Abstract
There is considerable evidence of interactions between adenosine A2A receptors and dopamine D2 receptors in striatal areas, and antagonists of the A2A receptor have been shown to reverse the motor effects of DA antagonists in animal models. The D2 antagonist haloperidol produces parkinsonism in humans, and also induces motor effects in rats, such as suppression of locomotion. The present experiments were conducted to study the ability of the adenosine A2A antagonist MSX-3 to reverse the locomotor effects of acute or subchronic administration of haloperidol in rats. Systemic (i.p.) injections of MSX-3 (2.5–10.0 mg/kg) were capable of attenuating the suppression of locomotion induced by either acute or repeated (i.e., 14 day) administration of 0.5 mg/kg haloperidol. Bilateral infusions of MSX-3 directly into the nucleus accumbens core (2.5 µg or 5.0 µg in 0.5 µl per side) produced a dose-related increase in locomotor activity in rats treated with 0.5 mg/kg haloperidol either acutely or repeatedly. There were no overall significant effects of MSX-3 infused directly into the dorsomedial nucleus accumbens shell or the ventrolateral neostriatum. These results indicate that antagonism of adenosine A2A receptors can attenuate the locomotor suppression produced by DA antagonism, and that this effect may be at least partially mediated by A2A receptors in the nucleus accumbens core. These studies suggest that adenosine and dopamine systems interact to modulate the locomotor and behavioral activation functions of nucleus accumbens core.
Keywords: Dopamine, Basal ganglia, Neostriatum, Caudate putamen, Antipsychotic, Parkinson’s disease;, D2 receptor
1. Introduction
Interactions between diverse neurotransmitter systems in the basal ganglia are thought to regulate several aspects of motor function [22,82]. Neostriatal depletions of dopamine (DA) are the immediate cause of motor dysfunction in patients with idiopathic Parkinson’s disease [35], while pharmacological blockade of DA transmission with DA receptor antagonists such as haloperidol leads to drug-induced parkinsonism [53]. The most common treatments for Parkinson’s disease generally involve dopaminergic strategies, including the DA precursor L-DOPA, as well as DA agonists such as bromocriptine, pergolide, or ropinirole [10,47,48]. Nevertheless, considerable research has implicated several other neurotransmitters in motor processes related to the basal ganglia, including acetylcholine [67,68], serotonin [11], glutamate [58,60], and GABA [12,45,77,80,81]. Within the last few years, evidence has begun to emerge indicating that brain adenosine neurons play an important role in regulating the motor functions of the basal ganglia [24,25,28,76]. Although several subtypes of adenosine receptors are involved in motor function, anatomical studies have demonstrated that the adenosine A2A receptor subtype is expressed to a high degree in striatal regions [15,27,33,49,76,78]. Adensonine A2A receptors in the striatum are largely expressed on enkephalin-positive striatopallidal neurons, which also contain DA D2 receptors [76]. Antagonism of adenosine A2A receptors produces motor effects in animal models [13,29,74,75], and it has been widely suggested that adenosine A2A antagonists could be used as an alternative for the treatment of parkinsonian symptoms [24,25,38,52,56,61]. Because of the interest in studying the neurochemical interactions involved in motor control, and identifying novel non-dopaminergic treatments for parkinsonism, it is important to characterize the effects of adenosine A2A antagonists in both human clinical trials and animal models.
A number of tests in rodents are used to study motor function, and adenonsine A2A antagonists have been assessed for their effects in various procedures. Haloperidol-induced rigidity was reversed by the A2A antagonist SCH 58261 [79]. Hauber et al. [32] observed that catalepsy induced by either DA D1 or D2 antagonists could be reversed by the selective A2A antagonist MSX-3. Drug-induced tremulous jaw movements, which are used as an animal model of parkinsonian tremor [67,69], were reduced by co-administration of adenosine A2A antagonists [18,74]. In addition, several studies have focused upon the effects of adenosine A2A antagonists on locomotor activity. The adenosine A2A antagonist KW-6002 reversed the hypolocomotion induced by the DA depleting agent reserpine [73]. The impairment of locomotion shown by D2 receptor deficient mice was rescued by the adenosine A2A antagonist KW-6002 [3]. Systemic injections of the adenosine A2A antagonist KF17837 (5.0–20.0 mg/kg) reversed the suppression of locomotion induced by subchronic injections of haloperidol [18].
The specific brain areas at which adenosine A2A receptor antagonists act to increase locomotion in animals with impaired dopaminergic function are unclear. There are A2A receptors present throughout the striatal complex, including subregions of neostriatum as well as nucleus accumbens [15,33,76,78]. Although Parkinson’s disease is generally associated with depletions of DA in the neostriatum [35], it also has been demonstrated that this disorder is accompanied by depletions of DA in nucleus accumbens [9,50]. Previous evidence indicates that adenosine A2A receptors in nucleus accumbens may be important for mediating the locomotor effects of A2A antagonists. The adenosine A2A agonist CGS 21680 was shown to suppress locomotion when injected directly into the nucleus accumbens [6,7,31]. Infusions of the adenosine A2A antagonist MSX-3 directly into the nucleus accumbens produced a dose-related increase in locomotor activity [59]. Moreover, there is considerable evidence indicating that interference with DA transmission in nucleus accumbens leads to a suppression of spontaneous locomotion [5,16,19,46].
The present experiments were conducted to study the ability of systemic or intra-accumbens injections of the selective adenosine A2A antagonist MSX-3 to reverse the locomotor effects of acute or subchronic administration of haloperidol in rats. Haloperidol was selected for these studies because it is a DA antagonist that is known to suppress locomotion in rats (e.g., ref. [18]), and to produce motor side effects in humans [8,53]. MSX-3 is a water-soluble pro-drug that is rapidly cleaved by phosphatases in vivo into MSX-2, which is the active antagonist of A2A receptors [30,34,57,71]. Experiments 1 and 2 studied the ability of systemic injections of MSX-3 to reverse the suppression of locomotion induced by acute or repeated subchronic administration of 0.5 mg/kg haloperidol. Repeated administration of haloperidol was used because this procedure has been employed previously for studies of adenosine A2A antagonists [18], and because repeated administration mimics the conditions seen when antipsychotic drugs such as haloperidol are used therapeutically. Experiments 3 and 4 studied the ability of intracranial administration of MSX-3 to increase locomotion in haloperidol-treated rats. Three brain areas were studied: nucleus accumbens core, dorsomedial nucleus accumbens shell, and ventrolateral neostriatum (VLS). Nucleus accumbens was investigated because, as described above, this brain area is involved in the regulation of locomotor activity. Although previous studies have examined the effects of local nucleus accumbens injections of MSX-3 on locomotor activity [59], these studies did not differentiate between core and shell subregions, and did not assess the effects of A2A antagonism in the presence of a DA antagonist. The VLS site was chosen as a control striatal site because this striatal subregion is thought to be involved in motor functions such as tremor [20,21,39,54,67,74] and skilled motor control [20,65,66], but is not thought to be important for locomotion [19,39,42]. The fifth experiment studied the effects of systemic and intracranial injections of MSX-3 in animals not treated with haloperidol, tested under the same conditions that were used in the previous experiments.
2. Materials and methods
2.1. Animals
A total of 417 male Sprague Dawley rats (Harlan Sprague Dawley, Indianapolis, IN) were used in the present experiments. The rats had no prior drug experience, and weighed 315–480 g at the beginning of the experiment, with ad libitum access to lab chow and water. The rats were group-housed in a colony that was maintained at approximately 23 °C and had a 12-h light/12-h dark cycle (lights on at 07:00 h). These studies were conducted in accordance with University of Connecticut and NIH guidelines for animal care and use.
2.2. Drugs
Haloperidol was obtained from Sigma Chemical Co. (St. Louis, MO). It was dissolved in 0.3% tartaric acid, which also was used as the vehicle control for haloperidol injections. MSX-3 free acid ((E)-phosphoric acid mono-[3-[8-[2-(3-methoxyphenyl)vinyl]-7-methyl-2,6-dioxo-1-prop-2-ynyl-1,2,6,7-tetrahydropurin-3-yl]propyl] ester disodium salt) was synthesized in the Müller laboratory (Pharmazeutisches Institut, Universität Bonn, Bonn, Germany). MSX-3 was dissolved in 0.9% saline, and the pH of the MSX-3 solution was adjusted by adding 1.0N NaOH until the drug was completely in solution (pH 7.1–7.4) as the disodium salt. Solutions of 0.9% saline also were used as the vehicle solution for control injections. The dose of haloperidol used (0.5 mg/kg), and the 14 day repeated administration procedures, were selected based upon previous studies [18,81]. The doses of MSX-3 were chosen based upon pilot experiments, as well as the results of previously published studies [30,59].
