Abstract
Growth-restricted fetuses are at risk for a variety of lifelong medical conditions. Preeclampsia, a life-threatening hypertensive disorder of pregnancy, is associated with fetuses who suffer from intrauterine growth restriction (IUGR). Recently, emerging evidence indicates that preeclamptic women harbor AT1 receptor agonistic autoantibodies (AT1-AAs) that contribute to the disease features. However, the exact role of AT1-AAs in IUGR and the underlying mechanisms have not been identified. We report that these autoantibodies are present in the cord blood of women with preeclampsia and retain the ability to activate AT1 receptors. Using an autoantibody-induced animal model of preeclampsia, we show that AT1-AAs cross the mouse placenta, enter fetal circulation, and lead to small fetuses with organ growth retardation. AT1-AAs also induce apoptosis in the placentas of pregnant mice, human villous explants, and human trophoblast cells. Finally, autoantibody-induced IUGR and placental apoptosis are diminished by either losartan or an autoantibody-neutralizing peptide. Thus, these studies identify AT1-AA as a novel causative factor of preeclampsia-associated IUGR and offer two possible underlying mechanisms: a direct detrimental effect on fetal development by crossing the placenta and entering fetal circulation, and indirectly through AT1-AA–induced placental damage. Our findings highlight AT1-AAs as important therapeutic targets.
Intrauterine growth restriction (IUGR) is generally defined as fetal growth in <10th percentile for gestational age (Cetin et al., 2004) and affects 7–15% of pregnancies (Alexander et al., 2003; Cetin and Alvino, 2009). Growth-restricted fetuses have a higher incidence of mortality and morbidity than fetuses of normal growth, and are at increased risk for future development of metabolic disorders such as hypertension, coronary heart disease, dyslipidemia, obesity, impaired glucose tolerance, type 2 diabetes mellitus, and many other diseases (Barker, 1998; Godfrey and Barker, 2000; Baum et al., 2003; Hales and Ozanne, 2003). Most cases of IUGR, particularly those with significant recurrent risks, are often considered the result of ischemic placental disease (Roberts and Post, 2008; Cetin and Alvino, 2009). However, the factors contributing to placental distress and IUGR remain largely unknown.
IUGR and ischemic placentas are frequently associated with a serious hypertensive disorder of pregnancy, preeclampsia (Kaufmann et al., 2003). When IUGR is observed, the preeclamptic mothers often have a poorly developed placenta characterized by shallow trophoblast invasion and inadequate spiral artery remodeling (Zhou et al., 1997a; Zhou et al., 1997b). Therefore, preeclampsia represents an appropriate disease model to investigate the molecular basis of placental damage and IUGR. Alterations in both the immune system and the renin–angiotensin system (RAS) are believed to contribute to the pathophysiology of preeclampsia (Redman and Sargent, 2005; Shah, 2006; Saito et al., 2007, Irani and Xia, 2008). Recently, these two mechanisms have been merged with reports that preeclamptic women harbor autoantibodies that activate the major angiotensin II (Ang II) receptor, AT1, and are hence termed AT1 receptor agonistic autoantibodies (AT1-AAs; Wallukat et al., 1999). Many features of preeclampsia can be explained by the ability of these autoantibodies to activate AT1 receptor on a variety of cell types (Xia et al., 2003; Thway et al., 2004; Bobst et al., 2005; Zhou et al., 2008a). We have recently shown that the introduction of these autoantibodies into pregnant mice resulted in hypertension, proteinuria, and other key features of preeclampsia (Zhou et al., 2008b). The autoantibody-induced features of preeclampsia were prevented by coinjection with losartan, an AT1 receptor antagonist, or a 7-aa epitope peptide that blocks autoantibody-induced AT1 receptor activation. These in vivo studies provide the first direct evidence of the pathophysiological role of AT1-AAs in the maternal features of preeclampsia, and suggest that this animal model will be an extremely valuable investigative tool to analyze the underlying pathogenic mechanisms of various abnormalities associated with the disease. Thus, we used this animal model of preeclampsia to address the exact contributory role of AT1-AAs in IUGR and its underlying mechanisms.
In this paper, we show that AT1-AAs exist in the cord blood of women with preeclampsia and in the fetal circulation of autoantibody-injected pregnant mice. We also observed that the autoantibody-induced preeclamptic model results in IUGR with impaired multiple organ development. Our findings indicate that these pathogenic autoantibodies enter the fetal circulation, where they may have a direct detrimental effect on fetal growth and maturation. Additionally, we found that AT1-AAs impair placental development, resulting in organs characterized by increased apoptosis. These results were corroborated with similar findings in human placental villous explants and in cultured human trophoblast cells exposed to the autoantibody. These studies demonstrate that an abnormal placenta may be another underlying mechanism for AT1-AA–induced IUGR. Finally, autoantibody-induced fetal growth restriction and placental apoptosis were largely corrected by coinjection with either losartan or an antibody-neutralizing 7-aa epitope peptide, indicating that autoantibody-mediated AT1 receptor activation was required. Overall, our studies reveal the detrimental role of AT1-AAs in IUGR and reveal two underlying mechanisms for this process. These novel findings point to possible adverse effects of AT1-AAs on babies born to mothers with preeclampsia and identify these autoantibodies as potentially important therapeutic targets.
RESULTS
Autoantibodies from preeclamptic women are present in cord blood and retain the ability to activate AT1 receptors
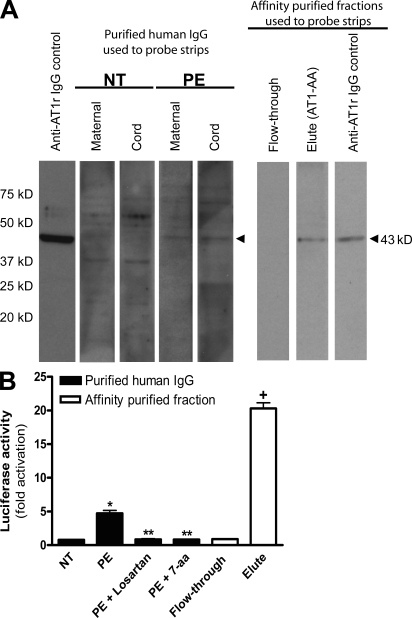
The placenta is a massive vascular organ that brings maternal and fetal circulatory systems into close proximity, facilitating the nutrient and gas exchange essential for fetal development (Georgiades et al., 2002). Maternal blood is supplied to the placenta via the uterine spiral arteries, and the fetus receives blood from the vessels present in the umbilical cord. Cord blood therefore represents a convenient source of fetal blood that is easily obtained at the time of parturition. To determine if AT1-AAs cross from the maternal to fetal circulation during pregnancy, we obtained maternal and cord blood from normotensive pregnant women and women with preeclampsia. Total IgG was isolated from serum and examined for the presence of AT1-AAs. The results of the Western blot (Fig. 1 A) show that total IgGs from maternal and cord sera of women with preeclampsia detected a band of 43 kD, corresponding to the AT1 receptor derived from cellular lysates enriched with the receptor that were transferred to a nitrocellulose membrane. In contrast, IgG from maternal and cord sera of women with normotensive pregnancies did not cross react with a protein of 43 kD (Fig. 1 A). To further confirm this result, an affinity chromatography strategy was used to specifically isolate AT1-AAs from the cord blood of fetuses from women with preeclampsia. The Western blot indicates that only the eluted fraction (AT1-AAs) could detect a band at 43 kD, corresponding to the AT1 receptor, whereas the flow-through fraction did not. These findings suggest that specific IgGs from women with preeclampsia that bind to the AT1 receptor can cross the placenta and enter the fetal circulation.
Figure 1.
AT1-AAs can pass through the human placenta and retain biological activity in fetal circulation. (A and B) Autoantibodies detected by Western blot in the sera of preeclamptic women could also be found in cord blood (A). Cellular lysate from CHO.AT1A cells containing stably integrated copies of a minigene encoding the AT1 receptor was run on a 10% SDS-PAGE gel and transferred to a nitrocellulose membrane. The AT1 receptor–rich membrane was cut into strips and each strip was individually probed by either an anti–AT1 receptor antibody (control), or purified antibodies derived from maternal serum or cord blood from normotensive (n = 6) or preeclamptic (n = 6) pregnancies. Also, to specifically detect AT1-AAs from the total IgG pool, an affinity purification strategy was used. The flow-through and eluted affinity-purified fractions of IgGs derived from the cord blood of babies from preeclamptic patients were also tested for their ability to bind to the AT1 receptor (n = 6). Only preeclamptic maternal sera, cord blood from babies of preeclamptic women, and the eluted affinity-purified fraction harbored autoantibodies recognizing the AT1 receptor at 43 kD. Then, cord blood IgGs were tested for biological activity (B) using an in vitro luciferase activity assay that is increased secondary to AT1 receptor activation. Only IgGs purified from the cord blood of babies from preeclamptic patients or the eluted affinity-purified fraction induced luciferase activity. This bioactivity could be blocked by co-culturing the reporter cell line with IgG derived from preeclamptic patients and losartan or the 7-aa epitope peptide (n = 5 for each variable in two independent experiments). Data are expressed as means ± SEM. *, P < 0.01 versus IgG derived from cord blood of a normotensive patient; **, P < 0.01 versus IgG derived from cord blood of a preeclamptic patient; +, P < 0.01 versus flow-through affinity-purified fraction. NT, normotensive; PE, preeclampsia.
