Abstract
AIM: To investigate the role of hepatopoietin Cn (HPPCn) in apoptosis of hepatocellular carcinoma (HCC) cells and its mechanism.
METHODS: Two human HCC cell lines, SMMC7721 and HepG2, were used in this study. Immunostaining, Western blotting and enzyme linked immunosorbent assay were conducted to identify the expression of HPPCn and the existence of an autocrine loop of HPPCn/HPPCn receptor in SMMC7721 and HepG2. Apoptotic cells were detected using fluorescein isothiocyanate (FITC)-conjugated Annexin V and propidium iodide.
RESULTS: The HPPCn was highly expressed in human HCC cells and secreted into culture medium (CM). FITC-labeled recombinant human protein (rhHPPCn) could specifically bind to its receptor on HepaG2 cells. Treatment with 400 ng/mL rhHPPCn dramatically increased the viability of HCC-derived cells from 48.1% and 36.9% to 85.6% and 88.4%, respectively (P < 0.05). HPPCn silenced by small-interfering RNA reduced the expression and secretion of HPPCn and increased the apoptosis induced by trichostatin A. Additionally, HPPCn could up-regulate the expression of myeloid cell leukemia-1 (Mcl-1) in HCC cells via mitogen-activated protein kinase (MAPK) and sphingosine kinase-1.
CONCLUSION: HPPCn is a novel hepatic growth factor that can be secreted to CM and suppresses apoptosis of HCC cells by up-regulating Mcl-1 expression.
Keywords: Hepatopoietin Cn, Autocrine, Hepatocellular carcinoma, Apoptosis, Myeloid cell leukemia-1
INTRODUCTION
Hepatocellular carcinoma (HCC) represents the fifth-most prevalent malignant disease affecting humans worldwide with an increasing incidence[1]. There is evidence that the protumorigenic growth factor signaling is dysregulated in human HCC affecting different signaling systems, such as insulin-like growth factor, hepatocyte-growth factor (HGF), transforming-growth factor α (TGF-α)/epidermal-growth factor (EGF), and TGF-β[2].
Hepatopoietin Cn (HPPCn) is a novel hepatic growth factor derived from a hepatocyte-stimulating substance, which was first reported by LaBrecque in 1975[3,4]. It has been shown that the HPPCn mRNA level increases in partially hepatectomized mice following liver injury[5]. Recombinant human protein (rhHPPCn) specifically stimulates cell proliferation in primary cultures of hepatocytes and HCC-derived cell lines (HepG2, SMMC7721) in vitro as well as liver regeneration following partial hepatectomy in vivo. In addition, rhHPPCn can protect hepatocytes against ethanol-induced injury[6].
Investigations of HPPCn have been mainly restricted to its remarkable activity in stimulating liver regeneration. However, its mechanism and potential effect on HCC are unclear. In this study, HPPCn was highly expressed in cytoplasm and nuclei of human HCC cells and secreted into the culture medium (CM), and fluorescein isothiocyanate (FITC)-labeled rhHPPCn could specifically bind to its receptor on HepaG2 cells, suggesting that there is an autocrine loop of HPPCn/HPPCn receptor in HCC-derived cell lines. Furthermore, exogenous rhHPPCn suppressed trichostatin A (TSA)-induced apoptosis of HCC cells and up-regulated myeloid cell leukemia-1 (Mcl-1) expression in HCC-derived cells via the mitogen-activated protein kinase (MAPK) or sphingosine kinase-1 (SPK1).
MATERIALS AND METHODS
Cell lines and cell culture
Two human HCC cell lines, SMMC7721 and HepG2, were used in this study. Cells were cultured in Roswell Park Memorial Institute (RPMI) 1640 medium (Sigma, Saint Louis, MO, USA) supplemented with 10% fetal calf serum, 2 mmol/L glutamine, 100 U/mL penicillin, and 100 mg/mL streptomycin.
Antibodies and other reagents
Recombinant human HPPCn and anti-HPPCn sera were produced as previously described[5]. Primary antibodies, including those against Mcl-1, phos-Erk1/2 (tyr204), phos-Stat3, non-activated Erk1/2, or Stat3, were obtained from Santa Cruz Company (Santa Cruz, CA, USA). Other reagents used in this study were monoclonal anti-mouse/goat/rabbit peroxidase conjugate (Sigma, St. Louis, MO), enhanced chemiluminescence (ECL) kit, and 3, 3’, 5, 5’ tetramethylbenzidine (TMB) substrate (Biozol, Eching, Germany).
Immune staining
Liver tissue samples were fixed with 4% (w/v) freshly prepared paraformaldehyde and cut into 4 μm-thick sections with a vibratome (Leica VT1000S; Germany). Non-specific protein binding sites were blocked using 2% bovine serum albumin in phosphate-buffered saline (PBS) for 1 h, and anti-HPPCn serum in a blocking solution was incubated overnight at 4°C in a humidified chamber. The sections were incubated with peroxidase-labeled secondary antibody, treated with 3,3’-diaminobenzidine and hydrogen peroxide, and observed under a microscope.
