Abstract
Summary: The licensure and recommendation of varicella vaccine in the mid-1990s in the United States have led to dramatic declines in varicella incidence and varicella-related deaths and hospitalizations. Varicella outbreaks remain common and occur increasingly in highly vaccinated populations. Breakthrough varicella in vaccinated individuals is characteristically mild, typically with fewer lesions that frequently do not progress to a vesicular stage. As such, the laboratory diagnosis of varicella has grown increasingly important, particularly in outbreak settings. In this review the impact of varicella vaccine on varicella-zoster virus (VZV) disease, arising complications in the effective diagnosis and monitoring of VZV transmission, and the relative strengths and limitations of currently available laboratory diagnostic techniques are all addressed. Since disease symptoms often resolve in outbreak settings before suitable test specimens can be obtained, the need to develop new diagnostic approaches that rely on alternative patient samples is also discussed.
INTRODUCTION
Varicella-zoster virus (VZV) causes two distinct diseases, varicella (chickenpox) and herpes zoster (HZ) (shingles). Varicella is a highly contagious disease that is transmitted by the airborne route from person to person; secondary attack rates for susceptible household contacts range from 61% to 100% (60, 113). During primary infection, VZV establishes a lifelong latent infection in the dorsal root ganglia and can subsequently reactivate from infected neurons to cause HZ (86). VZV can also be transmitted from patients with HZ; however, it is less contagious than that from patients with varicella. A household study reported that 16% of susceptible children <15 years old exposed to herpes zoster developed varicella (121). Unlike transmission from cases of varicella, transmission from cases of HZ appears to occur most commonly through direct contact with lesions, although there have been reports that suggested that airborne transmission occurs (65, 83, 117, 130, 131, 155).
The VZV genome consists of 125 kb of linear, double-stranded DNA comprising one long and one short unique region, each flanked by inverted repeats (33), and five internal repeat regions (R1 to R5) have been identified. The VZV genome contains at least 71 open reading frames (ORFs), three of which are duplicated in the inverted repeat regions. The functions of many of the proteins encoded by these genes have been characterized (10). In the prevaccine era, varicella was essentially a universally experienced infection of childhood in countries with a temperate climate; in the United States, approximately 98% of the population was seropositive for VZV by the age of 20 years. The development of a live-attenuated vaccine for varicella was first reported in 1974 and was derived by the serial and successive passage of a wild-type clinical VZV isolate in primary guinea pig embryo fibroblasts and human diploid cells (WI-38) (132, 134, 135). The Varivax varicella vaccine preparation was passaged several additional times in human MRC5 cells. A single-dose universal childhood varicella vaccination program was initiated in the United States in 1995 for children aged 12 to 18 months (22). According to the U.S. National Immunization Survey, single-dose varicella vaccine coverage for children aged 19 to 35 months had reached 89% in 2006 (24). Hospitalizations and deaths due to varicella have concomitantly declined by 80% to 90% (62, 97, 157).
Although varicella vaccination has dramatically reduced the incidence of both varicella disease and severe outcomes, several issues have emerged that could prevent or delay the introduction of broad varicella vaccination programs by other countries (88). Typically, mild breakthrough infection has been common among vaccinated children in day care and primary school outbreaks, and this observation led to a revised recommendation for a 2-dose schedule in 2006 (25). While this step is expected to improve levels of immunity to VZV in vaccinated persons, the increased cost burden associated with this measure could make vaccination cost-prohibitive in some countries. In addition, the reduced rate of VZV reexposure among adults with latent VZV infections has resulted in speculation that protective levels of VZV immunity might wane at an earlier age, leading to a younger average onset of herpes zoster (12). Finally, recent observations of vaccine-wild-type recombinant strains obtained from cases of herpes zoster in vaccinated children are expected to complicate the approach to performing surveillance for varicella vaccine adverse events.
In temperate climates, varicella typically occurred before the age of 4 years, and annual peaks occurred in late winter and early spring. Varicella seasonality has been suppressed as a consequence of high vaccine coverage rates in the United States, but it still evident in temperate countries with no or limited varicella vaccination policies. In tropical climates, the transmission dynamics of VZV are quite different, with no apparent seasonality, and a large fraction of the population (∼20%) remains susceptible into adulthood (76). The introduction of the vaccine into tropical countries could thus be anticipated to have an even higher public health impact than in temperate climates, since primary infection typically causes much more severe disease in adults and since women of child-bearing age would be expected to be at a higher risk for congenital varicella infection.
The purpose of this review is to provide a detailed overview of the impact of routine childhood varicella immunization in the United States, to describe the rationale for recommending a second dose, the potential threat of earlier-onset zoster, and the implications of recombination in superinfected vaccine recipients.
EPIDEMIOLOGY OF VARICELLA IN THE UNITED STATES
Prevaccine Epidemiology
Before the introduction of varicella vaccine in the United States in 1995, the number of annual varicella cases approximated the birth cohort of 4 million. This resulted in a per annum varicella incidence of 15.0 to 16.0 cases per 1,000 individuals (149), with significant yearly variations (51). The majority of cases occurred among children <15 years of age (85%), and the highest age-specific incidence occurred among children <5years of age. Prior to the recommendation for universal childhood varicella immunization in the United States, seroprevalence was high, with nearly all people being seropositive by the age of 40 years. Seroprevalence increased with age, ranging from 86% among children 6 to 11 years of age to >99.9% among adults 40 years of age or older (68). Although varicella was considered a benign disease, it caused an average of 11,000 to 13,500 hospitalizations (4.1 to >5.0 cases/100,000 individuals) and 100 to 150 deaths a year (0.04 to 0.06 cases/100,000 individuals). Hospitalization rates were about 4 times higher among adults, and mortality rates were much higher for adults (9, 32, 41, 97). HZ occurs much less commonly in VZV-infected children and is generally less severe, rarely leading to postherpetic neuralgia or hospitalization (53). Although serious clinical consequences from primary VZV infection were relatively uncommon, the societal cost, such as days of lost work, attributable to varicella were estimated to be over $1.5 billion annually, providing much of the rationale for universal varicella vaccination (158).
Postvaccine Epidemiology
In the United States, the varicella vaccination program has been associated with substantial reductions in varicella morbidity and mortality. Between 1997 and 2005, the national varicella vaccination coverage rate for children 19 to 35 months of age increased from 25.8% to 87.9% (20). In two varicella active surveillance sites located in Antelope Valley (AV), CA, and West Philadelphia (WP), PA, varicella disease had declined by about 90% in 2005 compared with rates in 1995, corresponding to vaccination coverage levels among children aged 19 to 35 months of 94% (AV) and 92% (WP). Declines in incidence occurred for all age groups, ranging from 57% to 95%; the highest reductions occurred for children <10 years of age for both sites (about 90%). An 80% decline in incidence was also observed among infants (ineligible for vaccination), and a 74% decline was observed among adults (who had low rates of vaccination), suggesting herd immunity (54). The peak age of varicella infection increased from 3 to 6 years of age in 1995 to 9 to 11 years of age in 2005, and the proportion of cases that were vaccinated increased from <1% to 60% over the same time period (54).
The impact of the varicella vaccination program on incidence, morbidity, and mortality was also reported by other data sources (18, 93, 154). For data from four states collected between 1990 and 1994 and in 2005, the incidences of varicella declined by 85% (88). In data from a health maintenance organization (HMO) collected between 1996 and 1999, the varicella incidence among children ≤18 years of age declined by 49.7%, with vaccination coverage of 73% among 2-year-old children (93). Finally, according to a Massachusetts phone survey study conducted between 1998 and 2003, the varicella incidence declined from 16.5 cases/1,000 individuals to 3.5 cases/1,000 individuals (79%) overall, with ≥66% decreases for all age groups except adults (27% decrease) (154). There have also been significant declines in rates of varicella-related deaths, hospitalizations, and ambulatory visits (32, 97, 112, 157). By 1999 to 2001, compared with 1990 to 1994, the national varicella death rate declined from an average of 145 deaths a year to 66 deaths a year. Considering varicella to be the underlying cause of death, the highest decline in the mortality rate was 92% for children 1 to 4 years of age, followed by 89% for individuals 5 to 49 years of age; infants <1 year of age experienced a 74% decline (97). Between 1994 to 1995 and 2002, varicella hospitalizations declined by 88%, and ambulatory visits declined by 59%, with >90% declines for children <10 years and adolescents 10 to 19 years of age (157). According to another study, hospitalizations declined by 64.9% between 1993 to 1996 and 2001 (32). A significant decrease in the number of varicella outbreaks was observed for the AV site during 2002 to 2005, compared with the years 1995 to 1998: 236 outbreaks were documented in the earlier period, which was reduced to 46 outbreaks in the later period. In addition, the median number of cases per outbreak declined from 15 to 9 (P < 0.001), and the mean duration of outbreaks declined from 44.5 to 30 days (P < 0.001). The median age of outbreak cases increased from 6 to 9 years (84). Thus, single-dose childhood varicella vaccination has had a dramatic impact on every facet of the varicella disease burden. In spite of this, outbreaks of varicella remain relatively common, and breakthrough disease, typically mild, has regularly been uniformly observed in day care centers and elementary schools (16, 42, 43, 63, 84, 103).
Breakthrough Varicella
Varicella vaccine is ∼80 to 85% effective in preventing any varicella disease and >95% effective in preventing severe disease. Therefore, about 15 to 20% of healthy vaccinated children will develop breakthrough varicella. Breakthrough varicella is defined as disease with symptom onset that occurs >42 days after vaccination. Most breakthrough disease in clinical trials was described as being mild, without fever and with fewer skin lesions (151). Breakthrough cases typically had <50 lesions that were atypical, with papules that did not progress to vesicles; they were also less likely to experience fever of ≥100°F or ≥102°F (12% and 3%, respectively) (8). Postlicensure data on the clinical presentation of breakthrough cases confirmed that cases are milder than cases for unvaccinated persons (43, 63, 125). Among vaccinated cases with skin lesions, 56% had <50 skin lesions, 33% had between 50 and 300 skin lesions, and 11% had over 300 skin lesions (125).
In 2005, 11 years after varicella vaccination commenced in the United States, breakthrough cases in the two (high-coverage) active surveillance sites accounted for 57% (AV) and 64% (WP) of reported varicella cases. Between 1997 and 2005, among vaccinated children 1 to 14 years of age, varicella was usually mild and modified. Compared with unvaccinated cases, vaccinated cases were one-third as likely to report fever and had one-half the duration of illness, were one-quarter as likely to have ≥50 lesions, and were about one-half as likely to develop complications.
Breakthrough varicella is also less readily transmissible than varicella in unvaccinated persons. The rate of spread of wild-type VZV from children with breakthrough disease to their vaccinated household contacts was reported to be 12% during prelicensure vaccine trials (146). In a postlicensure study, the rate of transmission from breakthrough cases to unvaccinated contacts was 37.1% (26/70; relative risk [RR], 0.52; 95% confidence interval [CI], 0.38 to 0.71), which was one-half the rate of transmission from unvaccinated varicella cases (71.5% [1,071/1,499]; RR, 1.00) (124). However, among breakthrough cases with >50 skin lesions, the transmission rate was comparable to that for unvaccinated cases. Breakthrough cases with <50 skin lesions were only one-third as contagious as unvaccinated cases (124).
Data from recent report describing 1,671 cases of breakthrough varicella in children aged 1 to 14 years occurring between 1997 and 2005 at two active surveillance sites were similar to data from previous studies in that most disease in vaccinated persons was milder and that vaccine recipients were less susceptible in outbreaks than unvaccinated children (25). However, vaccine recipients 8 to 14 years of age had a 2-fold-greater risk of developing moderate breakthrough varicella than 1- to 7-year-old vaccine recipients, suggesting a waning of immunity following a single dose. In contrast, another study of vaccinated children found no evidence of waning immunity (139). In addition, the findings of the outbreak study (25) were based on epidemiological data from cases that were not laboratory confirmed. It is therefore likely that the observations reflect a combination of both waning immunity and primary vaccine failure.
The observation of relatively high rates of susceptibility to breakthrough varicella (typically 20%) coupled with the observation that even very mild disease was capable of being transmitted provided two of the strongest arguments for a 2-dose schedule for children.
Varicella Vaccination and Herpes Zoster
Hope-Simpson first hypothesized that immunity to VZV may be maintained by the periodic internal reactivation of VZV, external boosting of immunity through exposures to varicella or HZ, or both (60). A number of studies have examined the impact of contacts with varicella cases or exposure to children, a proxy to varicella exposure, on the risk of herpes zoster. Two studies from England reported that exposure to varicella or to children reduces the risk of herpes zoster (12, 136). In a case-control study, the risk of zoster among those exposed to 5 or more varicella cases was 0.29 (95% CI, 0.10 to 0.84) compared to that among those without exposure to varicella cases (136). Another study found that living with children was associated with a lower risk of zoster, 0.75 (95% CI, 0.63 to 0.89) (12). The above-described findings were also reported for studies of children with leukemia who experienced a lower risk of zoster after household exposure to varicella than those without such exposure (46). On the other hand, in many studies, women were reported to have a higher incidence of herpes zoster than men (39, 60, 62, 101, 104, 136) despite the fact that women are more likely to have had exposure to young children with varicella. As such, the relationship between the frequency of reexposure to varicella and relative susceptibility to herpes zoster is not entirely clear.
