Abstract
Adenosine and dopamine receptors in striatal areas interact to regulate a number of different functions, including aspects of motor control and motivation. Recent studies indicate that adenosine A2A receptor antagonists can reverse the effects of dopamine (DA) D2 antagonists on instrumental tasks that provide measures of effort-related choice behavior. The present experiments compared the ability of the adenosine A2A antagonist KW6002, the nonselective adenosine antagonist caffeine, and the adenosine A1 receptor selective antagonist DPCPX, to reverse the behavioral effects of the DA D2 antagonist haloperidol. For these studies, a concurrent choice procedure was used in which rats could select between lever pressing on a fixed ratio 5 schedule for a preferred food or approaching and consuming a less preferred lab chow that was concurrently available in the chamber. Under baseline or control conditions, rats show a strong preference for lever pressing, and eat little of the chow; IP injections of 0.1 mg/kg haloperidol significantly reduced lever pressing and substantially increased chow intake. The adenosine A2A antagonist KW6002 (0.125–0.5 mg/kg IP) and the nonselective adenosine antagonist caffeine (5.0–20.0 mg/kg) significantly reversed the effects of haloperidol. However, the adenosine A1 antagonist DPCPX (0.1875–0.75 mg/kg IP) failed to reverse the effects of the D2 antagonist. The rank order of effect sizes in the reversal experiments was KW6002 > caffeine > DPCPX. None of these drugs had any effect on behavior when they were injected in the absence of haloperidol. These results indicate that the ability of an adenosine antagonist to reverse the effort-related effects of a D2 antagonist depends upon the subtype of adenosine receptor being blocked. Together with other recent results, these experiments indicate that there is a specific interaction between DA D2 and adenosine A2A receptors, which could be related to the co-localization of these receptors on the same population of striatal neurons.
Keywords: Operant, Reinforcement, Motivation, Behavioral economics, Reward, Decision making, Activation, Depression, Psychomotor slowing, Anergia
1. Introduction
Several lines of evidence indicate that dopamine (DA) and adenosine systems interact in the brain. Striatal areas such as neostriatum and nucleus accumbens are very rich in adenosine A2A receptors [13,18,19,33,70], and several papers have reported that there is a functional interaction between striatal DA D2 and adenosine A2A receptors [17-23,27,28,31]. This interaction frequently has been studied in regard to neostriatal motor functions that are related to parkinsonian symptoms [8,20,22,29,32,34,35,46,52,53,59,67,82]. Researchers also have characterized aspects of adenosine A2A receptor function related to cognitive processes [76] and motivation [26,44,50]. In particular, several recent studies have focused upon the functional significance of adenosine A2A receptors, and the interactions between adenosine and DA receptors, in relation to aspects of behavioral activation and effort-related processes [16,26,44,47,83].
Previous studies have shown that nucleus accumbens DA is a critical component of the brain circuitry involved in behavioral activation and effort-related behavioral processes. Nucleus accumbens DA depletions make rats highly sensitive to ratio requirements in operant schedules [1,7,45,73], and affect response allocation in tasks that measure effort-related choice behavior [58,61-63,66,68]. Some studies in this area have employed maze tasks to assess effort-related choice [9,24,47,65], while others have used a concurrent fixed ratio 5 (FR5)/chow-feeding procedure [38,58,68,73]. In the latter task, rats have a choice between responding on a FR5 lever-pressing schedule for a highly preferred food (i.e., high carbohydrate operant pellets) or approaching and consuming freely available food (i.e., less preferred rodent laboratory chow). Under baseline or control conditions, rats that are trained to respond on this procedure spend most of their time pressing the lever for the preferred food, and eat very little of the concurrently available chow. Low doses of DA antagonists alter choice behavior such that lever pressing for food is suppressed, but chow intake is substantially increased [12,38,58,68,72]. Nucleus accumbens is the DA terminal region most closely associated with these effects [10,11,38,49,68,73]. The actions of DA antagonists or accumbens DA depletions differ substantially from those produced by motivational manipulations such as pre-feeding [38], and appetite suppressant drugs [12,58,72]. These appetite-related manipulations all failed to increase chow intake under conditions that suppressed lever pressing.
Recent papers have reported that intra-accumbens injections of the adenosine A2A agonist CGS 21680 produced effects that resembled those of accumbens DA depletions or antagonism, i.e., they impaired performance of operant schedules that had high ratio requirements [44], and they decreased lever pressing and increased chow intake in rats responding on the concurrent choice procedure [26]. In addition, the adenosine A2A receptor antagonist MSX-3 has been reported to reverse the effects of DA D2 antagonists such as haloperidol and eticlopride on tasks that provide measures of effort-related choice behavior, such as the operant concurrent choice task [16,83] and the T-maze choice procedure [47]. The present studies were conducted to investigate the role of DA/adenosine A2A receptor interactions in effort-related choice behavior, using the concurrent lever-pressing/chow-feeding procedure. Specifically, these experiments were undertaken to determine if the ability of an adenosine receptor antagonist to reverse the effect of a DA D2 antagonist is dependent upon the particular subtype of adenosine receptor that was being blocked. In the first group of experiments, three drugs were assessed for their ability to reverse the effects of 0.1 mg/kg of the DA D2 antagonist haloperidol: the well-known adenosine A2A antagonist KW6002 (istradefylline; 0.125–0.5 mg/kg IP), the nonselective adenosine antagonist and minor stimulant caffeine (5.0–20.0 mg/kg), and the adenosine A1 antagonist DPCPX (0.1875–0.75 mg/kg IP). The fourth experiment studied the effects of the higher doses of KW6002, caffeine, and DPCPX in the absence of haloperidol. In view of the anatomical data demonstrating colocalization of DA D2 receptors and adenosine A2A receptors in striatum and nucleus accumbens, and the well-documented interactions between these receptors, it was hypothesized that the adenosine A2A antagonist would be more effective at reversing the effects of haloperidol than the A1 selective antagonist.
2. Materials and methods
2.1. Animals
A total of 33 adult male Sprague—Dawley rats (Harlan Sprague Dawley, Indianapolis, IN) were used in these experiments. They weighed 295–352 g at the beginning of the experiments, and were deprived to 85% of their free-feeding body weight for initial lever press training, but then were allowed modest growth (i.e., an additional 5–10%) throughout the course of the studies. All rats were housed in a climate-controlled animal colony maintained at 23 °C, with 12 h light—dark cycle (lights on 07:00 h), and had access to water ad libitum in their home cages. Animal protocols were approved by the University of Connecticut Institutional Animal Care and Use Committee, and the studies were conducted according to NIH guidelines.
2.2. Behavioral procedures
Behavioral sessions were conducted in operant conditioning chambers (28 cm × 23 cm × 23 cm; Med Associates). Rats were initially trained to lever press on a continuous reinforcement schedule (30-min sessions; 45-mg pellets, Bioserve, Frenchtown, NJ, were used for all operant behavior tests) and then were shifted to the FR5 schedule (30-min sessions, 5 days/week) and trained for several additional weeks. Rats were then trained on the concurrent FR5/chow-feeding procedure. With this task, weighed amounts of lab chow (Lab Diet, 5P00 Prolab RMH 3000, Purina Mills, St. Louis, MO; typically 15–20 g, three large pieces) were concurrently available on the floor of the chamber during the FR5 sessions. At the end of the session, rats were immediately removed from the chamber, and food intake was determined by weighing the remaining food (including spillage). Rats were trained until they attained stable levels of baseline lever pressing and chow intake (i.e., consistent responding over 1200 lever presses per 30 min), after which drug testing began. For most baseline days rats did not receive supplemental feeding, however, over weekends and after drug tests, rats usually received supplemental chow in the home cage. On baseline and drug treatment days, rats normally consumed all the operant pellets that were delivered from lever pressing during each session.