2.3. Locomotor activity
Locomotor activity was assessed in an automated motor activity chamber (28cm × 28cm × 28cm) that was placed inside a sound-proof shell. The floor of each chamber consisted of two movable wire mesh panels (27 cm × 13cm) mounted above the base of the chamber, which were balanced on a metal rod that passed through the center and was attached at either end to the sides of the chamber; this allowed for slight vertical movement of the floor panels. Movement of the panels was detected by microswitches mounted outside the chamber at the ends of the panels. The floor was thus divided into four quadrants, and a depression of each quadrant (i.e., 1/2 of each panel) would close the circuit on the microswitch attached to the panel. Each microswitch closure was counted as a single activity count, and activity counts were recorded by a computer for a 30 min period. Rats were not previously habituated, which was done so that low levels of activity in haloperidol-treated rats could be attributed to DA antagonism and not to prior habituation. Previous studies have used the same apparatus and procedure for measuring drug-induced changes in locomotion [17,77].
2.4. Cannula implantations and intracranial injections
Rats were anesthetized with a solution (1.0 ml/kg, i.p.) that contained ketamine (100 mg/ml) and xylazine (0.75 ml of a 20 mg/ml solution per 10.0 ml of ketamine solution). Bilateral guide cannulae (25 ga extra-thin wall stainless steel tubing, Small Parts) were chronically implanted 1.0mm dorsal to the target structure. The coordinates for the different placements were as follows (AP from interaural line; ML lateral from midline; DV from skull surface): nucleus accumbens core (AP + 2.8 mm, ML ± 1.8 mm, DV−6.8 mm), the dorsomedial shell (AP + 2.8 mm, ML ± 1.0 mm, DV−6.8 mm), or the VLS (AP + 1.4 mm, ML ± 4.0 mm, DV−6.2 mm) (incisor bar 5 mm above the intraural line). The dorsal coordinate was modified slightly based upon weight, such that 1.0–2.0 additional mm were added for rats weighing over 350 g. All rats were singlehoused after surgery, and were allowed 7–10 days recovery before testing. Stainless steel stylets were kept in the guide cannulae to maintain their integrity. On the drug test day, the intracranial injections were made via 30-gauge stainless steel injectors extending 1.0mm below the guide cannulae. The injectors were attached to 10 µl Hamilton syringes by PE-10 tubing. All injections were made at a volume of 0.5 µl per side (at a rate of 0.5 µl/min for 1 min). Injectors were left in place for 1 min after the infusion to allow for diffusion of the drug.
2.5. Experiments
2.5.1. Experiment 1. Effects of systemic MSX-3 on the suppression of locomotion induced by acute injections of haloperidol
A total of 117 rats were used for this experiment. Separate groups of rats were used to test each dose of MSX-3. On the test day, 101 rats received i.p. injections of 0.5 mg/kg haloperidol, while one group of animals (n = 16) received 0.3% tartaric acid. Thirty min after the first injection, the rats that had been given haloperidol also received i.p. injections of one of the following treatments with MSX-3 or vehicle (n = 16–19 per dose): saline vehicle, 0.625, 1.25, 2.5, 5.0, or 10.0 mg/kg MSX-3. The group that had received tartaric acid was injected with saline vehicle. After MSX-3 or vehicle injection, animals were returned to their home cage for 20 min. Rats were then placed in the motor activity chamber and tested for 30 min.
2.5.2. Experiment 2. Effects of systemic MSX-3 on the suppression of locomotion induced by repeated injections of haloperidol
A total of 99 rats were used, with separate groups of rats being used to test each dose of MSX-3. Eighty-seven rats received daily i.p. injections of 0.5 mg/kg haloperidol for 14 consecutive days, while one group of animals (n = 12) received 0.3% tartaric acid for 14 days. On day 14, the rats that received haloperidol were given i.p. injections of one of the following doses of MSX-3 (n = 12–16 per dose): saline vehicle, 0.625, 1.25, 2.5, 5.0, or 10.0 mg/kg MSX-3 (30 min after haloperidol injection). The group that had been receiving tartaric acid was injected with vehicle. After MSX-3 or vehicle injection, animals were returned to their home cage for 20 min. Rats were then placed in the motor activity chamber and tested for 30 min.
2.5.3. Experiment 3. Effects of intracranial injections of MSX-3 on the suppression of locomotion induced by acute injections of haloperidol
For this experiment, 93 rats were used, and separate groups of rats were used to test each dose of MSX-3 within each experiment. Rats were tested in three separate experiments, each of which involved different cannula placement sites (experiment 3a: nucleus accumbens core; experiment 3b: nucleus accumbens shell; experiment 3c: VLS), and bilateral implantations with stainless steel guide cannulae were conducted as described above. After 7–10 days of recovery, rats received i.p. injections of 0.5 mg/kg haloperidol 50 min before testing. Immediately before testing, animals received bilateral intracranial injections (see above) of one of the following doses of MSX-3 or vehicle: saline vehicle, 2.5 µg, or 5.0 µg MSX-3 per side. Animals were then placed in the motor activity chamber and tested for 30 min.
2.5.4. Experiment 4. Effects of intracranial injections of MSX-3 on the suppression of locomotion induced by repeated injections of haloperidol
Separate groups of rats were used to test each dose of MSX-3 within each experiment, and a total of 80 rats were used. Rats were tested in three separate experiments involving different cannula placement sites (experiment 4a: nucleus accumbens core; experiment 4b: nucleus accumbens shell; experiment 4c: VLS), and bilateral implantations with stainless steel guide cannulae were conducted as described above. After 7–10 days of recovery, rats received daily i.p. injections of 0.5 mg/kg haloperidol for 14 consecutive days. On day 14, the rats were given i.p. injections of 0.5 mg/kg haloperidol 50 min before testing. Immediately before testing, animals received bilateral intracranial injections of one of the following doses of MSX-3 or saline: saline vehicle, 2.5 µg, or 5.0 µg MSX-3 per side, as described above. Animals were then placed in the motor activity chamber and tested for 30 min.
2.5.5. Experiment 5. Effects of systemic and intracranial injections of MSX-3 on locomotion in animals not treated with haloperidol
Experiment 5A assessed the effects of systemic administration of the high dose (i.e., 10.0 mg/kg, i.p.) MSX-3 on locomotor activity, with rats tested under the same behavioral conditions as those used in experiments 1–2. Separate groups of naive rats were used to assess the effects of i.p. administration of saline (n = 7) and 10.0 mg/kg MSX-3 (n = 7). After MSX-3 or saline injection, animals were returned to their home cage for 20 min. Rats were then placed in the motor activity chamber and tested for 30 min. In experiment 5B, separate groups of rats were used to test the effects of acute intra-accumbens injections of saline (n = 7) and 5.0 µg MSX-3 (n = 7). The nucleus accumbens core was studied in this experiment based upon the results of experiments 3–4, and the same behavioral methods were used as those described above. Rats were implanted with cannulae in the nucleus accumbens core as described above. After 7–10 days of recovery, rats received bilateral intracranial injections of one of the following doses of either 0.5 µl saline or 5.0 µg MSX-3 per side, as described above. Animals were then placed in the motor activity chamber and tested for 30 min.