Two studies utilizing mathematical models predicted that if exposure to varicella is important in maintaining immunity to zoster, the zoster incidence will increase in the short to medium term (over 10 to 40 years and up to 70 years) as a result of the implementation of a varicella vaccination program; however, those studies also predicted a lower incidence in the long term assuming that vaccine recipients have a lower risk of developing zoster than those with a history of varicella (13, 49). One study estimated that boosting due to exposure to varicella could last up to an average of 20 years (95% CI, 7 to 41 years) (13).
Assessment of the impact of the varicella vaccination program on the incidence of zoster is challenging for several reasons: a lack of robust baseline data for the prevaccine time period for comparison purposes; the use of different methods for ascertaining cases, making comparisons difficult; and the complexity of teasing out the impact of other risk factors that may affect herpes zoster, particularly immunocompromising medical conditions and medications. This issue will be further complicated by the recommendation to vaccinate persons aged 60 years and older for herpes zoster (59). Achievement of moderate to high levels (40 to 60%) of zoster vaccine coverage would be expected to reduce zoster incidence by 20 to 30%, which would likely mask the effect due to reduced rates of reexposure. The impact will be even greater if the recommendation is extended to persons 50 years old and older, as expected. Several studies have documented increases in the incidence of zoster that started before use of varicella vaccine, as early as the period between 1945 and 1959 in the United States (111); HZ incidence rates increased from 1.12 per 1,000 person-years during 1945 to 1949 to 1.50 per 1,000 person-years during 1955 to 1959. Another study reported that between 1990 and 1992, the annual HZ incidence rate was 2.87 per 1,000 person-years (34), more than double the rate (1.31 per 1,000 person-years) reported previously (111). The extent to which periodic reactivation of VZV may substitute for lower rates of exogenous VZV reexposure has not yet been explored.
Postvaccination, several studies and surveillance data show no consistent trends in herpes zoster incidence. Two studies found no change; one found that the age-adjusted incidence of HZ remained stable at about 0.4 cases per 1,000 person-years from 1992 to 2002 as the incidence of age-adjusted varicella decreased from 2.63 cases/1,000 person-years in 1995 to 0.92 cases/1,000 person-years in 2002 (66). Another study found an overall incidence of herpes zoster in 2000 and 2001 of 3.2 (95% CI, 3.1 to 3.2) per 1,000 person-years, which is similar to a rate reported in an earlier study for 1990 to 1992 (34, 62). Two studies reported increases in incidence; during 1997 to 2003, a study found stable herpes zoster rates with significant increases in rates among children aged 10 to 17 years that were attributable to an increased use of oral steroids (93). Another study found an increase in herpes zoster rates between 1999 and 2003; as varicella decreased 66%, the rate of zoster increased from 2.77 per 1,000 person-years to 5.25 per 1,000 person-years. The highest increase was among those 25 to 44 years and over 65 years of age (154). One study reported increases in HZ incidence rates between 1996 and 2001, from 3.18 cases per 1,000 person-years in 1996 to 1997 to 4.11 cases per 1,000 person years in 2000 to 2001 (153).
PERFORMANCE OF VARICELLA VACCINE IN THE UNITED STATES
Varicella Vaccine Effectiveness
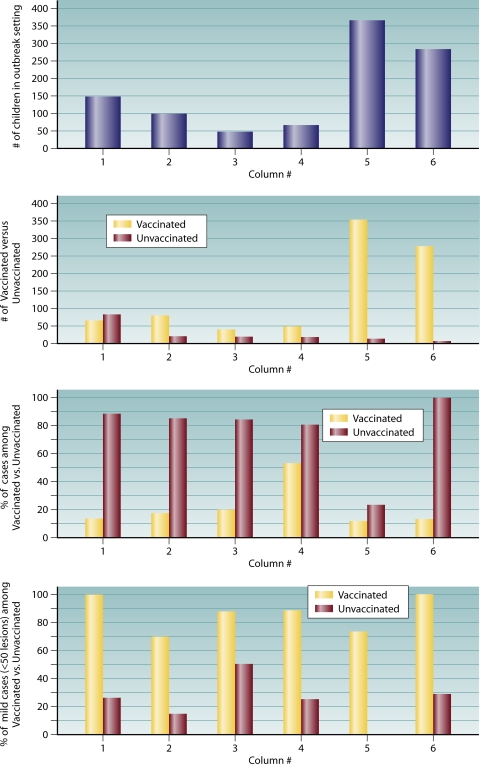
Data summarized from six publications of varicella outbreaks reported in the literature indicate that cases occurred among vaccinated children in every instance and that between 70 and 100% of those cases were modified (mild with few, often nonvesicular, lesions, typically without fever) (Fig. 1). All of the studies described varicella outbreaks among highly vaccinated populations of children in the United States: four occurred in a day care setting (15, 42, 43, 63), and two occurred in elementary schools (84, 103). In all instances, breakthrough disease occurred in vaccine recipients; however, in all instances, these breakthrough infections were characteristically milder, typically with fewer than 50 lesions. In addition, attack rates were substantially higher among unvaccinated children, with the exception of a day care outbreak described by Galil et al. (43), in which attack rates were only slightly higher among unvaccinated children.
FIG. 1.
Summary profile of day care and secondary school varicella outbreaks reported in the literature. Column 1, Izurieta et al. (63) (day care); column 2, Galil et al. (42) (day care); column 3, Buchholz et al. (16) (day care); column 4, Galil et al. (43) (day care); column 5, Lopez et al. (84) (elementary school); column 6, Parker et al. (103) (elementary school).
Prelicensure vaccine efficacy in healthy children in the United States, using vaccine preparations with variable potency, ranged from 92 to 100% (70). When the vaccine efficacy of one dose was compared that of two doses, and when children aged 1 to 12 years were monitored for 10 years, vaccine efficacy for one dose ranged from 90.2% to 94.4%. The efficacy for those who received two doses administered 3 months later ranged from 96.4% to 98.3% (69). Vaccine effectiveness against clinical varicella in postlicensure studies, mostly outbreak investigations, ranged from 44% to 100% (16, 18, 21, 29, 35, 42, 43, 56, 63, 73, 84, 89, 103, 124, 137, 139, 140); only one study assessed protection against laboratory-confirmed varicella (138, 139). Only two studies reported an effectiveness of less than 70%, 44% in a child care outbreak (43) and 56% in an elementary school outbreak (73).
Three studies designed to measure vaccine effectiveness reported ≥79% protection against any disease and ≥97% protection against severe disease. A cohort study of children attending day care centers reported vaccine effectivenesses of 83% (95% CI, 69% to 91%) against any disease and 100% against moderate to severe varicella (29). A case-control study in Connecticut showed vaccine effectivenesses of 85% (95% CI, 78% to 90%) against any laboratory-confirmed (PCR) varicella and 97% against moderately severe laboratory-confirmed varicella (95% CI, 93% to 99%) (140). Vaccine effectiveness dropped from 97% in year 1 to 85% in year 2 and remained at that level, at least during the 8-year period of follow-up (139). Finally, a household study for vaccinated and unvaccinated contacts aged 1 to 14 years exposed to unvaccinated primary household cases reported an effectiveness of 79% (95% CI, 70% to 85%) against any disease and an effectiveness of 100% against severe disease (124).
While the results from outbreak investigations supported high levels of protection from moderate to severe varicella, the observations also indicated that substantial residual susceptibility to infection remained for children receiving a single dose of vaccine. These observations were an important factor in the decision to move to a 2-dose schedule for varicella vaccination of children in the United States (88).
Risk Factors for Vaccine Failure
Many studies reported potential risk factors for varicella disease among vaccinated persons, impacting vaccine effectiveness. These include asthma, eczema, earlier age at vaccination, longer time since vaccination, receipt of varicella vaccine within 28 days of the measles-mumps-rubella (MMR) vaccine, and oral steroid use proximal to the development of breakthrough varicella (35, 43, 56, 63, 73, 137, 139, 141). However, few studies examined the simultaneous effects of these potential risk factors. Younger age at vaccination, ranging from <15 to 19 months, was associated with a 1.4- to 9-times-higher risk of breakthrough disease; however, only 2 studies controlled for the effect of other risk factors (56, 141). Other studies reported time since vaccination (>2 years and ≥5 years) as a risk factor; however, both age at vaccination and time since vaccination are highly correlated, and their independent association with the risk for breakthrough disease has not been extensively evaluated. One study that adjusted for multiple risk factors reported an increased risk for breakthrough disease in the 3 months immediately after prescription of oral steroids (adjusted relative risk [aRR], 2.4; 95% CI, 1.3 to 4.4) for vaccination before 15 months of age (aRR, 1.4; 95% CI, 1.1 to 1.9) and for varicella vaccination within 28 days of MMR vaccination (aRR, 3.1; 95% CI, 1.5 to 6.4) (141). Another study found that the effectiveness of the vaccine was lower only during the first year after vaccination (73% among children vaccinated at <15 months); however, the difference was not significant in the 8 years of follow-up (139). More recently, that same laboratory reported that measurements of VZV antibody in pre- and postimmunization sera from 148 healthy children with a median age of 12.5 months revealed a similar seroconversion rate of 76% (91). The observed differences in apparent vaccine failure rates may reflect something as simple as the end point cutoffs used to define protective immunity for the fluorescence antibody to membrane antigen (FAMA) and glycoprotein enzyme-linked immunosorbent assay (ELISA) (gpELISA) methods. In general, the range of observed risks for breakthrough disease of any severity is consistent with the rates of putative vaccine failure observed by various studies. Given that the majority of breakthrough infections are mild, with <50, typically maculopapular, lesions (often only a few lesions are observed), the issue of primary vaccine failure is a complex one, dependent, among other things, on whether failure is defined as absolute protection against any disease or if evidence of partial protection is taken into account.
Herpes Zoster in Vaccinated Individuals
During the primary infection with VZV, the virus migrates to the dorsal root and trigeminal ganglia, where it remains dormant. The virus reactivates later in life in about 15 to 30% of the population due to the waning of specific cell-mediated immunity (CMI), causing zoster, a unilateral, usually painful, vesicular rash illness. Herpes zoster is more common among the elderly and those with impaired cell-mediated immunity. Since the varicella vaccine is a live-attenuated virus that can establish latent infection in vaccine recipients, the issue of herpes zoster incidence among vaccine recipients is an important concern for the varicella vaccination program.
Early studies have demonstrated that the vaccine virus can become latent and later reactivate to cause herpes zoster in both healthy and immunocompromised persons. Postvaccination, both the vaccine strain and wild-type virus have been identified in zoster rashes of vaccinated children (125). Some studies reported that the incidence of herpes zoster among vaccinees is not higher, and may be lower, than that among healthy children with a history of varicella (107, 150). In the prevaccine era of 1960 to 1981, the herpes zoster rate among children was substantially lower among children aged <5 years (20 cases per 100,000 individuals) than adolescents aged 15 to 19 years (63 cases per 100,000 individuals) (54). In addition, active surveillance for herpes zoster in children <20 years of age from 2000 to 2006 has shown that the incidence of herpes zoster in children <10 years of age has declined 55%, from 74.8 cases/100,000 persons (95% CI, 55.3 to 101.2) to 33.3 cases/100,000 persons (95% CI, 20.9 to 52.8; P = 0.001). Moreover, vaccinated children <10 years of age had a 4- to 12-times-lower risk of HZ than did those with a history of varicella (28). However, during the same period, the incidence of HZ among those aged 10 to 19 years increased by 63%, from 59.5/100,000 persons (95% CI, 42.7 to 82.9) to 96.7/100,000 persons (95% CI, 75.7 to 123.6; P = 0.02). (28). However, since zoster is more common among persons 50 years of age or older, it will require a long time to assess the true risk of zoster among vaccinees.
Most of the available data on the risk of herpes zoster among vaccine recipients come from immunocompromised children, specifically leukemic children. In this group, children with a history of varicella disease have a higher risk of zoster than vaccinated children. In one study, 8 (15%) of 52 vaccinated leukemic children and 11 (18%) of 63 leukemic children with a history of varicella developed herpes zoster (67). In another study, none of 34 vaccinated children with leukemia developed herpes zoster, compared to 15 (21%) of 73 leukemic children with a history of varicella (15). In an NIAID collaborative study, the rate of HZ leukemic children was 2% for vaccinees and 15% for controls with a history of varicella. When two subgroups of 96 each were matched according to chemotherapeutic protocols, the incidence of HZ was 0.80 per 100 person-years among vaccinees and 2.46 per 100 person-years among those with a history of varicella (58, 72). Another study of 212 children who were immunized with varicella prior to a kidney transplantation procedure found that the risk of developing HZ was reduced by nearly one-half, with 13% of patients with a history of varicella prior to transplant compared with 7% among those vaccinated (14). Thus, all but one of those studies (67) found significantly lower rates of HZ for vaccinated children than for naturally infected children.
Several factors have been postulated for the lower risk of zoster among vaccinees than among those with a history of varicella; these include the attenuation of the vaccine virus, which may impact its ability to reactivate, and the absence of a rash postvaccination, as some researchers have speculated that the presence of a rash is important for the virus to travel to the dorsal root to establish latency (26). It was first observed in Japan that HZ occurred more frequently in children who had a vaccine-associated rash (16% of 83 children) than in those with no rash (2% of 249 children) (131). This observation was also reported in the NIAID collaborative study (58, 72), where zoster developed in 13 vaccinated children, 11 of whom had a skin rash due to varicella-zoster virus, either from the vaccine itself or from breakthrough varicella. The relative risk of zoster was 5.75 among children who had any type of rash after vaccination compared to those without any rash (58). Since vaccine rash and breakthrough are less likely to occur in healthy children, it is expected that the risk of zoster will also be lower among healthy vaccinees than among those who have a history of varicella.
