Abstract
Summary: In global terms, respiratory viral infection is a major cause of morbidity and mortality. Infancy, in particular, is a time of increased disease susceptibility and severity. Early-life viral infection causes acute illness and can be associated with the development of wheezing and asthma in later life. The most commonly detected viruses are respiratory syncytial virus (RSV), rhinovirus (RV), and influenza virus. In this review we explore the complete picture from epidemiology and virology to clinical impact and immunology. Three striking aspects emerge. The first is the degree of similarity: although the infecting viruses are all different, the clinical outcome, viral evasion strategies, immune response, and long-term sequelae share many common features. The second is the interplay between the infant immune system and viral infection: the immaturity of the infant immune system alters the outcome of viral infection, but at the same time, viral infection shapes the development of the infant immune system and its future responses. Finally, both the virus and the immune response contribute to damage to the lungs and subsequent disease, and therefore, any prevention or treatment needs to address both of these factors.
INTRODUCTION
The disease burden from respiratory infection is greater than that of any other cause of disease (232). In 2002, 18% of mortality for children younger than 5 years of age was caused by respiratory infections; diarrheal disease (15%) and malaria (11%) were the next greatest causes (352). Respiratory infections account not only for increased mortality but also for increased morbidity in this age group: between 22% (United Kingdom [242]) and 26.7% (Belgium [222]) of all hospitalizations and between 33.5% (Italy [287]) and 59% (United Kingdom [247]) of general practitioner consultations are due to respiratory viral infection. These respiratory infections occur with increased frequency in early life compared to adulthood (234), with approximately 5 to 6 infections per year (58).
Respiratory tract infections in the young also impose a large and intermittent burden on the health care infrastructure. For community-based care, the mean cost per acute respiratory infection (ARI) has been estimated to be between US$140 (86) and US$240 (184), with the cost depending in part on the infecting agent. (For comparison purposes, costs have been converted from original data into U.S. dollars using the values US$1 = AUD$1.3 = €0.75 = £0.5 [summer 2008].) These estimated costs include direct and indirect costs (e.g., loss of earnings of the caregivers). Hospitalization costs are estimated at an average of US$5,250 per case of respiratory syncytial virus (RSV) bronchiolitis (33, 86, 311). The total annual cost of respiratory infection of young children in Germany was estimated to be US$213 million (86).
FROM THE BEDSIDE: SYMPTOMS, SIGNS, AND TREATMENT
Upper Respiratory Tract Infection
Most respiratory virus infections in early childhood are confined to the upper respiratory tract, leading to symptoms of the common cold, with coryza, cough, and hoarseness. Upon examination, rhinitis and pharyngitis are found and are frequently associated with some conjunctival and tympanic vascular injections. In some cases, symptoms and signs of otitis media occur, such as earache, tenderness of the tragus upon pressure, and a red bulging tympanic membrane upon inspection. Upper respiratory tract infection (URTI) in infants is often accompanied by fever and may lead to lethargy and poor feeding. Specific treatment is usually neither available nor required. However, analgesics/antipyretics (e.g., paracetamol) and, in some cases, nasal decongestants may be helpful in reducing discomfort and symptoms, making feeding easier, and allowing an adequate supply of oral fluids.
Lower Respiratory Tract Infection
About one-third of infants with respiratory viral infections develop lower respiratory tract symptoms such as tachypnea, wheeze, severe cough, breathlessness, and respiratory distress. These symptoms may be accompanied by clinical signs including nasal flaring; jugular, intercostal, and thoracic indrawings; rarely cyanosis; and, on auscultation of the chest wheeze, crackles, crepitations, and inspiratory rhonchi or generally reduced breath sounds due to air trapping and peripheral hyperinflation of the lung. In RSV infection, recurrent episodes of apnea are a threat to infants less than 6 months of age.
Some clinicians make distinctions between bronchitis, bronchiolitis, and pneumonia to describe predominantly proximal (large)-airway disease, small (conducting)-airway disease, or involvement of the alveolar compartment. However, the lack of internationally agreed-upon definitions makes the use of these pathological descriptions as clinical diagnoses contentious and, since the treatment is the same regardless of these distinctions, probably irrelevant.
The treatment and care for viral lower respiratory tract infection (LRTI) depend on the assessment of the severity of respiratory compromise by using measurements of O2 saturation and of blood gases and the clinical assessment of the severity of respiratory distress and of respiratory exhaustion with decreased respiratory effort, increasing CO2 retention, and respiratory acidosis. In addition, the consequences of respiratory compromise, in particular the inability to feed and drink, determine the management of these infants. Additional risk factors and preexisting illness (e.g., chronic lung disease of prematurity or history of reactive-airway disease) also influence disease management.
Extrapulmonary manifestations of LRTI, which have been described for RSV infection, are observed rarely. They include seizures, hyponatremia, cardiac arrhythmias, cardiac failure, and hepatitis (87). Interestingly, viral RNA not only has been detected in the respiratory tract, where the epithelium is the primary site of infection and viral replication, but also is transiently present in peripheral blood mononuclear cells and, perhaps very rarely, in cerebrospinal fluid and cardiac muscle, raising the possibility of occasional extrapulmonary spread (87).
Viral Diagnosis of Infant LRTI
For infants with LRTI treated as outpatients, a virological diagnosis is often not sought. This is justifiable for healthy infants, since the virological diagnosis does not predict the severity or length of disease, nor does it usually lead to specific therapy. Most respiratory viruses can cause LRTI of various severities and with a wide range of manifestations, and for most respiratory viruses, clinically useful antiviral agents do not exist. However, the detection of a viral cause of LRTI can be useful since it reduces the use of antibiotics, which is unwarranted in most cases of viral LRTI. For infants at risk of severe LRTI (e.g., prematurity and congenital heart disease), an early viral diagnosis is useful. RSV infection of these infants often leads to severe LRTI, requiring close monitoring of disease and, in the case of deterioration, early hospitalization.
For 1 to 2% of infants, LRTI requires hospitalization (120). In these cases, a viral diagnosis should be sought upon presentation by antigen detection assays or PCR to inform decisions on the cohorting of patients and to prevent nosocomial infections.
Antiviral Drugs Currently in Use
For influenza A and B viruses, the neuraminidase inhibitors oseltamivir and zanamivir are licensed as antiviral drugs but only for patients between the ages of 1 and 5 years, respectively, and not for infants. These drugs can be used for postexposure prophylaxis and the treatment of influenza virus (IV) if they can be given within 48 h after exposure or 36 h after first symptoms. Neuraminidase inhibitors are recommended only for children with chronic morbidity who are at an increased risk of severe influenza-induced disease. Treatment shortens symptoms by about 1 day and may reduce disease severity (297). Neuraminidase inhibitors are not helpful for established influenza infection and do not improve severe LRTI. In a recent update, rimantadine and amantadine were no longer recommended by the National Institute for Clinical Excellence (NICE) for the treatment of influenza. However, with the emergence of H1N1 influenza A virus strains that are resistant to oseltamivir, combination treatments of oseltamivir and rimantadine or amantadine are currently (as of August 2009) recommended by the U.S. Centers for Disease Control and Prevention.
Ribavirin is an antiviral drug that is very effective against RSV in vitro and is licensed for use by inhalation for severe RSV bronchiolitis. However, due to teratogenic side effects, ribavirin cannot be administered as an aerosol in the presence of pregnant women (e.g., medical staff). In addition, regarding clinical use, ribavirin has generally been thought to be disappointing and to provide little or no benefit, possibly because once developed, the severe inflammation in RSV bronchiolitis may be maintained independently of the presence of live RSV virions. However, a recent study reported decreases in postbronchiolitic asthma and recurrent wheeze in 6-year-old children who were treated with ribavirin during RSV bronchiolitis (53). A Cochrane review found that published reports of trials of ribavirin lack the power to provide reliable estimates of its effects but suggested that ribavirin may reduce the duration of mechanical ventilation and hospitalization (341).
The anti-RSV antibody palivizumab, although not technically an antiviral drug, reduces the number of RSV cases requiring hospitalization for at-risk infants by 55% if given prophylactically (142a). In the original study, premature infants of ≤35 weeks of gestation with and without bronchopulmonary dysplasia were treated with palivizumab during their first winter season, resulting in 39% and 78% reductions in RSV-associated hospitalizations, respectively. Other high-risk groups for severe RSV LRTI for whom RSV immunoprophylaxis is recommended by the American Academy of Pediatrics include children under 2 years of age with chronic lung disease or with congenital heart disease (e.g., congestive heart failure, pulmonary hypertension, and cyanotic heart disease). Importantly, palivizumab does not have beneficial effects on established RSV bronchiolitis in immunocompetent infants and is therefore used for treatment only on an individual basis for immunocompromised patients. A new anti-RSV antibody derived from palivizumab with enhanced anti-RSV neutralizing activity, motavizumab, is currently being evaluated in clinical trials (275). Other antiviral drugs are in development for RSV and rhinovirus (RV) and are described below.
Bronchodilators, Corticosteroids, Antibiotics, and Other Treatments
In the absence of effective antivirals for severe infant LRTI, medical treatment is focused on drugs designed to overcome airway obstruction and the resulting respiratory distress. In analogy to asthma treatment, bronchodilators have been used widely, including β2 agonists, nebulized epinephrine, and antimuscarinics such as ipatropium bromide. However, in general, results of these interventions have been disappointing. Studies assessing the effects of these bronchodilators on lung function and clinical outcome yielded conflicting results. A recent Cochrane review concluded that bronchodilator treatment can improve clinical symptom scores in the short term in viral LRTI cases but that it does not reduce the duration of hospitalization and increases treatment cost (104). Studies published since the Cochrane review was performed support the argument that bronchodilators have no benefit for infant bronchiolitis (159, 193, 292). In line with this finding, the American Academy of Pediatrics recommends that inhaled bronchodilators should not be used routinely for the management of bronchiolitis (317). One possible exception is for LRTI with underlying reactive-airway disease and where wheeze is the hallmark symptom of LRTI, where short-acting β2 agonists may be effective for individual patients. However, short-acting β2 agonists need to be used cautiously in infants due to the risk of paradoxical β2 agonist reactions (38).
Another widely used approach was (and often still is) the use of corticosteroids in order to control airway inflammation and subsequent respiratory symptoms. Again, a multitude of studies using low-dose and high-dose inhaled corticosteroids as well as systemic application yielded conflicting results. The majority of those studies failed to demonstrate relevant reductions in LRTI symptoms, length of hospital stay, or need for mechanical ventilation. A meta-analysis of studies comparing systemic glucocorticoid treatment to placebo did not find any difference in the length of hospital stay or clinical score for infants and young children with LRTI from either group (252).
The effect may vary according to the infecting virus. There is little supporting evidence for the benefit of glucocorticoids for RSV bronchiolitis (40, 68) or the delayed effects of RSV bronchiolitis (93), although a recent study showed a possible partial effect of combined treatment with a bronchodilator and a glucocorticoid (262). One possible suggestion for their failure to relieve symptoms is that glucocorticoid administration in cases of RSV does not reduce cytokine production (307). There is limited information about glucocorticoids and influenza in infants, but for H5N1 infection of adults, there appeared to be no beneficial effect (128), and the data for severe acute respiratory syndrome (SARS) are inconclusive (316). However, glucocorticoids may have an effect on rhinovirus-induced recurrent wheezing (152, 191). Glucocorticoids may also be beneficial for the treatment of croup (17, 30), and croup is most often associated with parainfluenza virus (PIV), although the viral etiology was not demonstrated in those studies. Based on this evidence, the routine use of inhaled or systemic corticosteroids is not recommended for cases of RSV LRTI by most guidelines but may be of more use for defined cases of croup or RV infection.
The same idea holds for antibiotics. A recent Cochrane review found only one study comparing ampicillin to placebo that met the inclusion criteria. That study did not show any difference between the groups regarding the duration of illness or the number of deaths from LRTI (310). There has recently been particular interest in macrolide antibiotics, which are effective against atypical bacteria and which are also thought to have direct anti-inflammatory properties. A recent study of clarithromycin (320), which has been heavily criticized for inherent methodological defects (167, 171), reported a statistically significant reduction in the length of hospital stay and the need for supplemental oxygen and β2 agonist treatment. In contrast, a recent multicenter, randomized, double-blind, placebo-controlled trial of azithromycin for treatment of RSV LRTI failed to show any difference in the duration of hospitalization, oxygen supplementation, or nasal/gastric tube feeding or in RSV symptom scores (168). These data suggest that infants and young children with viral LRTI do not benefit from routine treatment with antibiotics. Other therapeutic approaches that have also failed to provide benefit to small children with viral LRTI include inhaled furosemide (20), recombinant DNase (32), or helium/oxygen inhalation (199) treatment.
Supportive Treatment and Inhalation of Hypertonic Saline
Given the lack of effective medications, current treatment for severe viral LRTI in infants relies on supportive measures only. These measures include supplementation of oxygen, monitoring of apnea, nasal/gastric tube feeding or intravenous fluids, and, if required, respiratory support with nasal bi-level positive-airway pressure (BiPAP) or intubation and mechanical ventilation. For infants with LRTI requiring mechanical ventilation, surfactant has been used. A Cochrane meta-analysis that included 3 studies that showed some reduction in the duration of mechanical ventilation and in the length of stay in intensive care concluded that there are no sufficiently powered data to provide reliable estimates of surfactant effects on ventilated infants with LRTI (340).
A promising new development is the use of hypertonic saline inhalation. A recent trial of this treatment, which has been successfully used for patients with cystic fibrosis, reported a reduction of 26% in the length of hospitalization for infants with acute viral bronchiolitis (182, 361). Provided that larger studies that focus on children under 6 months of age and exclude noneffective therapies confirm the beneficial effect of hypertonic saline inhalation, this treatment could become a new useful tool for the management of viral LRTI.
TO THE BENCH: EPIDEMIOLOGY AND GENETICS
Viral Diagnosis and Etiology
Comparison of the viral causes of infection provides a useful starting point for an understanding of illness following respiratory infection. It also provides data relevant for the development of prevention strategies. The following viruses (in no particular order) have been detected during acute respiratory infections (ARIs): adenovirus (AV), coronavirus (CoV), enterovirus (EV), human metapneumovirus (hMPV), influenza virus (IV), parainfluenza virus (PIV), rhinovirus (RV), and respiratory syncytial virus (RSV). There are also more recently identified viruses including bocavirus (BoV) and polyomaviruses.
There are four principal ways in which respiratory viruses are diagnosed: virus culture, serology, immunofluorescence/antigen detection, and nucleic acid/PCR-based tests. Virus culture refers to the practice of infecting cell lines with clinically obtained samples. Serology is where the blood is tested for either virus-specific antibodies or viral antigen by a functional assay. The disadvantage of both virus culture and serology is that they are labor-intensive and slow to produce results. Tissue cultures can take up to 10 days, and the antibody response to a viral infection can take 2 weeks to develop. Therefore, the infection can often be resolved before the infectious agent is defined. These two methods are no longer routinely used diagnostically but may have a role in epidemiological studies and when used to follow the course of an infection.
Antigen detection is based on the use of virus-specific monoclonal antibodies. There are a variety of diagnostic test kits based on antigen detection that are used for the rapid identification of virus. These tests use nasopharyngeal aspirate, nasopharyngeal wash, or nasal swab specimens as test material and detect viral antigen by use of either a conjugated enzyme or fluorescence. Antigen detection-based tests are still widely prevalent but are being replaced with nucleic acid-based tests; these tests have been reviewed in depth elsewhere (214). Nucleic acid tests are now being multiplexed, allowing the rapid detection of many viruses concurrently. Nucleic acid tests are significantly more sensitive than the other methods described above, and this may have an impact on which viruses are detected by studies.
The relative importance of individual viral agents in early life is open to debate. Certainly, RSV, RV, PIV, and influenza virus are predominant in the published data. However, as shown in Table 1, there are several factors limiting the ability to draw a definitive conclusion about which virus is the most common or important: differences in the way that data were collected (PCR versus immunoassay) between and within studies and the impact of assay sensitivity (214); differences in study design affecting age, recruitment criteria, and which viruses are studied; skewing of data historically, particularly the ease of in vitro detection of RSV compared to that of RV; changes following the wider introduction of reverse transcription (RT)-PCR; PCR diagnosis of virus that may not necessarily indicate that the virus is causing disease (353) (there is some evidence of viral RNA detection in asymptomatic children [335] and evidence of viral persistence [153]); and the predominance of hospital-based studies, which are skewed toward more severe illness.
TABLE 1.
Comparative studies of the relative prevalence of respiratory viral infection in children a
| Study authors (reference) | Recruitment criterion | Detection method(s) | Most prevalent virus(es) | Other viruses detected | Description |
|---|---|---|---|---|---|
| Bonzel et al. (31) | Children with acute ARI | PCR | RSV | BoV, RV | ∼20% viral coinfection; no conclusive effect on severity |
| Bosis et al. (34) | Children aged <1 yr hospitalized with wheeze | PCR | RSV | IV, CoV, hMPV, BoV | Association between RSV and wheezing |
| Bulut et al. (42) | Children aged <12 yr with AOM | PCR | RSV | RV, CoV, IV, PIV, AV | Also have data about bacterial infection, a more common cause of AOM |
| Cabello et al. (43) | Children aged <5 yr with ARI | Immunofluorescence | RSV/IV | PIV, AV | 65% viral, 13% bacterial, 22% coinfection |
| Calvo et al. (44) | Children aged <2 yr hospitalized with bronchiolitis | Immunofluorescence, viral culture, PCR | RSV | AV, RV, hMPV, IV, PIV, EV, CoV, CMV | |
| Calvo et al. (45) | Children aged <2 yr hospitalized with ARI | PCR | RSV | AV, RV, hMPV, IV, PIV, EV, CoV, CMV | 17.4% viral coinfection; coinfection associated with increased severity |
| Camps et al. (47) | Children aged <1 yr hospitalized with bronchiolitis or bronchopneumonia | Immunofluorescence, viral culture, PCR | RSV | hMPV, RV, AV, IV, PIV, CoV | |
| Canducci et al. (48) | Children aged <2 yr hospitalized with ARI | PCR | RSV | hMPV, CoV, BoV | 23% viral coinfection; single infection associated with increased severity |
| Chonmaitree et al. (58) | Children aged <3 yr with URTI or AOM | Viral culture, antigen detection, PCR | AV, RV (URTI); CoV, RSV, AV (AOM) | EV, CoV, PIV, RSV, IV | Age a critical factor in AOM |
| Chung et al. (59) | Children aged <6 yr with acute wheezing | Antigen detection, RT-PCR | RV | RSV, BoV, hMPV | 16% viral coinfection; no conclusive effect on severity |
| Cilla et al. (60) | Children aged <3 yr with community-acquired pneumonia | Immunofluorescence, viral culture, PCR | RSV | BoV, RV, hMPV, PIV, IV, CoV, AV | 27% viral coinfection; coinfection associated with increased severity |
| Cooper et al. (67) | Calls about fever, cough, difficulty breathing, and cold/flu | Database data | IV | RSV, RV, PIV | Modeled data from “NHS direct” calls; bacteria (especially S. pneumoniae) common |
| Costa et al. (69) | Children aged <5 yr with ARI | Immunofluorescence, PCR | RV | IV, PIV, AV, RSV | |
| Esposito et al. (94) | Children aged <15 yr in emergency room for any cause | PCR | IV | BoV, IV, RSV, hMPV, PIV, RV, AV, CoV | BoV not important cause of hospitalization on its own |
| Fabbiani et al. (95) | Children aged <15 yr with ARI | PCR | RV | RSV, AV, hMPV, BoV, CoV | |
| Forster et al. (101) | Children aged <3 yr with LRTI | PCR | RSV | PIV, IV | Office visit rate of ∼28.7%, hospitalization rate of ∼3% for all cases of LRTI |
| Hall et al. (120) | Children aged <5 yr with ARI | Viral culture, PCR | RSV | PIV, IV | RSV accounted for 15% of office visits and 20% of hospitalizations as a proportion of ARI |
| Heymann et al. (127) | Children aged <3 yr hospitalized with wheezing | Viral culture, antigen detection, PCR | RSV | AV, PIV, IV, RV, EV, CoV | In children aged <3 yr, viral infection was the main risk factor for wheeze (84%); incidence of RSV is greater in winter, and incidence of RV is greater in other months |
| Hon et al. (133) | Children admitted to PICU | Immunofluorescence | RSV | IV, PIV, AV | Age of RSV infection is lower |
| Jackson et al. (147) | Children aged <6 for prospective study for wheezing ARI | Viral culture, antigen detection, PCR | RV | RSV, hMPV, AV, IV, PIV, CoV | Link with RSV and RV wheeze and asthma |
| Jacques et al. (149) | Children aged <3 yr hospitalized with bronchiolitis | Immunofluorescence, viral culture, PCR | RSV | RV, EV, hMPV, PIV, IV, AV | ∼20 % viral coinfection; seasonality of viruses seen |
| Jartti et al. (151) | Children aged <1 yr with moderate-to-severe respiratory illnesses | Viral culture, antigen detection, PCR | RV | RSV, PIV, Echo, CoV, AV | Study investigated sequential, multiple infections throughout the yr |
| Jartti et al. (154) | Children aged <1 yr hospitalized with acute wheezing | Viral culture, antigen detection, PCR | RSV | hMPV, PIV, IV, AV, CoV | |
| Children aged <3 yr hospitalized with acute wheezing | EV/RV | ||||
| Klein et al. (165) | Children aged <2 yr born either premature or with congenital heart disease | PCR | RSV | hMPV, PIV, IV | |
| Konïg et al. (169) | Children aged <3 yr for prospective study | PCR, viral culture | RSV | hMPV, IV, PIV | hMPV and RSV coinfection associated with increased severity |
| Kusel et al. (180) | Children aged <1 yr with ARI | PCR | RV | RSV, CoV, PIV, IV, hMPV, AV | Extended findings reported (181) |
| Legg et al. (186) | Children aged <1 yr with ARI | PCR | Picornavirus | RSV, COV, PIV | ∼20% viral coinfection; no effect on severity |
| Lehtinen et al. (190) | Children aged <16 yr with acute wheezing | Viral culture, antigen detection, PCR | RV | RSV, EV | 18% of children had bacterial coinfection |
| Lemanske et al. (192) | Children aged <3 yr with ARI | Viral culture, antigen detection, PCR | RV | RSV, PIV, IV, AV | Link with RSV and RV wheeze and asthma |
| Louie et al. (208) | Children aged <18 yr hospitalized in PICU with LRTI | PCR | RV | hMPV, RSV, PIV | 47% viral or bacterial coinfection |
| Manning et al. (215) | Archived respiratory samples (67% from children aged <5 yr) | PCR | RSV | IV, BoV, PIV, AV | BoV common as a coinfection agent |
| Manoha et al. (216) | Children aged <3 yr with ARI | Immunofluorescence, PCR | RSV | hMPV, RV | |
| Mansbach et al. (217) | Children aged <2 yr with bronchiolitis | PCR | RSV | RV, hMPV, IV | |
| Marguet et al. (218) | Children aged <1 yr with bronchiolitis | PCR | RSV | RV, hMPV, CoV | RSV was associated with more severe disease |
| Mullins et al. (235) | Children aged <5 yr with ARI | Viral culture, PCR | RSV | RSV, hMPV | |
| Nicholson et al. (242) | Children aged <6 yr with ARI | PCR | RSV | hMPV, IV | Observation that IV is underreported |
| Papadopoulos et al. (251) | Children aged <18 mo with bronchiolitis | PCR | RSV | RV, AV, IV, PIV, CoV | 19.5% viral coinfection; RV is associated with increased disease severity |
| Pierangeli et al. (258) | Children aged <12 yr hospitalized with ARI | PCR | RSV | RSV, RV, IV, PIV, hMPV, AV | 4.8% viral coinfection |
| Regamey et al. (277) | Children aged <1 yr with ARI | PCR | RV, CoV | PIV, RSV, hMPV, BoV, IV, AV, EV | RSV infection led to more severe symptoms |
| Rihkanen et al. (279) | Children admitted to hospital with croup with median age of 1.9 yr | PCR | PIV | RSV, BoV, EV, IV, CoV, AV | Compared croup and wheeze; PIV was main agent in croup, and RSV was main agent in wheeze |
| Schanzer et al. (289) | Patients <19 yr old, based on hospital coding data | Various | PIV | RSV, IV | IV accounts for 1.5% of hospitalizations, rising to 7% in season |
| Stempel et al. (314) | Children aged <2 yr hospitalized with bronchiolitis | PCR, immunofluorescence | RSV | AV, hMPV, CoV, PIV, IV | 24% viral coinfection |
| Talbot et al. (323) | Children aged <5 yr hospitalized with LRTI | PCR | RV | RSV, IV, hMPV, CoV | Focus on CoV; CoV accounted for 1.8% of hospitalizations |
| Weigl et al. (348) | Children aged <16 yr with ARI | PCR | RV | RSV, hMPV, PIV, IV, EV, CoV | |
| Weinberg et al. (349) | Children aged <5 yr with ARI | Viral culture, PCR | RSV | IV, PIV | Focus on PIV; PIV accounted for 6.8% of hospitalizations |
| Wolf et al. (351) | Children aged < 5 yr with LRTI | Immunofluorescence, PCR | RSV | hMPV, IV, PIV, AV | hMPV coinfection did not increase severity |
The table shows a sample of the studies published comparing causes of infection. Studies were selected if they were recent (in the last 5 years), compared more than one virus, recruited children only (<18 years old), and were in readily available journals. Abbreviations: AV, adenovirus; BoV, bocavirus; CoV, coronavirus; EV, enterovirus; Echo, echovirus; hMPV, human metapneumovirus; IV, influenza virus; PIV, parainfluenza virus; RV, rhinovirus; RSV, respiratory syncytial virus; CMV, cytomegalovirus; ARI, acute respiratory illness; AOM, acute otitis media; URTI, upper respiratory tract infection; LRTI, lower respiratory tract infection; PICU, pediatric intensive care unit.
