Abstract
Drugs that interfere with cannabinoid CB1 receptor transmission suppress a number of food-related behaviors, and these compounds are currently being assessed for their potential utility as appetite suppressants. In addition to rimonabant (SR141716A), several other compounds have been evaluated, including AM251 and AM1387. Biochemical studies indicate that most of the drugs assessed thus far have been CB1 inverse agonists, and these drugs all act to suppress food intake and disrupt food-reinforced behavior. Behavioral tests involving intake of different diets (i.e., high fat, high carbohydrate, laboratory chow) indicate that consumption of all three food types is disrupted by CB1 inverse agonists, and that, expressed as a percent of baseline intake, the effect is roughly comparable across different diets. Although CB1 inverse agonists do not appear to produce severe motor impairments that disrupt feeding behavior, there is evidence that they can induce nausea and malaise. Recent studies have been undertaken to characterize the behavioral effects of CB1 receptor neutral antagonists such as AM4113 to determine if these drugs can reduce feeding and food-reinforced behaviors. Across a variety of different tests, AM4113 produces effects on food-motivated behavior that are very similar to those produced by CB1 inverse agonists. Moreover, this drug did not induce conditioned gaping in rats or vomiting in ferrets. These results suggest that CB1 receptor neutral antagonists may decrease appetite by blocking endogenous cannabinoid tone, and that these drugs may be less associated with nausea than is the case for CB1 inverse agonists.
Keywords: Appetite, Motivation, Operant, Feeding, THC, Rimonabant
1. Introduction
Cannabinoid CB1 receptor agonists, including delta-9-tetra-hydrocannabinol (THC), have a wide variety of behavioral effects, including actions on motor control [1–3], pain [1,3], and cognitive function [4–7]. These drugs also have been reported to exert effects upon processes related to food intake. Early reports suggested that consumption of marijuana could be accompanied by feelings of increased hunger and decreased satiety, as well as increases in food intake [8]. Initial laboratory experiments showed that CB1 agonists could increase eating [9,10] and enhance body weight gain [11]. Furthermore, CB1 agonists have been investigated for their potential as treatments for anorexia and wasting syndrome associated with chemotherapy and AIDS [12]. In animals, the effects of cannabinoid CB1 agonists on food intake depend greatly upon the dose [13]. Although some papers have reported that CB1 agonist administration decreases feeding, these studies have generally used higher doses that also produced catalepsy and suppressed locomotion (e.g., [14]). Several papers that employed moderate-to-low doses have shown that CB1 agonists can increase food intake [13,15–19].
Consistent with these observations that moderate doses of CB1 agonists could enhance food intake, it was suggested that CB1 antagonists such as SR 141716A (rimonabant; [20]) should have suppressive effects on food intake. Arnone et al. [21] observed that rimonabant decreased intake of high-sucrose food pellets. Rimonabant also was shown to decrease food intake, but not water intake, over the first few days of repeated administration in rats that had ad libitum access to lab chow and water [22]. Tolerance developed rapidly to the appetite suppression effect, although body weight was significantly decreased during the entire injection series, even at the lowest dose (2.5 mg/kg). In addition to these studies with food intake, food-reinforced operant responding also was demonstrated to be sensitive to rimonabant; this drug suppressed fixed ratio 15 responding in a dose-related manner, an effect that was partially reversed by coadministration of the CB1 agonist WIN55,212–2 [23]. These initial studies instigated a period of rapid development in this area, with a wide variety of methods being used for assessment of a growing number of compounds that interfered with cannabinoid CB1 transmission [18,22,24–28,55]. The present review is intended to provide a brief overview of some of the recent studies that have focused upon newly developed compounds, including AM251, AM1387, and AM4113 [29–33].
2. Effects of AM251 and AM1387 on food intake and food-reinforced behavior
Like rimonabant, AM251 and AM1387 can bind with relatively high affinity to CB1 receptors, and they have a modest degree of CB1 selectivity relative to CB2 receptors. Moreover, biochemical studies indicate that rimonabant, AM251, and AM1387 all act as inverse agonists, and exert actions on signal transduction mechanisms when administered in the absence of CB1 receptor stimulation (i.e., they inhibit GTPγS binding and increase cAMP production [31,34,35]). All three drugs have been assessed under comparable conditions in a series of studies measuring food-reinforced behavior and food intake. Several experiments examined the effects of rimonabant, AM 251 and AM1387 on food-reinforced responding using fixed ratio schedules with two different ratio requirements (i.e., fixed ratio 1(FR1) and 5 (FR5)). These particular ratio values were used because previous studies have indicated that FR1 and FR5 schedules can show differential sensitivity to various neurochemical or pharmacological conditions [36–38]. In fact, all three CB1 antagonists/inverse agonists suppressed performance on both schedules of reinforcement [29,31]. These effects occurred over roughly the same dose range for each schedule employed. In addition, the suppression of FR5 lever pressing was used to assess the duration of action for each compound. Both rimonabant and AM251 had a relatively long duration of action (t1/2: rimonabant—15.6 h; AM251—22.0 h [29]), while the half-life AM1387 was considerably shorter (t1/2=4.87 h [31]). These studies showed that AM251 and AM1387, like rimonabant, could suppress food reinforced behavior, and also demonstrated the utility of the operant procedures for assessing features of drug effects such as duration of action.
