Abstract
Alzheimer's disease (AD) pathology is estimated to develop many years before detectable cognitive decline. Fluid and imaging biomarkers may identify people in early symptomatic and even preclinical stages, possibly when potential treatments can best preserve cognitive function. We previously reported that cerebrospinal fluid (CSF) levels of amyloid-β42 (Aβ42) serve as an excellent marker for brain amyloid as detected by the amyloid tracer, Pittsburgh compound B (PIB). Using data from 189 cognitively normal participants, we now report a positive linear relationship between CSF tau/ptau181 (primary constituents of neurofibrillary tangles) with the amount of cortical amyloid. We observe a strong inverse relationship of cortical PIB binding with CSF Aβ42 but not for plasma Aβ species. Some individuals have low CSF Aβ42 but no cortical PIB binding. Together, these data suggest that changes in brain Aβ42 metabolism and amyloid formation are early pathogenic events in AD, and that significant disruptions in CSF tau metabolism likely occur after Aβ42 initially aggregates and increases as amyloid accumulates. These findings have important implications for preclinical AD diagnosis and treatment.
Keywords: amyloid, biomarker, cerebrospinal fluid, Pittsburgh compound B, preclinical Alzheimer's disease
INTRODUCTION
Alzheimer's disease (AD) is a progressive and fatal neurodegenerative disorder that currently affects ∼10.6 million people in the US and Europe, with projected estimates reaching 15.4 million by the year 2030 (Alzheimer's Association). Clinicopathological studies support the notion of a long ‘preclinical’ stage of the disease, with brain pathology (amyloid plaques and neurofibrillary tangles) estimated to begin ∼10–20 years prior to significant neuronal cell death and the consequent appearance of any behavioural signs or symptoms (Braak & Braak, 1997; Gomez-Isla et al, 1996; Hulette et al, 1998; Markesbery et al, 2006; Morris & Price, 2001; Price et al, 2001). Fluid and imaging biomarkers of this pathology are currently being sought in order to confirm early diagnoses and, importantly, to identify individuals in the preclinical stage so emerging therapies ultimately have a chance to preserve normal brain function (Craig-Schapiro et al, 2008).
We recently reported an inverse relationship between cortical amyloid deposits, as viewed by positron emission tomography (PET) imaging with the amyloid binding agent, Pittsburgh compound B (PIB) and the amount of cerebrospinal fluid (CSF) amyloid-β42 (Aβ42), the primary constituent of brain amyloid plaques (Fagan et al, 2006, 2007). Individuals with cortical amyloid (as detected by PET PIB) had low CSF Aβ42 whereas those without cortical amyloid had high CSF Aβ42. This relationship was observed independent of clinical status; several cognitively normal individuals had a CSF Aβ42/PIB profile indistinguishable from that of other individuals diagnosed clinically with early stage dementia of the Alzheimer type (DAT). These observations are consistent with the idea of preclinical AD and suggest that these measures may have clinical utility as antecedent biomarkers of the disease.
We have now obtained CSF and PIB data from 189 cognitively normal individuals ranging in age from 43 to 89 years. We explored the relationship between in vivo brain amyloid and CSF markers of proteins present in neurons and constituents of neurofibrillary tangles (tau and ptau181), as well as Aβ species in plasma, and investigated whether the CSF Aβ42/PIB relationship remains robust in this large cohort of non-demented individuals. The data we obtained shed further light on the potential utility of these measures as antecedent biomarkers of AD, and also provide insight into the normal time course of the pathophysiology of the disease as reflected in CSF, information that should aid in the design and evaluation of secondary prevention trials.
RESULTS
One hundred and eighty-nine research participants with a clinical dementia rating of 0 (CDR 0, indicating cognitively normal) (Morris, 1993) met the selection criterion of having a PIB scan within 2 years of CSF collection by lumbar puncture (LP). Combined PIB and biomarker data from 25 of these participants have been reported by us in previous studies (Fagan et al, 2006, 2009), whereas the remaining 164 are unique to the present study. The demographic characteristics of the present cohort are similar to what we have previously published with the exception of age (Table 1). By design, we have included a wide range of ages in the present study (43–89 years, normally distributed) so as to better capture the various biomarker correlations during the potential preclinical stage of AD (which is estimated to begin 10–20 years prior to cognitive symptoms). Therefore, the mean age of our cohort is younger (64.7 ± 10.4) than what we have reported on previously (71.41 ± 8.62; Fagan et al, 2009). In keeping with this age difference, CSF Aβ42 levels in the present study (652 ± 235 pg/ml) are higher than that reported by us in our studies of older individuals (572 ± 208 pg/ml) and, as expected, CSF tau (285 ± 151 pg/ml vs. 334 ± 180 pg/ml), and ptau181 (52 ± 23 pg/ml vs. 61 ± 27 pg/ml) levels are lower (Fagan et al, 2009). The absolute plasma Aβ values cannot be compared between our various studies because different methods were used to measure these analytes.
Table 1.
Participant demographic characteristics, psychometric performance and biomarker values
| CDR 0 participants | |
|---|---|
| N | 189 |
| Age at LP (year) | 64.7 (10.4) |
| Age range (year) | 43–89 |
| M/F (%F) | 62/127 (67%) |
| APOE ε4–/ε4+ (% ε4+) | 125/64 (34%) |
| Selective remembering (possible score, 0–48) | 31.2 (6.3) |
| Animal naming | 21.6 (5.3) |
| Trailmaking A (# of connections/s) | 0.916 (0.303) |
| Trailmaking B (# of connections/s) | 0.384 (0.151) |
| CSF Aβ38 | 1228 (514) |
| CSF Aβ40 | 8958 (4464) |
| CSF Aβ42 | 652 (235) |
| CSF tau | 285 (151) |
| CSF ptau181 | 52 (23) |
| Plasma Aβ1–40 | 217 (60) |
| Plasma Aβx–40 | 37 (11) |
| Plasma Aβ1–42 | 193 (44) |
| Plasma Aβx–42 | 25 (9) |
| PIB MCBP | 0.0931 (0.213) |
Fluid psychometric and PET PIB (MCBP) values are represented as means (standard deviations). CSF and plasma values are in pg/ml. PIB MCBP values are in arbitrary units (generated by Logan graphical analyses).
APOE, apolipoprotein E; CDR, clinical dementia rating; CSF, cerebrospinal fluid; LP, lumbar puncture; MCBP, mean cortical binding potential; PIB, Pittsburgh compound B.
Given the wide range of ages in the present cohort, we first investigated whether any of the biomarker measures correlated with age at the time of LP. As shown in Fig. 1, positive correlations were observed between age and cortical amyloid (represented by mean cortical PIB binding potential (MCBP); Mintun et al, 2006), CSF tau and ptau181 and plasma Aβ1–40. An inverse correlation was observed between age and CSF Aβ42, and no relationships between age and CSF Aβ38 or Aβ40 were found. Due to these age effects, all subsequent analyses were corrected for age.
