Abstract
Both class switch recombination (CSR) and somatic hypermutation (SHM) require transcription and the trans-acting factor activation-induced cytidine deaminase (AID), and must be up-regulated during antigen-dependent differentiation of B lymphocytes. To test the role of the heavy chain 3′ enhancers in both CSR and SHM, we used a BAC transgene of the entire heavy chain constant region locus. Using Cre-loxP recombination to delete a 28-kb region that contains the four known 3′ heavy chain enhancers, we isolated lines of BAC transgenic mice with an intact heavy chain locus and paired lines in the same chromosomal insertion site lacking the 3′ enhancers. Intact heavy chain transgenes undergo CSR to all heavy chain genes and mutate their transgenic VDJ exon. In paired transgenes lacking the 3′ enhancer region, CSR to most heavy chain genes is reduced to ∼1% of the levels for intact heavy chain loci; SHM is also reduced. Finally, we find that in B cells with a transgene lacking the 3′ enhancers, interchromosomal recombination between the transgenic VDJ exon and the endogenous heavy chain C genes is more easily detected than CSR within the transgene.
Class switch recombination (CSR) and somatic hypermutation (SHM) occur during antigen-driven differentiation of B lymphocytes. The heavy chain class switch is a DNA recombination event that occurs between a switch (S) region upstream of the Cµ gene and a second S region upstream of one of the γ, α, or ϵ heavy chain genes (Stavnezer, 2000; Manis et al., 2003). As a result of this deletional recombination event, the assembled VDJ exon is moved into physical and functional association with a new heavy chain gene, resulting in new effector functions of the expressed immunoglobulin. SHM introduces point mutations in the VDJ exon and several hundred basepairs downstream of the VDJ exon; however, the C region is spared (Storb and Stavnezer, 2002). The rate of SHM can be as high as 0.1% per nucleotide per cell division. Both CSR and SHM are dependent on the action of the B cell–specific activation-induced cytidine deaminase (AID; Muramatsu et al., 2000; Revy et al., 2000).
Both CSR and SHM are inactive in resting B cells, but are strongly induced during antigen-driven differentiation. The regulatory elements that control this dramatic up-regulation are poorly defined. Switch recombination is reduced, to a small extent, by deletion of the intronic µ enhancer (Bottaro et al., 1998; Sakai et al., 1999). It is clear that other elements must also play a role in the regulation of both CSR and SHM. The heavy chain 3′ enhancer region is a strong candidate for this regulation (Cogne and Birshtein, 2004). The region comprises a cluster of at least four DNase I hypersensitive sites (called HS3A; HS1,2; HS3B; and HS4), which are dispersed over a 28-kb region, beginning 4-kb downstream of the Cα gene. The heavy chain 3′ enhancers enhance transcription with a high level of B cell specificity and with substantial synergy among the four HS sites (Cogne and Birshtein, 2004). Consistent with a role in CSR, the enhancers can up-regulate the expression of “germline” transcripts from transgenic heavy chain genes (Collins and Dunnick, 1999; Laurencikiene et al., 2007). Germline transcripts for each heavy chain gene are initiated in an exon (termed “I”) upstream of the S region and continue through the S region and C region. Germline transcripts represent the first phase of CSR, the opening of the chromatin for a specific heavy chain gene (Stavnezer-Nordgren and Sirlin, 1986; Yancopoulos et al., 1986). HS3B and HS4 are known to play a role in CSR, as their deletion from the germline affects CSR to some genes profoundly (γ3 and γ2b), affects other genes by a reduction to ∼10% of wild-type values (γ2a, ϵ, and α), but affects CSR to γ1 and transcription of the Cµ gene by a minor increment (Pinaud et al., 2001). Unfortunately, it has not been possible to delete all four of the HS sites from the germline via ES cell technology, and so understanding of the regulation of CSR remains incomplete.
To study CSR, we use a 230-kb BAC that includes an inserted VDJ exon (encoding anti-arsonate [ARS] binding), all of the murine heavy chain S and C regions, and the known 3′ enhancers. The transgenic γ, ϵ, and α heavy chain genes undergo germline transcription and CSR with the same regulation as the endogenous genes. We had previously identified two truncated versions of this transgene that lacked the 3′ enhancers as well as the Cα gene, and showed that these truncated heavy chain transgenes could not undergo CSR to any of the γ genes, including γ1 (Dunnick et al., 2005). Both truncated heavy chain transgenes had deleted Cα, and one had deleted Cϵ; therefore, we could not test the effect of the deletion of the 3′ end of the locus on expression of these two isotypes. Furthermore, because of the spurious nature of the deletions, whether the defined HS sites or the deletion of additional elements within the 63-kb deletion were responsible for the phenotype, remained unclear.
Here, we have generated new transgenic constructs where the 28-kb region that comprises the 4 known HS sites corresponding to the 3′ heavy chain enhancers is flanked by loxP sites. After Cre-mediated deletion, we could compare intact transgenes to transgenes lacking the 3′ enhancer region, with the paired transgenes in the same chromosomal insertion site and with identical or similar copy number. The functional analysis of these transgenes has allowed us to evaluate the deletion of all four HS sites from the heavy chain locus on CSR to all isotypes. It has also allowed us to test the role of these elements in SHM of the transgenic V region. Given the inability to generate deletions at the genomic locus, this is the best available method for studying cis-acting elements that regulate heavy chain diversification.
RESULTS
B cell development in ARS/Igh transgenic mice lacking the heavy chain 3′ enhancers
The ARS/Igh11 transgene has five salient features (Fig. 1 A). First, it is derived from strain 129 DNA (Igha) and therefore differs from the endogenous C57BL/6 genes (Ighb) by many polymorphisms. Second, 4-bp insertions in Iγ1 and Iγ2a allow one to distinguish germline transcripts from the transgene from those from the endogenous genes (Dunnick et al., 2004, 2005). Third, the insertion of a Flag tag-encoding sequence at the C terminus of secreted version of Cγ2a allows the detection of transgene-specific IgG2a. Fourth, loxP sites were inserted 5′ of HS3A and 3′ of HS4. Fifth, two copies of the chicken β-globin insulator were inserted 3-kb 5′ of the anti-ARS VDJ exon. In the same targeting construct, a Not1 site was engineered into the BAC just 5′ to the insulators. Five lines of transgenic mice with the ARS/Igh11 construct were derived. In addition, a single mouse from line 761 survived with both the ARS/Igh11 transgene and the Ella Cre transgene. We could analyze her offspring as 761Δ mice, but could not obtain offspring with an intact ARS/Igh11 transgene. Hence, we had only this single mouse as a control. The transgene excludes endogenous heavy chain expression in all six types of mice because almost all B cells express transgenic IgMa only, and very few B cells express endogenous IgMb only (Fig. 1 B). This is in contrast to the versions of the ARS/Igh transgene we studied in the past, where allelic exclusion of the endogenous genes was incomplete in the majority of lines with a full-length heavy chain transgene (Gao et al., 2005). The improved allelic exclusion may be caused by the action of the chicken β-globin insulator. Alternatively, because we prepare the transgene by injection of the large Not1 fragment containing the bulk of the heavy chain locus, we eliminate a 6-kb Not1 fragment (from the 5′ end of ARS/Igh11 to the NotI site added with the chicken β-globin insulator; Fig. 1 A). In published versions of the heavy chain transgene, a D element in this 6-kb NotI fragment may undergo D to JH3 or JH4 joining within the transgene, deleting the transgenic VDJ exon and thus excluding transgenic expression (Dunnick et al., 2005; Gao et al., 2005).