Additional studies were conducted to characterize the effects of rimonabant, AM251 and AM1387 on intake of diets with differing macronutrient compositions. For several years, there has been intense interest in identifying the role that different types of food may play in modulating the appetite-related effects of drugs that act on CB1 receptors. In studies of cannabinoid-induced hyperphagia in humans, snacking on sweets between meals was reported to increase, but size of meals did not change [9]. In rats, stimulation of food intake with Δ9-THC was significantly greater for intake of a high-fat diet as compared to standard laboratory chow [17]. Although some researchers have reported that interference with CB1 transmission suppressed intake of sweet foods such as sucrose to a greater extent than intake of laboratory chow [21,24], other researchers have observed a substantial suppression of intake of standard diets such as laboratory chow [39,40]. For these reasons, rimonabant, AM251 and AM1387 were assessed for their effects on intake of three different foods [29,31]: a high-fat diet (HF; Diet # D12451, Research Diets, New Brunswick, New Jersey, 45% kcal from fat), a high-carbohydrate diet (HC; Diet # D12450B, Research Diets, New Brunswick, New Jersey, 67% kcal from carbohydrate) and standard laboratory chow (LC, 5P00 Prolab RMH 3000, PMI Nutrition International, St. Louis, Missouri). Food-deprived rats were trained to eat their assigned diet in test cages for three days a week, and after several weeks of training the drug treatment period began (1 drug treatment per week following two baseline days). Administration of all three drugs (rimonabant, AM251, AM1387) produced a dose-related suppression of intake of all three types of food. In the case of rimonabant and AM251, analysis of variance indicated that there was no drug treatment×diet interaction [29]. This suggests that the suppressive effects of these drugs on laboratory chow intake were not different from the suppressive effects of these drugs on intake of the other two foods. With AM1387, there was a significant interaction in terms of the raw gram quantity of food consumed [31]. Nevertheless, separate analyses indicated that all three drugs, including AM1387, significantly suppressed intake of laboratory chow, an effect similar to that reported in other studies [39,40].
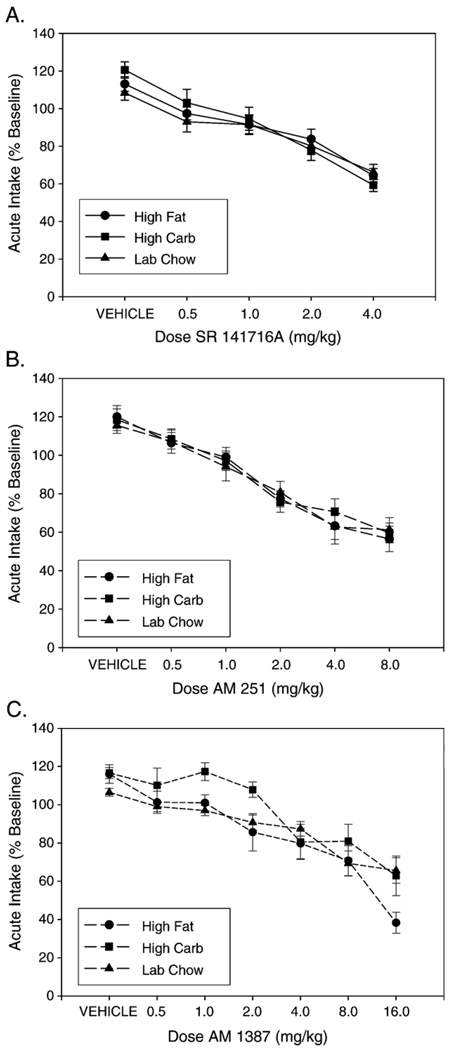
One of the features of these studies was that the baseline or control level of intake differed substantially for the three different foods; intake was highest for the high carbohydrate and high fat diets, and lowest for the laboratory chow [29,31]. In order to correct for these baseline differences, data were reanalyzed with food intake being expressed as a percent of the two previous baseline days. When the data were analyzed in this way, there were significant dose-related decreases in food intake with all three drugs, but no significant interactions; in fact, the dose–response curves for consumption of each food overlapped considerably [29,30] (see Fig. 1). Taken together, these results suggest that rimonabant, AM251 and AM1387 are not selectively suppressing feeding upon diets that are high in carbohydrates or fat. Rather, it seems that apparent differences in the effects of these drugs on intake of different foods may be due to differences in baseline consumption or scaling. Provided that the testing conditions generate substantial levels of chow intake, interference with CB1 transmission appears to suppress consumption of this particular food. Nevertheless, in considering the potential use of these drugs as appetite suppressants, it is worth emphasizing that the substantial feeding suppression seen in rats consuming calorically dense foods at high baseline rates suggests that CB1 inverse agonists could substantially reduce caloric intake in patients who consume large quantities of these foods. Moreover, it is possible that the intake of distinct types of foods can show different patterns of tolerance after repeated administration of CB1 inverse agonists [21].
Fig. 1.
Effect of CB1 receptor inverse agonists on intake of three different diets. Mean intake is expressed as percent of baseline consumption (defined as the mean consumption of the previous two non-injection sessions) of the three different diets during 30 min sessions. With the analysis of these data, expressed as a percent of baseline, there was no significant dose×group interaction for any drug; however a strong dose effect remained. A. Rimonabant (SR141716A). B. AM251. C. AM1387. (data are from McLaughlin et al. [29,31]).