2.6. Histology
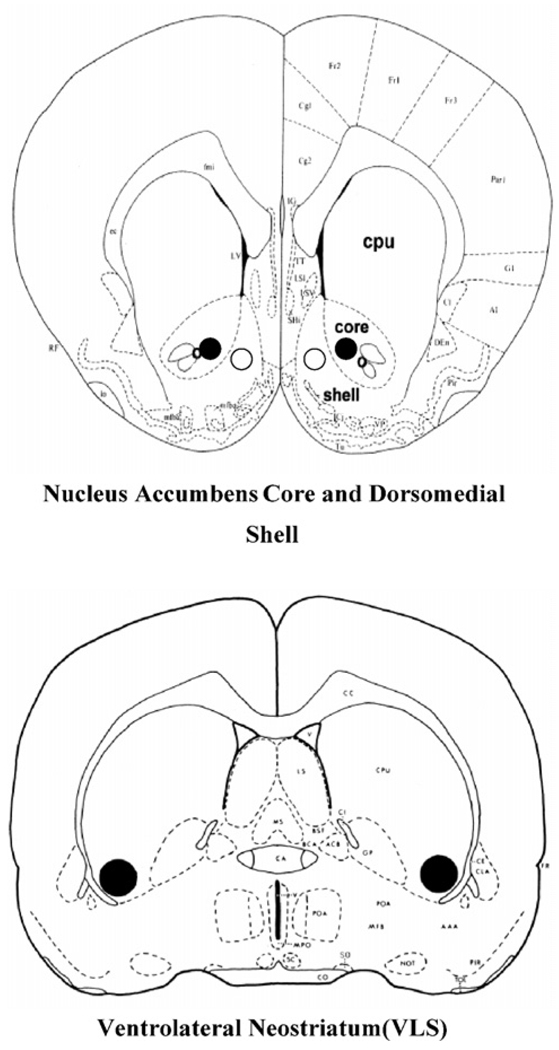
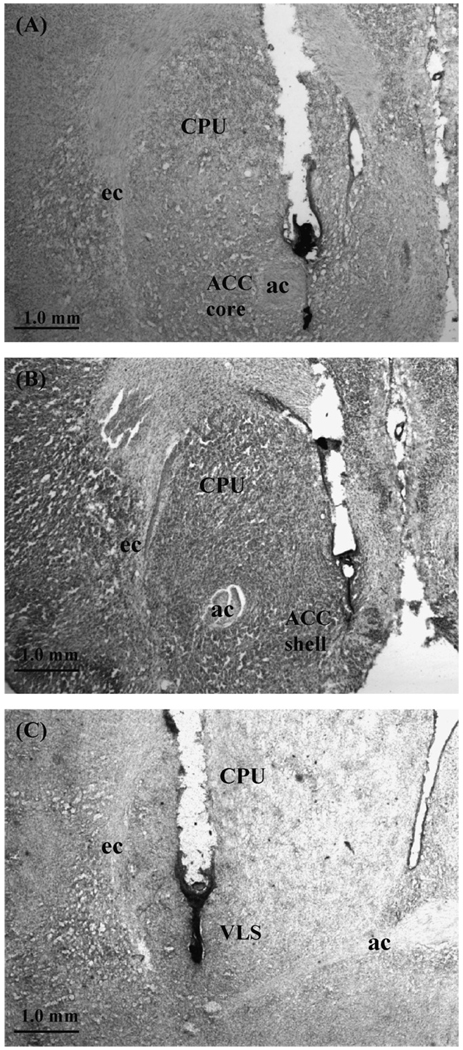
After experiments 3, 4 and 5 were completed, all animals in these experiments were intracardially perfused with 0.9% saline, followed by 3.7% formalin. Brains were then stored refrigerated in a formalin solution several days prior to slicing. The placements of the injectors were verified histologically by collecting consecutive 50 µm sections through the relevant brain areas. Sections were mounted on slides and stained with cresyl violet to aid in detection of the injector tracts. Slides were viewed microscopically to assess accuracy of implantation. Any animal with improper placement in either hemisphere (i.e., not in the target structures, such as accumbens core, or shell, or VLS; asymmetrical), or significant damage around the injection site, was not included in the statistical analyses of behavioral data (41.2% of all implantations were rejected). For analyses of core and shell placements, rats had to have bilateral and symmetrical placements solely within the core or the shell in order to be included, with no ambiguous placements in areas that separated the two subregions. See Fig. 1 and Fig. 2 for a drawings and photomicrographs of representative cannula placements in the target structures.
Figure 1.
Drawings depicting the location of representative cannula placements for experiments 3 and 4. Top: nucleus accumbens core (closed circles) and nucleus accumbens shell (dorsomedial region, open circles). Bottom: ventrolateral neostriatum (VLS; large closed circles).
Figure 2.
Photomicrographs of Nissl stained sections showing the locus surrounding representative cannula placements for experiments 3 and 4. (A) Nucleus accumbens core. (B) Nucleus accumbens shell (dorsomedial region). (C) Ventrolateral neostriatum (VLS). CPU = caudate/putamen; ACC = nucleus accumbens; ec = external capsule; ac = anterior commissure (scale bar = 1.0 mm).
2.7. Data analyses
Total number of locomotor activity counts were analyzed with betweengroups analysis of variance (ANOVA). Non-orthogonal planned comparisons using the overall error term were used to identify which doses significantly differed from vehicle plus haloperidol [44]. The Tukey test was used to make additional multiple comparisons in experiments 1 and 2. For experiments 3 and 4, effect size calculations (R2 values; see ref. [44]) were performed to assess the magnitude of the treatment effect (i.e., the size of the treatment effect expressed as the proportion of total variance accounted for by the treatment) across brain areas independently of the sample size.
3. Results
3.1. Experiment 1. Effects of systemic MSX-3 on the suppression of locomotion induced by acute injections of haloperidol
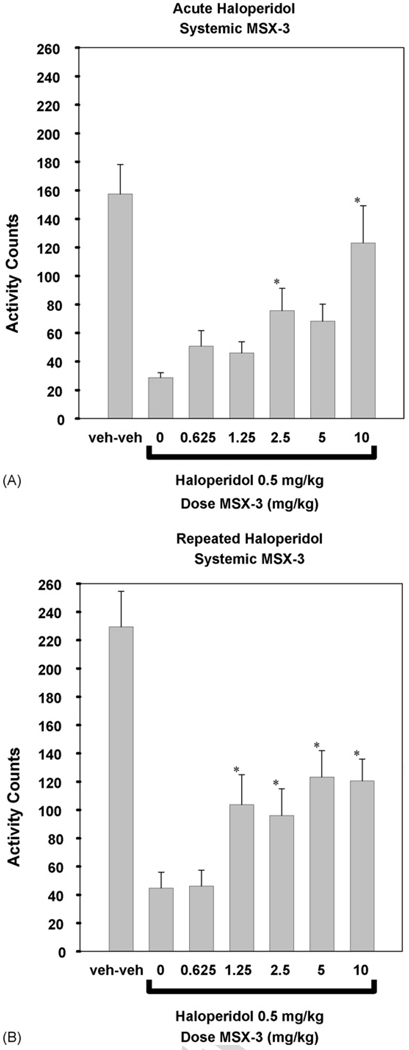
The effects of systemic injections of MSX-3 on the locomotor suppression induced by acute haloperidol are shown in Fig. 3A. ANOVA revealed a significant overall effect of drug treatment [n = 117; F(6, 110) = 9.204, p < 0.001]. Planned comparisons showed that the 2.5 and 10 mg/kg doses of MSX-3 significantly increased locomotor activity relative to haloperidol plus saline control (*differed from vehicle: p < 0.05). Post hoc comparisons (Tukey test, p < 0.05) also showed that there was no significant difference between the group that had received 10 mg/kg MSX-3 and the group that had not received haloperidol or MSX-3 (veh-veh), suggesting that the highest dose of MSX-3 produced a complete reversal of the locomotor suppression induced by acute haloperidol.
Figure 3.
Mean (±S.E.M.) number of locomotor activity counts during the 30 min session for rats that received treatment with tartaric acid vehicle plus saline vehicle, 0.5 mg/kg haloperidol plus vehicle, and 0.5 mg/kg haloperidol plus various doses of MSX-3 administered systemically. MSX-3 administered to haloperidol-treated rats significantly increased locomotion relative to treatment with haloperidol alone (*p < 0.05). (A) Acute haloperidol treatment (experiment 1; tartaric acid vehicle plus saline vehicle (n = 16), 0.5 mg/kg haloperidol plus vehicle (n = 18), and 0.5 mg/kg haloperidol plus various doses of MSX-3 administered i.p. 0.625 (n = 19), 1.25 (n = 16), 2.5 (n = 16), 5.0 (n = 16), or 10.0 (n = 16) mg/kg MSX-3). (B) Repeated haloperidol treatment (experiment 2; tartaric acid vehicle plus saline vehicle (n = 12), 0.5 mg/kg haloperidol plus vehicle (n = 15), and 0.5 mg/kg haloperidol plus various doses of MSX-3 administered i.p. 0.625 (n = 12), 1.25 (n = 15), 2.5 (n = 15), 5.0 (n = 14), or 10.0 (n = 16) mg/kg MSX-3).
3.2. Experiment 2. Effects of systemic MSX-3 on the suppression of locomotion induced by repeated injections of haloperidol
In Fig. 3B, the effects of systemic MSX-3 on the locomotor suppression induced by subchronic haloperidol are shown. ANOVA demonstrated that there was a significant overall effect of drug treatment [n = 99; F(6, 92) = 10.765, p < 0.001]. Planned comparisons revealed that the 1.25, 2.5, 5.0, and 10.0 mg/kg doses of MSX-3 significantly increased locomotor activity relative to haloperidol plus saline vehicle control (*differed from vehicle: p < 0.05). However, post hoc comparisons (Tukey test, p < 0.05) showed that all groups that had been treated with haloperidol and MSX-3 significantly differed from the group that had not received haloperidol or MSX-3 (veh-veh), indicating that MSX-3 produced only a partial reversal of the locomotor suppression induced by subchronic haloperidol.