To determine if the IgGs that enter the fetal circulation retain the ability to activate AT1 receptors, we incubated IgGs from cord blood with a reporter cell line in which AT1 receptor activation results in increased expression of a luciferase reporter gene. The results (Fig. 1 B) showed that luciferase activity was stimulated only when using IgG isolated from preeclamptic cord blood and that this activity was blocked by the presence of 100 nM losartan, an AT1 receptor antagonist. The activity was also inhibited by a 7-aa peptide that corresponds to an epitope on the second extracellular loop of the AT1 receptor that is recognized by autoantibodies from women with preeclampsia (Fig. 1 B). Similarly in the AT1-AA affinity purification experiment, only the eluted fraction of IgGs from the cord blood of babies born to preeclamptic women stimulated luciferase activity, whereas the flow-through fraction was unable to do so. Collectively, these findings demonstrated that AT1-AAs from the maternal circulation cross the placenta and enter fetal circulation, where they retain the ability to activate AT1 receptors.
Human AT1-AAs can cross the mouse placenta and enter fetal mouse circulation
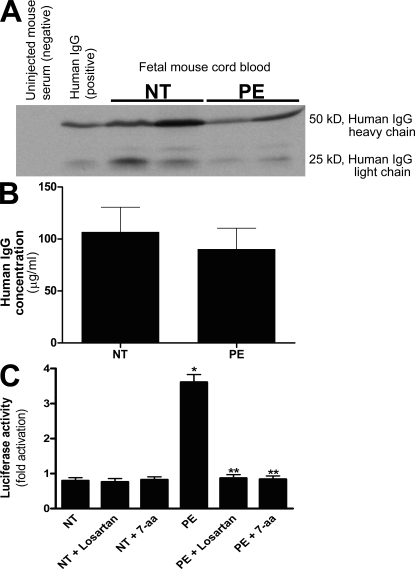
To determine if AT1-AAs can cross the mouse placenta and enter fetal circulation, we took advantage of the autoantibody-injected model of preeclampsia. Specifically, pregnant mice were injected with total IgG from normotensive pregnant women or from women with preeclampsia on embryonic day (E) 13 and E14. Blood was obtained from the dams and their fetuses upon sacrifice on E18 and examined for the presence of human IgG using Western blotting and ELISA. The results (Fig. 2, A and B) showed that similar amounts of human IgG were present in the maternal and fetal sera of antibody-injected pregnant mice. To determine if the preeclamptic IgG retained AT1 receptor agonistic activity after crossing the mouse placenta and entering fetal circulation, human IgG isolated from mouse fetal circulation was assayed for its ability to activate AT1 receptors using a reporter cell line in which AT1 receptor activation results in the activation of an NFAT-luciferase reporter gene. The results (Fig. 2 C) showed that IgG from the fetal blood of pregnant mice injected with preeclamptic IgG retained AT1 receptor agonistic activity. However, the fetuses of dams injected with IgG derived from normotensive patients harbored IgG that could not stimulate luciferase activity (Fig. 2 C). These data indicate that human AT1-AAs from women with preeclampsia cross the mouse placenta and enter fetal mouse circulation, where they retain the ability to activate AT1 receptors.
Figure 2.
Human IgG crosses the mouse placenta and can be detected in fetal mouse sera where it retains biological activity. (A–C) Human IgG could be detected in fetal mouse circulation by Western blotting (A) and ELISA (B). IgGs from normotensive or preeclamptic pregnant women were injected into pregnant mice at E13 and E14. Fetal sera were collected upon sacrifice on E18. Human IgG was detected in fetal mouse circulation (born to dams injected with human IgG) by Western blotting (A) using an anti–human IgG antibody, but not in the circulation of mice born to dams without human IgG injection. The concentration of human IgG in fetal mouse circulation was detected by an ELISA specific for human IgG (B). IgGs from both normotensive and preeclamptic pregnancies were detectable in fetal mice circulation, and the level of IgGs in fetal circulation between the two injected groups (n = 7 for each group in three independent experiments) was not significantly different (P > 0.05). However, the only group whose serum harbored antibodies that recognized the AT1 receptor and maintained the biological activity was the fetuses of preeclamptic IgG-injected mice (C). This was assessed by using a bioassay wherein AT1 receptor activation induces luciferase activity in transfected CHO-NFAT cells (n = 5 for each variable in two independent experiments). Data are expressed as means ± SEM. *, P < 0.01 versus normotensive IgG treatment; **, P < 0.01 versus preeclamptic IgG treatment. NT, normotensive; PE, preeclampsia.
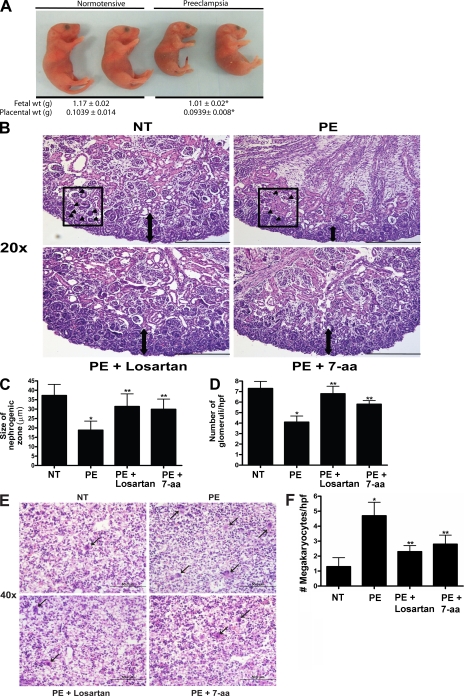
Injection of affinity-purified AT1-AAs into pregnant mice results in small fetuses and adversely affects kidney and liver development
Because of the process of passive immunity between mother and developing child, the potentially harmful autoantibodies associated with preeclampsia may enter fetal circulation and have a direct detrimental effect on the growing fetus. To investigate this possibility, affinity-purified AT1-AAs were injected into pregnant mice on E13 and E14, and the injected mice were examined 5 d later for the clinical signs of preeclampsia and their fetuses were examined for abnormalities of growth. The results (Fig. 3) show that autoantibody-injected mice bore fetuses of reduced weight (1.01 ± 0.02 g) as compared with dams injected with normotensive IgG (1.17 ± 0.02 g). Coinjecting AT1-AAs with either losartan or 7-aa epitope peptide restored fetal size to 1.119 ± 0.01 g and 1.151 ± 0.04 g, respectively. Furthermore, the fetuses exhibited renal and hepatic developmental delays. Histological analysis of fetal kidneys showed that the zone of nephrogenesis was narrowed and the number of glomeruli was decreased in the kidneys of fetuses born to mice injected with affinity-purified AT1-AAs (Fig. 3, B–D). These findings suggest that the renal development of these fetuses had been retarded (Alexander, 2003). Similarly, the livers of fetuses born to dams injected with AT1-AAs show developmental impediment. During normal mouse development, the fetal liver is the major site of embryonic blood production. From E10 to birth, the number of megakaryocyte progenitors begins to decrease with advancing gestational age (Matsumura and Sasaki, 1989). Our histological results showed that the injection of affinity-purified AT1-AAs from women with preeclampsia into pregnant mice is associated with a consistently elevated number of megakaryocytes in the fetal mouse liver (Fig. 3, E and F). This persistence of megakaryocytes suggests a delay in normal organ maturation. Thus, the autoantibody injection model of preeclampsia in pregnant mice has provided in vivo evidence that AT1-AAs can adversely affect fetal growth and organ development. Notably, both human and mouse studies indicate that AT1-AAs pass through the placenta and enter fetal circulation, where they retain biological activity. These findings reveal a previously unrecognized possible underlying mechanism of AT1-AA–induced IUGR: that the autoantibody may have direct detrimental effects on fetal development by crossing the placenta and entering fetal circulation.
Figure 3.
AT1 receptor activation results in decreased fetal weight and impaired organ development. AT1 receptor activation results in fetal abnormalities. (A) Injection of pregnant mice with IgGs from preeclamptic women resulted in fetuses weighing less than those born to pregnant mice injected with IgG derived from normotensive women. Only pups born in litters of six to eight were analyzed, and those depicted in the figure were selected from the center of the uterine horn (preeclamptic fetuses, n = 89; and normotensive fetuses, n = 80, collected and analyzed in four independent experiments). *, P < 0.05 versus normotensive IgG treatment. (B) Fetal mouse kidney histology (H&E staining). Bars, 50 µm. (C and D) As compared with the fetal kidneys from normotensive IgG injection litters, the kidneys from litters of preeclamptic IgG injection dams demonstrated a narrow nephrogenic zone (C) and a decreased number of glomeruli (D). The double arrows in B demarcate the nephrogenic zone. Arrowheads within the boxes indicate a glomerulus within a representative high power field. (E) Fetal mouse liver histology (H&E staining). Bars, 50 µm. (F) Compared with the livers of pups born to dams injected with normotensive IgG, the livers from pups of preeclamptic IgG injection dams showed an increased number of megakaryocytes. Arrows indicate megakaryocytes. When losartan (PE + Losartan) or the 7-aa peptide (PE + 7-aa) were coinjected into the dams, the fetal kidney and liver alterations were partially abolished (n = 12 for each variable for the histological analysis in four independent experiments). Data are expressed as means ± SEM. *, P < 0.05 versus normotensive IgG treatment; **, P < 0.05 versus preeclamptic IgG treatment. hpf, high power field; NT, normotensive; PE, preeclampsia.
