Abstract
Summary: Human fungal pathogens are associated with diseases ranging from dandruff and skin colonization to invasive bloodstream infections. The major human pathogens belong to the Candida, Aspergillus, and Cryptococcus clades, and infections have high and increasing morbidity and mortality. Many human fungal pathogens were originally assumed to be asexual. However, recent advances in genome sequencing, which revealed that many species have retained the genes required for the sexual machinery, have dramatically influenced our understanding of the biology of these organisms. Predictions of a rare or cryptic sexual cycle have been supported experimentally for some species. Here, I examine the evidence that human pathogens reproduce sexually. The evolution of the mating-type locus in ascomycetes (including Candida and Aspergillus species) and basidiomycetes (Malassezia and Cryptococcus) is discussed. I provide an overview of how sex is suppressed in different species and discuss the potential associations with pathogenesis.
INTRODUCTION
Fungi first appeared approximately 1.5 billion years ago (102, 103) and were among the earliest organisms domesticated by humans. There are approximately 1.5 million fungal species known, of which fewer than 100 are associated with human disease. Others cause disease in animals, such as the chytrid species Batrachochytrium dendrobatidis, which is devastating the global amphibian population (210). Plant fungal pathogens both destroy crops and generate mycotoxins (65). However, the vast majority of fungi are not associated with disease, and many are saprophytic.
Human fungal diseases became a serious problem only during the 20th and 21st centuries, as a result of increased efficiency in treating bacterial infections followed by a growth in the number immunodeficient patients and the increasing use of indwelling medical devices (202). The most common human fungal pathogens belong to the Basidiomycota and the Ascomycota (Fig. 1), including the major pathogens Candida albicans, Cryptococcus neoformans, and Aspergillus fumigatus. Members of these two phyla will therefore be discussed in this review.
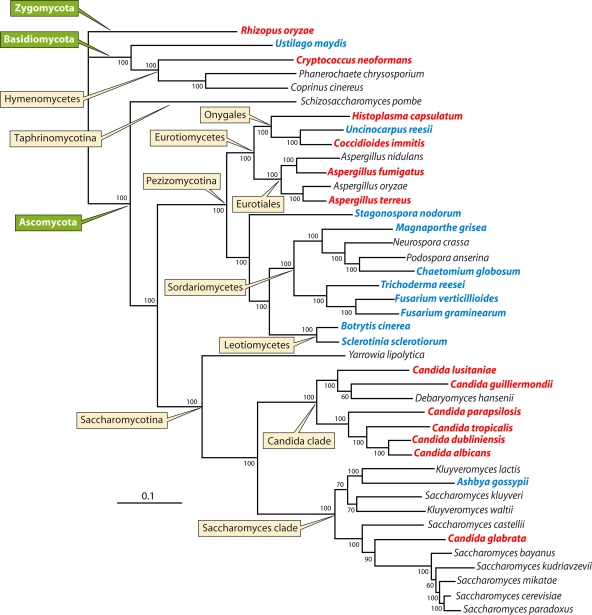
FIG. 1.
Fungal phylogeny. The tree shows the phylogeny of 42 fungi with fully sequenced genomes. Plant pathogens are indicated in blue, and human pathogens are in red. (Adapted from reference 74.)
Although mating and meiosis have been well described for many fungal species, a substantial number of pathogenic species have rarely or never been observed to undergo a full sexual cycle in the wild. For some, the existence of a possible cryptic sexual cycle was suggested initially from genomic analysis. It has been hypothesized that reproducing asexually allows the pathogen to proliferate in the human host, whereas the presence of a rare or cryptic sexual cycle under stressful conditions may promote adaptation (192). Here I will examine the evidence that sexual reproduction occurs in human pathogens. Analysis of related species will be used to discuss the evolution of sex and to ascertain if sex is specifically suppressed in pathogenic species. I will also discuss the direct contribution of sex pathways to virulence.
MATING IN THE ASCOMYCETES
Cell identity in the Ascomycetes is determined by the gene content at the mating-type (MAT), or mating-type-like (MTL) locus. The sequence at MAT may include more than one open reading frame, and these are therefore usually referred to as idiomorphs, rather than alleles (178). Mating is predominantly bipolar and occurs between two isolates differing in sequence at MAT (36).
Saccharomyces cerevisiae and Candida glabrata
Mating was first characterized at the molecular level in Saccharomyces cerevisiae (baker's yeast), and this organism is still among the best studied (7, 97). However, the mating system is not representative of the other ascomycetes. S. cerevisiae exists as three main cell types, a, α, and a/α. Mating occurs between haploid a and α cells, to generate an a/α diploid. Mating is pseudohomothallic; the a and α isolates may be genetically identical, and one arises from the other by switching the information present at the active MAT locus (97, 106, 107). Mating-type switching occurs through a gene conversion-like event, where information at the MAT locus near the centromere of chromosome III is replaced with information from one of the silent cassettes (HMR and HML) present at the ends of the chromosome. Cell identity is determined by the information expressed at MAT; MATα encodes two proteins (α1 and α2), whereas MATa encodes only a1. HMLα and HMRa contain sequences that are very similar to their MAT counterparts, but they are located in heterochromatin and are not expressed.
Switching between mating types is initiated by the activity of the HO endonuclease, which introduces a staggered double-stranded break at a specific recognition site within MAT. Switching occurs only in mother cells (i.e., cells that have undergone mitotic division at least once). This is largely regulated through the expression of the HO gene, which is repressed in daughter cells by asymmetric localization of Ash1 (22, 230). HO expression is also repressed in diploid cells by the a1/α2 repressor, and in haploid mother cells it is expressed only at G1, via the coordinated action of transcription factors and chromatin-remodeling machines (55, 175, 181, 188).
Cassette-based mating-type switching is not restricted to S. cerevisiae but is also found in closely related species. The asexual human fungal pathogen Candida glabrata contains an MTL and two silent cassettes (67, 236, 260). C. glabrata is a common cause of bloodstream infection worldwide, and only C. albicans (or sometimes Candida tropicalis) is more prevalent (124, 207). However, C. glabrata is a distant relative of the other Candida species (Fig. 1). Mating-type switching has been observed in only one clinical isolate of C. glabrata (31) and at a very low level in environmental isolates (37). In addition, one of the silent cassettes (the equivalent of HMRa) is located on a separate chromosome from MTL and HMLα.
Two studies have shown that the majority of global isolates of C. glabrata have the MTLa idiomorph (30, 236). Overall, the incidence of MTLa may be 4-fold higher than that of MTLα (30), although in isolates from Taiwan, MTLα idiomorphs dominate (148). MTLa and MTLα isolates are often found in the same clade, suggesting that they arose from single isolates by mating-type switching (30). However, the rate of switching from MTLα to MTLa is apparently very low (30). This may be because HMRa is on a different chromosome from MTL; in Kluyveromyces lactis, which has a similar arrangement of mating cassettes, switching from MATa to MATα occurs at a higher rate than the reciprocal switch (105). In addition, the HO recognition site in C. glabrata MTLα differs slightly from the canonical recognition site (37). This does not prevent switching in S. cerevisiae (190) but may reduce it in C. glabrata.
A reduction in mating-type switching does not, however, explain the lack of mating in C. glabrata. All isolates identified to date are haploid, and mating has never been observed in the laboratory (30, 37, 148, 186). Multilocus sequence typing (MLST) and variable number of tandem repeat (VNTR) analyses indicate that the population structure is mostly clonal, with a high level of linkage disequilibrium (30, 148). However, there is also evidence for recombination, although this is a rare event (62).
The C. glabrata genome contains all the genes known to be required for mating, from the regulators in the mating-type locus to the mating pheromones, receptors, and the pheromone response pathway (67, 260). In S. cerevisiae, expression of the MAT genes a1, α1, and α2 is cell type specific. In C. glabrata however, whereas expression of α1 and α2 is restricted to MTLα cells, a1 is expressed in both MTLa and MTLα cells (186). Expression of a1 results from a lack of silencing at HMRa, possibly because C. glabrata does not contain a homolog of SIR1, which is required for silencing in S. cerevisiae (67). Somewhat surprisingly, however, splicing of a1 occurs only in MTLa cells (186). It is therefore likely that differential splicing regulates that activity of the a1 protein.
Unlike in S. cerevisiae, in C. glabrata the expression of the pheromone receptors STE2 and STE3 is not cell specific (186). In addition, MTLa cells do not respond to the presence of α pheromone, even though the C. glabrata pheromone can induce expression in S. cerevisiae (186). In fact, C. glabrata cells of neither mating type respond to a- or α-factor (186). Whereas the C. glabrata α pheromone gene is intact, it appears that it is not expressed in a wide variety of isolates (186). The failure to express or to respond to pheromone is likely to be a major cause of the inability to mate.
Mating-type cassettes are found in the genome of Kluyveromyces delphensis, a close relative of C. glabrata that is apparently nonpathogenic and fully sexual (37). Cassettes are also present in the more distantly related species Saccharomyces castellii and Zygosaccharomyces rouxii (37). The sequenced isolate of Ashbya gossypii, a pathogen of cotton plants, contains three MATa-type cassettes at the HMR, MAT, and HML loci (60). However, it is likely that other combinations occur in nature, as rare a,a,a and α,α,α isolates of S. cerevisiae and α,α,α isolates of C. glabrata have also been reported (97, 236).
The silent cassettes are not present in the lineage containing the pathogenic yeast C. albicans and were acquired by the Saccharomyces group following the divergence from Candida (36, 37). Acquisition of the cassettes preceded the adoption of the HO endonuclease, which is not present in K. lactis or A. gossypii (36, 37, 67). HO belongs to a family of homing endonucleases (HEGs), which behave as mobile genetic elements (35). HEG open reading frames usually lie within their own recognition site; they introduce double-stranded breaks in other alleles, which induces copying of the HEG to the new location. HO is closely related to the VDE intein, which lies within the coding sequence of the Vma1 vacuolar (H)-ATPase (29, 86, 98). The VDE element is excised after translation. As well as the protein splicing and homing domains, HO also contains a zinc finger region at the C terminus. HO does not copy itself to other locations but instead introduces breaks within MAT. This probably increases the efficiency of switching of the cassette system (37, 67, 125).
It appears unlikely that mating is directly associated with virulence in the Saccharomyces group. The asymmetric distribution of MAT idiomorphs in the major pathogen C. glabrata is apparently not related to drug susceptibility or pathogenicity (148). C. glabrata is the only major human pathogen in the Saccharomyces clade (Fig. 1), and mating is clearly rare or even nonexistent. This is consistent with a general lack of mating in human fungal pathogens. However, more detailed comparative analysis with close asexual and sexual relatives is required before definitive conclusions can be drawn. There is some evidence that relatives of C. glabrata (such as Candida bracarensis and Candida nivariensis [4, 54]) are also rare human pathogens. The sexual state of these yeasts has not been characterized, but it has been reported that C. nivariensis does not generate ascospores (4). C. nivariensis may also be less susceptible to azole drugs (24). However, these species account for fewer than 0.2% of isolates previously characterized as C. glabrata (158), and so it is unclear whether the lack of a sexual cycle confers an advantage to these human pathogens.
Mating in the Candida Clade
Candida albicans.
The term Candida was originally used to indicate imperfect or asexual yeasts and has been applied to a number of species that are found in the same clade, such as Candida albicans, C. glabrata and Candida krusei. As described above, C. glabrata is more closely related to S. cerevisiae than to C. albicans, and C. krusei lies outside both the Saccharomyces and Candida groups. In addition, some sexual species such as Pichia stipitis and Debaryomyces hansenii share a more recent ancestry with C. albicans (74). We therefore use the term “Candida clade” to refer to a monophyletic group of related species that all translate the codon CUG as serine rather than leucine (Fig. 1) (38).
C. albicans is the best-characterized member of the Candida clade and is closely associated with the human host. It is a commensal, but it is also a major cause of oropharyngeal disease, vaginitis, and systemic bloodstream infections. Approximately 8% of nosocomial bloodstream infections in U.S. hospitals are caused by Candida species, and C. albicans is the most common (206). Virulence is correlated with the ability to grow as yeast, pseudohyphal, and hyphal forms (19). Until the first data emerged from the C. albicans sequencing project, it was generally accepted that this species was completely asexual. All known isolates are diploid, and they have never been observed to undergo meiosis. However, Hull and Johnson (115) identified an MTL that resembles the S. cerevisiae MAT locus, suggesting that mating may occur. This was followed by analyses that showed that most genes associated with the mating signal transduction pathway in S. cerevisiae are also conserved in C. albicans (49, 170).
The first C. albicans isolate sequenced was heterozygous at MTL and contained both MTLa and MTLα idiomorphs. MTLa encodes a homeodomain protein, a1, and an HMG domain protein, a2, which has been lost from the Saccharomyces group (37, 245). MTLα encodes α1 and α2. Both idiomorphs carry alleles of a poly(A) polymerase gene (PAP), an oxysterol binding protein gene (OBP), and a phosphatidylinositol kinase gene (PIK), with no known function in mating. Association of PAP, OBP, and PIK with MTL is an ancient event in the Candida clade (38).
Identification of the MTL idiomorphs was quickly followed by demonstration of mating. Hull et al. (116) deleted either MTLa or MTLα in auxotrophic strains and showed that tetraploid products were generated when potential mating pairs were introduced into mice. In contrast, Magee and Magee (171) induced loss of one of the chromosomes containing MTL, which is usually followed by duplication of the remaining chromosome. They then crossed auxotrophic isolates containing identical copies of either MTLa or MTLα. The frequency of mating observed, however, remained low, until Miller and Johnson (179) observed an association with a phenotypic transition from white to opaque cells.
Phenotype switching between colony variants is a common phenomenon among Candida species (233). The specific transition between white and opaque cells was first described by Slutsky et al. (231) for a clinical isolate called WO-1. Opaque cells are elongated, with “pimples” on the surface, and colonies of opaque cells absorb the dye phloxine B, whereas colonies of white cells do not (5). Miller and Johnson (179) showed that the white/opaque transition is repressed by the a1/α2 proteins encoded by MTL and that opaque cells mate with an efficiency that is 106 times higher than that of white cells. Regulation of the white/opaque transition is complex. The master regulator WOR1 (also known as TOS9) is absolutely required for switching (113, 235, 278) and is itself repressed by a1/α2. WOR1 regulates its own expression and also the expression of three other regulators, WOR2, EFG1, and CZF1 (279). This generates a series of interlocking feedback loops that results in high levels of expression of EFG1 in white cells and of WOR1 in opaque cells.
White/opaque switching is induced by several environmental signals, such as temperature (165), oxygen and carbon dioxide concentrations (112, 216), and genotoxic and oxidative stress (2). Opaque cells are favored at low temperatures, which facilitates mating on skin (139). At body temperature, opaque cells are stabilized by low oxygen and increased carbon dioxide, which also allow mating (112).
There is a clear association between white/opaque switching and virulence. White cells are more virulent than opaque cells in a mouse tail vein model (133), they secrete a chemoattractant for human polymorphonuclear leukocytes (83), and they are preferentially phagocytosed by mouse macrophages and Drosophila hemocyte-derived cells (165). Switching to opaque cells may help C. albicans to avoid the immune system. Initial analysis suggested that isolates that are heterozygous a/α at MTL (and cannot switch) are more virulent than a/a or α/α isolates (161). However, this may result from heterozygosity of alleles elsewhere on the chromosome carrying MTL, and the virulence of naturally occurring homozygous strains varies considerably (264).
Candida dubliniensis.
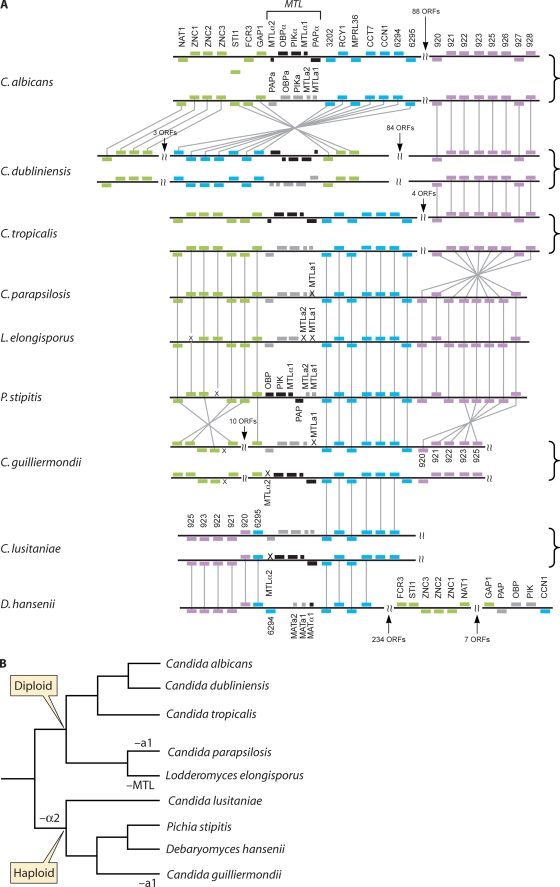
C. dubliniensis is very closely related to C. albicans and was identified as a distinct species only in the 1990s (242). It is, however, much less virulent and is most commonly associated with human immunodeficiency virus (HIV) patients (241). The MTL idiomorphs of C. dubliniensis are very similar in structure to those of C. albicans (213), though there has been an inversion of some of the surrounding region in C. dubliniensis (Fig. 2). Whereas the proportion of MTL homozygotes is low in C. albicans (143, 160), it is approximately 3-fold higher (33%) in C. dubliniensis (213). MTLa and MTLα isolates of C. dubliniensis can mate with each other, but surprisingly, they also mate with C. albicans, at even higher efficiencies (213). The biological significance of this observation is unclear.
FIG. 2.
(A) Organization of the mating-type-like locus in the Candida clade. MTLα-specific genes are shown in black and MTLa-specific genes in gray. Orthologs are indicated in color and are connected by gray lines. The MTL locus in C. dubliniensis is inverted relative to that in C. albicans. For heterothallic species, both idiomorphs are shown, joined by a curly bracket. No MTLα idiomorph of C. parapsilosis has been identified, and no mating genes have been identified in L. elongisporus. P stipitis, D. hansenii, and L. elongisporus are assumed to be homothallic. ORF, open reading frame. (Adapted from reference 38, with data from references 38, 67, 115, 120, 213, and 219.) (B) Gene losses are indicated on the Candida clade phylogeny.
Mating in the remainder of the Candida clade.