3.3. Experiments 3A–C. Effects of intracranial injections of MSX-3 on the suppression of locomotion induced by acute injections of haloperidol
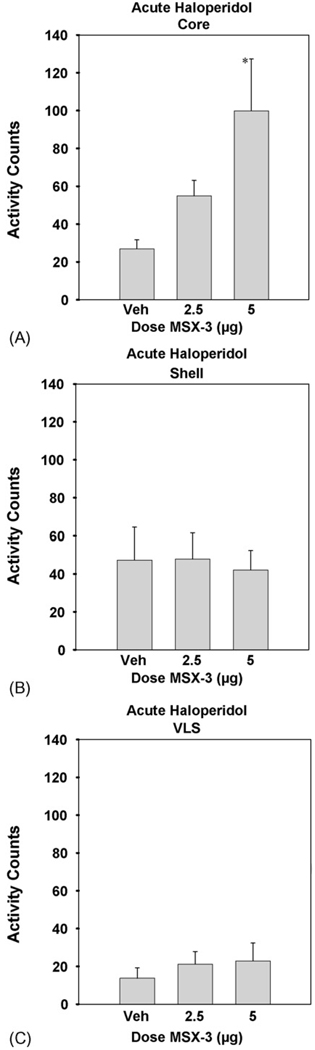
The effects of microinjections of MSX-3 into the nucleus accumbens core on locomotion in rats treated with acute haloperidol are shown in Fig. 4A. ANOVA demonstrated that there was a significant overall effect of drug treatment [n = 34; F(2, 31) = 4.45, p < 0.05]. Planned comparisons revealed that 5.0 µg of MSX-3 significantly increased locomotor activity relative to haloperidol plus saline vehicle control (*differed from vehicle: p < 0.01). The effects of microinjections of MSX-3 into the accumbens shell on the locomotor suppression induced by acute haloperidol are shown in Fig. 4B. ANOVA revealed no significant effect of treatment [n = 34; F(2, 31) = 0.056, n.s. at p = 0.05]. Fig. 4C depicts the effects of microinjections of MSX-3 into the VLS on the locomotor suppression induced by acute haloperidol. ANOVA revealed no significant effect of treatment [n = 25; F(2, 22) = 0.375, n.s. at p = 0.05].
Figure 4.
Mean (±S.E.M.) number of locomotor activity counts during the 30 min session for rats that received acute treatment with 0.5 mg/kg haloperidol plus saline vehicle, and 0.5 mg/kg haloperidol plus various doses of MSX-3 injected intracranially (2.5 and 5.0 µg per side). MSX-3 administered to haloperidol-treated rats significantly increased locomotion relative to treatment with haloperidol alone (*p < 0.05). (A) MSX-3 injected into nucleus accumbens core (experiment 3A; 0.5 mg/kg haloperidol plus saline vehicle (n = 11), and 0.5 mg/kg haloperidol plus 2.5 (n =11) and 5.0 (n = 12) µg MSX-3 per side). (B) MSX-3 injected into nucleus accumbens shell (experiment 3B; 0.5 mg/kg haloperidol plus saline vehicle (n =10), and 0.5 mg/kg haloperidol plus 2.5 (n = 12) and 5.0 (n = 12) µg MSX-3 per side). (C) MSX-3 injected into VLS (experiment 3C; 0.5 mg/kg haloperidol plus saline vehicle (n = 7), and 0.5 mg/kg haloperidol plus 2.5 (n = 10) and 5.0 (n = 8) µg MSX-3 per side).
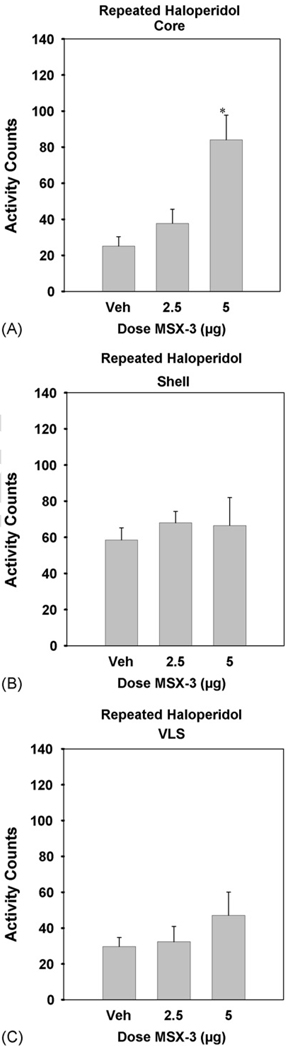
3.4. Experiments 4A–C. Effects of intracranial injections of MSX-3 on the suppression of locomotion induced by repeated injections of haloperidol
The effects of microinjections of MSX-3 into the accumbens core on motor activity in rats treated with repeated haloperidol are shown in Fig. 5A. ANOVA revealed that there was a significant overall treatment effect [n = 30; F(2, 27) = 10.39, p < 0.01]. Planned comparisons revealed that 5.0 µg of MSX-3 significantly increased locomotor activity relative to haloperidol plus saline vehicle control (*differed from vehicle: p < 0.01). Fig. 5B shows the effects of microinjections of MSX-3 into the accumbens shell on the locomotor suppression induced by repeated haloperidol. ANOVA revealed no significant effect of treatment [n = 29; F(2, 26) = 0.203, n.s. at p = 0.05]. The effects of microinjections of MSX-3 into the VLS on locomotion in rats treated with repeated haloperidol are shown in Fig. 5C. ANOVA demonstrated that there was no significant treatment effect [n = 21; F(2, 18) = 0.94, n.s. at p = 0.05].
Figure 5.
Mean (±S.E.M.) number of locomotor activity counts during the 30 min session for rats that received repeated treatment with 0.5 mg/kg haloperidol plus saline vehicle, and 0.5 mg/kg haloperidol plus various doses of MSX-3 injected intracranially (2.5 and 5.0 µg per side). MSX-3 administered to haloperidol-treated rats significantly increased locomotion relative to treatment with haloperidol alone (*p < 0.05). (A) MSX-3 injected into nucleus accumbens core (experiment 4A; 0.5 mg/kg haloperidol plus saline vehicle (n = 11), and 0.5 mg/kg haloperidol plus 2.5 (n = 8) and 5.0 (n = 11) µg MSX-3 per side). (B) MSX-3 injected into nucleus accumbens shell (experiment 4B; 0.5 mg/kg haloperidol plus saline vehicle (n =11), and 0.5 mg/kg haloperidol plus 2.5 (n = 7) and 5.0 (n = 11) µg MSX-3 per side). (C) MSX-3 injected into VLS (experiment 4C; 0.5 mg/kg haloperidol plus saline vehicle (n = 7), and 0.5 mg/kg haloperidol plus 2.5 (n = 6) and 5.0 (n = 8) µg MSX-3 per side).
3.5. comparisons of effect sizes across brain areas in experiments 3 and 4
Table 1 shows the calculated effect magnitudes (i.e., R2 values; ref. [44]) for all six intracranial experiments. Injections of MSX-3 into nucleus accumbens core produced a moderate effect size in experiment 3A (0.223) and a large effect size in experiment 4A (0.435). All other effects sizes were small, and none of them significantly differed from 0.
Table 1.
Effect size calculations for the intracranial experiments (3A–C and 4A–C)
| R2 values | |
|---|---|
| Acute haloperidol | |
| Nucleus accumbens core | 0.223* |
| Nucleus accumbens shell | 0.004 |
| Ventrolateral neostriatum | 0.033 |
| Repeated haloperidol | |
| Nucleus accumbens core | 0.435* |
| Nucleus accumbens shell | 0.015 |
| Ventrolateral neostriatum | 0.095 |
This table shows the results of the effect size calculations (R2 values, ref. [44]) obtained with injections into each brain region studied.
Effect size significantly different from 0, p<0.05.
3.6. Experiments 5A and B. Effects of systemic and intracranial injections of MSX-3 in rats not treated with haloperidol
Systemic administration of 10.0 mg/kg MSX-3, which was effective at stimulating locomotion in experiments 1–2, had no effect on locomotor activity in rats that were not haloperidol treated in experiment 5A. Mean (±S.E.M.) locomotor activity counts per 30 min were as follows: saline: 176.4 (±26.7), 10.0 mg/kg MSX-3: 223.4 (±46.6); (t = 0.87, d.f. = 12, n.s.). In experiment 5B, accumbens core injections at the dose of MSX-3 that was effective in experiments 3A and 4A (i.e., 5.0 µg per side) had no significant effect on locomotion in rats that were not pre-treated with haloperidol. Mean (±S.E.M.) locomotor activity counts per 30 min were as follows: saline: 252.4 (±23.9), 5.0 µg MSX-3: 267.6 (±39.1); (t = 0.33, d.f. = 12, n.s.).