LABORATORY METHODS FOR CONFIRMATION OF VZV DISEASE
Laboratory Diagnosis of Varicella
The highly modified nature typical of breakthrough disease, coupled with the dramatic reduction in incident cases resulting from universal vaccination, presents a serious challenge for clinical diagnosis. Such cases may be misdiagnosed by experienced physicians even in the context of an outbreak investigation. As a direct consequence, laboratory confirmation, particularly of varicella outbreaks, has become increasingly important to effectively monitor the impact and efficacy of varicella vaccination. Various approaches to confirming VZV disease, together with their strengths and limitations, are shown in Table 1.
TABLE 1.
Approaches to confirming VZV diseasec
| Diagnostic method | Reference(s) or source | Strength(s) | Limitation(s) | Description |
|---|---|---|---|---|
| Serology | ||||
| IgG ELISA (whole cell) | 135 | Ease of performance; reliably detects serum IgG responses to natural VZV infection; antigen easily prepared | Insufficient sensitivity to reliably detect seroconversion to vaccination | Must perform assay on both acute- and convalescent-phase specimens taken 2 or more weeks apart to confirm recent VZV exposure; 4-fold increase in titer can be difficult to demonstrate for individuals with preexisting VZV-specific high titers (has been observed for elderly populationsa) |
| IgG gpELISA | 64 | Ease of performance; reliably detects serum IgG responses to both natural infection and vaccination; performance standards comparable with those of FAMA | Neither the standard relied upon to calculate units of antibody nor the highly purified antigen preparation is readily available; the assay is not yet commercially available | |
| 135 | Ease of performance; reliably detects serum IgG responses to both natural infection and vaccination; performance standards comparable with those of FAMA | Uses adjusted OD cutoffs based on ROC curve analysis of defined true-positive and -negative (pre- and postvaccination) specimens; interassay/interoperator variation must be evaluated using a small defined set of sera as a control; highly purified antigen currently available only through a material transfer agreement with Merck & Co. | ||
| FAMA | 43, 44 | Reliably detects serum IgG responses to both natural infection and vaccination; performance comparable to that of gpELISA when performed by an experienced operator | Readout is subjective, and accurate determination of results, particularly for the detection of vaccine seroconversion, is dependent on a high level of operator experience; assay is cumbersome to perform compared to ELISA methods | |
| TRFIA | 79 | Reliably detects serum IgG responses to both natural infection and vaccination; performance comparable to those of gpELISA and FAMA in one reported study | Method requires specialized reader | |
| IgM capture | 135 | Relative ease of performance; eliminates possible problems from interfering antibody of non-IgM isotypes | Inherently broad specificity and low avidity/high valence of IgM make these assays susceptible to false-positive results | IgM is inconsistently observed even during PCR-confirmed primary infectionsb; IgM can be produced upon primary infection, reactivation, and reinfection (indicates recent exposure, if present, and not primary infection) |
| Direct IgM | Commercial assays available | Readily available; ease of performance | Potential performance issues due to incomplete removal of IgG and other non-IgM immunoglobulins | |
| IgG avidity | 77 | Relative ease of performance; definitive indicator of primary infection | Establishment of cutoff is somewhat arbitrary | |
| PCR | ||||
| Conventional PCR | 69 | Reliable and sensitive for detection of specific VZV DNA sequences | Unable to distinguish among strains or between vaccine and wild-type viruses as a standalone procedure | This approach is fine when the presence of VZV DNA only needs to be confirmed; also a useful method for obtaining amplicons for genotyping purposes |
| PCR-RFLP | 66 | Reliable and sensitive for the detection of specific VZV DNA sequences | Targeted to markers that are not specific to the vaccine strain; no longer reliable as a means for discriminating vaccine strain from wild-type viruses; more cumbersome and time-consuming than real-time methods; requires manipulation of product; increased potential for contamination of work area | The requirement for postamplification manipulation generally extends this approach into a 2-day procedure |
| 71 | Reliable and sensitive for detection of specific VZV DNA sequence; distinguishes vaccine strains from the wild type using a vaccine-specific marker | More cumbersome and time-consuming than real-time methods; requires manipulation of product; increased potential for contamination of work area | ||
| Real-time PCR | 34 | Reliable and sensitive for the detection of specific VZV DNA sequence; rapid, single-tube, closed-system technique with reduced risk of contamination of work area | Unable to distinguish among strains or between vaccine and wild-type viruses as a standalone procedure | These protocols are readily adaptable to melting-curve-based (FRET) analysis and can be performed in only a few hours when urgency demands it |
| 92 | Reliable and sensitive for detection of specific VZV DNA sequence; rapid, single-tube, closed-system technique with reduced risk of contamination of work area | Unable to distinguish among strains or between vaccine and wild-type viruses as a standalone procedure | ||
| FRET PCR | 74 | Reliable and sensitive for detection of specific VZV DNA sequence; rapid, single-tube, closed-system technique with reduced risk of contamination of work area; able to distinguish vaccine strain from wild-type strains using vaccine-specific markers | Melting-curve-based assays can be misleading; questionable results need to be confirmed by sequencing of the DNA products | |
| Others | ||||
| DFA | 106 | Reliably detects most varicella infections using material obtained from lesions; rapid same-day method | Substantially less sensitive than PCR; critically dependent on careful collection of material from lesion (must be swabbed aggressively enough to collect cells without causing bleeding; blood can lead to false-negative results due to blocking antibodies) | |
| EM | 29 | Can be used to confirm the presence of herpesvirus in material collected from lesions or other tissues; rapid same-day method | Is not capable of distinguishing VZV from other human herpesviruses; is not as sensitive as either DFA or PCR | |
| EM colloidal gold | 37 | Modification of EM method to permit discrimination of VZV in lesions or other tissues | Suffers from cross-reactivity problems with other alphaherpesviruses (HSV-1, HSV-2, and pseudorabies virus); same limit in sensitivity as conventional EM | |
| Standard viral culture | 106 | Can detect presence of VZV in freshly isolated specimens | Considerably less sensitive than EM, DFA, or PCR; typically detects only between 50 and 75% of infections using more sensitive techniques; critically dependent on immediate culture of freshly obtained specimen; requires multiple days (as much as a week) for result | |
| Shell vial culture | 106 | Modification of standard culture method; generally has substantially improved sensitivity (comparable to that of DFA) | Not as sensitive as PCR; requires multiple days to obtain a result | |
| Tzanck smear | 106 | Rapid; ease of performance; readily interpreted | Not specific for VZV (other alphaherpesviruses produce the same effect); less sensitive than PCR or DFA |
Since most patients with breakthrough varicella will have preexisting IgG antibody titers to VZV, conventional IgG determinations are of limited value unless measurements of acute- and convalescent-phase sera are performed. Evidence of VZV-specific IgM in serum indicates recent exposure to VZV but does not discriminate between primary infection, reinfection, and reactivation. Moreover, IgM antibodies are inconsistently observed even among cases with PCR-confirmed exposure to infectious VZV (148), and therefore, the absence of IgM antibody is no indication that recent VZV exposure has not taken place. IgG avidity testing is valuable for the confirmation of a primary infection, reflected by the presence of low-avidity IgG antibody, but most vaccine recipients with breakthrough infection are likely to have formed high-avidity antibody in response to vaccination.
At least 10 methods for detecting VZV-specific IgG are commercially available in the United States, and while the performance specifications of these methods vary widely, none is sufficiently sensitive to reliably detect seroconversion to the Oka vaccine (10; our unpublished observations). Several methods have been described to have sufficient sensitivity and specificity for that purpose, including fluorescence antibody to membrane antigen (FAMA), purified VZV glycoprotein ELISA (gpELISA), and time-resolved fluorescent immunoassay (TRFIA) (47, 57, 87, 87a, 122). Some care must be taken in setting end point cutoffs for the gpELISA method to prevent the detection of false-positive results. Seropositivity rates as high as 14% have been reported for children <12 months of age, some of which may be attributable to lingering maternal antibody but which also likely reflects the selection of a cutoff-adjusted optical density that is set too low (109). While TRFIA has been shown to have sensitivity and specificity comparable to those of gpELISA and FAMA, there is limited experience with this assay, and it does not appear to have been validated in a clinical setting. For FAMA, while it has been used in multiple studies, including those that demonstrated a reasonable correlation with protective immunity (139), the method is subjective relative to ELISA-type methods, and its performance is quite variable from one laboratory to another. Among these methods, only gpELISA and TRFIA could be readily adapted for the measurement of antibody avidity. Since FAMA relies on a stepwise dilution series end point, it is not possible to calculate readily comparable avidity indices using this approach. Evaluation of antibody avidity has already proven useful in a VZV outbreak setting (83).
The most reliable and sensitive laboratory method for confirming varicella is the detection of VZV DNA in samples (vesicular swabs or scabs) obtained from skin lesions by using PCR (102, 128). A number of VZV PCR methods have been reported in the literature (17, 36, 37, 71, 77, 81, 95, 102, 105, 128), including several that are designed to distinguish vaccine strain Oka from wild-type strains of VZV (17, 71, 77, 81, 105). While the PCR-restriction fragment length polymorphism (RFLP) protocol described by LaRussa et al. (71) was originally useful for the detection of vaccine virus, the method targets genotypic markers that are present in the Oka vaccine but that are also observed for wild-type strains now known to be circulating in areas where vaccine use is prevalent (78, 81, 122). A multiyear study of VZV encephalitis/meningitis in California determined that 15% (5/32 isolates) of clinical VZV isolates were of genotype J, only one of which was vaccine. The remaining 4 J isolates would have been determined to be a vaccine genotype using the ORF38/ORF54 method (D. S. Schmid, unpublished observations). As such, it is no longer reliable as a standalone method for differentiating vaccine virus from wild-type strains. VZV DNA can also often be detected in saliva but only during acute disease and less consistently than in lesion material (Schmid, unpublished). A number of other direct detection methods have been used to diagnose current VZV infection, including viral culture (30, 40, 95, 118), electron microscopy (EM) (40, 74, 142), direct fluorescent antibody assay (DFA) (30, 52, 118), Tzanck smear (31, 40, 95, 102, 118, 127), and loop-mediated isothermal amplification (LAMP) (100), but none is as sensitive as PCR. The reliabilities of these methods in declining order of sensitivity are PCR, LAMP, DFA, viral culture, EM, and Tzanck smear. Electron micrographs and Tzanck smears of material from lesions are not agent specific, confirming only the presence of a herpesvirus. An EM technique employing VZV-specific antibody labeled with colloidal gold renders the method VZV specific, although cross-reactivity with other alphaherpesviruses was also detected (142).
While PCR is quite effective at detecting VZV DNA in material from classical varicella vesicles or crusts from healed lesions, it is more problematic to obtain an adequate specimen for testing from the maculopapular lesions more typical of breakthrough disease. Further complicating this problem is the frequency with which suspected cases have already fully resolved by the time an outbreak investigation is initiated. Once lesions have resolved, the likelihood of obtaining a specimen that will contain viral DNA detectable by PCR is reduced effectively to zero. There are most likely several explanations for why varicella DNA is difficult to detect in mild disease: (i) less virus may be present in such lesions, which may account for their failure to progress to vesicles, and (ii) much of the disease that gets characterized as varicella in an outbreak setting may not be varicella at all, with lesions being due to arthropod bites or other etiologies.
Serology is of limited use in this context, as noted above. There are therefore no methods currently available that will reliably confirm recent infection of individuals for whom all clinical symptoms have resolved. Outbreaks in the United States typically involve a large percentage of breakthroughs in vaccinated persons, which is almost invariably mild disease. Overascertainment of cases in that context is a problem, since arthropod bites and other unrelated skin lesions could be mistaken for breakthrough varicella. Previously unexplored potential diagnostic markers, such as antigen-specific cell populations in whole blood and the detection of virally infected cells at the individual-cell level using flow cytometric approaches, enzyme-linked immunospot (ELISPOT), and other methods, need to be evaluated to identify new tools that would be useful for this purpose.
Assessment of Vaccine-Induced Protection against Varicella
Historically, the presence of antibodies to varicella-zoster virus has been used as a correlate of protection against varicella, although in general, there are no commercially available VZV serological methods with sufficient sensitivity to reliably detect seroconversion to varicella vaccination (11; our unpublished observations). This has been problematic since even low levels of circulating VZV-specific antibody, beneath the threshold detection level of all but a few methods, have been shown to correlate with protection against disease (1, 2, 4, 48, 69, 70, 96). The use of insensitive assays to evaluate vaccine seroconversion can lead to unnecessary vaccinations, and the failure to detect vaccine seroconversion in a substantial number of vaccinated persons can undermine public confidence in vaccine efficacy. That said, the presence of very low levels of VZV-specific antibody in 20 to 30% of vaccinated persons, who nonetheless appear to have some level of protection from infection, suggests that antibody may not be the ideal in vitro indicator of protection. As such, alternative measurements, such as antigen-specific cell-mediated immunity, need to be more aggressively investigated than they have been to this point.
