Abstract
Purpose
Our previous studies in a canine animal model demonstrated that the flexor tendon-to-bone insertion site has a poor capacity to heal. Magnesium based adhesives have the potential to improve tendon-to-bone healing. Therefore, we hypothesized that magnesium based bone adhesive (MBA) will improve the tendon-to-bone biomechanical properties initially and in the early period after repair.
Methods
Flexor digitorum profundus tendons were injured and repaired into bone tunnels in the distal phalanges of dogs. The bone tunnels were either filled with MBA prior to completing the repair or left empty (CTL). Histologic appearance, tensile properties, range of motion, and bone density were examined at time zero and 21 days after the repair.
Results
There was no histologic evidence of acute inflammation. There appeared to be more mast cells in the MBA group than in the CTL group. Chronic inflammatory infiltrate and fibrosis was slightly higher in the MBA group compared to the CTL group. Tensile properties at time zero were significantly higher in the MBA group compared to the CTL group. However, tensile properties were significantly lower in the MBA group compared to the CTL group at 21 days. Range of motion and bone density were significantly lower in the MBA and CTL groups compared to normal (i.e., uninjured) at 21 days; no differences were seen when comparing MBA to CTL.
Conclusions
We found that the initial biomechanical properties of flexor tendon-to-bone repairs can be improved with MBA. However, MBA use in vivo led to a decrease in the biomechanical properties of the repair. There was no effect of MBA on bone density or range of motion in the early period after repair. Our histologic analysis suggests that the poor healing in the MBA group may have been due to an allergic response or to increased chronic inflammation due to the foreign material.
Keywords: flexor tendon, insertion site, enthesis, animal model, hand
INTRODUCTION
Successful tendon-to-bone healing is critical for functional repair of a number of clinical conditions. Zone 1 flexor tendon injuries require repair of the tendon to the distal phalanx. Our previous studies in a clinically relevant canine animal model demonstrated that the flexor tendon-to-bone insertion site has a poor capacity to heal, with bone mineral density and biomechanical properties declining over the first 21 days of healing (1-4). In the rotator cuff, full thickness tears most commonly result from avulsion of the cuff tendon from the greater tuberosity, thereby requiring tendon-to-bone repair (5). Despite improvements in surgical technique and rehabilitation, tendon-to-bone repair techniques for the cuff have been plagued by a high rate of recurrent tears (ranging from 20 - 94%) (6,7). Typical anterior cruciate ligament reconstruction techniques use tendon grafts that must heal in tibial and femoral bone tunnels (8,9). Poor tendon-to-bone healing can lead to graft loosening and knee instability. Previous attempts to improve tendon-to-bone healing, including improved suture techniques (10,11), surface vs. tunnel repair (2,12), and biologics (13,14), have not been entirely successful. These clinical and experimental findings motivate our search for new treatment modalities for tendon-to-bone insertion repair. Our overall goal is to improve the properties of the healing tendon-to-bone insertion in the first three weeks of healing, a critical time interval where the repair is at risk for gap formation and failure.
Recent studies in other animal models have shown that magnesium-based adhesives have the potential to improve tendon-to-bone healing (15,16). The adhesive may improve the initial biomechanical properties of the repair and promote bone formation during healing. Based on these studies, we hypothesized that a magnesium-based bone adhesive, applied at the time of surgical repair in our canine flexor tendon-to-bone injury and repair model, will improve the tensile biomechanical of the repair initially and in the early period after repair.
MATERIALS AND METHODS
Animal model
Flexor digitorum profundus (FDP) tendons were injured and repaired into bone tunnels in the distal phalanx in canines using methods described below. Twenty six FDP tendon repairs were performed in cadaver dogs to determine time zero properties. Twenty four additional repairs were performed in vivo in twelve dogs. Animals were sacrificed 21 days post-surgery. Animal care complied with the guidelines of the Animal Studies Committee at the host institution, with the National Institutes of Health, and with all national laws on the care and use of laboratory animals.
Each dog had the second and fifth FDP tendons of their right forelimbs injured and repaired (17). The FDP tendon was approached via a v-shaped lateral incision and transected sharply at its insertion. The cut tendon end was grasped using a 4-strand modified Becker stitch (the ‘core’ suture; Supramid 3-0). A 5mm deep × 3mm diameter tunnel was drilled at the volar aspect of the distal phalanx. Two needle holes for the core suture were drilled through the distal phalanx out through the nail. Two further parallel needle holes were drilled through the side of the distal phalanx (medial side for the second digit and lateral side for the fifth digit) which communicates with the tunnel for the ‘supplemental’ suture (18). Each needle was placed retrograde through the hole and delivered proximally out of the tunnel. A 1mm diameter stainless steel bead was sutured to the end of the tendon to mark the position of the tendon in the bone tunnel. Beads were prefabricated with sutures passed through a small central drill hole. The bead was secured by suturing it to the end of the tendon. The core sutures were passed through the needle holes and left untied over the dorsal surface of the toenail.
