Abstract
Objective:
To compare the efficacy and safety of 1-mg and 4-mg doses of preservative-free intravitreal triamcinolone with standard care (grid photocoagulation in eyes without dense macular hemorrhage and deferral of photocoagulation until hemorrhage clears in eyes with dense macular hemorrhage) for eyes with vision loss associated with macular edema secondary to branch retinal vein occlusion (BRVO).
Methods:
Multicenter, randomized clinical trial of 411 participants.
Main Outcome Measure:
Gain in visual acuity letter score of 15 or more from baseline to month 12.
Results:
Twenty-nine percent, 26%, and 27% of participants achieved the primary outcome in the standard care, 1-mg, and 4-mg groups, respectively. None of the pairwise comparisons between the 3 groups was statistically significant at month 12. The rates of elevated intraocular pressure and cataract were similar for the standard care and 1-mg groups, but higher in the 4-mg group.
Conclusions:
There was no difference identified in visual acuity at 12 months for the standard care group compared with the triamcinolone groups; however, rates of adverse events (particularly elevated intraocular pressure and cataract) were highest in the 4-mg group.
Application to Clinical Practice:
Grid photocoagulation as applied in the SCORE Study remains the standard care for patients with vision loss associated with macular edema secondary to BRVO who have characteristics similar to participants in the SCORE-BRVO trial. Grid photocoagulation should remain the benchmark against which other treatments are compared in clinical trials for eyes with vision loss associated with macular edema secondary to BRVO.
Trial Registration:
clinicaltrials.gov Identifier: NCT00105027
Retinal vein occlusion disease is estimated to be the second most common cause of retinal vascular disease in the United States.1 The prevalence of retinal vein occlusion in the Blue Mountains Eye Study was 1.6%, with 69.5%(41of59)oftheseocclusionsclassified as branch retinal vein occlusion (BRVO).2 Retinal vein occlusion was bilateral in 5.1% of cases.2 The 15-year cumulative incidence of BRVO was 1.8% in the Beaver Dam Eye Study.3 In the Beaver Damcohort,centraland branch retinal vein occlusion accounted for 12% of eyes that developed severe vision loss during a 15-year period.4
Macular edema is a frequent cause of visual acuity loss from BRVO.1,5,6 The natural history of macular edema secondary to BRVO was delineated in the Branch Vein Occlusion Study (BVOS).1 One arm of the BVOS demonstrated a benefit with grid photocoagulation.1 Of 43 treated eyes available for follow-up at the 3-year visit, 28 (65%) had gained 2 or more lines of visual acuity from baseline and maintained this gain for at least 8 months compared with the same gain in 13 of 35 untreated eyes (37%). The BVOS also identified a subset of patients who derived limited benefit from grid photocoagulation. In the BVOS, 40% of treated eyes (n=43) had worse than 20/40 visual acuity at 3 years, and 12% of treated eyes had 20/200 or worse visual acuity at 3 years.1
During the last decade, a number of additional treatments for macular edema secondary to BRVO have been investigated. Such treatments include laser chorioretinal anastomosis, vitrectomy, arteriovenous sheathotomy, and intravitreal injection of antibodies targeted at vascular endothelial growth factor (VEGF).7-12 Numerous reports have suggested that intravitreal injection(s) of triamcinolone acetonide (hereafter referred to as intravit-real triamcinolone) is a potentially efficacious therapy for retinal thickening and vision loss in patients with macular edema secondary to BRVO but that some patients may develop steroid-related complications, such as elevated intraocular pressure (IOP) and cataract.13-26 Most of the information concerning intravitreal triamcinolone for macular edema secondary to BRVO is derived from case series that lacked long-term follow-up and adequate numbers of study participants; a randomized and controlled study has not been performed.13-26 Despite the shortcomings of these case series, intravitreal triamcinolone is currently in use as a treatment for BRVO.
The rationale for the use of corticosteroids to treat macular edema secondary to BRVO follows from the observation that the increase in retinal capillary permeability that results in macular edema may be caused by a breakdown of the blood-retina barrier mediated in part by VEGF, a 45-kDa glycoprotein.27-29 Therefore, attenuation of the effects of VEGF may reduce macular edema associated with BRVO.30,31 Corticosteroids have been demonstrated to inhibit the expression of VEGF and therefore may be an effective therapy for macular edema.32,33 Inflammation may also play a role in the pathology of BRVO, and the anti-inflammatory properties of corticosteroids may contribute to the attenuation of the disease process.34
Intravitreal triamcinolone is used by ophthalmologists in the clinical setting as a readily available pharmacologic agent (Kenalog 40; Bristol-Myers Squibb, Princeton, New Jersey, or Triesence; Alcon Inc, Fort Worth, Texas), though use for the treatment of macular edema is off label. Other formulations such as compounded preservative-free triamcinolone acetonide are also used in the clinical setting.
The Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) Study, sponsored by the National Eye Institute, is a clinical trial designed to compare 1-mg and 4-mg doses of intravitreal triamcinolone with standard care for vision loss associated with macular edema secondary to per-fused central retinal vein occlusion (CRVO) and BRVO.35,36 This article describes findings from the SCORE-BRVO trial. A companion article that compares intravitreal triamcinolone with observation for vision loss associated with macular edema secondary to CRVO is published concurrently (the SCORE-CRVOtrial).37 The 2 primary study objectives of the SCORE-BRVO trial are (1) to determine whether intravit-real triamcinolone at 1-mg and 4-mg doses produces greater visual benefit, with an acceptable safety profile,than grid photocoagulation, when appropriate, for the treatment of vision loss associated with macular edema secondary to BRVO; and (2) to compare the efficacy and safety of the 1-mg and 4-mg triamcinolone doses.
METHODS
DESIGN
The SCORE-BRVO trial was designed as a multicenter, prospective, randomized clinical trial and adhered to the tenets of the Declaration of Helsinki. Approval for the protocol was obtained from either a central (Jaeb Center for Health Research) or local institutional review board. Health Insurance Portability and Accountability Act–compliant informed consent forms were obtained before screening any participants. Study oversight was provided by an independent data and safety monitoring committee. Eligibility for the SCORE-BRVO trial was determined at the clinical sites (Table 1). For the purposes of the SCORE Study, participants identified as having hemiretinal vein occlusion according to the definition outlined in the protocol were included in the BRVO trial. All participants were randomized within 21 days of screening or rescreening. One eye per participant was enrolled in the trial. Participants and physicians were masked to the intravitreal triamcinolone dose (1 mg vs 4 mg) but not to the treatment assignment of standard care vs intravitreal triamcinolone. The prespecified primary efficacy evaluation was performed at month 12. The primary outcome measure was the proportion of participants who experienced a gain in visual acuity letter score of 15 or more from baseline to month 12 as assessed by the electronic Early Treatment Diabetic Retinopathy Study (E-ETDRS) method.38
Table 1.
Study Eye Major Inclusion and Exclusion Criteria
| Inclusion Criteria |
| Best-corrected E-ETDRS visual acuity letter score of ≤73 (approximate Snellen equivalent, 20/40 or worse) and ≥19 (approximate Snellen equivalent, 20/400 or better)a |
| Center-involved macular edema secondary to BRVO present on clinical examination |
| Mean central subfield retinal thickness of 2 OCT fast macular scans ≥250 μm |
| Media clarity, pupillary dilation, and subject cooperation sufficient for adequate fundus photographs |
| Exclusion Criteria |
| Macular edema due to a cause other than BRVO |
| An ocular condition such that visual acuity would not improve from resolution of the edema (eg, foveal atrophy) |
| Substantial cataract estimated to have reduced visual acuity by ≥3 lines |
| Prior treatment with intravitreal corticosteroids at any time or peribulbar steroid injection within 6 mo prior to randomization |
| History of focal/grid macular photocoagulation within 15 weeks (3.5 mo) or PRP within 4 mo prior to randomization or anticipated need for PRP within the 4 mo following randomization |
| Prior pars plana vitrectomy |
| Major ocular surgery (including cataract extraction) within prior 6 mo or anticipated within the next 6 mo following randomization |
| Yttrium aluminum garnet capsulotomy performed within 2 mo prior to randomization |
| IOP ≥25 mm Hg, open-angle glaucoma (either primary open-angle glaucoma or other cause of open-angle glaucoma), or steroid-induced IOP elevation that required IOP-lowering treatment or pseudoexfoliation |
| Aphakia |
Abbreviations: BRVO, branch retinal vein occlusion; E-ETDRS, electronic Early Treatment Diabetic Retinopathy Study; IOP, intraocular pressure; OCT, optical coherence tomography; PRP, panretinal photocoagulation.
The original lower limit of visual acuity was expanded from 34 letters to 24 or more letters 5 months after accrual began and then from 24 or more letters to 19 or more letters 12 months after accrual began.
RANDOMIZATION
Within baseline visual acuity strata of good (visual acuity letter score, 73-59; Snellen equivalent, 20/40 to 20/63); moderate (visual acuity letter score, 58-49; Snellen equivalent, 20/80 to 20/ 100); and poor (visual acuity letter score, 48-19; Snellen equivalent, 20/125 to 20/400) participants were randomly assigned centrally through a Web-based data entry system maintained at the Data Coordinating Center (The EMMES Corporation, Rockville, Maryland), with equal probability to receive standard care (referred to as the standard care group); 1 mg of intravitreal triamcinolone (referred to as the 1-mg triamcinolone group), or 4 mg of intravitreal triamcinolone (referred to as the 4-mg triamcinolone group) using a permuted blocks design with random block sizes. Standard care consisted of either grid photocoagulation if there was no dense macular hemorrhage or, if a dense macular hemorrhage was present, observation at 4-month intervals until the dense macular hemorrhage cleared sufficiently to allow grid photocoagulation to be performed. The decision as to what constituted a dense vs nondense hemorrhage was left to the investigator.
VISIT SCHEDULE
Study visits were planned for every 4 months through 36 months. At baseline and at each follow-up visit, best-corrected visual acuity letter score was measured at 3m by a masked, certified tester using the E-ETDRS method.38 A standardized refraction was performed at baseline, month 4, and at the annual visits (months 12, 24, and 36). All participants who received intravitreal triamcinolone had safety visits at 4 days (±3 days) and at 4 weeks (±1 week) following each injection; visual acuity, IOP measurement, and eye examination results, including those from a dilated fundus examination, were recorded in the study eye at these visits. At all other study visits, participants underwent an eye examination, E-ETDRS testing, IOP measurement, and an optical coherence tomography (OCT) scan (OCT2 or Stratus OCT; Carl Zeiss Meditech, Dublin, California). These tests were performed on both eyes at all visits, except for visits at months 8, 16, 20, 28, and 32, when OCT was performed only on the study eye. Stereoscopic color fundus photographs (7 fields) were taken of the study eye at baseline and at the annual visits. Three-field photographs of the study eye were taken at visits at months 4, 8, 16, 20, 28, and 32 and of the fellow eye at baseline and the annual visits. Lens opacities for both eyes were assessed at baseline and at the annual visits using the modified Age-Related Eye Disease Study grading method.39 Fluorescein angiography was performed at baseline and at the month 4, 12, and 24 visits. All images were sent to the reading center (University of Wisconsin Fundus Photograph Reading Center, Madison, Wisconsin) for analysis, where they were graded in a masked fashion. Blood pressure was measured at baseline and at the annual visits.