The following broad conclusions can be drawn about viral etiology and infant hospitalization due to respiratory infection (Table 1). First, influenza virus, adenovirus, hMPV, PIV, RV, and RSV can all cause bronchiolitis, necessitating hospitalization. Second, of these viruses, RSV has most commonly been reported to be the main cause of hospitalization due to bronchiolitis and increased disease severity, followed by RV and then by influenza virus. Third, viral coinfection is relatively common, occurring in about 20% of cases. However, there is no consensus on the effect of coinfection on disease severity. The effect may depend upon which viruses coinfect together. For example, when adenovirus or rhinovirus was detected during RSV infection, there was no increase in severity (3); however, coinfection with both hMPV and RSV increased the intensive care unit admission rate (295). Finally, the profile of viruses detected is changing due to the increasing use of nucleic acid-based diagnostic screens and the discovery of newly isolated viruses.
Knowledge of the infecting agent does not routinely alter treatment except insofar as a positive viral identification will reduce the inappropriate use of antibiotics and may allow the cohorting of patients to reduce nosocomial infection. More importantly, while knowledge of which virus is predominant is relevant for the design of vaccines and specific prophylactic treatments, what can be observed is the similarity of symptoms caused by a wide range of viral agents. It may therefore be more appropriate to focus on ways to target the symptoms and not the agent. This may be especially relevant when an excess immune response causes the disease or when there are multiple serologically distinct subtypes circulating.
“New Respiratory Viruses”
Recently, several “new” viruses have been characterized, in part triggered by new diagnostic technology, especially RT-PCR. Recently isolated respiratory viral agents include human metapneumovirus (hMPV) (337), found in samples from children with RSV-like bronchiolitis who were RSV negative; human bocavirus (BoV), discovered by a random PCR screen of respiratory tract samples (8); and two new polyomaviruses, WU (106) and KI (9). Two questions arise about these new viruses. First, are they truly new or only newly discovered? For example, hMPV was shown to have been circulating for at least 50 years (337). Second, what is the clinical impact of these viruses? hMPV certainly has clinical impact, and there is evidence to suggest that BoV is pathogenic (46, 225), but data from previously reported studies suggested that the new polyomaviruses are not pathogenic on their own (2, 244). The discovery of new agents of infection is important because they may play a role as coinfecting agents, altering disease severity. Newly discovered viruses may also be important in future outbreaks; for example, severe acute respiratory syndrome (SARS) was caused by a coronavirus (103, 254), and coronaviruses had previously been considered to be minimally pathogenic.
Who Gets Colds?
The likelihood of infection is determined by two factors: age (234) and exposure to infection. The severity of infection once it occurs is more complex and is determined by both environmental and genetic risk factors. The risk factors for severe RSV infection have been most thoroughly characterized (reviewed in reference 302), and they are as follows: (i) age when infected (120); (ii) increased exposure to an infectious agent, such as sibling order, day care attendance, birth season, hospitalization, and socioeconomic status (245); (iii) decreased body size due to gestational age, malnutrition, and birth weight; (iv) protection against virus due to breastfeeding and the amount of IgG in breast milk; and (v) factors affecting lung function, such as exposure to smoke and air pollution.
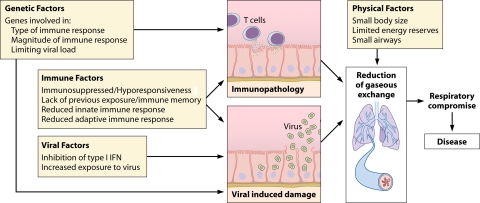
Young age acts as a metafactor reflecting the interplay of factors causing disease following viral infection (Fig. 1). Age has an effect on the size of the child, particularly airway size, transmission dynamics (due to multiple close contacts between small children), and immune experience, all of which contribute to an increased severity of infection. Furthermore, younger children have smaller energy reserves and are more likely to get exhausted by the effort of breathing—the ultimate cause of mortality in acute bronchiolitis. There are also critical differences in the infant immune system compared to that of adults (discussed below) that directly affect infection.
FIG. 1.
Interplay of factors that cause disease following respiratory viral infection and impact of infancy. The ultimate cause of illness/disease following respiratory viral infection is airway occlusion, which leads to a reduction in gaseous exchange, leading to respiratory distress. This airway occlusion can be either immune or virally mediated and most probably is a combination of both. Early life has an effect on both virus- and immune-mediated damage. The infant immune system is skewed to a hyporesponsive phenotype, with a reduced type I interferon response leading to a higher viral load. The adaptive immune response is also skewed and limited in its effect. These factors act in combination with small body size and small airways to further increase disease severity.
Genetics
As well as environmental risk factors, genetic risk factors have been identified. A large number of candidate gene association studies have been performed for both RSV (231) and SARS-CoV, but studies have also been performed for influenza virus and RV (Table 2). Significant correlations between genes of the immune system and the risk of severe respiratory viral infection have been observed. There is overlap among the different viruses, with several of the same genes having an association with disease, but this may reflect merely bias in the selection of candidate genes. This in turn reflects the core limitation of the candidate approach: it will answer only the question posed rather than revealing novel interactions (305). Candidate gene studies can be increased in power when supported by functional evidence of the effect of the polymorphism.
TABLE 2.
Genetic associations with respiratory viral infectiona
| Group and gene | Virus | Effect of studied SNP | Reference(s) |
|---|---|---|---|
| Inflammatory mediators | |||
| CCL5 | SARS-CoV | −28G allele, which increases NF-κB binding, increases the risk of fatality | 239 |
| IFN-α | RSV | −453T allele (no known function) association on limited genome-wide approach | 150 |
| IFN-γ | RSV | Increased IFN-γ production associated with increased disease severity | 108 |
| SARS-CoV | −874A allele, which reduces NF-κB binding and increases SARS susceptibility | 57 | |
| IL-4 | RSV | −589T allele, which increases transcription factor binding affinity, associated with increased risk | 56 |
| RSV | −589T allele study | 130 | |
| RSV | Study of 5q31 Th2 cytokine gene cluster | 102 | |
| RSV | No association with −589T SNP | 271 | |
| IL-6 | RV/RSV | −174C allele, which decreases IL-6 expression, associated with increased risk of OM | 11 |
| RSV | High-producing IL-6 alleles associated with shorter hospital stay | 108 | |
| IL-8 | RSV | −251A allele, which increases IL-8 expression, associated with increased risk of bronchiolitis | 136 |
| −251A allele, which increases IL-8 expression, associated with increased risk of wheeze | 110 | ||
| −781C/T allele, which alters promoter binding, associated with asthma but not RSV bronchiolitis | 273 | ||
| IL-9 | RSV | No association | 129 |
| IL-10 | RV/RSV | High-producing IL-10 allele associated with increased risk of OM | 11 |
| RSV | −592C allele (no known function) associated with bronchiolitis | 129 | |
| IV | No effect on vaccine response | 324 | |
| IL-13 | RSV | −1112T allele, which increases binding affinity, associated with increased risk of severe RSV infection | 271 |
| Study of 5q31 Th2 cytokine gene cluster | 102 | ||
| Arg130Gln, which alters IL-13Rα1 binding, associated with increased risk of persistent wheeze | 92 | ||
| IL-18 | RSV | 133G/C allele, which may alter STAT binding and IL-18 expression, associated with increased RSV bronchiolitis | 270 |
| SARS-CoV | −607T allele (no known function) associated with increased nasal shedding | 54 | |
| TGF-β | RSV | −509T allele, which increases TGF-β1 expression, associated with RSV wheeze | 131 |
| TNF | RSV | No association | 108, 129, 267 |
| SARS-CoV | −204C/T allele (no known function) associated with protective effect | 345 | |
| PGI2 | RSV | No association | 123 |
| Receptors | |||
| CCR5 | RSV | −2459/G (decreases expression) and 2554/T alleles associated with severe RSV bronchiolitis | 135 |
| CD14 | RSV | −550C/T allele, which increases levels of soluble CD14, associated with risk of severe RSV bronchiolitis | 143 |
| No association | 269, 322 | ||
| SARS-CoV | −155C/C allele, which decreases level of soluble CD14, increases risk of SARS-CoV | 356 | |
| CX3CR1 | RSV | Thr280Met (no known function) increases risk of RSV LRTI | 14 |
| E selectin | RSV | No association | 178 |
| FCεR1A | RSV | −66C allele (no known function) association on limited genome-wide approach | 150 |
| FCγRIIA | SARS-CoV | Arg131His (no known function) associated with increased disease severity | 357 |
| HLA | RSV | No association | 145 |
| IV | Increased frequency of HLA-DRB1*07 and decreased frequency of HLA-DQB1*0603-9/14 and DRB1*13 in nonresponders to IV subunit vaccine | 107 | |
| SARS-CoV | Association of HLA-B*4601 with severity of SARS infection | 201 | |
| ICAM1 | RSV | No association | 178 |
| ICAM3 | SARS-CoV | Asp143Gly (no known function) associated with decreased white blood cell counts | 50 |
| IL4RA | RSV | Gln551Arg, which may affect STAT6 binding; associated with increased RSV hospitalization | 130 |
| MBL | RSV | No association | 175 |
| SARS-CoV | Low-expression polymorphisms associated with SARS infection | 360 | |
| No association | 357 | ||
| Higher frequency of haplotypes associated with low or deficient serum levels of MBL in patients with SARS than in control subjects | 144 | ||
| TLR4 | RSV | Asp299Gly and Thr399Ile, which reduce TLR4 translocation, associated with severe RSV bronchiolitis | 322 |
| Asp259Gly increased risk of RSV infection | 269 | ||
| Asp299Gly and Thr399Ile were significantly overrepresented among infants with severe RSV bronchiolitis | 18 | ||
| No association | 143, 253 | ||
| VCAM1 | RSV | No association | 178 |
| Vitamin D receptor | RSV | FokI ff genotype, which reduces receptor expression associated with increased rates of ARI | 281 |
| Thr1Met association on limited genome-wide approach | 150 | ||
| Others | |||
| FGL2 | SARS-CoV | Gly57Gln (no known function) associated with increased nasal shedding | 54 |
| JUN | RSV | −750G/A allele (no known function) association on limited genome-wide approach | 150 |
| L-Sign (CD209L) | SARS-CoV | No association | 197 |
| Homozygous expression of tandem repeats in CLEC4M may bind virus to cells and has a protective advantage | 51 | ||
| MASP2 | SARS-CoV | No association | 346 |
| NOS2A | RSV | −2757A allele (no known function) association on limited genome-wide approach | 150 |
| SP-A | RSV | Gln223Lys, which may alter RSV binding, associated with severe RSV infection | 206 |
| SP-B | RSV | No association | 268 |
| SP-C | RSV | No association | 272 |
| SP-D | RSV | No association | 177 |
| Met11Thr, which may reduce opsonization, associated with increased risk of RSV bronchiolitis | 183 |
The table shows genes that have been studied in association with an increased risk or severity of respiratory viral infection. Many of the studies were not specific for infantile bronchiolitis, but due to the limited availability of published genetic study data, they have also been included here. Where known or speculated, the allele and its putative function have been included. Abbreviations: SNP, single nucleotide polymorphisms; OM, otitis media; ARI, acute respiratory illness; URTI, upper respiratory tract infection; LRTI, lower respiratory tract infection; TGF-β, transforming growth factor β; MBL, mannose binding lectin.
Two studies have taken a wider approach to look at the risk of RSV bronchiolitis (150, 300). Of these studies, the earlier study by Janssen et al. demonstrated that RSV susceptibility is complex, but the strongest associations were with polymorphisms in the genes of the innate immune response (150). This included the transcriptional regulator Jun, alpha interferon (IFN-α), nitric oxide synthase, and the vitamin D receptor. The more recent study of preterm children by the same group also indicated a critical association with innate immune system genes and bronchiolitis susceptibility (300).
Another approach is to use animal models to either define novel genes of interest or explore their function. There are differences in the susceptibility of inbred mouse strains to respiratory viral infection, which allow comparative studies. Differences have been seen in mouse strain susceptibility to RSV (122, 139, 265, 312). Alternatively, mouse models can be used to support the findings of human studies. For example, polymorphisms in surfactant protein A (SP-A) were associated with an increased risk of RSV bronchiolitis (206), and SP-A-deficient mice have an increased RSV viral load (194). The role of Toll-like receptor 4 (TLR4) in RSV infection was also clarified by a combination of mouse and human genetic studies. The initial importance of TLR4 was observed for mice (179), and studies were then performed by using human airway cells (233), leading to genetic studies of susceptibility (18, 143, 253, 269, 322).
There appear to be two loose groups of genes that are important for altering the outcome following respiratory viral infection. Genes in the first group are involved in the magnitude and type of the immune response but do not necessarily control viral load. Alleles that lead to an increased level of expression or efficacy of these genes increase the risk of severe disease, for example, the interleukin-4 (IL-4) −589T allele (56), the IL-8 −251A allele (136), and the IL-13 −1112T allele (271). The second group contains genes that are involved in the control of viral load. Alleles that lead to a decreased expression of these antiviral genes increase the risk of severe disease, for example, the TLR4 Asp299Gly polymorphism (322), the CD14 −155C allele (143), and the IL-6 −174C allele (11). This reflects the two arms that contribute to respiratory viral disease damage caused by the virus and damage caused by the immune system (Fig. 1).
VIROLOGY
Viral Detection by the Host
The initial detection of viruses by the immune system is critical for their control and for shaping the response required for clearing them. It is increasingly being recognized that there are highly conserved host receptors that recognize basic components of viruses, triggering an immune response. These viral components, termed pathogen-associated molecular patterns (PAMPs), are often constituents of the virus that cannot be evolved away from, e.g., the physical makeup of their genomes. Which pattern recognition receptors (PRRs) are involved in the detection of respiratory viruses, particularly in vivo, has not been clearly defined. This is reflected by the somewhat contradictory nature of the data reported thus far. However, viral detection and the upregulation of the type I interferon (IFN) response are clearly important. The importance of this system in acute viral respiratory infection of children is highlighted by studies of the genome-wide association of RSV bronchiolitis, which indicated a significant association between IFN-α single nucleotide polymorphisms (SNPs) and bronchiolitis (150, 300). Furthermore, bronchial epithelial cells from asthmatics, who are at an increased risk of severe viral infection, have been shown to have deficient type I IFN (347) and type III IFN (66) production. This increased susceptibility to infection may be important for both the development and exacerbations of asthma. As discussed below, infants have reduced type I IFN responses (195), which may lead to increased disease severity following viral infection.
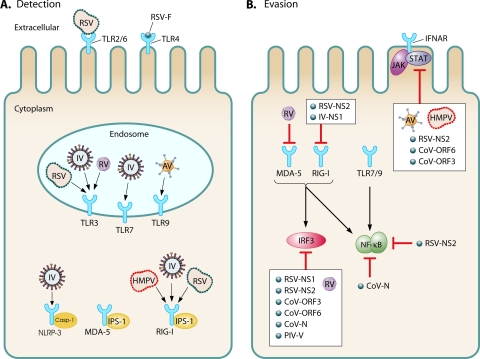
The detection of viruses can occur extracellularly, in the endosome, and in the cytoplasm (Fig. 2A). TLR2, TLR4, and TLR6 are all extracellular receptors that have been characterized principally for the detection of bacterial products, both lipopolysaccharides (LPSs) and lipoproteins. An increased RSV viral load was observed for mice that were deficient for TLR2 and TLR6 (236). RSV was shown to interact with TLR4, normally associated with LPS, via its F protein (179, 236). On some levels, this would seem counterintuitive since activating TLR4 would induce an antiviral immune response, and viral protein could evolve away from this. Indeed, it was suggested that IL-12 and not TLR4 is important for viral control (85). However, it may be that the interaction between TLR4 and RSV is necessary for another viral function, for example, entry, and the benefit of this outweighs the cost of activating the downstream immune response, or the virus has evolved secondary mechanisms to inhibit the downstream response to TLR4. An analogous situation may occur in SARS-CoV, which was demonstrated to use the C-type lectins DC and L-Sign for entry (121).
FIG. 2.
Viral detection and viral evasion. (A) Viral detection by the innate immune system. Respiratory viruses are detected extracellularly by TLR2, TLR4, and TLR6; in the endosome by TLR3, TLR7, and TLR9; and in the cytoplasm by RIG-I (retinoic acid-inducible gene I), MDA-5 (melanoma differentiation-associated gene 5), and NLRP3 (NLR family, pyrin domain-containing 3). (B) Viral evasion of the innate immune system. Viruses inhibit the pattern recognition receptors RIG-I and MDA-5 and the downstream molecules IRF3, NF-κB, and JAK/STAT. In some cases the viral protein that inhibits the response has been identified. Abbreviations: AV, adenovirus; CoV, SARS coronavirus; hMPV, human metapneumovirus; IV, influenza virus; PIV, parainfluenza virus, RSV, respiratory syncytial virus; RV, rhinovirus; TLR, Toll-like receptor; IFNAR, interferon alpha receptor; ORF, open reading frame; CASP-1, caspase 1; NS, nonstructural.
TLR3, TLR7, and TLR9 are located in the endosome and have been demonstrated to be important for the detection of virally associated genome components. TLR3 recognizes double-stranded RNA (dsRNA), TLR7 recognizes single-stranded RNA (ssRNA), and TLR9 recognizes unmethylated CpG repeats. The inhibition of TLR3 with small interfering RNA (siRNA) impaired CCL5 and CXCL10 production following RSV infection of human cell lines but did not alter viral load (283). Anti-TLR3 treatment increased the viral load in human cell lines following RV infection (126), and the use of a dominant negative form of TLR3 blocked the NF-κB response to influenza virus in human cell lines (118). In vivo, TLR3−/− mice have decreased inflammation and pathology but increased influenza viral loads (187). Alpha interferon production following influenza virus infection is reduced in murine TLR7−/− dendritic cells (DC) (81, 211). No association between RSV or RV and TLR7 has been observed; however, other members of the family Picornaviridae have been shown to interact with TLR7 in human cell lines (332). RSV infection also increases the levels of TLR3 (115) and TLR4 (233) in human primary airway cells and cell lines, which may sensitize cells to future infection. TLR9 was observed to be important for the detection of adenoviral vectors (16, 24, 49, 354).
The RIG-I-like receptor (RLR) family is a recently described group of intracellular proteins that are able to detect the viral genome in the cytoplasm. The prototypic member of this family, RIG-I, was shown to be involved in the antiviral response to influenza virus in mice (257) and to hMPV (198) and RSV (204) in human cell lines. RIG-I mRNA levels positively correlate with RSV viral load in infected children (288). Different members of the RLR family have different specificities for viruses: MDA-5 was shown to be important for the detection of the picornavirus encephalomyocarditis virus (EMCV) in mice (161) but not influenza virus (188) or hMPV (198) in human cell lines. The downstream adaptor protein for the RIG-I-like family, IPS1/MAVS/CARDIF, was shown to be critical for the detection of RSV using human cell lines (207, 249) and knockout mice (26).
There may be other methods of viral detection that are also important, for example, the NOD-like receptor inflammasome, which was recently demonstrated to be required for the immune response to influenza virus (10, 141, 328), and β-3 integrins have been shown to be important for the detection of adenovirus (83). Members of the C-type lectin family have been associated with an increased severity of infection, including surfactants (114) and mannose binding lectins (144, 278). The protein DAI (DNA-dependent activator of IFN-regulatory factors; DLM-1/ZBP1) is a cytosolic DNA sensor and may also be of importance for the detection of viruses (321), and it is likely that there are other DNA receptors that are critical for the detection of viruses.
Viral Evasion
The type I interferon (IFN) system is critical for the host defense against virus, and evading it is of critical importance to all viruses. All respiratory viruses have mechanisms to avoid the type I IFN response (Fig. 2B). While structurally and functionally diverse, the downstream result of these proteins is to improve conditions for viral replication in host cells. The host specificity of the virus is determined by its ability to evade the type I IFN system, which was demonstrated for both influenza virus (124) and RSV (36).
Viruses can hide their PAMPs; for example, the influenza virus NS1 protein conceals the viral genome from detection (200). RSV leader negative-strand RNA binds the La antigen, which inhibits the RIG-I detection of RSV (27). Alternatively, viral proteins actively subvert the function of pattern recognition receptors. Both influenza virus (257) and RSV (202) target RIG-I, and RV (22) targets MDA5. Downstream signaling to these receptors can be inhibited. RSV was shown to inhibit IFN production by plasmacytoid dendritic cells in response to CpG (TLR9) and resiquimod (TLR7) (290). SARS-CoV was shown to block NF-κB function (170), and interferon response factor 3 (IRF3) activation is inhibited by RV (174), RSV (309), SARS-CoV (170), and PIV (210). Of interest is the increase in NF-κB function following RSV infection (309), and this may contribute to the inhibition of apoptosis (28). Signaling from the IFN-α receptor by elements of the JAK-STAT pathway is inhibited by RSV (274), hMPV (82), SARS-CoV (170), and adenovirus (AV) (296).
The adaptive immune response is principally evaded by the mutation of viral proteins. Most respiratory viruses (barring adenovirus) have RNA genomes, and the combination of RNA polymerase leakiness and a high level of viral turnover means that there is a high rate of mutation (88). This is reflected in the large number of circulating viruses (for example, there are over 110 serologically distinct RVs) and results in multiple infections by the same virus family within and between seasons. The segmented genome of influenza virus further increases its ability to rapidly change genotypes. The evolution of the influenza virus genome is the driving factor behind the emergence of pandemic strains, as observed for the recent H1N1 swine flu pandemic (306). The coat proteins of viruses can also be altered by changing glycosylation patterns (343). By altering the glycosylation of H3N2 influenza virus, immune evasion increased without altering infectivity (1). Reinfection with genetically identical RSVs can also occur (294), suggesting that the memory response that it induces may be poor.
