Abstract
Summary: The coccidian parasite Cyclospora cayetanensis is recognized as an emerging pathogen that causes protracted diarrhea in humans. The first cases of Cyclospora infection were reported in the late 1970s and were observed among expatriates and travelers in regions where infections are endemic. Since then, Cyclospora has been considered a cause of traveler's diarrhea. Epidemiological investigations were reported and examined in areas of endemicity even before the true identity of Cyclospora was elucidated. Cyclospora was fully characterized in the early 1990s, but it was not until the 1995 Cyclospora outbreak in the United States and Canada that it caught the attention of the public and physicians. The biology, clinical presentation, epidemiology, diagnosis, treatment, and control of cyclosporiasis are reviewed, with a focus on diagnostic assays currently being used for clinical and environmental samples. Challenges and limitations in working with Cyclospora are also discussed.
INTRODUCTION
The risk of exposure to exotic and uncommon tropical diseases has increased in parallel with the globalization of the food supply, increased consumption of fresh foods, and increased travel. The rapid transport of fresh fruits and produce from developing countries has increased the chance that endemic parasites from other regions may come into contact with consumers from industrialized nations. Changes in nutritional habits have resulted in increased consumption of undercooked or raw foods, thus potentially exposing consumers to parasites that proper food processing would otherwise reduce or eliminate (135). As international travel becomes more frequent, so does the risk of acquiring microbes in industrialized nations where they are not endemic, as is the case for Cyclospora.
Enforcement of international food trade and implementation of methods using hazard analysis and critical control points (HACCP) may play a relevant role in the control of food-borne illnesses (20). As we review the biology, epidemiology, treatment, and control of Cyclospora, we demonstrate some of the challenges and discuss limitations associated with preventing or controlling food-borne pathogens.
BIOLOGICAL CHARACTERISTICS
History of Discovery
Some of the initial cases of Cyclospora infection were noted in the 1980s, when the AIDS epidemic emerged and Cryptosporidium was identified as one of the most important opportunistic infections among AIDS patient populations. Because of the increased use of acid-fast stains, Cyclospora oocysts were also observed. These oocysts were initially misdiagnosed as Cryptosporidium or assumed to be an artifact.
The first published report of Cyclospora infection in humans can probably be dated to 1979. Ashford (6) described coccidian organisms causing diarrhea in two children and a woman in Papua New Guinea and concluded that they could be a coccidian of the genus Isospora. In 1986, Soave et al. (176) described four travelers returning from Mexico and Haiti with flu-like illness and suggested that the causative agent was a new enteric pathogen. In subsequent reports, Cyclospora was described as a coccidian-like body (CLB), cyanobacterium-like body, blue-green alga, or large Cryptosporidium. In 1989, Naranjo and collaborators described 53 cases of CLB infection in Peru and concluded that CLB was an unidentified flagellate (129). In 1989, Hart et al. examined an AIDS patient in Chicago with chronic diarrhea and with no history of recent travel, and they found similar structures in the stool samples (80). Then, in the early 1990s, Long and collaborators (72, 109, 171) described blue-green algae as the causative agent of severe diarrhea in eight international travelers who had been to Mexico, South America, India, or Southeast Asia and also in expatriates living in Nepal. The spherical bodies observed did not have organelles with membranes, but the authors described lamellar structures similar to chloroplasts.
In 1991 and 1992, Ortega and collaborators (142) characterized this controversial organism as a new coccidian species capable of infecting humans and belonging to the genus Cyclospora. In reports published in 1993 (142) and 1994 (138), the name Cyclospora cayetanensis was proposed. The etymology of the nomen triviale was derived from Peruvian University Cayetano Heredia, Ortega's alma mater and the research base for the field studies that collected the Cyclospora-positive specimens (138). In 1995, molecular phylogenetic analysis of the small-subunit (SSU) rRNA gene suggested that Cyclospora was a parasite closely related to the Eimeria genus (157). Since then, more than 400 scientific articles have been published describing biological and molecular characteristics, epidemiology, therapy, and measures for control of Cyclospora.
Taxonomy
Cyclospora measures 8.6 μm (7.7 to 9.9 μm) in diameter. When this parasite is excreted in stools, it is an undifferentiated sphere containing a morula. The oocyst has a bilayered wall. A polar body and oocyst residuum are also present. It takes more than 1 week for the oocysts to sporulate. When sporulated, the oocyst has two sporocysts (4 by 6.3 μm) and stieda and substieda bodies. Each sporocyst contains two sporozoites (1.2 by 9 μm) that are folded in two (138).
Cyclospora belongs to the subphylum Apicomplexa, subclass Coccidiasina, and family Eimeriidae. Thirteen Cyclospora species have been described for vipers, moles, myriapodes, and rodents, including Cyclospora viperae, C. glomericola, C. babaulti, C. tropidonoti, C. anglomurinensis, C. caryolytica, C. talpae, C. ashtabulensis, C. megacephali, C. parascalopi, C. niniae, C. scinci, and C. zamenis (138, 169).
In 1995, Cyclospora-like oocysts were described for baboons and chimpanzees. The sporulation times were similar for oocysts of these Cyclospora spp. and those of C. cayetanensis (174). In 1999, phylogenetic analysis using the 18S rRNA gene demonstrated that the Cyclospora species from baboons was a different species, closely related to C. cayetanensis, and that these two Cyclospora species constituted a coherent clade within the Eimeria species (113). That same year, on the basis of SSU rRNA sequence analysis, Eberhard et al. (61) characterized and described three new species of Cyclospora in nonhuman primates: C. cercopitheci in green monkeys, C. colobi in colobus monkeys, and C. papionis in baboons. These species cannot be differentiated by light microscopy, as they are morphologically similar. Moreover, these four species of Cyclospora seem to be host specific.
Cyclospora-like structures have also been reported to infect cattle in China. Molecular analysis of an extended region of the SSU rRNA sequence described by Li et al. (106) suggested that these structures were a different species of Cyclospora. Of 168 specimens collected from dairy farms, 6 (4.7%) were positive for these Cyclospora-like organisms (193). Further studies are needed to conclusively determine the identity of this Cyclospora-like organism.
Life Cycle
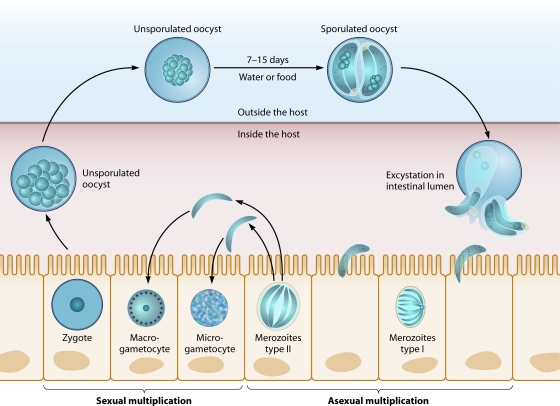
Individuals with Cyclospora infection excrete unsporulated oocysts in their feces (Fig. 1). These oocysts require 7 to 15 days to sporulate under ideal conditions (23 to 27°C) and presumably become infectious to a susceptible host. When food or water contaminated with infectious oocysts is ingested by a susceptible host, the oocysts excyst and sporozoites are released to infect epithelial cells of the duodenum and jejunum. Asexual multiplication results in type I and II meronts. The latter differentiate into sexual stages or gametocytes. The macrogametocyte is fertilized by the microgametocyte and produces a zygote. Oocysts are then formed and excreted into the environment as unsporulated oocysts. The extended period for oocysts to sporulate and become infectious raises questions as to where and how sporulation occurs. Unsuccessful attempts to infect animals or cells with sporulated oocysts suggest the need for a specific, unknown trigger to initiate infection (63).
FIG. 1.
Life cycle of Cyclospora cayetanensis. Unsporulated oocysts differentiate into sporulated oocysts, which undergo the excystation process. Sporozoites infect cells to form type I merozoites, and these form type II merozoites. The sexual-stage microgametocyte fertilizes the macrogametocyte to become a zygote and thus to differentiate as an unsporulated oocyst.
Thus far, C. cayetanensis infections have been identified only in humans. The role of other animal species as reservoirs or intermediary hosts has been examined, with conflicting results. Cyclospora oocysts have been found in feces of chickens (37, 62, 70), ducks (62, 197), and dogs (27, 37, 62, 194) collected in countries where Cyclospora is endemic (27, 62, 63). However, attempts to experimentally infect a variety of animals (63) and humans (5) have been unsuccessful. The presence of Cyclospora in stools from animals could be explained by eating or coprophagic habits of the surveyed animals. Nonetheless, it cannot yet be ruled out that animals may play a role in the dissemination of Cyclospora by contaminating water and food products. Two reports indicate that Cyclospora can be propagated in guinea pigs (191) and albino mice (162). These studies could not be reproduced, and further analyses are needed to determine the susceptibility of these animals to infection by Cyclospora and their suitability as hosts or reservoirs of C. cayetanensis.
Insects, rotifers, and free-living nematodes could also play a role in the dissemination of oocysts, a phenomenon that has been described for Cryptosporidium (67, 74, 75, 90, 179) and other food-borne pathogens. The nematode Caenorhabditis elegans can ingest and excrete infectious bacteria (23, 98, 99) as well as Cryptosporidium parvum oocysts. Although the oocysts of Cyclospora are larger and may not be ingested by C. elegans, other, larger species of free-living nematodes could have a significant role in Cyclospora oocyst dissemination. Therefore, the role of free-living nematodes and other mechanical vectors in the contamination of produce and other food crops needs to be examined further (90).
Cyclospora oocysts have been detected in nongastrointestinal samples. There are two reports of oocysts in the sputa of HIV patients with a history of pulmonary tuberculosis (55, 93), suggesting that Cyclospora could be considered an opportunistic pathogen. Travel to rural areas and ingestion of contaminated foods could be modes of infection. Accidental inhalation of oocysts has also been suggested (93).
CLINICAL PRESENTATION
Cyclospora infection is characterized by anorexia, nausea, flatulence, fatigue, abdominal cramping, diarrhea, low-grade fever, and weight loss (42, 68, 83, 140, 171). The clinical presentation is somewhat different in areas of endemicity, where asymptomatic infections are more frequent. Nevertheless, younger children have more severe clinical symptoms. In endemic settings, infections tend to be milder as children get older, as the duration of the infection is shorter and the severity of disease decreases. As in young children, the elderly may also present with a more severe illness (8, 11, 42).
In areas where Cyclospora is not endemic, infections are almost invariably symptomatic, and there are reports of severe clinical manifestations. An HIV-positive patient returning from a trip to Southeast Asia presented with excessive watery diarrhea and pronounced fatigue (127). There are infrequent reports of fatalities associated with Cyclospora infections. A 43-year-old otherwise healthy patient infected with Cyclospora had concomitant diarrhea and fever (21). During the course of the febrile illness, the patient suffered cardiac arrest. It was suggested that the cause could be a possible complication after febrile illness including Cyclospora infection that could potentially lead to fatal ventricular dysrhythmia.
Symptoms associated with cyclosporiasis are more severe in HIV/AIDS patients. Moderate weight losses (∼3.5 kg) were reported for non-AIDS patients (170, 171), whereas losses (∼7.2 kg) were more severe in AIDS patients (171). The median incubation period is about 7 days (68, 83); however, the average duration of diarrhea for HIV-positive patients is longer than that for HIV-negative patients (199 days and 57.2 days, respectively) (163, 172).
The time at which untreated expatriates in Nepal presented with diarrhea was at 19 to 57 days (171). Diarrhea lasted more than 3 weeks in people who contracted Cyclospora infection at a wedding in the United States (68). The reported frequency of bowel movements in immunocompetent people with diarrhea is 5 to 15 times a day. In addition to the explosive loss of fluids, d-xylose malabsorption has also been reported (44).
Biliary disease has also been reported after Cyclospora infections (50, 172). Acalculous cholecystitis was reported for HIV-positive and AIDS patients (172, 196) and resolved after initiation of treatment. These patients presented with right upper quadrant abdominal pain and elevated alkaline phosphatase levels (171).
Coinfection with Cyclospora, Cryptosporidium, and other parasites has been described for immunocompetent and immunocompromised individuals (9). Guillain-Barré syndrome (GBS) (158) and Reiter syndrome (41) have also been reported following Cyclospora infection. In the first case, 18 h after admission the patient was quadriparetic, areflexic, and mechanically ventilated. Circumstantial evidence suggested a Cyclospora-induced immune response resulting in severe GBS (158). In the second case, the patient had cyclosporiasis and was sulfa allergic and thus could not be treated with trimethoprim-sulfamethoxazole (TMP-SMX). Later, this patient developed ocular inflammation, inflammatory oligoarthritis, and sterile urethritis. Although Reiter syndrome could have been coincidental, the authors proposed Cyclospora as another infectious trigger for Reiter syndrome (41).
Histopathology
Cyclospora infects the small intestine, particularly the jejunum. Nine patients with gastrointestinal illness caused by CLBs (now called Cyclospora) in Nepal were examined endoscopically. They had histological evidence of small bowel injury. Five had moderate to marked erythema of the distal duodenum. Oocysts were observed in the duodenal aspirates. All patients had mild to moderate acute inflammation of the lamina propria, and neutrophils were observed in 5 of 9 cases. Additionally, diffuse chronic inflammation of mild to moderate degree was present in all samples. An increase of plasma cells in the lamina propria was also observed (44). Histopathological alterations of the epithelial tissue included focal vacuolization at the tips of the villi of the surface epithelium, loss of the brush border, and alteration of cells from a columnar to cuboid shape. The architecture of the tissues showed mild to moderately severe partial villous atrophy and crypt hyperplasia, characterized by shortened blunted villi and increased crypt length and mitosis. Interestingly, no parasitic vacuoles were observed in any of the biopsy sections examined (44). In 1996, Deluol et al. (52) described supranuclear intracytoplasmic vacuoles with 6 to 8 comma-shaped structures (merozoites) in a patient with Cyclospora infection (Fig. 1). The same year, Sun et al. (178) described the presence of multiple parasitic vacuoles containing asexual-stage Cyclospora organisms in intestinal biopsies of an HIV-positive patient who had just returned from the Dominican Republic. This patient had a low CD4+ cell count (224 cells/μl) and presented with chronic inflammation of the duodenum and stomach. Organisms were present in the supranuclear location of the mucosal villi and absent in the crypts. Histological examination of biopsies of the stomach, rectum, and transverse and sigmoid colon did not reveal any organisms. In 1997, Ortega et al. (140) demonstrated the presence of sexual-stage coccidian organisms in 17 patients with Cyclospora infection. The presence of sexual and asexual stages in the same host is suggestive that the life cycle can be completed within one host. Two types of meronts, the asexual stages, were observed. Type I had 8 to 12 merozoites, each measuring about 0.5 by 3 to 4 μm. Type II meronts had 4 merozoites, measuring 0.7 to 0.8 by 12 to 15 μm. Biopsies of these patients showed diffuse edema and infiltration of the villous mucosa by mixed inflammatory cells. Plasma cells and lymphocytes were notoriously present, and eosinophils were also numerous in 4 of 17 cases.
In 1999, Connor et al. (42) described additional lesions in three patients with cyclosporiasis. They reported an accumulation of myelin-like material (MLM) between the base and sides of the enterocytes. In one of the cases, these dense MLM appeared later in the infection, suggesting an ongoing inflammatory or immunologic process.
EPIDEMIOLOGY
Most of the information concerning the epidemiology of Cyclospora is from travelers (Table 1) and inhabitants of areas where this protozoan is endemic, such as Haiti, Guatemala, Peru, and Nepal (Table 2). Interestingly, some of these reports precede the true identification of C. cayetanensis.
TABLE 1.