3. Behavioral processes affected by reductions in CB1 transmission: patterns of food intake and food aversions
In assessing the effects of CB1 receptor inverse agonists on food-related behaviors, it is important to consider the wide variety of behavioral processes in addition to appetite that can modulate feeding behavior. For example, it would be critical to determine whether drug-induced reductions in feeding are due solely to decreased appetite, or if alternative effects such as motor slowing, incoordination, nausea or malaise play a role. Although rimonabant does not produce obvious signs of sedation or motor slowing, other effects such as reductions in spontaneous locomotion, as well as the induction of grooming, scratching and head twitching, have been observed at high doses [41,42]. This suggests that there could be subtle actions on motor control processes, such as forepaw coordination, that may impair feeding behavior. In attempting to assess the possible role that various aspects of motor function play in feeding behavior, it is worthwhile to emphasize that it is insufficient simply to obtain measures of gross locomotor activity. Considerable evidence indicates that impairments in locomotion can be quite distinct from impairments in motor control and food handling during food intake. For example, depletions of dopamine in the ventrolateral neostriatum do not suppress spontaneous locomotion, but do impair food intake, feeding efficiency and food handling [43–45]. In contrast, nucleus accumbens dopamine depletions suppress locomotion but do not impair feeding or food handling [44,45]. Thus, impairments in locomotor activity are easily dissociable from deficits in motor patterns related to food intake. For that reason, direct observations of feeding behavior are necessary to determine if CB1 receptor inverse agonists impair motor functions specifically related to feeding. In fact, AM251 failed to suppress feeding rate or disrupt forepaw usage during feeding at doses that substantially suppressed food intake [30]. These results indicate that CB1 inverse agonists are not simply reducing feeding by disrupting the motor patterns necessary for feeding behavior. Thus, although the neostriatum contains a relatively high concentration of CB1 receptors[46–47], the effects of CB1 inverse agonists do not closely resemble the effects of neostriatal dopamine depletions.
Some of the feeding-related effects produced by drugs that act on CB1 receptors may be due to actions such as food avoidance, food aversion, nausea or malaise. Considerable evidence indicates that CB1 agonists have anti-emetic effects [48–50]. CB1 receptors that are associated with triggering emetic responses are present in the brain stem dorsal vagal complex [51]. Rimonabant potentiated lithium chloride-induced conditioned rejection reactions in rats [52], and this drug also produced conditioned taste avoidance in the rat [53], emesis in the least shrew [54] and nausea in humans [53]. Although rats do not vomit, several studies have employed measures of conditioned gaping in rats, which is thought to be a selective marker of nausea in that species [56,57]. These gaping responses are elicited by treatments that produce vomiting in species capable of emesis [56], and conditions that act to attenuate toxin-induced vomiting in emetic species also attenuate toxin-induced conditioned gaping in rats [58,59] (for a review, see [52]). A recent paper from our laboratory demonstrated that doses of AM251 that reduce feeding are accompanied by the induction of conditioned gaping, as well as conditioned food avoidance [30]. This suggests that drug-induced food aversions may contribute to the feeding suppression produced by CB1 receptor inverse agonists.
4. Studies of the effects of CB1 receptor neutral antagonists
As noted above, biochemical studies have indicated that rimonabant, AM251, and AM1387 act as inverse agonists, exerting actions on signal transduction mechanisms in the absence of CB1 receptor stimulation (i.e., they inhibit GTPγS binding and increase cAMP production, see Fig. 2; [31,34,35]). There is some uncertainty about how important inverse agonist actions are for the food-related effects of these drugs. In one recent study, CB1 knockout and wild-type mice responded to a similar extent on a progressive ratio schedule reinforced with corn oil, while wild type mice treated with rimonabant decreased responding [60]. This suggests that rimonabant may exert inverse agonist effects in addition to simply blocking CB1 receptors [60]. The development of CB1 receptor neutral antagonists would be important for the field, because if such drugs decreased feeding it would indicate that there is a tonic influence of endogenous cannabinoid on food intake. Moreover, it is possible that CB1 receptor neutral antagonists might be devoid of effects on food avoidance or aversion.
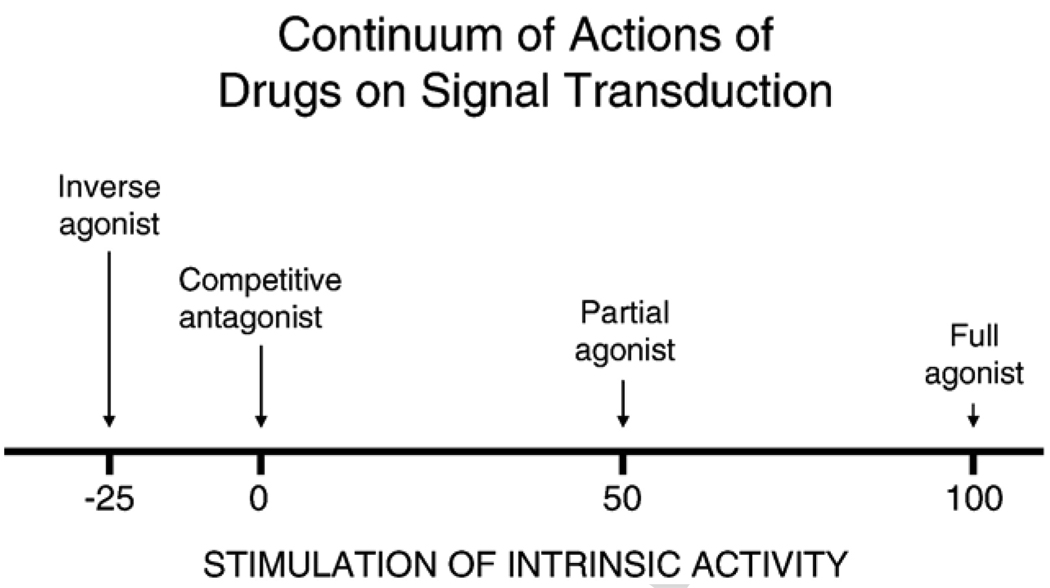
Fig. 2.