Within the Candida clade, C. tropicalis and C. parapsilosis are major human pathogens, whereas species such as C. guilliermondii and C. lusitaniae are much rarer causes of infection (206). C. tropicalis and C. parapsilosis are among the closest relatives of C. albicans with sequenced genomes, together with Lodderomyces elongisporus, which is very rarely associated with disease (Fig. 1) (159). The MTL idiomorphs of C. tropicalis are very similar in gene content and structure to C. albicans, but mating has never been tested in this organism (38) (Fig. 2). The sequenced isolate is heterozygous at MTL, and the distribution of MTL homozygotes is unknown. The MTLa idiomorph of C. parapsilosis was first described by Logue et al. (164). The structure is similar to that of C. albicans, except that the MTLa1 region is a pseudogene, with four stop codons in the most likely translation. The pseudogene is present in seven other isolates and also in the isolate used for genome sequencing (38, 164). Initial Southern blot analysis suggest that MTLα sequences lay elsewhere in the genome, but with the completion of the genome sequence it became clear that this most likely resulted from low-stringency hybridization (38). So far, no MTLα idiomorphs have been identified, though only 15 isolates have been tested (38, 164). It is, however, clear that if MTLα homozygotes or MTLa/α heterozygotes exist, they are at a low frequency. The loss of MTLa1 is likely to be a very recent event in C. parapsilosis, as it is still possible to identify potential a1/α2 binding sites in the promoters of putative target genes (38). In addition, the a1 gene is intact in the closely related species Candida orthopsilosis (243; G. Butler, unpublished data). C. parapsilosis undergoes phenotypic switching, but there is no known association with mating (140, 168).
L. elongisporus was originally designated Saccharomyces elongisporus and was assumed to be homothallic (self-mating) and sexual, based on the formation of one or two ascospores in asci (218, 252). Ascospores were also observed recently by Lockhart et al. (159). L. elongisporus was generally assumed to be nonpathogenic, but this may be related to underidentification in clinical samples. Lockhart et al. (159) showed that approximately 2% of isolates in a large collection categorized as C. parapsilosis are actually L. elongisporus. Because of the close relationship of L. elongisporus and C. parapsilosis, for some time it was assumed to be the sexual form of the species (118). It was therefore a considerable surprise when analysis of the genome sequence of L. elongisporus revealed that it has no obvious mating-type locus (38). The gene order surrounding the presumed MTL region is conserved with other species and contains PAPa, OBPa, and PIKa alleles, suggesting it was derived by gene loss from an ancestral MTLa idiomorph (Fig. 2). The loss is not restricted to the sequenced isolates, as the region between PIKa and the adjacent open reading frame is approximately 500 bp in other isolates also, which is insufficient to encode a1 and a2 (G. Butler, unpublished data). There is no evidence that the genes exist anywhere else in the sequenced genome.
Within the Saccharomycotina, cells of opposite mating type communicate via diffusible pheromones, called a-factor and α-factor, and pheromone receptors. α-factor pheromones have a conserved structure, produced from cleavage of a preprotein. The S. cerevisiae pheromone is encoded by two similar genes, which contain several copies of the mature peptide of 13 amino acids (131). The structure of α-factor is conserved in C. albicans (18, 162, 197) and is easily identified in the other Candida species (38). Mature a-factor in S. cerevisiae is generated by posttranslational modification of a 35-amino-acid protein that contains a C-terminal CAAX motif, or isoprenylation site (50). The cysteine residue in the CAAX motif is first farnesylated by the farnesyl transferase enzyme (Ram1 and Ram2), and the terminal three amino acids are removed by proteolysis, catalyzed by the Ste24 or Rce1 endoprotease (28). The small size and general lack of structure made it difficult to identify an orthologous a-factor in C. albicans, although the presence of the processing enzymes and the fact that their expression is induced by α-factor suggested that one did exist (18, 217). The C. albicans a-factor was eventually identified by searching for small encoded peptides with a terminal CAAX motif that were conserved in C. albicans and C. dubliniensis (61). This analysis was not possible until the availability of sequences of related genomes, as conceptual translation of the C. albicans genome generates more than 1,000 peptides between 26 and 46 amino acids in length with a terminal CAAX motif. However, only one is completely conserved between C. albicans and C. dubliniensis: a 42-amino-acid peptide terminating with the sequence CSVM. C. albicans and C. dubliniensis contain only one gene each encoding a-factor (called MFA1), and the position is conserved between C. albicans WO-1 and C. dubliniensis (61). Expression of MFA1 in C. albicans is induced by exposure to α-factor, and deleting the gene blocks mating in MTLa but not MTLα cells.
Similar approaches were used to identify a-factor genes in the other Candida species (38). The number of genes ranges from 1 in C. lusitaniae to 2 in C. guilliermondii, 3 in C. parapsilosis, and up to 12 in C. tropicalis. However, no a-factor sequences were identified in L. elongisporus (or in D. hansenii) (38). In addition, in L. elongisporus there is no obvious a-factor receptor, nor is there is an ortholog of the STE6/HST6 gene, which transports a-factor in both S. cerevisiae and C. albicans and is required for mating (170, 217). The presence of only one pheromone and one pheromone receptor, together with the lack of the MTL locus, suggests that L. elongisporus may have an autocrine-type system, where a single pheromone signals the cell that produces it. In the fission yeast Schizosaccharomyces pombe, expressing the M-factor receptor (one of the mating pheromone receptors) in cells that normally express the P-factor receptor results in activation of the mating response, although not to the extent that mating is observed (128). Pheromones in the ciliate Euplotes raikovi and the fungus Cryptococcus neoformans act both in an autocrine manner (to promote growth and differentiation) and also in a paracrine manner (as a sexual pheromone) (251). In basidiomycetes like Cr. neoformans, only a-type lipopeptide pheromones are produced; cells are distinguished by different alleles of the same pheromone (46). It is therefore possible to envisage a mechanism where cells can signal using one pheromone and one receptor, especially in a homothallic population. The very recent demonstration of same-sex mating in C. albicans is intriguing and suggests that similar mechanisms may exist in other yeasts (3; reviewed in reference 234). For L. elongisporus, further investigation is required to determine if this species does indeed mate and if it goes through meiosis, as four spore asci have never been observed.
Mating in the haploid Candida clade.
A subclade with the Candida group contains the species P. stipitis, D. hansenii, C. guilliermondii and C. lusitaniae, all of which are haploid and apparently fully sexual. P. stipitis and D. hansenii are each other's closest relatives (Fig. 2) and are homothallic. P. stipitis is a xylose-fermenting yeast that is closely related to endosymbionts of pallasid beetles, which degrade rotted hardwood (239). Mating occurs between identical isolates, and sporulation induced by growth on nutrient-deficient media generates asci containing two hat-shaped spores (177). Analysis of hybrids formed by protoplast fusion demonstrated segregation of auxotrophic markers in the spores, suggesting that they are the products of meiosis, although not all spores were haploid (177).
D. hansenii (anamorph, Candida famata) is a very rare human pathogen, which is often misidentified as C. guilliermondii (59). D. hansenii has a life cycle very similar to that of P. stipitis: it is haplontic (undergoes meiosis soon after conjugation) and usually produces a small number of spores, and there is evidence for recombination of auxotrophic markers (253).
C. guilliermondii and C. lusitaniae are distant relatives of P. stipitis and D. hansenii. They are also haploid, but have a heterothallic (outbreeding) life cycle; i.e., mating occurs between conspecific but genetically distinct isolates. Two mating types of C. guilliermondii (Pichia guilliermondii) were identified by Wickerham and Burton (259), and sporulation of the mating products generates asci, usually containing two spores. Opposite mating types of C. lusitaniae (Clavispora lusitaniae) were identified in 1979 (220) and were shown to undergo mating and sporulation, generating ascospores with between one and four spores. Assortment of markers was also reported (272). Reedy et al. have recently completed a very detailed analysis of meiosis in C. lusitaniae (219). They developed a restriction fragment length polymorphism map for three chromosomes and showed that there was a high degree of recombination in the spore progeny. SPO11, encoding a meiosis-specific topisomerase in S. cerevisiae, is required for recombination, suggesting that the cells are proceeding through meiosis. Similar to the case for the other species in the subclade, usually only two spores are generated per ascus, and whereas most are haploid, there is a significant level of aneuploidy (219).
The MTL loci for all the haploid Candida species have been sequenced (Fig. 2) (38, 67, 120, 219). In general, the genomic context has been conserved, and it is similar to that in C. albicans and related species. However, there are also notable differences. Homothallism appears to have originated by different mechanisms in P. stipitis and D. hansenii. The MTL in P. stipitis resembles the MTLα of the other species, as it contains α-like alleles of OBP, PIK, and PAP in the same order, followed by a1 and a2 genes. The simplest explanation is that this arose from recombination of ancestral MTLα and MTLa idiomorphs, placing the regulatory genes together. In D. hansenii, however, a2, a1, and α1 are together at one chromosomal position, with a-like alleles of PAP, OBP, and PIK at a second location (67). Examination of synteny conservation suggests that this structure may have arisen in two stages; first the regulatory genes were placed together as in P. stipitis, possibly at an MTLa-like idiomorph, followed by a gene rearrangement that separated the cell identity genes from the PAP, OBP, and PIK genes (Fig. 2) (38, 67).
C. lusitaniae and C. guilliermondii are heterothallic, and both MTL idiomorphs have been sequenced from each (Fig. 2, (38, 219). The gene order in C. guilliermondii is well conserved with that in the other Candida species; C. lusitaniae has undergone some rearrangement and more closely resembles the organization in D. hansenii. Reedy et al. (219) showed that α1 and a2 are required for mating and for cell identity in C. lusitaniae, similar to the case for C. albicans.
There are, however, some notable gene losses in the haploid Candida clade. None of the species contain an ortholog of the α2 protein, which forms a dimer with a1 in both S. cerevisiae and C. albicans. In S. cerevisiae, a1/α2 represses expression of haploid-specific genes and also induces meiosis and sporulation, at least partly by inducing expression of IME1 (180, 232). IME1 is a master regulator of meiosis gene expression in S. cerevisiae, but there is no ortholog present in any of the Candida species (38). In C. albicans, a1/α2 controls white/opaque switching, which is necessary for mating competency (122). α2 together with Mcm1 represses expression of a-specific genes in S. cerevisiae; this does not occur in C. albicans, where the activity of a2 is required for expression of a-specific genes (245). The loss of α2 in the haploid Candida clade may explain some of the differences observed in sporulation between these species and Saccharomyces. The haploid Candida species spend very little time as diploids (although diploids can be rescued from P. stipitis by transfer to rich medium before sporulation occurs [177]). There is therefore little requirement for repression of expression of haploid-specific genes, and loss of α2 may have driven the haplontic lifestyle of these species. Induction of sporulation is also likely to occur by a different mechanism, as IME1 is not present. This role may be partially provided by a1, which is required for sporulation in C. lusitaniae (219). However, a1 is not present in C. guilliermondii, which also undergoes sporulation (219).
The fact that the haploid Candida clade generates asci with fewer than four spores would suggest that meiosis is not complete. Two-spore asci have been routinely described for the four species with sequenced genomes. Two spores can be generated by apomixis, without meiosis or karyogamy (20). However, meiotic recombination has been clearly demonstrated in P. stipitis (177) and C. lusitaniae (219) and is strongly indicated in D. hansenii (253). Some four-spore asci are produced from engineered hybrids of P. stipitis (177), suggesting that it retains the ability to generate them. Several yeast species produce various numbers of ascospores. For example, members of the Kazachstania genus (relatives of Saccharomyces) generate from 1 to 16 asci (132). The number of spores produced by S. cerevisiae also depends on environmental conditions (20). It is therefore likely that the haploid Candida species undergo meiosis generating haploid spores and that two of the meiotic progeny are lost.
Evolution of meiosis.
Meiosis has never been observed in the diploid Candida clade that includes C. albicans. Mating between diploid isolates heterozygous at MTL produces a tetraploid product, which reverts to diploidy via random chromosome loss, in a parasexual cycle (17). Recombination occurs between homologous chromosomes, which is dependent on SPO11 (75). One of the first analyses of the C. albicans genome concluded that meiosis might not occur because certain key genes, such as IME1, are missing (249). However, subsequent analysis showed that most genes required for meiosis in S. cerevisiae and other fungi are present in all Candida species, and those that are missing from C. albicans are also missing from the fully sexual species (38). There is therefore no obvious reason why C. albicans cannot undergo meiosis.
Somewhat surprisingly, there has been additional gene loss in two of the sexual species, C. guilliermondii and C. lusitaniae. These have lost components of the synaptonemal and synapsis initiation complex (SIC), the Dmc1-dependent pathway for pairing of homologous chromosomes, and the major crossover formation pathway (38). Other sexual fungi, such as Cryptococcus neoformans and Neurospora crassa, also lack orthologs of the SIC, and S. pombe does not form a true synaptonemal complex (166, 204). The extent of gene loss in C. lusitaniae and C. guilliermondii is unique; however, meiosis does take place, at least in C. lusitaniae.
It is generally assumed that genes will decay if the pathway in which they act is no longer functional (99). Identification of meiotic genes in the parasite Giardia led to the suggestion that meiosis could occur, although it had never been observed (1, 214). Demonstration of recombination was difficult to obtain, as many Giardia isolates come from clonal lineages (163), until in 2007 Cooper et al. (53) obtained clear evidence of recombination between distinct populations of Giardia lamblia. Giardia lamblia is binucleate, and the two nuclei remain separate during mitosis in the noninfectious form of the parasite's life cycle (224). However, Poxleitner et al. (211) have recently demonstrated that during cyst formation, the nuclei fuse and exchange information via a mechanism that requires some meiotic gene products. This unusual parasexual cycle has been termed “diplomixis,” as there is no associated genome reduction through meiosis. The protist Trichomonas vaginalis also contains core meiotic genes, and it has been postulated that it uses a sexual or parasexual cycle (172). The search for a meiotic cycle in C. albicans has perhaps been overinfluenced by our understanding of meiosis in S. cerevisiae. The evidence from the haploid Candida clade is that sporulation is inefficient, producing fewer than four spores, and progeny are often aneuploid. The parasexual cycle in C. albicans may therefore be derived from meiosis in an ancestral organism that resembled the present haploid Candida clade, and some meiosis-associated genes (such as SPO11) have been adopted to drive homologous recombination without meiosis (75).
Role of the mating pheromone in virulence of C. albicans.
The white/opaque switching mechanism described for C. albicans is not required for mating in the haploid Candida clade. It is therefore interesting to consider whether switching has roles other than mating. Several groups have shown that exposure of C. albicans opaque cells to α-factor results in induction of expression of some cell surfaces and secreted proteins, as well as many genes associated with mating (18, 162). Although white cells are not competent for mating, substantial changes in gene expression are also induced following exposure to α-factor, including expression of much of the signal transduction pathway (162). Although “shmooing” of white cells does not occur and there is no general effect on the cell cycle, a general α-factor response was observed in all tested isolates of C. albicans (225).
Daniels et al. (57) showed that α-factor induces adhesiveness of white, and not opaque, cells, and as a result supports the formation of biofilms. The addition of minority opaque cells of the opposite mating type resulted in the formation of thicker biofilms. They then demonstrated that the white cell biofilm promoted chemotropism between minor opaque a/a and α/α cells and therefore enhanced mating. They therefore hypothesized that the white cell response evolved to support mating between opaque cells.
The white cell response to α-factor is mediated by the same signal transduction pathway as the opaque cell response, except for the final downstream targets (270). However, Yi et al. (271) observed that the α-pheromone receptor in C. albicans (Ste2) contains two unique regions, which are not present in the orthologous protein from S. cerevisiae. These sequences are found in C. albicans and C. dubliniensis, the only two species known to undergo white-to-opaque switching (213), and are not present in Ste2 sequences from any of the other sequenced Candida species (Fig. 3). The IC1 region, which encodes an intracellular loop, is required for the white cell cohesive response but is not necessary for mating of opaque cells (271).
FIG. 3.
Comparison of STE2 (α-factor receptor) sequences from the Candida clade and from S. cerevisiae. The IC1 and EC1 domains described by Yi et al. (271) are unique to C. albicans and C. dubliniensis. Motif IC1 is required for the white cell response to α pheromone.
The regulators of white-to-opaque switching (WOR1, WOR2, EFG1, and CZF1) are generally conserved in the other Candida species (38). However, the association of Wor1 with the transcriptional regulator Mcm1, and subsequent regulation of white-opaque switching, occurs only in the C. albicans and C. dubliniensis lineage (246). Zordan et al. (279) have hypothesized that white-opaque switching evolved during the association of C. albicans with the warm-blooded host, suggesting an association with virulence. Analysis of Wor1 (246) and Ste2 (271) supports the hypothesis that switching emerged in the ancestor of C. albicans and C. dubliniensis. It is not clear whether this ancestor was highly pathogenic (like C. albicans) or relatively attenuated (like C. dubliniensis), although recent evidence suggests that lack of pathogenesis in C. dubliniensis may result from gene loss or changes in regulatory pathways (66, 117, 184). White-opaque switching is not needed for pathogenesis in C. tropicalis and C. parapsilosis, which presumably also have a long association with the human host. However, it may confer an additional advantage on C. albicans.
Association of Wor1 with pathogenesis may be an ancient event predating the split of C, albicans and C. dubliniensis from other species and the evolution of white-opaque switching, because the WOR1 ortholog in the filamentous fungus Histoplasma capsulatum is required for the transition into the pathogenic yeast form (189).
However, it is also possible that Wor1 orthologs have a conserved role as regulators of morphological switches and that the associated with pathogenesis evolved independently in H. capsulatum and C. albicans.
MATING IN THE PEZIZOMYCOTINA
Several of the Pezizomycotina are associated with human disease, including the dimorphic pathogens (Penicillium marneffei, Histoplasma capsulatum, Coccidioides immitis, Coccidioides posadasii, Paraccoccidioides brasilienis, Blastomyces dermatidis, and Sporothrix schenckii). These species grow predominantly in mold form at low temperatures in the soil and undergo a switch to the pathogenic yeast form at body temperature (129). They cause disease ranging from the flu-like penicilliosis to rose-gardeners' disease, or sporothichosis. Many species are restricted to specific geographical locations; for example, P. marneffei is particularly prevalent in HIV patients in southeast Asia (250), and Coccidioides is endemic in the southwestern United States (199).
