Abstract
The Chlamydomonas reinhardtii genomic DNA database contains a predicted open reading frame (ORF-P) without an apparent stop-codon and unknown coding sequence, located in close proximity and immediately upstream of the TLA1 gene (GenBank Accession No. AF534570). The latter was implicated in the regulation of the light-harvesting chlorophyll antenna size of photosynthesis (Tetali et al. Planta 225:813–829, 2007). To provide currently lacking information on ORF-P and its potential participation in TLA1 gene expression, thus in the regulation of the chlorophyll antenna size, genetic and biochemical analyses were undertaken. The coding and UTR regions of the ORF-P were defined and delineated from those of the adjacent TLA1 gene. ORF-P is shown to encode a protein with a distinct RING-like zinc finger domain that is present in numerous eukaryotic proteins, believed to play a role in cellular ubiquitination, leading to regulation of cellular processes like signaling, growth, transcription, and DNA repair. It is further shown that the two genes share a 74-bp overlap between the 3′ UTR region of ORF-P and the 5′ UTR region of TLA1. However, they possess distinct start and stop codons and separate coding sequences, and transcribed as separate mRNAs without any trans-splicing between them. Complementation experiments showed that the TLA1 gene alone is sufficient to rescue the truncated chlorophyll antenna size phenotype of the tla1 mutant. Protein sequence alignments in C. reinhardtii and the colorless microalga Polytomella parva suggested that TLA1 defines the relationship between nucleus and organelle in microalgae, indirectly affecting the development of the chlorophyll antenna size.
Electronic supplementary material
The online version of this article (doi:10.1007/s00425-009-1083-3) contains supplementary material, which is available to authorized users.
Keywords: Chlamydomonas, Chlorophyll antenna size, Chl-deficient mutant, Photosynthesis, Polytomella parva, TLA1 gene
Introduction
The chlorophyll (Chl) antenna size of the photosystems is defined genetically by unknown genes and regulated molecularly by a mechanism that is not well understood. Up to 300 Chl (a and b) molecules can be associated with PSII, whereas the Chl antenna of PSI may contain up to 250 Chl molecules (Melis 2002, 2005). The number of Chl molecules associated with each photosystem is not fixed but could vary substantially depending on environmental, developmental, genetic, or physiological conditions (Bjorkman et al. 1972; Anderson 1986; Melis 1991). A long-term acclimation mechanism by plants and algae regulates the expression of nuclear chlorophyll a–b light-harvesting complex (Lhcb, Lhca ) and chlorophyllide a oxygenase (CAO) genes (Jansson et al. 1992; Ohtsuka et al. 1997), thereby attenuating the Chl antenna size. For example, upon growth under light-limiting conditions, up-regulation of the Lhc and CAO gene expression results in a larger Chl antenna size (Masuda et al. 2003). Conversely, under high irradiance, conferring excitation pressure to the photosynthetic apparatus (Maxwell et al. 1995; Huner et al. 1998; Wilson and Huner 2000), the reverse occurs. Earlier efforts to elucidate the molecular mechanism for the dynamic regulation of the Chl antenna size postulated involvement of the redox state of the plastoquinone pool (Escoubas et al. 1995) and/or the operation of a cytosolic signaling transduction pathway for the rapid (order of minutes) regulation of both Lhc and CAO gene expression by irradiance (Masuda et al. 2003). The latter implicated activation of a specific Ca2+/CaM-dependent protein kinase in this cytosolic signal transduction pathway. Acclimation of the Chl antenna size enables photosynthetic organisms to better balance rates of the light versus the carbon reactions, so as to optimize the overall efficiency of photosynthesis in diverse irradiance ecotypes (Melis 2009). The above-described Chl antenna size regulatory mechanism is highly conserved and functions in all organisms of oxygenic and anoxygenic photosynthesis (Anderson 1986; Nakada et al. 1995; Escoubas et al. 1995; Huner et al. 1998; Yakovlev et al. 2002; Masuda et al. 2002, 2003). Physiological and biochemical consequences of the function of this molecular mechanism for the regulation of the Chl antenna size are well understood (Anderson 1986; Melis 1991; Melis et al. 1999). However, unknown are still the nuclear genes that dynamically modulate the development and define the size of the Chl antenna in the chloroplast (Escoubas et al. 1995; Melis 1991, 1996, 2002, 2005).
A genetic approach toward identification of the genes and elucidation of the mechanism for the regulation of the Chl antenna size employed DNA-insertional mutagenesis and screening for the isolation of mutants with a “truncated light-harvesting Chl antenna size” (tla mutants) in the model organism Chlamydomonas reinhardtii. The tla1 mutant strain was isolated from such a DNA-insertional mutagenesis library. It possessed a smaller than wild-type Chl antenna size for both photosystems, with the PSII and PSI Chl antenna size of the mutant being 50 and 65% of those found in the wild type, respectively. It also showed lower levels of light-harvesting proteins and of Chl b relative to the wild type (Polle et al. 2003). The tla1 strain required a higher light intensity for the saturation of photosynthesis and showed greater solar conversion efficiency and a higher photosynthetic productivity than the wild type under mass culture conditions. Molecular and genetic analyses revealed that, in the tla1 mutant, the exogenous plasmid DNA was inserted at the end of the 5′ UTR and just prior to the ATG start codon of a hitherto unknown gene (termed TLA1), which encodes a protein of 213 amino acids. Expression of the TLA1 gene was substantially down-regulated as a consequence of this plasmid insertion (Tetali et al. 2007).
Work in this manuscript was necessitated by the presence of a C. reinhardtii genomic DNA “predicted open reading frame” (ORF-P), which lacked an apparent stop-codon, and was located in close proximity and immediately upstream of the TLA1 gene. Lack of ESTs for ORF-P, and lack of a stop codon, coupled with the close proximity of ORF-P to TLA1 raised questions as to whether ORF-P is expressed, whether a portion or all of the ORF-P extends into and might be part of the TLA1 gene, or whether trans-splicing between coding regions of ORF-P and TLA1 mRNA plays a role in the expression of the TLA1. These possibilities were not previously investigated (Tetali et al. 2007). The work provides new information on the genetic, molecular and functional characteristics of the TLA1 gene. It addressed the relationship of TLA1 with the upstream proximal ORF-P gene, reports on tla1 mutant rescue experiments performed separately with the ORF-P and TLA1 genes, and probes TLA1 protein similarities between two divergent microalgae, the green C. reinhardtii and the colorless Polytomella parva. Insights gained from this work advance our knowledge of C. reinhardtii genomic DNA organization in the vicinity of the TLA1 gene and provide evidence on the function of the TLA1 protein.
Materials and methods
Growth of the algae
Chlamydomonas reinhardtii CC425, the arginine-requiring strain (http://www.chlamy.org); tla1, the chlorophyll-deficient tla1 mutant (Polle et al. 2003); and tla1-complemented strains of the tla1 mutant (Tetali et al. 2007) were employed in this work. Strains were grown to the mid-exponential growth phase either in TAP [Tris acetate phosphate, pH 7.4], TAP + Arg (Sueoka 1960; Harris 1989), or in modified minimal media containing 40 mM Tris–HCl, pH 7.4, supplemented with 25 mM sodium bicarbonate with or without Arg (TBP medium, Polle et al. 2000) in flat 1-L Roux bottles at 25°C under continuous illumination at 200 μmol photons m−2 s−1, provided by cool-white fluorescent lamps. The cultures were stirred continuously to ensure mixing, a uniform illumination of the cells, and to prevent settling.
Cell density was estimated upon counting the number of cells per mL culture using a Neubauer ultraplane hemacytometer. Pigments from intact cells were extracted in 80% acetone and cell debris removed by centrifugation at 10,000g for 5 min. The absorbance of the supernatant was measured with a Shimadzu UV-160U spectrophotometer and the chlorophyll (Chl a and Chl b) concentration of the samples was determined according to Arnon (1949), with equations corrected as in Melis et al. (1987).
Nucleic acid extractions
Chlamydomonas genomic DNA was isolated using a combination of Qiagen’s (Valencia, CA, USA) DNeasy plant mini kit and phenol chloroform extraction. Chlamydomonas total RNA was isolated using Trizol Reagent (Invitrogen, Carlsbad, CA, USA). Small- and large-scale isolation of plasmid preps were done using the QIAprep spin miniprep and Hispeed plasmid Midi kit (Qiagen), respectively. Gel purifications of amplified PCR and RT-PCR products were performed using QIAEX II kit (Qiagen).