To examine the effect of the magnesium-based adhesive (MBA) (Osteocrete; Bone Solutions, Inc.), the bone tunnels were either filled with approximately 0.5mL of MBA prior to completing the repair (MBA group) or left empty (CTL group). MBA was prepared following manufacturer's instructions. Twelve grams of MBA were mixed with 3mL modified phosphate buffered saline in a plastic specimen cup. The mixture was manually stirred for 2 minutes. The material was then transferred into a 3mL syringe and injected into the bone tunnel (no needle was used). Approximately 0.5mL of the mixture was injected into each tunnel. The core sutures were then tied over the dorsal surface of the toenail, pulling the tendon stump into the bone tunnel. MBA which was leaked out of the tunnel was cleaned using gauze. A supplemental suture (Supramid 3-0) was added through the medial or lateral needle holes, grasping the tendon at the tunnel entrance with a simple stitch and completing the repair.
Post-operatively, forelimbs were placed in fiberglass casts at 70° wrist flexion and subjected to passive motion rehabilitation as described previously (2,3). Briefly, the volar portion of the cast was removed daily to allow for passive motion rehabilitation of the wrist and digits. The digits were passively flexed and extended with the wrist flexed at 70° for five minutes each day. Our previous studies on flexor tendon repair into a bone tunnel indicated that the structural properties declined over the first 21 days of healing, leaving the repair at risk for failure (2,3). We therefore chose to study the effect of loading on the early tendon-to-bone healing response (i.e., at 21 days).
Gap formation
Gap formation (N=7 per group) was quantified at sacrifice by determining the positions of the radio-opaque stainless steel beads (1mm in diameter) which were implanted at the time of surgery. In order to determine the initial position of the bead (i.e., at the time of surgery), calipers were used to measure the tunnel depth, the length of the Becker suture, and the length of the Becker suture which remained outside of the tunnel after the repair was completed. Based on repeated trials made on cadaver tendon-to-bone repairs, the precision of the tunnel measurement was 5.1% and the precision of the suture length measurement was 1.4%. At sacrifice, a radiograph of each specimen was taken (including a calibration bar to determine magnification) and the position of the bead was determined using image analysis software (Scion Image, Frederick, MD). “Gap” was calculated as the difference between the position of the bead at the time of surgery and the position of the bead at sacrifice relative to the tunnel entrance. These specimens were then evaluated biomechanically. Based on visual inspection after biomechanical testing and prior to histologic processing it was confirmed that all markers remained attached to the tendon ends.
Biomechanics
Group sample sizes for biomechanics outcomes were chosen a priori based on prior results (1,3,17) with the objective of being able to detect a difference of 20% in maximum force and a difference of 30% in rigidity with a significance level of 0.05 and a power of 0.8.
Range of motion (N=8 per group)- The second and fifth digits were disarticulated at the metacarpophalangeal joints. Specimens were blinded to group and timepoint before biomechanical assessment. Functional properties were assessed using a motion analysis system (PC Reflex, Qualisys) and a passive motion protocol described below (2,3). One reflective marker (2 mm diameter) was glued to the proximal end of the flexor tendon, a pair of reflective markers were pinned to the middle phalanx, and a second pair was pinned to the toe nail of each digit (which is attached to the distal phalanx). A pair of reflective ‘reference’ markers was attached to the holding fixture. The proximal phalanx was held in a vertical orientation and the coordinates of the markers were sampled first with the digit in a flexed position and then in an extended position. Flexion was produced by suspending a 1.5 N weight from the proximal stump of the flexor tendon and a 0.15 N counterweight from the extensor tendon. For extension, the 1.5 N weight was suspended from the extensor tendon and the 0.15 N weight from the flexor tendon. Based on differences between the flexed and extended positions two parameters were computed: range of motion of the distal interphalangeal (DIP) joint and tendon excursion. Based on our previous repeated trials, the precision of these measurements was 7% for flexion of the distal interphalangeal joint and 13% for tendon excursion (19).