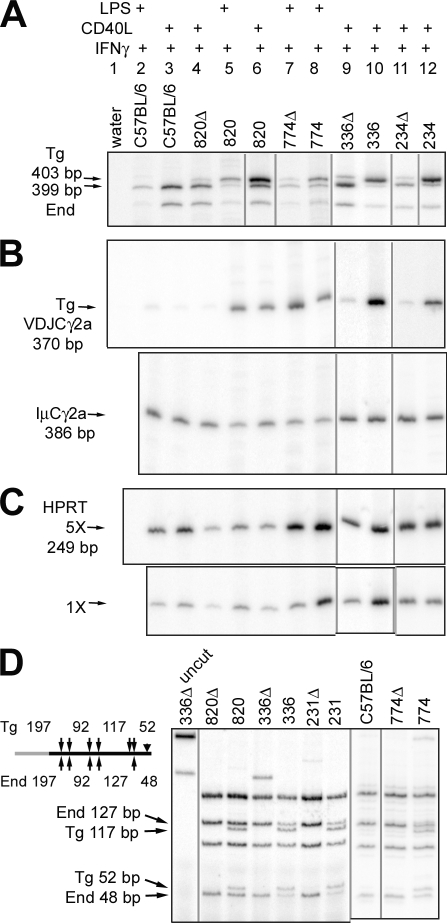
Figure 1.
Structure of the ARS/Igh11 transgene and transgenic µ expression. (A) Heavy chain variable region and constant region coding segments are shown as gray rectangles. Both the intronic enhancer (between the VDJ and Cμ exons) and the 3′ enhancers are shown as black circles. “2X INS” indicates two copies of the chicken β-globin insulator. Expression of Cre from the EIIa Cre transgene results in deletion of the 28-kb region containing the four 3′ enhancers (A, bottom), but spares 15 kb of sequence that flank the 3′ end of the enhancer region. 761* was not established as paired transgenic lines. We had only a mother with intact genes and 761Δ offspring from her, and so could test these mice in only a single experiment. (B) Analysis of surface Ig expression by flow cytometry. Line 336 B cells were tested with a different concentration of anti-B220, resulting in a lower apparent surface expression. Expression in lines 234, 234Δ, 231, 231Δ, 761, and 761Δ was similar to surface Ig expression in line 336. (C) The relative surface transgenic IgMa expression in lines 820 and 820Δ, and 774 and 774Δ, is shown as histograms. (D) Transgenic VDJCµ expression, as determined by RT-PCR, using fivefold dilutions of cDNA. Each experiment shown was repeated in one or two additional experiments.
We also prepared paired versions of the ARS/Igh11 transgenic lines by Cre-loxP deletion of the 28-kb fragment, including the four known 3′ enhancers (the “Δ” transgenic lines). In lines 231Δ, 234Δ, and 761Δ (not depicted), transgenic µ expression is similar to that of the paired, intact line. However, in lines 820Δ, 774Δ, and 336Δ (Fig. 1 D), both surface IgM and VDJCµ mRNA expression is reduced two- to fivefold compared with the intact lines (Fig. 1, B and D). However, neither B cell development nor allelic exclusion (Fig. 1 B and Fig. S1) is altered by deletion of the 3′ enhancers. Depending on the transgenic line, the deletion of the 3′ enhancers has a small or no effect on transgenic µ expression. We also determined copy number for 12 DNA segments across the ARS/Igh11 transgene, and found that paired intact and Δ versions of the transgene had identical (820, 774, 761, and 336) or similar copy numbers (Fig. S2). Lines 231Δ and 234Δ appeared to have one less transgene copy than their intact paired lines.
Specific deletion of the 28-kb 3′ enhancer region drastically reduces CSR compared with the levels of recombination in B cells with an intact ARS/Igh transgene
B cells from each transgenic line with an intact heavy chain transgene-secreted transgenic IgG1, IgG2a, and IgA after culture with the appropriate inducing agents (Fig. 2). The amount of antibody secreted varied, in part because of the exact inducing agent used, but mostly because of unavoidable variation in the B cell cultures. However, B cells from mice with deletion of the 3′ enhancers from the transgene (820Δ, 774Δ, etc.) rarely secreted amounts of transgenic IgG2a and IgG1 above that of control nontransgenic B cells (Fig. 2, A and B). The one exception to this was IgA; specifically, for two of the transgenic lines, 336Δ and 234Δ (each with two copies of the transgene), transgenic B cells sometimes expressed transgenic IgA amounts that were slightly in excess of the amounts secreted by control, nontransgenic B cells (Fig. 2 C). Supernatant fluids from B cells from both Δ mice and nontransgenic mice were shown to express significant amounts of endogenous Ig (Fig. S3) and supernatants from Δ mice expressed transgenic IgM (not depicted), demonstrating that these B cells underwent activation and differentiation.
Figure 2.
Transgenic IgG expression in vitro. Supernatants of cultured B cells (with cytokines as indicated in each panel) from various transgenic and nontransgenic mice were tested. (A) Transgenic Flag+ IgG2a expression; (B) transgenic IgG1a expression; and (C) transgenic AD8+ (idiotype+) IgA expression. Each data point represents an independent culture, derived from independent transgenic mice. In this and subsequent figures, the 774 line and 820 line were tested in separate experiments from the 336 line, the 234 line, the 231 line, and the 761 mice, as the latter four mice were established 1 yr later.
The 28-kb 3′ enhancer region is necessary for the production of CSR-associated transcripts
Before CSR, germline transcripts are initiated from each switch region (I) promoter and are thought to prepare the locus for recombination. After CSR, post-switch transcripts are expressed and translated into the switched heavy chain. Both germline transcripts and post-switch VDJCγ2a transcripts were expressed from the transgene in B cells with an intact ARS/Igh11 transgene (Fig. 3). In contrast to VDJ recombination during B cell development, CSR-associated germline transcription is biallelic (Delpy et al., 2003). Therefore, transgenic cells also expressed endogenous transcripts. Transgenic germline transcripts were distinguished from endogenous germline transcripts by migration polymorphisms (γ2a gene; Fig. 3 A) or by restriction site polymorphisms (see below). Using endogenous germline transcripts for normalization, B cells from enhancer-deleted mice express 10–20% of the amount of germline transcripts of B cells with intact transgenes. The residual germline transcription after deletion of the 3′ enhancers, particularly for the γ1 (Fig. 4 A) and α (Fig. 5 A) genes, probably results from the ability of these heavy chain genes (for example, as isolated transgenes) to express some germline transcripts (Xu and Stavnezer, 1992; Elenich et al., 1996; Collins and Dunnick, 1999; Laurencikiene et al., 2007). However, for ϵ, α, and the 4 γ heavy chain genes, the 28-kb 3′ enhancer region is necessary for the expression of physiological levels of germline transcripts.
Figure 3.