RACE analysis of ORF-P and TLA1 cDNAs
3′ and 5′ RACE analyses were performed on isolated RNA samples from the wild-type strain using the Gene Racer kit primers (Invitrogen) and respective TLA1/ORF-P gene-specific primers (Table 1 and 1S). The Hot Star Taq Plus PCR kit (Qiagen) was used for this PCR work. The amplified RACE PCR products were cloned into the pCR4-TOPO vector using the TOPO TA cloning kit for sequencing (Invitrogen). The cloned constructs were sequenced at the UC Berkeley DNA Sequencing Facility.
Table 1.
RT-PCR and 5′ and 3′ RACE primers used for RT-PCR and RACE analysis of TLA1 and ORF-P cDNA in the wild-type strain
| Primers | Expected product |
|---|---|
|
“Primer 6” (5′ GTACGACAAGGGCAATGATAATGG 3′) “Primer 7” (5′ CGTAGCAGAATGACGCGC 3′) Probing for the ORF-P gene and its cDNA |
772 bp genomic DNA product; 296 bp cDNA product |
|
“Primer 2” (5′ ATGACTTTCAGCTGCTCCGCTGACCAAA 3′) “Primer 3R” (5′ TTGTTGTCCAGCACCAGCAC 3′) Probing for TLA1 cDNA |
359 bp cDNA product; 768 bp genomic DNA product |
|
“Primer X” (5′ CGACTGGAGCACGAGGACACTGA 3′) and “Primer Y” (5′ TTAAGAGCGCGGTTTGGTCAGCGGAGCAGC 3′) Used for 5′ RACE of TLA1 cDNA |
207 bp |
|
“Primer A” (5′ CAACACCAGGGGCAACACCAGGGGCA 3′) and “Primer B” (5′ CGCTACGTAACGGCATGACAGTG 3′) Used for 3′ RACE of ORF-P cDNA |
847 bp |
|
“Primer X” (5′ CGACTGGAGCACGAGGACACTGA 3′) and “Primer C” (5′ CCATTATCATTGCCCTTGTCGTAC 3′) Used for 5′ RACE of ORF-P cDNA |
473 bp |
Cloning of full-length TLA1 gene for complementation of the tla1 mutant
The full-length TLA1 gene (genomic DNA sequence including two exons and a single intron) was amplified using “primer 2” and “primer 3R” (Exon-1 and Exon-2; Table 1) on extracted wild-type genomic DNA. The “primer 2” and “primer 3R” had NdeI and XbaI restriction sites added at the 5′ ends, respectively. The 768 bp genomic-DNA PCR product (TLA1 gene with the added base pair from the restriction sites) was digested with NdeI and XbaI and cloned into vector pSL18 (Fischer and Rochaix 2001; Pollock et al. 2003). Platinum Taq high fidelity DNA polymerase (Invitrogen) was used for the PCR amplification. A 1-kb plus DNA ladder was used as DNA size markers (Invitrogen). The pSL18 plasmid incorporates 5′ and 3′ UTR regions from the PsaD gene of C. reinhardtii, and also contains the ampicillin and paromomycin (Sizova et al. 2001) resistance genes as selectable markers (Fig. 1S). The latter are useful for screening transformants of Escherichia coli and C. reinhardtii on LB + ampicillin and TAP + paromomycin agar plates, respectively. The recombinant pSL18-TLA1 construct from one of the positive E. coli clones was sequenced to confirm the TLA1 gene sequence present. The sequenced pSL18-TLA1 construct was used to transform the tla1 mutant strain by the glass-bead method (Debuchy et al. 1989; Kindle 1990).
Isolation of tla1-complements
The tla1 mutant was transformed with the above-mentioned pSL18-TLA1 construct (Fig. 1S) and transformant lines were screened on TAP + 7.5 μM paromomycin-containing agar plates (Sizova et al. 2001). About 100 colonies were thus isolated and screened for strains showing high Chl/cell and low Chl a/Chl b ratios compared with that of the tla1 mutant. These paromomycin-resistant colonies, having a dark green coloration, were tested for the presence of the transforming TLA1 gene by PCR/RT-PCR and TLA1 protein levels by western blot analysis. Five different tla1-complemented lines were isolated on the basis of visual/microscopic inspection and Chl analysis of the cultures. From these, two lines with similar to the wild-type Chl content and light-harvesting Chl antenna size and were selected for further analysis.
PCR and RT-PCR analysis of ORF-P
Primers (Table 1) were designed on the basis of the ORF-P genomic DNA sequence in the database and used for PCR and RT-PCR reactions with the wild-type DNA and RNA, respectively. The amplified PCR and RT-PCR products were cloned in pCR4-TOPO vector using the TOPO TA cloning kit for sequencing (Invitrogen). The cloned constructs were sequenced at the UC Berkeley DNA sequencing facility.
Analysis of the TLA1 gene
Primers for PCR and RT-PCR reactions were designed on the basis of wild type, transforming, and mutant tla1 gene structure. These features are summarized below:
Wild-type TLA1 gene possess the following distinct DNA regions: 5′ UTR, Exon 1, Intron, Exon 2, 3′ UTR.
Mutant tla1 gene shows: 3′ end of pJD67 plasmid, Exon 1, Intron, Exon 2, 3′ UTR. (Note that the TLA1 mutant lacks the wild-type 5′ UTR region.)
Transforming TLA1 gene for mutant complementation contains: PsaD 5′ UTR, Exon 1, Intron, Exon 2, PsaD 3′ UTR.
tla1 mutant strains complemented with the wild-type TLA1 gene (tla1-complements) were first tested by PCR and RT-PCR to check for the presence and expression of two distinct TLA1 genes (transforming and endogenous). To test for the presence of the transforming TLA1 gene and transcripts, a PsaD 5′ UTR-specific forward primer (“primer 5”; Fig. 1S, Table 1S; supplemental material) and a reverse primer specific to the second exon of the TLA1 gene (“primer 4”; Fig. 1S, Table 1S; supplemental material) were used. To test for the presence of the wild-type TLA1 gene, PCR and RT-PCR were also performed on the genomic DNA and cDNA by using a forward primer specific to the 5′ UTR of the TLA1 gene (“primer 1”; Table 1S; supplemental material) and a reverse primer specific to the second exon of the TLA1 gene (“primer 3”; Fig. 1S, Table 1S; supplemental material). Exon-1-specific forward primer beginning with the start codon of the TLA1 gene (“primer 2”; Table 1S; supplemental material) and the Exon-2-specific reverse primer ending with the stop codon of the TLA1 gene (“primer 4”; Fig. 1S, Table 1S; supplemental material) were used to probe for the full-length TLA1 transcript.
The one-step RT-PCR kit (Qiagen) was used for RT-PCR experiments. A 1 kb plus DNA ladder was used as DNA size markers for all DNA gel electrophoresis (Invitrogen).
Cell protein analysis
Chlamydomonas reinhardtii cells were harvested, washed twice with fresh growth medium and resuspended in TEN buffer, containing 10 mM Tris–HCl, 10 mM EDTA and 150 mM NaCl, pH 8. Following sonication, the crude cell extract was incubated in the presence of solubilization buffer (Smith et al. 1990). Gel lanes were loaded with an equal amount of Chl, in the range 1–2 nmol Chl, as indicated. SDS-PAGE analysis was performed according to Laemmli (1970) on a 12.5% gel, using either the Fermentas prestained (Glen Burnie, MD, USA) or Benchmark unstained protein ladder (Invitrogen). A constant current of 15 mA was applied to the electrophoresis for a 3-h period. Gels were stained with 1% Coomassie brilliant Blue R for protein visualization.
Electrophoretic transfer of the SDS-PAGE resolved proteins onto nitrocellulose was carried out for 2 h at a constant current of 400 mA in the transfer buffer (25 mM Tris, 192 mM glycine and 20% methanol). The TLA1 immune serum was used to detect the TLA1 protein in the total cell extracts of wild type (CC425), tla1 mutant, and tla1-complements. The TLA1 immune serum was diluted with the antibody buffer [Tris-buffered saline, 0.005% Tween 20 and 1% bovine serum albumin (pH 7.4)] to a ratio of 1:3,000 before used as the primary probe. The secondary antibody employed for this western blot analysis was conjugated to horseradish peroxidase (BioRad, Hercules, CA, USA) and diluted to a ratio of 1:30,000 with the antibody buffer. Western blots were developed for visualization by using the Supersignal West chemiluminescent substrate kit (Pierce, Rockford, IL, USA). Image processing software (ImageJ, http://rsb.info.nih.gov/ij/) in conjunction with Microsoft Excel 2007, were used for optical density quantification of Lhcb bands from the western blot analysis experiments.
