Abstract
The inflammatory mediator thrombin proteolytically activates protease-activated receptor (PAR1) eliciting a transient, but reversible increase in vascular permeability. PAR1-induced dissociation of Gα subunit from heterotrimeric Gq and G12/G13 proteins is known to signal the increase in endothelial permeability. However, the role of released Gβγ is unknown. We now show that impairment of Gβγ function does not affect the permeability increase induced by PAR1, but prevents reannealing of adherens junctions (AJ), thereby persistently elevating endothelial permeability. We observed that in the naive endothelium Gβ1, the predominant Gβ isoform is sequestered by receptor for activated C kinase 1 (RACK1). Thrombin induced dissociation of Gβ1 from RACK1, resulting in Gβ1 interaction with Fyn and focal adhesion kinase (FAK) required for FAK activation. RACK1 depletion triggered Gβ1 activation of FAK and endothelial barrier recovery, whereas Fyn knockdown interrupted with Gβ1-induced barrier recovery indicating RACK1 negatively regulates Gβ1-Fyn signaling. Activated FAK associated with AJ and stimulated AJ reassembly in a Fyn-dependent manner. Fyn deletion prevented FAK activation and augmented lung vascular permeability increase induced by PAR1 agonist. Rescuing FAK activation in fyn−/− mice attenuated the rise in lung vascular permeability. Our results demonstrate that Gβ1-mediated Fyn activation integrates FAK with AJ, preventing persistent endothelial barrier leakiness.
A persistent increase in endothelial permeability during inflammatory conditions such as pneumonia, trauma, and burn leads to the life-threatening illness acute respiratory distress syndrome (Mehta and Malik, 2006; Liu and Matthay, 2008). Increased endothelial permeability occurs because of loss of cell–cell contacts and disruption of cell–extracellular matrix (ECM) adhesions (Yuan, 2002; Mehta and Malik, 2006). Focal adhesion kinase (FAK) and VE-cadherin play a fundamental role in establishing the endothelial barrier to macromolecules and liquid by maintaining intercellular adhesion and cell–ECM adhesivity (Nelson et al., 2004; van Buul et al., 2005; Wu, 2005; Mehta and Malik, 2006; Dejana et al., 2008; Rudini and Dejana, 2008). We have shown that thrombin, a serine protease generated early on during acute respiratory distress syndrome, plays a critical role in increasing endothelial permeability by inducing the loss of VE-cadherin homotypic adhesion and redistribution of focal adhesions dependent on FAK (Mehta et al., 2002; Kouklis et al., 2004; Holinstat et al., 2006). Interestingly, the thrombin-induced increase in endothelial permeability is reversed within 2–3 h, indicating activation of endogenous pathways that limit the persistent increase in endothelial permeability produced by thrombin (Kouklis et al., 2004; Holinstat et al., 2006; Kaneider et al., 2007).
Thrombin binds to endothelial cell surface receptor, protease-activating receptor 1 (PAR1) and PAR4 (Coughlin, 2000, 2005; Kataoka et al., 2003). We have shown that the permeability increasing effects of thrombin in lung endothelium are predominantly mediated through PAR1 because thrombin and selective PAR1 peptide agonists failed to induce endothelial contraction and lung microvascular permeability increase in mice lacking PAR1 (Vogel et al. 2000). PAR1 is a seven-transmembrane domain receptor that couples to heterotrimeric G proteins of the Gq and G12/13 families (Hung et al., 1992; Coughlin 1999). Upon ligation by thrombin, PAR1 signals the dissociation of the α-subunits of Gq and G12/13 from the Gβγ dimer. Gαq and Gα12/13 activate myosin light chain kinase and RhoA pathways, which by inducing endothelial cell contraction increase permeability (Goeckeler and Wysolmerski, 1995; Dudek and Garcia, 2001; Holinstat et al., 2003; McLaughlin et al., 2005; Knezevic et al., 2007; Singh et al., 2007; Gavard and Gutkind, 2008; Korhonen et al., 2009). However, the role of Gβγ after its dissociation from these heterotrimeric G proteins in the mechanism of PAR1-induced alteration in endothelial barrier function is unknown.
The Gβγ pathway has progressively emerged as a critical element of GPCR signaling (Clapham and Neer, 1997; Cabrera-Vera et al., 2003; Oldham and Hamm, 2008). Gβγ is known to induce cyclic AMP generation (Tang and Gilman, 1992; Taurin et al., 2007), Ca2+ signaling (Herlitze et al., 1996; Blackmer et al., 2001), oxidant generation (Niu et al., 2003), neurotransmission (Blackmer et al., 2005), chemotaxis (Neptune and Bourne, 1997; Jin et al., 2000), and caveolae-mediated transcytosis (Shajahan et al., 2004). The β subunit of Gβγ contains WD 40 repeats that are thought to mediate protein–protein interactions (Neer et al., 1994; Chen et al., 2004b). Studies show that Gβ interacts with receptor for activated C kinase 1 (RACK1; Dell et al., 2002; Chen et al., 2004a), p60cSrc (Luttrell et al., 1996; McGarrigle and Huang, 2007), and Fyn (Yaka et al., 2002; Thornton et al., 2004). Fyn, p60cSrc, and RACK1 are known to influence adherens junctions (AJ) and focal adhesions (Xing et al., 1994; Bockholt and Burridge, 1995; Thomas and Brugge, 1997; Roura et al., 1999; Owens et al., 2000; Mourton et al., 2001; Schaller, 2001; Piedra et al., 2003). We tested the hypothesis that, besides restraining Gα subunits, Gβγ orchestrates signaling to terminate endothelial permeability increase through its ability to coordinate intercellular and cell–matrix interactions.
To explore the function of Gβγ in regulating endothelial permeability, we interfered with expression of Gβγ, RACK1, or Fyn using small interfering RNA (siRNA) or Fyn knockout mice. We show that Gβγ plays a fundamental role in signaling endothelial barrier recovery. Moreover, we identified Fyn and FAK as the key downstream effectors of Gβγ. Fyn-mediated activation of FAK facilitated the association of FAK with p120-catenin and reannealing of AJ, which reversed the increased endothelial permeability responses produced by G protein–coupled receptor agonists.
RESULTS
Gβγ is required for reannealing AJ and for restoration of endothelial barrier function
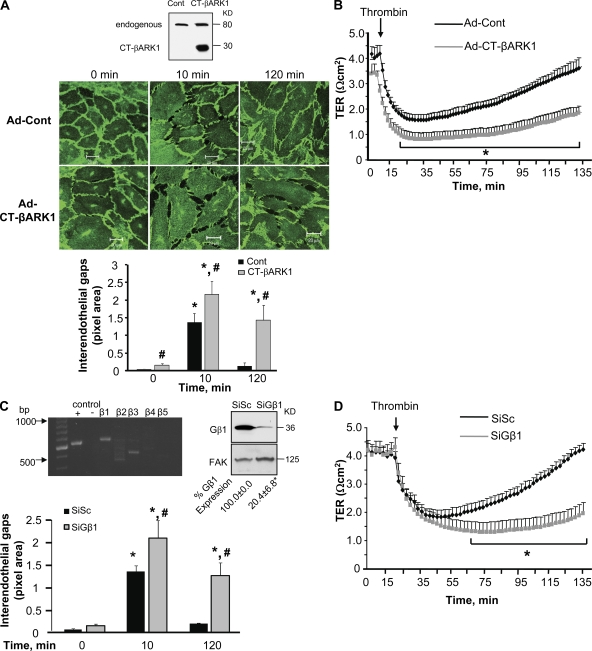
To address the contribution of Gβγ to the mechanism of increased endothelial permeability, we transduced the 194-aa C terminus of β-adrenergic receptor kinase 1 (CT-βARK-1; Drazner et al. 1997) in endothelial cells to inhibit Gβγ (Fig. 1 A, inset). Changes in endothelial permeability were determined by quantifying interendothelial gap area in endothelial monolayers immunostained with anti–VE-cadherin antibody (Ab) and dynamically by determining the decrease in transendothelial electrical resistance (TER) after thrombin challenge. Consistent with our previous study (Holinstat et al., 2006), thrombin rapidly disrupted AJ, induced gap formation (Fig. 1 A), and decreased TER (Fig. 1 B) in cells receiving control vector. AJ subsequently reannealed, as interendothelial gaps closed and TER returned to basal levels within 2 h (Fig. 1, A and B). Inhibition of Gβγ did not affect the acute disruption of AJ, interendothelial gap formation, or TER decrease induced by thrombin, but in contrast to control cells, these thrombin responses persisted without recovery to basal level (Fig. 1, A and B).
Figure 1.
Inhibition of Gβγ prevents reannealing of AJ and restoration of endothelial barrier permeability after thrombin stimulation. (A) Effect of Gβγ inhibition on adherens junction reassembly. (top) HPAE cells were infected with adenovirus (Ad) containing either control vector or CT-βARK1 for 6 h in serum-free medium, after which the medium was replaced with fresh complete medium without virus. After 34 h, cells were stimulated with 50 nM thrombin for indicated times, fixed, permeabilized, and stained with anti–VE cadherin Ab followed by incubation with Alexa Fluor–labeled secondary Ab as described in Materials and methods. (inset) The expression of CT-βARK in HPAE cell lysates was determined by immunoblotting with anti-GRK2 Ab. (bottom) Plot shows mean ± SD of interendothelial gaps formed in monolayers transduced with CT-βARK1 after thrombin stimulation at indicated times from three independent experiments. * indicates significant increase in gap area from that in unstimulated (zero time) monolayers transduced with control vector (Ad-control; P < 0.05); # indicates significant increase in gap area in monolayers transduced with CT-βARK1 compared with monolayers transduced with control vector after thrombin stimulation (P < 0.05). (B) Effect of Gβγ inhibition on endothelial permeability. HPAE monolayers infected with Ad-control vector or Ad-CT-βARK1 were stimulated with 50 nM thrombin, and changes in TER, a dynamic assessment of intercellular adhesion, were determined over time. Data represent mean ± SD from four experiments performed in duplicates. * indicates significance from Ad-control monolayer (P < 0.05). (C) Effect of Gβ1 knockdown on adherens junction assembly. (top left) Gβ expression in HPAE cells. RNA isolated from HPAE cells were reverse transcribed using various Gβ primers as described in Materials and methods. GAPDH primers were used as a positive control (+), whereas RNA without primers (−) was used as a negative control. Results are representative of findings from at least three similar experiments. (top right) HPAE cells transfected with either scrambled siRNA (SiSc) or Gβ1siRNA (SiGβ1) were immunoblotted with anti-Gβ1 (top) or anti-FAK (bottom) Abs to determine knock down of Gβ1. Mean ± SD densitometric values of Gβ1 expression normalized against FAK expression. Data represent experiments that were repeated three times. * indicates significance (P < 0.05). (bottom) Graph quantifies mean ± SD of interendothelial gaps formed in monolayers transfected with SiSc or SiGβ1 without or with thrombin stimulation from three independent experiments. * indicates significance from unstimulated SiSc-transfected monolayers (P < 0.05); # indicates significance from thrombin-stimulated SiSc transfected monolayers (P < 0.05). (D) Effect of Gβ1 knockdown on endothelial permeability. HPAE cells transduced with SiSc or SiGβ1 were stimulated with 50 nM thrombin and TER across the endothelial monolayer was recorded. Data represent mean ± SD from four experiments performed in duplicates (n = 4). * indicates significance from SiSc-transfected endothelial monolayers (P < 0.05).