4. Discussion
These experiments were conducted to study the ability of the adenosine A2A antagonist MSX-3 to reverse the locomotor effects of acute or subchronic administration of haloperidol in rats. Systemic injections of MSX-3 in a dose range of 2.5–10.0 mg/kg were capable of reversing the suppression of locomotion induced by either acute or repeated (i.e., 14 day) administration of 0.5 mg/kg haloperidol. Bilateral infusions of MSX-3 into the nucleus accumbens core (2.5 µg or 5.0 µg in 0.5 µl per side) produced a dose-related increase in locomotor activity in rats treated with 0.5 mg/kg haloperidol either acutely or repeatedly. There was not an overall significant effect of MSX-3 infused into either the dorsomedial nucleus accumbens shell or the VLS. In addition, there were no significant effects of systemic or intra-accumbens injections of MSX-3 (10.0 mg/kg and 5.0 µg per side, respectively) in rats that were not treated with haloperidol. Taken together, these results indicate that antagonism of adenosine A2A receptors can reverse the locomotor suppression produced by DA antagonism, and that a critical site for this effect is the nucleus accumbens core. These results have important implications for understanding the mechanisms underlying the motor effects of adenosine A2A receptor antagonists.
In the first two experiments, MSX-3 attenuated the suppression of locomotion induced by administration of haloperidol. This effect was evident whether the haloperidol was injected acutely (experiment 1) or with a repeated 14 day administration procedure (experiment 2). The stimulation of locomotion induced by MSX-3 was comparable in both experiments, although the control levels of activity were higher in the repeated haloperidol study. The present results are consistent with previous studies demonstrating that systemic administration of adenosine A2A antagonists can reverse the deficits in locomotion that were induced by reserpine [73], D2 receptor deficiency [3], and haloperidol [18]. In addition, these results involving locomotion are consistent with those of studies that employed other measures of motor dysfunction, including haloperidol-induced rigidity [79], catalepsy [30,32,40], and drug-induced tremulous jaw movements, which are used as an animal model of parkinsonian tremor [18,74]. Taken together, these results provide additional support for the hypothesis that adenosine A2A antagonism can reverse motor impairments induced by interference with DA transmission in animals. These findings with animal models are widely used to support the idea that adenosine A2A antagonists could represent a novel non-dopaminergic treatment strategy for idiopathic Parkinson’s disease [37,38,76]. Moreover, the ability of adenosine A2A antagonists to reverse motor dysfunctions induced by typical antipsychotics such as haloperidol suggests that adenosine A2A antagonists also could be used to treat antipsychotic-induced parkinsonism in humans [18].
Although studies involving systemic administration of drugs are important, they do not provide specific information about the anatomical locus mediating the effects of those drugs. For that reason, experiments 3 and 4 were conducted in order to study the effects of local intracranial administration of MSX-3 directly into distinct striatal subregions, including two sites in the nucleus accumbens and one in the neostriatum. Bilateral infusions of MSX-3 directly into the nucleus accumbens core produced a dose-related increase in locomotor activity in rats treated with 0.5 mg/kg haloperidol. This effect occurred when the haloperidol was injected acutely, and also when the 14 day repeated injection procedure was used. Effect size analyses indicated that MSX-3 produced moderate-to-large effects when injected into nucleus accumbens core. These positive effects obtained with MSX-3 injections into the core clearly demonstrate that nucleus accumbens is an effective site for the stimulation of locomotion by MSX-3 in haloperidol-treated rats, an observation that is consistent with previous studies showing that adenosine A2A receptors in nucleus accumbens are involved in the regulation of locomotion [6,7,31,59]. However, the robust and consistent effects observed after injections of MSX-3 into the nucleus accumbens core were not mimicked by injections into the dorsomedial shell. There was not an overall significant effect of MSX-3 infused directly into the dorsomedial shell in either experiment 3 or 4. The involvement of specific subregions of nucleus accumbens in locomotion appears to differ depending upon the particular drug class being studied [36,51,57]. The present results, indicating that adenosine A2A antagonism in the core was effective at stimulating locomotion in rats with impaired DA transmission while shell injections of MSX-3 were not, are consistent with previous reports indicating that the concentration of adenosine A2A receptors is higher in the core than in the shell [29,63]. Despite the fact that 5.0 µg MSX-3 injected into the nucleus accumbens core was able to stimulate locomotion in haloperidol-treated rats, this dose did not stimulate locomotion in the absence of haloperidol. This observation is consistent with the results from experiment 5A with systemic MSX-3, and also with previous findings. A systemic dose of KF 17837 that was capable of reversing the haloperidol-induced suppression of locomotion did not stimulate locomotion when administered alone [18]. In addition, doses of MSX-3 that reversed haloperidol-induced lever pressing had no effects when injected without the DA antagonist [23]. Although recent work has indicated that intra-accumbens injections of MSX-3 can increase locomotion [59], that paper used behavioral methods that were substantially different from those used in the present study (i.e., open-field activity in habituated animals). Moreover, previous studies showing that systemic injections of MSX-3 increased locomotion used younger rats than the present study [2,41,72]. Additional studies should investigate the role of factors such as dose, placement, age, degree of habituation, and behavioral conditions in modulating the locomotor effects of MSX-3 when administered alone. Furthermore, in view of data indicating that medial caudate-putamen is involved in the effects of adenosine A2A receptor agonists and antagonists on catalepsy [30–32], it is possible that this striatal region also is involved in locomotor effects of these drugs. For that reason, future mapping studies should explore the effects of drug injections into various placement sites within the region extending from the nucleus accumbens core to the anteromedial regions of caudate-putamen.
In contrast to the positive effects obtained from injections of MSX-3 into the nucleus accumbens core, no significant effects on locomotion were obtained after injections of MSX-3 into the VLS. Previous work has suggested minimal involvement of this neostriatal subregion in locomotor function. For example, depletions of DA in the VLS by local infusions of 6-hydroxydopamine failed to suppress locomotion [19,39], and intracranial injections of amphetamine into the VLS were reported to have no effect on locomotor activity [42]. Although the VLS does not appear to be an important site for the regulation of locomotion, it is important for other aspects of motor function. VLS DA depletions have been shown to impair skilled use of the forepaws for functions such as reaching, grasping, feeding, food handling and lever pressing [19,20,39,65,66]. In addition, the VLS is an important region of the neostriatum for the control of oral motor activity [43,62,64,67]. It has been suggested that the lateral striatum of the rat is the homologue of the putamen of primates [67], and anatomical studies have demonstrated that the VLS of the rat receives input from headrelated areas of motor cortex [55]. Neurochemical mechanisms in the VLS involving several neurotransmitters, including DA, acetylcholine, and adenosine, have been implicated in tremulous jaw movements [21,26,39,54,64,67,74]. These movements, which are induced by DA antagonists, DA depletions, and cholinomimetics, have been used as a rodent model of parkinsonian tremor [14,37,67,69]. Recently, it was demonstrated that the jaw movements induced by the anticholinesterase tacrine could be reversed by infusions of an adenosine A2A antagonist directly into the VLS [64]. Thus, despite the fact that the VLS has been implicated in several aspects of motor function and dysfunction, including skilled control of the forepaws and oral tremor, the present results indicate that the stimulation of locomotion by an adenosine A2A antagonist in animals with impaired DA transmission is related to actions on the nucleus accumbens, particularly the core subregion, but not to actions on the VLS.
In conjunction with other published studies, the present results emphasize that different striatal subregions are involved in distinct aspects of motor function. This principle is demonstrated clearly in the substantial literature showing that DA depletions or antagonism can have radically different effects depending upon the striatal locus being affected [4,19,65,66]. In addition, this principle has important implications for understanding the anatomical mechanisms underlying the motor effects of antiparkinsonian drugs, including adenosine A2A antagonists. Although antiparkinsonian drugs are typically given systemically, and the therapeutic target would generally be a broad improvement across diverse motor symptoms, it is nevertheless reasonable to argue that different therapeutic effects (i.e., increases in locomotion, decreases in rigidity or tremor) are related to actions on distinct striatal subcircuits. The present results would suggest that the nucleus accumbens core is a critical striatal site for the restoration of locomotion seen after administration of adenosine A2A antagonists. This observation is consistent with previous studies involving the locomotor functions of nucleus accumbens in both rodents [6,7,16,19,31,59] and primates [1]. Thus, it is possible that restoration of locomotor activity in human patients also is related to actions of antiparkinsonian drugs on the nucleus accumbens, at least in part. In contrast, motor dysfunctions such as tremor and skilled forelimb usage appear to involve other striatal subregions [67]. Additional research should focus on how adenosine A2A antagonists exert their effects by modifying distinct subcircuits within the overall striatal circuitry, including neostriatal areas adjacent to the nucleus accumbens. Moreover, future studies should investigate the extent to which adenosine A2A receptors in nucleus accumbens core are involved in other behavioral processes, such as psychomotor activation and energy-related functions [23,70], which are related to the motivational deficits seen in patients with Parkinson’s disease, depression, and other disorders.
Acknowledgements
This research was supported by a grant to J.D.S. from the United States NIH/NINDS. Many thanks to Joshua Orabone, Brian Mirante and Jamie Bunce for their technical assistance.