H. capsulatum reproduces asexually in the soil, generating microsporidia that are inhaled and infect the lung (262). The fungus can also reproduce sexually and is bipolar and heterothallic (136). The mating types were originally described as + or −. More recently, MAT in both H. capsulatum and the closely related species C. immitis and C. posadasii (32, 80) were described using the nomenclature of Turgeon and Yoder (248). In this system, the MATα-like idiomorph is termed MAT1-1 (and is equivalent to + in H. capsulatum) and the MATa-like idiomorph is MAT1-2.
In both H. capsulatum and the Coccidioides species, the MAT locus of the two mating types encodes either an α-box domain protein (MAT1-1-1) or an HMG domain protein (MAT1-2-1). In Coccidioides the MAT locus has been expanded and also contains mating-type specific alleles of APN2 and COX13. Mating has not yet been demonstrated in Coccidioides (though it is supported by population studies [34]), so it not clear if these genes have any functional role. It is possible that MAT loci have a tendency to expand and “capture” adjacent genes, and the Coccidioides structure may have arisen by a mechanism similar to that for the acquisition of PIK, PAP, and OBP by Candida species. A direct association of mating type with virulence has yet to be demonstrated, but an almost 7-fold predominance of MAT1-2 isolates of H. capsulatum in clinical samples suggests that there is one (138).
H. capsulatum and the Coccidioides species are closely related and belong to the Onygales order (Fig. 1), whereas P. marneffei is slightly more distant and is a member of the Eurotiales (the same group as the Aspergillus species). The population structure of P. marneffei is predominantly clonal, suggesting that it is asexual (73). In fact, P. marneffei is usually described as the best-studied and best-characterized asexual fungus (72). However, once again, analysis of the genome sequence provides evidence for a recent sexual cycle (261). Two MAT loci have been identified; MAT1-1 contains a MAT1-1-1 (α-box) gene and an unknown open reading frame, and MAT1-2 contains MAT1-2-1 (HMG domain) and a second open reading frame (MAT1-2-4). MAT1-1 and MAT1-2 idiomorphs are widely distributed among tested isolates, suggesting that the organism may have a hidden heterothallic sexual cycle or may have only recently lost the ability to mate. The structure of MAT in Penicillium chrysogenum, the main producer of the antibiotic penicillin, is very similar (108).
Aspergillus species are also part of the order Eurotiales, with the genus Aspergillus usually used to denote an asexual or anamorph form. A number of sexual species are closely related to the aspergilli, and by convention these are assigned to different genera, such as Neosartorya, Emericella, Petromyces, etc. (Fig. 4). It is becoming increasingly common, however, to use the term Aspergillus to refer to both sexual and nonsexual forms (84). A small number of species are associated with human infection. Aspergillus fumigatus (Neosartorya fumigati), which is associated with soil, decaying matter, and compost, is a major cause of infection of the lungs in immunocompromised patients, and it is also a primary pathogen and an allergen. Aspergillus flavus and Aspergillus terreus are much rarer causes of infection, and Aspergillus nidulans (Emericella nidulans), usually considered a model organism and nonpathogenic, has been isolated from patients with chronic granulomatous disease (229).
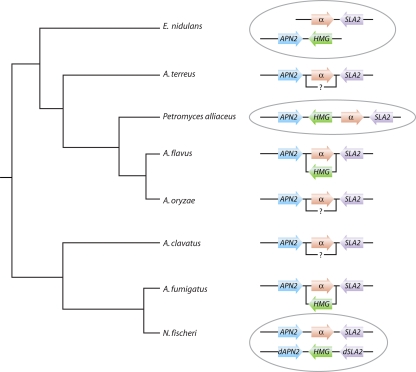
FIG. 4.
Organization of the mating-type-like locus in the aspergilli. The α-box domain proteins (MAT1-1-1) are shown in red and the HMG domain proteins (MAT1-2-1) in green. The MTL organization in homothallic species is circled in gray. Alternative idiomorphs in the heterothallic species are shown by an offset box below the α idiomorph. Some genes (such as MAT1-2-4 in A. fumigatus and other species) are omitted for clarity. A question mark indicates that the sequence of the MTLa-like idiomorph has not been sequenced, but isolates have been reported (64). (Based on data from references 82, 215, and 222 and http:/www.broadinstitute.org. The schematic phylogeny is based on data from references 68 and 205.)
Our understanding of mating in the aspergilli has been greatly advanced by the plethora of genome sequencing projects in the past few years. These include genomes of A. nidulans (82); two isolates of A. fumigatus and its close relative Neosartorya fischeri (68, 194); A. flavus (273) and its relative Aspergillus parasiticus, which are major causes of aflatoxin contamination in crops; A. terreus (http://www.broadinstitute.org/); Aspergillus clavatus (68); Aspergillus oryzae, an important food ingredient in Japan (169); and an industrial species, Aspergillus niger (200).
The first genomic analysis of mating in Aspergillus compared the MAT loci of the homothallic species A. nidulans with the equivalent structures in the apparently asexual species A. fumigatus and A. oryzae (Fig. 4) (82). The authors showed that both A. fumigatus and A. oryzae have structures reminiscent of sexual heterothallic species. Isolates contain either MAT1-1 or MAT1-2 idiomorphs, encoding an α-box protein (MAT1-1-1) or an HMG domain protein (MAT1-2-1), respectively. As in other filamentous ascomycetes, the MAT idiomorphs are flanked by SLA2 and APN2 genes (36).
The identification of two idiomorphs suggested that A. fumigatus and A. oryzae might have previously hidden sexual cycles. This was supported by an analysis of worldwide isolates of A. fumigatus, which indicated a 1:1 ratio of MAT1-1 to MAT1-2 idiomorphs (198). Evidence of sex was eventually obtained when cells of opposite mating type were successfully crossed following incubation on oatmeal agar for 6 months (195). The authors suggested that A. fumigatus should therefore also be assigned a teleomorph designation (Neosartorya fumigati), though it is likely that the former name will be more commonly used (101).
Idiomorph distribution is also approximately equal in A. flavus and in its close relative A. parasiticus (215). Crosses between isolates of A. parasiticus and of A. flavus with opposite mating types was reported in 2009 (109, 110). Like for A. fumigatus, successful crossing required long incubation times of 9 to 12 months.
The genome of the homothallic species A. nidulans contains both α-box and HMG domain genes, at different places in the genome (Fig. 4) (82). The association of the α-box gene with SLA2 and of the HMG domain with APN2 led to the suggestion that these regions were together in a homothallic ancestor and were separated by translocation in A. nidulans and by segregation in the heterothallic isolates (82). The suggestion that heterothallism arose from homothallism is not consistent with the proposal for evolution of mating proposed for Candida species (discussed above) or for mating in the more closely related Cochliobolus species (36, 247). As the genomes of more aspergilli were sequenced, it became clear that the heterothallic structure is more common than at first thought and that homothallism has arisen by more than one mechanism (Fig. 4). MAT1-1 and MAT1-2 idiomorphs have been identified in different isolates of Aspergillus sojae and supported by the genome sequences of A. terreus (64) and A. clavatus (http://www.broadinstitute.org/), suggesting that these species are heterothallic. In the homothallic species N. fischeri, both MAT1-1 and MAT1-2 idiomorphs are present in different parts of the genome (222). The MAT1-1 idiomorph is flanked by SLA2 and APN2 and is generally syntenic with A. fumigatus. The MAT1-2 region is flanked by portions of the SLA2 and APN2 genes and may have arisen by transposition. In contrast, the homothallic species Petromyces alliaceus has one MAT idiomorph, containing both α-box and HMG domain genes between APN2 and SLA2 (215) (Fig. 4). The variation in the structure of MAT in the homothallic species and the general conservation of gene order in the heterothallic isolates suggest that the ancestor of the aspergilli was heterothallic.
Role of VelvetA
It was generally assumed that sexual forms of the aspergilli are not associated with disease, as infection with Emericella, Neosartorya, Eurotium, and Petromyces is uncommon. However, as it is becoming increasingly clear that many of the Aspergillus species are likely to be heterothallic (although mating may be rare), it is possible that infection with homothallic, rather than heterothallic, species is uncommon (84). One possible explanation is that conidiation is reduced in homothallic isolates, and conidia are often the infectious particles (84).
In A. nidulans, asexual and sexual development is controlled by light, which inhibits sexual reproduction and is required for conidiation (183). The organism develops sexually in the dark. The VelvetA (veA) gene is required for light-dependent regulation, and mutations in veA allow conidiation in the dark (182). VeA also controls expression of secondary metabolism genes (123). The VeA protein is cytoplasmic in the light and accumulates in the nucleus in the dark (237). Red-light sensing via the cytochrome FphA is required for sexual development, and this may be how a signal is transmitted to VeA (21). However, other light receptor systems may also be involved (71).
The correlation between sexual development and secondary metabolism has only recently been unraveled. Most secondary metabolism genes are in clusters, and expression is cocoordinately regulated by the master regulator, LaeA (23). Bayram et al. (15) have shown that VeA and LaeA act together, in a complex that also contains a VeA-like protein called VelB. Expression of veA is increased in the dark, and the protein forms a complex with VelB in the cytoplasm, moves to the nucleus, and associates with LaeA. The trimeric complex is required for expression of secondary metabolite clusters; it is likely that a different protein (not LaeA) interacts with VeA and VelB to control sexual development. Several other VeA-like proteins and proteins that interact with VeA have been identified (43). In the light, veA expression is reduced, and VeA interacts only with VelB.
The VeA protein is conserved in other aspergilli and in other members of the Pezizomycotina, such as the dimorphic pathogen H. capsulatum and the Sordariomycetes Magnaporthe grisea, Fusarium verticillioides, and Neurospora crassa (43). The mechanism of action may not be identical however. Deletion of veA causes increased asexual development in A. flavus, whereas a similar deletion in A. parasiticus reduces conidiation (44, 63). Deletion of veA in A. fumigatus leads to reduced conidiation on nitrate-containing medium (130). In F. verticilliodes and N. crassa, deletion of the veA ortholog causes an increase in conidiation, independent of light (16, 147).
In H. capsulatum, the VeA-like proteins Ryp2 and Ryp3 control formation of vegetative spores, which can be inhaled into the host's lungs (258). At body temperature the fungus switches to infectious yeast-like growth. Ryp2 and Ryp3 are therefore important regulators of pathogenesis, but in H. capsulatum they respond to temperature rather than light. Interestingly, Ryp1, a master regulator of the transition to yeast growth (189) and an ortholog of Wor1 (discussed above), associates with the promoter of RYP2 and is regulated in a similar manner (258). The role of Ryp2 and Ryp3 in mating in H. capsulatum has not been investigated.
It has been hypothesized that inducing the sexual cycle in the dark may be advantageous in the soil-living organism A. nidulans, as the ascospores are more resistant to environmental challenge (64). A similar process may allow pathogens to resistant the challenging environment in the host during infection.
MATING IN THE BASIDIOMYCETES
Malassezia and Ustilago
Among the basidiomycetes, Cryptococcus species are the most common fungal pathogens, and these are described below. Malassezia species belong to a sister clade separate from Cryptococcus and are more closely related to the Ustilago plant pathogens. Malassezia species are not serious human pathogens; they are strongly associated with dandruff, although not all individuals with Malassezia on their skin have dandruff (51). The fungi have also been associated with skin diseases and, in rare instances, with systemic disease in neonates (14). The genome sequences of Malassezia globosa and part of Malassezia restricta were reported in 2007 (268).
The Ustilago genus and other related smut fungi include species with both bipolar and tetrapolar mating systems. The tetrapolar species U. maydis contains two unlinked loci, a and b, encoding pheromone and pheromone receptors and homeodomain transcription factors, respectively (69). In tetrapolar systems, mating occurs between cells that differ at both loci. This structure is common in the basidiomycetes, particularly in the homobasidiomycetes (mushroom fungi), which can have several thousand different mating types, and is assumed to promote outbreeding (79). The homeodomain proteins, which regulate cell type, fall into two classes; HD1 resembles the S. cerevisiae α2 protein, and HD2 resembles the S. cerevisiae a1 protein. Many basidiomycete MAT idiomorphs contain both HD1 and HD2 genes, but the HD1 proteins form heterodimers only with HD2 proteins from the mating partner. The pheromone and pheromone receptors are equivalent to the a-factor and a-factor receptor system in S. cerevisiae; no α-factor-like pheromones have been identified. Different idiomorphs express different alleles of the pheromone/receptor combination.
In the bipolar species Ustilago hordei, the a and b regions have been linked together to form a single large (430- to 500-kb) MAT locus, which also contains repetitive DNA and retrotransposons (9, 10). Recombination between the MAT alleles is reduced, possibly because of the repetitive DNA, as well as sequence rearrangements and indels. There have been several independent transitions between tetrapolar and bipolar mating systems in the smut fungi (reviewed in reference 111). The MAT locus of M. globosa resembles the bipolar structure of U. hordei (268). The M. globosa locus is smaller (∼170 kb), however, and does not contain repetitive DNA. Only one idiomorph has been identified in the sequenced isolate; it is assumed that mating occurs in nature with cells of the opposite mating type. This has not yet been observed, but it is supported by the identification of other mating-associated genes (such as orthologs of the pheromone signaling pathway) in M. globosa.
Mating in Ustilago and other smut fungi is directly related to virulence, as sexual reproduction is required to produce an infectious hyphal form which penetrates the plant (reviewed by Morrow and Fraser [185]). The fungus can sporulate only when inside the plant (11). It is not known if Malassezia has a similar life cycle. However, Cryptococcus isolates are often associated with trees (in particular, Eucalyptus) (33, 87, 100), and the recent demonstration that Cryptococcus can complete its life cycle when cultured on woody debris (25) suggests that there may be a general link between plants and mating in some of the Basidiomycota.
Cryptococcus neoformans
The human pathogens Cr. neoformans and its close relative Cr. gattii are two of at least six varieties that form the Cryptococcus group (70). Some have been distinguished by serotype for a long time, and it is gradually being recognized that these may represent distinct species. As well as Cr. gattii (serotypes B and C), which can be subdivided into four cryptic species, the group also includes Cr. neoformans var. neoformans (serotype D) and Cr. neoformans var. grubii (serotype A). Hybrids (AD) between Cr. neoformans var. neoformans and Cr. neoformans var. grubii have also been identified. Pathogenic Cryptococcus sp. are common in immunosuppressed hosts and are one of the major causes of human meningoencephalitis. They are also primary pathogens and a cause of pulmonary disease (201). The majority of clinical isolates (up to 95% of human infections) are serotype A, which are also more virulent in animal models (151).
Whereas most molecular analysis has been carried out with serotype A and D varieties of Cr. neoformans, the sibling species Cr. gattii is also a major pathogen, and caused an outbreak of infection on Victoria Island in British Columbia that is gradually spreading into the Pacific Northwest and beyond (39, 40, 78, 127).
The asexual and sexual life cycles of Cr. neoformans var. neoformans are well characterized. The sexual form was first described by Kwon-Chung (134) and was originally called Filobasidiella neoformans. Cells of opposite mating type (a and α) mate and fuse under nutrient-poor conditions. Nuclear fusion is delayed and occurs in basidia generated from filaments. Rounds of meiosis and mitosis produce chains of haploid basidiospores (135, 192).
The mating-type locus of Cryptococcus is very complex. Like U. hordei, Cryptococcus species are bipolar, with large (>100-kb) MAT idiomorphs that encompass both the pheromone/receptor region and homeodomain protein region of tetrapolar basidiomycetes at one site. However, the Cryptococcus MAT idiomorphs also contain genes involved in the pheromone response pathway and others required for meiosis and sporulation (77, 144). Up to 25% of the idiomorphs are composed of transposons and repetitive DNA. Unlike other basidiomycetes, the MAT idiomorphs contain only one homeodomain protein, i.e., HD1 in the MATα idiomorph and HD2 at MATa. Mating occurs between α and a isolates; however, in Cr. neoformans var. neoformans there is an approximately 45-fold-higher frequency of α isolates in environmental samples and 30-fold-higher incidence in clinical samples (137).
An initial analysis of Cr. neoformans var. grubii suggested that isolates contained MATα idiomorphs only (137, 145). A small number of MATa isolates were subsequently identified, predominantly of African origin (145, 154, 155, 256), leading to the suggestion that the sexual cycle is geographically limited in this species (153). However, the surprising identification of same-sex mating between α isolates in the lab (149) and between naturally occurring α isolates of Cr. neoformans var. neoformans and Cr. neoformans var. grubii (33, 150) indicated that a different form of sexual reproduction may be more commonplace. The diploids generated undergo meiosis and recombination. A recent survey of global populations of Cr. neoformans isolates revealed that approximately 8% are diploid and contain two MATα idiomorphs, with the majority derived from Cr. neoformans var. grubii (152). Fusion between identical cells is more common than fusion between unrelated isolates. Mating between α isolates of Cr. gattii has also been described (226). The existence of both haploids and diploids, plus the ability to undergo recombination via same-sex mating, may help Cryptococcus to adapt to changing conditions, such as during infection of the mammalian host (152) or by expanding its ecological range (45).
The direct impact of mating type on virulence is complex. In some backgrounds, congenic a and α cells (of both serotype A and serotype D) are equally virulent in animal models (191, 193). However, other studies suggest that α isolates in general are more virulent than a strains (12, 126, 145). Recently, Lin et al. (151) carried out a detailed analysis to investigate the effect of ploidy and mating type on virulence of serotype A and serotype AD isolates. Somewhat surprisingly, they found that diploid isolates are slightly less virulent than haploid isolates in a murine inhalation model. In AD hybrids, combinations of a only or of α and a idiomorphs had little effect on virulence of the diploids. However, hybrids containing two copies of the MATa idiomorphs were less virulent, supporting the hypothesis that mating type can be important for pathogenesis. It will take some time to identify which genes in MAT are important. Deleting the homeodomain gene SXI1α has no effect on virulence (114). However, the Cryptococcus MAT idiomorphs are large, and it is likely that many genes will be involved (see below).