2.3. Pharmacological agents and selection of doses
Haloperidol (Sigma Chemical Co., St. Louis, MO) was dissolved in a 0.3% tartaric acid solution (pH 4.0), and this tartaric acid solution also was used as the vehicle control for the haloperidol injections. The adenosine A2A antagonist KW6002 was generously provided by Lundbeck Pharmaceuticals (Copenhagen, Denmark), and was dissolved in DMSO and Tween-80 mixed with 0.9% saline (10:10:80% mixture). DPCPX (8-cyclopentyl-1,3-dipropylxanthine) was obtained from Tocris, and was dissolved in a 20% ethanol vehicle. In all experiments, drug treatments were administered IP (see below for descriptions of individual experiments and drug administration schedules).
The dose of haloperidol used to alter choice behavior (0.1 mg/kg IP) was based upon previous research [16,68]. Although higher doses of haloperidol can suppress food intake, this dose did not suppress intake of chow or operant pellets, and did not alter preference between them [68]. Doses of KW6002 and caffeine were determined by unpublished pilot data and on previous research [59]. In addition, a pilot study indicated that 40.0 mg/kg caffeine plus haloperidol actually decreased performance relative to haloperidol alone; for that reasons, a dose range of 5.0–20.0 mg/kg caffeine was selected. Several factors were considered for determining the dose range for DPCPX that was used. In part, it was based upon doses listed in published behavioral studies involving IP administration in rats [2,39,40,47,54]. Furthermore, extensive pilot studies also were performed. In one study, it was determined that 1.5 and 3.0 mg/kg DPCPX did not reverse the effects of haloperidol on lever pressing, and that the 3.0 mg/kg dose actually tended to impair lever pressing in haloperidol-treated rats; this is consistent with a recent T-maze study showing that 3.0 mg/kg DPCPX in combination with haloperidol actually led to worse performance than haloperidol alone [47]. In addition, it was observed that very low doses of DPCPX (i.e., 0.04–0.09 mg/kg IP) also failed to reverse the effects of haloperidol. Based upon all this pilot work, an intermediate dose range of DPCPX (0.1875–0.75 mg/kg) was selected.
2.4. Experimental procedures
Rats were trained on the concurrent FR5/chow-feeding procedure (as described above) before drug testing began, and each experiment employed different groups of rats. All five experiments used a within-groups design, with each rat receiving all combined IP drug treatments in their particular experiment in a randomly varied order (one treatment per week, with none of the treatment sequences repeated across different animals in the same experiment). Baseline (i.e., non-drug) sessions were conducted four additional days per week. The specific treatments and testing times for each experiment are listed below.
2.4.1. Experiment 1: ability of KW6002 to reverse the effort-related effects of haloperidol
On the test day, rats (n = 8) were injected with either tartaric acid vehicle (50 min before testing) plus DMSO/Tween vehicle IP (20 min before testing), 0.1 mg/kg haloperidol IP (50 min before testing) plus DMSO/Tween vehicle IP (20 min before testing), and 0.1 mg/kg haloperidol IP (50 min before testing) plus various doses of KW6002 injected IP (0.125, 0.25 and 0.5 mg/kg; 20 min before testing).
2.4.2. Experiment 2: ability of caffeine to reverse effort-related effects of haloperidol
For the drug test days, rats (n = 8) were injected with either tartaric acid vehicle (50 min before testing) plus saline vehicle IP (20 min before testing), 0.1 mg/kg haloperidol IP (50 min before testing) plus saline vehicle IP (20 min before testing), and 0.1 mg/kg haloperidol IP (50 min before testing) plus various doses of caffeine injected IP (5.0, 10.0 and 20.0 mg/kg; 20 min before testing).
2.4.3. Experiment 3: ability of DPCPX to reverse effort-related effects of haloperidol
Rats (n = 10) were injected on drug treatment days with either tartaric acid vehicle (50 min before testing) plus saline vehicle IP (30 min before testing), 0.1 mg/kg haloperidol IP (50 min before testing) plus saline vehicle IP (30 min before testing), and 0.1 mg/kg haloperidol IP (50 min before testing) plus various doses of DPCPX injected IP (0.1875, 0.375 and 0.75 mg/kg; 30 min before testing).
2.4.4. Experiment 4: effects of KW6002, caffeine and DPCPX in the absence of haloperidol
Rats (n = 5) received IP injections of either saline vehicle, DMSO/Tween vehicle, 20% ethanol vehicle, 0.5 mg/kg KW6002, 20.0 mg/kg caffeine, or 0.75 mg/kg DPCPX 30 min before testing.
2.5. Statistical analyses
Total number of lever presses and gram quantity of chow intake from the 30 min sessions were analyzed with repeated measures analysis of variance (ANOVA). When the overall ANOVA was significant, non-orthogonal planned comparisons using the overall error term were used to compare each treatment with the haloperidol plus vehicle control condition. For these comparisons, α level was kept at 0.05 because the number of comparisons was restricted to the number of treatments minus one (Ref. [37]; pp. 110–139). With this analysis, each condition that combined haloperidol plus adenosine antagonist was compared with its respective haloperidol plus vehicle condition using the planned comparisons. The present analyses used Systat 7.0, and the factorial ANOVA with trend analysis also tested for interactions across the linear, quadratic and cubic trends. Effect size calculations (R2 values; [37]) were performed to assess the magnitude of the treatment effect (i.e., the size of the treatment effect sum of squares expressed as the proportion of total sum of squares, which is a marker of the total variance accounted for by treatment variance; for example R2 = 0.3 reflects 30% of the variance explained) across experiments and measures.
3. Results
3.1. Experiments 1–3: KW6002, caffeine and DPCPX combined with haloperidol
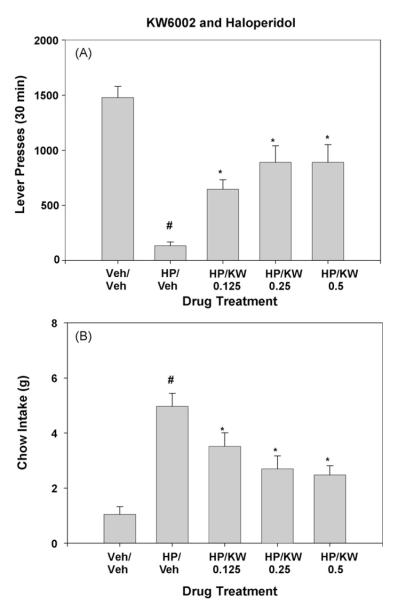
KW6002 significantly attenuated the effects of haloperidol on the concurrent lever-pressing/chow-feeding task. The overall treatment effect for lever pressing was statistically significant (Fig. 1A; [F(4,28) = 16.6, p < 0.01]). Planned comparisons revealed that haloperidol decreased lever pressing compared to injection of Veh/Veh (p < 0.01). KW6002 significantly increased responding in haloperidol-treated rats, with all three doses being significantly different from haloperidol plus vehicle (p < 0.05). The overall treatment effect for chow intake also was statistically significant (Fig. 1B; [F(4,28) = 11.2, p < 0.01]). Planned comparisons showed that haloperidol increased chow intake compared to injections of Veh/Veh (p < 0.01). KW6002 significantly decreased chow intake relative to haloperidol plus vehicle at all three doses (p < 0.05).