The γ subunit must dimerize with the β subunit in order for Gβγ to function normally (Jones et al., 2004). Approximately 12 γ isoforms and 5 β isoforms are reported in various cell types (Hurowitz et al., 2000; Robishaw and Berlot, 2004). Our semiquantitative RT-PCR analysis of human pulmonary arterial endothelial (HPAE) cells demonstrated that two β isoforms are expressed in endothelial cells, of which the β1 is the predominant isoform (Fig. 1 C, top left). Therefore, we targeted the β1 isoform to achieve the maximal disruption of the Gβγ dimer. Using siRNA, we depleted Gβ1 (Fig. 1 C, top right) and determined the effect of Gβ1 depletion on the endothelial barrier recovery response. We observed that in Gβ1 knockdown cells, thrombin induced an irreversible disruption of AJ (Fig. 1 C) resulting in a persistent increase in the duration of endothelial permeability responses (Fig. 1 D), confirming our findings that Gβγ is required to restore basal endothelial barrier function.
Gβγ mediates FAK activation
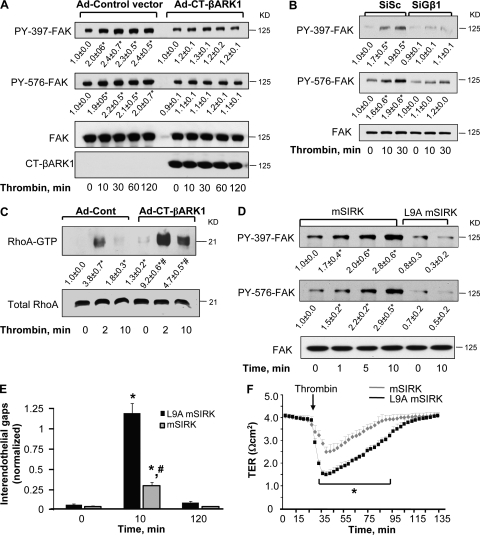
Based on our previous study showing that FAK, upon being phosphorylated at tyrosine residues Y397 and Y576, suppresses RhoA activation and thereby promotes restoration of basal endothelial barrier function (Holinstat et al., 2006), we addressed the possible role of Gβγ in regulating FAK activation. Endothelial cells transfected with CT-βARK-1 or Gβ1 siRNA were stimulated with thrombin for the indicated times and FAK activation was determined by using anti-Y397 or anti-Y576 phospho-FAK antibodies. We found that Gβ1γ was necessary for FAK activation because thrombin failed to increase FAK phosphorylation at 397 and 576 Tyr residues in endothelial cells expressing CT-βARK-1 (Fig. 2 A) or transfected with Gβ1 siRNA (Fig. 2 B). Inhibition of Gβγ increased basal RhoA activity and potentiated the increase in RhoA activation in response to thrombin (Fig. 2 C), in agreement with our previous study (Holinstat et al. 2006).
Figure 2.
Gβγ is required to induce FAK activity. (A and B) Effect of Gβγ inhibition on FAK activity. HPAE cells infected with Ad-CT-βARK1 (A) or transfected with SiGβ1 (B) were stimulated with 50 nM thrombin for indicated times. Lysates were immunoblotted with Y397 or Y576 residue specific anti–phospho-Abs or pan anti–FAK Abs to determine phosphorylation of FAK. Immunoblot with anti-GRK2 Ab was performed to confirm the expression of CT-βARK1. Data representative of at least three experiments. * indicates significant increase in phosphorylation (P < 0.05). (C) Effect of Gβγ inhibition on RhoA-GTPase activity. HPAE cells infected with indicated adenovirus were stimulated with 50 nM thrombin for 0, 2, or 10 min to determine RhoA activity. RhoA activity was measured by the increased amount of RhoA bound to GST-bound rhotekin fusion proteins, GTP-bound RhoA (top), compared with the total amount of RhoA in whole cell lysate (bottom). Data represent mean ± SD from three individual experiments. * indicates significance from Ad-control transducing unstimulated monolayer (P < 0.05); # indicates significance from thrombin stimulated Ad-control monolayers (P < 0.05). (D) Gβγ activation with mSIRK induces FAK phosphorylation. HPAE cells were stimulated with 10 µM mSIRK for indicated times. The peptide L9A-mSIRK (where substitution of residue Leu 9 for Ala decreases the binding affinity of this peptide to Gβγ by >100-fold) was used as a control. Cell lysates were immunoblotted with anti-phospho-Y397 or -Y576 residue–specific antibodies or pan anti-FAK Abs to determine FAK phosphorylation. Data represent mean ± SD from three individual experiments. * indicates significant increase in phosphorylation above time zero (P < 0.05). (E) Effect of Gβγ activation on adherens junction assembly. HPAE cells were preincubated with 10 µM mSIRK or mSIRK-L9A (control peptide) 15 min before stimulation with 50 nM thrombin, and after indicated times cells were fixed, permeabilized, and stained with anti–VE cadherin Ab. Plot shows mean ± SD of interendothelial gap formation after thrombin stimulation. Results were quantified from three independent experiments. * indicates significant increase in gap area in monolayers exposed to control peptide (P < 0.05). (F) Effect of Gβγ activation on endothelial permeability. HPAE cells were preincubated with 10 µM mSIRK or mSIRK-L9A 15 min before stimulation with 50 nM thrombin and TER across the endothelial monolayer was recorded. Data represent mean ± SD from four experiments performed in duplicate. * indicates significance decrease in TER in mSIRK-pretreated endothelial monolayers (P < 0.05).
We next used the cell-permeant Gβγ-activating peptide mSIRK (Goubaeva et al. 2003) to further substantiate the role of Gβγ in regulating FAK activation. Stimulation of HPAE cells with mSIRK induced FAK phosphorylation at both sites without thrombin stimulation; control peptide, i.e., L9A mSIRK, was ineffective (Fig. 2 D). mSIRK also counteracted the disruptive effect of thrombin on AJ (Fig. 2 E), and thereby markedly suppressed thrombin-induced increase in endothelial permeability (Fig. 2 F). These findings support the conclusion that up-regulation of FAK activity by Gβγ induces barrier repair by facilitating reannealing of AJ, which thus prevents persistent increase in endothelial permeability.
Thrombin induces the formation of Gβ1γ2–Fyn–FAK complex
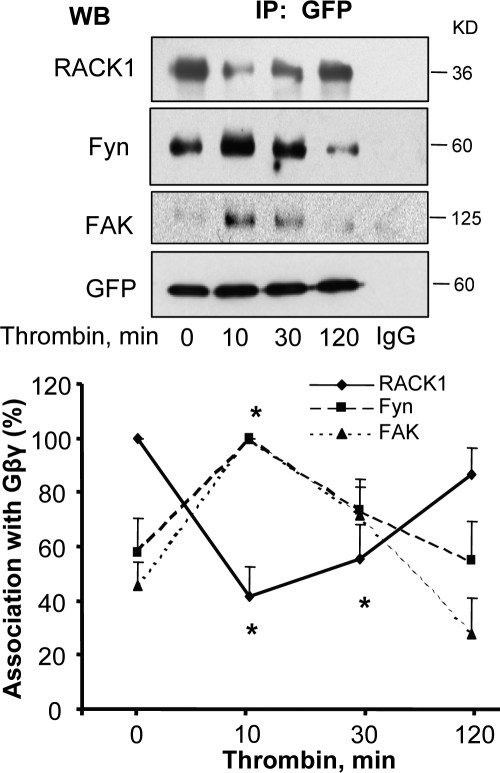
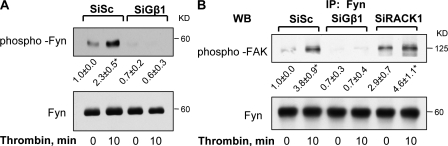
Gβ1 belongs to a large family of WD 40 proteins and shares 57% amino acid homology with its binding partner RACK1 (Chen et al., 2004b). RACK1 also binds Fyn, a Src family nonreceptor tyrosine kinase that phosphorylates FAK (Yaka et al., 2002). To address the mechanism of Gβ1 up-regulation of FAK activity, we determined whether thrombin induces formation of a signaling complex consisting of Gβ1, RACK1, and Fyn. To do this, we cotransduced endothelial cells with GFP-tagged Gβ1 and GFP-tagged Gγ2 and used coimmunoprecipitation assay to detect complex formation. We noted that in unstimulated endothelial cells, Gβ1γ2-associated with RACK1 and Fyn (Fig. 3). However, under these conditions, an association between Gβ1γ2 and FAK was not found. Thrombin potentiated the interaction of Gβ1γ2 with Fyn and FAK within 10–30 min, but induced a transient dissociation of RACK1 from Gβ1γ2 (Fig. 3) that correlated with FAK activation, initiation of AJ reannealing, and recovery of endothelial barrier function, as shown in Figs. 1 and 2.
Figure 3.
RACK1 dissociation from Gβ1γ2 leads to ternary complex formation between Gβ1γ2, Fyn, and FAK. HPAE cells cotransduced with GFP-tagged Gβ1 and GFP-tagged γ2 were stimulated with 50 nM thrombin for indicated times and lysates were immunoprecipitated with anti-GFP Ab. Immunocomplexes were immunoblotted with anti-RACK1, anti-Fyn, and anti-FAK Abs to determine complex formation. Immunoblot with anti-GFP Ab was performed to confirm GFP expression to indicate equal protein loading. (top) A representative immunoblot. (bottom) The plot of densitometry analysis of interaction. Data represent mean ± SD from four experiments. * indicates significance (P < 0.05).