This figure is a diagram showing the variations in intrinsic activity that can be shown by different classes of drugs that act on the same receptor site as the neurotransmitter. All drug classes shown have affinity for the receptor. Agonists bind to the receptor and stimulate the same intrinsic activity as the neurotransmitter. Full agonists stimulate maximal or near maximal levels of intrinsic activity, while partial agonists produce low-to-moderate levels of stimulation of the signal transduction mechanism (e.g. 50%, which is the arbitrary value listed above). Neutral or ‘silent’ antagonists bind to the receptor but do not instigate their own signal transduction effects. In contrast, inverse agonists stimulate signal transduction effects in the opposite direction from those stimulated by the agonist. For CB1 receptors, agonists inhibit cyclic-AMP production, while inverse agonists stimulate production of this second messenger [31,34,35]. In neuropharmacology, the designation of compounds as inverse agonists or neutral antagonists is based upon their signal transduction effects, which are studied at the cellular or tissue levels. Nevertheless, in behavioral pharmacology it also is important to determine if neutral antagonists block the effects of both inverse agonists and agonists at the behavioral level.
Recently, investigators have begun to characterize the behavioral effects of CB1 receptor neutral antagonists. Gardner and Mallet [61] reported that O-2050 suppressed food intake in non-deprived rats in a manner comparable to rimonabant. Additional studies have been performed with AM4113, which is a pyrazole analog that is structurally related to rimonabant and AM251 [32,33]. This drug blocked the analgesia and locomotor suppression induced by the CB1 agonist AM411 [32,33], and also was used in studies related to feeding. AM4113 produced many food-related effects similar to those previously shown by rimonabant, AM251 and AM1387. For example, AM4113 reduced food-reinforced FR1 and FR5 responding [32,33], and was actually more potent at suppressing FR1 lever pressing than it was at reducing FR5 performance. AM4113 reduced intake of high-fat, high-carbohydrate and laboratory chow diets, and as was the case with the inverse agonists, the relative degree of suppression of intake (i.e., expressed as percent baseline) for each type of food was roughly comparable [32,33]. Taken together, these data indicate that CB1 receptor neutral antagonists can suppress feeding and food-reinforced behavior. This suggests that blockade of CB1 receptor tone is sufficient to produce a suppression of food-motivated behaviors. Additional studies also should focus on thirst-motivated behavior, as evidence indicates that O-2050 also suppresses water intake [61].
Another important issue is whether or not the suppression of feeding produced by CB1 receptor neutral antagonists is accompanied by signs of nausea, malaise, or food aversion. A few recent studies have examined this critical question. AM4113, in contrast to the inverse agonist AM251, does not induce vomiting in ferrets [62]. In addition, doses of AM4113 that substantially suppress food intake do not induce conditioned gaping [33], which is a marker of nausea in rats. Recent clinical work has shown that nausea was one of the most common adverse effects reported in clinical trials with the antagonist/inverse agonist rimonabant [63,64]. Thus, if CB1 receptor neutral antagonists such as O-2050 and AM4113 can suppress feeding without producing food aversion effects in humans, it might mean that neutral antagonists offer some clinical advantages over drugs that also have inverse agonist properties.
5. Conclusions
In summary, drugs that interfere with cannabinoid CB1 receptor transmission exert suppressive effects on food intake. These effects occur across a variety of tasks, including those that involve food-reinforced behavior as well as feeding. Behavioral tests involving intake of different diets, including less preferred foods such as laboratory chow, indicate that food consumption is disrupted by CB1 inverse agonists such as rimonabant, AM251 and AM1387. Moreover, when the data are expressed as a percent of baseline intake, the effect is roughly comparable for each drug across different diets. CB1 inverse agonists do not appear to produce severe motor impairments (e.g., suppression of feeding rate or impairments in food handling) that disrupt feeding behavior. However, there is evidence that they can induce signs of nausea and malaise. Recent studies have been undertaken to characterize the behavioral effects of CB1 receptor neutral antagonists such as AM4113 to determine if these drugs can reduce feeding and food-reinforced behaviors. AM4113 produces effects on food-motivated behavior that are very similarto those produced by CB1 inverse agonists. Moreover, this drug did not induce conditioned gaping in rats or vomiting in ferrets. These results suggest that CB1 neutral antagonists may decrease appetite by blocking endogenous cannabinoid tone, and that these drugs may be less associated with nausea than CB1 inverse agonists. Future studies should determine if neutral antagonists such as AM4113 or O-2050 can act to block food-related effects of inverse agonists, as well as antagonizing actions produced by CB1 receptor stimulation.
