Abstract
Although Fc receptors (FcRs) for switched immunoglobulin (Ig) isotypes have been extensively characterized, FcR for IgM (FcμR) has defied identification. By retroviral expression and functional cloning, we have identified a complementary DNA (cDNA) encoding a bona fide FcμR in human B-lineage cDNA libraries. FcμR is defined as a transmembrane sialoglycoprotein of ∼60 kD, which contains an extracellular Ig-like domain homologous to two other IgM-binding receptors (polymeric Ig receptor and Fcα/μR) but exhibits an exclusive Fcμ-binding specificity. The cytoplasmic tail of FcμR contains conserved Ser and Tyr residues, but none of the Tyr residues match the immunoreceptor tyrosine-based activation, inhibitory, or switch motifs. Unlike other FcRs, the major cell types expressing FcμR are adaptive immune cells, including B and T lymphocytes. After antigen-receptor ligation or phorbol myristate acetate stimulation, FcμR expression was up-regulated on B cells but was down-modulated on T cells, suggesting differential regulation of FcμR expression during B and T cell activation. Although this receptor was initially designated as Fas apoptotic inhibitory molecule 3, or TOSO, our results indicate that FcμR per se has no inhibitory activity in Fas-mediated apoptosis and that such inhibition is only achieved when anti-Fas antibody of an IgM but not IgG isotype is used for inducing apoptosis.
IgM is the first Ig isotype to appear during phylogeny, ontogeny, and the immune response, and has been suggested as a first line of host defense to pathogens. Serum levels of IgM in mice raised under germ-free conditions are similar to those of mice maintained under conventional housing conditions (Haury et al., 1997). Thus, production of preimmune “natural” IgM antibody, primarily by CD5+ B-1 cells, is likely to be regulated by mechanisms unrelated to exogenous antigen specificity. In contrast, antigen-induced IgM production is mainly derived from conventional B-2 cells. The importance of both natural and induced IgM antibodies in immune responses has been established through recent studies of a mutant mouse strain in which B cells expressing surface IgM and IgD could switch and secrete IgG and IgA, but not IgM, antibodies (Boes et al., 1998a; Ehrenstein et al., 1998). These mutant mice had impaired control of viral and bacterial infections because of inefficient induction of protective IgG antibody responses (Boes et al., 1998b; Ochsenbein et al., 1999; Baumgarth et al., 2000). Thus, these findings indicate that IgM antibody can profoundly influence immune responses and suggest that some of these effects are mediated by binding to effector molecules such as Fc receptor (FcR) and complement via its carboxyl-constant regions.
Several FcRs, namely FcR for IgG (FcγRI/CD64, FcγRII/CD32, and FcγRIII/CD16), IgE (FcϵRI), and IgA (FcαR/CD89), have been characterized at both the protein and nucleic acid levels (Ravetch and Nimmerjahn, 2008). In contrast, FcR for IgM (FcμR) has defied genetic identification, although the existence of FcμR on B, T, NK, and phagocytic cells has been suggested for >30 yr with variable and conflicting results (Basten et al., 1972; Moretta et al., 1975; Lamon et al., 1976; Ferrarini et al., 1977; Moretta et al., 1977; Pichler and Knapp, 1977; Santana, 1977; Haegert, 1979; Reinherz et al., 1980; Uher et al., 1981; Sanders et al., 1987; Mathur et al., 1988a; Mathur et al., 1988b; Ohno et al., 1990; Nakamura et al., 1993; Pricop et al., 1993; Rabinowich et al., 1996). In addition to the aforementioned classical FcRs, several other receptors expressed on unique cell types also bind Ig molecules: (a) neonatal FcR for IgG (FcRn) on intestinal epithelium, placenta, and endothelium (Roopenian and Akilesh, 2007); (b) low affinity FcϵR (FcϵRII/CD23) on B cells and macrophages (Conrad, 1990); (c) polymeric Ig receptor (pIgR) on mucosal epithelium (Kaetzel, 2005); and (d) FcR for IgA and IgM (Fcα/μR; Shibuya et al., 2000) on follicular dendritic cells (Kikuno et al., 2007). Although the latter two receptors bind polymeric IgA and IgM, their biochemical features and cellular distribution are distinct from those of the FcμR that we have previously characterized on B and T cells in humans (Sanders et al., 1987; Ohno et al., 1990; Nakamura et al., 1993). In this paper, we have identified a cDNA encoding a bona fide FcμR that is defined as transmembrane protein of ∼60 kD expressed predominantly on B and T lymphocytes.
RESULTS
Molecular cloning of the FcμR
Our previous cellular and biochemical studies provided strong evidence for the existence of an FcμR that is expressed constitutively on chronic lymphocytic leukemia (CLL) B cells and inducibly on pre–B cell lines (Sanders et al., 1987; Ohno et al., 1990). To identify the gene encoding the putative FcμR, two different cDNA libraries from CLL B cells and a PMA-activated 697 pre–B cell line were constructed in a retroviral expression vector and then introduced into mouse T cell line BW5147. Transduced cells exhibiting IgM binding were enriched by FACS and subcloned. Many of the single cell–derived subclones from both cDNA libraries bound IgM (Fig. 1 A). RT-PCR analysis revealed that a DNA fragment of ∼2 kb was specifically amplified only from IgM-binding subclones (Fig. 1 B), and their nucleotide sequence analyses defined an identical 1,173-bp open reading frame (CLL- and PMA-activated 697 pre–B cell–derived FcμR cDNA available from GenBank/EMBL/DDBJ under accession nos. GQ160900 and GQ160901, respectively; Fig. S1). Basic local alignment search technique database analysis revealed that the isolated FcμR cDNA was identical to that of the previously described human Fas apoptotic inhibitory molecule 3 (FAIM3; available from GenBank/EMBL/DDBJ under accession no. NM_005449), except for one nucleotide difference at a position reported as a synonymous single nucleotide polymorphism. FAIM3 was identified in a similar retroviral cDNA library–based functional assay as a potent inhibitor of Fas/CD95-induced apoptotic signaling and was originally designated as TOSO, after a Japanese liquor drunk on New Year’s day to celebrate long life and eternal youth (Hitoshi et al., 1998). Interestingly, however, the apoptosis in this functional assay was induced by ligation of Fas with a mouse mAb of IgM isotype (CH11). This immediately raised the possibility that the CH11 mAb bound to the Fas via its Fabμ portion and to the FAIM3/TOSO via its Fcμ portion, thereby bringing them in close physical proximity in a process reminiscent of that described in FcγRIIb-mediated inhibition of BCR signaling by intact IgG anti-μ antibodies (Tony and Schimpl, 1980; Ravetch and Nimmerjahn, 2008).
Figure 1.
Isolation of IgM-binding subclones and identification of cDNA inserts. (A) Cells transduced by the retroviral expression construct containing CLL-derived (top) or PMA-activated 697 pre–B cell–derived (bottom) cDNA libraries were enriched for IgM binding by FACS and subcloned for limiting dilution. Three representative subclones from each library are shown for their IgM-binding activity or lack of binding, as determined by flow cytometry. (B) Agarose gel electrophoresis analysis of RT-PCR products. RNA isolated from nontransduced control BW5147 T cells (lane 1) and from IgM-binding (lanes 3–5 and 7–9) or IgM-nonbinding (lanes 2 and 6) subclones from CLL-derived (lanes 2–5) and PMA-activated 697 pre–B cell–derived (lanes 6–9) cDNA libraries were subjected to RT-PCR as described in Materials and methods. Amplified products were electrophoresed in 0.7% agarose and stained with ethidium bromide. Lane 10 is a PCR control without a first-strand cDNA template. HindIII-digested λ DNA was used as a size marker. The experiments were performed once for A and twice for B.
The FAIM3/TOSO gene encodes a bona fide FcμR
To reconcile the conflicting functions of FAIM3/TOSO and our functionally defined FcμR, an ∼1.2-kb cDNA containing the protein coding region of FAIM3/TOSO/FcμR was PCR amplified from PMA-activated 697 pre–B cells, subcloned along with a GFP cDNA into the bicistronic retroviral vector and then transduced into BW5147 T cells. The resultant GFP+ transductants clearly exhibited IgM binding (Fig. S2), thereby confirming that FAIM3/TOSO is an IgM receptor. The FcμR cDNA is predicted to encode a 390-aa type I membrane protein (17-aa signal peptide, 236-aa extracellular region, 19-aa transmembrane segment, and 118-aa cytoplasmic tail). The N-terminal half of the extracellular region contains a single V-set Ig-like domain with homology to both the pIgR and Fcα/µR (see next section), but the remaining extracellular region has no identifiable domain features (Fig. S3). There are no N-linked glycosylation motifs in the extracellular region, consistent with our previous biochemical characterization of the FcμR (Ohno et al., 1990). The mature core peptide is predicted to have an Mr of ∼41 kD and an isoelectric point (pI) of ∼9.9.
A quantitative inhibition immunofluorescence assay with various Ig isotypes and IgM fragments as inhibitors revealed that IgM and its Fc5µ fragments consisting mostly of Cμ3/Cμ4 domains inhibited the binding of a biotin-labeled human IgM to FcμR+ BW5147 T cells in a dose-dependent manner, whereas the Fabµ fragments and other human Ig isotypes (IgG1-4, IgA1,2, IgD, and IgE) did not, thereby confirming the Fcμ specificity of the FcμR (Fig. 2 A). Fig. 2 B shows a representative inhibition profile for IgM binding with an eightfold excess of inhibitors. The inability of FcμR to bind polymeric IgA clearly indicates that FcμR is distinct from pIgR and Fcα/µR, both of which are shown to bind IgM and polymeric IgA. Moreover, the lack of binding to aggregated IgG further confirms the unique IgM isotype specificity of this receptor. Interestingly, mouse IgM bound better to the human FcμR than human IgM, and essentially identical FcμR binding was observed with IgMκ and IgMλ ligands irrespective of the presence of Ca2+/Mg2+. The affinity of IgM/FcμR binding was estimated by Scatchard plot analysis using 125I-labeled human IgM and FcμR+ BW5147 T cells. Assuming a 1:1 stoichiometry of pentameric IgM ligand to FcμR, this analysis revealed a strikingly high binding affinity of 10.8 ± 9.2 nM (mean ± SD from four experiments with two different human IgM myeloma proteins). Pretreatment of FcμR+ cells with neuraminidase slightly enhanced IgM binding, suggesting a role of sialic acid in this interaction, as reported previously by others (Pricop et al., 1993). Higher concentrations (>100-fold) were required for binding of IgM monomers to the FcμR+ cells than IgM pentamers, indicating the importance of IgM ligand configuration (Fig. 2 C). Collectively, these results indicate that the previously identified FAIM3/TOSO is an authentic FcμR with exclusive and high affinity binding specificity for the Fc portion of IgM.