References
- 1.Annett LE, Ridley RM, Gamble SJ, Baker HF. Social withdrawal following amphetamine administration to marmosets. Psychopharmacology. 1989;99:222–229. doi: 10.1007/BF00442812. [DOI] [PubMed] [Google Scholar]
- 2.Antoniou K, Papadopoulou-Daifoti Z, Hyphantis T, Papathanasiou G, Bekris E, Marselos M, et al. A detailed behavioral analysis of the acute motor effects of caffeine in the rat: involvement of adenosine A1 and A2A receptors. Psychopharmacology. 2005;183:154–162. doi: 10.1007/s00213-005-0173-6. [DOI] [PubMed] [Google Scholar]
- 3.Aoyama S, Kase H, Borrelli E. Rescue of locomotor impairment in dopamine D2 receptor-deficient mice by an adenosine A2A receptor antagonist. J Neurosci. 2000;20:5848–5852. doi: 10.1523/JNEUROSCI.20-15-05848.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bakshi VP, Kelley AE. Dopaminergic regulation of feeding behavior:I.Differential effects of haloperidol microinjection in three striatal subregions. Psychobiology. 1991;19:223–232. [Google Scholar]
- 5.Baldo BA, Sadeghian K, Basso AM, Kelley AE. Effects of selective dopamine D1 or D2 receptor blockade within nucleus accumbens subregions on ingestive behavior and associated motor activity. Behav Brain Res. 2002;137:165–177. doi: 10.1016/s0166-4328(02)00293-0. [DOI] [PubMed] [Google Scholar]
- 6.Barraco RA, Martens KA, Parizon M, Normile HJ. Adenosine A2a receptors in the nucleus accumbens mediate locomotor depression. Brain Res Bull. 1993;31:397–404. doi: 10.1016/0361-9230(93)90233-2. [DOI] [PubMed] [Google Scholar]
- 7.Barraco RA, Martens KA, Parizon M, Normile HJ. Role of adenosine A2a receptors in the nucleus accumbens. Prog Neuropsychopharmacol Biol Psychiat. 1994;18:545–553. doi: 10.1016/0278-5846(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 8.Bezchlibnyk-Butler KZ, Remington GJ. Antiparkinsonian drugs in the treatment of neuroleptic-induced extrapyramidal symptoms. Can J Psychiat. 1994;39:74–84. doi: 10.1177/070674379403900203. [DOI] [PubMed] [Google Scholar]
- 9.Bokobza B, Ruberg M, Scatton B, Javoy-Agid F, Agid Y. [3H]spiperone binding, dopamine and HVA concentrations in Parkinson’s disease and supranuclear palsy. Eur J Pharmacol. 1984;99:167–175. doi: 10.1016/0014-2999(84)90238-3. [DOI] [PubMed] [Google Scholar]
- 10.Brunt ER, Brooks DJ, Korczyn AD, Montastruc JL, Stocchi F. A six-month multicentre, double-blind, bromocriptine-controlled study of the safety and efficacy of ropinirole in the treatment of patients with Parkinson’s disease not optimally controlled by L-dopa. J Neural Transm. 2002;109:489–502. doi: 10.1007/s007020200040. [DOI] [PubMed] [Google Scholar]
- 11.Carlson BB, Wisniecki A, Salamone JD. Local injections of the 5-hydroxytryptamine antagonist mianserin into substantia nigra pars reticulata block tremulous jaw movements in rats: studies with a putative model of Parkinsonian tremor. Psychopharmacology. 2003;165:229–237. doi: 10.1007/s00213-002-1247-3. [DOI] [PubMed] [Google Scholar]
- 12.Carlson BB, Behrstock S, Tobin AJ, Salamone JD. Brain implantations of engineered GABA-releasing cells suppress tremor in an animal model of Parkinsonism. Neuroscience. 2003;119:927–932. doi: 10.1016/s0306-4522(03)00218-5. [DOI] [PubMed] [Google Scholar]
- 13.Carta AR, Pinna A, Cauli O, Morelli M. Differential regulation of GAD67, enkephalin and dynorphin mRNAs by chronic-intermittent L-DOPA and A(2A) receptor blockade plus L-DOPA in dopamine-denervated rats. Synapse. 2002;44:166–174. doi: 10.1002/syn.10066. [DOI] [PubMed] [Google Scholar]
- 14.Cenci MA, Whishaw IQ, Schallert T. Animal models of neurological deficits: how relevant is the rat? Nat Rev Neurosci. 2002;3:574–579. doi: 10.1038/nrn877. [DOI] [PubMed] [Google Scholar]
- 15.Chen JF, Moratalla R, Impagnatiello F, Grandy DK, Cuellar B, Rubinstein M, et al. The role of the D2 dopamine receptor (D2R) in A2a adenenosinereceptor (A2aR) mediated behavioral and cellular responses as revealed by A2a and D2 receptor knockout mice. Proc Natl Acad Sci. 2001;98:1970–1975. doi: 10.1073/pnas.98.4.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Correa M, Carlson BB, Wisniecki A, Salamone JD. Nucleus accumbens dopamine and work requirements on interval schedules. Behav Brain Res. 2002;137:179–187. doi: 10.1016/s0166-4328(02)00292-9. [DOI] [PubMed] [Google Scholar]
- 17.Correa M, Arizzi MN, Betz A, Mingote S, Salamone JD. Locomotor stimulant effects of intraventricular injections of ethanol in rats: acute and repeated administration. Psychopharmacology. 2003;170:368–375. doi: 10.1007/s00213-003-1557-0. [DOI] [PubMed] [Google Scholar]
- 18.Correa M, Wisniecki A, Betz A, Dobson DR, O’Neill MF, O’Neill MJ, et al. The adenosine A2A antagonist KF 17837 reverses the locomotor suppression and tremulous jaw movements induced by haloperidol in rats: possible relevance to parkinsonism. Behav Brain Res. 2004;148:47–54. doi: 10.1016/s0166-4328(03)00178-5. [DOI] [PubMed] [Google Scholar]
- 19.Cousins MS, Sokolowski JD, Salamone JD. Different effects of nucleus accumbens and ventrolateral striatal dopamine depletions on instrumental response selection in the rat. Pharmacol Biochem Behav. 1993;46:943–951. doi: 10.1016/0091-3057(93)90226-j. [DOI] [PubMed] [Google Scholar]
- 20.Cousins MS, Salamone JD. Involvement of ventrolateral striatal dopamine in movement initiation and execution: a microdialysis and behavioral investigation. Neuroscience. 1996;70:849–859. doi: 10.1016/0306-4522(95)00407-6. [DOI] [PubMed] [Google Scholar]
- 21.Cousins MS, Finn M, Trevitt J, Carriero DL, Conlan A, Salamone JD. The role of ventrolateral striatal acetylcholine in the production of tacrineinduced jaw movements. Pharmacol Biochem Behav. 1999;62:439–447. doi: 10.1016/s0091-3057(98)00214-7. [DOI] [PubMed] [Google Scholar]
- 22.DeLong MR. Primate model of movement disorders of basal ganglia origin. Trends Neurosci. 1990;13:281–285. doi: 10.1016/0166-2236(90)90110-v. [DOI] [PubMed] [Google Scholar]
- 23.Farrar A, Pereira M, Velasco F, Hockemeyer J, Müller CE, Salamone JD. Adenosine A2A receptor antagonism reverses the effects of dopamine receptor antagonism on instrumental output and effort-related choice in the rat.Implications for studies of psychomotor slowing. Psychopharmacology. 2007 doi: 10.1007/s00213-006-0554-5. in press. [DOI] [PubMed] [Google Scholar]
- 24.Ferré S, Freidholm BB, Morelli M, Popoli P, Fuxe K. Adenosine-dopamine receptor-receptor interactions as an integrative mechanism in the basal ganglia. Trends Neurosci. 1997;20:482–487. doi: 10.1016/s0166-2236(97)01096-5. [DOI] [PubMed] [Google Scholar]
- 25.Ferré S, Popoli P, Gimenez-Llort L, Rimondini R, Müller CE, Stromberg I, et al. Adenosine/dopamine interaction: implications for the treatment of Parkinson’s disease. Parkinson Rel Disord. 2001;7:235–241. doi: 10.1016/s1353-8020(00)00063-8. [DOI] [PubMed] [Google Scholar]
- 26.Finn M, Jassen A, Baskin P, Salamone JD. Tremulous characteristic of vacuous jaw movements induced by pilocarpine and ventrolateral striatal dopamine depletions. Pharmacol Biochem Behav. 1997;57:243–249. doi: 10.1016/s0091-3057(96)00385-1. [DOI] [PubMed] [Google Scholar]
- 27.Fink JS, Weaver DR, Rivkees SA, Peterfreund RA, Pollack AE, Adler EM, et al. Molecular cloning of the rat A2 adenosine receptor: selective co-expression with D2 dopamine receptors in rat striatum. Brain Res. 1992;14:186–195. doi: 10.1016/0169-328x(92)90173-9. [DOI] [PubMed] [Google Scholar]