RACK1 negatively regulates Gβ1–Fyn interaction, FAK activation, AJ resealing, and restoration of endothelial barrier function
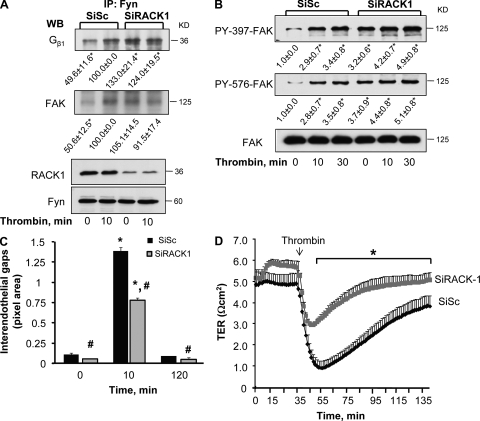
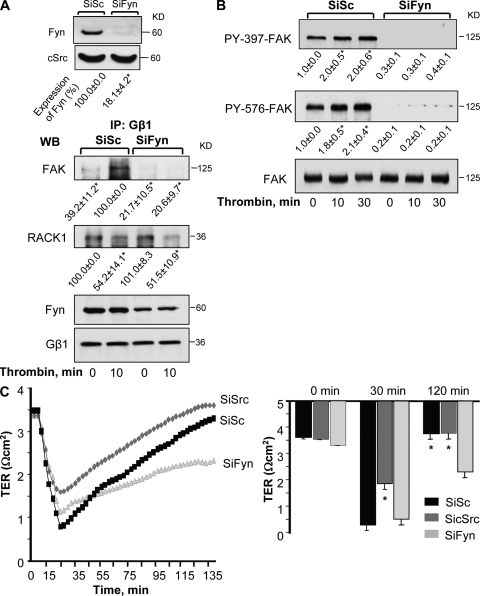
Next, we suppressed endogenous expression of RACK1 to assess the causal role of RACK1 in regulating Gβ1–Fyn interaction, FAK activation, and endothelial barrier permeability. We observed that knockdown of RACK1-potentiated basal interaction of Gβ1 with Fyn and FAK, which did not increase further after thrombin stimulation (Fig. 4 A). Suppression of endogenous RACK1 basally activated FAK (Fig. 4 B), and significantly accelerated reannealing of AJ within 10 min (Fig. 4 C), thereby speeding recovery of endothelial barrier function (Fig. 4 D). We noted a marked suppression of thrombin-induced disruption of endothelial barrier function in RACK1-deficient endothelial cells (Fig. 4 D).
Figure 4.
Effect of knockdown of RACK1 on Gβ1 function. (A) Knockdown of RACK1 increases Gβ1 interaction with Fyn and FAK. HPAE cells transduced with either SiSc or SiRACK1 were stimulated with 50 nM thrombin for indicated times. Lysates were immunoprecipitated with anti-Fyn Ab and immunoblotted with anti-Gβ1 or anti-FAK Abs to assess interaction. Immunoblot with anti-RACK1 Ab was performed to determine RACK1 knockdown while immunoblot with anti-Fyn Abs was performed to assess equal protein loading. Data represent results from three individual experiments. (B) Knock down of RACK1 increases basal FAK phosphorylation. Lysates from cells transduced by SiSc or SiRACK1 were immunoblotted with anti–phospho-Y397, anti–phospho-Y576, or anti-FAK Abs to determine changes in FAK phosphorylation. Data representative of three independent experiments. * indicates significant difference in densitometric values of phospho FAK over unstimulated SiSc-transfected cells at time zero (P < 0.05). (C) Effect of RACK1 knockdown on adherens junction reassembly. After 48 h, HPAE monolayers transfected with SiSc or SiRACK1 were stimulated with 50 nM thrombin for indicated times and stained with anti-VE cadherin and anti-RACK1 Abs, followed by incubation with appropriate Alexa Fluor–labeled secondary Ab. Plot quantifies mean ± SD of interendothelial gaps formed in monolayers from three experiments. * indicates significance from unstimulated monolayer (P < 0.05); # indicates significance from SiSc-transfected cells (P < 0.05). (D) Knockdown of RACK1 promotes endothelial barrier restoration after thrombin-induced increase in endothelial permeability. HPAE cells transduced with SiSc or SiRACK1 were stimulated with 50 nM of thrombin and changes in TER were recorded to assess the role of RACK1 knockdown on endothelial permeability. Data represent mean ± SD from at least three experiments performed in duplicates. * indicates significance (P < 0.05).
Gβ1 interaction with Fyn is required for FAK phosphorylation
We addressed the possibility that a physical interaction between Gβ1 and Fyn contributes to Fyn activation of FAK. We first determined whether thrombin phosphorylates Fyn in a Gβ1-dependent manner. HPAE cells transfected with Gβ1 siRNA or scrambled siRNA were stimulated with thrombin and lysed. Lysates were immunoprecipitated with anti-Fyn Ab, and tyrosine phosphorylation was assessed using phosphotyrosine Abs. Under control conditions (scrambled siRNA-transfected cells), we observed that thrombin challenge increased tyrosine phosphorylation of Fyn above basal (Fig. 5 A). However, basal Fyn phosphorylation was reduced to undetectable levels in Gβ1-depleted cells and did not increase after thrombin stimulation (Fig. 5 A).
Figure 5.
Gβ1–Fyn interaction is required for FAK activation. (A) Gβ1 is required for thrombin-induced Fyn phosphorylation. Lysates from HPAE cells transfected with either SiSc or SiGβ1 were immunoprecipitated with anti-Fyn Ab after which they were immunoblotted with anti-phosphotyrosine Abs to determine Fyn phosphorylation. Lower immunoblot with anti-Fyn Ab was perfomed to verify equal protein loading. Depicted blots are typical of three similar experiments. (B) Gβ1 is required for inducing Fyn kinase activity on FAK. HPAE cells were transduced with SiSc, SiGβ1, or SiRACK1, stimulated with 50 nM thrombin for 10 min, and immunoprecipitated with anti-Fyn Ab, followed by incubation with protein AG beads. Protein complexes on beads were then incubated with purified recombinant FAK in a buffer containing ATP for determination of Fyn kinase activity on FAK in vitro. After 30 min, the reaction was terminated by the addition of SDS sample buffer and FAK phosphorylation was determined using anti-phosphotyrosine Abs. Immunoblot with anti-Fyn Ab was performed to confirm equal protein loading. Data represent results from experiments that were repeated at least two times. Results indicate that complex formation between Gβ1 and Fyn is required for Fyn kinase activity on FAK.
To determine the effect of Gβ1 knockdown on the activity of Fyn kinase (which phosphorylates FAK), we performed an in vitro kinase assay using recombinant FAK as a substrate. Fyn immunocomplexes obtained from cells transfected with Gβ1 siRNA were incubated with recombinant FAK and tyrosine phosphorylation of recombinant FAK was determined using phosphotyrosine Abs. In parallel, Fyn kinase activity was determined in cells transfected with RACK1 siRNA. We observed that Fyn immunoprecipitated from endothelial cells transfected with control siRNA or RACK1 siRNA phosphorylated recombinant FAK (Fig. 5 B). However, Fyn immunoprecipitated from Gβ1-siRNA–transfected cells failed to phosphorylate FAK, demonstrating that Gβ1 is required to induce Fyn activation (Fig. 5 B).
Fyn is required for Gβ1-mediated FAK activation and endothelial barrier restoration
We suppressed endogenous expression of Fyn in endothelial cells to assess the specific role of Fyn in mediating Gβ1-induced FAK activation and endothelial barrier repair. Western blot analysis showed that suppression of Fyn expression did not alter the expression of Src family kinase p60cSrc (Fig. 6 A, inset). Depletion of Fyn did not alter the basal or thrombin-induced association of Gβ1 with RACK1. However, Gβ1 failed to interact with FAK in Fyn-depleted cells (Fig. 6 A), and under these conditions FAK phosphorylation on 397 or 576 tyrosine residues was not found (Fig. 6 B). Fyn knockdown did not alter transfer of Evans blue–conjugated albumin across the endothelial monolayer (0.11 ± 0.01 vs. 0.10 ± 0.01 µl/min) or basal TER (Fig. 6 C). However, suppression of endogenous Fyn expression resulted in irreversible increase in endothelial permeability after thrombin challenge (Fig. 6 C). In contrast, knockdown of p60cSrc significantly inhibited thrombin-induced increase in endothelial permeability (Fig. 6 C). These findings demonstrate that downstream of Gβ1, Fyn plays a key role in mediating FAK activation and endothelial barrier repair. Our data also suggest that Fyn and p60cSrc play contrasting roles in PAR1 regulation of endothelial permeability.
Figure 6.
Requirement for Fyn downstream of Gβ1 in inducing FAK activation and restoration of endothelial barrier function. (A) Effect of Fyn knockdown on Gβ1–FAK interaction. HPAE cells were transfected with either control (Sc) or Fyn siRNA, stimulated with 50 nM thrombin for indicated times, and lysed. Immunoprecipitation was performed with anti-Gβ1 Ab, followed by immunoblotting with anti-FAK and anti-RACK1 Abs to determine interaction. (top) Immunoblot with anti-Fyn and anti-cSrc Abs that confirms the specific knockdown of Fyn in cells transfected with SiFyn. Data representative of three experiments (n = 3). (B) Effect of Fyn knockdown on FAK phosphorylation. Lysates from HPAE cells transfected with either SiSc or SiFyn were immunoblotted with anti–phospho-Y397, anti–phospho-Y576, or anti-FAK Abs to determine FAK phosphorylation. Data represent mean ± SD from four experiments. * indicates significance (P < 0.05). (C) Effect of Fyn and cSrc knockdown on endothelial permeability. HPAE cells were stimulated with 50 nM thrombin and changes in TER were measured over time. Representative traces (left) showing changes in TER in endothelial cell monolayer transfected with control (SiSc), cSrc (SiSrc), or Fyn (SiFyn) siRNA. Graph (right) gives mean ± SD of changes in TER from three independent experiments that were performed in duplicates. * indicates significance (P < 0.05).
Genetic deletion of Fyn leads to increased lung microvascular permeability
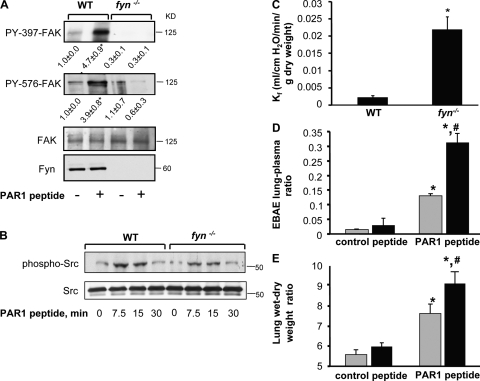
In another series of experiments, we investigated the role of Fyn in regulating endothelial barrier integrity of pulmonary microvessels using Fyn-deficient mice. First we determined whether activation of PAR1 by the specific activating peptide TFLLRN alters FAK activity in lungs. WT or fyn−/− mice received i.v. injection of either control peptide or PAR1-activating peptide (1 mg/kg). Lungs were homogenized to assess FAK phosphorylation. We observed that PAR1 agonist peptide increased FAK phosphorylation at tyrosine 397 and 576 residues in WT lungs (Fig. 7 A). However, in lungs of fyn−/− mice, FAK phosphorylation at tyrosine 397 and 576 was barely detectable under basal conditions and did not increase after PAR1 activation (Fig. 7 A), which is consistent with our findings in Fyn-depleted endothelial cells. Deletion of Fyn had no effect on either cSrc expression or cSrc phosphorylation induced by PAR1 (Fig. 7 B), implicating Fyn as the key enzyme regulating FAK activation.
Figure 7.