Figure 1. Cortical amyloid as detected by PET PIB and fluid biomarkers in CDR 0 participants (n = 189) as a function of age.
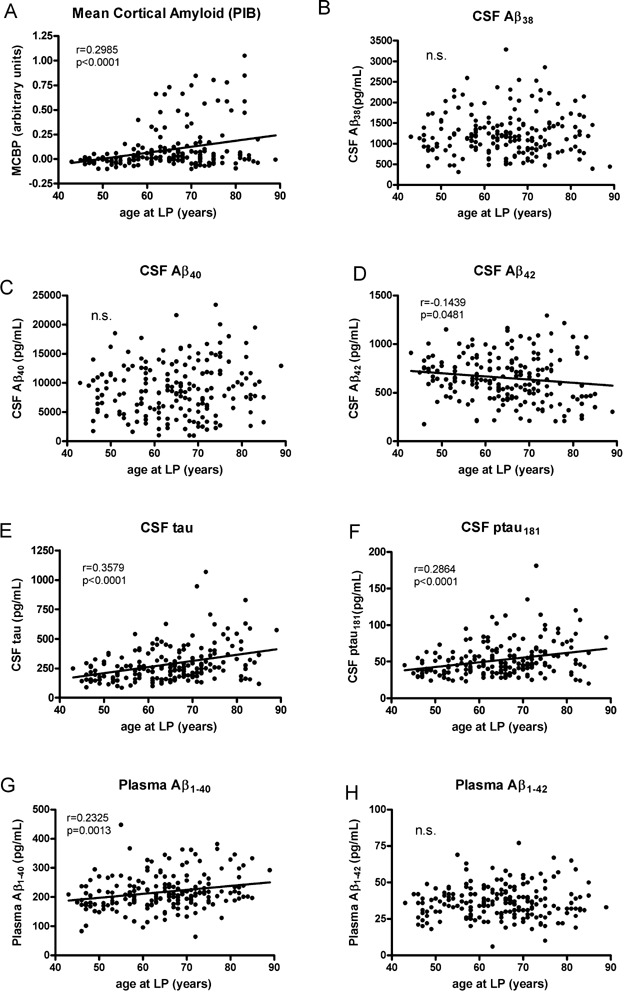
Levels of
- cortical amyloid are positively correlated with age in this CDR 0 cohort,
- The level of CSF Aβ38 is not correlated with age,
- nor is CSF Aβ40.
- CSF Aβ42 is negatively correlated with age.
- Positive correlations with age are observed for CSF tau,
- CSF ptau181 and
- plasma Aβ1–40.
- Plasma Aβ1–42 is not correlated with age in this cohort.
We next investigated whether the inverse relationship between CSF Aβ42 and cortical PIB binding that we had reported previously in a mixed cohort of mildly demented and non-demented individuals was observed in this cohort of cognitively normal individuals. Overall, 29 participants in this cohort had MCBP values greater than or equal to 0.18 whereas 160 participants had MCBPs below 0.18 (Fig 2A). In individuals with MCBP values greater than 0.18, PIB retention is visualized in the neocortex and appears qualitatively greater than background levels. We continued to observe a robust and linear relationship between CSF Aβ42 and cortical amyloid in this group of cognitively normal individuals (Fig 2B). Every participant with high PIB binding had CSF Aβ42 values <582 pg/ml; 86% had CSF Aβ42 values <500 pg/ml. A large majority (84%) of participants with low PIB binding had CSF Aβ42 values >500 pg/ml (Fig 2A). Consistent with our previous findings (Fagan et al, 2006, 2007), many of the CDR 0 participants within this broad age range had little or no cortical amyloid and high mean CSF Aβ42 levels (≥500 pg/ml) (Fig 2B). Twenty-five of the 189 CDR 0 participants displayed the typical AD biomarker phenotype in relation to Aβ, with high PIB binding and low CSF Aβ42 (Fig 2B). In many cases their PET PIB scans were indistinguishable from demented individuals with DAT (CDR > 0) (Fig 2C). In contrast to CSF Aβ42, CSF Aβ40 was not related to the presence or amount of cortical amyloid in these individuals (Fig 2D). Similarly, levels of CSF Aβ38 were not correlated with cortical amyloid load (Fig 2E), but the ratio of CSF Aβ38/Aβ42 was positively correlated with amyloid load (Fig 2F), likely due to the drop in CSF Aβ42 with amyloid deposition.
Figure 2. Cortical amyloid as detected by PET PIB and its relationship to CSF Aβ in CDR 0 participants (n = 189).
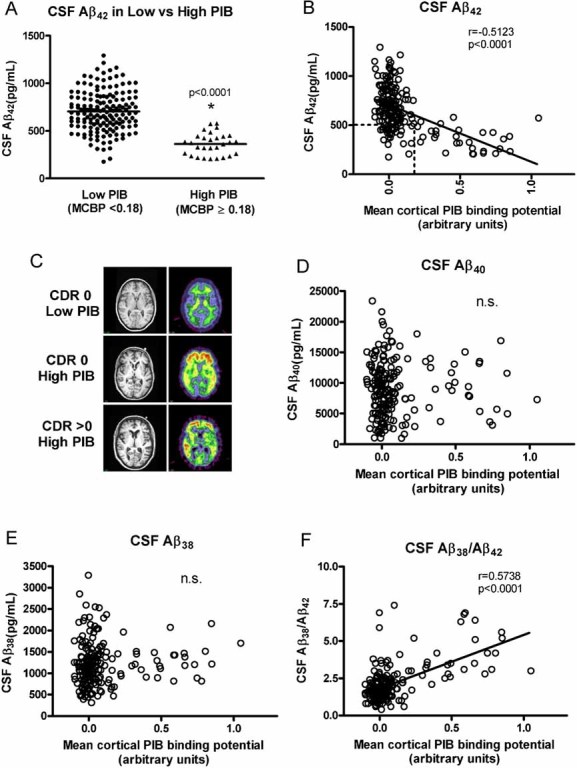
A. A high percentage (84%) of participants with low PIB values (MCBP < 0.18) had high CSF Aβ42 levels (mean (SD) = 705 pg/ml (211)) whereas the vast majority of participants (86%) in the cohort who had high PIB binding (MCBP ≥ 0.18) had low CSF Aβ42 (mean (SD) = 362 pg/ml (115)). Horizontal lines represent the group means, and these means are statistically different from each other (asterisk, p < 0.0001).
B. Relationship between CSF Aβ42 levels and cortical amyloid. Most participants had low MCBP values. The vast majority (86%) of participants with MCBPs ≥ 0.18 had low CSF Aβ42 levels. These CDR 0 participants are hypothesized to have preclinical AD. The box outlined by dashed lines identifies the 28 individuals who have low cortical PIB binding (MCBP < 0.18) with low CSF Aβ42. There is a linear relationship between CSF Aβ42 and the amount of cortical amyloid although CSF Aβ42 appears to drop and then stay low as the amyloid load increases.