- 28.Fuxe K, Ferreé S, Zoli M, Agnati LF. Integrated events in central dopamine transmission as analyzed at multiple levels. Evidence for intramembrane adenosine A2A/dopamine D2 and adenosine A1/dopamine D1 receptor interactions in the basal ganglia. Brain Res Rev. 1998;26:258–273. doi: 10.1016/s0165-0173(97)00049-0. [DOI] [PubMed] [Google Scholar]
- 29.Grondin R, Bedard PJ, Hadj Tahar A, Gregoire L, Mori A, Kase H. Antiparkinsonian effect of a new selective adenosine A2A receptor antagonist in MPTP-treated monkeys. Neurology. 1999;52:1673–1677. doi: 10.1212/wnl.52.8.1673. [DOI] [PubMed] [Google Scholar]
- 30.Hauber W, Nagel J, Sauer R, Müller CE. Motor effects induced by a blockade of adenosine A2A receptors in the caudate-putamen. Neuroreport. 1998;9:1803–1806. doi: 10.1097/00001756-199806010-00024. [DOI] [PubMed] [Google Scholar]
- 31.Hauber W, Munkle M. Motor depressant effects mediated by dopamine D2 and adenosine A2A receptors in the nucleus accumbens and the caudate-putamen. Eur J Pharmacol. 1997;323:127–131. doi: 10.1016/s0014-2999(97)00040-x. [DOI] [PubMed] [Google Scholar]
- 32.Hauber W, Neuscheler P, Nagel J, Müller CE. Catalepsy induced by a blockade of dopamine D1 or D2 receptors was reversed by a concomitant blockade of adenosine A2A receptors in the caudate putamen of rats. Eur J Neurosci. 2001;14:1287–1293. doi: 10.1046/j.0953-816x.2001.01759.x. [DOI] [PubMed] [Google Scholar]
- 33.Hettinger BD, Lee A, Linden J, Rosin DL. Ultrastructural localization of adenosine A2A receptors suggests multiple cellular sites for modulation of GABAergic neurons in rat striatum. J Comp Neurol. 2001;431:331–346. doi: 10.1002/1096-9861(20010312)431:3<331::aid-cne1074>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 34.Hockemeyer J, Burbiel JC, Muller CE. Multigram-scale syntheses, stability, and photoreactions of A2A adenosine receptor antagonists with 8-styrylxanthine structure: potential drugs for Parkinson’s disease. J Org Chem. 2004;69:3308–3318. doi: 10.1021/jo0358574. [DOI] [PubMed] [Google Scholar]
- 35.Hornykiewicz O. Dopamine in the basal ganglia. Its role and therapeutic implications (including the clinical use of L-DOPA) Br Med Bull. 1973;29:172–178. doi: 10.1093/oxfordjournals.bmb.a070990. [DOI] [PubMed] [Google Scholar]
- 36.Ikemoto S. Ventral striatal anatomy of locomotor activity induced by cocaine, D-amphetamine, dopamine and D1/D2 agonists. Neuroscience. 2002;113:939–955. doi: 10.1016/s0306-4522(02)00247-6. [DOI] [PubMed] [Google Scholar]
- 37.Ishiwari K, Betz A, Weber S, Felsted J, Salamone JD. Pimozide (Orap) shows a pattern of behavioral effects similar to the typical antipsychotic haloperidol: studies with tremulous jaw movements and lever pressing in rats. Pharmacol Biochem Behav. 2005 [Google Scholar]
- 38.Jenner P. Istradefylline, a novel adenosine A2A receptor antagonist, for the treatment of Parkinson’s disease. Exp Opin Investig Drugs. 2005;14:729–738. doi: 10.1517/13543784.14.6.729. [DOI] [PubMed] [Google Scholar]
- 39.Jicha G, Salamone JD. Vacuous jaw movements and feeding deficits in rats with ventrolateral striatal dopamine depletions: possible model of parkinsonian symptoms. J Neurosci. 1991;11:3822–3829. doi: 10.1523/JNEUROSCI.11-12-03822.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kanda T, Shiozaki S, Shimada J, Suzuki F, Nakamara J. KF 17837 a novel selective adenosine A2A receptor antagonist with anticataleptic activity. Eur J Pharm. 1994;256:262–268. doi: 10.1016/0014-2999(94)90551-7. [DOI] [PubMed] [Google Scholar]
- 41.Karcz-Kubicha M, Antoniou K, Tersmaa A, Quarta D, Solinas M, Justinova Z, et al. Involvement of adenosine A1 and A2A receptors in the motor effects of caffeine after its acute and chronic administration. Neuropsychopharm. 2003;28:1281–1291. doi: 10.1038/sj.npp.1300167. [DOI] [PubMed] [Google Scholar]
- 42.Kelley AE, Gauthier AM, Lang CG. Amphetamine microinjections into distinct striatal subregions cause dissociable effects on motor and ingestive behavior. Behav Brain Res. 1989;35:27–39. doi: 10.1016/s0166-4328(89)80005-1. [DOI] [PubMed] [Google Scholar]
- 43.Kelley AE, Bakshi VP, Delfs JM, Lang CG. Cholinergic stimulation of the ventrolateral striatum elicits mouth movements in rats: pharmacological and regional specificity. Psychopharmacology. 1989;99:542–549. doi: 10.1007/BF00589906. [DOI] [PubMed] [Google Scholar]
- 44.Keppel G. Design and analysis: a researchers handbook. Englewood Cliffs, NJ: Prentice-Hall; 1991. [Google Scholar]
- 45.Khisti RT, Chopde CT, Abraham E. GABAergic involvement in motor effects of adenosine A2A receptor agonist in mice. Neuropharmacology. 2000;39:1004–1015. doi: 10.1016/s0028-3908(99)00187-2. [DOI] [PubMed] [Google Scholar]
- 46.Koob GF, Riley SJ, Smith SC, Robbins TW. Effects of 6-hydroxydopamine lesions of the nucleus accumbens septi and olfactory tubercle on feeding, locomotor activity, and amphetamine anorexia in the rat. J Comp Physiol Psychol. 1978;92:917–927. doi: 10.1037/h0077542. [DOI] [PubMed] [Google Scholar]
- 47.Lang AE, Lees A. DA agonists–non-ergot derivatives: Ropinirole. Mov Disord. 2002;17:s98–s102. doi: 10.1002/mds.5570. [DOI] [PubMed] [Google Scholar]
- 48.Lang AE, Lees A. DA agonists–ergot derivatives: Pergolide. Mov Disord. 2002;17:s79–s82. doi: 10.1002/mds.5566. [DOI] [PubMed] [Google Scholar]
- 49.Lillrank SM, Lipska BK, Weinberger DR, Fredholm BB, Fuxe K, Ferre S. Adenosine and dopamine receptor antagonist binding in the rat ventral and dorsal striatum: lack of changes after a neonatal bilateral lesion of the ventral hippocampus. Neurochem Int. 1999;34:235–244. doi: 10.1016/s0197-0186(99)00008-x. [DOI] [PubMed] [Google Scholar]
- 50.Madras BK, Gracz LM, Fahey MA, Elmaleh D, Meltzer PC, Liang AY, et al. Altropane, a SPECT or PET imaging probe for dopamine neurons: III. Human dopamine transporter in postmortem normal and Parkinson’s diseased brain. Synapse. 1998;29:116–127. doi: 10.1002/(SICI)1098-2396(199806)29:2<116::AID-SYN3>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 51.Maldonado-Irizarry CS, Kelley AE. Differential behavioral effects following microinjection of an NMDA antagonist into nucleus accumbens subregions. Psychopharmacology. 1994;116:65–72. doi: 10.1007/BF02244872. [DOI] [PubMed] [Google Scholar]
- 52.Mally J, Stone TW. Potential of adenosine A2A antagonists in the treatment of movement disorders. CNS Drugs. 1998;10:311–320. [Google Scholar]
- 53.Marsden C, Duvoisin R, Jenner P, Parkes J, Pycock C, Tarsy D. Relationship between animal models and clinical parkinsonism. Adv Neurol. 1975;9:165–175. [PubMed] [Google Scholar]
- 54.Mayorga AJ, Trevitt JT, Conlan A, Ginutsos G, Salamone JD. Striatal and nigral D1 mechanisms involved in the antiparkinsonian effects of SKF 82958 (APB): studies of tremulous jaw movements in rats. Psychopharmacology. 1999;143:72–81. doi: 10.1007/s002130050921. [DOI] [PubMed] [Google Scholar]
- 55.McGeorge AJ, Faull RLM. Organization of the projection from the cerebral cortex to the striatum in the rat. Neuroscience. 1989;29:503–537. doi: 10.1016/0306-4522(89)90128-0. [DOI] [PubMed] [Google Scholar]
