Abstract
Leukocyte migration across endothelial cell borders (paracellular) and through endothelial cells (transcellular) appear to be distinct processes. During paracellular migration, membrane from a parajunctional reticulum of interconnected vesicles, the endothelial lateral border recycling compartment (LBRC), moves to surround the leukocyte in a kinesin-mediated, microtubule-dependent manner. We show that transcellular migration likewise requires targeted trafficking of LBRC membrane. We show that in addition to platelet/endothelial cell adhesion molecule (PECAM; CD31), CD99 and junctional adhesion molecule A (JAM-A), but apparently not vascular endothelial cell–specific cadherin (cadherin 5, CD144), are components of the LBRC. During transcellular migration, LBRC membrane invests the transmigrating leukocyte. Intracellular adhesion molecule 1 (ICAM-1) on the apical endothelial surface is enriched around adherent leukocytes. Depolymerization of microtubules has no effect on ICAM-1 enrichment but blocks targeted trafficking of LBRC membrane and transcellular migration by >90%. Similar to their effects on paracellular transmigration, antibodies against PECAM or CD99, but not JAM-A, block transcellular migration. We conclude that similar molecular mechanisms promote both para- and transcellular migration.
During the inflammatory response, leukocytes leave the bloodstream and cross the endothelium to reach inflamed tissue. Leukocyte extravasation is known to involve a well-characterized sequence of rolling, activation, and firm adhesion followed by locomotion to the endothelial junction, where leukocytes squeeze between adjacent cells in an ameboid fashion across the endothelial borders via a process called diapedesis (Butcher, 1991; Springer, 1994; Muller, 2002). Platelet/endothelial cell adhesion molecule (PECAM; CD31), CD99, and junctional adhesion molecule A (JAM-A) are molecules shown to mediate the migration of leukocytes across endothelial cell junctions (Muller, 2003; Ley et al., 2007; Muller, 2009).
Although it is well accepted that leukocytes cross the endothelium at the cell borders (paracellular route), there is increasing evidence that leukocytes can also pass directly through endothelial cells (transcellular route). Much of the original evidence was indirect, based on single transmission electron micrographs that appeared to show leukocytes deeply indenting endothelial cells and/or passing across endothelial cells through a membrane-lined channel next to an intact junction (Williamson and Grisham, 1961; Marchesi and Gowans, 1964; Bamforth et al., 1997). However, endothelial junctions are serpentine, and it was possible that the leukocyte was passing through a less structured junction (Muller, 2001). Recently, several in vitro models were established that produced reliable transcellular migration (Carman and Springer, 2004; Yang et al., 2005; Millán et al., 2006). Despite the progress made in uncovering some of the molecules involved in the transcellular route of diapedesis, it remains unclear why leukocytes that appear to use the same mechanisms for rolling and adhesion will transmigrate through the endothelial cell rather than at the junctions. Understanding the mechanisms underlying transcellular diapedesis will help answer this question.
In our previous studies, we showed that PECAM at the borders of endothelial cells enters a novel membrane compartment connected to the cell surface at the cell borders (Mamdouh et al., 2003). It is quite distinct from typical recycling endosomes, caveolae, and vesiculo-vacuolar organelles (Feng et al., 1996; Mamdouh et al., 2003). We called this interconnected reticulum of membrane the lateral border recycling compartment (LBRC; Mamdouh et al., 2008). Membrane from this compartment was found to cycle constitutively between the LBRC and the cell surface evenly along the borders of resting endothelial cells. When a leukocyte crosses the endothelial cell junction, the LBRC membrane is mobilized to the surface of the junction at the site of diapedesis and surrounds the leukocyte (Mamdouh et al., 2003; Mamdouh et al., 2008). This targeted recycling of LBRC membrane to the site of diapedesis is mediated by kinesin molecular motors along microtubules, and is required for paracellular diapedesis of all classes of leukocytes, even under conditions where transmigration could not be blocked by anti-PECAM mAb (Mamdouh et al., 2008). We hypothesized that this targeted recycling would provide more membrane surface area and unligated PECAM to facilitate leukocyte passage (Mamdouh et al., 2003; Mamdouh et al., 2008).
In this paper, we report that transcellular diapedesis of monocytes and neutrophils across human endothelial cells involves trafficking of the LBRC to the site of transcellular diapedesis. We also show that in addition to PECAM, the LBRC contains CD99 and JAM-A but not vascular endothelial cell–specific cadherin (VE-cadherin; cadherin 5, CD144). Similar to paracellular migration, trafficking of the LBRC in transcellular migration is microtubule dependent. Transcellular migration is likewise dependent on PECAM and CD99. We conclude that trans- and paracellular leukocyte migration involve similar mechanisms.
RESULTS
Transcellular migration recruits molecules normally found at the endothelial cell border
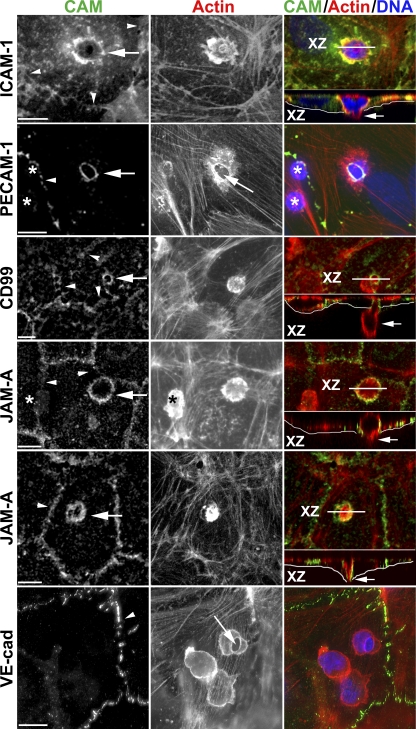
In our in vitro transmigration assay, under normal conditions of cytokine activation, almost all leukocytes cross the endothelial cells at the cell borders (i.e., paracellular migration). However, if we created a gradient of monocyte chemotactic protein 1 (MCP-1; CCL2) or fMLP by injecting 10 µl into the collagen gel underlying the endothelial monolayer, a substantial fraction of monocytes and neutrophils, respectively, crossed the monolayer by passing through the endothelial cells (i.e., transcellular migration). Monolayers were fixed within 10 min of cellular interaction to catch leukocytes in the act of transmigration. Cells were gently permeabilized and stained for intracellular adhesion molecule 1 (ICAM-1) and actin (to visualize the cellular interaction). As others have reported previously (Carman and Springer, 2004; Yang et al., 2005; Millán et al., 2006), we also found that transcellular migration of monocytes is concomitant with an enrichment of ICAM-1 around the crossing cells (Fig. 1). ICAM-1 was not only enriched at the luminal surface of endothelial cells at the site of transcellular diapedesis but followed the leukocyte as it crossed the endothelial cell body and formed a channel around it (Fig. 1, XZ insets). When transmigration was stopped at 10 min, up to 10% of leukocytes were caught in the act of transcellular migration. If the interaction was allowed to continue for 30 min, ∼30% of monocytes had migrated transcellularly.
Figure 1.
Enrichment of endothelial cell adhesion molecules around leukocytes migrating transcellularly. Representative confocal or deconvolved images of monocytes or PMNs caught in the act of transcellular migration are shown stained with mAbs for the indicated cell adhesion molecule (CAM; green in merge), phalloidin to mark actin (red in merge), and DAPI to mark nuclei (blue in merge). Rings of enrichment of ICAM-1, PECAM, CD99, and JAM-A were consistently seen surrounding the site at which leukocytes migrated through the endothelial cell (arrows; left). Each event was verified by scanning through the monolayer. Orthogonal (XZ) projections along the plane indicated by the white line (XZ) shown below the merged images demonstrate that the leukocyte was indeed crossing the endothelial cell (arrows; right). The thin white line underlies the basal surface of the endothelial cell. The JAM-A panels show a monocyte (top) and a PMN (bottom). Note in the PECAM panel and top JAM-A panel that several leukocytes are present on the endothelial cell but only the site of transcellular migration stains for PECAM or JAM-A, respectively, demonstrating that the PECAM and JAM-A stained around the leukocyte belongs to the endothelial cell. Asterisks indicate leukocytes not in the act of transmigration and not stained for PECAM or JAM-A. Arrows in the PECAM and VE-cadherin panels point to the site of transcellular migration where endothelial cell actin is pushed aside. Arrowheads mark cell borders. Data are representative of hundreds of transmigration events recorded in 3–10 independent experiments for each marker. Bars, 10 µm.