Enzyme linked immunosorbent assay (ELISA)
Cells (2 × 106) were seeded in a 75 cm2 tissue culture flask, cultured for 8 h, washed 3 times with a serum-free medium, and cultured for an additional 30 h in 10 mL of a serum-free medium. The supernatant liquid was used as a conditioned medium. To prepare a concentrated conditioned medium, the CM was concentrated to a final volume of 1 mL by ultra-filtration (Millipore, USA) with a 5000-MW-cut-off[7].
In order to detect HPPCn protein in CM, 100 μL of the harvested CM was dispensed in a 96-well ELISA plate and incubated overnight at 4°C. The ELISA plate was washed 3 times with PBS, incubated for 2 h with an anti-HPPCn antibody, washed with PBS and then with secondary antibody, incubated for 2 h followed by an additional 15 min in a TMB-substrate. Color intensity was measured with an ELISA-Reader (Dynatech Laboratories, Frankfurt, Germany).
Western blotting
Protein samples were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane. The nitrocellulose membrane was blocked with a 5% solution of non-fat milk in TBS containing 0.1% Tween 20 (TBST), incubated with primary antibody in 5% bovine serum albumin containing TBST at 4°C overnight, washed 3 times with TBST and incubated with secondary antibody in 5% milk containing TBST. After washed 5 times with TBST, the membrane was developed using the ECL method.
Cell-binding assay
FITC-labeled rhHPPCn was prepared by incubating FITC (Sigma, St. Louis, MO) with recombinant protein (1/20, w/w) in a borate buffer (0.05 mol/L Na2B4O7, pH 9.3) at 4°C for 12 h, followed by purification through a Sephadex G-25 column, according to its manufacturer’s instructions (Pharmacia Biotech, Alameda, CA)[8,9].
For the analysis of rhHPPCn binding to HepG2, cells (2 × 105-4 × 105) were stained with FITC-labeled rhHPPCn in RPMI with 1% BSA for 30 min at 37°C, washed with PBS containing 1% BSA to remove unbound proteins, resuspended and analyzed by flow cytometry using a FACScan flow cytometer (Becton Dickinson, Mountain View, CA, USA). Cells on the flow cytometer were gated by forward vs side scatter to eliminate dead cells.
Apoptosis assay
Apoptotic cells were detected using FITC-conjugated Annexin V (Caltag Laboratories, Burlingame, CA, USA) and propidium iodide (PI). Cells were washed twice with cold PBS and resuspended in an Annexin V-binding buffer containing 10 mmol/L 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), 140 mmol/L NaCl, and 5 mmol/L CaCl2 at a concentration of 1 × 106 cells/mL. The suspension (100 μL containing 1 × 105 cells), 5 μL of Annexin V-FITC and 10 μL of PI were added into a 5-mL culture tube. The tube was gently vibrated and incubated for 15 min in the dark at room temperature. After a binding buffer (400 μL) was added into the tube, the cells were analyzed by flow cytometry.
RNA interference
Small interfering RNA (siRNA) for human HPPCn was synthesized by Shanghai Gene Chemical Company (Shanghai, China). The sequences employed in silencing human HPPCn are sense: 5′-CGAACCUCACGCAUCUAAATT-3′ and antisense: 5′-UUUAGAUGCGUGAGGUUCGGA-3′. The sequences employed for non-silencing human HPPCn are sense: 5′-UUCUCCGAACGUGUCACGUTT-3′ and antisense: 5′-ACGUGACACGUUCGGAGAATT-3′. The HepG2 and SMMC7721 cells were transfected with siRNA using 1,2-dimyristyloxypropyl-3-dimethyl-hydroxy ethyl ammonium bromide-cholesterol (Invitrogen, San Diego, CA, USA) as recommended by its manufacturer.
Sphingosine (Sph) kinase activity assay
Cells were lysed by freeze-thawing (3 times). The lysates were cleared by centrifugation at 12 000 g for 30 min at 4°C. The protein concentration of cell lysates was measured by bicinchoninic acid assay (Pierce, Rockford, IL, USA). The SPK activity was assayed by incubating the cell lysates with Sph (Sigma, St Louis, MO, USA) and 32P-ATP (10 μCi, 50 mmol/L) for 30 min at 37°C. The products were separated by thin-layer chromatography on silica gel G60 (Merck KGaA, Darmstadt, Germany) using 1-butanol/methanol/acetic acid/water (80:20:10:20) and observed by autoradiography[10].
Statistics analysis
Results were presented as mean ± SD of three independent experiments. The data were analyzed by unpaired Student’s t test using SPSS version 10.0 (SPSS Inc, USA). P < 0.05 was considered statistically significant.
