Abstract
A potent 5-hydroxytryptamine (5-HT)2A receptor inverse agonist and antagonist, ACP-103 [N-(4-fluorophenylmethyl)-N-(1-methylpiperidin-4-yl)-N'-(4-(2-methylpropyloxy)phenylmethyl) carbamide (2R,3R)-dihydroxybutanedioate (2:1, active:salt)], was evaluated for its ability to reduce the primary motor symptom of tremor using tacrine-induced tremulous jaw movements in rats, which is an animal model of parkinsonian tremor. Furthermore, ACP-103 was evaluated for its ability to reduce levodopa-induced dyskinesias in monkeys rendered parkinsonian with MPTP [1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine]. ACP-103 reduced tacrine-induced tremulous jaw movements in rats. In addition, ACP-103 administered in combination with levodopa caused a dose-related reduction in dyskinesias in monkeys. These data suggest that ACP-103 may have the potential to reduce tremor and levodopa-induced dyskinesias in Parkinson's disease.
Keywords: Serotonin, 5-HT2A, Parkinson's disease, Rodent, Primate, Animal model, Dyskinesia, Tremor
1. Introduction
Parkinson's disease results from a loss of dopaminergic neurons in the substantia nigra pars compacta, and is characterized by a core set of neurological symptoms including tremor, bradykinesia, rigidity, and balance or posture disturbances (Obeso et al., 2000; Olanow and Koller, 1998). In addition to the primary motor disturbances, a significant percentage of Parkinson's disease patients can develop the disabling side effect of dyskinesias in response to chronic levodopa treatment (Brotchie, 2005; Jankovic, 2005). Intermittent, rather than continuous, exposure to levodopa is thought to mediate alterations in the motor response, such as wearing-off, on–off fluctuations, and involuntary movements (Chase and Oh, 2000). Activation of the medium spiny neurons in the dorsolateral striatum that project to the substantia nigra pars reticulata results in disinhibition of thalamocortical neurons and increased motor activity (Borgkvist and Fisone, 2007; Obeso et al., 2000; Olanow and Koller,1998). Overactivity of this “direct” striatal pathway may contribute to the expression of dyskinesias (Brotchie, 2005; Carta et al., 2005; Henry et al., 2003).
Serotonin (5-hyrdoxytryptamine; 5-HT) 5-HT2A receptors are localized in the lateral caudate–putamen nucleus, suggesting a major role for 5-HT2A receptors in motor function (Rodriguez et al., 1999). Interestingly, blockade of 5-HT2 family receptors, including 5-HT2A receptors, has been shown to affect motor functions related to parkinsonism. The 5-HT2A/C receptor antagonist mianserin was shown to be effective in reducing parkinsonian symptoms in haloperidol-induced monkeys (Korsgaard and Friis, 1986), and in human parkinsonian patients (Ikeguchi and Kuroda 1995). Ritanserin, another 5-HT2A receptor antagonist, improved motor function in patients with tremor-dominant Parkinson's disease (Auff et al., 1987). The atypical antipsychotic clozapine, which is known to bind to 5-HT2A receptors and act as an antagonist/inverse agonist (Richelson and Souder, 2000; Weiner et al., 2001), was shown to be effective at ameliorating tremor and other motor dysfunctions in patients with idiopathic Parkinson's disease (Pakkenberg and Pakkenberg, 1986; Bernardi and Del Zompo, 1990; Fisher et al., 1990; Friedman and Lannon, 1990; Arevalo and Gershanik, 1993; see review by Factor and Friedman, 1997). Ritanserin (Meco et al., 1988) and low dose clozapine (Durif et al., 2004; Pierelli et al., 1998) also reduced dyskinesias in patients with Parkinson's disease.
Levodopa-induced dyskinesias in MPTP-treated monkeys are a widely used animal model for levodopa-induced dyskinesias in humans (Jenner, 2003). The atypical antipsychotics with potent 5-HT2A receptor antagonism and inverse agonism (i.e., quetiapine and clozapine) have been shown to reduce levodopa-induced dyskinesias in this model (Baron and Dalton, 2003; Durif et al., 2004; Oh et al., 2002; Weiner et al., 2001). Tacrine-induced tremulous jaw movements in rats can be used as an experimental model of parkinsonian tremor (Cousins et al., 1997; Mayorga et al., 1997; Salamone et al., 1998,Salamone et al., 2005). The jaw movements induced by the cholinesterase inhibitor are in the same 3–7 Hz frequency range as the resting tremor common in Parkinson's disease (Mayorga et al., 1997; Cousins et al., 1999). Tremulous jaw movements have been used as a rodent model for assessing antiparkinsonian drugs with various pharmacological profiles, including dopamine agonists, muscarinic antagonists, and adenosine A2A antagonists (Cousins et al., 1997; Correa et al., 2004; Salamone et al.,1998,2005; Simola et al., 2004; Betz et al., 2007; Salamone et al., 2008). Several studies have shown that serotonergic mechanisms are involved in the regulation of tremulous jaw movements. 5-HT1A agonists have been shown to suppress tacrine-induced tremulous jaw movements (Zazpe et al., 2006). Atypical antipsychotics that act on 5-HT receptors, including clozapine, olanzapine and quetiapine, have all been reported to block tremulous jaw movements induced by pilocarpine, physostigmine or tacrine (Chesler and Salamone, 1996; Stewart et al., 1988; Trevitt et al., 1997,Trevitt et al., 1999; Betz et al., 2005; Zazpe et al., 2006). Similarly, several 5-HT2 receptor family antagonists, including seganserin, ritanserin and ketanserin, have been reported to decrease haloperidol-induced vacuous chewing in rats (Naidu and Kulkarni, 2001).Systemic and intracranial injections of the 5-HT2 receptor antagonist mianserin also were shown to reduce tacrine-induced tremulous jaw movements (Carlson et al., 2003). Taken together, these data suggest that tacrine-induced tremulous jaw movements are an appropriate animal model for the assessment of parkinsonian tremor, and that this model is sensitive to pharmacological manipulation, including sensitivity to 5-HT2 receptor family antagonism.