Acknowledgements
This work was supported by grants to J.S. and A.M. from NIH/NIDA.
References
- 1.Martin BR, Compton DR, Thomas BF, Prescott WR, Little PJ, Razdan RK, et al. Behavioral, biochemical, and molecular modeling evaluations of cannabinoid analogs. Pharmacol Biochem Behav. 1991;40:471–478. doi: 10.1016/0091-3057(91)90349-7. [DOI] [PubMed] [Google Scholar]
- 2.Carriero D, Aberman J, Lin SY, Hill A, Makriyannis A, Salamone JD. A detailed characterization of the effects of four cannabinoid agonists on operant lever pressing. Psychopharmacology. 1998;137:147–156. doi: 10.1007/s002130050604. [DOI] [PubMed] [Google Scholar]
- 3.McLaughlin PJ, Lu D, Winston KM, Thakur G, Swezey LA, Makriyannis A, et al. Behavioral effects of the novel cannabinoid full agonist AM411. Pharmacol Biochem Behav. 2005;81:78–88. doi: 10.1016/j.pbb.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 4.Darley CF, Tinklenberg JR, Hollister TE, Atkinson RC. Marihuana and retrieval from short-term memory. Psychopharmacologia. 1973;29:231–238. doi: 10.1007/BF00414037. [DOI] [PubMed] [Google Scholar]
- 5.Heishman SJ, Huestis MA, Henningfield JE, Cone EJ. Acute and residual effects of marijuana: profiles of plasma THC levels, physiological, subjective, and performance measures. Pharmacol Biochem Behav. 1990;37:561–565. doi: 10.1016/0091-3057(90)90028-g. [DOI] [PubMed] [Google Scholar]
- 6.Ilan AB, Smith ME, Gevins A. Effects of marijuana on neurophysiological signals of working and episodic memory. Psychopharmacology (Berl) 2004;176:214–222. doi: 10.1007/s00213-004-1868-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McLaughlin PJ, Brown CM, Winston KM, Thakur G, Lu D, Makriyannis A, et al. The novel cannabinoid agonist AM 411 produces a biphasic effect on accuracy in a visual target detection task in rats. Behav Pharmacol. 2005;16:477–486. doi: 10.1097/00008877-200509000-00022. [DOI] [PubMed] [Google Scholar]
- 8.Halikas JA, Weller RA, Morse CL, Hoffmann RG. A longitudinal study of marijuana effects. Int J Addict. 1985;20:701–711. doi: 10.3109/10826088509044290. [DOI] [PubMed] [Google Scholar]
- 9.Foltin RW, Brady JV, Fischman MF. Behavioral analysis of marijuana effects on food intake in humans. Pharmacol Biochem Behav. 1986;25:577–582. doi: 10.1016/0091-3057(86)90144-9. [DOI] [PubMed] [Google Scholar]
- 10.Foltin RW, Fischman MW, Byrne MF. Effects of smoked marijuana on food intake and body weight of humans living in a residential laboratory. Appetite. 1988;11:1–14. doi: 10.1016/s0195-6663(88)80017-5. [DOI] [PubMed] [Google Scholar]
- 11.Greenberg I, Kuehnle J, Mendelson JH, Bernstein JG. Effects of marihuana use on body weight and caloric intake in humans. Psychopharmacology. 1976;49:79–84. doi: 10.1007/BF00427475. [DOI] [PubMed] [Google Scholar]
- 12.Hao S, Avraham Y, Mechoulam R, Berry EM. Low dose anandamide affects food intake, cognitive function, neurotransmitter and corticosterone levels in diet-restricted mice. Eur J Pharmacol. 2000;392:147–156. doi: 10.1016/s0014-2999(00)00059-5. [DOI] [PubMed] [Google Scholar]
- 13.Giuliani D, Ottani A, Ferrari F. Effects of the cannabinoid receptor agonist, HU 210, on ingestive behaviour and body weight of rats. Eur J Pharmacol. 2000;391:275–279. doi: 10.1016/s0014-2999(00)00069-8. [DOI] [PubMed] [Google Scholar]
- 14.Drewnowski A, Grinker JA. Food and water intake, meal patterns and activity of obese and lean Zucker rats following chronic and acute treatment with delta9-tetrahydrocannabinol. Pharmacol Biochem Behav. 1978;9:619–630. doi: 10.1016/0091-3057(78)90213-7. [DOI] [PubMed] [Google Scholar]
- 15.Johansson JO, Jarbe TU, Henriksson BG. Acute and subchronic influences of tetrahydrocannabinols on water and food intake, body weight, and temperature in rats. TIT J Life Sci. 1975;5:17–27. [PubMed] [Google Scholar]
- 16.Williams CM, Rogers PJ, Kirkham TC. Hyperphagia in pre-fed rats following oral delta9-THC. Physiol Behav. 1998;65:343–346. doi: 10.1016/s0031-9384(98)00170-x. [DOI] [PubMed] [Google Scholar]
- 17.Koch JE. Δ9-THC stimulates food intake in Lewis rats: effects on chow, high-fat and sweet high-fat diets. Pharmacol Biochem Behav. 2001;68:539–543. doi: 10.1016/s0091-3057(01)00467-1. [DOI] [PubMed] [Google Scholar]
