Abstract
Partial-body biodosimetry is likely to be required after a radiological or nuclear exposure. Clinical signs and symptoms, distribution of dicentrics in circulating blood cells, organ-specific biomarkers, physical signals in teeth and nails all can provide indications of non-homogeneous exposures. Organ specific biomarkers may provide early warning regarding physiological systems at risk after radiation injury. Use of a combination of markers and symptoms will be needed for clinical insights for therapeutic approaches. Analysis of dicentrics, a marker specific for radiation injury, is the “Gold standard” of biodosimetry and can reveal partial-body exposures. Automation of sample processing for dicentric analysis can increase throughput with customization of off-the-shelf technologies for cytogenetic sample processing and information management. Automated analysis of the metaphase spreads is currently limited but improvements are in development. Our efforts bridge the technological gaps to allow the use of dicentric chromosome assay (DCA) for risk-based stratification of mass casualties. This article summarizes current knowledge on partial-body cytogenetic dose assessment synthesizing information leading to the proposal of an approach to triage dose prediction in radiation mass casualties, based on equivalent whole-body doses under partial-body exposure conditions and assesses the validity of using this model. An initial screening using only 20 metaphase spreads per subject can confirm irradiation above 2-Gy. A subsequent increase to 50 metaphases improves dose determination to allow risk stratification for clinical triage. Metaphases evaluated for inhomogeneous distribution of dicentrics can reveal partial-body exposures. We tested the validity of this approach in an in vitro model that simulates partial-body irradiation by mixing irradiated and un-irradiated lymphocytes in various proportions. Our preliminary results support the notion that this approach will be effective under a range of conditions including some partial-body exposures, but may have limitations with low doses or small proportions of irradiated body. Our studies address an important problem in the diagnosis of partial-body irradiation and dose assessment in mass casualties and propose a solution. However, additional work is needed to fully develop and validate the application of DCA to partial-body exposures.
Keywords: Partial-body biodosimetry, cytogenetics, triage, dicentric analysis
INTRODUCTION
1. Background
The current dogma of mitigating a nuclear catastrophe centers on a concept of “no loose nukes”, “no nascent nukes”, and “no new nuclear state”. These policies appear to have minimized the risks of a nuclear weapon detonation involving a large population with significant whole-body exposures. Nevertheless, the risk of nuclear or radiological exposure is still serious, with possible nuclear reactor or power plant accidents and potential terrorism using improvised nuclear devices (IND), radiological dispersal devices (RDD), or radiological exposure devices (RED) (Bader et al. 2008). This shift in the type of radiological or nuclear exposure increases the likelihood of partial-body exposures and the need for development and validation of appropriate assessment tools to improve treatment and to analyze health risks.
Two radiation accidents, Samut Prakaran (Bangkok, Thailand, Feb. 2000) and Cochabamba (Bolivia, April 2002) provide valuable lessons on potential partial-body exposure consequences from RDDs and REDs. In Samut Prakaran, Thailand, several groups of people were accidentally exposed to 60Co gamma-rays from a partially dismantled Gammatron teletherapy source that was sent for recycling as scrap metal. Those who procured the source, junkyard workers, and relatives of these workers, received significant radiation doses; scrap metal collectors received severe localized radiation injuries and burns (IAEA 2002; Jinaratana 2002). Similarly, the Bolivian radiological accident (Cochabamba, April 2002), where a faulty industrial radiography source containing 192Ir was sent in a passenger bus as cargo from Cochabamba to La Paz on a 400 km journey for eight hours exposing three workers, one supervisor, and 33 passengers (IAEA 2004), draws parallels with a potential terrorist use of RED. Because the sources in these accidents were localized, exposures were non-homogeneous.
In cases of unanticipated exposures, it is important to estimate radiation dose rapidly with a focus on assessment of partial-body exposure for immediate medical treatment. To identify the patient cohort suitable for therapeutic intervention, clinical triage is needed to provide approximate dose estimation using biological and clinical endpoints rather than precise dose estimations. New combinatorial approaches for assessment and treatment of partial-body exposures are evolving.
2. Partial-body exposure: current status
In May 2008, a workshop was held at the Armed Forces Radiobiology Research Institute, Bethesda, Maryland, to draw attention to the need for partial-body biodosimetry, to discuss current knowledge, and to identify the gaps to be filled. While organ-specific clinical signs and symptoms can be developed to determine the severity of radiation injury to organ systems at risk, analysis of dicentrics in circulating lymphocytes can provide a good estimate of the percent of total body exposed (Lloyd 1997). However, partial-body biodosimetry based on peripheral blood lymphocytes assumes that (i) exposure was uniform, (ii) exposure time was relatively short, and therefore, mixing of exposed and non-exposed pools is limited. Radiation elicits a stable electron paramagnetic resonance signal in teeth and nails that is independent of other intrinsic biological factors. Markers in skin, teeth and nails can allow assessment of localized exposures to specific areas of the body.
