the other early in the middle Eocene (U in Fig. 3). The Pakicetus-bearing interval must correspond to the higher of these, because shallow benthic foraminiferan Assilina spira of zone SB13 is reported below it in the Subathu section (9), Assilina exponens of zone SB14 is reported from the Kuldana Formation at its type section (21), and Orbitolites complanatus of zones SB12–16 is reported from the Shekhan Limestone underlying the Kuldana Formation at Panoba (13). The latter is a stratigraphic section through the Chorlakki fossil beds where the type cranium of Pakicetus inachus was found. This upper low sea stand is now calibrated at approximately 48 million years ago (Ma) using the time scale of Berggren et al. (10), meaning that Pakicetus is younger than previously thought.
The type specimen of Himalayacetus comes from interval IIIc of Mathur (9) in the type section of the Subathu Formation (Fig. 3). Intervals II and IV of Mathur both yield Nummulites atacicus of shallow benthic zone SB8. This constrains the age of H. subathuensis to between 53 and 54 Ma, or about 53.5 Ma.
ENVIRONMENT OF HIMALAYACETUS
Himalayacetus also is significant in showing that some whales probably were partially marine very early in the course of cetacean evolution. Himalayacetus came from a shallow oyster-bearing marine deposit, whereas Pakicetus and the other oldest pakicetids known previously came from continental red beds and were found in association with land mammals (1, 7).
Oxygen isotopes distinguish living freshwater from marine Cetacea (22, 23). Oxygen in a freshwater setting is fractionated—enriched in light 16O and depleted in heavy 18O—by comparison with oxygen in marine settings that retain more heavy 18O (Fig. 4). Oxygen isotopes of Eocene Pakicetus and Nalacetus were reported to have freshwater rather than marine values, which led Thewissen et al. (24) to conclude that pakicetids, the geologically oldest whales, were not marine.
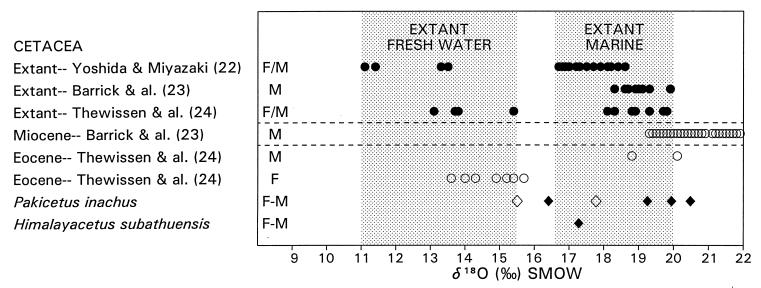
Figure 4.
Isotopic composition of oxygen in bone and tooth phosphate of living and fossil whales. Extant cetaceans living in fresh water have lighter isotopic values in the range of 11–15.5 (parts per thousand δ18O SMOW), whereas those living in marine waters have heavier isotopic values in the range of 16.5–20 (• and shaded areas). Miocene cetaceans have heavy values in the range of 19–22 (○). Few Eocene cetaceans have been reported, and these have been divided into freshwater and marine forms (○). Values for early-middle Eocene P. inachus range from 15.5 for juveniles (◊) to 20.5 for adults (⧫; A. Goswami and P.D.G., unpublished data). Middle early Eocene H. subathuensis tooth-enamel phosphate has a value of 17 ‰ δ18O Standard Mean Ocean Water (SMOW), which is heavier than oxygen found in phosphate of extant and Eocene freshwater cetaceans. Pakicetus and Himalayacetus are interpreted here as amphibious, spending time on land but ingesting both fresh and marine waters while feeding in rivers, estuaries, and shallow marine Tethys.
Interpretation is complicated because the isotopic composition of oxygen in bone and tooth phosphate of land mammals is often heavy (25), reflecting fractionation during respiration and other metabolic processes. Thus, the heavy values seen in Himalayacetus and at least some Pakicetus can be explained by respiration on land in animals that almost certainly still were amphibious, ingestion of some heavy oxygen in marine water during feeding, or both. Life on land is consistent with recovery of all Pakicetus in association with land mammals, and ingestion of heavy oxygen in marine water is consistent with discovery of Himalayacetus in association with marine oysters and other mollusks. Interpretation is still ambiguous, but two lines of evidence—recovery in marine strata and heavy oxygen isotopic composition—favor invasion of marine waters early in cetacean history. The origin of whales is commonly explained by the availability of fish as food in highly productive shallow marine waters of eastern Tethys (1), and early pakicetids are likely to have fed on fish in freshwater rivers, mixed estuaries, and shallow marine seas.