Participating study personnel, such as physician-investigators and study coordinators, were certified by the data coordinating center. Photographers and technicians who took the fundus photographs and OCT images for this study were certified by the reading center.
INTRAVITREAL INJECTIONS
The optimal dose of intraocular corticosteroid to maximize efficacy and simultaneously minimize adverse effects was not known when the SCORE Study was initiated. The most commonly used dose of triamcinolone for treating eyes with macular edema secondary to BRVO is 4 mg, delivered in a volume of 0.1 mL. However, the rationale for using this dose has been based on clinical feasibility (limiting volume of 0.1 mL of the first available triamcinolone formulation, Kenalog) rather than scientific principles. Clinical experience with other triamcinolone doses ranging from 1 to 20 mg is limited.40-42 Doses of intravitreal triamcinolone reported to be as high as 25 mg have been used in intravitreal injections.18,43
The SCORE-BRVO trial evaluated 1-mg and 4-mg doses of triamcinolone. The 4-mg dose was evaluated because it was the one used most commonly in clinical practice when the SCORE Study was initiated. The 1-mg dose was evaluated because this dose is likely to exceed the concentration necessary to saturate the glucocorticoid receptors in the cell cytoplasm.44,45 In addition, it was anticipated that, compared with the 4-mg dose, the 1-mg dose would have a lower risk of steroid-related adverse events. Insufficient data were available at the time of protocol development to warrant evaluating a dose higher than 4 mg.
A preservative-free, nondispersive formulation of triamcinolone was used in the current study in an effort to avoid the postinjection intraocular inflammation reported with Kenalog and some of the compounded triamcinolone formulations thought to be attributable to the excipients in Kenalog, endotoxins, or a particle dispersion phenomenon.46,47 The study drug was manufactured as a sterile, preservative-free, single-use, intravitreal injectable (Trivaris; Allergan, Inc, Irvine, California; 4-mg brand name TRIVARIS) in 1-mg and 4-mg doses. Both doses were administered in a volume of 0.05 mL. All study eyes assigned to intravit-real triamcinolone received a standardized ocular surface preparation procedure prior to the injection consisting of an eyelid speculum, topical anesthetic, administration of topical antibiotics on the day of injection, and asepsis with povidone iodine.48
Following the preparation procedure, either 1 mg or 4 mg of triamcinolone was injected into the vitreous cavity via the pars plana, 3 to 4 mm posterior to the limbus. The eyelid speculum was removed and indirect ophthalmoscopy was performed to confirm the intravitreal location of the triamcinolone and to confirm a perfused central retinal artery. Participants were instructed to use topical antibiotics 4 times daily for 3 days following the injection.
GRID PHOTOCOAGULATION PROCEDURE
For participants with BRVO assigned to standard care who were eligible for grid photocoagulation (ie, no dense baseline macular hemorrhage), investigators were asked to perform grid photocoagulation to treat both focal leaks, if any, and areas of diffuse retinal thickening. If the eye was not eligible for grid photocoagulation at randomization because of dense macular hemorrhage, the participant was reevaluated at 4-month intervals. Once the macular hemorrhage cleared, grid photocoagulation was performed. The following photocoagulation guidelines were provided in the study protocol:
Size: 50 to 100 μm
Exposure: 0.05 to 0.1 seconds Intensity: mild
Number: cover areas of diffuse retinal thickening and treat focal leaks if any are present
Placement: 1 to 2 burn-widths apart (500-3000 μm from center of fovea)
Wavelength: green to yellow
RETREATMENT CRITERIA
Retreatment criteria were identical for all 3 treatment arms. Participants were retreated at 4-month intervals (minimum, 105 days from the last treatment) according to the original treatment assigned at randomization, except when specific reasons not to retreat were encountered. However, even if these reasons for not retreating were present, investigators were not prohibited from retreating. The 3 reasons to consider deferral of retreatment were (1) if the treatment was successful (either the investigator believed that the macula was flat with an OCT-measured central subfield thickness of ≤225 μm, visual acuity letter score was ≥79 [Snellen equivalent, 20/25 or better] or there was substantial improvement in macular edema from the prior treatment and further improvement from the prior treatment might be expected); (2) if the treatment was contra-indicated because, in the judgment of the investigator, either the participant had a significant adverse effect from prior treatment (eg, IOP rise that required treatment) or maximum grid photocoagulation had already been performed; or (3) additional treatment was considered “apparently futile.” Treatment was considered apparently futile if a period of 8 or more months transpired during which there were 2 procedures (either 2 grid photocoagulation sessions or 2 intravitreal triamcinolone injections, according to the treatment assigned at randomization) but there was no evidence of at least borderline improvement. Borderline improvement was present if, when compared with findings at the beginning of the period, there was an increase in visual acuity letter score of 5 or more or there was a decrease in calculated retinal thickening (actual thickness minus mean normal thickness49) that was at least 50 μm and represented at least a 20% reduction in retinal thickening compared with the findings at the beginning of the period.
ALTERNATE TREATMENT CRITERIA
In the SCORE-BRVO trial, eyes could receive the alternate treatment when there was a loss from baseline in best-corrected visual acuity letter score of 15 or more that was present at 2 consecutive 4-month interval visits. The decrease in visual acuity had to be a result of persistent or recurrent macular edema (ie, not due to cataract or other abnormality) that was documented on OCT. If these criteria were met, eyes assigned to the macular grid photocoagulation group could receive (but were not required to receive) intravitreal triamcinolone (4-mg dose, study formulation), and eyes assigned to intravitreal triamcinolone could receive (but were not required to receive) grid photocoagulation. Study eyes that received an alternate treatment were analyzed in the arm to which they were randomized.
ASSESSMENT OF MACULAR EDEMA
The reading center graders, without knowledge of treatment assignment or participant clinical data, followed a standardized protocol to grade the area of macular edema and retinal hemorrhage using stereoscopic fundus photographs.50 The OCT scans were evaluated for both quantitative data (eg, central subfield thickness) using the macular fastmap scan, consisting of 6 radially oriented scans, and qualitative data (eg, presence or absence of vitreomacular traction, subretinal fluid, and cystoid spaces) using the 2-scan cross-hair images.51 Center point thickness was used for analysis instead of central subfield thickness, because this permitted correction of errors in the measurement of the inner and outer retinal boundaries. The correlation between center point thickness and central subfield thickness is 0.98.52 Fluorescein angiograms were graded for area of nonperfusion and leakage in disc areas.
STATISTICAL ANALYSIS
The primary efficacy outcome measure of the SCORE-BRVO trial is the proportion of participants experiencing a gain in E-ETDRS visual acuity letter score of 15 or more from baseline to month 12. The primary outcome measure was examined for each of 3 pairwise comparisons: (1) the 1-mg triamcinolone group vs the standard care group; (2) the 4-mg triamcinolone group vs the standard care group; and (3) the 1-mg triamcinolone group vs the 4-mg triamcinolone group.
The SCORE-BRVO trial was designed assuming efficacy of 35% in the standard care arm, estimated from the BVOS,1 and 53% in both the 1-mg and 4-mg triamcinolone arms. The original target sample size was 630 participants, to be divided equally among the 3 treatment arms. After 10% attrition, this would yield 90% power independently at α=.025 (2-tailed) for 2 of the 3 primary pairwise comparisons: (1) the 1-mg triamcinolone group vs the standard care group; and (2) the 4-mg triamcinolone group vs the standard care group. A priori, a treatment difference between the 1-mg and the 4-mg triamcinolone group was not expected. Slow recruitment prompted a downward revision of the sample size from 630 to 486 participants, granting 80% power. A common closeout date of February 28, 2009, was subsequently established to allow at least 12 months of follow-up on all participants.
The primary analysis of the SCORE-BRVO trial is based on an observed case analysis that analyzed participants based on the arm to which they were randomized (consistent with the intention-to-treat principle) and treating missing 12-month observations as missing completely at random. To be included in the primary analysis, a study participant must have had a visual acuity at 12 months within a window ranging from 2 months before the target date to 3 months after the target date, with the target date defined as 12 months after the date of randomization. The statistical significance of the 3 pairwise comparisons was calculated using closed testing procedures53 modified for sequential testing, with family-wide error controlled at no more than α=.05. Logistic regression modeled the effects of the treatment assignment on the primary outcome while adjusting for the stratification factor of baseline visual acuity (good, moderate, and poor) and baseline dense macular hemorrhage used in the design of the SCORE-BRVO trial. P values for the 3 primary treatment comparisons and for the simultaneous comparison of all 3 arms (1-mg triamcinolone vs 4-mg triamcinolone vs standard care) were calculated. P values were then adjusted to account for simultaneous inference at a single time point and for interim monitoring. For interim monitoring, an O'Brien-Fleming–type boundary using a Lan and DeMets α-spending function was specified, so that, for all but the final comparison, family-wide error would be no more than .005, and the amount of family-wide α spent at the final comparison would be between .045 and .05.
Additional analyses were performed to assess the consistency of the primary efficacy results and included a sensitivity analysis in which outcomes were assigned to participants without month 12 data so as to explore extreme possible estimates of treatment effects. A per-protocol analysis was also conducted, including only study eyes that have 12-month visual acuity data and excluded participants who, before 12 months, received an alternative treatment (ie, treatment cross-overs) or nonprotocol treatments; who did not meet the eligibility criteria; or who did not receive the treatment assigned at randomization.
Secondary statistical analyses were performed, with analysis of variance and Kruskal-Wallis tests for continuous data and χ2 tests for categorical data. For examination of changes from baseline in visual acuity for various subgroup analyses, 95% confidence intervals (CIs) for the mean changes were provided. Presentations of continuous data included median (interquartile range) instead of or in addition to mean (standard deviation) to give a description of the distribution of the data. Only the primary analysis was adjusted for multiple testing. Thus, P values and CIs for secondary findings are intended primarily to give a sense of the variability inherent in the data. Adverse events reported by the clinical centers were coded per the Medical Dictionary for Regulatory Activities coding system, version 11.0, by trained staff at the data coordinating center. Version 9.1.3 of SAS was used to conduct all statistical analyses. All analyses included data available as of April 1, 2009.
RESULTS
BASELINE CHARACTERISTICS
Between November 4, 2004, and February 29, 2008, 411 patients with BRVO were enrolled in the SCORE Study at 75 clinical sites (Table 2). The mean duration of macular edema (based on patient history or ophthalmologic diagnosis) prior to enrollment was 4 months; 37% of participants had macular edema for less than 3 months; and 82% had macular edema for 6 months or less. The mean baseline visual acuity letter score was 57 (Snellen equivalent, approximately 20/80), and eyes had a mean center point thickness of about 523 μm based on OCT. A more detailed description of the SCORE-BRVO participant population can be found elsewhere.48
Table 2.
