Abstract
Gastric motility studies are frequently conducted with anesthetized animal models. Some studies on the same animal species have reported differences in vagal control of the stomach that could not be explained solely by slightly different experimental conditions. A possible limitation in the comparison between similar studies relates to the use of different anesthetic agents. Furthermore, anesthetic effects may also limit generalizations between mechanistic studies of gastric motility and the gastric motility of conscious animals. In the present study, we used the [13C]-breath test following a liquid mixed-nutrient test meal (Ensure®, 1 ml) with the aim to investigate the rate of gastric emptying in animals that were either conscious or anesthetized with either Inactin® or urethane.
One week after determining the maximum 13CO2 concentration, time to peak [13C] recovery and gastric half emptying time in control, conscious rats, we repeated the experiment in the same rats anesthetized with Inactin® or urethane. Our data show that Inactin® anesthesia prolonged the time to peak [13C] recovery but did not significantly reduce the maximum 13CO2 concentration nor delay gastric half emptying time. Conversely, urethane anesthesia resulted in a significant slowing of all parameters of gastric emptying as measured by the maximum 13CO2 concentration, time to peak [13C] recovery and half emptying time.
Our data indicate that Inactin® anesthesia does not significantly affect gastric emptying while urethane anesthesia profoundly impairs gastric emptying. We suggest that Inactin®, not urethane, is the more suitable anesthetic for gastrointestinal research.
Keywords: [13C]-breath test, gastric emptying, gastric stasis, gastrointestinal motility, gastrointestinal transit
Introduction
The importance of vago-vagal reflex circuits mediating gastric motor function (motility) is well established1. The central, integrative, component of this reflex consists of the nucleus tractus solitarius (NTS) and the dorsal motor nucleus of the vagus (DMV) located within the medulla. Studies over the past decades have led to the generally accepted view that the motor output of the DMV regulates gastric motility through the opposing contributions of a cholinergic excitatory and a nonadrenergic, noncholinergic (NANC) inhibitory circuit1,2. However, mechanistic studies of the central control of gastric motility by the NTS and DMV frequently employ an anesthetized animal model for in vivo physiological and neuropharmacological studies3–8.
Anesthetic effects potentially limit generalizations between the conscious and anesthetized animal model. Furthermore, differences occurring between similar studies may relate to the use of different anesthetics. Both thiobutabarbital (Inactin®) and urethane are long-acting rodent anesthetics which have seen widespread use in gastrophysiology studies due to their presumptive sparing of autonomic function9. Cardiovascular parameters remain stable following Inactin®10 though changes in sodium balance have been reported11. Urethane anesthesia is presumed to spare autonomic function, although the degree of autonomic sparing may not be complete12,13. The potential effects of anesthetic choice upon brainstem autonomic reflexes are occasionally acknowledged in studies of gastrointestinal motility14, considered minimal15, or are purposefully exploited16. Surprisingly, the effects upon gastric motility of these commonly used anesthetics have not been adequately determined.
The controversy of anesthetic choice is only partially circumvented in studies of gastrointestinal motility that are performed in the conscious, behaving animal17–21. Commonly, these studies are based upon a single parameter of the entire gastric emptying process such as gastric contractility17,18 or are outcome based measurements such as post-mortem assays for gastric emptying of a meal challenge22–24. Non-invasive breath tests for gastric emptying utilizing [13C]- isotope-enriched substrates have been validated in a number of species including humans and rodents25–29 with the added benefit that this technique permits safe, repeatable, measurement of gastric emptying in the same animal.
Using the [13C]-breath test, the aims of the present study were 1) to establish baseline values of the rate of gastric emptying of a liquid, mixed-nutrient meal (Ensure®) in conscious animals; 2) to test the hypothesis whether sustained Inactin® or urethane anesthesia adversely delay gastric emptying rate.
Materials and Methods
All procedures were performed according to National Institutes of Health guidelines and were approved by the Institutional Animal Care and Use Committee at the Pennington Biomedical Research Center.