- 18.Williams CM, Kirkham TC. Anandamide induces overeating: Mediation by central cannabinoid (CB1) receptors. Psychopharmacology. 1999;143:315–317. doi: 10.1007/s002130050953. [DOI] [PubMed] [Google Scholar]
- 19.Williams CM, Kirkham TC. Observational analysis of feeding induced by Δ9-THC and anandamide. Physiol Behav. 2002;76:241–250. doi: 10.1016/s0031-9384(02)00725-4. [DOI] [PubMed] [Google Scholar]
- 20.Rinaldi-Carmona M, Barth F, Héaulme M, Shire D, Calandra B, Congy C, et al. SR141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Lett. 1994;350:240–244. doi: 10.1016/0014-5793(94)00773-x. [DOI] [PubMed] [Google Scholar]
- 21.Arnone M, Maruani J, Chaperon F, Thiebot MH, Poncelet M, Soubrie P, et al. Selective inhibition of sucrose and ethanol intake by SR141716, an antagonist of central cannabinoid (CB1) receptors. Psychopharmacology (Berl) 1997;132:104–106. doi: 10.1007/s002130050326. [DOI] [PubMed] [Google Scholar]
- 22.Colombo G, Agabio R, Diaz G, Lobina C, Reali R, Gessa GL. Appetite suppression and weight loss after the cannabinoid antagonist SR141716. Life Sci. 1998;63:PL113–PL117. doi: 10.1016/s0024-3205(98)00322-1. [DOI] [PubMed] [Google Scholar]
- 23.Freedland CS, Poston JS, Porrino LJ. Effects of SR141716A, a central cannabinoid receptor antagonist, on food-maintained responding. Pharmacol Biochem Behav. 2000;67:265–270. doi: 10.1016/s0091-3057(00)00359-2. [DOI] [PubMed] [Google Scholar]
- 24.Simiand J, Keane M, Keane PE, Soubrie P. SR141716, a CB1 cannabinoid receptor antagonist, selectively reduces sweet food intake in marmoset. Behav Pharmacol. 1998;9:179–181. [PubMed] [Google Scholar]
- 25.Hildebrandt AL, Kelly-Sullivan DM, Black SC. Antiobesity effects of chronic cannabinoid CB1 receptor antagonist treatment in diet-induced obese mice. Eur J Pharmacol. 2003;462:125–132. doi: 10.1016/s0014-2999(03)01343-8. [DOI] [PubMed] [Google Scholar]
- 26.Chen RZ, Huang RR, Shen CP, MacNeil DJ, Fong TM. Synergistic effects of cannabinoid inverse agonist am251 and opioid antagonist nalmefene on food intake in mice. Brain Res. 2004;999:227–230. doi: 10.1016/j.brainres.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 27.Shearman LP, Rosko KM, Fleischer R, Wang J, Xu S, Tong XS, et al. Antidepressant-like and anorectic effects of the cannabinoid CB1 receptor inverse agonist AM251 in mice. Behav Pharmacol. 2003;14:573–582. doi: 10.1097/00008877-200312000-00001. [DOI] [PubMed] [Google Scholar]
- 28.Wiley JL, Burston JJ, Leggett DC, Alekseeva OO, Razdan RK, Mahadevan A, et al. CB1 cannabinoid receptor-mediated modulation of food intake in mice. Br J Pharmacol. 2005;145:293–300. doi: 10.1038/sj.bjp.0706157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McLaughlin PJ, Winston K, Swezey L, Wisniecki A, Aberman J, Tardif DJ, et al. The cannabinoid CB1 antagonists SR141716A and AM251 suppress food intake and food-reinforced behavior in a variety of tasks in rats. Behav Pharmacol. 2003;14:583–588. doi: 10.1097/00008877-200312000-00002. [DOI] [PubMed] [Google Scholar]
- 30.McLaughlin PJ, Winston KM, Limebeer CL, Parker LA, Makriyannis A, Salamone JD. The cannabinoid CB1 antagonist AM251 produces food avoidance and behaviors associated with nausea but does not impair feeding efficiency in rats. Psychopharmacology (Berl) 2005;180:286–293. doi: 10.1007/s00213-005-2171-0. [DOI] [PubMed] [Google Scholar]
- 31.McLaughlin PJ, Qian L, Wood JT, Wisniecki A, Winston KM, Swezey LA, et al. Suppression of food intake and food-reinforced behavior produced by the novel CB1 receptor antagonist/inverse agonist AM1387. Pharmacol Biochem Behav. 2006;83:396–402. doi: 10.1016/j.pbb.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 32.Sink KS, McLaughlin PJ, Brown CM, Makriyannis A, Salamone JD. Effects of a novel cannabinoid CB1 neutral antagonist, AM4113, on feeding, food-reinforced behaviors, and behaviors associated with malaise. Program Number 456.5. Meeting Planner; Atlanta, GA: Society for Neuroscience; 2006. Online. [Google Scholar]
- 33.Sink KS, McLaughlin PJ, Brown CM, Limebeer CL, Parker LA, Xu W, Fan P, Wood JT, Makriyannis A, Salamone JD. The novel cannabinoid CB1 receptor neutral antagonist AM4113 suppresses food intake and food-reinforced behavior but does not induce signs of nausea in rats. Neuropsychopharmacology. doi: 10.1038/sj.npp.1301476. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Landsman RS, Burkey TH, Consroe P, Roeske WR, Yamamura HI. SR141716A is an inverse agonist at the human cannabinoid CB1 receptor. Eur J Pharmacol. 1997;334:R1–R2. doi: 10.1016/s0014-2999(97)01160-6. [DOI] [PubMed] [Google Scholar]