Case reports of Cyclospora infection in returning travelers
| Country of origin | Visited country or countries (no. of cases) | Reference |
|---|---|---|
| Australia | Indonesia (1) | 28 |
| Australia (Queensland) | Variousd | 121 |
| Indonesia (1) | 175 | |
| Belgium | Indonesia (2) | 110 |
| Canada | Indonesia (1) | 154 |
| Chile | Cuba (1) | 116 |
| France | Variousa | 24 |
| Variousb | 51 | |
| Indonesia (1) | 148 | |
| Nepal (2), Pakistan (1) | 188 | |
| Germany | Singapore/Java/Bali (2) | 147 |
| Greece | Morocco (1) | 97 |
| Ireland | Nepal (2), Pakistan (1) | 46 |
| Italy | Nepal (1), Indonesia (1) | 32 |
| Bolivia/Peru (1) | 59 | |
| The Netherlands | Variousc | 159 |
| Indonesia (4) | 187 | |
| New Zealand | Vietnam (1) | 131 |
| Spain | Variouse | 71 |
| Guatemala (7) | 153 | |
| Sweden | Variousf | 104 |
| Turkey | Greek Islands (1) | 183 |
| United Kingdom | Variousg | 16 |
| Dominican Republic (2) | 77 | |
| Varioush | 149 | |
| United States | Mexico (4), Thailand (1) | 18 |
| Haiti (1) | 43 | |
| Puerto Rico (1), Mexico (2) | 192 |
Turkey (2), India (2), Indonesia (1), and Madagascar (1).
Vietnam (2), India (3), Bali (2), Java (1), Pakistan (2), and Dominican Republic (3).
Sri Lanka (2), Thailand (1), Pakistan (2), Peru/Bolivia (1), Indonesia (1), India (1), and Southeast China (1).
Papua New Guinea (1), Australia (1), and Bali (3).
Dominican Republic (4), Mexico (2), Guatemala (1), India (4), Malaysia (1), Indonesia (5), Sri Lanka (1), Turkey (1), and Gabon (1).
Vietnam (1), Turkey (1), Lebanon (1), Colombia (1), and Eastern Europe (1).
Indonesia (11), Nepal (8), Solomon Islands (1), United Kingdom (3), Cambodia (2), Thailand (2), Far East (1), Southeast Asia (2), India (9), Southeast Asia/Africa (1), Morocco (1), Nigeria (1), Tanzania (1), Turkey (5), Mexico/Cuba (1), Bangladesh (4), India/Nepal/Thailand (2), Mexico/China (2), Bulgaria (1), Indonesia/Hong Kong (1), and Nepal/Hong Kong (1).
Indian Subcontinent (6), Cambodia (1), Mexico (1), Solomon Islands (1), and Australia (1).
TABLE 2.
Case reports of individuals with Cyclospora infection and without a reported travel history
| Country (city/state) of origin | No. of casesa | Reference |
|---|---|---|
| Argentina | 2 HIV+ | 185 |
| Argentina (Buenos Aires) | 1 HIV+ | 55 |
| Bangladesh (Dhaka) | 6 | 10 |
| Brazil (Rio de Janeiro) | 1 HIV+ | 167 |
| Cuba | 2 HIV+ | 164 |
| Guatemala (Guatemala City) | 4 with AIDS | 152 |
| Italy (Rome) | 1 | 120 |
| Italy | 2 with AIDS | 117 |
| Italy (Apulia) | 1 HIV+ | 25 |
| Malaysia | 4 HIV+ | 173 |
| Mexico (DF; Guerrero) | 2 | 150 |
| Papua New Guinea | 3 | 12 |
| South Africa | 3 | 119 |
| Turkey (Kayseri) | 6 HIV+ or HIV− | 195 |
| Turkey (Ankara) | 5 | 163 |
| United States (Chicago, IL) | 4 | 192 |
| United States (Massachusetts) | 3 | 133 |
HIV+, HIV positive; HIV−, HIV negative.
In Haiti, 34% of patients seeking attention for chronic diarrhea at the Gheskio AIDS Clinic in Port-au-Prince had Cyclospora infection; 60% had Cryptosporidium infection, and 15% had Isospora infection (156). In a cohort study of HIV-positive adults who had diarrhea for at least 3 weeks, 11% (51/450 patients) had Cyclospora infection (144). In a series of epidemiological investigations in a Haitian community, Cyclospora prevalence was found to be higher in February, at 20 of 167 individuals (12%), than in April, at 4 of 352 individuals (1.1%). The prevalence rate for children under 10 years of age was 22.5% in February, 3% in April, and 2.5% in January. In this study, exposure to water was the only variable significantly associated with infection (111).
Epidemiological studies were conducted in Guatemala (13) to assess risk factors for cyclosporiasis (14). The study included a 1-year outpatient surveillance in two health centers; raspberry farm cohorts at three farms, two of which were implicated in the 1996 outbreak in the United States; and a case-control study where the majority of participants were from health care facilities. A total of 5,552 specimens were screened for Cyclospora. Children were five times more likely to show cyclosporiasis than adults, and AIDS patients had significantly higher rates of infection (relative risk [RR], 87; 95% confidence interval [CI], 16.2 to 847). The overall prevalence of Cyclospora was 2.3%, and infection was more common in the warmer months, coinciding with the spring raspberry harvest. The Cyclospora detection rate occurred predominantly between May and August, with the June rate being the highest (6.7%). In addition, high levels of fecal contamination were observed in the rivers from May to July. Estimates of 15,000 or more oocysts per 10 liters were reported. The prevalence of Cyclospora was 3.5% in children aged 1.5 to 4 years (RR, 5.1; 95% CI, 2.8 to 9.1) and 3.8% in children aged 5 to 9 years (RR, 5.5; 95% CI, 3 to 10.1), according to surveillance studies (14). Similar results were obtained from the case-control studies, where the highest prevalence was in children aged 1.5 to 9 years (11.6%).
In another study in Guatemala, fecal samples were collected for 1 year (April 1999 to April 2000) and examined for the presence of Cyclospora (151). Of these samples, 43.5% were from raspberry farm workers, 23.4% were from malnourished children, and 33.1% were from HIV or AIDS patients. One of 111 malnourished children and 6 of 157 HIV/AIDS outpatients had Cyclospora infection. No cases were observed in farm workers. Differences between farm workers in the two Guatemalan studies may have been associated with age. Participants in a previous study (12) had a median age of 16 years, with a range of 9 to 73 years, whereas in the latter study the median age was 29 years, with a range of 15 to 61 years (13).
In Lima, Peru, two prospective cohort studies were done during 1988 to 1991 (142). The first study, focused on children from 1 to 2 1/2 years of age, revealed that the prevalence of Cyclospora was 18% (26/147 children). The average age at infection was 23.3 months, and 28% of the infected children had diarrhea. The second study evaluated children from 1 month to 1 1/2 years of age. The prevalence of Cyclospora was 6% (15/230 children), with an average age at infection of 15.4 months, and 11% of infected children had diarrhea. For comparison, the Cryptosporidium prevalence was 70% in the first study group and 42% in the second group. Differences in prevalence rates for Cyclospora in both studies reflect the age at which children were exposed to Cyclospora, most likely from foods (142).
A cross-sectional study was conducted in Lima from 1992 to 1994 (115). It enrolled children older than 2 years of age and demonstrated that the highest prevalence (2%) was among children aged 2 to 4 years. The prevalence peaked during the summer months, when 3 to 4% of children were infected, and decreased during the winter months. All adults (older than 18 years of age) examined in the area were negative for Cyclospora.
In another longitudinal study involving Peruvian children aged 1 to 10 years, conducted from February 1995 to December 1998, the number of infections per child-year was highest among participants aged 1 to 9 years (0.21 to 0.28 episode/child-year). Of the children with Cyclospora infection, 33% had at least one detected episode of infection, 30% had two infections, and 10% had more than three infections. After an initial episode of cyclosporiasis, diarrhea decreased significantly in the following infections (15).
An outbreak of Cyclospora infection was reported for adults in November 2004 in Peru. Among subjects living at a naval recruit training base, 127 of 274 (46.3%) individuals presented with diarrhea (27 cases were laboratory confirmed) (180). All ate their meals at the base dining hall. Analysis of the epidemiological curve suggested a common point source, with an incubation period of 2 to 6 days.
Cyclospora has also been reported for children living in marginal urban districts of Trujillo, Peru (145). The prevalence of Cyclospora in these children (1 to 9 years old) was 13% (45).
Nepal is another area where Cyclospora is endemic and where several studies have been performed to better understand the epidemiology of the parasite. Between 1989 and June 1991, 964 samples were obtained from travelers and expatriates (89). Cyclospora was identified in 108 (11%) people with gastrointestinal symptoms. The prevalence of Cyclospora in Nepalese children aged 6 to 60 months who also had diarrhea was 5%, while only 2% of asymptomatic children had cyclosporiasis (87).
In Turkey, Cyclospora was identified in 1.91% of fecal samples examined in a hospital (49). In Izmir and vicinity, Western Turkey, 23 of 4,986 immunocompetent patients were positive for Cyclospora. Of the positive patients, 23% were under 9 years old. The incidence was higher in summer and early autumn (July to November), when the ambient temperature was 20 to 25°C and the mean rainfall was less than 6 mm (hot and dry) (182). In another study, examining 3,180 hospital patients in the Dakahlia governorate, 3% had Cryptosporidium and 4.2% had Cyclospora infection.
Forty-nine specimens from immunocompromised patients (Hodgkin's lymphoma and acute lymphoplastic leukemia patients) with severe diarrhea were analyzed. Of those patients, 24.5% of patients contained Cyclospora and 14.3% contained mixed infections with Cryptosporidium (81).
In Ismalia, Egypt, Cyclospora was identified in the sputum of an HIV-positive patient with active pulmonary tuberculosis with purulent sputum and dyspnea (93). In the Egyptian Sharkia governorate, 340 samples were collected (65). Cyclospora was identified in 5.6% of individuals with diarrhea and 2.3% of individuals without diarrhea.
Cyclospora infections have also been described in Venezuela (34-36), Brazil (3, 73), Colombia (17), Cuba (53, 124, 130), and Argentina (185) (Table 2).
Susceptible Populations
There are defined patterns of susceptibility. In industrialized nations, most people are susceptible to infections. The susceptible populations in areas of endemicity, in contrast, are restricted to the very young and the very old. In developed nations, cyclosporiasis has been observed in tourists (0.6%) or expatriates visiting countries where the disease is endemic (Table 1), in the immunocompromised, and in immunocompetent hosts with no foreign travel history. C. cayetanensis was detected in 9.1 to 13.6% of clinical cases among expatriates living in a developing country; most of these cases were adults (69). Infections were clustered during the wet season, and feces contained 100 to 327,600 Cyclospora oocysts per gram. Lower rates of C. cayetanensis infection were observed in school children, with 0.6% of asymptomatic cases showing infection, at an incidence of two infections per 19.3 person-years.
In areas of endemicity, young children are more likely to develop clinical symptoms. The severity of symptoms and duration of infection tend to be milder after repeated infections, which could be suggestive of acquired immunity. Older children and adults may either be resistant to infections or have asymptomatic infections.
Reservoirs
The transmission of Cyclospora through domestic animals was suggested in early epidemiological surveys and studies conducted in areas of endemicity. A few reports have described the presence of Cyclospora oocysts in the feces of chickens (70, 168), ducks (197), and dogs (194). Other studies surveying feces of cattle, horses, pigs, goats, dogs, cats, guinea pigs, turkeys, chickens, ducks, and pigeons did not find Cyclospora in these animal species (27, 62, 141). Attempts to infect different animals (chickens, ducks, mice, gerbils, hamsters, rabbits, rats, sand rats, ferrets, pigs, dogs, monkeys, and baboons) with C. cayetanensis have been unsuccessful, suggesting host specificity (63). More recently, Chu et al. (37) identified C. cayetanensis in domestic animals by PCR, but whether these findings represent true or spurious infections remains to be clarified. Other studies described the presence of Cyclospora-like oocysts in zoo animals (nonhuman primates, carnivores, and artiodactyla) (146). The diagnostic methods used included examination of the fecal smear by direct and concentration methods followed by light microscopy observation of the fecal suspension. Giemsa and acid-fast stains were also included in the study.
Shellfish have been proposed to concentrate oocysts from contaminated waters. Controlled laboratory studies with freshwater clams (Corbicula fluminea) showed that 48 to 100% of the clams retained Cyclospora oocysts for up to 13 days (76).
Risk Factors and Prevalence of Cyclosporiasis
Epidemiological studies have shown that consumption of untreated water, lack of adequate sanitation, and the presence of animals in the household are associated with increased risk of Cyclospora infections (14, 197). The first report of waterborne transmission of Cyclospora described a 1990 outbreak that affected physicians and administrative staff in a Chicago hospital (91). The tap water in the physician's dormitory was implicated as the most likely source of contamination because stagnant water in a storage tank could have contaminated the water supply after a pump failure. Bird feces were noted on the storage tank brim as well as on the canvas cover of the storage tanks, although the examination of water samples did not reveal Cyclospora oocysts. A follow-up study of the same outbreak revealed that drinking tap water (RR, 11.6; 95% CI, 2.7 to 103) and attendance at a house staff party (RR, 13.7; 95% CI, 3.2 to 122) were significant risk factors, with an up to 9.4% attack rate for the groups that were exposed to them. We cannot rule out that birds could have transported Cyclospora oocysts and contaminated the cistern water. We must reconsider this outbreak from a different perspective and not rule out the possibility of a food-borne outbreak associated with the food served at the house staff party.
Studies conducted in Guatemala concluded that significant risk factors for cyclosporiasis were drinking untreated water (odds ratio [OR], 4.2; 95% CI, 1.4 to 12.5) and soil contact among children <2 years of age (OR, 19.8; 95% CI, 2.2 to 182). These studies also found that among 182 people in the cohort, four farm workers had asymptomatic cyclosporiasis (14).
Countries that have areas where Cyclospora is endemic are listed in Tables 1 and 3. In these countries, Cyclospora shows marked seasonality. A few cases in patients with no travel history were also reported in countries where cyclosporiasis is not considered endemic (Table 2).
TABLE 3.
Reported food- and waterborne Cyclospora outbreaks
| Date of outbreak | Location or country (city/state/province) | No. of clinically defined cases (no. of clusters) | Vehicleh | Source of vehicleh | Reference |
|---|---|---|---|---|---|
| July 1990 | United States (Illinois) | 21 (1) | Tap water | United States | 91 |
| June 1994 | Nepal (Pokhara) | 12 (1) | River and municipal water | Nepal | 155 |
| June 1995 | United States (Florida) | 38 (2) | Raspberries, country club | Guatemala, Chile | 82 |
| May-June 1995 | United States (New York) | 32 (1) | Food | ND | 82 |
| September 1995 | United States (Florida) | 38 | ND | ND | 31 |
| May 1996 | United States (Boston, MA) | 57 (1) | Berry dessert | Berriesa | 68 |
| May-June 1996 | United States/Canadab | 1,465 (55) | Raspberries | 83 | |
| April to June 1997 | United States/Canadac | 1,012 (41) | Raspberries | Guatemala | 84 |
| December 1997 | United States (Florida) | 12 (1) | Mesclun salad | Peru | 82 |
| July 1997 | United States (northern Virginia; Washington, DC) | 48 | Basil-pesto pasta salad | ND | 29 |
| June-July 1997 | United States (Washington, DC) | 341 (57) | Basil-pesto pasta salad | Multiple | 82 |
| March to May 1997 | U.S. cruise ship (Florida departure) | 220 (1) | Raspberries | Guatemala | 30 |
| September 1997 | United States (Virginia) | 21 (1) | Fruit plate | ND | 82 |
| May 1998 | United States (Georgia) | 17 (1) | Probably fruit salad | ND | 82 |
| May-June 1998 | Canada (Ontario) | 221 (13) | Berry garnish (raspberries) | Guatemala | 32 |
| August 1999 | United States (Missouri) | 62 (2) | Chicken pasta and tomato basil salad | Basil from Mexico and United States | 112 |
| May 1999 | Canada (Ontario) | 104 (1) | Dessert (berry)d | 82 | |
| May 1999 | United States (Florida) | 94 (1) | Fruits, berry | ND | 82 |
| December 2000 to January 2001 | Southwest Germany | 34 | Salads, leafy herbs | Variouse | 57 |
| June 2000 | United States (Pennsylvania) | 54 | Raspberries, wedding cake | Guatemala | 85 |
| April 2001 | Mexico (Monterrey) | 70 samples | Watercress | ND | 13 |
| January to June 2001 | Canada (British Columbia) | 17 (1) | Thai basil | United States | 86 |
| September 2001 | Indonesia (Bangor) | 14 (1) | ND | ND | 22 |
| April 2002 | Colombia (Medellin) | 31 (of 56 cases) | Salads and juice | ND | 23 |
| February 2004 | United States (Texas; Illinois) | 95 | ND | ND | CDC health update |
| June-July 2004 | United States (Pennsylvania) | 96 | Snow peas | Guatemala | 33 |
| November 2004 | Peru (Lima) | 127 | ND | Mealsf | 180 |
| April 2005 | United States (Florida) | 592 (6) | Fresh basil | Restaurants | 79 |
| March 2005 | Peru (Lima) | 45 | ND | Mealsf | 128 |
| September 2005 | Turkey (Izmir) | 35 | Water | Water | 8 |
| July-August 2007 | Turkey (Istanbul) | 286 | ND | ND | 143 |
| April 2009 | Cruise ship (multiple countries) | 160 | ND | ND | CDCg |
California strawberries, Florida blueberries, Guatemala blackberries, and Guatemala/Chile raspberries.