Transgenic expression of the γ2a heavy chain gene. (A) T cell—depleted splenocytes from various transgenic mice were cultured in activators and IFN-γ as indicated at the top of the figure. Germline transcripts from the endogenous and transgenes were distinguished by a migration polymorphism, which is due in part to a 4-bp insertion in transgenic Iγ2a. The 820 CD40L + IFN-γ sample was run on the same gel as the other seven samples on the left. However, because of the increased expression of all germline transcripts in this sample, the radioactivity was very high. Therefore, a different exposure of this lane is shown. (B) Post-switch transcripts. In this Figure, and in Fig. 4, Fig. 5, and Fig. S4, intact and Δ cDNA samples were first equalized for the expression of IμCH transcripts, and then the same concentrations of cDNA were tested for VDJCH expression. The expression patterns of germline and post-switch transcripts expressed by B cells from lines 231Δ and 231 were identical to those of lines 336, 336Δ, 234, and 234Δ (not depicted). (C) HPRT mRNA expression. Two concentrations of cDNA were tested by RT-PCR, representing one-fifth and one-twenty-fifth of the amount of cDNA from the indicated cultures tested for post-switch transcripts in B. (D) µ-γ2a CSR of the endogenous and transgenes was tested by DC-PCR. In the schematic, the gray portion depicts sequences 5′ of Sµ and the black portion depicts sequences 3′ of Sγ2a. Dde1 sites (arrows in schematic) differentiate DC-PCR products from the endogenous genes and transgene. A 4-bp insertion in the transgene (black triangle) also differentiates one transgenic fragment from its endogenous counterpart. Each experiment shown was repeated in one or two additional experiments. Black lines between lanes indicate that the order of the lanes has been rearranged from the original electronic file, or that the lanes are derived from an independent experiment.
Figure 4.
Transgenic expression of the γ1 heavy chain gene. (A) T cell–depleted splenocytes from the various transgenic mice were cultured in LPS or LPS+IL-4. Germline transcripts from the endogenous and transgenes were distinguished by a 4-bp insertion resulting in a TaqI restriction site (arrows in schematic). RNA samples were derived from cells cultured in LPS+IL-4. (B) Post-switch transcripts. Expression patterns for germline and post-switch transcripts from B cells from lines 234Δ and 234 were identical to those from lines 820Δ and 820 (not depicted). (C) HPRT mRNA expression. (D) µ-γ1 CSR, distinguishing the transgene and endogenous genes, was tested by DC-PCR. Note that the 231Δ sample has a larger amount of fragment from the endogenous genes. (E) The same amount of 820Δ LPS+IL-4 and 820 LPS+IL-4 tested in Part B, and three fivefold dilutions of the 820 cDNA, were tested for transgenic VDJCγ1 expression. Each experiment shown was repeated in one or two additional experiments. Black lines between lanes indicate that the order of the lanes has been rearranged from the original electronic file, or that the lanes are derived from an independent experiment.
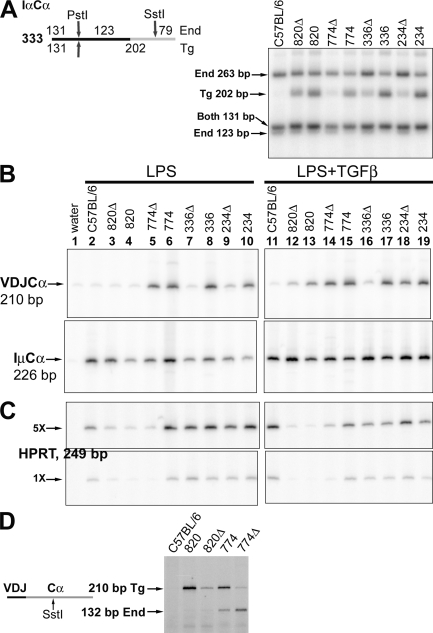
Figure 5.
Transgenic expression of the α heavy chain gene. (A) Expression of α germline transcripts. Germline transcripts from the transgene were distinguished from transcripts from the endogenous genes by digestion of the 333-bp IαCα PCR products with PstI and SstI, as illustrated at the top of the figure. The 263-bp endogenous fragment derives from an alternatively spliced version with a longer Iα exon. (B) Post-switch transcripts. The expression patterns of germline and post-switch transcripts for lines 231Δ and 231 were similar to those of lines 234Δ and 234 (not depicted). Cells from C57BL/6, 820Δ, and 820 were treated with CD40L, with or without TGFβ as indicated, instead of LPS. (C) HPRT transcripts. (D) Recombination between the transgenic VDJ and endogenous Cα in lines 774 and 774Δ. Transgenic and endogenous Cα sequences in mRNA, from B cells cultured in LPS+TGFβ, were distinguished by a SstI site found in the endogenous, but not the transgenic, Cα. Each experiment shown was repeated in one or two additional experiments.
Transgenic post-switch transcripts were distinguished from potential endogenous transcripts using a transgene-specific RT-PCR (Dunnick et al., 2005); note that nontransgenic C57BL/6 B cells produce only a remnant of a signal (Fig. 3 B). All lines with an intact ARS/Igh11 transgene expressed VDJCγ2a transcripts (Fig. 3 B). In contrast, transgenes lacking the 3′ enhancer region expressed barely detectable amounts of post-switch VDJCγ2a transcripts (lines 820Δ, 336Δ, and 234Δ; Fig. 3 B). As an exception, line 774Δ expressed post switch VDJCγ2a (and other VDJCH transcripts, see below). As we discuss in the section after next, line 774 inserted near the endogenous heavy chain locus, and the post-switch transcripts are the joining of transgenic VDJ to endogenous CH genes. All cultures produced IµCγ2a transcripts (Li et al., 1994), demonstrating that the nontransgenic and Δ cultures contained viable B cells, were activated in vitro, switched their endogenous γ2a genes, etc. This enhancer-dependent defect was not restricted to the γ2a genes: analogous results were obtained for the γ3, γ2b, and ϵ heavy chain genes (Fig. S4) as well as the transgenic γ1 (Fig. 4) and α (Fig. 5) genes. Therefore, the 28-kb 3′ enhancer region may be important for switch recombination in DNA. Alternatively, it is possible that deletion of the 3′ enhancers has no effect on DNA recombination, and the reduced amount of post-switch transcripts is the result of a lack of enhancement of those transcripts from a correctly switched locus (Shi and Eckhardt, 2001).
The 28-kb 3′ enhancer region is essential for DNA recombination during CSR
To assess the possibility that the lack of expression of transgenic post-switch transcripts from a heavy chain transgene with the deletion of the 28-kb 3′ enhancer region might be caused by a failure to undergo switch recombination in DNA, we tested for CSR in DNA by a modified direct circularization PCR (DC-PCR) technique (Chu et al., 1992). We digested the DC-PCR products with DdeI, which distinguishes the transgenic and endogenous γ2a genes (Fig. 3 D). Transgenic DC-PCR fragments were easily detected in DNA samples from B cells with intact transgenes. In addition, the amount of transgenic fragment (from one to three genes) was comparable to the amount of the endogenous fragment (from two genes). This indicates that CSR of the ARS/Igh transgene is more or less as efficient as CSR of the endogenous genes. However, specific removal of the 3′ enhancers from the integrated BAC transgene by Cre-recombination, clearly abrogated CSR in all B cell samples (including line 774Δ). Analogous results were obtained for CSR at the DNA level for the γ1 gene (Fig. 4 D). These results demonstrate that the 3′ enhancer region deletion has a profound effect on CSR at the level of DNA recombination.