Biomarkers that reflect changes in specific organ systems, such as gut, kidney, lung, skin, and bone marrow are also in development. For example, Flt3 ligand reflects changes in the hematopoietic system, citrulline indicates damage to the digestive tract, and several oxysterols are lipid metabolism and vascular markers. These biomarkers, used in conjunction with radiation cytogenetics and clinical manifestations of radiation injury, can indicate damage to the specific physiological systems in a radiation accident victim (Bertho et al. 2008). Biomarkers can provide information on the distribution of dose as well as the severity of risk to specific organs and might be able to predict delayed effects, which would allow the use of mitigators when available and the preparation of patients for upcoming health consequences.
3. “Gold standard” dicentric assay in partial-body exposures: current status
Dicentic chromosome aberration (DCA) assay, performed using human peripheral blood lymphocytes (HPBL), is the current ““Gold standard”” dose assessment method. The main strength of DCA is that it is radiation-specific (IAEA 2001). Regulatory compliance standards (Voisin et al. 2002; ISO 2004) and a technical manual (IAEA 2001) are already available for conducting the assay. More recently, International Standardization Organization’s (ISO) Workgroup 18 of Subcommittee 2 developed the standard, “Radiation Protection — Performance Criteria for Service Laboratories Performing Cytogenetic Triage for Assessment of Mass Casualties in Radiological and Nuclear Emergencies” (ISO 2008). This standard defines the process and identifies quality control standards for the use of cytogenetic methods to assess radiation dose rapidly. Recently, we determined DCA’s validity and accuracy among five international cytogenetic biodosimetry laboratories in an inter-laboratory comparison study performed according to ISO guidelines. For the laboratories the estimated coefficients of the fitted curves generally were within 99.7% confidence intervals and percent error in estimated doses were ±15% of the actual physical doses (Wilkins et al. 2008).
The DCA is gaining renewed importance for quickly assessing dose to potentially irradiated individuals in the early period after a radiological or nuclear incident, when a rapid determination of dose is required for proper medical management (Lloyd et al. 2000; Prasanna et al. 2003). The utility of the DCA to assess health risks and to guide medical treatment decisions has been demonstrated in several radiation accidents involving mass casualties, such as those in Chernobyl, Goiania, and Tokaimura. Estimated doses using the DCA correlate well with the severity of acute radiation syndrome (Sevan’kaev 2000). In the Chernobyl accident, an approximate dosimetry was achieved by rapid preliminary examination of 50 lymphocyte metaphases per person for several individuals (Pyatkin et al. 1989). More recently, dose estimation was done using the DCA in the Tokaimura criticality accident (Japan), in three severely exposed workers (Hayata et al. 2001) and 43 resident workers (Sasaki et al. 2001).
Lloyd (1997) has shown that the frequency of metaphase spreads without dicentric aberrations can be used to identify patients with partial-body exposure. Since DCA uses circulating blood lymphocytes, radiation-induced cytogenetic damage reflect the average total-body dose, independent of specific regions of the body that have been exposed. However, the overall dicentric frequency following a high dose exposure of a small part of the body can equal the overall frequency after exposure of large portion of the body to a lower dose, but the distribution of dicentrics among cells in metaphase will be different. With uniform whole-body exposures to low-LET radiations, the dicentrics follow a Poisson distribution; with significant partial-body exposures, the distribution is non-Poisson. Two statistical models described in the IAEA technical manual (IAEA 2001), Dolphin’s contaminated Poisson method and Sasaki’s QDR method, can be used to identify partial-body exposures and estimate dose from dicentric analysis.
Lloyd et al. (2000) described in vitro simulation of an accident with mass casualties receiving whole- or partial-body irradiation in the 0- to 8-Gy range. Clinical triage was accomplished by scoring as few as 20 metaphases per subject. However, Lloyd et al. (2000) suggested increasing the analyses to 50 metaphase spreads when there is evidence of partial-body exposures, as indicated by a non-Poisson distribution of dicentrics.
Dose estimation, irrespective of partial-body or whole-body exposure, is imperative to influence treatment and analyze health risks. Dose estimation requires construction of a calibration curve by the maximum likelihood method, a method not available in routine statistical software. Several laboratories have generic programs, which are not especially user-friendly, quality-controlled or widely available. With advances in computer application developments and inter-disciplinary contributions, two software tools, CABAS (Deperas et al. 2007) and Dose Estimate (Ainsbury and Lloyd, in press), now freely available, can construct calibration curves and estimate dose and confidence limits for whole-body as well as partial-body exposures.
