Abstract
Altered calcium mobilization has been implicated in the development of colonic dysmotility in inflammatory bowel disease. The aim of this study was to investigate the mechanisms by which disrupted intracellular Ca2+ signaling contributes to the impaired contractility of colon circular smooth muscles. Acute colitis was induced in C57Bl/6 mice with dextran sulfate sodium (DSS) in the drinking water for five days. Spontaneous and acetylcholine-evoked contractions, caffeine-evoked hyperpolarization, and SERCA2 and phospholamban expression were reduced compared to controls. Tetrodotoxin did not restore control levels of contractile activity. The amplitudes, but not the frequency, of intracellular Ca2+ waves were increased compared to controls. Caffeine abolished intracellular Ca2+ waves in control smooth muscle cells, but not in smooth muscle cells from DSS-treated mice. CaM kinase II activity and cytosolic levels of HDAC4 were increased, and IκBα levels were decreased in distal colon smooth muscles from DSS-treated mice. These results suggest that disruptions in intracellular Ca2+ mobilization due to down-regulation of SERCA2 and phospholamban expression lead to increased CaM kinase II activity and cytosolic HDAC4 that may contribute to the dysmotility of colonic smooth muscles in colitis by enhancing NF-κB activity.
Keywords: Ca2+ waves, CaM kinase II, colonic smooth muscle, DSS colitis, phospholamban, SERCA
Ulcerative colitis and Crohn's disease are inflammatory bowel diseases that involve chronic intestinal inflammation associated with dysmotility resulting in symptoms that include abdominal pain, weight loss, and bloody diarrhea [1-3]. Inflammation is associated with reduced colonic mixing patterns and impaired haustra formation, but increased propulsive motility from propagating contractions, causing rapid movement of the colonic contents to the rectum, accentuating the diarrhea [4-7]. Multiple mechanisms involving changes in enteric neurotransmission, afferent sensory input, and intrinsic smooth muscle contractility underlie the dysmotility [5].
Improper Ca2+ mobilization is implicated in the dysmotility of colon circular smooth muscle cells from ulcerative colitis patients and in animal models of colitis [8;9]. Impaired Ca2+ influx in smooth muscles from the inflamed colon has been reported to occur by reduced L-type Ca2+ channel expression or by decreased L-type Ca2+ channel activity with no change in expression [10;11]. In addition, SERCA2 expression in rat colon smooth muscle is decreased in colitis, suggesting that reduced SR Ca2+ uptake can also disrupt intracellular Ca2+ mobilization in smooth muscles from inflamed colon [12]. A variety of effectors, including Ca2+/calmodulin-dependent protein kinase II (CaMKII) and Ca2+-sensitive ion channels are regulated by SR Ca2+ dynamics [13-15]. HDAC4 is sequestered in the cytoplasm following CaMKII phosphorylation, allowing HDAC4 target genes to be de-repressed [16]. NF-κB-dependent gene expression requires transcriptional co-activators, including histone acetyltransferases (HATs) and histone deacetylases (HDACs) [17]. These findings suggest that CaMKII could play a role in colonic dysmotility by enhancing NF-κB-dependent gene transcription.
Understanding the molecular mechanisms underlying the disruptions in Ca2+ mobilization and the impaired contractile responses of colon smooth muscles to intestinal inflammation is important for understanding the myogenic basis of the dysmotility that is associated with colitis. In the present study, we assessed SR Ca2+ dynamics by evaluating intracellular Ca2+ wave properties and CaMKII activity following DSS-induced colitis. SERCA2, PLB, and γCaMKII protein expression, and cytosolic levels of HDAC4 and IκBα were also examined.
Methods
Inducing DSS-colitis
Male C57Bl/6 mice (6-8 week old, Charles River Laboratories, Wilmington, MA) were administered 3.5% w/v DSS (MW = 36,000-50,000 Da, MP Biomedicals LLC, Solon, OH) ad libitum in the drinking water for five days. Distal colons were utilized up to five days post-DSS treatment [18]. Mice were maintained and experiments carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All protocols were approved by the University of Nevada, Reno Institutional Animal Care and Use Committee.
Preparation of distal colon smooth muscles
Mice were anesthetized with isofluorane and euthanized by decapitation. The colons were removed, pinned to a Sylgard-lined dish containing oxygenated Krebs solution, and the mucosa and submucosa were removed by sharp dissection. The distal colon was identified as a 2 cm region beginning 0.5 cm aboral from the proximal colon mucosal striations.
Histological staining
Distal colon cross sections (5 μm) were fixed in 10% v/v neutral buffered formalin solution, embedded in paraffin, and H&E stained for light microscopy.
CaMKII assays
Total (Ca2+/CaM stimulated) and autonomous (Ca2+/CaM independent) CaMKII activity in distal colon smooth muscles were assayed using the specific CaMKII peptide substrate autocamtide-2 (KKALRRQETVDAL, 20 μM, BioMol, Plymouth Meeting, PA) [19].
SDS-PAGE and western blotting
The cytosolic and SR fractions were obtained from distal colon smooth muscles frozen in liquid nitrogen, and stored at -80°C [20]. Protein concentrations were determined by Bradford assay using bovine γ-globulin as standard. Cytosolic fractions were analyzed with anti- γCaMKII, HDAC4, and IκBαantibodies, and SR fractions were analyzed with anti- SERCA2, and PLB antibodies (Santa Cruz Biotechnology, Santa Cruz, CA). Immunostained protein bands were detected using ECL Advantage (GE HealthCare Biosciences, Piscataway, NJ), and visualized with a CCD camera-based detection system (Epi Chem II, UVP Laboratory Products, Upland, CA). The .tiff images were analyzed with Adobe Photoshop (CS2, V9.0.2, Adobe Systems, San Jose, CA) and densitometry was carried out using Un-Scan-It software (Silk Scientific, Orem, UT).
Co-immunoprecipitation
γCaMKII was immunoprecipitated from cytosolic fractions with anti-γCaMKII antibodies and Protein A/G agarose beads (Santa Cruz Biotechnology, Santa Cruz, CA). The beads were boiled in SDS-sample buffer, and the bound proteins were resolved by SDS-PAGE, transferred to nitrocellulose membranes, immunoblotted with anti-HDAC4 antibodies, and visualized by enhanced chemiluminescence as described above.