Tensile properties (N=12-14 for time zero groups, N=7 for injury and repair groups)-After range of motion testing, tendon-bone specimens were pulled in uniaxial tension until failure. Without interrupting the repair site, specimens were isolated from the digits that were operated on and tested in tension with a material testing machine (8500R; Instron Corp); the distal phalanx was held by a rigid clamp and the proximal end of the tendon held by a soft-tissue clamp (1-3). A 1N preload was applied and the tendon was loaded at a rate of 0.5%/s of the specimen length per second until failure. This slow rate of loading ensured quasi-static testing conditions and is consistent with our previous biomechanical studies (1-3). Force-elongation data were recorded using a computerized data acquisition system (Labview 7.0; National Instruments) and marker displacement data were recorded using a motion analysis system (PC-Reflex; Qualisys). The two markers, spaced ~10 mm apart and spanning the repair site, were tracked by the motion analysis system to determine repair-site elongation. From the force-elongation curves we determined the structural properties ultimate (i.e., maximum) load and repair-site stiffness (i.e., the slope of the linear portion of the force-elongation curve). Repair-site elongation was then normalized to compute the average repair-site strain, based on the initial marker distance. From the force-strain curves we determined the normalized properties repair-site rigidity (i.e., the slope of the linear portion of the force-strain curve), ultimate (i.e., maximum) strain, and repair-site strain at 20 N force (a physiologically relevant level of load).
Bone densitometry
Bone mineral density of the distal phalanges (N=9 per group) was assessed after biomechanical testing using peripheral quantitative computed tomography (pQCT; XCT Research M, Stratec) as described previously (1-3). Two transverse slices were obtained 1 and 2 mm distal to the FDP insertion site (0.5 mm thickness; 0.07 mm voxel size). Using the manufacturer's software (CALCBD routine) and a threshold of 280 mg/cm3 to segment bone from external soft tissues, we determined average bone mineral density (BMD, mg/cm3) for the two slices. The BMD determined by this method represents an “apparent” value, as the volume of interest is the entire cross-section of the distal phalanx, including the cortical and trabecular bone and the bone tunnel. Specimens were blinded to group before bone densitometry analysis.
Histology
After biomechanical testing, specimens for tendon histology (N=8 per group) were fixed in 4% paraformaldehyde for 24 h and embedded in paraffin. After bone densitometry, specimens for bone histology were fixed in 10% neutral buffered formalin for 48 h and embedded in polymethylmethacrylate. Longitudinal sections (5-8μm) were cut in the mid-sagittal plane and stained with Hematoxylin and Eosin, Giemsa, or Von Kossa. Sections were blinded and evaluated under bright-field illumination by a pathologist (NH) for the level of inflammation and numbers of inflammatory cells (mononuclear cells and polymorphonuclear leukocytes), the number of mast cells, the level of vascular proliferation, and the amount of mineral. Sections were graded for each of the outcomes as “-” (negative), “+” (low levels), “++” (intermediate levels) and “+++” (high levels). Histologic section grades were combined to produce a representative grade for each group.
Statistics
Data were first tested for normality using the Shapiro-Wilk test. To take advantage of the paired nature of our data and test the main hypotheses of our study, the ‘MBA’ group was compared to the ‘CTL’ group 21 days after repair using a paired t-test. In order to compare groups at time zero and to examine changes over time (where data are not paired), we performed an analysis of variance (ANOVA) followed by a Fisher's least squares differences post-hoc test. A p value less than 0.05 was deemed statistically significant. Any outliers were identified by two standard deviations from the mean and according to Studentized Residuals and Stem-and-Leaf analysis. All statistical analyses were performed using Systat 12 (Systat Software, Inc.).