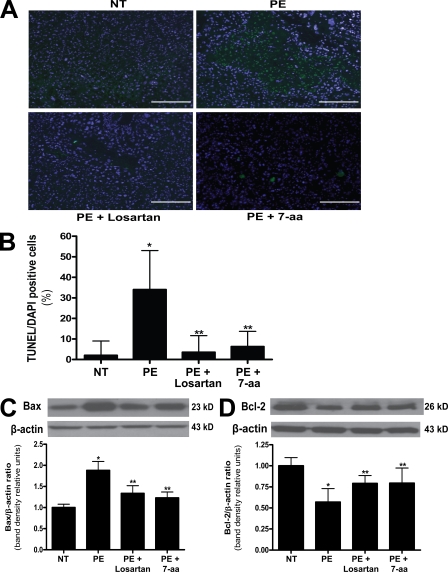
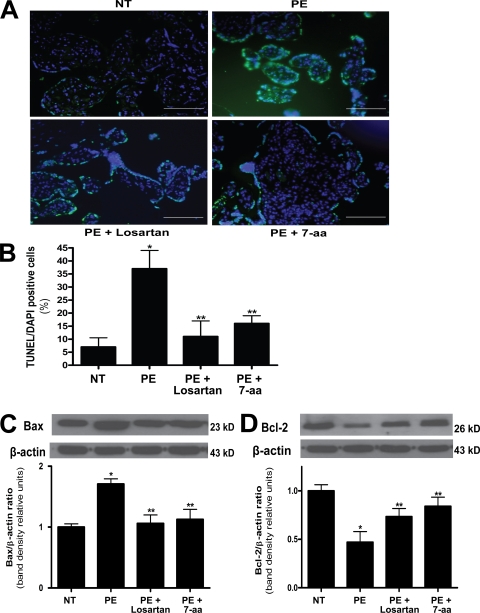
AT1-AA–increased placental apoptosis in pregnant mice and human villous explants
Placental health is vital for normal fetal development. In an effort to determine whether an impaired placenta is another potential underlying mechanism for AT1-AA–induced IUGR, we evaluated the size and morphology of placentas in the autoantibody-injected mouse model of preeclampsia. The results (Fig. 3) show that placentas from pregnant mice injected with AT1-AAs were significantly smaller (0.0939 ± 0.008 g) than placentas from mice injected with a comparable amount of total IgG from normotensive pregnant women (0.1039 ± 0.014 g). In addition, coinjection of AT1-AAs with either losartan or 7-aa epitope peptide restored placental size to 0.0991 ± 0.009 g and 0.105 ± 0.023 g, respectively. To determine whether increased apoptosis is a potential cause of the small placentas observed in AT1-AA–injected pregnant mice, we performed terminal deoxynucleotidyl transferase–mediated dUTP nick-end labeling (TUNEL) staining for apoptotic cells and found that the reduction in placental size was accompanied by an increase in apoptosis evident in the labyrinth zone of placentas from mice injected with AT1-AAs (Fig. 4, A and B). In addition, Bax, a proapoptotic marker, was increased, and Bcl-2, an antiapoptotic marker, was decreased as determined by Western blot analysis of mouse placenta protein extracts (Fig. 4, C and D). Coinjection of AT1-AAs with losartan or 7-aa epitope peptide significantly inhibited these features. Therefore, increased apoptosis may contribute to the reduction in placental size in pregnant mice injected with AT1-AAs. These findings also suggest that an impaired placenta may indirectly be another underlying mechanism of AT1-AA–induced IUGR in pregnant mice.
Figure 4.
Mouse placentas demonstrate increased apoptosis caused by AT1 receptor activation. The placentas of AT1-AA–injected pregnant mice have increased apoptosis. (A) TUNEL-stained mouse placental sections demonstrate increased apoptosis in the labyrinth zone of mice injected with IgG from preeclamptic women as compared with normotensive IgG-injected mice (green, TUNEL+; blue, DAPI nuclear stain). Bars, 500 µm. (B) This qualitative increase in apoptosis is quantified and corroborated with an increased apoptotic index (percentage of TUNEL-/DAPI-positive cells) as measured in the same mouse placental sections (n = 12 placentas for each variable collected over four independent experiments). (C and D) Western blot analysis of mouse placentas indicates that AT1 receptor activation leads to increased Bax (C) and decreased Bcl-2 (D; n = 6 for each variable collected over four independent experiments). Mice coinjected with IgG from preeclamptic women and either losartan or 7-aa epitope peptide have placentas that demonstrate less apoptotic features. Data are expressed as means ± SEM. *, P < 0.05 versus normotensive IgG treatment; **, P < 0.05 versus preeclamptic IgG treatment. NT, normotensive; PE, preeclampsia.
To evaluate the pathophysiologic significance of the role of AT1-AAs in impaired placental development in preeclampsia, human placenta villous explants from healthy term pregnancies were incubated with IgG from normotensive pregnant women or IgG from women with preeclampsia. After incubation with the Igs, placental villous explants were embedded and sectioned to perform TUNEL staining. The results (Fig. 5, A and B) show that the presence of IgGs from women with preeclampsia increased apoptosis in these explants. The autoantibody-induced increase in apoptosis was inhibited by coincubation with losartan or the 7-aa epitope peptide. The histological evidence for apoptosis was corroborated with Western blot analysis indicating an increase in Bax and a decrease in Bcl-2 proteins, resulting in a proapoptotic state in these explants (Fig. 5, C and D). Similarly, cotreatment with losartan or the 7-aa neutralizing epitope peptide partially abolished the alterations in Bax and Bcl-2 proteins induced by these autoantibodies. These studies demonstrate that AT1-AAs are capable of inducing apoptosis in human placenta explants through AT1 receptor activation and may potentially contribute to IUGR via this mechanism in humans.
Figure 5.
Human villous explants exhibit increased apoptosis caused by AT1 receptor activation. AT1 receptor activation induces human placental apoptosis. (A) Human villous explants cultured with IgG derived from preeclamptic patients demonstrate increased apoptosis as compared with explants incubated with IgG derived from normotensive sera. TUNEL-stained cultured human villous explants indicate that AT1-AAs, through AT1-receptor activation, increase apoptosis (green, TUNEL+; blue, DAPI nuclear stain). Bars, 500 µm. (B) Quantification of the increased apoptosis is reflected in an increased apoptotic index in the explants incubated with PE IgG (B). (C and D) Western blot analysis of explant proteins demonstrate increased Bax (C) and decreased Bcl-2 (D), indicating a proapoptotic state. Coincubation of the explants with preeclamptic IgG and losartan or 7-aa epitope peptide partially attenuated the increase in cell death. Explants of placentas from four different patients were cultured, and each variable was examined six times per placenta (n = 24). Data are expressed as means ± SEM. *, P < 0.05 versus normotensive IgG treatment; **, P < 0.05 versus preeclamptic IgG treatment. NT, normotensive; PE, preeclampsia.
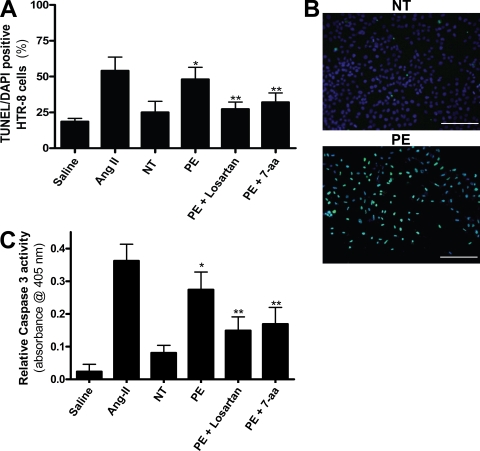
AT1 receptor activation induces apoptosis in human trophoblast cells
Many studies report that human trophoblast cells house the full machinery for apoptotic cell death (Allaire et al., 2000; Huppertz et al., 2006; Kadyrov et al., 2006). They also possess the AT1 receptor (Li et al., 1998; Xia et al., 2002). To further elucidate the mechanism by which cell death may occur in the placenta of a preeclamptic woman, we monitored the levels of apoptosis in trophoblasts after stimulation with IgG purified from preeclamptic or normotensive pregnant individuals. Interestingly, IgG purified from preeclamptic individuals induced an increase in programmed cell death in an immortalized human trophoblast line, HTR-8/SVneo (Fig. 6, A and B). This is in contrast to IgG from normotensive pregnant women, which did not raise the level of apoptosis as assessed by a TUNEL assay and apoptotic index. These findings reveal that AT1 receptor activation is capable of inducing apoptosis in trophoblast cells. Consistent with the findings in mouse placental sections and human placental villous explants, we found that the increased apoptosis in the human trophoblast cells by AT1-AAs was inhibited by coincubation with losartan or the 7-aa epitope peptide (Fig. 6, A and B). In addition, the activity of caspase 3, a rapidly activated cysteine protease essential for cell death, was measured in the cultured trophoblast cells to corroborate the TUNEL assay results. HTR-8/SVneo cells incubated with IgG from preeclamptic women exhibited a higher caspase 3 activity level over those incubated with IgG isolated from normotensive pregnant women (Fig. 6 C). The coincubation of the cultured trophoblast cells with AT1-AAs and losartan or the 7-aa epitope peptide reduced the caspase 3 activity significantly. These data support the findings obtained from the human placental villous explants that demonstrate increased apoptosis when exposed to the autoantibody (Fig. 5). Overall, these results provide strong in vitro evidence that autoantibodies isolated from the sera of preeclamptic women are capable of activating AT1 receptors on human trophoblast cells and inducing their programmed cell death.
Figure 6.