All experiments were conducted on male Wistar rats (n = 14; Harlan, Indianapolis, IN). Rats were ≥8 weeks of age upon entrance into the experiment and were double housed in a room maintained at 21–24°C and a 12:12-h light-dark cycle with food and water provided ad libitum. Following acquisition of the baseline values in conscious, unanesthetized conditions, rats were randomly assigned to receive either Inactin® or urethane. Body weights prior to baseline measurement were recorded and tested to ensure that no significant weight difference existed between groups.
Measurement of gastric emptying using [13C]-octanoic acid breath test
The liquid test meal used in this study was 1 ml of Ensure® (1.06 kcal, protein 16.4%, fat 10.1%, glucose 73.5%) combined with 5µl octanoic acid. Rats were gavaged with 1ml Ensure® in the days prior to the experiment in order to establish familiarity to the technique and the meal. Following an overnight fast (water ad libitum), the [13C]-breath test was performed using procedures described previously30. Briefly, animals were placed in a 7 L capacity metabolic chamber with a continuous flow of fresh air (0.65 l/min) in order to maintain CO2 levels near 0.5%. Samples were automatically withdrawn and analyzed by nondispersive Infra Red Isotope Analyzer (IRIS; Wagner Analysen Technik, Bremen, Germany). After two baseline measurements were taken, rats were briefly removed from the chamber and administered the test meal through a flexible polyethylene oro-gastric tube (PE-90). During all breath tests, rats were allowed 30 min for accommodation to the chamber before beginning the measurement. Air samples were collected at 5 min intervals for the first 1h of testing; subsequent air samples were taken at 15 min intervals for a total testing time of 240 min.
The [13C]-octanoic acid breath test was performed twice. The first test established the rate of gastric emptying for the conscious rat. Rats were returned to their home cage at the conclusion of the first 6 hr test. At 7 d after baseline measurement, rats were fasted a second time and randomly chosen to be anesthetized with thiobutabarbital (Inactin®; Sigma, St. Louis, MO; 100 mg/kg IP), or urethane (Sigma; 1.25g/kg IP). Following anesthesia, the rats were maintained at 37±1°C using a feedback-controlled warming pad in the testing chamber for 30 min before beginning the [13C]-octanoic acid breath test. After the first two breath analysis measurements were taken, rats were gavaged with the [13C]-octanoic acid labeled liquid meal. Following completion of the [13C]-octanoic acid breath test, anesthetized rats were euthanized.
Using the change in 13CO2 level over baseline for each air sample, the maximum concentration (Cmax; ‰) time to reach the maximum concentration in fractional dose/h (Tmax) and the gastric half emptying time (T1/2) were calculated by the IRIS software. The Results are represented as the mean±S.E.M. Statistical analysis was performed by using a two-sample t-test and significance differences were assumed when p<0.05.
Results
In conscious male Wistar rats (n=14), the maximum concentration of exhaled 13CO2 was 61.9±3.4 (Cmax; ‰). The time to reach maximum values (Tmax) of exhaled 13CO2 was 44.8±1.9 min. The calculated T1/2 for the gastric emptying of Ensure® was 76.9±3.1 min. Following this test, rats were randomly assigned to groups to receive either Inactin®- or urethane-anesthesia. There were no significant differences in Cmax, Tmax or the gastric emptying rate between group assignments.