4. Laboratory automation
The DCA is a mature technology and likely to find application in radiation mass casualties for diagnostic dose assessment and triage. However, bottlenecks in terms of cytogenetic expertise, processing of blood samples, lengthy sample preparation procedures, and laborious downstream dicentric analysis hamper its application in radiation mass casualties, in its current form. DCA involves two steps: (i) short-term peripheral blood culture for obtaining metaphase spreads on glass microscope slides and (ii) analysis of radiation-induced damage under a microscope by experts. Pursuant to identification by the Office of Science and Technology Policy and the Homeland Security Council that automation is one of the top six research priorities projects (Pellmar and Rockwell 2005), our laboratory focused on DCA’s automation. We performed an industrial work flow analysis for a cytogenetic biodosimetry laboratory. We developed an electronic sample tracking system that uses barcodes to provide unbroken chain-of-custody of information for every sample and increased efficiency, speed, and throughput, while minimizing data transcription errors (Martin et al. 2007). Also, our streamlined efforts to increase sample processing throughput to facilitate analysis via laboratory automation by customization of commercially available off-the-shelf technologies for cytogenetic sample processing and a Laboratory Information Management System (LIMS) to manage increased sample processing capacity and data is described. Our efforts bridge the technological gaps, which allow DCA’s use for risk-based stratification of mass casualties, facilitated by laboratory automation and information management. Strategy for automated high-throughput batch processing of samples in tandem, increasing the laboratory’s capacity to process over 1000 samples per week for preparing slides for cytogenetic analysis in radiation mass casualty event, is also proposed (Prasanna et al. accepted).
While our automated cytogenetic laboratory offers capability to rapidly process samples, downstream metaphase-spread analysis is still challenging until a reliable and “walk away analysis” system is built and validated (which we are pursuing). Automated analysis is currently limited to differentially locating metaphase spreads on microscope slides at best, and computer assisted manual scoring (Schunck et al. 2004, Prasanna et al. 2002). In the interim, these chromosome-aberration analysis throughput challenges can be overcome by (i) a physical transfer of slides to various satellite laboratories or laboratories in the network for manual analysis; our recently completed inter-laboratory comparison study demonstrated this possibility (Wilkins et al. 2008), (ii) alternatively, virtual high-resolution images of metaphase spreads acquired by metaphase finders can be digitally encrypted and transferred via a virtual private network for downstream analyses and assessment (Prasanna et al. accepted). Thus, automation of sample processing is still invaluable to increase cytogenetic analysis throughput.
In radiation mass casualties, an approach of estimating equivalent whole-body doses is likely to be used for risk-based stratification of exposed subjects, independent of whether exposure is uniform whole-body or partial-body. In this context, a triage dose prediction model for assessing equivalent whole-body doses in radiation mass casualties is proposed here. Also, we tested the validity of using this model to assess equivalent whole-body doses after partial-body exposures using a simulated in vitro partial-body irradiation of human blood.
MATERIALS AND METHODS
For the in vitro simulated partial-body irradiation study, blood was drawn from three healthy donors into vacutainers containing sodium heparin as an anticoagulant (BD Biosciences, USA). All donors provided informed consent on a form approved by the Uniformed Services University of the Health Sciences, Human Use Committee, Bethesda, MD, USA.
Dosimetry and irradiation was performed in the bilateral field of AFRRI’s 60Co gamma exposure facility as previously published (Wilkins et al. 2008). Blood was irradiated with 3-Gy and 5-Gy doses, at a dose rate of 0.6 Gy min−1. To simulate partial-body exposures, irradiated blood samples were mixed in various proportions with un-irradiated blood. Peripheral blood lymphocytes were isolated by centrifugation on a density gradient (Histopaque 1077, Sigma) and stimulated to proliferate for 24 hours at 37°C by incubation in bone marrow media (Gibco, USA) pre-mixed with 10 μl mL−1 of phytohemaglutinin (Murex Diagnostics, UK). Afterwards, the cell cycle was arrested by addition of 0.1 μg ml−1 colcemid (Gibco, USA) and incubated at 37°C for additional 24 hours. This established method of harvesting metaphases from lymphocyte cultures as previously described for our laboratory (Wilkins et al. 2008, Moroni et al. 2008) ensures that metaphases are harvested in their first-division cycle for analysis as recommended by the ISO (19238, 2004) for dose estimation. With this procedure, second-division cycle metaphases are not expected, and account for no more than 5% of the total. At completion of the 48 hours culture period, an automated metaphase harvester (Hanabi PII, ADSTEC Technologies, Japan) was used to swell (0.56% potassium chloride), fix (1: 3 acetic acid: methanol solution), and harvest metaphase enriched the cell suspension. The cell suspension was spread on clean glass slides using a metaphase spreader (Hanabi, ADSTEC Technologies, Japan) at 37 °C and 54% relative humidity. Slides were then stained with 4% Giemsa in phoshphate buffered saline for chromosome aberration analysis using light microscopy. Fifty metaphases were analyzed for radiation-induced dicentrics in accordance with IAEA (IAEA 2001) and ISO (ISO 2004) guidelines. DoseEstimate (v. 2) developed by by the Health Protection Agency (Ainsbury and Lloyd, 2009) was used to determine the nature of the distribution and estimate equivalent whole-body doses to dose-blinded samples in the in vitro simulated partial-body exposure experiment. AFRRI’s dose-response calibration curve obtained in the recently concluded inter-laboratory comparison study (Wilkins et al. 2008) was used for developing a triage dose prediction model. This calibration curve was constructed using the Chromosome Aberration Calculation Software (CABAS, v.2) developed by the Swietokryska Academy, Kielce, Poland (Deperas et al. 2007).