Fyn is required for limiting the increase in lung microvascular permeability. (A and B) Fyn deletion prevents PAR1 activation of FAK phosphorylation. Lungs from C57BL/6 (WT) and fyn −/− mice receiving control or PAR1 peptide (1 mg/kg) for indicated times were homogenized, and proteins immunoblotted with anti–phospho-Y397-FAK, anti–phospho-Y576-FAK, and anti-FAK Abs (A) or and anti–phospho-Y419Src and anti-cSrc Ab (B) to assess FAK (A) and Src (B) phosphorylation. Immunoblot with anti-Fyn and anti-cSrc show that deletion of Fyn (A) did not alter the expression of cSrc (B). Data representative of three independent experiments. (C) Changes in lung microvascular permeability in fyn−/− mice. In isogravimetric lungs intravascular pressure was raised by 10 cm H2O for 20 min and microvessel filtration coefficient (Kf) was calculated from the slope of the weight gain function normalized by lung dry weight, as described in Materials and methods. Plot gives mean ± SD of four experiments. In each experiment fyn −/− lung was paired with WT lung. * indicates significance (P < 0.05). (D–E) PAR1 agonist peptide augments the increase in lung microvascular permeability in fyn−/− mice. PAR-1 peptide (1 mg/kg) or control peptide was injected together with Evans blue albumin retroorbitally into mice (WT, grey column; fyn−/−, black bar). After 30 min, Evans blue albumin extravasation (EBAE) from lungs or plasma (D) and lung wet/dry weight ratio (E) were determined as described in Materials and methods to quantify lung microvascular protein permeability and lung edema formation, respectively. Data represent mean ± SD of four individual experiments where fyn −/− lungs were paired with WT lungs. * indicates significance from appropriate control peptide group (P < 0.05); # indicates significance from WT mice after PAR1 challenge (P < 0.05).
Next, we determined the microvessel filtration coefficient (Kf) in lungs isolated from WT and fyn−/− mice. We showed that basal Kf was significantly higher in fyn−/− lungs than WT lungs (Fig. 7 C). To confirm that deletion of Fyn in mouse lungs leads to pulmonary edema, we determined Evans blue albumin extravasation (EBAE) and lung wet-to-dry weight ratio after PAR1 activation. Lung vascular albumin permeability was the same in WT and fyn−/− mice receiving a scrambled PAR1 peptide (Fig. 7, D and E). However, injection of PAR1 peptide (i.v.) produced a significantly greater increase in EBAE (Fig. 7 D) and edema formation (Fig. 7 E) in the Fyn knockout, indicating that Fyn mitigates PAR1-agonist–induced pulmonary edema.
Fyn is required for FAK-mediated recovery of AJ
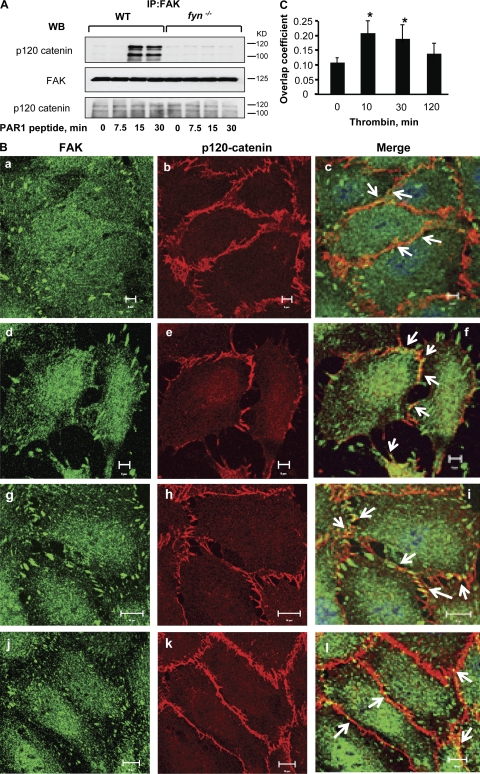
To assess whether Fyn-dependent activation of FAK induces the association of FAK with endothelial junctions, we obtained lysates from WT and fyn−/− mice stimulated with PAR1-activating peptide, immunoprecipitated FAK with anti-FAK Ab, and immunoblotted with anti–p120-catenin Ab. PAR1 activation markedly increased the association between FAK and p120-catenin (a marker of endothelial junctions) between 15 and 30 min in WT but not fyn−/− lungs (Fig. 8 A). Fyn deletion had no effect on p120-catenin expression (Fig. 8 A). The results indicate that Fyn-dependent phosphorylation of FAK induces association between FAK and AJ.
Figure 8.
Fyn facilitates the association of FAK with AJ. (A) FAK associates with p120 catenin. Lungs from WT or fyn−/− mice receiving control or PAR1 peptide for indicated times, were homogenized and lysates were immunoprecipitated with anti-FAK Abs, followed by immunoblotting with p120-catenin Ab. Data are representative of three independent experiments. (B and C) FAK colocalizes with p120-catenin. HPAE cells grown to confluence on coverslips were left unstimulated (a–c) or stimulated with 50 nM thrombin for 10 min (d–f), 30 min (g–i), or 120 min (j–l) and costained with anti–p120-catenin (red) and anti-FAK (green) antibodies to determine colocalization. Results are representative of at least three separate experiments. (C) Mean ± SD of four experiments. * indicate significant increase in colocalization between phosphoFAK and p120-catenin (P < 0.05).
To demonstrate that FAK and AJ associate in endothelial cells, we coimmunostained HPAE cells with anti-FAK and anti–p120-catenin antibodies. As expected, FAK localized at focal adhesions under basal conditions and to a greater extent after thrombin challenge (Fig. 8 B). FAK also colocalized with p120-catenin under basal conditions, and the association was significantly increased by thrombin (Fig. 8 B). No interaction was found between FAK and isotype-matched control IgG Ab (unpublished data).
FAK-397/-576 phosphorylation mimicking mutant rescues lung vascular permeability in fyn−/− mice
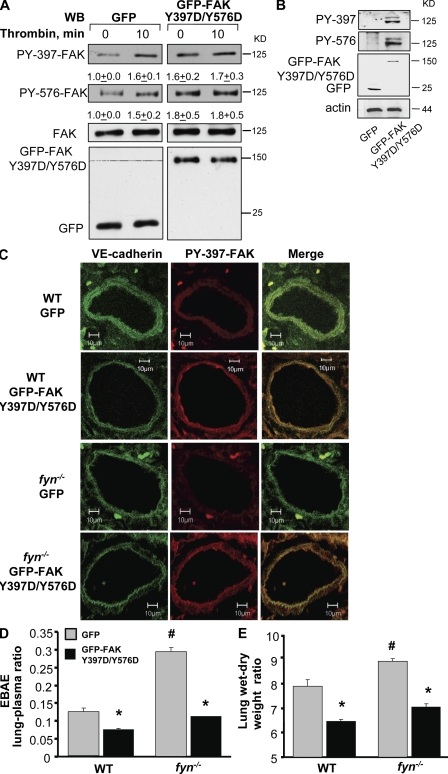
Because Fyn deletion prevented FAK phosphorylation at tyrosine residues 397 and 576 (Fig. 7 A), we generated a phosphorylation-mimicking mutant of FAK to test whether restoring FAK phosphorylation in the fyn−/− mouse lung could restore lung vascular permeability responses to PAR1 agonist to the level seen in WT mice. We replaced Tyr 397 and Tyr 576 with Asp and expressed the mutant construct in cultured endothelial cells. First, we confirmed that expression of FAK mutant Y397D/Y576D increased FAK phosphorylation. As shown in Fig. 9 A, transduction of the mutant construct increased basal FAK phosphorylation at Y397 and Y576 to levels seen in vector control cells after thrombin stimulation. Thrombin could not increase FAK phosphorylation further in cells transducing FAK phosphorylation mimicking mutant (Fig. 9 A).
Figure 9.
Transduction of phosphorylation mimicking FAK mutant rescues lung microvascular permeability in fyn−/− mice. (A) HPAE cells transducing control vector (GFP) or GFP-tagged FAK Y397D/Y576D mutants were left unstimulated or stimulated with thrombin for 10 min. Cell lysates were immunoblotted with Y397 or Y756 residue specific phospho-FAK antibodies or pan anti-FAK Abs to determine FAK phosphorylation. Mean ± SD represent values from three separate experiments. (B–E) fyn−/− and/or WT mice were injected with liposomes conjugated with either GFP-tagged phosphorylation mimicking mutant (FAK-Y397D/Y576D) or GFP via retroorbital injection. (B) Immunoblot showing expression of phosphomimicking FAK mutant in fyn−/− mice. Lysates from lungs transduced with GFP or GFP-tagged FAK-Y397D/Y576D mutant were immunoblotted with anti-phospho-Y397 anti-FAK Abs. Immunoblot represent data from at least two independent experiments. (C) Confocal images of lungs showing expression of GFP or GFP-tagged FAK-Y397D/Y576D mutant in pulmonary vessels. Lungs transduced with GFP or GFP-tagged FAK-Y397D/Y576D mutant were sectioned and coimmunostained with anti–Y397-phospho-FAK or VE-cadherin to determine whether mutants are expressed in endothelium. Images represent results from experiments that were performed independently at least twice. (D and E) Effect of transduction of FAK-Y397D/Y576D mutant on PAR1-induced lung vascular permeability. WT or fyn−/− mice expressing indicated mutants received PAR1 peptide i.v. along with Evans blue albumin to determine protein permeability. Lung wet/dry weight ratio was also determined as described in Materials and methods. Data represent mean ± SD of four experiments done paired wise. * indicates significance from control group (P < 0.05). All experiments were performed 48 h after injection of liposome encapsulated mutants.
Next we transduced the same mutant construct into WT and fyn−/− murine lung microvessels using cationic liposomes encapsulating GFP-tagged FAK-Y397D/-Y576D cDNA. We observed increased FAK phosphorylation in lungs of fyn−/− (Fig. 9, B and C) and WT mice (Fig. 9 C). Immunostaining of lung sections from WT or Fyn-null mice expressing the FAK-Y397D/-Y576D mutant with anti–VE cadherin and phosphospecific FAK antibodies showed that activated FAK was localized in pulmonary vascular endothelial cells (Fig. 9 C). We next determined transvascular albumin permeability and lung edema formation in WT or fyn−/− mice transduced with either control vector (GFP) or GFP-tagged FAK-Y397D/-Y576D cDNA. As shown in Fig. 9 (D and E), transduction of FAK-Y397D/Y576D in the lungs significantly decreased albumin leakage and edema formation in response to PAR1 activation in WT lungs. We also observed that restoring FAK phosphorylation reversed PAR-1–induced lung vascular permeability in fyn−/− lungs to the level observed in WT mice lungs (Fig. 9, D and E).