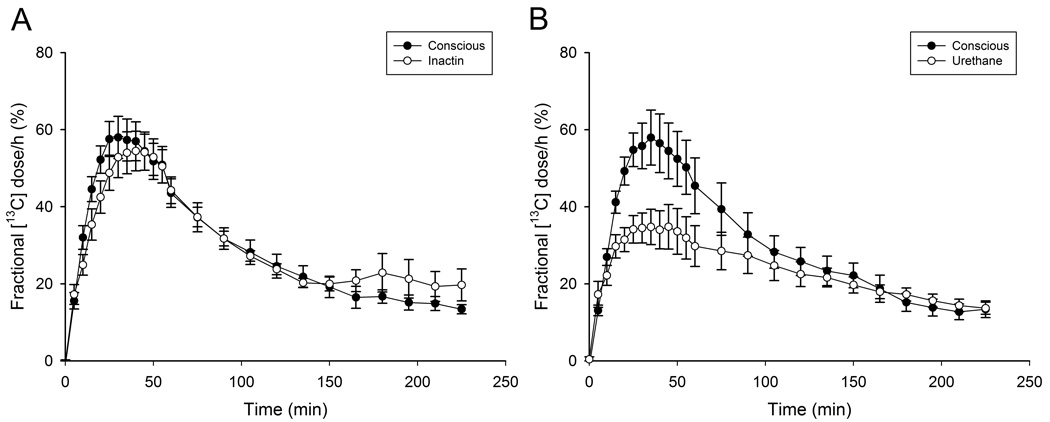
In a separate test 7 d later Fig. 1, the Tmax of animals anesthetized with Inactin® was significantly delayed (Table 1; p<0.05) while the values for Cmax and T1/2 were not significantly different from conscious values (Table 1; p>0.05). Following urethane anesthesia, Cmax, Tmax and T1/2 were all significantly delayed when compared to previous conscious values and those observed in Inactin® anesthetized animals (Table 1; p<0.05). These data indicate that the [13C]-octanoic acid breath test in conscious rats shows low between-animal variability in the rate of gastric emptying of a liquid, mixed nutrient meal. Unlike urethane, Inactin® anesthesia does not significantly depress maximum 13CO2 recovery or gastric half emptying time and indicates that it is a more suitable anesthetic for experiments in gastric emptying and motility.
Figure 1.
The within-subjects [13C] fractional dose curves for animals administered the breath test. A. animals without anesthesia (conscious) or following Inactin anesthesia 7 d later (n=7) or B) conscious animals that received urethane anesthesia 7 d later (n=7).
Table 1.
Gastric emptying parameters of [13C]-octanoate labeled Ensure prior to, and following anesthesia with either Inactin or urethane
| Conscious, prior to Inactin |
Inactin | Conscious, prior to Urethane |
Urethane | |
|---|---|---|---|---|
| Cmax (‰) | 59.4±5.0 | 56.7±4.6 | 59.7±6.3 | 36.9±2.2*,# |
| Tmax (min) | 44±3 | 51±2✪ | 45±3 | 54±2* |
| T1/2 (min) | 77±5 | 92±7 | 76±3 | 117±8*,# |
significantly different from conscious values prior to inactin anesthesia (p<0.05).
significantly different from conscious values prior to urethane anesthesia (p<0.05).
significantly different from inactin anesthesia (p<0.05).
Discussion
In the present study we have shown that Inactin® anesthesia does not significantly change gastric emptying rate compared to conscious animals. Conversely, urethane anesthesia significantly delays gastric emptying. Our data suggest that Inactin® is an acceptable long-acting anesthetic for studies of gastric emptying and motility which may extend to physiological studies of gastrointestinal functions. Conversely, urethane anesthesia inhibits gastric emptying significantly and may not be suitable for some gastrointestinal physiological studies.
Our conclusions are based upon the following lines of evidence. The sensitivity of our [13C]-breath test data from conscious animals is in general agreement with measures of gastric emptying reported for a similarly formulated liquid enteral meal31. Specifically, the time to peak 13CO2 level following the administration of [13C]-octanoic acid was nearly identical. Unfortunately, gastric half emptying time was not reported by those authors, thus limiting further comparisons. In our previous [13C]-breath test study30 we reported Tmax and T1/2 values for gastric emptying in surgically naïve animals fed a solid meal that contained the same molar amount of [13C]-octanoic acid. Similarly to our observations of liquid gastric emptying, the sensitivity of our [13C]-breath test for solid emptying was also in general agreement with published reports27. Comparison of our previous findings with our present data reveals that gastric emptying for a solid meal was approximately 50% slower, which is appropriate due to the differences in the transpyloric flow of liquid or solid meals32,33
Special attention must be paid to pulmonary sufficiency when utilizing the [13C]-breath test. Suppressed respiration as a result of anesthesia would predict a reduction in 13CO2 expression throughout the [13C]-breath test. Inactin® anesthesia does lead to an increase in CO2 blood gas values, though oxygen tension remains similar to conscious rats34 while respiratory function is considered stable under urethane anesthesia35,36. In our study both groups of anesthetized animals had calculated CO2 production levels (mmol/h*cm2) that were not significantly different from those of conscious controls (data not shown). Therefore, it is unlikely that diminished respiratory rates under urethane anesthesia account for the delay in gastric emptying seen during the [13C]-breath test.