Viruses also actively subvert the function of immune cells that are directly infected. In vitro infection of DC with RSV or hMPV reduces their antigen-presenting capacity, a change that may be linked with the inhibition of type I IFN (116, 117). However, other mechanisms may be utilized to suppress the antigen-presenting capacity, thereby blinding the immune system to the presence of virus. RV was demonstrated to induce IL-10 in DC (315), influenza virus was shown to inhibit DC function by both the hemagglutinin (HA) (243) and NS1 proteins (97).
Persistence
There is also some evidence, mostly from models, that respiratory viruses are able to cause persistent infection. Persistent and/or latent AV infection was demonstrated for children with established bronchiolitis (213) but was not found in children with chronic obstructive bronchitis (256). The persistence of RV RNA was detected in the lungs of hospitalized children (153). Persistence has also been demonstrated by using guinea pig (125), bovine (334), and mouse models of RSV (293) and hMPV (13, 205). However, the occurrence of persistent respiratory viral infection, particularly the persistence of RNA viruses, is controversial. Furthermore, the role of persistent infection and whether it is a function of the host or the virus are unclear. Persistent infection may provide a pool of virus for reinfection (173), or there may be a retention of a pool of viral antigen and/or genomic material to maintain adaptive immune memory (359).
In conclusion, viruses have evolved to evade the immune system, and this immune evasion is critical for viral host specificity and has an important impact on the host response to infection. The combination of viral immunosuppression and the hyporesponsiveness of the early-life immune response may increase the amount of virally mediated damage and therefore increase disease.
IMMUNOLOGY
Immunopathology versus Viral Pathology
A core question about respiratory viral infection is, how is disease caused? This is focused around a central debate, the relative impacts of virus and host on the pathogenesis of infection, and has a critical bearing on approaches to limit the effect of childhood infection. Unfortunately, it is very difficult to separate the effects of one component from those of the other. When the viral load is higher, disease is more severe, but when the viral load is higher, the proinflammatory stimuli are also greater, and therefore, the immune response is greater. This subject has been thoroughly reviewed by Collins and Graham (63), so we will touch upon it briefly here.
Some evidence suggests that damage caused to the lung by viral infection is the key factor. A recent study of the lungs of infants who died of RSV infection demonstrated the presence of virus but not lymphocytes (350). Of the nine children with RSV disease analyzed in that study, two suffered from Down's syndrome and five had heart disease (in infants, presumably congenital cardiac defects), both conditions which are often associated with some degree of immunodeficiency. This suggests that these findings may be particular to immunodeficient or immunosuppressed individuals, for whom RSV infection is known to be a major clinical problem, e.g., after bone marrow transplantation (229). There is also a correlation between viral load and disease severity in RSV (100) and hMPV (35) infections. Direct viral damage was demonstrated for some viruses. RSV can inhibit cilia movement, which might lead to airway blockade (362). RV increases mucus production (23), and in vitro cytotoxicity has been seen for RV infection (37). In fatal cases of SARS-CoV, viral infection damages primarily type 1 and, to a lesser extent, type 2 pneumocytes (240). Data from fatal influenza infection are confounded by the regular occurrence of bacterial coinfection (241), but inhibiting the cytokine response in a mouse model had no effect on H5N1 pathogenesis (286), and IL-1 knockout mice had worse pathology for influenza virus (291, 319).
However, there is also plenty of evidence to support the idea of immunopathology. An autopsy study of an RSV-infected child who died in a vehicle crash demonstrated substantial lymphocytosis (155). RSV-infected HIV-positive infants had increased viral shedding but decreased bronchiolitis (52). There are parallels between fatal SARS-CoV and H5N1 influenza virus infections: lung infiltration by macrophages is associated with disease (55). Data also demonstrate that the virus had cleared from the lungs of patients who died of SARS or fatal H5N1 infection (240, 333). Furthermore, in both SARS-CoV and H5N1 infections, antiviral drugs were used on fatally infected patients but did not alter disease outcome. Both RSV and RV are characterized by neutrophilic infiltrate (227, 261). The main neutrophil chemoattractant, IL-8 (CXCL8), was shown to be upregulated in the airways of RSV bronchiolitics (226) and asthmatic children during RV infection (327). For RV, CD8 T cells are closely associated with fatal asthma exacerbations (250). There is a requirement for synergistic studies of animals and humans, both of which give incomplete answers but can contribute insight into the whole. Data from animal studies mainly support the idea of immunopathology. For example, the depletion of T cells during primary viral infection of BALB/c mice inhibits disease (113). Until very recently, there was no RV mouse model available, but the recent development of such a model should allow complementary studies to be performed (23).
It is our view that immunopathology does play a role in disease, and this needs to be taken into consideration in the development of preventative treatments. However, there can be a sliding scale of the relative contributions of viral pathology and immunopathology where the end point of observable disease is the same.
Bacterial Coinfection
One interesting side effect of respiratory viral infection is increased susceptibility to bacterial coinfection. This has been reported for RSV (330) but is highly associated with influenza virus infections (282). Many of the fatalities due to the 1918-1919 flu pandemic were caused by secondary bacterial pneumonia (166). Viral infection enhances bacterial infection in two ways, altering physical barriers and altering immune system barriers. Viral infection (and the subsequent immune response) may damage the lung epithelia, increasing bacterial entry (263). The neuraminidase protein from influenza virus plays an active role in thinning mucus and exposing receptors on epithelial cells, leading to increased bacterial infectivity (255). Viral infection can also skew the immune response, allowing greater infection. Influenza infection can inhibit neutrophilia, leading to increased bacterial infection (61, 228). Viral infection was proposed to increase the expression of host receptors used by bacteria to enter cells, particularly platelet-activating receptor, a key factor for Streptococcus pneumoniae infection (338); however, other studies suggested that this is not the case (224). Bacterial coinfection often happens in the later stages of viral infection, during the dampening of the immune response. IL-10 is a key cytokine in the resolution of the immune response, but it can lead to increased bacterial infection (339). A general downregulation of pathogen sensing may also occur following viral infection, leading to an increased incidence of bacterial infection (80). The incidence and importance of subsequent bacterial coinfection have a considerable impact on the prescription of antibiotics (209).
Infant Immunology
The infant immune system is different from the adult immune system, and this has a critical impact on susceptibility to respiratory viral infection. There is a general naivety of the infant immune system: the lack of prior exposure to pathogens leads to a lack of immune memory. However, there is also a tendency toward hyporesponsive immune responses in early life, characterized by both reduced innate and adaptive immune responses (4). This is a critical adaptation to survive early-life exposure to previously unseen nonpathogenic antigens of both self and foreign origins. Infant immune responses are also characterized as being T-helper 2 (Th2) skewed; this is in part reflective of the immune response of the fetus. Pregnancy is strongly associated with Th2 cytokines, which is important for the avoidance of rejection (29). A Th1 environment can be associated with preeclampsia, a complication of pregnancy (19); furthermore, infections that skew the environment toward Th1 during pregnancy (e.g., Chlamydophila abortus in sheep) can cause abortions (91). This fetal Th2 skewing extends into early childhood and influences the immune responses to infection and possibly the development of asthma and allergy. Immune responses in pregnancy can also be dampened by other means, for example, an increased level of production of l-arginase leading to a local depletion of l-arginine and functional T-cell hyporesponsiveness (176). The removal of the baby from the uterine environment removes this immunosuppression, as observed by the similarity in neonatal immune responses compared by birth rather than gestational age.
The innate immune response of infants is much reduced compared to that of adults (195). Studies have demonstrated that there are reduced responses to innate stimuli, including model TLR ligands: LPS (196), poly(I:C) (79), and CpG oligonucleotides (78). The level of the response may depend upon the effector molecule that is being used as a readout: the level of IL-6 appears to be increased (15), while the level of tumor necrosis factor (TNF) (196) or type I IFNs (78, 79) is decreased. Interestingly, it was demonstrated that the level of expression of Toll-like receptors on cord blood dendritic cells is not different from that of adults (196, 342). However, the level of expression of proteins downstream of these receptors, including MyD88 (285), IRF3 (5), and IRF7 (74), is decreased in cord blood-derived DC. It is therefore possible that early-life innate responses are controlled by the suppression of adaptor proteins. This suppression at the “choke point” of signal transduction may be the most efficient way of globally limiting the immune response.
The level of the adaptive immune response in infants is also reduced. This may be a downstream consequence of the failure to initiate type I IFN responses and therefore minimal DC activation, but other mechanisms may be involved. Dendritic cell immaturity, specifically the reduced level of production of IL-12 (112), may lead to the reported skewing of the immune response to a Th2 phenotype. In addition, both CD4 and CD8 functions were reported to be deficient, which may in turn lead to reduced viral clearance and increased reinfection. B-cell and antibody responses to infant vaccination are especially poor, with weaker, shorter-lived responses (299). Although not fully understood, the failure to produce high levels of antibody has been linked to several aspects of the B-cell response. First, infants have immature B cells affecting the strength of the antibody response. Second, there is a failure of signaling for the crucial B-cell survival factors BAFF (B-cell activating factor of the TNF family) and APRIL (a proliferation-inducing ligand), with reduced levels of APRIL expression (25) and reduced levels of expression of the receptors TACI (transmembrane activator and calcium modulator and cyclophilin ligand interactor), BCMA, and BAFF-R (162) leading to the rapid waning of the antibody response observed. These factors have been identified as being determinants of the mucosal antibody response to RSV infection (276). Finally, the immaturity of dendritic cells in early life (112) might also influence the strength of B-cell responses; for example, there is poor follicular dendritic cell development in neonatal murine germinal centers (259). Although reduced in magnitude, infants do develop a memory response to infection, which reduces the effect of subsequent infections with the same virus.
The early-life immune response may also be actively suppressed. CD25+ CD4+ regulatory T cells (Tregs) were shown to inhibit the murine neonatal immune response to herpes simplex virus (96). Children born to mothers with placental Plasmodium falciparum infection are more susceptible to malaria (189), and this may be associated with the enhanced development of P. falciparum-specific Tregs in cord blood (39). B cells may play a role in the suppression of infant immune responses; a subset of B cells, CD5+ (B1a) B cells, was shown to be suppressive (318, 363).
Asthma
Another aspect of pediatric respiratory viral infection linked to the immune system is the development of asthma following viral bronchiolitis. Whether viral bronchiolitis is causative of wheezing or is indicative of a child prone to wheezing is unclear (260). The ultimate cause of asthma is likely to very heterogeneous, reflective of the heterogeneity of asthma itself, with contributions from the environment, infection, and the child's genotype (220). There is a strong connection between infant viral bronchiolitis and wheezing in later childhood (260). Links between infant infection with hMPV (105), RV (147, 192), and RSV (99, 301, 313) and later-life wheezing have been demonstrated. Blocking viral infection with drugs (53) or a prophylactic antibody (303) may reduce the incidence of asthma and wheeze in later life. However, other studies have shown that early-life viral infection is protective against asthma (142), and a recent study suggested that hospitalization with viral bronchiolitis does not cause asthma but may be an indicator of a genetic predisposition to asthma (329).
If viral infection is causative, what is the mechanism? The cytokine balance of the infant lung may have an impact on the development of asthma, and early-life respiratory viral infection may alter this (212, 221). Data from genetic studies (see above) suggested links between several cytokine genes and RSV severity (305). Again, animal models may contribute to our greater understanding of this issue. When neonatal BALB/c mice are infected with RSV, it predisposes them to more severe disease upon reinfection as adults (71), and this is linked to T cells (331), IL-13 (73), and IgE (72).
Viral infection was shown to be an important cause of acute exacerbations of wheezing (7, 154, 157). This enhanced allergic airway disease can also be observed following influenza virus (219), RSV (21), and RV (23) infections in mouse models. The mechanistic links between viral infections and asthma, however, are not well understood. While the immune response to viral infection is characterized as T-helper 1 (Th1) biased, allergic asthma is characterized as T-helper 2 (Th2) biased. Earlier work focused on the inflammatory infiltrate in asthma, but the airway epithelium is now appreciated to have a role in triggering and orchestrating the immune response (132). One epithelial product of particular interest is thymic stromal lymphopoietin (TSLP), an interleukin-7-like cytokine identified as being a murine B-cell-line growth factor (304). TSLP was described as having a role in the development and pathology of allergic asthma. Patients with asthma have higher levels of TSLP (355), and TSLP was demonstrated to be critical in mouse models of allergic airway disease (12, 365). Its probable role in asthma is to act as a Th2 amplification factor, inducing dendritic cells (DCs) to differentiate CD4 T cells to a Th2 phenotype (308) via OX40L (146) and activating mast cells (6).
The expression of TSLP has been observed following RSV infection (344) and RV infection (160), but it is not known how this occurs and what effect this has on subsequent allergic responses. The expression of TSLP is induced by TLR2 (185) and TLR3 (160) ligation via the NF-κB complex. This induction is potentiated by a Th2 environment (160, 164). The pattern recognition receptors (PRRs) that lead to TSLP induction are also associated with viral detection; for example, RSV can be detected by both TLR2 (236) and TLR3 (284). The possibility therefore arises that viral infection of epithelial cells in the context of a Th2-skewed background induces TSLP, leading to the amplification of the Th2-skewed response. This might have a role both in exacerbations of asthma and in the development of asthma in the context of neonatal Th2 skewing (4).
Challenges: Back to the Bedside
How should infant infection be controlled? In part, this depends upon the conclusions drawn from the immunopathology-versus-viral-pathology arguments. If viral pathology is the critical aspect, then specific, preventative treatments including vaccines and antiviral drugs are more appropriate. If, however, immunopathology is foremost, then methods to limit the immune system and careful assessment of vaccines for immunopathology are required.
What hope is there for a vaccine? There is an influenza virus vaccine, and this is now routinely administered to all children in the United States from 6 months to 18 years of age annually (64). This has been demonstrated to reduce the rate of influenza infection (264). The prevalence of influenza may lead to its routine use in other countries, especially in the aftermath of the pandemic H1N1 outbreak. An additional benefit of widespread immunization programs might be reduced viral carriage and therefore protection of nonimmunized groups as observed for Haemophilus influenzae type b (Hib) (162a). However, the mutation rate of influenza virus and the significant animal reservoir mean that there is a need for an annual vaccination program, and therefore, the cost of this may reduce the wider introduction of the vaccine.
For other viruses, there are a number of roadblocks to the development of a vaccine. There are the general challenges posed by the development of any new vaccine, both societal and scientific. These challenges are compounded by specific problems associated with pediatric vaccination caused by the limitations of the infant immune system (298). There are also virus-specific challenges; for example, with RSV, there is the specter of the formalin-inactivated RSV (FI-RSV) trial in the 1960s (163). Viral mutation rates may make conventional B-cell-based vaccines against respiratory viruses virtually impossible to create. One approach might be to focus upon T-cell epitopes, which were shown to be cross-reactive in RV (109). However, we might speculate that the use of T-cell-based vaccines may have drawbacks with regard to immunopathology; for example, RSV vaccines based on T-cell epitopes alone caused enhanced disease pathology (248). An alternative approach might be to vaccinate pregnant mothers and thus provide protection during the first few months of life (358), when the infant is most vulnerable, but to allow normal infection to take place after that.
What role could antiviral drugs play? There are some antiviral agents available: ribavirin for RSV and oseltamivir and zanamivir for influenza virus. There are also several new drugs in development, some of which have reached phase II clinical trials. These include ALN-RSV01 (Alnylam), an RNA interference (RNAi)-based drug; RSV-604 (Arrow/Novartis, United Kingdom), an N protein inhibitor of RSV (246); and plecoranil (Schering-Plough), an RV VP1 inhibitor (280). A particular problem with antivirals is that they are prone to inducing viral escape mutants, particularly for the highly plastic RNA viruses; for example, escape mutants associated with oseltamivir require only a single point mutation (76). A further problem with antiviral drugs is the timing of application; for example, anti-influenza virus drugs need to be applied during the first 48 h of illness to be effective.
Debate arises over the cost-effectiveness of preventative treatment. Vaccines represent the best cost-effectiveness, but apart from influenza virus vaccines, this option is not available. The alternative, passive immunization, e.g., with the monoclonal antibody palivizumab, is expensive; current costs in the United Kingdom are about £1,800 (US$3,600) per season for an infant of 3 to 4 kg of body weight: 5 monthly injections at 15 mg per kg, i.e., about a vial per month (based on 2008 figures) (158). Furthermore, anti-RSV antibody escape mutants have been isolated (364), and studies indicated that this treatment is cost-effective only for the highest-risk infants (89, 90). An improved antibody to replace palivizumab with increased affinity for the RSV F protein (motavizumab) has been tested in phase III clinical trials but has not yet been licensed at the time of writing.
Are there any alternative approaches? Potentially, toe use of anti-inflammatory drugs and treatments might be effective, especially if disease following respiratory infection is immune mediated. This is particularly attractive in light of the reemergence of the concept of hypercytokinemia, or the “cytokine storm.” This term was coined in 1993 to describe graft-verses-host disease (98). The term refers to systemic inflammatory disease caused by an excess of proinflammatory cytokines, in particular TNF. It has been associated with fatal cases of H5N1 influenza virus (75) and SARS-CoV (134) infection.
The advantage of a general anti-inflammatory approach is that it is not limited to a specific virus. However, opinion is mixed as to whether this would be effective. There is evidence for success in animal models. Broad-range anti-inflammatory treatments have been shown to reduce disease severity of influenza infection: gabexate (a synthetic protease inhibitor, which inhibits cytokines) reduced inflammation but did not alter survival (172), and gemfibrozil (another broad-range cytokine inhibitor) increased rates of survival for mice infected with influenza from 26% to 52% (41). However, the use of glucocorticoids has not been demonstrated to have any effect on RSV bronchiolitis (93, 252), although their anti-inflammatory effect may be too slowly mediated for a viral infection.
Specific treatments against proinflammatory mediators can have significant effects on reducing disease in animal models. Blocking or depleting of cytokines including TNF (140, 319), IL-4 (65), IL-13 (156), and IL-12 (326); chemokines, including CCL11 (eotaxin) (223), CCL5 (RANTES) (70), and the receptor CCR1 (230); and costimulatory markers, including ICOS (137) and OX40L (138) has been shown to be effective in reducing disease. However, other studies have shown that the inhibition of some mediators either has no effect or worsens disease, including IL-1 (286, 319), TNF (238), and CCL2 (monocyte chemoattractant protein 1 [MCP-1]) (77).
One issue with treatments that dampen the immune response is their nonspecific effect; for example, long-term anti-TNF treatment has been shown to lead to tuberculosis reactivation. Another issue is timing; immunity-dampening treatments during the early phase of infection might increase viral load and, therefore, virally induced damage. Ultimately, the most effective treatment might be treatment based on a combination of antiviral drugs used early in the disease course and anti-immunity drugs later on.
CONCLUSION
An important consideration in the development of control measures against respiratory viral infection, in particular vaccines, is early-life immunity development. The early-life immune system appears to be suppressed; how this suppression is relaxed over time and the role of infection in the development of normal immune responses are of critical importance. Infection/colonization with normal flora may be necessary in shaping normal immune responses. If this is the case, it has important implications for vaccination strategies: increases in vaccination coverage which reduce viral infection in early-life might have an impact on immunity development, leading to more severe infection/asthma in later life (111).
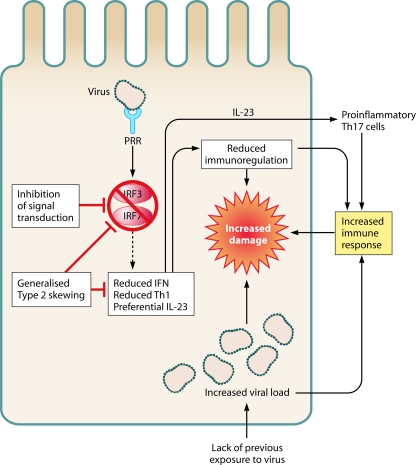
Another important consideration is a seeming paradox: given the hypothesis that disease following respiratory viral infection is immune mediated and infant immune responses are dampened, why do infants get more severe infections (Fig. 3) ? The first possibility is that all damage and disease are mediated directly by the virus, with no immune component. This higher viral load may be exacerbated by the lack of previous exposure and therefore the lack of protection against the infectious agent. However, as argued above, there is significant evidence that suggests that the immune system does play a role in disease following viral infection. It may be that because the response to pathogens is diminished, infection is more aggressive, leading to a higher viral load prior to the initiation of the immune response, and thus, the resulting response is greater in magnitude and causes more collateral damage.
FIG. 3.
The paradox of early-childhood immune hyporesponsiveness and virally induced immunopathology. Shown are possible mechanisms by which increased immunopathology may occur in the context of dampened responses to infection in infants. Similar levels of pattern recognition receptors are detectable for infant and adult leukocytes. However, the molecules that transduce the signal, e.g., IRF3 and IRF7, have reduced function. This leads to an altered immune response, with higher viral load, decreased immunoregulation (via IDO [indoleamine 2,3-dioxygenase]), and skewed cytokine production, all of which may increase damage caused by the immune response. Abbreviations: PRR, pattern recognition receptor; IRF, interferon response factor.
Alternatively, there may be a reduced regulation of the immune response in infants, leading to increased immunopathology. An engagement of the type I IFN response may be necessary for a controlled immune response that is sufficient to clear infection without much bystander damage (84). The expression of the immunoregulatory enzyme indoleamine-2,3-dioxygenase (IDO) was demonstrated to be upregulated by both type I (IFN-α/β) and type II (IFN-γ) interferons (266). Another regulatory molecule (nitric oxide) was shown to be induced by Th1 but not Th2 T cells (325) and may be absent in infants. Therefore, reduced signaling through the normal type I IFN pathway during infection in early infancy may lead to a more pathogenic immune response. Finally, cord blood-derived dendritic cells were shown to have a bias toward IL-23 production (336); this cytokine is associated with an increased development of proinflammatory Th17 T cells. All these factors may lead to a pathogenic rather than protective immune response.
In conclusion, respiratory viral infection is an important cause of morbidity and mortality in early life (Table 3). The disease that is seen in children is composed of both a virus- and an immune-mediated component. The relative contributions of these two factors vary among individuals and are influenced by the infecting virus, host genetics, and age. That there is an immune component is important in considerations regarding the development of vaccines and antiviral treatments.
TABLE 3.
Comparative features of respiratory viral infections during infancya
| Parameter | Virus |
||
|---|---|---|---|
| RSV | RV | Influenza virus | |
| Estimated occurrence rate | 688/1,000 (infected 1st yr), 826/1,000 (infected 2nd yr) | 0.51/child/mo | 5-30% outpatients, 0.1% inpatients |
| Seasonality | Winter (October-March) | Fall and Spring | Epidemics |
| Effect of glucocorticoids | None observed | Possible reduction in wheeze | None observed |
| Severity | Can cause bronchiolitis requiring hospitalization | Can cause bronchiolitis requiring hospitalization | Can cause bronchiolitis requiring hospitalization |
| Control | Specific antibody (palivizumab) | Nothing currently | Seasonal vaccine |
| Antiviral drug (ribavirin) | Antiviral drugs (oseltamivir and zanamivir) | ||
| Genetic risk factors | Antiviral genes reduce severity | Limited studies but suggestion that cytokine genes are important | Limited studies |
| Immune response genes increase severity | |||
| Host viral detection | RIG-I, TLR3, TLR4 | TLR3 | RIG-I, TLR3, TLR7, NLRP3, Casp-1 |
| Viral host evasion | Inhibits RIG-I, IRF-3, STAT | Inhibits MDA5, IRF-3 | Inhibits RIG-I |
| Immunopathology | Strong association | Evidence of role of IL-8 | Cytokine storm |
| Asthma | Associated with later-life wheeze and exacerbations | Associated with later-life wheeze and exacerbations | Associated with exacerbations |
The table compares the 3 most common viruses occurring in infants and summarizes various aspects described in detail in this review.