- 35.Mato S, Pazos A, Valdizan EM. Cannabinoid receptor antagonism and inverse agonism in response to SR141716A on cAMP production in human and rat brain. Eur J Pharmacol. 2002;443:43–46. doi: 10.1016/s0014-2999(02)01575-3. [DOI] [PubMed] [Google Scholar]
- 36.Salamone JD, Aberman JE, Sokolowski JD, Cousins MS. Nucleus accumbens dopamine and rate of responding: neurochemical and behavioral studies. Psychobiology. 1999;27:236–247. [Google Scholar]
- 37.Solinas M, Panlilio LV, Antoniou K, Pappas LA, Goldberg SR. The cannabinoid antagonist N-piperidinyl-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methylpyrazole-3-carbamoxide (SR 141716A) differentially alters the reinforcing effects of heroin under continuous reinforcement, fixed ratio, and progressive ratio schedules of drug self-administration in rats. J Pharmacol Exp Ther. 2003;306:93–102. doi: 10.1124/jpet.102.047928. [DOI] [PubMed] [Google Scholar]
- 38.Ishiwari K, Weber SM, Mingote S, Correa M, Salamone JD. Accumbens dopamine and the regulation of effort in food-seeking behavior: modulation of work output by different ratio or force requirements. Behav Brain Res. 2004;151:83–91. doi: 10.1016/j.bbr.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 39.Rowland NE, Mukherjee M, Robertson K. Effects of the cannabinoid receptor antagonist SR 141716, alone and in combination with dexfenfluramine or naloxone, on food intake in rats. Psychopharmacology. 2001;159:111–116. doi: 10.1007/s002130100910. [DOI] [PubMed] [Google Scholar]
- 40.Verty AN, McGregor IS, Mallet PE. Consumption of high carbohydrate, high fat, and normal chow is equally suppressed by a cannabinoid receptor antagonist in non-deprived rats. Neurosci Lett. 2004;354:217–220. doi: 10.1016/j.neulet.2003.10.035. [DOI] [PubMed] [Google Scholar]
- 41.Darmani NA, Pandya DK. Involvement of other neurotransmitters in behaviors induced by the cannabinoid CB1 receptor antagonist SR 141716A in naive mice. J Neural Transm. 2000;107:931–945. doi: 10.1007/s007020070043. [DOI] [PubMed] [Google Scholar]
- 42.Järbe TUC, Andrzejewski ME, DiPatrizio NV. Interactions between the CB1 receptor agonist Δ9-THC and the CB1 receptor antagonist SR-141716in rats: Open field revisited. Pharmacol Biochem Behav. 2002;73:911–919. doi: 10.1016/s0091-3057(02)00938-3. [DOI] [PubMed] [Google Scholar]
- 43.Jicha GA, Salamone JD. Vacuous jaw movements and feeding deficits in rats with ventrolateral striatal dopamine depletions: Possible relation to parkinsonian symptoms. J Neurosci. 1991;11:3822–3829. doi: 10.1523/JNEUROSCI.11-12-03822.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Salamone JD, Mahan K, Rogers S. Ventrolateral striatal dopamine depletions impair feeding and food handling in rats. Pharmacol Biochem Behav. 1993;44:605–610. doi: 10.1016/0091-3057(93)90174-r. [DOI] [PubMed] [Google Scholar]
- 45.Cousins MS, Sokolowski JD, Salamone JD. Different effects of nucleus accumbens and ventrolateral striatal dopamine depletions on instrumental response selection in the rat. Pharmacol Biochem Behav. 1993;46:943–951. doi: 10.1016/0091-3057(93)90226-j. [DOI] [PubMed] [Google Scholar]
- 46.Herkenham M. Cannabinoid receptor localization in brain:relationship to motor and reward systems. Ann N Y Acad Sci. 1992;654:19–32. doi: 10.1111/j.1749-6632.1992.tb25953.x. [DOI] [PubMed] [Google Scholar]
- 47.Egertova M, Elphick MR. Localisation of cannabinoid receptors in the rat brain using antibodies to the intracellular C-terminal tail of CB1. J Comp Neurol. 2000;422:159–171. doi: 10.1002/(sici)1096-9861(20000626)422:2<159::aid-cne1>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 48.Gonzalez-Rosales F, Walsh D. Intractable nausea and vomiting due to gastrointestinal mucosal metastases relieved by tetrahydrocannabinol (Dronabinol) J Pain Symptom Manage. 1997;14:311–314. doi: 10.1016/S0885-3924(97)00229-7. [DOI] [PubMed] [Google Scholar]