Twenty states, District of Columbia, and two Canadian provinces.
Thirteen states, District of Columbia, and one Canadian province.
Guatemala raspberries, U.S. strawberries, and frozen Chilean raspberries.
Lettuce from Southern France, mixed lettuce and herbs from Southern Italy, and German chives.
Naval recruit training base food.
ND, not determined.
Relevant Epidemiological Characteristics of the Parasite
It is not yet clear how oocysts are disseminated or transferred from one infected person to another. There is biological and epidemiological evidence suggesting that Cyclospora is an anthroponotic pathogen transmitted through the fecal-oral route. However, Cyclospora has some unique characteristics: it is noninfectious when excreted, requiring 7 to 15 days to sporulate and become infectious, and it is highly resistant to disinfectants commonly used in the food industry.
Where oocysts sporulate in natural environments is unknown, as are the environmental factors that favor or hinder this process. This knowledge may help in understanding the unique seasonality of the parasite as well as its geographical distribution. The time for sporulation contributes to challenges associated with trace-back outbreak investigations that attempt to identify sources of the outbreak as well as potential sites or times of contamination.
Cyclospora is highly resistant to disinfectants commonly used in food and water processing. This resistance may also be enhanced by the apparent higher binding affinities of the parasite for certain fresh produce. In the case of raspberries, which have been implicated in several outbreaks, the topography of the fruit is characterized by fine hair-like projections that facilitate the entrapment of the “sticky” Cyclospora oocysts. The microenvironment of the berry provides a favorable environment for parasite retention on the fruit. The stickiness of Cyclospora oocysts seems to be stronger than that of Cryptosporidium or Giardia oocysts. The adhesins responsible for this strong attachment to produce are unknown.
Evidence to date supports observations that C. cayetanensis is a parasite of humans, and no true infections have been observed naturally or described for other animal species (39). The lack of animal models in which to propagate this parasite has limited efforts to develop detection methods, understand its biology, and test control strategies. In all, Cyclospora has unique characteristics that have challenged our ability to understand its transmission dynamics in the human population.
Waterborne Outbreaks
Cyclospora oocysts have been detected in water for human consumption in several instances (Table 3). A study tested 27 sachets containing drinking water that were sold for human consumption in Accra, Ghana (102). Cryptosporidium oocysts were identified in 63% of the bags, while 59.3% contained Cyclospora oocysts, suggesting fecal contamination. Cyclospora was also identified in 5 of 12 water sources used for human consumption in rural areas near Guatemala City (58).
In Dakahlia Governorate, Egypt, 0.24% of 840 surveyed drinking water samples contained Cyclospora oocysts. Diagnosis was performed using modified Ziehl-Neelsen (MZN) and auramine-rhodamine stains (66). Although these stains are not specific for Cyclospora detection in environmental samples, they give an indication of the potential source of Cyclospora contamination.
In 2005, in a village close to Ismir, Turkey, 30 cases of abdominal pain, diarrhea, and nausea were reported for school-age children (2). Among 191 locals, 5% had Cyclospora infection (22.8% of patients were under 14 years old) and 8% had Cryptosporidium infection. Coinfection with Cryptosporidium and Cyclospora was identified in 1% of the samples. It was suggested that infections were most likely acquired by ingestion of contaminated water, but water analysis was not performed. In Pokhara, Nepal, a waterborne outbreak of Cyclospora involved 12 of 14 British soldiers and dependents who developed diarrheal illness (155). The drinking water consisted of a mixture of river and municipal water containing chlorine at a concentration of 0.3 to 0.8 ppm. Coliform bacteria were not detected in the water, suggesting that water chlorination was at acceptable levels, although it was not sufficient to inactivate Cyclospora oocysts. Cyclospora oocysts have also been detected in drinking water sources in Guatemala, Nepal, and Haiti (58, 113, 125, 168).
Food-Borne Outbreaks
In the United States, the estimated number of cases of cyclosporiasis per year attributed to food-borne transmission is 14,638 (with 4.1% of cases caused by parasites), with 15 cases of hospitalization per year (122). This estimate was done at a time when many cases of cyclosporiasis were being detected, mostly associated with imported berries. Raspberries, mesclun lettuce, basil, and snow peas, most of them imported from countries where Cyclospora is endemic, have been implicated in these outbreaks (28, 30, 32, 85), but in many instances the contaminated foods were not identified. For instance, in an outbreak that occurred in the United States and Canada in 1996, cyclosporiasis was associated with the consumption of raspberries (P < 0.05) (83), and raspberries were imported from Guatemala in 21 of 29 events for which documented data were available. Contaminated agricultural water was hypothesized as the source of Cyclospora. In this outbreak, the median attack rate among people who consumed raspberry-containing items was 93.3%, and the median incubation period was 7 days. Most cases of cyclosporiasis in the United States have been associated with consumption of contaminated imported foods. Based on the reported cases, Cyclospora does not appear to be endemic to the United States.
Raspberries were also implicated in a multistate outbreak in the United States in 1997 and in the Georgia and Pennsylvania outbreaks in 2000. In the Pennsylvania outbreak, a wedding cake was significantly associated with the illness (RR, 5.9; 95% CI, 3.6 to 10.5), and the presence of Cyclospora DNA was confirmed by PCR analysis of the raspberry filling (85). In this outbreak, the origin of raspberries was not identified. One farm in Guatemala, one Mexican farm, and U.S. farms could have provided the contaminated raspberries; however, the Guatemalan farm was suspected as most likely to be involved because it was the only farm whose raspberries were also implicated in the Georgia outbreak (85). One peculiarity of this outbreak was that the raspberries used to make the cake filling were exposed to freezing conditions, as was the cake before it was served at the event, suggesting that either Cyclospora is more resistant to freezing than other parasites or the raspberry topology played a protective role for this parasite.
In 1999, a chicken pasta salad containing basil (RR, 4.25; 95% CI, 1.80 to 10.01) and a tomato basil salad (RR, 2.95; 95% CI, 1.72 to 5.07) were associated with cyclosporiasis in two events in Missouri (112). In this outbreak, Cyclospora was identified in the chicken pasta salad and basil by PCR and microscopy. The fresh produce linked to this outbreak was provided by farms in Mexico and the United States. Snow peas imported from Guatemala were also associated with a Cyclospora outbreak in the United States (33). The snow peas were used to prepare the pasta salad, a food item that in this outbreak was significantly associated with cyclosporiasis (RR, 32; 95% CI, 5 to 219).
In 2001, an outbreak was documented in British Columbia, Canada, with 17 cases of cyclosporiasis. A case-control investigation for this outbreak reported that 11 of 12 (92%) cases had consumed Thai basil, compared to 3 of 16 controls. Interestingly, the Thai basil was imported from the United States (86).
Molecular Epidemiology
Characteristics of polymorphic areas of the genomes of microorganisms have been studied extensively to better understand their role in epidemiology. Tracing the source of infection can be facilitated by genomic comparison between isolates. Moreover, Cyclospora may be identified clearly and differentiated from other protozoan parasites and from other microorganisms involved in coinfections that result in food-borne or waterborne outbreaks.
The 18S rRNA gene has been used to differentiate Cyclospora from other apicomplexan parasites (157). In addition, sequence analysis of the 18S ribosomal DNA (rDNA) has shown that Cyclospora strains from humans and baboons belong to a distinct monophyletic group with 1.6% to 1.7% dissimilarity, whereas clones of Cyclospora isolates from humans and baboons are 0.78% and 0.73% dissimilar, respectively, among clones from each species (113). In a different study, C. papionis, C. colobi, and C. cercopitheci showed sequence identities of 98.4 to 98.7% with the human isolate at this region (61). However, it should be noted that the 18S rRNA gene is highly conserved and that multiple copies could explain low sequence variability; therefore, this region may not be useful to differentiate between isolates of C. cayetanensis or other Cyclospora species.
In studies conducted using the highly variable ITS1 region, C. cayetanensis and C. papionis were clearly differentiated (132). Considerable variability was determined for C. cayetanensis isolates (0 to 6.5%). Internal transcribed spacer (ITS) sequence variation was greater for U.S. isolates (8.3%) than for Guatemalan isolates (3.8%), but sequences were not associated with the geographical origin of the samples. The high sequence variability observed in samples obtained from persons in the United States without a recent travel history overseas suggests the possibility of an endemic focus for C. cayetanensis (132). The ITS1 sequences in five isolates obtained from the 1996 outbreak in the United States (Floridian isolates) were identical, while different ITS1 sequences were present in Guatemalan and Peruvian isolates. Seven clones of Guatemalan isolates were identical to Floridian isolates (1). In all cases, coinfection or the presence of multiple copies may explain variability in the ITS1 region.
Although current PCR protocols can be effective in detecting Cyclospora in different samples, these methods cannot distinguish between sporulated and unsporulated oocysts (82), limiting their use in biological or epidemiological studies. Use of PCR for the 18S rDNA region has also been reported to amplify oocysts of Cyclospora species other than C. cayetanensis; thus, the specificity of the assays needs to be improved (170).
Epidemiological studies would also be facilitated if there were serological screening tests for Cyclospora. These assays would be very useful in outbreak investigations, where asymptomatic or convalescent-phase cases are not usually reported (126).
DIAGNOSIS AND TREATMENT
Diagnosis
Samples suspected to contain Cyclospora can be examined using microscopy, molecular detection techniques, or sporulation studies. Samples can be stored in 2.5% potassium dichromate for sporulation or molecular detection or in 10% formalin for direct microscopy, concentration procedures, and staining or can be frozen for molecular testing and long-term archiving. Multiple stool samples from the same person at 2- to 3-day intervals may enhance the chance of detecting infections that have intermittent shedding and/or small numbers of oocysts (64). Cyclospora oocysts can be observed in wet preparations by light and epifluorescence microscopy. Fecal smears can be stained and observed using a light microscope or by molecular analysis, using frozen samples or samples stored in potassium dichromate.
Proficiency in identification of Cyclospora has been a challenge since the early 1990s. As a result, a pseudo-outbreak of Cyclospora was reported in the United States. During 1993 to 1998, in the United Kingdom, 58% of the participating laboratories correctly identified Cyclospora-positive samples in a wet preparation; 42% reported the wrong parasite or no parasite at all (25). Information that patients have obtained from the Internet and media reports has enhanced the reporting of food-borne outbreaks in the United States (126).
Light microscopy.
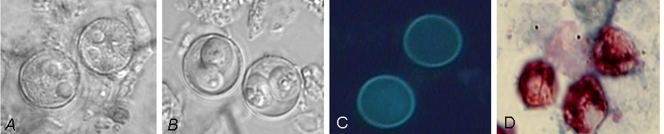
Cyclospora can be identified by phase-contrast microscopy or bright-field microscopy during examination of specimens for the ova and parasites. The oocysts measure 8 to 10 μm. Oocysts, when excreted, are spherical refractile bodies with a central morula (Fig. 2A). If the sample is stored at 23 to 30°C for 7 to 15 days, the oocysts will differentiate into sporulated oocysts that have two sporocysts (138) (Fig. 2B).
FIG. 2.
Detection of Cyclospora oocysts. The images show unsporulated (A) and sporulated (B) oocysts and oocysts detected by autofluorescence (C) or modified acid-fast stain (D).
Like other coccidia, e.g., Eimeria, Toxoplasma, Isospora, etc., in animals Cyclospora oocysts autofluoresce white-blue under an epifluorescence microscope, using a 330-380 DM excitation filter, or fluorescent green when using an excitation filter of 450-490 DM (Fig. 2C). The intensity of oocyst fluorescence may be influenced by the time and storage conditions of the fecal sample. This characteristic has been used to confirm the diagnosis of Cyclospora and also to purify the parasite from human fecal samples by flow cytometry. Detection of fluorescence has also been suggested as a useful alternative for screening large numbers of samples in the event of an outbreak (56).
Staining methods.
Cyclospora oocysts stain variably with MZN acid-fast stain. Some oocysts stain dark red, whereas others stain pale pink or do not take the stain at all. Safranin stains oocysts uniformly (98%) when the fecal smear and stain are heated by microwave treatment (189) (Fig. 2D). Similar results were obtained when slides were heated at 85°C for 5 min using a water bath (118). Other stains used in parasite detection, such as Giemsa, trichrome, and Gram-chromothrope stains, do not stain Cyclospora oocysts (189).
Molecular methods.
A nested PCR targeting a segment of the 18S rRNA gene (positions 685 to 978) was developed by Relman et al. (157) and has been used widely to examine clinical specimens in different settings and during outbreak investigations. The amplified product of about 300 bases cannot differentiate Cyclospora from Eimeria species. Digestion of the amplified products using the restriction enzyme MnlI (PCR-restriction fragment length polymorphism [PCR-RFLP]) followed by visualization by gel electrophoresis results in patterns that can discriminate these two genera of parasites. Compared to conventional microscopy assays, PCR is a more sensitive diagnostic tool (128).
Hussein (92) examined fecal samples of 140 children with diarrhea; 17.8% were positive by Kinyoun staining, 22.2% were positive by autofluorescence, 22.9% were positive by sporulation, and 25% were positive by PCR. Using single nucleotide polymorphisms (SNPs) of the 18S rRNA, one child was suspected of coinfection with a nonhuman Cyclospora strain.
Using MZN and modified acid-fast trichrome (MAFT) staining methods followed by confirmation using epifluorescence microscopy, Cyclospora was detected in patients with mild (16%) to severe (60%) disease (94). Using PCR and real-time PCR (RT-PCR), Cyclospora was identified in 100% of positive cases and 20% of controls (94).
Lalonde and Gajadhar (103) described a PCR technique for detecting Cyclospora oocysts by use of ITS2 DNA as a target. DNA was extracted using a QIAamp microkit (Qiagen) and a DNeasy blood and tissue kit (Qiagen) that included 8 cycles of a freeze-thaw process in ATL buffer and incubation in proteinase K followed by AL buffer. The amplified product of the PCR assay was a 116-bp segment.
Serological testing.
Serological assays to determine human exposure to Cyclospora are not yet available. However, attempts to determine a serological immune response were done using immunofluorescent-antibody (IFA) microscopy (38). Preliminary studies were conducted in China to determine cellular and humoral immune function in patients with cyclosporiasis. The assays used oocysts produced in guinea pigs. This animal model and serological testing need to be reproduced and validated by other scientists (191). A Western blot assay has been developed and can identify acute- from convalescent-phase cyclosporiasis (Y. R. Ortega, unpublished data). A serious limitation of this assay is the large number of oocysts needed and the lack of an animal model in which Cyclospora can be propagated.