Baseline Characteristics by Treatment Group
| No. (%) |
||||
|---|---|---|---|---|
| Total (N=411) |
Standard Carea (n=137) |
Intravitreal Triamcinolone |
||
| Characteristic | 1 mg (n=136) | 4 mg (n=138) | ||
| Demographic characteristics | ||||
| Age, y | ||||
| Mean (SD) | 67.4 (11.2) | 66.9 (11.5) | 67.2 (11.5) | 68.1 (10.6) |
| Range | 22-94 | 22-90 | 32-91 | 33-94 |
| Women | 202 (49) | 71 (52) | 68 (50) | 63 (46) |
| White race | 362 (88) | 124 (91) | 115 (85) | 123 (89) |
| Study eye characteristics | ||||
| E-ETDRS visual acuity letter score | ||||
| Mean (SD) | 57.0 (12.6) | 56.8 (13.0) | 58.2 (11.3) | 56.1 (13.4) |
| Range (Snellen equivalent) | ||||
| 73-59 (20/40-20/63) | 215 (52) | 70 (51) | 74 (54) | 71 (51) |
| 58-49 (20/80-20/100) | 100 (24) | 34 (25) | 35 (26) | 33 (24) |
| 48-19 (20/125-20/400) | 96 (23) | 33 (24) | 27 (20) | 34 (25) |
| Duration of macular edema, mo | ||||
| Mean (SD) | 4.4 (3.8) | 4.5 (4.2) | 4.1 (3.4) | 4.6 (3.9) |
| <3 | 152 (37) | 49 (36) | 52 (38) | 51 (37) |
| 3-6 | 185 (45) | 62 (45) | 63 (46) | 60 (43) |
| 7-12 | 57 (14) | 17 (12) | 18 (13) | 22 (16) |
| >12 | 17 (4) | 9 (7) | 3 (2) | 5 (4) |
| HRVO classification | 59 (14) | 23 (17) | 16 (12) | 20 (14) |
| Dense macular hemorrhage | 120 (29) | 39 (28) | 38 (28) | 43 (31) |
| Prior grid photocoagulation | 32 (8) | 15 (11) | 8 (6) | 9 (7) |
| Prior sector/panretinal photocoagulation | 4 (1) | 1 (1) | 1 (1) | 2 (1) |
| IOP, mean (SD), mm Hg | 15.1 (3.0) | 15.2 (3.1) | 15.0 (2.8) | 15.1 (3.1) |
| IOP-lowering medication | 13 (3.2) | 6 (4) | 4 (3) | 3 (2) |
| Phakic eye | 335 (82) | 115 (84) | 110 (81) | 110 (80) |
| Imaging data | ||||
| OCT center point thickness, mean (SD), μm | 525 (186) | 537 (198) | 521 (198) | 516 (160) |
| Total macular volume, mean (SD), mm3 | 9.7 (1.8) | 9.6 (1.8) | 10.0 (2.1) | 9.5 (1.6) |
| Area of retinal thickening within the grid, mean (SD), DAb | 7.5 (2.8) | 7.4 (2.7) | 7.8 (3.0) | 7.4 (2.8) |
| Area of retinal hemorrhage within the grid, mean (SD), DAb | 2.9 (2.4) | 3.2 (2.5) | 2.8 (2.3) | 2.9 (2.4) |
| Area of fluorescein leakage within the grid, mean (SD), DAb | 6.1 (2.4) | 6.2 (2.4) | 6.2 (2.3) | 6.0 (2.5) |
| >5 DA of capillary nonperfusion in the eyec | 41 (15) | 12 (13) | 17 (18) | 12 (12) |
| >10 DA of capillary nonperfusion in the eyec | 26 (9) | 6 (6) | 13 (14) | 7 (7) |
| Nonstudy eye E-ETDRS visual acuity letter score, mean (SD) | 82.5 (11.0) | 81.9 (13.2) | 83.7 (8.6) | 81.9 (10.7) |
| Other clinical characteristics | ||||
| Diabetes mellitus | 59 (14) | 18 (13) | 17 (13) | 24 (17) |
| Hypertension | 287 (70) | 99 (72) | 93 (68) | 95 (69) |
| Coronary artery disease | 75 (18) | 20 (15) | 27 (20) | 28 (20) |
| History of cancer | 78 (19) | 24 (18) | 24 (18) | 30 (22) |
Abbreviations: DA, disc area; E-ETDRS, electronic Early Treatment Diabetic Retinopathy Study; HRVO, hemiretinal vein occlusion; IOP, intraocular pressure; OCT, optical coherence tomography.
Standard care consisted of either grid photocoagulation if there was no dense macular hemorrhage or, if a dense macular hemorrhage was present, observation at 4-month intervals until the dense macular hemorrhage cleared sufficiently to allow grid photocoagulation to be performed.
The grid is defined as the 9 ETDRS subfields centered in the macula. The grid is 16 DAs in size.
Capillary nonperfusion in the eye was assessed in 96, 95, and 102 eyes at baseline in the standard care, 1-mg, and 4-mg groups, respectively.
FOLLOW-UP
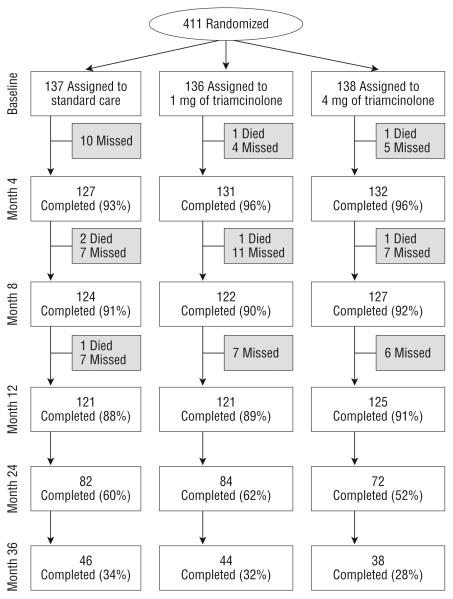
Figure 1 shows study follow-up of all participants at 4-month intervals through 12 months, and then annually through 36 months. The month 12 primary outcome visit was completed by 88%, 89%, and 91% of participants in the standard care, 1-mg, and 4-mg groups, respectively. At the time of study closeout, 58% of study participants had month 24 outcomes assessed and 31% had month 36 outcomes assessed.
Figure 1.
Flowchart of participants in the Standard Care vs Corticosteroid for Retinal Vein Occlusion–Branch Retinal Vein Occlusion Study. Missed visits include those who missed a visit but came back for other visits and those who prematurely withdrew from the study.
STUDY TREATMENTS
For eyes without a dense macular hemorrhage at baseline and randomized to the standard care group, the mean number of laser treatments prior to 12 months was 1.8 (95% CI, 1.6-2.0); 27% of these eyes received the maximum number of 3 treatments and 98% received at least 1 laser treatment prior to 12 months (Table 3). For the 39 eyes in the standard care group with a dense macular hemorrhage at baseline, the mean number of grid photocoagulation treatments prior to 12 months was 0.7 (95% CI, 0.5-1.0); 49% of these eyes received at least 1 grid photocoagulation treatment during the study period through 12 months, and 21% received 2 laser treatments (at months 4 and 8) prior to 12 months. Overall, 84% of participants in the standard care group received at least 1 grid photocoagulation treatment prior to month 12.
Table 3.
Protocol Treatments in Participants
| Standard Care, Grid Photocoagulationa |
Intravitreal Triamcinolone |
||||
|---|---|---|---|---|---|
| Characteristic | All (n=137) |
DMH at Baseline (n=39) |
No DMH at Baseline (n=98) |
1 mgb (n=136) |
4 mgb (n=138) |
| No. of treatments from baseline to prior to 12 mo, mean (95% CI) | 1.5 (1.3-1.7) | 0.7 (0.5-1.0) | 1.8 (1.6-2.0) | 2.2 (2.1-2.4) | 2.1 (2.0-2.3) |
| No. of treatments prior to 12 mo, No. (%) | |||||
| 3 | 26 (19) | 0 | 26 (27) | 63 (46) | 47 (34) |
| 2 | 37 (27) | 8 (21) | 29 (30) | 43 (32) | 62 (45) |
| 1 | 52 (38) | 11 (28) | 41 (42) | 30 (22) | 28 (20) |
| 0 | 22 (16) | 20 (51) | 2 (2) | 0 | 1 (1)c |
| Treatments received during visit, No. (%) | |||||
| Baseline | 137 (69) | 39 (3) | 98 (96) | 136 (100) | 138 (99) |
| 4 mo | 127 (47) | 36 (42) | 91 (49) | 132 (76) | 132 (60) |
| 8 mo | 125 (39) | 35 (31) | 90 (42) | 123 (56) | 128 (60) |
| 12 mo | 124 (23) | 37 (16) | 87 (25) | 121 (45) | 126 (31) |
| 16 mo | 111 (5) | 33 (6) | 78 (5) | 107 (35) | 105 (35) |
| 20 mo | 96 (7) | 28 (7) | 68 (7) | 92 (32) | 92 (23) |
| 24 mo | 82 (11) | 28 (7) | 54 (13) | 84 (26) | 73 (25) |
Abbreviations: CI, confidence interval; DMH, dense macular hemorrhage.
Standard care consisted of either grid photocoagulation if there was no DMH or, if a DMH was present, observation at 4-month intervals until the DMH cleared sufficiently to allow grid photocoagulation to be performed. There were 4 participants who underwent standard care, all without DMH at baseline, who switched groups and received 4 mg of triamcinolone per protocol guidelines prior to 12 months.
There were 7 participants who received 1 mg and 3 who received 4 mg of triamcinolone who switched groups and received grid photocoagulation per protocol guidelines prior to 12 months.
One participant was randomized to the 4-mg arm, but refused initial treatment and withdrew from the study.
Prior to month 12, the mean number of injections was similar in the triamcinolone groups, with 2.2 (95% CI, 2.1-2.4) in the 1-mg triamcinolone group and 2.1 in the 4-mg triamcinolone group (95% CI, 2.0-2.3). Success of the prior triamcinolone treatment was the primary reason for not administering additional injections prior to 12 months (69%). Other reasons cited for not administering retreatment prior to 12 months include futility of the treatment (12%), treatment contraindicated (4%), participant refusal (2%), and other (13%).
Few treatment protocol deviations were noted prior to 12 months. These entailed the administration of intravitreal injections of (1) an anti-VEGF drug in 1 participant in the 4-mg triamcinolone group and 1 participant in the standard care group; and (2) nonstudy-formulation triamcinolone in 1 participant in the 4-mg triamcinolone group and 3 participants in the standard care group. These participants were included in the primary analyses but not in the per-protocol analysis.