Figure 2.
Evaluation of the Ig isotype specificity of the FcμR. (A) FcμR cDNA–transduced BW5147 T cells were preincubated with various concentrations of inhibitor paraproteins of human origin (IgM, IgG1-4, IgA1-2, IgD, IgE, Fabμ, and Fc5μ) and incubated with 4 µg/ml of biotin-labeled human IgMκ. Bound biotinylated IgM was detected by addition of PE-labeled SA. Stained cells were analyzed by flow cytometry. Results are expressed as the percent mean fluorescence intensity (MFI) estimated as follows: 100 × ([X of IgM binding with inhibitors − X of background control]/[X of IgM binding without inhibitors − X of background control]), where X indicates the MFI values. Because there were no significant differences among each subclass of IgG and IgA, the results from all four IgG subclasses and two IgA subclasses have been combined as IgG and IgA, and the mean values are presented for simplicity. (B) Representative binding inhibition profiles. FcμR+ BW5147 T cells were incubated first with an eightfold excess of the indicated inhibitor proteins and then with 4 µg/ml of biotin-labeled human IgMκ. The dotted, dashed, and continuous lines indicate the immunofluorescence profiles for background controls, IgM binding without inhibitors, and IgM binding with the test inhibitors, respectively. (C) Control and FcμR+ BW5147 cells were incubated with culture supernatants containing the indicated concentrations of monomeric (m) or pentameric (p) IgM anti–mouse RBC mAb before developing with biotin-labeled anti–mouse κ mAb and APC-SA. These experiments were performed at least twice.
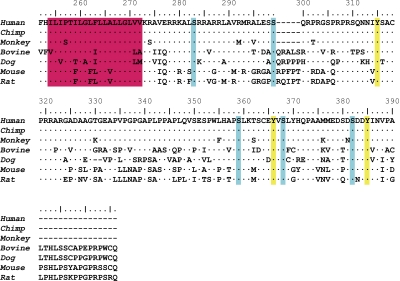
The Ig domain of FcμR is similar but distantly related to that of pIgR and Fcα/µR
FCMR is a single copy gene located on chromosome 1q32.2, adjacent to two other IgM-binding receptor genes, PIGR and FCAMR. The Ig-like domain of FAIM3/TOSO/FcμR is thought to be involved in the binding of agonistic IgM anti-Fas mAb (Hitoshi et al., 1998). A comparison of the protein sequence of the Ig-binding domains of FcμR, pIgR, and Fcα/μR to the pIgR structural data reported by Hamburger et al. (2004) provided some potential insight into ligand specificity (Fig. 3). In addition to a disulfide bond between Cys22 and Cys92 linking the two β sheets (B and F strands), a second disulfide bond between Cys38 and Cys46 linking the C and C′ strands is also conserved in all three receptors. Arg63 and Asp86 are also completely conserved, but Trp37 is found only in the pIgR and Fcα/μR. Several other residues (Gly6, Tyr24, Val29, Arg31, Lys35, Tyr55, and Leu101) are also conserved in pIgR and Fcα/μR but not in FcμR. A major difference between FcμR and the other two receptors is in the CDR1 region. The CDR1 of the pIgR from six different species consists of 9 aa (Pro25 to Thr33), and this is also the case in the Fcα/μR from two different species. In contrast, the corresponding region of the FcμR from seven different species consists of 5 aa and has a noncharged residue (Met, Leu, or Thr) at the position corresponding to Arg31, which has been shown to be solvent exposed and possibly to interact directly with polymeric IgA in the human pIgR (Hamburger et al., 2004). These results suggest a structural basis for the distinct mode of IgM interactions with FcμR versus pIgR and Fcα/μR.
Figure 3.
aa sequence alignment of IgM-binding receptors. The Ig-binding domains of pIgR, Fcα/μR, and FcμR from several species were aligned using the CLUSTAL W multiple alignment program (Thompson et al., 1994). aa identity is indicated by dots and gaps are indicated by dashes. Residues conserved in all three receptors and in pIgR and Fcα/μR are highlighted in yellow and red, respectively. The numbers indicate the aa position from the N terminus of the Ig-binding domain of human pIgR. These sequences are available from GenBank/EMBL/DDBJ under the following accession nos.: pIgR of human (hu; P01833), rabbit (rb; P01832), mouse (mo; O70570), rat (rt; P15083), bovine (bo; P81265), and chicken (ch; AAP69798); Fcα/μR of human (AAL51154) and mouse (NP_659209); and FcμR of human (NP_005440), chimpanzee (cm; XP_001165341), monkey (mn; XP_001084243), bovine (XP_588921), dog (do; XP_547385), mouse (NP_081252), and rat (Q5M871).
Conserved Ser and Tyr residues in the cytoplasmic tail of FcμR
A charged His residue is adjacent to or within the putative 19-aa transmembrane segment of FcμR from all species examined except for the bovine (Fig. 4). The 118-aa cytoplasmic tail is composed of a basic aa-rich region, a Pro-rich region, two conserved Cys residues, and an acidic aa-rich region in all seven different FcμRs. Of the Ser residues, five are completely conserved and an additional four are highly conserved among these FcμRs, and some of them are potential sites for protein kinase C (PKC) phosphorylation (R/K1-3-X0-2-S/T-X0-2-R/K1-3 or R/K-X-S-Z-R/K, where Z represents a hydrophobic aa residue) or casein kinase 2 phosphorylation (S/T-X2-D/E). Three Tyr residues are also completely conserved among these FcμRs, but none of them (I/V-Y315-S/T-A-C, S-C-E/D-Y361-V-S, and S-D-D-Y385-I/V-N-V/I) match the immunoreceptor tyrosine-based activation motif (D/E-X2-Y-X2-L/I-X6-8-Y-X2-L/I), inhibitory motif (I/V-X-Y-X2-L/V), or switch motif (T-X-Y-X2-V/I). However, if phosphorylated, the most carboxyl tyrosine is a potential binding site (pY-X-N-X) for the Src homology 2 domains of growth factor receptor–bound protein 2 and growth factor receptor–bound protein 2–related adaptor protein, as observed in transmembrane adaptor proteins, including linker for activation of T cells and non–T cell activation linker (Horejsí et al., 2004). These findings indicate a quite distinct feature of the FcμR cytoplasmic tail compared with other FcRs, in which the Ig ligand binding chains are usually devoid of conserved Tyr residues except for FcγRIIA and FcγRIIB.
Figure 4.
aa sequence alignment of the transmembrane and cytoplasmic regions of FcμRs. aa sequences of the transmembrane segments and cytoplasmic tails of FcμR from seven different species are aligned. aa identity is indicated by dots, and a deletion is indicated by dashes. The predicted transmembrane region is highlighted in red. Conserved serine and tyrosine residues are also highlighted in blue and yellow, respectively. The numbers indicate the aa position from the first Met residue of human FcμR. The GenBank/EMBL/DDBJ accession nos. for these FcμRs are the same as those in Fig. 3. Chimp, chimpanzee.
To determine whether these conserved Ser and Tyr residues are phosphorylated upon stimulation, FcμR+ BW5147 T cells were treated with a tyrosine phosphatase inhibitor, pervanadate, or with preformed IgM immune complexes to cross-link FcμR. The FcμR was immunoprecipitated from the lysates of resting or activated cells and analyzed by immunoblotting with antibodies specific for phosphotyrosine or the phosphoserine of PKC substrates. Phosphorylation of both serine and tyrosine residues was clearly demonstrated in pervanadate-treated cells but not in untreated cells (Fig. 5 A). Interestingly, the serine-phosphorylated FcμR migrated at ∼52 kD, whereas most of the tyrosine-phosphorylated FcμRs migrated at ∼60 kD and, to a lesser extent, at ∼52 kD. When these membranes were reprobed with anti-FcμR mAb specific for its extracellular epitope, we found that in resting cells FcμR was present as a major band of ∼60 kD, which is consistent with the Mr of the cell-surface FcμR (see Fig. 7), along with a minor band of ∼45 kD, but in pervanadate-treated cells the FcμR was resolved as a major band of ∼52 kD together with multiple minor species of various sizes. When FcμR was cross-linked with preformed immune complexes consisting of IgM and F(ab′)2 fragments of anti-μ mAb, phosphorylation of both serine and tyrosine residues of the ∼52 kD FcμR was also demonstrated as early as 3 min after ligation. The serine phosphorylation became more prominent at 30 min after ligation, whereas tyrosine phosphorylation was diminished by that time point (Fig. 5 B). In contrast to the effects seen with pervanadate treatment, tyrosine-phosphorylated ∼60-kD FcμR was not observed in the lysates of receptor-ligated cells. Reprobing of these membranes with anti-FcμR mAb revealed the presence of both ∼60- and ∼52-kD FcμR proteins as well as minor, but discrete, bands of ∼45 and ∼63 kD. Collectively, these findings suggest that the conserved serine and tyrosine residues seen in the cytoplasmic tail of FcμR are indeed potentially phosphorylated upon receptor ligation and that the phosphorylated FcμR protein migrates differently on SDS-PAGE compared with its unphosphorylated form.
Figure 5.
Tyrosine and serine phosphorylation of FcμR upon stimulation. (A and B) BW5147 T cells stably expressing human FcμR were incubated in the presence (+) or absence (−) of 100 µM pervanadate for 15 min (A) or with the preformed IgM immune complexes for the indicated time periods (min) at 37°C (B) before cell lysis. FcμR was immunoprecipitated from cleared lysates with anti-FcμR (HM14) or control (Cont.) mAb–coupled beads, resolved on SDS–10% PAGE under reducing conditions, transferred onto membranes, and immunoblotted with rabbit antibody specific for phosphoserine of PKC substrates along with HRP-labeled goat anti–rabbit Ig antibody (anti-PKC P-Ser) or with HRP-labeled antiphosphotyrosine mAb (anti–P-Tyr) before visualization by ECL. After dissociating blotted antibodies, membranes were reprobed with biotin-labeled anti-FcμR mAbs along with HRP-labeled SA (anti-FcμR). These experiments were performed at least three times. Mr is shown in kilodaltons.