Bioinformatic analysis
Webserver BLAST-P (http://www.ncbi.nlm.nih.gov/) was used for identifying TLA1 homologs using the non-redundant protein sequence database. ClustalW (http://www.ebi.ac.uk/Tools/clustalw2/index.html) was used for alignment of TLA1 homologous sequence from C. reinhardtii and P. parva.
Accession numbers
The GenBank Accession numbers for the TLA1 gene are AF534570 and AF534571. The GenBank Accession numbers for the RDP1 (ORF-P) gene are EU717142 and EU717143.
Results
Identification of an open reading frame (ORF-P)
Examination of the C. reinhardtii genomic DNA sequence in the JGI Chlre3, Scaffold 36 database (http://genome.jgi-psf.org/Chlre3/Chlre3.home.html), which contains the TLA1 gene (Polle et al. 2003; Tetali et al. 2007) revealed the occurrence of a putative gene (ORF-P) of about 792 bp (chlre3/scaffold 36: 146,926–147,461 bp). The predicted start codon of the ORF-P was about 1,882 bp upstream of the ATG start codon of the TLA1 gene (Fig. 1a). Three putative exons were identified to be 129, 158 and 29 bp long, respectively, with two putative introns of 249 and 227 bp long (Fig. 1a). The 316 bp of the putative ORF-P cDNA would normally encode a polypeptide of 105 amino acids, but ORF-P revealed no apparent stop codon in the JGI C. reinhardtii database, suggesting a longer product. Further, the 5′ and the 3′ un-translated regions of the ORF-P were not specified in the database. Due to lack of detailed information in the database, and lack of a corresponding EST, a question was raised as to whether the ORF-P-encoding sequence might be longer than the three putative exons described above, and whether it could be part of the TLA1 gene, which is immediately downstream of the ORF-P sequence (Fig. 1a). These questions pertain to the genetics and regulation of the TLA1 gene, and bear upon the mode of action of the TLA1 protein in the regulation of the Chl antenna size (Polle et al. 2003; Tetali et al. 2007). Accordingly, a detailed investigation was undertaken to better define ORF-P gene and product, and to clarify its relationship with the TLA1 gene.
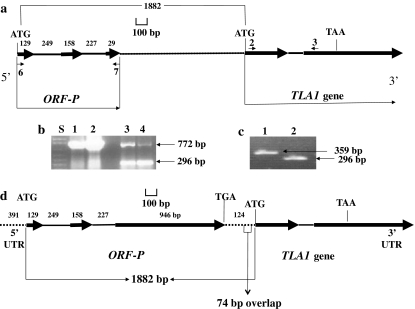
Fig. 1.
Identification of an incomplete predicted open reading frame (ORF-P) in the Chlamydomonas reinhardtii database. a Genomic map of the ORF-P showing predicted exons (thick black arrow) and introns (thin black lines in-between exons). The uncharacterized genomic DNA region between ORF-P and TLA1 is depicted by a dotted line. The small black arrows (6, 7; 2, 3) denote primers used for genomic DNA PCR and RT-PCR. The known start codon (ATG) and the stop codon (TAA) of the TLA1 gene are denoted. Numbers refer to the base pairs of the putative exons, introns and distance between the start codon of ORF-P and start codon of TLA1. b Lanes 1 and 2 show the genomic DNA PCR product (772 bp) obtained with ORF-P-specific primer 6 and primer 7 (Exon-1/Exon-3 of ORF-P; Table 1); lanes 3 and 4 show the RT-PCR product (296 bp) obtained with the same primer set. (Genomic DNA contamination in the RNA preparation gave rise to the 772 bp product in the RT-PCR reaction in lanes 3 and 4.). Lane S refers to 1 kb plus DNA ladder. c RT-PCR using TLA1 gene-specific Exon-1 “primer 2” and Exon-2 “reverse primer 3R” (359 bp product; lane 1, Table 1) and ORF-P gene-specific Exon-1 “primer 6” and Exon-3 “primer 7” (296 bp product; lane 2, Table 1). d Complete genomic DNA map of the C. reinhardtii ORF-P and TLA1 genes. The positional alignment of the TLA1 gene with respect to the ORF-P is shown. Thick black arrows denote exons, while thin black lines denote introns. Untranslated regions of the two genes are denoted by dotted lines. Note that the 3′ UTR of ORF-P overlaps the 5′ UTR of TLA1 by 74 bp. The start and stop codons of the ORF-P and TLA1 genes are labeled. Numbers denote the base pairs of the respective DNA regions, i.e., 5′ UTR, exons, introns and 3′ UTR of the ORF-P
Effort was directed toward testing for the expression of the ORF-P. An initial set of primers was designed based on the putative ORF-P genomic DNA sequence and used for PCR and RT-PCR analyses. When ORF-P Exon-1-specific “primer 6”, and ORF-P Exon-3-specific “primer 7” (Fig. 1a; Table 1), were used for PCR and RT-PCR, products of 772 and 296 bp, respectively, were obtained (Fig. 1b). These PCR products are consistent with those expected from the structure of the ORF-P putative gene. Moreover, nucleotide-sequencing analysis of the PCR and RT-PCR amplified products (Fig. 1b) confirmed the identity of these products and, therefore, the existence of three exons and two introns as components of the ORF-P gene. Figure 1c shows results of RT-PCR analysis performed with RNA from the same sample, but with TLA1 gene-specific primers, “primer 2” and “primer 3” (Exon-1/Exon-2, Fig. 1c, lane 1; Table 1) and ORF-P gene-specific primers, “primer 6” and “primer 7” (Exon-1/Exon-3, Fig. 1c, lane 2; Table 1). This side-by-side comparison showed RT-PCR products of 359 and 296 bp, respectively, providing evidence that both TLA1 and ORF-P are expressed under the customary and physiological C. reinhardtii growth conditions employed in this work. To facilitate comparison, Fig. 1d shows the updated complete map of the ORF-P:TLA1 C. reinhardtii genomic DNA region. Evidence for some of the features presented in this map is provided by detailed analysis, below.
RACE analyses of ORF-P and TLA1 cDNAs
Detailed RACE analyses for the ORF-P and TLA1 genes were undertaken to define coding and untranslated regions. RACE primers used in this work are listed in Table 1. It was found that the 5′ UTR of ORF-P is 391 bp long (Fig. 1d). These 5′ RACE results did not change the translational reading frame that was earlier deduced for ORF-P. In order to precisely define the 3′ UTR end of the ORF-P and the beginning of the 5′ UTR of the TLA1 gene, 3′ RACE and 5′ RACE analyses of the ORF-P and TLA1 cDNAs were performed, respectively. Results from these analyses showed that the putative TGA stop codon of ORF-P is 1,706 bp away from the start codon in the genomic sequence, and 1,230 bp away from the start codon in the cDNA sequence (Fig. 1d). These results suggested a putative ORF-P-encoded protein of 410 amino acids. The putative TGA stop codon of ORF-P is shown in Figs. 1d and 2a along with the 124 bp that comprise the 3′ UTR of this open reading frame. 5′ RACE analysis for the TLA1 gene revealed a 5′ UTR comprising 123 bp. The ATG start codon and upstream 5′ UTR of the TLA1 gene is also shown in Figs. 1d and 2a. There is overlap of 74 bp between the 3′ UTR of ORF-P and 5′ UTR of the TLA1 gene (Fig. 2a, gray highlighted nucleotides). It was also observed that the TGA stop codon of the ORF-P and the ATG start codon of the TLA1 gene are outside this overlap region.