Because PECAM-1 is a key player in leukocyte paracellular diapedesis, we wondered whether it had any role in transcellular diapedesis. To test this, we allowed monocytes or neutrophils to cross TNF-activated human umbilical vein endothelial cell (HUVEC) monolayers in the presence of exogenous MCP-1 or fMLP to stimulate transcellular migration as described, but stained for PECAM. As shown in Fig. 1, PECAM, which is normally expressed at the borders of endothelial cells, is now also found in the center of the cell surrounding the crossing leukocytes. Note that although PECAM is expressed on the surfaces of monocytes and neutrophils, its concentration is 10–20 times lower than on endothelial cells and is not detected on leukocytes at this level of sensitivity. Note the absence of staining on leukocytes not crossing the endothelial cell (asterisks). Other junctional molecules such as CD99 and JAM-A, which have also been shown to be key players in the paracellular diapedesis step, also formed rings that surrounded the crossing leukocyte (Fig. 1). These data strongly suggest that PECAM, CD99, and JAM-A are involved in the transcellular route of leukocyte diapedesis. In contrast, when we examined the localization of VE-cadherin during monocyte transcellular diapedesis, it remained along the endothelial border and was absent from the site of transcellular migration. This result suggests that VE-cadherin is not involved in leukocyte transcellular diapedesis (Fig. 1).
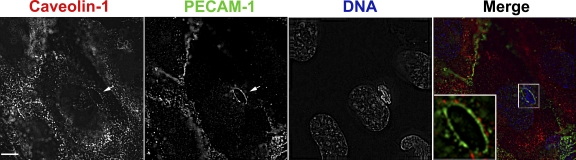
A previous study provided evidence that ICAM-1 was recruited into caveolae, which were relatively enriched at the site of transcellular migration (Millán et al., 2006), although others studying the same phenomenon found caveolae to be only partially (Carman and Springer, 2004) or not enriched (Carman et al., 2007). Similar to the latter study, we rarely detected caveolin-1 staining around leukocytes migrating transcellularly. When we did, however, the staining was weak and clearly outside the zone of PECAM staining that encircled the transmigrating leukocyte (Fig. 2). Because there was no overlap of the green and red fluorescence (Fig. 2, inset), the membranes bearing PECAM and caveolin-1 should be at least 200 nm (approximately four caveolae diameters) apart.
Figure 2.
Caveolae do not comprise the membrane that forms the transmigration pore. Leukocytes fixed in the act of transcellular migration were stained for caveolin-1 (red in merge), PECAM (green in merge), and DNA to label nuclei (blue in merge). PECAM forms a definite ring around the leukocyte (arrow), as seen in Fig. 1. In this image, several vesicular structures staining for caveolin-1 are seen distributed in a circular fashion around the transmigrating leukocyte as well (arrow). However, when the images are overlapped (merge), it is clear that the caveolin-1 staining is peripheral to and not overlapping with the PECAM staining. (inset) An enlargement of the transmigration pore displaying just the caveolin-1 and PECAM staining for clarity. In the inset, the brightness of the caveolin-1 staining was artificially raised to enable better visualization of caveolin-1. Data are representative of three independent experiments. Bar, 10 µm.
CD99 and JAM-A, but not VE-cadherin, are in the LBRC
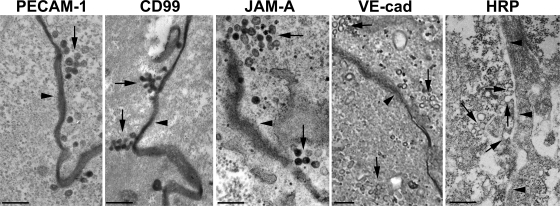
The unexpected presence of molecules from the lateral cell border (PECAM, JAM-A, and CD99) on the apical surface of the endothelial cell surrounding the leukocyte raised the question of how they got there. We previously described the LBRC, a novel membrane compartment in endothelial cells that is made of vesicle-like structures connected to each other and to the cell surface at the borders. 30% of total endothelial PECAM constitutively cycles between this compartment and the surface at the borders (Mamdouh et al., 2003; Mamdouh et al., 2008). Moreover, we showed that paracellular diapedesis of both monocytes, lymphocytes, and neutrophils requires mobilization of PECAM-containing membrane from the LBRC to the site of the junction where leukocytes are crossing (Mamdouh et al., 2008). We wondered whether the other junctional molecules that surrounded the leukocyte during the transcellular diapedesis also originate from the LBRC. We examined the localization of CD99, JAM-A, and VE-cadherin by immunoelectron microscopy (immuno-EM). Fig. 3 shows representative en face sections (i.e., cut in a plane parallel to the culture dish) of HUVEC monolayers labeled at 37°C with mAbs against PECAM, CD99, JAM-A, or VE-cadherin. Positive staining of PECAM, CD99, JAM-A, and VE-cadherin was seen, as expected, at the junction of HUVECs. More important, 50-nm vesicle-like structures connected to each other and to the cell surface appearing morphologically similar to the PECAM-containing LBRC were also seen labeled with anti-CD99 and anti–JAM-A antibodies. Moreover, these molecules were shown to be in the LBRC functionally, because they underwent constitutive recycling with the same time course as PECAM (unpublished data; Mamdouh et al., 2003; Mamdouh et al., 2008). However, despite examination of hundreds of cell profiles, such structures were never seen to be stained with anti–VE-cadherin antibody, even though VE-cadherin was detected in the junctions between HUVECs. These data suggest that the LBRC contains CD99 and JAM-A in addition to PECAM, but not VE-cadherin. This hypothesis was confirmed by ablation experiments (Ghosh et al., 1998; Lampson et al., 2001), which are based on quenching of Alexa Fluor 488 fluorescence by products of the horseradish peroxidase (HRP), 3,3′-diaminobenzidine (DAB), and H2O2 reaction. When HUVECs were incubated with Alexa Fluor 488–anti-CD99 and HRP–anti-PECAM, and then exposed to a mixture of DAB and H2O2, Alexa Fluor 488 fluorescence was completely quenched (99%), whereas the endocytic marker, HRP-transferrin, had no effect on Alexa Fluor 488–anti-CD99 fluorescence (unpublished data). The same results were seen when the HRP and Alexa Fluor labels were switched.
Figure 3.
CD99 and JAM-A, but not VE-cadherin, are in the LBRC. Endothelial cells were incubated with HRP-conjugated mAbs specific for PECAM, CD99, JAM-A, or VE-cadherin for 1 h at 37°C, and were fixed and reacted with diaminoabenzidine-H2O2 as previously described (Mamdouh et al., 2003). As a control for nonspecific labeling, free HRP was added at the concentration present on the antibodies (5.5 µM). En face sections were cut for electron microscopic analysis. Images shown are representative of at least 100 cell profiles taken from at least two independent experiments. In addition to being present along the cell border (dark electron-dense staining), PECAM is present in interconnected vesicular structures of the LBRC, as previously described (Mamdouh et al., 2003), as are CD99 and JAM-A. VE-cadherin is present at the cell borders (arrowhead) but not in the interconnected vesicular structures of the LBRC adjacent to it (arrows). Bars, 200 nm.
Targeted movement of LBRC membrane to the site of transcellular migration
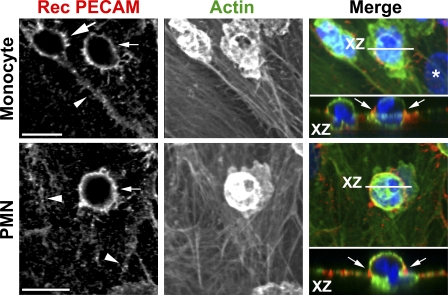
Because PECAM, CD99, and JAM-A, but not VE-cadherin, were recruited to the site of transcellular migration, we hypothesized that the LBRC might be recruited to surround the leukocyte in a similar manner to the way it is recruited to surround leukocytes undergoing paracellular diapedesis (Mamdouh et al., 2003; Mamdouh et al., 2008). The facts that the LBRC does not contain caveolin-1 (Mamdouh et al., 2003) nor appears to contain VE-cadherin (Fig. 3) and that neither was enriched around the transmigrating leukocyte (Figs. 1 and 2) were also consistent with this hypothesis. We next investigated whether there was targeted trafficking of LBRC membrane to the site of transcellular migration using recycling PECAM as a surrogate tracer of LBRC. In this experiment, we used a nonblocking Fab fragment to trace the recycling of LBRC during leukocyte diapedesis, as described previously (Mamdouh et al., 2003; Mamdouh et al., 2008). Fig. 4 shows Z-series confocal projections of representative images in which a monocyte (top) and a neutrophil (bottom) are crossing an endothelial cell far from the junctions, as shown in the XZ insets. Trafficking of LBRC membrane was detected at the site of leukocyte transcellular diapedesis in virtually every migration event we could document. The intensity of recycled PECAM at the diapedesis site was clearly higher than PECAM at the junctions (the latter represents constitutive recycling of LBRC membrane evenly along the endothelial borders that we have previously demonstrated takes place independently of leukocytes; Mamdouh et al., 2003; Mamdouh et al., 2008). These data suggest that transcellular migration, like paracellular diapedesis, involves a similar mechanism of LBRC membrane recruitment to the site of diapedesis. Because CD99 and JAM-A were also seen in an intracellular compartment structurally and functionally resembling the LBRC and were also recruited to the site of transcellular diapedesis, we speculate that leukocyte transcellular diapedesis involves the recruitment of membrane from the LRBC that contains not only PECAM but also CD99 and JAM-A. VE-cadherin, which is excluded from the LBRC, is absent from the site of leukocyte transcellular diapedesis (Fig. 1), as it is in the paracellular pathway (Allport et al., 2000; Shaw et al., 2001).