RESULTS
Gross observation and histology
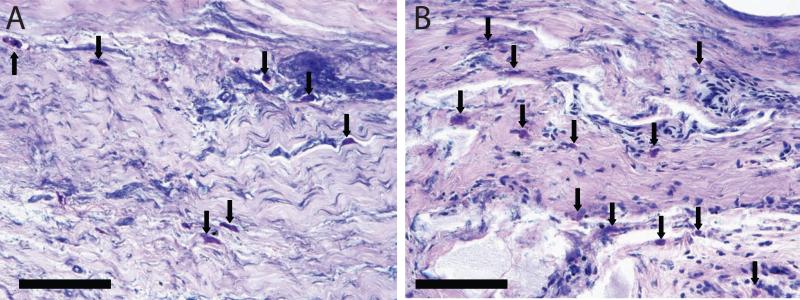
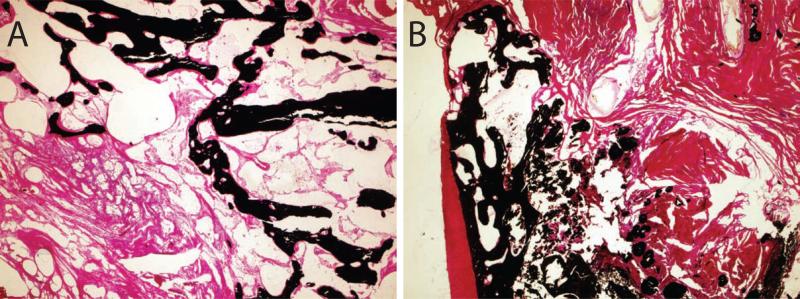
Gross examination of the skin incisions post-operatively revealed some dehiscence, effusion, and swelling in 7/12 of the animals within two weeks. These observations were generally more severe at the MBA treated sites. When examining tendon histology, there was no evidence of acute inflammation in any tendon sample (Table 1). There were more mast cells in the MBA group than in the CTL group (Table 1, Figure 1), although with a high variability from section to section. Analysis of mast cells in the bone histology revealed an average 19 mast cells per high power field in the CTL group compared to 25 mast cells per high powered field in the MBA group. Chronic inflammatory infiltrate and fibrosis was slightly higher in the MBA group than in the CTL group. There were no apparent differences in mineral content when comparing the MBA group to the CTL group (Figure 2). There was no evidence of acute inflammation in any bone histologic section. There was no evidence of regeneration of a four zone interface at the healing tendon-to-bone insertion (Figure 1).
Table 1.
Tendon histology grading results (− negative, + low levels, ++ intermediate levels, +++ high levels).
| Group | PMN | MONO | MAST | CALC | FOREIGN | FIBROSIS |
|---|---|---|---|---|---|---|
| CTL | − | + | + | − | + | − |
| MBA | − | ++ | +++ | + | ++ | + |
Key
PMN polymorphonuclear leukocytes
MONO mononuclear cells (histiocytes, lymphocytes, and plasma cells)
MAST mast cells
CALC calcification
FOREIGN foreign body
FIBROSIS fibrosis
Figure 1.
Mast cells (arrows) were seen in both the CTL (A) and MBA (B) groups. (Giemsa stain, scale bar 100μm, 40x objective).
Figure 2.
The amount of mineralized tissue (black stain) was similar in the CTL (A) and MBA (B) groups. (Von Kossa stain, scale bar 1mm, 4x objective).
Biomechanical Properties
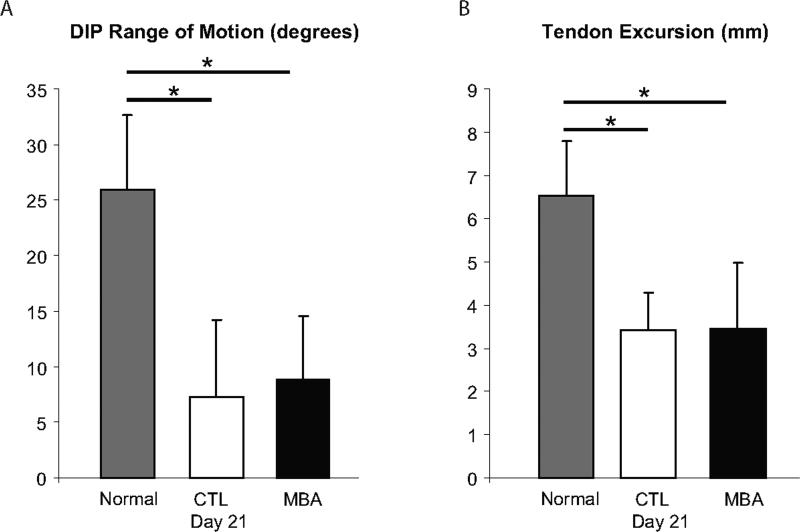
The tensile biomechanical properties stiffness and rigidity were significantly higher in the MBA group than in the CTL group at time zero (Figure 3). However, the biomechanical properties maximum load and stiffness were significantly lower in the MBA group than in the CTL group at 21 days. Tensile properties decreased significantly over time in both groups. Range of motion was significantly lower in the MBA and CTL groups than in normal tendons at 21 days (Figure 4); no difference was seen when comparing MBA to CTL.
Figure 3.