Lack of CSR in enhancer-deleted heavy chain transgenes can be partially rescued by recombination in trans with the endogenous locus
Enhancer deletion had abrogated the production of post-switch transcripts in all but one of our transgenic strains: in line 774, deletion of the 3′ enhancer region had a modest effect on the levels of post-switch transcripts (Figs. 3–5 and Fig. S4). We therefore wondered if the transgenic BAC in that line had inserted in a privileged location. Indeed, we noticed that, in a standard genetic cross, the 774 transgene cosegregated with the endogenous heavy chain locus in each of 13 offspring. Therefore, the ARS/Igh transgene in line 774 is inserted into murine chromosome 12, in linkage with the heavy chain locus. It does not replace the endogenous locus, as endogenous genes are retained in mice with an intact 774 transgene on one chromosome and a 774Δ transgene on the other homologue (unpublished data).
We therefore considered the possibility that the VDJCα transcripts observed in line 774Δ B cells were actually derived by a recombination event between the VDJ exon of the transgene and the Cα exons of the endogenous locus. To test this, we amplified VDJCα transcripts from various transgenic B cells, and then distinguished those with an endogenous Cα from those with a transgenic Cα by digestion with SstI (Fig. 5 D). At least 15% of the transgenic VDJ-containing transcripts from line 774 B cells are associated with endogenous Cα sequences, and almost all of the transcripts from line 774Δ B cells are associated with endogenous Cα sequences. Therefore, the 774 line is not an outlier with regard to production of post-switch transcripts: the enhancer-deleted version switches just as poorly within the transgene as any of the other deleted lines. The milder effect apparent on post-switch transcripts in previous figures is almost entirely caused by recombination with the endogenous CH genes. Consistent with this explanation, supernatant fluids from line 774Δ B cells do not secrete detectable transgenic IgG2a or IgG1 because detection requires a transgenic CH region (Fig. 2, A and B). Also consistent with this explanation, CSR within the 774Δ transgene at the DNA level cannot be detected (Fig. 3 D).
Products of trans-recombination are positively selected in vivo, during an immune response
To determine whether trans-recombination was functional in vivo, we amplified and sequenced the transgenic VDJ from RNA of B cells from immunized spleen, 21 d after immunization (Fig. S5) . As predicted from the in vitro data, transgenic VDJ recombination with the endogenous Cγ was frequent in line 774 (19 of 26 VDJ sequences had the endogenous Cγ) and 774Δ (17 of the 18 VDJ sequences had the endogenous Cγ). Furthermore, recombination of the transgenic 820 VDJ with the endogenous Cγ was infrequent (occurring only 4 out of 34 sequences). Surprisingly however, recombination of the transgenic 820Δ VDJ with the endogenous Cγ was frequent in the enhancer-deleted line; 22 of 33 total sequences had the transgenic VDJ associated with an endogenous Cγ. Apparently, during an immune response in vivo, there is a strong selection for the antibodies (on the cell surface or secreted) that result from the virtually undetectable recombination between the transgene and the endogenous genes in lines 820 and 820Δ in vitro (Fig. 5 D).
The 3′ enhancers are important for somatic hypermutation
Past work has shown that elements within the 3′ enhancer region (specifically the HS3B and HS4 sites) were dispensable for SHM (Le Morvan et al., 2003). To determine whether deletion of the entire 28-kb enhancer region had an effect on mutation, we immunized our transgenic mice with ARS-KLH, and isolated GL7+/Fas+ (germinal center) B cells from their spleens 12 d after immunization. DNA from sorted B cells was used to amplify the VDJ exon and 3′ flanking sequences, which were then cloned and sequenced. In contrast to most heavy chain transgenes which do not mutate at wild-type levels (Giusti and Manser, 1993; Unniraman and Schatz, 2007), our BAC transgenics were fully competent for SHM, accumulating mutations at a rate of 0.3–0.8% (Fig. 6). Deletion of the enhancers had a major effect on the frequency of mutations. For example, the frequency of mutation in 820 VDJ and flanking region dropped from ∼0.46–0.06%, in the absence of the enhancers; this is close to the background rate of mutation caused by polymerase error during amplification (∼0.02% or 2/10,000 nt). Similarly, the frequency of mutation near the VDJ in the 336 line dropped from 0.36% to 0.08%, and in the 774 line dropped from 0.72% to 0.23%. In each set of paired transgenic lines, the number of clones with multiple mutations was also reduced in the absence of the enhancers (Fig. 6).
Figure 6.
Somatic hypermutation of transgenic VDJ exons and flanking sequences. Part of the transgenic VDJ exon and 3′ flanking sequences (from germinal center B cells) were amplified, cloned, and sequenced. Mutations in the 3′ 171 bp of VDJH2 exon and 329 bp of 3′ flanking sequences are presented as pie charts, in which the sectors of the pie are proportional to the number of sequences with a given number of mutations. The number of sequences is shown in the center of the pie chart, and the number of mutations in some sectors is noted. For lines 820, 820Δ, 774, and 774Δ, the data represent a compilation of sequences from two sorted cell samples derived from independent mice. For lines 336 and 336Δ, the data are derived from one sorted cell sample each; a second set of sequences from the same cell sample yielded 0.21% mutations in line 336 DNA and 0.02% mutations in line 336Δ DNA. For all three pairs of transgenic lines tested, the mutation frequency is greater in intact versus the enhancer-deleted line, P < 0.0001 (two-tailed χ2 test, with Yate’s correction and one degree of freedom). Mutation frequencies in the intronic 329-bp were: line 820, 0.36% (27 mutations in 7557 nt); line 820Δ, 0.03% (4 mutations in 13,818 nt); line 774, 0.40% (43 mutations in 10,857 nt); line 774Δ, 0.11% (14 mutations in 12,831 nt); line 336, 0.16% (44 mutations in 27,636 nt); and 336Δ, 0.03% (9 mutations in 30,268 nt). For each set of paired transgenic lines, the difference in intronic mutations was significant at P < 0.0001 (two-tailed χ2 test, with Yate’s correction and one degree of freedom). In separate experiments, transgenic V region mutations were also determined in cloned mRNA (VDJCγ1, VDJCγ2a, and VDJCγ2b pooled sequences) from spleens of immunized line 820 mice (see text). The 820 VDJ exon had 1.67% mutations (128 mutations in 7680 nt sequenced from 30 clones). The 820 Cγ region had 0.1% mutations (10 mutations in 10,041 nt sequenced in the same 30 clones). By a two-tailed χ2 test, with Yate’s correction and one degree of freedom, the difference in mutation frequency in V and C is significant at P < 0.0001.
DISCUSSION
Several lines of work suggest that the 3′ enhancer region would control CSR. Chromatin interactions between heavy chain genes and HS1,2 suggest functional interactions (Wuerffel et al., 2007). Deletions or replacements of individual hypersensitive regions (Cogné et al., 1994; Manis et al., 1998; Seidl et al., 1999) implicate the 3′ regulatory locus in CSR. Additionally, insertions of foreign sequence in the locus also affect CSR, possibly by disrupting interactions between enhancer elements (Seidl et al., 1999). However, any conclusions are complicated by the fact that clean deletion of single elements reveals only a minimal phenotype (Manis et al., 1998; Seidl et al., 1999; Vincent-Fabert et al., 2009). Deletion of both HS3B and HS4 (Pinaud et al., 2001) from the germline demonstrates a role in CSR to some heavy chain genes. To pursue the role of the 3′ enhancer region, over the last 10 yr, several laboratories have attempted to delete all four elements from the mouse germline, but for reasons that remain unclear, this has not been achieved to date.