RESULTS
HPPCn acted as an autocrine factor for HCC cells in vitro
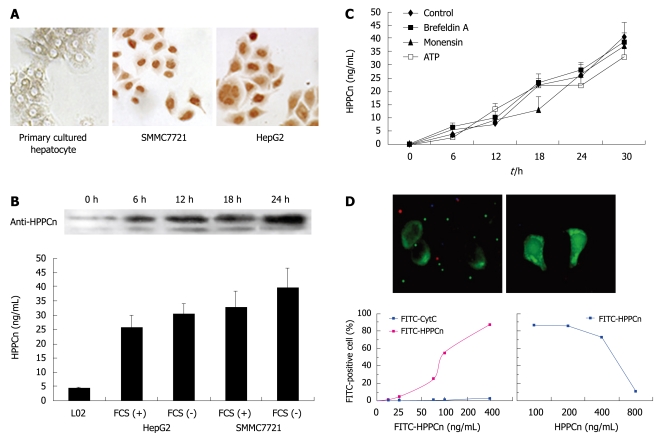
Sequence analysis revealed that HPPCn was a nuclear factor and a member of the leucine-rich acidic nuclear protein family. Since extracellular HPPCn can act as an autocrine factor for HCC cells in vitro, we studied its production, secretion, and mechanism of action. The intracellular localization of HPPCn was observed in human HCC cells with immune staining (Figure 1A). The HCC cells (HepG2 and SMMC7721) were strongly stained for HPPCn and the staining was mostly restricted to nuclei and cytoplasm. However, in primarily cultured hepatocytes, the HPPCn staining was weak and mainly restricted to cytoplasm of HCC cells. To determine whether HPPCn is secreted by HCC cells, we analyzed their CM by ELISA and Western blotting, which showed that HPPCn protein was released into CM by HepG2 and SMMC7721 (Figure 1B). The kinetics of HPPCn secretion by HCC cells is shown in Figure 1C. The HPPCn secretion, first detected at 6 h following culture, increased even at 18 and 30 h following culture, suggesting that brefeldin A and monensin, inhibitors of the endoplasmic reticulum (ER)-Golgi pathway, do not affect the HPPCn secretion[11]. The secretion of HPPCn triggered by ATP is negligible[12,13]. To eliminate the possibility of its leakage (as opposed to secretion) from HCC cells, we assessed the cellular damage with trypan blue staining, which showed that over 99% of the cells were still alive (data not shown), indicating that accumulation of HPPCn in the CM of those cells is caused by its secretion rather by its leakage from HCC cells. To test the binding of HPPCn to HCC cells, HepG2 cells were incubated with different concentrations of FITC-labeled rhHPPCn and cytochrome C (Cyt-C) at 37°C for 1 h and analyzed by flow cytometry. Fluorescence was found in membrane of HepG2 cells incubated with FITC-labeled HPPCn (Figure 1D) but not in membrane of those incubated with Cyt-C control (results not shown). Furthermore, HCC cells binding to FITC-labeled HPPCn and the density of the bound rhHPPCn tended to reach a plateau when the concentration of FITC-labeled HPPCn was higher than 400 ng/mL (Figure 1D), indicating that the binding of HCC cells to FITC-labeled HPPCn is saturated. In addition, we assessed the reversibility of bound HPPCn with a competitive inhibition curve (Figure 1D), which showed that approximately 30% of HCC cells were bound to FITC-labeled HPPCn following incubation in the presence of 100 ng/mL of unlabeled HPPCn and approximately 10% of HCC cells were bound to FITC-labeled HPPCn when the concentration of unlabeled rhHPPCn was higher than 800 ng/mL, indicating that HCC cells can express and secrete HPPCn and HPPCn can bind to HCC cells in a specific, saturated, and reversible manner and that an autocrine loop of HPPCn/HPPCn receptor is existed in HCC cells.
Figure 1.
Hepatopoietin Cn (HPPCn) acting as an autocrine factor for hepatocellular carcinoma (HCC) cells in vitro. A: Immunohistochemistry showing staining with anti-HPPCn antibody in primarily cultured hepatocytes and HCC cells; B: Western blotting and enzyme-linked immunosorbent assay (ELISA) showing the secretion of HPPCn into culture medium (CM) of HCC-derived cells; C: ELISA showing the kinetics of HPPCn secretion from HCC cells; D: Binding of HPPCn to HCC cells.
HPPCn suppressed apoptosis of HCC cells
HPPCn showed its effect on TSA-induced apoptosis of HCC cells. TSA was initially characterized by an anti-fungal drug and strongly inhibited TSA activity at nanomolar level[14]. It has been demonstrated that TSA induces apoptosis of human HCC cell lines (HepG2, Hep3B, and Huh-7) by inducing acetylation of p53 and histones (H2A, H2B, H3, and H4)[15-18], and inhibits HGF-induced invasion of HepG2 cells but does not affect increased phosphorylation of Erk and Akt in HGF-treated HepG2 cells[19].