AT1 receptor activation increases trophoblast cell apoptosis. AT1 receptor activation induces apoptosis in HTR-8/SVneo cells, a human trophoblast cell line. (A–C) HTR-8/SVneo cells cultured with IgG derived from preeclamptic patients demonstrate increased apoptosis as compared with explants incubated with IgG derived from normotensive sera. A cell death index (A) based on TUNEL-stained HTR-8/SVneo cells (B) indicates that AT1 receptor activation increases apoptosis. Caspase 3 activity (C) is also increased in HTR-8/SVneo cells cultured with IgG from preeclamptic patients as compared with those cultured with IgG from normotensive patients. Coincubation of PE IgG with losartan or 7-aa epitope peptide reduces the amount of apoptosis as well as caspase 3 activity (green, TUNEL+; blue, DAPI nuclear stain; n = 12 for each variable in three independent experiments). Data are expressed as means ± SEM. *, P < 0.05 versus normotensive IgG treatment; **, P < 0.05 versus preeclamptic IgG treatment. NT, normotensive; PE, preeclampsia. Bars, 500 µm.
Inhibition of AT1 receptor activation or neutralization of AT1-AAs reduced the IUGR and placental apoptosis seen in autoantibody-injected pregnant mice: a novel therapeutic strategy
Lastly, to determine the direct pathogenic role of AT1-AAs and the benefit of inhibiting AT1 receptor activation in IUGR, we coinjected losartan, a specific AT1 receptor antagonist, with AT1-AAs into pregnant mice at E13 and E14. We found that the coinjection of losartan attenuated the reduced placental size associated with autoantibody-induced placental apoptosis and the increased expression of Bax and the decreased expression of Bcl-2 (Fig. 4). At the same time, the coinjection of AT1-AAs and losartan into pregnant mice also ameliorated the reduction in fetal size and developmental impairment of fetal kidneys and liver (Fig. 3). Collectively, these results demonstrate that impaired fetal growth and increased placental apoptosis were mediated by autoantibody-induced AT1 receptor activation and suggest that blocking autoantibody-mediated AT1 receptor activation may be a novel effective therapy for treating the IUGR associated with preeclampsia.
Although losartan is contraindicated during early pregnancy because of its fetotoxic effects (Spence et al., 1995; Saji et al., 2001), drugs aimed at neutralizing the effects of AT1-AAs would not be expected to have harmful effects on the developing fetus. Because the 7-aa neutralizing peptide blocked the apoptosis induced by AT1-AAs in cultured human villous explants (Fig. 5), we speculated that the epitope peptide may be effective in blocking the placental apoptosis and IUGR seen in AT1-AA–injected pregnant mice. For this reason, we conducted experiments in which pregnant mice were coinjected with AT1-AAs and excess 7-aa epitope peptide on E13 and E14 to block the ability of the autoantibody to activate AT1 receptors. 5 d later, on E18, pregnant mice were sacrificed and their pups and placentas were collected. The results show that the 7-aa epitope peptide blocked placental apoptosis and alterations of apoptotic proteins, and small fetuses with impaired organ development (Fig. 3). The neutralizing effects of losartan and 7-aa epitope peptide indicate that AT1-AAs contribute to the IUGR associated with preeclampsia and suggest that blockade of the detrimental autoantibody effects may yield a novel therapeutic strategy for this life-threatening disease.
DISCUSSION
In this study, we discovered that biologically active autoantibodies cross the placenta and enter fetal circulation in preeclamptic patients. We used an autoantibody injection mouse model of preeclampsia to identify the detrimental role of AT1-AAs in IUGR. We show that these autoantibodies may contribute to fetal growth restriction via two previously unrecognized mechanisms: (a) directly, by crossing the mouse placenta and entering fetal mouse circulation as we see in humans, and (b) indirectly, via AT1-AA–induced placental impairment characterized by increased apoptosis. Finally, autoantibody-induced fetal growth restriction and placental apoptosis were largely corrected by either losartan or an antibody-neutralizing 7-aa epitope peptide, indicating that autoantibody-induced AT1 receptor activation was required. Thus, our studies reveal the detrimental role of AT1-AAs in preeclampsia-associated IUGR, offer underlying mechanisms for this process, and suggest a novel therapeutic strategy based on these autoantibody-induced mechanisms.
During the third trimester of pregnancy, antibodies generated by the mother cross the placenta and enter fetal circulation. The transfer of Ig from mother to fetus is a naturally occurring process by which the mother confers immunity to the developing fetus. This process of passive immunity puts the growing fetus at risk if the mother carries autoantibodies that are harmful. A well-known example of this is Graves’ disease, where maternal autoantibodies that activate the thyroid-stimulating hormone receptor pass through the placenta, enter the fetal circulation, and cause hyperthyroidism in the fetus and newborn (Chistiakov, 2003). Other autoimmune diseases, such as systemic lupus erythematosus, Sjögren’s syndrome, and antiphospholipid syndrome, are associated with the pathogenic autoantibodies that result in severe complications of pregnancy, often resulting in severe IUGR and fetal loss (Siamopoulou-Mavridou et al., 1988; Deleze et al., 1989; Julkunen et al., 1995; Holers et al., 2002). Because preeclampsia is also associated with the presence of pathogenic autoantibodies (i.e., AT1-AAs) in the maternal circulation, it is possible that these autoantibodies may be transferred to the fetus and cause harm. Using an autoantibody injection model of preeclampsia in the pregnant mouse, we have shown in this study that AT1-AAs are transported from the maternal circulation through the placenta into the fetal circulation, where they can be physically and biologically detected. The research reported in this paper shows that the presence of these autoantibodies in the fetal mouse circulation is associated with smaller fetuses who suffer from renal and liver abnormalities. Thus, these autoantibodies may also have direct detrimental effects on the fetuses of women with preeclampsia.
In this paper, we have shown that AT1-AAs induce apoptosis in cultured human trophoblast cells, in human villous explants, and in the placentas of autoantibody-injected pregnant mice. Thus, the impaired placenta is likely another underlying mechanism responsible for AT1-AA–induced IUGR. In both the cardiac and renal systems, Ang II has been shown to increase cell death through various proapoptotic pathways (Ruiz-Ortega et al., 1998; Kalra et al., 2002). In addition, trophoblasts house the AT1 receptor as well as all the machinery necessary to undergo apoptosis (DiFederico et al., 1999; Huppertz et al., 2006). Therefore, it was not unexpected that AT1-AAs were capable of inducing trophoblast cell death via AT1 receptor activation. Poor placental development is associated with a proapoptotic placental environment that includes increased production of Bax and decreased production of Bcl-2 (Huppertz and Kingdom, 2004). These features contribute to a suboptimal environment for placental development and result in an impaired placenta. Autoantibody-induced AT1 receptor activation may contribute to impaired placental development in other ways, such as decreased angiogenesis (Zhou et al., 2007), decreased trophoblast invasion (Xia et al., 2003), increased production of reactive oxygen species (Sedeek et al., 2003; Sedeek et al., 2008), and increased thrombosis (Shaarawy and Didy, 1996; Bobst et al., 2005). These reports are in agreement with double-transgenic mouse studies that show that increased placental renin production leads to increased Ang II in the maternal circulation and results in impaired, highly apoptotic placentas and pups suffering from IUGR (Takimoto et al., 1996; Saito et al., 2004). Although preeclampsia in humans is not associated with increased Ang II levels (Langer et al., 1998), as in this double-transgenic mouse model, human preeclampsia is associated with the presence of autoantibodies, AT1-AAs, that mimic the physiological action of Ang II (Wallukat et al., 1999). Therefore, the additive actions of Ang II and AT1-AAs may result in elevated AT1 receptor activation and could contribute to impaired placental development and IUGR. This concept is supported by the observations reported in this paper and suggest that autoantibody-induced AT1 receptor activation likely contributes to impaired placental development and is an underlying mechanism for the IUGR observed in preeclampsia.
AT1 receptor activation is also related to another factor associated with the small placentas and IUGR of severe preeclampsia: reduced aa transport. 20–40% of the energy needed for fetal growth is supplied from aa’s found in the maternal circulation (Bauer et al., 1998). If the aa transporter systems of the placenta are impaired, the developing fetus is nutritionally starved and is at risk for growth defects. Numerous aa transport systems are Na+ dependent and couple the uptake of aa’s with that of Na+ into the cell. The Na+-K+-ATPase is highly abundant in most cell types, including placental syncytiotrophoblasts (Johansson et al., 2000), where they maintain a low intracellular Na+ concentration by transporting Na+ outside the cells and provide a Na+ gradient, the essential driving force for Na+-dependent aa transport systems. Recent studies have not only shown that the Na+-K+-ATPase is down-regulated in cases of IUGR (Johansson et al., 2003) but that the inhibitory effect on Na+-dependent aa transport systems may be through AT1 receptor signaling. Of particular interest is a study by Shibata et al. (2006) that reports that Ang II inhibits system A aa transporter activity in human placental villous fragments through AT1 receptor activation. The system A aa transporter is a Na+-dependent aa transporter that mediates transport of the small neutral aa’s alanine, serine, glutamine, and glycine by human syncytiotrophoblasts. The authors show that Ang II, via AT1 receptor signaling, decreases system A activity by suppressing Na+-K+-ATPase activity in human placental villi. Presumably, other Na+-dependent aa transport systems would also be inhibited by enhanced AT1 receptor activation. Shibata et al. (2006) suggest that their findings may account for the adverse affects of enhanced AT1 receptor activation on fetal growth. They specifically suggest that one possible source of excess AT1 receptor activation in IUGR associated with preeclampsia is the presence of AT1-AAs in the maternal circulation. The research we report in this paper adds support to their speculation.