DISCUSSION
Thrombin, which is generated during vascular injury, acutely increases endothelial permeability by activating PAR1 on endothelial cells (Coughlin, 2000; Landis, 2007; McLaughlin et al., 2007; Suzuki et al., 2009). PAR1 produces these effects by stimulating dissociation of the heterotrimeric G protein complexes into functional Gα and Gβγ subunits (Swift et al., 2000; Rahman et al., 2002; McLaughlin et al., 2005). Several lines of evidence favor the conclusion that the dissociated Gαq and Gα12/Gα13 subunit increases endothelial permeability by signaling the activation of myosin light chain kinase and RhoA pathways, which in turn leads to endothelial cell contraction and disruption of cell–cell adhesion (Dudek and Garcia, 2001; Mehta et al., 2001; Holinstat et al., 2003; Singh et al., 2007; Knezevic et al., 2007; Singh et al., 2007; Gavard and Gutkind, 2008; Korhonen et al., 2009). However, such studies have not defined the role of the released Gβγ unit under these conditions. Impairing Gβγ function using CT-βARK1 or Gβ1 siRNA allowed us to address the functional role of Gβγ in the mechanism of PAR1-induced increase in endothelial permeability. These results showed that inhibition of Gβγ did not augment thrombin-induced increase in endothelial permeability because of dysregulation of α subunit activity, and hence exacerbated RhoA signaling (Holinstat et al., 2003; Holinstat et al., 2006; Mehta and Malik, 2006), but prevented recovery of endothelial barrier function. We also showed that direct stimulation of Gβγ by mSIRK, bypassing the α-subunit activation, accelerated the recovery of endothelial barrier function after thrombin challenge. Thus, Gβγ functions to promote recovery of barrier function through orchestration of signaling involving Fyn activation of FAK.
Studies show that five Gβ isoforms exist in various cell types (Schwindinger and Robishaw, 2001; Robishaw and Berlot, 2004; Thompson et al., 2008). However, our data show that Gβ1 is the predominant isoform expressed in human endothelial cells. Because Gβ dimerization with Gγ is required for the dimer to function (Jones et al. 2004), we showed that targeted suppression of endogenous Gβ1 (∼80%) prolonged the increase in permeability caused by thrombin, as contrasted with full recovery normally seen within 2 h in control cells. Together, these findings further support the conclusion that the primary function of Gβγ is to restore endothelial barrier function.
Gβ1 belongs to a large family of WD40 repeat proteins and interacts with several proteins, including RACK1 (Chen et al., 2004b). RACK1 also binds Fyn (Yaka et al., 2002). Fyn induces FAK activity by phosphorylating Y397 and Y576 residues in FAK. We have shown that thrombin induces a sustained phosphorylation of FAK at Y397 and Y576 (Holinstat et al., 2006). We also showed that activation of FAK is required to reverse the increase in endothelial permeability (Holinstat et al., 2006). Thus, we considered a model in which Gβ1 interacts with Fyn, inducing Fyn activation, which in turn up-regulates FAK activity, subsequently restoring endothelial barrier function. We noted that under basal conditions Gβ1 coimmunoprecipitated with RACK1 and Fyn, but not FAK. The results of this study further showed that thrombin induced the interaction between Gβ1, Fyn, and FAK, coinciding with Fyn and FAK activation and restoration of endothelial barrier function. However, the formation of this ternary complex required dissociation of Gβ1 from RACK1 because knockdown of RACK1 enhanced interaction between Gβ1, Fyn, and FAK in the absence of thrombin stimulation and augmented FAK activity. Also, Gβ1, but not RACK1, was required for Fyn activation, which in turn induced FAK activity. We also showed that Gβ1 failed to interact with FAK in Fyn-depleted cells. Our data show that there is a basal interaction between Gβγ, Fyn, and RACK1. We interpret these findings to mean that Gβγ exists in a dynamic equilibrium with RACK1, Fyn, and PAR1 receptor. Thrombin stimulation results in an increased association of Gβγ with Fyn and FAK at the expense of release of Gβγ from RACK1, and presumably from the PAR1 receptor, as GPCR activation leads to dissociation of Gα subunit from Gβγ (Cabrera-Vera et al., 2003; Oldham and Hamm, 2008). Additionally, our data show that upon stimulation the equilibrium is shifted away from the receptor, and definitely from RACK1, favoring Gβγ association with Fyn and FAK. However, our studies cannot delineate whether Gβγ forms a single complex with RACK1 and Fyn/FAK or if these two form separate complexes. Thus, our findings suggest that RACK1 negatively regulates Gβ1 restoration of barrier function by preventing Gβ1–Fyn interaction and restricting Fyn up-regulation of FAK activity. Supporting this conclusion are our findings that depletion of RACK1 enhanced Gβ1 interaction with Fyn, thereby stimulating Fyn activation of FAK activity, with the predictable consequences of counteracting PAR1-induced barrier dysfunction and of accelerating the recovery of barrier function to basal levels. The mechanism by which thrombin facilitates the dissociation of RACK1 from Gβ1 and Fyn is not clear. Evidence indicates that RACK1 acts as a negative regulator of Gβ1 and Fyn (Dell et al., 2002; Thornton et al., 2004). Gβ1 and Fyn share binding sites on RACK1 (Yaka et al., 2002; Thornton et al., 2004), and in some studies RACK1 also heteromerizes with Gβ1 (Dell et al. 2002). PKC and phosphatases such as SHIP were shown to modify RACK1 function by inducing its posttranslational modification (Ron et al., 1994; Nishio et al., 2007). Because thrombin activates PKC and SHIP (Mehta et al., 2001; Tiruppathi et al., 2002; Dyson et al., 2003), a possible scenario is that posttranslational modification of RACK1 decreases the affinity of RACK1 for Fyn and Gβ1, thus enabling them to interact with FAK to signal barrier restoration.
Our data also showed that the permeability increasing effects of thrombin partially overlapped with the process of barrier recovery such that the 10-min time point reflects both processes, whereas the 30-min time points predominantly reflect recovery. It is therefore possible that Gβγ-Fyn signaling may serve to protect against the “acute” phase of thrombin-induced permeability. However, we showed that FAK activity increased at 10 min, reached a maximum at 30 min, and persisted for 2 h. Interfering with Gβγ or Fyn function inhibited FAK phosphorylation as early as 10 min, and therefore at all succeeding time points, and also prevented barrier recovery to basal levels. These results indicate the importance of Gβγ-Fyn signaling in FAK-dependent reannealing of endothelial junctions.
We found that activated PAR1 failed to induce phosphorylation of FAK at tyrosine residues 397 and 576 in Fyn knockdown cells and in fyn−/− lung preparations. However, Fyn deletion did not alter thrombin-induced p60cSrc phosphorylation. These findings implied that Fyn is required for FAK activation in endothelial cells. Studies showed that several members of the Src family of tyrosine kinases, including Fyn, can activate FAK (Xing et al., 1994; Bockholt and Burridge, 1995; Thomas and Brugge, 1997; Schaller, 2001; Brunton et al., 2005; Mitra and Schlaepfer, 2006). In this regard, our experimental observations that expression of p60cSrc, as well as p60Src phosphorylation, was normal in Fyn-depleted endothelial cells, and Fyn-null lungs showed that p60cSrc could not substitute for Fyn in inducing FAK activation.
Because FAK organizes focal adhesions, a likely scenario could be that FAK restores endothelial barrier function by its effect on cell–ECM interaction. Intriguingly, our data show that a pool of FAK colocalized with the AJ marker p120-catenin under basal conditions, and colocalization increased further when the endothelial barrier function was restored. FAK activation or junctional reassembly was not evident when endogenous expression of Fyn was suppressed. Moreover, PAR1 stimulation in lungs from Fyn-deficient mice did not induce FAK activation, or FAK interaction with p120-catenin, and thus induced an exaggerated lung vascular permeability increase. Studies show that p120-catenin is required for maintaining AJ integrity and for repairing the endothelial barrier (Iyer et al., 2004; Mehta and Malik, 2006; Dejana et al., 2008; Rudini and Dejana, 2008). Our findings that interaction of activated FAK with p120-catenins subsequent to Fyn activation of FAK facilitates reannealing of AJ leading to reestablishment of the basal endothelial barrier suggest that the Fyn–FAK pathway plays a critical role in restoring endothelial barrier function. We have demonstrated that tyrosine phosphorylation of p190RhoGAP by FAK is crucial in down-regulating RhoA activity, which in turn facilitates the restoration of barrier function after thrombin challenge (Holinstat et al., 2006). The findings of this study indicate that FAK can also interact with AJ components and reanneal AJ.
Our findings also demonstrate the contrasting roles of p60cSrc and Fyn in regulating endothelial permeability. We showed that knockdown of p60cSrc suppressed the increase in endothelial permeability induced by PAR1. These findings are consistent with the observations in p60cSrc-null mice that were protected from VEGF-induced vascular leak (Eliceiri et al., 1999). However, Fyn knockdown resulted in a persistent increase in endothelial permeability. Because we showed that basal pulmonary microvascular permeability is markedly increased in fyn−/− mouse lungs, it is indicated that activation of FAK regulates basal endothelial barrier function in vivo, as previously described (Holinstat et al., 2006). We also showed that Fyn was required for suppressing the lung vascular permeability increase after PAR1 activation because fyn−/− lungs failed to recover and therefore developed persistent edema. We conclude that the absence of FAK activation in the endothelium of fyn−/− lungs impaired endothelial barrier function. This scenario is likely because the transduction of a phosphomimicking FAK mutant rescued lung vascular function in fyn−/− lungs to near normal values. Thus, these findings demonstrate that Gβγ-Fyn dependent pathway for activation of FAK stimulates reannealing of junctions. Additionally, studies have shown that activation of SPHK1 is required for restoring endothelial barrier function (Li et al., 2008; Tauseef et al., 2008). Whether these two pathways act in concert remains to be investigated, but it is likely that multiple mechanisms are engaged to correct endothelial barrier defects.
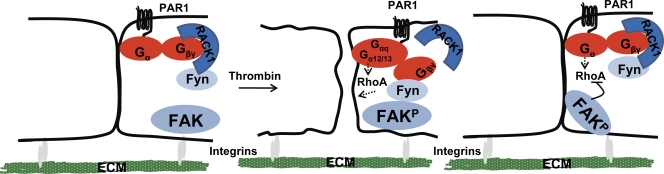
In conclusion, our results indicate that Gβγ plays an essential role in preventing the PAR1-induced long-lived increase in endothelial permeability. We propose a model whereby thrombin-mediated dissociation of RACK1 from Gβγ results in Gβγ and Fyn complex formation that leads to Fyn activation and phosphorylation of FAK (Fig. 10). A pool of activated FAK interacts with p120 catenin, where it induces reannealing of AJ downstream of Fyn. FAK also associates with p190RhoGAP (Holinstat et al., 2006) which suppresses endothelial contraction by down-regulating RhoA activity. Together, these events promote stabilization of cell–ECM attachment and intercellular adhesion, resulting in the restoration of endothelial permeability. Development of targets that specifically modify this pathway could therefore lead to therapeutic strategies to protect against persistent lung vascular barrier leakiness.
Figure 10.
Model of Gβγ -mediated adherens junction assembly. After increases in endothelial permeability via Gαq and Gα12/13 pathways, thrombin dissociates RACK1 from Gβ1, enabling it to interact with Fyn that leads to Fyn activation. Fyn phosphorylates FAK which localizes to intercellular junctions where it induces reannealing of AJ. FAK also associates with p190RhoGAP (not depicted), which suppresses endothelial contraction by down-regulating RhoA activity. Together, these events promote stabilization of cell–ECM attachment and intercellular adhesion resulting in the restoration of endothelial permeability.