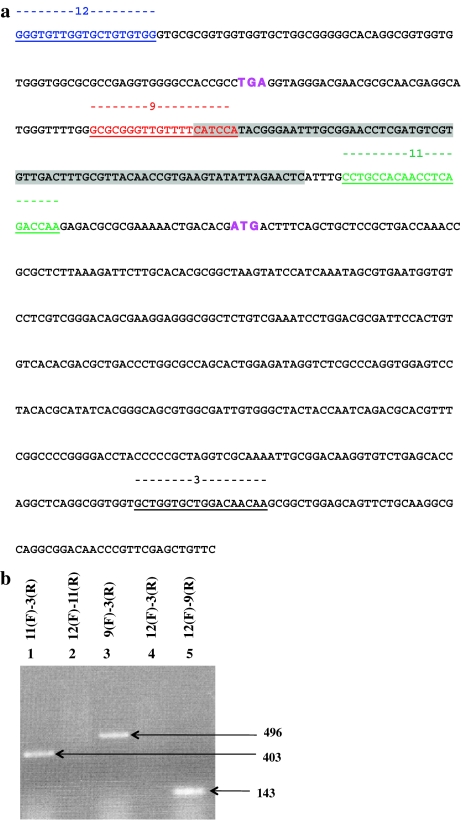
Fig. 2.
cDNA sequence of the ORF-P 3′ and TLA1 5′ ends. a Overlap between the cDNA sequence of ORF-P and TLA1 is highlighted in gray. Gene-specific primers used for RT-PCR are color coded and underlined. Shown in uppercase bold and underlined fonts are “primer 12F” (blue), “primer 9F” (red), “primer 11F” (green), and “primer 3F” (black). The stop codon TGA of ORF-P and start codon ATG of TLA1 are shown in uppercase bold magenta font. b RT-PCR results using combinations of ORF-P- and TLA1-specific primers. Lane 1 shows 403-bp RT-PCR product obtained with TLA1 forward “primer 11F” and second exon-specific reverse “primer 3R”. Lane 2 shows no RT-PCR result with ORF-P 3′ UTR-specific forward “primer 12F” and TLA1 5′ UTR-specific reverse “primer 11R” (reverse sequence of “primer 11F”). Lane 3 shows 496-bp RT-PCR product obtained with TLA1 5′ UTR-specific forward “primer 9F” and second exon-specific reverse “primer 3R”. Lane 4 shows no RT-PCR result with ORF-P 3′ UTR-specific forward “primer 12F” and TLA1 second exon-specific reverse “primer 3R”. Lane 5 shows 143 bp RT-PCR product obtained with ORF-P 3′ UTR-specific forward “primer 12F” and TLA1 5′ UTR-specific reverse “primer 9R” (reverse sequence of “primer 9F”). F and R stand for forward primer and reverse primer, respectively
Transcriptional analysis of ORF-P and TLA1 genes
To investigate the possibility of trans-splicing between the ORF-P and TLA1 mRNAs, RT-PCR analyses were performed with a combination of ORF-P- and TLA1-specific forward (F) and reverse (R) primers, with the following outcomes:
TLA1 5′ UTR-specific forward “primer 11F” and TLA1 second exon-specific reverse “primer 3R” (Fig. 2a) yielded a 403-bp product (Fig. 2b, lane 1).
ORF-P 3′ UTR-specific forward “primer 12F” (Fig. 2a) and TLA1 5′ UTR-specific reverse “primer 11R” (reverse sequence of “primer 11”) generated no product (Fig. 2b, lane 2), suggesting that the two genes generate distinct and separate mRNAs.
TLA1 5′ UTR-specific forward “primer 9F” and TLA1 second exon-specific reverse “primer 3R” (Fig. 2a) resulted in a 496 bp RT-PCR product (Fig. 2b, lane 3).
ORF-P 3′ UTR-specific forward “primer 12F” and TLA1 second exon-specific reverse “primer 3R” (Fig. 2a) generated no product (Fig. 2b, lane 4), consistent with the notion that ORF-P and TLA1 generate two distinct and separate mRNAs.
ORF-P 3′ UTR-specific forward “primer 12F” and TLA1 5′ UTR-specific reverse “primer 9R” (reverse sequence of “primer 9F”, Fig. 2a) produced a PCR product of 143 bp (Fig. 2b, lane 5), consistent with the notion of an overlap in the 3′ UTR of ORF-P with the 5′ UTR of TLA1.
The resulting detailed genomic DNA map and gene structure of ORF-P and TLA1 is shown in Fig. 1d. Thick black arrows represent exons and thin black lines show introns. It is seen that exon 3 of the ORF-P is 1,070 bp long including 124 bp of the 3′ UTR of the ORF-P. The positional alignment of the TLA1 gene with respect to the ORF-P and the 74-bp overlap between the two genes is also indicated (Fig. 1d).
Characterization of the full-length ORF-P
The above analysis showed that the full-length ORF-P genomic DNA and cDNA are 2,224 and 1,748 kb long, respectively (Fig. 1d). A C. reinhardtii EST database search with the ORF-P cDNA did not show significant homology to any existing sequences. This indicates that ORF-P is not highly expressed, or it is not expressed under the conditions that were used to grow the cells from which mRNA was extracted to make the cDNAs for the generation of the EST database.
The predicted 1,748 kb ORF-P cDNA, when translated in the +2 reading frame, yields a putative protein of 410 amino acids (Fig. 3a). Protein BLAST analysis using the Swiss Prot protein sequences database revealed that this putative protein contains a domain highly similar to eukaryotic really interesting new gene (RING) zinc finger proteins. In general, zinc finger proteins mediate protein–protein interactions with proteins involved in ubiquitination and 26S proteasome degradation pathways or DNA transcription and repair (Capili et al. 2001; Dominguez et al. 2004). Figure 3b shows the canonical RING motif also called the C3HC4 motif. In this motif, one zinc ion is bound to three cysteines and a histidine, while the other zinc ion is bound to four cysteines. The tetrahedral coordination is atypical and referred to as a “cross-brace” motif. Figure 3b compares the consensus sequence of a zinc finger domain (Fig. 3b upper; Mladek et al. 2003 http://www.ebi.ac.uk/interpro/IEntry?ac=IPR001841) with the corresponding sequence of the putative ORF-P gene product (Fig. 3b lower), showing that the latter deviates from the canonical zinc finger motif at only one location with regard to the spacing between two cysteine residues (highlighted in red). Given the zinc finger domain and canonical C3HC4 motif in the protein product of the ORF-P gene, we named this protein RDP1 (RING-like domain protein 1) and renamed ORF-P as the CrRDP1 gene. Henceforth, we shall be referring to RDP1 instead of ORF-P as the name of this novel gene.
Fig. 3.
Deduced amino acid sequence of the ORF-P-encoded, RDP1 protein. a The RDP1 gene encodes a protein of 410 amino acids. The C3HC4 RING zinc finger domain is shown in bold. b Comparison of the conventional C3HC4 RING zinc finger motif (upper) with that present in RDP1 (lower). C and H denote conserved cysteine and histidine residues, respectively, and X represents generic amino acids. The non-canonical residues are highlighted in red. Note that the spacing between the fourth and fifth cysteine is one amino acid in RDP1 in contrast to two amino acids in the conventional C3HC4 RING zinc finger
An interesting feature of the putative RDP1 amino acid sequence (Fig. 3a) is the occurrence of numerous stretches of homopolypeptide repeats of poly-alanine, poly-glutamine and poly-glycine in the RDP1 protein sequence. Similar homopolypeptide repeats also occur in other C. reinhardtii proteins, including the psaA trans-splicing factors Raa1 and Raa3, Nac2, Mbb1 and Mca1, which are involved in RNA processing and stability, or Tbc2 which seems to be involved in translation (Boudreau et al. 2000; Lown et al. 2001; Rivier et al. 2001; Auchincloss et al. 2002; Merendino et al. 2006). However, in RDP1, the repetitive sequences are longer, more frequent, and involve diverse residues (Ala, Gln and Gly). A polyglycine tract in plant protein Toc-75 (a component of the protein import machinery in the outer chloroplast envelope) has been shown to be important for targeting this protein to the outer envelope (Inoue and Keegstra 2003; Faux et al. 2007). The functional significance of these repetitive stretches is largely unknown (Merendino et al. 2006). They may play a structural role and may occur in parts of proteins where the precise sequence is not important so that it has drifted during evolution (Katti et al. 2000).
Delineation of the function of TLA1 and RDP1 genes in C. reinhardtii
To test whether TLA1 alone is responsible for the regulation of the Chl antenna size in chloroplasts, without involvement by the RDP1 gene, we constructed a plasmid upon cloning the full-length TLA1 genomic DNA (758 bp), coupled with the constitutive PsaD promoter, in vector pSL18 (Fig. 1S, Table 1S, supplementary material). This construct was used to complement the tla1 mutant in order to clarify whether the TLA1 gene by itself is necessary and sufficient to rescue the tla1 mutation. Transformant colonies were selected on TAP agar plates in the presence of 7.5 μM paromomycin and screened for dark green coloration.