The FAMA test, gpELISA, and TRFIA have all been used to establish respective correlates of protection based on VZV-specific antibody titers (44, 69, 87a, 114, 152). For the FAMA assay, an end point titration dilution of ≥4 correlated with protection in a study of vaccinees with documented household exposures to VZV (45). Greater than 95% of vaccinated children who had titers of ≥5 gpELISA units/ml 6 weeks after immunization were protected from breakthrough varicella during a 7-year follow-up study (75). Children with titers of <5 gpELISA units/ml were 3 times more likely to develop breakthrough varicella over the study period. The cutoff for protection for TRFIA is 150 mIU/ml, which was established by comparing gpELISA to an evaluated standard using receiver operator characteristic (ROC) curve analysis. Nonetheless, as noted above, studies of varicella outbreaks in recent years have revealed sometimes surprisingly high levels of breakthrough infection among varicella vaccinees, although the disease is characteristically milder. Other putative markers of protection, notably VZV-specific cell-mediated immunity, have not been well studied.
Assessment of Duration of Protective Immunity
A number of studies have shown that protective levels of anti-VZV antibody can persist for long periods in persons who have received a single dose of varicella vaccine (2, 4, 9, 48, 64, 69, 70, 96, 126). However, many of the evaluations have been performed using the gpELISA method at a cutoff value of 5 geometric mean titer (GMT) units/ml, which may overestimate protection. In addition, even vaccinated individuals with no detectable specific antibody may retain detectable levels of antigen-specific T cells, suggesting that other measures of immunity, e.g., cell-mediated immunity, may be more relevant measures of protection (85). A study of 277 children monitored for 9 years found that nearly 100% retained titers judged to be protective at the end of the study period (69). A small study of vaccinated children in Japan showed that VZV titers were comparable to initial IgG response titers after 7 to 10 years and for 100% of 25 study participants followed up after 20 years (5). A third early study of persistence of humoral immunity to varicella vaccine reported an initial decline in titers (1 to 2 years postvaccination) but reported titers comparable to or exceeding 1-dose vaccine responses for most vaccinees 1 to 4 years after immunization and frequently enhanced titers for persons tested 10 years after vaccination (64). However, those studies monitored small numbers of vaccine recipients and were undertaken during a period of a high incidence of varicella, where periodic subclinical reinfection likely resulted in the boosting of VZV antibody titers. Possible but undemonstrated factors attributable to varicella vaccine failure include lot-to-lot variability in the vaccine, disruptions of the cold chain, errors in administering the vaccine, and genetic variability among recipients. Children receiving two doses of varicella vaccine developed improved immunological responses compared with those of one-dose recipients that persisted at presumed protective levels for 9 years of follow-up. However, the difference between the two groups was only marginal at the end of the study period (69). An 8-year study of 1,017 vaccinated children ≥13 months old (339 case subjects and 678 controls) concluded that the level of protection conferred by vaccine declined measurably during the first year postvaccination and then remained relatively stable during the remaining 7 years of the study (139). Case subjects were children with PCR-confirmed cases of varicella. The decline in protection was most pronounced among children who had been vaccinated before the age of 15 months. Nonetheless, the overall vaccine effectiveness was high (87%), and the majority of breakthrough disease was mild (severity score of ≤7).
Varicella vaccination provides less robust immunity in adults than it does in children (19). Among 120 varicella-seronegative health care workers (median age, 26 years), 12 developed varicella 6 months to 8.4 years following vaccination (114). Exposures leading to breakthrough illness were twice as common for household contacts rather than hospital contacts, and most of the illness was mild to moderate (median, 40 lesions).
The duration of the CMI response to vaccine has been less extensively studied and has generally been limited to measurements of T-cell proliferation (94, 145, 147, 156). While proliferation assays provide an indication that antigen-specific T cells are present in detectable numbers, they relate nothing about the nature of the T-cell response (e.g., Th1 versus Th2, cytotoxic activity, and cytokines produced, etc.). In addition, studies of the duration of varicella vaccine CMI have had relatively short periods of follow-up, generally not exceeding 5 to 6 years postvaccination. This is a problem since a number of studies have established that T-cell responses to VZV (similar to those of other virus infections) probably play the dominant role in containing the virus during reinfections and reactivations (3, 89, 129). Studies of CMI of vaccinated children (single dose) demonstrated that VZV-specific T-cell proliferation could be detected for 26 of 29 children (90%) a year following vaccination and for 52 of 60 children (87%) 5 years after vaccination (156). Interestingly, the mean stimulation index (SI) nearly doubled, from 12.1 to 22.1 at 1 year versus 5 years postvaccination. Based on measurements of T-cell proliferation, two additional studies provided evidence that children who receive two doses of varicella vaccine had significantly higher CMI responses than did single-dose recipients (145, 146). Vaccinated adults displayed similar T-cell proliferation profiles, with 16 of 17 persons (94%) being positive after 1 year (mean SI, 9.9) and 16 of 17 persons still being positive at the end of 5 years (mean SI, 22.4) (156). T-cell cytokine responses were also comparable between adult and child vaccine recipients, with the Th1 cytokines gamma interferon (IFN-γ) (peak levels at days 5 to 7) and interleukin-2 (IL-2) (peak levels at days 2 to 4) and the Th2 cytokine IL-10 (peak levels at days 5 to 7) all being produced at significant levels (156). Skin testing for delayed-type hypersensitivity to VZV has been little explored, although several Japanese studies established that the test is specific for VZV (without herpes simplex virus [HSV] cross-reactivity) and may be a useful marker for waning VZV immunity (6, 115, 133). About one-third of 60 subjects aged 50 years or older initially had negative or weakly positive VZV delayed-type hypersensitivity (DTH) reactions, but all subjects developed strong reactions measured 5 to 7 weeks postimmunization (133). Skin tests based on several purified VZV glycoprotein preparations (gB, gE/gI, and gH/gL) all effectively measured boosts in VZV CMI for four adults aged 27 to 44 years (115). There are no published reports evaluating the cytotoxic T-cell response to varicella vaccine, although our laboratory has observed robust granzyme B production in an adult vaccine recipient that was comparable to that of naturally infected persons in VZV-stimulated, cultured peripheral blood lymphocytes (our unpublished observations). While this is obviously not a generalizable finding, it suggests another potential marker that could be useful for the evaluation of CMI activity in vaccine recipients.
GENETICS OF VARICELLA VACCINE
Genetic Basis of Vaccine Attenuation
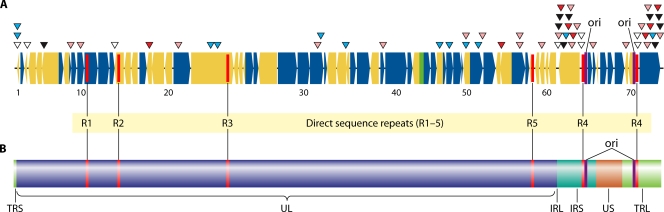
The DNA sequence data revealed that just 42 single nucleotide polymorphisms (SNP) account for the attenuated phenotype of the vaccine (50). Sixteen of the SNP in ORF62 and ORF64 of the internal short repeats (IRS) are repeated in the ORF69 and ORF71 genes, which are duplicated in the terminal repeat short (TRS) region (Fig. 2). Among these markers, 6 occur in noncoding regions, 16 cause no changes in the encoded protein, and only 20 lead to an amino acid substitution. Fifteen of the markers are present in the vaccine as a mixture of vaccine-associated and wild-type bases; i.e., at that locus there are substrains present in the vaccine that carry either the wild-type marker or the vaccine marker. This observation indicated that the vaccine contains a mixture of strains that have variable vaccine-associated SNP contents (50). Twelve of the SNP, including 8 of those that confer an amino acid change, are located in a single open reading frame, ORF62 (repeated in ORF71, the duplicate copy of ORF62). Finally, several short-length differences occurred in the tandem repeat regions R1, R3, and R4 and in the origin of replication; these can vary in copy number among circulating wild-type strains, and it is unknown whether they make any contribution to pathogenicity. As such, 20 vaccine-associated SNP (∼0.002% of the complete genome) located in 11 open reading frames have the highest probability of contributing to the attenuation of Oka varicella vaccine. This is not a foregone conclusion, however. Vaccine SNP occurring in noncoding regions could affect the performance of genetic regulatory elements, and those occurring in coding regions that do not alter the amino acid sequence could affect translation rates if some species of tRNA are present at much lower levels than others. Several studies of clinical isolates obtained from documented vaccine-attributable cases of varicella and zoster have shown that a subset of these markers are present as the wild-type SNP in 60 to 100% of all such isolates (10, 79, 110) (Table 2). Table 2 includes both markers leading to an amino acid shift and those that do not. It is hoped that a systematic study of these single-locus base variations will lead to a better understanding of the virulence factors of VZV and of the basis for attenuation for the vaccine preparation. One as-yet-unresolved discrepancy between the reported SNP analyses of vaccine adverse-event isolates is the detection of mixtures of isolates in some lesions by Loparev et al. (79). If the observation of mixtures of strains in many isolates is correct, it suggests that coinfection of a single neuron during latency may be common or, alternatively, that reactivation occurs simultaneously among multiple neurons in the same dorsal root ganglion.
FIG. 2.
(A) Model of the VZV genome illustrating the single-base differences between pOka (parental strain Oka) and vOka (vaccine strain Oka). White triangles, SNP located in noncoding region; black triangles, SNP present uniformly among vaccine strains leading to amino acid substitutions; red triangles, SNP present uniformly among vaccine strains that does not lead to amino acid substitutions; pink triangles, SNP present as a mixed base in vaccine preparations and vaccine-associated SNP that leads to amino acid substitutions; blue triangles, SNP present as a mixed base in vaccine preparation that does not lead to amino acid substitutions. Dark blue ORFs are transcribed from the sense strand; yellow ORFs are transcribed from the antisense strand. (B) Display of VZV genome architecture. TRS, terminal repeat short; TLR, terminal repeat long; UL, unique long; US, unique short; IRL, internal repeat long; IRS, internal repeat short; ori, origin of replication.
TABLE 2.
Studies of documented vaccine-attributable cases of varicella and zoster
| ORF(s) | Position | % Wild-type markera |
|
|---|---|---|---|
| Breuer laboratory | Schmid laboratory | ||
| 9A | 10900 | 90 | 82 |
| 10 | 12779 | 90 | 100 |
| 21 | 31732 | 100 | 100 |
| 50 | 87306 | 80 | 92 |
| 52 | 90535 | 100 | 87 |
| 55 | 97748 | 80 | 67 |
| 55 | 97796 | 80 | 94 |
| 59 | 101089 | 70 | 41 |
| 62 and 71 | 105310 | 74 | 44 |
| 62 and 71 | 105356 | 80 | ND |
| 62 and 71 | 107599 | 90 | 95 |
| 62 and 71 | 107797 | 100 | 100 |
| 62 and 71 | 108838 | 100 | 52 |
| 64 and 69 | 111650 | 100 | 59 |
ND, not determined.
Potential Role of VZV Recombination in Varicella Vaccine Pathogenicity and VZV Evolution
Evidence for intraspecies and interstrain genetic recombination has been observed for a variety of human herpesviruses including cytomegalovirus (CMV) (55), Epstein-Barr virus (92, 144), human herpesvirus 8 (HHV-8) (108), herpes simplex virus (98, 138), and, relevant to this review, varicella-zoster virus (7, 99, 106, 116, 120, 143), and recombination has clearly played an important role in the evolution of this family of viruses (90). Studies of strain variation among globally distributed wild-type strains of VZV have identified 7 genotypes, 5 of which have been confirmed as stably circulating phylogenetic groups through both extensive targeted sequence analysis and complete genomic sequences (E1, E2, J, M1, and M2) (7, 78, 80, 82, 106). Two other provisional genotypes (M3 and M4) require additional study (78, 123). Phylogenetic analysis of complete sequences from representative strains for 4 of the established genotypes (E1, J, M1, and M2) suggested that the M1 and M2 genotypes arose through recombination events between E1 and J strains (99). This relationship has been observed by several different groups, leading to speculation that recombination between vaccine and wild-type strains could occur in vaccinated individuals (7, 106, 116). Our laboratory recently documented the occurrence of Oka vaccine-wild-type recombinant strains isolated from vaccinated children, most of which were obtained from children initially diagnosed as cases of vaccine zoster (119). The two isolates studied in detail have been shown to be clonal, and since previous observations suggested that isolates from vaccine varicella and zoster are often mixtures of vaccine strains (79), the detection of two clonal recombinants implies a selective preference for reactivation, enhanced replication, and/or an enhanced ability to traverse the nerve. Admittedly, this is a limited finding and will require more study before any firm conclusions regarding the nature of these isolates can be made. In all, 15 of 134 isolates (11%) previously documented by the National VZV Laboratory at the CDC, Atlanta, GA, as being varicella vaccine adverse events had DNA sequence characteristics consistent with vaccine-wild-type recombination. There are no apparent differences in pathogenicity among circulating wild-type VZV viruses, and as such, recombination with an attenuated vaccine virus is unlikely to result in viruses with enhanced pathogenicity. However, wild-type VZV isolates of different genotypes have not been systematically characterized for differences in pathogenicity, and the possibility that differences exist cannot be ruled out. Nonetheless, there is currently no evidence that the occurrence of varicella vaccine-wild-type recombination would require a change in varicella vaccine recommendations or policy. It is, however, conceivable that such recombinants could restore essentially wild-type pathogenicity, leading to a possible transmission and circulation of such strains among unvaccinated persons and greatly complicating vaccine surveillance. It can no longer be considered adequate to confirm vaccine adverse events using the targeted analysis of nucleotide variability at several single nucleotide polymorphisms (SNP). Extensive SNP analysis combined with routine genotyping will now be necessary to be certain that disease is attributable to Oka vaccine. Finally, the occurrence of vaccine-wild-type recombination might be expected to increase substantially with the administration of zoster vaccine, which is formulated at a virus concentration more than 14-fold higher than that of childhood varicella vaccine and which is being used to immunize persons known to have preexistenting latent VZV infections.