C. MRI (left) and PET PIB (right) images of a representative low PIB (MCBP = 0.0270) CDR 0 participant (top panel), a high PIB (MCBP = 0.7790) CDR 0 participant (middle panel), and a high PIB (MCBP = 0.7812) CDR > 0 participant (bottom panel). The amount of cortical PIB binding (yellow-red corresponds to high binding) in the high PIB CDR 0 participant and the high PIB CDR > 0 participant is comparable, whereas there is only background PIB binding (green) in white matter tracks in the low PIB CDR 0 participant.
D,E. No relationship between CSF Aβ40 (D) and CSF Aβ38 (E) levels and cortical amyloid was observed in this cognitively normal cohort (r = −0.0287, p = 0.6963; r = 0.06851, p = 0.3515, respectively).
F. A negative correlation was found between cortical amyloid and the CSF Aβ38/Aβ42 ratio. All Pearson correlation coefficients are corrected for age. n.s., not significant.
Twenty-eight CDR 0 individuals showed a mismatch, appearing in the ‘lower quadrant’ of Fig 2B (whose boundaries are indicated by the square outlined in a dashed line in Fig 2B) in that they had little or no cortical PIB binding (MCPB < 0.18) but had low CSF Aβ42 (<500 pg/ml). The mean interval between LP and PIB scans for individuals in this quadrant did not differ statistically from the mean intervals of those participants in the other quadrants (i.e. low PIB/high CSF Aβ42 and high PIB/low CSF Aβ42) (p = 0.4693). In addition, this low PIB/low CSF Aβ42 group (‘lower quadrant’, n = 28) did not differ from the low PIB/high CSF Aβ42 group (‘upper quadrant’, n = 132) in the frequency of the ε4 allele of APOE (39% vs. 27%, respectively, p = 0.2049), nor did it differ from the high PIB/low CSF Aβ42 (‘PIB-positive quadrant’) group (39% vs. 62%, respectively), but it did approach statistical significance (p = 0.0854). The ε4+ frequency in the high PIB/low CSF Aβ42 (‘PIB-positive quadrant’) group did, however, differ significantly from that of the low PIB/high CSF Aβ42 (‘upper quadrant’) group (p = 0.0003). We did not observe any significant associations between quadrant membership and performance on any of the psychometric tests when adjusted for age (Selective Reminding, p = 0.2486; Animal Naming, p = 0.1209; Trailmaking A, p = 0.8561; Trailmaking B, p = 0.2817). The groups also did not differ in the percentage of self-reported presence or absence of heart disease, diabetes, history of stroke and/or TIAs, prior head trauma (with loss of consciousness) or NSAID use (all p > 0.05, Fisher's exact test). However, the low PIB/low CSF Aβ42 group (‘lower quadrant’) had a greater frequency of reported hypertension than the low PIB/high CSF Aβ42 group (‘upper quadrant’) (50% vs. 29.6%, respectively, p = 0.0469) and a greater frequency of arthritis than the low PIB/high CSF Aβ42 group (14.3% vs. 3.03%, respectively, p = 0.0323). The biological significance of these findings, if any, remains unclear but warrants further investigation. Overall, longitudinal PIB follow-up of the participants in this lower quadrant will be required to understand whether their low CSF Aβ42 represents Aβ aggregation in diffuse (PIB-negative) plaques, oligomeric forms prior to substantial fibrillar (PIB-positive) Aβ deposition or simply reflects the low end of the normal spectrum of CSF Aβ42 levels. It is interesting to note that one of the individuals in this quadrant (having no cortical PIB binding but low CSF Aβ42) has come to autopsy 2 years after LP and PIB testing. This participant was CDR 0 at the time of LP and PIB (6 months apart) but received a CDR rating of 0.5 (very mild dementia) just prior to death. Subsequent histological analysis of the brain revealed abundant diffuse but few neuritic (amyloid) plaques, (Cairns et al, 2009) consistent with the first proposed hypothesis.
Despite the strong relationship between PIB binding and CSF Aβ42, we observed no relationship between cortical amyloid load and plasma levels of Aβ42, Aβx–42, Aβ40 or Aβx–40 (Fig. 3). Our previous study reported the same results in a much smaller, clinically mixed cohort. Furthermore, for the present study we used the xMAP plasma kit (Inno-Bia Plasma Aβ Forms Multiplex Assay) which generates reliable values in the lower pg/ml range required for plasma measures (Blennow et al, 2009; Lachno et al, 2009). We obtained reliable values (with low coefficients of variability) for all but five samples; these five had very low levels of Aβx–42 that were below the level of detection so they were assigned a value of 0 pg/ml.
Figure 3. Cortical amyloid as detected by PET PIB and its relationship to plasma Aβ42 and Aβ40 species in CDR 0 participants (n = 189).
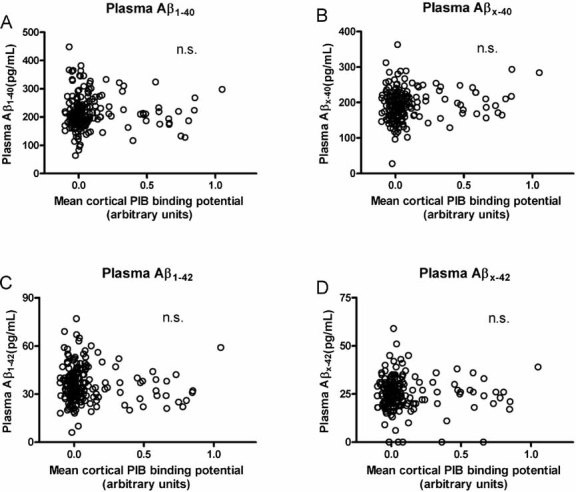
No relationship was observed between mean cortical PIB binding and plasma
- Aβ1–40 (r = −0.0724, p = 0.3234),
- Aβx–40 (r = 0.04583, p = 0.5323),
- Aβ1–42 (r = −0.1015, p = 0.1658) or
- Aβx–42 (r = −0.03869, p = 0.5981). Five participants had levels of plasma Aβx–42 below the level of detection so they are represented as having 0 pg/ml. All Pearson correlation coefficients are corrected for age. n.s., not significant.
Importantly, analysis of this CDR 0 cohort revealed a novel pattern of increases in CSF tau (and ptau181) with increasing cortical amyloid deposition (Fig 4A,B). Elevations in CSF tau in general did not appear to occur substantially in participants with an MCBP less than 0.5 but did increase in many, but not all, participants with binding potentials 0.5 and greater. Regression analyses correcting for age revealed a linear relationship between CSF tau (and ptau181) and PIB binding (Fig 4A,B). In addition, the ratios of CSF tau/Aβ42 and ptau181/Aβ42 also increased linearly with amyloid deposition and the correlations were particularly robust (Fig 4C,D). Similar to what we observed for CSF tau and ptau181, the ratios of tau and ptau181 to CSF Aβ42 were generally not elevated until substantial PIB binding values were reached.