RESULTS AND DISCUSSION
Radiation accidents involving high doses are relatively rare. Accidents involving partial-body irradiations with severe complications are more frequent than those with uniform whole-body irradiations. For risk-based stratification, in radiation mass casualties, an approach of estimating equivalent whole-body doses is likely to be used. Therefore, it is necessary to test whether estimation of equivalent whole-body doses under partial-body exposure conditions is valid. We used an in vitro simulated partial-body irradiation model by mixing irradiated and un-irradiated lymphocytes in various proportions for validation. Similar in vitro simulated mixed culture model was previously used in cytogenetic studies (Lloyd et al. 2000, Lloyd et al. 1987, Blakely et al. 1995).
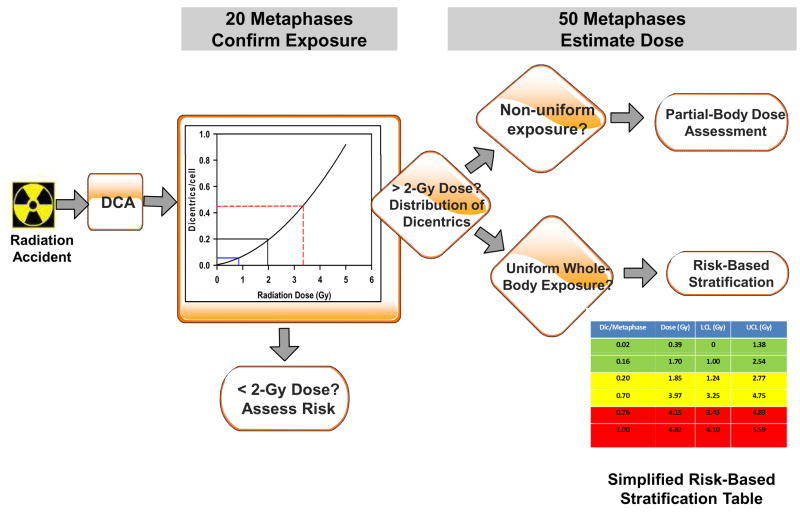
Our laboratory’s approach for using DCA for rapid risk-based stratification of radiation exposed population is proposed in Fig. 1. Accordingly, following receipt of blood samples from a radiation accident, DCA is performed. Initial screening involves confirmation of irradiation above 2-Gy by analysis of only 20 metaphases instead of the typical 500–1000 metaphases scored during routine analysis (IAEA 2001; ISO 2004). Presence of four dicentrics in 20 metaphase spreads (0.2 dicentrics per cell) indicates a potential dose of approximately 2-Gy with a lower confidence limit (LCL) of 0.85 Gy. For cases with confirmed ≥2-Gy dose, analysis is increased to 50 metaphases. Metaphases are evaluated for indications of homogeneous or inhomogeneous distribution of dicentrics (Lloyd et al. 2000; Prasanna et al. 2003); partial-body exposures are indicated by variation from the expected dose-dependent distribution of number of dicentric per cell. In cases of uniform whole-body exposures, samples are categorized based on a risk-based stratification table (simplified table is shown in Fig. 1 for clarity) into “not life-threatening (green)”, “potentially life-threatening (yellow)”, and “significantly life-threatening (red)” cohorts, as indicated (Lloyd et al. 2000, Prasanna et al. 2003) in the embedded table (Fig. 1). Assignment of victims to risk-based stratified cohorts will enable prioritization of samples for medical care and to establish sample scoring priority if additional (500 metaphase spreads) cytogenetic analysis is required. Construction of the calibration curve shown in Fig. 1 is based on an analysis of 1000 metaphase spreads. This calibration curve for our laboratory previously published (Wilkins et al. 2008), was fitted by a linear quadratic model, described by the equation, y = c+αD+βD2 using the CABAS software (Deperas et al. 2007). The coefficients of this fitted curve are α = 0.0293±0.0078, β = 0.0369 ±0.0036, and c = 0.0032 ±0.0016, with standard deviations as indicated. Distribution of dicentrics in cell population at all uniform doses conforms to a Poisson distribution. For non-uniform exposure, the distribution of dicentrics will be over-dispersed. For these cases, the triage dose prediction model will be used to predict the equivalent whole-body dose (Fig. 1).
Figure 1.