TIME OF ORIGIN OF WHALES
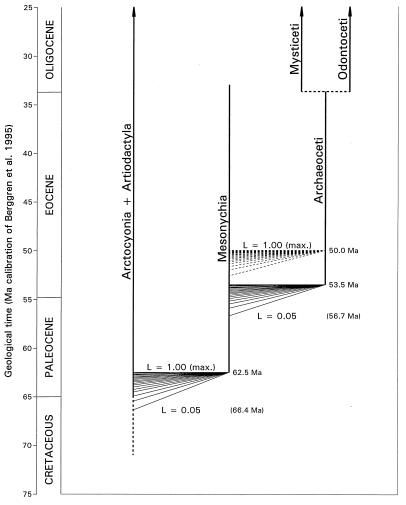
The difference between the age of H. subathuensis and the age of the oldest pakicetids known previously is illustrated graphically in Fig. 5 in the context of hypothesized phylogenetic relationships. Following Van Valen (26), archaeocetes are generally regarded as descendants of Mesonychia, with which they were long confused (14, 27). Archaeoceti, and hence Cetacea, now are known from strata about 3.5 million years older than any known previously.
Figure 5.
Outline of phylogenetic relationships of extant Mysticeti and Odontoceti to Eocene Archaeoceti, and through Archaeoceti to earlier Mesonychia and Arctocyonia + Artiodactyla. Discovery of the oldest archaeocete, H. subathuensis, reported here extends the time of origin of archaeocetes based on fossils from about 50 to 53.5 Ma (maximum relative likelihood L = 1.00). Acceptance of a relative likelihood L as small as 0.05 extends the time of origin of whales to a reasonable limit of 56.7 Ma near or just before the beginning of Eocene evolutionary time. The fan of solid lines at 53.5 Ma shows likelihoods decreasing from maximum in increments of 0.05 (fan of dashed lines at 50.0 reflects pre-Himalayacetus values; ref. 30). The times of divergence of extant sister groups Artiodactyla and Cetacea are unaffected by discovery of Himalayacetus and remain in the range of ≈62.5 Ma (maximum-likelihood L = 1.00) to 66.4 Ma (L = 0.05), or about the beginning of Cenozoic evolutionary time.
The origin of a taxon must be at least as early in geological time as the oldest fossil representing the group, and hypotheses of earlier origin can be evaluated in terms of their relative likelihoods (28–30). Previous analysis of 42 independent sites around the world documenting archaeocetes in the time range between 36 and 49.5 Ma yielded a maximum-likelihood time of origin of Cetacea of 49.5 Ma (30), and a 95% confidence limit (28) or relative likelihood L = 0.05 for this estimate of 51.6 Ma (30). Conversion to the current Berggren et al. (10) time scale extends these values by about 0.5 million years (fan of dashed lines at 50 Ma reflecting likelihoods decreasing in 0.05 increments).
Discovery of H. subathuensis alters the maximum-likelihood time of origin of whales to 53.5 Ma and extends the L = 0.05 confidence limit to 56.7 Ma (Fig. 5), straddling the Paleocene–Eocene boundary (fan of solid lines at 53.5 Ma reflecting likelihoods decreasing in 0.05 increments). The time of divergence of extant Cetacea from extant Artiodactyla is unchanged and lies in the range of 62.5–66.4 Ma, at or near the beginning of the Cenozoic.
The range extension of Archaeoceti back in time represented by Himalayacetus had a prior expectation or relative likelihood on the order of only 0.01 (10−2) before Himalayacetus was found, making this extension unlikely and hence unusually important (although the diversity of pakicetids in the Kuldana fauna and the diversity of other archaeocetes in slightly younger faunas clearly required an earlier origin).
Some systematists using molecular genetic clocks suggest divergence of Cetacea from other orders of mammals in the Mesozoic as early as 100 Ma (31), but the quantified likelihood of such a hypothesis is vanishingly small (L ≈ 10−9) from the point of view of known fossils and radiometric calibration of the geologic time scale. Resolution of discrepant interpretations of conflicting evidence will be important for understanding both the history of whales (and other mammals) and the dynamics of evolutionary change.
Acknowledgments
We thank E. Buffetaut, A. Goswami, P. L. Koch, J.-J. Jaeger, J. R. O’Neil, L. Roe, A. Sahni, and J. G. M. Thewissen for discussion and access to comparative specimens. A. Goswami analyzed oxygen isotopes of Himalayacetus, Pakicetus, and other Eocene archaeocetes and land mammals at the University of Michigan. S. B. Bhatia provided information on Subathu biostratigraphy from the unpublished dissertation of H. Bagi. William J. Sanders completed preparation of the holotype, and Bonnie Miljour drew the illustrations in Figs. 1 and 2. Research on archaeocete whales of Eocene Tethys is funded by National Science Foundation Grant EAR-9714923 to P.D.G.