Tensile biomechanical properties (A: maximum load, B: stiffness, C: rigidity) were significantly higher in the MBA group than in the CTL group at time zero. However, biomechanical properties were significantly lower in the MBA group than in the CTL group at 21 days. Tensile properties decreased over time in both groups. (* p < 0.05, # p = 0.10).
Figure 4.
Range of motion (A: DIP angular rotation, B: FDP tendon excursion) was significantly lower in the MBA and CTL groups than in normal tendons at 21 days; no difference was seen when comparing MBA to CTL. (* p < 0.05).
Bone mineral density
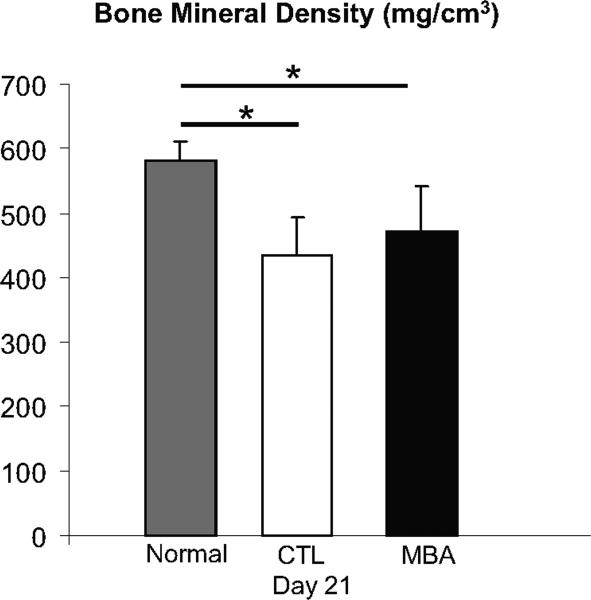
Bone density was significantly lower in the MBA and CTL groups than in normal tendons at 21 days (Figure 5); no difference was seen when comparing MBA to CTL.
Figure 5.
Bone density was significantly lower in the MBA and CTL groups than in normal tendons at 21 days (Figure 5); no difference was seen when comparing MBA to CTL. (* p < 0.05).
DISCUSSION
We found that the initial biomechanical properties of flexor tendon-to-bone surgical repairs can be improved with use of MBA. However, MBA use in vivo led to a decrease in the biomechanical properties of the tendon-to-bone repair. There was no effect of MBA on bone density or range of motion in the early period after repair. Our histologic analysis suggests that the poor healing response in the MBA group may have been due to an allergic response or to increased mast cell mediated chronic inflammation from the foreign material. While the MBA tested in this study improved the initial properties of the repair, further in vivo study is necessary to determine the cause of its negative effect on early healing.
Consistent with previous reports of tendon-to-bone healing (1-3,13,17,20-23), a four zone interface was not regenerated at the healing insertion. Instead, a disorganized fibrous zone formed at the attachment of tendon to bone in both the control group and the MBA group. This is consisted with studies examining rotator cuff tendon-to-bone healing (20-22), tendon graft healing in a tunnel for anterior cruciate ligament reconstruction (13,23), and flexor tendon-to-bone healing (1-3,17).
There are two prior studies examining the effect of magnesium-based adhesives for bone and tendon-to-bone repair (15,16). Gullota et al examined the effect of MBA on tendon-to-bone healing in a rabbit anterior cruciate ligament reconstruction model (16). They showed improved histologic appearance of the tendon-to-bone interface and increased bone mineral density after six weeks of healing. It was unclear, however, whether the increased bone mineral density was due to the MBA itself, which is radiopaque, due to increased bone formation, or due to decreased bone resorption. Biomechanically, this study showed improvements in one biomechanical measure (failure load) at one timepoint. Time zero biomechanical properties were not reported. Presumably, the adhesive nature of this bone cement improved biomechanical properties at time zero (i.e., at the time of repair), as in our study. It is unclear, therefore, why the biomechanical properties were not increased in the MBA group at 3 weeks, and were only increased in one outcome measure at 6 weeks. It is also unclear why the histologic and bone mineral density changes did not result in a more apparent functional (i.e., biomechanical) improvement. Nevertheless, the results from Gullota et al demonstrate the potential for improved tendon-to-bone repair using bone cement adhesives.
Similar results were presented by Waselau et al in a bone osteotomy model (15). Triangular fragments of bone were repaired with either a magnesium phosphate cement, a calcium phosphate cement, or left empty. Radiographically, magnesium phosphate cement held the fragments closer to the parent bone and produced larger calluses. However, as in our study, there were no improvements in the functional (i.e., biomechanical properties) due to the MBA.