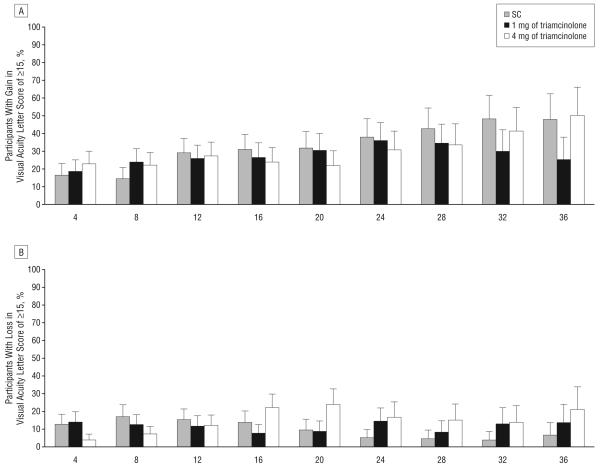
VISUAL ACUITY OUTCOMES
The primary outcome of the SCORE-BRVO trial, the percentage of participants with a gain in visual acuity letter score of 15 or more from baseline to month 12, was similar in all 3 groups: 28.9%, 25.6%, and 27.2% in the standard care, and 1-mg and 4-mg triamcinolone groups, respectively (Table 4 and Figure 2A). The odds ratios (ORs) for all 3 comparisons were close to 1 and P>.05 (1-mg triamcinolone group compared with standard care group, OR, 0.9; 95% CI, 0.5-1.6; P=.89; and 4-mg triamcinolone group compared with standard care group, OR, 0.9; 95% CI, 0.5-1.6; P=.89; 4-mg triamcinolone group compared with 1-mg triamcinolone group, OR, 1.0; 95% CI, 0.5-1.7; P=.89). All 3 groups had a similar gain of approximately 4 to 6 in mean visual acuity letter score from baseline to month 12 (Table 4). At month 12, there was a similar percentage of eyes in all 3 study groups with a loss in visual acuity letter score of 15 or more, approximately 11% to 15% (Table 4 and Figure 2B). The sensitivity of the primary efficacy conclusions to missing data was investigated, and no pattern of outcomes among missing participants overturns the conclusion that 1 mg or 4 mg of intravitreal triamcinolone is not superior to standard care at 12 months. Furthermore, the per-protocol analysis gave results that were qualitatively similar to the primary analysis.
Table 4.
Change in Visual Acuity Letter Score at Month 12
| % |
|||
|---|---|---|---|
| Standard Carea (n=121) |
Intravitreal Triamcinolone |
||
| Change in Visual Acuity Letter Score |
1 mg (n=121) |
4 mg (n=125) |
|
| Change at month 12, letters | |||
| ≥15 Gainb | 28.9 | 25.6 | 27.2 |
| 10-14 Gain | 9.9 | 17.4 | 12.8 |
| 5-9 Gain | 14.0 | 17.4 | 12.8 |
| No change, ±4 | 22.3 | 20.7 | 23.2 |
| 5-9 Loss | 5.0 | 4.1 | 5.6 |
| 10-14 Loss | 5.0 | 3.3 | 6.4 |
| ≥15 Loss | 14.9 | 11.6 | 12.0 |
| Mean (95% CI)c | 4.2 (1.1 to 7.3) |
5.7 (2.8 to 8.6) |
4.0 (0.9 to 7.2) |
| Median (interquartile range) | 6.0 (−4.0 to 16.0) |
8.0 (−3.0 to 15.0) |
5.0 (−4.0 to 15.0) |
Abbreviation: CI, confidence interval.
Standard care consisted of either grid photocoagulation if there was no dense macular hemorrhage or, if a dense macular hemorrhage was present, observation at 4-month intervals until the dense macular hemorrhage cleared sufficiently to allow grid photocoagulation to be performed.
Pairwise comparisons with a gain in visual acuity letter score of 15 or more: 1-mg vs standard care group, odds ratio, 0.9; 95% CI, 0.5-1.6; P=.89; 4-mg vs standard care group, odds ratio, 0.9; 95% CI, 0.5-1.6; P=.89; 4-mg vs 1-mg group, odds ratio, 1.0; 95% CI, 0.5-1.7; P=.89. The odds ratios are adjusted for visual acuity and presence of dense macular hemorrhage at baseline. Confidence intervals are not adjusted for simultaneous testing or interim monitoring.
Analysis of variance comparing the means among the 3 groups at month 12, P=.70.
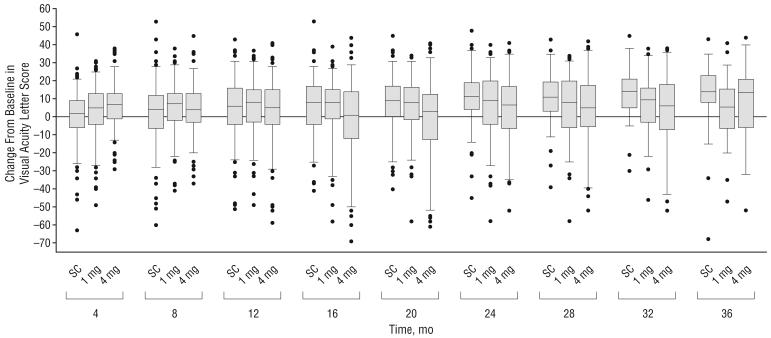
Figure 2.
Change from baseline in electronic Early Treatment Diabetic Retinopathy Study visual acuity at each 4-month follow-up visit. The histograms show the percentages of participants with a gain (A) or loss (B) in visual acuity letter score of 15 or more from baseline. Error bars represent upper 95% confidence limits. C, Box plot with whiskers representing the fifth and 95th percentiles; the line in the box represents the median; dots, values outside the whiskers; SC, standard care; 1 mg and 4 mg, doses of intravitreal triamcinolone acetonide.
The percentages with a gain or loss in visual acuity letter score of 15 or more from baseline and mean changes from baseline in visual acuity letter score are presented by study group and scheduled follow-up visit in Table 5 and Figure 2. The similarity of visual acuity results across the 3 study groups that is evident at month 12 was not present at month 4 for the comparison of the mean change in visual acuity letter score, which was greater in the 4-mg triamcinolone group compared with the other 2 groups (P=.002, based on analysis of variance). After month 12, mean change from baseline in visual acuity letter score is greater in the standard care group compared with the 2 triamcinolone groups (P<.05 at months 16, 20, 24, and 32, based on analysis of variance).
Table 5.
Change From Baseline Visual Acuity Letter Score by Follow-up Visit and Treatment Group
| Mean Change From Baseline in Visual Acuity Letter Score (95% CI) |
Gain in Visual Acuity Letter Score of ≥15, % (95% CI) |
Loss in Visual Acuity Letter Score of ≥15, % |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Follow-up Visit |
No. of Participants, Standard Carea/ 1-mg/4-mg Groups |
Standard Carea |
Intravitreal Triamcinolone |
Standard Carea |
Intravitreal Triamcinolone |
Standard Carea |
Intravitreal Triamcinolone |
|||
| 1 mg | 4 mg | 1 mg | 4 mg | 1 mg | 4 mg | |||||
| Month 4b | 127/131/132 | 0.7 (−1.9 to 3.3) | 2.6 (−0.1 to 5.2) | 6.9 (4.8 to 9.0) | 17 (10-23) | 18 (12-25) | 23 (16-30) | 13 | 14 | 4 |
| Month 8 | 124/122/127 | 1.6 (−1.6 to 4.8) | 5.0 (2.2 to 7.7) | 4.3 (1.8 to 6.9) | 15 (8-21) | 24 (16-31) | 22 (15-29) | 17 | 12 | 7 |
| Month 12 | 121/121/125 | 4.2 (1.1 to 7.3) | 5.7 (2.8 to 8.6) | 4.0 (0.9 to 7.2) | 29 (21-37) | 26 (18-33) | 27 (19-35) | 15 | 12 | 12 |
| Month 16 | 110/107/105 | 5.5 (2.4 to 8.7) | 5.9 (2.8 to 9.1) | −0.8 (−5.0 to 3.3) | 31 (22-40) | 26 (18-34) | 24 (16-32) | 14 | 7 | 22 |
| Month 20 | 95/92/92 | 8.1 (5.0 to 11.3) | 6.4 (3.1 to 9.8) | −0.2 (−4.7 to 4.2) | 32 (22-41) | 30 (21-40) | 22 (13-30) | 9 | 9 | 24 |
| Month 24 | 82/84/72 | 11.3 (8.1 to 14.5) | 6.3 (2.3 to 10.3) | 4.0 (−0.7 to 8.8) | 38 (27-48) | 36 (25-46) | 31 (20-41) | 5 | 14 | 17 |
| Month 28 | 68/73/60 | 10.9 (7.3 to 14.4) | 6.1 (1.9 to 10.3) | 4.4 (−1.0 to 9.7) | 43 (31-54) | 34 (23-45) | 33 (21-45) | 4 | 8 | 15 |
| Month 32 | 54/54/51 | 13.4 (9.7 to 17.0) | 6.3 (1.5 to 11.1) | 5.1 (−1.1 to 11.3) | 48 (35-61) | 30 (17-42) | 41 (28-55) | 4 | 13 | 14 |
| Month 36 | 46/44/38 | 12.9 (7.4 to 18.4) | 4.4 (−1.1 to 9.9) | 8.0 (0.9 to 15.2) | 48 (33-62) | 25 (12-38) | 50 (34-66) | 7 | 14 | 21 |
Abbreviation: CI, confidence interval.
Standard care consisted of either grid photocoagulation if there was no dense macular hemorrhage or, if a dense macular hemorrhage was present, observation at 4-month intervals until the dense macular hemorrhage cleared sufficiently to allow grid photocoagulation to be performed.
Analysis of variance comparing the means among the 3 groups at month 4: P=.002.
Analyses examining 12-month visual acuity outcomes for prespecified baseline subgroups categorizing dense macular hemorrhage status at baseline, grid photocoagulation status prior to enrollment, duration of macular edema, visual acuity letter score, and OCT-measured center point thickness demonstrated consistency of results with those observed in the overall 12-month analysis, with no sub-group showing a statistically significant treatment effect (Table 6). An analysis limited to eyes that were pseudophakic at baseline also demonstrated no statistically significant difference among the treatment groups with respect to change in visual acuity over time (P=.44, based on analysis of variance).
Table 6.