Figure 7.
Biochemical characterization of FcμR molecules. (A and B) GPI-PLC treatments. BW5147 T cells stably expressing human FcμR (A) and PMA-activated 697 pre–B cells (B) were incubated with PBS (blue) or 10 U/ml GPI-PLC (red) for 30 min at 30°C, and then examined for the expression of FcμR by anti-FcμR mAb or IgM ligand binding along with the expression of Thy-1 and CD11a (A) or of CD73 (ecto-5′-nucleotidase) and CD19 (B). A control sample was kept on ice during this treatment without GPI-PLC (green). Note the significant reduction in MFI of Thy-1 and CD73 but not of CD11a, CD19, FcμR, and IgM-binding profiles after GPI-PLC treatment. (C) SDS-PAGE analysis of cell-surface proteins. Plasma membrane proteins on control (BW) and FcμR-bearing BW5147 T cells (FcμR) were labeled with biotin, quenched, and incubated with mouse γ1κ control (Cont.) or anti-FcμR (HM14) mAbs or mouse IgMκ ligand before washing and solubilization in 1% NP-40 lysis buffer containing protease inhibitors. The mAb-bound cell-surface proteins were captured by addition of beads coupled with rat anti–mouse κ mAb (187.1 clone) and resolved on SDS–10% PAGE under nonreducing (not depicted) and reducing conditions, followed by transfer onto membranes, blotting with HRP-SA, and visualization by ECL. The same results were obtained with the HM7 anti-FcμR mAbs. The arrow indicates FcμR. The experiments were performed 3 times for A and B and >10 times for C.
FcμR per se has no antiapoptotic activity
To determine whether FcμR inhibits Fas-mediated apoptosis as originally described for FAIM3/TOSO (Hitoshi et al., 1998), retroviral constructs containing both FcμR and GFP cDNAs or only the GFP cDNA were transduced into the apoptosis-prone Jurkat human T cell line. Cells expressing comparable levels of GFP were enriched from each transductant by FACS, and the FcμR/GFP transductant was found to express relatively high levels of cell-surface FcμR as determined by both receptor-specific mAbs (see next section) and IgM ligand binding (Fig. 6 A). The resultant FcμR+GFP+ or GFP+ Jurkat cells and nontransduced Jurkat cells as an additional control were then subjected to apoptosis assays using agonistic anti-Fas mAbs of the IgM or IgG3 isotype. Cross-linkage of Fas with the IgM antibody induced robust early (annexin V+/7-aminoactinomycin D [7-AAD]−) and late (annexin V+/7-AAD+) apoptotic cells as well as dead cells (annexin V−/7-AAD+) in the nontransduced Jurkat cells and the GFP+ cells, but not in the FcμR+/GFP+ cells (Fig. 6 B). This result is consistent with the previously reported antiapoptotic activity of FAIM3/TOSO (Hitoshi et al., 1998). It should be noted, however, that addition of control IgM of either human or mouse origin at a 100-fold molar excess into these cultures did not make the FcμR+/GFP+ cells susceptible to IgM anti-Fas mAb-induced apoptosis, suggesting that the simultaneous dual binding to Fas and FcμR (i.e., cis interaction) is dominant over the single binding to FcμR (i.e., trans interaction) in this apoptosis model (Fig. S4). Unlike the effect seen with IgM anti-Fas mAb, ligation of Fas receptor with the IgG3 antibody induced apoptosis in all three cell types, including the FcμR+GFP+ cells. Notably, ligation of FcμR and Fas with the corresponding mAbs either in the absence (i.e., separate ligation of each receptor) or presence of a common secondary reagent (i.e., coligation of both receptors) had no demonstrable effects on the IgG3 anti-Fas mAb–induced apoptosis of FcμR+GFP+ cells. Essentially identical results using IgM versus IgG3 anti-Fas mAb were also obtained with EBV-transformed B cell lines expressing both endogenous FcμR and Fas on their cell surface (unpublished data). Collectively, these findings indicate that FcμR has no intrinsic activity to inhibit Fas-mediated apoptosis, but they raise the interesting possibility that IgM anti-Fas autoantibody, if present in individuals with autoimmune disorders, could interrupt Fas-mediated signaling via FcμR in vivo.
Figure 6.
Role of FcμR in Fas-mediated apoptosis of Jurkat T cells. (A) Jurkat cells transduced without (none) or with the bicistronic retroviral construct containing GFP cDNA only (GFP) or both FcμR and GFP cDNAs (FcμR/GFP) were incubated with biotin-labeled isotype-matched control mAb (left), HM14 anti-FcμR mAb (middle), or human IgM (right), and then with APC-SA before analysis by FACSCalibur. Note the comparable levels of GFP in both GFP and FcμR/GFP transductants, and the expression of FcμR on the FcμR/GFP transductant as determined by anti-FcμR reactivity and IgM ligand binding. (B) These three cell lines were incubated at 37°C for 24 h with agonistic anti–human Fas mAbs of mouse IgMκ (CH11 clone; 10 ng/ml) or IgG3κ isotype (2R2 clone; 0.3 µg/ml). Cells were stained with 7-AAD and APC-labeled annexin V before identification of early (annexin V+/7-AAD−) and late (annexin V+/7-AAD+) apoptotic and dead (annexin V−/7-AAD+) cells by FACSCalibur. Note the resistance of FcμR/GFP transductant to IgM but not IgG3 anti-Fas mAb–induced apoptosis. Numbers indicate percentages of cells. These experiments were performed more than three times.
To determine if FcμR could also affect apoptosis mediated through the BCR, the same retroviral constructs as used for Jurkat cells were transduced into a mouse immature B cell line, WEHI231, and a human germinal center B cell line, Ramos, both of which are negative for FcμR expression and are known to undergo apoptosis after BCR cross-linking. However, unlike the Jurkat T cell line, no cell lines of either type that stably expressed both FcμR and GFP were obtained after multiple attempts in different laboratories (unpublished data), whereas control GFP+ cell lines were easily established. Even after enriching GFPhi cells by FACS or by antibiotic selection, the established cell lines were found to express low levels of GFP and no cell-surface expression of FcμR. Flow cytometric analysis of these B cell lines shortly after transduction revealed that forced expression of FcμR resulted in down-modulation of their cell-surface IgM, presumably because of its ligation with the FcμR, thereby leading to loss of the FcμR+GFP+ cell population. Thus, these findings suggest that the ectopic expression of FcμR on WEHI231 and Ramos B cell lines triggers BCR-mediated apoptosis as a consequence of direct interaction between the FcμR and membrane-bound IgM molecules.
FcμR is an ∼60-kD transmembrane protein
Two hybridoma mAbs specific for human FcμR, HM7 (γ2bκ), and HM14 (γ1κ) were established from mice immunized with FcμR+ BW5147 T cells and were used along with the IgM ligand for biochemical characterization of the receptor. The HM7 mAb appeared to recognize an epitope near the IgM ligand binding site, because HM7 antibody binding was significantly inhibited by preincubation of FcμR+ cells with IgM, whereas HM14 binding was not (unpublished data). Our earlier biochemical analysis revealed that FcμR on B-lineage cells could be attached to the plasma membrane via a glycosylphosphatidylinositol (GPI) linkage (Ohno et al., 1990; Nakamura et al., 1993), but the structure predicted by the cDNA is of a transmembrane protein. We thus reexamined this issue using a highly purified GPI-specific phospholipase C (GPI-PLC). After GPI-PLC treatment, the surface expression of the GPI-anchored Thy-1 on FcμR+ BW5147 T cells was reduced by ∼65%, whereas surface FcμR levels were unaffected as determined by staining with both anti-FcμR mAbs and the IgM ligand (Fig. 7 A). As expected, levels of the control transmembrane glycoprotein CD11a were also unaffected. We extended this analysis to the 697 pre–B cell line. Consistent with our previous IgM-binding results (Ohno et al., 1990), these cells do not constitutively express cell-surface FcμR, but its expression could be induced by PMA treatment (Fig. S5). After GPI-PLC treatment, the surface levels of FcμR and CD19 on PMA-activated 697 pre–B cells were unchanged, whereas the expression of GPI-anchored CD73 was reduced by ∼50% (Fig. 7 B). Thus, these findings indicate that FcμR is an authentic transmembrane protein, consistent with the predicted structure encoded by the FcμR cDNA.
To determine the Mr of FcμR, we performed SDS-PAGE analysis of biotinylated cell-surface proteins that were precipitated from membrane lysates with anti-FcμR mAbs and IgM ligands. A major protein with an Mr of ∼60 kD was precipitated from the FcμR-bearing but not control BW5147 T cells with both probes (Fig. 7 C). The same Mr estimate was obtained under both reducing and nonreducing conditions, indicating that there are no interchain disulfide linkages of FcμR with itself or other proteins. Removal of sialic acid residues with neuraminidase from the ∼60-kD FcμR resulted in a decrease in Mr to ∼50 kD. The cell-surface FcμR isolated from PMA-activated 697 pre–B cells and normal adult blood mononuclear cells (MNCs) had an identical Mr of ∼60 kD, consistent with our previous size estimates (Sanders et al., 1987; Ohno et al., 1990; Nakamura et al., 1993). An additional minor band of ∼40 kD was occasionally identified in the precipitates from membrane lysates of FcμR+ BW5147 T cells with anti-FcμR mAbs irrespective of detergents used (NP-40, digitonin, or CHAPS). The molecular identity of this 40-kD protein is presently unknown. Although the predicted pI of FcμR is ∼9.9, the ∼60-kD FcμR was resolved into a spot with a pI of ∼5 by two-dimensional gel electrophoresis analysis (unpublished data), consistent with our previous finding that FcμR is sialylated (Ohno et al., 1990).
FcμR is predominantly expressed by both B and T lymphocytes
To determine the cellular distribution of FcμR, we first conducted RT-PCR analysis of various tissues and a panel of representative cell lines. FcμR transcripts were restricted to hematopoietic and lymphoid tissues, including the blood, bone marrow, tonsils, spleen, and appendix. FcμR transcripts were detected in both CD4+ and CD8+ T cells from blood as well as in all subsets of tonsillar B cells, although the transcript levels appeared highest in the follicular and memory B cells (Fig. S6, top). Among the cell lines, 697 pre–B cells expressed FcμR transcripts, although they did not constitutively express cell-surface FcμR protein (Fig. S5). Another pro–/pre–B cell line (REH) and some B cell lines (Ramos and the EVB-transformed line BDB-14.4) also contained FcμR mRNA (Fig. S6, bottom).