Figure 4.
Targeted trafficking of membrane from the LBRC to surround leukocytes undergoing transcellular migration. PECAM in the LBRC was labeled with Fab fragments of a nonblocking mAb as a surrogate marker for LBRC trafficking to the endothelial surface, as previously described (see Materials and methods; Mamdouh et al., 2003; Mamdouh et al., 2008). Staining of recycled PECAM (Rec PECAM; red in merge) surrounding monocytes (top) and a PMN (bottom) demonstrates movement of membrane from the LBRC to the endothelial surface. Recycled PECAM is seen around the leukocytes migrating transcellularly (small arrows) far from the endothelial border (arrowheads). Recycling LBRC enrichment is as great around the monocyte migrating transcellularly as it is around the monocyte migrating paracellularly (large arrow). Actin staining (green in merge) shows relative positions of the cells. Orthogonal (XZ) projection demonstrates leukocytes in the act of transcellular migration. Arrows (right) indicate recycled PECAM around leukocytes. The asterisk indicates an endothelial cell nucleus. These images are representative of >200 transcellular migration events captured in >12 independent experiments. Bars, 10 µm.
The targeted trafficking assay that we have used to document LBRC movement in paracellular migration (Mamdouh et al., 2003; Mamdouh et al., 2008) and transcellular migration (Fig. 4) is a functional assay. The transmigration takes place while the cells are incubated in fluorescently labeled secondary antibody. Membrane from the LBRC becomes labeled only when it reaches the surface of the cell. Monolayers are washed and fixed before microscopic examination. The rings of fluorescence around the leukocyte represent membrane from the LBRC in contact with the leukocyte at the time of fixation.
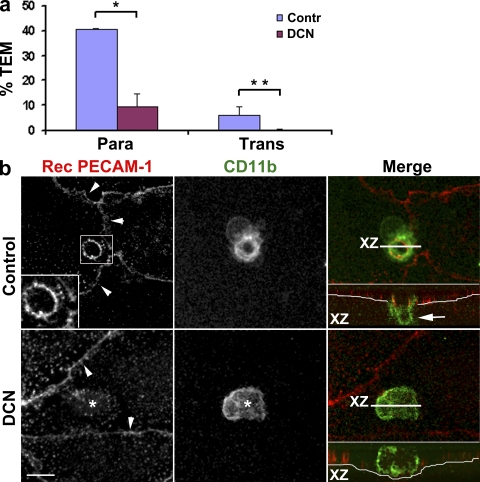
These data collectively support the idea that both the para- and transcellular routes involve similar mechanisms. They both involve targeted recycling of LBRC membrane containing PECAM, CD99, and JAM-A, but not VE-cadherin, to the site of diapedesis. We have recently shown that microtubules and kinesins are critical for targeted recycling of the LBRC and thus for leukocyte paracellular migration (Mamdouh et al., 2008). If the same mechanisms apply to trans- and paracellular transmigration, microtubules would also be required for guiding LBRC trafficking for the transcellular route. We pretreated HUVEC monolayers with 10 µM demecolcine (DCN; colcemide) for 1 h as previously described (Mamdouh et al., 2008), washed monolayers free of drug, and examined transcellular migration of monocytes in control and DCN-treated cells. This treatment was shown to reproducibly depolymerize microtubules without affecting endothelial cell adhesion molecule distribution, calcium signaling, actin or intermediate filament distribution, or monolayer permeability over the course of the experiment (Mamdouh et al., 2008). Controls run with each experiment verified the effectiveness of DCN treatment and the integrity of the endothelial monolayers (Mamdouh et al., 2008). Transendothelial migration (TEM) in this experiment was performed for a short period of time (10 min), which enabled us to catch leukocytes in the act of diapedesis. We stained the cells with Alexa Fluor 488–phalloidin to visualize both endothelial cells and leukocytes and the junctions with Alexa Fluor 546–anti–VE-cadherin. Hundreds of fields were examined by confocal microscopy to quantify the number of monocytes crossing the endothelial cell at the junctions and far from them (Fig. 5 a). Under these conditions, in control cells most monocytes were crossing the monolayer at the cell borders; however, ∼13% of cells undergoing diapedesis were taking the transcellular route. Consistent with previous data (Mamdouh et al., 2008), DCN treatment blocked paracellular diapedesis by 75%. The same treatment blocked transcellular diapedesis almost completely (>90% block).
Figure 5.
Depolymerizing microtubules blocks transcellular migration and targeted trafficking of the LBRC. (a) Endothelial monolayers were treated with DMSO carrier (Contr) or DCN under conditions that selectively and reproducibly depolymerized only endothelial microtubules (Mamdouh et al., 2008). Monocytes were allowed to transmigrate for 10 min, and the monolayers were then fixed and stained for VE-cadherin to mark cell junctions and phalloidin to stain actin. The number of adherent cells undergoing paracellular (Para) or transcellular (Trans) migration was scored as described in Materials and methods. Because this was an early time point, designed to catch leukocytes at the site of their transmigration, the absolute levels were not yet maximal. Paracellular transmigration was blocked by >75% by microtubule depolymerization, as expected (Mamdouh et al., 2008), and transcellular migration was blocked by >90%. Values are means ± SEM derived from observations of a total of 1,418 leukocytes on control monolayers and 1,690 leukocytes on DCN-treated monolayers in five independent experiments. *, P = 0.000304 for paracellular transmigration; **, P = 0.00092 for transcellular migration. (b) Targeted trafficking of LBRC membrane was visualized in similarly treated monolayers using anti-CD11b to visualize the leukocytes. A ring of LBRC membrane surrounds the leukocyte undergoing transcellular migration in the control monolayer, as seen in Fig. 4. (inset) A leukocyte clearly migrating transcellularly. However, in DCN-treated monolayers (bottom), monocytes adhere (*) but no targeted trafficking of LBRC membrane is seen. Arrowheads show endothelial cell borders marked by constitutive recycling. The thin white line in the orthogonal projection indicates the abluminal surface of endothelial cells. The arrow indicates the portion of leukocytes below the endothelium. Data are representative of five independent experiments. Bar, 10 µm.
These data show that intact microtubules are as critical for transcellular diapedesis as they are for the paracellular pathway. Moreover, dissolution of microtubules abolished the targeted trafficking of LBRC membrane to the site of transcellular diapedesis (Fig. 5 b) just as it abolishes the targeted recycling of LBRC membrane to the cell border in paracellular diapedesis (Mamdouh et al., 2008). In the absence of functional microtubules, there is no targeted trafficking of LBRC membrane and no transmigration, either paracellularly or transcellularly.
Transcellular migration requires PECAM and CD99
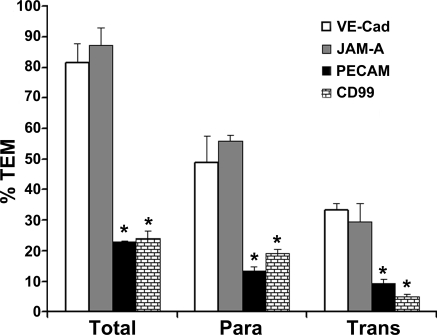
Paracellular transmigration of human neutrophils and monocytes, which requires targeted recycling of LBRC membrane (Mamdouh et al., 2003; Mamdouh et al., 2008), can be blocked by mAbs against PECAM and CD99 but not JAM-A (Muller et al., 1993; Liu et al., 2000; Schenkel et al., 2002; Schenkel et al., 2004). This raised the question of whether transcellular migration similarly could be blocked by these antibodies. Transmigration assays were performed in the presence of antibodies against PECAM, CD99, JAM-A, or VE-cadherin and examined by confocal microscopy, scoring for leukocytes that had undergone para- or transcellular diapedesis (Fig. 6). To be able to distinguish leukocytes blocked in the act of transmigration from those that were actually going through (especially for CD99, which arrests leukocytes part way through the junction; Schenkel et al., 2002; Lou et al., 2007), we ran the transmigration assay for 30 min to allow ample time for unblocked leukocytes to move across the endothelial monolayer. As a result, the control transmigration rate was ∼80%, with up to 33% of the leukocytes migrating transcellularly (compare with the 10-min assay in Fig. 4 in which only ∼45% of control leukocytes transmigrated.) As previously reported, paracellular diapedesis was effectively blocked (by ∼75% compared with control) by antibody against PECAM or CD99 but not against JAM-A or VE-cadherin (Muller et al., 1993; Liu et al., 2000; Schenkel et al., 2002; Schenkel et al., 2004). Interestingly, transcellular diapedesis was also blocked by antibody against PECAM or CD99 but not against JAM-A or VE-cadherin, and transcellular diapedesis was inhibited to about the same extent as was paracellular diapedesis. In pilot experiments, the block in transcellular migration was confirmed by live-cell imaging using differential interference contrast optics (Schenkel et al., 2004; Lou et al., 2007).