Figure 4. Cortical amyloid as detected by PET PIB and its relationship to CSF tau and ptau181 and the ratios of CSF tau/Aβ42 and ptau181/Aβ42 in CDR 0 participants (n = 189).
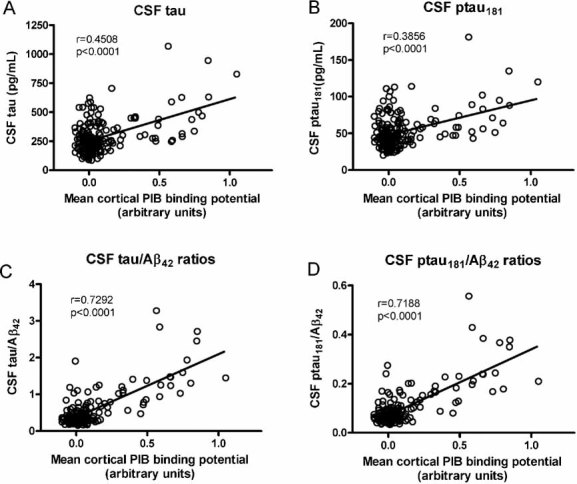
A linear relationship is observed between the amount of cortical amyloid and
- the levels of CSF tau
- the levels of CSF ptau181
- the ratios of CSF tau/Aβ42 and
- the ratios of the ptau181/Aβ42. The correlations between the CSF tau(s)/Aβ42 ratios and MCBP remain significant even when the statistical outlier (high PIB, high ratio) is omitted from the analysis (tau/Aβ42, r = 0.74227, p < 0.0001; ptau181/Aβ42, r = 0.73510, p < 0.0001). All Pearson correlation coefficients are corrected for age.
All participants in this cognitively normal cohort were administered a common battery of psychometric tests, including Selective Reminding (a measure of episodic memory) (Grober et al, 1988), Animal Naming (assesses semantic memory) (Goodglass & Kaplan, 1983) and a speeded visuospatial test with two parts: Trailmakings A and B (Armitage, 1946). None of the biomarker measures showed significant associations with performance on any of the psychometric tests, with the exception of negative correlations between Trailmaking A and CSF Aβ38 (r = −0.14621, p = 0.0464) and plasma Aβ1–40 (r = −0.24069, p = 0.0009). Due to the number of statistical tests conducted overall, however, some statistically significant differences could be due to chance.
DISCUSSION
The data presented here are part of an ongoing longitudinal study investigating fluid and imaging measures as possible antecedent (preclinical) biomarkers of AD. Importantly, they shed light on what may be the pathophysiology of the earliest events in the disease process and their relationship with CSF biomarkers. Consistent with our previous, smaller studies which included both non-demented and demented individuals (Fagan et al, 2006, 2007), we observed a robust relationship between cortical PIB binding and levels of CSF Aβ42 but not CSF Aβ40 (or Aβ38) in this large cohort of cognitively normal participants. While this relationship is linear, visual inspection of the graphs gives the impression of CSF Aβ42 levels dropping early in the disease process and staying low as the amount of cortical amyloid increases. The lack of correlation we observed between CSF Aβ38 and the amount of cortical amyloid is consistent with other studies suggesting this Aβ species does not change with dementia status (Mehta & Pirttila, 2005; Welge et al, 2009). However, these studies suggested that the ratio of Aβ38 to Aβ42, as opposed to Aβ38 alone, may have better specificity for distinguishing AD from controls or other non-AD dementias, although not all studies have reported this (Schoonenboom et al, 2005). The positive correlation we observed between cortical amyloid and the ratio of Aβ38 to Aβ42 is likely driven by the drop in Aβ42, as suggested by others (Mehta & Pirttila, 2005).
We were also now able to measure, with great sensitivity and reliability, a number of Aβ species in plasma, including Aβ1–40, Aβx–40, Aβ1–42 and Aβx–42. However, the level of these species did not in any way relate to the presence or amount of amyloid in the brain. Previous studies investigating the utility of plasma Aβ species have reported mixed results. While plasma Aβ42 has been reported to be neither specific nor sensitive for a clinical diagnosis of mild cognitive impairment (MCI) or AD (Fukumoto et al, 2003), nor in predicting the probability of progression from MCI to AD (Hansson et al, 2008), other studies have reported that the ratio of plasma Aβ42/Aβ40 may be useful as an antecedent marker for identifying risk for developing cognitive impairment in cognitively normal elders (Graff-Radford et al, 2007). Others have reported alterations in the direction of change in plasma Aβ species over the course of the disease (Schupf et al, 2008). Using the same protocol as we used in the present study, a very recent study reported significant decreases in the levels of plasma Aβ42 and the Aβ42/Aβ40 ratio in those who were classified as having AD or MCI that would progress to AD (with subjects preselected based on clinical and CSF biomarker profiles) compared to those who did not have the ‘abnormal’ profile (Lewczuk et al, 2009). However, this decrease was not particularly robust, on the order of 10–15%, with great overlap between the groups. Thus, while the mechanism(s) underlying potential changes in Aβ metabolism in plasma is still unclear, our cross-sectional data indicate that Aβ42 levels in plasma do not reflect the amount of amyloid in the brain in cognitively normal individuals (nor are they related to the level of Aβ42 in the CSF, data not shown).
In this large cohort we now observed a new grouping of participants; those who had low CSF Aβ42 levels in the absence of cortical PIB binding. It is unlikely that this is simply an APOE effect (Sunderland et al, 2004) since the frequency of the ε4 allele did not differ between the low PIB groups with low versus high CSF Aβ42. Instead, these data suggest that CSF Aβ42 may drop prior to amyloid becoming detectable by PIB, or this drop may reflect the presence of diffuse plaques and/or oligomeric Aβ species, consistent with the participant in the lower (low PIB/low CSF Aβ42) quadrant who has come to autopsy with abundant diffuse, but few amyloid (neuritic), plaques (Cairns et al, 2009). However, we cannot rule out the possibility that an absence of PIB binding in the face of low CSF Aβ42 simply reflects amyloid-free individuals who are at the low end of the normal spectrum of CSF Aβ42 levels. Longitudinal follow-up (with clinical, PIB and CSF measures) of such subjects will be important for understanding the biological relevance of this pattern.