Other examples of IUGR, including fetal loss, involve the deleterious effects of autoantibodies at the maternal–fetal interface. One of the best-studied examples is that of patients with antiphospholipid syndrome, characterized by thrombosis and recurrent fetal loss (Salmon and Girardi, 2008). It is reported that recurrent fetal loss occurs in ∼1% of pregnant women and that up to 20% of these women have antiphospholipid antibodies (Girardi et al., 2003). Using an antibody injection model of fetal loss in pregnant mice, Girardi et al. have shown that these autoantibodies are directed at targets in the decidua and lead to the activation of complement, the recruitment of neutrophils, and the enhanced production of tissue factor, TNF, antiangiogenic factors, and reactive oxygen species, all of which lead to fetal loss (Redecha et al., 2007). It is interesting to note that these same features are also induced by AT1-AAs in a variety of systems (Sedeek et al., 2003; Zhou et al., 2007; Sedeek et al., 2008). Thus, these autoantibody-induced models of IUGR including fetal loss (perhaps resulting from a severe impairment of placental development) have shown that immunological factors, including components of the innate and adaptive arms of the immune system, contribute to pregnancy loss and IUGR. In each case, the sequence of events leading to IUGR is initiated by a maternal autoantibody. Together, AT1-AAs and antiphospholipid antibodies may account for one third of the cases of IUGR and pregnancy loss (Holers et al., 2002). The role of autoantibodies in other cases of IUGR, impaired placental development, and/or fetal loss warrants further investigation.
Losartan and the 7-aa epitope peptide were used to assess the specificity of the observed autoantibody-induced effects. Coinjection of AT1-AAs with the AT1 receptor blocker losartan resulted in diminished placental destruction and fetal abnormalities, indicating that effects were mediated by autoantibody-induced AT1 receptor activation. Consistent with the in vivo experiments, coincubation of losartan and AT1-AAs reduced apoptosis in the human villous explant and cell-culture systems. Similar findings were observed by coinjection of AT1-AAs and the 7-aa epitope peptide. It is unlikely that this peptide would be stable in the circulation of the injected mice if it were not in a complex with the autoantibody, which is why it is coinjected simultaneously. It will be necessary to synthesize stable derivatives of this peptide for use in preclinical studies and potential clinical trials. More research is needed to exploit the therapeutic potential suggested by the result of our peptide-blocking experiments. Collectively, the ability of losartan and 7-aa epitope peptide to reduce harmful autoantibody effects provide additional evidence that AT1-AAs contribute to the IUGR and placental impairment associated with preeclampsia. More importantly, the ability of the 7-aa epitope peptide to neutralize the effects of AT1-AAs represents a potential therapeutic approach to block the autoantibody-mediated AT1 receptor activation seen in preeclampsia and the associated IUGR.
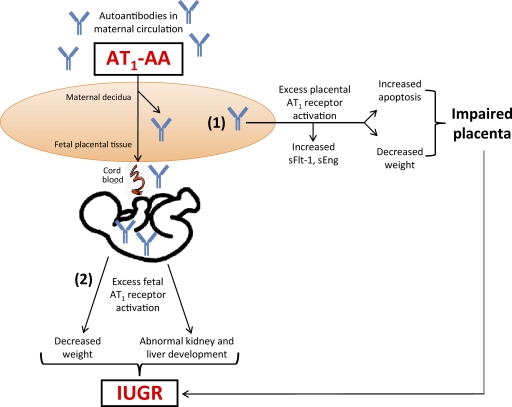
In conclusion, our study demonstrates that AT1-AAs may contribute to IUGR through a direct detrimental effect by activation of AT1 receptors on multiple fetal organs, and indirectly by inducing small placentas characterized by increased trophoblast apoptosis (Fig. 7). Future work will have to delineate between the placental effects and the direct harmful action of the autoantibody on the fetus. However, there is no doubt that our studies identify AT1-AA as a detrimental factor that contributes to IUGR. Furthermore, we have shown that the blockade of excessive AT1 receptor stimulation by losartan or the 7-aa epitope peptide not only reduced the placental apoptosis observed in mouse and human placentas but also the fetal abnormalities seen in the autoantibody-injected mouse model. Thus, our work identified the detrimental role of AT1-AAs in preeclampsia-associated IUGR and revealed underlying mechanisms for this process. Selective neutralization of these autoantibodies would enhance our ability to intervene in IUGR without fetotoxicity, and suggests that targeting AT1-AAs is a potentially important therapeutic strategy for the treatment of preeclampsia and its devastating clinical fetal complications.
Figure 7.
Schematic of the working model of AT1 receptor–mediated apoptosis: placental and fetal consequences. AT1 receptor activation via AT1-AAs, found in the serum of preeclamptic women, leads to (1) placental damage (2) and fetal abnormalities. sEng, soluble endoglin; sFlt-1, soluble fms-like tyrosine kinase 1.
MATERIALS AND METHODS
Patients.
Patients admitted to Memorial Hermann Hospital were identified by the obstetrics faculty of the University of Texas Medical School at Houston Health Science Center. Patients were diagnosed with severe preeclampsia based on the blood pressure criteria set by the National High Blood Pressure Education Program Working Group (2000; n = 12). Inclusion criteria were a blood pressure reading of 160 mmHg systolic or higher or 110 mmHg diastolic or higher in women who were normotensive before 20 wk gestation age (WGA). Blood pressure measurements were taken on two occasions at least 6 h apart. An absolute level of proteinuria measuring 0.3 g of protein or higher in a 24-h urine specimen was another requirement for diagnosis. This value normally corresponds to ≥30 mg/dl (≥1 + reading on dipstick) in a random urine determination with no evidence of urinary tract infection. These women had no previous history of hypertension. The working group report does not strictly define early or late onset subclassifications. However, our cohort of preeclamptic patients included women with symptom onset <30 WGA (n = 4) and those ≥30 WGA (n = 8). Preeclamptic sera were used as a source for AT1-AA, and similar concentrations of the autoantibody were found in both groups. Control patients were normotensive pregnant individuals with uncomplicated pregnancies who were undergoing normal term deliveries (n = 12). The clinical characteristics of the study patients are presented in Table I. Blood samples were drawn, allowed to clot, and subsequently centrifuged at 18,000 g at 4°C for 10 min. Sera were collected and stored at −80°C. The research protocol, including the informed consent form, was approved by the University of Texas Health Science Center at Houston institutional Committee for the Protection of Human Subjects.
Table I.
Clinical characteristics of patients
| Normotensive (n = 12) | Preeclamptic (n = 12) | |
| Maternal age (yr) | 24 ± 4.71 | 28.42 ± 6.13 |
| Gestation age (wk) | 38.63 ± 0.98 | 31.21 ± 2.8 |
| Primigravid (%) | 33 | 25 |
| Systolic blood pressure (mmHg) | 118.2 ± 4.97 | 181.3 ± 16.93* |
| Diastolic blood pressure (mmHg) | 67.33 ± 6.21 | 106.8 ± 13.22* |
| Proteinuria (mg/24 h) | <300 | 554.5 ± 800.7 |
| Fetal weight (g) | 3,347 ± 429 | 1,437 ± 390 |
| Weight by gestational age (percentile) | 58.1 ± 31 | 20.2 ± 18* |
| Number of babies born at <10th percentile (n [%]) | 1 (8%) | 6 (50%)* |
| Luciferase activity of patient serum (fold induction) | 1.64 ± 0.97 | 7.68 ± 0.34* |
This table illustrates the key differences between the two patient populations used in the experiments. The preeclamptic women demonstrate increased blood pressure, proteinuria, and luciferase activity, which reflects the biological activity of AT1-AA. 6 out of the 12 babies (50%) born to the preeclamptic cohort weighed <10th percentile for their gestational age, whereas only 1 out of 12 of babies (8%) born to the normotensive cohort were considered IUGR. *, P < 0.05.
Reagents.
Cell-culture medium, antibiotics, and FBS were purchased from Invitrogen. Ang II was purchased from Sigma-Aldrich. Losartan was a generous gift from Merck & Co., Inc. A short 7-aa peptide corresponding to an epitope on the second extracellular loop of the human AT1 receptor (AFHYESQ) was purchased from Baylor College of Medicine (Houston, TX).
Preparation of IgG fraction from patient sera.
IgG fractions were isolated from patient sera as previously described (Zhou et al., 2008b) using GammaBind G Sepharose (GE Healthcare). Typically, 200 µl of patient sera was applied to the column matrix and eluted in 1.8 ml of buffer according to the manufacturer’s recommended protocol. The isolated IgG was assayed for the presence of AT1-AA using the luciferase reporter cell line described in Bioassay to determine the presence of AT1-AA. According to this assay, nearly all of the women with severe preeclampsia (25 out of 26) harbored AT1-AAs, whereas less than one third of the normotensive pregnant women possessed AT1-AAs, and in these women the autoantibody concentration was significantly reduced (Siddiqui et al., In press). IgG fractions from individual patients were used separately for the experiments reported in this paper. Individual patient IgG preparations were not pooled.
Affinity purification of AT1-AA from human IgG.