Figure 6.
Blocking PECAM or CD99 blocks transcellular migration. Transmigration assays conducted in the presence of antibodies against PECAM, CD99, JAM-A, or VE-cadherin and analyzed as described in Materials and methods. The mAb against VE-cadherin was used as a negative control, because it has been shown not to inhibit transmigration (Muller et al., 1993; Schenkel et al., 2002). Leukocytes were scored as nontransmigrated, transmigrated paracellularly (Para), or transmigrated transcellularly (Trans). Under these conditions, ∼80% of control leukocytes transmigrated: ∼50% paracellularly and 30% transcellularly. Antibodies against PECAM and CD99 blocked both para- and transcellular diapedesis significantly, whereas antibody against JAM-A had no effect. Data are means ± SEM from three separate experiments in which >250 leukocytes were counted for each condition. *, P < 10−5 compared with VE-cadherin control or JAM-A.
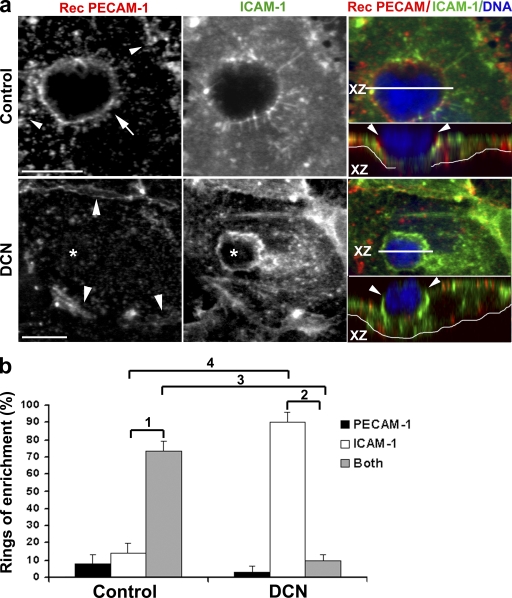
Finally, we wished to understand the relative timing of the involvement of ICAM-1, an apical surface molecule, and molecules within the LBRC in transcellular migration. Clustering of ICAM-1 has been observed to precede paracellular migration (Shaw et al., 2004) and be important for transcellular migration (Yang et al., 2005). Would disruption of microtubules affect the clustering of ICAM-1, or is it independent and/or “upstream” of this process? We performed a recycling experiment as previously described using PECAM as a tracer for the LBRC (Mamdouh et al., 2003; Mamdouh et al., 2008) in control and DCN-treated cells. We fixed the cells and stained them for ICAM-1. Representative images are shown in Fig. 7. In a typical control monolayer, the z-series confocal projection showed a monocyte crossing the middle of an endothelial cell, as shown in the XZ inset. PECAM and ICAM-1 are both enriched around the crossing monocytes, consistent with the data shown in Fig. 1. In DCN-pretreated cells, the LBRC cannot move to the site of leukocyte migration; consequently, monocytes are blocked and cannot cross the body of endothelial cells. The ring of ICAM-1 around these monocytes indicates that they are actively engaged with the endothelial cell. However, despite robust enrichment of ICAM-1 around the leukocyte, in the absence of targeted trafficking from the LBRC, transcellular migration did not occur. These data are representative of hundreds of interactions on 50 individual co-cultures in five independent experiments.
Figure 7.
Disruption of microtubules prevents targeted trafficking of LBRC but not ICAM-1 enrichment. (a) Endothelial monolayers treated as in Fig. 6 were used in experiments to promote transcellular migration. Monolayers were stained to demonstrate LBRC recycling (Rec PECAM-1; red in merge), ICAM-1 (green in merge), or DNA (blue in merge). Control cells show enrichment of ICAM-1 around monocytes migrating transcellularly (orthogonal projection) as well as membrane trafficking from the LBRC, as seen in the merge. In the orthogonal images, the thin white line underlies the endothelial monolayer. In DCN-treated monolayers, the monocyte is still firmly attached to the endothelial surface surrounded by a zone of enrichment of ICAM-1. However, depolymerization of microtubules prevents targeted trafficking from the LBRC, and the monocyte cannot transmigrate. These images are representative of several hundred leukocyte–endothelial cell interactions viewed on 50 separate monolayers in five independent experiments. Bars, 10 µm. (b) Quantification of data shown in panel a. Leukocytes making productive contact with the endothelial monolayer, as defined by being surrounded by a ring of enrichment of either ICAM-1 or recycled PECAM, or both, were enumerated. Data are expressed as the percentage of leukocytes surrounded only by recycled PECAM, only by ICAM-1, or by both recycled PECAM and ICAM-1. Data are means ± SEM. p-values are highly significant: 1, P = 1.9 × 10−5; 2, P = 7.4 × 10−13; 3, P = 5.86 × 10−7; 4, P = 7.07 × 10−10.
To quantify these data in an objective way, we examined all monocytes interacting with the endothelial monolayer in these experiments. Because it was difficult to determine whether a leukocyte deeply indenting the apical surface of the endothelial cell was in the process of transmigration, we scored any monocyte with a ring of either ICAM-1 or recycled PECAM. In the control monolayers, 73 ± 5.6% of monocytes were surrounded by rings of both ICAM-1 and recycled PECAM. Most monocytes that were surrounded only by ICAM-1 were on the apical surface and had not penetrated the endothelial cell, whereas most monocytes surrounded only by a ring of recycled PECAM had almost completed transcellular migration (unpublished data). This suggested that virtually every monocyte caught in the more advanced stages of diapedesis was surrounded by a ring of recycled PECAM. In contrast, when microtubules were depolymerized and monocytes could not transmigrate, 90 ± 6% of leukocytes were surrounded only by a ring of ICAM-1 and virtually none with a ring of recycled PECAM only (Fig. 7). These data demonstrate that microtubule depolymerization blocks recycling of the LBRC and transcellular migration. These results show that ICAM-1 enrichment is an upstream event that is perhaps necessary but certainly not sufficient for transcellular migration.
DISCUSSION
Previous studies performed in vivo and in vitro have shown that leukocytes are capable of crossing the endothelium by both para- and transcellular routes (Muller, 2001; Ley et al., 2007; Carman and Springer, 2008). At first glance, these routes appear quite distinct. However, the results we report in this paper show that they have much more in common than originally thought. In both cases, the leukocyte undergoing transmigration is surrounded by membrane recruited from the LBRC. This membrane compartment contains PECAM, JAM-A, and CD99, but appears to exclude VE-cadherin (Figs. 1 and 3). When the LBRC membrane surrounds leukocytes undergoing transcellular migration, it provides them with a “parajunctional junction,” a channel of membrane lined with the molecules it needs to interact with as it crosses the endothelial cell. Trafficking of LBRC membrane for both para- and transcellular migration requires intact microtubules (Fig. 5), which might be expected for membrane vesicles moving rapidly over relatively long distances. Both para- and transcellular migration of myeloid cells are also dependent on PECAM and CD99 (Fig. 6).
Our data clearly show that membrane from the LBRC surrounds leukocytes migrating transcellularly. We are presently unable to directly demonstrate how it gets there. Because of technical limitations, we cannot satisfactorily resolve targeted recycling of LBRC membrane in living cells in real time. However, we favor the idea that, as in the case of paracellular transmigration, LBRC membrane is targeted to the site of transmigration in a microtubule-dependent fashion. There are four possible routes that the membrane could take: (a) it could traffic directly to the site of transcellular migration; (b) it could recycle constitutively back to the endothelial cell border and diffuse along the surface to the site of transcellular migration; (c) it could recycle to the apical surface and diffuse to the site of transcellular migration; and (d) it could recycle to the cell border or the apical surface, and then be reendocytosed and targeted (by a non-LBRC pathway) to the site of transcellular migration.
The data in Figs. 5 and 7 clearly show that depolymerization of microtubules blocks the accumulation of LBRC membrane around the leukocyte (and hence blocks transmigration.) We have shown that the same treatment has no effect on constitutive recycling of LBRC membrane back to the cell border (Mamdouh et al., 2008). Furthermore, depolymerization of microtubules under the conditions we use has no effect on endocytosis. Finally, we have shown that the LBRC is distinct from typical recycling endosomes and caveolae (Mamdouh et al., 2003). If membrane from the LBRC were getting to the site of transcellular migration by any of routes b–d, it would not have been blocked by DCN and it would have accumulated under the leukocyte to form a ring in the experiments of Figs. 5 and 7. Therefore, our data rule out any other possibility except direct targeting to the site of transcellular migration.