As expected, the adhesive properties of the cement used in our study resulted in significantly improved biomechanical properties at the time of repair. Our previous studies indicate that the highest risk for failure in flexor tendon suture is in the first three weeks after surgical repair (1-4). Therefore, improving the initial properties and hence reducing the risk of post-repair rupture is an attractive treatment approach. Our previous work has focused on enhancing the initial strength of the repair through the addition of sutures and anchors. There is a limit, however, to the amount of suture that can be passed through the flexor tendon before the grasping strength and the healing potential of the tendon is compromised. Therefore, improving the initial strength of the repair using an adhesive, which does not require cutting through the tendon, is an attractive repair option for flexor tendon-to-bone repair.
Surprisingly, we found that MBA was consistently detrimental to the biomechanical properties 21 days after the surgical repair. A paired analysis comparing MBA to the adjacent CTL group at 21 days showed statistically significant decreases in most biomechanical measures. The mechanical properties of the MBA and the CTL group were significantly lower than the time zero properties. While a decrease in mechanical properties in the early period after tendon-to-bone repair is common (1-3,17), the magnitude of the decrease in our study was greater than expected. The biomechanical properties at 21 days in the current study are significantly lower than those in another recently published study using the same animal model (17). This suggests a reaction to the MBA that affected not only the MBA repair site, but also the CTL repair site in the nearby digit. Indeed, our histologic analysis revealed the presence of mast cells in tissues throughout the operated paw, including at the tendon and bone repair sites for MBA and CTL. The number of mast cells was slightly higher in the MBA group than in the CTL group, although there was high variability in these counts from section to section. The large numbers of mast cells seen in the current study is a strong indicator of an allergic or chronic inflammatory reaction (24). This is unexpected since the composition of magnesium phosphate cement should be well tolerated in vivo (15,16). However, many implanted biomaterials trigger acute and chronic inflammatory responses that are driven by mast cells (25-28). These cells and their granular products, especially histamine, are important in recruitment of inflammatory cells to biomaterial implants. For example, a study using implanted polyethylene terephthalate disks in mice showed that degranulation of mast cells occurred adjacent to the implants, suppression of histamine diminished inflammation, and mast cell reconstitution of mast cell-deficient mice restored “normal” inflammatory responses to biomaterial implants (25). Related to the current study, analysis of earlier timepoints is necessary to examine if the allergic reaction originated at the MBA surgical site.
There were a number of limitations to our study. First, gross examination of the skin incisions post-operatively revealed dehiscence, effusion, and swelling in the majority of the animals within two weeks. This is highly atypical in our experience with the canine flexor digitorum profundus tendon model. The incidence of problems was similar at MBA and CTL incisions. The wound closure issues and the presence of mast cells in both groups weakens the paired statistical comparison, as a negative reaction at one repair site may have influenced the properties of the adjacent tendon repair in the same paw. However, we can compare the MBA group to a separate group of animals from a recent study which received the identical surgical injury, repair, and post-operative rehabilitation without MBA (17). A comparison of the biomechanical properties of the MBA group to the CTL group in this separate group of animals reveals an even stronger negative effect of MBA at 21 days of healing. For example, the ultimate load and stiffness for the previous study were 43.4 N and 8.2 N/mm, respectively. In comparison, the ultimate load and stiffness for the MBA group in current study were 11.7 N and 2.0 N/mm, respectively.
The samples for histology were processed after biomechanical testing for the tendons and after biomechanical testing and densitometry for the bones. This resulted in some artifact in the sections, presumably due to the freeze-thaw cycle the sample underwent before fixation. Histologic sectioning after biomechanical testing also resulted in separate tendon and bone samples. Therefore, we were unable to examine the healing tendon-bone interface. Nevertheless, we were able to attain high quality tendon and bone histologic sections that could be assed reproducibly.
In conclusion, while magnesium-based adhesives hold promise for enhancing tendon-to-bone repair, a concept supported by our time zero biomechanical results, caution should be used when applying this novel biomaterial in vivo. The negative effect of MBA at 21 days of healing indicates a possible allergic or inflammatory reaction to the foreign material. Our study highlights the importance of following positive in vitro results with rigorous in vivo examination. Further biocompatibility studies are necessary before magnesium based adhesives are used clinically.
Acknowledgements
This study was funded by Bone Solutions Incorporated.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: This study was funded by Bone Solutions Incorporated and examines their product, a magnesium-based adhesive with the trade name ‘Osteocrete’.