ACP-103 [N-(4-fluorophenylmethyl)-N-(1-methylpiperidin-4-yl)-N'-(4-(2-methylpropyloxy)phenylmethyl)carbamide (2R,3R)-dihydroxybutanedioate (2:1)] is a potent 5-HT2A receptor inverse agonist that can antagonize serotonin agonists at 5-HT2A receptors (Vanover et al., 2006). Moreover, ACP-103 is highly selective for 5-HT2A receptors, lacking affinity for other receptors in a broad profile screen including 65 different molecular targets; the only other receptor for which ACP-103 demonstrates affinity is 5-HT2C, and ACP-103 is approximately 30-fold selective for 5-HT2A receptors over 5-HT2C receptors depending on the assay (Vanover et al., 2006). ACP-103 is being developed as a therapy for treatment-induced dysfunction in Parkinson's disease and other neuropsychiatric disorders. The purpose of the present study was to evaluate whether ACP-103 might improve tremor and levodopa-induced dyskinesias in animal models.
2. Methods
2.1. Subjects
All experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals. Male Sprague–Dawley rats (Harlan Sprague Dawley, Indianapolis, Ind., USA; n=11) were used in the tremulous jaw movement (TJM) experiments. The rats weighed 290–350 g at the beginning of the experiment, and had ad libitum access to water and standard lab chow. All rats were group housed in a colony maintained at approximately 23 °C, with a 12-h light/dark cycle. Five cynomolgus monkeys weighing between 3.5 and 7.5kg were used for the dyskinesia experiment. These monkeys were housed individually, under stable room conditions, with a 12-h light/dark cycle. All were fed a standard biscuit diet twice daily supplemented with fruit and had ad libitum access to water.
2.2. Procedures
Observations of tremulous jaw movements in rats were made in a 27×17.5×17 cm clear Plexiglas chamber with a wire mesh floor. Tremulous jaw movements were defined as rapid vertical deflections of the lower jaw that resembled chewing but were not directed at any particular stimulus (Salamone et al., 1998). Each individual deflection of the jaw was recorded using a mechanical hand counter. Jaw movements were recorded by an observer who was unaware of the experimental treatment conditions, and the observer was trained to demonstrate inter-rater reliability with a second observer over a number of pilot test sessions (r=0.92; P<0.05). To induce tremulous jaw movements, each rat received an i.p. injection of 5.0 mg/kg of the anticholinesterase tacrine 10 min before testing. Rats were placed in the observation chamber immediately after tacrine injection for a 10-min habituation period. The rats subsequently were observed for tremulous jaw movements during a 5-min session. The effects of ACP-103 (5.0–40.0 mg/kg, i.p., 20 min before tacrine; n = 11) on tacrine-induced tremulous jaw movements were evaluated. Rats were tested once a week for 5 weeks, during the light phase of the light/dark cycle. Over the course of the experiment, each rat received all treatments in a randomly varied order. Consistent with earlier studies, vehicle levels of tremulous jaw movement activity were consistent across the repeated weeks of the study.
For the dyskinesia experiment, each monkey was injected subcutaneously with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) once a week at a dose of 0.5–1 mg/kg until definite parkinsonian features, including tremor, appeared (scores of 4–5 points on a Disability Scale where the normal state extends from 0–2 points and maximum disability is 10 points) (Bibbiani et al., 2001). The Disability Scale included observational assessments of posture, mobility, climbing, gait, eating, social interactions, grooming, and tremor. Due to individual variability in response, the average cumulative MPTP dose was calculated. The average cumulative MPTP dose was 4.1 mg/kg (range 2.0–8.35) to induce parkinsonism. Following observation for two additional months to ensure stable disability, monkeys that had equal parkinsonian severity (disability score of 4.5 points) were selected for study.
Monkeys were treated with subcutaneous doses of levodopa until discernable dyskinesias appeared; subsequently, a “dyskinetic” dose of levodopa was individually determined for each animal (mean dose 22.6 mg/kg; range 17.1 mg/kg–29 mg/kg) and administered three times a week to maintain dyskinesias. Levodopa was dissolved in saline together with the peripheral decarboxylase inhibitor benser-azide at a dose of 15 mg/kg (mean total dose 75.6 mg; range 48 mg–112.5). On test days, monkeys were transferred to an observation room and allowed to habituate to their environment before testing. ACP-103 (0.6–1.8 mg/kg, s.c.) or vehicle was administered to the monkeys and both the severity of dyskinesias and the parkinsonian symptoms were scored using the Laval scales for motor disability and involuntary movements (Bibbiani et al., 2001). Observations began immediately after injection of ACP-103 and continued for 3 h or until there was a return to baseline dyskinesia scores. Doses of ACP-103 were administered to different monkeys in a mixed, pseudo-random order. Testing with ACP-103 occurred no more than twice weekly with at least 3 days between tests. Monkeys were only tested with ACP-103 when their levels of dyskinesias had returned to baseline levels.