Twelve-Month Change From Baseline in Visual Acuity Letter Score Among Subgroups
| % |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of Participants, Standard Care/ 1-mg/4-mg Groups |
Mean Change in Visual Acuity Letter Score From Baseline (95% CI) |
Gain in Visual Acuity Letter Score of ≥15 |
Loss in Visual Acuity Letter Score of ≥15 |
|||||||
| Standard Carea |
Intravitreal Triamcinolone |
Standard Carea |
Intravitreal Triamcinolone |
Standard Carea |
Intravitreal Triamcinolone |
|||||
| Characteristic | 1 mg | 4 mg | 1 mg | 4 mg | 1 mg | 4 mg | ||||
| Baseline visual acuity letter score (Snellen equivalent) | ||||||||||
| 73-59 (20/40-20/63) | 61/66/61 | 2.0 (−1.5 to 5.5) | 2.5 (−0.9 to 5.8) | −1.7 (−6.1 to 2.6) | 21 | 15 | 15 | 15 | 15 | 18 |
| 58-49 (20/80-20/100) | 32/32/30 | 1.5 (−6.5 to 9.4) | 8.4 (2.1 to 14.8) | 4.8 (−2.3 to 12.0) | 27 | 32 | 30 | 21 | 6 | 13 |
| 48-19 (20/125-20/400) | 28/23/34 | 12.2 (5.7 to 18.7) | 11.6 (3.6 to 19.5) | 13.6 (8.9 to 18.3) | 45 | 48 | 47 | 7 | 10 | 0 |
| Baseline center point thickness, μm | ||||||||||
| <500 | 58/61/60 | 5.4 (1.8 to 9.0) | 7.4 (3.3 to 11.4) | 6.9 (2.4 to 11.3) | 23 | 25 | 37 | 7 | 8 | 10 |
| ≥500 | 63/59/64 | 3.2 (−1.9 to 8.3) | 3.9 (−0.3 to 8.2) | 1.3 (−3.3 to 5.8) | 33 | 27 | 19 | 22 | 15 | 14 |
| Dense macular hemorrhage at baseline | ||||||||||
| No | 84/87/85 | 2.4 (−1.2 to 6.0) | 4.7 (1.1 to 8.3) | 4.8 (1.5 to 8.2) | 22 | 23 | 29 | 14 | 11 | 11 |
| Yes | 37/34/40 | 8.4 (2.2 to 14.5) | 8.3 (3.6 to 13.0) | 2.3 (−4.7 to 9.2) | 43 | 32 | 23 | 16 | 12 | 15 |
| Prior grid photocoagulation | ||||||||||
| No | 107/113/116 | 4.1 (0.6 to 7.5) | 6.6 (3.7 to 9.5) | 4.3 (1.0 to 7.6) | 29 | 27 | 28 | 17 | 11 | 11 |
| Yes | 14/8/9 | 5.4 (−0.1 to 11.0) | −6.6 (−20.4 to 7.2) | 0.3 (−13.2 to 13.9) | 21 | 0 | 22 | 0 | 25 | 22 |
| Duration of macular edema at baseline, mo | ||||||||||
| ≤3 | 70/60/60 | 7.8 (3.6 to 12.0) | 8.1 (4.1 to 12.0) | 2.0 (−2.3 to 6.4) | 38 | 30 | 20 | 15 | 10 | 13 |
| >3 | 51/61/65 | −0.6 (−5.1 to 3.8) | 3.4 (−0.8 to 7.7) | 5.8 (1.3 to 10.4) | 15 | 21 | 34 | 13 | 13 | 11 |
| Pseudophakic at baseline | 20/21/25 | −3.2 (−14.1 to 7.8) | 2.5 (−8.0 to 13.0) | 4.8 (−2.0 to 11.6) | 20 | 29 | 28 | 35 | 24 | 4 |
Abbreviation: CI, confidence interval.
Standard care consisted of either grid photocoagulation if there was no dense macular hemorrhage or, if a dense macular hemorrhage was present, observation at 4-month intervals until the dense macular hemorrhage cleared sufficiently to allow grid photocoagulation to be performed.
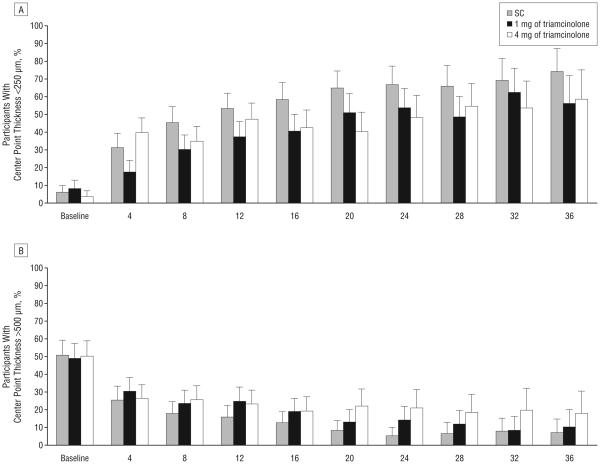
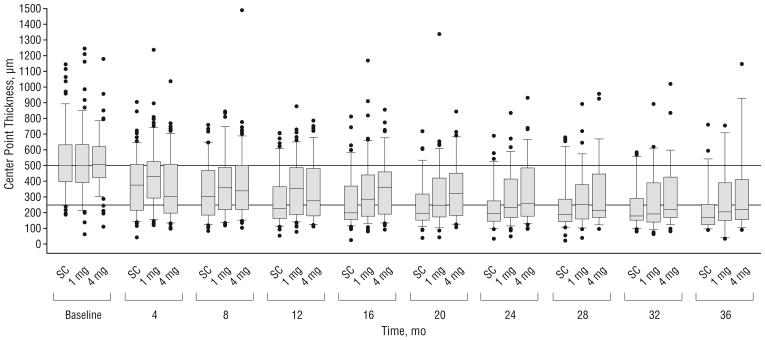
IMAGING OUTCOMES
All 3 study groups showed OCT-measured center point thickness decreases from baseline throughout follow-up (Table 7 and Figure 3). At the month 4 visit, the median decrease was greater in the 4-mg triamcinolone group (142-μm decrease) than the 1-mg group (77-μm decrease) and the standard care group (113-μm decrease; P=.008, Kruskal-Wallis test). At the month 12 visit, the median decrease from baseline in OCT-measured center point thickness was similar among the 3 treatment groups.
Table 7.
OCT-Measured Center Point Thickness
| Center Point Thickness, μm |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of Participants, Standard Care/1-mg/ 4-mg Groups |
Median (IQR) |
Median Change From Baseline (IQR) |
<250 μm, % |
|||||||
| Standard Carea |
Intravitreal Triamcinolone |
Standard Carea |
Intravitreal Triamcinolone |
Standard Carea |
Intravitreal Triamcinolone |
|||||
| Visit | 1 mg | 4 mg | 1 mg | 4 mg | 1 mg | 4 mg | ||||
| Baseline | 136/135/135 | 501 (399-631) | 496 (391-631) | 506 (421-621) | 6 | 8 | 4 | |||
| Month 4b | 125/126/129 | 375 (215-507) | 429 (291-525) | 302 (196-508) | −113 (−265 to −8) | −77 (−182 to 11) | −142 (−287 to −23) | 31 | 17 | 39 |
| Month 8 | 119/116/121 | 300 (184-469) | 356 (218-489) | 339 (218-501) | −189 (−359 to −48) | −118 (−265 to 6) | −125 (−222 to −42) | 45 | 30 | 33 |
| Month 12 | 120/113/112 | 228 (163-364) | 354 (183-486) | 274 (180-481) | −224 (−411 to −67) | −149 (−279 to −18) | −170 (−304 to −63) | 53 | 37 | 45 |
| Month 16 | 104/101/94 | 200 (155-370) | 283 (177-441) | 361 (189-457) | −257 (−420 to −118) | −174 (−309 to −46) | −167 (−305 to −66) | 58 | 40 | 40 |
| Month 20 | 88/86/77 | 195 (151-318) | 245 (170-419) | 320 (181-450) | −272 (−410 to −163) | −227 (−332 to −65) | −176 (−293 to −67) | 64 | 50 | 36 |
| Month 24 | 79/78/62 | 193 (146-274) | 233 (169-413) | 257 (177-484) | −271 (−417 to −202) | −212 (−379 to −66) | −201 (−333 to −110) | 67 | 52 | 45 |
| Month 28 | 62/68/55 | 187 (141-285) | 253 (159-377) | 210 (169-447) | −288 (−444 to −194) | −223 (−343 to −60) | −222 (−344 to −125) | 65 | 46 | 52 |
| Month 32 | 52/48/41 | 178 (151-291) | 191 (139-388) | 220 (169-426) | −266 (−441 to −186) | −236 (−394 to −64) | −211 (−315 to −108) | 69 | 59 | 48 |
| Month 36 | 43/39/34 | 171 (124-252) | 203 (150-389) | 220 (157-410) | −312 (−458 to −209) | −245 (−370 to −79) | −250 (−300 to −81) | 73 | 51 | 54 |
Abbreviations: IQR, interquartile range; OCT, optical coherence tomography.
Standard care consisted of either grid photocoagulation if there was no dense macular hemorrhage or, if a dense macular hemorrhage was present, observation at 4-month intervals until the dense macular hemorrhage cleared sufficiently to allow grid photocoagulation to be performed.
Kruskal-Wallis test comparing the distribution among the 3 groups at month 4, P=.008.
Figure 3.
Optical coherence tomography–measured center point thickness at each 4-month follow-up visit. The histograms show the percentages of participants with center point thickness smaller than 250 μm (A) and larger than 500 μm (B) throughout follow-up. Error bars represent upper 95% confidence limits. C, Box plots, with whiskers representing the fifth and 95th percentiles; the line in the box represents the median; dots, values outside the whiskers; SC, standard care; 1 mg and 4 mg, doses of intravitreal triamcinolone acetonide. Horizontal reference lines at 250 and 500 μm are presented.
Changes in OCT-measured center point thickness from baseline in all 3 study groups showed moderate negative correlation with changes from baseline in visual acuity letter score over time. The Pearson correlation coefficients were −0.23, −0.28, and −0.32 at month 4 and −0.19, −0.30, and −0.10 at month 12 for the standard care, 1-mg, and 4-mg study groups, respectively. For all 3 study groups, disc areas of fluorescein leakage within the grid were similar at baseline and follow-up visits (Table 8). The percentages of eyes with greater than 10 disc areas of capillary nonperfusion within the eye at month 12 was about 15% in all 3 groups; 23% to 31% of eyes had greater than 5 disc areas of nonperfusion at month 12.
Table 8.
Area of Fluorescein Leakage and Capillary Nonperfusion by Fluorescein Angiogram
| Capillary Nonperfusion Within the Eye, DAb |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Participants, Standard Care/ 1-mg/4-mg Groups |
Fluorescein Leakage Within the Grid, Median (IQR), DAa |
No. of Participants, Standard Care/ 1-mg/4-mg Groups |
>5 DA, % |
>10 DA, % |
|||||||
| Standard Carec |
Intravitreal Triamcinolone |
Standard Carec |
Intravitreal Triamcinolone |
Standard Carec |
Intravitreal Triamcinolone |
||||||
| Visit | 1 mg | 4 mg | 1 mg | 4 mg | 1 mg | 4 mg | |||||
| Baseline | 137/134/137 | 6 (5-8) | 6 (5-7) | 6 (4-7) | 96/95/102 | 13 | 18 | 12 | 6 | 14 | 7 |
| Month 4 | 121/123/130 | 6 (3-7) | 6 (4-7) | 6 (3-7) | 101/101/104 | 18 | 25 | 20 | 12 | 15 | 9 |
| Month 12 | 118/113/115 | 5 (2-6) | 5 (3-7) | 5 (2-7) | 104/100/93 | 29 | 31 | 23 | 15 | 16 | 14 |
| Month 24 | 79/76/66 | 3 (1-6) | 4 (2-6) | 5 (2-6) | 74/62/53 | 26 | 34 | 26 | 12 | 19 | 17 |
Abbreviations: DA, disc area; IQR, interquartile range.