Fig. 1.
Effects of the adenosine A2A antagonist KW6002 in combination with 0.1 mg/kg haloperidol. (A) Mean (±S.E.M.) number of lever presses after treatment with vehicle or haloperidol plus various doses of KW6002 are shown. (B) Mean (±S.E.M.) intake of lab chow (in grams) after treatment with vehicle or haloperidol plus various doses of KW6002 are shown. Veh/Veh (vehicle plus vehicle), HP/Veh (0.1 mg/kg haloperidol plus vehicle), HP/KW 0.125 (0.1 mg/kg haloperidol plus 0.125 mg/kg KW6002), HP/KW 0.25 (0.1 mg/kg haloperidol plus 0.125 mg/kg KW6002), HP/KW 0.5 (0.1 mg/kg haloperidol plus 0.5 mg/kg KW6002). #p < 0.01, haloperidol different from vehicle/vehicle, planned comparison; *p < 0.05, different from vehicle plus haloperidol, planned comparison.
In experiment 2, caffeine also significantly attenuated the effects of haloperidol on the concurrent lever-pressing/chow-feeding task. The overall treatment effect for lever pressing was statistically significant (Fig. 2A; [F(4,28) = 28.8, p < 0.01]). Planned comparisons demonstrated that haloperidol decreased lever pressing compared to injection of Veh/Veh (p < 0.01). Caffeine significantly increased responding in haloperidol-treated rats, with all three doses being significantly different from haloperidol plus vehicle (p < 0.05). The overall treatment effect for chow intake also was statistically significant (Fig. 2B; [F(4,28) = 11.3, p < 0.01). Haloperidol increased chow intake compared to injections of Veh/Veh (p < 0.01). Caffeine significantly decreased chow intake relative to haloperidol plus vehicle at the 10.0 and 20.0 mg/kg doses (p < 0.05).
Fig. 2.
Effects of the nonselective adenosine antagonist caffeine in combination with 0.1 mg/kg haloperidol. (A) Mean (±S.E.M.) number of lever presses after treatment with vehicle or haloperidol plus various doses of caffeine are shown. (B) Mean (±S.E.M.) intake of lab chow (in grams) after treatment with vehicle or haloperidol plus various doses of caffeine are shown. Veh/Veh (vehicle plus vehicle), HP/Veh (0.1 mg/kg haloperidol plus vehicle), HP/CAF 5.0 (0.1 mg/kg haloperidol plus 5.0 mg/kg caffeine), HP/CAF 10.0 (0.1 mg/kg haloperidol plus 10.0 mg/kg caffeine), HP/CAF 20.0 (0.1 mg/kg haloperidol plus 20.0 mg/kg caffeine). #p < 0.01, haloperidol different from vehicle/vehicle, planned comparison; *p < 0.05, different from vehicle plus haloperidol, planned comparison.
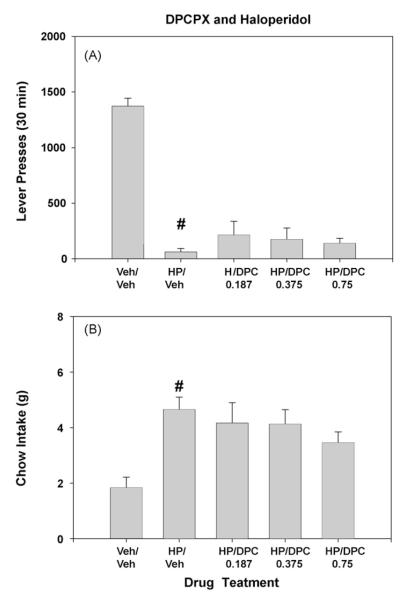
The results of the third experiment showed that DPCPX failed to significantly alter responding in haloperidol-treated rats. The treatment effect for lever pressing was statistically significant (Fig. 3A; [F(4,36) = 32.3, p < 0.001]), and as in the other experiments, haloperidol decreased lever pressing compared to injection of Veh/Veh (p < 0.01). However, DPCPX combined with haloperidol had no significant effects compared to haloperidol plus vehicle. Similarly, the overall treatment effect for chow intake also was statistically significant (Fig. 3B; [F(4,36) = 7.5, p < 0.01]), and planned comparisons revealed that haloperidol increased chow intake compared to injections of Veh/Veh (p < 0.01), but there were no significant differences between haloperidol/Veh and haloperidol plus any dose of DPCPX.
Fig. 3.
Effects of the adenosine A1 antagonist DPCPX in combination with 0.1 mg/kg haloperidol. (A) Mean (±S.E.M.) number of lever presses after treatment with vehicle or haloperidol plus various doses of DPCPX are shown. (B) Mean (±S.E.M.) intake of lab chow (in grams) after treatment with vehicle or haloperidol plus various doses of DPCPX are shown. Veh/Veh (vehicle plus vehicle), HP/Veh (0.1 mg/kg haloperidol plus vehicle), HP/DPC 0.187 (0.1 mg/kg haloperidol plus 0.1875 mg/kg DPCPX), HP/DPC 0.37 (0.1 mg/kg haloperidol plus 0.375 mg/kg DPCPX), HP/PDC 0.75 (0.1 mg/kg haloperidol plus 0.75 mg/kg DPCPX). #p < 0.01, haloperidol different from vehicle/vehicle, planned comparison.
3.2. Comparisons across the reversal experiments: effect sizes
In order to make comparisons between the effects of the different adenosine antagonists across multiple experiments, data from the Veh/Veh condition were excluded, and additional ANOVAs were performed. Effect size analyses were conducted based upon these separate ANOVAs performed on each drug reversal experiment (1–3) for both lever pressing and chow intake. There were marked differences in effect sizes between the three reversal experiments (see Table 1). KW6002 had the highest effect size, caffeine was intermediate, and DPCPX had a very small effect size.
Table 1.