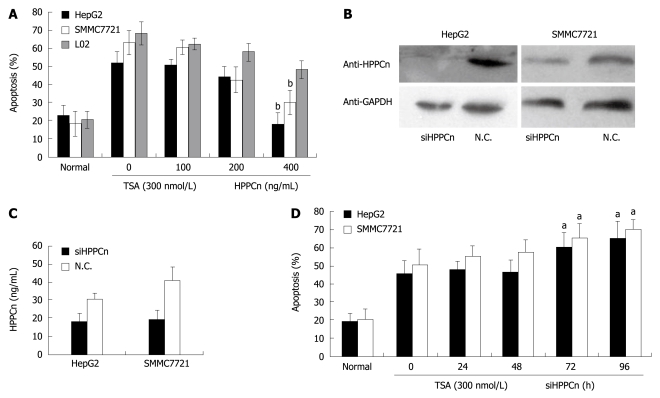
The viability of HepG2, SMMC7721 and L02 cells was 48.11%, 36.9% and 31.9%, respectively, 24 h after treatment with 300 nmol/L of TSA (Figure 2A), and increased to 85.6% and 88.4%, respectively, in HCC-derived cells after treatment with 400 ng/mL rhHPPCn (P < 0.02). In normal hepatocytes, the protective effect of HPPCn was moderate. HPPCn silenced by small-interfering RNA reduced the expression and secretion of HPPCn (Figure 2B and C) and enhanced TSA-induced apoptosis of HCC cells (Figure 2D), indicating that exogenous HPPCn suppresses apoptosis of HCC cells while HPPCn silenced by siRNA increases the apoptosis of HCC cells.
Figure 2.
HPPCn suppressing apoptosis of HCC-derived cells. A: Flow cytometry showing dose-dependent suppressing activity of recombinant human protein (rhHPPCn) on apoptosis of HCC cells incubated with 300 nmol/L trichostatin A (TSA) for 24 h prior to treatment with indicated concentrations of rhHPPCn; B: Western blotting showing hybridization to anti-HPPCn antibody and anti-GAPDH in HepG2 and SMMC7721 transfected with specific siRNA; C: ELISA showing the secretion of HPPCn into CM of HepG2 and SMMC7721; D: Flow cytometry showing the percentages of apoptotic cells incubated with 300 nmol/L TSA for 24 h prior to transfection with specific siRNA for an indicated period of time. The data are expressed as mean ± SD of 3 independent experiments. aP < 0.05, bP < 0.01 vs group without HPPCn/siHPPCn treatment. N.C.: Negative control.
HPPCn induced Mcl-1 expression in HCC cells via SPK/S1P and MEK/ERK pathways
It has been recently demonstrated that Mcl-1, an anti-apoptotic member of the Bcl-2 protein family that interferes with mitochondrial activation, plays a role in the survival of HCC cells[20]. The specific up-regulation of Mcl-1 expression in HCC cells could inhibit HCC cell apoptosis induced by chemotherapeutic drugs including histone deacetylase inhibitors[21]. However, Mcl-1 knockdown significantly augments the apoptosis sensitivity of HCC cells toward chemotherapy in combination with PI3K inhibitors[22,23]. In addition, certain growth factors (such as HGF and EGF) can induce Mcl-1 expression in HCC cells by activating the PI3K/Akt and MEK/ERK pathways[24,25].
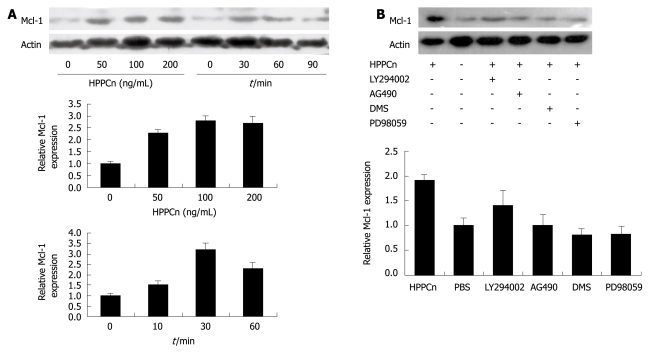
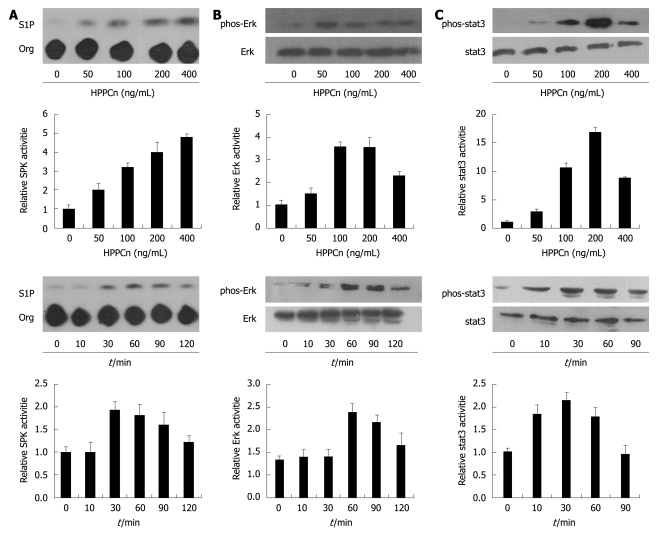
In order to test whether HPPCn influences Mcl-1 expression in HCC cells, HepG2 cells were treated with rhHPPCn at different concentrations for different periods of time. HPPCn induced Mcl-1 expression in a dose- and time-dependent manner (Figure 3A). Furthermore, treatment with rhHPPCn activated the SPK, Stat3, and ERK pathways in HepG2 cells (Figure 4). Thereafter, we determined if the blockage of these pathways can inhibit HPPCn-induced Mcl-1 expression. Cells were pre-incubated with LY294002, PD98059 AG490, or DMS for 1 h, and treated with HPPCn for an additional 3 h. Kinase inhibited by specific inhibitors abrogated the effect of HPPCn on Mcl-1 expression (Figure 3B), demonstrating that HPPCn can activate the signaling pathways involved in survival of HCC cells and up-regulate Mcl-1 expression via the MAPK and SPK pathways in HCC-derived cells.