Illustration of rapid risk-based stratification of radiation-exposed population by the dicentric assay after whole-body and partial-body exposures. Following receipt of blood samples from a radiation accident, DCA is performed. For confirmation of irradiation, initial screening involves analysis of only 20 metaphase spreads. Radiation dose >2-Gy is confirmed by the presence of four dicentrics in 20 metaphases with a lower confidence limit of 0.85 Gy. The data from previously published work for AFRRI was used for the shown calibration curve (Wilkins et al. 2008). For cases with confirmed ≥2-Gy dose, analysis is increased to 50 metaphases for evaluation of homogeneity of dicentric distribution. Partial-body exposures are indicated by variation from the expected dose-dependent distribution of number of dicentric per cell. In cases of uniform whole-body exposures, samples are categorized based on a risk-based stratification table (simplified table is shown in Fig. 1 for clarity) into “not life-threatening (green)”, “potentially life-threatening (yellow)”, and “significantly life-threatening (red)” cohorts. Calibration curve and risk-based stratification table shown in the algorithm are obtained from actual data published for our laboratory (Wilkins et al. 2008).
Tables 1 and 2 show equivalent whole-body doses estimated after analysis of 50 metaphases under in vitro simulated partial-body irradiation conditions, for 3-Gy and 5-Gy doses, along with LCL and upper confidence limit (UCL) based on exact Poisson error on yield, distribution of dicentrics, dispersion coefficients (variance/mean), u values, and nature of distribution.
Table 1.
Equivalent whole-body dose estimation after simulated in vitro partial-body exposure to a 3-Gy dose. Different proportions of 3-Gy irradiated blood were mixed with unirradiated blood at various fractions. Radiation doses were estimated to the fractions along with lower and upper confidence limits (LCL and UCL) based on previously published (Wilkins et al. 2008) coefficients of AFRRI’s calibration curve using DoseEstimate software (v.2, Ainsbury and Lloyd, 2009). Determination of LCL and UCL was based on exact Poisson error on yield. Dispersion index and u values were also calculated using the DoseEstimate software (v.2).
| Irradiated fraction (%) | Expected dose(Gy) | Estimated dose(Gy, LCL, UCL) | Distribution of dicentrics | Dispersion coefficient ± S.E. | u | Poisson? | ||
|---|---|---|---|---|---|---|---|---|
| D0 | D1 | D2 | ||||||
| 25 | 0.75 | 0.64 ± 0.35 (0.04, 1.54) | 51 | 2 | - | 0.98±0.14 | −0.139 | yes |
| 50 | 1.5 | 1.99 ± 0.34 (1.33, 2.65) | 42 | 9 | 1 | 0.99±0.19 | −0.057 | yes |
| 75 | 2.25 | 2.25 ± 0.34 (1.56, 3.08) | 39 | 9 | 2 | 1.07±0.19 | 0.356 | yes |
| 100 | 3.0 | 3.21 ± 0.31 (2.50, 4.01) | 30 | 16 | 4 | 0.87±0.20 | −0.654 | yes |
Table 2.
Equivalent whole-body dose estimation after simulated in vitro partial-body exposure to a 5-Gy dose. Different proportions of 5-Gy irradiated blood were mixed with unirradiated blood at various fractions. Radiation doses were estimated to the fractions along with lower and upper confidence limits (LCL and UCL) based on the previously published (Wilkins et al. 2008) co-efficients of AFRRI’s calibration curve using DoseEstimate software (v.2, Ainsbury and Lloyd, 2009). Determination of LCL and UCL was based on exact Poisson error on yield. Dispersion index and u values were also calculated using the DoseEstimate software (v.2).
| Irradiated Fraction (%) | Expected dose (Gy) | Estimated dose (Gy, LCL, UCL) | Distribution of dicentrics | Dispersion Co-efficient | u | Poisson? | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| D0 | D1 | D2 | D3 | D4 | D5 | ||||||
| 25 | 1.25 | 1.37±0.35 (0.16, 9.57) | 50 | 1 | 0 | 0 | 0 | 1 | 4.30±0.18 | 18.26 | N |
| 50 | 2.50 | 0.95±0.33 (0.25, 2.61) | 56 | 2 | 1 | - | - | - | 1.46±0.16 | 2.84 | N |
| 75 | 3.75 | 3.18±0.30 (1.10, 8.99) | 40 | 5 | 5 | 2 | 4 | - | 2.27±0.19 | 6.76 | N |
| 100 | 5.0 | 4.76±0.22 (4.07, 5.51) | 21 | 17 | 10 | 3 | - | - | 0.94±0.19 | −0.29 | Y |
Expected physical doses, derived from the percent irradiated volume, fell within the 95% LCL and upper confidence limits (UCL) of estimated equivalent whole-body doses for all fractions at both radiation doses.. However, in one case (5-Gy, 50% irradiated fraction) the underestimation was quite large (0.95-Gy estimated dose versus 2.5-Gy expected dose, LCL 0.25-Gy and UCL 2.61-Gy) and placed the victim in the ‘wrong’ category (“not life-threatening” instead of “potentially life-threatening”) using the triage dose prediction model. For one additional case, 3-Gy, 50% irradiated sample, although the estimated whole-body equivalent dose was within the 95% LCL and UCL of expected dose, the value was borderline between “not-life threatening” and “potentially life threatening” categories. Although our results are preliminary, we propose that in cases of non-uniform exposures, estimation of equivalent whole-body doses may be considered for rapid risk-based stratification. As our results are preliminary, further studies involving larger number of samples are warranted, before integration of this partial-body dose assessment approach for treatment.