Outstanding Questions
Outstanding questions in this field of research include the following. (i) What drives viral lung disease: virus-induced damage or the immune system? (ii) Does viral infection cause wheezing in later life, or is it an early marker of a wheezy child? (iii) What is the most appropriate way to control viral infection, and does it even need to be controlled? (iv) If disease following respiratory viral infection is indeed immune mediated, how do the immature immune responses in early childhood contribute to the development of severe LRTI?
ADDENDUM
While the manuscript was in preparation, a novel influenza virus H1N1 strain emerged (A/California/7/2009). On 11 June 2009, the WHO raised the pandemic alert level to 6, signifying the first influenza pandemic since 1968. As of 18 September 2009, the WHO estimated over 296,471 cases of H1N1 infection (with the caveat that “countries are no longer required to test and report individual cases; the number of cases reported actually understates the real number of cases”) and at least 3,486 fatalities. The U.S. Centers for Disease Control and Prevention (CDC) reported that the rates are similar to or lower than those for seasonal influenza and that the number of deaths is within the bounds of what is expected for this time of year, with 9,079 cases and 593 deaths (dated 4 Sept 2009). The Health Protection Agency in the United Kingdom reported 13,471 cases and 78 deaths (dated 17 September 2009). The influenza pandemic does not appear at this point to be any more skewed toward the pediatric population than seasonal influenza, although there may be an increased severity in pregnancy. Two recent studies of hospitalized children from the United Kingdom indicated that a large proportion of the children had preexisting disorders (31 out of 77 cases [119] and 32 out of 58 cases [203]; in the latter study, 9 out of 58 cases died, all of which had preexisting disorders).
The situation is changing rapidly, and therefore, anything written here, although accurate at the time of writing, may be incorrect by the time of publication. This pandemic does raise important points of note, particularly about the socioeconomic effect of respiratory infections. One important observation is about the use of antiviral drugs. Specifically, oseltamivir use has greatly increased and has moved from a preventative control medicine prescribed to those in contact with individuals with confirmed influenza virus infection to being prescribed to anyone with suspected influenza. A second point is the use of vaccines; a vaccine has been developed, but the production of this vaccine may reduce the capacity to produce the annual seasonal influenza virus vaccine. The distribution of both vaccines and antiviral drugs has been skewed toward richer countries (62). Another concern is the impact on what is already seen as a fragile economic recovery, by affecting consumer confidence and spending. It will be of great interest to see how this pandemic plays out and what effect it has on strategic health care planning for the future.
Acknowledgments
We thank Peter Openshaw, Cecilia Johansson, and Charlotte Weller (Imperial College London) for proofreading and advice.
Biography
 John S. Tregoning (Ph.D.) is the lecturer in infection and immunity at St. George's University of London, United Kingdom. Dr. Tregoning received his B.A. from the University of Cambridge, majoring in genetics, and his Ph.D. from Imperial College London, in biochemistry. He was a postdoctoral research fellow at the Department of Respiratory Medicine, Imperial College London, studying the delayed effects of neonatal respiratory syncytial virus infection. The Tregoning group is focused on the development of the immune system in early life. The infant (<1 year old) immune system is a highly important and interesting area—immunologically, infants are not simply little adults. Infants are highly susceptible to infectious disease but resistant to vaccination. Furthermore, early-life infection has links to the development (or not) of asthma and allergy in later life.
John S. Tregoning (Ph.D.) is the lecturer in infection and immunity at St. George's University of London, United Kingdom. Dr. Tregoning received his B.A. from the University of Cambridge, majoring in genetics, and his Ph.D. from Imperial College London, in biochemistry. He was a postdoctoral research fellow at the Department of Respiratory Medicine, Imperial College London, studying the delayed effects of neonatal respiratory syncytial virus infection. The Tregoning group is focused on the development of the immune system in early life. The infant (<1 year old) immune system is a highly important and interesting area—immunologically, infants are not simply little adults. Infants are highly susceptible to infectious disease but resistant to vaccination. Furthermore, early-life infection has links to the development (or not) of asthma and allergy in later life.
 Jürgen Schwarze, F.R.C.P.C.H., qualified in medicine from Freiburg University (Germany) in 1988. He trained in pediatrics in Germany at Marburg and Ulm Universities (1990 to 1994) and subspecialized in respiratory medicine and allergy at Bochum University (1998 to 2002). After five years as a Wellcome Trust Senior Fellow at Imperial College London (2002 to 2007), he moved to his current post as the Edward Clark Chair of Child Life and Health at the University of Edinburgh in 2007. Based on the clinical experience of RSV bronchiolitis as a common and often severe infectious disease of early childhood for which vaccination or specific treatment is not available, Professor Schwarze has been interested in immune responses in RSV infection and subsequent reactive-airway disease since 1994, when he started working as a postdoctoral fellow at National Jewish Medical and Research Center in Denver, CO (1994 to 1998).
Jürgen Schwarze, F.R.C.P.C.H., qualified in medicine from Freiburg University (Germany) in 1988. He trained in pediatrics in Germany at Marburg and Ulm Universities (1990 to 1994) and subspecialized in respiratory medicine and allergy at Bochum University (1998 to 2002). After five years as a Wellcome Trust Senior Fellow at Imperial College London (2002 to 2007), he moved to his current post as the Edward Clark Chair of Child Life and Health at the University of Edinburgh in 2007. Based on the clinical experience of RSV bronchiolitis as a common and often severe infectious disease of early childhood for which vaccination or specific treatment is not available, Professor Schwarze has been interested in immune responses in RSV infection and subsequent reactive-airway disease since 1994, when he started working as a postdoctoral fellow at National Jewish Medical and Research Center in Denver, CO (1994 to 1998).
REFERENCES
- 1.Abe, Y., E. Takashita, K. Sugawara, Y. Matsuzaki, Y. Muraki, and S. Hongo. 2004. Effect of the addition of oligosaccharides on the biological activities and antigenicity of influenza A/H3N2 virus hemagglutinin. J. Virol. 78:9605-9611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abedi, K. B., P. J. Vallely, C. E. Corless, M. Al Hammadi, and P. E. Klapper. 2008. Age-related pattern of KI and WU polyomavirus infection. J. Clin. Virol. 43:123-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aberle, J. H., S. W. Aberle, E. Pracher, H. P. Hutter, M. Kundi, and T. Popow-Kraupp. 2005. Single versus dual respiratory virus infections in hospitalized infants: impact on clinical course of disease and interferon-gamma response. Pediatr. Infect. Dis. J. 24:605-610. [DOI] [PubMed] [Google Scholar]
- 4.Adkins, B., C. LeClerc, and S. Marshall-Clarke. 2004. Neonatal adaptive immunity comes of age. Nat. Rev. Immunol. 4:553-564. [DOI] [PubMed] [Google Scholar]
- 5.Aksoy, E., V. Albarani, M. Nguyen, J. F. Laes, J. L. Ruelle, D. De Witt, F. Willems, M. Goldman, and S. Goriely. 2007. Interferon regulatory factor 3-dependent responses to lipopolysaccharide are selectively blunted in cord blood cells. Blood 109:2887-2893. [DOI] [PubMed] [Google Scholar]
- 6.Allakhverdi, Z., M. R. Comeau, H. K. Jessup, B. R. Yoon, A. Brewer, S. Chartier, N. Paquette, S. F. Ziegler, M. Sarfati, and G. Delespesse. 2007. Thymic stromal lymphopoietin is released by human epithelial cells in response to microbes, trauma, or inflammation and potently activates mast cells. J. Exp. Med. 204:253-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allander, T., T. Jartti, S. Gupta, H. G. Niesters, P. Lehtinen, R. Osterback, T. Vuorinen, M. Waris, A. Bjerkner, A. Tiveljung-Lindell, B. G. Van Den Hoogen, T. Hyypia, and O. Ruuskanen. 2007. Human bocavirus and acute wheezing in children. Clin. Infect. Dis. 44:904-910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allander, T., M. T. Tammi, M. Eriksson, A. Bjerkner, A. Tiveljung-Lindell, and B. Andersson. 2005. Cloning of a human parvovirus by molecular screening of respiratory tract samples. Proc. Natl. Acad. Sci. U. S. A. 102:12891-12896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allander, T., K. Andreasson, S. Gupta, A. Bjerkner, G. Bogdanovic, M. A. A. Persson, T. Dalianis, T. Ramqvist, and B. Andersson. 2007. Identification of a third human polyomavirus. J. Virol. 81:4130-4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allen, I. C., M. A. Scull, C. B. Moore, E. K. Holl, E. McElvania-TeKippe, D. J. Taxman, E. H. Guthrie, R. J. Pickles, and J. P. Ting. 2009. The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity 30:556-565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alper, C. M., B. Winther, J. O. Hendley, and W. J. Doyle. 2009. Cytokine polymorphisms predict the frequency of rhinovirus and RSV infections in children. Eur. Arch. Otorhinolaryngol. 266:199-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al Shami, A., R. Spolski, J. Kelly, A. Keane-Myers, and W. J. Leonard. 2005. A role for TSLP in the development of inflammation in an asthma model. J. Exp. Med. 202:829-839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alvarez, R., K. S. Harrod, W. J. Shieh, S. Zaki, and R. A. Tripp. 2004. Human metapneumovirus persists in BALB/c mice despite the presence of neutralizing antibodies. J. Virol. 78:14003-14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amanatidou, V., G. Sourvinos, S. Apostolakis, A. Tsilimigaki, and D. A. Spandidos. 2006. T280M variation of the CX3C receptor gene is associated with increased risk for severe respiratory syncytial virus bronchiolitis. Pediatr. Infect. Dis. J. 25:410-414. [DOI] [PubMed] [Google Scholar]
- 15.Angelone, D. F., M. R. Wessels, M. Coughlin, E. E. Suter, P. Valentini, L. A. Kalish, and O. Levy. 2006. Innate immunity of the human newborn is polarized toward a high ratio of IL-6/TNF-alpha production in vitro and in vivo. Pediatr. Res. 60:205-209. [DOI] [PubMed] [Google Scholar]
- 16.Appledorn, D. M., S. Patial, A. McBride, S. Godbehere, N. van Rooijen, N. Parameswaran, and A. Amalfitano. 2008. Adenovirus vector-induced innate inflammatory mediators, MAPK signaling, as well as adaptive immune responses are dependent upon both TLR2 and TLR9 in vivo. J. Immunol. 181:2134-2144. [DOI] [PubMed] [Google Scholar]
- 17.Ausejo, M., A. Saenz, B. Pham, J. D. Kellner, D. W. Johnson, D. Moher, and T. P. Klassen. 1999. The effectiveness of glucocorticoids in treating croup: meta-analysis. BMJ 319:595-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Awomoyi, A. A., P. Rallabhandi, T. I. Pollin, E. Lorenz, M. B. Sztein, M. S. Boukhvalova, V. G. Hemming, J. C. G. Blanco, and S. N. Vogel. 2007. Association of TLR4 polymorphisms with symptomatic respiratory syncytial virus infection in high-risk infants and young children. J. Immunol. 179:3171-3177. [DOI] [PubMed] [Google Scholar]
- 19.Banerjee, S., A. Smallwood, J. Moorhead, A. E. Chambers, A. Papageorghiou, S. Campbell, and K. Nicolaides. 2005. Placental expression of interferon-gamma (IFN-gamma) and its receptor IFN-gamma R2 fail to switch from early hypoxic to late normotensive development in preeclampsia. J. Clin. Endocrinol. Metab. 90:944-952. [DOI] [PubMed] [Google Scholar]
- 20.Bar, A., I. Srugo, I. Amirav, C. Tzverling, G. Naftali, and A. Kugelman. 2008. Inhaled furosemide in hospitalized infants with viral bronchiolitis: a randomized, double-blind, placebo-controlled pilot study. Pediatr. Pulmonol. 43:261-267. [DOI] [PubMed] [Google Scholar]
- 21.Barends, M., A. Boelen, L. de Rond, J. Kwakkel, T. Bestebroer, J. Dormans, H. Neijens, and T. Kimman. 2002. Influence of respiratory syncytial virus infection on cytokine and inflammatory responses in allergic mice. Clin. Exp. Allergy 32:463-471. [DOI] [PubMed] [Google Scholar]
- 22.Barral, P. M., J. M. Morrison, J. Drahos, P. Gupta, D. Sarkar, P. B. Fisher, and V. R. Racaniello. 2007. MDA-5 is cleaved in poliovirus-infected cells. J. Virol. 81:3677-3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bartlett, N. W., R. P. Walton, M. R. Edwards, J. Aniscenko, G. Caramori, J. Zhu, N. Glanville, K. J. Choy, P. Jourdan, J. Burnet, T. J. Tuthill, M. S. Pedrick, M. J. Hurle, C. Plumpton, N. A. Sharp, J. N. Bussell, D. M. Swallow, J. Schwarze, B. Guy, J. W. Almond, P. K. Jeffery, C. M. Lloyd, A. Papi, R. A. Killington, D. J. Rowlands, E. D. Blair, N. J. Clarke, and S. L. Johnston. 2008. Mouse models of rhinovirus-induced disease and exacerbation of allergic airway inflammation. Nat. Med. 14:199-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Basner-Tschakarjan, E., E. Gaffal, M. O'Keeffe, D. Tormo, A. Limmer, H. Wagner, H. Hochrein, and T. Tuting. 2006. Adenovirus efficiently transduces plasmacytoid dendritic cells resulting in TLR9-dependent maturation and IFN-alpha production. J. Gene Med. 8:1300-1306. [DOI] [PubMed] [Google Scholar]
- 25.Belnoue, E., M. Pihlgren, T. L. McGaha, C. Tougne, A. F. Rochat, C. Bossen, P. Schneider, B. Huard, P. H. Lambert, and C. A. Siegrist. 2008. APRIL is critical for plasmablast survival in the bone marrow and poorly expressed by early-life bone marrow stromal cells. Blood 111:2755-2764. [DOI] [PubMed] [Google Scholar]
- 26.Bhoj, V. G., Q. Sun, E. J. Bhoj, C. Somers, X. Chen, J. P. Torres, A. Mejias, A. M. Gomez, H. Jafri, O. Ramilo, and Z. J. Chen. 2008. MAVS and MyD88 are essential for innate immunity but not cytotoxic T lymphocyte response against respiratory syncytial virus. Proc. Natl. Acad. Sci. U. S. A. 105:14046-14051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bitko, V., A. Musiyenko, M. A. Bayfield, R. J. Maraia, and S. Barik. 2008. Cellular La protein shields nonsegmented negative-strand RNA viral leader RNA from RIG-I and enhances virus growth by diverse mechanisms. J. Virol. 82:7977-7987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bitko, V., O. Shulyayeva, B. Mazumder, A. Musiyenko, M. Ramaswamy, D. C. Look, and S. Barik. 2007. Nonstructural proteins of respiratory syncytial virus suppress premature apoptosis by an NF-κB-dependent, interferon-independent mechanism and facilitate virus growth. J. Virol. 81:1786-1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bjorksten, B. 1999. The intrauterine and postnatal environments. J. Allergy Clin. Immunol. 104:1119-1127. [DOI] [PubMed] [Google Scholar]
- 30.Bjornson, C. L., T. P. Klassen, J. Williamson, R. Brant, C. Mitton, A. Plint, B. Bulloch, L. Evered, and D. W. Johnson. 2004. A randomized trial of a single dose of oral dexamethasone for mild croup. N. Engl. J. Med. 351:1306-1313. [DOI] [PubMed] [Google Scholar]
- 31.Bonzel, L., T. Tenenbaum, H. Schroten, O. Schildgen, S. Schweitzer-Krantz, and O. Adams. 2008. Frequent detection of viral coinfection in children hospitalized with acute respiratory tract infection using a real-time polymerase chain reaction. Pediatr. Infect. Dis. J. 27:589-594. [DOI] [PubMed] [Google Scholar]
- 32.Boogaard, R., A. R. Hulsmann, L. van Veen, A. A. Vaessen-Verberne, Y. N. Yap, A. J. Sprij, G. Brinkhorst, B. Sibbles, T. Hendriks, S. W. Feith, C. R. Lincke, A. E. Brandsma, P. L. Brand, W. C. Hop, M. de Hoog, and P. J. Merkus. 2007. Recombinant human deoxyribonuclease in infants with respiratory syncytial virus bronchiolitis. Chest 131:788-795. [DOI] [PubMed] [Google Scholar]
- 33.Bos, J. M., E. Rietveld, H. A. Moll, E. W. Steyerberg, W. Luytjes, J. C. Wilschut, R. De Groot, and M. J. Postma. 2007. The use of health economics to guide drug development decisions: determining optimal values for an RSV-vaccine in a model-based scenario-analytic approach. Vaccine 25:6922-6929. [DOI] [PubMed] [Google Scholar]
- 34.Bosis, S., S. Esposito, H. G. Niesters, G. V. Zuccotti, G. Marseglia, M. Lanari, G. Zuin, C. Pelucchi, A. D. Osterhaus, and N. Principi. 2008. Role of respiratory pathogens in infants hospitalized for a first episode of wheezing and their impact on recurrences. Clin. Microbiol. Infect. 14:677-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bosis, S., S. Esposito, A. D. Osterhaus, E. Tremolati, E. Begliatti, C. Tagliabue, F. Corti, N. Principi, and H. G. Niesters. 2008. Association between high nasopharyngeal viral load and disease severity in children with human metapneumovirus infection. J. Clin. Virol. 42:286-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bossert, B., and K. K. Conzelmann. 2002. Respiratory syncytial virus (RSV) nonstructural (NS) proteins as host range determinants: a chimeric bovine RSV with NS genes from human RSV is attenuated in interferon-competent bovine cells. J. Virol. 76:4287-4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bossios, A., S. Psarras, D. Gourgiotis, C. Skevaki, A. Constantopoulos, P. Saxoni-Papageorgiou, and N. Papadopoulos. 2005. Rhinovirus infection induces cytotoxicity and delays wound healing in bronchial epithelial cells. Respir. Res. 6:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brand, P. L. P., E. Baraldi, H. Bisgaard, A. L. Boner, J. A. Castro-Rodriguez, A. Custovic, J. de Blic, J. C. de Jongste, E. Eber, M. L. Everard, U. Frey, M. Gappa, L. Garcia-Marcos, J. Grigg, W. Lenney, P. Le Souef, S. McKenzie, P. J. F. M. Merkus, F. Midulla, J. Y. Paton, G. Piacentini, P. Pohunek, G. A. Rossi, P. Seddon, M. Silverman, P. D. Sly, S. Stick, A. Valiulis, W. M. C. Van Aalderen, J. H. Wildhaber, G. Wennergren, N. Wilson, Z. Zivkovic, and A. Bush. 2008. Definition, assessment and treatment of wheezing disorders in preschool children: an evidence-based approach. Eur. Respir. J. 32:1096-1110. [DOI] [PubMed] [Google Scholar]
- 39.Brustoski, K., U. Moller, M. Kramer, F. C. Hartgers, P. G. Kremsner, U. Krzych, and A. J. Luty. 2006. Reduced cord blood immune effector-cell responsiveness mediated by CD4+ cells induced in utero as a consequence of placental Plasmodium falciparum infection. J. Infect. Dis. 193:146-154. [DOI] [PubMed] [Google Scholar]
- 40.Buckingham, S. C., H. S. Jafri, A. J. Bush, C. M. Carubelli, P. Sheeran, R. D. Hardy, M. G. Ottolini, O. Ramilo, and J. P. DeVincenzo. 2002. A randomized, double-blind, placebo-controlled trial of dexamethasone in severe respiratory syncytial virus (RSV) infection: effects on RSV quantity and clinical outcome. J. Infect. Dis. 185:1222-1228. [DOI] [PubMed] [Google Scholar]
- 41.Budd, A., L. Alleva, M. Alsharifi, A. Koskinen, V. Smythe, A. Mullbacher, J. Wood, and I. Clark. 2007. Increased survival after gemfibrozil treatment of severe mouse influenza. Antimicrob. Agents Chemother. 51:2965-2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bulut, Y., M. Guven, B. Otlu, G. Yenisehirli, I. Aladag, A. Eyibilen, and S. Dogru. 2007. Acute otitis media and respiratory viruses. Eur. J. Pediatr. 166:223-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cabello, C., M. E. Manjarrez, R. Olvera, J. Villalba, L. Valle, and I. Paramo. 2006. Frequency of viruses associated with acute respiratory infections in children younger than five years of age at a locality of Mexico City. Mem. Inst. Oswaldo Cruz 101:21-24. [DOI] [PubMed] [Google Scholar]
- 44.Calvo, C., M. L. Garcia-Garcia, C. Blanco, F. Pozo, I. C. Flecha, and P. Perez-Brena. 2007. Role of rhinovirus in hospitalized infants with respiratory tract infections in Spain. Pediatr. Infect. Dis. J. 26:904-908. [DOI] [PubMed] [Google Scholar]
- 45.Calvo, C., M. L. Garcia-Garcia, C. Blanco, M. C. Vazquez, M. E. Frias, P. Perez-Brena, and I. Casas. 2008. Multiple simultaneous viral infections in infants with acute respiratory tract infections in Spain. J. Clin. Virol. 42:268-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Calvo, C., M. L. Garcia-Garcia, F. Pozo, O. Carvajal, P. Perez-Brena, and I. Casas. 2008. Clinical characteristics of human bocavirus infections compared with other respiratory viruses in Spanish children. Pediatr. Infect. Dis. J. 27:677-680. [DOI] [PubMed] [Google Scholar]
- 47.Camps, M., S. Ricart, V. Dimova, N. Rovira, C. Munoz-Almagro, J. J. Garcia, M. Pons-Odena, M. A. Marcos, and T. Pumarola. 2008. Prevalence of human metapneumovirus among hospitalized children younger than 1 year in Catalonia, Spain. J. Med. Virol. 80:1452-1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Canducci, F., M. Debiaggi, M. Sampaolo, M. C. Marinozzi, S. Berre, C. Terulla, G. Gargantini, P. Cambieri, E. Romero, and M. Clementi. 2008. Two-year prospective study of single infections and co-infections by respiratory syncytial virus and viruses identified recently in infants with acute respiratory disease. J. Med. Virol. 80:716-723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cerullo, V., M. P. Seiler, V. Mane, N. Brunetti-Pierri, C. Clarke, T. K. Bertin, J. R. Rodgers, and B. Lee. 2007. Toll-like receptor 9 triggers an innate immune response to helper-dependent adenoviral vectors. Mol. Ther. 15:378-385. [DOI] [PubMed] [Google Scholar]
- 50.Chan, K. Y., J. C. Ching, M. S. Xu, A. N. Cheung, S. P. Yip, L. Y. Yam, S. T. Lai, C. M. Chu, A. T. Wong, Y. Q. Song, F. P. Huang, W. Liu, P. H. Chung, G. M. Leung, E. Y. Chow, E. Y. Chan, J. C. Chan, H. Y. Ngan, P. Tam, L. C. Chan, P. Sham, V. S. Chan, M. Peiris, S. C. Lin, and U. S. Khoo. 2007. Association of ICAM3 genetic variant with severe acute respiratory syndrome. J. Infect. Dis. 196:271-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chan, V. S., K. Y. Chan, Y. Chen, L. L. Poon, A. N. Cheung, B. Zheng, K. H. Chan, W. Mak, H. Y. Ngan, X. Xu, G. Screaton, P. K. Tam, J. M. Austyn, L. C. Chan, S. P. Yip, M. Peiris, U. S. Khoo, and C. L. Lin. 2006. Homozygous L-SIGN (CLEC4M) plays a protective role in SARS coronavirus infection. Nat. Genet. 38:38-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chandwani, S., W. Borkowsky, K. Krasinski, R. Lawrence, and R. Welliver. 1990. Respiratory syncytial virus infection in human immunodeficiency virus-infected children. J. Pediatr. 117:251-254. [DOI] [PubMed] [Google Scholar]
- 53.Chen, C. H., Y. T. Lin, Y. H. Yang, L. C. Wang, J. H. Lee, C. L. Kao, and B. L. Chiang. 2008. Ribavirin for respiratory syncytial virus bronchiolitis reduced the risk of asthma and allergen sensitization. Pediatr. Allergy Immunol. 19:166-172. [DOI] [PubMed] [Google Scholar]
- 54.Chen, W. J., J. Y. Yang, J. H. Lin, C. S. Fann, V. Osyetrov, C. C. King, Y. M. Chen, H. L. Chang, H. W. Kuo, F. Liao, and M. S. Ho. 2006. Nasopharyngeal shedding of severe acute respiratory syndrome-associated coronavirus is associated with genetic polymorphisms. Clin. Infect. Dis. 42:1561-1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cheung, C. Y., L. L. Poon, I. H. Ng, W. Luk, S. F. Sia, M. H. Wu, K. H. Chan, K. Y. Yuen, S. Gordon, Y. Guan, and J. S. Peiris. 2005. Cytokine responses in severe acute respiratory syndrome coronavirus-infected macrophages in vitro: possible relevance to pathogenesis. J. Virol. 79:7819-7826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Choi, E. H., H. J. Lee, T. Yoo, and S. J. Chanock. 2002. A common haplotype of interleukin-4 gene IL4 is associated with severe respiratory syncytial virus disease in Korean children. J. Infect. Dis. 186:1207-1211. [DOI] [PubMed] [Google Scholar]
- 57.Chong, W. P., W. K. Ip, G. H. Tso, M. W. Ng, W. H. Wong, H. K. Law, R. W. Yung, E. Y. Chow, K. L. Au, E. Y. Chan, W. Lim, J. S. Peiris, and Y. L. Lau. 2006. The interferon gamma gene polymorphism +874 A/T is associated with severe acute respiratory syndrome. BMC Infect. Dis. 6:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chonmaitree, T., K. Revai, J. J. Grady, A. Clos, J. A. Patel, S. Nair, J. Fan, and K. J. Henrickson. 2008. Viral upper respiratory tract infection and otitis media complication in young children. Clin. Infect. Dis. 46:815-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chung, J. Y., T. H. Han, S. W. Kim, C. K. Kim, and E. S. Hwang. 2007. Detection of viruses identified recently in children with acute wheezing. J. Med. Virol. 79:1238-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cilla, G., E. Onate, E. G. Perez-Yarza, M. Montes, D. Vicente, and E. Perez-Trallero. 2008. Viruses in community-acquired pneumonia in children aged less than 3 years old: high rate of viral coinfection. J. Med. Virol. 80:1843-1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Colamussi, M. L., M. R. White, E. Crouch, and K. L. Hartshorn. 1999. Influenza A virus accelerates neutrophil apoptosis and markedly potentiates apoptotic effects of bacteria. Blood 93:2395-2403. [PubMed] [Google Scholar]