REFERENCES
- 1.Thomopoulos S, Matsuzaki H, Zaegel M, Gelberman RH, Silva MJ. Alendronate prevents bone loss and improves tendon-to-bone repair strength in a canine model. J Orthop Res. 2007;25:473–479. doi: 10.1002/jor.20293. [DOI] [PubMed] [Google Scholar]
- 2.Silva MJ, Thomopoulos S, Kusano N, Zaegel MA, Harwood FL, Matsuzaki H, et al. Early healing of flexor tendon insertion site injuries: Tunnel repair is mechanically and histologically inferior to surface repair in a canine model. J Orthop Res. 2006;24:990–1000. doi: 10.1002/jor.20084. [DOI] [PubMed] [Google Scholar]
- 3.Silva MJ, Boyer MI, Ditsios K, Burns ME, Harwood FL, Amiel D, et al. The insertion site of the canine flexor digitorum profundus tendon heals slowly following injury and suture repair. Journal of Orthopaedic Research. 2002;20:447–453. doi: 10.1016/S0736-0266(01)00139-5. [DOI] [PubMed] [Google Scholar]
- 4.Boyer MI, Harwood F, Ditsios K, Amiel D, Gelberman RH, Silva MJ. Two-portal repair of canine flexor tendon insertion site injuries: histologic and immunohistochemical characterization of healing during the early postoperative period. Journal of Hand Surgery - American Volume. 2003;28:469–474. doi: 10.1053/jhsu.2003.50091. [DOI] [PubMed] [Google Scholar]
- 5.Iannotti JP, Naranja RJ, Gartsman GM. Orthopaedic Knowledge Update: Shoulder and Elbow. American Academy of Orthopaedic Surgeons; Rosemont IL: 1994. Surgical treatment of the intact cuff and repairable cuff defect: Arthroscopic and open techniques. pp. 151–155. [Google Scholar]
- 6.Harryman DT, 2nd, Mack LA, Wang KY, Jackins SE, Richardson ML, Matsen FA., 3rd Repairs of the rotator cuff. Correlation of functional results with integrity of the cuff. Journal of Bone & Joint Surgery. 1991;73:982–989. [PubMed] [Google Scholar]
- 7.Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. Journal of Bone & Joint Surgery - American Volume. 2004;86-A:219–224. doi: 10.2106/00004623-200402000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Arnoczky SP. Biology of ACL reconstructions: what happens to the graft? Instructional Course Lectures. 1996;45:229–233. [PubMed] [Google Scholar]
- 9.Fu FH, Bennett CH, Lattermann C, Ma CB. Current trends in anterior cruciate ligament reconstruction. Part I: Biology and biomechanics of reconstruction. Am J Sports Med. 1999;27:821–830. doi: 10.1177/03635465990270062501. [DOI] [PubMed] [Google Scholar]
- 10.Boyer MI, Ditsios K, Gelberman RH, Leversedge F, Silva M. Repair of flexor digitorum profundus tendon avulsions from bone: an ex vivo biomechanical analysis. Journal of Hand Surgery - American Volume. 2002;27:594–598. doi: 10.1053/jhsu.2002.33708. [DOI] [PubMed] [Google Scholar]
- 11.Ahmad CS, Stewart AM, Izquierdo R, Bigliani LU. Tendon-bone interface motion in transosseous suture and suture anchor rotator cuff repair techniques. Am J Sports Med. 2005;33:1667–1671. doi: 10.1177/0363546505278252. [DOI] [PubMed] [Google Scholar]
- 12.St Pierre P, Olson EJ, Elliott JJ, O'Hair KC, McKinney LA, Ryan J. Tendon-healing to cortical bone compared with healing to a cancellous trough. A biomechanical and histological evaluation in goats. Journal of Bone & Joint Surgery - American Volume. 1995;77:1858–1866. doi: 10.2106/00004623-199512000-00010. [DOI] [PubMed] [Google Scholar]
- 13.Rodeo SA, Suzuki K, Deng XH, Wozney J, Warren RF. Use of recombinant human bone morphogenetic protein-2 to enhance tendon healing in a bone tunnel. American Journal of Sports Medicine. 1999;27:476–488. doi: 10.1177/03635465990270041201. [DOI] [PubMed] [Google Scholar]
- 14.Rodeo SA, Potter HG, Kawamura S, Turner AS, Kim HJ, Atkinson BL. Biologic augmentation of rotator cuff tendon-healing with use of a mixture of osteoinductive growth factors. J Bone Joint Surg Am. 2007;89:2485–2497. doi: 10.2106/JBJS.C.01627. [DOI] [PubMed] [Google Scholar]