The grid is defined as the 9 Early Treatment Diabetic Retinopathy Study subfields centered in the macula. The grid is 16 DAs in size.
Within the eye is 210 DAs.
Standard care consisted of either grid photocoagulation if there was no dense macular hemorrhage or, if a dense macular hemorrhage was present, observation at 4-month intervals until the dense macular hemorrhage cleared sufficiently to allow grid photocoagulation to be performed.
SAFETY OUTCOMES
Intraocular pressure–lowering medication was initiated in more eyes through 12 months in the 4-mg triamcinolone group (41%) compared with the 1-mg triamcinolone (7%) and standard care (2%) groups (χ2 test adjusting for multiple testing: standard care vs 1 mg of triamcinolone, P=.03; standard care vs 4 mg of triamcinolone, P<.001; and 1 mg vs 4 mg of triamcinolone, P<.001) (Table 9). Trabeculectomy or tube shunt surgery was not performed up to 12 months. Between 12 and 24 months, 1 participant in the 4-mg group received a trabeculectomy and 1 participant in the 4-mg group received a tube shunt to control IOP unrelated to neovascular glaucoma.
Table 9.
Adverse Ocular Events
| No. |
|||
|---|---|---|---|
| Standard Carea (n=137) |
Intravitreal Triamcinolone |
||
| Characteristic | 1 mg (n=136) |
4 mg (n=138) |
|
| Events Through 12 Months | |||
| Elevated IOP/glaucoma | |||
| Initiation of IOP-lowering medication, No. (%)b | 3 (2) | 11 (8) | 57 (41) |
| IOP>35 mm Hg | 1 | 2 | 14 |
| IOP>10 mm Hg above baseline | 1 | 12 | 50 |
| Laser peripheral iridotomy | 0 | 0 | 1 |
| Trabeculectomy | 0 | 0 | 0 |
| Tube shunt | 0 | 0 | 0 |
| Cataractc | |||
| Lens opacity onset or progression,d No. (%) | 15 (13) | 27 (25) | 38 (35) |
| Cataract surgery | 3 | 0 | 4 |
| ≥1 of the following other ocular adverse events: | 8 | 4 | 8 |
| Infectious endophthalmitis | 0 | 0 | 1 |
| Noninfectious endophthalmitis | 0 | 0 | 0 |
| Retinal detachment | 1 | 1 | 0 |
| HRVO to CRVO transition | 0 | 0 | 1 |
| BRVO to CRVO transition | 1 | 0 | 0 |
| Iris neovascularization or neovascular glaucoma | 1 | 1 | 2 |
| Retinal neovascularization | 5 | 1 | 3 |
| Vitreous hemorrhage | 2 | 1 | 3 |
| Other ocular surgical procedures | |||
| YAG capsulotomy | 1 | 0 | 1 |
| Sector/panretinal scatter photocoagulation | 5 | 1 | 4 |
| Pars plana vitrectomy | 1 | 0 | 1 |
|
| |||
| Selected Events at 12-24 Months | |||
| Glaucoma procedures | |||
| Argon laser trabeculoplasty | 0 | 0 | 2 |
| Laser peripheral iridotomy | 0 | 1 | 0 |
| Trabeculectomy | 0 | 0 | 1 |
| Tube shunt | 0 | 0 | 1 |
| Cataract surgery | 6 | 8 | 35 |
Abbreviations: BRVO, branch retinal vein occlusion; CRVO, central retinal vein occlusion; HRVO, hemiretinal vein occlusion; IOP, intraocular pressure; YAG, yttrium-aluminum-garnet.
Standard care consisted of either grid photocoagulation if there was no dense macular hemorrhage or, if a dense macular hemorrhage was present, observation at 4-month intervals until the dense macular hemorrhage cleared sufficiently to allow grid photocoagulation to be performed.
Percents are of the total sample size. P<.001 based on an overall χ2 test. Pairwise comparisons, adjusting for multiple testing: standard care vs 1-mg group, P=.03; standard care vs 4-mg group, P<.001; and 1-mg vs 4-mg group, P<.001.
Standard care group, 115; 1-mg group, 110; and 4-mg group, 110 phakic eyes at baseline.
P<.001 based on overall χ2 test. Pairwise comparison, adjusting for multiple testing: standard care vs 1-mg group, P=.03; standard care vs 4-mg group, P<.001; and 1-mg vs 4-mg group, P=.10.
Among eyes that were phakic at baseline, the estimate of new-onset lens opacity or progression of an existing opacity based on clinical assessment through month 12 in the standard care group was 13% compared with 25% and 35% in the 1-mg and 4-mg triamcinolone groups, respectively (χ2 test adjusting for multiple testing: standard care vs 1 mg of triamcinolone, P=.03; standard care vs 4 mg of triamcinolone, P<.001; and 1 mg vs 4 mg of triamcinolone, P=.10) (Table 9). Through month 12, cataract surgery was reported for 3 participants in the standard care group vs none in the 1-mg triamcinolone group and 4 in the 4-mg triamcinolone group. Cataract surgery was more frequent between months 12 and 24 in the 4-mg group, with 35 study eyes receiving such surgery, compared with 8 in the 1-mg study group and 6 in the standard care group (log-rank test between 1 and 2 years, standard care vs 1 mg of triamcinolone, P=.59; standard care vs 4 mg of triamcinolone, P<.001; and 1 mg vs 4 mg of triamcinolone, P<.001).
Through month 12, there were no reports of infectious endophthalmitis in the standard care group or 1-mg triamcinolone group and 1 case in the 4-mg triamcinolone group 3 days after the third injection. This participant's vitreous culture grew coagulase-negative staphylococcus. The participant's visual acuity letter score returned to 59 five weeks after the endophthalmitis diagnosis (the pre-endophthalmitis visual acuity letter score was 54), and the participant's visual acuity letter score at 36 months was 65 (visual acuity letter score at randomization, 65). Surgical procedures through 12 months, including sector/ panretinal scatter photocoagulation, pars plana vitrectomy, and yttrium-aluminum-garnet capsulotomy, were uncommon.
Minor ocular adverse events related to the injection procedure were evaluated. Vitreous floaters and conjunctival hemorrhage were reported in a similar percentage of participants in both triamcinolone groups through month 12: 31% of the 1-mg triamcinolone group and 26% of 4-mg triamcinolone group had vitreous floaters and 30% of the 1-mg triamcinolone group and 33% of the 4-mg triamcinolone group had conjunctival hemorrhage. Silicone oil droplets in the vitreous were reported through 12 months in 33% of eyes treated with 1 mg of triamcinolone and 12% of eyes treated with 4 mg of triamcinolone. A separate report provides more detailed information on the incidence of intravitreal silicone oil in the SCORE Study, which decreased precipitously following the introduction of a luer cone needle design in place of a staked-on needle design.54 No adverse events were reported as a result of treatment with grid photocoagulation.
Reports of systemic adverse events were similar among the SCORE-BRVO trial groups. The Medical Dictionary for Regulatory Activities system/organ class of infection and infestations had the highest incidence through month 12, with 10%, 16%, and 15% of participants reporting at least 1 event in the standard care, 1-mg, and 4-mg groups, respectively. Seven deaths occurred prior to 12 months of follow-up (3 in the standard care group, 2 in the 1-mg triamcinolone group, and 2 in the 4-mg triamcinolone group), and 9 more deaths occurred after 12 months of follow-up (2 in the standard care group and 7 in the 4-mg triamcinolone group).
COMMENT
The results of the SCORE-BRVO trial demonstrate no significant differences among the 3 treatment groups for a gain in visual acuity letter score of 15 or more at 12 months, though an early positive treatment response of a gain in visual acuity letter score of 15 or more was observed at month 4 in the 4-mg triamcinolone group compared with the 1-mg triamcinolone and standard care groups. Additionally, at month 12 there was a similar gain of 4 to 5 in mean visual acuity letter score from baseline and a similar likelihood of a loss in visual acuity letter score of 15 or more in all 3 treatment groups. After month 12 and through month 36, the mean improvement from baseline visual acuity letter score was greatest in the standard care group compared with the 2 triamcinolone groups.
With respect to OCT-measured center point thickness, all 3 groups showed a decrease from baseline to month 12. Analogous to the visual acuity results, only at month 4 did the 4-mg triamcinolone group demonstrate a greater treatment effect on center point thickness than the 1-mg and standard care groups; at all other times investigated (months 8-36), the standard care group demonstrated the greatest overall median decrease in center point thickness from baseline. There was only moderate correlation between changes from baseline in OCT-measured thickness and changes from baseline in visual acuity letter score, which is consistent with previously reported observations of a modest correlation between OCT-measured thickness and visual acuity in patients with macular edema secondary to CRVO,52 BRVO,52 or diabetic retinopathy.55
The rates of adverse events were higher in the 4-mg triamcinolone group compared with the 1-mg and standard care groups. There was a dose-dependent higher frequency of initiating IOP-lowering medications in the triamcinolone groups compared with the standard care group. No participant was treated with trabeculectomy or tube shunt surgery through 12 months. The proportion of eyes that were phakic at baseline and had new-onset lens opacity or progression of an existing opacity through 12 months based on assessment at the clinical center was greater in the 2 triamcinolone groups compared with the standard care group. Most cataract surgeries were performed during the second year of the study and occurred with the highest frequency in the 4-mg group. Intravitreal silicone oil droplets were observed in 23% of eyes treated with intravitreal triamcinolone, but no adverse effects were attributable to the silicone oil droplets. Intravitreal silicone oil associated with the use of siliconized syringes is a recognized occurrence.56,57
The rate of infectious endophthalmitis in the SCORE-BRVO trial was 0.1% (1 of 914) per injection. The lack of any report of noninfectious endophthalmitis in the SCORE-BRVO trial, which included an evaluation of all participants within 1 week postinjection to evaluate for complications, may be due to the preservative-free, micronized, nondispersive triamcinolone formulation (the triamcinolone crystals were suspended in a hyaluronate matrix gel) used in this trial.
Cataract development is unlikely to have masked a significant treatment benefit of triamcinolone over standard care in the overall results. First, an analysis of eyes pseudophakic at baseline demonstrated no statistically significant difference among the treatment groups with respect to change in visual acuity over time. Second, the protocol specified that cataract surgery was to be performed at any time that such surgery was indicated clinically, a point that was emphasized in the SCORE Study by a memorandum sent to all study investigators, encouraging them to facilitate cataract surgery for any study participants in whom this was indicated clinically. Third, most cataract surgeries in SCORE-BRVO participants occurred between 12 and 24 months, suggesting that this is when the cataracts became clinically significant. Despite more cataract extractions having been performed between 12 and 24 months in the 4-mg triamcinolone group than in the other 2 groups, there was no significant difference in visual acuity outcome that showed a benefit of triamcinolone over standard care at any time other than month 4. Lastly, the results from the SCORE-CRVO trial demonstrate a strong treatment effect of intravitreal triamcinolone relative to observation. Thus, in the SCORE-BRVO trial, it is unlikely that the triamcinolone groups fared no better than the standard care group because of a masking of treatment effect by cataract (as there is no reason to suspect that participants with BRVO are more susceptible to cataract than participants with CRVO) but rather as a result of triamcinolone being compared with an equally efficacious therapy—grid photocoagulation.