- 62.Collin, N., X. de Radiguéz, and the World Health Organization H1N1 Vaccine Task Force. 2009. Vaccine production capacity for seasonal and pandemic (H1N1) 2009 influenza. Vaccine 27:5184-5186. [DOI] [PubMed] [Google Scholar]
- 63.Collins, P. L., and B. S. Graham. 2008. Viral and host factors in human respiratory syncytial virus pathogenesis. J. Virol. 82:2040-2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Committee on Infectious Diseases. 2008. Prevention of influenza: recommendations for influenza immunization of children, 2008-2009. Pediatrics 122:1135-1141. [DOI] [PubMed] [Google Scholar]
- 65.Connors, M., N. A. Giese, A. B. Kulkarni, C.-Y. Firestone, H. C. Morse III, and B. R. Murphy. 1994. Enhanced pulmonary histopathology induced by respiratory syncytial virus (RSV) challenge of formalin-inactivated RSV-immunized BALB/c mice is abrogated by depletion of interleukin-4 (IL-4) and IL-10. J. Virol. 68:5321-5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Contoli, M., S. D. Message, V. Laza-Stanca, M. R. Edwards, P. A. Wark, N. W. Bartlett, T. Kebadze, P. Mallia, L. A. Stanciu, H. L. Parker, L. Slater, A. Lewis-Antes, O. M. Kon, S. T. Holgate, D. E. Davies, S. V. Kotenko, A. Papi, and S. L. Johnston. 2006. Role of deficient type III interferon-lambda production in asthma exacerbations. Nat. Med. 12:1023-1026. [DOI] [PubMed] [Google Scholar]
- 67.Cooper, D. L., G. E. Smith, W. J. Edmunds, C. Joseph, E. Gerard, and R. C. George. 2007. The contribution of respiratory pathogens to the seasonality of NHS direct calls. J. Infect. 55:240-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Corneli, H. M., J. J. Zorc, P. Mahajan, K. N. Shaw, R. Holubkov, S. D. Reeves, R. M. Ruddy, B. Malik, K. A. Nelson, J. S. Bregstein, K. M. Brown, M. N. Denenberg, K. A. Lillis, L. B. Cimpello, J. W. Tsung, D. A. Borgialli, M. N. Baskin, G. Teshome, M. A. Goldstein, D. Monroe, J. M. Dean, and N. Kuppermann. 2007. A multicenter, randomized, controlled trial of dexamethasone for bronchiolitis. N. Engl. J. Med. 357:331-339. [DOI] [PubMed] [Google Scholar]
- 69.Costa, L. F., J. Yokosawa, O. C. Mantese, T. F. Oliveira, H. L. Silveira, L. L. Nepomuceno, L. S. Moreira, G. Dyonisio, L. M. Rossi, R. C. Oliveira, L. Z. Ribeiro, and D. A. Queiroz. 2006. Respiratory viruses in children younger than five years old with acute respiratory disease from 2001 to 2004 in Uberlandia, MG, Brazil. Mem. Inst. Oswaldo Cruz 101:301-306. [DOI] [PubMed] [Google Scholar]
- 70.Culley, F. J., A. M. Pennycook, J. S. Tregoning, J. S. Dodd, G. Walzl, T. N. Wells, T. Hussell, and P. J. Openshaw. 2006. Role of CCL5 (RANTES) in viral lung disease. J. Virol. 80:8151-8157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Culley, F. J., J. Pollott, and P. J. Openshaw. 2002. Age at first viral infection determines the pattern of T cell-mediated disease during reinfection in adulthood. J. Exp. Med. 196:1381-1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dakhama, A., Y. M. Lee, H. Ohnishi, X. Jing, A. Balhorn, K. Takeda, and E. W. Gelfand. 2008. Virus-specific IgE enhances airway responsiveness on reinfection with respiratory syncytial virus in newborn mice. J. Allergy Clin. Immunol. 123:138.e5-145.e5 [DOI] [PubMed] [Google Scholar]
- 73.Dakhama, A., J. W. Park, C. Taube, A. Joetham, A. Balhorn, N. Miyahara, K. Takeda, and E. W. Gelfand. 2005. The enhancement or prevention of airway hyperresponsiveness during reinfection with respiratory syncytial virus is critically dependent on the age at first infection and IL-13 production. J. Immunol. 175:1876-1883. [DOI] [PubMed] [Google Scholar]
- 74.Danis, B., T. C. George, S. Goriely, B. Dutta, J. Renneson, L. Gatto, P. Fitzgerald-Bocarsly, A. Marchant, M. Goldman, F. Willems, and D. De Wit. 2008. Interferon regulatory factor 7-mediated responses are defective in cord blood plasmacytoid dendritic cells. Eur. J. Immunol. 38:507-517. [DOI] [PubMed] [Google Scholar]
- 75.de Jong, M. D., C. P. Simmons, T. T. Thanh, V. M. Hien, G. J. Smith, T. N. Chau, D. M. Hoang, N. V. Chau, T. H. Khanh, V. C. Dong, P. T. Qui, B. V. Cam, D. Q. Ha, Y. Guan, J. S. Peiris, N. T. Chinh, T. T. Hien, and J. Farrar. 2006. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat. Med. 12:1203-1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.de Jong, M. D., T. T. Tran, H. K. Truong, M. H. Vo, G. J. Smith, V. C. Nguyen, V. C. Bach, T. Q. Phan, D. Q. Ha, Y. Guan, J. S. Peiris, T. H. Tran, and J. Farrar. 2005. Oseltamivir resistance during treatment of influenza A (H5N1) infection. N. Engl. J. Med. 353:2667-2672. [DOI] [PubMed] [Google Scholar]
- 77.Dessing, M. C., K. F. van der Sluijs, S. Florquin, and T. van der Pol. 2007. Monocyte chemoattractant protein 1 contributes to an adequate immune response in influenza pneumonia. Clin. Immunol. 125:328-336. [DOI] [PubMed] [Google Scholar]
- 78.De Wit, D., V. Olislagers, S. Goriely, F. Vermeulen, H. Wagner, M. Goldman, and F. Willems. 2004. Blood plasmacytoid dendritic cell responses to CpG oligodeoxynucleotides are impaired in human newborns. Blood 103:1030-1032. [DOI] [PubMed] [Google Scholar]
- 79.De Wit, D., S. Tonon, V. Olislagers, S. Goriely, M. Boutriaux, M. Goldman, and F. Willems. 2003. Impaired responses to Toll-like receptor 4 and Toll-like receptor 3 ligands in human cord blood. J. Autoimmun. 21:277-281. [DOI] [PubMed] [Google Scholar]
- 80.Didierlaurent, A., J. Goulding, S. Patel, R. Snelgrove, L. Low, M. Bebien, T. Lawrence, L. S. van Rijt, B. N. Lambrecht, J. C. Sirard, and T. Hussell. 2008. Sustained desensitization to bacterial Toll-like receptor ligands after resolution of respiratory influenza infection. J. Exp. Med. 205:323-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Diebold, S. S., T. Kaisho, H. Hemmi, S. Akira, and C. Reis e Sousa. 2004. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science 303:1529-1531. [DOI] [PubMed] [Google Scholar]
- 82.Dinwiddie, D. L., and K. S. Harrod. 2008. Human metapneumovirus inhibits IFN-alpha signaling through inhibition of STAT1 phosphorylation. Am. J. Respir. Cell Mol. Biol. 38:661-670. [DOI] [PubMed] [Google Scholar]
- 83.Di Paolo, N. C., E. A. Miao, Y. Iwakura, K. Murali-Krishna, A. Aderem, R. A. Flavell, T. Papayannopoulou, and D. M. Shayakhmetov. 2009. Virus binding to a plasma membrane receptor triggers interleukin-1 alpha-mediated proinflammatory macrophage response in vivo. Immunity 31:110-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Durbin, J. E., A. Fernandez-Sesma, C. K. Lee, T. D. Rao, A. B. Frey, T. M. Moran, S. Vukmanovic, A. Garcia-Sastre, and D. E. Levy. 2000. Type I IFN modulates innate and specific antiviral immunity. J. Immunol. 164:4220-4228. [DOI] [PubMed] [Google Scholar]
- 85.Ehl, S., R. Bischoff, T. Ostler, S. Vallbracht, J. Schulte-Monting, A. Poltorak, and M. Freudenberg. 2004. The role of Toll-like receptor 4 versus interleukin-12 in immunity to respiratory syncytial virus. Eur. J. Immunol. 34:1146-1153. [DOI] [PubMed] [Google Scholar]
- 86.Ehlken, B., G. Ihorst, B. Lippert, A. Rohwedder, G. Petersen, M. Schumacher, and J. Forster. 2005. Economic impact of community-acquired and nosocomial lower respiratory tract infections in young children in Germany. Eur. J. Pediatr. 164:607-615. [DOI] [PubMed] [Google Scholar]
- 87.Eisenhut, M. 2006. Extrapulmonary manifestations of severe respiratory syncytial virus infection—a systematic review. Crit. Care 10:R107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Elena, S. F., and R. Sanjuan. 2005. Adaptive value of high mutation rates of RNA viruses: separating causes from consequences. J. Virol. 79:11555-11558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Elhassan, N. O., T. P. Stevens, M. E. Sorbero, A. W. Dick, R. Guillet, and C. B. Hall. 2003. Guidelines for palivizumab prophylaxis: are they based on infant's risk of hospitalization for respiratory syncytial viral disease? Pediatr. Infect. Dis. J. 22:939-943. [DOI] [PubMed] [Google Scholar]
- 90.Embleton, N. D., C. Harkensee, and M. C. Mckean. 2005. Palivizumab for preterm infants. Is it worth it? Arch. Dis. Child. Fetal Neonatal Ed. 90:F286-F289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Entrican, G. 2002. Immune regulation during pregnancy and host-pathogen interactions in infectious abortion. J. Comp. Pathol. 126:79-94. [DOI] [PubMed] [Google Scholar]
- 92.Ermers, M. J., B. Hoebee, H. M. Hodemaekers, T. G. Kimman, J. L. Kimpen, and L. Bont. 2007. IL-13 genetic polymorphism identifies children with late wheezing after respiratory syncytial virus infection. J. Allergy Clin. Immunol. 119:1086-1091. [DOI] [PubMed] [Google Scholar]
- 93.Ermers, M. J., M. M. Rovers, J. B. van Woensel, J. L. Kimpen, and L. J. Bont. 2009. The effect of high dose inhaled corticosteroids on wheeze in infants after respiratory syncytial virus infection: randomised double blind placebo controlled trial. BMJ 338:b897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Esposito, S., S. Bosis, H. G. Niesters, E. Tremolati, C. Sabatini, A. Porta, E. Fossali, A. D. Osterhaus, and N. Principi. 2008. Impact of human bocavirus on children and their families. J. Clin. Microbiol. 46:1337-1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fabbiani, M., C. Terrosi, B. Martorelli, M. Valentini, L. Bernini, C. Cellesi, and M. G. Cusi. 2009. Epidemiological and clinical study of viral respiratory tract infections in children from Italy. J. Med. Virol. 81:750-756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fernandez, M. A., F. K. Puttur, Y. M. Wang, W. Howden, S. I. Alexander, and C. A. Jones. 2008. T regulatory cells contribute to the attenuated primary CD8+ and CD4+ T cell responses to herpes simplex virus type 2 in neonatal mice. J. Immunol. 180:1556-1564. [DOI] [PubMed] [Google Scholar]
- 97.Fernandez-Sesma, A., S. Marukian, B. J. Ebersole, D. Kaminski, M. S. Park, T. Yuen, S. C. Sealfon, A. Garcia-Sastre, and T. M. Moran. 2006. Influenza virus evades innate and adaptive immunity via the NS1 protein. J. Virol. 80:6295-6304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ferrara, J. L., S. Abhyankar, and D. G. Gilliland. 1993. Cytokine storm of graft-versus-host disease: a critical effector role for interleukin-1. Transplant. Proc. 25:1216-1217. [PubMed] [Google Scholar]
- 99.Fjaerli, H. O., T. Farstad, G. Rod, G. K. Ufert, P. Gulbrandsen, and B. Nakstad. 2005. Acute bronchiolitis in infancy as risk factor for wheezing and reduced pulmonary function by seven years in Akershus County, Norway. BMC Pediatr. 5:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fodha, I., A. Vabret, L. Ghedira, H. Seboui, S. Chouchane, J. Dewar, N. Gueddiche, A. Trabelsi, N. Boujaafar, and F. Freymuth. 2007. Respiratory syncytial virus infections in hospitalized infants: association between viral load, virus subgroup, and disease severity. J. Med. Virol. 79:1951-1958. [DOI] [PubMed] [Google Scholar]
- 101.Forster, J., G. Ihorst, C. H. Rieger, V. Stephan, H. D. Frank, H. Gurth, R. Berner, A. Rohwedder, H. Werchau, M. Schumacher, T. Tsai, and G. Petersen. 2004. Prospective population-based study of viral lower respiratory tract infections in children under 3 years of age (the PRI.DE study). Eur. J. Pediatr. 163:709-716. [DOI] [PubMed] [Google Scholar]
- 102.Forton, J. T., K. Rowlands, N. Hanchard, M. Herbert, D. P. Kwiatkowski, and J. Hull. 2009. Genetic association study for RSV bronchiolitis in infancy at the 5q31 cytokine cluster. Thorax 64:345-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fouchier, R. A., T. Kuiken, M. Schutten, G. van Amerongen, G. J. van Doornum, B. G. Van Den Hoogen, M. Peiris, W. Lim, K. Stohr, and A. D. Osterhaus. 2003. Aetiology: Koch's postulates fulfilled for SARS virus. Nature 423:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gadomski, A. M., and A. L. Bhasale. 2006. Bronchodilators for bronchiolitis. Cochrane Database Syst. Rev. 2006:CD001266. [DOI] [PubMed] [Google Scholar]
- 105.Garcia-Garcia, M. L., C. Calvo, I. Casas, T. Bracamonte, A. Rellan, F. Gozalo, T. Tenorio, and P. Perez-Brena. 2007. Human metapneumovirus bronchiolitis in infancy is an important risk factor for asthma at age 5. Pediatr. Pulmonol. 42:458-464. [DOI] [PubMed] [Google Scholar]
- 106.Gaynor, A. M., M. D. Nissen, D. M. Whiley, I. M. Mackay, S. B. Lambert, G. Wu, D. C. Brennan, G. A. Storch, T. P. Sloots, and D. Wang. 2007. Identification of a novel polyomavirus from patients with acute respiratory tract infections. PLoS Pathog. 3:e64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gelder, C. M., R. Lambkin, K. W. Hart, D. Fleming, O. M. Williams, M. Bunce, K. I. Welsh, S. E. Marshall, and J. Oxford. 2002. Associations between human leukocyte antigens and nonresponsiveness to influenza vaccine. J. Infect. Dis. 185:114-117. [DOI] [PubMed] [Google Scholar]
- 108.Gentile, D. A., W. J. Doyle, A. Zeevi, J. Howe-Adams, S. Kapadia, J. Trecki, and D. P. Skoner. 2003. Cytokine gene polymorphisms moderate illness severity in infants with respiratory syncytial virus infection. Hum. Immunol. 64:338-344. [DOI] [PubMed] [Google Scholar]
- 109.Gern, J. E., E. C. Dick, E. A. Kelly, R. Vrtis, and B. Klein. 1997. Rhinovirus-specific T cells recognize both shared and serotype-restricted viral epitopes. J. Infect. Dis. 175:1108-1114. [DOI] [PubMed] [Google Scholar]
- 110.Goetghebuer, T., K. Isles, C. Moore, A. Thomson, D. Kwiatkowski, and J. Hull. 2004. Genetic predisposition to wheeze following respiratory syncytial virus bronchiolitis. Clin. Exp. Allergy 34:801-803. [DOI] [PubMed] [Google Scholar]
- 111.Goriely, S., and M. Goldman. 2007. From tolerance to autoimmunity: is there a risk in early life vaccination? J. Comp. Pathol. 137(Suppl. 1):S57-S61. [DOI] [PubMed] [Google Scholar]
- 112.Goriely, S., B. Vincart, P. Stordeur, J. Vekemans, F. Willems, M. Goldman, and D. De Wit. 2001. Deficient IL-12(p35) gene expression by dendritic cells derived from neonatal monocytes. J. Immunol. 166:2141-2146. [DOI] [PubMed] [Google Scholar]
- 113.Graham, B. S., L. A. Bunton, P. F. Wright, and D. T. Karzon. 1991. Role of T lymphocyte subsets in the pathogenesis of primary infection and rechallenge with respiratory syncytial virus in mice. J. Clin. Invest. 88:1026-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Griese, M. 2002. Respiratory syncytial virus and pulmonary surfactant. Viral Immunol. 15:357-363. [DOI] [PubMed] [Google Scholar]
- 115.Groskreutz, D. J., M. M. Monick, L. S. Powers, T. O. Yarovinsky, D. C. Look, and G. W. Hunninghake. 2006. Respiratory syncytial virus induces TLR3 protein and protein kinase R, leading to increased double-stranded RNA responsiveness in airway epithelial cells. J. Immunol. 176:1733-1740. [DOI] [PubMed] [Google Scholar]
- 116.Guerrero-Plata, A., A. Casola, G. Suarez, X. Yu, L. Spetch, M. E. Peeples, and R. P. Garofalo. 2006. Differential response of dendritic cells to human metapneumovirus and respiratory syncytial virus. Am. J. Respir. Cell Mol. Biol. 34:320-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Guerrero-Plata, A., D. Kolli, C. Hong, A. Casola, and R. P. Garofalo. 2009. Subversion of pulmonary dendritic cell function by paramyxovirus infections. J. Immunol. 182:3072-3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Guillot, L., R. Le Goffic, S. Bloch, N. Escriou, S. Akira, M. Chignard, and M. Si-Tahar. 2005. Involvement of Toll-like receptor 3 in the immune response of lung epithelial cells to double-stranded RNA and influenza A virus. J. Biol. Chem. 280:5571-5580. [DOI] [PubMed] [Google Scholar]
- 119.Hackett, S., L. Hill, J. Patel, N. Ratnaraja, A. Ifeyinwa, M. Farooqi, U. Nusgen, P. Debenham, D. Gandhi, N. Makwana, E. Smit, and S. Welch. 2009. Clinical characteristics of paediatric H1N1 admissions in Birmingham, UK. Lancet 374:605. [DOI] [PubMed] [Google Scholar]
- 120.Hall, C. B., G. A. Weinberg, M. K. Iwane, A. K. Blumkin, K. M. Edwards, M. A. Staat, P. Auinger, M. R. Griffin, K. A. Poehling, D. Erdman, C. G. Grijalva, Y. Zhu, and P. Szilagyi. 2009. The burden of respiratory syncytial virus infection in young children. N. Engl. J. Med. 360:588-598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Han, D. P., M. Lohani, and M. W. Cho. 2007. Specific asparagine-linked glycosylation sites are critical for DC-SIGN- and L-SIGN-mediated severe acute respiratory syndrome coronavirus entry. J. Virol. 81:12029-12039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hancock, G. E., P. W. Tebbey, C. A. Scheuer, K. S. Pryharski, K. M. Heers, and N. A. LaPierre. 2003. Immune responses to the nonglycosylated ectodomain of respiratory syncytial virus attachment glycoprotein mediate pulmonary eosinophilia in inbred strains of mice with different MHC haplotypes. J. Med. Virol. 70:301-308. [DOI] [PubMed] [Google Scholar]
- 123.Hashimoto, K., K. Ishibashi, T. Gebretsadik, T. V. Hartert, A. Yamamoto, T. Nakayama, K. Ohashi, H. Sakata, Y. Kawasaki, M. Katayose, H. Sakuma, H. Suzuki, M. Hosoya, R. S. Peebles, Jr., and T. Suzutani. 2008. Functional polymorphism of the promoter region of the prostacyclin synthase gene and severity of RSV infection in hospitalized children. J. Med. Virol. 80:2015-2022. [DOI] [PubMed] [Google Scholar]
- 124.Hayman, A., S. Comely, A. Lackenby, L. C. Hartgroves, S. Goodbourn, J. W. McCauley, and W. S. Barclay. 2007. NS1 proteins of avian influenza A viruses can act as antagonists of the human alpha/beta interferon response. J. Virol. 81:2318-2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hegele, R. G., S. Hayashi, A. M. Bramley, and J. C. Hogg. 1994. Persistence of respiratory syncytial virus genome and protein after acute bronchiolitis in guinea pigs. Chest 105:1848-1854. [DOI] [PubMed] [Google Scholar]
- 126.Hewson, C. A., A. Jardine, M. R. Edwards, V. Laza-Stanca, and S. L. Johnston. 2005. Toll-like receptor 3 is induced by and mediates antiviral activity against rhinovirus infection of human bronchial epithelial cells. J. Virol. 79:12273-12279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Heymann, P. W., H. T. Carper, D. D. Murphy, T. A. Platts-Mills, J. Patrie, A. P. McLaughlin, E. A. Erwin, M. S. Shaker, M. Hellems, J. Peerzada, F. G. Hayden, T. K. Hatley, and R. Chamberlain. 2004. Viral infections in relation to age, atopy, and season of admission among children hospitalized for wheezing. J. Allergy Clin. Immunol. 114:239-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hien, N. D., N. H. Ha, N. T. Van, N. T. Ha, T. T. Lien, N. Q. Thai, V. D. Trang, T. Shimbo, Y. Takahashi, Y. Kato, A. Kawana, S. Akita, and K. Kudo. 2009. Human infection with highly pathogenic avian influenza virus (H5N1) in northern Vietnam, 2004-2005. Emerg. Infect. Dis. 15:19-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hoebee, B., L. Bont, E. Rietveld, M. van Oosten, H. M. Hodemaekers, N. J. Nagelkerke, H. J. Neijens, J. L. Kimpen, and T. G. Kimman. 2004. Influence of promoter variants of interleukin-10, interleukin-9, and tumor necrosis factor-alpha genes on respiratory syncytial virus bronchiolitis. J. Infect. Dis. 189:239-247. [DOI] [PubMed] [Google Scholar]
- 130.Hoebee, B., E. Rietveld, L. Bont, M. Oosten, H. M. Hodemaekers, N. J. Nagelkerke, H. J. Neijens, J. L. Kimpen, and T. G. Kimman. 2003. Association of severe respiratory syncytial virus bronchiolitis with interleukin-4 and interleukin-4 receptor alpha polymorphisms. J. Infect. Dis. 187:2-11. [DOI] [PubMed] [Google Scholar]
- 131.Hoffjan, S., I. Ostrovnaja, D. Nicolae, D. L. Newman, R. Nicolae, R. Gangnon, L. Steiner, K. Walker, R. Reynolds, D. Greene, D. Mirel, J. E. Gern, R. F. Lemanske, Jr., and C. Ober. 2004. Genetic variation in immunoregulatory pathways and atopic phenotypes in infancy. J. Allergy Clin. Immunol. 113:511-518. [DOI] [PubMed] [Google Scholar]
- 132.Holgate, S. T. 2007. The epithelium takes centre stage in asthma and atopic dermatitis. Trends Immunol. 28:248-251. [DOI] [PubMed] [Google Scholar]
- 133.Hon, K. L., E. Leung, J. Tang, C. M. Chow, T. F. Leung, K. L. Cheung, and P. C. Ng. 2008. Premorbid factors and outcome associated with respiratory virus infections in a pediatric intensive care unit. Pediatr. Pulmonol. 43:275-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Huang, K. J., I. J. Su, M. Theron, Y. C. Wu, S. K. Lai, C. C. Liu, and H. Y. Lei. 2005. An interferon-gamma-related cytokine storm in SARS patients. J. Med. Virol. 75:185-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hull, J., K. Rowlands, E. Lockhart, C. Moore, M. Sharland, and D. Kwiatkowski. 2003. Variants of the chemokine receptor CCR5 are associated with severe bronchiolitis caused by respiratory syncytial virus. J. Infect. Dis. 188:904-907. [DOI] [PubMed] [Google Scholar]
- 136.Hull, J., A. Thomson, and D. Kwiatkowski. 2000. Association of respiratory syncytial virus bronchiolitis with the interleukin 8 gene region in UK families. Thorax 55:1023-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Humphreys, I. R., L. Edwards, R. J. Snelgrove, A. J. Rae, A. J. Coyle, and T. Hussell. 2006. A critical role for ICOS co-stimulation in immune containment of pulmonary influenza virus infection. Eur. J. Immunol. 36:2928-2938. [DOI] [PubMed] [Google Scholar]
- 138.Humphreys, I. R., G. Walzl, L. Edwards, A. Rae, S. Hill, and T. Hussell. 2003. A critical role for OX40 in T cell-mediated immunopathology during lung viral infection. J. Exp. Med. 198:1237-1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hussell, T., A. Georgiou, T. E. Sparer, S. Matthews, P. Pala, and P. J. M. Openshaw. 1998. Host genetic determinants of vaccine-induced eosinophilia during respiratory syncytial virus infection. J. Immunol. 161:6215-6222. [PubMed] [Google Scholar]
- 140.Hussell, T., A. Pennycook, and P. J. M. Openshaw. 2001. Inhibition of tumour necrosis factor reduces the severity of virus-specific lung immunopathology. Eur. J. Immunol. 31:2566-2573. [DOI] [PubMed] [Google Scholar]
- 141.Ichinohe, T., H. K. Lee, Y. Ogura, R. Flavell, and A. Iwasaki. 2009. Inflammasome recognition of influenza virus is essential for adaptive immune responses. J. Exp. Med. 206:79-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Illi, S., E. von Mutius, S. Lau, R. Bergmann, B. Niggemann, C. Sommerfeld, and U. Wahn. 2001. Early childhood infectious diseases and the development of asthma up to school age: a birth cohort study. BMJ 322:390-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142a.IMpact-RSV Study Group. 1998. Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics 102:531-537. [PubMed] [Google Scholar]
- 143.Inoue, Y., N. Shimojo, Y. Suzuki, E. J. Campos Alberto, A. Yamaide, S. Suzuki, T. Arima, T. Matsuura, M. Tomiita, M. Aoyagi, A. Hoshioka, A. Honda, A. Hata, and Y. Kohno. 2007. CD14-550 C/T, which is related to the serum level of soluble CD14, is associated with the development of respiratory syncytial virus bronchiolitis in the Japanese population. J. Infect. Dis. 195:1618-1624. [DOI] [PubMed] [Google Scholar]
- 144.Ip, W., K. Chan, H. Law, G. Tso, E. Kong, W. Wong, Y. To, R. Yung, E. Chow, K. Au, E. Chan, W. Lim, J. Jensenius, M. Turner, J. Peiris, and Y. Lau. 2005. Mannose-binding lectin in severe acute respiratory syndrome coronavirus infection. J. Infect. Dis. 191:1697-1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Isaacs, D., C. J. Taylor, A. Ting, and A. J. McMichael. 1989. HLA class I antigens in severe RSV bronchiolitis. Tissue Antigens 34:210-212. [DOI] [PubMed] [Google Scholar]
- 146.Ito, T., Y. H. Wang, O. Duramad, T. Hori, G. J. Delespesse, N. Watanabe, F. X. Qin, Z. Yao, W. Cao, and Y. J. Liu. 2005. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J. Exp. Med. 202:1213-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Jackson, D. J., R. E. Gangnon, M. D. Evans, K. A. Roberg, E. L. Anderson, T. E. Pappas, M. C. Printz, W. M. Lee, P. A. Shult, E. Reisdorf, K. T. Carlson-Dakes, L. P. Salazar, D. F. Dasilva, C. J. Tisler, J. E. Gern, and R. F. Lemanske, Jr. 2008. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am. J. Respir. Crit. Care Med. 178:667-672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Reference deleted.