To study the role of the entire 3′ enhancer region in antibody diversification processes, we have developed a BAC transgenic system from which we can delete all four 3′ enhancer elements. For all our BAC ARS/Igh transgenes tested, CSR (and SHM) is similar, qualitatively and quantitatively, to that of the endogenous locus. CSR is quantitatively similar in different transgenic lines (Figs. 2–5 and Fig. S4), indicating that insertion site effects are relatively minor (line 774 being a special exception). At the same time, in all cases, enhancer deletion has a pronounced effect on CSR, at the level of DNA recombination. Comparison of the amount of switch recombination in the “Δ” lines to dose–response curves for both RT-PCR (Fig. 4 E and Fig. S4 D) and ELISA (Fig. S3) indicates that for most isotypes, CSR for the transgenes lacking the 3′ enhancers is less than 1% the level of CSR for intact transgenes. The only exception is the α heavy chain, where enhancer-deleted transgenic lines produce germline transcripts and post-switch transcripts above background levels (Fig. 5 A and B, compare lanes 12, 14, and 18 to lane 11). For transgenic lines 336, 234, and 231 (two or more copies), transgenic IgA above background levels can be detected in the supernatants of cells with an enhancer-deleted transgene (Fig. 2 C and not depicted). These results suggest that transcription and recombination of the α heavy chain gene may depend on the 3′ enhancer region to a lesser extent than other heavy chain genes. Perhaps some ability to enhance CSR to the α heavy chain gene is encoded outside the 28-kb 3′ enhancer region, and is most easily revealed when extra copies of the heavy chain transgene can insulate one of the α heavy chain genes. Alternatively, in a head-to-tail transgene configuration, the intronic µ enhancer of the downstream transgene might enhance CSR to the upstream α gene. Even though enhancer region deletion has a milder effect on CSR to IgA, its absence significantly affects both transcription and recombination to this isotype. In addition, CSR to the α gene is dramatically affected by deletion of the 3′ enhancer region in single copy mice (Figs. 2 C and Fig. 5, B and D), and CSR to all other isotypes is practically nil, regardless of the transgene or copy number. Taken together, our experiments demonstrate that the 28-kb 3′ enhancer region controls germline transcription and switch recombination to all heavy chain genes.
Remarkably, CSR within transgenes lacking the 3′ enhancers is so inefficient that intra- or interchromosomal recombination events predominate after induction of CSR. For example, recombination events between the VDJ of the 774Δ transgene with the endogenous genes are easily detected (Fig. 5 D and Results). If the 774/774Δ insertion site is within the VH cluster, and in the same orientation as the endogenous genes, recombination of the transgenic VDJ with endogenous CH must involve much greater distances than switch recombination within the transgene (because the VDJ exon must pass over all the transgenic CH to reach the endogenous CH). If the 774/774Δ insertion site is in the opposite orientation to the endogenous genes, recombination of the transgenic VDJ with the endogenous CH genes must involve an inversion event. Finally, if the transgenic insertion is downstream of Cα in either orientation, recombination of the transgenic VDJ and endogenous CH is more complex than CSR within the transgene. The VDJ and CH exons would be in the nonphysiological order, and may be inverted relative to one another. Selection during an immune response in vivo reveals the same recombination products in line 820Δ, where recombination between the transgenic VDJ and endogenous CH genes (on different chromosomes!) is easier to detect than CSR within the 820Δ transgene. Similar recombination events between the VDJ exon of heavy chain transgenes on one chromosome with endogenous heavy chain genes on another chromosome have been described in multiple reports (Durdik et al., 1989; Gerstein et al., 1990; Giusti and Manser, 1993). The physical interactions that lead to these inter-chromosomal recombination events may be mediated by an interaction of the endogenous 3′ enhancers with the transgenic intronic μ enhancer, VH promoter, or Sµ region (Wuerffel et al., 2007). The inter-chromosomal events involving transgenes may also mimic CSR between allelic chromosomes (Knight et al., 1995; Reynaud et al., 2005). The 3′ enhancers seem to favor CSR between heavy chain genes within a single locus. An important experimental question for the future is whether the 3′ enhancers have an additional function to suppress inter-chromosomal translocation events.
There has been considerable speculation about the potential role of the 3′ enhancer region in SHM. Studies in transgenic mice where the various hypersensitive sites of the 3′ enhancers were ectopically placed on a regular transgene, have indicated a role for HS3B and HS4 (but not HS1,2) in the recruitment of SHM to the transgene (Terauchi et al., 2001). However, these data are complicated by the fact that regular transgenes are generally poor hypermutation targets, and also by the possibility that inclusion of HS3B and HS4 simply increased the transcriptional efficiency of the transgene, as it is well known that the rates of mutation fluctuate with the rates of transcription. This possibility was not controlled for in these transgenic studies (Terauchi et al., 2001). Other investigators, studying mice where the HS3B and HS4 elements were deleted from the germline, have reached the opposite conclusion, namely that joint deletion of these two elements does not affect SHM of the endogenous IgH locus, thus suggesting that CSR and SHM can be uncoupled with regard to their dependence on cis-regulatory elements (Le Morvan et al., 2003).
Our studies presented herein demonstrate that the 28-kb 3′ enhancer region is necessary for wild-type levels of SHM. We find that deletion of the enhancer region significantly reduces SHM to almost background levels. The actual reduction in SHM caused by deletion of the 3′ enhancers is likely to be even greater than we observe here (Fig. 6). In vivo, recombination events between the transgenic VDJ and endogenous locus dominate in mice whose transgene lacks the 3′ enhancers (Results). Unfortunately, we cannot determine if the DNA we amplified and sequenced for investigation of SHM is linked to a transgenic CH gene or an endogenous CH gene because potential recombination events between the transgenic VDJ and endogenous CH are likely to occur outside the region we amplify. However, some of the mutations from Δ mice we report (Fig. 6) are likely to derive from transgenic VDJ exon associated with endogenous CH genes. Hence, some of the mutations we assign to the Δ transgenes should be associated with the endogenous genes, and not counted as mutations in an enhancer-deleted transgene. Recombination of the transgenic VDJ with the endogenous genes is probably the reason the mutation frequency in line 774Δ remains high at 0.23% (Fig. 6).
Some or all of the reduction in SHM is probably secondary to a reduction in transcription rate of the VDJ exon; the reduction in SHM (Fig. 6) and transcription (Fig. 1 D) are similar in magnitude. As we argue above, the true reduction in SHM may be greater. Therefore, it is also possible that the 3′ enhancer region has a function in SHM independent of transcriptional enhancement (Kothapalli et al., 2008; Blagodatski et al., 2009). Results from line 336Δ would be consistent with this notion. This transgenic line suffers a twofold reduction in surface IgM expression caused by deletion of the 3′ enhancers (Fig. 1 D), but suffers sixfold reduction in SHM (Fig. 6).