2.3. Drugs
ACP-103 was synthesized by ACADIA Pharmaceuticals. For the tremor experiment, ACP-103 was dissolved in a mixture of 9:1 mixture of 0.9% saline and 10% Tween 80 (Fisher Scientific; Hampton, NH), and this solution also served as the vehicle control. Tacrine (Sigma-Aldrich; St. Louis, MO) was dissolved in 0.9% saline. For the dyskinesia experiment, ACP-103 was dissolved in 0.9% saline,1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) HCl (Research Biochemicals Intl.; Natick, MA) and levodopa (Sigma-Aldrich; St. Louis, MO) were dissolved in 0.9% saline.
2.4. Data analysis
TJM data (i.e., total number of movements in the 5-min session) and dyskinesia data (i.e., severity score) were analyzed using repeated-measures analysis of variance (ANOVA), with dose as the repeated measure. Planned comparisons using the overall error term from the ANOVA were used to assess the differences between each combined drug condition versus the tacrine plus vehicle control condition, keeping the total number of comparisons to the number of conditions minus one (Keppel, 1991).
3. Results
3.1. Tremulous jaw movements in rats
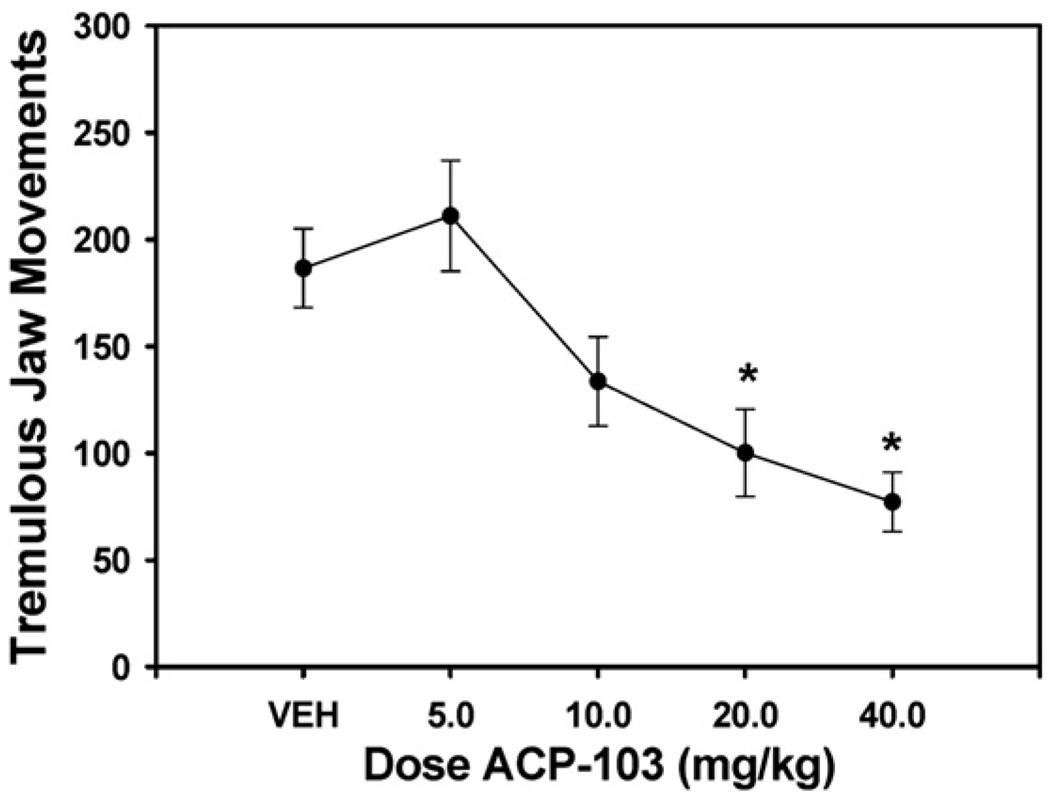
As shown in Fig. 1, systemic injections of ACP-103 produced a significant overall reduction of tacrine-induced tremulous jaw movements [n=11; F(4,40)=7.16, P<0.01; see Fig. 1]. Planned comparisons showed that the 20.0 and 40.0 mg/kg doses of ACP-103 co-administered with tacrine significantly reduced tremulous jaw movements relative to the tacrine plus vehicle control condition (20.0 mg/kg, F(1,40)=8.38, P<0.05; 40.0 mg/kg, F(1,40)=13.4, P<0.05).
Fig. 1.
Anti-tremor effect of ACP-103. The number of tremulous jaw movements is shown as a function of ACP-103 dose in combination with tacrine. Mean (±SEM) number of tremulous jaw movements (n =11) is shown. Statistical difference from tacrine alone (P<0.05) is indicated by asterisks.