THE PHEROMONE RESPONSE PATHWAY
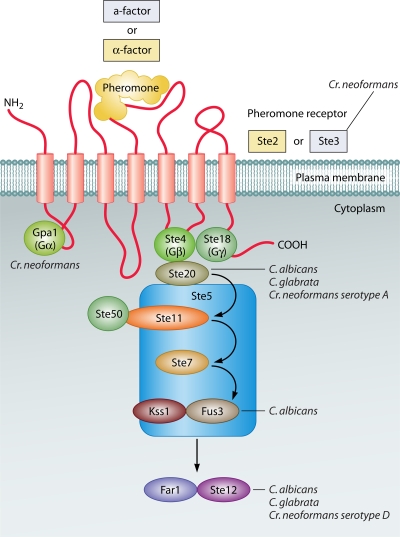
The pheromone response pathway is one of several mitogen-activated protein (MAP) kinase pathways in fungi (276). The pathway is best characterized in S. cerevisiae, but it is well conserved across all fungi (228) (Fig. 5). The pheromones (a- or α-factor) bind to one of two G-protein-coupled transmembrane receptors, resulting in dissociation of the Gα subunit and activation of the MAP kinase cascade. The pathway includes Ste20, a p21-activated protein kinase; Ste11 (a MAP kinase kinase kinase); Ste7 (a MAP kinase kinase); and two MAP kinases (Fus3 and Kss1). Ste11, Ste7, Fus3, and Kss1 are supported by the Ste5 scaffold protein. MAP kinases are activated by phosphorylation on both threonine and tyrosine residues. The signal is then transferred to the cyclin-dependent kinase inhibitor Far1 and the transcription factor Ste12, which regulate expression of genes required for mating. Many components of the pheromone response pathway are also involved in regulating pseudohyphal and filamentous growth in S. cerevisiae.
FIG. 5.
The pheromone response pathway in fungi. The pathway in S. cerevisiae is shown; there are differences in other fungi, but the core MAP kinase pathway is conserved. Genes in which disruptions affect virulence of human pathogens are indicated.
Components of the pheromone response pathway are highly conserved in pathogenic fungi, including species that are apparently asexual or parasexual (38, 170, 260). There is substantial evidence that the pathway is directly associated with pathogenesis in at least some fungi. In Fusarium graminearum, a causative agent of head-blight in wheat, disruption of the GPMK1 gene, an ortholog of FUS3, results in sterility and loss of virulence (121). Components of the mating MAP kinase cascade are also required for virulence of U. maydis, the rice blast fungus Magnaporthe grisea, and several other plant pathogenic fungi (276).
In human pathogens the association of the pheromone response pathway with virulence is less clear. In Fusarium oxysporum, a cause of wilt disease in plants and also an emerging opportunistic human pathogen, disrupting MAP kinase reduces virulence in plants but not in a mouse model (196). In C. albicans, however, the CEK1/CEK2 (KSS1/FUS3) MAP kinase pathway is required for mating, for the yeast-to-hypha transition, and for virulence (49, 157, 170, 221). Deletion of CST20 (an ortholog of STE20) and CEK1 (encoding a MAP kinase) reduces virulence in animal models (56, 96, 142), although deletion of other components (such as HST7 [STE7]) does not (142) (Fig. 5). The transcription factor Cph1 (an ortholog of Ste12) acts together with Efg1, and cph1/efg1 double mutants do not filament and are avirulent in many models of infection (156). STE20 and STE12 are also required for virulence of C. glabrata (41, 42).
In Cr. neoformans, many of the components of the pheromone signal transduction pathway are associated with the MAT locus, as discussed above. STE7 and CPK1 (MAP kinase) are not, and whereas mutants with gene disruptions cannot mate, there is no obvious effect on virulence (58). Most of the G-protein subunits are not required for virulence; however, a double mutant of gpa2 and gpa3 (encoding Gα subunits) is avirulent (146). Of the genes within the mating idiomorph, deleting STE11α has no effect on virulence (58), whereas deleting STE20α reduces virulence in serotype A but not serotype D strains (257) and deleting STE12α reduces virulence in serotype D (48, 274). The pheromone receptor genes (STE3a or STE3α) are also required for virulence (47, 52).
In human pathogenic fungi, it appears that components of the pheromone response pathway, rather that mating per se, are important for virulence. Zhao et al. (276) suggested that the mating MAP kinase pathway is more important for virulence in plant fungal pathogens, as they need to overcome physical host barriers, such as strong cell walls. In the human pathogens it appears likely that other MAP kinase pathways, such as the cell wall integrity pathway and the osmoregulatory pathway, are more important for virulence (221, 276).
ADVANTAGES AND DISADVANTAGES OF SEX
Evolutionary theory suggests that sex is expensive and inefficient, as it requires the formation of nonidentical haploid gametes, which must fuse to regenerate a diploid (89). Even in fungi, the need to maintain two mating partners reduces fitness (265, 266). However, sex emerged early in evolution, and most eukaryotes undergo a sexual cycle (176, 187). The benefits of sex, including recombination and adaptation, therefore greatly outweigh the efficiencies of mitotic reproduction (13). Homothallic species do not need to maintain mating partners, but the theoretical benefits of same-cell mating (as opposed to mitotic recombination) are not immediately obvious. However, homothallic species are also capable of outcrossing, and even a small amount of cross-fertilization is sufficient to maintain diversity.
The benefits of adaptation in fungi have been best characterized in studies of S. cerevisiae. Goddard et al. (88) generated strains carrying disruptions in the meiotic genes SPO11 and SPO13, which as a result can reproduce only asexually. They detected no change in fitness in sexual and asexual derivatives grown in rich media in laboratory conditions. Both adapted to harsh environments, but the sexual isolates had a higher increased fitness than the asexual ones. The results from similar experiments by Grimberg and Zeyl (95) were less clear-cut but suggested that sex is advantageous in harsh environments such as during infection. Subsequent analysis of large populations showed that recombination generates substrates for positive selection, as well as removing deleterious combinations of alleles (203). Recombination is also important for overcoming host resistance by the wheat pathogen Mycosphaerella graminicola (275).
In at least some human pathogenic fungi, the sexual process itself directly influences virulence. In Cr. neoformans the basidiospores are infectious propagules (85, 240, 255, 277). The spores are surrounded by a thick coat containing glucuronoxylomannan, which is also present in capsules, and are protected from environmental stresses such as high temperature, desiccation, and oxidative stress (26). Ascospores generated by sexual Aspergillus species are also resistant to environmental stress, which may be important for infection and for surviving in stressful environments such as in the soil (64, 209). Sexual reproduction (including homothallism) may be as important for the generation of resistant spores, as for the advantages of recombination.
CONCLUSIONS: ARE PATHOGENIC FUNGI GENERALLY ASEXUAL?
The signs of lack of sex, according to Schurko et al. (227), include (i) degeneration of sex genes, (ii) increased heterozygosity among alleles, (iii) a lack of transposable elements, and (iv) linkage disequilibria. As discussed in this review, genes at the mating locus and in the pheromone response pathway are highly conserved, even in apparently asexual fungi. The presence of a coding sequence, however, does not mean that the gene is correctly transcribed and translated. In C. glabrata, for example, the expression of the pheromone receptor genes is not regulated by cell type, and the cells are insensitive to the α-factor pheromone (186).
The best example supporting increased heterozygosity in asexual lineages came from an analysis of the genomes of bdelloid rotifers (173); however, it later emerged that these evolved from a degenerate tetraploid, which possibly arose from hybridization of two related but different species (174). The apparently asexual species C. parapsilosis has much lower levels of heterozygosity than its relatives with a parasexual cycle, whereas L. elongisporus, long believed to be homothallic, has high levels of heterozygosity (38, 141). The levels of heterozygosity depend on recombination that takes place in C. albicans even though no meiosis has been observed (75), and so this may not be an accurate indication of sex.
Uncontrolled proliferation of transposons may lead to extinction in asexual species (6). Ancient asexual species are therefore likely to evolve mechanisms to prevent proliferation and are predicted to be free of transposons (263). It is difficult to see how this hypothesis can be used to predict sex in fungi. All the Candida species, parasexual and fully sexual, contain retrotransposons, though the families differ between species (38, 90-93). Sex may have only recently been lost, or the transposons may have a beneficial function.
One of the best indications of recombination is independent assortment of alleles. Again, however, the data can be inconclusive, making it difficult to assess the importance of low levels of recombination. Most analysis of C. albicans suggests that the population is clonal (76, 167, 244, 267, 269). However, low levels of recombination are detected, even within isolates from the same clade (27, 81, 94). It is therefore likely that mating is rare. Analysis of A. fumigatus indicates that diversity levels are very low, particularly compared to those in its sexual relatives N. fischeri and Neosartorya spinosa (8, 223). However, evidence for low levels of recombination was reported (212, 254) and later was validated by the demonstration of a sexual cycle (195). Even P. marneffei, a highly clonal species, is being reevaluated following the description of its MAT locus (261). It is therefore difficult to use population data to conclude that species are fully asexual.
Nielsen and Heitman (192) hypothesized that the rare sexual reproduction in the major human fungal pathogens (C. albicans, A. fumigatus, and Cr. neoformans) allows them to proliferate in the host, while allowing recombination in stressful conditions. This is supported by similar studies of protozoa that infect humans (104). As more genomic data from fungal species become available, it is apparent that rare or cryptic sexual cycles are more common than previously realized. For example, the sexual cycle of the major pathogen A. fumigatus does not differ significantly from that of A. flavus (a rarer pathogen) or A. parasiticus (a plant pathogen) (109, 110). It is likely that many of the isolates of Aspergillus and Penicillium are heterothallic and mate in a similar manner, whether pathogenic or not (64, 108).
Our hypotheses are also influenced by the species with sequenced genomes. It has been suggested that up to 95% of fungal species have still to be described (119), and of the approximately 100,000 species that have been named, about 17,000 have no characterized sexual cycle (266). Those that are completely sequenced are heavily biased toward pathogens of both animals and plants (74). For example, the Candida clade contains many species that have not been associated with infection, some of which may also be parasexual (238). In the aspergilli, the sexual state of over 100 known species has not yet been described (208). Whereas there is substantial evidence that sex is advantageous in allowing adaptation to hostile environments, our interpretations of the role of mating in virulence, and the apparent avoidance of sex in human pathogens, may change significantly as more data become available, particularly from sister species.
Acknowledgments
Thanks go to K. Wolfe and members of the Butler lab for comments on the manuscript.
Research in the Butler lab is supported by Science Foundation Ireland.
Biography
 Geraldine Butler received her B.A.(Mod) and Ph.D. degrees from Trinity College Dublin, graduating in 1989. She then worked as postdoctoral fellow at the University of Michigan, where she studied the regulation of metallothionein expression in Saccharomyces cerevisiae. She returned to Ireland in 1992 to take up a position in the Department of Biochemistry at University College Dublin, and she is now an Associate Professor of Genetics and a Principal Investigator in the Conway Institute at UCD. Geraldine's interest in pathogenic fungi began during her postdoctoral work, and she took advantage of a sabbatical visit to the University of New South Wales in Sydney in 2000 to begin working with Candida glabrata. More recently she has developed an interest in genomics and evolution, and in particular she has investigated the evolution of mating in Candida species.
Geraldine Butler received her B.A.(Mod) and Ph.D. degrees from Trinity College Dublin, graduating in 1989. She then worked as postdoctoral fellow at the University of Michigan, where she studied the regulation of metallothionein expression in Saccharomyces cerevisiae. She returned to Ireland in 1992 to take up a position in the Department of Biochemistry at University College Dublin, and she is now an Associate Professor of Genetics and a Principal Investigator in the Conway Institute at UCD. Geraldine's interest in pathogenic fungi began during her postdoctoral work, and she took advantage of a sabbatical visit to the University of New South Wales in Sydney in 2000 to begin working with Candida glabrata. More recently she has developed an interest in genomics and evolution, and in particular she has investigated the evolution of mating in Candida species.
REFERENCES
- 1.Adam, R. D. 2001. Biology of Giardia lamblia. Clin. Microbiol. Rev. 14:447-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alby, K., and R. Bennett. 2009. Stress-induced phenotypic switching in Candida albicans. Mol. Biol. Cell. 20:3178-3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alby, K., D. Schaefer, and R. J. Bennett. 2009. Homothallic and heterothallic mating in the opportunistic pathogen Candida albicans. Nature 460:890-893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alcoba-Florez, J., S. Mendez-Alvarez, J. Cano, J. Guarro, E. Perez-Roth, and M. del Pilar Arevalo. 2005. Phenotypic and molecular characterization of Candida nivariensis sp. nov., a possible new opportunistic fungus. J. Clin. Microbiol. 43:4107-4111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson, J., R. Mihalik, and D. R. Soll. 1990. Ultrastructure and antigenicity of the unique cell wall pimple of the Candida opaque phenotype. J. Bacteriol. 172:224-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arkhipova, I., and M. Meselson. 2005. Deleterious transposable elements and the extinction of asexuals. Bioessays 27:76-85. [DOI] [PubMed] [Google Scholar]
- 7.Astell, C. R., L. Ahlstrom-Jonasson, M. Smith, K. Tatchell, K. A. Nasmyth, and B. D. Hall. 1981. The sequence of the DNAs coding for the mating-type loci of Saccharomyces cerevisiae. Cell 27:15-23. [DOI] [PubMed] [Google Scholar]
- 8.Bain, J. M., A. Tavanti, A. D. Davidson, M. D. Jacobsen, D. Shaw, N. A. Gow, and F. C. Odds. 2007. Multilocus sequence typing of the pathogenic fungus Aspergillus fumigatus. J. Clin. Microbiol. 45:1469-1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bakkeren, G., G. Jiang, R. L. Warren, Y. Butterfield, H. Shin, R. Chiu, R. Linning, J. Schein, N. Lee, G. Hu, D. M. Kupfer, Y. Tang, B. A. Roe, S. Jones, M. Marra, and J. W. Kronstad. 2006. Mating factor linkage and genome evolution in basidiomycetous pathogens of cereals. Fungal Genet. Biol. 43:655-666. [DOI] [PubMed] [Google Scholar]
- 10.Bakkeren, G., J. Kamper, and J. Schirawski. 2008. Sex in smut fungi: structure, function and evolution of mating-type complexes. Fungal Genet. Biol. 45(Suppl. 1):S15-S21. [DOI] [PubMed] [Google Scholar]
- 11.Banuett, F., and I. Herskowitz. 1996. Discrete developmental stages during teliospore formation in the corn smut fungus, Ustilago maydis. Development 122:2965-2976. [DOI] [PubMed] [Google Scholar]
- 12.Barchiesi, F., M. Cogliati, M. C. Esposto, E. Spreghini, A. M. Schimizzi, B. L. Wickes, G. Scalise, and M. A. Viviani. 2005. Comparative analysis of pathogenicity of Cryptococcus neoformans serotypes A, D and AD in murine cryptococcosis. J. Infect. 51:10-16. [DOI] [PubMed] [Google Scholar]
- 13.Barton, N. H., and B. Charlesworth. 1998. Why sex and recombination? Science 281:1986-1990. [PubMed] [Google Scholar]
- 14.Batra, R., T. Boekhout, E. Gueho, F. J. Cabanes, T. L. Dawson, Jr., and A. K. Gupta. 2005. Malassezia Baillon, emerging clinical yeasts. FEMS Yeast Res. 5:1101-1113. [DOI] [PubMed] [Google Scholar]
- 15.Bayram, O., S. Krappmann, M. Ni, J. W. Bok, K. Helmstaedt, O. Valerius, S. Braus-Stromeyer, N. J. Kwon, N. P. Keller, J. H. Yu, and G. H. Braus. 2008. VelB/VeA/LaeA complex coordinates light signal with fungal development and secondary metabolism. Science 320:1504-1506. [DOI] [PubMed] [Google Scholar]
- 16.Bayram, O., S. Krappmann, S. Seiler, N. Vogt, and G. H. Braus. 2008. Neurospora crassa ve-1 affects asexual conidiation. Fungal Genet. Biol. 45:127-138. [DOI] [PubMed] [Google Scholar]
- 17.Bennett, R. J., and A. D. Johnson. 2003. Completion of a parasexual cycle in Candida albicans by induced chromosome loss in tetraploid strains. EMBO J. 22:2505-2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bennett, R. J., M. A. Uhl, M. G. Miller, and A. D. Johnson. 2003. Identification and characterization of a Candida albicans mating pheromone. Mol. Cell Biol. 23:8189-8201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berman, J., and P. E. Sudbery. 2002. Candida albicans: a molecular revolution built on lessons from budding yeast. Nat. Rev. Genet. 3:918-930. [DOI] [PubMed] [Google Scholar]
- 20.Bilinski, C. A., N. Marmiroli, and J. J. Miller. 1989. Apomixis in Saccharomyces cerevisiae and other eukaryotic micro-organisms. Adv. Microb. Physiol. 30:23-52. [DOI] [PubMed] [Google Scholar]
- 21.Blumenstein, A., K. Vienken, R. Tasler, J. Purschwitz, D. Veith, N. Frankenberg-Dinkel, and R. Fischer. 2005. The Aspergillus nidulans phytochrome FphA represses sexual development in red light. Curr. Biol. 15:1833-1838. [DOI] [PubMed] [Google Scholar]
- 22.Bobola, N., R. P. Jansen, T. H. Shin, and K. Nasmyth. 1996. Asymmetric accumulation of Ash1p in postanaphase nuclei depends on a myosin and restricts yeast mating-type switching to mother cells. Cell 84:699-709. [DOI] [PubMed] [Google Scholar]
- 23.Bok, J. W., S. A. Balajee, K. A. Marr, D. Andes, K. F. Nielsen, J. C. Frisvad, and N. P. Keller. 2005. LaeA, a regulator of morphogenetic fungal virulence factors. Eukaryot. Cell 4:1574-1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borman, A. M., R. Petch, C. J. Linton, M. D. Palmer, P. D. Bridge, and E. M. Johnson. 2008. Candida nivariensis, an emerging pathogenic fungus with multidrug resistance to antifungal agents. J. Clin. Microbiol. 46:933-938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Botes, A., T. Boekhout, F. Hagen, H. Vismer, J. Swart, and A. Botha. 2009. Growth and mating of Cryptococcus neoformans var. grubii on woody debris. Microb. Ecol. 57:757-765. [DOI] [PubMed] [Google Scholar]
- 26.Botts, M. R., S. S. Giles, M. A. Gates, T. R. Kozel, and C. M. Hull. 2009. Isolation and characterization of Cryptococcus neoformans spores reveal a critical role for capsule biosynthesis genes in spore biogenesis. Eukaryot. Cell 8:595-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bougnoux, M. E., C. Pujol, D. Diogo, C. Bouchier, D. R. Soll, and C. d'Enfert. 2008. Mating is rare within as well as between clades of the human pathogen Candida albicans. Fungal Genet. Biol. 45:221-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boyartchuk, V. L., M. N. Ashby, and J. Rine. 1997. Modulation of Ras and a-factor function by carboxyl-terminal proteolysis. Science 275:1796-1800. [DOI] [PubMed] [Google Scholar]
- 29.Bremer, M. C., F. S. Gimble, J. Thorner, and C. L. Smith. 1992. VDE endonuclease cleaves Saccharomyces cerevisiae genomic DNA at a single site: physical mapping of the VMA1 gene. Nucleic Acids Res. 20:5484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brisse, S., C. Pannier, A. Angoulvant, T. de Meeus, L. Diancourt, O. Faure, H. Muller, J. Peman, M. A. Viviani, R. Grillot, B. Dujon, C. Fairhead, and C. Hennequin. 2009. Uneven distribution of mating types among genotypes of Candida glabrata isolates from clinical samples. Eukaryot. Cell 8:287-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brockert, P. J., S. A. Lachke, T. Srikantha, C. Pujol, R. Galask, and D. R. Soll. 2003. Phenotypic switching and mating type switching of Candida glabrata at sites of colonization. Infect. Immun. 71:7109-7118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bubnick, M., and A. G. Smulian. 2007. The MAT1 locus of Histoplasma capsulatum is responsive in a mating type-specific manner. Eukaryot. Cell 6:616-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bui, T., X. Lin, R. Malik, J. Heitman, and D. Carter. 2008. Isolates of Cryptococcus neoformans from infected animals reveal genetic exchange in unisexual, alpha mating type populations. Eukaryot. Cell 7:1771-1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burt, A., D. A. Carter, G. L. Koenig, T. J. White, and J. W. Taylor. 1996. Molecular markers reveal cryptic sex in the human pathogen Coccidioides immitis. Proc. Natl. Acad. Sci. U. S. A. 93:770-773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burt, A., and V. Koufopanou. 2004. Homing endonuclease genes: the rise and fall and rise again of a selfish element. Curr. Opin. Genet. Dev. 14:609-615. [DOI] [PubMed] [Google Scholar]
- 36.Butler, G. 2007. The evolution of MAT: the ascomycetes, p. 3-18. In J. Heitman, J. W. Kronstad, J. W. Taylor, and L. A. Casselton (ed.), Sex in fungi. ASM Press, Washington, DC.