Concentration procedures.
Cyclospora oocysts can be concentrated prior to microscopic observation or as a primary step in parasite purification, and ethyl acetate-formalin sedimentation has been used frequently. If the goal is to concentrate viable oocysts, a modification of this procedure includes replacing the formalin with saline solution. Other procedures have been used in which ethyl acetate is replaced by the FeKal CON-trate system (108). After this initial concentration step, oocysts can also be purified using discontinuous sucrose gradients. Whenever highly purified oocysts are needed, usually for research purposes, an additional purification step using cesium chloride can be used (Ortega, personal observation).
Other concentration/purification protocols have also been described. A modified aqueous detachment solution containing 0.563 mM H2Na2P2O7 and 42.8 mM NaCl followed by a discontinuous sucrose gradient with diatrizoate meglumine (Renocal) in 0.25 M sucrose yielded cleaner oocysts than did the use of sucrose gradients (160). In the event that the samples were preserved in SAF fixative, it is recommended that equal volumes of SAF-fixed samples and 10% KOH be subjected to vortex homogenization and centrifugation with 0.85% saline solution (12). The addition of KOH yields larger numbers of oocysts than those obtained using SAF-fixed samples alone. A discontinuous Percoll gradient was reported to recover more oocysts than ethyl acetate and discontinuous sucrose gradients (123). The Percoll solution was composed of Percoll and 1.5 M NaCl in a 9:1 ratio. A discontinuous gradient was then prepared using equal volumes of 60% Percoll solution as the top layer, with 77.7% Percoll solution at the bottom. The fecal suspension was then placed on top of the bilayer. The Percoll gradient was centrifuged at 250 × g for 15 min. The sample yielded more positive samples than did Sheather's sucrose and Formol-ether. This study also described the application of the method for recovering other parasites, such as Cryptosporidium, Giardia, and Entamoeba, and other protozoan oocysts and cysts (123).
Viability
It is important to know if parasites or oocysts in foods or water can cause infection if ingested. It is also important to determine parasite viability when testing inactivation strategies that should neutralize the infectious potential of the parasite. Currently, there are no animal models or in vitro cultivation methods to determine the infectious potential of Cyclospora. Two alternatives have been proposed to determine the viability of Cyclospora oocysts. The first method is to determine if unsporulated oocysts sporulate followed by excystation. Although feasible, sporulation/excystation methods are not practical, and the procedure has not been fully validated (137, 165, 166). The second alternative is electrorotation, which has been reported to correlate well with vital dyes and morphological indicators for Giardia oocysts (47, 48). Two parameters were considered, namely, direction of rotation and rotational velocity. This technique has been proposed to determine oocyst viability and compares favorably to oocyst sporulation detection. The reader should keep in mind that oocyst sporulation does not necessarily correlate with oocyst infectivity. The ideal method would correlate sporulation with infectivity by use of animal models. As noted above, in spite of major efforts, attempts to infect animals with Cyclospora oocysts have been unsuccessful.
Treatment
The therapy of choice for treatment of cyclosporiasis is TMP-SMX. Children with cyclosporiasis stopped excreting oocysts after a 3-day treatment with TMP-SMX at 5-25 mg/kg of body weight/day (114). In a later study, 63 children enrolled in a double-blind, placebo-controlled trial received the same 3-day course of therapy. The mean time with detectable Cyclospora oocysts was 4.8 ± 1.2 days for the treated group, compared to 12.1 ± 6.1 days for the placebo group (115). In Nepal, adult expatriates with Cyclospora infection participated in a randomized double-blinded study with TMP-SMX given at 160-800 mg twice a day (BID) for 7 days (88). At that time, 6% of the treated group was still excreting oocysts. In these patients, the infection resolved after extending treatment for an additional week. The eradication of Cyclospora in stools was directly correlated with clinical improvement (88). In Haiti, 43 patients with Cyclospora infection were treated with TMP-SMX (144). Recurrent symptomatic cyclosporiasis was observed in 43% of the patients. As a secondary prophylaxis treatment, these patients received TMP-SMX three times a week for 1 month, with success. Other antibiotics, such as azithromycin (40), norfloxacin, tinidazole, nalidixic acid, diloxanide fluorate, and quinacrine (171), have also been tested for Cyclospora treatment, all without success. In a randomized control trial, ciprofloxacin (500 mg BID for 7 days) was tested in a randomized control trial of HIV-positive patients with cyclosporiasis (186). Diarrhea ceased in all patients receiving TMP-SMX at 160-800 mg BID (median time to cessation of diarrhea was 3 days) and in 10 of 11 patients receiving ciprofloxacin (median time to cessation, 4 days). After 7 days, all patients receiving TMP-SMX were negative for Cyclospora upon stool examination, whereas four of the patients receiving ciprofloxacin were still positive. After 10 weeks of secondary prophylaxis using either TMP-SMX or ciprofloxacin, all patients treated with TMP-SMX remained negative and one of seven had recurrence after 4 weeks. No side effects were observed in any of the patients in the study. Ciprofloxacin can be used as an alternative therapy, especially in patients who are allergic to sulfa products (186); however, anecdotal evidence of treatment failure with ciprofloxacin has also been reported (198). A 7-day regimen with nitazoxanide has been proposed as an effective alternative for treatment of cyclosporiasis in a patient with sulfa allergy (54, 198).
DETECTION AND CONTROL IN ENVIRONMENTAL SAMPLES
Water
There are reports that suggest waterborne transmission of Cyclospora. An elderly man with Cyclospora infection was in contact with a sewage backup in the basement of a house, and another person in the same house later developed persistent diarrhea that was suspected to have been caused by Cyclospora (78). In another suspected waterborne case, a patient without travel history reported having cleaned a salt water aquarium by oral siphoning 4 days prior to developing symptoms (192). In a hospital-based outbreak in Chicago, drinking water was associated with Cyclospora infections (91).
Water used either for irrigation or for processing vegetables has been found to contain Cyclospora oocysts. A study by Tram et al. (181) found that 11.8% (34/288 samples) of market water and herb samples and 8.4% (24/287 samples) of farm samples were positive for Cyclospora. Oocysts were isolated from all varieties of farm-grown herbs examined (basil, coriander, lettuce, marjoram, and Vietnamese mint). Cyclospora contamination was observed before the rainy season (November to April) but not during the rainy season (May to October). Interestingly, no human cases were detected in a hospital-based surveillance study performed in the same period.
Cyclospora has also been detected in wastewater (168, 177). Eight of 11 water samples from a primary oxidation lagoon in Peru contained Cyclospora oocysts (177). These samples were concentrated using Envirocheck capsules and Hannifin filters. Environmental water collected from rivers and lakes in Vietman, Guatemala, and Egypt was also positive for Cyclospora (14, 65, 125). In California, water from the Santa Clara River was positive for Cyclospora by the Relman PCR protocol; however, examination of the same samples using other PCR-RFLP methods yielded negative results (170). These contradictions suggest that the nested PCR method gives false-positive results, as amplification of other coccidia present in river water or environmental samples occurs.
One of the possible vehicles of Cyclospora contamination of produce could result from use of contaminated water for application of pesticides. Fungicides (50% captan WP, 50% benomyl WP, and 75% zineb WP) and insecticides (25% malathion WP and 47.5% diazinon 4E) do not affect oocyst sporulation (165). Extended incubation (1 week) in Benomyl, however, reduces sporulation.
Cyclospora can be isolated from irrigation and drinking water by filtration, using Hannifin polypropylene cartridge filters or Envirocheck capsules (177). Particles trapped in the filters are released using an elution buffer (1% sodium dodecyl sulfate, 1% Tween 80, NaCl, KH2PO4, Na2HPO4·12H2O, KCl, and antifoam A). After centrifugation, pellets are stored in 2.5% potassium dichromate and examined for the presence of Cyclospora. Autofluorescence, phase-contrast microscopy, and PCR-RFLP have been used successfully to test water samples (166). Inactivation of Cyclospora oocysts can be accomplished by freezing at −20°C for at least 2 days or at −70°C for 60 min. Inactivation also occurs by heating oocysts in water at 70°C for 15 min. Sporulation at 50°C was minimal (0.01 to 0.03%). Microwave heating inactivates Cyclospora oocysts in water when the temperature reaches 80°C or higher (139). These temperatures can be reached in shorter periods than those with conventional heating.
Foods
Fresh produce samples in Phnom Penh, Cambodia, were examined for the presence of Cyclospora. High concentrations of thermotolerant coliforms (ThC), intestinal helminth eggs, and protozoa were found in a wastewater-fed lake where water spinach was grown. Water spinach samples contained Cyclospora (8%), Giardia cysts (56%), Cryptosporidium (17%), and 105 to 107 ThC/100 ml (190). Calvo et al. (24) detected Cyclospora in vegetables in local agricultural markets in the Central Valley of Costa Rica. The highest prevalence of fecal coliforms was identified during the rainy season. Cyclospora was identified only in lettuce during the dry season. In Peru, Cyclospora was identified in vegetables surveyed on three occasions (141). Of 110 vegetables analyzed, 2 (1.8%) contained Cyclospora in one survey, and of 62 vegetables sampled in a second survey, 1 (1.6%) contained Cyclospora. Lettuce, mint, and black mint were contaminated with Cyclospora oocysts. Sherchand et al. (168) found Cyclospora in vegetables and wastewater in Nepal.
Since Cyclospora has been associated with outbreaks of food-borne infections, methods for food sample preparation and detection of the parasite have been a research priority. Robertson et al. (161) used lectin (wheat germ agglutinin)-coated paramagnetic beads to recover Cyclospora oocysts from fruits and vegetables. The oocyst recovery rate was 12% for raspberries and 4% for bean sprouts. Although recovery efficiencies were not improved, this method resulted in cleaner samples that were of smaller volume. Other studies used water, saline solution, glycine buffer, pH 5.5 (103), and elution buffers to remove oocysts from food products.
Oocyst detection methods for foods and environmental samples include direct observation using phase-contrast, bright-field, and differential interference contrast (DIC) microscopy. Epifluorescence microscopy has also been used. Other coccidian oocysts also autofluoresce and stain with acid-fast stain, so these procedures have to be used in combination with other diagnostic assays. Using Toxoplasma as a surrogate for Cyclospora, Kniel et al. (100) determined that Toxoplasma oocysts attach better to raspberries than to blueberries, probably because of the fine hair-like projections on raspberries.
In combination with oocyst extraction from foods, molecular assays are a useful diagnostic tool. The PCR protocol developed by Relman et al. (157) was modified to improve the specificity and sensitivity, primarily to overcome PCR inhibitors and other contaminants present in food matrices. Jinneman et al. (95) proposed extracting DNA from Cyclospora oocysts by using six freeze-thaw cycles. In this protocol, skim milk (50 mg/ml) was included in the PCR mixtures to overcome the effects of PCR inhibitors. Although the primers described by Relman et al. (157) work well for examining human samples, nonspecific amplification was noted when examining environmental samples, which may include parasites from other animal species (95). This limitation was overcome by adding an RFLP step, using the MnlI endonuclease. Later, an oligonucleotide-ligation assay (OLA) was developed for differentiating Cyclospora and Eimeria (96). This procedure could be automated, allowing a larger number of samples to be tested and simplifying data analysis. This assay uses two oligonucleotides, with one being a biotinylated capture probe (5′) and the other being a digoxigenin-labeled reporter probe (3′), which can detect single-base-pair differences.
A quantitative real-time PCR assay with the capacity to detect one oocyst in 5 μl of reaction mixture has been reported (184). Another PCR-based method using a different procedure to prepare the DNA template has been described (136). Produce was washed, and 50 to 100 μl of the wash was loaded onto Flinders Technology Associates (FTA) filters. The filters were allowed to dry at 56°C, and sections of the FTA filters were cut and used directly in PCRs. These filters are time- and cost-effective and alleviate difficulties in template preparation when using washes from produce and environmental samples that are not too viscous. The detection limit using these filters was 10 to 30 oocysts per 100 g of fresh raspberries (136). A nested PCR using SNP primers was also developed and seems to offer a more rapid and sensitive alternative to the PCR-RFLP assay (134).
Another PCR method that appears to be as sensitive and specific as the nested PCR described by Relman et al. (157) was described by Lalonde and Gajadhar. They used the ITS2 gene, with a reported detection limit of 1 to 10 oocysts with the use of a QIAmp DNA extraction microkit (103).
Control
Cyclosporiasis can be acquired by the ingestion of contaminated foods or water. Direct person-to-person transmission is unlikely, as the excreted oocysts are not infectious and require more than 7 days outside the host to sporulate. Strategies to prevent food-borne and waterborne contamination should therefore target the reduction or prevention of human infections and strive for improved sanitation. Proper hygiene habits and food washing and sanitizing may reduce, but would not be expected to eliminate, the risk of acquiring infections. It has been demonstrated that these practices do not completely remove Cyclospora oocysts from contaminated produce (161). Good agricultural practices would indeed contribute to reducing the burden of parasite contamination at the farm level. These practices would involve the use of properly treated irrigation water and the use of pathogen-free water for washing produce.
Several practices have been tested for the ability to inactivate or reduce the number of viable parasites in foods and in water. Because of the lack of animal or in vitro infectivity models, oocyst sporulation has been used as an indicator of viability. Methods that rely on temperature and time of storage have been evaluated for killing parasites. For dairy substrates, storage at −15°C for 24 h did not inactivate Cyclospora oocysts. For basil or water, storage at −20°C for 2 days, 50°C for 1 h, and 37°C for up to 4 days did not prevent Cyclospora sporulation; however, extreme temperatures (70°C, −70°C, and 100°C) were effective in preventing oocysts from sporulating (137). Temperatures frequently used for produce storage, e.g., 4 to 23°C, do not affect sporulation of Cyclospora. Microwave heating of Cyclospora oocysts can inactivate oocysts; however, more time is required to kill Cyclospora oocysts than to kill Cryptosporidium oocysts. Short exposures to a high temperature (96°C for 45 s) did not completely prevent the sporulation of Cyclospora (139). Chemicals have been tested for the ability to interfere with the sporulation of Cyclospora. Gaseous chlorine dioxide at 4.1 mg/liter does not affect the sporulation of Cyclospora; however, this treatment does inactivate Cryptosporidium and microsporidia (137).
The lack of in vivo or in vitro methods to test viability has prompted researchers to use surrogate parasites, such as Eimeria and Toxoplasma, to evaluate other treatments. Gamma irradiation (137Cs) of sporulated and unsporulated Toxoplasma oocysts was evaluated as a model system for inactivation of Cyclospora oocysts (60). Toxoplasma oocysts treated with ≥0.4 kGy could sporulate, excyst, and infect cells but did not cause infections in mice. It was recommended that 0.5 kGy be used to kill coccidian oocysts on fruits and vegetables (60). Inactivation of Eimeria acervulina oocysts was achieved by freezing, heating, and irradiation at 1 kGy and higher (105).
High hydrostatic pressure (550 MPa at 40°C for 2 min) and UV light (up to 261 mW/cm2) treatments of produce contaminated with E. acervulina as a Cyclospora surrogate were evaluated on experimentally inoculated basil and raspberries (101). Both treatments yielded smaller numbers of animals infected with E. acervulina but did not completely inactivate the oocysts recovered from these food matrices. Toxoplasma oocysts (VEG strain) inoculated onto raspberries were rendered noninfectious to mice when a high-pressure processing treatment of 340 MPa for 60 s was applied (107).
CONCLUSIONS AND RECOMMENDATIONS
Cyclospora infection is frequently identified in travelers from areas of endemicity. These infections should therefore be considered in all travelers with diarrhea. Testing for Cyclospora must be requested specifically, as standard examinations for ova and parasites may not look for Cyclospora oocysts. Adequate processing of water and foods and abstaining from consumption of raw produce when traveling to areas of endemicity will aid in reducing the risk of acquiring Cyclospora infection. Potable water and cooked foods that are not subsequently contaminated will reduce the incidence of Cyclospora in local children and travelers.