Finally, we report the novel finding of a positive linear correlation between CSF tau and ptau181 with the amount of cortical amyloid in cognitively normal individuals. However, neither the amount of amyloid nor the levels of CSF Aβ42 or tau/ptau correlated with psychometric test performance. Although we did observe a significant correlation between Trailmaking A and plasma Aβ1–40, this finding awaits replication since it could be due to chance considering the large number of statistical tests conducted overall. Interestingly, those who developed elevated CSF tau, ptau181 or an elevated tau(s)/Aβ42 ratio appear to be individuals who have already developed a substantial amyloid load. Together, these human data are consistent with the idea that in the natural progression of AD neuropathology, changes in brain Aβ42 metabolism and amyloid formation occur early in disease pathogenesis, and that disruptions in CSF tau metabolism occur in larger amounts after Aβ42 aggregates and amyloid accumulates. Neuropathological studies have demonstrated the presence of tangles in certain brain regions (e.g. entorhinal cortex) as early as the fifth decade of life (Braak & Braak, 1997). The relationship between tangles and CSF tau is not clear in non-demented individuals. Longitudinal data within individuals would be required to prove the sequence of neuropathological events during the natural course of preclinical AD. However, our data showing increases in CSF tau primarily in individuals who already have substantial amyloid (as detected by PET PIB) is consistent with results from transgenic mice in which Aβ aggregation/deposition appears to exacerbate tauopathy and neurodegeneration regardless of the actual sequence of events (Gotz et al, 2001; Lewis et al, 2001; Oddo et al, 2003a,b). These data are also consistent with recent reports suggesting that PIB accumulation occurs with mild brain atrophy (Bakkour et al, 2009; Dickerson et al, 2009; Fagan et al, 2009) and prior to more marked atrophy (Jack et al, 2009). If confirmed, this sequence has important implications for the detection of AD pathology and treatment. A correlation between levels of tau/ptau, presumed markers of neurodegeneration, and the amount of cortical amyloid in cognitively normal individuals suggests that increasing amyloid deposition is driving neurodegeneration in the preclinical period of AD, and that treatments aimed at lowering or preventing amyloid accumulation may have the potential to also reduce neurodegeneration as reflected by CSF tau/ptau in the preclinical period. It will be important to see if reducing amyloid burden or halting its accumulation correlates with prevention of cognitive decline.
We observed many non-demented individuals with the typical Aβ-related AD biomarker pattern (high PIB binding and low CSF Aβ42). The fact that one can be cognitively normal (CDR 0) and have cortical amyloid speaks to the feasibility of PET PIB and/or CSF Aβ42 levels as being good identifiers of preclinical AD cases, but only if one can show that these individuals eventually go on to dement (Morris et al, 2009). Additional clinical follow-up is needed before strong conclusions can be drawn from this group of individuals. It is striking that, at least in some individuals, the brain can withstand a substantial amount of amyloid before they are detected as being clinically impaired, if they become impaired at all. It is likely that there exist additional genetic and/or epigenetic (environmental) factors that dictate or modify the brain's response to amyloid. Perhaps those are the substrates of ‘cognitive reserve’. Regardless, it is becoming clear that an increase in CSF tau and ptau species, in the presence of cortical amyloid, likely marks a tipping point, as enough neurofibrillary tangle formation/neuritic dystrophy and neuronal cell death has taken place to result in observable cognitive decline. Consistent with this idea, the CSF tau/Aβ42 ratio has been reported by several groups, including our own, to predict future cognitive decline in cognitively normal elders (Fagan et al, 2007; Li et al, 2007) and in those with MCI/very mild dementia (Hansson et al, 2006; Mattsson et al, 2009; Snider et al, 2009).
The ultimate goal of AD research is to develop treatments that will cure the disease or, at the least, delay its onset and/or slow its progression. Many strategies are being employed to this end (Rafii & Aisen, 2009; Salloway et al, 2008). As a complementary objective, AD biomarker research aims to develop markers (fluid, imaging, genetic, cognitive, etc.) that will identify the presence of AD pathology with high sensitivity and specificity, especially in the preclinical and very early disease stages so new treatments will have the best chance of preserving normal brain function. However, in the shorter term, we propose that such biomarkers will be very useful for identifying early stage patients to enroll in clinical trials and, in certain cases, to evaluate treatment efficacy. Many clinical trials testing various AD targets have been carried out but, unfortunately, have been considered failures since the test treatment did not prevent or slow cognitive decline (for review, see Rafii & Aisen, 2009). Given that so much AD pathology (plaques, neurofibrillary tangles, inflammation and neuronal dysfunction) is already present in the apparent preclinical stages (Aizenstein et al, 2008; Fagan et al, 2006, 2007; Hulette et al, 1998; Mintun et al, 2006; Price & Morris, 1999), it seems logical that future secondary prevention trials should employ promising biomarkers (e.g. PET PIB, CSF Aβ42, CSF tau/Aβ42) to enrich treatment groups for non-demented, preclinical cases. Such a strategy would decrease the number of patients necessary to be enrolled, as well as potentially shorten the trial duration, thus getting us closer to finding a true treatment for this devastating disease.
MATERIALS AND METHODS
Participants
Participants were cognitively normal, community-dwelling volunteers enrolled in longitudinal studies of healthy aging and dementia through the Washington University Alzheimer Disease Research Center (ADRC). Participants were 43–89 years of age and in good general health, having no neurological, psychiatric or systemic medical illness that could contribute importantly to dementia, nor medical contraindication to LP for CSF collection or PET with the amyloid imaging agent PIB (Klunk et al, 2004). Cognitive status was determined in accordance with standard protocols and criteria (Berg et al, 1998; Morris et al, 1988). A CDR (Morris, 1993) of 0 indicated no dementia. All CDR 0 participants who had an LP and a PIB scan within 2 years of each other were included in the analysis (n = 189). The scan could be before or after the LP. In cases where participants had more than one LP or PIB scan, we analysed values from the tests most closely together in time. In this study, we defined a participant's cognitive status from the clinical assessment just prior to LP. All studies were approved by the Human Studies Committee at Washington University, and written informed consent was obtained from all participants. APOE genotyping was kindly performed by the Washington University ADRC Genetics Core under the direction of Dr Alison Goate.
Psychometric assessments
Psychometric tests that assess a broad spectrum of abilities across multiple cognitive domains were administered to all participants by trained psychometricians usually a week or two after the clinical assessment. Participants in the present study receive one of two test batteries depending on the purpose of the study in which they were initially enrolled. The tests examined in this report were common to the two batteries. Selective Reminding test (sum of three free recall trials) measures episodic memory (Grober et al, 1988). Animal Naming (name as many animals as possible in 1 min) assesses semantic memory (Goodglass & Kaplan, 1983). The final test is a speeded visuospatial test with two parts: Trailmakings A and B (number of connections per second for each part) (Armitage, 1946).
The paper explained
PROBLEM
AD pathology is estimated to develop many years before detectable cognitive decline. Once symptoms are apparent, the brain has already experienced substantial neuronal and synaptic loss. Thus there is a great need to develop biomarkers that can identify people in the very earliest symptomatic and even ‘preclinical’ stages, prior to any cognitive impairment, when potential treatments will have the best opportunity to preserve cognitive function.