Next, we examined cell-surface FcμR expression by immunofluorescence analysis using receptor-specific mAbs and IgM ligands. In normal adult blood samples, FcμR was clearly expressed on CD19+ B cells and on the CD4+ and CD8+ T cells, although there was no discrete demarcation between FcμR+ and FcμR− T cells (Fig. 8 A). The intensity of staining with the HM14 mAb was higher than with the HM7 mAb (unpublished data), an observation consistent with the finding that the HM7 epitope is sensitive to IgM ligand binding; given its high affinity, the FcμR is likely to be occupied by IgM in vivo. Clearly, mAb reactivity was a more sensitive assay for the detection of FcμR than ligand binding using biotin-labeled human IgM, although the sensitivity of the ligand-binding assay could be increased by using mouse IgMκ, biotin-labeled rat anti–mouse κ mAb and streptavidin (SA)-PE. In addition to B and T cells, CD56+/CD3− NK cells also expressed FcμR at relatively low density. Other blood cell types, CD14+ monocytes, CD13+ granulocytes, erythrocytes, and platelets, did not express FcμR at detectable levels (Fig. S7).
Figure 8.
Immunofluorescence analysis of cell-surface FcμR expression in various tissues. (A–D) MNCs from blood (A and B), tonsils (C), and bone marrow (D) were first incubated with aggregated human IgG to block FcγRs and then with biotin-labeled HM14 anti-FcμR mAb along with the appropriate fluorochrome-labeled mAbs specific for CD19, IgM, IgD, CD38, CD3, CD4, CD8, or CD56. For IgM ligand binding, mouse IgMκ and biotin-labeled rat anti–mouse κ mAbs were sequentially added to MNCs without preincubation with aggregated IgG. The bound biotin-labeled reagents were detected by addition of SA-PE. Essentially the same results were obtained with the HM7 anti-FcμR mAb (not depicted). Because the results of the FcμR expression by CD3+/CD8+ and CD3+/CD4− T cells were essentially the same, the CD8 data were omitted for simplicity. The cell populations indicated by the red boxes were gated and examined for their reactivity with the HM14 anti-FcμR mAb and IgM ligand. Biotin-labeled irrelevant mAbs of the γ1κ (for HM14) or γ2bκ (for HM7) isotype were used as controls. The analysis was performed with freshly prepared cell preparations (A and D; labeled Fresh) or with cells cultured overnight in IgM-free media (O/N; B and C). Because the immunofluorescence profiles of freshly prepared tonsillar B cells with anti-FcμR mAbs and isotype-matched control mAbs as well as these of overnight-cultured B cells with the isotype-matched control mAbs were all essentially the same, only the results of freshly prepared and overnight-cultured B cells with anti-FcμR mAbs are shown in C (top) for simplicity. CD19+ B cells in tonsils (C) were analyzed for FcμR expression as follicular/naive (IgD+/CD38−), pregerminal center (preGC; IgD+/CD38+), germinal center (GC; IgD−/CD38+), and memory (IgD−/CD38−) cells. The frequency (%) of FcμR+ cells in each cell type among 10 different blood samples was 62 ± 18 for CD19+ B cells, 62 ± 13 for CD4+ T cells, 43 ± 23 for CD8+ T cells, and 19 ± 11 for CD56+ NK cells (means ± SD). The frequencies (%) of FcμR+ cells over the background staining with isotype-matched control mAbs in three tonsillar samples were 31 ± 7 for follicular/naive, 15 ± 3 for preGC, 10 ± 3 for GC, and 30 ± 12 for memory B cells, and 34 ± 6 for CD4+ T and 51 ± 7 for CD8+ T cells (means ± SD). The experiments were performed >10 times for A and B, 3 times for C, and 2 times for D.
Notably, overnight culture of blood MNCs in IgM-free media enhanced FcμR expression especially by T cells (Fig. 8 B), consistent with our previous IgM-binding data (Nakamura et al., 1993). Curiously, this enhancement was more evident for the cell preparations from the tonsils and spleen than from blood. Freshly isolated tonsillar MNCs, including B and T cells, had no reactivity with either anti-FcμR mAbs or IgM ligands, but after overnight culture, there was clear-cut expression of FcμR on the surface of the CD19+ B cells and the CD4+ and CD8+ T cells (Fig. 8 C). Most follicular (IgD+/CD38−) and memory (IgD−/CD38−) B cells expressed FcμR, whereas only a small subpopulation of the germinal center (IgD−/CD38+) and pregerminal center (IgD+/CD38+) B cells expressed FcμR, consistent with our RT-PCR data. Many CD4+ T cells and the majority of CD8+ T cells clearly expressed cell-surface FcμR after culture. After overnight culture, the proportion of FcμR+ B, CD4+ T, CD8+ T, and NK cells in the spleen was similar to that in blood samples (unpublished data). In adult bone marrow, a small subpopulation (∼21%) of the CD19+/surface IgM− pro–/pre–B cells expressed low levels of FcμR on their cell surface, whereas ∼42% of the CD19+/surface IgM+ B cells expressed slightly higher levels of FcμR, indicating that FcμR expression begins at the pro–/pre–B cell stage in B-lineage differentiation (Fig. 8 D). No FcμR expression was observed on myeloid cells even after overnight culture in IgM-free media. In contrast to the FcμR phenotype of humans, our initial immunofluorescence analysis of mouse splenocytes with a receptor-specific mAb revealed that FcμR was expressed by B220+ B cells but not by CD3+ T cells or Mac-1+ macrophages (unpublished data).
To further examine the effects of cellular activation on surface FcμR expression, blood MNCs were activated with various stimuli. Treatments of blood B cells with anti-µ mAb or PMA for 24 h resulted in an ∼2.2-fold increase in the cell-surface FcμR level in comparison to that on B cells cultured in media only (unpublished data). In contrast, treatment of blood T cells with anti-CD3 mAb or PMA for 24–72 h reduced the cell-surface FcμR level by ∼90%, suggesting that signaling through antigen receptors on B and T cells has distinct modulating effects on FcμR expression. Consistent with previous observations (Ferrarini et al., 1977; Pichler and Knapp, 1977; Sanders et al., 1987), there was enhanced FcμR expression by CLL B cells from three randomly selected patient blood samples (Fig. S8). Collectively, these findings indicate that, in striking contrast to other FcRs, FcμR is predominantly expressed by cells of the adaptive immune system. Moreover, the cell-surface levels of FcμR are sensitive to IgM ligand concentration, tissue milieu, and cellular activation status.
DISCUSSION
We have identified for the first time a bona fide FcμR cDNA in humans. By using receptor-specific mAbs and IgM ligands, FcμR is defined as an ∼60-kD transmembrane sialoglycoprotein. Its predicted structure consists of a single V-set Ig-like domain with homology to the Ig-binding domains of pIgR and Fcα/µR, an additional extracellular region with no known domain features, a transmembrane segment containing a charged His residue, and a relatively long cytoplasmic tail carrying conserved Tyr and Ser residues. Pentameric IgM and its Fc5µ fragments bound cell-surface FcμR on transductants, but the Fabµ fragments and other Ig isotypes did not, thereby confirming the Fcμ specificity of this receptor. The affinity of the FcμR for its IgM ligand is strikingly high, ∼10 nM. Despite the initial designation of FcμR as an antiapoptotic protein FAIM3/TOSO, FcμR per se had no inhibitory activity in Fas-mediated apoptosis, and such inhibition was only achieved when agonistic anti-Fas antibody of an IgM but not IgG isotype was used for inducing apoptosis. The cell types expressing FcμR were predominantly B and T cells and not phagocytes; hence, the cellular distribution of FcμR is quite distinct from that of other FcRs. The surface FcμR levels on those lymphocytes were susceptible to IgM ligand concentration, tissue milieu. and cellular activation.
The FCMR gene is found to be in an appropriate location on chromosome 1q32.2 adjacent to two other IgM-binding receptor genes (PIGR and FCAMR). Although the ligand-binding domains of these three receptors are similar to each other, FcμR seems to be the most distantly related among the group based on the following findings. (a) Many residues are well conserved in pIgR and Fcα/µR but not in FcμR. (b) The length of the CDR1 region, which is predicted to contact the Ig ligands, is shorter in FcμR (5 aa) than in pIgR and Fcα/µR (9 aa). (c) Within the CDR1 region, there are two charged residues: Arg31 conserved in both pIgR and Fcα/µR, and His32 conserved in all three receptors. Although Arg31 is predicted to be solvent exposed and to interact directly with polymeric IgA (Hamburger et al., 2004), FcμRs from seven different species have a noncharged residue at the corresponding position. (d) FcμR recognizes only the IgM isotype, whereas both pIgR and Fcα/µR in humans bind polymeric IgA and IgM (Kaetzel, 2005; Kikuno et al., 2007). These findings thus suggest that the interaction of FcμR with its IgM ligand is distinct from that of pIgR and Fcα/µR with IgM and polymeric IgA. In this regard, the finding that Fc5μ fragments mostly consisting of the Cμ3/Cμ4 domains inhibit IgM binding to FcμR suggests that FcμR recognizes a molecular configuration on IgM that is conferred by the Cμ3/Cμ4 domains. In contrast, pIgR recognizes the C-terminal domain Cμ4 (Kaetzel, 2005).
The finding that the FcμR cDNAs identified in two cDNA libraries from PMA-activated 697 pre–B cells and CLL B cells encode a transmembrane but not a GPI-linked protein was unexpected, because in our previous biochemical analysis, the FcμR expressed on such pre–B cells was sensitive to GPI-PLC, whereas the FcμR on blood T cells was resistant (Ohno et al., 1990; Nakamura et al., 1993). An intensive search for an alternatively spliced transcript encoding a GPI-linked form of FcμR was unsuccessful but led to identification of an FcμR splice variant lacking the transmembrane exon that may encode a soluble form of FcμR with an Mr of ∼34 kD (unpublished data). Reexamination of the susceptibility of FcμR to GPI-PLC treatment yielded an unequivocal result: the expression of FcμR on both PMA-activated 697 pre–B cells and T cell transductants was unchanged after GPI-PLC treatment, whereas the surface expression of GPI-anchored CD73 or Thy-1/CD90 was reduced by 50–65%. Thus, the discrepancy is likely caused by the fact that the GPI-PLC available for our studies in 1990 contained residual contaminating protease activity.