Mechanical responses
Contractile activity from control and DSS-colitis smooth muscle strips was measured using standard myobath techniques, with each strip attached to a Fort 10 isometric strain gauge (WPI, Sarasota, FL) in parallel with the circular muscles [20]. A resting force of 6 mN was applied and tissues were equilibrated for ∼1 h. Responses were recorded using Acqknowledge 3.2.7 software (BIOPAC Systems).
Intracellular microelectrode recordings
Distal colon smooth muscles from control and DSS-colitis mice were perfused with Krebs (37.5 ± 0.5°C, pH 7.3-7.4) bubbled with 97% O2-3% CO2 and 1 μM nicardipine (to control tissue movement) [20]. Smooth muscle cells were impaled with glass microelectrodes filled with 3M KCl having resistances between 50-80 MΩ. Transmembrane potential was measured with a high input impedance amplifier (model S-7071, WPI). Electrical signals were recorded with Axoscope 9.2 data acquisition software (Axon Instruments, Union City, CA), and printed using Clampfit analysis software (Axon Instruments).
Visualization and image analysis of intracellular Ca2+ waves
Distal colon smooth muscles were pinned down with longitudinal muscle facing up in a bath containing oxygenated Krebs at 36.5 ± 0.5°C. The tissue was equilibrated for 1 h, incubated with 10 μM fluo-4 AM (Molecular Probes, Eugene, OR) for 20 min at room temperature, and then perfused with oxygenated Krebs for 15-20 min at 36.5 ± 0.5°C to allow for de-esterification [15;21;22]. Intracellular Ca2+ waves were recorded from 150 μm × 150 μm regions of interest (ROIs) of intact smooth muscles from day 4 post-DSS treatment and from age-matched control mice [22]. Exposure matching was used to compare control and drug effects because subsequent exposure of fluo-4AM to the excitation light will decrease the amplitude and frequency of the intracellular waves [15]. Image sequences were analyzed with custom software (Volumetry G6a, by G.W. Hennig) to generate spatiotemporal (ST) maps of intracellular Ca2+ waves [21]. Frequency (Hz), amplitude (intensity units, iu), and area under the curve (AUC) of waves from individual cells were calculated [21]. The amplitudes of intracellular Ca2+ waves were measured using the F/Favg ratio as intensities of Ca2+-induced fluorescence was constantly oscillating, preventing Fo from being determined [15;21].
Materials
Tetrodotoxin (TTX), 2-aminoethoxydiphenylborate (2-APB), and acetylcholine (ACh) were obtained from Sigma (St. Louis, MO), caffeine from Calbiochem (La Jolla, CA), and western blot materials, including nitrocellulose paper, gel components, and Bradford assay materials from BioRad (Hercules, CA). Paraffin, alcohols, dyes, and xylene for histological staining were purchased from Richard Allan Scientific (Kalamazoo, MI).
Statistical Analysis
Averages from experiments were analyzed for statistical significance using Sigma Stat software (Access Softek Inc.). Mouse weights, contractile data, and CaMKII activity before and after DSS treatment were analyzed by One-Way ANOVA and data are expressed as means ± SD. Western blot data was normalized to controls or to the average of controls and tested for significance with t-test. Intracellular wave data were analyzed with One-Way ANOVA, data are expressed as means ± SEM, and P < 0.05 was considered significant.
Results
Mechanical responses
The frequencies of spontaneous phasic contractions of muscle strips from control and DSS-colitis mice were not significantly different (0.5 ± 0.1 cycles per minute (cpm) vs. 0.4 ± 0.07 cpm, respectively, n=7, P > 0.05). The amplitudes of spontaneous phasic contractions of muscle strips from mice with DSS-colitis were significantly decreased (2.7 ± 0.8 mN vs. 1.6 ± 0.4 mN, for control and DSS-colitis mice, respectively, n=7, P < 0.05) (Figure 1A). ACh (1μM) increased the amplitudes of phasic contractions of control smooth muscle strips from 2.7 ± 0.8 mN to 6.9 ± 0.4 mN (n=5, P < 0.001), and also increased the frequency of phasic contractions from 0.5 ± 0.1 cpm to 0.8 ± 0.1 cpm (n=6, P < 0.001) (Figure 1B). ACh increased the amplitudes of phasic contractions of smooth muscle strips from DSS-colitis mice from 1.6 ± 0.4 mN to 3.6 ± 1.3 mN (n=7, P < 0.001), but did not significantly increase the frequency of spontaneous contractions (0.4 ± 0.07 cpm vs 0.5 ± 0.1 cpm n=7, P > 0.05) (Figure 1B). The amplitude of 3.6 ± 1.3 mN from DSS-affected colons is significantly lower than the amplitude of 6.9 ± 0.4 mN from control smooth muscle strips in the presence of 1μM ACh (P < 0.001).
Figure 1. Impaired mechanical responses of distal colon smooth muscles from DSS-treated mice.
A. Representative traces of the spontaneous phasic contractile activity of distal colon smooth muscle strips from control and DSS-treated (day 4) mice. B. Representative traces of the mechanical responses of distal colon smooth muscle strips from control and DSS-treated (day 4) mice to 1 μM ACh. C. Representative traces of the spontaneous phasic contractile activity of distal colon smooth muscle strips from control and DSS-treated (day 4) mice in the presence of 0.3 μM TTX.
TTX increased the amplitudes of phasic contractions of control smooth muscles from 2.7 ± 0.8 mN to 3.6 ± 1.9 mN (n=8, P < 0.05) and increased the frequency of phasic contractions from 0.5 ± 0.1 cpm to 0.8 ± 0.1 cpm (n=5, P < 0.001) (Figure 1C). The amplitudes of the phasic contractions of smooth muscle strips from DSS-colitis mice were not significantly increased by TTX (1.6 ± 0.4 mN vs. 1.5 ± 0.4 mN in the absence and presence of TTX, respectively, n=8) (Figure 1C). The amplitude of 1.5 ± 0.4 mN from DSS-affected colons is significantly lower than the amplitude of 3.6 ± 1.9 mN from control smooth muscle strips (P < 0.05). TTX increased the frequency of phasic contractions in DSS-colitis mice from 0.4 ± 0.07 cpm to 0.6 ± 0.04 cpm (n=7, P < 0.05). The frequency of 0.6 ± 0.04 cpm from DSS-affected colons is significantly lower than the frequency of 0.8 ± 0.1 cpm from control smooth muscle strips in the presence of TTX (P < 0.05).