The ability of the 7-aa epitope peptide to block autoantibody-induced receptor activation suggests a physical association between the autoantibodies and AT1 receptors. We took advantage of this presumed physical association as the basis of an affinity purification strategy to obtain highly enriched preparations of AT1-AAs. For this purpose, we prepared a glutathione S-transferase (GST) fusion protein containing a 27-aa peptide encoding the entire second extracellular loop of the AT1 receptor. The construction and expression of the GST-27mer AT1 receptor fusion protein and its subsequent use in AT1-AA affinity purification was performed as previously described (Zhou et al., 2008b). In brief, a pGEX-4T-1 GST expression vector (Promega) containing the GST-27mer-AT1 receptor fusion protein encoding 27 aa’s corresponding to the second extracellular loop of the human AT1 receptor (available from GenBank/EMBL/DDBJ under accession no. NM_009585.2) was used to transform BL21 DE3 Escherichia coli cells (Stratagene). After induction and collection of cells, the GST-27mer-AT1 receptor fusion protein was isolated by glutathione beads (GE Healthcare) and its expression was confirmed by Western blotting. The GST-27mer AT1 receptor fusion protein was then linked to agarose beads by a microlink protein coupling kit (Thermo Fisher Scientific) according the manufacturer’s protocol and used in an affinity chromatography column. Total IgG isolated from preeclamptic or normotensive patient sera was loaded on the affinity chromatography column and incubated for 3 h at room temperature. The flow-through fraction was collected and the bound IgG (AT1-AA) was then eluted by centrifugation. The affinity of AT1-AA for the GST-27mer–AT1 receptor fusion protein was exploited in an affinity purification scheme resulting in a 40-fold enrichment of AT1-AA in the eluted fraction. Only the eluted fraction (affinity-purified AT1-AA) was able to bind to AT1 receptors transferred to a nitrocellulose membrane. In contrast, the affinity chromatography flow-through fraction could not bind to the 43-kD AT1 receptor and was not detected by Western blot analysis. To confirm which fraction retained biological activity, we used an established luciferase activity assay. Only the preeclamptic eluted fraction (containing AT1-AAs) could stimulate luciferase activity, whereas the other fractions could not. This fraction was used in the subsequent experimentation.
Bioassay to determine the presence of AT1-AA.
Chinese hamster ovary (CHO) cells stably transfected with rat Ang II receptor type 1A (CHO.AT1A) were provided by T.S. Elton (The Ohio State University, Columbus, OH). Cells were maintained and cultured in RPMI 1640 medium containing 5% FBS, 1% antibiotics, 8.75 g/liter proline and 100 µg/ml gentamycin and hygromycin at 37°C and 5% CO2. Cells were stably transfected with an NFAT-luciferase reporter construct containing four copies of the NFAT binding element driving the expression of a luciferase reporter gene. 105 CHO.AT1A cells containing stably integrated copies of a minigene encoding the rat AT1 receptor and a 4× NFAT-driven luciferase construct were plated on 24-well plates overnight. The next day, cells were changed to serum-free medium and treated with IgG (1:10 dilution) for 24 h. The treated cells were lysed in 100 µl of passive lysis buffer (Promega) at room temperature for 45 min. Luciferase activity (measured in relative light units) was assessed using 20 µl of lysate with a Dual-Luciferase Reporter Assay System (Promega) according to the manufacturer’s protocol.
Introduction of antibody into mice.
C57BL/6J pregnant mice (18–22 g; Harlan) were used. Mice were anesthetized with 50 mg/kg sodium pentobarbital i.p., and 20 µg of affinity-purified IgG was introduced into pregnant mice via orbital sinus injection at E13 and again on E14. We chose E13 because this stage of mouse pregnancy is comparable to early onset preeclampsia in humans and is a time at which we can reliably determine if a mouse is pregnant. In some cases, the autoantibody was simultaneously coinjected with either 8 mg/kg losartan i.v. or 50 mg/kg of the 7-aa epitope peptide i.v. Mouse systolic blood pressure was measured by tail cuff (AD Instruments). The dams were sacrificed at E18 when fetal mouse organs and blood (which was pooled from the same littermates) were collected. Only fetuses born in litters of six to eight pups were analyzed in fetal mouse experiments. All animal studies were reviewed and approved by the Animal Welfare Committee of the University of Texas Health Science Center at Houston.
Western blotting.
The presence of AT1-AAs in human serum was analyzed by Western blotting as previously described (Zhou et al., 2008b). In brief, 30 µg/well of proteins from CHO-NFAT cells stably transfected with the AT1 receptor gene were run on 10% SDS-PAGE gel and transferred to a nitrocellulose membrane. The membrane was cut to strips and blocked. The strips were probed with purified human IgG (1:10 dilution) and mouse anti–human IgG conjugated with horseradish peroxidase (1:5,000 dilution; Jackson ImmunoResearch Laboratories). One strip was probed with anti-AT1 receptor antibody (1:1,000 dilution; Santa Cruz Biotechnology, Inc.) and goat anti–rabbit IgG conjugated with horseradish peroxidase (1:5,000 dilution; Jackson ImmunoResearch Laboratories) as positive controls. Human IgG in fetal mouse circulation was also analyzed by Western blotting. 12 µl of fetal mouse serum was run on 10% SDS-PAGE gel and transferred to a nitrocellulose membrane. The membrane was probed with mouse anti–human IgG antibody. For the assessment of apoptotic markers in mouse and human placentas, rabbit anti–human or anti–mouse primary antibodies against Bax, a 23-kD proapoptotic protein, and Bcl-2, a 26-kD antiapoptotic protein, were used in a 1:200 dilution (Santa Cruz Biotechnology, Inc.). β-Actin, a 43-kD housekeeping gene, was used as a loading control (Santa Cruz Biotechnology, Inc.). Relative densiometry was assessed using the Storm 840 Phosphorimager and the accompanying ImageQuant TL analysis software (both from GE Healthcare). All signals of Western blotting were detected by the ECL kit (GE Healthcare).
ELISA.
For determination of human IgG concentration in fetal mouse circulation, fetal mouse serum was diluted 300-fold, and the concentration of human IgG in the serum was quantified by a commercial ELISA kit (Thermo Fisher Scientific).
Human placental explants.
Human placentas were obtained from normotensive pregnant patients whose pregnancies were uncomplicated and underwent an elective term Caesarean section at Memorial Hermann Hospital. The culture system described was adapted from Ahmad and Ahmed (2004). Upon delivery, the placentas were placed on ice and submerged in phenol red–free DMEM containing 0.2% BSA and 1% antibiotics. Within 30 min of delivery, chorionic villous explant fragments were carefully dissected from the placenta and transferred to 24-well plates for an overnight equilibration period at 37°C. The explants were incubated with saline, 100 nM Ang II, or IgG from either preeclamptic or normotensive pregnant women (1:10 dilution). Some experiments involved the coincubation of IgG with 5 µM losartan or 1 µM of the 7-aa blocking epitope peptide, which was added to the serum-free culture medium at the same time as the human IgG. After 24 h, the media was siphoned and stored at −80°C, and the villous explants were either lysed for use in Western blot analysis or fixed in 10% formalin for embedding in paraffin wax and further histological or immunohistochemical analysis.
Human trophoblast cell culture.
To assess the level of AT1 receptor–mediated apoptosis, HTR-8/SVneo cells, an immortalized human trophoblast cell line (Graham et al., 1993), were plated at 2 × 104 cells per well in 8-well chamber slides overnight (Laboratory-Tek; Thermo Fisher Scientific). The next day, serum-free media was used and cells were incubated with 100 nM Ang II or IgG from either preeclamptic or normotensive pregnant women (1:10 dilution) and cultured for an additional day. For some experiments, 5 µM losartan or 1 µM of the 7-aa epitope peptide was added to the chamber well at the same time as the human IgG. The next day, cells were either lysed for caspase 3 activity analysis or permanently fixed to the slide for subsequent staining.
TUNEL assay and index.
HTR-8/SVneo cells manipulated as described in the previous section or 4-µm sections of mouse or human placental tissue collected from the experiments described earlier were permanently fixed onto a glass slide, deparaffinized, and rehydrated through an alcohol gradient by standard techniques. Cells or tissue were permeabilized using cold, fresh 0.1% Triton X-100 in 0.1% sodium citrate and stained by TUNEL using a commercial kit (Roche) according to the manufacturer’s protocol. TUNEL-positive cells are identified by cellular morphology (cell shrinkage, membrane blebbing, and nuclear fragmentation) and positive green staining under 515–565 nm of fluorescent light. Negative control sections were treated in a similar fashion but lacking the terminal deoxynucleotidyl transferase enzyme. To identify healthy cells with normal nuclear morphology, after TUNEL staining was complete a DAPI nuclear stain was added and visualized as blue when excited at 360 nm of fluorescent light (Vector Laboratories). For each TUNEL and DAPI-stained cell or tissue section, green apoptotic and blue healthy nuclei were blindly counted in 10 random microscopic fields. Quantification of the apoptotic index (the number of apoptotic nuclei per total nuclei × 100) was assessed blindly in 10 random microscopic fields per sample using Image Pro Plus 6.3 software (Media Cybernetics).
Caspase 3 activity assay.
To measure the activity of caspase 3, a sensitive commercial assay was used (Millipore). HTR-8/SVneo cells were cultured as described. 0.5 × 106 cells were pelleted and lysed to obtain the cytosolic extract, on which the activity assay was performed according to the manufacturer’s protocol. Relative absorbance was measured on a spectrophotometer at a wavelength of 405 nm, which correlates to the caspase 3 activity level.
Histological analysis.