Unlike other FcRs, the FcμR has a relatively long cytoplasmic tail containing three conserved tyrosine and five to nine conserved serine residues. We found that some of these tyrosine and serine residues are targets for phosphorylation after FcμR ligation with IgM immune complexes or pervanadate treatment. Intriguingly, the phosphorylated FcμR was found to migrate on SDS-PAGE faster than the unphosphorylated form. One possible explanation for this is that such phosphorylation may cause a global structural change of FcμR leading to increased mobility on SDS-PAGE. Although phosphorylated proteins usually migrate slower than their unphosphorylated forms, CD45 on a myeloid cell line in fact exhibits enhanced mobility on SDS-PAGE after PMA-induced phosphorylation (Buzzi et al., 1992). Another explanation may be proteolytic cleavage in the cytoplasmic tail of FcμR after receptor ligation, as has been observed in the FcγRIIA on platelets (Gardiner et al., 2008). FcγRIIA ligation on platelets leads to activation of both the metalloprotease that targets the collagen receptor GPVI to shed its ectodomain and the intracellular calpain that cleaves the cytoplasmic tail of FcγRIIA to remove the immunoreceptor tyrosine-based activation motif–containing stub, suggesting a novel mechanism for platelet dysfunction by FcγRIIA after immunological insult including IgG autoantibodies to platelets. The precise mechanism for the enhanced migration of phosphorylated FcμR awaits further investigation.
Nucleotide sequence analysis indicated that FcμR and FAIM3/TOSO are identical, but we have clearly shown that the antiapoptotic activity of FcμR in Fas-bearing Jurkat cells is only observed when agonistic anti-Fas mAb of an IgM but not IgG3 isotype is used and that the FcμR expression itself does not prevent Fas-mediated apoptosis. Addition of a 100-fold molar excess of control IgM into these cultures did not convert the FcμR+GFP+ cells from resistance to sensitivity to IgM anti-Fas mAb–induced apoptosis. Even when FcμR and Fas on FcμR+ cells were brought into close physical proximity by ligation with a common secondary antibody, FcμR did not inhibit Fas-mediated apoptosis. Notably, Hitoshi et al. (1998) found that a FAIM3/TOSO deletion mutant lacking most of its cytoplasmic tail could still inhibit apoptosis mediated by IgM anti-Fas mAb, implying that FAIM3/TOSO might act indirectly through noncovalent association with another cell-surface protein. This might be relevant to our finding that an additional membrane protein of ∼40 kD often coprecipitated with the ∼60-kD FcμR from membrane lysates of FcμR+ cells and that there is a charged His residue, which could be involved in electrostatic association with other proteins, adjacent to the transmembrane segment of FcμR. We therefore propose that the original designation of this gene as FAIM3/TOSO should be reconsidered and that renaming it FCMR would be more appropriate in keeping with its true physiological role.
Another reason to rename this gene is that several groups (Pallasch et al., 2008; Proto-Siqueira et al., 2008) have recently reported that FAIM3/TOSO is overexpressed in CLL, a heterogeneous leukemia thought to originate from antigen-stimulated B cells that escape normal cell-death mechanisms. The interpretation of this finding by both groups is that the resistance of CLL B cells to death mechanisms may result from the enhanced expression of “antiapoptotic” FAIM3/TOSO molecules. However, enhanced FcμR expression by CLL B cells had been consistently observed by many investigators using either rosetting or immunofluorescence methods (Ferrarini et al., 1977; Pichler and Knapp, 1977; Sanders et al., 1987). Although the mechanism for enhanced FcμR expression on CLL cells is unclear, it may result from chronic antigenic stimulation as supported by (a) reduced levels of membrane IgM, IgD, and CD79/Igα/Igβ on CLL cells and (b) polyreactivity of CLL-derived IgM molecules (Chiorazzi et al., 2005). In this regard, our finding that treatment of normal blood B cells with anti-μ antibody down-modulates membrane IgM and up-regulates FcμR cell-surface expression is consistent with the hypothesis that CLL cells are being activated by certain common antigens; thereby, antigen-driven proliferation may provide an alternative mode of survival of the leukemic cells.
The finding that the major cell types expressing FcμR are the adaptive immune cells, both B and T lymphocytes, is remarkable, because FcRs for the switched Ig isotypes (FcγRs, FcϵRI, and FcαR) are expressed by various hematopoietic cells, including phagocytes, and are thought to be central mediators coupling the innate and adaptive immune responses (Nimmerjahn and Ravetch, 2008). Intriguingly, FcμR is the only FcR constitutively expressed on T cells, which are generally negative for the expression of other FcRs. The expression of FcμR by both CD4+ and CD8+ T cells is consistent with an early report that T cells forming rosettes with IgM-coated erythrocytes included both cell types (Reinherz et al., 1980). For B cells, FcμR is the only IgM-binding receptor expressed. Although the initial report indicated that Fcα/µR is expressed on B cells (Shibuya et al., 2000), our subsequent analyses revealed that the major cell type expressing Fcα/µR was a follicular dendritic cell in both humans (Kikuno et al., 2007) and mice (unpublished data). A small subpopulation of blood CD56+/CD3− NK cells was also found to express FcμR, consistent with the results previously reported by others (Pricop et al., 1993; Rabinowich et al., 1996). The physiological relevance of such restricted cellular expression of FcμR may be related to unique features of the IgM ligand, such as its early appearance during immune responses, the pentameric configuration of its secreted form, and its potency in complement activation.
Many investigators had previously noticed the instability of IgM binding by B, T, and NK cells (Moretta et al., 1977; Nakamura et al., 1993; Pricop et al., 1993). We also found that the cell-surface FcμR levels were sensitive to extracellular IgM concentration, tissue milieu, and cellular activation status. This vulnerability could explain why FcμR was limited to an operationally defined entity for such a long time. As is the case with many other receptors, the detection of FcμR with IgM ligands was much less efficient than with anti-FcμR mAbs. Short-term culture in IgM-free media enhanced the cell-surface expression of FcμR on T cells and, to a lesser extent, on B and NK cells. Remarkably, this phenomenon was much more pronounced with cells from tonsils and spleen; cell-surface FcμR was not detectable on freshly isolated B and T cells from these organs but easily demonstrated after overnight culture in IgM-free media. Many other cell-surface antigens were detectable in those freshly isolated preparations, ruling out an artifact of tissue manipulation. To our knowledge, the IgM concentration in the interstitial spaces of such intact tissues has never been determined. If this in vivo down-modulation of FcμR is solely dependent on the extracellular concentration of IgM and not on the tissue microenvironment (e.g., proteases) or cellular activation status, then the interstitial IgM concentration in secondary lymphoid tissues is perhaps higher than in blood. In this regard, it is noteworthy that IgM-producing plasma cells are in the immediate vicinity of B and T cells within these lymphoid tissues.
Although both B and T cells express FcμR, there is a striking difference in their response after antigen receptor ligation. FcμR expression on B cells was up-regulated after treatment with anti-µ mAb, whereas its expression on T cells was down-modulated after treatment with anti-CD3 mAb. The response to PMA was also different in the B and T cells, suggesting that the difference might be attributed to the downstream events involving PKC. The role of PKC in internalization of cell-surface receptors including TCR has been clearly demonstrated (Cantrell et al., 1985; Minami et al., 1987; Bonefeld et al., 2003). PKC is a conserved family of 11 serine/threonine protein kinases, and most cell types express multiple isozymes of PKC (Spitaler and Cantrell, 2004). PKC α, β, δ, ϵ, η, θ, and ζ are known to be present in lymphocytes. Interestingly, the disruption of the gene encoding a single PKC isozyme expressed in both B and T cells (e.g., PKCβ−/−, PKCζ−/−, and PKCδ−/−) often caused a selective immunological abnormality in only one of the cell types, suggesting compensatory or complementary functions of other PKC isozymes in the other cell type (Spitaler and Cantrell, 2004). Based on the consensus sequence motifs, there are several potential Ser residues available for phosphorylation by PKC in the human FcμR, particularly R-R-K-A-L-S283-R-R or A-P-S359-L-K. Thus, it seems possible that FcμR expressed on B and T cells may have distinct influences on their respective antigen receptor–mediated signaling.
With regard to the function of FcμR on B cells, it has been shown that passive administration of IgM antibody, in contrast to IgG, enhances the subsequent antibody response to relevant antigenic challenge (Hjelm et al., 2006). Recent studies with mice unable to produce the soluble form of IgM have clearly demonstrated the importance of secreted IgM in development of protective IgG antibody responses to viral and bacterial infections, presumably through both complement and FcμR systems (Boes et al., 1998a; Boes et al., 1998b; Ehrenstein et al., 1998; Ochsenbein et al., 1999; Baumgarth et al., 2000). The complement cleavage products C3dg and C3d attach covalently to antigen and cross-link both CD21 (CR2) and BCR on the membrane of B cells, thereby facilitating the B cell response to low concentrations of antigen despite the typically low affinity of BCR during the primary immune response (Fearon and Carter, 1995; Fearon and Carroll, 2000). The enhancing or adjuvant activity of C3d has been demonstrated with various antigens, although recently the inhibitory activity of C3d has also been reported for certain antigens (Bergmann-Leitner et al., 2006). Given that IgM antibody is a first line of host defense, it is reasonable to propose that FcμR may contribute to enhancement of B cell responses by interacting with BCR and CD21/CD19/CD81 via IgM–antigen–C3d complexes. Another potential role for FcμR on B cells is antigen presentation. The functional significance of FcμR on T cells has been the subject of considerable speculation (Moretta et al., 1977; Mathur et al., 1988b; Nakamura et al., 1993). It seems possible that FcμR on T cells may interact with the IgM BCR or IgM/antigen complexes on B cells to facilitate T and B cell interactions, thereby enhancing B cell activation. FcμR may also trigger cytotoxic T cells in IgM antibody–dependent cell-mediated cytotoxicity. The true physiological roles of FcμR, however, will become apparent with further studies, including analysis of the immunological phenotypes in FcμR-deficient mice. Although the molecular nature of the FcμR has long been elusive, its final unveiling in this study reveals a receptor full of intriguing aspects and opens new avenues of investigation.
MATERIALS AND METHODS
Construction of retroviral cDNA libraries.