Effect of caffeine on intracellular Ca2+ waves
The frequency, amplitude, and AUC of Ca2+ waves were measured from 3-4 individual myocytes from 5 separate preparations (Figure 2A, B). The frequencies of Ca2+ waves in myocytes from control or DSS-colitis mice were not significantly different (1.1 ± 0.06 Hz and 1.1 ± 0.06 Hz, respectively, n=15, P > 0.05) (Figure 2C, D, and G). The amplitudes and AUC of Ca2+ waves in myocytes from mice with DSS-colitis were increased by 33% (32 ± 5 iu vs. 24 ± 3 iu, n=15, P > 0.05), and 50% (12 ± 2 iu vs. 8 ± 1 iu, n=15, P > 0.05), respectively compared to control mice (Figure 2H, I). Additional differences in Ca2+ waves became apparent in the presence of 1mM caffeine. The Ca2+ wave frequencies were decreased by 71% (1.1 ± 0.06 Hz to 0.3 ± 0.01 Hz, n=15, P < 0.001), and 39% (1.1 ± 0.06 Hz to 0.7 ± 0.04 Hz, n=15, P < 0.001), in control myocytes, and myocytes from mice with DSS-colitis, respectively (Figure 2E, F, and G). The frequency of 0.7 ± 0.04 Hz is significantly greater than the frequency of 0.3 ± 0.01 Hz (P < 0.001) from controls. Caffeine reduced the amplitudes of Ca2+ waves by 79% in control myocytes, (24 ± 3 iu to 5 ± 1 iu, n=15, P < 0.001), and by 50% in myocytes from mice with DSS-colitis (32 ± 5 iu to 16 ± 3 iu, n=15, P < 0.05). The amplitude of 16 ± 3 iu is significantly greater than the amplitude of 5 ± 1 iu from controls (P < 0.001) (Figure 2H). Caffeine reduced the AUC of Ca2+ waves by 62% in control myocytes (8 ± 1 iu to 3 ± 1 iu, n=15, P < 0.05), and by 42% in myocytes from mice with DSS-colitis (12 ± 2 iu to 7 ± 1 iu, n=15, P < 0.05). The AUC of 12 ± 2 iu is significantly greater than the AUC of 7 ± 1 iu from controls (P < 0.05) (Figure 2I). Ca2+ waves were abolished by 2-APB (30 μM) but were unaffected by ryanodine (10 μM) (data not shown), indicating that intracellular Ca2+ waves are mediated by inositol 1,4,5-trisphosphate receptors (IP3R) [23].
Figure 2. Altered intracellular Ca2+ wave properties in distal colon smooth muscles from DSS-treated mice.
Representative Z-compressed images showing the region of interest (ROI) in distal colon smooth muscles from control (A) and DSS-treated mice (day 4) (B) from which intracellular Ca2+ waves were recorded and spatio-temporal maps constructed. Representative spatio-temporal maps of intracellular Ca2+ waves in distal colon smooth muscles from control and DSS-treated mice (day 4) in the absence (C, D) or presence (E, F) of 1mM caffeine for 10 min. horizontal scale = 2 sec; vertical scale = 10 μm. Bar graphs of the frequencies (G), amplitudes (H), and AUC (I) of intracellular Ca2+ waves without and with caffeine. open bars, control; filled bars, day 4 post-DSS treatment; *P < 0.05 compared to − caffeine, #P < 0.05 compared to control + caffeine.
Effect of caffeine on RMP
Intracellular Ca2+ waves regulate membrane potential and contribute to smooth muscle excitability [23;24]. Therefore, we examined the RMP in the presence of 1mM caffeine in control and DSS-colitis smooth muscles. The effects of caffeine were fully reversible upon washout and were insensitive to 0.3 μM TTX (data not shown). The RMPs of smooth muscle cells from control (-46.6 ± 3 mV, n=10) and DSS-colitis mice (-47.3 ± 2 mV, n=14) were not significantly different (P > 0.05). Caffeine induced a larger hyperpolarization (ΔRMP) in smooth muscles from DSS-colitis mice compared to controls (16.0 ± 2 mV vs. 12 ± 1 mV, n=6, *P < 0.05) (Figure 3A, B).
Figure 3. Effect of caffeine on electrical responses of distal colon smooth muscles from control and DSS-treated mice.
A. Representative microelectrode recordings of the hyper-polarization of distal colon smooth muscle membrane potentials from control and DSS-treated mice (day 4) in response to 1mM caffeine. B. Bar graph of the ΔRMP from control and DSS-treated mice in response to 1mM caffeine. *P < 0.05.
DSS-colitis decreases SERCA2 and PLB expression
The results shown in Figures 2 and 3 suggest that DSS-colitis alters SR Ca2+ mobilization in distal colon smooth muscles. SERCA2 and PLB expression progressively decreased in smooth muscles from DSS-colitis mice (Figure 4A, C). SERCA2 expression became significantly lower than control at day 2, and reached plateau values 30%-35% of control at days 4 and 5 post-DSS treatment (Figure 4B) (n=5, *P < 0.05). PLB expression became significantly lower than control at day 3, and reached plateau values 50%-60% of control at days 4 and 5 post-DSS treatment (Figure 4D) (n=5, *P < 0.05). Smooth muscle myosin heavy chain expression was unaffected by DSS-colitis (lower panels, Figure 4A, C; 5 μg protein per lane).
Figure 4. SERCA2 and PLB expression is decreased in distal colon smooth muscles from DSS-treated mice.
Representative western blots of SERCA2 (A), and PLB (C) in distal colon smooth muscles SR fractions from control (Con) and DSS-treated mice on days 1-5 post-DSS-treatment. Each lane contains 50 μg of protein. Lower panels are immunoblots of smooth muscle myosin heavy chain (5μg protein per lane). Densitometry of SERCA2 (B), and PLB (D) western blots from control (Con) and DSS-treated mice at days 1-5 post-DSS treatment. Control intensity values from the densitometry analyses were normalized to 1. (n=5 western blots from 5 different sets of control and DSS-treated mice, *P < 0.05 compared to control).