Additional diagnostic assays for clinical and environmental samples need to be developed and implemented. These assays need to be of low cost, sensitive, and simple to use, so they can be used widely in developing countries. In vitro or in vivo systems for propagation of Cyclospora are much needed to better understand the biology and epidemiology of Cyclospora.
Although large food- or waterborne outbreaks of Cyclospora infections have not been reported recently in the United States, sporadic cases are still reported. The safety of imported foods needs to be monitored. Because treatments for inactivation and complete removal of Cyclospora oocysts from contaminated produce have not been developed, good agricultural practices and use of filtered or otherwise decontaminated irrigation water should be implemented in countries where crops are grown for local consumption and exportation. Because globalization of food systems has occurred, developed countries need to take an active role in helping countries where disease is endemic to improve practices to minimize the risk of unsafe foods.
Part of the U.S. food supply is outsourced, in many instances from areas where Cyclospora is endemic. Based on information from the Department of Commerce, U.S. Census Bureau, in 2005 approximately 16% of the total U.S. vegetable supply was imported (M. P. Doyle, personal communication). Leading export countries included Mexico, Canada, Peru, The Netherlands, China, Costa Rica, and Guatemala. Increases in spinach and lettuce imports between 2000 and 2005 were 314% and 303%, respectively (Doyle, personal communication). With this in mind, food safety training worldwide is an absolutely necessity if progress is to be made in providing safe food and water to consumers internationally.
Acknowledgments
This work was supported in part by a grant from the United States Department of Agriculture and by a Center for Food Safety seed grant.
Biography
 Ynés R. Ortega is an Associate Professor at the Center for Food Safety, University of Georgia (UGA), Griffin, GA. She completed her undergraduate studies at Cayetano Heredia University, Peru, her Ph.D. at the University of Arizona, and her M.P.H. at Johns Hopkins University. She is a guest researcher at the Centers for Disease Control and Prevention (CDC), Adjunct Faculty of the Center for Tropical and Emerging Global Diseases, UGA, Associate at Johns Hopkins University Bloomberg School of Public Health, and Visiting Professor at the Cayetano Heredia University in Peru. She is a science advisor for the FDA Southeast Region and a member of the Gorgas Memorial Institute Research Award Committee and the Editorial Board of the Journal for Food Protection, and she serves as a reviewer for 11 journals. Her research interests include isolation, identification, genotyping, and control of parasites in human and animal populations, the environment, foods, and water. She is also interested in the host response to parasitic infections and in the development of diagnostic assays. Dr. Ortega has published over 50 refereed articles and book chapters and is editor of a book on food-borne parasites. She has been an invited speaker on parasites at scientific meetings in the United States, Canada, Mexico, Colombia, Asia, and Europe.
Ynés R. Ortega is an Associate Professor at the Center for Food Safety, University of Georgia (UGA), Griffin, GA. She completed her undergraduate studies at Cayetano Heredia University, Peru, her Ph.D. at the University of Arizona, and her M.P.H. at Johns Hopkins University. She is a guest researcher at the Centers for Disease Control and Prevention (CDC), Adjunct Faculty of the Center for Tropical and Emerging Global Diseases, UGA, Associate at Johns Hopkins University Bloomberg School of Public Health, and Visiting Professor at the Cayetano Heredia University in Peru. She is a science advisor for the FDA Southeast Region and a member of the Gorgas Memorial Institute Research Award Committee and the Editorial Board of the Journal for Food Protection, and she serves as a reviewer for 11 journals. Her research interests include isolation, identification, genotyping, and control of parasites in human and animal populations, the environment, foods, and water. She is also interested in the host response to parasitic infections and in the development of diagnostic assays. Dr. Ortega has published over 50 refereed articles and book chapters and is editor of a book on food-borne parasites. She has been an invited speaker on parasites at scientific meetings in the United States, Canada, Mexico, Colombia, Asia, and Europe.
 Roxana Sanchez received her bachelor's degree in veterinary medicine in 1996 at the Universidad Nacional Mayor de San Marcos, Lima, Peru. In 2002, she received her D.V.M. degree, and in 2004, she received the title of Specialist in Veterinary Economics and Epidemiology, both from the same institution. She worked as the Head of the Microbiology Laboratory of Laser SRL, a private avian pathology laboratory in Peru specializing in diagnostics of avian and swine pathogens and detection of spoilage microorganisms and mycotoxins in poultry products and feed ingredients. Currently, she is a Ph.D. candidate at the University of Georgia. Her work is focused on the identification of new molecular targets for the diagnosis, therapeutics, control, and differentiation of Cryptosporidium spp.
Roxana Sanchez received her bachelor's degree in veterinary medicine in 1996 at the Universidad Nacional Mayor de San Marcos, Lima, Peru. In 2002, she received her D.V.M. degree, and in 2004, she received the title of Specialist in Veterinary Economics and Epidemiology, both from the same institution. She worked as the Head of the Microbiology Laboratory of Laser SRL, a private avian pathology laboratory in Peru specializing in diagnostics of avian and swine pathogens and detection of spoilage microorganisms and mycotoxins in poultry products and feed ingredients. Currently, she is a Ph.D. candidate at the University of Georgia. Her work is focused on the identification of new molecular targets for the diagnosis, therapeutics, control, and differentiation of Cryptosporidium spp.
REFERENCES
- 1.Adam, R. D., Y. R. Ortega, R. H. Gilman, and C. R. Sterling. 2000. Intervening transcribed spacer region 1 variability in Cyclospora cayetanensis. J. Clin. Microbiol. 38:2339-2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aksoy, U., C. Akisu, S. Sahin, S. Usluca, G. Yalcin, F. Kuralay, and A. M. Oral. 2007. First reported waterborne outbreak of cryptosporidiosis with Cyclospora co-infection in Turkey. Euro Surveill. 12:E070215. [DOI] [PubMed] [Google Scholar]
- 3.Alarcon, R. S., N. Amato, V. E. Gakiya, and R. C. Bezerra. 2007. Observations on Blastocystis hominis and Cyclospora cayetanensis in routine parasitological examinations. Rev. Soc. Bras. Med. Trop. 40:253-255. [DOI] [PubMed] [Google Scholar]
- 4.Albert, M. J., I. Kabir, T. Azim, A. Hossain, M. Ansaruzzaman, and L. Unicomb. 1994. Diarrhea associated with Cyclospora sp. in Bangladesh. Diagn. Microbiol. Infect. Dis. 19:47-49. [DOI] [PubMed] [Google Scholar]
- 5.Alfano-Sobsey, E. M., M. L. Eberhard, J. R. Seed, D. J. Weber, K. Y. Won, E. K. Nace, and C. L. Moe. 2004. Human challenge pilot study with Cyclospora cayetanensis. Emerg. Infect. Dis. 10:726-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ashford, R. W. 1979. Occurrence of an undescribed coccidian in man in Papua New Guinea. Ann. Trop. Med. Parasitol. 73:497-500. [DOI] [PubMed] [Google Scholar]
- 7.Ayala-Gaytan, J. J., C. az-Olachea, P. Riojas-Montalvo, and C. Palacios-Martinez. 2004. Cyclosporidiosis: clinical and diagnostic characteristics of an epidemic outbreak. Rev. Gastroenterol. Mex. 69:226-229. [PubMed] [Google Scholar]
- 8.Behera, B., B. R. Mirdha, G. K. Makharia, S. Bhatnagar, S. Dattagupta, and J. C. Samantaray. 2008. Parasites in patients with malabsorption syndrome: a clinical study in children and adults. Dig. Dis. Sci. 53:672-679. [DOI] [PubMed] [Google Scholar]
- 9.Bellagra, N., F. Ajana, C. Coignard, M. Caillaux, and Y. Mouton. 1998. Co-infection with Cryptosporidium sp. and Cyclospora sp. in an AIDS stage HIV patient. Ann. Biol. Clin. (Paris) 56:476-478. [PubMed] [Google Scholar]
- 10.Bendall, R. P., and P. L. Chiodini. 1995. The epidemiology of human Cyclospora infection in the UK, p. 26-29. In W. B. Betts, D. P. Casemore, C. Fricker, H. Smith, and J. Watkins (ed.), Protozoan parasites and water. The Royal Society of Chemistry, London, United Kingdom.
- 11.Bendall, R. P., S. Lucas, A. Moody, G. Tovey, and P. L. Chiodini. 1993. Diarrhoea associated with cyanobacterium-like bodies: a new coccidian enteritis of man. Lancet 341:590-592. [DOI] [PubMed] [Google Scholar]
- 12.Berlin, O. G., S. M. Novak, R. K. Porschen, E. G. Long, G. N. Stelma, and F. W. Schaeffer III. 1994. Recovery of Cyclospora organisms from patients with prolonged diarrhea. Clin. Infect. Dis. 18:606-609. [DOI] [PubMed] [Google Scholar]
- 13.Bern, C., M. J. Arrowood, M. Eberhard, and J. H. Maguire. 2002. Cyclospora in Guatemala: further considerations. J. Clin. Microbiol. 40:731-732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bern, C., B. Hernandez, M. B. Lopez, M. J. Arrowood, M. A. de Mejia, A. M. De Merida, A. W. Hightower, L. Venczel, B. L. Herwaldt, and R. E. Klein. 1999. Epidemiologic studies of Cyclospora cayetanensis in Guatemala. Emerg. Infect. Dis. 5:766-774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bern, C., Y. Ortega, W. Checkley, J. M. Roberts, A. G. Lescano, L. Cabrera, M. Verastegui, R. E. Black, C. Sterling, and R. H. Gilman. 2002. Epidemiologic differences between cyclosporiasis and cryptosporidiosis in Peruvian children. Emerg. Infect. Dis. 8:581-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blans, M. C., B. U. Ridwan, J. J. Verweij, M. Rozenberg-Arska, and J. Verhoef. 2005. Cyclosporiasis outbreak, Indonesia. Emerg. Infect. Dis. 11:1453-1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Botero-Garces, J., M. N. Montoya-Palacio, J. I. Barguil, and A. Castano-Gonzalez. 2006. An outbreak of Cyclospora cayetanensis in Medellin, Colombia. Rev. Salud Publica (Bogota) 8:258-268. [DOI] [PubMed] [Google Scholar]
- 18.Bouree, P., A. Lancon, F. Bisaro, and G. Bonnot. 2007. Six human cyclosporiasis: with general review. J. Egypt. Soc. Parasitol. 37:349-360. [PubMed] [Google Scholar]
- 19.Brandonisio, O., P. Maggi, M. A. Panaro, A. Marangi, R. Marzio, and G. Angarano. 1993. A Cyanobacterium-like body found in the stools of an HIV+ patient with diarrhoea. Eur. J. Epidemiol. 9:453-454. [DOI] [PubMed] [Google Scholar]
- 20.Buisson, Y., J. L. Marie, and B. Davoust. 2008. These infectious diseases imported with food. Bull. Soc. Pathol. Exot. 101:343-347. [DOI] [PubMed] [Google Scholar]
- 21.Burrell, C., S. Reddy, G. Haywood, and R. Cunningham. 2007. Cardiac arrest associated with febrile illness due to U.K. acquired Cyclospora cayetanensis. J. Infect. 54:e13-e15. [DOI] [PubMed] [Google Scholar]
- 22.Butcher, A. R., R. Lumb, E. Coulter, and D. J. Nielsen. 1994. Coccidian/cyanobacterium-like body associated diarrhea in an Australian traveler returning from overseas. Pathology 26:59-61. [DOI] [PubMed] [Google Scholar]
- 23.Caldwell, K. N., B. B. Adler, G. L. Anderson, P. L. Williams, and L. R. Beuchat. 2003. Ingestion of Salmonella enterica serotype Poona by a free-living nematode, Caenorhabditis elegans, and protection against inactivation by produce sanitizers. Appl. Environ. Microbiol. 69:4103-4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calvo, M., M. Carazo, M. L. Arias, C. Chaves, R. Monge, and M. Chinchilla. 2004. Prevalence of Cyclospora sp., Cryptosporidium sp., microsporidia and fecal coliform determination in fresh fruit and vegetables consumed in Costa Rica. Arch. Latinoam. Nutr. 54:428-432. [PubMed] [Google Scholar]
- 25.Cann, K. J., R. M. Chalmers, G. Nichols, and S. J. O'Brien. 2000. Cyclospora infections in England and Wales: 1993 to 1998. Commun. Dis. Public Health 3:46-49. [PubMed] [Google Scholar]