In agreement with previous reports of transcellular migration (Carman and Springer, 2004; Yang et al., 2005; Millán et al., 2006; Carman et al., 2007), we found enrichment of ICAM-1 and JAM-A, and the absence of VE-cadherin, at the site of leukocyte migration (Fig. 1). We found, as reported in those other transcellular models, that uniformly expressed ICAM-1 on the luminal surface of HUVECs redistributed during leukocyte transcellular migration and was concentrated at the site of diapedesis. We also showed that ICAM-1 was enriched in the membrane channel that surrounded the crossing monocyte as it went through the endothelial cell body (Fig. 1). However, for transcellular migration, as for paracellular migration, we (Mamdouh et al., 2008) and others (Shaw et al., 2004; Yang et al., 2005) do not observe the docking structures or transmigratory cups formed by projections of ICAM-1 above the plane of the endothelial cell membrane (Barreiro et al., 2002; Carman and Springer, 2004). Even if the XZ projections shown in Figs. 4 and 7, for example, are summed across the entire width of the leukocyte, the ICAM and PECAM staining does not rise significantly above the surface of the endothelial cell (unpublished data). Previous documentation of PECAM at the site of transcellular migration has been inconsistent, even from the same group (Carman and Springer, 2004; Carman et al., 2007). However, we clearly see PECAM enrichment (Fig. 1) and document that the source of that PECAM is the LBRC (Fig. 4).
Similar to the observations of Carman et al. (Carman and Springer, 2004; Carman et al., 2007) but in contrast to a previous study from Millán et al. (2006), we generally did not see any enrichment in caveolin-1 at the site of diapedesis, which is consistent with the finding that the membrane of the LBRC does not contain caveolin-1 (Mamdouh et al., 2003). We did observe transcellular migration events in which caveolin-1 staining appeared to be modestly enriched above background in the vicinity of the transmigration pore (Fig. 2). However, when these cells were costained with anti-PECAM, it was clear that the caveolin-1 staining (presumably marking caveolae) was peripheral to the ring of LBRC membrane and not fused with it. The observations of Millán et al. (2006) were made on lymphoblasts, whereas we report on PMNs and monocytes; however, the observations of Carman et al. (Carman and Springer, 2004; Carman et al., 2007) were also made on lymphoblasts. Caveolae and other organelles have low diffusion rates and can have membrane and cytoskeletal tethers, which could impair their ability to be displaced by a migrating leukocyte. This could give the impression that they are relatively enriched around the transmigration pore. Because we did not attempt to detect them, we cannot exclude the possibility that membrane from other subcellular compartments, including the apical surface, is involved in transcellular migration. However, our data demonstrate that the LBRC is a critical functional component of the transcellular channel.
The apparent lack of VE-cadherin in the LBRC could explain an old observation made during paracellular migration: When a leukocyte crosses the endothelial border, VE-cadherin at the border appears to move out of the way (Allport et al., 2000; Shaw et al., 2001). As the transmigrating leukocyte is surrounded by membrane coming from the LBRC, VE-cadherin at the cell border may be either diluted or pushed out of the way. VE-cadherin has been shown to undergo endocytosis via a clathrin-dependent pathway (Xiao et al., 2005); it is apparently not internalized into the LBRC.
We grow our endothelial monolayers on hydrated fibrillar collagen gels rather than directly on glass or plastic to allow them to form a physiological basal lamina, which is important for endothelial monolayer stability and polarity. This precludes the use of shear flow in our transmigration studies. However, like paracellular migration (Mamdouh et al., 2008), transcellular migration is not noticeably affected by shear flow (Carman et al., 2007).
Using transfected cell lines, Yang et al. (2005) showed that overexpression of ICAM-1 promoted transcellular migration, implicating a role for ICAM-1 in this process. We show that disruption of microtubules had no effect on ICAM-1 enrichment around adherent leukocytes (Fig. 7), even though it blocked targeted recycling from the LBRC (Figs. 5 and 7) and both para- and transcellular migration (Figs. 5 and 7). Therefore, although enrichment of ICAM-1 may help promote transmigration and may even be a necessary prerequisite, it is not sufficient to promote transmigration in the absence of a functional LBRC.
Paracellular migration involves an elaborate series of rolling, adhesion, and locomotion events designed to bring the leukocyte close to the endothelial border. Transcellular migration appears to use the same initial steps. However, for some reason the leukocytes migrate through the cell rather than at the border. Carman and Springer (2008) speculated that the leukocytes take the path of least resistance across the endothelium. If junctions are very tight, e.g., as in the blood–brain barrier, migrating across the cell at a thin point may be easier, as seen in cerebral inflammation (Lossinsky and Shivers, 2004; Wolburg et al., 2005). However, the postcapillary venules that are the sites of most inflammatory diapedesis have very leaky junctions. In fact, they are specialized for permeability, because this is the site of fluid reuptake from the interstitium (Simionescu and Simionescu, 1983). Endothelial cells in culture, where transcellular migration has been best demonstrated, form a monolayer of low electrical resistance even under the most optimal culture conditions (Furie et al., 1984).
We hypothesize that at least for low-resistance endothelia, transcellular migration may occur when leukocytes are highly and/or directly activated. The first indisputable evidence of neutrophil transcellular migration in vivo came from studies in which emigration was stimulated by direct injection of fMLP, a PMN chemoattractant and β2 integrin activator, into the skin of guinea pigs (Feng et al., 1998). The authors presented a collection of electron microscope serial sections in which PMNs were shown to pass entirely across an endothelial cell without ever contacting a recognizable junction (Feng et al., 1998). Although the absolute frequency of transcellular migration was not addressed in this study, it demonstrated that transcellular migration was possible in vivo. The published in vitro studies of transcellular migration used MCP-1, platelet-activating factor, or stromal cell–derived factor 1 (CXCL12) to stimulate migration of monocytes, neutrophils, and T cells, respectively (Carman and Springer, 2004), or used mitogen-activated T lymphoblasts (Millán et al., 2006; Carman and Springer, 2008). Furthermore, these agents were added to the apical side of the endothelium, where they would activate leukocytes but not provide a chemotactic gradient. In our standard TEM assay system, paracellular migration predominates and transcellular migration is almost never seen across cytokine-activated HUVECs. However, when we applied chemokine or chemoattractant in the present study, 10–30% of leukocytes migrated transcellularly, similar to levels observed in the published studies (Carman and Springer, 2004; Yang et al., 2005; Millán et al., 2006; Carman et al., 2007). As observed by the authors of those studies, the rate of transcellular migration may be underestimated because we only scored leukocytes crossing endothelial cells >3 µm from the junction as transcellular events. Many of the studies that provide evidence for transcellular migration in vivo often show electron micrographs of leukocytes passing through the cell within a micrometer or two of an intact endothelial junction (Lossinsky and Shivers, 2004; Wolburg et al., 2005). This is exactly where the LBRC is situated and would put it in a prime location for its role in promoting trans- as well as paracellular migration.
Transcellular migration is also dependent on PECAM and CD99 (Fig. 6). Because expression of PECAM and CD99 on the endothelial cell surface is essentially restricted to cell borders, this was not necessarily expected. Homophilic interaction between leukocyte PECAM and endothelial PECAM appears to be the trigger for targeted recycling of the LBRC during paracellular migration of neutrophils and monocytes (Mamdouh et al., 2003; Mamdouh et al., 2008). Polyclonally activated lymphocytes, whose migration is PECAM independent, still require targeted recycling of the LBRC for paracellular migration (Mamdouh et al., 2008). We hypothesize that these activated lymphocytes are capable of sending a signal to the endothelial cells to recruit the LBRC that bypasses the PECAM requirement (Mamdouh et al., 2008). It is reasonable to expect that activated myeloid cells migrating transcellularly transmit a similar signal because there would be no PECAM to interact with, rendering transcellular migration PECAM (and CD99) independent. This hypothesis may still hold true for the initial recruitment of LBRC membrane. However, once transcellular migration starts, it is apparently PECAM and CD99 dependent. The fact that PECAM and CD99 are both in the LBRC and are required for both para- and transcellular migration raises the question of whether PECAM regulates a step in transcellular migration before the CD99-dependent step, as it does in paracellular migration (Schenkel et al., 2002; Lou et al., 2007). This is a goal for future research. Our immuno-EM and fluorescence quenching experiments show that PECAM and CD99 share the same distribution, but give no quantitative information on how they may be spatially organized along the cell border.
We and others find no role for JAM-A in the paracellular transmigration of human PMNs or monocytes (Fig. 6; Liu et al., 2000; Schenkel et al., 2004; Shaw et al., 2004). Although JAM-A appears to be in the LBRC, we are unable to block transcellular migration using the same mAb (Fig. 6). The role for JAM-A in leukocyte emigration has been reported to be through interaction with LFA-1 on the leukocyte when JAM-A is on the apical surface of the endothelial cells (Ostermann et al., 2002). Under our culture conditions, JAM-A remains at the cell border or in the LBRC. Furthermore, interactions with JAM-A on the apical surface would be more relevant to the adhesion step that precedes transmigration than to the transmigration step itself.