- 149.Jacques, J., M. Bouscambert-Duchamp, H. Moret, J. Carquin, V. Brodard, B. Lina, J. Motte, and L. Andreoletti. 2006. Association of respiratory picornaviruses with acute bronchiolitis in French infants. J. Clin. Virol. 35:463-466. [DOI] [PubMed] [Google Scholar]
- 150.Janssen, R., L. Bont, C. L. Siezen, H. M. Hodemaekers, M. J. Ermers, G. Doornbos, R. Slot, C. Wijmenga, J. J. Goeman, J. L. Kimpen, H. C. van Houwelingen, T. G. Kimman, and B. Hoebee. 2007. Genetic susceptibility to respiratory syncytial virus bronchiolitis is predominantly associated with innate immune genes. J. Infect. Dis. 196:826-834. [DOI] [PubMed] [Google Scholar]
- 151.Jartti, T., W. M. Lee, T. Pappas, M. Evans, R. F. Lemanske, Jr., and J. E. Gern. 2008. Serial viral infections in infants with recurrent respiratory illnesses. Eur. Respir. J. 32:314-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Jartti, T., P. Lehtinen, T. Vanto, T. Vuorinen, J. Hartiala, H. Hiekkanen, P. Malmberg, M. Makela, and O. Ruuskanen. 2007. Efficacy of prednisolone in children hospitalized for recurrent wheezing. Pediatr. Allergy Immunol. 18:326-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Jartti, T., P. Lehtinen, T. Vuorinen, M. Koskenvuo, and O. Ruuskanen. 2004. Persistence of rhinovirus and enterovirus RNA after acute respiratory illness in children. J. Med. Virol. 72:695-699. [DOI] [PubMed] [Google Scholar]
- 154.Jartti, T., P. Lehtinen, T. Vuorinen, R. Osterback, B. van den Hoogen, A. D. Osterhaus, and O. Ruuskanen. 2004. Respiratory picornaviruses and respiratory syncytial virus as causative agents of acute expiratory wheezing in children. Emerg. Infect. Dis. 10:1095-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Johnson, J. E., R. A. Gonzales, S. J. Olson, P. F. Wright, and B. S. Graham. 2007. The histopathology of fatal untreated human respiratory syncytial virus infection. Mod. Pathol. 20:108-119. [DOI] [PubMed] [Google Scholar]
- 156.Johnson, T. R., R. A. Parker, J. E. Johnson, and B. S. Graham. 2003. IL-13 is sufficient for respiratory syncytial virus G glycoprotein-induced eosinophilia after respiratory syncytial virus challenge. J. Immunol. 170:2037-2045. [DOI] [PubMed] [Google Scholar]
- 157.Johnston, S. L., P. K. Pattemore, G. Sanderson, S. Smith, F. Lampe, L. Josephs, P. Symington, S. O'Toole, S. H. Myint, D. A. J. Tyrrell, and S. T. Holgate. 1995. Community study of role of viral infections in exacerbations of asthma in 9-11 year old children. BMJ 310:1225-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Joint Formulary Committee. 2008. British national formulary, 54th ed. British Medical Association and Royal Pharmaceutical Society of Great Britain, London, United Kingdom.
- 159.Karadag, B., O. Ceran, G. Guven, E. Dursun, I. O. Ipek, F. Karakoc, R. H. Ersu, A. Bozaykut, S. Inan, and E. Dagli. 2008. Efficacy of salbutamol and ipratropium bromide in the management of acute bronchiolitis—a clinical trial. Respiration 76:283-287. [DOI] [PubMed] [Google Scholar]
- 160.Kato, A., S. Favoreto, Jr., P. C. Avila, and R. P. Schleimer. 2007. TLR3- and Th2 cytokine-dependent production of thymic stromal lymphopoietin in human airway epithelial cells. J. Immunol. 179:1080-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kato, H., O. Takeuchi, S. Sato, M. Yoneyama, M. Yamamoto, K. Matsui, S. Uematsu, A. Jung, T. Kawai, K. J. Ishii, O. Yamaguchi, K. Otsu, T. Tsujimura, C. S. Koh, C. Reis e Sousa, Y. Matsuura, T. Fujita, and S. Akira. 2006. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature 441:101-105. [DOI] [PubMed] [Google Scholar]
- 162.Kaur, K., S. Chowdhury, N. S. Greenspan, and J. R. Schreiber. 2007. Decreased expression of tumor necrosis factor family receptors involved in humoral immune responses in preterm neonates. Blood 110:2948-2954. [DOI] [PubMed] [Google Scholar]
- 162a.Kelly, D. F., E. R. Moxon, and A. J. Pollard. 2004. Haemophilus influenzae type b conjugate vaccines. Immunology 113:163-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kim, H. W., J. G. Canchola, C. D. Brandt, G. Pyles, R. M. Chanock, K. Jensen, and R. H. Parrott. 1969. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am. J. Epidemiol. 89:422-434. [DOI] [PubMed] [Google Scholar]
- 164.Kinoshita, H., T. Takai, T. A. Le, S. Kamijo, X. L. Wang, H. Ushio, M. Hara, J. Kawasaki, A. T. Vu, T. Ogawa, H. Gunawan, S. Ikeda, K. Okumura, and H. Ogawa. 2009. Cytokine milieu modulates release of thymic stromal lymphopoietin from human keratinocytes stimulated with double-stranded RNA. J. Allergy Clin. Immunol. 123:179-186. [DOI] [PubMed] [Google Scholar]
- 165.Klein, M. I., S. Coviello, G. Bauer, A. Benitez, M. E. Serra, M. P. Schiatti, M. F. Delgado, G. A. Melendi, L. Novalli, H. G. Pena, R. A. Karron, S. R. Kleeberger, and F. P. Polack. 2006. The impact of infection with human metapneumovirus and other respiratory viruses in young infants and children at high risk for severe pulmonary disease. J. Infect. Dis. 193:1544-1551. [DOI] [PubMed] [Google Scholar]
- 166.Klugman, K. P., and S. A. Madhi. 2007. Pneumococcal vaccines and flu preparedness. Science 316:49-50. [DOI] [PubMed] [Google Scholar]
- 167.Kneyber, M. C., and J. L. Kimpen. 2007. Antibiotics in RSV bronchiolitis: still no evidence of effect. Eur. Respir. J. 29:1285. [DOI] [PubMed] [Google Scholar]
- 168.Kneyber, M. C., J. B. van Woensel, E. Uijtendaal, C. S. Uiterwaal, and J. L. Kimpen. 2008. Azithromycin does not improve disease course in hospitalized infants with respiratory syncytial virus (RSV) lower respiratory tract disease: a randomized equivalence trial. Pediatr. Pulmonol. 43:142-149. [DOI] [PubMed] [Google Scholar]
- 169.Konïg, B., W. Konig, R. Arnold, H. Werchau, G. Ihorst, and J. Forster. 2004. Prospective study of human metapneumovirus infection in children less than 3 years of age. J. Clin. Microbiol. 42:4632-4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kopecky-Bromberg, S. A., L. Martinez-Sobrido, M. Frieman, R. A. Baric, and P. Palese. 2007. Severe acute respiratory syndrome coronavirus open reading frame (ORF) 3b, ORF 6, and nucleocapsid proteins function as interferon antagonists. J. Virol. 81:548-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Korppi, M. 2007. Macrolides and bronchiolitis in infants. Eur. Respir. J. 29:1283-1284. [DOI] [PubMed] [Google Scholar]
- 172.Kosai, K., M. Seki, K. Yanagihara, S. Nakamura, S. Kurihara, K. Izumikawa, H. Kakeya, Y. Yamamoto, T. Tashiro, and S. Kohno. 2008. Gabexate mesilate suppresses influenza pneumonia in mice through inhibition of cytokines. J. Int. Med. Res. 36:322-328. [DOI] [PubMed] [Google Scholar]
- 173.Kostova, T. 2007. Persistence of viral infections on the population level explained by an immunoepidemiological model. Math. Biosci. 206:309-319. [DOI] [PubMed] [Google Scholar]
- 174.Kotla, S., T. Peng, R. E. Bumgarner, and K. E. Gustin. 2008. Attenuation of the type I interferon response in cells infected with human rhinovirus. Virology 374:399-410. [DOI] [PubMed] [Google Scholar]
- 175.Kristensen, I. A., S. Thiel, R. Steffensen, S. Madhi, G. Sorour, and J. Olsen. 2004. Mannan-binding lectin and RSV lower respiratory tract infection leading to hospitalization in children: a case-control study from Soweto, South Africa. Scand. J. Immunol. 60:184-188. [DOI] [PubMed] [Google Scholar]
- 176.Kropf, P., D. Baud, S. E. Marshall, M. Munder, A. Mosley, J. M. Fuentes, C. R. Bangham, G. P. Taylor, S. Herath, B. S. Choi, G. Soler, T. Teoh, M. Modolell, and I. Muller. 2007. Arginase activity mediates reversible T cell hyporesponsiveness in human pregnancy. Eur. J. Immunol. 37:935-945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Krueger, M., B. Puthothu, E. Gropp, J. Heinze, S. Braun, and A. Heinzmann. 2006. Amino acid variants in surfactant protein D are not associated with bronchial asthma. Pediatr. Allergy Immunol. 17:77-81. [DOI] [PubMed] [Google Scholar]
- 178.Krueger, M., B. Puthothu, J. Heinze, J. Forster, and A. Heinzmann. 2006. Genetic polymorphisms of adhesion molecules in children with severe RSV-associated diseases. Int. J. Immunogenet. 33:233-235. [DOI] [PubMed] [Google Scholar]
- 179.Kurt-Jones, E. A., L. Popova, L. Kwinn, L. M. Haynes, L. P. Jones, R. A. Tripp, E. E. Walsh, M. W. Freeman, D. T. Golenbock, L. J. Anderson, and R. W. Finberg. 2000. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat. Immunol. 1:398-401. [DOI] [PubMed] [Google Scholar]
- 180.Kusel, M. M., N. H. de Klerk, P. G. Holt, T. Kebadze, S. L. Johnston, and P. D. Sly. 2006. Role of respiratory viruses in acute upper and lower respiratory tract illness in the first year of life: a birth cohort study. Pediatr. Infect. Dis. J. 25:680-686. [DOI] [PubMed] [Google Scholar]
- 181.Kusel, M. M., N. H. de Klerk, T. Kebadze, V. Vohma, P. G. Holt, S. L. Johnston, and P. D. Sly. 2007. Early-life respiratory viral infections, atopic sensitization, and risk of subsequent development of persistent asthma. J. Allergy Clin. Immunol. 119:1105-1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kuzik, B. A., S. A. Al Qadhi, S. Kent, M. P. Flavin, W. Hopman, S. Hotte, and S. Gander. 2007. Nebulized hypertonic saline in the treatment of viral bronchiolitis in infants. J. Pediatr. 151:266-270. [DOI] [PubMed] [Google Scholar]
- 183.Lahti, M., J. Lofgren, R. Marttila, M. Renko, T. Klaavuniemi, R. Haataja, M. Ramet, and M. Hallman. 2002. Surfactant protein D gene polymorphism associated with severe respiratory syncytial virus infection. Pediatr. Res. 51:696-699. [DOI] [PubMed] [Google Scholar]
- 184.Lambert, S. B., K. M. Allen, R. C. Carter, and T. M. Nolan. 2008. The cost of community-managed viral respiratory illnesses in a cohort of healthy preschool-aged children. Respir. Res. 9:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Lee, H. C., and S. F. Ziegler. 2007. Inducible expression of the proallergic cytokine thymic stromal lymphopoietin in airway epithelial cells is controlled by NFkappaB. Proc. Natl. Acad. Sci. U. S. A. 104:914-919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Legg, J. P., J. A. Warner, S. L. Johnston, and J. O. Warner. 2005. Frequency of detection of picornaviruses and seven other respiratory pathogens in infants. Pediatr. Infect. Dis. J. 24:611-616. [DOI] [PubMed] [Google Scholar]
- 187.Le Goffic, R., V. Balloy, M. Lagranderie, L. Alexopoulou, N. Escriou, R. Flavell, M. Chignard, and M. Si-Tahar. 2006. Detrimental contribution of the Toll-like receptor (TLR) 3 to influenza A virus-induced acute pneumonia. PLoS Pathog. 2:e53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Le Goffic, R., J. Pothlichet, D. Vitour, T. Fujita, E. Meurs, M. Chignard, and M. Si-Tahar. 2007. Influenza A virus activates TLR3-dependent inflammatory and RIG-I-dependent antiviral responses in human lung epithelial cells. J. Immunol. 178:3368-3372. [DOI] [PubMed] [Google Scholar]
- 189.Le Hesran, J. Y., M. Cot, P. Personne, N. Fievet, B. Dubois, M. Beyeme, C. Boudin, and P. Deloron. 1997. Maternal placental infection with Plasmodium falciparum and malaria morbidity during the first 2 years of life. Am. J. Epidemiol. 146:826-831. [DOI] [PubMed] [Google Scholar]
- 190.Lehtinen, P., T. Jartti, R. Virkki, T. Vuorinen, M. Leinonen, V. Peltola, A. Ruohola, and O. Ruuskanen. 2006. Bacterial coinfections in children with viral wheezing. Eur. J. Clin. Microbiol. Infect. Dis. 25:463-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Lehtinen, P., A. Ruohola, T. Vanto, T. Vuorinen, O. Ruuskanen, and T. Jartti. 2007. Prednisolone reduces recurrent wheezing after a first wheezing episode associated with rhinovirus infection or eczema. J. Allergy Clin. Immunol. 119:570-575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Lemanske, R. F., Jr., D. J. Jackson, R. E. Gangnon, M. D. Evans, Z. Li, P. A. Shult, C. J. Kirk, E. Reisdorf, K. A. Roberg, E. L. Anderson, K. T. Carlson-Dakes, K. J. Adler, S. Gilbertson-White, T. E. Pappas, D. F. Dasilva, C. J. Tisler, and J. E. Gern. 2005. Rhinovirus illnesses during infancy predict subsequent childhood wheezing. J. Allergy Clin. Immunol. 116:571-577. [DOI] [PubMed] [Google Scholar]
- 193.Levin, D. L., A. Garg, L. J. Hall, S. Slogic, J. D. Jarvis, and J. C. Leiter. 2008. A prospective randomized controlled blinded study of three bronchodilators in infants with respiratory syncytial virus bronchiolitis on mechanical ventilation. Pediatr. Crit. Care Med. 9:598-604. [DOI] [PubMed] [Google Scholar]
- 194.LeVine, A. M., J. Gwozdz, J. Stark, M. Bruno, J. Whitsett, and T. Korfhagen. 1999. Surfactant protein-A enhances respiratory syncytial virus clearance in vivo. J. Clin. Invest. 103:1015-1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Levy, O. 2007. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nat. Rev. Immunol. 7:379-390. [DOI] [PubMed] [Google Scholar]
- 196.Levy, O., K. A. Zarember, R. M. Roy, C. Cywes, P. J. Godowski, and M. R. Wessels. 2004. Selective impairment of TLR-mediated innate immunity in human newborns: neonatal blood plasma reduces monocyte TNF-alpha induction by bacterial lipopeptides, lipopolysaccharide, and imiquimod, but preserves the response to R-848. J. Immunol. 173:4627-4634. [DOI] [PubMed] [Google Scholar]
- 197.Li, H., N. L. Tang, P. K. Chan, C. Y. Wang, D. S. Hui, C. Luk, R. Kwok, W. Huang, J. J. Sung, Q. P. Kong, and Y. P. Zhang. 2008. Polymorphisms in the C-type lectin genes cluster in chromosome 19 and predisposition to severe acute respiratory syndrome coronavirus (SARS-CoV) infection. J. Med. Genet. 45:752-758. [DOI] [PubMed] [Google Scholar]
- 198.Liao, S., X. Bao, T. Liu, S. Lai, K. Li, R. P. Garofalo, and A. Casola. 2008. Role of retinoic acid inducible gene-I in human metapneumovirus-induced cellular signalling. J. Gen. Virol. 89:1978-1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Liet, J. M., B. Millotte, M. Tucci, S. Laflammme, J. Hutchison, D. Creery, T. Ducruet, and J. Lacroix. 2005. Noninvasive therapy with helium-oxygen for severe bronchiolitis. J. Pediatr. 147:812-817. [DOI] [PubMed] [Google Scholar]
- 200.Lin, D., J. Lan, and Z. Zhang. 2007. Structure and function of the NS1 protein of influenza A virus. Acta Biochim. Biophys. Sin. (Shanghai) 39:155-162. [DOI] [PubMed] [Google Scholar]
- 201.Lin, M., H. K. Tseng, J. A. Trejaut, H. L. Lee, J. H. Loo, C. C. Chu, P. J. Chen, Y. W. Su, K. H. Lim, Z. U. Tsai, R. Y. Lin, R. S. Lin, and C. H. Huang. 2003. Association of HLA class I with severe acute respiratory syndrome coronavirus infection. BMC Med. Genet. 4:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Ling, Z., K. C. Tran, and M. N. Teng. 2009. The human respiratory syncytial virus nonstructural NS2 protein antagonizes the activation of beta interferon transcription by interacting with RIG-I. J. Virol. 83:3734-3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Lister, P., F. Reynolds, R. Parslow, A. Chan, M. Cooper, A. Plunkett, S. Riphagen, and M. Peters. 2009. Swine-origin influenza virus H1N1, seasonal influenza virus, and critical illness in children. Lancet 374:605-607. [DOI] [PubMed] [Google Scholar]
- 204.Liu, P., K. Li, R. P. Garofalo, and A. R. Brasier. 2008. Respiratory syncytial virus induces RelA release from cytoplasmic 100-kDa NF-kappa B2 complexes via a novel retinoic acid-inducible gene-I·NF-kappa B-inducing kinase signaling pathway. J. Biol. Chem. 283:23169-23178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Liu, Y., D. L. Haas, S. Poore, S. Isakovic, M. Gahan, S. Mahalingam, Z. F. Fu, and R. A. Tripp. 2009. Human metapneumovirus establishes persistent infection in the lungs of mice and is reactivated by glucocorticoid treatment. J. Virol. 83:6837-6848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Lofgren, J., M. Ramet, M. Renko, R. Marttila, and M. Hallman. 2002. Association between surfactant protein A gene locus and severe respiratory syncytial virus infection in infants. J. Infect. Dis. 185:283-289. [DOI] [PubMed] [Google Scholar]
- 207.Loo, Y. M., J. Fornek, N. Crochet, G. Bajwa, O. Perwitasari, L. Martinez-Sobrido, S. Akira, M. A. Gill, A. Garcia-Sastre, M. G. Katze, and M. Gale, Jr. 2008. Distinct RIG-I and MDA5 signaling by RNA viruses in innate immunity. J. Virol. 82:335-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Louie, J. K., A. Roy-Burman, L. Guardia-Labar, E. J. Boston, D. Kiang, T. Padilla, S. Yagi, S. Messenger, A. M. Petru, C. A. Glaser, and D. P. Schnurr. 2009. Rhinovirus associated with severe lower respiratory tract infections in children. Pediatr. Infect. Dis. J. 28:337-339. [DOI] [PubMed] [Google Scholar]
- 209.Low, D. 2008. Reducing antibiotic use in influenza: challenges and rewards. Clin. Microbiol. Infect. 14:298-306. [DOI] [PubMed] [Google Scholar]
- 210.Lu, L. L., M. Puri, C. M. Horvath, and G. C. Sen. 2008. Select paramyxoviral V proteins inhibit IRF3 activation by acting as alternative substrates for inhibitor of kappaB kinase epsilon (IKKe)/TBK1. J. Biol. Chem. 283:14269-14276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Lund, J. M., L. Alexopoulou, A. Sato, M. Karow, N. C. Adams, N. W. Gale, A. Iwasaki, and R. A. Flavell. 2004. Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc. Natl. Acad. Sci. U. S. A. 101:5598-5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Macaubas, C., N. H. de Klerk, B. J. Holt, C. Wee, G. Kendall, M. Firth, P. D. Sly, and P. G. Holt. 2003. Association between antenatal cytokine production and the development of atopy and asthma at age 6 years. Lancet 362:1192-1197. [DOI] [PubMed] [Google Scholar]
- 213.Macek, V., J. Sorli, S. Kopriva, and J. Marin. 1994. Persistent adenoviral infection and chronic airway obstruction in children. Am. J. Respir. Crit. Care Med. 150:7-10. [DOI] [PubMed] [Google Scholar]
- 214.Mahony, J. B. 2008. Detection of respiratory viruses by molecular methods. Clin. Microbiol. Rev. 21:716-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Manning, A., V. Russell, K. Eastick, G. H. Leadbetter, N. Hallam, K. Templeton, and P. Simmonds. 2006. Epidemiological profile and clinical associations of human bocavirus and other human parvoviruses. J. Infect. Dis. 194:1283-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Manoha, C., S. Espinosa, S. L. Aho, F. Huet, and P. Pothier. 2007. Epidemiological and clinical features of hMPV, RSV and RVs infections in young children. J. Clin. Virol. 38:221-226. [DOI] [PubMed] [Google Scholar]
- 217.Mansbach, J. M., A. J. McAdam, S. Clark, P. D. Hain, R. G. Flood, U. Acholonu, and C. A. Camargo, Jr. 2008. Prospective multicenter study of the viral etiology of bronchiolitis in the emergency department. Acad. Emerg. Med. 15:111-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Marguet, C., M. Lubrano, M. Gueudin, P. Le Roux, A. Deschildre, C. Forget, L. Couderc, D. Siret, M. D. Donnou, M. Bubenheim, A. Vabret, and F. Freymuth. 2009. In very young infants severity of acute bronchiolitis depends on carried viruses. PLoS One 4:e4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Marsland, B. J., C. B. Scanga, M. Kopf, and G. Le Gros. 2004. Allergic airway inflammation is exacerbated during acute influenza infection and correlates with increased allergen presentation and recruitment of allergen-specific T-helper type 2 cells. Clin. Exp. Allergy 34:1299-1306. [DOI] [PubMed] [Google Scholar]