RESULTS
We analysed CSF samples and in parallel determined the amount of cortical amyloid as evidenced by retention of the in vivo amyloid binding agent, PIB, in 189 cognitively normal research participants (age 43–89 years). We observed a positive linear relationship between the levels of CSF tau and ptau181 (primary constituents of neurofibrillary tangles) with the amount of cortical amyloid. We also observed a strong inverse relationship between cortical PIB binding and CSF Aβ42 (the primary constituent of amyloid plaques), but not plasma Aβ species, demonstrating that a low level of CSF Aβ42 is an excellent marker of brain amyloid even in the absence of cognitive symptoms.
IMPACT
The data obtained shed light on the potential utility of PIB amyloid imaging and CSF Aβ42, tau and ptau181 as antecedent (‘preclinical’) biomarkers of AD and also provide insight into the normal time course of the pathophysiology of the disease as reflected in the CSF. These findings have important implications for preclinical AD diagnosis and treatment, and should aid in the design and evaluation of secondary prevention trials in AD.
CSF and plasma collection and processing
CSF (20–30 ml) was collected at 8:00 am after overnight fasting as previously described (Fagan et al, 2006). Samples were gently inverted to avoid possible gradient effects, briefly centrifuged at low speed to pellet any cellular debris and aliquoted into polypropylene tubes prior to freezing at −84 °C (Fagan et al, 2006). Fasted blood was obtained at the time of LP and collected into polypropylene tubes containing EDTA. Plasma was prepared by standard centrifugation methods prior to aliquoting and freezing at −84 °C.
CSF and plasma biomarker assessment
CSF samples were analysed for Aβ42, total tau and phospho-tau181 (ptau181) by enzyme-linked immunosorbant assay (ELISA) (Innotest™, Innogenetics, Ghent, Belgium). CSF Aβ40 was assayed by ELISA (Cirrito et al, 2003), and plasma Aβ1–40, Aβx–40 and Aβ1–42 and Aβx–42 were analysed by xMAP (bead-based) ELISA (Inno-Bia Plasma Aβ Forms Multiplex Assay™; Innogenetics, Ghent, Belgium). CSF Aβ38 was analysed by an antibody-based electrochemiluminescence assay according to the manufacturer's recommendations (MSD 96 well Multi-Array Human (6E10) Abeta 38 Ultra-Sensitive Kit™, Meso Scale Discovery, Gaithersburg, MD). For all biomarker measures, samples were continuously kept on ice, and assays were performed on sample aliquots after a single thaw following initial freezing.
In vivo amyloid imaging with PIB
All participants underwent in vivo amyloid imaging via PET PIB as previously described (Mintun et al, 2006) within 2 years of LP. The cerebellum was chosen as a region with very low specific PIB binding for use as a reference region, and Logan graphical analyses were performed to calculate the mean cortical PIB distribution volume (Logan et al, 1996) for each participant. The value for MCBP was defined by averaging the distribution volumes for the prefrontal cortex, precuneus, lateral temporal cortex and gyrus rectus.
Statistical analyses
All statistical analyses were carried out using SAS (SAS Institute, Inc., Cary, NC). The relationships between MCBP and the fluid measures and between the biomarker measures and psychometric test scores were determined by Pearson correlations, adjusting for age. Chi-square was used to determine whether APOE ε4 status differed across the PIB/CSF Aβ42 quadrants (low PIB/high CSF Aβ42 vs. low PIB/low CSF Aβ42 vs. high PIB/low CSF Aβ42). Due to small expected frequencies in some cells, Fisher's exact test was used to test whether there were associations between various comorbidities and quadrant membership. One-way ANOVA was used to test whether the interval between PIB scan and LP differed across the quadrants. General linear models were used to examine whether quadrant membership was significantly associated with scores on the four psychometric tests, after adjusting for age. Statistical significance was defined as p < 0.05.
Author contributions
A. M. F. oversaw the CSF studies, performed some of the CSF analyses, created the final dataset and graphs, evaluated and interpreted the data and wrote the paper. M. A. M. oversaw the PIB studies, assisted in data evaluation and interpretation, provided critical comments during the writing of the paper and obtained funding. A. R. S. processed the CSF samples, performed some of the CSF analyses and managed the CSF database. P. A. contributed to the analysis of the PIB data. C. M. R. performed the statistical analyses. R. H. M. contributed to the PIB studies. D. M. managed the PIB. database. J. C. M. oversaw the clinical studies, performed some of the clinical evaluations, assisted in data evaluation and interpretation, provided critical comments during the writing of the paper and obtained funding. D. M. H. performed some of the LPs, assisted in data evaluation and interpretation, provided critical comments during the writing of the paper and obtained funding.
Acknowledgments
We gratefully acknowledge the contributions of Ms S. Sathyan, Dr J. Christiansen, Dr R. Perrin and Dr J. Ladenson, our lumbar puncture physicians, and the Clinical, Psychometrics, Imaging and Biostatistics Cores of the Washington University Alzheimer's Disease Research Center. Supported by grants: P50 AG05681 (J. C. M.), P01 AG03991 (J. C. M.), P01 AG026276 (J. C. M.) and P30 NS057105 (D. M. H.) from the National Institute of Aging of the National Institutes of Health; P30 NS048056 (M. A. M.) from the National Institute of Neurological Disorders and Stroke; UL1 RR024992, National Center for Research Resources, NIH Roadmap for Medical Research (M. A. M.), the Dana Foundation (M. A. M.) and the Charles and Joanne Knight Alzheimer Research Initiative (J. C. M.).
The authors declare that they have no conflict of interest.