Figure 3.
HPPCn inducing Mcl-1 expression via the MEK/ERK and SPK/S1P pathways. A: HPPCn induces Mcl-1 expression in a dose- and time-dependent manner and the densities of signals were determined by densitometry; B: Western blotting showing hybridization to anti-Mcl-1 antibody and anti-β-actin in HepG2 after the cells were treated with HPPCn for 3 h prior to pre-incubation with DMS, PD98059, LY294002, or AG490 for 1 h.
Figure 4.
Effects of rhHPPCn on the activities of SPK (A), Jak-Stat3 (B), and Erk1/2 (C). HepG2 cells were treated for 30 min with the indicated concentration of rhHPPCn and 200 ng/mL rhHPPCn for the indicated periods of time. The phosphorylation of Stat3 and Erk1/2 were determined by Western blotting with antibodies against both species of Stat3 and Erk1/2. The densities of signals were determined by densitometry.
DISCUSSION
HPPCn is a novel hepatic growth factor with a specific proliferation stimulating activity and suppresses apoptosis of HCC cells as an autocrine factor. In this study, the potential nuclear localization sequences and immune staining suggested that HPPCn was a nuclear factor and localized in nuclei of HCC cells. ELASA and Western blotting showed that the secretion of HPPCn into CM was not influenced by brefeldin A or monensin, two inhibitors of ER-Golgi pathway. Furthermore, FITC-labeled rhHPPCn binding to HCC cells was specific, saturated and reversible, suggesting that HPPCn acts as an autocrine growth factor in HCC cells in vitro.
It has been reported there are some autocrine loops in HCC cells, such as basic fibroblast growth factor (bFGF)/flg, TGF-α/erbB2[26-28], and augmenter of liver regeneration (ALR)[7]. However, the action of bFGF and TGF-α is neither hepatocyte-specific nor liver-specific. It has been shown that ALR protects hepatocytes in Eck’s fistula but not cultured hepatocytes and hepatic cell lines against atrophy[29,30]. Thus, identification of HPPCn autocrine mechanism in HCC cells might elucidate the pivotal process of hepatic carcinogenesis.
It is interesting to note that HPPCn resembles HCC-derived growth factor (HDGF) and high-mobility group box 1 protein (HMGB1). Extracellular HDGF can stimulate the proliferation of cultured hepatoma cells, fibroblasts, smooth muscle cells, and endothelial cells. However, it has been reported that nuclear localization is a prerequisite for the mitogenic activity of intracellular HDGF[31,32]. Similarly, as a nuclear protein, HMGB1 promotes the interaction between DNA and other proteins, and regulates several families of DNA-binding proteins. It has been reported that HMGB1 relocalizes in LPS-activated monocytes from nuclei to secretory organelles, and activates endothelial cells, promotes angiogenesis, and initiates inflammation as a cytokine[13,33]. Another similarity between HPPCn, HMGB1, and HDGF is their secondary structure. Motifs within their N-terminal domains, referred to as LRR, HMG box, and HATH, are involved in protein-protein interaction. In addition, these proteins contain 2 or 3 nuclear localization sequences but lack of any signal peptide-like hydrophobic region, indicating that HPPCn is a bifunctional nuclear protein and acts as a growth factor for cells[34,35].
HPPCn activates signaling pathways involved in survival of HCC cells and up-regulates Mcl-1 expression via MAPK, SPK1 in HCC cells. It has been recently demonstrated that Mcl-1, an anti-apoptotic member of the Bcl-2 protein family which interferes with mitochondrial activation, plays a role in the survival of HCC cells. Furthermore, some growth factors, including HGF and EGF, induce Mcl-1 expression in HCC cells by activating the PI3K/Akt and MEK/ERK pathways contributing to the poor prognosis of HCC patients, suggesting that Mcl-1 is one of the important molecules involved in HPPCn action.
In conclusion, HPPCn is a novel hepatic growth factor that can be secreted to CM and suppresses apoptosis of HCC cells by up-regulating Mcl-1expression.