How is the LBRC, a membrane compartment that is contiguous with the lateral borders of endothelial cells, redirected to the site of transcellular migration? This is not clear and is obviously the subject for future work. However, we speculate that a signal from the leukocyte may trigger it, perhaps the same signal that is given at the cell border to recruit the LBRC to promote paracellular migration. This might occur if the leukocyte were so activated that it transmitted the signal prematurely before reaching the lateral border. The fact that transcellular migration is more common among highly and directly activated leukocytes is consistent with this hypothesis. The clustering of ICAM-1 that precedes LBRC recruitment suggests that it might be involved in transmitting this signal through interactions with the leukocyte. Transcellular migration may also occur if the leukocyte has difficulty reaching the junction, such as in cells in which CD11b is nonfunctional (Schenkel et al., 2004) or deficient (Phillipson et al., 2006) or in cells that are unable to polarize (Gerard et al., 2009), or in vasculature in which the junction is particularly tight, such as the blood–brain barrier (Lossinsky and Shivers, 2004; Wolburg et al., 2005). A mechanism in which homophilic adhesion molecules such as PECAM, CD99, and JAM-A tightly engage leukocytes as they pass through the endothelial cell could allow leukocyte passage without plasma leakage. The LBRC could provide such a route.
Carman et al. (2007) showed leukocytes appearing to probe the endothelial surface with lamellipodia (podosomes), often deeply invaginating the surface of the endothelial cell. They were highly enriched for actin filaments (Feng et al., 1998; Carman et al., 2007), which were required for their function (Carman et al., 2007). Actin polymerization and lamellapodia formation are events triggered in leukocytes by integrin activation, so prominent podosomes in cells migrating transcellularly could be manifestations of high levels of leukocyte activation. The podosomes were hypothesized to initiate the formation of the transcellular channel. In previous studies, such podosomes were observed invaginating the endothelial surface away from the junctions even under conditions where leukocytes crossed at the cell borders (Furie et al., 1987; Migliorisi et al., 1987), as well as under conditions where leukocytes are blocked in their attempts to cross at the junctions (Fig. 4 a in Liao et al., 1995). These lamellapodia may be a general mechanism for leukocytes to crawl across the endothelial surface. However, if they have a role in promoting transmigration, it is conceivable that they signal the recruitment of LBRC membrane to the leukocytes when they are at the cell borders as well as when they are not. In this case, the mechanisms of para- and transcellular migration would have even more in common.
At least one potential mechanistic difference between para- and transcellular migration remains. Because the LBRC is connected to the lateral endothelial cell surface at the cell borders, fusion of the LBRC membrane with the plasma membrane is not necessarily required to bring the LBRC in contact with the leukocyte for paracellular TEM. On the other hand, to bring this membrane compartment in contact with the leukocyte on the apical surface for transcellular migration would require membrane fusion. In fact, Carman et al. (2007) provide evidence that membrane fusion is required for transcellular migration. However, because the LBRC vesicles are connected to each other (Mamdouh et al., 2003) rather than multiple fusion events involving dozens or hundreds of vesicles, the entire surface area of the LBRC could be brought to surround the leukocyte with perhaps only two fusion events necessary: one at the apical surface and one at the basal surface of the endothelial cell.
In conclusion, we report for the first time that leukocyte transcellular diapedesis requires targeted trafficking of LBRC membrane, which contains PECAM-1, JAM-A, and CD99, but not VE-cadherin, to surround the leukocyte. Antibodies that block PECAM and CD99 function inhibit transcellular migration. We also show that transcellular diapedesis and LBRC trafficking are microtubule dependent, but that ICAM-1 clustering around the crossing leukocyte is an upstream event that is independent of microtubules and LBRC recycling. Our data demonstrate that trans- and paracellular diapedesis involve similar mechanisms and may, in fact, have more mechanistic similarities than differences. If this is true, it is conceivable that many transmigration events that occur close to endothelial borders may be partially transcellular and partially paracellular, with leukocytes moving between and through endothelial cells depending on the rapidity and ease of LBRC recruitment. In that case, the distinction between trans- and paracellular migration would break down, and the major question for future research in this area would be, what recruits the LBRC to the leukocyte?
MATERIALS AND METHODS
Reagents.
Hybridomas producing the mAbs R6.5 (anti–ICAM-1) and OKM1 (anti-CD11b) were obtained from American Type Culture Collection. The mAbs hec1 (anti–VE-cadherin) and hec2 (anti-CD99) were produced from hybridomas generated in the laboratory, as described previously (Muller et al., 1989). P1.1 (nonblocking anti–PECAM-1 mAb) was column purified on protein A–Sepharose (GE Healthcare) from ascites provided by P.J. Newman (BloodCenter of Wisconsin, Milwaukee, WI). Fab fragments of P1.1 were cut using papain (Thermo Fisher Scientific), followed by column purification on protein A–Sepharose. Purity of Fab fragments was confirmed by SDS-PAGE. 1H29A (anti–JAM-A) was purified on protein A–Sepharose from ascites provided by C. Parkos (Emory University, Atlanta, GA). Rabbit anti-PECAM antibody 177 was produced and purified as previously described (Lou et al., 2007). Rabbit anti–caveolin-1 antibody was purchased from BD. Unlabeled and rhodamine-conjugated goat anti–mouse F(ab′)2 antibodies used for recycling experiments of PECAM were purchased from Jackson ImmunoResearch Laboratories. Antibodies for immunofluorescence were coupled to Alexa Fluor 488, Alexa Fluor 546, or Alexa Fluor 633 according to the manufacturer’s protocol (Invitrogen). Alexa Fluor 488–phalloidin and Alexa Fluor 546–phalloidin were purchased from Invitrogen. DCN was purchased from EMD. DAB was purchased from Sigma-Aldrich.
Cells.
HUVECs were isolated from fresh human umbilical cords as previously described (Muller et al., 1989) and grown in M199 medium (Invitrogen) supplemented with 20% heat-inactivated normal human serum and 100 U/ml penicillin–streptomycin at 37°C in a humidified atmosphere of 5% CO2. HUVECs at passage two were cultured on thick hydrated collagen type I (Vitrogen; Cohesion Technologies) gels set in 96-well plates (Muller and Weigl, 1992) or on glass coverslip dishes (Carolina Biological Supply) coated with 5 µg/ml fibronectin (Sigma-Aldrich). HUVEC monolayers were activated with 20 ng/ml TNF for >4 h before each experiment (Mamdouh et al., 2008). PMNs were isolated from healthy volunteers by density gradient centrifugation in Ficoll-Paque (GE Healthcare). Neutrophils were prepared from heparinized peripheral blood by density sedimentation in a discontinuous gradient of Ficoll and Histopaque-1119 (Sigma-Aldrich), as previously described (Muller and Weigl, 1992). All experimental protocols were approved by the Institutional Review Boards of the Weill Medical College of Cornell University and the Feinberg School of Medicine of Northwestern University.
Recycling experiments and transcellular migration.
Recycling of PECAM was performed on TNF-activated HUVECs as described previously (Mamdouh et al., 2003; Mamdouh et al., 2008). In brief, HUVECs plated on collagen gels were incubated with Fab fragment P1.1 antibody for 1 h at 37°C to label PECAM. They were subsequently washed, chilled, and incubated with an excess of a unlabeled goat anti–mouse F(ab′)2 IgG for 1 h on ice to saturate the primary antibody bound to surface PECAM. Cells were washed, and 10 µl of 20 ng/ml MCP-1 or 10−7 M fMLP was injected into the collagen to create a chemokine gradient for monocytes and neutrophils, respectively. Medium 199 containing rhodamine-conjugated goat anti–mouse F(ab′)2 IgG and 2 × 105 PBMCs or neutrophils were added to HUVECs. Cells were kept on ice for 15 min and warmed at 37°C for 10 min in a CO2 incubator. They were subsequently washed and fixed in freshly prepared 2% paraformaldehyde for 10 min at room temperature.
In some experiments, microtubules were depolymerized as previously described using 10 µM DCN under conditions that did not affect the distribution of PECAM, ICAM-1, or VE-cadherin, or otherwise noticeably perturb the cells (Mamdouh et al., 2008). This drug was washed out before addition of leukocytes. Control experiments (not depicted; Mamdouh et al., 2008) demonstrated that there was no carryover of DCN to affect leukocyte microtubules.
Immunofluorescence microscopy.