MATERIALS AND METHODS
Materials.
HPAE cells and endothelial growth medium (EBM-2) were obtained from Lonza. Human α-thrombin was obtained from Enzyme Research Laboratories. Nucleofector kit and electroporation system were obtained from Lonza. Superfect transfection reagent was purchased from QIAGEN. Alexa Fluor–phallodin, anti–Alexa Fluor–568 and –488 antibodies, DAPI, and ProLong Gold antifade were obtained from Invitrogen. Electrodes for transendothelial resistance measurements were obtained from Applied Biosciences. 12-mm-diam transwell plates and 0.4-µm polyester membranes were purchased from Corning. Primers for Gβ 1–5 subunits, siRNA for Gβ1, Fyn, cSrc, and control scrambled siRNA, as well as transfection reagent for siRNA, were purchased from Santa Cruz Biotechnology, Inc. RACK1 siRNA (5′-CCAUCAAGCUAUGGAAUACTT-3′ sense and 5′-GUAUUCCAUAGCUUGAUGGTT-3′ antisense) were purchased from Sigma-Aldrich. Anti-FAK, anti–phospho FAK PY576, anti-RhoA, anti-RACK1, anti-Gβ1, anti-Fyn, anti-cSrc, anti-VE-cadherin, anti-GRK2, anti-GFP, anti-p120, anti-phosphotyrosine Abs (PY20, PY99, and PY350), and protein A/G agarose beads were purchased from Santa Cruz Biotechnology, Inc. Anti–phospho FAK PY397 was purchased from Invitrogen, anti–β catenin Ab was obtained from Abcam. mSIRK and mSIRK-L9A were purchased from Calbiochem. GST-rhotekin-Rho binding domain beads were purchased from Cytoskeleton. Ad-CT-βARK (C terminus of β-adrenoreceptor kinase) was a gift from R. Minshall (University of Illinois, Chicago, IL, and GFP-tagged Gβ1 and Gγ2 were gifts from J. McLaughlin (University of Illinois, Chicago, IL).
Animals.
All animal studies were approved by the Institutional Animal Care and Use Committee of the University of Illinois. fyn−/− male mice obtained from The Jackson Laboratory were backcrossed to C57BLk/6J background. C57BLk/6J mice were used as WT controls. All experiments were performed on 6–8-wk-old male mice.
Endothelial cell culture.
HPAE cells were cultured as previously described (Knezevic et al., 2007; Singh et al., 2007).
Transfection of cells.
siRNA or cDNA were transduced into cells by electroporation or using transfection reagents, as previously described (Singh et al., 2007). For adenoviral vector-mediated gene transduction, cells grown to confluence were infected with Ad-control vector or Ad-CT-βARK in serum-free medium. 6 h after infection, the medium was replaced with fresh complete medium without virus. In all experiments, cells were used between 32 and 36 h after virus infection. Control vector (Ad-control vector) or Ad-CT-βARK was generated as previously described (Drazner et al., 1997).
RT-PCR.
Total RNA was isolated from HPAE cells with Trizol reagent (Invitrogen) according to the manufacturer's instructions. RNA (1 µg) was used for semiquantitative RT-PCR to assess the expression of Gβ1, Gβ2, Gβ3, Gβ4, and Gβ5 subunits using primers designed for the human mRNA sequences (Santa Cruz Biotechnology, Inc.) with the aid of the Invitrogen SuperScript RT-PCR kit. We used the following conditions: an initial 94°C denaturing for 2 min, followed by 25 cycles at 95°C for 15 s each, annealing at 55°C for 30 s, and extension at 72°C for 30 s. The last cycle was followed by a 7-min reaction at 72°C. RT-PCR products were separated by electrophoresis on a 2% agarose gel.
Coimmunoprecipitation.
HPAE cells grown in 100-mm dishes were lysed and equal amounts of protein were immunoprecipitated with appropriate Abs overnight at 4°C, followed by the addition of protein A/G agarose beads for 4 h at 4°C, as described previously (Holinstat et al., 2006).
In vitro kinase assay.
HPAE cells transduced with appropriate siRNA were stimulated with thrombin for the indicated times. Lysates were and immunoprecipitated with anti-Fyn Ab, followed by incubation with protein A/G agarose beads to form immunocomplexes. Immunocomplexes were used for determining Fyn in vitro kinase activity, using purified FAK as the substrate as previously described (Holinstat et al., 2006).
RhoGTPase activity.
HPAE cells transducing indicated cDNA were stimulated with 50 nM thrombin, and RhoA activity was measured using the GST-rhotekin-Rho binding domain, as previously described (Holinstat et al., 2006).
Immunofluorescence.
Cells transduced with cDNA or siRNA were stimulated with 50 nM thrombin for the indicated times, fixed, and stained with appropriate Abs, as previously described (Knezevic et al., 2007; Singh et al., 2007). Cells were visualized with 63× 1.2 NA objective on a LSM 510 confocal microscope (Carl Zeiss, Inc.). Interendothelial gap area was quantified using a MetaMorph software (Molecular Devices).
Endothelial permeability assessment.
For TER measurements, HPAE cells were seeded on 8-well gold-coated electrodes, where they formed confluent monolayers. TER is expressed as specific electrical resistance (Ω cm2; Mehta et al., 2002). Albumin permeability of HPAE monolayers grown on transwell plates was measured by quantifying the flux of Evans blue–labeled albumin (EBA) from luminal to abluminal chamber (Tauseef et al., 2008).
Liposomal delivery of cDNA in the mouse lung.
Cationic liposomes were made using a mixture of dimethyldioctadecyl-ammonium bromide and cholesterol in chloroform, as described previously (Holinstat et al., 2006). GFP or GFP-FAK-Y397D/Y576D (50 µg) were mixed with 100 µl of liposomes. The mixture of liposomes and cDNA were injected intravenously (via retroorbital injection) into WT or fyn−/− mice. After 48 h, mouse lungs were used for determining lung microvascular permeability or protein expression.
Immunohistochemistry.
Formalin fixed 4-µm-thick lung sections were immunostained with appropriate Abs using manufacture protocol (Bethyl Laboratories). Lung sections were visualized with a LSM510 confocal microscope (Carl Zeiss, Inc.).
Assessment of lung capillary leakage.
EBA (20 mg/kg) was injected retroorbitally 30 min before sacrifice to assess vascular leak, as previously described (Peng et al., 2004; Tauseef et al., 2008). Blood was collected from the right ventricle into heparinized syringes and plasma was separated by centrifugation at 1,300 g for 10 min. Lungs homogenates were prepared as previously described (Tauseef et al., 2008). Lung homogenates and plasma were incubated with 2 volumes of formamide (18 h, 60°C), centrifuged at 5,000 g for 30 min, and the optical density of the supernatant was determined spectrophotometrically at 620 and 740 nm (to correct for hemoglobin). EBA extravasation is given as lung/plasma ratio.
Pulmonary microvascular permeability.
Fyn−/− and WT mice were anesthetized with an i.p. injection of ketamine (100 mg/kg) and xylazine (2.5 g/kg). Microvessel permeability in the lung was measured by determining microvascular filtration coefficient (Kf) and isogravimetric lung water determinations as previously described (Vogel et al. 2000; Holinstat et al. 2006).
Lung weight determination.
Left lungs from the same mice used for Evans blue albumin extravasation were excised and completely dried in the oven at 60°C overnight for calculation of lung wet/dry ratio (Barnard et al. 1995).
Statistical analysis.
Comparisons between experimental groups were made by one-way ANOVA and post-hoc test. Differences in mean values were considered significant at P < 0.05.
Acknowledgments
We acknowledge Drs. Asrar B. Malik and Stephen M. Vogel for their constructive criticism during the preparation of this manuscript; Yulia Komarova for her advice in analysis of interendothelial gap formation; Tiffany Sharma and Debra Salvi for constructing FAKY387D/Y576D mutant; and Ms. Vidisha Kini for technical help. We also thank Dr. Xiaoping Dufor his gift of fyn−/− mice.
This work was supported by National Institutes of Health grants HL71794 and HL84153.
The authors have no conflicting financial interests.