One consequence of urethane anesthesia is a rapid elevation of blood glucose levels37,38. This hyperglycemia is only observed when urethane is administered intraperitoneally rather than intrarterial or subcutaneously36. Hyperglycemia inhibits gastric emptying of both liquid and solid meals39,40 due, in part, to reduced antro-pyloric coordination39 and a reduction in gastric tone that is proposed to involve the activation of nitric oxide- and VIP-containing neurons41. Conversely, studies of Inactin® anesthetized rats that include blood glucose data do not indicate any change in blood glucose concentration42,43. Therefore, our data suggest that urethane is a less desirable long-acting anesthetic than Inactin® for in vivo studies of gastric motility due the potential confounding variable of hyperglycemia.
In summary, the use of any anesthetic regimen duly raises concerns regarding the interpretation of physiological data. Our data from Inactin® anesthetized rats suggests that, unlike urethane anesthesia, the components of gastric emptying necessary for the delivery of the [13C]-labeled test meal into the duodenum (e.g., gastric contractions, antropyloric coordination, duodenal feedback) more closely resemble those of the conscious animal. Our study also demonstrates the applicability of the enteral delivery of a mixed-nutrient test meal to study pre- and post-prandial gastric physiology and pathophysiology in an anesthetized animal model. This will permit more detailed mechanistic studies directed at the actions of feeding-associated regulatory peptides and the central control of gastric reflexs.
Acknowledgements
The authors wish to acknowledge our gratitude to Drs. R.A. Travagli and K.N. Browning for their assistance and extensive commentary during the development of this project and preparation of the manuscript.
Funding
This work was supported by NINDS grant #49177.
Footnotes
Competing interests
The authors have no competing interests.
Reference List
- 1.Travagli RA, Hermann GE, Browning KN, Rogers RC. Brainstem circuits regulating gastric function. Annu Rev Physiol. 2006;68:279–305. doi: 10.1146/annurev.physiol.68.040504.094635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang HY, Mashimo H, Goyal RK. Musings on the wanderer: what's new in our understanding of vago-vagal reflex? IV. Current concepts of vagal efferent projections to the gut. Am J Physiol Gastrointest Liver Physiol. 2003;284(3):G357–G366. doi: 10.1152/ajpgi.00478.2002. [DOI] [PubMed] [Google Scholar]
- 3.Holmes GM, Tong M, Travagli RA. Effects of brainstem cholecystokinin-8s on gastric tone and esophageal-gastric reflex. Am J Physiol Gastrointest Liver Physiol. 2009;296(3):G621–G631. doi: 10.1152/ajpgi.90567.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takahashi T, Owyang C. Characterization of vagal pathways mediating gastric accommodation reflex in rats. J Physiol. 1997 Oct 15;504(Pt 2):479–488. doi: 10.1111/j.1469-7793.1997.479be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Viard E, Zheng Z, Wan S, Travagli RA. Vagally mediated, nonparacrine effects of cholecystokinin-8s on rat pancreatic exocrine secretion. Am J Physiol Gastrointest Liver Physiol. 2007 Aug 1;293(2):G493–G500. doi: 10.1152/ajpgi.00118.2007. [DOI] [PubMed] [Google Scholar]