It is unlikely that a triamcinolone benefit over standard care went undetected, particularly because the trends favored the standard care group. One sensitivity analysis shows that, even after imputing favorable outcomes for all the missing month 12 outcomes in the 2 triamcinolone groups and unfavorable outcomes in the standard care group, the month 12 results would still not favor the triamcinolone groups, providing more evidence of no treatment difference among the 3 study groups. Other sensitivity analyses produced similar results. The SCORE Study protocol encouraged frequent retreatment (see the “Methods” section). Thus, participants in this study were not likely to have been undertreated.
Twelve-month data are important in a disease with an acute nature, and 12 months may be sufficient follow-up, but comparison of results among the study groups after 12 months is another topic of interest; a shortcoming of the current study is the lack of definitive data at these later scheduled visits, owing either to missed visits or to participants not having long enough follow-up to complete the 24- or 36-month visits because of the common closeout. However, the 128 participants in the SCORE-BRVO trial observed at 36 months, 82 of whom were in the triamcinolone arms, exceed the size of the cohort of participants studied in the BVOS (group III),1 which followed 78 eyes (including 43 eyes treated with grid photocoagulation and 35 untreated) for as long as 36 months. In the SCORE-BRVO trial, the data available in the month 36 visit do not suggest a treatment benefit of intravitreal triamcinolone relative to standard care; in fact, the trend in the SCORE-BRVO trial at month 36 suggests that standard care may be superior to intravitreal triamcinolone (Table 5 and Figure 2).
The month 12 results of the SCORE-BRVO trial demonstrate that treatment with 1 mg of triamcinolone, 4 mg oftriamcinolone,orstandardcareishistoricallycomparable with what was observed in the treated arm of the BVOS (grid photocoagulation) and that all 3 treatments are likely associated with a better visual outcome than the natural history of macular edema secondary to BRVO as demonstrated in the untreated arm of the BVOS. The results of the SCORE-BRVO trial also underscore the importance of grid photocoagulation in managing vision loss due to macular edema associated with BRVO across a wide range of visual acuities and retinal thicknesses, even in eyes with prior grid photocoagulation treatment for macular edema secondary to BRVO. It is important to note that an eye with prior grid photocoagulation was enrolled in this trial only if the investigatorjudgedtheeyetohavethepotentialtobenefitfrom additional photocoagulation. Notably, whether or not laser photocoagulation was performed prior to randomization, there was no difference in treatment effect among the 3 groups, though the number of participants entering the trial having undergone grid photocoagulation was small (Table 6). Given the equal efficacy for visual acuity (gain, mean change, and loss), the superior adverse event profile of standard care over 4 mg of triamcinolone and the at least theoretical safety advantage of standard care over 1 mg of intravitreal triamcinolone, standard care is recommended over either dose of intravitreal triamcinolone.
Despite the findings from the SCORE-BRVO trial, there is a need for improved treatments in the future, because, at 12 months, less than one-third of study eyes in the standard care group had a gain in visual acuity letter score of 15 or more, and about half of study eyes in the standard care group still had central retinal thickening. The fact that the 4-mg triamcinolone group had a greater positive treatment response on visual acuity and retinal thickness at 4 months, while the standard care group demonstrated a more delayed but progressive improvement in visual acuity and retinal thickness over time, raises the possibility that combining grid photocoagulation with intravitreal triamcinolone may produce a greater benefit than either of these treatments alone; this could be evaluated in a future clinical trial.
In conclusion, there was no significant difference in terms of visual acuity outcomes (gain, mean change, or loss) among the groups treated with standard care, 1 mg of intravitreal triamcinolone, or 4 mg of intravitreal triamcinolone for vision loss associated with macular edema secondary to BRVO at 12 months. The results of the SCORE-BRVO trial suggest that standard care and intravitreal triamcinolone in either the 1-mg or 4-mg dose are all likely associated with a better visual outcome than the natural history of macular edema secondary to BRVO. The rates of adverse events were higher in the 4-mg triamcinolone group than in the standard care group. The rates of adverse events with respect to cataract surgery and elevated IOP were similar between the standard care and 1-mg groups, but the potential for added risks of procedure-related complications in the 1-mg group (as exemplified by the 1 case of endophthalmitis reported in the 4-mg group) suggests a superior safety profile for the standard care group. Thus, current study results (up to 12 months and possibly up to 36 months) support grid photocoagulation as the continued standard of care for patients with decreased visual acuity associated with macular edema secondary to BRVO who have similar characteristics to the cohort in this clinical trial. The results of this trial also support the idea that grid photocoagulation, as applied in the current study, should be the benchmark against which other treatments for vision loss associated with macular edema secondary to BRVO are compared in clinical trials.
Acknowledgments
Funding/Support: This study was supported by the National Eye Institute (National Institutes of Health, Department of Health and Human Services)grants 5U10EY014351, 5U10EY014352, and 5U10EY014404; and in part by Allergan Inc.
Footnotes
Financial Disclosure: None reported.
REFERENCES
- 1.The Branch Vein Occlusion Study Group Argon laser photocoagulation for macular edema in branch vein occlusion. Am J Ophthalmol. 1984;98(3):271–282. doi: 10.1016/0002-9394(84)90316-7. [DOI] [PubMed] [Google Scholar]
- 2.Mitchell P, Smith W, Chang A. Prevalence and associations of retinal vein occlusion in Australia: The Blue Mountains Eye Study. Arch Ophthalmol. 1996;114(10):1243–1247. doi: 10.1001/archopht.1996.01100140443012. [DOI] [PubMed] [Google Scholar]
- 3.Klein R, Moss SE, Meuer SM, Klein BEK. The 15-year cumulative incidence of retinal vein occlusion: The Beaver Dam Eye Study. Arch Ophthalmol. 2008;126(4):513–518. doi: 10.1001/archopht.126.4.513. [DOI] [PubMed] [Google Scholar]
- 4.Klein R, Klein BEK, Lee KE, Cruickshanks KJ, Gangnon RE. Changes in visual acuity in a population over a 15-year period: The Beaver Dam Eye Study. Am J Ophthalmol. 2006;142(4):539–549. doi: 10.1016/j.ajo.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 5.Chang MA, Fine HF, Bass E, et al. Patients' preferences in choosing therapy for retinal vein occlusions. Retina. 2007;27(6):789–797. doi: 10.1097/IAE.0b013e31802c0a34. [DOI] [PubMed] [Google Scholar]
- 6.McIntosh RL, Mohamed Q, Saw SM, Wong TY. Interventions for branch retinal vein occlusion: an evidence-based systematic review. Ophthalmology. 2007;114(5):835–854. doi: 10.1016/j.ophtha.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 7.Kumagai K, Furukawa M, Ogino N, Uemura A, Larson E. Long-term outcomes of vitrectomy with or without arteriovenous sheathotomy in branch retinal vein occlusion. Retina. 2007;27(1):49–54. doi: 10.1097/01.iae.0000221996.77421.69. [DOI] [PubMed] [Google Scholar]
- 8.Shah GK, Sharma S, Fineman MS, Federman J, Brown MM, Brown GC. Arteriovenous adventitial sheathotomy for the treatment of macular edema associated with branch retinal vein occlusion. Am J Ophthalmol. 2000;129(1):104–106. doi: 10.1016/s0002-9394(99)00287-1. [DOI] [PubMed] [Google Scholar]
- 9.Opremcak EM, Bruce RA. Surgical decompression of branch retinal vein occlusion via arteriovenous crossing sheathotomy: a prospective review of 15 cases. Retina. 1999;19(1):1–5. doi: 10.1097/00006982-199901000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Fekrat S, Goldberg MF, Finkelstein D. Laser-induced chorioretinal venous anastomosis for nonischemic central or branch retinal vein occlusion. Arch Ophthalmol. 1998;116(1):43–52. doi: 10.1001/archopht.116.1.43. [DOI] [PubMed] [Google Scholar]
- 11.Bavbek T, Yenice O, Toygar O. Problems with attempted chorioretinal venous anastomosis by laser for nonischemic CRVO and BRVO. Ophthalmologica. 2005;219(5):267–271. doi: 10.1159/000086109. [DOI] [PubMed] [Google Scholar]
- 12.Costa RA, Jorge R, Calucci D, Melo LA, Jr, Cardillo JA, Scott IU. Intravitreal bevacizumab (Avastin) for central and hemicentral retinal vein occlusions: IBeVO study. Retina. 2007;27(2):141–149. doi: 10.1097/IAE.0b013e31802eff83. [DOI] [PubMed] [Google Scholar]
- 13.Cekic O, Chang S, Tseng JJ, et al. Intravitreal triamcinolone treatment for macular edema associated with central retinal vein occlusion and hemiretinal vein occlusion. Retina. 2005;25(7):846–850. doi: 10.1097/00006982-200510000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Ozkiris A, Evereklioglu C, Erkilic K, Ilhan O. The efficacy of intravitreal triamcinolone acetonide on macular edema in branch retinal vein occlusion. Eur J Ophthalmol. 2005;15(1):96–101. doi: 10.1177/112067210501500115. [DOI] [PubMed] [Google Scholar]
- 15.Lee H, Shah GK. Intravitreal triamcinolone as primary treatment of cystoid macular edema secondary to branch retinal vein occlusion. Retina. 2005;25(5):551–555. doi: 10.1097/00006982-200507000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Krepler K, Ergun E, Sacu S, et al. Intravitreal triamcinolone acetonide in patients with macular oedema due to branch retinal vein occlusion: a pilot study. Acta Ophthalmol Scand. 2005;83(5):600–604. doi: 10.1111/j.1600-0420.2005.00490.x. [DOI] [PubMed] [Google Scholar]
- 17.Hayashi K, Hayashi H. Intravitreal versus retrobulbar injections of triamcinolone for macular edema associated with branch retinal vein occlusion. Am J Ophthalmol. 2005;139(6):972–982. doi: 10.1016/j.ajo.2004.12.087. [DOI] [PubMed] [Google Scholar]
- 18.Jonas JB, Akkoyun I, Kamppeter B, Kreissig I, Degenring RF. Branch retinal vein occlusion treated by intravitreal triamcinolone acetonide. Eye. 2005;19(1):65–71. doi: 10.1038/sj.eye.6701395. [DOI] [PubMed] [Google Scholar]