The kidneys and livers of fetal mice were harvested and fixed in a zinc solution (BD). The tissues were processed according to standard protocols. In brief, the tissues were fixed in a zinc solution for 24–48 h at room temperature, and then washed with PBS twice for 30 min, dehydrated, infiltrated, and embedded in paraffin. 4-µm serial sections were obtained and stained with hematoxylin and eosin (H&E) by standard techniques. The number of glomeruli in the fetal kidney samples was assessed blindly by counting and averaging the number of glomeruli in 10 random high power microscopic fields per sample.
Statistical analysis.
All data are expressed as means ± SEM. Data were analyzed for statistical significance using Prism 4 software (GraphPad Software, Inc.). Student’s t tests (paired or unpaired as appropriate) were applied in a two-group analysis. Differences between the means of multiple groups were compared by the one-way analysis of variance, followed by a Tukey’s multiple comparisons test. P < 0.05 was considered significant and was the threshold to reject the null hypothesis.
Acknowledgments
We would like to acknowledge Dr. C. Carreno for aiding in the collection of human placental tissue at Memorial Hermann Hospital.
Support for this work was provided by National Institutes of Health grants HL076558 (Y. Xia) and HD34130, March of Dimes grant 6-FY06-323, and the Texas Higher Education Coordinating Board (R.E. Kellems).
The authors have no conflicting financial interests.
Footnotes
Abbreviations used:
- Ang II
- angiotensin II
- AT1-AA
- AT1 receptor agonistic autoantibody
- CHO
- Chinese hamster ovary
- IUGR
- intrauterine growth restriction
- TUNEL
- terminal deoxynucleotidyl transferase–mediated dUTP nick-end labeling
References
- Ahmad S., Ahmed A. 2004. Elevated placental soluble vascular endothelial growth factor receptor-1 inhibits angiogenesis in preeclampsia. Circ. Res. 95:884–891 10.1161/01.RES.0000147365.86159.f5 [DOI] [PubMed] [Google Scholar]
- Alexander B.T. 2003. Intrauterine growth restriction and reduced glomerular number: role of apoptosis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 285:R933–R934 [DOI] [PubMed] [Google Scholar]
- Alexander G.R., Kogan M., Bader D., Carlo W., Allen M., Mor J. 2003. US birth weight/gestational age-specific neonatal mortality: 1995-1997 rates for whites, hispanics, and blacks. Pediatrics. 111:e61–e66 10.1542/peds.111.1.e61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allaire A.D., Ballenger K.A., Wells S.R., McMahon M.J., Lessey B.A. 2000. Placental apoptosis in preeclampsia. Obstet. Gynecol. 96:271–276 10.1016/S0029-7844(00)00895-4 [DOI] [PubMed] [Google Scholar]
- Barker D.J. 1998. In utero programming of chronic disease. Clin. Sci. (Lond.). 95:115–128 10.1042/CS19980019 [DOI] [PubMed] [Google Scholar]
- Bauer M.K., Harding J.E., Bassett N.S., Breier B.H., Oliver M.H., Gallaher B.H., Evans P.C., Woodall S.M., Gluckman P.D. 1998. Fetal growth and placental function. Mol. Cell. Endocrinol. 140:115–120 10.1016/S0303-7207(98)00039-2 [DOI] [PubMed] [Google Scholar]
- Baum M., Ortiz L., Quan A. 2003. Fetal origins of cardiovascular disease. Curr. Opin. Pediatr. 15:166–170 10.1097/00008480-200304000-00005 [DOI] [PubMed] [Google Scholar]
- Bobst S.M., Day M.C., Gilstrap L.C., III, Xia Y., Kellems R.E. 2005. Maternal autoantibodies from preeclamptic patients activate angiotensin receptors on human mesangial cells and induce interleukin-6 and plasminogen activator inhibitor-1 secretion. Am. J. Hypertens. 18:330–336 10.1016/j.amjhyper.2004.10.002 [DOI] [PubMed] [Google Scholar]
- Cetin I., Alvino G. 2009. Intrauterine growth restriction: implications for placental metabolism and transport. A review. Placenta. 30(Suppl. A):S77–S82 [DOI] [PubMed] [Google Scholar]
- Cetin I., Foidart J.M., Miozzo M., Raun T., Jansson T., Tsatsaris V., Reik W., Cross J., Hauguel-de-Mouzon S., Illsley N., et al. 2004. Fetal growth restriction: a workshop report. Placenta. 25:753–757 10.1016/j.placenta.2004.02.004 [DOI] [PubMed] [Google Scholar]
- Chistiakov D.A. 2003. Thyroid-stimulating hormone receptor and its role in Graves’ disease. Mol. Genet. Metab. 80:377–388 10.1016/j.ymgme.2003.09.001 [DOI] [PubMed] [Google Scholar]
- Deleze M., Alarcón-Segovia D., Valdes-Macho E., Oria C.V., Ponce de Leon S. 1989. Relationship between antiphospholipid antibodies and recurrent fetal loss in patients with systemic lupus erythematosus and apparently healthy women. J. Rheumatol. 16:768–772 [PubMed] [Google Scholar]
- DiFederico E., Genbacev O., Fisher S.J. 1999. Preeclampsia is associated with widespread apoptosis of placental cytotrophoblasts within the uterine wall. Am. J. Pathol. 155:293–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiades P., Ferguson-Smith A.C., Burton G.J. 2002. Comparative developmental anatomy of the murine and human definitive placentae. Placenta. 23:3–19 10.1053/plac.2001.0738 [DOI] [PubMed] [Google Scholar]
- Girardi G., Berman J., Redecha P., Spruce L., Thurman J.M., Kraus D., Hollmann T.J., Casali P., Caroll M.C., Wetsel R.A., et al. 2003. Complement C5a receptors and neutrophils mediate fetal injury in the antiphospholipid syndrome. J. Clin. Invest. 112:1644–1654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey K.M., Barker D.J. 2000. Fetal nutrition and adult disease. Am. J. Clin. Nutr. 71(Suppl.):1344S–1352S [DOI] [PubMed] [Google Scholar]
- Graham C.H., Hawley T.S., Hawley R.G., MacDougall J.R., Kerbel R.S., Khoo N., Lala P.K. 1993. Establishment and characterization of first trimester human trophoblast cells with extended lifespan. Exp. Cell Res. 206:204–211 10.1006/excr.1993.1139 [DOI] [PubMed] [Google Scholar]
- Hales C.N., Ozanne S.E. 2003. For debate: Fetal and early postnatal growth restriction lead to diabetes, the metabolic syndrome and renal failure. Diabetologia. 46:1013–1019 10.1007/s00125-003-1131-7 [DOI] [PubMed] [Google Scholar]
- Holers V.M., Girardi G., Mo L., Guthridge J.M., Molina H., Pierangeli S.S., Espinola R., Xiaowei L.E., Mao D., Vialpando C.G., Salmon J.E. 2002. Complement C3 activation is required for antiphospholipid antibody-induced fetal loss. J. Exp. Med. 195:211–220 10.1084/jem.200116116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppertz B., Kingdom J.C. 2004. Apoptosis in the trophoblast—role of apoptosis in placental morphogenesis. J. Soc. Gynecol. Investig. 11:353–362 10.1016/j.jsgi.2004.06.002 [DOI] [PubMed] [Google Scholar]
- Huppertz B., Kadyrov M., Kingdom J.C. 2006. Apoptosis and its role in the trophoblast. Am. J. Obstet. Gynecol. 195:29–39 10.1016/j.ajog.2005.07.039 [DOI] [PubMed] [Google Scholar]
- Irani R.A., Xia Y. 2008. The functional role of the renin-angiotensin system in pregnancy and preeclampsia. Placenta. 29:763–771 10.1016/j.placenta.2008.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson M., Jansson T., Powell T.L. 2000. Na(+)-K(+)-ATPase is distributed to microvillous and basal membrane of the syncytiotrophoblast in human placenta. Am. J. Physiol. Regul. Integr. Comp. Physiol. 279:R287–R294 [DOI] [PubMed] [Google Scholar]
- Johansson M., Karlsson L., Wennergren M., Jansson T., Powell T.L. 2003. Activity and protein expression of Na+/K+ ATPase are reduced in microvillous syncytiotrophoblast plasma membranes isolated from pregnancies complicated by intrauterine growth restriction. J. Clin. Endocrinol. Metab. 88:2831–2837 10.1210/jc.2002-021926 [DOI] [PubMed] [Google Scholar]
- Julkunen H., Kaaja R., Kurki P., Palosuo T., Friman C. 1995. Fetal outcome in women with primary Sjögren’s syndrome. A retrospective case-control study. Clin. Exp. Rheumatol. 13:65–71 [PubMed] [Google Scholar]
- Kadyrov M., Kingdom J.C., Huppertz B. 2006. Divergent trophoblast invasion and apoptosis in placental bed spiral arteries from pregnancies complicated by maternal anemia and early-onset preeclampsia/intrauterine growth restriction. Am. J. Obstet. Gynecol. 194:557–563 10.1016/j.ajog.2005.07.035 [DOI] [PubMed] [Google Scholar]
- Kalra D., Sivasubramanian N., Mann D.L. 2002. Angiotensin II induces tumor necrosis factor biosynthesis in the adult mammalian heart through a protein kinase C-dependent pathway. Circulation. 105:2198–2205 10.1161/01.CIR.0000015603.84788.47 [DOI] [PubMed] [Google Scholar]