- 6.Tack J. Neurophysiologic mechanisms of gastric reservoir function. In: Johnson LR, Barrett KE, Ghishan FK, Merchant JL, Said HM, Wood JD, editors. Physiology of the gastrointestinal tract. 4th ed. New York: Elsevier Academic Press; 2006. pp. 927–933. [Google Scholar]
- 7.Cruz MT, Murphy EC, Sahibzada N, Verbalis JG, Gillis RA. A reevaluation of the effects of stimulation of the dorsal motor nucleus of the vagus on gastric motility in the rat. Am J Physiol Regul Integr Comp Physiol. 2007 Jan 1;292(1):R291–R307. doi: 10.1152/ajpregu.00863.2005. [DOI] [PubMed] [Google Scholar]
- 8.Kobashi M, Yanagihara M, Fujita M, Mitoh Y, Matsuo R. Fourth ventricular administration of ghrelin induces relaxation of the proximal stomach in the rat. Am J Physiol Regul Integr Comp Physiol. 2009 Feb 1;296(2):R217–R223. doi: 10.1152/ajpregu.00878.2007. [DOI] [PubMed] [Google Scholar]
- 9.Gaertner DJ, Hallman TM, Hankenson FC, Batchelder MA. Anesthesia and Analgesia for Laboratory Rodents. In: Richard E.Fish, Marilyn JB, Peggy JD, Alicia ZK., editors. Anesthesia and Analgesia in Laboratory Animals. Second Edition. San Diego: Academic Press; 2008. pp. 239–297. [Google Scholar]
- 10.Buelke-Sam J, Holson JF, Bazare JJ, Young JF. Comparative stability of physiological parameters during sustained anesthesia in rats. Lab Anim Sci. 1978;28(2):157–162. [PubMed] [Google Scholar]
- 11.Metz C, Ise T, H+ñberle D. Chloralose/ketamine anaesthesia preserves a form of postprandial sodium chloride balance in Wistar rats. Pflugers Arch. 1996;432(5):944–947. doi: 10.1007/s004240050220. [DOI] [PubMed] [Google Scholar]
- 12.Jong WM, Zuurbier CJ, De Winter RJ, Van Den Heuvel DA, Reitsma PH, Ten CH, et al. Fentanyl-fluanisone-midazolam combination results in more stable hemodynamics than does urethane alpha-chloralose and 2,2,2-tribromoethanol in mice. Contemp Top Lab Anim Sci. 2002 May;41(3):28–32. [PubMed] [Google Scholar]
- 13.Sun MK, Reis DJ. Urethane directly inhibits chemoreflex excitation of medullary vasomotor neurons in rats. Eur J Pharmacol. 1995 Oct 6;293(3):237–243. doi: 10.1016/0926-6917(95)00023-2. [DOI] [PubMed] [Google Scholar]
- 14.Zhou SY, Lu YX, Yao H, Owyang C. Spatial organization of neurons in the dorsal motor nucleus of the vagus synapsing with intragastric cholinergic and nitric oxide/VIP neurons in the rat. Am J Physiol Gastrointest Liver Physiol. 2008 May 1;294(5):G1201–G1209. doi: 10.1152/ajpgi.00309.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rogers RC, Travagli RA, Hermann GE. Noradrenergic neurons in the rat solitary nucleus participate in the esophageal-gastric relaxation reflex. Am J Physiol Regul Integr Comp Physiol. 2003 Aug;285(2):R479–R489. doi: 10.1152/ajpregu.00155.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herman MA, Cruz MT, Sahibzada N, Verbalis J, Gillis RA. GABA signaling in the nucleus tractus solitarius sets the level of activity in dorsal motor nucleus of the vagus cholinergic neurons in the vagovagal circuit. Am J Physiol Gastrointest Liver Physiol. 2009 Jan 1;296(1):G101–G111. doi: 10.1152/ajpgi.90504.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ariga H, Nakade Y, Tsukamoto K, Imai K, Chen C, Mantyh C, et al. Ghrelin accelerates gastric emptying via early manifestation of antro-pyloric coordination in conscious rats. Regulatory Peptides. 2008 Feb 7;146(1–3):112–116. doi: 10.1016/j.regpep.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 18.Ariga H, Imai K, Chen C, Mantyh C, Pappas TN, Takahashi T. Fixed feeding potentiates interdigestive gastric motor activity in rats: importance of eating habits for maintaining interdigestive MMC. Am J Physiol Gastrointest Liver Physiol. 2008 Mar 1;294(3):G655–G659. doi: 10.1152/ajpgi.00484.2007. [DOI] [PubMed] [Google Scholar]