3.2. Dyskinesia in monkeys
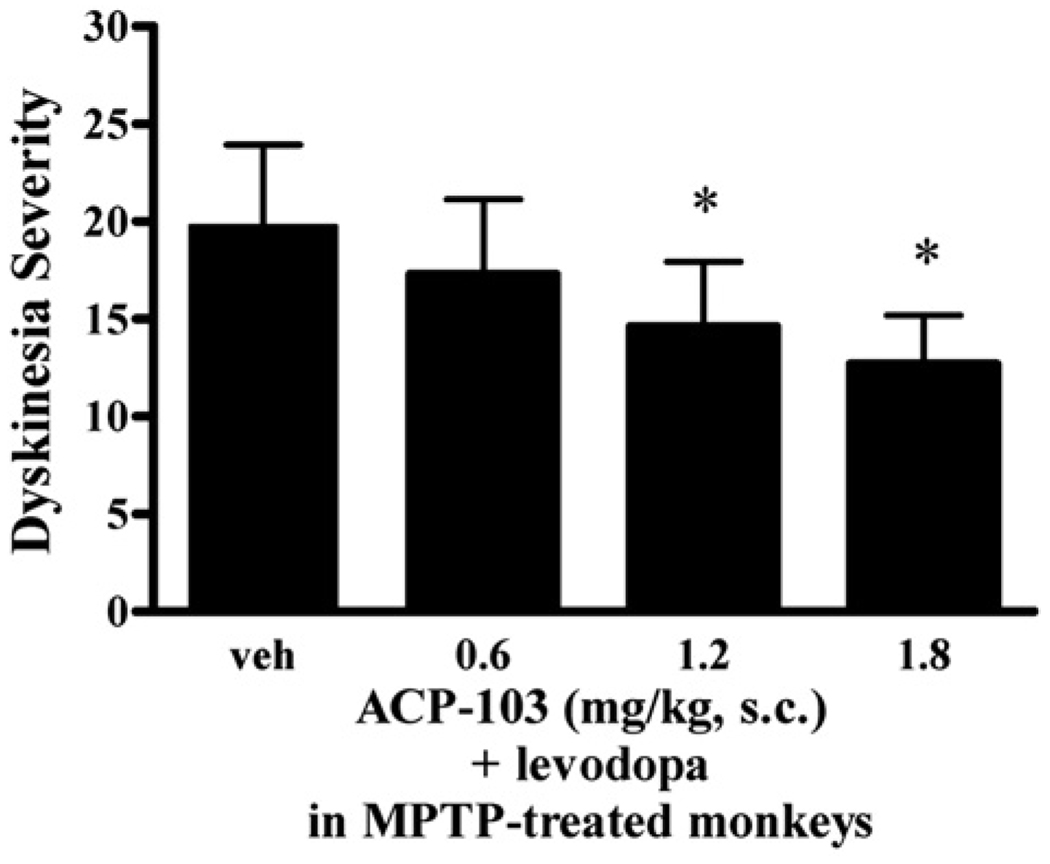
ACP-103 was administered alone and in combination with a dyskinetic dose of levodopa. In combination with levodopa, ACP-103 caused a dose-related reduction in dyskinesias [F(3,19)=14.50, P=0.0003; Fig. 2]. The low dose of ACP-103 (0.6 mg/kg) reduced levodopa-induced dyskinesias by 12%, the intermediate dose (1.2 mg/kg) by 26% and the high dose (1.8 mg/kg) by 36%. Doses of 1.2 and 1.8 mg/kg were statistically different from vehicle (P<0.01). ACP-103 did not show any side effects, such as somnolence, ataxia, changes in demeanor, or other overt behavioral signs at the selected doses. When given alone, no effect (less than a 2-point average change on the Disability Scale) was noted on the parkinsonian symptoms as assessed by total score on the Disability Scale assessing posture, mobility, climbing, gait, eating, social interactions, grooming and tremor. When administered in combination of levodopa, ACP-103 did not affect the antiparkinsonian response; ACP-103 caused less than a 2-point average change in levodopa-induced reduction in parkinsonian symptoms. Anything less than a 2-point change in the total Disability score is not considered clinically meaningful (Bibbiani et al., 2001).
Fig. 2.
Anti-dyskinetic effect of ACP-103. Dyskinesia severity score is shown as a function of ACP-103 dose in combination with levodopa in MPTP-treated monkeys. The means and standard errors of the means for each group (n=5) are shown. Statistical difference from vehicle (P<0.01) is indicated by asterisks.
4. Discussion
The 5-HT2A receptor inverse agonist/antagonist ACP-103 reduced tacrine-induced tremulous jaw movements in rats. This finding is consistent with previous studies showing that other drugs that act as 5-HT2A receptor inverse agonist/antagonists, including clozapine, olanza-pine, quetiapine and mianserin, also could reduce tacrine-induced tremulous jaw movements (Chesler and Salamone, 1996; Trevitt et al., 1997,Trevitt et al., 1999; Carlson et al., 2003). These data suggest that 5-HT2A receptor inverse agonists and antagonists may have potential for reducing resting tremor, a common motor symptom in Parkinson's disease. ACP-103 reduced levodopa-induced dyskinesias in MPTP-treated monkeys, consistent with an anti-dyskinetic efficacy in Parkinson's disease. It should be noted that a lack of effect on tremor was observed in the primates. However, baseline levels of tremor were not optimized in primates to be able to measure pharmacological change in this experiment, and the overall Disability Scale included only a crude observational assessment of tremor as present or absent in the context of 8 different disability features being measured. Additional experiments are warranted to determine if ACP-103 reduces tremor in primates as is suggested by the rodent tremulous jaw model.