Two independent lines of putative complemented tla1 mutant strains were randomly selected from a batch of five isolated transformants and further analyzed upon growth autotrophically in minimal TBP liquid media. Figure 4 shows that wild type (Fig. 4a) and complemented strains (Fig. 4c, d) had a dark green coloration when compared with the lighter green phenotype of the tla1 mutant (Fig. 4b). The tla1-comp1 and tla1-comp2 strains were tested for their Chl/cell and Chl a/Chl b ratios, and compared with those of the wild type and the original tla1 strain. Table 2 shows the Chl antenna characteristics of the strains examined. Chl/cell in the wild type (3.2 × 10−15 mol/cell) was substantially greater than that in the tla1 mutant (1.1 × 10−15 mol/cell). The complemented strains had Chl/cell values (2.9–3.0 × 10−15 mol/cell) i.e., comparable to that of the wild type. Table 2 also shows that wild-type C. reinhardtii had a Chl a/Chl b ratio of 2.6, whereas the tla1 mutant had a Chl a/Chl b ratio of 6. The putative tla1-complemeted strains had much lower Chl a/Chl b ratios, ranging between 2.7 and 2.8. A lower Chl a/Chl b ratio suggests assembly of peripheral subunits of the Chl a–b light-harvesting complex, underlying an enlarged photosystem Chl antenna size (Polle et al. 2001, 2003). These results provide evidence for the return of the wild-type levels of Chl in the tla1-complemented strains.
Fig. 4.
Phenotype of wild type, tla1 mutant and tla1-complemented strains of C. reinhardtii. Minimal media (TBP) liquid cultures of wild type (a), tla1 mutant (b) and two complemented strains, tla1-comp1 (c) and tla1-comp2 (d) are shown. Cell densities of the four cultures were approximately, 6 × 106 cells/mL. The tla1 mutant strain was complemented with a single copy of the wild-type TLA1 gene. The tla1 mutant showed a pale green coloration phenotype (b), indicative of the low-level chlorophyll concentration in these cells, whereas the wild type (a) and putative complemented strains tla1-comp1 (c) and tla1-comp2 (d) had a dark green coloration similar to that of the wild type
Table 2.
Chl content per cell, Chl a/Chl b ratios, and LHC protein amount in C. reinhardtii wild type (WT), tla1 mutant and tla1 strains complemented with the TLA1 gene (tla1-comp1 and tla1-comp2)
| Strain | Chl (×10−15 mol/cell) | Chl a/Chl b ratio | Lhcb amount (%) |
|---|---|---|---|
| WT (cw15) | 3.2 | 2.6 | 100 |
| tla1 | 1.1 | 6.0 | 22 |
| tla1-comp1 | 2.9 | 2.7 | 65 |
| tla1-comp2 | 3 | 2.8 | 80 |
Quantification of the LHC band from the scanned western blots was achieved with image processing software (ImageJ). Statistical error (±SD) was <10% of the values shown
In contrast, complementation of the tla1 mutant strain with a pSL18 construct containing the full-length 2.2 kb RDP1 genomic DNA, also cloned under the control of the PsaD promoter, failed to rescue the mutation, as evidenced by inability of such transformants to restore wild-type levels of Chl/cell and Chl a/Chl b ratio in the mutant strain (not shown). These results suggest that the TLA1 gene alone is responsible for the regulation of the Chl antenna size of photosynthesis in C. reinhardtii. The functional role of RDP1 was not further investigated in the context of this work.
PCR and RT-PCR analysis of tla1-complemented strains
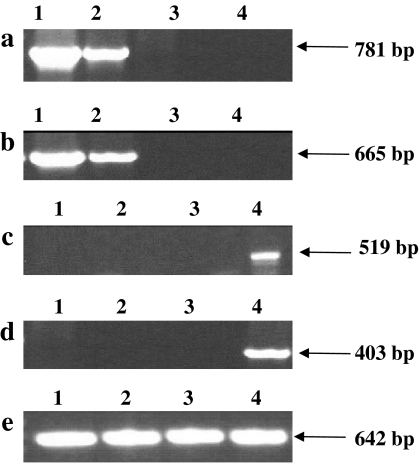
A more detailed molecular and biochemical analysis was undertaken, entailing a comparative and quantitative evaluation of tla1-comp1, tla1-comp2, tla1 mutant and wild type. When PCR and RT-PCR were performed on the genomic DNA using forward PsaD 5′ UTR “primer 5” and reverse TLA1 Exon-2 “primer 4” (Fig. 1S and Table 1S supplementary material), strains tla1-comp1 and tla1-comp2 yielded a 781-bp PCR product (Fig. 5a) and a 665-bp RT-PCR product (Fig. 5b), whereas the tla1 mutant and the wild type failed to yield a product, consistent with the absence of the transforming PsaD-TLA1 gene. When forward TLA1 5′ UTR “primer 1” and reverse TLA1 Exon-2 “primer 3” (Table 1S and Fig. 1S, supplementary material) were used in the PCR and RT-PCR reactions, the wild type produced a 519-bp genomic PCR product (Fig. 5c) and a 403 bp RT-PCR product (Fig. 5d), while the tla1 mutant, tla1-comp1 and tla1-comp2 did not generate any product, consistent with the absence of the 5′ UTR of the TLA1 gene in the latter strains. As a control, RT-PCR was employed to test for the presence of the intact full-length TLA1 transcript in the two complemented strains, using forward TLA1 Exon-1 “primer 2” and reverse TLA1 Exon-2 “primer 4” (Table 1S and Fig. 1S, supplementary material). Figure 5e shows that all strains gave a product of 642 bp, as all strains contain the coding region of the TLA1 gene. These results offer evidence of successful complementation of the tla1 mutant by the pSL18-TLA1 construct and expression of the TLA1 gene in the two independent transformant lines. These results further corroborate the notion that TLA1 is solely responsible for the functional complementation of the tla1 mutant.
Fig. 5.
PCR and RT-PCR analysis of wild type, tla1 and two tla1-complemented strains. Lane 1 tla1-comp1, lane 2 tla1-comp2, lane 3 tla1 mutant, lane 4 wild type. a Genomic DNA PCR product of 781 bp was obtained with forward PsaD 5′ UTR “primer 5” and reverse TLA1 Exon-2 “primer 4” (Fig. 1S and Table 1S; supplementary material). No products were obtained with the tla1 mutant (lane 3) or wild type samples (lane 4). b RT-PCR products of 665 bp were obtained with forward PsaD 5′ UTR “primer 5” and reverse (TLA1 Exon-2 “primer 4” (Fig. 1S and Table 1S; supplementary material) from the cDNA of the tla1-complements (lanes 1 and 2). No products were obtained from the cDNA of the tla1 mutant (lane 3) or wild type samples (lane 4). c Genomic DNA PCR product of 519 bp was obtained with forward TLA1 5′ UTR “primer 1” and reverse Exon-2 “primer 3” from the wild type sample only (lane 4). d RT-PCR product of 403 bp was obtained with forward TLA1 5′ UTR “primer 1” and reverse Exon-2 “primer 3” with the wild type cDNA only lane 4). e RT-PCR products of 642 bp were obtained with all samples when using forward Exon-1 “primer 2” and reverse Exon-2 “primer 4”. Genomic DNA and RNA for PCR and RT-PCR were extracted from cells grown photosynthetically in minimal TBP liquid media
Western blot analysis of tla1-complemented strains
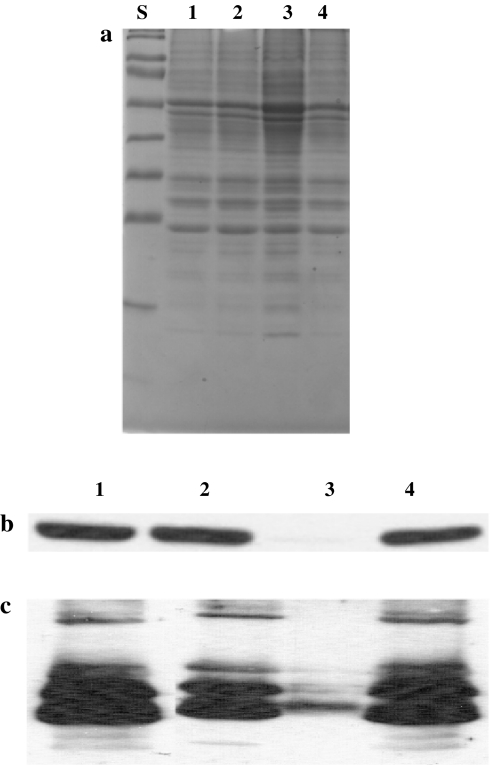
Total protein extracts from the tla1-comp1, tla1-comp2, tla1 mutant and wild type strains were resolved on a 12.5% SDS-PAGE gel, lanes loaded on an equal chlorophyll basis (Fig. 6a). Western blot analysis of the total cell extract from these samples with specific polyclonal antibodies raised against the recombinant TLA1 and Lhcb proteins showed that the amount of the TLA1 protein and the Lhcb proteins in the two complements (Fig. 6b, c, lanes 1 and 2, see also Table 2) were comparable to those in the wild type (Fig. 6b, c, lane 4; Table 2), whereas the amount of the TLA1 protein and of the Lhcb proteins in the tla1 mutant were substantially lower than those of the other three (Fig. 6b, c, lane 3; Table 2). Levels of Lhca paralleled those of the Lhcb in the wild type, tla1 mutant, and tla1-complemented strains (not shown). These results are consistent with earlier findings from this lab (Polle et al. 2003; Tetali et al. 2007) and provide evidence that a limited translation of the TLA1 gene takes place in the tla1 mutant. However, this is in no way comparable to the levels of translation seen in the wild type or tla1-comp strains. The substantially reduced rate of translation and level of TLA1 protein in the tla1 mutant was attributed to the absence of the native 5′ UTR of the TLA1 gene in this strain, as exogenous plasmid insertion occurred immediately prior to the TLA1 ATG codon (Tetali et al. 2007), thus interfering with the ability of this strain to carry out high rates of TLA1 mRNA translation. The absence of the 5′ UTR from the native TLA1 gene in the tla1 mutant did not apparently affect the molecular weight of the TLA1 protein, which is the same in wild type and tla1 mutant (Fig. 6b, lane 3).