- 15.Waselau M, Samii VF, Weisbrode SE, Litsky AS, Bertone AL. Effects of a magnesium adhesive cement on bone stability and healing following a metatarsal osteotomy in horses. Am J Vet Res. 2007;68:370–378. doi: 10.2460/ajvr.68.4.370. [DOI] [PubMed] [Google Scholar]
- 16.Gulotta LV, Kovacevic D, Ying L, Ehteshami JR, Montgomery S, Rodeo SA. Augmentation of tendon-to-bone healing with a magnesium-based bone adhesive. Am J Sports Med. 2008;36:1290–1297. doi: 10.1177/0363546508314396. [DOI] [PubMed] [Google Scholar]
- 17.Thomopoulos S, Zampiakis E, Das R, Silva MJ, Gelberman RH. The effect of muscle loading on flexor tendon-to-bone healing in a canine model. J Orthop Res. 2008;26:1611–1617. doi: 10.1002/jor.20689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dovan TT, Gelberman RH, Kusano N, Calcaterra M, Silva MJ. Zone I flexor digitorum profundus repair: an ex vivo biomechanical analysis of tendon to bone repair in cadavera. J Hand Surg [Am] 2005;30:258–266. doi: 10.1016/j.jhsa.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 19.Silva MJ, Brodt MD, Boyer MI, Morris TS, Dinopoulos H, Amiel D, et al. Effects of increased in vivo excursion on digital range of motion and tendon strength following flexor tendon repair. J Orthop Res. 1999;17:777–783. doi: 10.1002/jor.1100170524. [DOI] [PubMed] [Google Scholar]
- 20.Thomopoulos S, Hattersley G, Rosen V, Mertens M, Galatz L, Williams GR, et al. The localized expression of extracellular matrix components in healing tendon insertion sites: an in situ hybridization study. Journal of Orthopaedic Research. 2002;20:454–463. doi: 10.1016/S0736-0266(01)00144-9. [DOI] [PubMed] [Google Scholar]
- 21.Thomopoulos S, Williams GR, Soslowsky LJ. Tendon to bone healing: differences in biomechanical, structural, and compositional properties due to a range of activity levels. Journal of Biomechanical Engineering. 2003;125:106–113. doi: 10.1115/1.1536660. [DOI] [PubMed] [Google Scholar]
- 22.Galatz LM, Sandell LJ, Rothermich SY, Das R, Mastny A, Havlioglu N, et al. Characteristics of the rat supraspinatus tendon during tendon-to-bone healing after acute injury. J Orthop Res. 2006;24:541–550. doi: 10.1002/jor.20067. [DOI] [PubMed] [Google Scholar]
- 23.Rodeo SA, Arnoczky SP, Torzilli PA, Hidaka C, Warren RF. Tendon-healing in a bone tunnel. A biomechanical and histological study in the dog. Journal of Bone & Joint Surgery - American Volume. 1993;75:1795–1803. doi: 10.2106/00004623-199312000-00009. [DOI] [PubMed] [Google Scholar]
- 24.Prussin C, Metcalfe DD. 4. IgE, mast cells, basophils, and eosinophils. J Allergy Clin Immunol. 2003;111:S486–494. doi: 10.1067/mai.2003.120. [DOI] [PubMed] [Google Scholar]
- 25.Tang L, Jennings TA, Eaton JW. Mast cells mediate acute inflammatory responses to implanted biomaterials. Proc Natl Acad Sci U S A. 1998;95:8841–8846. doi: 10.1073/pnas.95.15.8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rezzani R, Rodella L, Tartaglia GM, Paganelli C, Sapelli P, Bianchi R. Mast cells and the inflammatory response to different implanted biomaterials. Arch Histol Cytol. 2004;67:211–217. doi: 10.1679/aohc.67.211. [DOI] [PubMed] [Google Scholar]
- 27.Al-Saffar N, Iwaki H, Revell PA. Direct activation of mast cells by prosthetic biomaterial particles. J Mater Sci Mater Med. 1998;9:849–853. doi: 10.1023/a:1008952329788. [DOI] [PubMed] [Google Scholar]
- 28.Mussel RL, De Sa Silva E, Costa AM, Mandarim-De-Lacerda CA. Mast cells in tissue response to dentistry materials: an adhesive resin, a calcium hydroxide and a glass ionomer cement. J Cell Mol Med. 2003;7:171–178. doi: 10.1111/j.1582-4934.2003.tb00216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]