- 37.Butler, G., C. Kenny, A. Fagan, C. Kurischko, C. Gaillardin, and K. H. Wolfe. 2004. Evolution of the MAT locus and its Ho endonuclease in yeast species. Proc. Natl. Acad. Sci. U. S. A. 101:1632-1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Butler, G., M. D. Rasmussen, M. F. Lin, M. A. Santos, S. Sakthikumar, C. A. Munro, E. Rheinbay, M. Grabherr, A. Forche, J. L. Reedy, I. Agrafioti, M. B. Arnaud, S. Bates, A. J. Brown, S. Brunke, M. C. Costanzo, D. A. Fitzpatrick, P. W. de Groot, D. Harris, L. L. Hoyer, B. Hube, F. M. Klis, C. Kodira, N. Lennard, M. E. Logue, R. Martin, A. M. Neiman, E. Nikolaou, M. A. Quail, J. Quinn, M. C. Santos, F. F. Schmitzberger, G. Sherlock, P. Shah, K. A. Silverstein, M. S. Skrzypek, D. Soll, R. Staggs, I. Stansfield, M. P. Stumpf, P. E. Sudbery, T. Srikantha, Q. Zeng, J. Berman, M. Berriman, J. Heitman, N. A. Gow, M. C. Lorenz, B. W. Birren, M. Kellis, and C. A. Cuomo. 2009. Evolution of pathogenicity and sexual reproduction in eight Candida genomes. Nature 459:657-662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Byrnes, E. J., III, R. J. Bildfell, S. A. Frank, T. G. Mitchell, K. A. Marr, and J. Heitman. 2009. Molecular evidence that the range of the Vancouver Island outbreak of Cryptococcus gattii infection has expanded into the Pacific Northwest in the United States. J. Infect. Dis. 199:1081-1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Byrnes, E. J., III, W. Li, Y. Lewit, J. R. Perfect, D. A. Carter, G. M. Cox, and J. Heitman. 2009. First reported case of Cryptococcus gattii in the Southeastern U. S. A.: implications for travel-associated acquisition of an emerging pathogen. PLoS One 4:e5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Calcagno, A. M., E. Bignell, T. R. Rogers, M. Canedo, F. A. Muhlschlegel, and K. Haynes. 2004. Candida glabrata Ste20 is involved in maintaining cell wall integrity and adaptation to hypertonic stress, and is required for wild-type levels of virulence. Yeast 21:557-568. [DOI] [PubMed] [Google Scholar]
- 42.Calcagno, A. M., E. Bignell, P. Warn, M. D. Jones, D. W. Denning, F. A. Muhlschlegel, T. R. Rogers, and K. Haynes. 2003. Candida glabrata STE12 is required for wild-type levels of virulence and nitrogen starvation induced filamentation. Mol. Microbiol. 50:1309-1318. [DOI] [PubMed] [Google Scholar]
- 43.Calvo, A. M. 2008. The VeA regulatory system and its role in morphological and chemical development in fungi. Fungal Genet. Biol. 45:1053-1061. [DOI] [PubMed] [Google Scholar]
- 44.Calvo, A. M., J. Bok, W. Brooks, and N. P. Keller. 2004. veA is required for toxin and sclerotial production in Aspergillus parasiticus. Appl. Environ. Microbiol. 70:4733-4739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Campbell, L. T., and D. A. Carter. 2006. Looking for sex in the fungal pathogens Cryptococcus neoformans and Cryptococcus gattii. FEMS Yeast Res. 6:588-598. [DOI] [PubMed] [Google Scholar]
- 46.Casselton, L. A., and N. S. Olesnicky. 1998. Molecular genetics of mating recognition in basidiomycete fungi. Microbiol. Mol. Biol. Rev. 62:55-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chang, Y. C., G. F. Miller, and K. J. Kwon-Chung. 2003. Importance of a developmentally regulated pheromone receptor of Cryptococcus neoformans for virulence. Infect. Immun. 71:4953-4960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chang, Y. C., B. L. Wickes, G. F. Miller, L. A. Penoyer, and K. J. Kwon-Chung. 2000. Cryptococcus neoformans STE12alpha regulates virulence but is not essential for mating. J. Exp. Med. 191:871-882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen, J., S. Lane, and H. Liu. 2002. A conserved mitogen-activated protein kinase pathway is required for mating in Candida albicans. Mol. Microbiol. 46:1335-1344. [DOI] [PubMed] [Google Scholar]
- 50.Chen, P., S. K. Sapperstein, J. D. Choi, and S. Michaelis. 1997. Biogenesis of the Saccharomyces cerevisiae mating pheromone a-factor. J. Cell Biol. 136:251-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen, T. A., and P. B. Hill. 2005. The biology of Malassezia organisms and their ability to induce immune responses and skin disease. Vet. Dermatol. 16:4-26. [DOI] [PubMed] [Google Scholar]
- 52.Chung, S., M. Karos, Y. C. Chang, J. Lukszo, B. L. Wickes, and K. J. Kwon-Chung. 2002. Molecular analysis of CPRalpha, a MATalpha-specific pheromone receptor gene of Cryptococcus neoformans. Eukaryot. Cell 1:432-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cooper, M. A., R. D. Adam, M. Worobey, and C. R. Sterling. 2007. Population genetics provides evidence for recombination in Giardia. Curr. Biol. 17:1984-1988. [DOI] [PubMed] [Google Scholar]
- 54.Correia, A., P. Sampaio, S. James, and C. Pais. 2006. Candida bracarensis sp. nov., a novel anamorphic yeast species phenotypically similar to Candida glabrata. Int. J. Syst. Evol. Microbiol. 56:313-317. [DOI] [PubMed] [Google Scholar]
- 55.Cosma, M. P., T. Tanaka, and K. Nasmyth. 1999. Ordered recruitment of transcription and chromatin remodeling factors to a cell cycle- and developmentally regulated promoter. Cell 97:299-311. [DOI] [PubMed] [Google Scholar]
- 56.Csank, C., K. Schroppel, E. Leberer, D. Harcus, O. Mohamed, S. Meloche, D. Y. Thomas, and M. Whiteway. 1998. Roles of the Candida albicans mitogen-activated protein kinase homolog, Cek1p, in hyphal development and systemic candidiasis. Infect. Immun. 66:2713-2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Daniels, K. J., T. Srikantha, S. R. Lockhart, C. Pujol, and D. R. Soll. 2006. Opaque cells signal white cells to form biofilms in Candida albicans. EMBO J. 25:2240-2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Davidson, R. C., C. B. Nichols, G. M. Cox, J. R. Perfect, and J. Heitman. 2003. A MAP kinase cascade composed of cell type specific and non-specific elements controls mating and differentiation of the fungal pathogen Cryptococcus neoformans. Mol. Microbiol. 49:469-485. [DOI] [PubMed] [Google Scholar]
- 59.Desnos-Ollivier, M., M. Ragon, V. Robert, D. Raoux, J. C. Gantier, and F. Dromer. 2008. Debaryomyces hansenii (Candida famata), a rare human fungal pathogen often misidentified as Pichia guilliermondii (Candida guilliermondii). J. Clin. Microbiol. 46:3237-3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dietrich, F. S., S. Voegeli, S. Brachat, A. Lerch, K. Gates, S. Steiner, C. Mohr, R. Pohlmann, P. Luedi, S. Choi, R. A. Wing, A. Flavier, T. D. Gaffney, and P. Philippsen. 2004. The Ashbya gossypii genome as a tool for mapping the ancient Saccharomyces cerevisiae genome. Science 304:304-307. [DOI] [PubMed] [Google Scholar]
- 61.Dignard, D., A. L. El-Naggar, M. E. Logue, G. Butler, and M. Whiteway. 2007. Identification and characterization of MFA1, the gene encoding Candida albicans a-factor pheromone. Eukaryot. Cell 6:487-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dodgson, A. R., C. Pujol, M. A. Pfaller, D. W. Denning, and D. R. Soll. 2005. Evidence for recombination in Candida glabrata. Fungal Genet. Biol. 42:233-243. [DOI] [PubMed] [Google Scholar]
- 63.Duran, R. M., J. W. Cary, and A. M. Calvo. 2007. Production of cyclopiazonic acid, aflatrem, and aflatoxin by Aspergillus flavus is regulated by veA, a gene necessary for sclerotial formation. Appl. Microbiol. Biotechnol. 73:1158-1168. [DOI] [PubMed] [Google Scholar]
- 64.Dyer, P. S. 2007. Sexual reproduction and significance of MAT in the aspergilli, p. 123-142. In J. Heitman, J. W. Kronstad, J. W. Taylor, and L. A. Casselton (ed.), Sex in fungi. ASM Press, Washington, DC.
- 65.Egan, M. J., and N. J. Talbot. 2008. Genomes, free radicals and plant cell invasion: recent developments in plant pathogenic fungi. Curr. Opin. Plant Biol. 11:367-372. [DOI] [PubMed] [Google Scholar]
- 66.Enjalbert, B., G. P. Moran, C. Vaughan, T. Yeomans, D. M. Maccallum, J. Quinn, D. C. Coleman, A. J. Brown, and D. J. Sullivan. 2009. Genome-wide gene expression profiling and a forward genetic screen show that differential expression of the sodium ion transporter Ena21 contributes to the differential tolerance of Candida albicans and Candida dubliniensis to osmotic stress. Mol. Microbiol. 72:216-228. [DOI] [PubMed] [Google Scholar]
- 67.Fabre, E., H. Muller, P. Therizols, I. Lafontaine, B. Dujon, and C. Fairhead. 2005. Comparative genomics in hemiascomycete yeasts: evolution of sex, silencing, and subtelomeres. Mol. Biol. Evol. 22:856-873. [DOI] [PubMed] [Google Scholar]
- 68.Fedorova, N. D., N. Khaldi, V. S. Joardar, R. Maiti, P. Amedeo, M. J. Anderson, J. Crabtree, J. C. Silva, J. H. Badger, A. Albarraq, S. Angiuoli, H. Bussey, P. Bowyer, P. J. Cotty, P. S. Dyer, A. Egan, K. Galens, C. M. Fraser-Liggett, B. J. Haas, J. M. Inman, R. Kent, S. Lemieux, I. Malavazi, J. Orvis, T. Roemer, C. M. Ronning, J. P. Sundaram, G. Sutton, G. Turner, J. C. Venter, O. R. White, B. R. Whitty, P. Youngman, K. H. Wolfe, G. H. Goldman, J. R. Wortman, B. Jiang, D. W. Denning, and W. C. Nierman. 2008. Genomic islands in the pathogenic filamentous fungus Aspergillus fumigatus. PLoS Genet. 4:e1000046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Feldbrugge, M., J. Kamper, G. Steinberg, and R. Kahmann. 2004. Regulation of mating and pathogenic development in Ustilago maydis. Curr. Opin. Microbiol. 7:666-672. [DOI] [PubMed] [Google Scholar]
- 70.Findley, K., M. Rodriguez-Carres, B. Metin, J. Kroiss, A. Fonseca, R. Vilgalys, and J. Heitman. 2009. Phylogeny and phenotypic characterization of pathogenic Cryptococcus species and closely related saprobic taxa in the Tremellales. Eukaryot. Cell 8:353-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fischer, R. 2008. Developmental biology. Sex and poison in the dark. Science 320:1430-1431. [DOI] [PubMed] [Google Scholar]
- 72.Fisher, M. C. 2007. The evolutionary implications of anasexual lifestyle manifested by Penicillium marneffei, p. 201-212. In J. Heitman, J. W. Kronstad, J. W. Taylor, and L. A. Casselton (ed.), Sex in fungi. ASM Press, Washington, DC.
- 73.Fisher, M. C., W. P. Hanage, S. de Hoog, E. Johnson, M. D. Smith, N. J. White, and N. Vanittanakom. 2005. Low effective dispersal of asexual genotypes in heterogeneous landscapes by the endemic pathogen Penicillium marneffei. PLoS Pathog. 1:e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fitzpatrick, D. A., M. E. Logue, J. E. Stajich, and G. Butler. 2006. A fungal phylogeny based on 42 complete genomes derived from supertree and combined gene analysis. BMC Evol. Biol. 6:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Forche, A., K. Alby, D. Schaefer, A. D. Johnson, J. Berman, and R. J. Bennett. 2008. The parasexual cycle in Candida albicans provides an alternative pathway to meiosis for the formation of recombinant strains. PLoS Biol. 6:e110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Forche, A., G. Schonian, Y. Graser, R. Vilgalys, and T. G. Mitchell. 1999. Genetic structure of typical and atypical populations of Candida albicans from Africa. Fungal Genet. Biol. 28:107-125. [DOI] [PubMed] [Google Scholar]
- 77.Fraser, J. A., S. Diezmann, R. L. Subaran, A. Allen, K. B. Lengeler, F. S. Dietrich, and J. Heitman. 2004. Convergent evolution of chromosomal sex-determining regions in the animal and fungal kingdoms. PLoS Biol. 2:e384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fraser, J. A., S. S. Giles, E. C. Wenink, S. G. Geunes-Boyer, J. R. Wright, S. Diezmann, A. Allen, J. E. Stajich, F. S. Dietrich, J. R. Perfect, and J. Heitman. 2005. Same-sex mating and the origin of the Vancouver Island Cryptococcus gattii outbreak. Nature 437:1360-1364. [DOI] [PubMed] [Google Scholar]
- 79.Fraser, J. A., Y. P. Hsueh, K. M. Findley, and J. Heitman. 2007. Evolution of the mating-type locus: the basidiomycetes, p. 19-34. In J. Heitman, J. W. Kronstad, J. W. Taylor, and L. A. Casselton (ed.), Sex in fungi. ASM Press, Washington, DC.
- 80.Fraser, J. A., J. E. Stajich, E. J. Tarcha, G. T. Cole, D. O. Inglis, A. Sil, and J. Heitman. 2007. Evolution of the mating type locus: insights gained from the dimorphic primary fungal pathogens Histoplasma capsulatum, Coccidioides immitis, and Coccidioides posadasii. Eukaryot. Cell 6:622-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fundyga, R. E., T. J. Lott, and J. Arnold. 2002. Population structure of Candida albicans, a member of the human flora, as determined by microsatellite loci. Infect. Genet. Evol. 2:57-68. [DOI] [PubMed] [Google Scholar]
- 82.Galagan, J. E., S. E. Calvo, C. Cuomo, L. J. Ma, J. R. Wortman, S. Batzoglou, S. I. Lee, M. Basturkmen, C. C. Spevak, J. Clutterbuck, V. Kapitonov, J. Jurka, C. Scazzocchio, M. Farman, J. Butler, S. Purcell, S. Harris, G. H. Braus, O. Draht, S. Busch, C. D'Enfert, C. Bouchier, G. H. Goldman, D. Bell-Pedersen, S. Griffiths-Jones, J. H. Doonan, J. Yu, K. Vienken, A. Pain, M. Freitag, E. U. Selker, D. B. Archer, M. A. Penalva, B. R. Oakley, M. Momany, T. Tanaka, T. Kumagai, K. Asai, M. Machida, W. C. Nierman, D. W. Denning, M. Caddick, M. Hynes, M. Paoletti, R. Fischer, B. Miller, P. Dyer, M. S. Sachs, S. A. Osmani, and B. W. Birren. 2005. Sequencing of Aspergillus nidulans and comparative analysis with A. fumigatus and A. oryzae. Nature 438:1105-1115. [DOI] [PubMed] [Google Scholar]
- 83.Geiger, J., D. Wessels, S. R. Lockhart, and D. R. Soll. 2004. Release of a potent polymorphonuclear leukocyte chemoattractant is regulated by white-opaque switching in Candida albicans. Infect. Immun. 72:667-677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Geiser, D. M. 2009. Sexual structures in Aspergillus: morphology, importance and genomics. Med. Mycol. 47(Suppl. 1):S21-S26. [DOI] [PubMed] [Google Scholar]
- 85.Giles, S. S., T. R. Dagenais, M. R. Botts, N. P. Keller, and C. M. Hull. 2009. Elucidating the pathogenesis of spores from the human fungal pathogen Cryptococcus neoformans. Infect. Immun. 77:3491-3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gimble, F. S., and J. Thorner. 1992. Homing of a DNA endonuclease gene by meiotic gene conversion in Saccharomyces cerevisiae. Nature 357:301-306. [DOI] [PubMed] [Google Scholar]
- 87.Girish Kumar, C. P., D. Prabu, H. Mitani, Y. Mikami, and T. Menon. Environmental isolation of Cryptococcus neoformans and Cryptococcus gattii from living trees in Guindy National Park, Chennai, South India. Mycoses, in press. [DOI] [PubMed]
- 88.Goddard, M., H. Godfray, and A. Burt. 2005. Sex increases the efficacy of natural selection in experimental yeast populations. Nature 434:636-640. [DOI] [PubMed] [Google Scholar]
- 89.Goddard, M. R. 2007. Why bother with sex?, p. 489-506. In J. Heitman, J. W. Kronstad, J. W. Taylor, and L. A. Casselton (ed.), Sex in fungi. ASM Press, Wasington, DC.