Effect size calculations (R2 values) for the reversal experiments.
| Drug | Lever pressing | Chow intake |
|---|---|---|
| KW6002 | 0.49 | 0.40 |
| Caffeine | 0.21 | 0.11 |
| DPCPX | 0.06 | 0.08 |
3.3. Experiment 4: effects of KW6002, caffeine and DPCPX alone
Experiment 4 was a control study that assessed the effects of each vehicle condition used in experiments 1–3, as well as the highest doses of KW6002, caffeine and DPCPX used, in the absence of haloperidol. The means (±S.E.M.) number of lever presses were as follows: saline vehicle: 1250.6 (±120.2); 20% ethanol/saline vehicle: 1300.0 (±121.4); DMSO/Tween vehicle: 1227.4 (±165.7); 0.5 mg/kg KW6002: 1327 (±179.8); caffeine: 970.6 (±65.4); 0.75 mg/kg DPCPX: 1567.4 (±95.6). There were no significant differences between these conditions as determined by repeated measures ANOVA (F(5,20) = 2.4, n.s.). The means (±S.E.M.) gram quantity of chow intake was as follows: saline vehicle: 2.82 (±0.65); 20% ethanol/saline vehicle: 2.54 (±0.80); DMSO/Tween vehicle: 2.02 (±0.89); 0.5 mg/kg KW6002: 2.10 (±0.89); caffeine: 2.96 (±0.27); 0.75 mg/kg DPCPX: 1.80 (±0.70). As with lever pressing, there were no significant differences between these conditions in terms of chow intake (F(5,20) = 2.6, n.s.). With both measures, there was a tendency for caffeine alone to decrease responding and increase chow intake, and for DPCPX to increase lever pressing and decrease chow intake. The three vehicle conditions did not significantly differ from each other.
4. Discussion
In the experiments presented above, a concurrent choice lever-pressing/chow-feeding task was used to investigate the interaction between adenosine receptor antagonists with different profiles of selectivity and the DA D2 family antagonist haloperidol. Injection of haloperidol without co-administration of an adenosine antagonist produced a well-documented shift in response allocation; 0.1 mg/kg haloperidol significantly decreased lever pressing and increased chow intake in all experiments. These results are consistent with previously reported findings from experiments that employed systemic administration of numerous DA antagonists with varying degrees of receptor selectivity [12,58,68,72], as well as local DA antagonism in nucleus accumbens [38,49] and accumbens DA depletions [10,11,68,73]. Previous research has shown that the shift from lever pressing to chow intake in rats responding on this task occurs after DA depletions in the nucleus accumbens, but not after depletions in anteroventromedial neostriatum [11]. Moreover, DA depletions in ventrolateral neostriatum produced severe motor impairments that disrupted both lever pressing and feeding [11]. Based upon these results, as well as the outcome of numerous control experiments investigating the effects of various appetite and effort-related manipulations [12,58,66,68,69,72], and studies employing a discrete-trial T-maze choice task [9,47,65], this pattern of findings has been interpreted to indicate that DA antagonists and nucleus accumbens DA depletions are not acting to blunt appetite for food or to suppress primary or unconditioned food reinforcement [3,36,61,68,69]. Rather, these results are widely seen as consistent with the suggestion that DA antagonists and accumbens DA depletions are altering behavioral activation, instrumental response output, response allocation, or effort-related processes [4,25,48,51,56,61,68,69,72]. Of course, accumbens DA must participate in effort-related processes in concert with other structures and neurotransmitters [15,16,30,71,80,81], and for that reason the present studies investigated the ability of adenosine antagonists to reverse the effects of the D2 antagonist haloperidol.
In the first experiment, the adenosine A2A antagonist KW6002 was able to produce a substantial attenuation of the behavioral effects of the D2 antagonist haloperidol. Combined administration of KW6002 with haloperidol led to very large increases in lever pressing and decreases in chow intake compared to haloperidol alone. These data are consistent with recent studies indicating that the adenosine A2A antagonist MSX-3 was able to reverse the effects of the DA antagonists haloperidol [16] and eticlopride [83] in rats responding on the concurrent lever-pressing/chow intake task, and to reverse the suppression of an instrumental barrier climbing response in rats treated with haloperidol [47]. Taken together, these results indicate that adenosine and DA systems interact in the regulation of effort-related functions. Moreover, these observations are consistent with recent reports that local administration of an adenosine A2A agonist into the nucleus accumbens can produce behavioral effects that resemble those of DA antagonists or accumbens DA depletions [26,44].
The nonselective adenosine antagonist caffeine also was able to significantly reduce the effects of haloperidol in experiment 2. Although the effect sizes in the caffeine experiment were generally smaller than those produced by KW6002, it is nevertheless true that this minor stimulant did produce statistically significant effects in haloperidol-treated rats. The present results with caffeine are consistent with previous reports indicating that nonselective adenosine antagonists can reverse the behavioral effects of DA antagonists [79]. Furthermore, the modest effects of caffeine are consistent with reports indicating that caffeine, despite its some-what mixed pattern of binding to adenosine receptors, does show some degree of preference for adenosine A1 receptors over A2A receptors [23]. In marked contrast to the ability of MSX-3, KW6002, and caffeine to reverse the effects of the D2 antagonist haloperidol, the selective adenosine A1 antagonist DPCPX did not significantly alter the actions produced by haloperidol. In the dose range tested, DPCPX failed to produce significant changes in lever pressing or chow intake in haloperidol-treated rats. Moreover, pilot experiments indicated that neither higher nor lower doses of DPCPX were effective (see Section 2.3 above). The present results are consistent with several previous studies showing differences between the behavioral effects of adenosine A1 and A2A receptor antagonists [42,43,55]. DPCPX also was reported to be relatively ineffective compared to selective adenosine A2A antagonists at producing signs of antiparkinsonian actions in monkeys [79] and rats [6]. Furthermore, the present observations are consistent with a recent report indicating that DPCPX in an IP dose range of 0.75–3.0 mg/kg failed to reverse the effects of 0.15 mg/kg haloperidol in a T-maze task; in fact, the 3.0 mg/kg dose of DPCPX only served to further impair responding in haloperidol-treated rats [47].
The present data, together with other recent results, indicate that there are differential effects of adenosine A1 and A2A receptor antagonists in terms of the extent to which these drugs can reverse the impact of DA D2 antagonism. Drugs that can exert functional antagonism of A2A receptors, including selective A2A antagonists such as MSX-3 and KW6002, as well as nonselective adenosine antagonists such as caffeine, can attenuate the effects of DA D2 antagonists (see Refs. [16,47,83]; experiments 1 and 2 above). In contrast, the adenosine A1 receptor antagonist DPCPX was not able to do so ([47]; experiment 3 above). In the present studies there were substantial differences in effect sizes between the three reversal experiments (see Table 1); KW6002 had the highest effect size, caffeine was intermediate, and DPCPX had a very small effect size. Furthermore, a recent paper has demonstrated that the adenosine A2A antagonist MSX-3 was able to produce a complete reversal of the effects of the highly selective D2 family antagonist eticlopride in rats responding on the concurrent lever-pressing/feeding task, while it produced only a marginal reversal of the effects of the highly selective D1 antagonist ecopipam [83]. When considered together, this pattern of results points to a rather important and selective interaction between DA D2 and adenosine A2A receptors. The neurochemical basis of this interaction is not completely clear, however, it is likely that it is related to the pattern of cellular localization of adenosine A1 and A2A receptors in striatal areas, including the nucleus accumbens [17]. Adenosine A2A receptors tend to be co-localized on striatal and accumbens medium spiny neurons with DA D2 receptors (i.e., enkephalin positive medium spiny neurons), and these receptors converge onto the same signal transduction mechanisms and show the capacity for forming heterodimers [17,23,27,31,75]. Therefore, it is reasonable to suggest that adenosine A2A receptor antagonists are so effective in reversing the effort-related actions of D2 antagonists such as haloperidol and eticlopride because of the direct interaction between adenosine A2A and DA D2 receptors located on the same medium spiny neurons. This suggestion is consistent with studies showing that adenosine A2A receptor antagonists can reverse the expression of Fos-like immunoreactivity that is induced by D2 antagonists in medium spiny neurons [52]. In contrast, adenosine A1 receptors are more likely to be co-localized with DA D1 receptors [17], which could help to explain why it is more difficult for adenosine A1 receptor antagonists to reverse the effects of D2 receptor blockade. Although DPCPX has generally failed to reverse the behavioral actions of haloperidol, it is possible that DPCPX would be able to reverse T-maze or lever-pressing effects if they were induced by a D1 antagonist. Future research will investigate this possibility.