- 49.Simoneau II, Hamza MS, Mata HP, Siegel EM, Vanderah TW, Porreca F, et al. The cannabinoid agonist WIN55,212–2 suppresses opioid-induced emesis in ferrets. Anesthesiology. 2001;94:882–887. doi: 10.1097/00000542-200105000-00029. [DOI] [PubMed] [Google Scholar]
- 50.Darmani NA, Johnson JC. Central and peripheral mechanisms contribute to the antiemetic actions of delta-9-tetrahydrocannabinol against 5-hydroxytryptophan-induced emesis. Eur J Pharmacol. 2004;488:201–212. doi: 10.1016/j.ejphar.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 51.Van Sickle MD, Oland LD, Mackie K, Davison JS, Sharkey KA. Delta9-tetrahydrocannabinol selectively acts on CB1 receptors in specific regions of dorsal vagal complex to inhibit emesis in ferrets. Am J Physiol Gastrointest Liver Physiol. 2003;285:G566–G576. doi: 10.1152/ajpgi.00113.2003. [DOI] [PubMed] [Google Scholar]
- 52.Parker LA, Mechoulam R, Schlievert C, Abbott L, Fudge ML, Burton P. Effects of cannabinoids on lithium-induced conditioned rejection reactions in a rat model of nausea. Psychopharmacology (Berl) 2003;166:156–162. doi: 10.1007/s00213-002-1329-2. [DOI] [PubMed] [Google Scholar]
- 53.De Vry J, Schreiber R, Eckel G, Jentzsch KR. Behavioral mechanisms underlying inhibition of food-maintained responding by the cannabinoid receptor antagonist/inverse agonist SR141716A. Eur J Pharmacol. 2004;483:55–63. doi: 10.1016/j.ejphar.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 54.Darmani NA. Delta-9-tetrahydrocannabinol and synthetic cannabinoids prevent emesis produced by the cannabinoid CB1 receptor antagonist/inverse agonist SR 141716A. Neuropsychopharmacology. 2001;24:198–203. doi: 10.1016/S0893-133X(00)00197-4. [DOI] [PubMed] [Google Scholar]
- 55.Despres JP, Golay A, Sjostrom L. Effects of rimonabant on metabolic risk factors in overweight patients with dyslipidemia. N Engl J Med. 2005;353:2121–2134. doi: 10.1056/NEJMoa044537. [DOI] [PubMed] [Google Scholar]
- 56.Parker LA, Limebeer CL, Simpson GR. Chlordiazepoxide-induced conditioned place and taste aversion learning in rats. Pharmacol Biochem Behav. 1998;59:33–37. doi: 10.1016/s0091-3057(97)00333-x. [DOI] [PubMed] [Google Scholar]
- 57.Parker LA, Limebeer CL. Conditioned gaping in rats: a selective measure of nausea. Auton Neurosci. 2006;129:36–41. doi: 10.1016/j.autneu.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 58.Limebeer CL, Parker LA. The antiemetic drug ondansetron interferes with lithium-induced conditioned rejection reactions, but not lithium-induced taste avoidance in rats. J Exp Psychol Anim Behav Process. 2000;26:371–384. doi: 10.1037//0097-7403.26.4.371. [DOI] [PubMed] [Google Scholar]
- 59.Limebeer CL, Parker LA, Fletcher PJ. 5,7-dihydroxytryptamine lesions of the dorsal and median raphe nuclei interfere with lithium-induced conditioned gaping, but not conditioned taste avoidance, in rats. Behav Pharmacol. 2004;118:1391–1399. doi: 10.1037/0735-7044.118.6.1391. [DOI] [PubMed] [Google Scholar]
- 60.Ward SJ, Dykstra LA. The role of CB1 receptors in sweet versus fat reinforcement: Effect of CB1 receptor deletion, CB1 receptor antagonism (SR141716A) and CB1 receptor agonism (CP-55940) Behav Pharmacol. 2005;16:381–388. doi: 10.1097/00008877-200509000-00010. [DOI] [PubMed] [Google Scholar]
- 61.Gardner A, Mallet PE. Suppression of feeding, drinking, and locomotion by a putative cannabinoid receptor ‘silent antagonist’. Eur J Pharmacol. 2006;530:103–106. doi: 10.1016/j.ejphar.2005.11.032. [DOI] [PubMed] [Google Scholar]
- 62.Chambers AP, Vemuri K, Pittman QJ, Makriyannis A, Sharkey KA. Antagonist vs. inverse agonist activity at CB1 receptors: effects on food intake and emesis. Program Number 457.15. Meeting Planner; Atlanta, GA: Society for Neuroscience; 2006. Online. [Google Scholar]
- 63.Pi-Sunyer FX, Aronne LJ, Heshmati HM, Devin J, Rosenstock J RIO-North America Study Group. Effect of rimonabant, a cannabinoid-1 receptor blocker, on weight and cardiometabolic risk factors in overweight or obese patients: RIO-North America: a randomized controlled trial. JAMA. 2006;295:761–775. doi: 10.1001/jama.295.7.761. [DOI] [PubMed] [Google Scholar]
- 64.Van Gaal LF, Rissanen AM, Scheen AJ, Ziegler O, Rossner S RIO-Europe Study Group. Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the RIO-Europe study. Lancet. 2005;365:1363–1364. doi: 10.1016/S0140-6736(05)66374-X. [DOI] [PubMed] [Google Scholar]