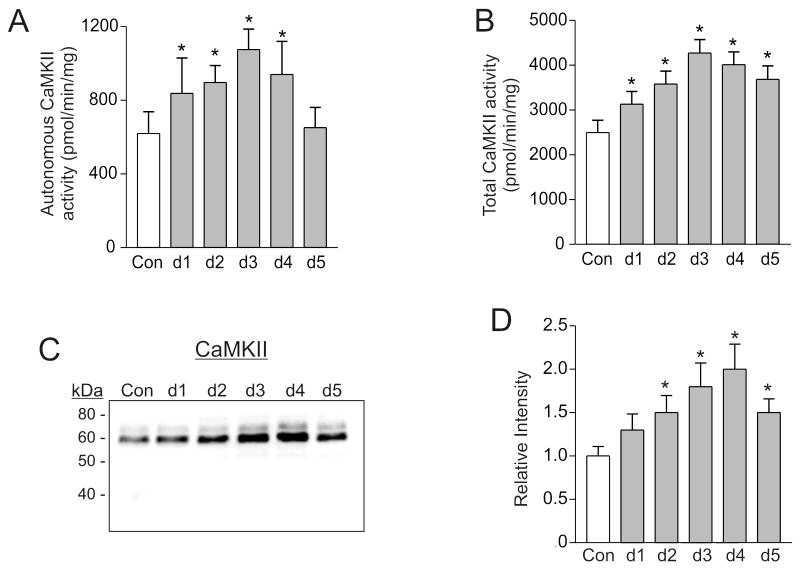
DSS-colitis increases CaMKII activity and expression
Increased intracellular Ca2+ wave frequencies in antrum smooth muscles activates CaMKII [15]. Since DSS-colitis increased the amplitudes and AUC of Ca2+ waves (Figure 2), CaMKII activity in distal colon smooth muscles from control, and DSS-colitis mice was measured. CaMKII is activated in smooth muscles from DSS-colitis mice, as indicated by the increased autonomous activity on days 1-5 post-DSS treatment compared to controls (Figure 5A) (n=7, *P < 0.05). Unexpectedly, total CaMKII activity was also increased on days 1-5 post-DSS treatment (Figure 5B), suggesting that CaMKII expression is increased (n=7, *P < 0.05) [25]. Western blot and densitometry analysis shows that γCaMKII expression was significantly increased in distal colon smooth muscles from mice with DSS-colitis on days 1-5 post-DSS treatment compared to controls (Figure 5C, D).
Figure 5. CaMKII activity and expression are increased in distal colon smooth muscles from DSS-treated mice.
Autonomous (A) and total (B) CaMKII activity in distal colon smooth muscle homogenates from control (Con) and DSS-treated mice at days 1-5 post-DSS treatment (n=7, *P < 0.05 compared to control). Representative western blot of γCaMKII (C) in distal colon smooth muscle cytosolic fractions from control and DSS-treated mice on days 1-5 post-DSS treatment. 50 μg protein per lane. Densitometry of γCaMKII western blots from control (Con) and DSS-treated mice on days 1-5 post-DSS treatment (D). Control intensity values from the densitometry analyses were normalized to 1. (n=5 western blots from 5 different sets of control and DSS-treated mice, *P < 0.05 compared to control).
DSS-colitis decreases cytosolic IκBα levels
Pro-inflammatory cytokines activate NF-κB by proteolysis of the inhibitory IκBα subunit [26]. Thus, decreased levels of IκBα are an indicator of NF-κB activation [27]. Western blot and densitometry analysis shows that cytosolic IκBα levels were significantly decreased compared to control levels (Figure 6A, B; n=4, *P < 0.05) on days 3-5 post-DSS treatment.
Figure 6. IκBα levels are decreased in distal colon smooth muscles from DSS-treated mice.
A. Representative western blot of IκBα in distal colon smooth muscle cytosolic fractions from control and DSS-treated mice on days 1-5 post-DSS treatment. 50 μg protein per lane. B. Densitometry of IκBα western blots from control (Con) and DSS-treated mice on days 1-5 post-DSS treatment. Control intensity values from the densitometry analyses were normalized to 1. (n=4 western blots from 4 different sets of control and DSS-treated mice, *P < 0.05 compared to control).
CaMKII increases cytosolic HDAC4 levels in DSS-colitis
The findings that DSS-colitis elevated CaMKII activity and expression in distal colon smooth muscles prompted us to examine a CaMKII substrate that is a key component of inflammatory signaling pathways. HDAC4 translocates to the cytoplasm following phosphorylation by CaMKII, neutralizing the suppression of NF-κB-dependent gene transcription by HDAC4 [16]. Western blot and densitometry analysis shows that cytosolic HDAC4 levels were significantly increased compared to controls (Figure 7A, B; n=4, *P < 0.05) on days 1-5 post-DSS treatment, when CaMKII activity and expression was also increased (Figure 5). HDAC4 phosphorylation by CaMKII depends on the binding of activated CaMKII to HDAC4 [27]. Co-immunoprecipitation experiments show that γCaMKII binding to HDAC4 was increased on day 3 post-DSS treatment (Figure 7C), when HDAC4 cytosolic levels and CaMKII autonomous activity were also increased.
Figure 7. Cytosolic HDAC4 levels and HDAC4-γCaMKII interaction are increased in distal colon smooth muscles from DSS-treated mice.
A. Representative western blot of HDAC4 in distal colon smooth muscle cytosolic fractions from control and DSS-treated mice on days 1-5 post-DSS treatment. 50 μg protein per lane. B. Densitometry of HDAC4 western blots from control (Con) and DSS-treated mice on days 1-5 post-DSS treatment. Control intensity values from the densitometry analyses were normalized to 1. (n=4 western blots from 4 different sets of control and DSS-treated mice, *P < 0.05 compared to control). C. Distal colon smooth muscle cytosolic fractions from control and DSS-treated mice on day 3 post-DSS treatment were immunoprecipitated with anti-γCaMKII antibodies and then immunoblotted with anti-HDAC4 antibodies. Representative western blot of four experiments.