CONCLUSIONS
The varicella vaccination program in the United States has resulted in dramatic declines in rates of varicella disease in all age groups including infants and adults. As expected with a successful childhood vaccination program, the greatest decline occurred among children <10 years of age targeted by vaccination. This decline has resulted in a higher proportion of cases occurring for older children 10 to 14 years of age, stressing the importance of catch-up vaccination.
Postlicensure varicella vaccine effectivenesses varied, with the lowest estimates being reported for outbreak investigations. This may be due to the fact that outbreaks that come to public health attention are more likely to represent situations where the vaccine failed and, as such, could represent extreme estimates of vaccine effectiveness (38). Outbreaks where the vaccine is performing well are unlikely to be investigated. Although some risk factors for vaccine failures have been identified, most studies were unable to examine these risk factors simultaneously due to small numbers of cases.
Although breakthrough varicella is most often mild and less infectious, a recent study found an increasing severity of breakthrough cases with time of vaccination indicating a possible waning of immunity (25); in addition, it was predicted that there would be an accumulation of vaccinated adolescents who had not seroconverted after one dose and would therefore be susceptible to varicella as young adults. The reduction in varicella disease would limit the opportunity for these persons to contract varicella at an earlier age, when varicella is less severe. Given the above-described considerations, these persons would potentially be at a high risk of developing severe varicella. A routine 2-dose vaccine policy for varicella-containing vaccines for children was recommended in the United States in 2006. In 1996, one dose of varicella vaccine was recommended by the Advisory Committee on Immunization Practices (ACIP) (22, 88) for routine use in healthy children 12 to 18 months of age; healthy, susceptible children 19 months through 12 years of age; as well as persons in close contact with those at high risk for serious varicella. Updated recommendations in 1999 (23) progressively expanded these recommendations to cover all susceptible persons. While it is not expected that the 2nd dose would provide complete protection, it is expected that the number of breakthrough cases will decrease significantly, with a subsequent decrease in rates of transmission of the virus.
The recent data on zoster incidence in vaccinees suggest that the risk is lower than among those with a history of varicella. However, long-term follow-up of vaccinated, healthy children will be required to provide detailed data on risks throughout the life span. Studies of herpes zoster incidence in the future, when vaccinated children are older adults, are likely to provide useful information.
To date, only one of four studies has shown an increase in zoster incidence after varicella vaccination in the United States. While it is plausible that a sufficient number of varicella exposures can reduce the risk of zoster in select populations, it remains unclear whether such levels of exposure play an epidemiologically important role in reducing the risk of zoster among the general population of older adults who are at the highest risk of the disease and, if so, how long such effects would last in the elderly. Persons living or interacting with children may have different underlying health conditions compared to persons without exposure to children, which may be a confounder in these studies. This issue is also complicated by the introduction of a zoster vaccine that reduces the risk for developing zoster by 50% in persons ≥60 years of age. Even modest coverage levels with this vaccine could substantially mask other trends in zoster incidence, particularly if its use is recommended for younger persons (50 to 59 years of age).
Finally, the limitations of currently available diagnostic techniques for ascertaining vaccine-modified cases of varicella are a problem requiring urgent attention, since accurate evaluations of efforts to quantify vaccine impact will critically rely on better tools than are available now.
Acknowledgments
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the funding agency.
Biography
 D. Scott Schmid is the team leader for herpesvirus laboratory activities at the Centers for Disease Control and Prevention. He has nearly 30 years of experience and expertise in the immunology, molecular epidemiology, and laboratory diagnosis of human herpesvirus infections. In 1998 he established the National VZV Laboratory at the CDC as a reference laboratory to assist in monitoring varicella vaccine impact in the United States, to verify varicella vaccine adverse events, to evaluate varicella outbreaks, and to confirm severe varicella disease. Dr. Schmid's primary focus has been on VZV since 1998, but his team also conducts research on congenital CMV and HHV-8. Dr. Schmid has served as an author or coauthor of more than 110 scientific manuscripts, reviews, and book chapters. He received a B.A. in biology from Kent State University (1974), an M.S. in microbiology from Arizona State University (1981), and a Ph.D. in microbiology from the University of Tennessee at Knoxville (1983). He completed postdoctoral work at Yale University School of Medicine (1982 to 1986), at which point he began his career with the CDC in Atlanta, GA.
D. Scott Schmid is the team leader for herpesvirus laboratory activities at the Centers for Disease Control and Prevention. He has nearly 30 years of experience and expertise in the immunology, molecular epidemiology, and laboratory diagnosis of human herpesvirus infections. In 1998 he established the National VZV Laboratory at the CDC as a reference laboratory to assist in monitoring varicella vaccine impact in the United States, to verify varicella vaccine adverse events, to evaluate varicella outbreaks, and to confirm severe varicella disease. Dr. Schmid's primary focus has been on VZV since 1998, but his team also conducts research on congenital CMV and HHV-8. Dr. Schmid has served as an author or coauthor of more than 110 scientific manuscripts, reviews, and book chapters. He received a B.A. in biology from Kent State University (1974), an M.S. in microbiology from Arizona State University (1981), and a Ph.D. in microbiology from the University of Tennessee at Knoxville (1983). He completed postdoctoral work at Yale University School of Medicine (1982 to 1986), at which point he began his career with the CDC in Atlanta, GA.
 Aisha O. Jumaan is the Director for the HPV Vaccines: Evidence for Impact Project at PATH. Dr. Jumaan has over 20 years of experience and expertise in viral and other vaccine-preventable diseases, cervical and breast cancer research, and surveillance. She worked at the U.S. Centers for Disease Control and Prevention from 1995 to 2007, most recently as the team leader for herpesvirus epidemiology activities, specifically for the varicella and zoster vaccination program. Previous team lead and senior epidemiologist positions within the CDC have been with the National Immunization Program, the Division of Cancer Control and Prevention, the Nutrition Division, and the Agency for Toxic Substances and Disease Registry. She also held the position of assistant professor at the Rollins School of Public Health, Epidemiology Department, at Emory University. Dr. Jumaan is author and coauthor of a number of peer-reviewed publications. Dr. Jumaan earned her doctorate in epidemiology at the University of North Carolina, Chapel Hill; an M.P.H. degree, also in epidemiology, from Emory University, Atlanta, GA; and her undergraduate degree in biology from Mills College, Oakland, CA. In addition, she holds a certificate for Women in Development from the Royal Tropical Institute, The Netherlands. She is fluent in both Arabic and English.
Aisha O. Jumaan is the Director for the HPV Vaccines: Evidence for Impact Project at PATH. Dr. Jumaan has over 20 years of experience and expertise in viral and other vaccine-preventable diseases, cervical and breast cancer research, and surveillance. She worked at the U.S. Centers for Disease Control and Prevention from 1995 to 2007, most recently as the team leader for herpesvirus epidemiology activities, specifically for the varicella and zoster vaccination program. Previous team lead and senior epidemiologist positions within the CDC have been with the National Immunization Program, the Division of Cancer Control and Prevention, the Nutrition Division, and the Agency for Toxic Substances and Disease Registry. She also held the position of assistant professor at the Rollins School of Public Health, Epidemiology Department, at Emory University. Dr. Jumaan is author and coauthor of a number of peer-reviewed publications. Dr. Jumaan earned her doctorate in epidemiology at the University of North Carolina, Chapel Hill; an M.P.H. degree, also in epidemiology, from Emory University, Atlanta, GA; and her undergraduate degree in biology from Mills College, Oakland, CA. In addition, she holds a certificate for Women in Development from the Royal Tropical Institute, The Netherlands. She is fluent in both Arabic and English.
REFERENCES
- 1.Ampofo, K., L. Saiman, P. LaRussa, S. Steinberg, P. Annunziato, and A. Gershon. 2002. Persistence of immunity to live attenuated varicella vaccine in healthy adults. Clin. Infect. Dis. 34:774-779. [DOI] [PubMed] [Google Scholar]
- 2.Arbeter, A. M., S. E. Starr, and S. A. Plotkin. 1986. Varicella vaccine studies in healthy children and adults. Pediatrics 78:748-756. [PubMed] [Google Scholar]
- 3.Arvin, A. M. 1996. Immune responses to varicella-zoster virus. W. B. Saunders, Philadelphia, PA. [DOI] [PubMed]
- 4.Asano, Y., T. Nagai, T. Miyata, T. Yazaki, S. Ito, K. Yamanishi, and M. Takahashi. 1985. Long-term protective immunity of recipients of the OKA strain of live varicella vaccine. Pediatrics 75:667-671. [PubMed] [Google Scholar]
- 5.Asano, Y., S. Suga, T. Yoshikawa, I. Kobayashi, T. Yazaki, M. Shibata, K. Tsuzuki, and S. Ito. 1994. Experience and reason: twenty year follow-up of protective immunity of the Oka strain live varicella vaccine. Pediatrics 94:524-526. [PubMed] [Google Scholar]
- 6.Baba, K., K. Shiraki, T. Kanesaki, K. Yamanishi, P. L. Ogra, H. Yabuuchi, and M. Takahashi. 1987. Specificity of skin test with varicella-zoster virus antigen in varicella-zoster and herpes simplex virus infections. J. Clin. Microbiol. 25:2193-2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barrett-Muir, W., F. T. Scott, P. Aaby, J. John, P. Matondo, Q. L. Chaudry, M. Siquera, A. Poulsen, K. Yamanishi, and J. Breuer. 2003. Genetic variation of varicella-zoster virus: evidence for geographical separation of strains. J. Med. Virol. 70(Suppl.):S42-S47. [DOI] [PubMed] [Google Scholar]
- 8.Bernstein, H. H., E. P. Rothstein, Pennridge Pediatric Associates, B. M. Watson, K. S. Reisinger, M. M. Blatter, C. O. Wellman, S. A. Chartrand, I. Cho, A. Ngail, and C. J. White. 1993. Clinical survey of natural varicella compared with breakthrough varicella after immunization with live attenuated Oka/Merck varicella vaccine. Pediatrics 92:833-837. [PubMed] [Google Scholar]
- 9.Black, S., P. Ray, H. Shinefield, P. Saddler, and A. Nikas. 2008. Lack of association between age at varicella vaccination and risk of breakthrough varicella, within the Northern California Kaiser Permanente Medical Care Program. J. Infect. Dis. 197(Suppl. 2):S139-S142. [DOI] [PubMed] [Google Scholar]
- 10.Breuer, J., and D. S. Schmid. 2008. Vaccine Oka variants and sequence variability in vaccine-related skin lesions. J. Infect. Dis. 197(Suppl. 2):S54-S57. [DOI] [PubMed] [Google Scholar]
- 11.Breuer, J., D. S. Schmid, and A. A. Gershon. 2008. Use and limitations of varicella-zoster virus-specific serological testing to evaluate breakthrough in vaccinees and to screen for susceptibles. J. Infect. Dis. 197(Suppl. 2):S147-S151. [DOI] [PubMed] [Google Scholar]
- 12.Brisson, M., N. J. Gay, W. J. Edmunds, and N. J. Andrews. 2002. Exposure to varicella boosts immunity to herpes-zoster: implications for mass vaccination against chickenpox. Vaccine 20:2500-2507. [DOI] [PubMed] [Google Scholar]
- 13.Brisson, M., W. J. Edmunds, N. J. Gay, B. Law, and G. De Serres. 2000. Modelling the impact of immunization on the epidemiology of varicella zoster virus. Epidemiol. Infect. 125:651-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Broyer, M., and M. J. Tete. 1997. Varicella and zoster in children after kidney transplantation: long-term results of vaccination. Pediatrics 99:35-39. [DOI] [PubMed] [Google Scholar]
- 15.Brunell, P. A., J. Taylor-Wiedeman, C. F. Geiser, L. Frierson, and E. Lydick. 1986. Risk of herpes zoster in children with leukemia: varicella vaccine compared with history of chickenpox. Pediatrics 77:53-56. [PubMed] [Google Scholar]
- 16.Buchholz, U., R. Moolenaar, C. Peterson, and L. Mascola. 1999. Varicella outbreaks after vaccine licensure: should they make you chicken? Pediatrics 104:561-563. [DOI] [PubMed] [Google Scholar]
- 17.Campsall, S. A., N. H. C. Au, J. S. Prendiville, D. P. Speert, R. Tan, and E. E. Thomas. 2004. Detection and genotyping of varicella-zoster virus by TaqMan allelic discrimination real-time PCR. J. Clin. Microbiol. 42:1409-1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.CDC. 2003. Decline in annual incidence of varicella—selected states, 1990-2001. MMWR Morb. Mortal. Wkly. Rep. 52:884-885. [PubMed] [Google Scholar]
- 19.CDC. 1997. Immunization of health care workers: recommendations of Advisory Committee on Immunization Practices (ACIP) and the Hospital Infection Control Practices Advisory Committee (HICPAC). MMWR Morb. Mortal. Wkly. Rep. 46:1-42. [PubMed] [Google Scholar]
- 20.CDC. 2006. National, state, and urban area vaccination coverage among children aged 19-35 months—United States, 2005. MMWR Morb. Mortal. Wkly. Rep. 55:988-993. [PubMed] [Google Scholar]
- 21.CDC. 2004. Outbreak of varicella among vaccinated children—Michigan, 2003. MMWR Morb. Mortal. Wkly. Rep. 53:389-392. [PubMed] [Google Scholar]
- 22.CDC. 1996. Prevention of varicella: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recommend. Rep. 45(RR11):1-36. [PubMed] [Google Scholar]
- 23.CDC. 1999. Prevention of varicella: updated recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recommend. Rep. 48(RR6):1-5. [PubMed] [Google Scholar]
- 24.CDC. 2009. Immunization coverage in the United States—National Immunization Survey (NIS). Centers for Disease Control and Prevention, Atlanta, GA. http://www.cdc.gov/vaccines/stats-surv/imz-coverage.htm#nis.