- Kaufmann P., Black S., Huppertz B. 2003. Endovascular trophoblast invasion: implications for the pathogenesis of intrauterine growth retardation and preeclampsia. Biol. Reprod. 69:1–7 10.1095/biolreprod.102.014977 [DOI] [PubMed] [Google Scholar]
- Langer B., Grima M., Coquard C., Bader A.M., Schlaeder G., Imbs J.L. 1998. Plasma active renin, angiotensin I, and angiotensin II during pregnancy and in preeclampsia. Obstet. Gynecol. 91:196–202 10.1016/S0029-7844(97)00660-1 [DOI] [PubMed] [Google Scholar]
- Li X., Shams M., Zhu J., Khalig A., Wilkes M., Whittle M., Barnes N., Ahmed A. 1998. Cellular localization of AT1 receptor mRNA and protein in normal placenta and its reduced expression in intrauterine growth restriction. Angiotensin II stimulates the release of vasorelaxants. J. Clin. Invest. 101:442–454 10.1172/JCI119881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura G., Sasaki K. 1989. Megakaryocytes in the yolk sac, liver and bone marrow of the mouse: a cytometrical analysis by semithin light microscopy. J. Anat. 167:181–187 [PMC free article] [PubMed] [Google Scholar]
- National High Blood Pressure Education Program Working Group 2000. Report of the National High Blood Pressure Education Program Working Group on high blood pressure in pregnancy. Am. J. Obstet. Gynecol. 183:S1–S22 [PubMed] [Google Scholar]
- Redecha P., Tilley R., Tencati M., Salmon J.E., Kirchhofer D., Mackman N., Girardi G. 2007. Tissue factor: a link between C5a and neutrophil activation in antiphospholipid antibody induced fetal injury. Blood. 110:2423–2431 10.1182/blood-2007-01-070631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman C.W., Sargent I.L. 2005. Latest advances in understanding preeclampsia. Science. 308:1592–1594 10.1126/science.1111726 [DOI] [PubMed] [Google Scholar]
- Roberts D.J., Post M.D. 2008. The placenta in pre-eclampsia and intrauterine growth restriction. J. Clin. Pathol. 61:1254–1260 10.1136/jcp.2008.055236 [DOI] [PubMed] [Google Scholar]
- Ruiz-Ortega M., Bustos C., Hernández-Presa M.A., Lorenzo O., Plaza J.J., Egido J. 1998. Angiotensin II participates in mononuclear cell recruitment in experimental immune complex nephritis through nuclear factor-kappa B activation and monocyte chemoattractant protein-1 synthesis. J. Immunol. 161:430–439 [PubMed] [Google Scholar]
- Saito S., Shiozaki A., Nakashima A., Sakai M., Sasaki Y. 2007. The role of the immune system in preeclampsia. Mol. Aspects Med. 28:192–209 10.1016/j.mam.2007.02.006 [DOI] [PubMed] [Google Scholar]
- Saito T., Ishida J., Takimoto-Ohnishi E., Takamine S., Shimizu T., Sugaya T., Kato H., Matsuoka T., Nangaku M., Kon Y., et al. 2004. An essential role for angiotensin II type 1a receptor in pregnancy-associated hypertension with intrauterine growth retardation. FASEB J. 18:388–390 [DOI] [PubMed] [Google Scholar]
- Saji H., Yamanaka M., Hagiwara A., Ijiri R. 2001. Losartan and fetal toxic effects. Lancet. 357:363 10.1016/S0140-6736(00)03648-5 [DOI] [PubMed] [Google Scholar]
- Salmon J.E., Girardi G. 2008. Antiphospholipid antibodies and pregnancy loss: a disorder of inflammation. J. Reprod. Immunol. 77:51–56 10.1016/j.jri.2007.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedeek M.H., Llinas M.T., Drummond H., Fortepiani L., Abram S.R., Alexander B.T., Reckelhoff J.F., Granger J.P. 2003. Role of reactive oxygen species in endothelin-induced hypertension. Hypertension. 42:806–810 10.1161/01.HYP.0000084372.91932.BA [DOI] [PubMed] [Google Scholar]
- Sedeek M., Gilbert J.S., LaMarca B.B., Sholook M., Chandler D.L., Wang Y., Granger J.P. 2008. Role of reactive oxygen species in hypertension produced by reduced uterine perfusion in pregnant rats. Am. J. Hypertens. 21:1152–1156 10.1038/ajh.2008.239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaarawy M., Didy H.E. 1996. Thrombomodulin, plasminogen activator inhibitor type 1 (PAI-1) and fibronectin as biomarkers of endothelial damage in preeclampsia and eclampsia. Int. J. Gynaecol. Obstet. 55:135–139 10.1016/S0020-7292(96)02755-5 [DOI] [PubMed] [Google Scholar]
- Shah D.M. 2006. The role of RAS in the pathogenesis of preeclampsia. Curr. Hypertens. Rep. 8:144–152 10.1007/s11906-006-0011-1 [DOI] [PubMed] [Google Scholar]
- Shibata E., Powers R.W., Rajakumar A., von Versen-Höynck F., Gallaher M.J., Lykins D.L., Roberts J.M., Hubel C.A. 2006. Angiotensin II decreases system A amino acid transporter activity in human placental villous fragments through AT1 receptor activation. Am. J. Physiol. Endocrinol. Metab. 291:E1009–E1016 10.1152/ajpendo.00134.2006 [DOI] [PubMed] [Google Scholar]
- Siamopoulou-Mavridou A., Manoussakis M.N., Mavridis A.K., Moutsopoulos H.M. 1988. Outcome of pregnancy in patients with autoimmune rheumatic disease before the disease onset. Ann. Rheum. Dis. 47:982–987 10.1136/ard.47.12.982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui A.H., Irani R.A., Thway T.M., Blackwell S.C., Ramin S.M., Kellems R.E., Xia Y. 2009. Angiotensin receptor agonistic autoantibody is highly prevalent in preeclampsia and its titer correlates with disease severity. Hypertension. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence S.G., Allen H.L., Cukierski M.A., Manson J.M., Robertson R.T., Eydelloth R.S. 1995. Defining the susceptible period of developmental toxicity for the AT1-selective angiotensin II receptor antagonist losartan in rats. Teratology. 51:367–382 10.1002/tera.1420510603 [DOI] [PubMed] [Google Scholar]
- Takimoto E., Ishida J., Sugiyama F., Horiguchi H., Murakami K., Fukamizu A. 1996. Hypertension induced in pregnant mice by placental renin and maternal angiotensinogen. Science. 274:995–998 10.1126/science.274.5289.995 [DOI] [PubMed] [Google Scholar]
- Thway T.M., Shlykov S.G., Day M.C., Sanborn B.M., Gilstrap L.C., III, Xia Y., Kellems R.E. 2004. Antibodies from preeclamptic patients stimulate increased intracellular Ca2+ mobilization through angiotensin receptor activation. Circulation. 110:1612–1619 10.1161/01.CIR.0000142855.68398.3A [DOI] [PubMed] [Google Scholar]
- Wallukat G., Homuth V., Fischer T., Lindschau C., Horstkamp B., Jüpner A., Baur E., Nissen E., Vetter K., Neichel D., et al. 1999. Patients with preeclampsia develop agonistic autoantibodies against the angiotensin AT1 receptor. J. Clin. Invest. 103:945–952 10.1172/JCI4106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y., Wen H.Y., Kellems R.E. 2002. Angiotensin II inhibits human trophoblast invasion through AT1 receptor activation. J. Biol. Chem. 277:24601–24608 10.1074/jbc.M201369200 [DOI] [PubMed] [Google Scholar]
- Xia Y., Wen H., Bobst S., Day M.C., Kellems R.E. 2003. Maternal autoantibodies from preeclamptic patients activate angiotensin receptors on human trophoblast cells. J. Soc. Gynecol. Investig. 10:82–93 10.1016/S1071-5576(02)00259-9 [DOI] [PubMed] [Google Scholar]
- Zhou Y., Damsky C.H., Fisher S.J. 1997a. Preeclampsia is associated with failure of human cytotrophoblasts to mimic a vascular adhesion phenotype. One cause of defective endovascular invasion in this syndrome? J. Clin. Invest. 99:2152–2164 10.1172/JCI119388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Fisher S.J., Janatpour M., Genbacev O., Dejana E., Wheelock M., Damsky C.H. 1997b. Human cytotrophoblasts adopt a vascular phenotype as they differentiate. A strategy for successful endovascular invasion? J. Clin. Invest. 99:2139–2151 10.1172/JCI119387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C.C., Ahmad S., Mi T., Xia L., Abbasi S., Hewett P.W., Sun C., Ahmed A., Kellems R.E., Xia Y. 2007. Angiotensin II induces soluble fms-like tyrosine kinase-1 release via calcineurin signaling pathway in pregnancy. Circ. Res. 100:88–95 10.1161/01.RES.0000254703.11154.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C.C., Ahmad S., Mi T., Abbasi S., Xia L., Day M.C., Ramin S.M., Ahmed A., Kellems R.E., Xia Y. 2008a. Autoantibody from women with preeclampsia induces soluble Fms-like tyrosine kinase-1 production via angiotensin type 1 receptor and calcineurin/nuclear factor of activated T-cells signaling. Hypertension. 51:1010–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C.C., Zhang Y., Irani R.A., Zhang H., Mi T., Popek E.J., Hicks M.J., Ramin S.M., Kellems R.E., Xia Y. 2008b. Angiotensin receptor agonistic autoantibodies induce pre-eclampsia in pregnant mice. Nat. Med. 14:855–862 10.1038/nm.1856 [DOI] [PMC free article] [PubMed] [Google Scholar]