To visualize the distribution of adhesion molecules during leukocyte transmigration, leukocytes were allowed to transmigrate for 10 min across HUVEC monolayers as described, and were then washed and fixed in freshly prepared 2% paraformaldehyde. Specific antibodies for each adhesion molecule were used either directly conjugated to Alexa Fluor dyes or detected with a fluorescent secondary antibody. To label actin, cells were permeabilized after fixation with 0.2% Triton X-100 (Sigma-Aldrich) for 10 min and incubated with Alexa Fluor 488– or Alexa Fluor 546–labeled phalloidin for 10 min at room temperature.
Images were collected using a confocal inverted microscope (Axiovert 100M) equipped with an LSM 510 laser scanning unit and a 63 × 1.4 or a 100 × 1.46 NA plan Apochromat objective (all from Carl Zeiss, Inc.). The LSM 510 was equipped with a 25-mW argon laser emitting at 488 nm, a 1-mW helium/neon laser emitting at 543 nm, a 5-mW helium/neon laser emitting at 633 nm, and a UV laser emitting at 405 nm. Image processing and quantification were performed using image processing software (MetaMorph; MDS Analytical Technologies). Alternatively, stacks of optical sections through the specimen were acquired on a restoration workstation (Delta Vision 3D; Applied Precision) equipped with an inverted microscope (model IX70; Olympus) using a 100× objective lens. Images were deconvolved using SoftWorx software (Applied Precision). The figures show either representative optical sections through the region of interest or projections of the whole stack using the maximum intensity method.
Quantification of trans- and paracellular diapedesis.
10 µl of 20 ng/ml MCP-1 was injected into the collagen bearing TNF-activated HUVECs before adding 2 × 105 PBMCs in cold M199 containing 0.1% human serum albumin. Cells were allowed to settle for 15 min on ice and warmed for 10 min at 37° in a CO2 incubator. Cells were washed, fixed in 2% paraformaldehyde, and stained for VE-cadherin and actin. Fixed samples were examined by confocal and wide-field microscopy. Image stacks obtained by confocal microscopy and deconvolved images acquired by wild-field microscopy were analyzed for quantification of diapedesis. Monocytes crossing endothelial cells >3 µm from the junctions were scored as transcellular diapedesis. Total monocytes associated with endothelial cells in each field (i.e., monocytes on top and under HUVECs, plus those in the act of diapedesis) were counted. Percent TEM was calculated by dividing the number of cells in the act of diapedesis by the total number of monocytes in each field.
In experiments to determine whether blockade of PECAM, CD99, or JAM-A would affect transcellular migration, experiments were performed as described, except that leukocytes were mixed with antibodies against those molecules or VE-cadherin (as a negative control) before adding them to the endothelial monolayers. Transmigration was allowed to proceed for 30 min, and the monolayers were fixed and processed as described. Alexa Fluor 488–conjugated secondary antibodies were used to detect the cell junctions. Actin was visualized with Alexa Fluor 546–phalloidin. Leukocytes found directly below or crossing at cell borders were scored as paracellular migration events; those detected under or in the act of crossing the endothelial cell body >3 µm from the junctions were scored as transcellular migration events. Percent TEM was calculated as the number of transmigrated monocytes divided by the total monocytes in the field.
immuno-EM.
P1.1, 1H29A, hec1, and hec2 IgGs were conjugated to HRP as previously described (Mamdouh et al., 2003) . HUVEC monolayers plated on fibronectin-coated coverslip dishes were incubated with each of the HRP-conjugated antibodies separately for 1 h at 37°C or with the equivalent concentration of free HRP (5.5 µg/ml). Cells were rinsed in PBS and fixed in glutaraldehyde (EM Sciences) in 0.1 M sodium cacodylate buffer for 15 min. Cells were washed and exposed to a mixture of DAB and H2O2 according to the method of Graham and Karnovsky (1966). The cells were postfixed in 4% glutaraldehyde and embedded in plastic, and en face sections were cut and examined on an electron microscope (JSM 100CX II; JEOL).
Fluorescence quenching experiments.
These experiments were performed as previously described (Ghosh et al., 1998; Lampson et al., 2001). In brief, endothelial cells grown on coverslip dishes were incubated with 20 µg/ml of Alexa Fluor 488–conjugated anti-CD99 mAb and 20 µg/ml of HRP-conjugated anti-PECAM mAb for 1 h at 37° to allow ample time for delivery to the LBRC. In control experiments, chromophores were reversed or HRP-transferrin (the gift of T. McGraw, Weill Cornell Medical College, New York, NY) was used to label a defined endocytic compartment in place of the HRP–anti-PECAM or HRP–anti-CD99. Cells were chilled, washed extensively, and incubated on ice for 30 min in darkness with 250 µg/ml DAB and 0.0025% H2O2. DAB diffuses into the cell and the HRP on the conjugated antibody catalyzes the oxidation of DAB in the presence of H2O2. Oxidized DAB reaction product polymerizes and quenches the fluorescence of molecules in its vicinity. Because the polymerized DAB precipitates locally and does not diffuse across membranes, it will only quench fluorescence of the Alexa Fluor–labeled mAb if the antibodies (and hence the antigens) are in the same compartment.
Statistics.
All data were analyzed by pairwise comparison using a two-tailed t test assuming unequal variances, with the Bonferroni correction for multiple comparisons as appropriate.
Acknowledgments
We wish to thank R.M. Liebman for excellent technical assistance, A. Beaulieu for performing the fluorescence quenching experiments, L. Cohen-Gould for help with electron microscopy, Dr. K. van Leyen for assistance purifying HRP-conjugated antibodies, and Dr. D. Sullivan for helpful comments on the manuscript.
This work was supported by grants R37 HL064774 and R01 HL046849 from the National Heart, Lung, and Blood Institute to W.A. Muller.
The authors have no competing financial interests to declare.
Footnotes
Abbreviations used:
- DAB
- 3,3′-diaminobenzidine
- DCN
- demecolcine
- HRP
- horseradish peroxidase
- HUVEC
- human umbilical vein endothelial cell
- ICAM-1
- intracellular adhesion molecule 1
- immuno-EM
- immunoelectron microscopy
- JAM-A
- junctional adhesion molecule A
- LBRC
- lateral border recycling compartment
- MCP-1
- monocyte chemotactic protein 1
- PECAM
- platelet/endothelial cell adhesion molecule
- TEM
- transendothelial migration
- VE-cadherin
- vascular endothelial cell–specific cadherin
References
- Allport J.R., Muller W.A., Luscinskas F.W. 2000. Monocytes induce reversible focal changes in vascular endothelial cadherin complex during transendothelial migration under flow. J. Cell Biol. 148:203–216 10.1083/jcb.148.1.203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamforth S.D., Lightman S.L., Greenwood J. 1997. Ultrastructural analysis of interleukin-1 β-induced leukocyte recruitment to the rat retina. Invest. Ophthalmol. Vis. Sci. 38:25–35 [PubMed] [Google Scholar]
- Barreiro O., Yanez-Mo M., Serrador J.M., Montoya M.C., Vicente-Manzanares M., Tejedor R., Furthmayr H., Sanchez-Madrid F. 2002. Dynamic interaction of VCAM-1 and ICAM-1 with moesin and ezrin in a novel endothelial docking structure for adherent leukocytes. J. Cell Biol. 157:1233–1245 10.1083/jcb.200112126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher E.C. 1991. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 67:1033–1036 10.1016/0092-8674(91)90279-8 [DOI] [PubMed] [Google Scholar]
- Carman C.V., Springer T.A. 2004. A transmigratory cup in leukocyte diapedesis both through individual vascular endothelial cells and between them. J. Cell Biol. 167:377–388 10.1083/jcb.200404129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carman C.V., Springer T.A. 2008. Trans-cellular migration: cell-cell contacts get intimate. Curr. Opin. Cell Biol. 20:533–540 10.1016/j.ceb.2008.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carman C.V., Sage P.T., Sciuto T.E., de la Fuente M.A., Geha R.S., Ochs H.D., Dvorak H.F., Dvorak A.M., Springer T.A. 2007. Transcellular diapedesis is initiated by invasive podosomes. Immunity. 26:784–797 10.1016/j.immuni.2007.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng D., Nagy J.A., Hipp J., Dvorak H.F., Dvorak A.M. 1996. Vesiculo-vacuolar organelles and the regulation of venule permeability to macromolecules by vascular permeability factor, histamine, and serotonin. J. Exp. Med. 183:1981–1986 10.1084/jem.183.5.1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng D., Nagy J.A., Pyne K., Dvorak H.F., Dvorak A.M. 1998. Neutrophils emigrate from venules by a transendothelial cell pathway in response to FMLP. J. Exp. Med. 187:903–915 10.1084/jem.187.6.903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furie M.B., Cramer E.B., Naprstek B.L., Silverstein S.C. 1984. Cultured endothelial cell monolayers that restrict the transendothelial passage of macromolecules and electrical current. J. Cell Biol. 98:1033–1041 10.1083/jcb.98.3.1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furie M.B., Naprstek B.L., Silverstein S.C. 1987. Migration of neutrophils across monolayers of cultured microvascular endothelial cells. An in vitro model of leucocyte extravasation. J. Cell Sci. 88:161–175 [DOI] [PubMed] [Google Scholar]