In summary, the present data are consistent with previous studies showing that adenosine A2A antagonists can reverse the effects of DA D2 antagonists. This observation has relevance for the potential antiparkinsonian effects of adenosine A2A antagonists [8,32,59,67], and also for understanding activational aspects of motivation and effort-related processes [16,47,83]. These findings support the hypothesis that DA and adenosine systems in the brain, possibly in nucleus accumbens, interact in the regulation of instrumental response output and effort-related choice behavior [16,26,44,61]. Furthermore, the present results illustrate the specific nature of the interaction between adenosine A2A and DA D2 receptors, which is likely to be related to the co-localization of these receptors on the same population of striatal and accumbens neurons. Characterization of the neurochemical mechanisms involved in regulating behavioral activation and effort-based choice behavior can shed light on these important facets of motivation, and also may serve to illustrate the relation between activational aspects of motivation (i.e., vigor, persistence and work output) and higher-order processes involved in motor control [61]. Activational aspects of motivated behavior are highly adaptive because they enable organisms to surmount work-related response costs or obstacles that limit access to significant stimuli [57,60,68,78]. In addition, impairments in behavioral activation can be maladaptive. Symptoms such as anergia, psychomotor slowing, and fatigue, which reflect psychopathologies related to behavioral activation in humans, are fundamental aspects of depression and other psychiatric and neurological disorders [5,14,41,61,64,74,77,84]. Research in this area may promote our understanding of the neural mechanisms involved in clinical psychopathologies related to behavioral activation and effort [61,64]. Furthermore, it is possible that A2A receptor antagonists could be beneficial for ameliorating the motivational effects of D2 antagonists that are used clinically, and also for treating other energy-related disorders [64].
Acknowledgement
This work was supported by a grant to J.S. from the National Institute of Mental Health (MH078023).
References
- [1].Aberman JE, Salamone JD. Nucleus accumbens dopamine depletions make rats more sensitive to high ratio requirements but do not impair primary food reinforcement. Neuroscience. 1999;92:545–52. doi: 10.1016/s0306-4522(99)00004-4. [DOI] [PubMed] [Google Scholar]
- [2].Aubel B, Kayser V, Farré A, Hamon M, Bourgoin S. Evidence for adenosine- and serotonin-mediated antihyperalgesic effects of cizolirtine in rats suffering from diabetic neuropathy. Neuropharmacology. 2007;52:487–96. doi: 10.1016/j.neuropharm.2006.08.017. [DOI] [PubMed] [Google Scholar]
- [3].Baldo BA, Kelley AE. Discrete neurochemical coding of distinguishable motivational processes: insights from nucleus accumbens control of feeding. Psychopharmacology. 2007;191:439–59. doi: 10.1007/s00213-007-0741-z. [DOI] [PubMed] [Google Scholar]
- [4].Barbano MF, Cador M. Opioids for hedonic experience and dopamine to get ready for it. Psychopharmacology. 2007;191:497–506. doi: 10.1007/s00213-006-0521-1. [DOI] [PubMed] [Google Scholar]
- [5].Capuron L, Pagnoni G, Demetrashvili MF, Lawson DH, Fornwalt FB, Woolwine B, et al. Basal ganglia hypermetabolism and symptoms of fatigue during interferon-alpha therapy. Neuropsychopharmacology. 2007;32:2384–92. doi: 10.1038/sj.npp.1301362. [DOI] [PubMed] [Google Scholar]
- [6].Collins LE, Galtieri DL, Hockemeyer J, Müller CE, Salamone JD. Society for Neuroscience; Washington, DC: 2008. Systemic injections of the adenosine A2A antagonist MSX-3, but not of the adenosine A1 antagonist DPCPX, suppress the tremulous jaw movements induced by a cholinomimetic: possible relevance to parkinsonian tremor. Program No. 341.7 neuroscience meeting planner. 2008. Online. [Google Scholar]
- [7].Correa M, Carlson BB, Wisniecki A, Salamone JD. Nucleus accumbens dopamine and work requirements on interval schedules. Behav Brain Res. 2002;137:179–87. doi: 10.1016/s0166-4328(02)00292-9. [DOI] [PubMed] [Google Scholar]
- [8].Correa M, Wisniecki A, Betz A, Dobson DR, O’Neill MF, O’Neill MJ, et al. The adenosine A2A antagonist KF17837 reverses the locomotor suppression and tremulous jaw movements induced by haloperidol in rats: possible relevance to parkinsonism. Behav Brain Res. 2004;148:47–54. doi: 10.1016/s0166-4328(03)00178-5. [DOI] [PubMed] [Google Scholar]
- [9].Cousins MS, Atherton A, Turner L, Salamone JD. Nucleus accumbens dopamine depletions alter relative response allocation in a T-maze cost/benefit task. Behav Brain Res. 1996;74:189–97. doi: 10.1016/0166-4328(95)00151-4. [DOI] [PubMed] [Google Scholar]
- [10].Cousins MS, Salamone JD. Nucleus accumbens dopamine depletions in rats affect relative response allocation in a novel cost/benefit procedure. Pharmacol Biochem Behav. 1994;49:85–91. doi: 10.1016/0091-3057(94)90460-x. [DOI] [PubMed] [Google Scholar]
- [11].Cousins MS, Sokolowski JD, Salamone JD. Different effects of nucleus accumbens and ventrolateral striatal dopamine depletions on instrumental response selection in the rat. Pharmacol Biochem Behav. 1993;46:951–3. doi: 10.1016/0091-3057(93)90226-j. [DOI] [PubMed] [Google Scholar]
- [12].Cousins MS, Wei W, Salamone JD. Pharmacological characterization of performance on a concurrent lever pressing/feeding choice procedure: effects of dopamine antagonist, cholinomimetic, sedative and stimulant drugs. Psychopharmacology. 1994;116:529–37. doi: 10.1007/BF02247489. [DOI] [PubMed] [Google Scholar]
- [13].DeMet EM, Chicz-DeMet A. Localization of adenosine A2A-receptors in rat brain with [3H]ZM-241385. Naunyn Schmiedebergs Arch Pharmacol. 2002;366:478–81. doi: 10.1007/s00210-002-0613-3. [DOI] [PubMed] [Google Scholar]
- [14].Demyttenaere K, De Fruyt J, Stahl SM. The many faces of fatigue in major depressive disorder. Int J Neuropsychopharmacol. 2005;8:93–105. doi: 10.1017/S1461145704004729. [DOI] [PubMed] [Google Scholar]