- 220.Martinez, F. D. 2005. Heterogeneity of the association between lower respiratory illness in infancy and subsequent asthma. Proc. Am. Thorac. Soc. 2:157-161. [DOI] [PubMed] [Google Scholar]
- 221.Martinez, F. D., D. A. Stern, A. L. Wright, L. M. Taussig, and M. Halonen. 1998. Differential immune responses to acute lower respiratory illness in early life and subsequent development of persistent wheezing and asthma. J. Allergy Clin. Immunol. 102:915-920. [DOI] [PubMed] [Google Scholar]
- 222.Massin, M. M., J. Montesanti, P. Gerard, and P. Lepage. 2006. Spectrum and frequency of illness presenting to a pediatric emergency department. Acta Clin. Belg. 61:161-165. [DOI] [PubMed] [Google Scholar]
- 223.Matthews, S. P., J. S. Tregoning, A. J. Coyle, T. Hussell, and P. J. Openshaw. 2005. Role of CCL11 in eosinophilic lung disease during respiratory syncytial virus infection. J. Virol. 79:2050-2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.McCullers, J. A., A. R. Iverson, R. McKeon, and P. J. Murray. 2008. The platelet activating factor receptor is not required for exacerbation of bacterial pneumonia following influenza. Scand. J. Infect. Dis. 40:11-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.McIntosh, K. 2006. Human bocavirus: developing evidence for pathogenicity. J. Infect. Dis. 194:1197-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.McNamara, P. S., B. F. Flanagan, C. A. Hart, and R. L. Smyth. 2005. Production of chemokines in the lungs of infants with severe respiratory syncytial virus bronchiolitis. J. Infect. Dis. 191:1225-1232. [DOI] [PubMed] [Google Scholar]
- 227.McNamara, P. S., P. Ritson, A. Selby, C. A. Hart, and R. L. Smyth. 2003. Bronchoalveolar lavage cellularity in infants with severe respiratory syncytial virus bronchiolitis. Arch. Dis. Child. 88:922-926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.McNamee, L. A., and A. G. Harmsen. 2006. Both influenza-induced neutrophil dysfunction and neutrophil-independent mechanisms contribute to increased susceptibility to a secondary Streptococcus pneumoniae infection. Infect. Immun. 74:6707-6721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Meissner, H. C. 2003. Selected populations at increased risk from respiratory syncytial virus infection. Pediatr. Infect. Dis. J. 22:S40-S44. [DOI] [PubMed] [Google Scholar]
- 230.Miller, A. L., C. Gerard, M. Schaller, A. D. Gruber, A. A. Humbles, and N. W. Lukacs. 2006. Deletion of CCR1 attenuates pathophysiologic responses during respiratory syncytial virus infection. J. Immunol. 176:2562-2567. [DOI] [PubMed] [Google Scholar]
- 231.Miyairi, I., and J. P. DeVincenzo. 2008. Human genetic factors and respiratory syncytial virus disease severity. Clin. Microbiol. Rev. 21:686-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Mizgerd, J. P. 2006. Lung infection—a public health priority. PLoS Med. 3:e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Monick, M. M., T. O. Yarovinsky, L. S. Powers, N. S. Butler, A. B. Carter, G. Gudmundsson, and G. W. Hunninghake. 2003. Respiratory syncytial virus up-regulates TLR4 and sensitizes airway epithelial cells to endotoxin. J. Biol. Chem. 278:53035-53044. [DOI] [PubMed] [Google Scholar]
- 234.Monto, A. S., and B. M. Ullman. 1974. Acute respiratory illness in an American community. The Tecumseh Study. JAMA 227:164-169. [PubMed] [Google Scholar]
- 235.Mullins, J. A., D. D. Erdman, G. A. Weinberg, K. Edwards, C. B. Hall, F. J. Walker, M. Iwane, and L. J. Anderson. 2004. Human metapneumovirus infection among children hospitalized with acute respiratory illness. Emerg. Infect. Dis. 10:700-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Murawski, M. R., G. N. Bowen, A. M. Cerny, L. J. Anderson, L. M. Haynes, R. A. Tripp, E. A. Kurt-Jones, and R. W. Finberg. 2009. Respiratory syncytial virus activates innate immunity through Toll-like receptor 2. J. Virol. 83:1492-1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Reference deleted.
- 238.Neuzil, K. M., Y. W. Tang, and B. S. Graham. 1996. Protective role of TNF-alpha in respiratory syncytial virus infection in vitro and in vivo. Am. J. Med. Sci. 311:201-204. [DOI] [PubMed] [Google Scholar]
- 239.Ng, M. W., G. Zhou, W. P. Chong, L. W. Lee, H. K. Law, H. Zhang, W. H. Wong, S. F. Fok, Y. Zhai, R. W. Yung, E. Y. Chow, K. L. Au, E. Y. Chan, W. Lim, J. S. Peiris, F. He, and Y. L. Lau. 2007. The association of RANTES polymorphism with severe acute respiratory syndrome in Hong Kong and Beijing Chinese. BMC Infect. Dis. 7:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Nicholls, J. M., J. Butany, L. L. Poon, K. H. Chan, S. L. Beh, S. Poutanen, J. S. Peiris, and M. Wong. 2006. Time course and cellular localization of SARS-CoV nucleoprotein and RNA in lungs from fatal cases of SARS. PLoS Med. 3:e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Nicholls, J. M., and J. S. Peiris. 2008. Avian influenza: update on pathogenesis and laboratory diagnosis. Respirology 13(Suppl. 1):S14-S18. [DOI] [PubMed] [Google Scholar]
- 242.Nicholson, K. G., T. McNally, M. Silverman, P. Simons, J. D. Stockton, and M. C. Zambon. 2006. Rates of hospitalisation for influenza, respiratory syncytial virus and human metapneumovirus among infants and young children. Vaccine 24:102-108. [DOI] [PubMed] [Google Scholar]
- 243.Noone, C. M., E. A. Lewis, A. B. Frawely, R. W. Newman, B. P. Mahon, K. H. Mills, and P. A. Johnson. 2005. Novel mechanism of immunosuppression by influenza virus haemagglutinin: selective suppression of interleukin 12 p35 transcription in murine bone marrow-derived dendritic cells. J. Gen. Virol. 86:1885-1890. [DOI] [PubMed] [Google Scholar]
- 244.Norja, P., I. Ubillos, K. Templeton, and P. Simmonds. 2007. No evidence for an association between infections with WU and KI polyomaviruses and respiratory disease. J. Clin. Virol. 40:307-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Okiro, E. A., M. Ngama, A. Bett, P. A. Cane, G. F. Medley, and N. D. James. 2008. Factors associated with increased risk of progression to respiratory syncytial virus-associated pneumonia in young Kenyan children. Trop. Med. Int. Health 13:914-926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Olszewska, W., and P. Openshaw. 2009. Emerging drugs for respiratory syncytial virus infection. Expert Opin. Emerg. Drugs 14:207-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Olszewska, W., M. Zambon, and P. J. M. Openshaw. 2002. Development of vaccines against common colds. Br. Med. Bull. 62:99-111. [DOI] [PubMed] [Google Scholar]
- 248.Openshaw, P. J. M., and J. S. Tregoning. 2005. Immune responses and disease enhancement during respiratory syncytial virus infection. Clin. Microbiol. Rev. 18:541-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Opitz, B., A. Rejaibi, B. Dauber, J. Eckhard, M. Vinzing, B. Schmeck, S. Hippenstiel, N. Suttorp, and T. Wolff. 2007. IFNbeta induction by influenza A virus is mediated by RIG-I which is regulated by the viral NS1 protein. Cell. Microbiol. 9:930-938. [DOI] [PubMed] [Google Scholar]
- 250.O'Sullivan, S., L. Cormican, J. L. Faul, S. Ichinohe, S. L. Johnston, C. M. Burke, and L. W. Poulter. 2001. Activated, cytotoxic CD8(+) T lymphocytes contribute to the pathology of asthma death. Am. J. Respir. Crit. Care Med. 164:560-564. [DOI] [PubMed] [Google Scholar]
- 251.Papadopoulos, N. G., M. Moustaki, M. Tsolia, A. Bossios, E. Astra, A. Prezerakou, D. Gourgiotis, and D. Kafetzis. 2002. Association of rhinovirus infection with increased disease severity in acute bronchiolitis. Am. J. Respir. Crit. Care Med. 165:1285-1289. [DOI] [PubMed] [Google Scholar]
- 252.Patel, H., R. Platt, J. M. Lozano, and E. E. Wang. 2004. Glucocorticoids for acute viral bronchiolitis in infants and young children. Cochrane Database Syst. Rev. 2004:CD004878. [DOI] [PubMed] [Google Scholar]
- 253.Paulus, S. C., A. F. Hirschfeld, R. E. Victor, J. Brunstein, E. Thomas, and S. E. Turvey. 2007. Common human Toll-like receptor 4 polymorphisms—role in susceptibility to respiratory syncytial virus infection and functional immunological relevance. Clin. Immunol. 123:252-257. [DOI] [PubMed] [Google Scholar]
- 254.Peiris, J. S., S. T. Lai, L. L. Poon, Y. Guan, L. Y. Yam, W. Lim, J. Nicholls, W. K. Yee, W. W. Yan, M. T. Cheung, V. C. Cheng, K. H. Chan, D. N. Tsang, R. W. Yung, T. K. Ng, and K. Y. Yuen. 2003. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet 361:1319-1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Peltola, V. T., K. G. Murti, and J. A. McCullers. 2005. Influenza virus neuraminidase contributes to secondary bacterial pneumonia. J. Infect. Dis. 192:249-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Pichler, M., G. Herrmann, H. Schmidt, P. Ahrens, and S. Zielen. 2001. Persistent adenoviral infection and chronic obstructive bronchitis in children: is there a link? Pediatr. Pulmonol. 32:367-371. [DOI] [PubMed] [Google Scholar]
- 257.Pichlmair, A., O. Schulz, C. P. Tan, T. I. Naslund, P. Liljestrom, F. Weber, and C. Reis e Sousa. 2006. RIG-I-mediated antiviral responses to single-stranded RNA bearing 5′-phosphates. Science 314:997-1001. [DOI] [PubMed] [Google Scholar]
- 258.Pierangeli, A., M. Gentile, P. Di Marco, P. Pagnotti, C. Scagnolari, S. Trombetti, L. Lo Russo, V. Tromba, C. Moretti, F. Midulla, and G. Antonelli. 2007. Detection and typing by molecular techniques of respiratory viruses in children hospitalized for acute respiratory infection in Rome, Italy. J. Med. Virol. 79:463-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Pihlgren, M., C. Tougne, P. Bozzotti, A. Fulurija, M. A. Duchosal, P. H. Lambert, and C. A. Siegrist. 2003. Unresponsiveness to lymphoid-mediated signals at the neonatal follicular dendritic cell precursor level contributes to delayed germinal center induction and limitations of neonatal antibody responses to T-dependent antigens. J. Immunol. 170:2824-2832. [DOI] [PubMed] [Google Scholar]
- 260.Piippo-Savolainen, E., and M. Korppi. 2008. Wheezy babies—wheezy adults? Review on long-term outcome until adulthood after early childhood wheezing. Acta Paediatr. 97:5-11. [DOI] [PubMed] [Google Scholar]
- 261.Pizzichini, M. M., E. Pizzichini, A. Efthimiadis, A. J. Chauhan, S. L. Johnston, P. Hussack, J. Mahony, J. Dolovich, and F. E. Hargreave. 1998. Asthma and natural colds. Inflammatory indices in induced sputum: a feasibility study. Am. J. Respir. Crit. Care Med. 158:1178-1184. [DOI] [PubMed] [Google Scholar]
- 262.Plint, A. C., D. W. Johnson, H. Patel, N. Wiebe, R. Correll, R. Brant, C. Mitton, S. Gouin, M. Bhatt, G. Joubert, K. J. Black, T. Turner, S. Whitehouse, and T. P. Klassen. 2009. Epinephrine and dexamethasone in children with bronchiolitis. N. Engl. J. Med. 360:2079-2089. [DOI] [PubMed] [Google Scholar]
- 263.Plotkowski, M.-C., E. Puchelle, G. Beck, J. Jacquot, and C. Hannoun. 1986. Adherence of type 1 streptococcus pnuemonia to tracheal epithelium of mice infected with influenza A/PR8 virus. Am. Rev. Respir. Dis. 134:1040-1044. [DOI] [PubMed] [Google Scholar]
- 264.Poehling, K. A., H. K. Talbot, J. V. Williams, Y. Zhu, J. Lott, L. Patterson, K. M. Edwards, and M. R. Griffin. 2009. Impact of a school-based influenza immunization program on disease burden: comparison of two Tennessee counties. Vaccine 27:2695-2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Prince, G. A., R. L. Horswood, J. Berndt, S. C. Suffin, and R. M. Chanock. 1979. Respiratory syncytial virus infection in inbred mice. Infect. Immun. 26:764-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Puccetti, P. 2007. On watching the watchers: IDO and type I/II IFN. Eur. J. Immunol. 37:876-879. [DOI] [PubMed] [Google Scholar]
- 267.Puthothu, B., S. Bierbaum, M. V. Kopp, J. Forster, J. Heinze, M. Weckmann, M. Krueger, and A. Heinzmann. 2008. Association of TNF-alpha with severe respiratory syncytial virus infection and bronchial asthma. Pediatr. Allergy Immunol. 20:157-163. [DOI] [PubMed] [Google Scholar]
- 268.Puthothu, B., J. Forster, J. Heinze, A. Heinzmann, and M. Krueger. 2007. Surfactant protein B polymorphisms are associated with severe respiratory syncytial virus infection, but not with asthma. BMC Pulm. Med. 7:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Puthothu, B., J. Forster, A. Heinzmann, and M. Krueger. 2006. TLR-4 and CD14 polymorphisms in respiratory syncytial virus associated disease. Dis. Markers 22:303-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Puthothu, B., M. Krueger, J. Forster, J. Heinze, M. Weckmann, and A. Heinzmann. 2007. Interleukin (IL)-18 polymorphism 133C/G is associated with severe respiratory syncytial virus infection. Pediatr. Infect. Dis. J. 26:1094-1098. [DOI] [PubMed] [Google Scholar]
- 271.Puthothu, B., M. Krueger, J. Forster, and A. Heinzmann. 2006. Association between severe respiratory syncytial virus infection and IL13/IL4 haplotypes. J. Infect. Dis. 193:438-441. [DOI] [PubMed] [Google Scholar]
- 272.Puthothu, B., M. Krueger, J. Heinze, J. Forster, and A. Heinzmann. 2006. Haplotypes of surfactant protein C are associated with common paediatric lung diseases. Pediatr. Allergy Immunol. 17:572-577. [DOI] [PubMed] [Google Scholar]
- 273.Puthothu, B., M. Krueger, J. Heinze, J. Forster, and A. Heinzmann. 2006. Impact of IL8 and IL8-receptor alpha polymorphisms on the genetics of bronchial asthma and severe RSV infections. Clin. Mol. Allergy 4:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Ramaswamy, M., L. Shi, M. M. Monick, G. W. Hunninghake, and D. C. Look. 2004. Specific inhibition of type I interferon signal transduction by respiratory syncytial virus. Am. J. Respir. Cell Mol. Biol. 30:893-900. [DOI] [PubMed] [Google Scholar]
- 275.Ramilo, O. 2009. Evolution of prophylaxis: MoAb, siRNA, vaccine, and small molecules. Paediatr. Respir. Rev. 10(Suppl. 1):23-25. [DOI] [PubMed] [Google Scholar]
- 276.Reed, J. L., T. P. Welliver, G. P. Sims, L. McKinney, L. Velozo, L. Avendano, K. Hintz, J. Luma, A. J. Coyle, and R. C. Welliver, Sr. 2009. Innate immune signals modulate antiviral and polyreactive antibody responses during severe respiratory syncytial virus infection. J. Infect. Dis. 199:1128-1138. [DOI] [PubMed] [Google Scholar]
- 277.Regamey, N., L. Kaiser, H. L. Roiha, C. Deffernez, C. E. Kuehni, P. Latzin, C. Aebi, and U. Frey. 2008. Viral etiology of acute respiratory infections with cough in infancy: a community-based birth cohort study. Pediatr. Infect. Dis. J. 27:100-105. [DOI] [PubMed] [Google Scholar]
- 278.Ribeiro, L. Z., R. A. Tripp, L. M. Rossi, P. V. Palma, J. Yokosawa, O. C. Mantese, T. F. Oliveira, L. L. Nepomuceno, and D. A. Queiroz. 2008. Serum mannose-binding lectin levels are linked with respiratory syncytial virus (RSV) disease. J. Clin. Immunol. 28:166-173. [DOI] [PubMed] [Google Scholar]
- 279.Rihkanen, H., E. Ronkko, T. Nieminen, K. L. Komsi, R. Raty, H. Saxen, T. Ziegler, M. Roivainen, M. Soderlund-Venermo, A. L. Beng, T. Hovi, and A. Pitkaranta. 2008. Respiratory viruses in laryngeal croup of young children. J. Pediatr. 152:661-665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Rohde, G. 2009. Drug targets in rhinoviral infections. Infect. Disord. Drug Targets 9:126-132. [DOI] [PubMed] [Google Scholar]
- 281.Roth, D. E., A. B. Jones, C. Prosser, J. L. Robinson, and S. Vohra. 2008. Vitamin D receptor polymorphisms and the risk of acute lower respiratory tract infection in early childhood. J. Infect. Dis. 197:676-680. [DOI] [PubMed] [Google Scholar]
- 282.Rothberg, M. B., S. D. Haessler, and R. B. Brown. 2008. Complications of viral influenza. Am. J. Med. 121:258-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Rudd, B. D., E. Burstein, C. S. Duckett, X. Li, and N. W. Lukacs. 2005. Differential role for TLR3 in respiratory syncytial virus-induced chemokine expression. J. Virol. 79:3350-3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Rudd, B. D., J. J. Smit, R. A. Flavell, L. Alexopoulou, M. A. Schaller, A. Gruber, A. A. Berlin, and N. W. Lukacs. 2006. Deletion of TLR3 alters the pulmonary immune environment and mucus production during respiratory syncytial virus infection. J. Immunol. 176:1937-1942. [DOI] [PubMed] [Google Scholar]
- 285.Sadeghi, K., A. Berger, M. Langgartner, A. R. Prusa, M. Hayde, K. Herkner, A. Pollak, A. Spittler, and E. Forster-Waldl. 2007. Immaturity of infection control in preterm and term newborns is associated with impaired Toll-like receptor signaling. J. Infect. Dis. 195:296-302. [DOI] [PubMed] [Google Scholar]
- 286.Salomon, R., E. Hoffmann, and R. G. Webster. 2007. Inhibition of the cytokine response does not protect against lethal H5N1 influenza infection. Proc. Natl. Acad. Sci. U. S. A. 104:12479-12481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Sauro, A., F. Barone, G. Blasio, L. Russo, and L. Santillo. 2006. Do influenza and acute respiratory infective diseases weigh heavily on general practitioners' daily practice? Eur. J. Gen. Pract. 12:34-36. [DOI] [PubMed] [Google Scholar]
- 288.Scagnolari, C., F. Midulla, A. Pierangeli, C. Moretti, E. Bonci, R. Berardi, D. De Angelis, C. Selvaggi, P. Di Marco, E. Girardi, and G. Antonelli. 2009. Gene expression of nucleic acid-sensing pattern recognition receptors in children hospitalized for respiratory syncytial virus-associated acute bronchiolitis. Clin. Vaccine Immunol. 16:816-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Schanzer, D. L., J. M. Langley, and T. W. Tam. 2006. Hospitalization attributable to influenza and other viral respiratory illnesses in Canadian children. Pediatr. Infect. Dis. J. 25:795-800. [DOI] [PubMed] [Google Scholar]
- 290.Schlender, J., V. Hornung, S. Finke, M. Gunthner-Biller, S. Marozin, K. Brzozka, S. Moghim, S. Endres, G. Hartmann, and K. K. Conzelmann. 2005. Inhibition of Toll-like receptor 7- and 9-mediated alpha/beta interferon production in human plasmacytoid dendritic cells by respiratory syncytial virus and measles virus. J. Virol. 79:5507-5515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Schmitz, N., M. Kurrer, M. F. Bachmann, and M. Kopf. 2005. Interleukin-1 is responsible for acute lung immunopathology but increases survival of respiratory influenza virus infection. J. Virol. 79:6441-6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Schuh, S., A. L. Coates, P. Dick, D. Stephens, A. Lalani, E. Nicota, M. Mokanski, S. Khaikin, and U. Allen. 2008. A single versus multiple doses of dexamethasone in infants wheezing for the first time. Pediatr. Pulmonol. 43:844-850. [DOI] [PubMed] [Google Scholar]
- 293.Schwarze, J., D. R. O'Donnell, A. Rohwedder, and P. J. Openshaw. 2004. Latency and persistence of respiratory syncytial virus despite T cell immunity. Am. J. Respir. Crit. Care Med. 169:801-805. [DOI] [PubMed] [Google Scholar]