ACP-103 exhibits effects similar to antipsychotic drugs in rodent models at lower doses (Vanover et al., 2006) than those shown to have an anti-tremor effect in the present study. It is unclear whether ACP-103 is more potent in producing effects similar to antipsychotic drugs than in reducing tremor, or if the differing experimental conditions across these studies make it difficult to permit direct potency comparisons. One hypothesis is that underlying dopaminergic tone may influence potency of 5-HT2A receptor pharmacological manipulations and the different experimental conditions may involve different levels of underlying dopaminergic tone. Interestingly, Bishop et al. (2004) suggested that 5-HT2A receptor mediated signaling in the striatum is stronger under conditions of dopamine depletion. Therefore, dopaminergic tone may have influenced the potency of ACP-103 across experimental conditions. If this is true across species, ACP-103 may exhibit different potencies in patients with Parkinson's disease than in patients with other disorders, such as schizophrenia. On the other hand, other differences in experimental conditions may explain differences in behavioral potencies. For example, route of administration, pretreatment times, vehicles used for dilutions, species and strain of animals, and housing conditions can influence behavioral results. In the present experiment with the primates, the individually titrated levodopa doses may have been more sensitive to pharmacological manipulation than other animal models that use a consistent high dose that might require a higher dose to overcome. Lastly, ACP-103 is highly selective for 5-HT2A receptors at low doses, but interactions at 5-HT2C receptors at high doses cannot be excluded. Therefore, some behavioral effects that require higher doses of ACP-103 may require some 5-HT2C receptor antagonism.
Striatal medium spiny neurons are thought to contribute to the pathogenesis of parkinsonism and levodopa-induced dyskinesias (Chase and Oh, 2000). It has been shown that agonist action at the pre-synaptic 5-HT1A autoreceptors can attenuate levodopa-stimulated dopaminergic neurotransmission in the striatum (Kannari et al., 2001) and reduce dyskinesias in animal models (Bibbiani et al., 2001) and in patients with Parkinson's disease (Bara-Jimenez et al., 2005). The 5-HT1A receptor agonist, 8-OH-DPAT, has been reported to decrease haloperidol-induced motoric behavioral effects such as tongue thrusts and head shakes in rats (Naidu and Kulkarni, 2001). 5-HT1A receptors and 5-HT2A receptors can be highly co-localized and appear to function in opposite directions, such that a post-synaptic 5-HT2A receptor antagonist can have neurochemical and behavioral effects similar to pre-synaptic 5-HT1A receptor agonists (Amargós-Bosch et al., 2004; Fink and Göthert, 2007). Thus, it is reasonable to suggest that post-synaptic or heterosynaptic antagonism of 5-HT2A receptors in the striatum may mediate the reduction of dyskinesias by ACP-103. Consistent with this hypothesis, ACP-103 has been shown to antagonize 5-HT2A receptor agonist induced behaviors, consistent with a post-synaptic 5-HT2A receptor antagonist effect in vivo (Vanover et al., 2006).
Tremulous jaw movements (Salamone et al., 1998) and rhythmic motor output such as tremor in Parkinson's disease (Buzsaki et al., 1990) are dependent upon striatal mechanisms, and also upon substantia nigra pars reticulata, which is a critical basal ganglia output area. The rank order binding potency of antipsychotics for blocking tremulous jaw movements (i.e., risperidone, olanzapine, clozapine, quetiapine, thioridazine; Trevitt et al., 1997,Trevitt et al., 1999; Betz et al., 2005) is consistent with the rank order of affinity of these drugs for binding to 5-HT2A receptors (Richelson and Souder, 2000; Seeman et al., 1997; Weiner et al., 2001), suggesting that 5-HT2A receptors mediate this behavioral effect. This observation is consistent with the present data showing that ACP-103 could suppress tacrine-induced tremulous jaw movements. Furthermore, there is additional evidence for a role of serotonin in tremor; the 5-HT2 receptor antagonist mianserin injected directly into the substantia nigra pars reticulata, but not into the ventrolateral striatum, blocked tremulous jaw movements in rats (Carlson et al., 2003). Given the high expression of 5-HT2A receptors in the dorsolateral caudate–putamen and substantia nigra pars reticulata (Bubser et al., 2001; Cornea-Hebert et al., 1999; Dwivedi and Pandey, 1998; Hamada et al., 1998; Rodriguez et al., 1999) and the role that these areas play in mediating levodopa-induced dyskinesias (Brotchie, 2005) and cholinomimetic-stimulated tremor (Carlson et al., 2003), it is possible that the anti-tremor and anti-dyskinetic effects of ACP-103 involve actions upon one or both of these structures.
Acknowledgement
This research was partially supported by a grant to JDS from the United States NIH/NINDS.