- 90.Goodwin, T. J., D. E. Dalle Nogare, M. I. Butler, and R. T. Poulter. 2003. Ty3/gypsy-like retrotransposons in Candida albicans and Candida dubliniensis: Tca3 and Tcd3. Yeast 20:493-508. [DOI] [PubMed] [Google Scholar]
- 91.Goodwin, T. J., J. E. Ormandy, and R. T. Poulter. 2001. L1-like non-LTR retrotransposons in the yeast Candida albicans. Curr. Genet. 39:83-91. [DOI] [PubMed] [Google Scholar]
- 92.Goodwin, T. J., and R. T. Poulter. 2000. Multiple LTR-retrotransposon families in the asexual yeast Candida albicans. Genome Res. 10:174-191. [DOI] [PubMed] [Google Scholar]
- 93.Goodwin, T. J., and R. T. Poulter. 1998. The CARE-2 and rel-2 repetitive elements of Candida albicans contain LTR fragments of a new retrotransposon. Gene 218:85-93. [DOI] [PubMed] [Google Scholar]
- 94.Graser, Y., M. Volovsek, J. Arrington, G. Schonian, W. Presber, T. G. Mitchell, and R. Vilgalys. 1996. Molecular markers reveal that population structure of the human pathogen Candida albicans exhibits both clonality and recombination. Proc. Natl. Acad. Sci. U. S. A. 93:12473-12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Grimberg, B., and C. Zeyl. 2005. The effects of sex and mutation rate on adaptation in test tubes and to mouse hosts by Saccharomyces cerevisiae. Evolution 59:431-438. [PubMed] [Google Scholar]
- 96.Guhad, F. A., H. E. Jensen, B. Aalbaek, C. Csank, O. Mohamed, D. Harcus, D. Y. Thomas, M. Whiteway, and J. Hau. 1998. Mitogen-activated protein kinase-defective Candida albicans is avirulent in a novel model of localized murine candidiasis. FEMS Microbiol. Lett. 166:135-139. [DOI] [PubMed] [Google Scholar]
- 97.Haber, J. E. 1998. Mating-type gene switching in Saccharomyces cerevisiae. Annu. Rev. Genet. 32:561-599. [DOI] [PubMed] [Google Scholar]
- 98.Haber, J. E., and K. H. Wolfe. 2005. Function and evolution of HO and VDE endonucleases in fungi. Nucleic Acids Mol. Biol. 16:161-175. [Google Scholar]
- 99.Hall, A. R., and N. Colegrave. 2008. Decay of unused characters by selection and drift. J. Evol. Biol. 21:610-617. [DOI] [PubMed] [Google Scholar]
- 100.Halliday, C. L., and D. A. Carter. 2003. Clonal reproduction and limited dispersal in an environmental population of Cryptococcus neoformans var. gattii isolates from Australia. J. Clin. Microbiol. 41:703-711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hawksworth, D. L. 2009. Separate name for fungus's sexual stage may cause confusion. Nature 458:29. [DOI] [PubMed] [Google Scholar]
- 102.Heckman, D. S., D. M. Geiser, B. R. Eidell, R. L. Stauffer, N. L. Kardos, and S. B. Hedges. 2001. Molecular evidence for the early colonization of land by fungi and plants. Science 293:1129-1133. [DOI] [PubMed] [Google Scholar]
- 103.Hedges, S. B., J. E. Blair, M. L. Venturi, and J. L. Shoe. 2004. A molecular timescale of eukaryote evolution and the rise of complex multicellular life. BMC Evol. Biol. 4:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Heitman, J. 2006. Sexual reproduction and the evolution of microbial pathogens. Curr. Biol. 16:R711-R725. [DOI] [PubMed] [Google Scholar]
- 105.Herman, A., and H. Roman. 1966. Allele specific determinants of homothallism in Saccharomyces lactis. Genetics 53:727-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hicks, J., and J. N. Strathern. 1977. Interconversion of mating type in S. cerevisiae and the cassette model for gene transfer. Brookhaven Symp. Biol. May 12-20:233-242. [PubMed] [Google Scholar]
- 107.Hicks, J. B., J. N. Strathern, and I. Herskowitz. 1977. The cassette model of mating type interconversion, p. 457-462. In M. N. Hall and P. Linder (ed.), DNA insertion elements, plasmids and episomes. Cold Spring Harbor Laboratory, Plainview, NY.
- 108.Hoff, B., S. Poggeler, and U. Kuck. 2008. Eighty years after its discovery, Fleming's Penicillium strain discloses the secret of its sex. Eukaryot. Cell 7:465-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Horn, B. W., G. G. Moore, and I. Carbone. 2009. Sexual reproduction in Aspergillus flavus. Mycologia 101:423-429. [DOI] [PubMed] [Google Scholar]
- 110.Horn, B. W., J. H. Ramirez-Prado, and I. Carbone. 2009. Sexual reproduction and recombination in the aflatoxin-producing fungus Aspergillus parasiticus. Fungal Genet. Biol. 46:169-175. [DOI] [PubMed] [Google Scholar]
- 111.Hsueh, Y. P., and J. Heitman. 2008. Orchestration of sexual reproduction and virulence by the fungal mating-type locus. Curr. Opin. Microbiol. 11:517-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Huang, G., T. Srikantha, N. Sahni, S. Yi, and D. R. Soll. 2009. CO(2) regulates white-to-opaque switching in Candida albicans. Curr. Biol. 19:330-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Huang, G., H. Wang, S. Chou, X. Nie, J. Chen, and H. Liu. 2006. Bistable expression of WOR1, a master regulator of white-opaque switching in Candida albicans. Proc. Natl. Acad. Sci. U. S. A. 103:12813-12818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hull, C. M., G. M. Cox, and J. Heitman. 2004. The alpha-specific cell identity factor Sxi1alpha is not required for virulence of Cryptococcus neoformans. Infect. Immun. 72:3643-3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hull, C. M., and A. D. Johnson. 1999. Identification of a mating type-like locus in the asexual pathogenic yeast Candida albicans. Science 285:1271-1275. [DOI] [PubMed] [Google Scholar]
- 116.Hull, C. M., R. M. Raisner, and A. D. Johnson. 2000. Evidence for mating of the “asexual” yeast Candida albicans in a mammalian host. Science 289:307-310. [DOI] [PubMed] [Google Scholar]
- 117.Jackson, A. P., J. A. Gamble, T. Yeomans, G. P. Moran, D. Saunders, D. Harris, M. Aslett, J. F. Barrell, G. Butler, F. Citiulo, D. C. Coleman, P. W. de Groot, T. J. Goodwin, M. A. Quail, J. McQuillan, C. A. Munro, A. Pain, R. T. Poulter, M. A. Rajandream, H. Renauld, M. J. Spiering, A. Tivey, N. A. Gow, B. Barrell, D. J. Sullivan, and M. Berriman. 2009. Comparative genomics of the fungal pathogens Candida dubliniensis and C. albicans. Genome Res. 19:2231-2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.James, S. A., M. D. Collins, and I. N. Roberts. 1994. The genetic relationship of Lodderomyces elongisporus to other ascomycete yeast species as revealed by small-subunit rRNA gene sequences. Lett. Appl. Microbiol. 19:308-311. [DOI] [PubMed] [Google Scholar]
- 119.James, T. Y., F. Kauff, C. L. Schoch, P. B. Matheny, V. Hofstetter, C. J. Cox, G. Celio, C. Gueidan, E. Fraker, J. Miadlikowska, H. T. Lumbsch, A. Rauhut, V. Reeb, A. E. Arnold, A. Amtoft, J. E. Stajich, K. Hosaka, G. H. Sung, D. Johnson, B. O'Rourke, M. Crockett, M. Binder, J. M. Curtis, J. C. Slot, Z. Wang, A. W. Wilson, A. Schussler, J. E. Longcore, K. O'Donnell, S. Mozley-Standridge, D. Porter, P. M. Letcher, M. J. Powell, J. W. Taylor, M. M. White, G. W. Griffith, D. R. Davies, R. A. Humber, J. B. Morton, J. Sugiyama, A. Y. Rossman, J. D. Rogers, D. H. Pfister, D. Hewitt, K. Hansen, S. Hambleton, R. A. Shoemaker, J. Kohlmeyer, B. Volkmann-Kohlmeyer, R. A. Spotts, M. Serdani, P. W. Crous, K. W. Hughes, K. Matsuura, E. Langer, G. Langer, W. A. Untereiner, R. Lucking, B. Budel, D. M. Geiser, A. Aptroot, P. Diederich, I. Schmitt, M. Schultz, R. Yahr, D. S. Hibbett, F. Lutzoni, D. J. McLaughlin, J. W. Spatafora, and R. Vilgalys. 2006. Reconstructing the early evolution of Fungi using a six-gene phylogeny. Nature 443:818-822. [DOI] [PubMed] [Google Scholar]
- 120.Jeffries, T. W., I. V. Grigoriev, J. Grimwood, J. M. Laplaza, A. Aerts, A. Salamov, J. Schmutz, E. Lindquist, P. Dehal, H. Shapiro, Y. S. Jin, V. Passoth, and P. M. Richardson. 2007. Genome sequence of the lignocellulose-bioconverting and xylose-fermenting yeast Pichia stipitis. Nat. Biotechnol. 25:319-326. [DOI] [PubMed] [Google Scholar]
- 121.Jenczmionka, N. J., F. J. Maier, A. P. Losch, and W. Schafer. 2003. Mating, conidiation and pathogenicity of Fusarium graminearum, the main causal agent of the head-blight disease of wheat, are regulated by the MAP kinase gpmk1. Curr. Genet. 43:87-95. [DOI] [PubMed] [Google Scholar]
- 122.Johnson, A. 2003. The biology of mating in Candida albicans. Nat. Rev. Microbiol. 1:106-116. [DOI] [PubMed] [Google Scholar]
- 123.Kato, N., W. Brooks, and A. M. Calvo. 2003. The expression of sterigmatocystin and penicillin genes in Aspergillus nidulans is controlled by veA, a gene required for sexual development. Eukaryot. Cell 2:1178-1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kaur, R., R. Domergue, M. L. Zupancic, and B. P. Cormack. 2005. A yeast by any other name: Candida glabrata and its interaction with the host. Curr. Opin. Microbiol. 8:378-384. [DOI] [PubMed] [Google Scholar]
- 125.Keeling, P. J., and A. J. Roger. 1995. The selfish pursuit of sex. Nature 375:283. [DOI] [PubMed] [Google Scholar]
- 126.Keller, S. M., M. A. Viviani, M. C. Esposto, M. Cogliati, and B. L. Wickes. 2003. Molecular and genetic characterization of a serotype A MATa Cryptococcus neoformans isolate. Microbiology 149:131-142. [DOI] [PubMed] [Google Scholar]
- 127.Kidd, S. E., F. Hagen, R. L. Tscharke, M. Huynh, K. H. Bartlett, M. Fyfe, L. Macdougall, T. Boekhout, K. J. Kwon-Chung, and W. Meyer. 2004. A rare genotype of Cryptococcus gattii caused the cryptococcosis outbreak on Vancouver Island (British Columbia, Canada). Proc. Natl. Acad. Sci. U. S. A. 101:17258-17263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kitamura, K., T. Nakamura, F. Miki, and C. Shimoda. 1996. Autocrine response of Schizosaccharomyces pombe haploid cells to mating pheromones. FEMS Microbiol. Lett. 143:41-45. [DOI] [PubMed] [Google Scholar]
- 129.Klein, B. S., and B. Tebbets. 2007. Dimorphism and virulence in fungi. Curr. Opin. Microbiol. 10:314-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Krappmann, S., O. Bayram, and G. H. Braus. 2005. Deletion and allelic exchange of the Aspergillus fumigatus veA locus via a novel recyclable marker module. Eukaryot. Cell 4:1298-1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kurjan, J., and I. Herskowitz. 1982. Structure of a yeast pheromone gene (MF alpha): a putative alpha-factor precursor contains four tandem copies of mature alpha-factor. Cell 30:933-943. [DOI] [PubMed] [Google Scholar]
- 132.Kurtzman, C. P. 2003. Phylogenetic circumscription of Saccharomyces, Kluyveromyces and other members of the Saccharomycetaceae, and the proposal of the new genera Lachancea, Nakaseomyces, Naumovia, Vanderwaltozyma and Zygotorulaspora. FEMS Yeast Res. 4:233-245. [DOI] [PubMed] [Google Scholar]
- 133.Kvaal, C., S. A. Lachke, T. Srikantha, K. Daniels, J. McCoy, and D. R. Soll. 1999. Misexpression of the opaque-phase-specific gene PEP1 (SAP1) in the white phase of Candida albicans confers increased virulence in a mouse model of cutaneous infection. Infect. Immun. 67:6652-6662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kwon-Chung, K. J. 1975. A new genus, Filobasidiella, the perfect state of Cryptococcus neoformans. Mycologia 67:1197-1200. [PubMed] [Google Scholar]
- 135.Kwon-Chung, K. J. 1976. Morphogenesis of Filobasidiella neoformans, the sexual state of Cryptococcus neoformans. Mycologia 68:821-833. [PubMed] [Google Scholar]
- 136.Kwon-Chung, K. J. 1972. Sexual stage of Histoplasma capsulatum. Science 175:326. [DOI] [PubMed] [Google Scholar]
- 137.Kwon-Chung, K. J., and J. E. Bennett. 1978. Distribution of a and alpha mating types of Cryptococcus neoformans among natural and clinical isolates. Am. J. Epidemiol. 108:337-340. [DOI] [PubMed] [Google Scholar]
- 138.Kwon-Chung, K. J., R. J. Weeks, and H. W. Larsh. 1974. Studies on Emmonsiella capsulata (Histoplasma capsulatum). II. Distribution of the two mating types in 13 endemic states of the United States. Am. J. Epidemiol. 99:44-49. [DOI] [PubMed] [Google Scholar]
- 139.Lachke, S. A., S. R. Lockhart, K. J. Daniels, and D. R. Soll. 2003. Skin facilitates Candida albicans mating. Infect. Immun. 71:4970-4976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Laffey, S. F., and G. Butler. 2005. Phenotype switching affects biofilm formation by Candida parapsilosis. Microbiology 151:1073-1081. [DOI] [PubMed] [Google Scholar]
- 141.Lasker, B. A., G. Butler, and T. J. Lott. 2006. Molecular genotyping of Candida parapsilosis group I clinical isolates by analysis of polymorphic microsatellite markers. J. Clin. Microbiol. 44:750-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Leberer, E., D. Harcus, I. D. Broadbent, K. L. Clark, D. Dignard, K. Ziegelbauer, A. Schmidt, N. A. Gow, A. J. Brown, and D. Y. Thomas. 1996. Signal transduction through homologs of the Ste20p and Ste7p protein kinases can trigger hyphal formation in the pathogenic fungus Candida albicans. Proc. Natl. Acad. Sci. U. S. A. 93:13217-13222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Legrand, M., P. Lephart, A. Forche, F. M. Mueller, T. Walsh, P. T. Magee, and B. B. Magee. 2004. Homozygosity at the MTL locus in clinical strains of Candida albicans: karyotypic rearrangements and tetraploid formation. Mol. Microbiol. 52:1451-1462. [DOI] [PubMed] [Google Scholar]
- 144.Lengeler, K. B., D. S. Fox, J. A. Fraser, A. Allen, K. Forrester, F. S. Dietrich, and J. Heitman. 2002. Mating-type locus of Cryptococcus neoformans: a step in the evolution of sex chromosomes. Eukaryot. Cell 1:704-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lengeler, K. B., P. Wang, G. M. Cox, J. R. Perfect, and J. Heitman. 2000. Identification of the MATa mating-type locus of Cryptococcus neoformans reveals a serotype A MATa strain thought to have been extinct. Proc. Natl. Acad. Sci. U. S. A. 97:14455-14460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Li, L., G. Shen, Z. G. Zhang, Y. L. Wang, J. K. Thompson, and P. Wang. 2007. Canonical heterotrimeric G proteins regulating mating and virulence of Cryptococcus neoformans. Mol. Biol. Cell 18:4201-4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Li, S., K. Myung, D. Guse, B. Donkin, R. H. Proctor, W. S. Grayburn, and A. M. Calvo. 2006. FvVE1 regulates filamentous growth, the ratio of microconidia to macroconidia and cell wall formation in Fusarium verticillioides. Mol. Microbiol. 62:1418-1432. [DOI] [PubMed] [Google Scholar]
- 148.Lin, C. Y., Y. C. Chen, H. J. Lo, K. W. Chen, and S. Y. Li. 2007. Assessment of Candida glabrata strain relatedness by pulsed-field gel electrophoresis and multilocus sequence typing. J. Clin. Microbiol. 45:2452-2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lin, X., C. M. Hull, and J. Heitman. 2005. Sexual reproduction between partners of the same mating type in Cryptococcus neoformans. Nature 434:1017-1021. [DOI] [PubMed] [Google Scholar]