- [15].Farrar AM, Font L, Pereira M, Mingote SM, Bunce JG, Chrobak JJ, et al. Forebrain circuitry involved in effort-related choice: injections of the GABAA agonist muscimol into ventral pallidum alters response allocation in food-seeking behavior. Neuroscience. 2008;152:321–30. doi: 10.1016/j.neuroscience.2007.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Farrar AM, Pereira M, Velasco F, Hockemeyer J, Muller CE, Salamone JD. Adenosine A(2A) receptor antagonism reverses the effects of dopamine receptor antagonism on instrumental output and effort-related choice in the rat: implications for studies of psychomotor slowing. Psychopharmacology. 2007;191:579–86. doi: 10.1007/s00213-006-0554-5. [DOI] [PubMed] [Google Scholar]
- [17].Ferré S. Adenosine—dopamine interactions in the ventral striatum. Implications for the treatment of schizophrenia. Psychopharmacology. 1997;133:107–20. doi: 10.1007/s002130050380. [DOI] [PubMed] [Google Scholar]
- [18].Ferre S, Ciruela F, Borycz J, Solinas M, Quarta D, Antoniou K, et al. Adenosine A1—A2A receptor heteromers: new targets for caffeine in the brain. Front Biosci. 2008;13:2391–9. doi: 10.2741/2852. [DOI] [PubMed] [Google Scholar]
- [19].Ferré S, Ciruela F, Canals M, Marcellino D, Burgueno J, Casado V, et al. Adenosine A2A—dopamine D2 receptor—receptor heteromers. Targets for neuro-psychiatric disorders. Parkinsonism Relat Disord. 2004;10:265–71. doi: 10.1016/j.parkreldis.2004.02.014. [DOI] [PubMed] [Google Scholar]
- [20].Ferré S, Fredholm BB, Morelli M, Popoli P, Fuxe K. Adenosine—dopamine receptor—receptor interactions as an integrative mechanism in the basal ganglia. Trends Neurosci. 1997;20:482–7. doi: 10.1016/s0166-2236(97)01096-5. [DOI] [PubMed] [Google Scholar]
- [21].Ferré S, O’Connor WT, Snaprud P, Ungerstedt U, Fuxe K. Antagonistic interaction between adenosine A2A receptors and dopamine D2 receptors in the ventral striopallidal system. Implications for the treatment of schizophrenia. Neuroscience. 1994;63:765–73. doi: 10.1016/0306-4522(94)90521-5. [DOI] [PubMed] [Google Scholar]
- [22].Ferré S, Popoli P, Giménez-Llort L, Rimondini R, Müller CE, Strömberg I, et al. Adenosine/dopamine interaction: implications for the treatment of Parkinson’s disease. Parkinsonism Relat Disord. 2001;7:235–41. doi: 10.1016/s1353-8020(00)00063-8. [DOI] [PubMed] [Google Scholar]
- [23].Ferré S. An update on the mechanisms of the psychostimulant effects of caffeine. J Neurochem. 2008;105:1067–79. doi: 10.1111/j.1471-4159.2007.05196.x. [DOI] [PubMed] [Google Scholar]
- [24].Floresco SB, Ghods-Sharifi S. Amygdala-prefrontal cortical circuitry regulates effort-based decision making. Cereb Cortex. 2007;17:251–60. doi: 10.1093/cercor/bhj143. [DOI] [PubMed] [Google Scholar]
- [25].Floresco SB, Tse MT, Ghods-Sharifi S. Dopaminergic and glutamatergic regulation of effort- and delay-based decision making. Neuropsychopharmacology. 2008;33:1966–79. doi: 10.1038/sj.npp.1301565. [DOI] [PubMed] [Google Scholar]
- [26].Font L, Mingote S, Farrar AM, Pereira M, Worden L, Stopper C, et al. Intra-accumbens injections of the adenosine A(2A) agonist CGS 21680 affect effort-related choice behavior in rats. Psychopharmacology. 2008;199:515–26. doi: 10.1007/s00213-008-1174-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Fuxe K, Agnati LF, Jacobsen K, Hillion J, Canals M, Torvinen M, et al. Receptor heteromerization in adenosine A2A receptor signaling: relevance for striatal function and Parkinson’s disease. Neurology. 2003;61:S19–23. doi: 10.1212/01.wnl.0000095206.44418.5c. [DOI] [PubMed] [Google Scholar]
- [28].Fuxe K, Ferré S, Genedani S, Franco R, Agnati LF. Adenosine receptor—dopamine receptor interactions in the basal ganglia and their relevance for brain function. Physiol Behav. 2007;92:210–7. doi: 10.1016/j.physbeh.2007.05.034. [DOI] [PubMed] [Google Scholar]
- [29].Hauber W, Munkel M. Motor depressant effects mediated by dopamine D2 and adenosine A2A receptors in the nucleus accumbens and the caudate-putamen. Eur J Pharmacol. 1997;323:127–31. doi: 10.1016/s0014-2999(97)00040-x. [DOI] [PubMed] [Google Scholar]
- [30].Hauber W, Sommer S. Prefrontostriatal circuitry regulates effort-related decision making. Cereb Cortex. doi: 10.1093/cercor/bhn241. in press. [DOI] [PubMed] [Google Scholar]
- [31].Hillion J, Canals M, Torvinen M, Casado V, Scott R, Terasmaa A, et al. Coaggregation, cointernalization, and codesensitization of adenosine A2A receptors and dopamine D2 receptors. J Biol Chem. 2002;277:18091–7. doi: 10.1074/jbc.M107731200. [DOI] [PubMed] [Google Scholar]
- [32].Ishiwari K, Madson LJ, Farrar AM, Mingote SM, Valenta JP, DiGianvittorio MD, et al. Injections of the selective adenosine A2A antagonist MSX-3 into the nucleus accumbens core attenuate the locomotor suppression induced by haloperidol in rats. Behav Brain Res. 2007;178:190–9. doi: 10.1016/j.bbr.2006.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Jarvis MF, Williams M. Direct autoradiographic localization of adenosine A2A receptors in the rat brain using the A2A-selective agonist, [3H]CGS 21680. Eur J Pharmacol. 1989;168:243–6. doi: 10.1016/0014-2999(89)90571-2. [DOI] [PubMed] [Google Scholar]
- [34].Jenner P. Dopamine agonists, receptor selectivity and dyskinesia induction in Parkinson’s disease. Curr Opin Neurol. 2003;16(Suppl 1):S3–7. doi: 10.1097/00019052-200312001-00002. [DOI] [PubMed] [Google Scholar]
- [35].Jenner P, Istradefylline A novel adenosine A2A receptor antagonist, for the treatment of Parkinson’s disease. Expert Opin Investig Drugs. 2005;14:729–38. doi: 10.1517/13543784.14.6.729. [DOI] [PubMed] [Google Scholar]
- [36].Kelley AE, Baldo BA, Pratt WE, Will MJ. Corticostriatal-hypothalamic circuitry and food motivation: integration of energy, action and reward. Physiol Behav. 2005;86:773–95. doi: 10.1016/j.physbeh.2005.08.066. [DOI] [PubMed] [Google Scholar]
- [37].Keppel G. Design and analysis: a researcher’s handbook. Prentice-Hall, Englewood Cliffs; New Jersey: 1991. pp. 110–139. [Google Scholar]
- [38].Koch M, Schmid A, Schnitzler HU. Role of nucleus accumbens dopamine D1 and D2 receptors in instrumental and Pavlovian paradigms of conditioned reward. Psychopharmacology. 2000;152:67–73. doi: 10.1007/s002130000505. [DOI] [PubMed] [Google Scholar]