- Gerard A., van der Kammen R.A., Janssen H., Ellenbroek S.I., Collard J.G. 2009. The Rac activator Tiam1 controls efficient T-cell trafficking and route of transendothelial migration. Blood. 113:6138–6147 [DOI] [PubMed] [Google Scholar]
- Ghosh R.N., Mallet W.G., Soe T.T., McGraw T.E., Maxfield F.R. 1998. An endocytosed TGN38 chimeric protein is delivered to the TGN after trafficking through the endocytic recycling compartment in CHO cells. J. Cell Biol. 142:923–936 10.1083/jcb.142.4.923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham R.C., Jr., Karnovsky M.J. 1966. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J. Histochem. Cytochem. 14:291–302 [DOI] [PubMed] [Google Scholar]
- Lampson M.A., Schmoranzer J., Zeigerer A., Simon S.M., McGraw T.E. 2001. Insulin-regulated release from the endosomal recycling compartment is regulated by budding of specialized vesicles. Mol. Biol. Cell. 12:3489–3501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley K., Laudanna C., Cybulsky M.I., Nourshargh S. 2007. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat. Rev. Immunol. 7:678–689 10.1038/nri2156 [DOI] [PubMed] [Google Scholar]
- Liao F., Huynh H.K., Eiroa A., Greene T., Polizzi E., Muller W.A. 1995. Migration of monocytes across endothelium and passage through extracellular matrix involve separate molecular domains of PECAM-1. J. Exp. Med. 182:1337–1343 10.1084/jem.182.5.1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Nusrat A., Schnell F.J., Reaves T.A., Walsh S., Pochet M., Parkos C.A. 2000. Human junction adhesion molecule regulates tight junction resealing in epithelia. J. Cell Sci. 113:2363–2374 [DOI] [PubMed] [Google Scholar]
- Lossinsky A.S., Shivers R.R. 2004. Structural pathways for macromolecular and cellular transport across the blood-brain barrier during inflammatory conditions. Review. Histol. Histopathol. 19:535–564 [DOI] [PubMed] [Google Scholar]
- Lou O., Alcaide P., Luscinskas F.W., Muller W.A. 2007. CD99 is a key mediator of the transendothelial migration of neutrophils. J. Immunol. 178:1136–1143 [DOI] [PubMed] [Google Scholar]
- Mamdouh Z., Chen X., Pierini L.M., Maxfield F.R., Muller W.A. 2003. Targeted recycling of PECAM from endothelial surface-connected compartments during diapedesis. Nature. 421:748–753 10.1038/nature01300 [DOI] [PubMed] [Google Scholar]
- Mamdouh Z., Kreitzer G.E., Muller W.A. 2008. Leukocyte transmigration requires kinesin-mediated microtubule-dependent membrane trafficking from the lateral border recycling compartment. J. Exp. Med. 205:951–966 10.1084/jem.20072328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchesi V.T., Gowans J.L. 1964. The migration of lymphocytes through the endothelium of venules in lymph nodes: an electron microscope study. Proc. R. Soc. Lond. B Biol. Sci. 159:283–290 10.1098/rspb.1964.0002 [DOI] [PubMed] [Google Scholar]
- Migliorisi G., Folkes E., Pawlowski N., Cramer E.B. 1987. In vitro studies of human monocyte migration across endothelium in response to leukotriene B4 and f-Met-Leu-Phe. Am. J. Pathol. 127:157–167 [PMC free article] [PubMed] [Google Scholar]
- Millán J., Hewlett L., Glyn M., Toomre D., Clark P., Ridley A.J. 2006. Lymphocyte transcellular migration occurs through recruitment of endothelial ICAM-1 to caveola- and F-actin-rich domains. Nat. Cell Biol. 8:113–123 10.1038/ncb1356 [DOI] [PubMed] [Google Scholar]
- Muller W.A. 2001. Migration of leukocytes across endothelial junctions: some concepts and controversies. Microcirculation. 8:181–193 10.1038/sj.mn.7800078 [DOI] [PubMed] [Google Scholar]
- Muller W.A. 2002. Leukocyte-endothelial cell interactions in the inflammatory response. Lab. Invest. 82:521–533 10.1038/labinvest.3780446 [DOI] [PubMed] [Google Scholar]
- Muller W.A. 2003. Leukocyte-endothelial-cell interactions in leukocyte transmigration and the inflammatory response. Trends Immunol. 24:326–333 10.1016/S1471-4906(03)00117-0 [DOI] [PubMed] [Google Scholar]
- Muller W.A. 2009. Mechanisms of transendothelial migration of leukocytes. Circ. Res. 105:223–230 10.1161/CIRCRESAHA.109.200717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller W.A., Weigl S.A. 1992. Monocyte-selective transendothelial migration: dissection of the binding and transmigration phases by an in vitro assay. J. Exp. Med. 176:819–828 10.1084/jem.176.3.819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller W.A., Ratti C.M., McDonnell S.L., Cohn Z.A. 1989. A human endothelial cell–restricted, externally disposed plasmalemmal protein enriched in intercellular junctions. J. Exp. Med. 170:399–414 10.1084/jem.170.2.399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller W.A., Weigl S.A., Deng X., Phillips D.M. 1993. PECAM-1 is required for transendothelial migration of leukocytes. J. Exp. Med. 178:449–460 10.1084/jem.178.2.449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostermann G., Weber K.S.C., Zernecke A., Schröder A., Weber C. 2002. JAM-1 is a ligand of the β(2) integrin LFA-1 involved in transendothelial migration of leukocytes. Nat. Immunol. 3:151–158 10.1038/ni755 [DOI] [PubMed] [Google Scholar]
- Phillipson M., Heit B., Colarusso P., Liu L., Ballantyne C.M., Kubes P. 2006. Intraluminal crawling of neutrophils to emigration sites: a molecularly distinct process from adhesion in the recruitment cascade. J. Exp. Med. 203:2569–2575 10.1084/jem.20060925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkel A.R., Mamdouh Z., Chen X., Liebman R.M., Muller W.A. 2002. CD99 plays a major role in the migration of monocytes through endothelial junctions. Nat. Immunol. 3:143–150 10.1038/ni749 [DOI] [PubMed] [Google Scholar]
- Schenkel A.R., Mamdouh Z., Muller W.A. 2004. Locomotion of monocytes on endothelium is a critical step during extravasation. Nat. Immunol. 5:393–400 10.1038/ni1051 [DOI] [PubMed] [Google Scholar]
- Shaw S.K., Bamba P.S., Perkins B.N., Luscinskas F.W. 2001. Real-time imaging of vascular endothelial-cadherin during leukocyte transmigration across endothelium. J. Immunol. 167:2323–2330 [DOI] [PubMed] [Google Scholar]
- Shaw S.K., Ma S., Kim M.B., Rao R.M., Hartman C.U., Froio R.M., Yang L., Jones T., Liu Y., Nusrat A., et al. 2004. Coordinated redistribution of leukocyte LFA-1 and endothelial cell ICAM-1 accompany neutrophil transmigration. J. Exp. Med. 200:1571–1580 10.1084/jem.20040965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simionescu N., Simionescu M. 1983. The cardiovascular system. Histology: Cell and Tissue Biology. Weiss L., editor Elsevier Science Publishing Co. Inc., New York: 371–433 [Google Scholar]
- Springer T.A. 1994. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 76:301–314 10.1016/0092-8674(94)90337-9 [DOI] [PubMed] [Google Scholar]
- Williamson J.R., Grisham J.W. 1961. Electron microscopy of leukocytic margination and emigration in acute inflammation in dog pancreas. Am. J. Pathol. 39:239–256 [PMC free article] [PubMed] [Google Scholar]
- Wolburg H., Wolburg-Buchholz K., Engelhardt B. 2005. Diapedesis of mononuclear cells across cerebral venules during experimental autoimmune encephalomyelitis leaves tight junctions intact. Acta Neuropathol. 109:181–190 10.1007/s00401-004-0928-x [DOI] [PubMed] [Google Scholar]
- Xiao K., Garner J., Buckley K.M., Vincent P.A., Chiasson C.M., Dejana E., Faundez V., Kowalczyk A.P. 2005. p120-Catenin regulates clathrin-dependent endocytosis of VE-cadherin. Mol. Biol. Cell. 16:5141–5151 10.1091/mbc.E05-05-0440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Froio R.M., Sciuto T.E., Dvorak A.M., Alon R., Luscinskas F.W. 2005. ICAM-1 regulates neutrophil adhesion and transcellular migration of TNF-alpha-activated vascular endothelium under flow. Blood. 106:584–592 10.1182/blood-2004-12-4942 [DOI] [PMC free article] [PubMed] [Google Scholar]