Abstract
Drugs that interfere with cannabinoid CB1 transmission suppress food-motivated behaviors, and may be clinically useful as appetite suppressants. Several CB1 receptor inverse agonists, such as rimonabant and AM251, as well as the CB1 receptor neutral antagonist, AM4113, have been assessed for their effects on food-motivated behavior. One important criterion for establishing if a drug may be useful clinically is the determination of its oral bioavailability. The present studies compared the effects of AM4113 and a novel CB1 antagonist, AM6527, on the suppression of food-reinforced behavior following intraperitoneal (IP) and oral administration. AM4113 and AM6527 both suppressed lever pressing after IP injections. The ED50 for the effect on FR5 responding was 0.78 mg/kg for IP AM4113, and 0.5763 mg/kg for IP AM6527. AM6527 also was effective after oral administration (ED50 = 1.49 mg/kg), however, AM 4113 was ineffective up to oral doses of 32.0 mg/kg. AM 4113 may be very useful as a research tool, but its lack of oral activity suggests that this drug might not be effective if orally administered in humans. In contrast, AM 6527 is an orally active CB1 antagonist, which may be useful for clinical research on the appetite suppressant effects of CB1 antagonists.
Keywords: Appetite, Motivation, Operant, Feeding, THC, Rimonabant
1. Introduction
Considerable evidence indicates that cannabinoid systems are involved in the regulation of feeding and food-motivated behaviors. CB1 receptor agonists such as delta-9 tetrahydrocannabinol, anandamide and 2-AG have been shown to elevate levels of food intake (Jamshidi and Taylor, 2001; Kirkham et al., 2002; Williams and Kirkham, 1999). Conversely, food intake is impaired by CB1 receptor inverse agonists such as rimonabant (SR141716A), AM251 and AM1387 (Arnone et al., 1997; Colombo et al., 1998; Simiand et al., 1998; Williams and Kirkham 1999; McLaughlin et al., 2003, 2005, 2006; Salamone et al., 2007), and by CB1 antagonists including AM4113 and O-2050 (Gardner and Mallet, 2006; Salamone et al., 2007; Sink et al., 2008a,b). Drugs that interfere with CB1 receptor transmission also have been shown to impair food-reinforced behavior (Freedland et al., 2000; McLaughlin et al., 2003, 2006; Ward and Dyskstra, 2005; Salamone et al., 2007; Sink et al., 2008a,b). Based upon these actions on food intake and food-reinforced behavior, as well as other metabolic effects, it has been suggested that drugs that interfere with cannabinoid CB1 transmission could be useful as treatments for obesity (Pi-Sunyer et al, 2006; Van Gaal et al., 2005).
One of the important criteria for establishing whether or not a drug may be useful clinically is the determination of the oral bioavailability of that drug (Jogani et al., 2007; Ohta et al., 2007). Several previous studies have shown that rimonabant is orally active (Huestis et al, 2001; Costa et al., 2005; Davis and Nomikos, 2008). The present studies were conducted to compare the effects of AM4113 and a novel CB1 antagonist, AM6527, on the suppression of food-reinforced behavior following intraperitoneal (IP) and oral administration. AM4113 is a recently described CB1 receptor antagonist that lacks CB1-mediated signal transduction effects in human CB1 transfected cell lines, and is a pyrazole-3-carboxamide analog of SR141716A (Sink et al., 2008a). It was recently reported that this drug effectively reduces feeding and food-reinforced lever pressing behavior in a manner similar to AM251, but in contrast to the inverse agonist AM251, AM4113 did not induce behaviors associated with nausea and malaise (Sink et al., 2008a). Unlike inverse agonists such as rimonabant and AM251, AM4113 also failed to produce nausea in ferrets (Chambers et al., 2007). Because of this reduced propensity for inducing aversive side effects such as nausea, it is important to characterize the effects of antagonists such as AM4113, and the determination of the oral bioavailability of this drug is a critical part of that evaluation. The other CB1 antagonist evaluated in the present study was AM6527. This is the first behavioral evaluation of the effects of AM6527 (see receptor binding data below), and neither AM6527 nor AM4113 have been evaluated for their oral bioavailability. In the present studies, both drugs were assessed using food-reinforced lever pressing on a fixed ratio 5 (FR5) schedule of reinforcement. This procedure was used because of the large body of previous data showing that it is highly sensitive to the effects of drugs that interfere with CB1 receptor transmission (McLaughlin et al., 2003, 2006; Salamone et al., 2007; Sink et al., 2008a,b). Both drugs were initially studied for their effects after IP administration, and two additional experiments investigated the effects of these drugs after oral administration.