- [39].Lobato KR, Binfaré RW, Budni J, Rosa AO, Santos AR, Rodrigues AL. Involvement of the adenosine A1 and A2A receptors in the antidepressant-like effect of zinc in the forced swimming test. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:994–9. doi: 10.1016/j.pnpbp.2008.01.012. [DOI] [PubMed] [Google Scholar]
- [40].Maione S, de Novellis V, Cappellacci L, Palazzo E, Vita D, Luongo L, et al. The antinociceptive effect of 2-chloro-2′-C-methyl-N6-cyclopentyladenosine (2′-Me-CCPA), a highly selective adenosine A1 receptor agonist, in the rat. Pain. 2007;131:281–92. doi: 10.1016/j.pain.2007.01.013. [DOI] [PubMed] [Google Scholar]
- [41].Majer M, Welberg LA, Capuron L, Pagnoni G, Raison CL, Miller AH. IFN-alpha-induced motor slowing is associated with increased depression and fatigue in patients with chronic hepatitis C. Brain Behav Immun. 2008;22:870–80. doi: 10.1016/j.bbi.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Mandryk M, Fidecka S, Poleszak E, Malec D. Participation of adenosine system in the ketamine-induced motor activity in mice. Pharmacol Rep. 2005;57:55–60. [PubMed] [Google Scholar]
- [43].Marston HM, Finlayson K, Maemoto T, Olverman HJ, Akahane A, Sharkey J, et al. Pharmacological characterization of a simple behavioral response mediated selectively by central adenosine A1 receptors, using in vivo and in vitro techniques. J Pharmacol Exp Ther. 1998;285:1023–30. [PubMed] [Google Scholar]
- [44].Mingote S, Font L, Farrar AM, Vontell R, Worden L, Stopper CM, et al. Nucleus accumbens adenosine A2A receptors regulate exertion of effort by acting on the ventral striatopallidal pathway. J Neuroscience. 2008;28:9037–46. doi: 10.1523/JNEUROSCI.1525-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Mingote S, Weber SM, Ishiwari K, Correa M, Salamone JD. Ratio and time requirements on operant schedules: effort-related effects of nucleus accumbens dopamine depletions. Eur J Neurosci. 2005;21:1749–57. doi: 10.1111/j.1460-9568.2005.03972.x. [DOI] [PubMed] [Google Scholar]
- [46].Morelli M, Pinna A. Interaction between dopamine and adenosine A2A receptors as a basis for the treatment of Parkinson’s disease. Neurol Sci. 2002;22:71–2. doi: 10.1007/s100720170052. [DOI] [PubMed] [Google Scholar]
- [47].Mott AM, Nunes EJ, Collins LE, Port RG, Sink KS, Hockemeyer J, et al. The adenosine A2A MSX-3 reverses the effects of the dopamine antagonist haloperidol on effort-related decision making in a t-maze cost/benefit procedure. Psychopharmacology. doi: 10.1007/s00213-008-1441-z. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Niv Y, Daw ND, Joel D, Dayan P. Tonic dopamine: opportunity costs and the control of response vigor. Psychopharmacology. 2007;191:507–20. doi: 10.1007/s00213-006-0502-4. [DOI] [PubMed] [Google Scholar]
- [49].Nowend KL, Arizzi M, Carlson BB, Salamone JD. D1 or D2 antagonism in nucleus accumbens core or dorsomedial shell suppresses lever pressing for food but leads to compensatory increases in chow consumption. Pharmacol Biochem Behav. 2001;69:373–82. doi: 10.1016/s0091-3057(01)00524-x. [DOI] [PubMed] [Google Scholar]
- [50].O’Neill M, Brown VJ. The effect of the adenosine A2A antagonist KW6002 on motor and motivational processes in the rat. Psychopharmacology. 2006;184:46–55. doi: 10.1007/s00213-005-0240-z. [DOI] [PubMed] [Google Scholar]
- [51].Phillips PE, Walton ME, Jhou TC. Calculating utility: preclinical evidence for cost-benefit analysis by mesolimbic dopamine. Psychopharmacology. 2007;191:483–95. doi: 10.1007/s00213-006-0626-6. [DOI] [PubMed] [Google Scholar]
- [52].Pinna A, Wardas J, Cozzolino A, Morelli M. Involvement of adenosine A2A receptors in the induction of c-fos expression by clozapine and haloperidol. Neuropsychopharmacol. 1999;20:44–51. doi: 10.1016/S0893-133X(98)00051-7. [DOI] [PubMed] [Google Scholar]
- [53].Pinna A, Wardas J, Simola N, Morelli M. New therapies for the treatment of Parkinson’s disease: adenosine A2A receptor antagonists. Life Sci. 2005;77:3259–67. doi: 10.1016/j.lfs.2005.04.029. [DOI] [PubMed] [Google Scholar]
- [54].Prediger RD, Fernandes D, Takahashi RN. Blockade of adenosine A2A receptors reverses short-term social memory impairments in spontaneously hypertensive rats. Behav Brain Res. 2005;159:197–205. doi: 10.1016/j.bbr.2004.10.017. [DOI] [PubMed] [Google Scholar]
- [55].Prediger RD, Takahashi RN. Modulation of short-term social memory in rats by adenosine A1 and A(2A) receptors. Neurosci Lett. 2005;376:160–5. doi: 10.1016/j.neulet.2004.11.049. [DOI] [PubMed] [Google Scholar]
- [56].Robbins TW, Everitt BJ. A role for mesencephalic dopamine in activation: commentary on Berridge (2006) Psychopharmacology. 2007;191:433–7. doi: 10.1007/s00213-006-0528-7. [DOI] [PubMed] [Google Scholar]
- [57].Salamone JD. Behavioral pharmacology of dopamine systems: a new synthesis. In: Willner P, Scheel-Kruger J, editors. The mesolimbic dopamine system: from motivation to action. Cambridge University Press; Cambridge, England: 1991. pp. 599–613. [Google Scholar]
- [58].Salamone JD, Arizzi M, Sandoval MD, Cervone KM, Aberman JE. Dopamine antagonists alter response allocation but do not suppress appetite for food in rats: contrast between the effects of SKF 83566, raclopride and fenfluramine on a concurrent choice task. Psychopharmacology. 2002;160:371–80. doi: 10.1007/s00213-001-0994-x. [DOI] [PubMed] [Google Scholar]
- [59].Salamone JD, Betz AJ, Ishiwari K, Felsted J, Madson L, Mirante B, et al. Tremorolytic effects of adenosine A2A antagonists: implications for parkinsonism. Front Biosci. 2008;13:3594–605. doi: 10.2741/2952. [DOI] [PubMed] [Google Scholar]
- [60].Salamone JD, Correa M. Motivational views of reinforcement: implications for understanding the behavioral functions of nucleus accumbens dopamine. Behav Brain Res. 2002;137:3–25. doi: 10.1016/s0166-4328(02)00282-6. [DOI] [PubMed] [Google Scholar]
- [61].Salamone JD, Correa M, Farrar A, Mingote SM. Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacology. 2007;191:461–82. doi: 10.1007/s00213-006-0668-9. [DOI] [PubMed] [Google Scholar]