Fig. 6.
SDS-PAGE and western blot analysis of Chlamydomonas reinhardtii proteins. Lane 1 tla1-comp1, lane 2 tla1-comp2, lane 3 tla1 mutant, lane 4 wild type. a SDS-PAGE of total cell protein extracts. Lanes were loaded on an equal Chl basis (1.5 nmol Chl per lane). Lane S shows the pre-stained low molecular weight markers. b, c Western blot analysis of C. reinhardtii total cell protein extracts. Lanes, loaded as in a, were probed with TLA1-specific (b) and Lhcb-specific polyclonal antibodies (c). Note the substantially lower levels of the TLA1 and Lhcb proteins in lane 3 (tla1 mutant) relative to that in the other samples
TLA1 protein sequence analysis
The C. reinhardtii TLA1 gene has homologs in many eukaryotic organisms, including algae, plants, Drosophila and even human (Tetali et al. 2007). The highest identity known to date, however, is between the C. reinhardtii TLA1 gene and its counterpart in the colorless microalga Polytomella parva (Chlorophyceae). Borza et al. (2007) presented a “conceptual translation” of a cDNA from this microalga, yielding a polypeptide matching the TLA1 sequence from C. reinhardtii. Figure 7 shows a ClustalW amino acid sequence alignment of the two putative TLA1 proteins, revealing a 50% identity and a substantially higher degree of homology. P. parva is noteworthy in this respect as it retains a functional chloroplast, in spite of the apparent absence of thylakoids and chlorophyll. Retention and expression of the TLA1 gene homolog in a colorless microalga supports the hypothesis that TLA1 is defining the relationship between nucleus and organelle, and probably playing a role in the control of organelle development (Tetali et al. 2007; see also discussion below).
Fig. 7.
ClustalW generated amino acid sequence alignment of the TLA1 protein from Chlamydomonas reinhardtii and Polytomella parva. The sequence alignment revealed a 50% identity and 67% homology between the two proteins
Discussion
Chloroplasts have the ability to regulate the size of the functional Chl antenna in response to the level of irradiance (Bjorkman et al. 1972; Anderson 1986). For example, when high-light acclimated plants and algae are shifted to low-light, enlargement of the Chl antenna size occurs with a concomitant increase in cellular Chl, LHC apoproteins and a decrease in the Chl a/Chl b ratio, as Chl b accumulates in the photosystems (Sukenik et al. 1988; Webb and Melis 1995; Tanaka and Melis 1997; Neidhardt et al. 1998). At the molecular level, these changes are preceded by a rapid up-regulation of the transcription of CAO and Lhcb genes (LaRoche et al. 1991; Webb and Melis 1995; Masuda et al. 2003), suggesting that increase in the Chl antenna size occurs by coordinate induction in Chl biosynthesis and expression of Lhcb and CAO genes. In an alternative approach, application of powerful gene silencing RNA interference technologies in the model microalga C. reinhardtii resulted in the generation of a viable mutant, termed stm3LR3, which had a significantly reduced content of Lhcb and Lhca proteins (Mussgnug et al. 2007).
Regulation of the Chl antenna size is of practical importance as it defines the quantum yield and productivity of photosynthesis, especially under mass culture conditions (Melis 2007, 2009; Mitra and Melis 2008). In spite of a substantial number of physiological and biochemical studies on this phenomenon (Anderson 1986; Melis 1991, 1996), genetic determinants of the Chl antenna size and the signal transduction pathway for the regulation of this phenomenon are mostly unknown. To date, only two genes, namely TLA1 (Polle et al. 2003; Tetali et al. 2007) and NAB1 (Mussgnug et al. 2005), have been identified as players in the regulation of the photosynthetic Chl antenna size. TLA1, the first gene to be identified in the regulation of the chlorophyll antenna size of photosynthesis, encodes a protein of 213 amino acids that has homologs in diverse groups of eukaryotic organisms, ranging from microalgae to vascular plants, insects and mammals. Although all TLA1-like proteins from the different organisms show highly conserved domains in their amino acid sequence, these domains do not match any known protein motifs (Tetali et al. 2007). Conserved domain architecture retrieval tool (CDART) analysis categorized the family of TLA1 protein as a novel protein family, i.e., uncharacterized protein family (UPF0172). On the other hand, the NAB1 protein is a cytosolic putative nucleic acid-binding protein that binds RNA, thought to play a role in controlling the expression of the light-harvesting antenna proteins of PSII at the posttranscriptional level (Mussgnug et al. 2005). Unlike TLA1, down-regulation of the NAB1 gene leads to enlargement of the Chl antenna size in C. reinhardtii, presumably due to an increase in the amount of Lhcb proteins in the cell (Mussgnug et al. 2005).