Discussion
We used the murine DSS-colitis model to investigate the mechanisms by which disrupted intracellular Ca2+ signaling contributes to the impaired contractility of colon circular smooth muscles. Although the DSS-colitis model is generally considered an acute injury model, the colitis shares clinical and histopathologic characteristics with ulcerative colitis [28;29]. The responsiveness of the DSS-colitis model to sulphasalazine, olsalazine, and recently infliximab, further supports its utility as a model of ulcerative colitis [29]. The reduced contractility of colon smooth muscles in animal models of colitis is well documented [3;5;7;8;10;30;31]. Similarly, we found that the spontaneous phasic contractions and ACh-evoked contractions of distal colon circular smooth muscles were diminished by DSS-colitis. The neuronal blocker TTX increased the frequency of spontaneous phasic contractions of smooth muscle strips from DSS-colitis mice, suggesting the presence of ongoing inhibitory neural activity. However, TTX did not increase the amplitudes of spontaneous or ACh-evoked contractions, indicating the presence of a myogenic defect independent of any neurogenic defect. Recent findings showing increased carbachol-evoked contractions of distal colon circular smooth muscles from DSS-treated female Balb/c mice are in contrast to these findings, and may reflect differences due to the time and dose of DSS administered, and strain and gender differences in the manifestation of DSS-colitis [32].
The mechanisms by which impaired calcium mobilization contributes to the reduced contractility of smooth muscles from ulcerative colitis patients, and animal models of colitis are not fully understood [8;9;12;33]. Reduced activity and expression of L-type Ca2+ channels, or interference with Ca2+ release from intracellular stores has been suggested [9;11;12]. In this study we measured intracellular Ca2+ waves to investigate SR Ca2+ mobilization. We found that the Ca2+ wave frequencies in control strips and strips from mice with DSS-colitis were essentially identical. In contrast, the amplitudes and the AUCs were increased (33% and 50% respectively) in distal colon smooth muscles from DSS-treated mice, although statistical analyses indicated these increases were not significant. The lack of statistical significance may be due to variability in wave amplitudes and AUCs observed between individual mice, as well as differences in basal Ca2+ levels which could affect the dynamic range of the Ca2+ dye. Since caffeine diminishes intracellular Ca2+ wave activity by SR Ca2+ store depletion, we examined the effect of caffeine on Ca2+ waves [21]. Caffeine decreased Ca2+ wave activity in smooth muscles from untreated mice, as indicated by reductions of 71%, 79%, and 62%, respectively, in the frequency, amplitude, and AUC of intracellular Ca2+ waves. In contrast, the frequency, amplitude, and AUC of Ca2+ waves in distal colon smooth muscles from DSS-colitis mice were decreased by 39%, 50%, and 42%, respectively, indicating that intracellular Ca2+ mobilization is altered by DSS-colitis. Intracellular Ca2+ waves result from the spatio-temporal recruitment of Ca2+ puffs from IP3Rs [21;23]. Our findings (data not shown) that 2-APB, but not ryanodine, abolished intracellular Ca2+ waves support the conclusion that the waves originate from IP3R-mediated Ca2+ release. IP3Rs are stimulated at low Ca2+ concentrations (< 300nM) but inhibited by higher Ca2+ concentrations (> 300nM) [34]. The increased amplitude and AUC of Ca2+ waves, and persistence of waves in the presence of caffeine suggest that the altered Ca2+ mobilization in smooth muscles from mice with DSS colitis may elevate basal cytosolic Ca2+ concentration to within the range that activates IP3Rs. We measured the effects of caffeine on membrane potential and contraction to examine this possibility. Caffeine hyperpolarized smooth muscles from control, and DSS-colitis mice by 16mV and 12mV, respectively, supporting the idea that the persistence of Ca2+ waves in the presence of caffeine blunted the hyperpolarization, in contrast to control smooth muscles in which Ca2+ wave activity was markedly reduced. It is unclear how a reduction in Ca2+ waves could result in hyperpolarization, since IP3-mediated SR Ca2+ release events (Ca2+puffs) increase spontaneous transient outward currents (STOCs) and hyperpolarize colon smooth muscle cells [23;35;36]. Decreased intracellular Ca2+ wave activity may also result in a reduction in the activities of Ca2+-sensitive non-selective cation channels, facilitating hyperpolarization, although additional studies are necessary to test this possibility.
TNBS-colitis reduces SERCA2 expression in rat colon smooth muscle, suggesting that an altered balance between cytoplasmic Ca2+ and SR Ca2+ contributes to the defects in intracellular Ca2+ mobilization [12]. We found that SERCA2 and PLB expression in distal colon smooth muscles were reduced by DSS-colitis, suggesting that SR Ca2+ uptake is impaired. This is not due to a generalized decrease in protein expression as smooth muscle myosin heavy chain expression was unchanged. These findings suggest that reduced levels of SERCA2 and PLB contribute to the altered caffeine-evoked Ca2+ mobilization of colon smooth muscle cells observed in DSS-colitis. The findings that intracellular Ca2+ waves persist in the presence of caffeine suggest that the intrinsic regulation of smooth muscle intracellular Ca2+ homeostasis is altered by DSS-colitis, and thus could contribute to the altered contractile responses of colonic smooth muscles. These findings also suggest that down-regulation of SERCA2 and PLB expression, and disruptions in intracellular Ca2+ mobilization occur independently of the manner in which colitis is induced and may be a common defect contributing to impaired contractility.
Similar to the frequencies, the amplitudes and AUCs of Ca2+ waves were increased, but not significantly, in colon smooth muscles from DSS-colitis mice. Autonomous CaMKII activity in distal colon smooth muscles from DSS-colitis mice was increased, suggesting that the increased amplitudes of Ca2+ waves activated CaMKII [37;38]. Unexpectedly, total CaMKII activity was also increased. Elevated Ca2+ levels typically do not change total CaMKII activity because it is dependent on the amount of CaMKII [25]. Changes in total CaMKII activity usually indicate changes in CaMKII expression [25]. Western blot analysis confirmed that γCaMKII expression was increased in colon smooth muscles from mice with DSS-colitis. Murine colon smooth muscle cells express the δ and γ CaMKII isoforms [19]. CaMKII activation by TNF-α has been reported [39] [40], but there are no reports of TNF-α affecting CaMKII protein expression. However, the murine γCaMKII promoter contains at least one NF-κB response element sequence (5′-GGGCTGCACC-3′), providing a potential mechanism for the increased γCaMKII expression in distal colon smooth muscles during DSS-colitis.