- 294.Scott, P. D., R. Ochola, M. Ngama, E. A. Okiro, D. James Nokes, G. F. Medley, and P. A. Cane. 2006. Molecular analysis of respiratory syncytial virus reinfections in infants from coastal Kenya. J. Infect. Dis. 193:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Semple, M. G., A. Cowell, W. Dove, J. Greensill, P. S. McNamara, C. Halfhide, P. Shears, R. L. Smyth, and C. A. Hart. 2005. Dual infection of infants by human metapneumovirus and human respiratory syncytial virus is strongly associated with severe bronchiolitis. J. Infect. Dis. 191:382-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Shi, L., M. Ramaswamy, L. J. Manzel, and D. C. Look. 2007. Inhibition of Jak1-dependent signal transduction in airway epithelial cells infected with adenovirus. Am. J. Respir. Cell Mol. Biol. 37:720-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Shun-Shin, M., M. Thompson, C. Heneghan, R. Perera, A. Harnden, and D. Mant. 2009. Neuraminidase inhibitors for treatment and prophylaxis of influenza in children: systematic review and meta-analysis of randomised controlled trials. BMJ 339:b3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Siegrist, C. A. 2007. The challenges of vaccine responses in early life: selected examples. J. Comp. Pathol. 137(Suppl. 1):S4-S9. [DOI] [PubMed] [Google Scholar]
- 299.Siegrist, C. A., and R. Aspinall. 2009. B-cell responses to vaccination at the extremes of age. Nat. Rev. Immunol. 9:185-194. [DOI] [PubMed] [Google Scholar]
- 300.Siezen, C. L., L. Bont, H. M. Hodemaekers, M. J. Ermers, G. Doornbos, R. Van't Slot, C. Wijmenga, H. C. van Houwelingen, J. L. Kimpen, T. G. Kimman, B. Hoebee, and R. Janssen. 2009. Genetic susceptibility to respiratory syncytial virus bronchiolitis in preterm children is associated with airway remodeling genes and innate immune genes. Pediatr. Infect. Dis. J. 28:333-335. [DOI] [PubMed] [Google Scholar]
- 301.Sigurs, N., P. M. Gustafsson, R. Bjarnason, F. Lundberg, S. Schmidt, F. Sigurbergsson, and B. Kjellman. 2005. Severe respiratory syncytial virus bronchiolitis in infancy and asthma and allergy at age 13. Am. J. Respir. Crit. Care Med. 171:137-141. [DOI] [PubMed] [Google Scholar]
- 302.Simoes, E. A. 2003. Environmental and demographic risk factors for respiratory syncytial virus lower respiratory tract disease. J. Pediatr. 143:S118-S126. [DOI] [PubMed] [Google Scholar]
- 303.Simoes, E. A., J. R. Groothuis, X. Carbonell-Estrany, C. H. Rieger, I. Mitchell, L. M. Fredrick, and J. L. Kimpen. 2007. Palivizumab prophylaxis, respiratory syncytial virus, and subsequent recurrent wheezing. J. Pediatr. 151:34-42. [DOI] [PubMed] [Google Scholar]
- 304.Sims, J. E., D. E. Williams, P. J. Morrissey, K. Garka, D. Foxworthe, V. Price, S. L. Friend, A. Farr, M. A. Bedell, N. A. Jenkins, N. G. Copeland, K. Grabstein, and R. J. Paxton. 2000. Molecular cloning and biological characterization of a novel murine lymphoid growth factor. J. Exp. Med. 192:671-680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Singh, A. M., P. E. Moore, J. E. Gern, R. F. Lemanske, Jr., and T. V. Hartert. 2007. Bronchiolitis to asthma: a review and call for studies of gene-virus interactions in asthma causation. Am. J. Respir. Crit. Care Med. 175:108-119. [DOI] [PubMed] [Google Scholar]
- 306.Smith, G. J. D., D. Vijaykrishna, J. Bahl, S. J. Lycett, M. Worobey, O. G. Pybus, S. K. Ma, C. L. Cheung, J. Raghwani, S. Bhatt, J. S. M. Peiris, Y. Guan, and A. Rambaut. 2009. Origins and evolutionary genomics of the 2009 swine-origin H1N1 influenza A epidemic. Nature 459:1122-1125. [DOI] [PubMed] [Google Scholar]
- 307.Somers, C. C., N. Ahmad, A. Mejias, S. C. Buckingham, C. Carubelli, K. Katz, N. Leos, A. M. Gomez, J. P. DeVincenzo, O. Ramilo, and H. S. Jafri. 2009. Effect of dexamethasone on respiratory syncytial virus-induced lung inflammation in children: results of a randomized, placebo controlled clinical trial. Pediatr. Allergy Immunol. 20:477-485. [DOI] [PubMed] [Google Scholar]
- 308.Soumelis, V., P. A. Reche, H. Kanzler, W. Yuan, G. Edward, B. Homey, M. Gilliet, S. Ho, S. Antonenko, A. Lauerma, K. Smith, D. Gorman, S. Zurawski, J. Abrams, S. Menon, T. McClanahan, R. R. Waal-Malefyt, F. Bazan, R. A. Kastelein, and Y. J. Liu. 2002. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat. Immunol. 3:673-680. [DOI] [PubMed] [Google Scholar]
- 309.Spann, K. M., K. C. Tran, and P. L. Collins. 2005. Effects of nonstructural proteins NS1 and NS2 of human respiratory syncytial virus on interferon regulatory factor 3, NF-kappaB, and proinflammatory cytokines. J. Virol. 79:5353-5362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Spurling, G. K., K. Fonseka, J. Doust, and C. Del Mar. 2007. Antibiotics for bronchiolitis in children. Cochrane Database Syst. Rev. 2007:CD005189. [DOI] [PubMed] [Google Scholar]
- 311.Stang, P., N. Brandenburg, and B. Carter. 2001. The economic burden of respiratory syncytial virus-associated bronchiolitis hospitalizations. Arch. Pediatr. Adolesc. Med. 155:95-96. [DOI] [PubMed] [Google Scholar]
- 312.Stark, J. M., S. A. McDowell, V. Koenigsknecht, D. R. Prows, J. E. Leikauf, V. Le, and G. D. Leikauf. 2002. Genetic susceptibility to respiratory syncytial virus infection in inbred mice. J. Med. Virol. 67:92-100. [DOI] [PubMed] [Google Scholar]
- 313.Stein, R. T., D. Sherrill, W. J. Morgan, C. J. Holberg, M. Halonen, L. M. Taussig, A. L. Wright, and F. D. Martinez. 1999. Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years. Lancet 354:541-545. [DOI] [PubMed] [Google Scholar]
- 314.Stempel, H. E., E. T. Martin, J. Kuypers, J. A. Englund, and D. M. Zerr. 2009. Multiple viral respiratory pathogens in children with bronchiolitis. Acta Paediatr. 98:123-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Stockl, J., H. Vetr, O. Majdic, G. Zlabinger, E. Kuechler, and W. Knapp. 1999. Human major group rhinoviruses downmodulate the accessory function of monocytes by inducing IL-10. J. Clin. Invest. 104:957-965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Stockman, L. J., R. Bellamy, and P. Garner. 2006. SARS: systematic review of treatment effects. PLoS Med. 3:e343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Subcommittee on Diagnosis and Management of Bronchiolitis. 2006. Diagnosis and management of bronchiolitis. Pediatrics 118:1774-1793. [DOI] [PubMed] [Google Scholar]
- 318.Sun, C. M., E. Deriaud, C. LeClerc, and R. Lo-Man. 2005. Upon TLR9 signaling, CD5+ B cells control the IL-12-dependent Th1-priming capacity of neonatal DCs. Immunity 22:467-477. [DOI] [PubMed] [Google Scholar]
- 319.Szretter, K. J., S. Gangappa, X. Lu, C. Smith, W. J. Shieh, S. R. Zaki, S. Sambhara, T. M. Tumpey, and J. M. Katz. 2007. Role of host cytokine responses in the pathogenesis of avian H5N1 influenza viruses in mice. J. Virol. 81:2736-2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Tahan, F., A. Ozcan, and N. Koc. 2007. Clarithromycin in the treatment of RSV bronchiolitis: a double-blind, randomised, placebo-controlled trial. Eur. Respir. J. 29:91-97. [DOI] [PubMed] [Google Scholar]
- 321.Takaoka, A., Z. Wang, M. K. Choi, H. Yanai, H. Negishi, T. Ban, Y. Lu, M. Miyagishi, T. Kodama, K. Honda, Y. Ohba, and T. Taniguchi. 2007. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature 448:501-505. [DOI] [PubMed] [Google Scholar]
- 322.Tal, G., A. Mandelberg, I. Dalal, K. Cesar, E. Somekh, A. Tal, A. Oron, S. Itskovich, A. Ballin, S. Houri, A. Beigelman, O. Lider, G. Rechavi, and N. Amariglio. 2004. Association between common Toll-like receptor 4 mutations and severe respiratory syncytial virus disease. J. Infect. Dis. 189:2057-2063. [DOI] [PubMed] [Google Scholar]
- 323.Talbot, H. K., J. E. Crowe, Jr., K. M. Edwards, M. R. Griffin, Y. Zhu, G. A. Weinberg, P. G. Szilagyi, C. B. Hall, A. B. Podsiad, M. Iwane, and J. V. Williams. 2009. Coronavirus infection and hospitalizations for acute respiratory illness in young children. J. Med. Virol. 81:853-856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Tang, Y. W., H. Li, H. Wu, Y. Shyr, and K. M. Edwards. 2007. Host single-nucleotide polymorphisms and altered responses to inactivated influenza vaccine. J. Infect. Dis. 196:1021-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Taylor-Robinson, A. W., F. Y. Liew, A. Severn, D. Xu, S. J. McSorley, P. Garside, J. Padron, and R. S. Phillips. 1994. Regulation of the immune response by nitric oxide differentially produced by T helper type 1 and T helper type 2 cells. Eur. J. Immunol. 24:980-984. [DOI] [PubMed] [Google Scholar]
- 326.Tekkanat, K. K., H. Maassab, A. A. Berlin, P. M. Lincoln, H. L. Evanoff, M. H. Kaplan, and N. W. Lukacs. 2001. Role of interleukin-12 and stat-4 in the regulation of airway inflammation and hyperreactivity in respiratory syncytial virus infection. Am. J. Pathol. 159:631-638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Teran, L. M., S. L. Johnston, J. M. Schroder, M. K. Church, and S. T. Holgate. 1997. Role of nasal interleukin-8 in neutrophil recruitment and activation in children with virus-induced asthma. Am. J. Respir. Crit. Care Med. 155:1362-1366. [DOI] [PubMed] [Google Scholar]
- 328.Thomas, P. G., P. Dash, J. R. Aldridge, Jr., A. H. Ellebedy, C. Reynolds, A. J. Funk, W. J. Martin, M. Lamkanfi, R. J. Webby, K. L. Boyd, P. C. Doherty, and T. D. Kanneganti. 2009. The intracellular sensor NLRP3 mediates key innate and healing responses to influenza A virus via the regulation of caspase-1. Immunity 30:566-575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Thomsen, S. F., S. V. Sluis, L. G. Stensballe, D. Posthuma, A. Skytthe, K. O. Kyvik, D. L. Duffy, V. Backer, and H. Bisgaard. 2009. Exploring the association between severe respiratory syncytial virus infection and asthma: a registry-based twin study. Am. J. Respir. Crit. Care Med. 179:1091-1097. [DOI] [PubMed] [Google Scholar]
- 330.Thorburn, K., S. Harigopal, V. Reddy, N. Taylor, and H. K. van Saene. 2006. High incidence of pulmonary bacterial co-infection in children with severe respiratory syncytial virus (RSV) bronchiolitis. Thorax 61:611-615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Tregoning, J. S., Y. Yamaguchi, J. Harker, B. Wang, and P. J. Openshaw. 2008. The role of T cells in the enhancement of RSV infection severity during adult re-infection of neonatally sensitized mice. J. Virol. 82:4115-4124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Triantafilou, K., E. Vakakis, G. Orthopoulos, M. A. Ahmed, C. Schumann, P. M. Lepper, and M. Triantafilou. 2005. TLR8 and TLR7 are involved in the host's immune response to human parechovirus 1. Eur. J. Immunol. 35:2416-2423. [DOI] [PubMed] [Google Scholar]
- 333.Uiprasertkul, M., P. Puthavathana, K. Sangsiriwut, P. Pooruk, K. Srisook, M. Peiris, J. M. Nicholls, K. Chokephaibulkit, N. Vanprapar, and P. Auewarakul. 2005. Influenza A H5N1 replication sites in humans. Emerg. Infect. Dis. 11:1036-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Valarcher, J. F., H. Bourhy, A. Lavenu, N. Bourges-Abella, M. Roth, O. Andreoletti, P. Ave, and F. Schelcher. 2001. Persistent infection of B lymphocytes by bovine respiratory syncytial virus. Virology 291:55-67. [DOI] [PubMed] [Google Scholar]
- 335.van Benten, I., L. Koopman, B. Niesters, W. Hop, B. van Middelkoop, L. de Waal, K. van Drunen, A. Osterhaus, H. Neijens, and W. Fokkens. 2003. Predominance of rhinovirus in the nose of symptomatic and asymptomatic infants. Pediatr. Allergy Immunol. 14:363-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Vanden Eijnden, S., S. Goriely, D. De Wit, M. Goldman, and F. Willems. 2006. Preferential production of the IL-12(p40)/IL-23(p19) heterodimer by dendritic cells from human newborns. Eur. J. Immunol. 36:21-26. [DOI] [PubMed] [Google Scholar]
- 337.Van Den Hoogen, B. G., J. C. De Jong, J. Groen, T. Kuiken, R. De Groot, R. A. Fouchier, and A. D. Osterhaus. 2001. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat. Med. 7:719-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.van der Sluijs, K. F., L. J. R. van Elden, M. Nijhuis, R. Schuurman, S. Florquin, T. Shimizu, S. Ishii, H. M. Jansen, R. Lutter, and T. van der Poll. 2006. Involvement of the platelet-activating factor receptor in host defense against Streptococcus pneumoniae during postinfluenza pneumonia. Am. J. Physiol. Lung Cell. Mol. Physiol. 290:L194-L199. [DOI] [PubMed] [Google Scholar]
- 339.van der Sluijs, K. F., L. J. van Elden, M. Nijhuis, R. Schuurman, J. M. Pater, S. Florquin, M. Goldman, H. M. Jansen, R. Lutter, and T. van der Poll. 2004. IL-10 is an important mediator of the enhanced susceptibility to pneumococcal pneumonia after influenza infection. J. Immunol. 172:7603-7609. [DOI] [PubMed] [Google Scholar]
- 340.Ventre, K., M. Haroon, and C. Davison. 2006. Surfactant therapy for bronchiolitis in critically ill infants. Cochrane Database Syst. Rev. 2006:CD005150. [DOI] [PubMed] [Google Scholar]
- 341.Ventre, K., and A. G. Randolph. 2007. Ribavirin for respiratory syncytial virus infection of the lower respiratory tract in infants and young children. Cochrane Database Syst. Rev. 2007:CD000181. [DOI] [PubMed] [Google Scholar]
- 342.Viemann, D., G. Dubbel, S. Schleifenbaum, E. Harms, C. Sorg, and J. Roth. 2005. Expression of Toll-like receptors in neonatal sepsis. Pediatr. Res. 58:654-659. [DOI] [PubMed] [Google Scholar]
- 343.Vigerust, D. J., and V. L. Shepherd. 2007. Virus glycosylation: role in virulence and immune interactions. Trends Microbiol. 15:211-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Wang, H., Z. Su, and J. Schwarze. 2008. Healthy but not RSV-infected lung epithelial cells profoundly inhibit T-cell activation. Thorax 64:283-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Wang, S., M. Wei, Y. Han, K. Zhang, L. He, Z. Yang, B. Su, Z. Zhang, Y. Hu, and W. Hui. 2008. Roles of TNF-alpha gene polymorphisms in the occurrence and progress of SARS-Cov infection: a case-control study. BMC Infect. Dis. 8:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Wang, Y., J. Yan, Y. Shi, P. Li, C. Liu, Q. Ma, R. Yang, X. Wang, L. Zhu, X. Yang, and C. Cao. 2009. Lack of association between polymorphisms of MASP2 and susceptibility to SARS coronavirus infection. BMC Infect. Dis. 9:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Wark, P. A., S. L. Johnston, F. Bucchieri, R. Powell, S. Puddicombe, V. Laza-Stanca, S. T. Holgate, and D. E. Davies. 2005. Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J. Exp. Med. 201:937-947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Weigl, J. A., W. Puppe, C. U. Meyer, R. Berner, J. Forster, H. J. Schmitt, and F. Zepp. 2007. Ten years' experience with year-round active surveillance of up to 19 respiratory pathogens in children. Eur. J. Pediatr. 166:957-966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Weinberg, G. A., C. B. Hall, M. K. Iwane, K. A. Poehling, K. M. Edwards, M. R. Griffin, M. A. Staat, A. T. Curns, D. D. Erdman, and P. G. Szilagyi. 2009. Parainfluenza virus infection of young children: estimates of the population-based burden of hospitalization. J. Pediatr. 154:694-699. [DOI] [PubMed] [Google Scholar]
- 350.Welliver, T. P., R. P. Garofalo, Y. Hosakote, K. H. Hintz, L. Avendano, K. Sanchez, L. Velozo, H. Jafri, S. Chavez-Bueno, P. L. Ogra, L. McKinney, J. L. Reed, and R. C. Welliver, Sr. 2007. Severe human lower respiratory tract illness caused by respiratory syncytial virus and influenza virus is characterized by the absence of pulmonary cytotoxic lymphocyte responses. J. Infect. Dis. 195:1126-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Wolf, D. G., D. Greenberg, D. Kalkstein, Y. Shemer-Avni, N. Givon-Lavi, N. Saleh, M. D. Goldberg, and R. Dagan. 2006. Comparison of human metapneumovirus, respiratory syncytial virus and influenza A virus lower respiratory tract infections in hospitalized young children. Pediatr. Infect. Dis. J. 25:320-324. [DOI] [PubMed] [Google Scholar]
- 352.World Health Organization. 2005. Burden of Disease Project. World Health Organization, Geneva, Switzerland.
- 353.Wright, P. F., A. M. Deatly, R. A. Karron, R. B. Belshe, J. R. Shi, W. C. Gruber, Y. Zhu, and V. B. Randolph. 2007. Comparison of results of detection of rhinovirus by PCR and viral culture in human nasal wash specimens from subjects with and without clinical symptoms of respiratory illness. J. Clin. Microbiol. 45:2126-2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Yamaguchi, T., K. Kawabata, N. Koizumi, F. Sakurai, K. Nakashima, H. Sakurai, T. Sasaki, N. Okada, K. Yamanishi, and H. Mizuguchi. 2007. Role of MyD88 and TLR9 in the innate immune response elicited by serotype 5 adenoviral vectors. Hum. Gene Ther. 18:753-762. [DOI] [PubMed] [Google Scholar]
- 355.Ying, S., B. O'Connor, J. Ratoff, Q. Meng, K. Mallett, D. Cousins, D. Robinson, G. Zhang, J. Zhao, T. H. Lee, and C. Corrigan. 2005. Thymic stromal lymphopoietin expression is increased in asthmatic airways and correlates with expression of Th2-attracting chemokines and disease severity. J. Immunol. 174:8183-8190. [DOI] [PubMed] [Google Scholar]
- 356.Yuan, F. F., I. Boehm, P. K. Chan, K. Marks, J. W. Tang, D. S. Hui, J. J. Sung, W. B. Dyer, A. F. Geczy, and J. S. Sullivan. 2007. High prevalence of the CD14-159CC genotype in patients infected with severe acute respiratory syndrome-associated coronavirus. Clin. Vaccine Immunol. 14:1644-1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Yuan, F. F., J. Tanner, P. K. Chan, S. Biffin, W. B. Dyer, A. F. Geczy, J. W. Tang, D. S. Hui, J. J. Sung, and J. S. Sullivan. 2005. Influence of FcgammaRIIA and MBL polymorphisms on severe acute respiratory syndrome. Tissue Antigens 66:291-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Zaman, K., E. Roy, S. E. Arifeen, M. Rahman, R. Raqib, E. Wilson, S. B. Omer, N. S. Shahid, R. F. Breiman, and M. C. Steinhoff. 2008. Effectiveness of maternal influenza immunization in mothers and infants. N. Engl. J. Med. 359:1555-1564. [DOI] [PubMed] [Google Scholar]
- 359.Zammit, D. J., L. S. Cauley, Q. M. Pham, and L. Lefrancois. 2005. Dendritic cells maximize the memory CD8 T cell response to infection. Immunity 22:561-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Zhang, H., G. Zhou, L. Zhi, H. Yang, Y. Zhai, X. Dong, X. Zhang, X. Gao, Y. Zhu, and F. He. 2005. Association between mannose-binding lectin gene polymorphisms and susceptibility to severe acute respiratory syndrome coronavirus infection. J. Infect. Dis. 192:1355-1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Zhang, L., R. A. Mendoza-Sassi, C. Wainwright, and T. P. Klassen. 2008. Nebulized hypertonic saline solution for acute bronchiolitis in infants. Cochrane Database Syst. Rev. 2008:CD006458. [DOI] [PubMed] [Google Scholar]
- 362.Zhang, L., M. E. Peeples, R. C. Boucher, P. L. Collins, and R. J. Pickles. 2002. Respiratory syncytial virus infection of human airway epithelial cells is polarized, specific to ciliated cells, and without obvious cytopathology. J. Virol. 76:5654-5666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Zhang, X., E. Deriaud, X. Jiao, D. Braun, C. LeClerc, and R. Lo-Man. 2007. Type I interferons protect neonates from acute inflammation through interleukin 10-producing B cells. J. Exp. Med. 204:1107-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Zhao, X., E. Liu, F. P. Chen, and W. M. Sullender. 2006. In vitro and in vivo fitness of respiratory syncytial virus monoclonal antibody escape mutants. J. Virol. 80:11651-11657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Zhou, B., M. R. Comeau, T. De Smedt, H. D. Liggitt, M. E. Dahl, D. B. Lewis, D. Gyarmati, T. Aye, D. J. Campbell, and S. F. Ziegler. 2005. Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nat. Immunol. 6:1047-1053. [DOI] [PubMed] [Google Scholar]