COMMENTS
Background
There is evidence that protumorigenic growth factor signaling is dysregulated in human hepatocellular carcinoma (HCC) affecting different signaling systems such as insulin-like growth factor, hepatocyte-growth factor, transforming-growth factor α (TGF-α)/epidermal-growth factor, and TGF-β.
Research frontiers
Hepatopoietin Cn (HPPCn) is a novel hepatic growth factor derived from a hepatocyte-stimulating substance (HSS). Recombinant human protein (rhHPPCn) specifically stimulates cell proliferation in primary culture of hepatocytes and HCC-derived cell lines (HepG2, SMMC7721) in vitro as well as liver regeneration following partial hepatectomy in vivo. In addition, rhHPPCn has been demonstrated to be capable of protecting hepatocytes against ethanol-induced injury.
Innovations and breakthroughs
In this study, HPPCn was highly expressed in cytoplasm and nuclei of human HCC cells and secreted into the culture medium. Fluorescin isothiocyante-labeled rhHPPCn could specifically bind to its receptor on HepaG2 cells, suggesting that an autocrine loop of HPPCn/HPPCn receptor is existed in HCC-derived cell lines. Exogenous rhHPPCn suppressed trichostatin A-induced apoptosis of HCC cells. Moreover, HPPCn up-regulated the expression of myeloid cell leukemia-1 (Mcl-1) in HCC-derived cells via the mitogen-activated protein kinase or sphingosine kinase-1.
Applications
The mechanism of HPPCn underlying liver regeneration the authors observed in this contributes to the diagnosis and treatment of liver diseases.
Terminology
HSS: A hepatic stimulator substance isolated from rat liver and human fetal liver can increase synthesis of hepatic DNA in vivo and in vitro as shown by 3H-TdR incorporation. However, its composition still remains unknown.
Peer review
The manuscript written by Chang et al describes autocrine growth of human HCC cells inhibited by HPPCn. The study showed that HPPCn could suppress apoptosis of HCC cells by up-regulating the expression of Mcl-1 and by activating the PI3K/AKT and MEK/ERK pathways, suggesting that HPPCn plays an important role in the hepatocarcinogenesis. The data are interesting and the experiments are well organized.
Footnotes
Supported by (in part) Grants From the National Natural Science Foundation of China, No. 30800558 and No. 30930041 and the Chinese Major Special Science & Technology Project for Development of Major New Drugs, No. 2009ZX09103-617
Peer reviewers: Yukihiro Shimizu, MD, PhD, Kyoto Katsura Hospital, 17 Yamada-Hirao, Nishikyo, Kyoto 615-8256, Japan; Ezio Laconi, MD, PhD, Professor of General Pathology, Department of Sciences and Biomedical Technologies, Unit of Experimental Pathology, University of Cagliari, Via Porcell, 4 - IV Piano, 09125 - Cagliari, Italy; Qin Su, Professor, Department of Pathology, Cancer Hospital and Cancer Institute, Chinese Academy of Medical Sciences and Peking Medical College, PO Box 2258, Beijing 100021, China
S- Editor Tian L L- Editor Wang XL E- Editor Ma WH
References
- 1.El-Serag HB. Hepatocellular carcinoma: an epidemiologic view. J Clin Gastroenterol. 2002;35:S72–S78. doi: 10.1097/00004836-200211002-00002. [DOI] [PubMed] [Google Scholar]
- 2.Breuhahn K, Longerich T, Schirmacher P. Dysregulation of growth factor signaling in human hepatocellular carcinoma. Oncogene. 2006;25:3787–3800. doi: 10.1038/sj.onc.1209556. [DOI] [PubMed] [Google Scholar]
- 3.LaBrecque DR, Pesch LA. Preparation and partial characterization of hepatic regenerative stimulator substance (SS) from rat liver. J Physiol. 1975;248:273–284. doi: 10.1113/jphysiol.1975.sp010973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.LaBrecque DR, Steele G, Fogerty S, Wilson M, Barton J. Purification and physical-chemical characterization of hepatic stimulator substance. Hepatology. 1987;7:100–106. doi: 10.1002/hep.1840070121. [DOI] [PubMed] [Google Scholar]
- 5.Cui CP, Zhang DJ, Shi BX, Du SJ, Wu DL, Wei P, Zhong GS, Guo ZK, Liu Y, Wang LS, et al. Isolation and functional identification of a novel human hepatic growth factor: hepatopoietin Cn. Hepatology. 2008;47:986–995. doi: 10.1002/hep.22126. [DOI] [PubMed] [Google Scholar]