Footnotes
Abbreviations used:
- Ab
- antibody
- AJ
- adherens junctions
- CT-βARK-1
- C terminus of β-adrenergic receptor kinase 1
- EBA
- Evans blue–labeled albumin
- EBAE
- Evans blue albumin extravasation
- ECM
- extracellular matrix
- FAK
- focal adhesion kinase
- HPAE
- human pulmonary arterial endothelial
- PAR1
- protease-activating receptor 1
- RACK1
- receptor for activated C kinase 1
- siRNA
- small interfering RNA
- TER
- transendothelial electrical resistance
References
- Barnard J.W., Biro M.G., Lo S.K., Ohno S., Carozza M.A., Moyle M., Soule H.R., Malik A.B. 1995. Neutrophil inhibitory factor prevents neutrophil-dependent lung injury. J. Immunol. 155:4876–4881 [PubMed] [Google Scholar]
- Blackmer T., Larsen E.C., Takahashi M., Martin T.F., Alford S., Hamm H.E. 2001. G protein betagamma subunit-mediated presynaptic inhibition: regulation of exocytotic fusion downstream of Ca2+ entry. Science. 292:293–297 10.1126/science.1058803 [DOI] [PubMed] [Google Scholar]
- Blackmer T., Larsen E.C., Bartleson C., Kowalchyk J.A., Yoon E.J., Preininger A.M., Alford S., Hamm H.E., Martin T.F. 2005. G protein betagamma directly regulates SNARE protein fusion machinery for secretory granule exocytosis. Nat. Neurosci. 8:421–425 [DOI] [PubMed] [Google Scholar]
- Bockholt S.M., Burridge K. 1995. An examination of focal adhesion formation and tyrosine phosphorylation in fibroblasts isolated from src-, fyn-, and yes- mice. Cell Adhes. Commun. 3:91–100 10.3109/15419069509081279 [DOI] [PubMed] [Google Scholar]
- Brunton V.G., Avizienyte E., Fincham V.J., Serrels B., Metcalf C.A., III, Sawyer T.K., Frame M.C. 2005. Identification of Src-specific phosphorylation site on focal adhesion kinase: dissection of the role of Src SH2 and catalytic functions and their consequences for tumor cell behavior. Cancer Res. 65:1335–1342 10.1158/0008-5472.CAN-04-1949 [DOI] [PubMed] [Google Scholar]
- Cabrera-Vera T.M., Vanhauwe J., Thomas T.O., Medkova M., Preininger A., Mazzoni M.R., Hamm H.E. 2003. Insights into G protein structure, function, and regulation. Endocr. Rev. 24:765–781 10.1210/er.2000-0026 [DOI] [PubMed] [Google Scholar]
- Chen S., Dell E.J., Lin F., Sai J., Hamm H.E. 2004a. RACK1 regulates specific functions of Gbetagamma. J. Biol. Chem. 279:17861–17868 10.1074/jbc.M313727200 [DOI] [PubMed] [Google Scholar]
- Chen S., Spiegelberg B.D., Lin F., Dell E.J., Hamm H.E. 2004b. Interaction of Gbetagamma with RACK1 and other WD40 repeat proteins. J. Mol. Cell. Cardiol. 37:399–406 10.1016/j.yjmcc.2004.04.019 [DOI] [PubMed] [Google Scholar]
- Clapham D.E., Neer E.J. 1997. G protein beta gamma subunits. Annu. Rev. Pharmacol. Toxicol. 37:167–203 10.1146/annurev.pharmtox.37.1.167 [DOI] [PubMed] [Google Scholar]
- Coughlin S.R. 1999. How the protease thrombin talks to cells. Proc. Natl. Acad. Sci. USA. 96:11023–11027 10.1073/pnas.96.20.11023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlin S.R. 2000. Thrombin signalling and protease-activated receptors. Nature. 407:258–264 10.1038/35025229 [DOI] [PubMed] [Google Scholar]
- Coughlin S.R. 2005. Protease-activated receptors in hemostasis, thrombosis and vascular biology. J. Thromb. Haemost. 3:1800–1814 10.1111/j.1538-7836.2005.01377.x [DOI] [PubMed] [Google Scholar]
- Dejana E., Orsenigo F., Lampugnani M.G. 2008. The role of adherens junctions and VE-cadherin in the control of vascular permeability. J. Cell Sci. 121:2115–2122 10.1242/jcs.017897 [DOI] [PubMed] [Google Scholar]
- Dell E.J., Connor J., Chen S., Stebbins E.G., Skiba N.P., Mochly-Rosen D., Hamm H.E. 2002. The betagamma subunit of heterotrimeric G proteins interacts with RACK1 and two other WD repeat proteins. J. Biol. Chem. 277:49888–49895 10.1074/jbc.M202755200 [DOI] [PubMed] [Google Scholar]
- Drazner M.H., Peppel K.C., Dyer S., Grant A.O., Koch W.J., Lefkowitz R.J. 1997. Potentiation of beta-adrenergic signaling by adenoviral-mediated gene transfer in adult rabbit ventricular myocytes. J. Clin. Invest. 99:288–296 10.1172/JCI119157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudek S.M., Garcia J.G. 2001. Cytoskeletal regulation of pulmonary vascular permeability. J. Appl. Physiol. 91:1487–1500 [DOI] [PubMed] [Google Scholar]
- Dyson J.M., Munday A.D., Kong A.M., Huysmans R.D., Matzaris M., Layton M.J., Nandurkar H.H., Berndt M.C., Mitchell C.A. 2003. SHIP-2 forms a tetrameric complex with filamin, actin, and GPIb-IX-V: localization of SHIP-2 to the activated platelet actin cytoskeleton. Blood. 102:940–948 10.1182/blood-2002-09-2897 [DOI] [PubMed] [Google Scholar]
- Eliceiri B.P., Paul R., Schwartzberg P.L., Hood J.D., Leng J., Cheresh D.A. 1999. Selective requirement for Src kinases during VEGF-induced angiogenesis and vascular permeability. Mol. Cell. 4:915–924 10.1016/S1097-2765(00)80221-X [DOI] [PubMed] [Google Scholar]
- Gavard J., Gutkind J.S. 2008. Protein kinase C-related kinase and ROCK are required for thrombin-induced endothelial cell permeability downstream from Galpha12/13 and Galpha11/q. J. Biol. Chem. 283:29888–29896 10.1074/jbc.M803880200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeckeler Z.M., Wysolmerski R.B. 1995. Myosin light chain kinase-regulated endothelial cell contraction: the relationship between isometric tension, actin polymerization, and myosin phosphorylation. J. Cell Biol. 130:613–627 10.1083/jcb.130.3.613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goubaeva F., Ghosh M., Malik S., Yang J., Hinkle P.M., Griendling K.K., Neubig R.R., Smrcka A.V. 2003. Stimulation of cellular signaling and G protein subunit dissociation by G protein betagamma subunit-binding peptides. J. Biol. Chem. 278:19634–19641 10.1074/jbc.M300052200 [DOI] [PubMed] [Google Scholar]
- Herlitze S., Garcia D.E., Mackie K., Hille B., Scheuer T., Catterall W.A. 1996. Modulation of Ca2+ channels by G-protein beta gamma subunits. Nature. 380:258–262 10.1038/380258a0 [DOI] [PubMed] [Google Scholar]
- Holinstat M., Mehta D., Kozasa T., Minshall R.D., Malik A.B. 2003. Protein kinase Calpha-induced p115RhoGEF phosphorylation signals endothelial cytoskeletal rearrangement. J. Biol. Chem. 278:28793–28798 10.1074/jbc.M303900200 [DOI] [PubMed] [Google Scholar]
- Holinstat M., Knezevic N., Broman M., Samarel A.M., Malik A.B., Mehta D. 2006. Suppression of RhoA activity by focal adhesion kinase-induced activation of p190RhoGAP: role in regulation of endothelial permeability. J. Biol. Chem. 281:2296–2305 10.1074/jbc.M511248200 [DOI] [PubMed] [Google Scholar]
- Hung D.T., Wong Y.H., Vu T.K., Coughlin S.R. 1992. The cloned platelet thrombin receptor couples to at least two distinct effectors to stimulate phosphoinositide hydrolysis and inhibit adenylyl cyclase. J. Biol. Chem. 267:20831–20834 [PubMed] [Google Scholar]
- Hurowitz E.H., Melnyk J.M., Chen Y.J., Kouros-Mehr H., Simon M.I., Shizuya H. 2000. Genomic characterization of the human heterotrimeric G protein alpha, beta, and gamma subunit genes. DNA Res. 7:111–120 10.1093/dnares/7.2.111 [DOI] [PubMed] [Google Scholar]
- Iyer S., Ferreri D.M., DeCocco N.C., Minnear F.L., Vincent P.A. 2004. VE-cadherin-p120 interaction is required for maintenance of endothelial barrier function. Am. J. Physiol. Lung Cell. Mol. Physiol. 286:L1143–L1153 10.1152/ajplung.00305.2003 [DOI] [PubMed] [Google Scholar]
- Jin T., Zhang N., Long Y., Parent C.A., Devreotes P.N. 2000. Localization of the G protein betagamma complex in living cells during chemotaxis. Science. 287:1034–1036 10.1126/science.287.5455.1034 [DOI] [PubMed] [Google Scholar]
- Jones M.B., Siderovski D.P., Hooks S.B. 2004. The G betagamma dimer as a novel source of selectivity in G-protein signaling: GGL-ing at convention. Mol. Interv. 4:200–214 10.1124/mi.4.4.4 [DOI] [PubMed] [Google Scholar]
- Kaneider N.C., Leger A.J., Agarwal A., Nguyen N., Perides G., Derian C., Covic L., Kuliopulos A. 2007. ‘Role reversal’ for the receptor PAR1 in sepsis-induced vascular damage. Nat. Immunol. 8:1303–1312 10.1038/ni1525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka H., Hamilton J.R., McKemy D.D., Camerer E., Zheng Y.W., Cheng A., Griffin C., Coughlin S.R. 2003. Protease-activated receptors 1 and 4 mediate thrombin signaling in endothelial cells. Blood. 102:3224–3231 10.1182/blood-2003-04-1130 [DOI] [PubMed] [Google Scholar]
- Knezevic N., Roy A., Timblin B., Konstantoulaki M., Sharma T., Malik A.B., Mehta D. 2007. GDI-1 phosphorylation switch at serine 96 induces RhoA activation and increased endothelial permeability. Mol. Cell. Biol. 27:6323–6333 10.1128/MCB.00523-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korhonen H., Fisslthaler B., Moers A., Wirth A., Habermehl D., Wieland T., Schütz G., Wettschureck N., Fleming I., Offermanns S. 2009. Anaphylactic shock depends on endothelial Gq/G11. J. Exp. Med. 206:411–420 10.1084/jem.20082150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouklis P., Konstantoulaki M., Vogel S., Broman M., Malik A.B. 2004. Cdc42 regulates the restoration of endothelial barrier function. Circ. Res. 94:159–166 10.1161/01.RES.0000110418.38500.31 [DOI] [PubMed] [Google Scholar]
- Landis R.C. 2007. Protease activated receptors: clinical relevance to hemostasis and inflammation. Hematol. Oncol. Clin. North Am. 21:103–113 10.1016/j.hoc.2006.11.005 [DOI] [PubMed] [Google Scholar]
- Li X., Stankovic M., Bonder C.S., Hahn C.N., Parsons M., Pitson S.M., Xia P., Proia R.L., Vadas M.A., Gamble J.R. 2008. Basal and angiopoietin-1-mediated endothelial permeability is regulated by sphingosine kinase-1. Blood. 111:3489–3497 10.1182/blood-2007-05-092148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K.D., Matthay M.A. 2008. Advances in critical care for the nephrologist: acute lung injury/ARDS. Clin. J. Am. Soc. Nephrol. 3:578–586 10.2215/CJN.01630407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luttrell L.M., Hawes B.E., van Biesen T., Luttrell D.K., Lansing T.J., Lefkowitz R.J. 1996. Role of c-Src tyrosine kinase in G protein-coupled receptor- and Gbetagamma subunit-mediated activation of mitogen-activated protein kinases. J. Biol. Chem. 271:19443–19450 [DOI] [PubMed] [Google Scholar]
- McGarrigle D., Huang X.Y. 2007. GPCRs signaling directly through Src-family kinases. Sci. STKE. 392:pe35. [DOI] [PubMed] [Google Scholar]