- 19.Cekiç O, Chang S, Tseng JJ, et al. Intravitreal triamcinolone injection for treatment of macular edema secondary to branch retinal vein occlusion. Retina. 2005;25(7):851–855. doi: 10.1097/00006982-200510000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Karacorlu M, Ozdemir H, Karacorlu SA. Resolution of serous macular detachment after intravitreal triamcinolone acetonide treatment of patients with branch retinal vein occlusion. Retina. 2005;25(7):856–860. doi: 10.1097/00006982-200510000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Yepremyan M, Wertz FD, Tivnan T, Eversman L, Marx JL. Early treatment of cystoid macular edema secondary to branch retinal vein occlusion with intravitreal triamcinolone acetonide. Ophthalmic Surg Lasers Imaging. 2005;36(1):30–36. [PubMed] [Google Scholar]
- 22.Chen SDM, Sundaram V, Lochhead J, Patel CK. Intravitreal triamcinolone for the treatment of ischemic macular edema associated with branch retinal vein occlusion. Am J Ophthalmol. 2006;141(5):876–883. doi: 10.1016/j.ajo.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 23.Özkiris A, Evereklioglu C, Erkilic K, Dogan H. Intravitreal triamcinolone acetonide for treatment of persistent macular oedema in branch retinal vein occlusion. Eye. 2006;20(1):13–17. doi: 10.1038/sj.eye.6701803. [DOI] [PubMed] [Google Scholar]
- 24.Cheng KC, Wu W. Intravitreal triamcinolone acetonide for patients with macular edema due to branch retinal vein occlusion. Kaohsiung J Med Sci. 2006;22(7):321–330. doi: 10.1016/S1607-551X(09)70318-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oh JY, Seo JH, Ahn JK, Heo JW, Chung H. Early versus late intravitreal triamcinolone acetonide for macular edema associated with branch retinal vein occlusion. Korean J Ophthalmol. 2007;21(1):18–20. doi: 10.3341/kjo.2007.21.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cakir M, Dogan M, Bayraktar Z, et al. Efficacy of intravitreal triamcinolone for the treatment of macular edema secondary to branch retinal vein occlusion in eyes with or without grid laser photocoagulation. Retina. 2008;28(3):465–472. doi: 10.1097/IAE.0b013e318154b9d1. [DOI] [PubMed] [Google Scholar]
- 27.Aiello LP, Bursell SE, Clermont A, et al. Vascular endothelial growth factor-induced retinal permeability is mediated by protein kinase C in vivo and suppressed by an orally effective beta-isoform-selective inhibitor. Diabetes. 1997;46(9):1473–1480. doi: 10.2337/diab.46.9.1473. [DOI] [PubMed] [Google Scholar]
- 28.Antonetti DA, Barber AJ, Hollinger LA, Wolpert EB, Gardner TW. Vascular endothelial growth factor induces rapid phosphorylation of tight junction proteins occludin and zonula occluden 1: a potential mechanism for vascular permeability in diabetic retinopathy and tumors. J Biol Chem. 1999;274(33):23463–23467. doi: 10.1074/jbc.274.33.23463. [DOI] [PubMed] [Google Scholar]
- 29.Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF. Tumor cells secrete a vascular permeability factor (VPF) that promotes accumulation of ascites fluid. Science. 1983;219(4587):983–985. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- 30.Vinores SA, Youssri AI, Luna JD, et al. Upregulation of vascular endothelial growth factor in ischemic and non-ischemic human and experimental retinal disease. Histol Histopathol. 1997;12(1):99–109. [PubMed] [Google Scholar]
- 31.Pe'er J, Folberg R, Itin A, Gnessin H, Hemo I, Keshet E. Vascular endothelial growth factor upregulation in human central retinal vein occlusion. Ophthalmology. 1998;105(3):412–416. doi: 10.1016/S0161-6420(98)93020-2. [DOI] [PubMed] [Google Scholar]
- 32.Zhang X, Bao S, Lai D, Rapkins RW, Gillies MC. Intravitreal triamcinolone acetonide inhibits breakdown of the blood-retinal barrier through differential regulation of VEGF-A and its receptors in early diabetic rat retinas. Diabetes. 2008;57(4):1026–1033. doi: 10.2337/db07-0982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang K, Wang Y, Gao L, Li X, Li M, Guo J. Dexamethasone inhibits leukocyte accumulation and vascular permeability in retina of streptozotocin-induced diabetic rats via reducing vascular endothelial growth factor and intercellular adhesion molecule-1 expression. Biol Pharm Bull. 2008;31(8):1541–1546. doi: 10.1248/bpb.31.1541. [DOI] [PubMed] [Google Scholar]
- 34.Lee HB, Pulido JS, McCannel CA, Buettner H. Role of inflammation in retinal vein occlusion. Can J Ophthalmol. 2007;42(1):131–133. doi: 10.1139/i06-101. [DOI] [PubMed] [Google Scholar]
- 35.Flynn HW, Jr, Scott IU. Intravitreal triamcinolone acetonide for macular edema associated with diabetic retinopathy and venous occlusive disease: it's time for clinical trials. Arch Ophthalmol. 2005;123(2):258–259. doi: 10.1001/archopht.123.2.258. [DOI] [PubMed] [Google Scholar]
- 36.Blumenkranz MS. New therapy for central retinal vein occlusion: are intravitreal steroids a possible answer? Arch Ophthalmol. 2005;123(2):259–261. doi: 10.1001/archopht.123.2.259. [DOI] [PubMed] [Google Scholar]
- 37.The SCORE Study Research Group A randomized trial comparing the efficacy and safety of intravitreal triamcinolone with observation to treat vision loss associated with macular edema secondary to central retinal vein occlusion: The Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) Study Report 5. Arch Ophthalmol. 2009;127(9):1101–1114. doi: 10.1001/archophthalmol.2009.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beck RW, Moke PS, Turpin AH, et al. A computerized method of visual acuity testing: adaptation of the Early Treatment of Diabetic Retinopathy Study testing protocol. Am J Ophthalmol. 2003;135(2):194–205. doi: 10.1016/s0002-9394(02)01825-1. [DOI] [PubMed] [Google Scholar]
- 39.Age-Related Eye Disease Study Research Group The age-related eye disease study (AREDS) system for classifying cataracts from photographs: AREDS Report No. 4. Am J Ophthalmol. 2001;131(2):167–175. doi: 10.1016/s0002-9394(00)00732-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chieh JJ, Roth DB, Liu M, et al. Intravitreal triamcinolone acetonide for diabetic macular edema. Retina. 2005;25(7):828–834. doi: 10.1097/00006982-200510000-00002. [DOI] [PubMed] [Google Scholar]
- 41.Kim JE, Pollack JS, Miller DG, Mittra RA, Spaide RF, Isis Study Group ISIS-DME: a prospective, randomized, dose-escalation intravitreal steroid injection study for refractory diabetic macular edema. Retina. 2008;28(5):735–740. doi: 10.1097/IAE.0b013e318163194c. [DOI] [PubMed] [Google Scholar]
- 42.Jonas JB, Kreissig I, Spandau UH, Harder B. Infectious and noninfectious endophthalmitis after intravitreal high-dosage triamcinolone acetonide. Am J Ophthalmol. 2006;141(3):579–580. doi: 10.1016/j.ajo.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 43.Jonas JB, Kreissig I, Degenring RF. Intravitreal triamcinolone acetonide as treatment of macular edema in central retinal vein occlusion. Graefes Arch Clin Exp Ophthalmol. 2002;240(9):782–783. doi: 10.1007/s00417-002-0529-0. [DOI] [PubMed] [Google Scholar]
- 44.Then Bergh F, Grasser A, Trenkwalder C, Backmund H, Holsboer F, Rupprecht R. Binding characteristics of the glucocorticoid receptor in peripheral blood lymphocytes in multiple sclerosis. J Neurol. 1999;246(4):292–298. doi: 10.1007/s004150050349. [DOI] [PubMed] [Google Scholar]
- 45.Schottelius A, Wedel S, Weltrich R, et al. Higher expression of glucocorticoid receptor in peripheral mononuclear cells in inflammatory bowel disease. Am J Gastroenterol. 2000;95(8):1994–1999. doi: 10.1111/j.1572-0241.2000.02188.x. [DOI] [PubMed] [Google Scholar]
- 46.Chen SD, Lochhead J, Patel CK. Diffuse intraocular dispersion of triamcinolone particles as a cause of sterile endophthalmitis [letter] Arch Ophthalmol. 2004;122(11):1733. doi: 10.1001/archopht.122.11.1733-a. [DOI] [PubMed] [Google Scholar]
- 47.Moshfeghi DM, Kaiser PK, Bakri SJ, et al. Presumed sterile endophthalmitis following intravitreal triamcinolone acetonide injection. Ophthalmic Surg Lasers Imaging. 2005;36(1):24–29. [PubMed] [Google Scholar]
- 48.Ip MS, Oden NL, Scott IU, et al. SCORE Study Investigator Group SCORE Study Report 3: study design and baseline characteristics. Ophthalmology. doi: 10.1016/j.ophtha.2009.03.022. published online ahead of print July 18, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Massin P, Erginay A, Haouchine B, Mehidi AB, Paques M, Gaudric A. Retinal thickness in healthy and diabetic subjects measures using optical coherence tomography mapping software. Eur J Ophthalmol. 2002;12(2):102–108. doi: 10.1177/112067210201200205. [DOI] [PubMed] [Google Scholar]
- 50.Fundus Photograph Reading Center Imaging Procedures for the Standard Care vs COrticosteroid for REtinal Vein Occlusion (SCORE) Study. National Eye Institute; Bethesda, MD: 2008. (NTIS order No. PB2008-113740). [Google Scholar]
- 51.Domalpally A, Blodi BA, Scott IU, et al. SCORE Study Investigator Group SCORE Study Report 4: grading methodology for optical coherence tomography images. Arch Ophthalmol. 2009 doi: 10.1001/archophthalmol.2009.277. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scott IU, VanVeldhuisen PC, Oden NL, et al. SCORE Study Investigator Group SCORE Study Report 1: baseline association between central retinal thickness and visual acuity in patients with retinal vein occlusion. Ophthalmology. 2009;116(3):504–512. doi: 10.1016/j.ophtha.2008.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marcus R, Peritz E, Gabriel KR. On closed testing procedures with special reference to ordered analysis of variance. Biometrika. 1976;63:655–660. [Google Scholar]
- 54.Scott IU, Oden NL, VanVeldhuisen PC, et al. SCORE Study Report 7: incidence of intravitreal silicone oil droplets associated with staked-on versus luer cone syringe design. Am J Ophthalmol. doi: 10.1016/j.ajo.2009.06.004. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Browning DJ, Glassman AR, Aiello LP, et al. Diabetic Retinopathy Clinical Research Network. Relationship between optical coherence tomography-measured central retinal thickness and visual acuity in diabetic macular edema. Ophthalmology. 2007;114(3):525–536. doi: 10.1016/j.ophtha.2006.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Freund KB, Laud K, Eandi CM, Spaide RF. Silicone oil droplets following intravitreal injection. Retina. 2006;26(6):701–703. doi: 10.1097/01.iae.0000223177.08438.2b. [DOI] [PubMed] [Google Scholar]
- 57.Bakri SJ, Ekdawi NS. Intravitreal silicone oil droplets after intravitreal drug injections. Retina. 2008;28(7):996–1001. doi: 10.1097/IAE.0b013e31816c6868. [DOI] [PubMed] [Google Scholar]