- 26.Caramello, P., T. Brancale, B. Forno, A. Lucchini, A. Macor, G. Mazzucco, C. Tettoni, and A. Ullio. 1995. Clinical and diagnostic aspects of travelers' diarrhea due to Cyclospora organisms. J. Travel Med. 2:232-234. [DOI] [PubMed] [Google Scholar]
- 27.Carollo, M. C., N. Amato, V. L. M. Braz, and D. W. Kim. 2001. Detection of Cyclospora sp. oocysts in the feces of stray dogs in greater Sao Paulo (Sao Paulo State, Brazil). Rev. Soc. Bras. Med. Trop. 34:597-598. [DOI] [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention. 1996. Update: outbreaks of Cyclospora cayetanensis infection—United States and Canada, 1996. MMWR Morb. Mortal. Wkly. Rep. 45:611-612. [PubMed] [Google Scholar]
- 29.Centers for Disease Control and Prevention. 1997. Outbreak of cyclosporiasis—Northern Virginia-Washington, D.C.-Baltimore, Maryland, metropolitan area, 1997. MMWR Morb. Mortal. Wkly. Rep. 46:689-692. [PubMed] [Google Scholar]
- 30.Centers for Disease Control and Prevention. 1997. Outbreaks of cyclosporiasis—United States, 1997. MMWR Morb. Mortal. Wkly. Rep. 46:451-452. [PubMed] [Google Scholar]
- 31.Centers for Disease Control and Prevention. 1997. Outbreaks of pseudo-infection with Cyclospora and Cryptosporidium—Florida and New York City, 1995. MMWR Morb. Mortal. Wkly. Rep. 46:354-358. [PubMed] [Google Scholar]
- 32.Centers for Disease Control and Prevention. 1998. Outbreak of cyclosporiasis—Ontario, Canada, May 1998. MMWR Morb. Mortal. Wkly. Rep. 47:806-809. [PubMed] [Google Scholar]
- 33.Centers for Disease Control and Prevention. 2004. Outbreak of cyclosporiasis associated with snow peas—Pennsylvania, 2004. MMWR Morb. Mortal. Wkly. Rep. 53:876-878. [PubMed] [Google Scholar]
- 34.Chacin-Bonilla, L., F. Barrios, and Y. Sanchez. 2007. Epidemiology of Cyclospora cayetanensis infection in San Carlos Island, Venezuela: strong association between socio-economic status and infection. Trans. R. Soc. Trop. Med. Hyg. 101:1018-1024. [DOI] [PubMed] [Google Scholar]
- 35.Chacin-Bonilla, L., J. Estevez, F. Monsalve, and L. Quijada. 2001. Cyclospora cayetanensis infections among diarrheal patients from Venezuela. Am. J. Trop. Med. Hyg. 65:351-354. [DOI] [PubMed] [Google Scholar]
- 36.Chacin-Bonilla, L., Y. M. de Mejia, and J. Estevez. 2003. Prevalence and pathogenic role of Cyclospora cayetanensis in a Venezuelan community. Am. J. Trop. Med. Hyg. 68:304-306. [PubMed] [Google Scholar]
- 37.Chu, D. M., J. B. Sherchand, J. H. Cross, and P. A. Orlandi. 2004. Detection of Cyclospora cayetanensis in animal fecal isolates from Nepal using an FTA filter-base polymerase chain reaction method. Am. J. Trop. Med. Hyg. 71:373-379. [PubMed] [Google Scholar]
- 38.Clarke, S. C., and M. McIntyre. 1997. An attempt to demonstrate a serological immune response in patients infected with Cyclospora cayetanensis. Br. J. Biomed. Sci. 54:73-74. [PubMed] [Google Scholar]
- 39.Colley, D. G. 1996. Widespread foodborne cyclosporiasis outbreaks present major challenges. Emerg. Infect. Dis. 2:354-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Connor, B. A. 1997. Cyclospora infection: a review. Ann. Acad. Med. Singapore 26:632-636. [PubMed] [Google Scholar]
- 41.Connor, B. A., E. J. Johnson, and R. Soave. 2001. Reiter syndrome following protracted symptoms of Cyclospora infection. Emerg. Infect. Dis. 7:453-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Connor, B. A., J. Reidy, and R. Soave. 1999. Cyclosporiasis: clinical and histopathologic correlates. Clin. Infect. Dis. 28:1216-1222. [DOI] [PubMed] [Google Scholar]
- 43.Connor, B. A., and D. R. Shlim. 1995. Foodborne transmission of Cyclospora. Lancet 346:1634. [DOI] [PubMed] [Google Scholar]
- 44.Connor, B. A., D. R. Shlim, J. V. Scholes, J. L. Rayburn, J. Reidy, and R. Rajah. 1993. Pathologic changes in the small bowel in nine patients with diarrhea associated with a coccidia-like body. Ann. Intern. Med. 119:377-382. [DOI] [PubMed] [Google Scholar]
- 45.Cordova Paz, S. O., V. F. Vargas, V. A. Gonzalez, C. G. Perez, S. Velasco, Jr., M. Sanchez-Moreno, G. Rodriguez, and M. J. Rosales Lombardo. 2006. Intestinal parasitism in Peruvian children and molecular characterization of Cryptosporidium species. Parasitol. Res. 98:576-581. [DOI] [PubMed] [Google Scholar]
- 46.Crowley, B., C. Path, C. Moloney, and C. T. Keane. 1996. Cyclospora species—a cause of diarrhoea among Irish travelers to Asia. Ir. Med. J. 89:110-112. [PubMed] [Google Scholar]
- 47.Dalton, C., A. D. Goater, J. P. Burt, and H. V. Smith. 2004. Analysis of parasites by electrorotation. J. Appl. Microbiol. 96:24-32. [DOI] [PubMed] [Google Scholar]
- 48.Dalton, C., A. D. Goater, R. Pethig, and H. V. Smith. 2001. Viability of Giardia intestinalis cysts and viability and sporulation state of Cyclospora cayetanensis oocysts determined by electrorotation. Appl. Environ. Microbiol. 67:586-590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Degirmenci, A., N. Sevil, K. Gunes, A. Yolasigmaz, and N. Turgay. 2007. Distribution of intestinal parasites detected in the parasitology laboratory of the Ege University Medical School Hospital, in 2005. Turkiye Parazitol. Derg. 31:133-135. [PubMed] [Google Scholar]
- 50.de Górgolas, M., J. Fortes, and M. L. Fernandez Guerrero. 2001. Cyclospora cayetanensis cholecystitis in a patient with AIDS. Ann. Intern. Med. 134:166. [DOI] [PubMed] [Google Scholar]
- 51.Deluol, A. M., C. Junod, J. L. Poirot, F. Heyer, Y. N′go, and J. Cosnes. 1994. Travellers diarrhea associated with Cyclospora sp. J. Eukaryot. Microbiol. 41:32S. [PubMed] [Google Scholar]
- 52.Deluol, A. M., M. F. Teilhac, J. L. Poirot, F. Heyer, L. Beaugerie, and F. P. Chatelet. 1996. Cyclospora sp.: life cycle studies in patient by electron-microscopy. J. Eukaryot. Microbiol. 43:128S-129S. [DOI] [PubMed] [Google Scholar]
- 53.de Paz, V. C., M. B. Bringuez, B. V. Viamonte, C. L. Suarez, A. M. Rodriguez, and Z. A. Martinex. 2003. Diagnosis of coccidia and microspores in specimens of diarrheal feces from Cuban HIV seropositive patients: first report of microspores in Cuba. Rev. Cubana Med. Trop. 55:14-18. [PubMed] [Google Scholar]
- 54.Diaz, E., J. Mondragon, E. Ramirez, and R. Bernal. 2003. Epidemiology and control of intestinal parasites with nitazoxanide in children in Mexico. Am. J. Trop. Med. Hyg. 68:384-385. [PubMed] [Google Scholar]
- 55.Di Gliullo, A. B., M. S. Cribari, A. J. Bava, J. S. Cicconetti, and R. Collazos. 2000. Cyclospora cayetanensis in sputum and stool samples. Rev. Inst. Med. Trop. Sao Paulo 42:115-117. [DOI] [PubMed] [Google Scholar]
- 56.Dixon, B. R., J. M. Bussey, L. J. Parrington, and M. Parenteau. 2005. Detection of Cyclospora cayetanensis oocysts in human fecal specimens by flow cytometry. J. Clin. Microbiol. 43:2375-2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Doller, P. C., K. Dietrich, N. Filipp, S. Brockmann, C. Dreweck, R. Vonthein, C. Wagner-Wiening, and A. Wiedenmann. 2002. Cyclosporiasis outbreak in Germany associated with the consumption of salad. Emerg. Infect. Dis. 8:992-994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dowd, S. E., D. John, J. Eliopolus, C. P. Gerba, J. Naranjo, R. Klein, B. Lopez, M. de Mejía, C. E. Mendoza, and I. L. Pepper. 2003. Confirmed detection of Cyclospora cayetanensis, Encephalitozoon intestinalis and Cryptosporidium parvum in water used for drinking. J. Water Health 1:117-123. [PubMed] [Google Scholar]
- 59.Drenaggi, D., O. Cirioni, A. Giacometti, A. Fiorentini, and G. Scalise. 1998. Cyclosporiasis in a traveler returning from South America. J. Travel Med. 5:153-155. [DOI] [PubMed] [Google Scholar]
- 60.Dubey, J. P., D. W. Thayer, C. A. Speer, and S. K. Shen. 1998. Effect of gamma irradiation on unsporulated and sporulated Toxoplasma gondii oocysts. Int. J. Parasitol. 28:369-375. [DOI] [PubMed] [Google Scholar]
- 61.Eberhard, M. L., A. J. da Silva, B. G. Lilley, and N. J. Pieniazek. 1999. Morphologic and molecular characterization of new Cyclospora species from Ethiopian monkeys: C. cercopitheci sp.n., C. colobi sp.n., and C. papionis sp.n. Emerg. Infect. Dis. 5:651-658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Eberhard, M. L., E. K. Nace, and A. R. Freeman. 1999. Survey for Cyclospora cayetanensis in domestic animals in an endemic area in Haiti. J. Parasitol. 85:562-563. [PubMed] [Google Scholar]
- 63.Eberhard, M. L., Y. R. Ortega, D. E. Hanes, E. K. Nace, R. Q. Do, M. G. Robl, K. Y. Won, C. Gavidia, N. L. Sass, K. Mansfield, A. Gozalo, J. Griffiths, R. Gilman, C. R. Sterling, and M. J. Arrowood. 2000. Attempts to establish experimental Cyclospora cayetanensis infection in laboratory animals. J. Parasitol. 86:577-582. [DOI] [PubMed] [Google Scholar]
- 64.Eberhard, M. L., N. J. Pieniazek, and M. J. Arrowood. 1997. Laboratory diagnosis of Cyclospora infections. Arch. Pathol. Lab. Med. 121:792-797. [PubMed] [Google Scholar]
- 65.el-Karamany, E. M., T. I. Zaher, and M. M. el-Bahnasawy. 2005. Role of water in the transmission of cyclosporiasis in Sharkia Governorate, Egypt. J. Egypt. Soc. Parasitol. 35:953-962. [PubMed] [Google Scholar]
- 66.Elshazly, A. M., H. M. Elsheikha, D. M. Soltan, K. A. Mohammad, and T. A. Morsy. 2007. Protozoal pollution of surface water sources in Dakahlia Governorate, Egypt. J. Egypt. Soc. Parasitol. 37:51-64. [PubMed] [Google Scholar]
- 67.Fayer, R., J. M. Trout, E. Walsh, and R. Cole. 2000. Rotifers ingest oocysts of Cryptosporidium parvum. J. Eukaryot. Microbiol. 47:161-163. [DOI] [PubMed] [Google Scholar]
- 68.Fleming, C. A., D. Caron, J. E. Gunn, and M. A. Barry. 1998. A foodborne outbreak of Cyclospora cayetanensis at a wedding: clinical features and risk factors for illness. Arch. Intern. Med. 158:1121-1125. [DOI] [PubMed] [Google Scholar]
- 69.Fryauff, D. J., R. Krippner, P. Prodjodipuro, C. Ewald, S. Kawengian, K. Pegelow, T. Yun, C. von Heydwolff-Wehnert, B. Oyofo, and R. Gross. 1999. Cyclospora cayetanensis among expatriate and indigenous populations of West Java, Indonesia. Emerg. Infect. Dis. 5:585-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Garcia-Lopez, H. L., L. E. Rodriguez-Tovar, and C. E. Medina-de la Garza. 1996. Identification of Cyclospora in poultry. Emerg. Infect. Dis. 2:356-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gascon, J., M. Corachan, J. A. Bombi, M. E. Valls, and J. M. Bordes. 1995. Cyclospora in patients with traveller's diarrhea. Scand. J. Infect. Dis. 27:511-514. [DOI] [PubMed] [Google Scholar]
- 72.Gascon, J., M. Corachan, M. E. Valls, A. Gene, and J. A. Bombi. 1993. Cyanobacteria-like body (CLB) in travelers with diarrhea. Scand. J. Infect. Dis. 25:253-257. [DOI] [PubMed] [Google Scholar]
- 73.Goncalves, E. M., I. H. Uemura, V. L. Castilho, and C. E. Corbett. 2005. Retrospective study of the occurrence of Cyclospora cayetanensis at Clinical Hospital of the University of Sao Paulo Medical School, SP. Rev. Soc. Bras. Med. Trop. 38:326-330. [DOI] [PubMed] [Google Scholar]
- 74.Graczyk, T. K., R. Fayer, R. Knight, B. Mhangami-Ruwende, J. M. Trout, A. J. da Silva, and N. J. Pieniazek. 2000. Mechanical transport and transmission of Cryptosporidium parvum oocysts by wild filth flies. Am. J. Trop. Med. Hyg. 63:178-183. [DOI] [PubMed] [Google Scholar]
- 75.Graczyk, T. K., B. H. Grimes, R. Knight, B. Szostakowska, W. Kruminis-Lozowska, M. Racewicz, L. Tamang, A. J. DaSilva, and P. Myjak. 2004. Mechanical transmission of Cryptosporidium parvum oocysts by flies. Wiad. Parazytol. 50:243-247. [PubMed] [Google Scholar]
- 76.Graczyk, T. K., Y. R. Ortega, and D. B. Conn. 1998. Recovery of waterborne oocysts of Cyclospora cayetanensis by Asian freshwater clams (Corbicula fluminea). Am. J. Trop. Med. Hyg. 59:928-932. [DOI] [PubMed] [Google Scholar]
- 77.Green, S. T., M. W. McKendrick, A. H. Mohsen, M. L. Schmid, and S. F. Prakasam. 2000. Two simultaneous cases of Cyclospora cayetanensis enteritis returning from the Dominican Republic. J. Travel Med. 7:41-42. [DOI] [PubMed] [Google Scholar]
- 78.Hale, D., W. Aldeen, and K. Carroll. 1994. Diarrhea associated with Cyanobacteria-like bodies in an immunocompetent host. An unusual epidemiological source. JAMA 271:144-145. [PubMed] [Google Scholar]
- 79.Hammond, R. 2005. Cyclospora outbreak in Florida, 2005, abstr. S15. Foodborne Threats Health Policies Pract. Surveill. Prev. Outbreak Invest. Int. Coord. Workshop, Washington, DC, 25 to 26 October 2005.