- 25.Chaves, S. S., J. Zhang, R. Civen, B. M. Watson, T. Carbajal, D. Perella, and J. F. Seward. 2008. Varicella disease among vaccinated persons: clinical and epidemiological characteristics, 1997-2005. J. Infect. Dis. 197:S127-S131. [DOI] [PubMed] [Google Scholar]
- 26.Chen, J. J., Z. Zhu, A. A. Gershon, and M. D. Gershon. 2004. Mannose 6-phosphate receptor dependence of varicella zoster virus infection in vitro and in the epidermis during varicella and zoster. Cell 119:915-926. [DOI] [PubMed] [Google Scholar]
- 27.Reference deleted.
- 28.Civen, R., S. S. Chaves, A. Jumaan, H. Wu, L. Mascola, P. Gargiullo, and J. F. Seward. 2009. The incidence and clinical characteristics of herpes zoster among children and adolescents after implementation of varicella vaccination. Pediatr. Infect. Dis. J. 28:954-959. [DOI] [PubMed] [Google Scholar]
- 29.Clements, D. A., S. P. Moreira, P. M. Coplan, C. L. Bland, and E. B. Walter. 1999. Postlicensure study of varicella vaccine effectiveness in a day-care setting. Pediatr. Infect. Dis. J. 18:1047-1050. [DOI] [PubMed] [Google Scholar]
- 30.Coffin, S. E., and R. L. Hodinka. 1995. Utility of direct immunofluorescence and virus culture for detection of varicella-zoster virus in skin lesions. J. Clin. Microbiol. 33:2792-2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cohen, P. R. 1994. Tests for detecting herpes simplex virus and varicella-zoster virus. Dermatol. Clin. 12:51-68. [PubMed] [Google Scholar]
- 32.Davis, M. M., M. S. Patel, and A. Gebremariam. 2004. Decline in varicella-related hospitalizations and expenditures for children and adults after introduction of varicella vaccine in the United States. Pediatrics 114:786-792. [DOI] [PubMed] [Google Scholar]
- 33.Davison, A. J., and J. E. Scott. 1986. The complete DNA sequence of varicella-zoster virus. J. Gen. Virol. 67:1759-1816. [DOI] [PubMed] [Google Scholar]
- 34.Donahue, J. G., P. W. Choo, J. E. Manson, and R. Platt. 1995. The incidence of herpes zoster. Arch. Intern. Med. 155:1605-1609. [PubMed] [Google Scholar]
- 35.Dworkin, M. S., C. E. Jennings, J. Roth-Thomas, J. E. Lang, C. Stukenberg, and J. R. Lumpkin. 2002. An outbreak of varicella among children attending preschool and elementary school in Illinois. Clin. Infect. Dis. 35:102-104. [DOI] [PubMed] [Google Scholar]
- 36.Engelman, I., D. R. Petzold, A. Kosinska, B. G. Hepkema, T. F. Schulz, and A. Heim. 2008. Rapid quantitative PCR assays for the simultaneous detection of herpes simplex virus, varicella-zoster virus, cytomegalovirus, Epstein-Barr virus, and human herpesvirus 6 DNA in blood and other clinical specimens. J. Med. Virol. 80:467-477. [DOI] [PubMed] [Google Scholar]
- 37.Espy, M. J., R. Teo, T. K. Ross, K. A. Suien, A. D. Wold, J. R. Uhl, and T. F. Smith. 2000. Diagnosis of varicella-zoster virus infections in the clinical laboratory by LightCycler PCR. J. Clin. Microbiol. 38:3187-3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fine, P. E., and E. R. Zell. 1994. Outbreaks in highly vaccinated populations: implications for studies of vaccine performance. Am. J. Epidemiol. 139:77-90. [DOI] [PubMed] [Google Scholar]
- 39.Fleming, D. M., K. W. Cross, W. A. Cobb, and R. S. Chapman. 2004. Gender difference in the incidence of shingles. Epidemiol. Infect. 132:1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Folkers, E., J. Vreeswijk, A. P. Oranje, F. Waganaar, and J. N. Duivenvoorden. 1991. Improved detection of HSV by electron microscopy: comparison with viral culture and cytodiagnosis. J. Virol. Methods 34:273-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Galil, K., C. Brown, F. Lin, and J. Seward. 2002. Hospitalizations for varicella in the United States, 1988 to 1999. Pediatr. Infect. Dis. J. 21:931-935. [DOI] [PubMed] [Google Scholar]
- 42.Galil, K., E. Fair, N. Mountcastle, P. Britz, and J. Seward. 2002. Younger age at vaccination may increase risk of varicella vaccine failure. J. Infect. Dis. 186:102-105. [DOI] [PubMed] [Google Scholar]
- 43.Galil, K., B. Lee, T. Strine, C. Carraher, A. L. Baughman, M. Eaton, J. Montero, and J. Seward. 2002. Outbreak of varicella at a day-care center despite vaccination. N. Engl. J. Med. 347:1909-1915. [DOI] [PubMed] [Google Scholar]
- 44.Gershon, A., J. Chen, P. LaRussa, and S. Steinberg. 2007. Varicella-zoster virus: virology, p. 1537-1548. In P. R. Murray, E. J. Baron, J. H. Jorgensen, M. L. Landry, and M. A. Pfaller (ed.), Manual of clinical microbiology, 9th ed. ASM Press, Washington, DC.
- 45.Gershon, A. A., P. S. LaRussa, and S. P. Steinberg. 1994. Detection of antibodies to varicella zoster virus using a latex agglutination assay. Clin. Diagn. Virol. 2:271-277. [DOI] [PubMed] [Google Scholar]
- 46.Gershon, A. A., P. LaRussa, S. Steinberg, N. Mervish, S. H. Lo, and P. Meier. 1996. The protective effect of immunologic boosting against zoster: an analysis in leukemic children who were vaccinated against chickenpox. J. Infect. Dis. 173:450-453. [DOI] [PubMed] [Google Scholar]
- 47.Gershon, A. A., S. Piomelli, M. Karpatkin, E. Smithwick, and S. Steinberg. 1978. Antibody to varicella-zoster virus after passive immunization against chickenpox. J. Clin. Microbiol. 8:733-735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gershon, A. A., S. Steinberg, and the NIAID Collaborative Varicella Vaccine Study Group. 1990. Live attenuated varicella vaccine: protection in healthy adults in comparison to leukemic children. J. Infect. Dis. 161:661-666. [DOI] [PubMed] [Google Scholar]
- 49.Gidding, H. F., M. Brisson, C. R. Macintyre, and M. A. Burgess. 2005. Modelling the impact of vaccination on the epidemiology of varicella zoster virus in Australia. Aust. N. Z. J. Public Health 29:544-551. [DOI] [PubMed] [Google Scholar]
- 50.Gomi, Y., H. Sunamachi, Y. Mori, K. Nagaike, M. Takahashi, and K. Yamanishi. 2002. Comparison of the complete DNA sequences of the Oka varicella vaccine and its parental virus. J. Virol. 76:11447-11459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gordon, J. E. 1962. Chickenpox: an epidemiologic review. Am. J. Med. Sci. 224:362-389. [PubMed] [Google Scholar]
- 52.Greaves, C. A., C. F. Lee, C. I. Bustamante, and J. D. Meyers. 1988. Use of murine monoclonal antibodies for laboratory diagnosis of varicella-zoster virus infection. J. Clin. Microbiol. 26:1623-1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guess, H. A., D. D. Broughton, L. J. Melton, and L. T. Kurland. 1985. Epidemiology of herpes zoster in children and adolescents: a population-based study. Pediatrics 76:512-517. [PubMed] [Google Scholar]
- 54.Guris, D., A. O. Jumaan, L. Mascola, B. M. Watson, J. X. Zhang, S. S. Chaves, P. Gargiullo, D. Perella, R. Civen, and S. F. Seward. 2008. Changing varicella epidemiology in active surveillance sites—United States, 1995-2005. J. Infect. Dis. 197(Suppl. 2):S71-S75. [DOI] [PubMed] [Google Scholar]
- 55.Haberland, M., U. Meyer-König, and F. T. Hufert. 1999. Variation within the glycoprotein B gene of human cytomegalovirus is due to homologous recombination. J. Gen. Virol. 80:1495-1500. [DOI] [PubMed] [Google Scholar]
- 56.Haddad, M. B., M. B. Hill, A. T. Pavia, C. E. Green, A. O. Jumaan, A. K. De, and R. T. Rolfs. 2005. Vaccine effectiveness during a varicella outbreak among schoolchildren: Utah, 2002-2003. Pediatrics 115:1488-1493. [DOI] [PubMed] [Google Scholar]
- 57.Hammond, O., Y. Wang, T. Green, J. Antonello, R. Kuhn, C. Motley, P. Stump, B. Rich, N. Chirmule, and R. D. Marchese. 2006. The optimization and validation of the glycoprotein ELISA assay for quantitative varicella-zoster virus (VZV) antibody detection. J. Med. Virol. 78:1679-1687. [DOI] [PubMed] [Google Scholar]
- 58.Hardy, I., A. A. Gershon, S. Steinberg, and P. LaRussa. 1991. The incidence of zoster after immunization with live attenuated varicella vaccine. A study in children with leukemia. N. Engl. J. Med. 325:1545-1550. [DOI] [PubMed] [Google Scholar]
- 59.Harpaz, R., I. R. Ortega-Sanchez, J. F. Seward, and the Advisory Committee on Immunization Practices. 2008. Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recommend. Rep. 57(RR5):1-30. [PubMed] [Google Scholar]
- 60.Hope-Simpson, R. E. 1965. The nature of herpes zoster: a long-term study and a new hypothesis. Proc. R. Soc. Med. 58:9-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reference deleted.