References
- Amargós-Bosch M, Bortolozzi A, Puig MV, Serrats J, Adell A, Celada P, et al. Co-expression and in vivo interaction of serotonin1A and serotonin2A receptors in pyramidal neurons of prefrontal cortex. Cereb Cortex. 2004 Mar;14(3):281–299. doi: 10.1093/cercor/bhg128. [DOI] [PubMed] [Google Scholar]
- Arevalo GJ, Gershanik OS. Modulatory effect of clozapine on levodopa response in Parkinson's disease: a preliminary study. Mov Dis. 1993;8:349–354. doi: 10.1002/mds.870080317. [DOI] [PubMed] [Google Scholar]
- Auff E, Birkmayer W, Brücke T, Deecke L, Emich C, Goldenberg G, et al. Ritanserin in the treatment of tremor-dominant Parkinson's disease: a preliminary study. New Trends Clin Neuropharmacol. 1987;1:149–158. [Google Scholar]
- Bara-Jimenez W, Bibbiani F, Morris MJ, Dimitrova T, Sherzai A, Mouradian MM, et al. Effects of serotonin 5-HT1A agonist in advanced Parkinson's disease. Mov Disord. 2005;20:932–936. doi: 10.1002/mds.20370. [DOI] [PubMed] [Google Scholar]
- Baron MS, Dalton WB. Quetiapine as treatment for dopaminergic-induced dyskinesias in Parkinson's disease. Mov Disord. 2003;18:1208–1209. doi: 10.1002/mds.10551. [DOI] [PubMed] [Google Scholar]
- Bernardi F, Del Zompo M. Clozapine in idiopathic Parkinson's disease. Neurology. 1990;40:1151. doi: 10.1212/wnl.40.7.1151. [DOI] [PubMed] [Google Scholar]
- Betz A, Ishiwari K, Wisniecki A, Huyn N, Salamone JD. Quetiapine (Seroquel) shows a pattern of behavioral effects similar to the atypical antipsychotics clozapine and olanzapine: studies with tremulous jaw movements in rats. Psychopharmacology. 2005;179:383–392. doi: 10.1007/s00213-004-2046-9. [DOI] [PubMed] [Google Scholar]
- Betz AJ, McLaughlin PJ, Burgos M, Weber SM, Salamone JD. The muscarinic receptor antagonist tropicamide suppresses tremulous jaw movements in a rodent model of parkinsonian tremor: possible role of M4 receptors. Psychopharmacology. 2007;194:347–359. doi: 10.1007/s00213-007-0844-6. [DOI] [PubMed] [Google Scholar]
- Bibbiani F, Oh JD, Chase TN. Serotonin 5-HT1A agonist improves motor complications in rodent and primate parkinsonian models. Neurology. 2001;57:829–1834. doi: 10.1212/wnl.57.10.1829. [DOI] [PubMed] [Google Scholar]
- Bishop C, Tessmer JL, Ullrich T, Rice KC, Walker PD. Serotonin 5-HT2A receptors underlie increased motor behaviors induced in dopamine-depleted rats by intrastriatal 5-HT2A/2C agonism. J Pharmacol Exp Ther. 2004;310:687–694. doi: 10.1124/jpet.104.066365. [DOI] [PubMed] [Google Scholar]
- Borgkvist A, Fisone G. Psychoactive drugs and regulation of cAMP/PKA/DARPP-32 cascade in striatal medium spiny neurons. Neurosci Biobehav Rev. 2007;31:79–88. doi: 10.1016/j.neubiorev.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Brotchie JM. Nondopaminergic mechanisms in levodopa-induced dyskinesia. Mov Disord. 2005;20:919–931. doi: 10.1002/mds.20612. [DOI] [PubMed] [Google Scholar]
- Bubser M, Backstrom JR, Sanders-Bush E, Roth BL, Deutch AY. Distribution of serotonin 5-HT(2A) receptors in afferents of the rat striatum. Synapse. 2001;39:297–304. doi: 10.1002/1098-2396(20010315)39:4<297::AID-SYN1012>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Smith A, Berger S, Fisher LJ, Gage FH. Petit mal epilepsy and parkinsonian tremor: hypothesis of a common pacemaker. Neuroscience. 1990;36:1–14. doi: 10.1016/0306-4522(90)90345-5. [DOI] [PubMed] [Google Scholar]
- Carlson BB, Wisniecki A, Salamone JD. Local injections of the 5-hydroxytryptamine antagonist mianserin into substantia nigra pars reticulata block tremulous jaw movements in rats: studies with a putative model of Parkinsonian tremor. Psychopharmacology. 2003;165:229–237. doi: 10.1007/s00213-002-1247-3. [DOI] [PubMed] [Google Scholar]
- Carta AR, Tronci E, Pinna A, Morelli M. Different responsiveness of striatonigral and striatopallidal neurons to l-DOPA after a subchronic intermittent l-DOPA treatment. Eur J Neurosci. 2005;21:1196–1204. doi: 10.1111/j.1460-9568.2005.03944.x. [DOI] [PubMed] [Google Scholar]
- Chase TN, Oh JD. Striatal mechanisms and pathogenesis of parkinsonian signs and motor complications. Ann Neurol. 2000;47 Suppl:122–129. [PubMed] [Google Scholar]
- Chesler EJ, Salamone JD. Effects of acute and repeated clozapine injections on cholinomimetic-induced vacuous jaw movements. Pharmacol Biochem Behav. 1996;54:619–624. doi: 10.1016/0091-3057(95)02280-5. [DOI] [PubMed] [Google Scholar]