NF-κB activation contributes to impaired smooth muscle contractility [6;30;41]. Proteolytic degradation of IκBα allows NF-κB to translocate into the nucleus and initiate gene transcription [42]. Thus, reduced IκBα levels strongly suggest NF-κB activation [43]. Our findings show that cytosolic IκBα levels in distal colon smooth muscles from mice with DSS-colitis are significantly reduced on days 3-5 post-DSS treatment. These findings are similar to those of Shi et al., showing that IκBα is decreased and NF-κB is activated in canine colon smooth muscles in response to ethanol-acetic acid colitis [44]. These results suggest that, similar to other experimental models of IBD, DSS colitis results in activation of NF-κB-dependent gene transcription in distal colon smooth muscles.
NF-κB-dependent gene expression is regulated, in part, by transcriptional co-activators, including HATs and HDACs [17]. The retention of HDAC4 in the cytoplasm by CaMKII binding and phosphorylation allows its target genes to be de-repressed and transcribed [16]. Although indirect, increased cytosolic HDAC4 levels are an indicator of phosphorylation by CaMKII [27]. The sub-cellular localization of HDAC4 was examined to determine whether the increased CaMKII activity resulted in increased phosphorylation of an endogenous CaMKII substrate that plays a role in regulating NF-κB activity. HDAC4 protein levels in the cytosolic fraction of distal colon smooth muscles from mice with DSS-colitis were significantly increased, suggesting that increased CaMKII activity resulted in increased HDAC4 phosphorylation and cytosolic localization. Since binding of activated CaMKII to HDAC4 is required to sequester HDAC4 in the cytosol [16;27], we utilized a co-immunoprecipitation approach and found that HDAC4 binding to γCaMKII is increased in distal colon smooth muscle homogenates from mice with DSS-colitis. These findings further support the conclusion that increased CaMKII activity resulted in increased HDAC4 phosphorylation and cytosolic localization. The finding that cytosolic HDAC4 and binding to CaMKII is increased suggests that NF-κB-dependent gene expression may be de-repressed in distal colon smooth muscles from mice with DSS-colitis.
Higher regulatory systems (enteric motor and sensory neurons, interstitial cells of Cajal, and hormones) control and coordinate gut motility [45]. However, GI smooth muscle cells are equipped with intrinsic regulatory pathways that can amplify or oppose signaling from these higher regulatory systems. The smooth muscle cells of the gut wall are the final effectors of force development and work; thus it is important to understand the mechanisms which mucosal inflammation impairs colon circular smooth muscle contractility. In summary, we have found that alterations in the activities and expression of a subset of proteins involved in intracellular Ca2+ mobilization by colonic mucosal inflammation disrupts distal colon smooth muscle function. The results of the muscle tension and Ca2+ imaging studies link impairments in intracellular Ca2+ mobilization and contractile responses with changes in SERCA2, PLB, and γCaMKII protein expression in distal colon smooth muscles from mice with DSS-colitis. IκBα levels were reduced in colon smooth muscles from DSS-colitis mice, suggesting that NF-κB is activated. HDAC4 levels and binding to CaMKII were increased, suggesting that CaMKII activation and HDAC4 phosphorylation may facilitate NF-κB-dependent gene transcription that contributes to the reduced contractility of colon smooth muscles as a result of mucosal inflammation.
Supplementary Material
Supplemental Figure. Gross morphology and histological analysis of the colon from DSS-treated mice. A. Representative photographs of large intestines from untreated control mice (top panel), and DSS-treated mice (day 1 post-DSS treatment, bottom panel). B. Representative H&E stained paraffin-embedded distal colon cross-sections from control and days 1-5 post-DSS treatment; m = mucosa, sm = submucosa, cm = circular smooth muscle (10× magnification). Note expansion of the submucosal layer as early as day 1 post DSS-treatment, and mucosal ulceration in day 5. Changes in mucosal structure can be seen on all days.
Acknowledgments
We would like to thank Minkyung Kim and Kenton Sanders for helpful discussions and use of Ca2+ imaging equipment, and the University of Nevada School of Medicine Department of Pathology for help with the paraffin sections and H&E staining. This work was supported by National Institutes of Health grants RR018751, DK41315, and a Dr. V.A. Salvadorini Pathology Research Fellowship (SQ). Competing interests: the authors have no competing interests.
Abbreviations
- 2-APB
2-aminoethoxydiphenyl borate
- ACh
acetylcholine
- CaM
calmodulin
- CaMKII
Ca2+/calmodulin-dependent protein kinase II
- cpm
cycles per minute
- DSS
dextran sulfate sodium
- GI
gastrointestinal
- HAT
histone acetyltransferase
- HDAC
histone deacetylase
- IP3R
inositol 1,4,5-trisphosphate receptor
- iu
intensity units
- PLB
phospholamban
- RMP
resting membrane potential
- ROI
region of interest
- SERCA2
SR Ca2+-ATPase
- SR
sarcoplasmic reticulum
- ST maps
spatiotemporal maps
- STOCs
spontaneous transient outward currents
- TTX
tetrodotoxin
References
- 1.Ardizzone S, Bianchi PG. Biologic therapy for inflammatory bowel disease. Drugs. 2005;65:2253–86. doi: 10.2165/00003495-200565160-00002. [DOI] [PubMed] [Google Scholar]
- 2.Rufo PA, Bousvaros A. Current therapy of inflammatory bowel disease in children. Paediatr Drugs. 2006;8:279–302. doi: 10.2165/00148581-200608050-00002. [DOI] [PubMed] [Google Scholar]