- 80.Hart, A. S., M. T. Ridinger, R. Soundarajan, C. S. Peters, A. L. Swiatlo, and F. E. Kocka. 1990. Novel organism associated with chronic diarrhoea in AIDS. Lancet 335:169-170. [DOI] [PubMed] [Google Scholar]
- 81.Helmy, M. M., L. A. Rashed, and H. S. Abdel-Fattah. 2006. Co-infection with Cryptosporidium parvum and Cyclospora cayetanensis in immunocompromised patients. J. Egypt. Soc. Parasitol. 36:613-627. [PubMed] [Google Scholar]
- 82.Herwaldt, B. L. 2000. Cyclospora cayetanensis: a review, focusing on the outbreaks of cyclosporiasis in the 1990s. Clin. Infect. Dis. 31:1040-1057. [DOI] [PubMed] [Google Scholar]
- 83.Herwaldt, B. L., and M. L. Ackers. 1997. An outbreak in 1996 of cyclosporiasis associated with imported raspberries. The Cyclospora Working Group. N. Engl. J. Med. 336:1548-1556. [DOI] [PubMed] [Google Scholar]
- 84.Herwaldt, B. L., and M. J. Beach. 1999. The return of Cyclospora in 1997: another outbreak of cyclosporiasis in North America associated with imported raspberries. The Cyclospora Working Group. Ann. Intern. Med. 130:210-220. [DOI] [PubMed] [Google Scholar]
- 85.Ho, A. Y., A. S. Lopez, M. G. Eberhart, R. Levenson, B. S. Finkel, A. J. da Silva, J. M. Roberts, P. A. Orlandi, C. C. Johnson, and B. L. Herwaldt. 2002. Outbreak of cyclosporiasis associated with imported raspberries, Philadelphia, Pennsylvania, 2000. Emerg. Infect. Dis. 8:783-788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hoang, L. M., M. Fyfe, C. Ong, J. Harb, S. Champagne, B. Dixon, and J. Isaac-Renton. 2005. Outbreak of cyclosporiasis in British Columbia associated with imported Thai basil. Epidemiol. Infect. 133:23-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hoge, C. W., P. Echeverria, R. Rajah, J. Jacobs, S. Malthouse, E. Chapman, L. M. Jimenez, and D. R. Shlim. 1995. Prevalence of Cyclospora species and other enteric pathogens among children less than 5 years of age in Nepal. J. Clin. Microbiol. 33:3058-3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hoge, C. W., D. R. Shlim, M. Ghimire, J. G. Rabold, P. Pandey, A. Walch, R. Rajah, P. Gaudio, and P. Echeverria. 1995. Placebo-controlled trial of co-trimoxazole for Cyclospora infections among travelers and foreign residents in Nepal. Lancet 345:691-693. [DOI] [PubMed] [Google Scholar]
- 89.Hoge, C. W., D. R. Shlim, R. Rajah, J. Triplett, M. Shear, J. G. Rabold, and P. Echeverria. 1993. Epidemiology of diarrhoeal illness associated with coccidian-like organism among travelers and foreign residents in Nepal. Lancet 341:1175-1179. [DOI] [PubMed] [Google Scholar]
- 90.Huamanchay, O., L. Genzlinger, M. Iglesias, and Y. R. Ortega. 2004. Ingestion of Cryptosporidium oocysts by Caenorhabditis elegans. J. Parasitol. 90:1176-1178. [DOI] [PubMed] [Google Scholar]
- 91.Huang, P., J. T. Weber, D. M. Sosin, P. M. Griffin, E. G. Long, J. J. Murphy, F. Kocka, C. Peters, and C. Kallick. 1995. The first reported outbreak of diarrheal illness associated with Cyclospora in the United States. Ann. Intern. Med. 123:409-414. [DOI] [PubMed] [Google Scholar]
- 92.Hussein, E. M. 2007. Molecular identification of Cyclospora spp. using multiplex PCR from diarrheic children compared to other conventional methods. J. Egypt. Soc. Parasitol. 37:585-598. [PubMed] [Google Scholar]
- 93.Hussein, E. M., A. H. Abdul-Manaem, and S. L. el-Attary. 2005. Cyclospora cayetanensis oocysts in sputum of a patient with active pulmonary tuberculosis, case report in Ismailia, Egypt. J. Egypt. Soc. Parasitol. 35:787-793. [PubMed] [Google Scholar]
- 94.Hussein, E. M., A. A. El-Moamly, H. A. Dawoud, H. Fahmy, H. E. El-Shal, and N. A. Sabek. 2007. Real-time PCR and flow cytometry in detection of Cyclospora oocysts in fecal samples of symptomatic and asymptomatic pediatrics patients. J. Egypt. Soc. Parasitol. 37:151-170. [PubMed] [Google Scholar]
- 95.Jinneman, K. C., J. H. Wetherington, W. E. Hill, A. M. Adams, J. M. Johnson, B. J. Tenge, N. L. Dang, R. L. Manger, and M. M. Wekell. 1998. Template preparation for PCR and RFLP of amplification products for the detection and identification of Cyclospora sp. and Eimeria spp. oocysts directly from raspberries. J. Food Prot. 61:1497-1503. [DOI] [PubMed] [Google Scholar]
- 96.Jinneman, K. C., J. H. Wetherington, W. E. Hill, C. J. Omiescinski, A. M. Adams, J. M. Johnson, B. J. Tenge, N. L. Dang, and M. M. Wekell. 1999. An oligonucleotide-ligation assay for the differentiation between Cyclospora and Eimeria spp. polymerase chain reaction amplification products. J. Food Prot. 62:682-685. [DOI] [PubMed] [Google Scholar]
- 97.Kansouzidou, A., C. Charitidou, T. Varnis, N. Vavatsi, and F. Kamaria. 2004. Cyclospora cayetanensis in a patient with travelers' diarrhea: case report and review. J. Travel Med. 11:61-63. [DOI] [PubMed] [Google Scholar]
- 98.Kenney, S. J., G. L. Anderson, P. L. Williams, P. D. Millner, and L. R. Beuchat. 2005. Persistence of Escherichia coli O157:H7, Salmonella Newport, and Salmonella Poona in the gut of a free-living nematode, Caenorhabditis elegans, and transmission to progeny and uninfected nematodes. Int. J. Food Microbiol. 101:227-236. [DOI] [PubMed] [Google Scholar]
- 99.Kenney, S. J., G. L. Anderson, P. L. Williams, P. D. Millner, and L. R. Beuchat. 2006. Migration of Caenorhabditis elegans to manure and manure compost and potential for transport of Salmonella Newport to fruits and vegetables. Int. J. Food Microbiol. 106:61-68. [DOI] [PubMed] [Google Scholar]
- 100.Kniel, K. E., D. S. Lindsay, S. S. Sumner, C. R. Hackney, M. D. Pierson, and J. P. Dubey. 2002. Examination of attachment and survival of Toxoplasma gondii oocysts on raspberries and blueberries. J. Parasitol. 88:790-793. [DOI] [PubMed] [Google Scholar]
- 101.Kniel, K. E., A. E. Shearer, J. L. Cascarino, G. C. Wilkins, and M. C. Jenkins. 2007. High hydrostatic pressure and UV light treatment of produce contaminated with Eimeria acervulina as a Cyclospora cayetanensis surrogate. J. Food Prot. 70:2837-2842. [DOI] [PubMed] [Google Scholar]
- 102.Kwakye-Nuako, G., P. Borketey, I. Mensah-Attipoe, R. Asmah, and P. yeh-Kumi. 2007. Sachet drinking water in Accra: the potential threats of transmission of enteric pathogenic protozoan organisms. Ghana Med. J. 41:62-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lalonde, L. F., and A. A. Gajadhar. 2008. Highly sensitive and specific PCR assay for reliable detection of Cyclospora cayetanensis oocysts. Appl. Environ. Microbiol. 74:4354-4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lebbad, M., and E. Linder. 1993. Newly discovered organism behind diarrhea. All patients had recently been abroad. Lakartidningen 90:951-952. [PubMed] [Google Scholar]
- 105.Lee, M. B., and E. H. Lee. 2001. Coccidial contamination of raspberries: mock contamination with Eimeria acervulina as a model for decontamination treatment studies. J. Food Prot. 64:1854-1857. [DOI] [PubMed] [Google Scholar]
- 106.Li, G., S. Xiao, R. Zhou, W. Li, and H. Wadeh. 2007. Molecular characterization of Cyclospora-like organism from dairy cattle. Parasitol. Res. 100:955-961. [DOI] [PubMed] [Google Scholar]
- 107.Lindsay, D. S., D. Holliman, G. J. Flick, D. G. Goodwin, S. M. Mitchell, and J. P. Dubey. 2008. Effects of high pressure processing on Toxoplasma gondii oocysts on raspberries. J. Parasitol. 94:757-758. [DOI] [PubMed] [Google Scholar]
- 108.Long, E. G., A. T. Tsin, and B. A. Robinson. 1985. Comparison of the FeKal CON-Trate system with the formalin-ethyl acetate technique for detection of intestinal parasites. J. Clin. Microbiol. 22:210-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Long, E. G., E. H. White, W. W. Carmichael, P. M. Quinlisk, R. Raja, B. L. Swisher, H. Daugharty, and M. T. Cohen. 1991. Morphologic and staining characteristics of a cyanobacterium-like organism associated with diarrhea. J. Infect. Dis. 164:199-202. [DOI] [PubMed] [Google Scholar]
- 110.Lontie, M., K. Degroote, J. Michiels, J. Bellers, E. Mangelschots, and J. Vandepitte. 1995. Cyclospora sp.: a coccidian that causes diarrhoea in travelers. Acta Clin. Belg. 50:288-290. [DOI] [PubMed] [Google Scholar]
- 111.Lopez, A. S., J. M. Bendik, J. Y. Alliance, J. M. Roberts, A. J. da Silva, I. N. Moura, M. J. Arrowood, M. L. Eberhard, and B. L. Herwaldt. 2003. Epidemiology of Cyclospora cayetanensis and other intestinal parasites in a community in Haiti. J. Clin. Microbiol. 41:2047-2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lopez, A. S., D. R. Dodson, M. J. Arrowood, P. A. Orlandi, Jr., A. J. da Silva, J. W. Bier, S. D. Hanauer, R. L. Kuster, S. Oltman, M. S. Baldwin, K. Y. Won, E. M. Nace, M. L. Eberhard, and B. L. Herwaldt. 2001. Outbreak of cyclosporiasis associated with basil in Missouri in 1999. Clin. Infect. Dis. 32:1010-1017. [DOI] [PubMed] [Google Scholar]
- 113.Lopez, F. A., J. Manglicmot, T. M. Schmidt, C. Yeh, H. V. Smith, and D. A. Relman. 1999. Molecular characterization of Cyclospora-like organisms from baboons. J. Infect. Dis. 179:670-676. [DOI] [PubMed] [Google Scholar]
- 114.Madico, G., R. H. Gilman, E. Miranda, L. Cabrera, and C. R. Sterling. 1993. Treatment of Cyclospora infections with co-trimoxazole. Lancet 342:122-123. [DOI] [PubMed] [Google Scholar]
- 115.Madico, G., J. McDonald, R. H. Gilman, L. Cabrera, and C. R. Sterling. 1997. Epidemiology and treatment of Cyclospora cayetanensis infection in Peruvian children. Clin. Infect. Dis. 24:977-981. [DOI] [PubMed] [Google Scholar]
- 116.Madrid, V., E. Torrejon, N. Rivera, and M. Madrid. 1998. Cyclosporosis. Report of a clinical case in Concepcion, Chile. Rev. Med. Chil. 126:559-562. [PubMed] [Google Scholar]
- 117.Maggi, P., O. Brandonisio, A. M. Larocca, M. Rollo, M. A. Panaro, A. Marangi, R. Marzo, G. Angarano, and G. Pastore. 1995. Cyclospora in AIDS patients: not always an agent of diarrhoeic syndrome. New Microbiol. 18:73-76. [PubMed] [Google Scholar]
- 118.Maratim, A. C., K. K. Kamar, A. Ngindu, C. N. Akoru, L. Diero, and J. Sidle. 2002. Safranin staining of Cyclospora cayetanensis oocysts not requiring microwave heating. Br. J. Biomed. Sci. 59:114-115. [PubMed] [Google Scholar]
- 119.Markus, M. B., and J. A. Frean. 1993. Occurrence of human Cyclospora infection in sub-Saharan Africa. S. Afr. Med. J. 83:862-863. [PubMed] [Google Scholar]
- 120.Masucci, L., R. Graffeo, M. Siciliano, A. Franceschelli, F. Bugli, and G. Fadda. 2008. First Italian case of cyclosporiasis in an immunocompetent woman: local acquired infection. New Microbiol. 31:281-284. [PubMed] [Google Scholar]
- 121.McDougall, R. J., and M. W. Tandy. 1993. Coccidian/cyanobacterium-like bodies as a cause of diarrhea in Australia. Pathology 25:375-378. [DOI] [PubMed] [Google Scholar]
- 122.Mead, P. S., L. Slutsker, V. Dietz, L. F. McCaig, J. S. Bresee, C. Shapiro, P. M. Griffin, and R. V. Tauxe. 1999. Food-related illness and death in the United States. Emerg. Infect. Dis. 5:607-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Medina-de la Garza, C. E., H. L. Garcia-Lopez, M. C. Salinas-Carmona, and D. J. Gonzalez-Spencer. 1997. Use of discontinuous Percoll gradients to isolate Cyclospora oocysts. Ann. Trop. Med. Parasitol. 91:319-321. [DOI] [PubMed] [Google Scholar]
- 124.Mendoza, D., F. A. Nunez, A. Escobedo, L. Pelayo, M. Fernandez, D. Torres, and R. A. Cordovi. 2001. Intestinal parasitic infections in 4 child day-care centers located in San Miguel del Padron municipality, Havana City, 1998. Rev. Cubana Med. Trop. 53:189-193. [PubMed] [Google Scholar]
- 125.Miegeville, M., V. Koubi, L. C. Dan, J. P. Barbier, and P. D. Cam. 2003. Cyclospora cayetanensis presence in aquatic surroundings in Hanoi (Vietnam). Environmental study (well water, lakes and rivers). Bull. Soc. Pathol. Exot. 96:149-152. [PubMed] [Google Scholar]
- 126.Mohle-Boetani, J. C., S. B. Werner, S. H. Waterman, and D. J. Vugia. 2000. The impact of health communication and enhanced laboratory-based surveillance on detection of cyclosporiasis outbreaks in California. Emerg. Infect. Dis. 6:200-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mosimann, M., X. M. Nguyen, and H. Furrer. 1999. Excessive watery diarrhea and pronounced fatigue due to Cyclospora cayetanensis infection in an HIV infected traveler returning from the tropics. Schweiz. Med. Wochenschr. 129:1158-1161. [PubMed] [Google Scholar]
- 128.Mundaca, C. C., P. A. Torres-Slimming, R. V. Araujo-Castillo, M. Moran, D. J. Bacon, Y. Ortega, R. H. Gilman, and D. L. Blazes. 2008. Use of PCR to improve diagnostic yield in an outbreak of cyclosporiasis in Lima, Peru. Trans. R. Soc. Trop. Med. Hyg. 102:712-717. [DOI] [PubMed] [Google Scholar]
- 129.Naranjo, J., C. R. Sterling, and R. Gilman. 1989. Cryptosporidium muris-like objects from fecal samples of Peruvians, abstr. 324. Abstr. 38th Annu. Meet. Am. Soc. Trop. Med. Hyg., Honolulu, HI.
- 130.Nunez, F. A., O. M. Gonzalez, J. R. Bravo, A. A. Escobedo, and I. Gonzalez. 2003. Intestinal parasitosis in children admitted to the Pediatric Teaching Hospital of Cerro, Havana City, Cuba. Rev. Cubana Med. Trop. 55:19-26. [PubMed] [Google Scholar]
- 131.Ockelford, A., J. Dowling, and A. J. Morris. 1997. Cyclospora cayetanensis diarrhoea in a traveler. N. Z. Med. J. 110:404. [PubMed] [Google Scholar]
- 132.Olivier, C., S. van de Pas, P. W. Lepp, K. Yoder, and D. A. Relman. 2001. Sequence variability in the first internal transcribed spacer region within and among Cyclospora species is consistent with polyparasitism. Int. J. Parasitol. 31:1475-1487. [DOI] [PubMed] [Google Scholar]
- 133.Ooi, W. W., S. K. Zimmerman, and C. A. Needham. 1995. Cyclospora species as a gastrointestinal pathogen in immunocompetent hosts. J. Clin. Microbiol. 33:1267-1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Orlandi, P. A., L. Carter, A. M. Brinker, A. J. da Silva, D. M. Chu, K. A. Lampel, and S. R. Monday. 2003. Targeting single-nucleotide polymorphisms in the 18S rRNA gene to differentiate Cyclospora species from Eimeria species by multiplex PCR. Appl. Environ. Microbiol. 69:4806-4813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Orlandi, P. A., D. M. Chu, J. W. Bier, and G. J. Jackson. 2002. Parasites in the food supply. Food Technol. 56:72. [Google Scholar]
- 136.Orlandi, P. A., and K. A. Lampel. 2000. Extraction-free, filter-based template preparation for rapid and sensitive PCR detection of pathogenic parasitic protozoa. J. Clin. Microbiol. 38:2271-2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ortega, Y. R., A. Mann, M. P. Torres, and V. Cama. 2008. Efficacy of gaseous chlorine dioxide as a sanitizer against Cryptosporidium parvum, Cyclospora cayetanensis, and Encephalitozoon intestinalis on produce. J. Food Prot. 71:2410-2414. [DOI] [PubMed] [Google Scholar]
- 138.Ortega, Y. R., R. H. Gilman, and C. R. Sterling. 1994. A new coccidian parasite (Apicomplexa: Eimeriidae) from humans. J. Parasitol. 80:625-629. [PubMed] [Google Scholar]
- 139.Ortega, Y. R., and J. Liao. 2006. Microwave inactivation of Cyclospora cayetanensis sporulation and viability of Cryptosporidium parvum oocysts. J. Food Prot. 69:1957-1960. [DOI] [PubMed] [Google Scholar]
- 140.Ortega, Y. R., R. Nagle, R. H. Gilman, J. Watanabe, J. Miyagui, H. Quispe, P. Kanagusuku, C. Roxas, and C. R. Sterling. 1997. Pathologic and clinical findings in patients with cyclosporiasis and a description of intracellular parasite life-cycle stages. J. Infect. Dis. 176:1584-1589. [DOI] [PubMed] [Google Scholar]
- 141.Ortega, Y. R., C. R. Roxas, R. H. Gilman, N. J. Miller, L. Cabrera, C. Taquiri, and C. R. Sterling. 1997. Isolation of Cryptosporidium parvum and Cyclospora cayetanensis from vegetables collected in markets of an endemic region in Peru. Am. J. Trop. Med. Hyg. 57:683-686. [DOI] [PubMed] [Google Scholar]
- 142.Ortega, Y. R., C. R. Sterling, R. H. Gilman, V. A. Cama, and F. Diaz. 1993. Cyclospora species—a new protozoan pathogen of humans. N. Engl. J. Med. 328:1308-1312. [DOI] [PubMed] [Google Scholar]
- 143.Ozdamar, M. T., and S. Turkoglu, and E. Hakko. 2008. Outbreak of cyclosporiasis in Istanbul, Turkey, during an extremely dry and warm summer, p. 988. Abstr. 18th Cong. Clin. Microbiol. Infect. Dis., Barcelona, Spain.