Our observations on the nature of dicentric distributions at 3-Gy and 5-Gy irradiated fractions, show a Poisson distribution at the lower dose fractions and non-Poisson distribution at the higher dose fractions, as revealed by the dispersion co-efficients and u values. It is evident that at a relatively lower dose of 3-Gy, analysis 50 metaphases is inadequate to precisely identify partial-body exposures. However, precise identification of partial-body exposures is dependent on, in addition to number of metaphases analyzed, at least three other factors: (i) calibration curve, (ii) fraction of the body irradiated, and (iii) dose to the irradiated fraction. From our 5-Gy data (Table 2), it is evident that fractions of 25–75% are identifiable as partial-body irradiations, for the number of metaphases analyzed, based on non-Poisson distribution. Therefore, it is imperative under partial-body exposure conditions, not only to estimate equivalent whole-body doses, but also consider the nature of dicentric distributions for clinical management, as previously suggested by Lloyd (1997). Early identification of partial-body exposures is essential in order to determine whether cytokine therapy is beneficial.
Estimates of whole-body equivalent doses using the triage dose prediction model was accurate in all cases. Only in one case the true dose was seriously underestimated, placing the victim in the “wrong category”. Lloyd et al. (2000) has previously shown that for simulated partial-body irradiation, scoring of 50 metaphases provided satisfactory agreement between the true and the estimated dose and the percentage of irradiated fraction, at 3-Gy and above. Our preliminary results involved relatively a small number of samples and need to be further confirmed using a larger sample size, but they appear to support the notion that detection of partial-body irradiation, particularly at higher doses, is possible with only 50 metaphases. However, accurate estimation of the fraction of irradiated body could be satisfactorily determined only for large fraction (≥25%) and doses >3-Gy, using the Qdr or the Dolphin methods. The triage dose prediction model is an initial step towards rapidly assigning victims to risk-based categories. Additional testing and assessment of probability for true-positive and true-negative cases are required.
CONCLUSIONS AND FUTURE DIRECTIONS
1. Mass casualty biodosimetry
In the event of a mass-casualty radiological or nuclear event, biodosimetry will be important for both triage and clinical care decisions. Although most research efforts on biomarkers focus on whole-body irradiation, partial-body exposures are actually more likely in real cases. Non-homogeneous exposures can have a major impact on clinical outcomes. Treatment will depend on knowledge of an individual’s injury due to the absorbed dose and dose distribution. Treatment based on whole-body dose assessments may not be appropriate for partial-body exposures, especially when local doses are high. Rapid diagnosis will be essential to protect organ systems that are seriously impacted by radiation including bone marrow, the gastrointestinal tract and skin.
2. Dicentric analysis
Since dicentric analysis is the “Gold standard”, it is very likely to be used for dose assessments of radiation injuries in mass casualties. Studies (Lloyd et al. 2000) demonstrate that analysis of only 20–50 metaphase spreads can provide sufficient information for rapid triage of casualties. We have shown that this approach will be effective under a range of conditions including some partial-body exposures, but may not accurately determine the percentage of irradiated fraction for low-dose exposure or for small proportion of irradiated body. The development of automated processing and analysis will allow broader application of the DCA by laboratories without extensive expertise. Although applications to partial-body exposures have been well established, the approaches require additional validation. Studies with partial-body exposures in animal models can define the strengths and limitations of the approach, including assessment of the impact of patterns of non-homogenous exposures and the impact of confounding factors such as protective drugs. Animal models also provide the opportunity to learn how to integrate other biomarkers and clinical symptoms with DCA under controlled conditions. This would provide us with the knowledge of how to treat casualties in the event of a radiological or nuclear exposure. Patient populations also provide an opportunity to refine our understanding of how to apply dicentric analysis for partial-body exposures in a diverse population.
3. Partial-body biodosimetry
Many approaches to partial-body biodosimetry are available, each with their own advantages and disadvantages. Clinical decisions will require multiple, integrated endpoints. Using all the approaches (biomarkers, cytogenetics, clinical signs and symptoms and cutaneous and peripheral signals) might overcome the gaps inherent in each technique. No single measurement will be adequate to provide a reliable diagnosis or to predict organ involvement.