- 56.Morelli M, Pinna A. Interaction between dopamine and adenosine A2A receptors as a basis for the treatment of Parkinson’s disease. Neurol Sci. 2001;22:71–72. doi: 10.1007/s100720170052. [DOI] [PubMed] [Google Scholar]
- 57.Müller CE, Sauer R, Maurish Y, Fülle F, Nagel J, Hauber W. Water-soluble prodrug of potent A2A-selective adenosne receptor antagonists. Drug Dev Res. 1998;45:190–197. [Google Scholar]
- 58.Murray TK, Messenger MJ, Ward MA, Woodhouse S, Osborne DJ, Duty S, et al. Evaluation of the mGluR2/3 agonist LY379268 in rodent models of Parkinson’s disease. Pharmacol Biochem Behav. 2002;73:455–466. doi: 10.1016/s0091-3057(02)00842-0. [DOI] [PubMed] [Google Scholar]
- 59.Nagel J, Shladebach H, Kock M, Schwienbacher I, Müller CE, Hauber W. Effects of an adenosine A2A receptor blockade in the nucleus accumbens on locomotion, feeding, and prepulse inhibition in rats. Synapse. 2003;49:279–286. doi: 10.1002/syn.10240. [DOI] [PubMed] [Google Scholar]
- 60.Nash JE, Brotchie JM. A common signaling pathway for striatal NMDA and adenosine A2A receptors: implications for the treatment of Parkinson’s disease. J Neurosci. 2000;20:7782–7789. doi: 10.1523/JNEUROSCI.20-20-07782.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pinna A, Wardas J, Simola N, Morelli M. New therapies for the treatment of Parkinson’s disease: adenosine A2A receptor antagonists. Life Sci. 2005;77:3259–3267. doi: 10.1016/j.lfs.2005.04.029. [DOI] [PubMed] [Google Scholar]
- 62.Pisa M. Motor somatotopy in the striatum of rat: manipulation, biting and gait. Behav Brain Res. 1988;27:21–35. doi: 10.1016/0166-4328(88)90106-4. [DOI] [PubMed] [Google Scholar]
- 63.Rosin DL, Robeva A, Woodard RL, Guyenet PG, Linden J. Immunohistochemical localization of adenosine A2A receptors in the rat central nervous system. J Comp Neurol. 1998;401:163–186. [PubMed] [Google Scholar]
- 64.Salamone JD, Johnson CJ, McCullough LD, Steinpreis RE. Lateral striatal cholinergic mechanisms involved in oral motor activities in the rat. Psychopharmacology. 1990;102:529–534. doi: 10.1007/BF02247136. [DOI] [PubMed] [Google Scholar]
- 65.Salamone JD, Mahan K, Rogers S. Ventrolateral striatal dopamine depletions impair feeding and food handling in rats. Pharmacol Biochem Behav. 1993;44:605–610. doi: 10.1016/0091-3057(93)90174-r. [DOI] [PubMed] [Google Scholar]
- 66.Salamone JD, Kurth PA, McCullough LD, Sokolowski JD, Cousins JD. The role of brain dopamine in response initiation: effects of haloperidol and regionally specific dopamine depletions on local rate of instrumental responding. Brain Res. 1993;628:218–226. doi: 10.1016/0006-8993(93)90958-p. [DOI] [PubMed] [Google Scholar]
- 67.Salamone JD, Mayorga AJ, Trevitt JT, Cousins MS, Conlan A, Nawab A. Tremulous jaw movements in rats; a model of parkinsonian tremor. Prog Neurobiol. 1998;56:591–611. doi: 10.1016/s0301-0082(98)00053-7. [DOI] [PubMed] [Google Scholar]
- 68.Salamone J, Correa M, Carlson B, Wisniecki A, Mayorga A, Nisenbaum E, et al. Neostriatal muscarinic receptor subtypes involved in the generation of tremulous jaw movements in rodents. Implications for cholinergic involvement in parkinsonism. Life Sci. 2001;68:2579–2584. doi: 10.1016/s0024-3205(01)01055-4. [DOI] [PubMed] [Google Scholar]
- 69.Salamone JD, Carlson BB, Rios C, Lentini E, Correa M, Wisniecki A, et al. Dopamine agonists suppress cholinomimetic-induced tremulous jaw movements in an animal model of Parkinsonism: tremorolytic effects of pergolide, ropinirole and CY 208–243. Behav Brain Res. 2005;156:173–179. doi: 10.1016/j.bbr.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 70.Salamone JD, Correa M, Mingote SM, Weber SM, Farrar AM. Nucleus accumbens dopamine and the forebrain circuitry involved in behavioral activation and effort-related decision making: implications for understanding anergia and psychomotor slowing in depression. Curr Psychiatry Rev. 2006:2. [Google Scholar]
- 71.Sauer R, Maurinsh J, Reith U, Fulle F, Klotz KN, Müller CE. Water-soluble phosphate prodrugs of 1-propargyl-8-styrylxanthine derivatives, A(2A)-selective adenosine receptor antagonists. J Med Chem. 2000;43:440–448. doi: 10.1021/jm9911480. [DOI] [PubMed] [Google Scholar]
- 72.Schindler CW, Karcz-Kubicha M, Thorndike EB, Müller CE, Tella SR, Goldberg SR, et al. Lack of adenosine A1 and dopamine D2 receptormediated modulation of the cardiovascular effects of the adenosine A2A receptor agonist CGS 21680. Eur J Pharmacol. 2004;484:269–275. doi: 10.1016/j.ejphar.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 73.Shiozaki S, Ichikawa S, Nakamura J, Kitamura S, Yamada K, Kuwana Y. Actions of adenosine A2A receptor antagonist KW-6002 on drug-induced catalepsy and hypokinesia caused by reserpine or MPTP. Psychopharmacology. 1999;147:90–95. doi: 10.1007/s002130051146. [DOI] [PubMed] [Google Scholar]
- 74.Simola N, Fenu S, Baraldi PG, Tabrizi MA, Morelli M. Blockade of adenosine A2A receptors antagonizes parkinsonian tremor in the rat tacrine model by an action on specific striatal regions. Exp Neurol. 2004;189:182–188. doi: 10.1016/j.expneurol.2004.05.027. [DOI] [PubMed] [Google Scholar]
- 75.Stromberg I, Popoli P, Muller CE, Ferre S, Fuxe K. Electrophysiological and behavioural evidence for an antagonistic modulatory role of adenosine A2A receptors in dopamine D2 receptor regulation in dopamine-denervated striatum. Eur J Neurosci. 2000;12:4033–4037. doi: 10.1046/j.1460-9568.2000.00288.x. [DOI] [PubMed] [Google Scholar]
- 76.Svenningsson P, Le Moine C, Fisone G, Fredholm BB. Distribution, biochemistry and function of striatal adenosine A2A receptors. Prog Neurobiol. 1999;59:355–396. doi: 10.1016/s0301-0082(99)00011-8. [DOI] [PubMed] [Google Scholar]
- 77.Trevitt JT, Carlson BB, Correa M, Keene A, Morales M, Salamone JD. Interactions between D1 receptors and GABA mechanisms in substantia nigra pars reticulata of the rat: neurochemical and behavioral studies. Psychopharmacology. 2002;159:229–237. doi: 10.1007/s002130100908. [DOI] [PubMed] [Google Scholar]
- 78.Wang WF, Ishiwata K, Nonaka H, Ishii S, Kiyosawa M, Shimada J, et al. Carbon-11-labeled KF21213: a highly selective ligand for mapping CNS adenosine A(2A) receptors with positron emission tomography. Nucl Med Biol. 2000;27:541–546. doi: 10.1016/s0969-8051(00)00126-8. [DOI] [PubMed] [Google Scholar]
- 79.Wardas J, Konieczny J, Lorenc-Koci E. SCH 58261, an A(2A) adenosine receptor antagonist, counteracts parkinsonian-like muscle rigidity in rats. Synapse. 2001;41:160–171. doi: 10.1002/syn.1070. [DOI] [PubMed] [Google Scholar]
- 80.Wichmann T, Kliem MA, DeLong MR. Antiparkinsonian and behavioral effects of inactivation of the substantia nigra pars reticulata in hemiparkinsonian primates. Exp Neurol. 2001;167:410–424. doi: 10.1006/exnr.2000.7572. [DOI] [PubMed] [Google Scholar]
- 81.Wisniecki A, Correa M, Arizzi MN, Ishiwari K, Salamone JD. Motor effects of GABAA antagonism in globus palidus: studies of locomotion and tremulous jaw movements. Psychopharmacology. 2003;170:140–149. doi: 10.1007/s00213-003-1521-z. [DOI] [PubMed] [Google Scholar]
- 82.Young AB, Penney JB. Biochemical and functional organization of the basal ganglia. In: Jankowic J, Tolosa E, editors. Parkinson’s disease and movement disorders. Baltimore: Williams and Wilkins; 1993. pp. 1–12. [Google Scholar]