Our results cannot rule out additional regulatory elements in the heavy chain locus, outside the limits of the ARS/Igh transgene that further regulate CSR and/or SHM. However, they do establish that the 3′ regulatory region is necessary for both CSR and SHM in the context of the Ig locus. Finally, they reveal an unexpected role of the 3′ enhancers in suppressing interchromosomal recombination between endogenous and transgenic Ig loci, thus hinting at the possibility that the 3′ enhancers are crucial in suppressing chromosomal translocations that could arise during CSR.
MATERIALS AND METHODS
Transgenic constructs and mice.
Two copies of the chicken β-globin insulator were inserted at the EcoR1 site 3-kb 5′ of the assembled anti-ARS VDJ. Using oligonucleotide-mediated modification, a NotI site was inserted inside the BamH1 site of a 2.4-kb EcoRI–BamHI fragment (with two copies of the insulator) from pJC13-1 (Saitoh et al., 2000). This fragment was built into a targeting vector in pSV1.RecA (Yang et al., 1997) with a 1-kb BamHI fragment containing JH1 and JH2 for 5′ homology and a 5.2-kb EcoRI fragment containing the anti-ARS VDJH2, JH3, and JH4 (Durdik et al., 1989) for 3′ homology. The orientation and order of the various fragments was confirmed by appropriate restriction digests of the final targeting vector.
For insertion of a Flag tag at a position three codons in front of the C terminus of Cγ2a, 5′ and 3′ homology regions were amplified with the inserted Flag tag codons (Table S1) and were brought together by overlap extension (Ho et al., 1989). The resulting targeting sequences were transferred into pSV1.RecA using SalI sites provided by intermediate cloning vectors. For insertion of a loxP site 5′ of HS3A, 5′ and 3′ homology regions were identified from the sequence by Garrett et al. (2005), and were amplified with an inserted loxP site (Table S1). The two homology regions were brought together using overlap extension (Ho et al., 1989). The resulting targeting sequences were transferred to pSV1.RecA using HindIII sites ligated as linkers to the ends of the targeting fragments. For insertion of a loxP site 3′ of HS4, the 5′ and 3′ homology regions were amplified with an inserted loxP site (Table S1). The two homology regions were brought together by digestion with HindIII and ligation, and the resulting targeting sequences were transferred to pSV1.RecA using the SalI sites at the ends.
Each of four targetings (previous two paragraphs) was completed in two stages. First, the targeting vector was cointegrated into the ARS/Igh BAC using 5′ or 3′ homology regions, expression of RecA, and selection for resistance to tetracycline at high temperature (Yang et al., 1997). Second, excision of the targeting vector was selected by resistance to fusaric acid (Yang et al., 1997). Presence of the engineered insertion in the resulting BAC was verified by Southern hybridizations and PCR. In each case, the homology regions were cloned and sequenced to verify that no mutations had been introduced during PCR amplification. The mutations to create SalI sites, at the ends of the homology regions, were not transferred into the final ARS/Igh BAC, as homologous recombination must take place inside them.
The Not1 insert of this BAC (designated ARS/Igh11) was prepared as described at http://www.med.umich.edu/tamc/BACcol.html, and injected into fertilized C57BL/6 x SJL F2 oocytes. Transgenic lines were prepared by breeding founders to C57BL/6 mice. All experiments involving mice were approved by the University of Michigan Committee on the Use and Care of Animals, protocol 08147. Deletion of the 28-kb 3′ enhancer region was accomplished by breeding females with both the ARS/Igh11 transgene and the EIIa Cre transgene (Lakso et al., 1996). Intact and Cre-loxP-deleted lines of transgenic mice were characterized structurally by evaluation of twelve DNA segments spanning the ARS/Igh11 transgene (Fig. S2). Amplification and analysis of Cϵ genomic sequences (Dunnick et al., 2005) and of HS1,2 and HS3B genomic sequences (Dunnick et al., 2004) have been previously described. The amplification and analysis of seven other gene segments are detailed in Table S3. The Sγ1 region (SstI) and Sα region (SstI) were analyzed by Southern hybridization, using published probes (Hummel et al., 1987). Allelic exclusion and B cell development was evaluated using commercially available monoclonal antibody reagents (BD) and a FACSCanto with autocompensation.
Detection of CSR.
Secreted IgG1 (anti-IgG1a coating), IgG2a (anti-Flag coating), and IgA (anti-IgA coating) were detected in supernatants from 1-ml cultures of 100,000 transgenic B cells for 7 d. For analysis of transgenic IgA, rat monoclonal AD8 (transgenic idiotype; Hornbeck and Lewis, 1983) was used as an intermediate antibody. Developing reagents were anti-IgG1, anti-IgG2a, or anti–rat IgG conjugated to alkaline phosphatase. For analysis of nucleic acids, RNA or DNA was prepared from 3-d cultures of B cells at 1.5 million per ml (Dunnick et al., 2004). Transgene-specific germline transcripts and post-switch transcripts were detected by RT-PCR and restriction enzyme digestion which used polymorphisms between the transgenes and endogenous genes (Dunnick et al., 2004, 2005). PCRs were done for 30–35 cycles with extension for 1 min at 72 and denaturation for 1 min at 95. RT-PCR for germline, transgenic VDJCH, Iµ, HPRT transcripts, and for DC-PCR products for a recombined γ1 gene were as published (Dunnick et al., 2005). Germline transcripts for the α gene and DC-PCR products for a recombined γ2a gene were amplified and analyzed as outlined in Table S2, Fig. 3 and Fig. 5.
Detection of SHM.
For detection of SHM in genomic sequences, transgenic mice were immunized with 0.2 ml SRBC (Dong et al., 2001) to which ARS-KLH had been conjugated (Gronowicz et al., 1976). 12 d later splenocytes were depleted of red blood cells and germinal center B cells (B220+, GL7+, and fas+) were sorted. We then prepared genomic DNA from these cells using standard procedures, and amplified 0.8-kb of sequence containing the transgenic VDJ exon and downstream JH intron, using primers 5′-CCTATGATCAGTGTCCTCTCCACACTCC-3′ (within transgenic VH) and 5′-GGACTCACCTGCAGAGACAGTGACCAG-3′ (JH4). For detection of SHM in mRNA, transgenic mice were immunized with 100 µg ARS-KLH in complete Freund’s adjuvant i.p. 21 d later, RNA was prepared from T cell–depleted splenocytes of the immunized mice. VDJCγ sequences were amplified using 5′-GGTGAAACAGAGGCCTGGTC-3′ (amplifying 256 bp of transgenic VDJ, starting in the framework region 2) and 5′-GCATGATGGGAAGTTCACTGACTG-3′ (amplifying 544 bp of Cγ1 sequences), 5′-GCTGGGCCAGGTGCTCGAGGTT-3′ (amplifying 230 bp of Cγ2a), or 5′-TGCTGGGCATTTGCATGGAG-3′ (amplifying 343 bp of the transgenic Cγ2a). The amplified sequences were cloned and sequenced.
Online supplemental material.
Fig. S1 presents the analysis of B cell subsets in transgenic mice. Fig. S2 presents the analysis of transgene content in various lines of transgenic mice. Fig. S3 presents the dose–response characteristics of ELISAs and controls for total Ig expression. Fig. S4 presents expression of the γ3, γ2b, and ϵ heavy chain genes, as well as some of the dose-response characteristics of the RT-PCRs. Fig. S5 presents anti-ARS expression after immunization of transgenic mice. Tables S1–S3 describe primers and amplification conditions for various PCRs used in transgene construction or in detection of various DNA segments or RNAs. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20091280/DC1.