- 19.Fujimiya M, Itoh E, Kihara N, Yamamoto I, Fujimura M, Inui A. Neuropeptide Y induces fasted pattern of duodenal motility via Y2 receptors in conscious fed rats. Am J Physiol Gastrointest Liver Physiol. 2000 Jan 1;278(1):G32–G38. doi: 10.1152/ajpgi.2000.278.1.G32. [DOI] [PubMed] [Google Scholar]
- 20.Janssen P, Nielsen MA, Hirsch I, Svensson D, Gillberg PGr, Hultin L. A novel method to assess gastric accommodation and peristaltic motility in conscious rats. Scand J Gastroenterol. 2008 Jan;43(1):34–43. doi: 10.1080/00365520701580066. [DOI] [PubMed] [Google Scholar]
- 21.Uchida M, Mogami O. Usefulness of Breath Test for Evaluating Pancreatic Exocrine Function Using N-Benzoyl-L-tyrosyl-1-13C-L-alanine Sodium in Non-invasive and Conscious Rats. Biol Pharm Bull. 2008;31(5):785–788. doi: 10.1248/bpb.31.785. [DOI] [PubMed] [Google Scholar]
- 22.Nakade Y, Tsukamoto K, Pappas TN, Takahashi T. Central glucagon like peptide-1 delays solid gastric emptying via central CRF and peripheral sympathetic pathway in rats. Brain Res. 2006 Sep 21;1111(1):117–121. doi: 10.1016/j.brainres.2006.06.090. [DOI] [PubMed] [Google Scholar]
- 23.Maher J, Johnson AC, Newman R, Mendez S, Hoffmann TJ, Foreman R, et al. Effect of spinal cord stimulation in a rodent model of post-operative ileus. Neurogastroenterol Motil. 2009;21:672–677. doi: 10.1111/j.1365-2982.2008.01237.x. [DOI] [PubMed] [Google Scholar]
- 24.Gondim FA, Rodrigues CL, da Graca JR, Camurca FD, de Alencar HM, dos Santos AA, et al. Neural mechanisms involved in the delay of gastric emptying and gastrointestinal transit of liquid after thoracic spinal cord transection in awake rats. Autonomic Neuroscience. 2001;87:52–58. doi: 10.1016/s1566-0702(00)00261-7. [DOI] [PubMed] [Google Scholar]
- 25.Ghoos YF, Maes BD, Geypens BJ, Mys G, Hiele MI, Rutgeerts PJ, et al. Measurement of gastric emptying rate of solids by means of a carbon-labeled octanoic acid breath test. Gastroenterol. 1993 Jun;104(6):1640–1647. doi: 10.1016/0016-5085(93)90640-x. [DOI] [PubMed] [Google Scholar]
- 26.Maes BD, Mys G, Geypens BJ, Evenepoel P, Ghoos YF, Rutgeerts PJ. Gastric emptying flow curves separated from carbon-labeled octanoic acid breath test results. Am J Physiol. 1998 Jul;275(1 Pt 1):G169–G175. doi: 10.1152/ajpgi.1998.275.1.G169. [DOI] [PubMed] [Google Scholar]
- 27.Schoonjans R, Van Vlem B, Van Heddeghem N, Vandamme W, Vanholder R, Lameire N, et al. The 13C-octanoic acid breath test: validation of a new noninvasive method of measuring gastric emptying in rats. Neurogastroenterol Motil. 2002;14(3):287–293. doi: 10.1046/j.1365-2982.2002.00334.x. [DOI] [PubMed] [Google Scholar]
- 28.Choi KM, Zhu J, Stoltz GJ, Vernino S, Camilleri M, Szurszewski JH, et al. Determination of gastric emptying in nonobese diabetic mice. Am J Physiol Gastrointest Liver Physiol. 2007;293(5):G1039–G1045. doi: 10.1152/ajpgi.00317.2007. [DOI] [PubMed] [Google Scholar]