- Cornea-Hebert V, Riad M, Wu C, Singh SK, Descarries L. Cellularand subcellular distribution of the serotonin 5-HT2A receptor in the central nervous system of adult rat. J Comp Neurol. 1999;409:187–209. doi: 10.1002/(sici)1096-9861(19990628)409:2<187::aid-cne2>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Correa M, Wisniecki A, Betz A, Dobson DR, O'Neill MF, O'Neill MJ, et al. The adenosine A2A antagonist KF 17837 reverses the locomotor suppression and tremulous jaw movements induced by haloperidol in rats: possible relevance to parkinsonism. Behav Brain Res. 2004;148:47–54. doi: 10.1016/s0166-4328(03)00178-5. [DOI] [PubMed] [Google Scholar]
- Cousins MS, Carriero DL, Salamone JD. Tremulous jaw movements induced by the acetylcholinesterase inhibitor tacrine: effects of antiparkinsonian drugs. Eur J Pharmacol. 1997;322:137–145. doi: 10.1016/s0014-2999(97)00008-3. [DOI] [PubMed] [Google Scholar]
- Cousins MS, Finn MF, Carriero DL, Conlan A, Salamone JD. The role of ventrolateral striatal acetylcholine in the production of tacrine-induced tremulous jaw movements: a neurochemical and behavioral study. Pharmacol Biochem Behav. 1999;62:439–447. doi: 10.1016/s0091-3057(98)00214-7. [DOI] [PubMed] [Google Scholar]
- Durif F, Debilly B, Galitzky M, Morand D, Viallet F, Borg M, et al. Clozapine improves dyskinesias in Parkinson's disease. Neurology. 2004;62:381–388. doi: 10.1212/01.wnl.0000110317.52453.6c. [DOI] [PubMed] [Google Scholar]
- Dwivedi Y, Pandey GN. Quantitation of 5-HT2A receptor mRNA in human postmortem brain using competitive RT-PCR. NeuroReport. 1998;9:3761–3765. doi: 10.1097/00001756-199812010-00001. [DOI] [PubMed] [Google Scholar]
- Factor SA, Friedman JH. The emerging role of clozapine in the treatment of movement disorders. Mov Dis. 1997;12:483–496. doi: 10.1002/mds.870120403. [DOI] [PubMed] [Google Scholar]
- Fink KB, Göthert M. 5-HT receptor regulation of neurotransmitter release. Pharmacol Rev. 2007 Nov 30; doi: 10.1124/pr.107.07103. [electronic publication] [DOI] [PubMed] [Google Scholar]
- Fisher PA, Baas H, Hefner R. Treatment of parkinsonian tremor with clozapine. J Neurotrans. 1990;2:233–238. doi: 10.1007/BF02257654. [DOI] [PubMed] [Google Scholar]
- Friedman JH, Lannon MC. Clozapine-responsive tremor in Parkinson's disease. Mov Dis. 1990;5:225–229. doi: 10.1002/mds.870050307. [DOI] [PubMed] [Google Scholar]
- Hamada S, Senzaki K, Hamaguchi-Hamada K, Tabuchi K, Yamamoto H, Yamamoto T, et al. Localization of 5-HT2A receptor in rat cerebral cortex and olfactory system revealed by immunohistochemistry using two antibodies raised in rabbit and chicken. Brain Res Mol Brain Res. 1998;54:199–211. doi: 10.1016/s0169-328x(97)00322-7. [DOI] [PubMed] [Google Scholar]
- Henry B, Duty S, Fox SH, Crossman AR, Brotchie JM. Increased striatal pre-proenkephalin B expression is associated with dyskinesia in Parkinson's disease. Exp Neurol. 2003;183:458–468. doi: 10.1016/s0014-4886(03)00064-5. [DOI] [PubMed] [Google Scholar]
- Ikeguchi K, Kuroda A. Mianserin treatment of patients with psychosis induced by antiparkinsonian drugs. Eur Arch Psychiatry Clin Neurosci. 1995;244:320–324. doi: 10.1007/BF02190411. [DOI] [PubMed] [Google Scholar]
- Jankovic J. Motor fluctuations and dyskinesias in Parkinson's disease: clinical manifestations. Mov Disord. 2005;20 Suppl11:S11–S16. doi: 10.1002/mds.20458. [DOI] [PubMed] [Google Scholar]
- Jenner P. The MPTP-treated primate as a model of motor complications in PD. Neurology. 2003;61 Suppl3:S4–S11. doi: 10.1212/wnl.61.6_suppl_3.s4. [DOI] [PubMed] [Google Scholar]
- Kannari K, Yamoto H, Shen H, Tomiyama M, Suda T, Matsunaga M. Activation of 5-HT (1A) but not 5-HT(1B) receptors attenuates an increase in extracellular dopamine derived from exogenously administered l-DOPA in the striatum with nigrostriatal denervation. J Neurochem. 2001;76:1346–1353. doi: 10.1046/j.1471-4159.2001.00184.x. [DOI] [PubMed] [Google Scholar]
- Keppel G. Design and analysis: a researcher's handbook. Prentice Hall. 1991 Prentice Hall. [Google Scholar]
- Korsgaard S, Friis T. Effects of mianserin in neuroleptic-induced parkinsonism. Psycho-pharmacology. 1986;88:109–111. doi: 10.1007/BF00310523. [DOI] [PubMed] [Google Scholar]
- Mayorga AJ, Carriero DL, Cousins MS, Gianutsos G, Salamone JD. Tremulous jaw movements produced by acute tacrine administration: possible relation to Parkinsonian side effects. Pharmacol Biochem Behav. 1997;56:273–279. doi: 10.1016/s0091-3057(96)00225-0. [DOI] [PubMed] [Google Scholar]
- Meco G, Marini S, Lestingi L, Linfante I, Modarelli FT, Agnoli A. Controlled single-blind crossover study of ritanserin and placebo in L-dopa-induced dyskinesias in Parkinson's disease. Curr Ther Res. 1988;43:262–270. [Google Scholar]
- Naidu PS, Kulkarni SK. Effect of 5-HT1A and 5-HT2A/2C receptor modulation on neuroleptic-induced vacuous chewing movements. Eur J Pharmacol. 2001;428:81–86. doi: 10.1016/s0014-2999(01)01284-5. [DOI] [PubMed] [Google Scholar]