Inter-individual variability, the quality of radiation and dose rate can affect the appearance of biomarkers. Differences in health status, diets, pharmaceutical intake, environmental exposures, as well as differences in gender and age can modulate an individual’s response to radiation and the results of biodosimetry assays. In addition, combined exposures of radiation with chemical toxicants or traumatic injuries can further confound the results of dose assessment assays.
Partial-body and non-homogeneous exposures are further complicated by the existence of multi-organ interactions. Exposure of one organ system may influence the response of another organ system even if it is not in the original field of exposure (Moulder and Cohen 2005; Van der Meeren et al. 2005). As we increase our understanding of the underlying mechanisms of radiation injury, indicators regarding the progression of the syndrome might become more evident.
Acknowledgments
Financial support was provided by an interagency agreement (Y1-AI-5045-04) between the National Institute of Allergies and Infectious Diseases, NIH, USA, and the Armed Forces Radiobiology Research Institute, Uniformed Services University of the Health Sciences, USA. Special thanks to Dr. V. Nagy (AFRRI) for dosimetry and U. Subramanian and K. Krasnopolsky, for technical assistance.
References
- Ainsbury E, Lloyd DC. Dose estimation for radiation biodosimetry. Health Phys. doi: 10.1097/01.HP.0000346305.84577.b4. (in press) [DOI] [PubMed] [Google Scholar]
- Bader J, Nemhauser J, Coleman NC, Chang HF, Mashayekhi B, Vanderwagen W, Simon S, Knebel A, Hrdina C, Phillips SJ. [Accessed 26 Oct. 2008];Radiation Event Medical Management [online] Available at: http://www.remm.nlm.gov.
- Bertho J, Roy L, Souidi M, Benderitter M, Gueguen Y, Lataillade JJ, Prat M, Fagot T, De Revel T, Gourmelon P. New biological indicators to evaluate and monitor radiation-induced damage: an accident case report. Radiat Res. 2008;169:543–550. doi: 10.1667/RR1259.1. [DOI] [PubMed] [Google Scholar]
- Blakely WF, Prasanna PGS, Kolanko CJ, Pyle MD, Mosbrook DM, Loats AS, Rippeon TL, Loats H. Application of the premature chromosome condensation assay in simulated partial-body radiation exposures: evaluation of the use of an automated metaphase-finder. Stem Cells. 1995;13(Suppl 1):223–30. [PubMed] [Google Scholar]
- Deperas J, Szluinska M, Deperas-Kaminska M, Edwards AA, Lloyd DC, Lindholm C, Romm H, Roy L, Moss R, Morand J, Wojcik A. CABAS - a freely available PC program for fitting calibration curves in chromosome aberration dosimetry. Radiat Prot Dosim. 1997;124:115–123. doi: 10.1093/rpd/ncm137. [DOI] [PubMed] [Google Scholar]
- Hayata I, Kanda R, Minamihisamatsu M, Furukawa M, Sasaki MS. Cytognetic dose estimation for 3 severely exposed patients in the JCO criticality accident in Tokaimura. J Radiat Res. 2001;42:S149–155. doi: 10.1269/jrr.42.s149. [DOI] [PubMed] [Google Scholar]
- International Atomic Energy Agency. Cytogenetic analysis for radiation dose assessment: A manual. Vienna, Austria: 2001. Technical Report 405. [Google Scholar]
- International Atomic Energy Agency. The radiological accident in Samut Prakaran. IAEA; Vienna, Austria: 2002. [Google Scholar]
- International Atomic Energy Agency. The Radiological Accident in Cochabamba. IAEA; Vienna, Austria: 2004. [Google Scholar]
- International Standardization Organization. Radiation protection - Performance criteria for service laboratories performing biological dosimetry by cytogenetics. Geneva: ISO Office; 2004. ISO19238. [Google Scholar]
- International Standardization Organization. Radiation protection - Performance criteria for service laboratories performing cytogenetic triage for assessment of mass casualties in radiological and nuclear emergencies - General principles. Geneva: ISO Office; 2008. ISO 21243. [Google Scholar]
- Jinaratana V. The Medical Basis for Accident Preparedness: The Clinical Care of Victims. In: Ricks RC, Berger ME, O’Hara FM Jr, editors. The radiological accident in Thailand. Boca Raton, FL: Parthenon Publishing; 2002. pp. 283–301. [Google Scholar]
- Lloyd DC. Chromosomal analysis to assess radiation dose. Stem Cells. 1997;15(Suppl 2):195–201. doi: 10.1002/stem.5530150727. [DOI] [PubMed] [Google Scholar]
- Lloyd DC, Edwards AA, Moquet JE, Guerreo-Carbajal YC. The role of cytogenetics in early triage of radiation casualties. Applied Radiation Isotopes. 2000;52:1107–1112. doi: 10.1016/s0969-8043(00)00054-3. [DOI] [PubMed] [Google Scholar]
- Lloyd DC, Edwards AA, Prosser JS, Barjaktarovic N, Brown JK, Horvat D, Ismail SR, Koteles GE, Almassy Z, Krepinsky A, Kucerova M, Littlefield LG, Mukherjee U, Natarajan AT, Sasaki MS. A collaborative exercise on cytogenetic dosimetry for simulated whole and partial-body accidental irradiation. Mutat Res. 1987;179:197–208. doi: 10.1016/0027-5107(87)90310-1. [DOI] [PubMed] [Google Scholar]
- Martin PR, Berdychevski RE, Subramanian U, Blakely WF, Prasanna PGS. Sample tracking in an automated cytogenetic biodosimetry laboratory for radiation mass casualties. Radiat Measurements. 2007;42:1119–1124. doi: 10.1016/j.radmeas.2007.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroni MM, Krasnopolsky K, Subramanian U, Martin PR, Doherty KM, Prasanna PGS. Does cell culture type and blood transport temperature affect dicentric yield and radiation dose assessment? [Accessed 4 Feb. 2009];J Med CBR Def. 2008 Oct;6 Available at: http://www.jmedcbr.org/issue_0601/Prasanna/Prasanna_10_08.html.