- 3.Sato K, Ohkura S, Kitahara Y, et al. Involvement of CPI-17 downregulation in the dysmotility of the colon from dextran sodium sulphate-induced experimental colitis in a mouse model. Neurogastroenterol Motil. 2007;19:504–14. doi: 10.1111/j.1365-2982.2007.00911.x. [DOI] [PubMed] [Google Scholar]
- 4.Ajaj WM, Lauenstein TC, Pelster G, et al. Magnetic resonance colonography for the detection of inflammatory diseases of the large bowel: quantifying the inflammatory activity. Gut. 2005;54:257–63. doi: 10.1136/gut.2003.037085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Myers BS, Martin JS, Dempsey DT, Parkman HP, Thomas RM, Ryan JP. Acute experimental colitis decreases colonic circular smooth muscle contractility in rats. Am J Physiol. 1997;273:G928–G936. doi: 10.1152/ajpgi.1997.273.4.G928. [DOI] [PubMed] [Google Scholar]
- 6.Pazdrak K, Shi XZ, Sarna SK. TNFα suppresses human colonic circular smooth muscle cell contractility by SP1- and NF-κB-mediated induction of ICAM-1. Gastroenterology. 2004;127:1096–109. doi: 10.1053/j.gastro.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 7.Snape WJ, Jr, Williams R, Hyman PE. Defect in colonic smooth muscle contraction in patients with ulcerative colitis. Am J Physiol. 1991;261:G987–G991. doi: 10.1152/ajpgi.1991.261.6.G987. [DOI] [PubMed] [Google Scholar]
- 8.Shi XZ, Sarna SK. Impairment of Ca(2+) mobilization in circular muscle cells of the inflamed colon. AJP - Gastrointestinal and Liver Physiology. 2000;278:G234–G242. doi: 10.1152/ajpgi.2000.278.2.G234. [DOI] [PubMed] [Google Scholar]
- 9.Vrees MD, Pricolo VE, Potenti FM, Cao W. Abnormal motility in patients with ulcerative colitis: the role of inflammatory cytokines. Arch Surg. 2002;137:439–45. doi: 10.1001/archsurg.137.4.439. [DOI] [PubMed] [Google Scholar]
- 10.Kinoshita K, Sato K, Hori M, Ozaki H, Karaki H. Decrease in activity of smooth muscle L-type Ca2+ channels and its reversal by NF-kappaB inhibitors in Crohn's colitis model. AJP - Gastrointestinal and Liver Physiology. 2003;285:G483–G493. doi: 10.1152/ajpgi.00038.2003. [DOI] [PubMed] [Google Scholar]
- 11.Liu X, Rusch NJ, Striessnig J, Sarna SK. Down-regulation of L-type calcium channels in inflamed circular smooth muscle cells of the canine colon. Gastroenterology. 2001;120:480–9. doi: 10.1053/gast.2001.21167. [DOI] [PubMed] [Google Scholar]
- 12.Al-Jarallah A, Oriowo MA, Khan I. Mechanism of reduced colonic contractility in experimental colitis: role of sarcoplasmic reticulum pump isoform-2. Mol Cell Biochem. 2007;298:169–78. doi: 10.1007/s11010-006-9363-8. [DOI] [PubMed] [Google Scholar]
- 13.Amberg GC, Baker SA, Koh SD, et al. Characterization of the A-type potassium current in murine gastric antrum. The Journal of Physiology Online. 2002;544:417–28. doi: 10.1113/jphysiol.2002.025171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim M, Perrino BA. CaM kinase II activation and phospholamban phosphorylation by SNP in murine gastric antrum smooth muscles. AJP - Gastrointestinal and Liver Physiology. 2007;292:G1045–G1054. doi: 10.1152/ajpgi.00203.2006. [DOI] [PubMed] [Google Scholar]
- 15.Kim M, Hennig GW, Smith TK, Perrino BA. Phospholamban knockout increases CaM kinase II activity and intracellular Ca2+ wave activity and alters contractile responses of murine gastric antrum. AJP - Cell Physiology. 2008;294:C432–C441. doi: 10.1152/ajpcell.00418.2007. [DOI] [PubMed] [Google Scholar]
- 16.McKinsey TA. Derepression of pathological cardiac genes by members of the CaM kinase superfamily. Cardiovascular Research. 2007;73:667–77. doi: 10.1016/j.cardiores.2006.11.036. [DOI] [PubMed] [Google Scholar]
- 17.Calao M, Burny A, Quivy V, Dekoninck A, Van Lint C. A pervasive role of histone acetyltransferases and deacetylases in an NF-[kappa]B-signaling code. Trends in Biochemical Sciences. 2008;33:339–49. doi: 10.1016/j.tibs.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 18.Hoebler C, Gaudier E, De CP, Rival M, Cherbut C. MUC genes are differently expressed during onset and maintenance of inflammation in dextran sodium sulfate-treated mice. Dig Dis Sci. 2006;51:381–9. doi: 10.1007/s10620-006-3142-y. [DOI] [PubMed] [Google Scholar]
- 19.Lorenz JM, Riddervold MH, Beckett EAH, Baker SA, Perrino BA. Differential autophosphorylation of Ca2+/calmodulin-dependent protein kinase II from phasic and tonic smooth muscle tissues. Am J Physiol. 2002;283:C1399–C1413. doi: 10.1152/ajpcell.00020.2002. [DOI] [PubMed] [Google Scholar]
- 20.Kim M, Cho SY, Han IS, Koh SD, Perrino BA. CaM kinase II and phospholamban contribute to caffeine-induced relaxation of murine gastric fundus smooth muscle. AJP - Cell Physiology. 2005;288:C1202–C1210. doi: 10.1152/ajpcell.00299.2004. [DOI] [PubMed] [Google Scholar]
- 21.Hennig GW, Smith CB, O'Shea DM, Smith TK. Patterns of intracellular and intercellular Ca2+ waves in the longitudinal muscle layer of the murine large intestine in vitro. J Physiol. 2002;543:233–53. doi: 10.1113/jphysiol.2002.018986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee HT, Hennig GW, Fleming NW, et al. Septal interstitial cells of Cajal conduct pacemaker activity to excite muscle bundles in human jejunum. Gastroenterology. 2007;133:907–17. doi: 10.1053/j.gastro.2007.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bayguinov O, Hagen B, Bonev AD, Nelson MT, Sanders KM. Intracellular calcium events activated by ATP in murine colonic myocytes. Am J Physiol Cell Physiol. 2000;279:C126–C135. doi: 10.1152/ajpcell.2000.279.1.C126. [DOI] [PubMed] [Google Scholar]
- 24.Nelson MT, Cheng H, Rubart M, et al. Relaxation of arterial smooth muscle by calcium sparks. Science. 1995;270:633–7. doi: 10.1126/science.270.5236.633. [DOI] [PubMed] [Google Scholar]