- [62].Salamone JD, Correa M, Mingote S, Weber S. Accumbens dopamine and the regulation of effort in food-seeking behavior: implications for studies of natural motivation and psychiatry. J Pharmacol Exp Ther. 2003;305:1–8. doi: 10.1124/jpet.102.035063. [DOI] [PubMed] [Google Scholar]
- [63].Salamone JD, Correa M, Mingote SM, Weber SM. Beyond the reward hypothesis: alternative functions of nucleus accumbens dopamine. Curr Opin Pharmacol. 2005;5:34–41. doi: 10.1016/j.coph.2004.09.004. [DOI] [PubMed] [Google Scholar]
- [64].Salamone JD, Correa M, Mingote SM, Weber SM, Farrar AM. Nucleus accumbens dopamine and the forebrain circuitry involved in behavioral activation and effort-related decision making: implications for understanding anergia and psychomotor slowing in depression. Curr Psychiat Rev. 2006;2:267–80. [Google Scholar]
- [65].Salamone JD, Cousins MS, Bucher S. Anhedonia or anergia? Effects of haloperidol and nucleus accumbens dopamine depletion on instrumental response selection in a T-maze cost/benefit procedure. Behav Brain Res. 1994;65:221–9. doi: 10.1016/0166-4328(94)90108-2. [DOI] [PubMed] [Google Scholar]
- [66].Salamone JD, Cousins MS, Snyder BJ. Behavioral functions of nucleus accumbens dopamine: empirical and conceptual problems with the anhedonia hypothesis. Neurosci Biobehav Rev. 1997;21:341–59. doi: 10.1016/s0149-7634(96)00017-6. [DOI] [PubMed] [Google Scholar]
- [67].Salamone JD, Ishiwari K, Betz AJ, Farrar AM, Mingote SM, Font L, et al. Dopamine/adenosine interactions related to locomotion and tremor in animal models: possible relevance to parkinsonism. Parkinsonism Relat Disord. 2008;14:S130–4. doi: 10.1016/j.parkreldis.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Salamone JD, Steinpreis RE, McCullough LD, Smith P, Grebel D, Mahan K. Haloperidol and nucleus accumbens dopamine depletion suppress lever pressing for food but increase free food consumption in a novel food choice procedure. Psychopharmacology. 1991;104:515–21. doi: 10.1007/BF02245659. [DOI] [PubMed] [Google Scholar]
- [69].Salamone JD, Correa M, Font L, Pennarola A, Farrar AM, Mingote S. Nucleus accumbens and the neurochemical interactions regulating effort-related processes. In: David H, editor. The nucleus accumbens: neurotransmitters and related behaviours. 2008. pp. 195–212. [Google Scholar]
- [70].Schiffmann SN, Jacobs O, Vanderhaeghen JJ. Striatal restricted adenosine A2A receptor (RDC8) is expressed by enkephalin but not by substance P neurons: an in situ hybridization histochemistry study. J Neurochem. 1991;57:1062–71. doi: 10.1111/j.1471-4159.1991.tb08257.x. [DOI] [PubMed] [Google Scholar]
- [71].Schweimer J, Saft S, Hauber W. Involvement of catecholamine neurotransmission in the rat anterior cingulate in effort-related decision making. Behav Neurosci. 2005;119:1687–92. doi: 10.1037/0735-7044.119.6.1687. [DOI] [PubMed] [Google Scholar]
- [72].Sink KS, Vemuri VK, Olszewska T, Makriyannis A, Salamone JD. Cannabinoid CB1 antagonists and dopamine antagonists produce different effects on a task involving response allocation and effort-related choice in food-seeking behavior. Psychopharmacology. 2008;196:565–74. doi: 10.1007/s00213-007-0988-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Sokolowski JD, Salamone JD. The role of nucleus accumbens dopamine in lever pressing and response allocation: effects of 6-OHDA injected into core and dorsomedial shell. Pharmacol Biochem Behav. 1998;59:557–66. doi: 10.1016/s0091-3057(97)00544-3. [DOI] [PubMed] [Google Scholar]
- [74].Stahl SM. The psychopharmacology of energy and fatigue. J Clin Psychiatry. 2002;63:7–8. doi: 10.4088/jcp.v63n0102. [DOI] [PubMed] [Google Scholar]
- [75].Svenningsson P, Le Moine C, Fisone G, Fredholm BB. Distribution, biochemistry and function of striatal adenosine A2A receptors. Prog Neurobiol. 1999;59:355–96. doi: 10.1016/s0301-0082(99)00011-8. [DOI] [PubMed] [Google Scholar]
- [76].Takahashi RN, Pamplona FA, Prediger RD. Adenosine receptor antagonists for cognitive dysfunction: a review of animal studies. Front Biosci. 2008;13:2614–32. doi: 10.2741/2870. [DOI] [PubMed] [Google Scholar]
- [77].Tylee A, Gastpar M, Lepine JP, Mendlewicz J. DEPRES II (depression research in European society II): a patient survey of the symptoms, disability and current management of depression in the community. Int Clin Psychopharmacol. 1999;14:139–51. doi: 10.1097/00004850-199905002-00001. [DOI] [PubMed] [Google Scholar]
- [78].Van den Bos R, van der Harst J, Jonkman S, Schilders M, Spruijt B. Rats assess costs and benefits according to an internal standard. Behav Brain Res. 2006;171:350–4. doi: 10.1016/j.bbr.2006.03.035. [DOI] [PubMed] [Google Scholar]
- [79].Varty GB, Hodgson RA, Pond AJ, Grzelak ME, Parker EM, Hunter JC. The effects of adenosine A2A receptor antagonists on haloperidol-induced movement disorders in primates. Psychopharmacology. 2008;200:393–401. doi: 10.1007/s00213-008-1214-8. [DOI] [PubMed] [Google Scholar]
- [80].Walton ME, Bannerman DM, Alterescu K, Rushworth MF. Functional specialization within medial frontal cortex of the anterior cingulate for evaluating effort-related decisions. J Neurosci. 2003;23:6475–9. doi: 10.1523/JNEUROSCI.23-16-06475.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Walton ME, Kennerley SW, Bannerman DM, Phillips PE, Rushworth MF. Weighing up the benefits of work: behavioral and neural analyses of effort-related decision making. Neural Netw. 2006;19:1302–14. doi: 10.1016/j.neunet.2006.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Wardas J, Konieczny J, Lorenc-Koci E. SCH 58261, an A2A adenosine receptor antagonist, counteracts parkinsonian-like muscle rigidity in rats. Synapse. 2001;41:160–71. doi: 10.1002/syn.1070. [DOI] [PubMed] [Google Scholar]
- [83].Worden LT, Shariari M, Farrar AM, Sink KS, Hockemeyer J, Muller C, et al. The adenosine A2A antagonist MSX-3 reverses the effort-related effects of dopamine blockade: differential interaction with D1 and D2 family antagonists. Psychopharmacology. 2009;203:489–99. doi: 10.1007/s00213-008-1396-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Yurgelun-Todd DA, Sava S, Dahlgren MK. Mood disorders. Neuroimaging Clin N Am. 2007;17:511–21. doi: 10.1016/j.nic.2007.08.001. [DOI] [PubMed] [Google Scholar]