The mechanism by which the TLA1 gene regulates the Chl antenna size of photosynthesis is not presently understood. A current hypothesis, under investigation in this lab, postulates that TLA1 defines the relationship between nucleus and organelle in eukaryotes. According to this hypothesis, TLA1 regulates chloroplast development in C. reinhardtii, directly or indirectly affecting rates of Chl biosynthesis and the Chl antenna size of the photosystems. This hypothesis of a role in the relationship between nucleus and organelle explains the ubiquitous presence of this gene in eukaryotes only, including non-photosynthetic tissues (e.g. Drosophila and human). In the latter, TLA1-like genes may regulate the relationship between nucleus and mitochondria in these systems. In the tla1 mutant, lower levels of the TLA1 protein resulted in less total Chl (Chl a and Chl b) per cell, lower levels of Lhcb and CAO gene expression, and diminished abundance of LHC polypeptides, translating into a slow-down in the development of the photosynthetic apparatus, apparently as a consequence of down-regulation of translation of the TLA1 mRNA. It is tempting to postulate that expression of the TLA1 gene promotes the development of organelles (plastids and mitochondria) in eukaryotic cells via a currently unknown signal transduction pathway that entails communication with and/or control of organelles. This contention is supported by the fact that P. parva, a colorless alga, belonging to the Chlorophyceae family possesses the TLA1 gene, although it lacks thylakoids and chlorophyll (Borza et al. 2007). Signal peptide analysis of the TLA1 protein sequence failed to indicate a transit peptide, suggesting that TLA1 is localized in the nucleus or cytoplasm (Tetali et al. 2007). Conversely, analysis of COX4NB, a TLA1 homolog from human, also indicated localization to the cytoplasm, mitochondria, and/or nucleus (Bachman et al. 1999). Biochemical investigations leading to the localization of the TLA1 protein in Chlamydomonas are currently under way in this laboratory.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. 1S TLA1 gene construct for complementation of the tla1 mutant. pSL18-TLA1 genomic DNA construct that was used for the complementation of the tla1 mutant. The full-length TLA1 gene (thick black arrow with the small white box) was cloned in the pSL18 vector under control of the Chlamydomonas reinhardtii PsaD promoter (dotted box) and terminator (black line, PsaD 3’ UTR). Upstream from the TLA1 gene, the construct also contained the AphVIII (light gray arrow) and Amp r (dark gray arrow) genes, encoding paromomycin and ampicillin resistance, respectively. The AphVIII gene was under the control of the HspA (white box) and RbcS2 promoter (gray box). Primer 5, used for PCR and RT-PCR reactions (see also Table 2), is denoted by a small black arrow on the map of the pSL18-TLA1 construct. Primers 3, 4 and 5, used for PCR and RT-PCR reactions (see also Table 1S), are denoted by small black arrows on the map of the pSL18-TLA1 construct. (PDF 39 kb)
Acknowledgments
The work was supported by the US Department of Energy Hydrogen Program grant # DE-FG36-05GO15041.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Abbreviations
- Chl
Chlorophyll
- LHC
Light harvesting complex
- MSA
Multiple sequence alignment
- PS
Photosystem
- RACE
Rapid amplification of cDNA ends
- RING
Really interesting new gene
- RDP1
RING like domain protein 1
- TLA
Truncated Light-harvesting chlorophyll Antenna
- UTR
Untranslated region
References
- Anderson JM. Photoregulation of the composition, function and structure of thylakoid membranes. Annu Rev Plant Physiol. 1986;37:93–136. doi: 10.1146/annurev.pp.37.060186.000521. [DOI] [Google Scholar]
- Arnon D. Copper enzymes in isolated chloroplasts. Polyphenol oxidase in Beta vulgaris. Plant Physiol. 1949;24:1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auchincloss AH, Zerges W, Perron K, Girars-Bascou J, Rochaix JD. Characterization of Tbc2, a nucleus encoded factor specifically required for translation of the chloroplast psbC mRNA in Chlamydomonas reinhardtii. J Cell Biol. 2002;157:953–962. doi: 10.1083/jcb.200201060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachman NJ, Wu W, Schmidt TR, Grossman LI, Lomax MI. The 5′ region of the COX4 gene contains a novel overlapping gene NOC4. Mamm Genome. 1999;10:506–512. doi: 10.1007/s003359901031. [DOI] [PubMed] [Google Scholar]
- Bjorkman O, Boardman NK, Anderson JM, Thorne SW, Goodchild DJ, Pyliotis NA (1972) Effect of light intensity during growth of Atriplex patula on the capacity of photosynthetic reactions, chloroplast components and structure. Carnegie Inst Yearbook 71:115–135
- Borza T, Rajeswaran A, Lee RW (2007) The colorless plastid of the green alga Polytomella parva: a repertoire of its functions. GenBank Accession No. ABH10987
- Boudreau E, Nickelsen J, Lemaire SD, Ossenbuhl F, Rochaix JD. The Nac2 gene of Chlamydomonas encodes a chloroplast TPR-like protein involved in psbD mRNA stability. EMBO J. 2000;19:3366–3376. doi: 10.1093/emboj/19.13.3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capili AD, Schultz DC, Rauscher IF, Borden KLB. Solution structure of the PHD domain from the KAP-1 co-repressor: structural determinants for PHD, RING and LIM zinc-binding domains. EMBO J. 2001;20:165–177. doi: 10.1093/emboj/20.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debuchy R, Purton S, Rochaix JD. The arginosuccinate lyase gene of Chlamydomonas reinhardtii: an important tool for nuclear transformation and for correlating the genetic and molecular maps of the ARG7 locus. EMBO J. 1989;8:2803–2809. doi: 10.1002/j.1460-2075.1989.tb08426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez C, Folkers GE, Boelens R. Biological introduction: RING domain protein. Contributions to Handbook of Metalloproteins. 2004;3:338–351. [Google Scholar]
- Escoubas JM, Lomas M, LaRoche J, Falkowski PG. Light intensity regulation of cab gene transcription is signaled by the redox state of the plastoquinone pool. Proc Natl Acad Sci USA. 1995;92:10237–10241. doi: 10.1073/pnas.92.22.10237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faux NG, Bottomley SP, Lesk AM, Irving JA, Morrison JR, Garcia de la Banda MG, Whisstock JC. Functional insights from the distribution and role of homopeptide repeat- containing proteins. Genome Res. 2007;15:537–551. doi: 10.1101/gr.3096505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer N, Rochaix JD. The flanking regions of PsaD drive efficient gene expression in the nucleus of the green alga Chlamydomonas reinhardtii. Mol Genet Genomics. 2001;265:888–894. doi: 10.1007/s004380100485. [DOI] [PubMed] [Google Scholar]
- Harris EH. The Chlamydomonas source book: a comprehensive guide to biology and laboratory use. San Diego: Academic Press; 1989. [DOI] [PubMed] [Google Scholar]
- Huner NPA, Oquist G, Sarhan F. Energy balance and acclimation to light and cold. Trends Plant Sci. 1998;3:224–230. doi: 10.1016/S1360-1385(98)01248-5. [DOI] [Google Scholar]
- Inoue K, Keegstra K. A polyglycine stretch is necessary for proper targeting of the protein translocation channel precursor to the outer envelope membrane of chloroplasts. Plant J. 2003;34:661–669. doi: 10.1046/j.1365-313X.2003.01755.x. [DOI] [PubMed] [Google Scholar]
- Jansson S, Pichersky E, Bassi R, Green BR, Ikeuchi M, Melis A, Simpson DJ, Spangfort M, Staehelin LA, Thornber JP. A nomenclature for the genes encoding the chlorophyll a/b-binding proteins of higher plants. Plant Mol Biol Rep. 1992;10:242–253. doi: 10.1007/BF02668357. [DOI] [Google Scholar]
- Katti MV, Sami-Subbu Ranjekar PK, Gupta VS. Amino acid repeat patterns in protein sequences: their diversity and structural–functional implications. Protein Sci. 2000;9:1203–1209. doi: 10.1110/ps.9.6.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindle KL. High-frequency nuclear transformation of Chlamydomonas reinhardtii. Proc Natl Acad Sci USA. 1990;87:1228–1232. doi: 10.1073/pnas.87.3.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- LaRoche J, Mortain-Bertrand A, Falkowski PG. Light-intensity-induced changes in cab mRNA and light-harvesting complex II apoprotein levels in the unicellular chlorophyte Dunaliella tertiolecta. Plant Physiol. 1991;97:147–153. doi: 10.1104/pp.97.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lown FJ, Watson AT, Purton S. Chlamydomonas nuclear mutants that fail to assemble respiratory or photosynthetic electron transfer complexes. Biochem Soc Trans. 2001;29:452–455. doi: 10.1042/BST0290452. [DOI] [PubMed] [Google Scholar]
- Masuda T, Polle JEW, Melis A. Biosynthesis and distribution of chlorophyll among the photosystems during recovery of the green alga Dunaliellasalina from irradiance stress. Plant Physiol. 2002;128:603–614. doi: 10.1104/pp.010595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda T, Tanaka A, Melis A. Chlorophyll antenna size adjustments by irradiance in Dunaliella salina involve coordinate regulation of chlorophyll a oxygenase (CAO) and Lhcb gene expression. Plant Mol Biol. 2003;51:757–771. doi: 10.1023/A:1022545118212. [DOI] [PubMed] [Google Scholar]