- 29.Uchida M, Endo N, Shimizu K. Simple and noninvasive breath test using 13C-acetic acid to evaluate gastric emptying in conscious rats and its validation by metoclopramide. J Pharmacol Sci. 2005;98(4):388–395. doi: 10.1254/jphs.fp0050153. [DOI] [PubMed] [Google Scholar]
- 30.Creekmore EQ, Tong M, Holmes GM. Time-course of recovery of gastric emptying and motility in rats with experimental spinal cord injury. Neurogastroenterol Motil. 2009 doi: 10.1111/j.1365-2982.2009.01347.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Uchida M, Shimizu aK. 13C-Acetic Acid Is More Sensitive Than 13C-Octanoic Acid for Evaluating Gastric Emptying of Liquid Enteral Nutrient Formula by Breath Test in Conscious Rats. Biol Pharm Bull. 2007;30(3):487–489. doi: 10.1248/bpb.30.487. [DOI] [PubMed] [Google Scholar]
- 32.Behrns KE, Sarr MG. Diagnosis and management of gastric emptying disorders. Adv Surg. 1994;27:233–255. [PubMed] [Google Scholar]
- 33.Malagelada J-R, Azpiroz F. Determinants of gastric emptying and transit in the small intestine. In: Wood J, editor. Handbook of physiology. 2nd ed. Bethesda: American Physiological Society; 1989. pp. 909–937. [Google Scholar]
- 34.Sigmon DH, Florentino-Pineda I, Van Dyke RA, Beierwaltes WH. Halothane Impairs the Hemodynamic Influence of Endothelium-derived Nitric Oxide. Anesthesiology. 1995;82(1):135–143. doi: 10.1097/00000542-199501000-00018. [DOI] [PubMed] [Google Scholar]
- 35.Koblin DD. Urethane: Help or Hindrance? Anesth Analg. 2002 Feb 1;94(2):241–242. doi: 10.1097/00000539-200202000-00002. [DOI] [PubMed] [Google Scholar]
- 36.Maggi CA, Meli A. Suitability of urethane anesthesia for physiopharmacological investigations in various systems. Part 1: General considerations. Experientia. 1986 Feb 15;42(2):109–114. doi: 10.1007/BF01952426. [DOI] [PubMed] [Google Scholar]
- 37.Takeuchi K, Niida H, Ohuchi T, Okabe S. Influences of urethane anesthesia on indomethacin-induced gastric mucosal lesions in rats. Dig Dis Sci. 1994 Dec 1;39(12):2536–2542. doi: 10.1007/BF02087687. [DOI] [PubMed] [Google Scholar]
- 38.Reinert H. Urethane hyperglycaemia and hypothalamic activation. Nature. 1964 Nov 28;204:889–891. doi: 10.1038/204889a0. [DOI] [PubMed] [Google Scholar]
- 39.Ishiguchi T, Tada H, Nakagawa K, Yamamura T, Takahashi T. Hyperglycemia impairs antro-pyloric coordination and delays gastric emptying in conscious rats. Auton Neurosci. 2002 Jan 10;95(1–2):112–120. doi: 10.1016/s1566-0702(01)00383-6. [DOI] [PubMed] [Google Scholar]
- 40.Chang FY, Lee SD, Yeh GH, Wang P. Influence of blood glucose levels on rat liquid gastric emptying. Dig Dis Sci. 1996 Mar 25;41(3):528–532. doi: 10.1007/BF02282333. [DOI] [PubMed] [Google Scholar]
- 41.Zhou SY, Lu YX, Owyang C. Gastric relaxation induced by hyperglycemia is mediated by vagal afferent pathways in the rat. Am J Physiol Gastrointest Liver Physiol. 2008 May 1;294(5):G1158–G1164. doi: 10.1152/ajpgi.00067.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hindlycke M, Jansson L. Glucose tolerance and pancreatic islet blood flow in rats after intraperitoneal administration of different anesthetic drugs. Ups J Med Sci. 1992;97(1):27–35. doi: 10.3109/03009739209179279. [DOI] [PubMed] [Google Scholar]
- 43.Jansson L, Andersson A, Bodin B, Källskog Ö. Pancreatic islet blood flow during euglycaemic, hyperinsulinaemic clamp in anaesthetized rats. Acta Physiologica. 2007 Apr;189(4):319–324. doi: 10.1111/j.1748-1716.2006.01666.x. [DOI] [PubMed] [Google Scholar]