- 144.Pape, J. W., R. I. Verdier, M. Boncy, J. Boncy, and W. D. Johnson, Jr. 1994. Cyclospora infection in adults infected with HIV. Clinical manifestations, treatment, and prophylaxis. Ann. Intern. Med. 121:654-657. [DOI] [PubMed] [Google Scholar]
- 145.Perez, C. G., S. O. Cordova Paz, V. F. Vargas, S. Velasco, Jr., B. L. Sempere, M. M. Sanchez, and M. J. Rosales. 2008. Prevalence of enteroparasites and genotyping of Giardia lamblia in Peruvian children. Parasitol. Res. 103:459-465. [DOI] [PubMed] [Google Scholar]
- 146.Perez, C. G., P. A. Hitos, D. Romero, M. M. Sanchez, A. Pontes, A. Osuna, and M. J. Rosales. 2008. Intestinal parasitism in the animals of the zoological garden “Pena Escrita” (Almunecar, Spain). Vet. Parasitol. 156:302-309. [DOI] [PubMed] [Google Scholar]
- 147.Petry, F., J. Hofstatter, B. K. Schulz, G. Deitrich, M. Jung, and P. Schirmacher. 1997. Cyclospora cayetanensis: first imported infections in Germany. Infection 25:167-170. [DOI] [PubMed] [Google Scholar]
- 148.Pinel, C., S. Blachier, C. Jambon, M. P. Brenier-Pinchart, R. Grillot, and P. Ambroise-Thomas. 2001. Cyclosporiasis: an emerging intestinal protozoan infection. Presse Med. 30:23. [PubMed] [Google Scholar]
- 149.Pollok, R. C., R. P. Bendall, A. Moody, P. L. Chiodini, and D. R. Churchill. 1992. Traveller's diarrhoea associated with cyanobacterium-like bodies. Lancet 340:556-557. [DOI] [PubMed] [Google Scholar]
- 150.Ponce-Macotela, M., C. Cob-Sosa, and M. N. Martinez-Gordillo. 1996. Cyclospora in 2 Mexican children. Rev. Invest. Clin. 48:461-463. [PubMed] [Google Scholar]
- 151.Pratdesaba, R. A., M. Gonzalez, E. Piedrasanta, C. Merida, K. Contreras, C. Vela, F. Culajay, L. Flores, and O. Torres. 2001. Cyclospora cayetanensis in three populations at risk in Guatemala. J. Clin. Microbiol. 39:2951-2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Pratdesaba, R. A., T. Velaquez, and M. F. Torres. 1994. Occurrence of Isospora belli and cyanobacterium-like bodies in Guatemala. Ann. Trop. Med. Parasitol. 88:449-450. [DOI] [PubMed] [Google Scholar]
- 153.Puente, S., A. Morente, T. Garcia-Benayas, M. Subirats, J. Gascon, and J. M. Gonzalez-Lahoz. 2006. Cyclosporiasis: a point source outbreak acquired in Guatemala. J. Travel Med. 13:334-337. [DOI] [PubMed] [Google Scholar]
- 154.Purych, D. B., I. L. Perry, D. Bulawka, K. T. Kowalewska-Grochowska, and B. L. Oldale. 1995. A case of Cyclospora infection in an Albertan traveler. Can. Commun. Dis. Rep. 21:88-91. [PubMed] [Google Scholar]
- 155.Rabold, J. G., C. W. Hoge, D. R. Shlim, C. Kefford, R. Rajah, and P. Echeverria. 1994. Cyclospora outbreak associated with chlorinated drinking water. Lancet 344:1360-1361. [DOI] [PubMed] [Google Scholar]
- 156.Raccurt, C. P., B. Fouche, P. Agnamey, J. Menotti, T. Chouaki, A. Totet, and J. W. Pape. 2008. Presence of Enterocytozoon bieneusi associated with intestinal coccidia in patients with chronic diarrhea visiting an HIV center in Haiti. Am. J. Trop. Med. Hyg. 79:579-580. [PubMed] [Google Scholar]
- 157.Relman, D. A., T. M. Schmidt, A. Gajadhar, M. Sogin, J. Cross, K. Yoder, O. Sethabutr, and P. Echeverria. 1996. Molecular phylogenetic analysis of Cyclospora, the human intestinal pathogen, suggests that it is closely related to Eimeria species. J. Infect. Dis. 173:440-445. [DOI] [PubMed] [Google Scholar]
- 158.Richardson, R. F., Jr., B. F. Remler, B. Katirji, and M. H. Murad. 1998. Guillain-Barre syndrome after Cyclospora infection. Muscle Nerve 21:669-671. [DOI] [PubMed] [Google Scholar]
- 159.Rijpstra, A. C., and J. J. Laarman. 1993. Repeated findings of unidentified small Isospora-like coccidia in faecal specimens from travelers returning to The Netherlands. Trop. Geogr. Med. 45:280-282. [PubMed] [Google Scholar]
- 160.Riner, D. K., A. S. Mullin, S. Y. Lucas, J. H. Cross, and H. D. Lindquist. 2007. Enhanced concentration and isolation of Cyclospora cayetanensis oocysts from human fecal samples. J. Microbiol. Methods 71:75-77. [DOI] [PubMed] [Google Scholar]
- 161.Robertson, L. J., B. Gjerde, and A. T. Campbell. 2000. Isolation of Cyclospora oocysts from fruits and vegetables using lectin-coated paramagnetic beads. J. Food Prot. 63:1410-1414. [DOI] [PubMed] [Google Scholar]
- 162.Sadaka, H. A., and M. A. Zoheir. 2001. Experimental studies on cyclosporiosis. J. Egypt. Soc. Parasitol. 31:65-77. [PubMed] [Google Scholar]
- 163.Sancak, B., Y. Akyon, and S. Erguven. 2006. Cyclospora infection in five immunocompetent patients in a Turkish university hospital. J. Med. Microbiol. 55:459-462. [DOI] [PubMed] [Google Scholar]
- 164.Santana, A. M., F. A. Nunez Fernandez, A. J. Perez, B. M. Barrero, and V. B. Velazquez. 2000. Emergence of a new pathogen: Cyclospora cayetanensis in patients infected with human immunodeficiency virus. Rev. Cubana Med. Trop. 52:66-69. [PubMed] [Google Scholar]
- 165.Sathyanarayanan, L., and Y. Ortega. 2004. Effects of pesticides on sporulation of Cyclospora cayetanensis and viability of Cryptosporidium parvum. J. Food Prot. 67:1044-1049. [DOI] [PubMed] [Google Scholar]
- 166.Sathyanarayanan, L., and Y. Ortega. 2006. Effects of temperature and different food matrices on Cyclospora cayetanensis oocyst sporulation. J. Parasitol. 92:218-222. [DOI] [PubMed] [Google Scholar]
- 167.Schubach, T. M., E. S. Neves, A. C. Leite, A. Q. Araujo, and H. de Moura. 1997. Cyclospora cayetanensis in an asymptomatic patient infected with HIV and HTLV-1. Trans. R. Soc. Trop. Med. Hyg. 91:175. [DOI] [PubMed] [Google Scholar]
- 168.Sherchand, J. B., J. H. Cross, M. Jimba, S. Sherchand, and M. P. Shrestha. 1999. Study of Cyclospora cayetanensis in health care facilities, sewage water and green leafy vegetables in Nepal. Southeast Asian J. Trop. Med. Public Health 30:58-63. [PubMed] [Google Scholar]
- 169.Shields, J. M., and B. H. Olson. 2003. Cyclospora cayetanensis: a review of an emerging parasitic coccidian. Int. J. Parasitol. 33:371-391. [DOI] [PubMed] [Google Scholar]
- 170.Shields, J. M., and B. H. Olson. 2003. PCR-restriction fragment length polymorphism method for detection of Cyclospora cayetanensis in environmental waters without microscopic confirmation. Appl. Environ. Microbiol. 69:4662-4669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Shlim, D. R., M. T. Cohen, M. Eaton, R. Rajah, E. G. Long, and B. L. Ungar. 1991. An alga-like organism associated with an outbreak of prolonged diarrhea among foreigners in Nepal. Am. J. Trop. Med. Hyg. 45:383-389. [DOI] [PubMed] [Google Scholar]
- 172.Sifuentes-Osornio, J., G. Porras-Cortes, R. P. Bendall, F. Morales-Villarreal, G. Reyes-Teran, and G. M. Ruiz-Palacios. 1995. Cyclospora cayetanensis infection in patients with and without AIDS: biliary disease as another clinical manifestation. Clin. Infect. Dis. 21:1092-1097. [DOI] [PubMed] [Google Scholar]
- 173.Sinniah, B., B. Rajeswari, S. Johari, K. Ramakrishnan, S. W. Yusoff, and M. Rohela. 1994. Cyclospora sp. causing diarrhea in man. Southeast Asian J. Trop. Med. Public Health 25:221-223. [PubMed] [Google Scholar]
- 174.Smith, H. V., C. A. Paton, R. W. Girdwood, and M. M. Mtambo. 1996. Cyclospora in non-human primates in Gombe, Tanzania. Vet. Rec. 138:528. [PubMed] [Google Scholar]
- 175.Smith, P. M. 1993. Traveller's diarrhoea associated with a cyanobacterium-like body. Med. J. Aust. 158:724. [DOI] [PubMed] [Google Scholar]
- 176.Soave, R., J. P. Dubey, L. J. Ramos, and M. Tummings. 1986. A new intestinal pathogen? Clin. Res. 34:533A. [Google Scholar]
- 177.Sturbaum, G. D., Y. R. Ortega, R. H. Gilman, C. R. Sterling, L. Cabrera, and D. A. Klein. 1998. Detection of Cyclospora cayetanensis in wastewater. Appl. Environ. Microbiol. 64:2284-2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Sun, T., C. F. Ilardi, D. Asnis, A. R. Bresciani, S. Goldenberg, B. Roberts, and S. Teichberg. 1996. Light and electron microscopic identification of Cyclospora species in the small intestine. Evidence of the presence of asexual life cycle in human host. Am. J. Clin. Pathol. 105:216-220. [DOI] [PubMed] [Google Scholar]
- 179.Szostakowska, B., W. Kruminis-Lozowska, M. Racewicz, R. Knight, L. Tamang, P. Myjak, and T. K. Graczyk. 2004. Cryptosporidium parvum and Giardia lamblia recovered from flies on a cattle farm and in a landfill. Appl. Environ. Microbiol. 70:3742-3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Torres-Slimming, P. A., C. C. Mundaca, M. Moran, J. Quispe, O. Colina, D. J. Bacon, A. G. Lescano, R. H. Gilman, and D. L. Blazes. 2006. Outbreak of cyclosporiasis at a naval base in Lima, Peru. Am. J. Trop. Med. Hyg. 75:546-548. [PubMed] [Google Scholar]
- 181.Tram, N. T., L. M. Hoang, P. D. Cam, P. T. Chung, M. W. Fyfe, J. L. Isaac-Renton, and C. S. Ong. 2008. Cyclospora spp. in herbs and water samples collected from markets and farms in Hanoi, Vietnam. Trop. Med. Int. Health 13:1415-1420. [DOI] [PubMed] [Google Scholar]
- 182.Turgay, N., A. Yolasigmaz, D. D. Erdogan, F. Y. Zeyrek, and A. Uner. 2007. Incidence of cyclosporiasis in patients with gastrointestinal symptoms in western Turkey. Med. Sci. Monit. 13:CR34-CR39. [PubMed] [Google Scholar]
- 183.Turgay, N., A. Yolasigmaz, and A. Uner. 2006. A human case of cyclosporiasis after traveling in the subtropics. Turkiye Parazitol. Derg. 30:83-85. [PubMed] [Google Scholar]
- 184.Varma, M., J. D. Hester, F. W. Schaefer III, M. W. Ware, and H. D. Lindquist. 2003. Detection of Cyclospora cayetanensis using a quantitative real-time PCR assay. J. Microbiol. Methods 53:27-36. [DOI] [PubMed] [Google Scholar]
- 185.Velasquez, J. N., S. Carnevale, M. Cabrera, L. Kuo, A. Chertcoff, M. Mariano, J. P. Bozzini, C. Etchart, R. Argento, and C. di Risio. 2004. Cyclospora cayetanensis in patients with AIDS and chronic diarrhea. Acta Gastroenterol. Latinoam. 34:133-137. [PubMed] [Google Scholar]
- 186.Verdier, R. I., D. W. Fitzgerald, W. D. Johnson, Jr., and J. W. Pape. 2000. Trimethoprim-sulfamethoxazole compared with ciprofloxacin for treatment and prophylaxis of Isospora belli and Cyclospora cayetanensis infection in HIV-infected patients. A randomized, controlled trial. Ann. Intern. Med. 132:885-888. [DOI] [PubMed] [Google Scholar]
- 187.Verweij, J. J., D. Laeijendecker, E. A. Brienen, L. van Lieshout, and A. M. Polderman. 2003. Detection of Cyclospora cayetanensis in travellers returning from the tropics and subtropics using microscopy and real-time PCR. Int. J. Med. Microbiol. 293:199-202. [DOI] [PubMed] [Google Scholar]
- 188.Villard, O., R. Himy, C. Brogard, and M. Kremer. 1993. Diarrhea syndrome associated with the presence of Cyanobacterium-like bodies. Gastroenterol. Clin. Biol. 17:401-402. [PubMed] [Google Scholar]
- 189.Visvesvara, G. S., H. Moura, E. Kovacs-Nace, S. Wallace, and M. L. Eberhard. 1997. Uniform staining of Cyclospora oocysts in fecal smears by a modified safranin technique with microwave heating. J. Clin. Microbiol. 35:730-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Vuong, T. A., T. T. Nguyen, L. T. Klank, D. C. Phung, and A. Dalsgaard. 2007. Faecal and protozoan parasite contamination of water spinach (Ipomoea aquatica) cultivated in urban wastewater in Phnom Penh, Cambodia. Trop. Med. Int. Health 12(Suppl. 2):73-81. [DOI] [PubMed] [Google Scholar]
- 191.Wang, K. X., C. P. Li, J. Wang, and Y. Tian. 2002. Cyclospora cayetanensis in Anhui, China. World J. Gastroenterol. 8:1144-1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Wurtz, R. M., F. E. Kocka, C. S. Peters, C. M. Weldon-Linne, A. Kuritza, and P. Yungbluth. 1993. Clinical characteristics of seven cases of diarrhea associated with a novel acid-fast organism in the stool. Clin. Infect. Dis. 16:136-138. [DOI] [PubMed] [Google Scholar]
- 193.Xiao, S. M., G. Q. Li, R. Q. Zhou, W. H. Li, and J. W. Yang. 2007. Combined PCR-oligonucleotide ligation assay for detection of dairy cattle-derived Cyclospora sp. Vet. Parasitol. 149:185-190. [DOI] [PubMed] [Google Scholar]
- 194.Yai, L. E., A. R. Bauab, M. P. Hirschfeld, M. L. de Oliveira, and J. T. Damaceno. 1997. The first two cases of Cyclospora in dogs, Sao Paulo, Brazil. Rev. Inst. Med. Trop. Sao Paulo 39:177-179. [DOI] [PubMed] [Google Scholar]
- 195.Yazar, S., S. Yalcln, and I. Sahin. 2004. Human cyclosporiosis in Turkey. World J. Gastroenterol. 10:1844-1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Zar, F. A., E. El-Bayoumi, and M. M. Yungbluth. 2001. Histologic proof of acalculous cholecystitis due to Cyclospora cayetanensis. Clin. Infect. Dis. 33:E140-E141. [DOI] [PubMed] [Google Scholar]
- 197.Zerpa, R., N. Uchima, and L. Huicho. 1995. Cyclospora cayetanensis associated with watery diarrhoea in Peruvian patients. J. Trop. Med. Hyg. 98:325-329. [PubMed] [Google Scholar]
- 198.Zimmer, S. M., A. N. Schuetz, and C. Franco-Paredes. 2007. Efficacy of nitazoxanide for cyclosporiasis in patients with sulfa allergy. Clin. Infect. Dis. 44:466-467. [DOI] [PubMed] [Google Scholar]