- 62.Insinga, R. P., R. F. Itzler, J. M. Pellissier, P. Saddier, and A. A. Nikas. 2005. The incidence of herpes zoster in a United States administrative database. J. Gen. Intern. Med. 20:748-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Izurieta, H. S., P. M. Strebel, and P. A. Blake. 1997. Postlicensure effectiveness of varicella vaccine during an outbreak in a child care center. JAMA 278:1495-1499. [PubMed] [Google Scholar]
- 64.Johnson, C. E., T. Stancin, D. Fattlar, L. P. Rome, and M. L. Kumar. 1997. A long-term prospective study of varicella vaccine in healthy children. Pediatrics 100:761-766. [DOI] [PubMed] [Google Scholar]
- 65.Josephson, A., and M. E. Gombert. 1988. Airborne transmission of nosocomial varicella from localized zoster. Pediatrics 158:238-241. [DOI] [PubMed] [Google Scholar]
- 66.Jumaan, A. O., O. Yu, L. A. Jackson, K. Bohlke, K. Galil, and J. F. Seward. 2005. Incidence of herpes zoster, before and after varicella-vaccination associated decreases in the incidence of varicella, 1992-2002. J. Infect. Dis. 191:2002-2007. [DOI] [PubMed] [Google Scholar]
- 67.Kamiya, H., T. Kato, M. Isaji, S. Torigoe, K. Oitani, M. Ito, T. Ihara, M. Sakurai, and M. Takahashi. 1984. Immunization of acute leukemic children with a live varicella vaccine (Oka strain). Biken J. 27:99-102. [PubMed] [Google Scholar]
- 68.Kilgore, P. E., D. Kruszon-Moran, J. F. Seward, A. Jumaan, F. P. L. Van Loon, B. Forghani, G. M McQuillen, M. Wharton, L. J. Fehrs, C. K. Cossen, and S. C. Hadler. 2003. Varicella in Americans from NHANES III: implications for control through routine immunization. J. Med. Virol. 70(Suppl. 1):S111-S118. [DOI] [PubMed] [Google Scholar]
- 69.Kuter, B., H. Matthews, H. Shinefield, S. Black, P. Dennehy, B. Watson, K. Reisinger, L. L. Kim, L. Lupinacci, J. Hartzel, I. Chan, and the Study Group for Varivax. 2004. Ten year follow-up of healthy children who received one or two injections of varicella vaccine. Pediatr. Infect. Dis. J. 23:132-137. [DOI] [PubMed] [Google Scholar]
- 70.Kuter, B. J., R. E. Weibel, H. A. Guess, H. Matthews, D. H. Morton, B. J. Neff, P. J. Provost, B. A. Watson, S. E. Starr, and S. A. Plotkin. 1991. Oka/Merck varicella vaccine in healthy children: final report of a 2-year efficacy study and 7-year follow-up studies. Vaccine 9:643-647. [DOI] [PubMed] [Google Scholar]
- 71.LaRussa, P., O. Lungu, I. Hardy, A. Gershon, S. P. Steinberg, and S. Silverstein. 1992. Restriction fragment length polymorphism of polymerase chain reaction products from vaccine and wild-type varicella-zoster virus isolates. J. Virol. 66:1016-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lawrence, R., A. A. Gershon, R. Holzman, S. P. Steinberg, and the NIAID Varicella Vaccine Collaborative Study Group. 1988. The risk of zoster after varicella vaccination in children with leukemia. N. Engl. J. Med. 318:543-548. [DOI] [PubMed] [Google Scholar]
- 73.Lee, B. R., S. L. Feaver, C. A. Miller, C. W. Hedberg, and K. R. Ehresmann. 2004. An elementary school outbreak of varicella attributed to vaccine failure: policy implications. J. Infect. Dis. 190:477-483. [DOI] [PubMed] [Google Scholar]
- 74.Levin, M. J., G.-Y. Cai, M. D. Manchak, and L. I. Pizer. 2003. Varicella-zoster virus DNA in cells isolated from human trigeminal ganglia. J. Virol. 77:6979-6987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li, S., A. S. F. Chan, H. Matthews, J. F. Heyse, C. Chan, B. J. Kuter, K. M. Kaplan, S. J. R. Vessey, and J. C. Sadoff. 2002. Inverse relationship between six week postvaccination varicella antibody response to vaccine and likelihood of long term breakthrough infection. Pediatr. Infect. Dis. J. 21:337-342. [DOI] [PubMed] [Google Scholar]
- 76.Lolekha, S., W. Tanthiphabha, P. Sornchai, P. Kosuwan, S. Sutra, B. Warachit, S. Chup-Upprakarn, Y. Hutagalung, J. Weil, and H. L. Bock. 2001. Effect of climatic factors and population density on varicella zoster virus epidemiology within a tropical country. Am. J. Trop. Med. Hyg. 64:131-136. [DOI] [PubMed] [Google Scholar]
- 77.Loparev, V. N., T. Argaw, P. R. Krause, M. Takayama, and D. S. Schmid. 2000. Improved identification and differentiation of varicella-zoster virus (VZV) wild-type strains and an attenuated varicella vaccine strain using a VZV open reading frame 62-based PCR. J. Clin. Microbiol. 38:3156-3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Loparev, V. N., E. Rubtcova, V. Bostik, D. Govil, C. J. Birch, J. D. Druce, D. S. Schmid, and M. C. Croxson. 2007. Identification of five major and two minor genotypes of varicella-zoster virus strains: a practical two-amplicon approach used to genotype clinical isolates in Australia and New Zealand. J. Virol. 81:12758-12765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Loparev, V. N., E. Rubtcova, J. F. Seward, M. J. Levin, and D. S. Schmid. 2007. DNA sequence variability in isolates recovered from patients with postvaccination rash or herpes zoster caused by Oka varicella vaccine. J. Infect. Dis. 195:502-510. [DOI] [PubMed] [Google Scholar]
- 80.Loparev, V., E. Martro, E. Rubtcova, C. Rodrigo, J.-C. Piette, E. Caumes, J.-P. Vernant, D. S. Schmid, and A.-M. Fillet. 2007. Toward universal varicella-zoster virus (VZV) genotyping: diversity of VZV strains from France and Spain. J. Clin. Microbiol. 45:559-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Loparev, V. N., K. McCaustland, B. P. Holloway, P. R. Krause, M. Takayama, and D. S. Schmid. 2000. Rapid genotyping of varicella-zoster virus vaccine and wild-type strains with fluorophore-labeled hybridization probes. J. Clin. Microbiol. 38:4315-4319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Loparev, V. N., A. Gonzalez, M. Deleon-Carnes, G. Tipples, H. Fickenscher, E. G. Torfason, and D. S. Schmid. 2004. Global identification of three major genotypes of varicella-zoster virus: longitudinal clustering and strategies for genotyping. J. Virol. 78:8349-8358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lopez, A. S., A. Burnett-Hartman, R. Nambler, L. Ritz, P. Owens, V. N. Loparev, D. Guris, and D. S. Schmid. 2008. Transmission of a newly characterized strain of varicella-zoster virus from a patient with herpes zoster in a long-term care facility, West Virginia, 2004. J. Infect. Dis. 197:646-653. [DOI] [PubMed] [Google Scholar]
- 84.Lopez, A. S., D. Guris, L. Zimmerman, L. Gladden, T. Moore, D. T. Haselow, V. N. Loparev, D. S. Schmid, A. O. Jumaan, and S. L. Snow. 2006. One dose of varicella vaccine does not prevent school outbreaks: is it time for a second dose? Pediatrics 117:e1070-e1077. [DOI] [PubMed] [Google Scholar]
- 85.Ludwig, G., F. B. Kraus, R. Allwinn, S. Keim, H. W. Doerr, and S. Buxbaum. 2006. Loss of varicella zoster virus antibodies despite detectable cell mediated immunity after vaccination. Infection 34:222-226. [DOI] [PubMed] [Google Scholar]
- 86.Lungu, O., P. W. Annunziato, A. Gershon, S. M. Staugaitis, D. Josefson, P. LaRussa, and S. J. Silverstein. 1995. Reactivated and latent varicella-zoster virus in human dorsal root ganglia. Proc. Natl. Acad. Sci. U. S. A. 92:10980-10984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Maple, P. A., J. Gray, J. Breuer, G. Kafatos, S. Parker, and D. Brown. 2006. Performance of a time-resolved fluorescence immunoassay for measuring varicella-zoster virus immunoglobulin G levels in adults and comparison with commercial immunoassays and Merck glycoprotein enzyme immunoassay. Clin. Vaccine Immunol. 13:214-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87a.Maple, P. A. C., J. Gray, K. Brown, and D. Brown. 23 January 2009. Performance characteristics of a quantitative, standardized varicella IgG time-resolved fluorescence immunoassay (VZV TRFIA) for measuring antibody following natural infection. J. Virol. Methods [ePub ahead of print.] doi. 10.1016/j.jviromet.2008.12.007. [DOI] [PubMed]
- 88.Marin, M., D. Guris, S. S. Chaves, S. Schmid, J. F. Seward, and the Advisory Committee on Immunization Practices. 2007. Prevention of varicella: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recommend. Rep. 56(RR4):1-40. [PubMed] [Google Scholar]
- 89.Marin, M., H. Q. Nguyen, J. Keen, A. O. Jumaan, P. M. Mellen, E. B. Hayes, K. F. Gensheimer, J. Gunderman-King, and J. F. Seward. 2005. Importance of catch-up vaccination: experience from a varicella outbreak, Maine, 2002-2003. Pediatrics 115:900-905. [DOI] [PubMed] [Google Scholar]
- 90.McGeoch, D. J., F. J. Rixon, and A. J. Davison. 2006. Topics in herpesvirus genomics and evolution. Virus Res. 117:90-104. [DOI] [PubMed] [Google Scholar]
- 91.Michalik, D. E., S. P. Steinberg, P. S. LaRussa, K. M. Edwards, P. F. Wright, A. M. Arvin, H. A. Gans, and A. A. Gershon. 2008. Primary vaccine failure after 1 dose of varicella vaccine in healthy children. J. Infect. Dis. 197:944-949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Midgley, R. S., N. W. Blake, Q. Y. Yao, D. Croom-Carter, S. T. Cheung, S. F. Leung, A. T. C. Chan, P. J. Johnson, D. Huang, A. B. Rickenson, and S. P. Lee. 2000. Novel intertypic recombinants of Epstein-Barr virus in the Chinese population. J. Virol. 74:1544-1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mullooly, J. P., K. Riedlinger, C. Chun, S. Weinmann, and H. Houston. 2005. Incidence of herpes zoster, 1997-2002. Epidemiol. Infect. 133:245-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nader, S., R. Bergen, M. Sharp, and A. M. Arvin. 1995. Age-related differences in cell-mediated immunity to varicella-zoster virus in children and adults immunized with live attenuated varicella vaccine. J. Infect. Dis. 171:13-17. [DOI] [PubMed] [Google Scholar]
- 95.Nahass, G. T., B. A. Goldstein, W. Y. Zhu, U. Serfling, N. S. Penneys, and C. L. Leonardi. 1992. Comparison of Tzanck smear, viral culture, and DNA diagnostic methods in detection of herpes simplex and varicella-zoster infection. JAMA 268:2541-2544. [PubMed] [Google Scholar]
- 96.Ngai, A. L., B. O. Staehle, B. J. Kuter, N. M. Cyanovich, I. Cho, H. Matthews, P. Keller, A. M. Arvin, B. Watson, C. J. White, and the Varivax Study Group. 1996. Safety and immunogenicity of one vs. two injections of Oka/Merck varicella vaccine in healthy children. Pediatr. Infect. Dis. J. 15:49-54. [DOI] [PubMed] [Google Scholar]
- 97.Nguyen, H. Q., A. O. Jumaan, and J. F. Seward. 2005. Decline in mortality due to varicella after implementation of varicella vaccination in the United States. N. Engl. J. Med. 352:450-458. [DOI] [PubMed] [Google Scholar]
- 98.Norberg, P., M. J. Kasubi, L. Haarr, T. Bergström, and J. A. Liljeqvist. 2007. Divergence and recombination of clinical herpes simplex virus type 2 isolates. J. Virol. 81:13158-13167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Norberg, P., J.-Å. Liljeqvist, T. Bergström, S. Sammons, D. S. Schmid, and V. N. Loparev. 2006. Complete-genome phylogenetic approach to varicella-zoster virus evolution: genetic divergence and evidence for recombination. J. Virol. 80:9569-9576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Okamoto, S., T. Yoshikawa, M. Ihira, K. Suzuki, and K. Shimokawa. 2004. Rapid detection of varicella-zoster virus infection by a loop-mediated isothermal amplification method. J. Med. Virol. 74:677-682. [DOI] [PubMed] [Google Scholar]
- 101.Opstelten, W., G. A. Van Essen, F. Schellevis, T. J. Verheij, and K. G. Moons. 2006. Gender as an independent risk factor for herpes zoster: a population-based prospective study. Ann. Epidemiol. 16:692-695. [DOI] [PubMed] [Google Scholar]
- 102.Ozcan, A., M. Senol, H. Saglam, M. Seyhaw, R. Durmaz, E. Aktas, and I. H. Ozerol. 2007. Comparison of the Tzanck test and polymerase chain reaction in the diagnosis of cutaneous herpes simplex and varicella-zoster virus infections. Int. J. Dermatol. 46:1177-1179. [DOI] [PubMed] [Google Scholar]
- 103.Parker, A. A., M. A. Reynolds, J. Leung, M. Anderson, A. Rey, J. R. Ortega-Sanchez, D. S. Schmid, D. Guris, and K. F. Gensheimer. 2008. Challenges to implementing second-dose varicella vaccination during an outbreak in the absence of a routine 2-dose vaccination requirement—Maine, 2006. J. Infect. Dis. 197(Suppl. 2):S101-S107. [DOI] [PubMed] [Google Scholar]
- 104.Parker, C. J., K. Morgan, and M. E. Dewey. 1997. Physical illness and disability among elderly people in England and Wales: the Medical Research Council Cognitive Function and Ageing Study. J. Epidemiol. Community Health 51:494-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Parker, S. P., M. Quinlivan, Y. Taha, and J. Breuer. 2006. Genotyping of varicella-zoster virus and the discrimination of Oka vaccine strains by TaqMan real-time PCR. J. Clin. Microbiol. 44:3911-3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Peters, G. A., S. D. Tyler, C. Grose, A. Severini, M. J. Gray, C. Upton, and G. A. Tipples. 2006. A full-genome phylogenetic analysis of varicella-zoster virus reveals a novel origin of replication-based genotyping scheme and evidence of recombination between major circulating clades. J. Virol. 80:9850-9860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Plotkin, S. A., S. E. Starr, K. Connor, and D. Morton. 1989. Zoster in normal children after varicella vaccine. J. Infect. Dis. 159:1000-1001. [DOI] [PubMed] [Google Scholar]
- 108.Poole, L. J., J.-C. Zong, D. M. Ciufo, D. J. Alcendor, J. S. Cannon, R. Ambinder, J. M. Orenstein, M. S. Reitz, and G. S. Hayward. 1999. Comparison of genetic variability at multiple loci across the genomes of the major subtypes of Kaposi's sarcoma-associated herpesvirus reveals evidence for recombination and for two distinct types of open reading frame K15 alleles at the right-hand end. J. Virol. 73:6646-6660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Provost, P. J., D. L. Krah, B. J. Kuter, D. H. Morton, T. L. Schofield, E. H. Wasmuth, C. J. White, W. J. Miller, and R. W. Ellis. 1991. Antibody assays suitable for assessing immune responses to live varicella vaccine. Vaccine 9:111-116. [DOI] [PubMed] [Google Scholar]
- 110.Quinlivan, M. L., A. A. Gershon, S. P. Steinberg, and J. Breuer. 2004. Rashes occurring after immunization with a mixture of viruses in the Oka vaccine are derived from single clones of virus. J. Infect. Dis. 190:793-796. [DOI] [PubMed] [Google Scholar]
- 111.Ragozzino, M. W., L. J. Melton III, L. T. Kurland, C. P. Chu, and H. O. Perry. 1982. Population-based study of herpes zoster and its sequelae. Medicine (Baltimore) 61:310-316. [DOI] [PubMed] [Google Scholar]
- 112.Ratner, A. J. 2002. Varicella-related hospitalizations in the vaccine era. Pediatr. Infect. Dis. J. 21:927-930. [DOI] [PubMed] [Google Scholar]
- 113.Ross, A. H. 1962. Modification of chickenpox in family contacts by administration of gamma globulin. N. Engl. J. Med. 267:369-376. [DOI] [PubMed] [Google Scholar]
- 114.Saiman, L., P. LaRussa, S. P. Steinberg, J. Zhou, K. Baron, S. Whittier, P. Della-Latta, and A. A. Gershon. 2001. Persistence of immunity to varicella-zoster virus vaccination among health care workers. Infect. Control Hosp. Epidemiol. 22:279-283. [DOI] [PubMed] [Google Scholar]
- 115.Sato, H., J. Yamamura, S. Kageyama, M. Kurokawa, and K. Shiraki. 2003. Superiority of varicella skin test antigen over purified varicella-zoster virus glycoproteins in monitoring booster response to Oka varicella vaccine. Vaccine 22:15-20. [DOI] [PubMed] [Google Scholar]
- 116.Sauerbrei, A., and P. Wutzler. 2007. Different genotype pattern of varicella-zoster virus obtained from patients with varicella and zoster in Germany. J. Med. Virol. 79:1025-1031. [DOI] [PubMed] [Google Scholar]
- 117.Sawyer, M. H., C. J. Chamberlin, Y. N. Wu, N. Aintablian, and M. R. Wallace. 1994. Detection of varicella zoster virus DNA in air samples from hospital rooms. J. Infect. Dis. 169:91-94. [DOI] [PubMed] [Google Scholar]
- 118.Schirm, J., J. J. Meulenberg, G. W. Pastoor, P. C. van Voorst Vader, and F. P. Schröder. 1989. Rapid detection of varicella-zoster virus in clinical specimens using monoclonal antibodies on shell vials and smears. J. Med. Virol. 28:1-6. [DOI] [PubMed] [Google Scholar]
- 119.Schmid, D. S., V. Bostik, J. Folster, N. Jensen, P. Norberg, B. Bankamp, and K. Hummel. 2008. Evidence for recombination between Oka vaccine and wild type strains among clinical varicella-zoster virus (VZV) isolates, abstr. 803. Abstr. 33rd Int. Herpesvirus Wkshp.