- 6.Cui CP, Wei P, Liu Y, Zhang DJ, Wang LS, Wu CT. The protective role of Hepatopoietin Cn on liver injury induced by carbon tetrachloride in rats*. Hepatol Res. 2009;39:200–206. doi: 10.1111/j.1872-034X.2008.00447.x. [DOI] [PubMed] [Google Scholar]
- 7.Li Y, Xing G, Wang Q, Li M, Wei H, Fan G, Chen J, Yang X, Wu C, Chen H, et al. Hepatopoietin acts as an autocrine growth factor in hepatoma cells. DNA Cell Biol. 2001;20:791–795. doi: 10.1089/104454901753438606. [DOI] [PubMed] [Google Scholar]
- 8.Liang H, Reich CF, Pisetsky DS, Lipsky PE. The role of cell surface receptors in the activation of human B cells by phosphorothioate oligonucleotides. J Immunol. 2000;165:1438–1445. doi: 10.4049/jimmunol.165.3.1438. [DOI] [PubMed] [Google Scholar]
- 9.Rakowicz-Szulczynska EM, Rodeck U, Herlyn M, Koprowski H. Chromatin binding of epidermal growth factor, nerve growth factor, and platelet-derived growth factor in cells bearing the appropriate surface receptors. Proc Natl Acad Sci USA. 1986;83:3728–3732. doi: 10.1073/pnas.83.11.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duan HF, Wang H, Yi J, Liu HJ, Zhang QW, Li LB, Zhang T, Lu Y, Wu CT, Wang LS. Adenoviral gene transfer of sphingosine kinase 1 protects heart against ischemia/reperfusion-induced injury and attenuates its postischemic failure. Hum Gene Ther. 2007;18:1119–1128. doi: 10.1089/hum.2007.036. [DOI] [PubMed] [Google Scholar]
- 11.Rubartelli A, Bajetto A, Allavena G, Wollman E, Sitia R. Secretion of thioredoxin by normal and neoplastic cells through a leaderless secretory pathway. J Biol Chem. 1992;267:24161–24164. [PubMed] [Google Scholar]
- 12.Hamon Y, Luciani MF, Becq F, Verrier B, Rubartelli A, Chimini G. Interleukin-1beta secretion is impaired by inhibitors of the Atp binding cassette transporter, ABC1. Blood. 1997;90:2911–2915. [PubMed] [Google Scholar]
- 13.Gardella S, Andrei C, Ferrera D, Lotti LV, Torrisi MR, Bianchi ME, Rubartelli A. The nuclear protein HMGB1 is secreted by monocytes via a non-classical, vesicle-mediated secretory pathway. EMBO Rep. 2002;3:995–1001. doi: 10.1093/embo-reports/kvf198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoshida M, Horinouchi S, Beppu T. Trichostatin A and trapoxin: novel chemical probes for the role of histone acetylation in chromatin structure and function. Bioessays. 1995;17:423–430. doi: 10.1002/bies.950170510. [DOI] [PubMed] [Google Scholar]
- 15.Svechnikova I, Ammerpohl O, Ekström TJ. p21waf1/Cip1 partially mediates apoptosis in hepatocellular carcinoma cells. Biochem Biophys Res Commun. 2007;354:466–471. doi: 10.1016/j.bbrc.2006.12.222. [DOI] [PubMed] [Google Scholar]
- 16.Yamashita Y, Shimada M, Harimoto N, Rikimaru T, Shirabe K, Tanaka S, Sugimachi K. Histone deacetylase inhibitor trichostatin A induces cell-cycle arrest/apoptosis and hepatocyte differentiation in human hepatoma cells. Int J Cancer. 2003;103:572–576. doi: 10.1002/ijc.10699. [DOI] [PubMed] [Google Scholar]
- 17.Chiba T, Yokosuka O, Fukai K, Kojima H, Tada M, Arai M, Imazeki F, Saisho H. Cell growth inhibition and gene expression induced by the histone deacetylase inhibitor, trichostatin A, on human hepatoma cells. Oncology. 2004;66:481–491. doi: 10.1159/000079503. [DOI] [PubMed] [Google Scholar]
- 18.Carlisi D, Vassallo B, Lauricella M, Emanuele S, D’Anneo A, Di Leonardo E, Di Fazio P, Vento R, Tesoriere G. Histone deacetylase inhibitors induce in human hepatoma HepG2 cells acetylation of p53 and histones in correlation with apoptotic effects. Int J Oncol. 2008;32:177–184. doi: 10.3892/ijo.32.1.177. [DOI] [PubMed] [Google Scholar]
- 19.Matsumoto Y, Motoki T, Kubota S, Takigawa M, Tsubouchi H, Gohda E. Inhibition of tumor-stromal interaction through HGF/Met signaling by valproic acid. Biochem Biophys Res Commun. 2008;366:110–116. doi: 10.1016/j.bbrc.2007.11.089. [DOI] [PubMed] [Google Scholar]
- 20.Schulze-Bergkamen H, Fleischer B, Schuchmann M, Weber A, Weinmann A, Krammer PH, Galle PR. Suppression of Mcl-1 via RNA interference sensitizes human hepatocellular carcinoma cells towards apoptosis induction. BMC Cancer. 2006;6:232. doi: 10.1186/1471-2407-6-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fleischer B, Schulze-Bergkamen H, Schuchmann M, Weber A, Biesterfeld S, Müller M, Krammer PH, Galle PR. Mcl-1 is an anti-apoptotic factor for human hepatocellular carcinoma. Int J Oncol. 2006;28:25–32. [PubMed] [Google Scholar]