ABBREVIATION
- Ma
million years ago
References
- 1.Gingerich P D, Wells N A, Russell D E, Shah S M I. Science. 1983;220:403–406. doi: 10.1126/science.220.4595.403. [DOI] [PubMed] [Google Scholar]
- 2.Kumar K, Sahni A. J Vert Paleontol. 1985;5:153–168. [Google Scholar]
- 3.Thewissen J G M, Hussain S T. Nature (London) 1993;361:444–445. doi: 10.1038/361444a0. [DOI] [PubMed] [Google Scholar]
- 4.Sahni A, Jolly A. Kaupia, Darmstädter Beiträge zur Naturgeschichte, Darmstadt. 1993;3:209–222. [Google Scholar]
- 5.Srivastava R, Kumar K. Palaeogeog Palaeoclimatol Palaeoecol. 1996;122:185–211. [Google Scholar]
- 6.Zhou X, Zhai R, Gingerich P D, Chen L. J Vert Paleontol. 1995;15:387–400. [Google Scholar]
- 7.Thewissen J G M, Hussain S T. Bull Carnegie Mus Nat Hist. 1998;34:220–238. [Google Scholar]
- 8.Gingerich P D, Russell D E. Cont Mus Paleontol Univ Mich. 1990;28:1–20. [Google Scholar]
- 9.Mathur N S. Recent Res Geol. 1978;5:96–112. [Google Scholar]
- 10.Berggren W A, Kent D V, Swisher C C, Aubry M-P. In: Geochronology, Time Scales, and Global Stratigraphic Correlations: A Unified Temporal Framework for an Historical Geology. Berggren W A, Kent D V, Aubry M-P, Hardenbol J A, editors. Tulsa, OK: Soc. Econ. Geol. Mineral.; 1995. , Special Vol. 54, pp. 129–212. [Google Scholar]
- 11.Hardenbol J A, Thierry J, Farley M B, Jacquin T, Graciansky P-C d, Vail P R. In: Sequence Stratigraphy of European Basins. Graciansky P-C d, Hardenbol J A, Jacquin T, Vail P R, Farley M B., editors. Soc. Econ. Paleontol. Mineral. Special Publication; 1999. , in press. [Google Scholar]
- 12.Serra-Kiel J, Hottinger L, Caus E, Drobne K, Ferràndez C, Jauhri A K, Less G, Pavlovec R, Pignatti J S J M, Schaub H, et al. Bull Soc Géol Fr. 1998;169:281–299. [Google Scholar]
- 13.Weiss W. Zitteliana (Munich) 1993;20:223–252. [Google Scholar]
- 14.Dehm R, Oettingen-Spielberg T z. Abhandlungen der Bayerische Akademie der Wissenschaften, Mathematisch-Naturwissenschaftliche Klasse, München, Neue Folge. 1958;91:1–54. [Google Scholar]
- 15.Thewissen J G M, Madar S I, Hussain S T. Courier Forschungsinstitut Senckenberg, Frankfurt am Main. 1996;191:1–86. [Google Scholar]
- 16.West R M. J Paleontol. 1980;54:508–533. [Google Scholar]
- 17.Gingerich P D, Russell D E. Cont Mus Paleontol Univ Mich. 1981;25:235–246. [Google Scholar]
- 18.Wells N A. Special Publication of the International Association of Sedimentologists. 1983;6:393–403. [Google Scholar]
- 19.Vail P R, Hardenbol J A. Oceanus. 1979;22:71–79. [Google Scholar]
- 20.Haq B u, Hardenbol J A, Vail P R. Science. 1987;235:1156–1167. doi: 10.1126/science.235.4793.1156. [DOI] [PubMed] [Google Scholar]
- 21.Latif M A. Jahrbuch der Geologischen Bundesanstalt, Sonderband, Wien. 1970c;15:63–66. [Google Scholar]
- 22.Yoshida N, Miyazaki N. J Geophys Res. 1991;96:815–820. [Google Scholar]
- 23.Barrick R E, Fischer A G, Kolodny Y, Luz B, Bohaska D J. Palaios. 1992;7:521–531. [Google Scholar]
- 24.Thewissen J G M, Roe L J, O’Neil J R, Hussain S T, Sahni A, Bajpai S. Nature (London) 1996;381:379–380. [Google Scholar]
- 25.Fricke H C, Clyde W C, O’Neil J R, Gingerich P D. Earth Planet Sci Lett. 1998;160:193–208. [Google Scholar]
- 26.Van Valen L M. Bull Am Mus Nat Hist. 1966;132:1–126. [Google Scholar]
- 27.Szalay F S, Gould S J. Bull Am Mus Nat Hist. 1966;132:127–174. [Google Scholar]
- 28.Strauss D J, Sadler P M. Math Geol. 1989;21:411–427. [Google Scholar]
- 29.Edwards A W F. Likelihood, Expanded Edition. Baltimore: Johns Hopkins Univ. Press; 1992. pp. 1–275. [Google Scholar]
- 30.Gingerich P D, Uhen M D. Palaeontologia Electronica. 1998;1(2):1–45. http://www-odp.tamu.edu/paleo/1998_2/ging_uhen/issue2.htm [ http://www-odp.tamu.edu/paleo/1998_2/ging_uhen/issue2.htm]. ]. [Google Scholar]
- 31.Hedges S B, Parker P H, Sibley C G, Kumar S. Nature (London) 1996;381:226–229. doi: 10.1038/381226a0. [DOI] [PubMed] [Google Scholar]