Acknowledgments
We thank Dr. Michael Berton for discussions and for hosting WD during his sabbatical leave, during which these experiments were initiated. We also thank Drs. Douglas Engel, David Ferguson, and Thomas Waldschmidt for advice during the course of this investigation. We thank Dr. Engel for the use of his laboratory’s CHEF gel apparatus and for recombinant DNA constructs, and Drs. F.W. Alt, G. Felsenfeld, N. Heintz, J. Manis, and E. Selsing for gifts of constructs, reagents, and mice. We acknowledge Galina Gavrilina and Maggie Van Keuren for preparation of transgenic mice and the Transgenic Animal Model Core of the University of Michigan’s Biomedical Research Core Facilities.
This work was supported by the National Institutes of Health grants (AI076057 to W.A. Dunnick and CA098495 to F.N. Papavasiliou). Transgenic core support was provided by The University of Michigan Cancer Center (CA46592).
The authors have no conflicting financial interests.
Footnotes
Abbreviations used:
- AID
- activation-induced cytidine deaminase
- ARS
- arsonate
- CSR
- class switch recombination
- DC-PCR
- direct circularization PCR
- HS
- DNAse I hypersensitive site
- SHM
- somatic hypermutation
References
- Blagodatski A., Batrak V., Schmidl S., Schoetz U., Caldwell R.B., Arakawa H., Buerstedde J.-M. 2009. A cis-acting diversification activator both necessary and sufficient for AID-mediated hypermutation. PLoS Genet. 5:e1000332 10.1371/journal.pgen.1000332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottaro A., Young F., Chen J., Serwe M., Sablitzky F., Alt F.W. 1998. Deletion of the IgH intronic enhancer and associated matrix-attachment regions decreases, but does not abolish, class switching at the mu locus. Int. Immunol. 10:799–806 10.1093/intimm/10.6.799 [DOI] [PubMed] [Google Scholar]
- Chu C.C., Paul W.E., Max E.E. 1992. Quantitation of immunoglobulin mu-gamma 1 heavy chain switch region recombination by a digestion-circularization polymerase chain reaction method. Proc. Natl. Acad. Sci. USA. 89:6978–6982 10.1073/pnas.89.15.6978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogne M., Birshtein B.K. 2004. Regulation of class switch recombination. Molecular Biology of B Cells. Honjo T., Alt F.W., Neuberger M., Academic Press, San Diego, CA: 289–305 [Google Scholar]
- Cogné M., Lansford R., Bottaro A., Zhang J., Gorman J., Young F., Cheng H.-L., Alt F.W. 1994. A class switch control region at the 3′ end of the immunoglobulin heavy chain locus. Cell. 77:737–747 10.1016/0092-8674(94)90057-4 [DOI] [PubMed] [Google Scholar]
- Collins J.T., Dunnick W.A. 1999. Cutting edge: IFN-γ regulated germline transcripts are expressed from γ2a transgenes independently of the heavy chain 3′ enhancers. J. Immunol. 163:5758–5762 [PubMed] [Google Scholar]
- Delpy L., Le Bert M., Cogné M., Khamlichi A.A. 2003. Germ-line transcription occurs on both the functional and the non-functional alleles of immunoglobulin constant heavy chain genes. Eur. J. Immunol. 33:2108–2113 10.1002/eji.200323969 [DOI] [PubMed] [Google Scholar]
- Dong C., Temann U.-A., Flavell R.A. 2001. Cutting edge: critical role of inducible costimulator in germinal center reactions. J. Immunol. 166:3659–3662 [DOI] [PubMed] [Google Scholar]
- Dunnick W.A., Shi J., Graves K.A., Collins J.T. 2004. Germline transcription and switch recombination of a transgene containing the entire H chain constant region locus: effect of a mutation in a STAT6 binding site in the γ 1 promoter. J. Immunol. 173:5531–5539 [DOI] [PubMed] [Google Scholar]
- Dunnick W.A., Shi J., Graves K.A., Collins J.T. 2005. The 3′ end of the heavy chain constant region locus enhances germline transcription and switch recombination of the four γ genes. J. Exp. Med. 201:1459–1466 10.1084/jem.20041988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durdik J., Gerstein R.M., Rath S., Robbins P.F., Nisonoff A., Selsing E. 1989. Isotype switching by a microinjected mu immunoglobulin heavy chain gene in transgenic mice. Proc. Natl. Acad. Sci. USA. 86:2346–2350 10.1073/pnas.86.7.2346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elenich L.A., Ford C.S., Dunnick W.A. 1996. The γ 1 heavy chain gene includes all of the cis-acting elements necessary for expression of properly regulated germ-line transcripts. J. Immunol. 157:176–182 [PubMed] [Google Scholar]
- Gao N., Dang T., Dunnick W.A., Collins J.T., Blazar B.R., Yuan D. 2005. Receptors and counterreceptors involved in NK-B cell interactions. J. Immunol. 174:4113–4119 [DOI] [PubMed] [Google Scholar]
- Garrett F.E., Emelyanov A.V., Sepulveda M.A., Flanagan P., Volpi S., Li F., Loukinov D., Eckhardt L.A., Lobanenkov V.V., Birshtein B.K. 2005. Chromatin architecture near a potential 3′ end of the igh locus involves modular regulation of histone modifications during B-Cell development and in vivo occupancy at CTCF sites. Mol. Cell. Biol. 25:1511–1525 10.1128/MCB.25.4.1511-1525.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstein R.M., Frankel W.N., Hsieh C.-L., Durdik J.M., Rath S., Coffin J.M., Nisonoff A., Selsing E. 1990. Isotype switching of an immunoglobulin heavy chain transgene occurs by DNA recombination between different chromosomes. Cell. 63:537–548 10.1016/0092-8674(90)90450-S [DOI] [PubMed] [Google Scholar]
- Giusti A.M., Manser T. 1993. Hypermutation is observed only in antibody H chain V region transgenes that have recombined with endogenous immunoglobulin H DNA: implications for the location of cis-acting elements required for somatic mutation. J. Exp. Med. 177:797–809 10.1084/jem.177.3.797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronowicz E., Coutinho A., Melchers F. 1976. A plaque assay for all cells secreting Ig of a given type or class. Eur. J. Immunol. 6:588–590 10.1002/eji.1830060812 [DOI] [PubMed] [Google Scholar]
- Ho S.N., Hunt H.D., Horton R.M., Pullen J.K., Pease L.R. 1989. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 77:51–59 10.1016/0378-1119(89)90358-2 [DOI] [PubMed] [Google Scholar]
- Hornbeck P.V., Lewis G.K. 1983. Idiotype connectance in the immune system. I. Expression of a cross-reactive idiotype on induced anti-p-azophenylarsonate antibodies and on endogenous antibodies not specific for arsonate. J. Exp. Med. 157:1116–1136 10.1084/jem.157.4.1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel M., Berry J.K., Dunnick W. 1987. Switch region content of hybridomas: the two spleen cell Igh loci tend to rearrange to the same isotype. J. Immunol. 138:3539–3548 [PubMed] [Google Scholar]