For more information
Alzheimer's Association
Alzheimer's Disease Research Center at Washington University School of Medicine
Alzheimer Research Forum
Alzheimer's Disease Education and Referral Center
References
- Aizenstein H, Nebes R, Saxton J, Price J, Mathis C, Tsopelas N, Ziolko S, James J, Snitz B, Houck P, et al. Frequent amyloid deposition without significant cognitive impairment among the elderly. Arch Neurol. 2008;65:1509–1517. doi: 10.1001/archneur.65.11.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armitage S. An analysis of certain psychological tests used in the evaluation of brain injury. Psych Mono. 1946;60:1–48. [Google Scholar]
- Bakkour A, Morris J, Dickerson B. The cortical signature of prodromal AD: regional thinning predicts mild AD dementia. Neurology. 2009;72:1048–1055. doi: 10.1212/01.wnl.0000340981.97664.2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg L, McKeel DW, Miller JP, Storandt M, Rubin EH, Morris JC, Baty J, Coats M, Norton J, Goate AM, et al. Clinicopathologic studies in cognitively healthy aging and Alzheimer's disease—relation of histologic markers to dementia severity, age, sex, and apoE genotype. Arch Neurol. 1998;55:326–335. doi: 10.1001/archneur.55.3.326. [DOI] [PubMed] [Google Scholar]
- Blennow K, Meyer GD, Hansson O, Minthon L, Wallin A, Zetterberg H, Lewczuk P, Vanderstichele H, Vanmechelen E, Kornhuber J, et al. Evolution of Abeta42 and Abeta40 Levels and Abeta42/Abeta40 ratio in plasma during progression of Alzheimer's disease: a multicenter assessment. J Nutr Health Aging. 2009;13:205–208. doi: 10.1007/s12603-009-0059-0. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Frequency of stages of Alzheimer-related lesions in different age categories. Neurobiol Aging. 1997;18:351–357. doi: 10.1016/s0197-4580(97)00056-0. [DOI] [PubMed] [Google Scholar]
- Cairns N, Ikonomovic M, Benzinger T, Storandt M, Fagan A, Shah A, Reinwald L, Carter D, Felton A, Holtzman D, et al. PiB-PET detection of cerebral Aβ may lag clinical, cognitive, and CSF markers of Alzheimer's disease: a case report. Arch Neurol. 2009 doi: 10.1001/archneurol.2009.279. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirrito J, May P, O'Dell M, Taylor J, Parsadanian M, Cramer J, Audia J, Nissen J, Bales K, Paul S, et al. In vivo assessment of brain interstitial fluid with microdialysis reveals plaque-associated changes in amyloid-β metabolism and half-life. J Neurosci. 2003;23:8844–8853. doi: 10.1523/JNEUROSCI.23-26-08844.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig-Schapiro R, Fagan A, Holtzman D. Biomarkers of Alzheimer's disease. Neurobiol Dis. 2008;35:128–140. doi: 10.1016/j.nbd.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson B, Bakkour A, Salat D, Feczko E, Pacheco J, Greve D, Grodstein F, Wright C, Blacker D, Rosas H, et al. The cortical signature of Alzheimer's disease: regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid-positive individuals. Cereb Cortex. 2009;19:497–510. doi: 10.1093/cercor/bhn113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan A, Head D, Shah A, Marcus D, Mintun M, Morris J, Holtzman D. Decreased cerebrospinal fluid Aβ42 correlates with brain atrophy in cognitively normal elderly. Ann Neurol. 2009;65:175–183. doi: 10.1002/ana.21559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan A, Mintun M, Mach R, Lee S-Y, Dence C, Shah A, LaRossa G, Spinner M, Klunk W, Mathis C, et al. Inverse relation between in vivo amyloid imaging load and CSF Aβ42 in humans. Ann Neurol. 2006;59:512–519. doi: 10.1002/ana.20730. [DOI] [PubMed] [Google Scholar]
- Fagan A, Roe C, Xiong C, Mintun M, Morris J, Holtzman D. Cerebrospinal fluid tau/Aβ42 ratio as a prediction of cognitive decline in nondemented older adults. Arch Neurol. 2007;64:343–349. doi: 10.1001/archneur.64.3.noc60123. [DOI] [PubMed] [Google Scholar]
- Fukumoto H, Tennis M, Locascio J, Hyman B, Growdon J, Irizarry M. Age but not diagnosis is the main predictor of plasma amyloid beta-protein levels. Arch Neurol. 2003;60:958–964. doi: 10.1001/archneur.60.7.958. [DOI] [PubMed] [Google Scholar]
- Gomez-Isla T, Price J, McKeel D, Morris J, Growdon J, Hyman B. Profound loss of layer II entorhinal cortex neurons occurs in very mild Alzheimer's disease. J Neurosci. 1996;16:4491–4500. doi: 10.1523/JNEUROSCI.16-14-04491.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodglass H, Kaplan E. Boston Naming Test scoring booklet. Philadelphia: Lea & Febiger; 1983. [Google Scholar]
- Gotz J, Chen F, Dorpe JV, Nitsch R. Formation of neurofibrillary tangles in P301l tau transgenic mice induced by Abeta 42 fibrils. Science. 2001;293:1491–1495. doi: 10.1126/science.1062097. [DOI] [PubMed] [Google Scholar]
- Graff-Radford N, Crook J, Lucas J, Boeve B, Knopman D, Ivnik R, Smith G, Younkin L, Petersen R, Younkin S. Association of low plasma Aβ42/Aβ40 ratios with increased imminent risk for mild cognitive impairment and Alzheimer disease. Arch Neurol. 2007;64:354–362. doi: 10.1001/archneur.64.3.354. [DOI] [PubMed] [Google Scholar]
- Grober E, Buschke H, Crystal H, Bang S, Dresner R. Screening for dementia by memory testing. Neurology. 1988;3:900–903. doi: 10.1212/wnl.38.6.900. [DOI] [PubMed] [Google Scholar]
- Hansson O, Zetterberg H, Buchhave P, Londos E, Blennow K, Minthon L. Association between CSF biomarkers and incipient Alzheimer's disease in patients with mild cognitive impairment: a follow-up study. Lancet Neurol. 2006;5:228–234. doi: 10.1016/S1474-4422(06)70355-6. [DOI] [PubMed] [Google Scholar]
- Hansson O, Zetterberg H, Vanmechelen E, Vanderstichele H, Andreasson U, Londos E, Wallin A, Minthon L, Blennow K. Evaluation of plasma Abeta(40) and Abeta(42) as predictors of conversion to Alzheimer's disease in patients with mild cognitive impairment. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.03.027. in press, doi: 10.1016/j.neurobiolaging.2008.03.027. [DOI] [PubMed] [Google Scholar]
- Hulette CM, Welsh-Bohmer KA, Murray MG, Saunders AM, Mash DC, McIntyre LM. Neuropathological and neuropsychological changes in “normal” aging: evidence for preclinical Alzheimer disease in cognitively normal individuals. J Neuropathol Exp Neurol. 1998;57:1168–1174. doi: 10.1097/00005072-199812000-00009. [DOI] [PubMed] [Google Scholar]