- Maxwell DP, Falk S, Huner NPA. Photosystem II excitation pressure and development of resistance to photoinhibition. 1. Light harvesting complex II abundance and zeaxanthin content in Chlorella vulgaris. Plant Physiol. 1995;107:687–694. doi: 10.1104/pp.107.3.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis A. Dynamics of photosynthetic membrane composition and function. Biochim Biophys Acta. 1991;1058:87–106. doi: 10.1016/S0005-2728(05)80225-7. [DOI] [Google Scholar]
- Melis A. Excitation energy transfer: functional and dynamic aspects of Lhc (cab) proteins. In: Ort DR, Yocum CF, editors. Oxygenic photosynthesis: the light reactions. Dordrecht: Kluwer Academic Publishers; 1996. pp. 523–538. [Google Scholar]
- Melis A. Green alga hydrogen production: progress, challenges and prospects. Int J Hydrogen Energy. 2002;27:1217–1228. doi: 10.1016/S0360-3199(02)00110-6. [DOI] [Google Scholar]
- Melis A. Bioengineering of green algae to enhance photosynthesis and hydrogen production. In: Collins AF, Critchley C, editors. Artificial photosynthesis: from basic biology to industrial application. Weinheim: Wiley-VCH; 2005. pp. 229–240. [Google Scholar]
- Melis A. Photosynthetic H2 metabolism in Chlamydomonas reinhardtii (unicellular green algae) Planta. 2007;226:1075–1086. doi: 10.1007/s00425-007-0609-9. [DOI] [PubMed] [Google Scholar]
- Melis A (2009) Solar energy conversion efficiencies in photosynthesis: Minimizing the chlorophyll antennae to maximize efficiency. Plant Sci 177:272–280
- Melis A, Spangfort M, Andersson B. Light-absorption and electron-transport balance between PSII and PSI in spinach chloroplasts. Photochem Photobiol. 1987;45:129–136. doi: 10.1111/j.1751-1097.1987.tb08413.x. [DOI] [Google Scholar]
- Melis A, Neidhardt J, Benemann JR. Dunaliella salina (Chlorophyta) with small chlorophyll antenna sizes exhibit higher photosynthetic productivities and photon use efficiencies than normally pigmented cells. J Appl Phycol. 1999;10:515–552. doi: 10.1023/A:1008076231267. [DOI] [Google Scholar]
- Merendino L, Perron K, Rahire M, Howald I, Rochaix JD, Goldschmidt-Clermont M. A novel multifunctional factor involved in trans-splicing of chloroplast introns in Chlamydomonas. Nucleic Acids Res. 2006;34:262–274. doi: 10.1093/nar/gkj429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra M, Melis A. Optical properties of microalgae for enhanced biofuels production. Opt Express. 2008;16:21807–21820. doi: 10.1364/OE.16.021807. [DOI] [PubMed] [Google Scholar]
- Mladek C, Guger K, Hauser M. Identification and characterization of the ARIADNE gene family in Arabidopsis. A group of putative E3 ligases. Plant Physiol. 2003;131:27–40. doi: 10.1104/pp.012781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mussgnug JH, Wobbe L, Elles I, Claus C, Hamilton M, Fink A, Kahmann U, Kapazoglou A, Mullineaux CW, Hippler M, Nickelsen J, Nixon PJ, Kruse O. NAB1 is an RNA binding protein involved in the light-regulated differential expression of the light-harvesting antenna of Chlamydomonas reinhardtii. Plant Cell. 2005;17:3409–3421. doi: 10.1105/tpc.105.035774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mussgnug JH, Thomas-Hall S, Rupprecht J, Foo A, Klassen V, McDowall A, Schenk PM, Kruse O, Hankamer B. Engineering photosynthetic light capture: impacts on improved solar energy to biomass conversion. Plant Biotech J. 2007;5:802–814. doi: 10.1111/j.1467-7652.2007.00285.x. [DOI] [PubMed] [Google Scholar]
- Nakada E, Asada Y, Arai T, Miyake J. Light penetration into cell suspensions of photosynthetic bacteria and relation to hydrogen production. J Ferment Bioeng. 1995;80:53–57. doi: 10.1016/0922-338X(95)98176-L. [DOI] [Google Scholar]
- Neidhardt J, Benemann JR, Zhang L, Melis A. Photosystem-II repair and chloroplast recovery from irradiance stress: relationship between chronic photoinhibition, light-harvesting chlorophyll antenna size and photosynthetic productivity in Dunaliella salina (green algae) Photosynth Res. 1998;56:175–184. doi: 10.1023/A:1006024827225. [DOI] [Google Scholar]
- Ohtsuka T, Ito H, Tanaka A. Conversion of chlorophyll b to chlorophyll a and the assembly of chlorophyll with apoproteins by isolated chloroplasts. Plant Physiol. 1997;113:137–147. doi: 10.1104/pp.113.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polle JEW, Benemann JR, Tanaka A, Melis A. Photosynthetic apparatus organization and function in wild type and a Chl b-less mutant of Chlamydomonas reinhardtii. Dependence on carbon source. Planta. 2000;211:335–344. doi: 10.1007/s004250000279. [DOI] [PubMed] [Google Scholar]
- Polle JEW, Niyogi KK, Melis A. Absence of lutein, violaxanthin and neoxanthin affects the functional chlorophyll antenna size of photosystem-II but not that of photosystem-I in the green alga Chlamydomonas reinhardtii. Plant Cell Physiol. 2001;42:482–491. doi: 10.1093/pcp/pce058. [DOI] [PubMed] [Google Scholar]
- Polle JEW, Kanakagiri S, Melis A. tla1, a DNA insertional transformant of the green alga Chlamydomonas reinhardtii with a truncated light-harvesting chlorophyll antenna size. Planta. 2003;217:49–59. doi: 10.1007/s00425-002-0968-1. [DOI] [PubMed] [Google Scholar]
- Pollock SV, Colombo SL, Prout DL, Jr, Godfrey AC, Moroney JV. Rubisco activase is required for optimal photosynthesis in the green alga Chlamydomonas reinhardtii in a low-CO2 atmosphere. Plant Physiol. 2003;133:1854–1861. doi: 10.1104/pp.103.032078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivier C, Goldschmidt-Clermont M, Rochaix JD. Identification of an RNA-protein complex involved in chloroplast group II intron trans-splicing in Chlamydomonas reinhardtii. EMBO J. 2001;20:1765–1773. doi: 10.1093/emboj/20.7.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sizova I, Fuhrmann M, Hegemann P. A Streptomyces rimosus aphVIII gene coding for a new type phosphotransferase provides stable antibiotic resistance to Chlamydomonas reinhardtii. Gene. 2001;277:221–229. doi: 10.1016/S0378-1119(01)00616-3. [DOI] [PubMed] [Google Scholar]
- Smith BM, Morrissey PJ, Guenther JE, Nemson JA, Harrison MA, Allen JF, Melis A. Response of the photosynthetic apparatus in Dunaliella salina (green algae) to irradiance stress. Plant Physiol. 1990;93:1433–1440. doi: 10.1104/pp.93.4.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sueoka N. Mitotic replication of deoxyribonucleic acids in Chlamydomonas reinhardtii. Proc Natl Acad Sci USA. 1960;46:83–91. doi: 10.1073/pnas.46.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukenik A, Bennett J, Falkowski PG. Changes in the abundance of individual apoproteins of light-harvesting chlorophyll a/b-protein complexes of photosystem I and II with growth irradiance in the marine chlorophyte Dunaliella tertiolecta. Biochim Biophys Acta. 1988;932:206–215. doi: 10.1016/0005-2728(88)90157-0. [DOI] [Google Scholar]
- Tanaka A, Melis A. Irradiance-dependent changes in the size and composition of the chlorophyll a–b light-harvesting complex in the green alga Dunaliella salina. Plant Cell Physiol. 1997;38:17–24. [Google Scholar]
- Tetali SD, Mitra M, Melis A. A Development of the light-harvesting chlorophyll antenna in the green alga Chlamydomonas reinhardtii is regulated by the novel TLA1 gene. Planta. 2007;225:813–829. doi: 10.1007/s00425-006-0392-z. [DOI] [PubMed] [Google Scholar]
- Webb MR, Melis A. Chloroplast response in Dunaliella salina to irradiance stress. Effect on thylakoid membrane assembly and function. Plant Physiol. 1995;107:885–893. doi: 10.1104/pp.107.3.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson KE, Huner NPA. The role of growth rates, redox-state of the plastoquinone pool and the trans-thylakoid pH in photoacclimation of Chlorella vulgaris to growth irradiance and temperature. Planta. 2000;212:93–102. doi: 10.1007/s004250000368. [DOI] [PubMed] [Google Scholar]
- Yakovlev AG, Taisova AS, Fetisova ZG. Light control over the size of an antenna unit building block as an efficient strategy for light harvesting in photosynthesis. FEBS Lett. 2002;512:129–132. doi: 10.1016/S0014-5793(02)02238-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. 1S TLA1 gene construct for complementation of the tla1 mutant. pSL18-TLA1 genomic DNA construct that was used for the complementation of the tla1 mutant. The full-length TLA1 gene (thick black arrow with the small white box) was cloned in the pSL18 vector under control of the Chlamydomonas reinhardtii PsaD promoter (dotted box) and terminator (black line, PsaD 3’ UTR). Upstream from the TLA1 gene, the construct also contained the AphVIII (light gray arrow) and Amp r (dark gray arrow) genes, encoding paromomycin and ampicillin resistance, respectively. The AphVIII gene was under the control of the HspA (white box) and RbcS2 promoter (gray box). Primer 5, used for PCR and RT-PCR reactions (see also Table 2), is denoted by a small black arrow on the map of the pSL18-TLA1 construct. Primers 3, 4 and 5, used for PCR and RT-PCR reactions (see also Table 1S), are denoted by small black arrows on the map of the pSL18-TLA1 construct. (PDF 39 kb)