- Moulder JE, Cohen EP. Radiation induced multi-organ involvement and failure: the contribution of radiation effects on the renal system. BJR Suppl. 2005;27:82–88. [Google Scholar]
- Pellmar TC, Rockwell S The Radiological/Nuclear Threat Countermeasures Working Group. Priority list of research areas for radiological nuclear threat countermeasures. Radiat Res. 2005;163:115–123. doi: 10.1667/rr3283. [DOI] [PubMed] [Google Scholar]
- Prasanna PGS, Ramakumar A, Moroni M, Martin PR, Subramanian U, Krasnopolsky K, Pathak R. High throughput sample processing and laboratory information management for cytogenetic biodosimetry. Int J Radiat Biol. (accepted) [Google Scholar]
- Prasanna PGS, Loats H, Gerstenberg HM, Torres BN, Shehata CW, Duffy KL, Floura RS, Khusen AW, Jackson WE, Blakely WF. Application of a metaphase finder system. Fort Belvoir, VA, USA: Defense Technical Information Center, Fort Belvoir, VA; 2002. AFRRI’s gamma-ray, x-ray, and fission-neutron calibration curves for the dicentric assay. [Google Scholar]
- Prasanna PGS, Subramanian U, Greenhill RG, Jacocks JM, Jackson WE, Blakely WF. Radiation Safety Aspects of Homeland Security and Emergency Response. San Antonio, TX: Health Physics Society; 2003. Cytogenetic biodosimetry strategy for potential radiation mass casualties. (cdrom publication) [Google Scholar]
- Pyatkin EK, Nugis VY, Chrikov AA. Absorbed dose estimation according to the results of cytogenetic investigations of lymphocyte cultures of persons who suffered in the accident at Chernobyl atomic power station. Radiation Medicine. 1989;4:52–58. [Google Scholar]
- Sasaki MS, Hayata I, Kamada N, Kodama N, Kodama S. Chromosome aberration analysis in persons exposed to low-level radiation from JCO criticality accident in Tokaimura. J Radiat Res. 2001;42:S107–116. doi: 10.1269/jrr.42.s107. [DOI] [PubMed] [Google Scholar]
- Schunck C, Johannes T, Varga D, Lorch T, Plesch A. New developments in automated cytogenetic imaging: unattended scoring of dicentric chromosomes, micronuclei, single cell gel electrophoresis, and fluorescence signals. Cytogenet Genome Res. 2004;104:383–389. doi: 10.1159/000077520. [DOI] [PubMed] [Google Scholar]
- Sevan’kaev AV. Results of cytogenetic studies of the consequences of the Chernobyl accident. Radiat Biol Radioecol. 2000;40:589–95. [PubMed] [Google Scholar]
- Van der Meeren A, Monti P, Vandamme M, Squiban C, Wysocki J, Griffiths N. Abdominal radiation exposure elicits inflammatory responses and abscopal effects in the lungs of mice. Radiat Res. 2005;163:144–52. doi: 10.1667/rr3293. [DOI] [PubMed] [Google Scholar]
- Voisin P, Barquinero F, Blakely WF, Lindholm C, Lloyd DC, Luccioni C, Miller S, Palitti F, Prasanna PGS, Stephan G, Thierens H, Turai I, Wilkinson D, Wojcik A. Towards a standardization of biological dosimetry by cytogenetics. Cell Mol Biol. 2002;48:501–504. [PubMed] [Google Scholar]
- Wilkins RC, Romm H, Kao TC, Awa AA, Yoshida MA, Livingston GK, Jenkins MS, Oestreicher U, Pellmar TC, Prasanna PGS. Interlaboratory comparison of the dicentric chromosome assay for radiation biodosimetry in mass casualty events. Radiat Res. 2008;169:551–560. doi: 10.1667/RR1272.1. [DOI] [PubMed] [Google Scholar]