The cDNA libraries were constructed by a cDNA synthesis kit (Agilent Technologies) using poly(A)+ RNA isolated by an Oligotex mRNA purification kit (QIAGEN) from (a) blood MNCs from a patient with CLL and (b) the 697 pre–B cell line preactivated with 10 nM PMA for 8 h, as previously described (Kubagawa et al., 1997). The EcoRI/XhoI-digested and size-fractionated cDNAs were ligated into the pMXsΔN/S retrovirus vector, in which the 1,328-bp NotI/SalI fragment containing an IRES and a GFP cDNA was removed from the original pMXsIG vector (Kitamura et al., 2003). The ligated cDNA constructs were used to transform XL2-Blue MRF′ ultracompetent cells (Agilent Technologies). The titer of the cDNA library was ∼105 and ∼106 CFU/µg mRNA for CLL and 697 pre–B cells, respectively, and the mean size of the insert DNA in these libraries was ∼1.6 kb.
Transfection, transduction, and screening.
The cDNA libraries were transfected into the ecotropic retroviral packaging cell line BOSC23 with FuGENE 6 (Roche). 2 d later, the culture supernatants containing viruses were collected and filtered, and polybrene (Sigma-Aldrich) was added to a final concentration of 10 µg/ml before infecting the mouse thymoma line BW5147 at a ratio of ∼3 × 105 cells/ml of supernatants. After 2 d, infected BW5147 T cells were incubated with biotin-labeled human IgMκ and then with antibiotin microbeads (Miltenyi Biotec) or PE-labeled SA (SouthernBiotech) before sorting IgM-binding cells by MACS or FACS, respectively. Enrichment of IgM-binding cells was repeated three times for MACS and once for FACS within the interval of ∼3 d, and the final FACS-sorted cells were cloned by limiting dilution.
Identification of cDNA inserts and sequencing.
Total RNA isolated from single-cell derived, IgM-binding and -nonbinding subclones was converted to first-strand cDNA with a primer (5′-CCCTTTTTCTGGAGACTAAAT-3′) corresponding to the 3′ vector sequence flanking the cloning site and SuperScript II RT (Invitrogen). The resultant first-strand cDNAs were used as template DNAs in PCR amplification with PrimeSTAR HS DNA polymerase (Takara Bio Inc.) and a set of primers corresponding to the 5′ and 3′ flanking vector sequences of the cloning site, as previously described (Arase et al., 2001). Amplified PCR products were subcloned into the ZeroBlunt TOPO vector (Invitrogen) before sequencing analysis was performed at our institutional sequencing core facility using a DNA analyzer (model 3730xl) and DNA Sequencing Analysis Software (version 5.2; both from Applied Biosystems).
Preparation of FcμR stable transductants.
Total RNAs isolated from PMA-activated 697 pre–B cells, CLL B cells, and tonsils were similarly converted to first-strand cDNA with an oligo(dT)18 primer, and the resultant first-strand cDNAs were used as template DNAs for amplification of FcμR cDNA with a set of primers (forward, 5′-AGATCTAGAAGGGACAATGGACT-3′, and reverse, 5′-GAATTCTCAGGCAGGAACATTGATGT-3′; underlined portions indicate BglII and EcoRI sites). Amplified products of the expected size of ∼1.2 kb were subcloned into the BamHI and EcoRI sites of the pMXsPIE retroviral vector that contains a GFP cDNA and Streptomyces alboniger puromycin-N-acetyltransferase cDNA (a gift of A. Mui; DNAX, San Francisco, CA; Ehrhardt et al., 1999). After confirming sequence identity, the ligated FcμR cDNA construct and the empty vector were similarly transduced in BW5147 T cells, and the GFP+ cells in both transductants were enriched by FACS and in the presence of 1 µg/ml puromycin. For FcμR+ Jurkat cells, both the FcμR/GFP and GFP-only constructs were transfected into the 293T-A amphotropic packaging cell line before transducing the human Jurkat T cell line. GFP+ cells were enriched three times by FACS before establishing the stable cell lines. In some experiments, both FcμR/GFP and GFP constructs were similarly transfected into an appropriate packaging cell line and transduced into WEHI231 mouse B cells and Ramos human B cells.
Ig ligands and binding assay.
Human IgM myeloma proteins were purified from serum samples by euglobulin fractionation and Sephacryl S-300 gel filtration column chromatography (GE Healthcare; Ohno et al., 1990). The Fc5μ and Fabμ fragments were prepared from a human IgMκ by hot trypsin digestion (Plaut and Tomasi, 1970; Ohno et al., 1990; Nakamura et al., 1993). Other human myeloma Igs of each isotype (γ1, γ2, γ3, γ4, α1, α2, δ, and ϵ) and mouse myeloma IgM were purchased from EMD and Sigma-Aldrich. The purity of IgM, its fragments, and other myeloma Igs was confirmed by SDS-PAGE under both reducing and nonreducing conditions. Protein concentration was determined by absorbance at 280 nm with an extinction coefficient of 1.4 as 1 mg/ml. For the binding inhibition assay, FcμR+ BW5147 T cells were incubated with various concentrations of Ig preparations along with a constant amount of biotin-labeled IgMκ, washed, and incubated with PE-labeled SA to determine the bound IgM.
Production of hybridoma mAbs.
BALB/c mice were hyperimmunized subcutaneously with BW5147 T cells expressing human FcμR, and regional lymph node cells were fused with the Ag8.653 plasmacytoma line, as previously described (Kikuno et al., 2007). Hybridoma clones producing IgG mAbs reactive with FcμR+ BW5147 T cells, but not with Fcα/μR+ BW5147 T cells, control BW5147 T cells, and pIgR+ FT-29 cells were selected and subcloned by limiting dilution. Two human FcμR-specific mAbs, HM7 (γ2bκ) and HM14 (γ1κ), were selected in this study. Their F(ab′)2 fragments were prepared by digestion with lysyl endopeptidase (Yamaguchi et al., 1995) and pepsin (Maruyama et al., 1985) for HM7 and HM14, respectively.
Flow cytometric analysis of cells.
Blood MNCs were isolated by Ficoll-Hypaque density gradient centrifugation. Granulocytes were isolated from erythrocyte pellets by differential sedimentation in 1.5% dextran in PBS. MNCs were also prepared from long bone, tonsil, and spleen tissues obtained from our institutional tissue procurement service. Approval for use of these human materials in this investigation was obtained from the University of Alabama at Birmingham Institutional Review Board. Cells were first incubated with aggregated human IgG to block FcγRs and then stained with biotin-labeled anti-FcμR mAbs along with fluorochrome-labeled mAbs specific for CD3, CD4, CD8, CD19, CD14, CD56, CD10, or CD13. PE-labeled SA was used as a developing reagent for biotinylated mAbs. Controls included isotype-matched irrelevant mAbs labeled with the corresponding fluorochromes or biotin. In some experiments, biotin-labeled F(ab′)2 fragments of anti-FcμR mAbs were used. Stained cells were analyzed with a FACSCalibur instrument (BD). For GPI-PLC treatment, 106 cells were incubated for 45 min at 30°C in 10 mM Hepes/HBSS (without Ca2+ and Mg2+) containing 10 U/ml GPI-PLC (Sigma-Aldrich). After treatment, cells were washed and examined for FcμR and other cell-surface antigens by FACSCalibur. For neuraminidase treatment, 5 × 106 cells/ml in HBSS were incubated with 50 U/ml neuraminidase (New England Biolabs, Inc.) at 37°C for 45 min before washing and immunofluorescence analysis.
Cell-surface biotinylation and immunoprecipitation analysis.
Plasma membrane proteins on 107 viable cells were labeled with 1 ml sulfo-NHS-LC-biotin (0.1 mg/ml; Thermo Fisher Scientific) in 0.15 M NaCl/0.1 M Hepes (pH 8) for 30 min at 25°C. After washing, biotinylated cells were incubated with 10 µl anti-FcμR or isotype-matched control mAbs or mouse IgMκ ligand (50 µg/ml) for 20 min on ice, washed, and lysed in 200 µl of 1% NP-40 lysis buffer containing protease inhibitors (Sanders et al., 1987; Ohno et al., 1990; Nakamura et al., 1993). Cleared lysates were either transferred to 96-well plates precoated with 20 µg/ml of rat anti–mouse κ mAb (clone 187.1; Yelton et al., 1981) or incubated with rat anti–mouse κ mAb–coupled beads, and the bound materials were dissociated and separated by SDS-PAGE under reducing and nonreducing conditions, followed by transfer to membranes, blotting with horseradish peroxidase (HRP)–SA, and visualization by ECL (GE Healthcare), as previously described (Kikuno et al., 2007). In some experiments, the anti-FcμR mAb–bound materials were resuspended in 7 M urea/2 M thiourea/4% CHAPS/40 mM dithiothreitol/0.5% ampholite (pH 3–10)/40 mM Tris-HCl (pH 8.8) and subjected to two-dimensional gel electrophoresis analysis, as previously described (Ohno et al., 1990).
Immunoblot analysis.
To determine the phosphorylation status of Tyr and Ser residues in FcμR, 3 × 107 cells serum starved for 1.5 h in RPMI 1640/20 mM Hepes media were treated with 100 µM pervanadate for 15 min at 37°C, lysed in 1 ml of 1% NP-40 lysis buffer with protease/phosphatase inhibitors, and immunoprecipitated with Sepharose 4B beads coupled to HM14 anti-FcμR mAb or AM3 anti-Fcα/μR mAb as an isotype-matched control. The bound materials were dissociated with 0.1 M glycine-HCl buffer (pH 2.8) in 0.5% NP-40, immediately neutralized with 1 M Tris, and resolved on SDS–10% PAGE before transfer onto membranes. After soaking with 5% nonfat milk, membranes were immunoblotted with HRP-labeled antiphosphotyrosine mAb (4G10; Millipore) or rabbit antibody specific for phosphoserine of PKC substrates (Cell Signaling Technology) along with HRP-labeled goat anti–rabbit Ig antibody (SouthernBiotech) as a developing reagent. For receptor ligation, serum-starved cells were incubated with 50 µl of the preformed IgM immune complexes, an equal mixture of human IgMκ myeloma protein (100 µg/ml) and F(ab′)2 fragments of anti–human μ mAb with specificity for the Cμ1 domain (50 µg/ml), at 37°C for 0, 3, and 30 min before solubilizing in 200 µl of 1% NP-40 lysis buffer. The cleared lysates were subjected to immunoprecipitation with HM14 or AM3 mAb–coupled beads, and the bound materials were similarly analyzed by immunoblotting. Immunoblotted membranes were visualized by ECL. After dissociating the blotting antibodies, the membranes were reblotted with biotin-labeled anti-FcμR mAbs (HM14 and HM7) to confirm the phosphorylation of Tyr and Ser residues of FcμR.