- 22.Kern MA, Haugg AM, Koch AF, Schilling T, Breuhahn K, Walczak H, Fleischer B, Trautwein C, Michalski C, Schulze-Bergkamen H, et al. Cyclooxygenase-2 inhibition induces apoptosis signaling via death receptors and mitochondria in hepatocellular carcinoma. Cancer Res. 2006;66:7059–7066. doi: 10.1158/0008-5472.CAN-06-0325. [DOI] [PubMed] [Google Scholar]
- 23.Schulze-Bergkamen H, Brenner D, Krueger A, Suess D, Fas SC, Frey CR, Dax A, Zink D, Büchler P, Müller M, et al. Hepatocyte growth factor induces Mcl-1 in primary human hepatocytes and inhibits CD95-mediated apoptosis via Akt. Hepatology. 2004;39:645–654. doi: 10.1002/hep.20138. [DOI] [PubMed] [Google Scholar]
- 24.Ortiz C, Caja L, Sancho P, Bertran E, Fabregat I. Inhibition of the EGF receptor blocks autocrine growth and increases the cytotoxic effects of doxorubicin in rat hepatoma cells: role of reactive oxygen species production and glutathione depletion. Biochem Pharmacol. 2008;75:1935–1945. doi: 10.1016/j.bcp.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 25.Tacchini L, De Ponti C, Matteucci E, Follis R, Desiderio MA. Hepatocyte growth factor-activated NF-kappaB regulates HIF-1 activity and ODC expression, implicated in survival, differently in different carcinoma cell lines. Carcinogenesis. 2004;25:2089–2100. doi: 10.1093/carcin/bgh227. [DOI] [PubMed] [Google Scholar]
- 26.Stonāns I, Stonāne E, Russwurm S, Deigner HP, Böhm KJ, Wiederhold M, Jäger L, Reinhart K. HepG2 human hepatoma cells express multiple cytokine genes. Cytokine. 1999;11:151–156. doi: 10.1006/cyto.1998.0366. [DOI] [PubMed] [Google Scholar]
- 27.Nussbaum T, Samarin J, Ehemann V, Bissinger M, Ryschich E, Khamidjanov A, Yu X, Gretz N, Schirmacher P, Breuhahn K. Autocrine insulin-like growth factor-II stimulation of tumor cell migration is a progression step in human hepatocarcinogenesis. Hepatology. 2008;48:146–156. doi: 10.1002/hep.22297. [DOI] [PubMed] [Google Scholar]
- 28.Maret A, Galy B, Arnaud E, Bayard F, Prats H. Inhibition of fibroblast growth factor 2 expression by antisense RNA induced a loss of the transformed phenotype in a human hepatoma cell line. Cancer Res. 1995;55:5075–5079. [PubMed] [Google Scholar]
- 29.Francavilla A, Barone M, Van Thiel DH, Mazzaferro V, Prelich JG, Starzl TE. Further steps of hepatic stimulatory substance purification. Dig Dis Sci. 1991;36:674–680. doi: 10.1007/BF01297037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hagiya M, Francavilla A, Polimeno L, Ihara I, Sakai H, Seki T, Shimonishi M, Porter KA, Starzl TE. Cloning and sequence analysis of the rat augmenter of liver regeneration (ALR) gene: expression of biologically active recombinant ALR and demonstration of tissue distribution. Proc Natl Acad Sci USA. 1995;92:3076. doi: 10.1073/pnas.92.7.3076d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoshida K, Tomita Y, Okuda Y, Yamamoto S, Enomoto H, Uyama H, Ito H, Hoshida Y, Aozasa K, Nagano H, et al. Hepatoma-derived growth factor is a novel prognostic factor for hepatocellular carcinoma. Ann Surg Oncol. 2006;13:159–167. doi: 10.1245/ASO.2006.11.035. [DOI] [PubMed] [Google Scholar]
- 32.Everett AD, Stoops T, McNamara CA. Nuclear targeting is required for hepatoma-derived growth factor-stimulated mitogenesis in vascular smooth muscle cells. J Biol Chem. 2001;276:37564–37568. doi: 10.1074/jbc.M105109200. [DOI] [PubMed] [Google Scholar]
- 33.Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005;5:331–342. doi: 10.1038/nri1594. [DOI] [PubMed] [Google Scholar]
- 34.Seo SB, McNamara P, Heo S, Turner A, Lane WS, Chakravarti D. Regulation of histone acetylation and transcription by INHAT, a human cellular complex containing the set oncoprotein. Cell. 2001;104:119–130. doi: 10.1016/s0092-8674(01)00196-9. [DOI] [PubMed] [Google Scholar]
- 35.Opal P, Garcia JJ, McCall AE, Xu B, Weeber EJ, Sweatt JD, Orr HT, Zoghbi HY. Generation and characterization of LANP/pp32 null mice. Mol Cell Biol. 2004;24:3140–3149. doi: 10.1128/MCB.24.8.3140-3149.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]