2. Methods
2.1. Animals
For the behavioral experiments, adult male Sprague–Dawley rats (Harlan, Indianapolis, IN) were housed in a colony room on a 12-h light–dark cycle (lights on during 0700–1900). All experiments were conducted during the light part of the cycle. Rats were food-deprived to 85% of their free-feeding body weight and weighed daily. All animal protocols were approved by the University of Connecticut Institutional Animal Care and Use Committee and the methods used were in accordance with NIH guidelines.
2.2. Drugs and selection of doses
AM4113 and AM6527 were synthesized at the Center for Drug Discovery, Northeastern University. AM4113 is a pyrazole-3-carboxamide analog of SR141716A, and AM6527 a pyrazole-3-carboxamide analog of AM251. For intraperitoneal (IP) injection, AM4113 or AM6527 was suspended in a vehicle of 15% dimethylsulfoxide (DMSO), 15% Tween 80, and 70% saline (0.9%) and administered in a volume of 1.0 mL/kg. For oral administration, drug was suspended in a vehicle of Tween 80 and 0.9% saline in a ratio of 1:9, and administered in a volume of 2.0 mL/kg. The doses and lead times for AM4113 used in the IP experiments were based upon previous research (Sink et al., 2008a). Pilot studies were used to determine these values for AM6527, and to determine pretreatment times for oral administration of both drugs. Because AM6527 was found to be slightly more potent than AM4113 in the IP study (see Experiment 1 below), the oral dose progression for AM6527 covered a lower range than the oral doses used for AM4113 (Experiments 2 and 3). Oral administration of both drugs was performed using a gavage.
2.3. Rat brain CB1 and human CB2 binding assay
AM6527 was assessed for its affinity for CB1 and CB2 receptors using membrane preparations from rat brain or HEK293 cells expressing hCB2, respectively, as previously described using [3H]CP-55,940 (Morse et al., 1995; Lan et al., 1999; McLaughlin et al., 2006; Sink et al., 2008a). Results from the competition assays were analyzed using nonlinear regression to determine the IC50 values for the ligand (Prizm by GraphPad Software, Inc.); Ki values were calculated from the IC50 (Cheng and Prusoff, 1973). Each experiment was performed in triplicate and Ki values determined from at least three independent experiments. These methods are identical to those used previously to assess the binding characteristics of AM4113 and AM251 (Sink et al., 2008a).
2.4. Behavioral procedures
Rats were trained in operant chambers (internal dimensions: 20 × 21 × 28 cm, Med Associates) 30 min/day, 5 days/week for the duration of these experiments. After magazine training, all rats were trained for 2 weeks on an FR1 schedule in which each response was reinforced with one 45 mg sucrose pellet (Research Diets, New Brunswick, NJ). Following the 2 weeks of training on FR1, rats were trained for several more weeks on an FR5 schedule, receiving a single pellet for every fifth lever press. Operant conditioning test sessions were controlled by a MEDPC program, which also gathered the data.
2.5. Behavioral experiments
Following the initial training period, rats were injected with drug and then tested once a week on Thursdays. For experiment 1, AM4113 (n = 8) and AM6527 (n = 8) were injected IP at doses of 1.0, 2.0, 4.0, or 8.0 mg/kg or vehicle. Pretreatment time for these two drugs was 30 min. For experiments 2 and 3, rats were given drug or vehicle orally 1 h before testing. In experiment 2, rats (n = 8) were given vehicle or 8.0, 16.0, or 32.0 mg/kg AM4113. In experiment 3, rats (n = 8) received vehicle or AM6527 at doses of 4.0, 8.0, or 16.0 mg/kg. Within each experiment, all drug treatments were given to each rat using a repeated measures design, with each rat receiving all treatments in a randomly varied order over the successive weeks of the experiment. Different dose ranges were used in experiments 2 and 3 because the results of experiment 1 indicated that AM6527 was slightly more potent than AM4113 at suppressing lever pressing after IP administration.