- 120.Schmidt-Chanasit, J., S. Ölschläger, S. Günther, G. Jaeger, K. Bleymehl, S. G. Schäd, G. Heckel, R. G. Ulrich, and H. W. Doerr. 13 August 2008. Molecular analysis of varicella-zoster virus strains circulating in Tanzania demonstrates the presence of genotype M1. J. Clin. Microbiol. doi: 10.1128/JCM.01057-08. [DOI] [PMC free article] [PubMed]
- 121.Seiler, H. E. 1949. A study of herpes zoster particularly in its relationship to chickenpox. J. Hyg. (Lond.) 47:253-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sengupta, N., Y. Taha, F. T. Scott, M. E. Leedham-Green, M. Quinlivan, and J. Breuer. 2007. Varicella-zoster-virus genotypes in East London: a prospective study in patients with herpes zoster. J. Infect. Dis. 196:1014-1020. [DOI] [PubMed] [Google Scholar]
- 123.Sergeev, N., E. Rubtcova, V. Chizikov, D. S. Schmid, and V. N. Loparev. 2006. New mosaic subgenotype of varicella-zoster virus in the USA: VZV detection and genotyping by oligonucleotide-microarray. J. Virol. Methods 136:8-16. [DOI] [PubMed] [Google Scholar]
- 124.Seward, J. F., J. X. Zhang, T. J. Maupin, L. Mascola, and A. O. Jumaan. 2004. Contagiousness of varicella in vaccinated cases: a household contact study. JAMA 292:704-728. [DOI] [PubMed] [Google Scholar]
- 125.Sharrar, R. G., P. LaRussa, S. A. Galea, S. P. Steinberg, A. R. Sweet, R. M. Keatley, M. E. Wells, W. P. Stephenson, and A. A. Gershon. 2001. The postmarketing safety profile of varicella vaccine. Vaccine 19:916-923. [DOI] [PubMed] [Google Scholar]
- 126.Shinefield, H. R., S. Black, and B. J. Kuter. 2008. Varicella immunogenicity with 1- and 2-dose regimens of measles-mumps-rubella-varicella vaccine. J. Infect. Dis. 197(Suppl. 2):S152-S155. [DOI] [PubMed] [Google Scholar]
- 127.Solomon, A. R. 1986. The Tzanck smear. Viable and valuable in the diagnosis of herpes simplex, zoster and varicella. J. Dermatol. 18:218-221. [DOI] [PubMed] [Google Scholar]
- 128.Stránská, R., R. Schuurman, M. de Vos, and A. M. van Loon. 2004. Routine use of a highly automated and internally controlled real-time PCR assay for the diagnosis of herpes simplex and varicella-zoster virus infections. J. Clin. Virol. 30:39-44. [DOI] [PubMed] [Google Scholar]
- 129.Straus, S. E. 1994. The biology of varicella-zoster virus infection. Ann. Neurol. 35(Suppl.):S4-S8. [DOI] [PubMed] [Google Scholar]
- 130.Suzuki, K., T. Yoshikawa, A. Tomitaka, K. Suzuki, K. Matsunaga, and Y. Asano. 2002. Detection of varicella-zoster virus DNA in throat swabs of patients with herpes zoster and on air purifier filters. J. Med. Virol. 66:567-570. [DOI] [PubMed] [Google Scholar]
- 131.Suzuki, K., T. Yoshikawa, A. Tomitaka, K. Matsunaga, and Y. Asano. 2004. Detection of aerosolized varicella-zoster virus DNA in patients with localized herpes zoster. J. Infect. Dis. 189:1009-1012. [DOI] [PubMed] [Google Scholar]
- 132.Takahashi, M., K. Baba, K. Horiuchi, H. Kamiya, and Y. Asano. 1990. A live varicella vaccine. Adv. Exp. Med. Biol. 278:49-58. [DOI] [PubMed] [Google Scholar]
- 133.Takahashi, M., H. Kamiya, Y. Asano, K. Shiraki, K. Baba, T. Otsuka, T. Hirota, and K. Yamanishi. 2001. Immunization of the elderly to boost immunity against varicella-zoster virus (VZV) as assessed by VZV skin test reaction. Arch. Virol. Suppl. 17:161-172. [DOI] [PubMed] [Google Scholar]
- 134.Takahashi, M., T. Otsuka, Y. Okuno, Y. Asano, and T. Yazaki. 1974. Live vaccine used to prevent the spread of varicella in children in hospital. Lancet ii:1288-1290. [DOI] [PubMed] [Google Scholar]
- 135.Takahashi, M., Y. Okuno, T. Otsuka, J. Osame, and A. Takamizawa. 1975. Development of a live attenuated varicella vaccine. Biken J. 18:25-33. [PubMed] [Google Scholar]
- 136.Thomas, S. L., J. G. Wheeler, and A. J. Hall. 2002. Contacts with varicella or with children and protection against herpes zoster in adults: a case-control study. Lancet 360:678-682. [DOI] [PubMed] [Google Scholar]
- 137.Tugwell, B. D., L. E. Lee, H. Gillette, E. M. Lorber, K. Hedberg, and P. R. Cieslak. 2004. Chickenpox outbreak in a highly vaccinated school population. Pediatrics 113:455-459. [DOI] [PubMed] [Google Scholar]
- 138.Umene, K., S. Oohashi, M. Yoshida, and Y. Fukumaki. 2008. Diversity of the a sequence of herpes simplex virus type 1 developed during evolution. J. Gen. Virol. 89:841-852. [DOI] [PubMed] [Google Scholar]
- 139.Vázquez, M. D., P. S. LaRussa, A. A. Gershon, L. M. Niccolai, C. E. Muehlenbein, S. P. Steinberg, and E. D. Shapiro. 2004. Effectiveness over time of varicella vaccine. JAMA 291:851-855. [DOI] [PubMed] [Google Scholar]
- 140.Vázquez, M. D., P. S. LaRussa, A. A. Gershon, S. P. Steinberg, K. F. Freudigman, and E. Shapiro. 2001. The effectiveness of the varicella vaccine in clinical practice. N. Engl. J. Med. 344:955-960. [DOI] [PubMed] [Google Scholar]
- 141.Verstraeten, T., A. O. Jumaan, J. P. Mullooly, J. F. Seward, H. S. Izurieta, F. DeStefano, S. B. Black, R. T. Chen, and the Vaccine Safety Datalink Research Group. 2003. A retrospective cohort study of the association of varicella vaccine failure with asthma, steroid use, age at vaccination, and measles-mumps-rubella vaccination. Pediatrics 112:e98-e103. [DOI] [PubMed] [Google Scholar]
- 142.Vreeswijk, J., E. Folkers, F. Waganaar, and J. G. Kapsanberg. 1988. The use of colloidal gold immunoelectron microscopy to diagnose varicella-zoster virus (VZV) infections by rapid discrimination between VZV, HSV-1 and HSV-2. J. Virol. Methods 22:255-271. [DOI] [PubMed] [Google Scholar]
- 143.Waganaar, T. R., V. T. K. Chow, C. Buranathai, P. Thawatsupha, and C. Grose. 2003. The out of Africa model of varicella-zoster virus evolution: single nucleotide polymorphisms and private alleles distinguish Asian clades from European/North American clades. Vaccine 21:1072-1081. [DOI] [PubMed] [Google Scholar]
- 144.Walling, D. M., A. G. Perkins, J. Webster-Cyriaque, L. Resnick, and N. Raab-Traub. 1994. The Epstein-Barr virus EBNA-2 gene in oral hairy leukoplakia: strain variation, genetic recombination, and transcriptional expression. J. Virol. 68:7918-7926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Watson, B., C. Boardman, D. Laufer, S. Piercy, N. Tustin, D. Olaleye, A. Cnaan, and S. E. Starr. 1995. Humoral and cell-mediated immune responses in healthy children after one or two doses of varicella vaccine. Clin. Infect. Dis. 20:316-319. [DOI] [PubMed] [Google Scholar]
- 146.Watson, B. M., S. A. Piercy, S. A. Plotkin, and S. E. Starr. 1993. Modified chickenpox in children immunized with the Oka/Merck varicella vaccine. Pediatrics 91:17-22. [PubMed] [Google Scholar]
- 147.Watson, B., E. Rothstein, H. Bernstein, A. Arbeter, A. Arvin, S. Chartrand, D. Clements, M. L. Kumar, K. Reisinger, M. Blatter, et al. 1995. Safety and cellular and humoral immune responses of a booster dose of varicella vaccine 6 years after primary immunization. J. Infect. Dis. 172:217-219. [DOI] [PubMed] [Google Scholar]
- 148.Weinmann, S., C. Chun, J. P. Mullooly, K. Riedlinger, H. Houston, V. N. Loparev, D. S. Schmid, and J. F. Seward. 2008. Characteristics of breakthrough varicella in children. J. Infect. Dis. 197(Suppl. 2):S132-S138. [DOI] [PubMed] [Google Scholar]
- 149.Wharton, M. 1996. The epidemiology of varicella-zoster virus infections. Infect. Dis. Clin. North Am. 10:571-581. [DOI] [PubMed] [Google Scholar]
- 150.White, C. J. 1996. Clinical trials of varicella vaccine in healthy children. Infect. Dis. Clin. North Am. 10:595-608. [DOI] [PubMed] [Google Scholar]
- 151.White, C. J., B. J. Kuter, C. S. Hildebrand, K. L. Isganitis, H. Matthews, G. B. Calandra, W. J. Miller, P. J. Provost, R. W. Ellis, and R. J. Gerety. 1991. Varicella vaccine (VARIVAX) in healthy children and adolescents: results from clinical trials, 1987 to 1989. Pediatrics 87:604-610. [PubMed] [Google Scholar]
- 152.White, C. J., B. J. Kuter, A. Ngai, C. S. Hildebrand, K. L. Isganitis, C. M. Patterson, A. Capra, W. J. Miller, D. L. Krah, P. J. Provost, R. W. Ellis, and G. B. Callandra. 1992. Modified cases of chickenpox after varicella vaccination: correlation of protection with antibody response. Pediatr. Infect. Dis. J. 11:19-23. [DOI] [PubMed] [Google Scholar]
- 153.Yawn, B. P., R. A. Yawn, and E. Lydick. 1997. Community impact of childhood varicella infections. J. Pediatr. 130:759-765. [DOI] [PubMed] [Google Scholar]
- 154.Yih, W. K., D. R. Brooks, S. M. Lett, A. O. Jumaan, Z. Zhang, K. M. Clements, and J. F. Seward. 2005. The incidence of varicella and herpes zoster in Massachusetts as measured by the Behavioral Risk Factor Surveillance System (BRFSS) during a period of increasing varicella vaccine coverage, 1998-2003. BMC Public Health 5:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yoshikawa, T., M. Ihira, K. Suzuki, S. Suga, A. Tomitaka, H. Ueda, and Y. Asano. 2001. Rapid contamination of the environment with varicella-zoster virus DNA from a patient with herpes zoster. J. Med. Virol. 63:64-66. [PubMed] [Google Scholar]
- 156.Zerboni, L., S. Nader, K. Aoki, and A. M. Arvin. 1998. Analysis of the persistence of humoral and cellular immunity in children and adults immunized with varicella vaccine. J. Infect. Dis. 177:1701-1704. [DOI] [PubMed] [Google Scholar]
- 157.Zhou, F., R. Harpaz, A. O. Jumaan, C. A. Winston, and A. Shefer. 2005. Impact of varicella vaccination on health care utilization. JAMA 294:797-802. [DOI] [PubMed] [Google Scholar]
- 158.Zhou, F., I. R. Ortega-Sanchez, D. Guris, A. Shefer, T. Lieu, and J. F. Seward. 2008. An economic analysis of the universal varicella vaccination program in the United States. J. Infect. Dis. 197:S156-S164. [DOI] [PubMed] [Google Scholar]