- Obeso JA, Rodríguez-Oroz MC, Rodríguez M, Lanciego JL, Artieda J, Gonzalo N, et al. Pathophysiology of the basal ganglia in Parkinson's disease. Trends Neurosci. 2000;23(10 Suppl):S8–S19. doi: 10.1016/s1471-1931(00)00028-8. [DOI] [PubMed] [Google Scholar]
- Oh JD, Bibbiani F, Chase TN. Quetiapine attenuates levodopa-induced motor complications in rodent and primate Parkinsonian models. Exp Neurol. 2002;177:557–564. doi: 10.1006/exnr.2002.8009. [DOI] [PubMed] [Google Scholar]
- Olanow CW, Koller WC. An algorithm (decision tree) for the management of Parkinson's disease: treatment guidelines. Neurology. 1998;50:S1. doi: 10.1212/wnl.50.3_suppl_3.s1. [DOI] [PubMed] [Google Scholar]
- Pakkenberg H, Pakkenberg B. Clozapine in the treatment of tremor. Acta Neurol Scand. 1986;73:295–297. doi: 10.1111/j.1600-0404.1986.tb03279.x. [DOI] [PubMed] [Google Scholar]
- Pierelli F, Adipietro A, Soldati G, Fattapposta F, Pozzessere G, Scoppetta C. Low dosage clozapine effects on L-dopa induced dyskinesias in parkinsonian patients. Acta Neurol Scand. 1998;97:295–299. doi: 10.1111/j.1600-0404.1998.tb05955.x. [DOI] [PubMed] [Google Scholar]
- Richelson E, Souder T. Binding of antipsychotic drugs to human brain receptors focus on newer generation compounds. Life Sci. 2000;68:29–39. doi: 10.1016/s0024-3205(00)00911-5. [DOI] [PubMed] [Google Scholar]
- Rodriguez JJ, Garcia DR, Pickel VM. Subcellular distribution of 5-hydroxytryptamine2Aand N-methyl-D-aspartate receptors within single neurons in rat motor and limbic striatum. J Comp Neurol. 1999;413:219–231. doi: 10.1002/(sici)1096-9861(19991018)413:2<219::aid-cne4>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Mayorga AJ, Trevitt JT, Cousins MS, Conlan A, Nawab A. Tremulous jaw movements in rats:a model of Parkinsonian tremor. Prog Neurobiol. 1998;56:591–611. doi: 10.1016/s0301-0082(98)00053-7. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Carlson BB, Rios C, Lentini E, Correa M, Wisniecki A, et al. Dopamine agonists suppress cholinomimetic-induced tremulous jaw movements in an animal model of parkinsonism: tremorolytic effects of pergolide, ropinirole and CY 208–243. Behav Brain Res. 2005;156:173–179. doi: 10.1016/j.bbr.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Betz AJ, Ishiwari K, Felsted J, Madson L, Mirante B, et al. Tremorolytic effects of adenosine A2A antagonists: implications for parkinsonism. Front Biosci. 2008;13:3594–3605. doi: 10.2741/2952. [DOI] [PubMed] [Google Scholar]
- Seeman P, Tallerico T, Corbett R, Van Tol HH, Kamboj RK. Role of dopamine D2, D4 and serotonin(2A) receptors in antipsychotic and anticataleptic action. J Psychophar-macol. 1997;11:15–17. doi: 10.1177/026988119701100104. [DOI] [PubMed] [Google Scholar]
- Simola N, Fenu S, Baraldi PG, Tabrizi MA, Morelli M. Blockade of adenosine A2A receptors antagonizes parkinsonian tremor in the rat tacrine model by an action on specific striatal regions. Exp Neurol. 2004;189:182–188. doi: 10.1016/j.expneurol.2004.05.027. [DOI] [PubMed] [Google Scholar]
- Stewart BR, Jenner P, Marsden CD. The pharmacological characterization of pilocarpine-induced chewing in the rat. Psychopharmacology. 1988;96:55–62. doi: 10.1007/BF02431533. [DOI] [PubMed] [Google Scholar]
- Trevitt JT, Lyons M, Aberman J, Carriero D, Finn M, Salamone JD. Effects of clozapine, thioridazine, risperidone and haloperidol on behavioral tests related to extrapyr-amidal motor function. Psychopharmacology. 1997;132:74–81. doi: 10.1007/s002130050322. [DOI] [PubMed] [Google Scholar]
- Trevitt JT, Carlson BB, Salamone JD. Behavioral assessment of atypical antipsychotics inrats: studies of the effects of olanzapine (Zyprexa) Psychopharmacology. 1999;145:309–316. doi: 10.1007/s002130051063. [DOI] [PubMed] [Google Scholar]
- Vanover KE, Weiner DM, Makhay, Veinbergs I, Gardell LR, Lameh J, et al. Pharmacological and behavioral profile of N-(4-fluorophenylmethyl)-N-(1-methylpiperidin-4-yl)-N′-(4-(2-methylpropyloxy)phenylmethyl)carbamide (2R,3R)-dihydroxybutanedioate (2:1) (ACP-103), a novel 5-HT2A receptor inverse agonist. J Pharmacol Exp Ther. 2006;317:910–918. doi: 10.1124/jpet.105.097006. [DOI] [PubMed] [Google Scholar]
- Weiner DM, Burstein ES, Nash N, Croston GE, Currier EA, Vanover KE, et al. 5-Hydroxytriptamine2A receptor inverse agonists as antipsychotics. J Pharmacol Exp Ther. 2001;299:268–276. [PubMed] [Google Scholar]
- Zazpe A, Artaiz I, Innerárity A, Del Olmo E, Castro E, Labeaga L, et al. In vitro and in vivo characterization of F-97013-GD, a partial 5-HT1A agonist with antipsychotic-and antiparkinsonian-like properties. Neuropharmacology. 2006;51:129–140. doi: 10.1016/j.neuropharm.2006.03.008. [DOI] [PubMed] [Google Scholar]