2.6. Statistical analyses
Statistical analysis was performed using SPSS 14.0. Experiment 1 utilized a drug dose factorial analysis of variance (ANOVA) with repeated measures on the dose factor. ANOVA with repeated measures on the dose variable was employed to analyze data from experiments 2 and 3. Nonorthogonal planned comparisons (Keppel, 1982) were used to compare each drug treatment with vehicle. The overall ANOVA mean square error term was used in these calculations, and the number of comparisons was restricted to the number of drug conditions minus one. ED50 and 95% confidence intervals for the drug effect on the FR5 schedule was estimated using curvilinear regression analysis (GraphPad Prism), employing an exponential decay function.
3. Results
3.1. Receptor binding data for AM6527
CB1 and CB2 receptor binding data for AM6527 are shown in Table 1. AM6527 showed a relatively high affinity for CB1 receptors (4.88 nM), but a much lower affinity for CB2 receptors (463.0 nM). These results indicate that AM6527 shows approximately 100-fold selectivity for CB1 receptors relative to CB2 receptors. AM6527 was also profiled against a variety of neurotransmitter related receptors, ion-channels, enzymes and peptides and showed no affinity for any of these non-cannabinergic targets up to a concentration of 10 µM (data not shown).
Table 1.
Receptor binding data for AM6527
| Binding assay | AM6527 |
|---|---|
| CB1 binding Ki | 4.88 ± 1.50 |
| 95% confidence | (2.77, 8.79) |
| R2-value | 0.932 |
| CB2 binding Ki | 463 ± 158 |
| 95% confidence | (325, 659) |
| R2-value | 0.974 |
Values for Ki are in nM ± standard deviation of four (CB1) or three (CB2) assays done in triplicate (shown with 95% confidence intervals and R2-value).
3.2. Experiment 1
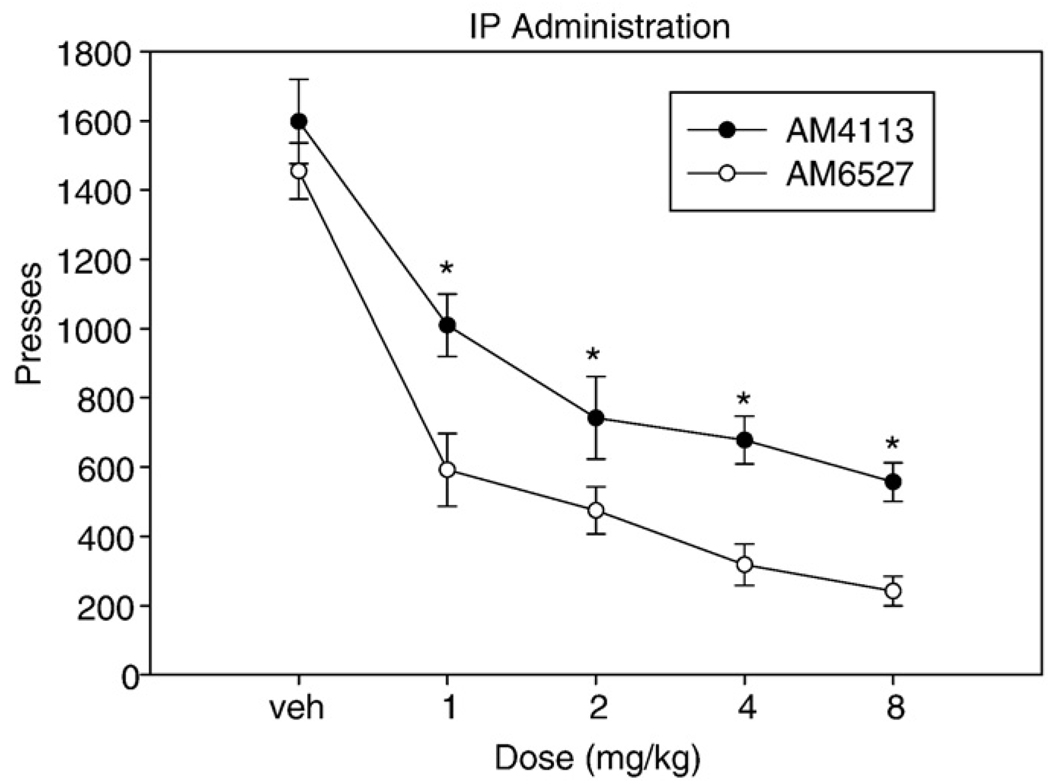
Fig. 1 depicts the effects of IP administration of AM4113 and AM6527 on FR5 responding. Factorial ANOVA with repeated measures on dose revealed a significant overall effect of dose on lever pressing [F(4,56) = 70.4, p < 0.001]. There were also significant differences between drug groups [F(1,14) = 16.8, p = 0.001], but no drug by dose interaction [F(4,56) = 0.942, n.s.]. Nonorthogonal planned comparisons revealed that every dose produced a significant decrease in lever pressing when compared to vehicle control (p < 0.05), and separate analyses showed that both AM4113 and AM6527 significantly suppressed FR5 responding compared to vehicle (p < 0.001). The ED50 for the effect on FR5 responding was 0.78 mg/kg (R2 = 0.68) for IP AM4113, and 0.58 mg/kg (R2 = 0.82) for IP AM6527.