- 150.Lin, X., A. P. Litvintseva, K. Nielsen, S. Patel, A. Floyd, T. G. Mitchell, and J. Heitman. 2007. Alpha AD alpha hybrids of Cryptococcus neoformans: evidence of same-sex mating in nature and hybrid fitness. PLoS Genet. 3:1975-1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lin, X., K. Nielsen, S. Patel, and J. Heitman. 2008. Impact of mating type, serotype, and ploidy on the virulence of Cryptococcus neoformans. Infect. Immun. 76:2923-2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lin, X., S. Patel, A. P. Litvintseva, A. Floyd, T. G. Mitchell, and J. Heitman. 2009. Diploids in the Cryptococcus neoformans serotype a population homozygous for the alpha mating type originate via unisexual mating. PLoS Pathog. 5:e1000283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Litvintseva, A. P., X. Lin, I. Templeton, J. Heitman, and T. G. Mitchell. 2007. Many globally isolated AD hybrid strains of Cryptococcus neoformans originated in Africa. PLoS Pathog. 3:e114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Litvintseva, A. P., R. E. Marra, K. Nielsen, J. Heitman, R. Vilgalys, and T. G. Mitchell. 2003. Evidence of sexual recombination among Cryptococcus neoformans serotype A isolates in sub-Saharan Africa. Eukaryot. Cell 2:1162-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Litvintseva, A. P., R. Thakur, R. Vilgalys, and T. G. Mitchell. 2006. Multilocus sequence typing reveals three genetic subpopulations of Cryptococcus neoformans var. grubii (serotype A), including a unique population in Botswana. Genetics 172:2223-2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Liu, H. 2002. Co-regulation of pathogenesis with dimorphism and phenotypic switching in Candida albicans, a commensal and a pathogen. Int. J. Med. Microbiol. 292:299-311. [DOI] [PubMed] [Google Scholar]
- 157.Liu, H. 2001. Transcriptional control of dimorphism in Candida albicans. Curr. Opin. Microbiol. 4:728-735. [DOI] [PubMed] [Google Scholar]
- 158.Lockhart, S. R., S. A. Messer, M. Gherna, J. A. Bishop, W. G. Merz, M. A. Pfaller, and D. J. Diekema. 2009. Identification of Candida nivariensis and Candida bracarensis in a large global collection of Candida glabrata isolates: comparison to the literature. J. Clin. Microbiol. 47:1216-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lockhart, S. R., S. A. Messer, M. A. Pfaller, and D. J. Diekema. 2008. Lodderomyces elongisporus masquerading as Candida parapsilosis as a cause of bloodstream infections. J. Clin. Microbiol. 46:374-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Lockhart, S. R., C. Pujol, K. J. Daniels, M. G. Miller, A. D. Johnson, M. A. Pfaller, and D. R. Soll. 2002. In Candida albicans, white-opaque switchers are homozygous for mating type. Genetics 162:737-745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lockhart, S. R., W. Wu, J. B. Radke, R. Zhao, and D. R. Soll. 2005. Increased virulence and competitive advantage of a/{alpha} over a/a or {alpha}/{alpha} offspring conserves the mating system of Candida albicans. Genetics 169:1883-1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lockhart, S. R., R. Zhao, K. J. Daniels, and D. R. Soll. 2003. Alpha-pheromone-induced “shmooing” and gene regulation require white-opaque switching during Candida albicans mating. Eukaryot. Cell 2:847-855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Logsdon, J. M., Jr. 2008. Evolutionary genetics: sex happens in Giardia. Curr. Biol. 18:R66-R68. [DOI] [PubMed] [Google Scholar]
- 164.Logue, M. E., S. Wong, K. H. Wolfe, and G. Butler. 2005. A genome sequence survey shows that the pathogenic yeast Candida parapsilosis has a defective MTLa1 allele at its mating type locus. Eukaryot. Cell 4:1009-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lohse, M. B., and A. D. Johnson. 2008. Differential phagocytosis of white versus opaque Candida albicans by Drosophila and mouse phagocytes. PLoS One 3:e1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lorenz, A., J. L. Wells, D. W. Pryce, M. Novatchkova, F. Eisenhaber, R. J. McFarlane, and J. Loidl. 2004. S. pombe meiotic linear elements contain proteins related to synaptonemal complex components. J. Cell Sci. 117:3343-3351. [DOI] [PubMed] [Google Scholar]
- 167.Lott, T. J., B. P. Holloway, D. A. Logan, R. Fundyga, and J. Arnold. 1999. Towards understanding the evolution of the human commensal yeast Candida albicans. Microbiology 145:1137-1143. [DOI] [PubMed] [Google Scholar]
- 168.Lott, T. J., R. J. Kuykendall, S. F. Welbel, A. Pramanik, and B. A. Lasker. 1993. Genomic heterogeneity in the yeast Candida parapsilosis. Curr. Genet. 23:463-467. [DOI] [PubMed] [Google Scholar]
- 169.Machida, M., K. Asai, M. Sano, T. Tanaka, T. Kumagai, G. Terai, K. Kusumoto, T. Arima, O. Akita, Y. Kashiwagi, K. Abe, K. Gomi, H. Horiuchi, K. Kitamoto, T. Kobayashi, M. Takeuchi, D. W. Denning, J. E. Galagan, W. C. Nierman, J. Yu, D. B. Archer, J. W. Bennett, D. Bhatnagar, T. E. Cleveland, N. D. Fedorova, O. Gotoh, H. Horikawa, A. Hosoyama, M. Ichinomiya, R. Igarashi, K. Iwashita, P. R. Juvvadi, M. Kato, Y. Kato, T. Kin, A. Kokubun, H. Maeda, N. Maeyama, J. Maruyama, H. Nagasaki, T. Nakajima, K. Oda, K. Okada, I. Paulsen, K. Sakamoto, T. Sawano, M. Takahashi, K. Takase, Y. Terabayashi, J. R. Wortman, O. Yamada, Y. Yamagata, H. Anazawa, Y. Hata, Y. Koide, T. Komori, Y. Koyama, T. Minetoki, S. Suharnan, A. Tanaka, K. Isono, S. Kuhara, N. Ogasawara, and H. Kikuchi. 2005. Genome sequencing and analysis of Aspergillus oryzae. Nature 438:1157-1161. [DOI] [PubMed] [Google Scholar]
- 170.Magee, B. B., M. Legrand, A. M. Alarco, M. Raymond, and P. T. Magee. 2002. Many of the genes required for mating in Saccharomyces cerevisiae are also required for mating in Candida albicans. Mol. Microbiol. 46:1345-1351. [DOI] [PubMed] [Google Scholar]
- 171.Magee, B. B., and P. T. Magee. 2000. Induction of mating in Candida albicans by construction of MTLa and MTLalpha strains. Science 289:310-313. [DOI] [PubMed] [Google Scholar]
- 172.Malik, S., A. Pightling, L. Stefaniak, A. Schurko, and J. Logsdon. 2008. An expanded inventory of conserved meioic genes provides evidence for sex in Trichomonas vaginalis. PLoS One 3:e2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Mark Welch, D., and M. Meselson. 2000. Evidence for the evolution of bdelloid rotifers without sexual reproduction or genetic exchange. Science 288:1211-1215. [DOI] [PubMed] [Google Scholar]
- 174.Mark Welch, D. B., J. L. Mark Welch, and M. Meselson. 2008. Evidence for degenerate tetraploidy in bdelloid rotifers. Proc. Natl. Acad. Sci. U. S. A. 105:5145-5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Mathias, J. R., S. E. Hanlon, R. A. O'Flanagan, A. M. Sengupta, and A. K. Vershon. 2004. Repression of the yeast HO gene by the MATalpha2 and MATa1 homeodomain proteins. Nucleic Acids Res. 32:6469-6478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Maynard Smith, J. 1978. The evolution of sex. Oxford University Press, Oxford, United Kingdom.
- 177.Melake, T., V. V. Passoth, and U. Klinner. 1996. Characterization of the genetic system of the xylose-fermenting yeast Pichia stipitis. Curr. Microbiol. 33:237-242. [DOI] [PubMed] [Google Scholar]
- 178.Metzenberg, R. L., and N. L. Glass. 1990. Mating type and mating strategies in Neurospora. Bioessays 12:53-59. [DOI] [PubMed] [Google Scholar]
- 179.Miller, M. G., and A. D. Johnson. 2002. White-opaque switching in Candida albicans is controlled by mating-type locus homeodomain proteins and allows efficient mating. Cell 110:293-302. [DOI] [PubMed] [Google Scholar]
- 180.Mitchell, A. P., S. E. Driscoll, and H. E. Smith. 1990. Positive control of sporulation-specific genes by the IME1 and IME2 products in Saccharomyces cerevisiae. Mol. Cell. Biol. 10:2104-2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Mitra, D., E. J. Parnell, J. W. Landon, Y. Yu, and D. J. Stillman. 2006. SWI/SNF binding to the HO promoter requires histone acetylation and stimulates TATA-binding protein recruitment. Mol. Cell. Biol. 26:4095-4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Mooney, J. L., D. E. Hassett, and L. N. Yager. 1990. Genetic analysis of suppressors of the veA1 mutation in Aspergillus nidulans. Genetics 126:869-874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Mooney, J. L., and L. N. Yager. 1990. Light is required for conidiation in Aspergillus nidulans. Genes Dev. 4:1473-1482. [DOI] [PubMed] [Google Scholar]
- 184.Moran, G., C. Stokes, S. Thewes, B. Hube, D. C. Coleman, and D. Sullivan. 2004. Comparative genomics using Candida albicans DNA microarrays reveals absence and divergence of virulence-associated genes in Candida dubliniensis. Microbiology 150:3363-3382. [DOI] [PubMed] [Google Scholar]
- 185.Morrow, C. A., and J. A. Fraser. 2009. Sexual reproduction and dimorphism in the pathogenic basidiomycetes. FEMS Yeast Res. 9:161-177. [DOI] [PubMed] [Google Scholar]
- 186.Muller, H., C. Hennequin, J. Gallaud, B. Dujon, and C. Fairhead. 2008. The asexual yeast Candida glabrata maintains distinct a and alpha haploid mating types. Eukaryot. Cell 7:848-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Muller, H. J. 1932. Some genetic aspects of sex. Am. Nat. 66:118-138. [Google Scholar]
- 188.Nasmyth, K. 1993. Regulating the HO endonuclease in yeast. Curr. Opin. Genet. Dev. 3:286-294. [DOI] [PubMed] [Google Scholar]
- 189.Nguyen, V. Q., and A. Sil. 2008. Temperature-induced switch to the pathogenic yeast form of Histoplasma capsulatum requires Ryp1, a conserved transcriptional regulator. Proc. Natl. Acad. Sci. U. S. A. 105:4880-4885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Nickoloff, J. A., J. D. Singer, and F. Heffron. 1990. In vivo analysis of the Saccharomyces cerevisiae HO nuclease recognition site by site-directed mutagenesis. Mol. Cell. Biol. 10:1174-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Nielsen, K., G. M. Cox, P. Wang, D. L. Toffaletti, J. R. Perfect, and J. Heitman. 2003. Sexual cycle of Cryptococcus neoformans var. grubii and virulence of congenic a and alpha isolates. Infect. Immun. 71:4831-4841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Nielsen, K., and J. Heitman. 2007. Sex and virulence of human pathogenic fungi. Adv. Genet. 57:143-173. [DOI] [PubMed] [Google Scholar]
- 193.Nielsen, K., R. E. Marra, F. Hagen, T. Boekhout, T. G. Mitchell, G. M. Cox, and J. Heitman. 2005. Interaction between genetic background and the mating-type locus in Cryptococcus neoformans virulence potential. Genetics 171:975-983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Nierman, W. C., A. Pain, M. J. Anderson, J. R. Wortman, H. S. Kim, J. Arroyo, M. Berriman, K. Abe, D. B. Archer, C. Bermejo, J. Bennett, P. Bowyer, D. Chen, M. Collins, R. Coulsen, R. Davies, P. S. Dyer, M. Farman, N. Fedorova, T. V. Feldblyum, R. Fischer, N. Fosker, A. Fraser, J. L. Garcia, M. J. Garcia, A. Goble, G. H. Goldman, K. Gomi, S. Griffith-Jones, R. Gwilliam, B. Haas, H. Haas, D. Harris, H. Horiuchi, J. Huang, S. Humphray, J. Jimenez, N. Keller, H. Khouri, K. Kitamoto, T. Kobayashi, S. Konzack, R. Kulkarni, T. Kumagai, A. Lafon, J. P. Latge, W. Li, A. Lord, C. Lu, W. H. Majoros, G. S. May, B. L. Miller, Y. Mohamoud, M. Molina, M. Monod, I. Mouyna, S. Mulligan, L. Murphy, S. O'Neil, I. Paulsen, M. A. Penalva, M. Pertea, C. Price, B. L. Pritchard, M. A. Quail, E. Rabbinowitsch, N. Rawlins, M. A. Rajandream, U. Reichard, H. Renauld, G. D. Robson, S. Rodriguez de Cordoba, J. M. Rodriguez-Pena, C. M. Ronning, S. Rutter, S. L. Salzberg, M. Sanchez, J. C. Sanchez-Ferrero, D. Saunders, K. Seeger, R. Squares, S. Squares, M. Takeuchi, F. Tekaia, G. Turner, C. R. Vazquez de Aldana, J. Weidman, O. White, J. Woodward, J. H. Yu, C. Fraser, J. E. Galagan, K. Asai, M. Machida, N. Hall, B. Barrell, and D. W. Denning. 2005. Genomic sequence of the pathogenic and allergenic filamentous fungus Aspergillus fumigatus. Nature 438:1151-1156. [DOI] [PubMed] [Google Scholar]
- 195.O'Gorman, C. M., H. T. Fuller, and P. S. Dyer. 2009. Discovery of a sexual cycle in the opportunistic fungal pathogen Aspergillus fumigatus. Nature 457:471-474. [DOI] [PubMed] [Google Scholar]
- 196.Ortoneda, M., J. Guarro, M. P. Madrid, Z. Caracuel, M. I. Roncero, E. Mayayo, and A. Di Pietro. 2004. Fusarium oxysporum as a multihost model for the genetic dissection of fungal virulence in plants and mammals. Infect. Immun. 72:1760-1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Panwar, S. L., M. Legrand, D. Dignard, M. Whiteway, and P. T. Magee. 2003. MFalpha1, the gene encoding the alpha mating pheromone of Candida albicans. Eukaryot. Cell 2:1350-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Paoletti, M., C. Rydholm, E. U. Schwier, M. J. Anderson, G. Szakacs, F. Lutzoni, J. P. Debeaupuis, J. P. Latge, D. W. Denning, and P. S. Dyer. 2005. Evidence for sexuality in the opportunistic fungal pathogen Aspergillus fumigatus. Curr. Biol. 15:1242-1248. [DOI] [PubMed] [Google Scholar]
- 199.Pappagianis, D. 1988. Epidemiology of coccidioidomycosis. Curr. Top. Med. Mycol. 2:199-238. [DOI] [PubMed] [Google Scholar]
- 200.Pel, H. J., J. H. de Winde, D. B. Archer, P. S. Dyer, G. Hofmann, P. J. Schaap, G. Turner, R. P. de Vries, R. Albang, K. Albermann, M. R. Andersen, J. D. Bendtsen, J. A. Benen, M. van den Berg, S. Breestraat, M. X. Caddick, R. Contreras, M. Cornell, P. M. Coutinho, E. G. Danchin, A. J. Debets, P. Dekker, P. W. van Dijck, A. van Dijk, L. Dijkhuizen, A. J. Driessen, C. d'Enfert, S. Geysens, C. Goosen, G. S. Groot, P. W. de Groot, T. Guillemette, B. Henrissat, M. Herweijer, J. P. van den Hombergh, C. A. van den Hondel, R. T. van der Heijden, R. M. van der Kaaij, F. M. Klis, H. J. Kools, C. P. Kubicek, P. A. van Kuyk, J. Lauber, X. Lu, M. J. van der Maarel, R. Meulenberg, H. Menke, M. A. Mortimer, J. Nielsen, S. G. Oliver, M. Olsthoorn, K. Pal, N. N. van Peij, A. F. Ram, U. Rinas, J. A. Roubos, C. M. Sagt, M. Schmoll, J. Sun, D. Ussery, J. Varga, W. Vervecken, P. J. van de Vondervoort, H. Wedler, H. A. Wosten, A. P. Zeng, A. J. van Ooyen, J. Visser, and H. Stam. 2007. Genome sequencing and analysis of the versatile cell factory Aspergillus niger CBS 513.88. Nat. Biotechnol. 25:221-231. [DOI] [PubMed] [Google Scholar]
- 201.Perfect, J. R. 2006. Cryptococcus neoformans: a sugar-coated killer, p. 281-303. In J. Heitman, S. G. Filler, J. E. Edwards, and A. P. Mitchell (ed.), Molecular principles of fungal pathogenesis. ASM Press, Washington, DC.
- 202.Perfect, J. R., and A. Casadevall. 2006. Fungal molecular pathogenesis: what can it do and why do we need it?, p. 3-11. In J. Heitman, S. G. Filler, J. E. Edwards, and A. P. Mitchell (ed.), Fungal pathogenesis. ASM Press, Washington, DC.
- 203.Perlstein, E. O., E. J. Deeds, O. Ashenberg, E. I. Shakhnovich, and S. L. Schreiber. 2007. Quantifying fitness distributions and phenotypic relationships in recombinant yeast populations. Proc. Natl. Acad. Sci. U. S. A. 104:10553-10558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Perry, J., N. Kleckner, and G. V. Borner. 2005. Bioinformatic analyses implicate the collaborating meiotic crossover/chiasma proteins Zip2, Zip3, and Spo22/Zip4 in ubiquitin labeling. Proc. Natl. Acad. Sci. U. S. A. 102:17594-17599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Peterson, S. W. 2008. Phylogenetic analysis of Aspergillus species using DNA sequences from four loci. Mycologia 100:205-226. [DOI] [PubMed] [Google Scholar]
- 206.Pfaller, M. A., and D. J. Diekema. 2007. Epidemiology of invasive candidiasis: a persistent public health problem. Clin. Microbiol. Rev. 20:133-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Pfaller, M. A., and D. J. Diekema. 2004. Twelve years of fluconazole in clinical practice: global trends in species distribution and fluconazole susceptibility of bloodstream isolates of Candida. Clin. Microbiol. Infect. 10(Suppl. 1):11-23. [DOI] [PubMed] [Google Scholar]
- 208.Pitt, J. H., R. A. Samson, and J. C. Frisvad. 2000. List of accepted species and their synonyms in the family Trichocomaceae, p. 9-72. In R. A. Samson and J. I. Pitt (ed.), Integration of modern taxonomic methods for Penicilium and Aspergillus classification. Harwood Acaemic Publishers, Amsterdam, The Netherlands.