- Jack C, Lowe V, Weigand S, Wiste H, Senjem M, Knopman D, Shiung M, Gunter J, Boeve B, Kemp B, et al. Serial PIB and MRI in normal, mild cognitive impairment and Alzheimer's disease: implications for sequence of pathological events in Alzheimer's disease. Brain. 2009;132:1355–1365. doi: 10.1093/brain/awp062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klunk W, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt D, Bergström M, Savitcheva I, Huang G-F, Estrada S, et al. Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Ann Neurol. 2004;55:306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- Lachno D, Vanderstichele H, Groote GD, Kostanjevecki V, Meyer GD, Siemers E, Willey M, Bourdage J, Konrad R, Dean R. The influence of matrix type, diurnal rhythm and sample collection and processing on the measurement of plasma beta-amyloid isoforms using the Inno-Bia Plasma Abeta Forms Multiplex Assay. J Nutr Health Aging. 2009;13:220–225. doi: 10.1007/s12603-009-0062-5. [DOI] [PubMed] [Google Scholar]
- Lewczuk P, Kornhuber J, Vanmechelen E, Peters O, Heuser I, Maier W, Jessen F, Bürger K, Hampel H, Frölich L, et al. Amyloid beta peptides in plasma in early diagnosis of Alzheimer's disease: a multicenter study with multiplexing. Exp Neurol. 2009 doi: 10.1016/j.expneurol.2009.07.024. (in press), PMID:19664622. [DOI] [PubMed] [Google Scholar]
- Lewis J, Dickson D, Lin W, Chisholm L, A AAC, Jones G, Yen S, Sahara N, Skipper L, Yager D, et al. Enhanced neurofibrillary degeneration in transgenic mice expressing mutant tau and APP. Science. 2001;293:1487–1491. doi: 10.1126/science.1058189. [DOI] [PubMed] [Google Scholar]
- Li G, Sokal I, Quinn J, Leverenz J, Brodey M, Schellenberg G, Kaye J, Raskind M, Zhang J, Peskind E, et al. CSF tau/Aβ42 ratio for increased risk of mild cognitive impairment: a follow-up study. Neurology. 2007;69:631–639. doi: 10.1212/01.wnl.0000267428.62582.aa. [DOI] [PubMed] [Google Scholar]
- Logan J, Fowler J, Volkow N, Wang G, Ding Y, Alexoff D. Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab. 1996;16:834–840. doi: 10.1097/00004647-199609000-00008. [DOI] [PubMed] [Google Scholar]
- Markesbery W, Schmitt F, Kryscio R, Davis D, Smith C, Wekstein D. Neuropathologic substrate of Mild Cognitive Impairment. Arch Neurol. 2006;63:38–46. doi: 10.1001/archneur.63.1.38. [DOI] [PubMed] [Google Scholar]
- Mattsson N, Zetterberg H, Hansson O, Andreasen N, Parnetti L, Jonsson M, Herukka S, Van der Flier WM, Blankenstein M, Ewers M, et al. CSF biomarkers and incipient Alzheimer disease in patients with mild cognitive impairment. JAMA. 2009;302:385–393. doi: 10.1001/jama.2009.1064. [DOI] [PubMed] [Google Scholar]
- Mehta P, Pirttila T. Increased cerebrospinal fluid Abeta38/Abeta42 ratio in Alzheimer's disease. Neurodegener Dis. 2005;2:242–245. doi: 10.1159/000090363. [DOI] [PubMed] [Google Scholar]
- Mintun M, LaRossa G, Sheline Y, Dence C, Lee S-Y, Mach R, Klunk W, Mathis C, DeKosky S, Morris J. [11C]PIB in a nondemented population: potential antecedent marker of Alzheimer disease. Neurology. 2006;67:446–452. doi: 10.1212/01.wnl.0000228230.26044.a4. [DOI] [PubMed] [Google Scholar]
- Morris J, McKeel D, Fulling K, Torack R, Berg L. Validation of clinical diagnostic criteria for Alzheimer's disease. Ann Neurol. 1988;24:17–22. doi: 10.1002/ana.410240105. [DOI] [PubMed] [Google Scholar]
- Morris J, Price J. Pathologic correlates of nondemented aging, mild cognitive impairment, and early stage Alzheimer's disease. J Mol Neurosci. 2001;17:101–118. doi: 10.1385/jmn:17:2:101. [DOI] [PubMed] [Google Scholar]
- Morris J, Roe C, Grant E, Head D, Storandt M, Goate A, Fagan A, Holtzman D, Mintun M. PIB imaging predicts progression from cognitively normal to symptomatic Alzheimer's disease. Arch Neurol. 2009 doi: 10.1001/archneurol.2009.269. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC. The Clinical Dementia Rating (CDR). Current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Kitazawa M, Tseng B, LaFerla F. Amyloid deposition precedes tangle formation in a triple transgenic model of Alzheimer's disease. Neurobiol Aging. 2003a;24:1063–1070. doi: 10.1016/j.neurobiolaging.2003.08.012. [DOI] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Shepherd J, Murphy M, Golde T, Kayed R, Metherate R, Mattson M, Akbari Y, LaFerla F. Triple-transgenic model of Alzheimer's disease with plaques and tangles: intracellular Aß and synaptic dysfunction. Neuron. 2003b;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- Price J, Ko A, Wade M, Tsou S, McKeel D, Morris J. Neuron number in the entorhinal cortex and CA1 in preclinical Alzheimer's disease. Arch Neurol. 2001;58:1395–1402. doi: 10.1001/archneur.58.9.1395. [DOI] [PubMed] [Google Scholar]
- Price JL, Morris JC. Tangles and plaques in nondemented aging and “preclinical” Alzheimer's disease. Ann Neurol. 1999;45:358–368. doi: 10.1002/1531-8249(199903)45:3<358::aid-ana12>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Rafii M, Aisen P. Recent developments in Alzheimer's disease therapeutics. BMC Med. 2009 doi: 10.1186/1741-7015-7-7. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salloway S, Mintzer J, Weiner M, Cummings J. Disease-modifying therapies in Alzheimer's disease. Alzheimers Dement. 2008;4:65–79. doi: 10.1016/j.jalz.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Schoonenboom N, Mulder C, Kamp GV, Mehta S, Scheltens P, Blankenstein M, Mehta P. Amyloid β 38, 40, and 42 species in cerebrospinal fluid: more of the same? Ann Neurol. 2005;58:139–142. doi: 10.1002/ana.20508. [DOI] [PubMed] [Google Scholar]
- Schupf N, Tang M, Fukuyama H, Manly J, Andrews H, Mehta P, Ravetch J, Mayeux R. Peripheral Abeta subspecies as risk biomarkers of Alzheimer's disease. Proc Natl Acad Sci USA. 2008;105:14052–14057. doi: 10.1073/pnas.0805902105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider B, Fagan A, Roe C, Shah A, Grant E, Xiong C, Morris J, Holtzman D. Cerebrospinal fluid biomarkers and rate of cognitive decline in very mild dementia of the Alzheimer type. Arch Neurol. 2009;66:638–645. doi: 10.1001/archneurol.2009.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunderland T, Mirza N, Putnam K, Linker G, Bhupali D, Durham R, Soares H, Kimmel L, Friedman D, Bergeson J, et al. Cerebrospinal fluid beta-amyloid -42 and tau in control subjects at risk for Alzheimer's disease: the effect of APOE epsilon4 allele. Biol Psychiatry. 2004;56:670–676. doi: 10.1016/j.biopsych.2004.07.021. [DOI] [PubMed] [Google Scholar]
- Welge V, Fiege O, Lewczul P, Mollenhauer B, Esselmann H, Klafki H-W, Wolf S, Trenkwalder C, Otto M, Kornhuber J, et al. Combined CSF tau, p-tau181 and amyloid-β 38/40/42 for diagnosing Alzheimer's disease. J Neural Transm. 2009;116:203–212. doi: 10.1007/s00702-008-0177-6. [DOI] [PubMed] [Google Scholar]