- Knight K.L., Kingzette M., Crane M.A., Zhai S.-K. 1995. Transchromosomally derived Ig heavy chains. J. Immunol. 155:684–691 [PubMed] [Google Scholar]
- Kothapalli N., Norton D.D., Fugmann S.D. 2008. Cutting edge: a cis-acting DNA element targets AID-mediated sequence diversification to the chicken Ig light chain gene locus. J. Immunol. 180:2019–2023 [DOI] [PubMed] [Google Scholar]
- Lakso M., Pichel J.G., Gorman J.R., Sauer B., Okamoto Y., Lee E., Alt F.W., Westphal H. 1996. Efficient in vivo manipulation of mouse genomic sequences at the zygote stage. Proc. Natl. Acad. Sci. USA. 93:5860–5865 10.1073/pnas.93.12.5860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurencikiene J., Tamosiunas V., Severinson E. 2007. Regulation of epsilon germline transcription and switch region mutations by IgH locus 3′ enhancers in transgenic mice. Blood. 109:159–167 10.1182/blood-2006-02-005355 [DOI] [PubMed] [Google Scholar]
- Li S.C., Rothman P.B., Zhang J., Chan C., Hirsh D., Alt F.W. 1994. Expression of I mu-C gamma hybrid germline transcripts subsequent to immunoglobulin heavy chain class switching. Int. Immunol. 6:491–497 10.1093/intimm/6.4.491 [DOI] [PubMed] [Google Scholar]
- Manis J.P., van der Stoep N., Tian M., Ferrini R., Davidson L., Bottaro A., Alt F.W. 1998. Class switching in B cells lacking 3′ immunoglobulin heavy chain enhancers. J. Exp. Med. 188:1421–1431 10.1084/jem.188.8.1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manis J.P., Tian M., Alt F.W. 2003. Mechanism and control of class-switch recombination. Trends Immunol. 23:31–39 10.1016/S1471-4906(01)02111-1 [DOI] [PubMed] [Google Scholar]
- Morvan C.L., Pinaud E., Decourt C., Cuvillier A., Cogné M. 2003. The immunoglobulin heavy-chain locus hs3b and hs4 3′ enhancers are dispensable for VDJ assembly and somatic hypermutation. Blood. 102:1421–1427 10.1182/blood-2002-12-3827 [DOI] [PubMed] [Google Scholar]
- Muramatsu M., Kinoshita K., Fagarasan S., Yamada S., Shinkai Y., Honjo T. 2000. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 102:553–563 10.1016/S0092-8674(00)00078-7 [DOI] [PubMed] [Google Scholar]
- Pinaud E., Khamlichi A.A., Le Morvan C., Drouet M., Nalesso V., Le Bert M., Cogné M. 2001. Localization of the 3′ IgH locus elements that effect long-distance regulation of class switch recombination. Immunity. 15:187–199 10.1016/S1074-7613(01)00181-9 [DOI] [PubMed] [Google Scholar]
- Revy P., Muto T., Levy Y., Geissmann F., Plebani A., Sanal O., Catalan N., Forveille M., Dufourcq-Labelouse R., Gennery A., et al. 2000. Activation-induced cytidine deaminase (AID) deficiency causes the autosomal recessive form of the Hyper-IgM syndrome (HIGM2). Cell. 102:565–575 10.1016/S0092-8674(00)00079-9 [DOI] [PubMed] [Google Scholar]
- Reynaud S., Delpy L., Fleury L., Dougier H.-L., Sirac C., Cogné M. 2005. Interallelic class switch recombination contributes significantly to class switching in mouse B cells. J. Immunol. 174:6176–6183 [DOI] [PubMed] [Google Scholar]
- Saitoh N., Bell A.C., Recillas-Targa F., West A.G., Simpson M., Pikaart M., Felsenfeld G. 2000. Structural and functional conservation at the boundaries of the chicken β-globin domain. EMBO J. 19:2315–2322 10.1093/emboj/19.10.2315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai E., Bottaro A., Alt F.W. 1999. The Ig heavy chain intronic enhancer core region is necessary and sufficient to promote efficient class switch recombination. Int. Immunol. 11:1709–1713 10.1093/intimm/11.10.1709 [DOI] [PubMed] [Google Scholar]
- Seidl K.J., Manis J.P., Bottaro A., Zhang J., Davidson L., Kisselgof A., Oettgen H., Alt F.W. 1999. Position-dependent inhibition of class-switch recombination by PGK-neor cassettes inserted into the immunoglobulin heavy chain constant region locus. Proc. Natl. Acad. Sci. USA. 96:3000–3005 10.1073/pnas.96.6.3000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X., Eckhardt L.A. 2001. Deletional analyses reveal an essential role for the hs3b/hs4 IgH 3′ enhancer pair in an Ig-secreting but not an earlier-stage B cell line. Int. Immunol. 13:1003–1012 10.1093/intimm/13.8.1003 [DOI] [PubMed] [Google Scholar]
- Stavnezer J. 2000. Molecular processes that regulate class switching. Curr. Top. Microbiol. Immunol. 245:127–168 [DOI] [PubMed] [Google Scholar]
- Stavnezer-Nordgren J., Sirlin S. 1986. Specificity of immunoglobulin heavy chain switch correlates with activity of germline heavy chain genes prior to switching. EMBO J. 5:95–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storb U., Stavnezer J. 2002. Immunoglobulin genes: generating diversity with AID and UNG. Curr. Biol. 12:R725–R727 10.1016/S0960-9822(02)01247-2 [DOI] [PubMed] [Google Scholar]
- Terauchi A., Hayashi K., Kitamura D., Kozono Y., Motoyama N., Azuma T. 2001. A pivotal role for DNase I-sensitive regions 3b and/or 4 in the induction of somatic hypermutation of IgH genes. J. Immunol. 167:811–820 [DOI] [PubMed] [Google Scholar]
- Unniraman S., Schatz D.G. 2007. Strand-biased spreading of mutations during somatic hypermutation. Science. 317:1227–1230 10.1126/science.1145065 [DOI] [PubMed] [Google Scholar]
- Vincent-Fabert C., Truffinet V., Fiancette R., Cogné N., Cogné M., Denizot Y. 2009. Ig synthesis and class switching do not require the presence of the hs4 enhancer in the 3′ IgH regulatory region. J. Immunol. 182:6926–6932 10.4049/jimmunol.0900214 [DOI] [PubMed] [Google Scholar]
- Wuerffel R., Wang L., Grigera F., Manis J., Selsing E., Perlot T., Alt F.W., Cogne M., Pinaud E., Kenter A.L. 2007. S-S synapsis during class switch recombination is promoted by distantly located transcriptional elements and activation-induced deaminase. Immunity. 27:711–722 10.1016/j.immuni.2007.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M.Z., Stavnezer J. 1992. Regulation of transcription of immunoglobulin germ-line γ 1 RNA: analysis of the promoter/enhancer. EMBO J. 11:145–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancopoulos G.D., DePinho R.A., Zimmerman K.A., Lutzker S.G., Rosenberg N., Alt F.W. 1986. Secondary genomic rearrangement events in pre-B cells: VHDJH replacement by a LINE-1 sequence and directed class switching. EMBO J. 5:3259–3266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X.W., Model P., Heintz N. 1997. Homologous recombination based modification in Escherichia coli and germline transmission in transgenic mice of a bacterial artificial chromosome. Nat. Biotechnol. 15:859–865 10.1038/nbt0997-859 [DOI] [PubMed] [Google Scholar]