- 209.Pontecorvo, G., J. A. Roper, L. M. Hemmons, K. D. Macdonald, and A. W. Bufton. 1953. The genetics of Aspergillus nidulans. Adv. Genet. 5:141-238. [DOI] [PubMed] [Google Scholar]
- 210.Pounds, J. A., M. R. Bustamante, L. A. Coloma, J. A. Consuegra, M. P. Fogden, P. N. Foster, E. La Marca, K. L. Masters, A. Merino-Viteri, R. Puschendorf, S. R. Ron, G. A. Sanchez-Azofeifa, C. J. Still, and B. E. Young. 2006. Widespread amphibian extinctions from epidemic disease driven by global warming. Nature 439:161-167. [DOI] [PubMed] [Google Scholar]
- 211.Poxleitner, M. K., M. L. Carpenter, J. J. Mancuso, C. J. Wang, S. C. Dawson, and W. Z. Cande. 2008. Evidence for karyogamy and exchange of genetic material in the binucleate intestinal parasite Giardia intestinalis. Science 319:1530-1533. [DOI] [PubMed] [Google Scholar]
- 212.Pringle, A., D. M. Baker, J. L. Platt, J. P. Wares, J. P. Latge, and J. W. Taylor. 2005. Cryptic speciation in the cosmopolitan and clonal human pathogenic fungus Aspergillus fumigatus. Evolution 59:1886-1899. [PubMed] [Google Scholar]
- 213.Pujol, C., K. J. Daniels, S. R. Lockhart, T. Srikantha, J. B. Radke, J. Geiger, and D. R. Soll. 2004. The closely related species Candida albicans and Candida dubliniensis can mate. Eukaryot. Cell 3:1015-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Ramesh, M. A., S. B. Malik, and J. M. Logsdon, Jr. 2005. A phylogenomic inventory of meiotic genes; evidence for sex in Giardia and an early eukaryotic origin of meiosis. Curr. Biol. 15:185-191. [DOI] [PubMed] [Google Scholar]
- 215.Ramirez-Prado, J. H., G. G. Moore, B. W. Horn, and I. Carbone. 2008. Characterization and population analysis of the mating-type genes in Aspergillus flavus and Aspergillus parasiticus. Fungal Genet. Biol. 45:1292-1299. [DOI] [PubMed] [Google Scholar]
- 216.Ramirez-Zavala, B., O. Reuss, Y. N. Park, K. Ohlsen, and J. Morschhauser. 2008. Environmental induction of white-opaque switching in Candida albicans. PLoS Pathog. 4:e1000089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Raymond, M., D. Dignard, A. M. Alarco, N. Mainville, B. B. Magee, and D. Y. Thomas. 1998. A Ste6p/P-glycoprotein homologue from the asexual yeast Candida albicans transports the a-factor mating pheromone in Saccharomyces cerevisiae. Mol. Microbiol. 27:587-598. [DOI] [PubMed] [Google Scholar]
- 218.Recca, J., and E. Mrak. 1952. Yeasts occuring in citrus products. Food Technol. 6:450-454. [Google Scholar]
- 219.Reedy, J. L., A. M. Floyd, and J. Heitman. 2009. Mechanistic plasticity of sexual reproduction and meiosis in the Candida pathogenic species complex. Curr. Biol. 19:891-899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Rodrigues de Miranda, L. 1979. Clavispora, a new yeast genus of the Saccharomycetales. Antonie Van Leeuwenhoek 45:479-483. [DOI] [PubMed] [Google Scholar]
- 221.Roman, E., D. M. Arana, C. Nombela, R. Alonso-Monge, and J. Pla. 2007. MAP kinase pathways as regulators of fungal virulence. Trends Microbiol. 15:181-190. [DOI] [PubMed] [Google Scholar]
- 222.Rydholm, C., P. S. Dyer, and F. Lutzoni. 2007. DNA sequence characterization and molecular evolution of MAT1 and MAT2 mating-type loci of the self-compatible ascomycete mold Neosartorya fischeri. Eukaryot. Cell 6:868-874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Rydholm, C., G. Szakacs, and F. Lutzoni. 2006. Low genetic variation and no detectable population structure in Aspergillus fumigatus compared to closely related Neosartorya species. Eukaryot. Cell 5:650-657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Sagolla, M. S., S. C. Dawson, J. J. Mancuso, and W. Z. Cande. 2006. Three-dimensional analysis of mitosis and cytokinesis in the binucleate parasite Giardia intestinalis. J. Cell Sci. 119:4889-4900. [DOI] [PubMed] [Google Scholar]
- 225.Sahni, N., S. Yi, C. Pujol, and D. R. Soll. 2009. The white cell response to pheromone is a general characteristic of Candida albicans strains. Eukaryot. Cell 8:251-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Saul, N., M. Krockenberger, and D. Carter. 2008. Evidence of recombination in mixed-mating-type and alpha-only populations of Cryptococcus gattii sourced from single eucalyptus tree hollows. Eukaryot. Cell 7:727-734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Schurko, A. M., M. Neiman, and J. M. Logsdon, Jr. 2009. Signs of sex: what we know and how we know it. Trends Ecol. Evol. 24:208-217. [DOI] [PubMed] [Google Scholar]
- 228.Schwartz, M. A., and H. D. Madhani. 2004. Principles of MAP kinase signaling specificity in Saccharomyces cerevisiae. Annu. Rev. Genet. 38:725-748. [DOI] [PubMed] [Google Scholar]
- 229.Segal, B. H., E. S. DeCarlo, K. J. Kwon-Chung, H. L. Malech, J. I. Gallin, and S. M. Holland. 1998. Aspergillus nidulans infection in chronic granulomatous disease. Medicine (Baltimore) 77:345-354. [DOI] [PubMed] [Google Scholar]
- 230.Sil, A., and I. Herskowitz. 1996. Identification of asymmetrically localized determinant, Ash1p, required for lineage-specific transcription of the yeast HO gene. Cell 84:711-722. [DOI] [PubMed] [Google Scholar]
- 231.Slutsky, B., J. Buffo, and D. R. Soll. 1985. High-frequency switching of colony morphology in Candida albicans. Science 230:666-669. [DOI] [PubMed] [Google Scholar]
- 232.Smith, H. E., S. S. Su, L. Neigeborn, S. E. Driscoll, and A. P. Mitchell. 1990. Role of IME1 expression in regulation of meiosis in Saccharomyces cerevisiae. Mol. Cell. Biol. 10:6103-6113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Soll, D. R. 2002. Phenotypic switching, p. 123-142. In R. A. Calderone (ed.), Candida and candidiasis. ASM Press, Washington, DC.
- 234.Soll, D. R., C. Pujol, and T. Srikantha. 2009. Sex: deviant mating in yeast. Curr. Biol. 19:R509-R511. [DOI] [PubMed] [Google Scholar]
- 235.Srikantha, T., A. R. Borneman, K. J. Daniels, C. Pujol, W. Wu, M. R. Seringhaus, M. Gerstein, S. Yi, M. Snyder, and D. R. Soll. 2006. TOS9 regulates white-opaque switching in Candida albicans. Eukaryot. Cell 5:1674-1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Srikantha, T., S. A. Lachke, and D. R. Soll. 2003. Three mating type-like loci in Candida glabrata. Eukaryot. Cell 2:328-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Stinnett, S. M., E. A. Espeso, L. Cobeno, L. Araujo-Bazan, and A. M. Calvo. 2007. Aspergillus nidulans VeA subcellular localization is dependent on the importin alpha carrier and on light. Mol. Microbiol. 63:242-255. [DOI] [PubMed] [Google Scholar]
- 238.Sugita, T., and T. Nakase. 1999. Non-universal usage of the leucine CUG codon and the molecular phylogeny of the genus Candida. Syst. Appl. Microbiol. 22:79-86. [DOI] [PubMed] [Google Scholar]
- 239.Suh, S. O., C. J. Marshall, J. V. McHugh, and M. Blackwell. 2003. Wood ingestion by passalid beetles in the presence of xylose-fermenting gut yeasts. Mol. Ecol. 12:3137-3145. [DOI] [PubMed] [Google Scholar]
- 240.Sukroongreung, S., K. Kitiniyom, C. Nilakul, and S. Tantimavanich. 1998. Pathogenicity of basidiospores of Filobasidiella neoformans var. neoformans. Med. Mycol. 36:419-424. [PubMed] [Google Scholar]
- 241.Sullivan, D. J., G. P. Moran, and D. C. Coleman. 2005. Candida dubliniensis: ten years on. FEMS Microbiol. Lett. 253:9-17. [DOI] [PubMed] [Google Scholar]
- 242.Sullivan, D. J., T. J. Westerneng, K. A. Haynes, D. E. Bennett, and D. C. Coleman. 1995. Candida dubliniensis sp. nov.: phenotypic and molecular characterization of a novel species associated with oral candidosis in HIV-infected individuals. Microbiology 141:1507-1521. [DOI] [PubMed] [Google Scholar]
- 243.Tavanti, A., A. D. Davidson, N. A. Gow, M. C. Maiden, and F. C. Odds. 2005. Candida orthopsilosis and Candida metapsilosis spp. nov. to replace Candida parapsilosis groups II and III. J. Clin. Microbiol. 43:284-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Tavanti, A., N. A. Gow, M. C. Maiden, F. C. Odds, and D. J. Shaw. 2004. Genetic evidence for recombination in Candida albicans based on haplotype analysis. Fungal Genet. Biol. 41:553-562. [DOI] [PubMed] [Google Scholar]
- 245.Tsong, A. E., M. G. Miller, R. M. Raisner, and A. D. Johnson. 2003. Evolution of a combinatorial transcriptional circuit: a case study in yeasts. Cell 115:389-399. [DOI] [PubMed] [Google Scholar]
- 246.Tuch, B. B., D. J. Galgoczy, A. D. Hernday, H. Li, and A. D. Johnson. 2008. The evolution of combinatorial gene regulation in fungi. PLoS Biol. 6:e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Turgeon, B. G., and R. Debuchy. 2007. Cochiobolus and Podospora: mechanisms of sex determination and the evolution of reproductive lifestyle, p. 93-121. In J. Heitman, J. W. Kronstad, J. W. Taylor, and L. A. Casselton (ed.), Sex in fungi. ASM Press, Washington, DC.
- 248.Turgeon, B. G., and O. C. Yoder. 2000. Proposed nomenclature for mating type genes of filamentous ascomycetes. Fungal Genet. Biol. 31:1-5. [DOI] [PubMed] [Google Scholar]
- 249.Tzung, K. W., R. M. Williams, S. Scherer, N. Federspiel, T. Jones, N. Hansen, V. Bivolarevic, L. Huizar, C. Komp, R. Surzycki, R. Tamse, R. W. Davis, and N. Agabian. 2001. Genomic evidence for a complete sexual cycle in Candida albicans. Proc. Natl. Acad. Sci. U. S. A. 98:3249-3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Ustianowski, A. P., T. P. Sieu, and J. N. Day. 2008. Penicillium marneffei infection in HIV. Curr. Opin. Infect. Dis. 21:31-36. [DOI] [PubMed] [Google Scholar]
- 251.Vallesi, A., P. Ballarini, B. Di Pretoro, C. Alimenti, C. Miceli, and P. Luporini. 2005. Autocrine, mitogenic pheromone receptor loop of the ciliate Euplotes raikovi: pheromone-induced receptor internalization. Eukaryot. Cell 4:1221-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.van der Walt, J. P. 1966. Lodderomyces, a new genus of the Saccharomycetacea. Antonie Van Leeuwenhoek 32:1-5. [DOI] [PubMed] [Google Scholar]
- 253.van der Walt, J. P., M. B. Taylor, and N. V. Liebenberg. 1977. Ploidy, ascus formation and recombination in Torulaspora (Debaryomyces) hansenii. Antonie Van Leeuwenhoek 43:205-218. [DOI] [PubMed] [Google Scholar]
- 254.Varga, J., and B. Toth. 2003. Genetic variability and reproductive mode of Aspergillus fumigatus. Infect. Genet. Evol. 3:3-17. [DOI] [PubMed] [Google Scholar]
- 255.Velagapudi, R., Y. P. Hsueh, S. Geunes-Boyer, J. R. Wright, and J. Heitman. 2009. Spores as infectious propagules of Cryptococcus neoformans. Infect. Immun. 77:4345-4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Viviani, M. A., M. C. Esposto, M. Cogliati, M. T. Montagna, and B. L. Wickes. 2001. Isolation of a Cryptococcus neoformans serotype A MATa strain from the Italian environment. Med. Mycol. 39:383-386. [DOI] [PubMed] [Google Scholar]
- 257.Wang, P., C. B. Nichols, K. B. Lengeler, M. E. Cardenas, G. M. Cox, J. R. Perfect, and J. Heitman. 2002. Mating-type-specific and nonspecific PAK kinases play shared and divergent roles in Cryptococcus neoformans. Eukaryot. Cell 1:257-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Webster, R. H., and A. Sil. 2008. Conserved factors Ryp2 and Ryp3 control cell morphology and infectious spore formation in the fungal pathogen Histoplasma capsulatum. Proc. Natl. Acad. Sci. U. S. A. 105:14573-14578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Wickerham, L. J., and K. A. Burton. 1954. A clarification of the relationship of Candida guilliermondii to other yeasts by a study of their mating types. J. Bacteriol. 68:594-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Wong, S., M. A. Fares, W. Zimmermann, G. Butler, and K. H. Wolfe. 2003. Evidence from comparative genomics for a complete sexual cycle in the ‘asexual’ pathogenic yeast Candida glabrata. Genome Biol. 4:R10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Woo, P. C., K. T. Chong, H. Tse, J. J. Cai, C. C. Lau, A. C. Zhou, S. K. Lau, and K. Y. Yuen. 2006. Genomic and experimental evidence for a potential sexual cycle in the pathogenic thermal dimorphic fungus Penicillium marneffei. FEBS Lett. 580:3409-3416. [DOI] [PubMed] [Google Scholar]
- 262.Woods, J. P. 2006. Molecular determinants of Histoplasma capsulatum pathogenesis, p. 321-346. In J. Heitman, S. G. Filler, and J. E. Edwards (ed.), Molecular principles of fungal pathogenesis. ASM Press, Washington, DC.
- 263.Wright, S., and D. Finnegan. 2001. Genome evolution: sex and the transposable element. Curr. Biol. 11:R296-R299. [DOI] [PubMed] [Google Scholar]
- 264.Wu, W., S. R. Lockhart, C. Pujol, T. Srikantha, and D. R. Soll. 2007. Heterozygosity of genes on the sex chromosome regulates Candida albicans virulence. Mol. Microbiol. 64:1587-1604. [DOI] [PubMed] [Google Scholar]
- 265.Xu, J. 2005. Cost of interacting with sexual partners in a facultative sexual microbe. Genetics 171:1597-1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Xu, J. 2007. Origin, evolution, and extinction of asexual fungi, p. 461-475. In J. Heitman, J. W. Kronstad, J. W. Taylor, and L. A. Casselton (ed.), Sex in fungi. ASM Press, Washington, DC.
- 267.Xu, J., C. M. Boyd, E. Livingston, W. Meyer, J. F. Madden, and T. G. Mitchell. 1999. Species and genotypic diversities and similarities of pathogenic yeasts colonizing women. J. Clin. Microbiol. 37:3835-3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Xu, J., C. W. Saunders, P. Hu, R. A. Grant, T. Boekhout, E. E. Kuramae, J. W. Kronstad, Y. M. Deangelis, N. L. Reeder, K. R. Johnstone, M. Leland, A. M. Fieno, W. M. Begley, Y. Sun, M. P. Lacey, T. Chaudhary, T. Keough, L. Chu, R. Sears, B. Yuan, and T. L. Dawson, Jr. 2007. Dandruff-associated Malassezia genomes reveal convergent and divergent virulence traits shared with plant and human fungal pathogens. Proc. Natl. Acad. Sci. U. S. A. 104:18730-18735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Xu, J., R. Vilgalys, and T. G. Mitchell. 1999. Lack of genetic differentiation between two geographically diverse samples of Candida albicans isolated from patients infected with human immunodeficiency virus. J. Bacteriol. 181:1369-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Yi, S., N. Sahni, K. J. Daniels, C. Pujol, T. Srikantha, and D. R. Soll. 2008. The same receptor, G protein, and mitogen-activated protein kinase pathway activate different downstream regulators in the alternative white and opaque pheromone responses of Candida albicans. Mol. Biol. Cell 19:957-970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Yi, S., N. Sahni, C. Pujol, K. J. Daniels, T. Srikantha, N. Ma, and D. R. Soll. 2009. A Candida albicans-specific region of the alpha-pheromone receptor plays a selective role in the white cell pheromone response. Mol. Microbiol. 71:925-947. [DOI] [PubMed] [Google Scholar]
- 272.Young, L. Y., M. C. Lorenz, and J. Heitman. 2000. A STE12 homolog is required for mating but dispensable for filamentation in Candida lusitaniae. Genetics 155:17-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Yu, J., G. A. Payne, W. C. Nierman, M. Machida, J. W. Bennett, B. C. Campell, J. F. Robens, D. Bhatnagar, R. A. Dean, and T. E. Cleveland. 2008. Aspergillus flavus genomics as a tool for studying the mechanism of aflatoxin formation. Food Addit. Contam. 15:1-6. [DOI] [PubMed] [Google Scholar]
- 274.Yue, C., L. M. Cavallo, J. A. Alspaugh, P. Wang, G. M. Cox, J. R. Perfect, and J. Heitman. 1999. The STE12alpha homolog is required for haploid filamentation but largely dispensable for mating and virulence in Cryptococcus neoformans. Genetics 153:1601-1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Zhan, J., C. C. Mundt, and B. A. McDonald. 2007. Sexual reproduction facilitates the adaptation of parasites to antagonistic host environments: evidence from empirical study in the wheat-Mycosphaerella graminicola system. Int. J. Parasitol. 37:861-870. [DOI] [PubMed] [Google Scholar]
- 276.Zhao, X., R. Mehrabi, and J. R. Xu. 2007. Mitogen-activated protein kinase pathways and fungal pathogenesis. Eukaryot. Cell 6:1701-1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Zimmer, B. L., H. O. Hempel, and N. L. Goodman. 1984. Pathogenicity of the basidiospores of Filobasidiella neoformans. Mycopathologia 85:149-153. [DOI] [PubMed] [Google Scholar]
- 278.Zordan, R. E., D. J. Galgoczy, and A. D. Johnson. 2006. Epigenetic properties of white-opaque switching in Candida albicans are based on a self-sustaining transcriptional feedback loop. Proc. Natl. Acad. Sci. U. S. A. 103:12807-12812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Zordan, R. E., M. G. Miller, D. J. Galgoczy, B. B. Tuch, and A. D. Johnson. 2007. Interlocking transcriptional feedback loops control white-opaque switching in Candida albicans. PLoS Biol. 5:e256. [DOI] [PMC free article] [PubMed] [Google Scholar]