Apoptosis assay.
4 × 105 cells/ml were cultured for 24 h in RPMI 1640 containing 10% FCS, penicillin/streptomycin, and 5 × 10−5 M 2-ME in the presence or absence of either of the agonistic anti-Fas mAbs, CH11 (10 ng/ml; mouse µk isotype; Millipore) or 2R2 (300 ng/ml; mouse γ3κ; Invitrogen), washed twice with PBS, and incubated with 7-AAD and allophycocyanin (APC)-labeled annexin V for detecting apoptotic cells according to the manufacturer’s recommendation (BD). In some experiments, 100-fold molar excess of human or mouse IgM myeloma protein as a ligand was added in these cultures. In other experiments, cells were preincubated with the 2R2 anti-Fas mAb (300 ng/ml) and either F(ab′)2 fragments or the intact form of the HM14 anti-FcμR mAb (50 µg/ml) for 20 min at 4°C, washed, and cultured in the presence or absence of F(ab′)2 fragments of goat anti–mouse κ antibodies (50 µg/ml) overnight at 37°C.
Scatchard plot analysis.
2 × 106 FcμR+ BW5147 T cells were incubated in triplicate with serial dilutions of 125I-labeled IgM with a specific activity of ∼1.6 × 1017 cpm/mol in 30 µl PBS containing 3% FCS and 0.2% sodium azide for 1.5 h at room temperature before washing and aspirating unbound IgM by centrifugation. Some tubes contained a 200-fold molar excess of cold IgM to determine the amounts of nonspecific binding of 125I-labeled IgM to cells. The numbers of IgM molecules specifically bound per cell were plotted on the x axis against the ratio of bound to free IgM on the y axis, and the apparent dissociation constant was obtained by dividing the number of receptors per cell by the bound/free ratio at the y-axis intercept, as previously described (Lowenthal et al., 2001).
Online supplemental material.
Fig. S1 shows the nucleotide sequence of the human FcμR cDNA. Fig. S2 shows the definition of FAIM3/TOSO as an FcμR. Fig. S3 shows the predicted protein structure of human FcμR. Fig. S4 shows the effects of FcμR ligation on anti-Fas antibody–mediated apoptosis in Jurkat T cells. Fig. S5 shows the expression of cell-surface FcμR on 697 pre–B cell line before and after PMA stimulation. Fig. S6 shows FCMR gene expression analyzed by RT-PCR. Fig. S7 shows the lack of FcμR expression by monocytes, granulocytes, erythrocytes, and platelets. Fig. S8 shows enhanced FcμR expression on CLL cells. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20091107/DC1.
Acknowledgments
This paper is dedicated to our former friends and FcμR investigators, E.W. Lamon and C.E. Grossi.
We thank S. Izui, S.K. Sanders, I.C.M. MacLennan, T. Kawakami, and S.J. Frank for valuable suggestions; T. Motohashi and A. Sasada for excellent technical support; P.D. Burrows and J.E. Volanakis for critically reading the manuscript; M.G. Salazar for DNA sequencing (AI27767); H. Kim and G.D. Robinson for two-dimensional gel electrophoresis (CA13148); our institutional tissue procurement staff for providing human tissues (CA13148); D.W. Garber, J. Novak, R.T. Brown, and J.K. Adams for providing the facility for iodination experiments; and J.B. Bennett for manuscript preparation. E. Takayama, T. Kitamura, and H. Kubagawa set up the retroviral expression procedure; H. Kubagawa and I. Torii cloned the FcμR cDNA; H. Kubagawa and D.-W. Kang produced anti-FcμR mAbs; S. Oka performed immunofluorescence assays; Y. Kubagawa and H. Kubagawa conducted biochemical and signaling analyses; H. Kubagawa, S. Oka, H. Mori, and J.-Y. Wang performed apoptosis experiments; H. Takatsu and H. Ohno performed mouse FcμR expression analysis; G.L. Gartland performed the FACS sort; L.F. Bertoli provided CLL patients’ materials; and H. Kubagawa wrote the manuscript.
This work was supported in part by National Institute of Allergy and Infectious Diseases/National Institutes of Health grants AI52243 and AI42127 (to H. Kubagawa).
The authors have no conflicting financial interests.
Footnotes
Abbreviations used:
- 7-AAD
- 7-aminoactinomycin D
- APC
- allophycocyanin
- CLL
- chronic lymphocytic leukemia
- FAIM3
- Fas apoptotic inhibitory molecule 3
- FcR
- Fc receptor
- GPI
- glycosylphosphatidylinositol
- HRP
- horseradish peroxidase
- MFI
- mean fluorescence intensity
- MNC
- mononuclear cell
- pI
- isoelectric point
- pIgR
- polymeric Ig receptor
- PKC
- protein kinase C
- PLC
- phospholipase C
- SA
- streptavidin
References
- Arase H., Saito T., Phillips J.H., Lanier L.L. 2001. Cutting edge: the mouse NK cell-associated antigen recognized by DX5 monoclonal antibody is CD49b (α 2 integrin, very late antigen-2). J. Immunol. 167:1141–1144 [DOI] [PubMed] [Google Scholar]
- Basten A., Warner N.L., Mandel T. 1972. A receptor for antibody on B lymphocytes. II. Immunochemical and electron microscopy characteristics. J. Exp. Med. 135:627–642 10.1084/jem.135.3.627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgarth N., Herman O.C., Jager G.C., Brown L.E., Herzenberg L.A., Chen J. 2000. B-1 and B-2 cell–derived immunoglobulin M antibodies are nonredundant components of the protective response to influenza virus infection. J. Exp. Med. 192:271–280 10.1084/jem.192.2.271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann-Leitner E.S., Leitner W.W., Tsokos G.C. 2006. Complement 3d: from molecular adjuvant to target of immune escape mechanisms. Clin. Immunol. 121:177–185 10.1016/j.clim.2006.07.001 [DOI] [PubMed] [Google Scholar]
- Boes M., Esau C., Fischer M.B., Schmidt T., Carroll M., Chen J. 1998a. Enhanced B-1 cell development, but impaired IgG antibody responses in mice deficient in secreted IgM. J. Immunol. 160:4776–4787 [PubMed] [Google Scholar]
- Boes M., Prodeus A.P., Schmidt T., Carroll M.C., Chen J. 1998b. A critical role of natural immunoglobulin M in immediate defense against systemic bacterial infection. J. Exp. Med. 188:2381–2386 10.1084/jem.188.12.2381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonefeld C.M., Rasmussen A.B., Lauritsen J.P., von Essen M., Ødum N., Andersen P.S., Geisler C. 2003. TCR comodulation of nonengaged TCR takes place by a protein kinase C and CD3 γ di-leucine-based motif-dependent mechanism. J. Immunol. 171:3003–3009 [DOI] [PubMed] [Google Scholar]
- Buzzi M., Lu L., Lombardi A.J., Jr., Posner M.R., Brautigan D.L., Fast L.D., Frackelton A.R., Jr 1992. Differentiation-induced changes in protein-tyrosine phosphatase activity and commensurate expression of CD45 in human leukemia cell lines. Cancer Res. 52:4027–4035 [PubMed] [Google Scholar]
- Cantrell D.A., Davies A.A., Crumpton M.J. 1985. Activators of protein kinase C down-regulate and phosphorylate the T3/T-cell antigen receptor complex of human T lymphocytes. Proc. Natl. Acad. Sci. USA. 82:8158–8162 10.1073/pnas.82.23.8158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiorazzi N., Rai K.R., Ferrarini M. 2005. Chronic lymphocytic leukemia. N. Engl. J. Med. 352:804–815 10.1056/NEJMra041720 [DOI] [PubMed] [Google Scholar]
- Conrad D.H. 1990. FcϵRII/CD23: the low affinity receptor for IgE. Annu. Rev. Immunol. 8:623–645 [DOI] [PubMed] [Google Scholar]
- Ehrenstein M.R., O’Keefe T.L., Davies S.L., Neuberger M.S. 1998. Targeted gene disruption reveals a role for natural secretory IgM in the maturation of the primary immune response. Proc. Natl. Acad. Sci. USA. 95:10089–10093 10.1073/pnas.95.17.10089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrhardt G.R., Leslie K.B., Lee F., Wieler J.S., Schrader J.W. 1999. M-Ras, a widely expressed 29-kD homologue of p21 Ras: expression of a constitutively active mutant results in factor-independent growth of an interleukin-3-dependent cell line. Blood. 94:2433–2444 [PubMed] [Google Scholar]
- Fearon D.T., Carroll M.C. 2000. Regulation of B lymphocyte responses to foreign and self-antigens by the CD19/CD21 complex. Annu. Rev. Immunol. 18:393–422 10.1146/annurev.immunol.18.1.393 [DOI] [PubMed] [Google Scholar]
- Fearon D.T., Carter R.H. 1995. The CD19/CR2/TAPA-1 complex of B lymphocytes: linking natural to acquired immunity. Annu. Rev. Immunol. 13:127–149 10.1146/annurev.iy.13.040195.001015 [DOI] [PubMed] [Google Scholar]
- Ferrarini M., Hoffman T., Fu S.M., Winchester R., Kunkel H.G. 1977. Receptors for IgM on certain human B lymphocytes. J. Immunol. 119:1525–1529 [PubMed] [Google Scholar]
- Gardiner E.E., Karunakaran D., Arthur J.F., Mu F.T., Powell M.S., Baker R.I., Hogarth P.M., Kahn M.L., Andrews R.K., Berndt M.C. 2008. Dual ITAM-mediated proteolytic pathways for irreversible inactivation of platelet receptors: de-ITAM-izing FcgammaRIIa. Blood. 111:165–174 10.1182/blood-2007-04-086983 [DOI] [PubMed] [Google Scholar]
- Haegert D.G. 1979. Phagocytic peripheral blood monocytes from rabbits and humans express membrane receptors specific for IgM molecules: evidence that incubation with neuraminidase exposes cryptic IgM (Fc) receptors. Clin. Exp. Immunol. 35:484–490 [PMC free article] [PubMed] [Google Scholar]