- McLaughlin J.N., Shen L., Holinstat M., Brooks J.D., Dibenedetto E., Hamm H.E. 2005. Functional selectivity of G protein signaling by agonist peptides and thrombin for the protease-activated receptor-1. J. Biol. Chem. 280:25048–25059 10.1074/jbc.M414090200 [DOI] [PubMed] [Google Scholar]
- McLaughlin J.N., Patterson M.M., Malik A.B. 2007. Protease-activated receptor-3 (PAR3) regulates PAR1 signaling by receptor dimerization. Proc. Natl. Acad. Sci. USA. 104:5662–5667 10.1073/pnas.0700763104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta D., Malik A.B. 2006. Signaling mechanisms regulating endothelial permeability. Physiol. Rev. 86:279–367 10.1152/physrev.00012.2005 [DOI] [PubMed] [Google Scholar]
- Mehta D., Rahman A., Malik A.B. 2001. Protein kinase C-alpha signals rho-guanine nucleotide dissociation inhibitor phosphorylation and rho activation and regulates the endothelial cell barrier function. J. Biol. Chem. 276:22614–22620 10.1074/jbc.M101927200 [DOI] [PubMed] [Google Scholar]
- Mehta D., Tiruppathi C., Sandoval R., Minshall R.D., Holinstat M., Malik A.B. 2002. Modulatory role of focal adhesion kinase in regulating human pulmonary arterial endothelial barrier function. J. Physiol. 539:779–789 10.1113/jphysiol.2001.013289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra S.K., Schlaepfer D.D. 2006. Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr. Opin. Cell Biol. 18:516–523 10.1016/j.ceb.2006.08.011 [DOI] [PubMed] [Google Scholar]
- Mourton T., Hellberg C.B., Burden-Gulley S.M., Hinman J., Rhee A., Brady-Kalnay S.M. 2001. The PTPmu protein-tyrosine phosphatase binds and recruits the scaffolding protein RACK1 to cell-cell contacts. J. Biol. Chem. 276:14896–14901 10.1074/jbc.M010823200 [DOI] [PubMed] [Google Scholar]
- Neer E.J., Schmidt C.J., Nambudripad R., Smith T.F. 1994. The ancient regulatory-protein family of WD-repeat proteins. Nature. 371:297–300 10.1038/371297a0 [DOI] [PubMed] [Google Scholar]
- Nelson C.M., Pirone D.M., Tan J.L., Chen C.S. 2004. Vascular endothelial-cadherin regulates cytoskeletal tension, cell spreading, and focal adhesions by stimulating RhoA. Mol. Biol. Cell. 15:2943–2953 10.1091/mbc.E03-10-0745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neptune E.R., Bourne H.R. 1997. Receptors induce chemotaxis by releasing the betagamma subunit of Gi, not by activating Gq or Gs. Proc. Natl. Acad. Sci. USA. 94:14489–14494 10.1073/pnas.94.26.14489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishio M., Watanabe K., Sasaki J., Taya C., Takasuga S., Iizuka R., Balla T., Yamazaki M., Watanabe H., Itoh R., et al. 2007. Control of cell polarity and motility by the PtdIns(3,4,5)P3 phosphatase SHIP1. Nat. Cell Biol. 9:36–44 10.1038/ncb1515 [DOI] [PubMed] [Google Scholar]
- Niu J., Profirovic J., Pan H., Vaiskunaite R., Voyno-Yasenetskaya T. 2003. G Protein betagamma subunits stimulate p114RhoGEF, a guanine nucleotide exchange factor for RhoA and Rac1: regulation of cell shape and reactive oxygen species production. Circ. Res. 93:848–856 10.1161/01.RES.0000097607.14733.0C [DOI] [PubMed] [Google Scholar]
- Oldham W.M., Hamm H.E. 2008. Heterotrimeric G protein activation by G-protein-coupled receptors. Nat. Rev. Mol. Cell Biol. 9:60–71 10.1038/nrm2299 [DOI] [PubMed] [Google Scholar]
- Owens D.W., McLean G.W., Wyke A.W., Paraskeva C., Parkinson E.K., Frame M.C., Brunton V.G. 2000. The catalytic activity of the Src family kinases is required to disrupt cadherin-dependent cell-cell contacts. Mol. Biol. Cell. 11:51–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng X., Hassoun P.M., Sammani S., McVerry B.J., Burne M.J., Rabb H., Pearse D., Tuder R.M., Garcia J.G. 2004. Protective effects of sphingosine 1-phosphate in murine endotoxin-induced inflammatory lung injury. Am. J. Respir. Crit. Care Med. 169:1245–1251 10.1164/rccm.200309-1258OC [DOI] [PubMed] [Google Scholar]
- Piedra J., Miravet S., Castaño J., Pálmer H.G., Heisterkamp N., García de Herreros A., Duñach M. 2003. p120 Catenin-associated Fer and Fyn tyrosine kinases regulate beta-catenin Tyr-142 phosphorylation and beta-catenin-alpha-catenin Interaction. Mol. Cell. Biol. 23:2287–2297 10.1128/MCB.23.7.2287-2297.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman A., True A.L., Anwar K.N., Ye R.D., Voyno-Yasenetskaya T.A., Malik A.B. 2002. Galpha(q) and Gbetagamma regulate PAR-1 signaling of thrombin-induced NF-kappaB activation and ICAM-1 transcription in endothelial cells. Circ. Res. 91:398–405 10.1161/01.RES.0000033520.95242.A2 [DOI] [PubMed] [Google Scholar]
- Robishaw J.D., Berlot C.H. 2004. Translating G protein subunit diversity into functional specificity. Curr. Opin. Cell Biol. 16:206–209 10.1016/j.ceb.2004.02.007 [DOI] [PubMed] [Google Scholar]
- Ron D., Chen C.H., Caldwell J., Jamieson L., Orr E., Mochly-Rosen D. 1994. Cloning of an intracellular receptor for protein kinase C: a homolog of the beta subunit of G proteins. Proc. Natl. Acad. Sci. USA. 91:839–843 10.1073/pnas.91.3.839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roura S., Miravet S., Piedra J., García de Herreros A., Duñach M. 1999. Regulation of E-cadherin/Catenin association by tyrosine phosphorylation. J. Biol. Chem. 274:36734–36740 10.1074/jbc.274.51.36734 [DOI] [PubMed] [Google Scholar]
- Rudini N., Dejana E. 2008. Adherens junctions. Curr. Biol. 18:R1080–R1082 10.1016/j.cub.2008.09.018 [DOI] [PubMed] [Google Scholar]
- Schaller M.D. 2001. Biochemical signals and biological responses elicited by the focal adhesion kinase. Biochim. Biophys. Acta. 1540:1–21 10.1016/S0167-4889(01)00123-9 [DOI] [PubMed] [Google Scholar]
- Schwindinger W.F., Robishaw J.D. 2001. Heterotrimeric G-protein betagamma-dimers in growth and differentiation. Oncogene. 20:1653–1660 10.1038/sj.onc.1204181 [DOI] [PubMed] [Google Scholar]
- Shajahan A.N., Tiruppathi C., Smrcka A.V., Malik A.B., Minshall R.D. 2004. Gbetagamma activation of Src induces caveolae-mediated endocytosis in endothelial cells. J. Biol. Chem. 279:48055–48062 10.1074/jbc.M405837200 [DOI] [PubMed] [Google Scholar]
- Singh I., Knezevic N., Ahmmed G.U., Kini V., Malik A.B., Mehta D. 2007. Galphaq-TRPC6-mediated Ca2+ entry induces RhoA activation and resultant endothelial cell shape change in response to thrombin. J. Biol. Chem. 282:7833–7843 10.1074/jbc.M608288200 [DOI] [PubMed] [Google Scholar]
- Suzuki H., Motley E.D., Eguchi K., Hinoki A., Shirai H., Watts V., Stemmle L.N., Fields T.A., Eguchi S. 2009. Distinct roles of protease-activated receptors in signal transduction regulation of endothelial nitric oxide synthase. Hypertension. 53:182–188 10.1161/HYPERTENSIONAHA.108.125229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swift S., Sheridan P.J., Covic L., Kuliopulos A. 2000. PAR1 thrombin receptor-G protein interactions. Separation of binding and coupling determinants in the galpha subunit. J. Biol. Chem. 275:2627–2635 10.1074/jbc.275.4.2627 [DOI] [PubMed] [Google Scholar]
- Tang W.J., Gilman A.G. 1992. Adenylyl cyclases. Cell. 70:869–872 10.1016/0092-8674(92)90236-6 [DOI] [PubMed] [Google Scholar]
- Taurin S., Hogarth K., Sandbo N., Yau D.M., Dulin N.O. 2007. Gbetagamma-mediated prostacyclin production and cAMP-dependent protein kinase activation by endothelin-1 promotes vascular smooth muscle cell hypertrophy through inhibition of glycogen synthase kinase-3. J. Biol. Chem. 282:19518–19525 10.1074/jbc.M702655200 [DOI] [PubMed] [Google Scholar]
- Tauseef M., Kini V., Knezevic N., Brannan M., Ramchandaran R., Fyrst H., Saba J., Vogel S.M., Malik A.B., Mehta D. 2008. Activation of sphingosine kinase-1 reverses the increase in lung vascular permeability through sphingosine-1-phosphate receptor signaling in endothelial cells. Circ. Res. 103:1164–1172 10.1161/01.RES.0000338501.84810.51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas S.M., Brugge J.S. 1997. Cellular functions regulated by Src family kinases. Annu. Rev. Cell Dev. Biol. 13:513–609 10.1146/annurev.cellbio.13.1.513 [DOI] [PubMed] [Google Scholar]
- Thompson M.D., Cole D.E., Jose P.A. 2008. Pharmacogenomics of G protein-coupled receptor signaling: insights from health and disease. Methods Mol. Biol. 448:77–107 10.1007/978-1-59745-205-2_6 [DOI] [PubMed] [Google Scholar]
- Thornton C., Tang K.C., Phamluong K., Luong K., Vagts A., Nikanjam D., Yaka R., Ron D. 2004. Spatial and temporal regulation of RACK1 function and N-methyl-D-aspartate receptor activity through WD40 motif-mediated dimerization. J. Biol. Chem. 279:31357–31364 10.1074/jbc.M402316200 [DOI] [PubMed] [Google Scholar]
- Tiruppathi C., Minshall R.D., Paria B.C., Vogel S.M., Malik A.B. 2002. Role of Ca2+ signaling in the regulation of endothelial permeability. Vascul. Pharmacol. 39:173–185 10.1016/S1537-1891(03)00007-7 [DOI] [PubMed] [Google Scholar]
- van Buul J.D., Anthony E.C., Fernandez-Borja M., Burridge K., Hordijk P.L. 2005. Proline-rich tyrosine kinase 2 (Pyk2) mediates vascular endothelial-cadherin-based cell-cell adhesion by regulating beta-catenin tyrosine phosphorylation. J. Biol. Chem. 280:21129–21136 10.1074/jbc.M500898200 [DOI] [PubMed] [Google Scholar]
- Vogel S.M., Gao X., Mehta D., Ye R.D., John T.A., Andrade-Gordon P., Tiruppathi C., Malik A.B. 2000. Abrogation of thrombin-induced increase in pulmonary microvascular permeability in PAR-1 knockout mice. Physiol. Genomics. 4:137–145 [DOI] [PubMed] [Google Scholar]
- Wu M.H. 2005. Endothelial focal adhesions and barrier function. J. Physiol. 569:359–366 10.1113/jphysiol.2005.096537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Z., Chen H.C., Nowlen J.K., Taylor S.J., Shalloway D., Guan J.L. 1994. Direct interaction of v-Src with the focal adhesion kinase mediated by the Src SH2 domain. Mol. Biol. Cell. 5:413–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaka R., Thornton C., Vagts A.J., Phamluong K., Bonci A., Ron D. 2002. NMDA receptor function is regulated by the inhibitory scaffolding protein, RACK1. Proc. Natl. Acad. Sci. USA. 99:5710–5715 10.1073/pnas.062046299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan S.Y. 2002. Protein kinase signaling in the modulation of microvascular permeability. Vascul. Pharmacol. 39:213–223 10.1016/S1537-1891(03)00010-7 [DOI] [PubMed] [Google Scholar]