- 25.Hudmon A, Schulman H. Structure-function of the multifunctional Ca2+/calmodulin-dependent protein kinase II. Biochem J. 2002;364:593–611. doi: 10.1042/BJ20020228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Catley MC, Sukkar MB, Chung KF, et al. Validation of the Anti-Inflammatory Properties of Small-Molecule I{kappa}B Kinase (IKK)-2 Inhibitors by Comparison with Adenoviral-Mediated Delivery of Dominant-Negative IKK1 and IKK2 in Human Airways Smooth Muscle. Molecular Pharmacology. 2006;70:697–705. doi: 10.1124/mol.106.023150. [DOI] [PubMed] [Google Scholar]
- 27.Backs J, Song K, Bezprozvannaya S, Chang S, Olson EN. CaM kinase II selectively signals to histone deacetylase 4 during cardiomyocyte hypertrophy. J Clin Invest. 2006;116:1853–64. doi: 10.1172/JCI27438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cooper HS, Murthy SN, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993;69:238–49. [PubMed] [Google Scholar]
- 29.Howarth GS, Xian CJ, Read LC. Insulin-like growth factor-I partially attenuates colonic damage in rats with experimental colitis induced by oral dextran sulphate sodium. Scand J Gastroenterol. 1998;33:180–90. doi: 10.1080/00365529850166923. [DOI] [PubMed] [Google Scholar]
- 30.Ohama T, Hori M, Momotani E, et al. Intestinal inflammation downregulates smooth muscle CPI-17 through induction of TNF-alpha and causes motility disorders. AJP - Gastrointestinal and Liver Physiology. 2007;292:G1429–G1438. doi: 10.1152/ajpgi.00315.2006. [DOI] [PubMed] [Google Scholar]
- 31.Snape WJ., Jr The role of a colonic motility disturbance in ulcerative colitis. Keio J Med. 1991;40:6–8. [PubMed] [Google Scholar]
- 32.Ihara E, Beck PL, Chappellaz M, Wong J, Medlicott SA, Macdonald JA. Mitogen-activated protein kinase pathways contribute to hypercontractility and increased Ca2+ sensitization in murine experimental colitis. Mol Pharmacol. 2009 doi: 10.1124/mol.108.049858. [DOI] [PubMed] [Google Scholar]
- 33.Akbarali HI, Pothoulakis C, Castagliuolo I. Altered Ion Channel Activity in Murine Colonic Smooth Muscle Myocytes in an Experimental Colitis Model. Biochemical and Biophysical Research Communications. 2000;275:637–42. doi: 10.1006/bbrc.2000.3346. [DOI] [PubMed] [Google Scholar]
- 34.Iino M. Biphasic Ca2+ dependence of inositol 1,4,5-trisphosphate-induced Ca release in smooth muscle cells of the guinea pig taenia caeci. The Journal of General Physiology. 1990;95:1103–22. doi: 10.1085/jgp.95.6.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bayguinov O, Hagen B, Sanders KM. Muscarinic stimulation increases basal Ca2+ and inhibits spontaneous Ca2+ transients in murine colonic myocytes. Am J Physiol. 2001;280:C689–C700. doi: 10.1152/ajpcell.2001.280.3.C689. [DOI] [PubMed] [Google Scholar]
- 36.Bayguinov O, Hagen B, Kenyon JL, Sanders KM. Coupling strength between localized Ca2+ transients and K+ channels is regulated by protein kinase C. AJP - Cell Physiology. 2001;281:C1512–C1523. doi: 10.1152/ajpcell.2001.281.5.C1512. [DOI] [PubMed] [Google Scholar]
- 37.DeKoninck P, Schulman H. Sensitivity of CaM kinase II to the frequency of Ca2+ oscillations. Science. 1998;279:227–30. doi: 10.1126/science.279.5348.227. [DOI] [PubMed] [Google Scholar]
- 38.Meyer T, Hanson PI, Schulman H. Calmodulin trapping by calcium-calmodulin-dependent protein kinase. Science. 1992;256:1199–202. doi: 10.1126/science.256.5060.1199. [DOI] [PubMed] [Google Scholar]
- 39.Lee CW, Lin CC, Luo SF, et al. Tumor necrosis factor-alpha enhances neutrophil adhesiveness: induction of vascular cell adhesion molecule-1 via activation of Akt and CaM kinase II and modifications of histone acetyltransferase and histone deacetylase 4 in human tracheal smooth muscle cells. Mol Pharmacol. 2008;73:1454–64. doi: 10.1124/mol.107.038091. [DOI] [PubMed] [Google Scholar]
- 40.Schumann MA, Gardner P, Raffin TA. Recombinant human tumor necrosis factor alpha induces calcium oscillation and calcium-activated chloride current in human neutrophils. The role of calcium/calmodulin-dependent protein kinase. Journal of Biological Chemistry. 1993;268:2134–40. [PubMed] [Google Scholar]
- 41.Egger B, Bajaj-Elliott M, MacDonald TT, Inglin R, Eysselein VE, Büchler MW. Characterisation of Acute Murine Dextran Sodium Sulphate Colitis: Cytokine Profile and Dose Dependency. Digestion. 2000;62:240–8. doi: 10.1159/000007822. [DOI] [PubMed] [Google Scholar]
- 42.Viatour P, Merville MP, Bours V, Chariot A. Phosphorylation of NF-κB and IκB proteins: implications in cancer and inflammation. Trends Biochem Sci. 2005;30:43–52. doi: 10.1016/j.tibs.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 43.Gao Z, Hwang D, Bataille F, et al. Serine phosphorylation of insulin receptor substrate 1 by inhibitor kappa B kinase complex. J Biol Chem. 2002;277:48115–21. doi: 10.1074/jbc.M209459200. [DOI] [PubMed] [Google Scholar]
- 44.Shi XZ, Lindholm PF, Sarna SK. NF-kappa B activation by oxidative stress and inflammation suppresses contractility in colonic circular smooth muscle cells. Gastroenterology. 2003;124:1369–80. doi: 10.1016/s0016-5085(03)00263-4. [DOI] [PubMed] [Google Scholar]
- 45.Sanders KM. Regulation of smooth muscle excitation and contraction. Neurogastroenterology & Motility. 2008;20:39–53. doi: 10.1111/j.1365-2982.2008.01108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure. Gross morphology and histological analysis of the colon from DSS-treated mice. A. Representative photographs of large intestines from untreated control mice (top panel), and DSS-treated mice (day 1 post-DSS treatment, bottom panel). B. Representative H&E stained paraffin-embedded distal colon cross-sections from control and days 1-5 post-DSS treatment; m = mucosa, sm = submucosa, cm = circular smooth muscle (10× magnification). Note expansion of the submucosal layer as early as day 1 post DSS-treatment, and mucosal ulceration in day 5. Changes in mucosal structure can be seen on all days.