- Hamburger A.E., West A.P., Jr., Bjorkman P.J. 2004. Crystal structure of a polymeric immunoglobulin binding fragment of the human polymeric immunoglobulin receptor. Structure. 12:1925–1935 10.1016/j.str.2004.09.006 [DOI] [PubMed] [Google Scholar]
- Haury M., Sundblad A., Grandien A., Barreau C., Coutinho A., Nobrega A. 1997. The repertoire of serum IgM in normal mice is largely independent of external antigenic contact. Eur. J. Immunol. 27:1557–1563 10.1002/eji.1830270635 [DOI] [PubMed] [Google Scholar]
- Hitoshi Y., Lorens J., Kitada S.I., Fisher J., LaBarge M., Ring H.Z., Francke U., Reed J.C., Kinoshita S., Nolan G.P. 1998. Toso, a cell surface, specific regulator of Fas-induced apoptosis in T cells. Immunity. 8:461–471 10.1016/S1074-7613(00)80551-8 [DOI] [PubMed] [Google Scholar]
- Hjelm F., Carlsson F., Getahun A., Heyman B. 2006. Antibody-mediated regulation of the immune response. Scand. J. Immunol. 64:177–184 10.1111/j.1365-3083.2006.01818.x [DOI] [PubMed] [Google Scholar]
- Horejsí V., Zhang W., Schraven B. 2004. Transmembrane adaptor proteins: organizers of immunoreceptor signalling. Nat. Rev. Immunol. 4:603–616 10.1038/nri1414 [DOI] [PubMed] [Google Scholar]
- Kaetzel C.S. 2005. The polymeric immunoglobulin receptor: bridging innate and adaptive immune responses at mucosal surfaces. Immunol. Rev. 206:83–99 10.1111/j.0105-2896.2005.00278.x [DOI] [PubMed] [Google Scholar]
- Kikuno K., Kang D.W., Tahara K., Torii I., Kubagawa H.M., Ho K.J., Baudino L., Nishizaki N., Shibuya A., Kubagawa H. 2007. Unusual biochemical features and follicular dendritic cell expression of human Fcα/μ receptor. Eur. J. Immunol. 37:3540–3550 10.1002/eji.200737655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura T., Koshino Y., Shibata F., Oki T., Nakajima H., Nosaka T., Kumagai H. 2003. Retrovirus-mediated gene transfer and expression cloning: powerful tools in functional genomics. Exp. Hematol. 31:1007–1014 [PubMed] [Google Scholar]
- Kubagawa H., Burrows P.D., Cooper M.D. 1997. A novel pair of immunoglobulin-like receptors expressed by B cells and myeloid cells. Proc. Natl. Acad. Sci. USA. 94:5261–5266 10.1073/pnas.94.10.5261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamon E.W., Andersson B., Whitten H.D., Hurst M.M., Ghanta V. 1976. IgM complex receptors on subpopulations of murine lymphocytes. J. Immunol. 116:1199–1203 [PubMed] [Google Scholar]
- Lowenthal J.W., Malek T.R., Saragovi H. 2001. Measurement of lymphokine receptors. Curr. Protoc. Immunol. 6:1–15 [DOI] [PubMed] [Google Scholar]
- Maruyama S., Kubagawa H., Cooper M.D. 1985. Activation of human B cells and inhibition of their terminal differentiation by monoclonal anti-μ antibodies. J. Immunol. 135:192–199 [PubMed] [Google Scholar]
- Mathur A., Lynch R.G., Köhler G. 1988a. Expression, distribution and specificity of Fc receptors for IgM on murine B cells. J. Immunol. 141:1855–1862 [PubMed] [Google Scholar]
- Mathur A., Lynch R.G., Köhler G. 1988b. The contribution of constant region domains to the binding of murine IgM to Fcμ receptors on T cells. J. Immunol. 140:143–147 [PubMed] [Google Scholar]
- Minami Y., Samelson L.E., Klausner R.D. 1987. Internalization and cycling of the T cell antigen receptor. Role of protein kinase C. J. Biol. Chem. 262:13342–13347 [PubMed] [Google Scholar]
- Moretta L., Ferrarini M., Durante M.L., Mingari M.C. 1975. Expression of a receptor for IgM by human T cells in vitro. Eur. J. Immunol. 5:565–569 10.1002/eji.1830050812 [DOI] [PubMed] [Google Scholar]
- Moretta L., Webb S.R., Grossi C.E., Lydyard P.M., Cooper M.D. 1977. Functional analysis of two human T-cell subpopulations: help and suppression of B-cell responses by T cells bearing receptors for IgM or IgG. J. Exp. Med. 146:184–200 10.1084/jem.146.1.184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T., Kubagawa H., Ohno T., Cooper M.D. 1993. Characterization of an IgM Fc-binding receptor on human T cells. J. Immunol. 151:6933–6941 [PubMed] [Google Scholar]
- Nimmerjahn F., Ravetch J.V. 2008. Fcg receptors as regulators of immune responses. Nat. Rev. Immunol. 8:34–47 10.1038/nri2206 [DOI] [PubMed] [Google Scholar]
- Ochsenbein A.F., Fehr T., Lutz C., Suter M., Brombacher F., Hengartner H., Zinkernagel R.M. 1999. Control of early viral and bacterial distribution and disease by natural antibodies. Science. 286:2156–2159 10.1126/science.286.5447.2156 [DOI] [PubMed] [Google Scholar]
- Ohno T., Kubagawa H., Sanders S.K., Cooper M.D. 1990. Biochemical nature of an Fcμ receptor on human B-lineage cells. J. Exp. Med. 172:1165–1175 10.1084/jem.172.4.1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallasch C.P., Schulz A., Kutsch N., Schwamb J., Hagist S., Kashkar H., Ultsch A., Wickenhauser C., Hallek M., Wendtner C.M. 2008. Overexpression of TOSO in CLL is triggered by B-cell receptor signaling and associated with progressive disease. Blood. 112:4213–4219 10.1182/blood-2008-05-157255 [DOI] [PubMed] [Google Scholar]
- Pichler W.J., Knapp W. 1977. Receptors for IgM-coated erythrocytes on chronic lymphatic leukemia cells. J. Immunol. 118:1010–1015 [PubMed] [Google Scholar]
- Plaut A.G., Tomasi T.B., Jr 1970. Immunoglobulin M: pentameric Fcμ fragments released by trypsin at higher temperatures. Proc. Natl. Acad. Sci. USA. 65:318–322 10.1073/pnas.65.2.318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pricop L., Rabinowich H., Morel P.A., Sulica A., Whiteside T.L., Herberman R.B. 1993. Characterization of the Fcμ receptor on human natural killer cells. Interaction with its physiologic ligand, human normal IgM, specificity of binding, and functional effects. J. Immunol. 151:3018–3029 [PubMed] [Google Scholar]
- Proto-Siqueira R., Panepucci R.A., Careta F.P., Lee A., Clear A., Morris K., Owen C., Rizzatti E.G., Silva W.A., Jr., Falcão R.P., et al. 2008. SAGE analysis demonstrates increased expression of TOSO contributing to Fas-mediated resistance in CLL. Blood. 112:394–397 10.1182/blood-2007-11-124065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinowich H., Manciulea M., Metes D., Sulica A., Herberman R.B., Corey S.J., Whiteside T.L. 1996. Physical and functional association of Fcμ receptor on human natural killer cells with the ζ- and FcϵRI γ-chains and with src family protein tyrosine kinases. J. Immunol. 157:1485–1491 [PubMed] [Google Scholar]
- Ravetch J.V., Nimmerjahn F. 2008. Fc receptors and their role in immune regulation and inflammation. Fundamental Immunology. Sixth edition Paul W.E., editor Lippincott Williams & Wilkins, Philadelphia: 684–705 [Google Scholar]
- Reinherz E.L., Moretta L., Roper M., Breard J.M., Mingari M.C., Cooper M.D., Schlossman S.F. 1980. Human T lymphocyte subpopulations defined by Fc receptors and monoclonal antibodies. A comparison. J. Exp. Med. 151:969–974 10.1084/jem.151.4.969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roopenian D.C., Akilesh S. 2007. FcRn: the neonatal Fc receptor comes of age. Nat. Rev. Immunol. 7:715–725 10.1038/nri2155 [DOI] [PubMed] [Google Scholar]
- Sanders S.K., Kubagawa H., Suzuki T., Butler J.L., Cooper M.D. 1987. IgM binding protein expressed by activated B cells. J. Immunol. 139:188–193 [PubMed] [Google Scholar]
- Santana V. 1977. Receptors for IgM on murine lymphoid cells. Immunology. 32:273–278 [PMC free article] [PubMed] [Google Scholar]
- Shibuya A., Sakamoto N., Shimizu Y., Shibuya K., Osawa M., Hiroyama T., Eyre H.J., Sutherland G.R., Endo Y., Fujita T., et al. 2000. Fcα/μ receptor mediates endocytosis of IgM-coated microbes. Nat. Immunol. 1:441–446 10.1038/80886 [DOI] [PubMed] [Google Scholar]
- Spitaler M., Cantrell D.A. 2004. Protein kinase C and beyond. Nat. Immunol. 5:785–790 10.1038/ni1097 [DOI] [PubMed] [Google Scholar]
- Thompson J.D., Higgins D.G., Gibson T.J. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673–4680 10.1093/nar/22.22.4673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tony H.P., Schimpl A. 1980. Stimulation of murine B cells with anti-Ig antibodies: dominance of a negative signal mediated by the Fc receptor. Eur. J. Immunol. 10:726–729 10.1002/eji.1830100914 [DOI] [PubMed] [Google Scholar]
- Uher F., Dobronyi I., Gergel J. 1981. IgM-Fc receptor-mediated phagocytosis of rat macrophages. Immunology. 42:419–425 [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi Y., Kim H., Kato K., Masuda K., Shimada I., Arata Y. 1995. Proteolytic fragmentation with high specificity of mouse immunoglobulin G. Mapping of proteolytic cleavage sites in the hinge region. J. Immunol. Methods. 181:259–267 10.1016/0022-1759(95)00010-8 [DOI] [PubMed] [Google Scholar]
- Yelton D.E., Desaymard C., Scharff M.D. 1981. Use of monoclonal anti-mouse immunoglobulin to detect mouse antibodies. Hybridoma. 1:5–11 [DOI] [PubMed] [Google Scholar]