Fig. 1.
The effects of IP administration of AM4113 and AM6527 on FR5 lever pressing. Mean (±SEM) number of lever presses (FR5 schedule) during the 30-min session for rats that received treatment with vehicle or drug at 1.0, 2.0, 4.0, or 8.0 mg/kg. Both AM4113 and AM6527 significantly decreased responding compared to vehicle (*p < 0.05, both drugs different from their respective vehicle treatments).
3.3. Experiments 2 and 3
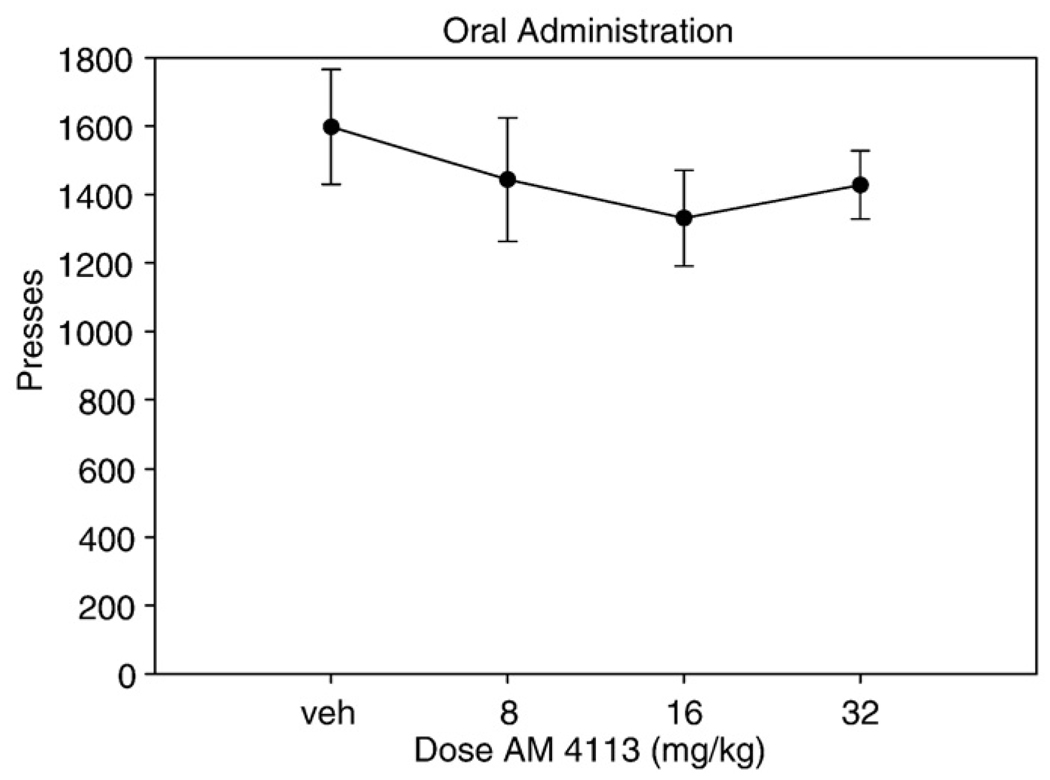
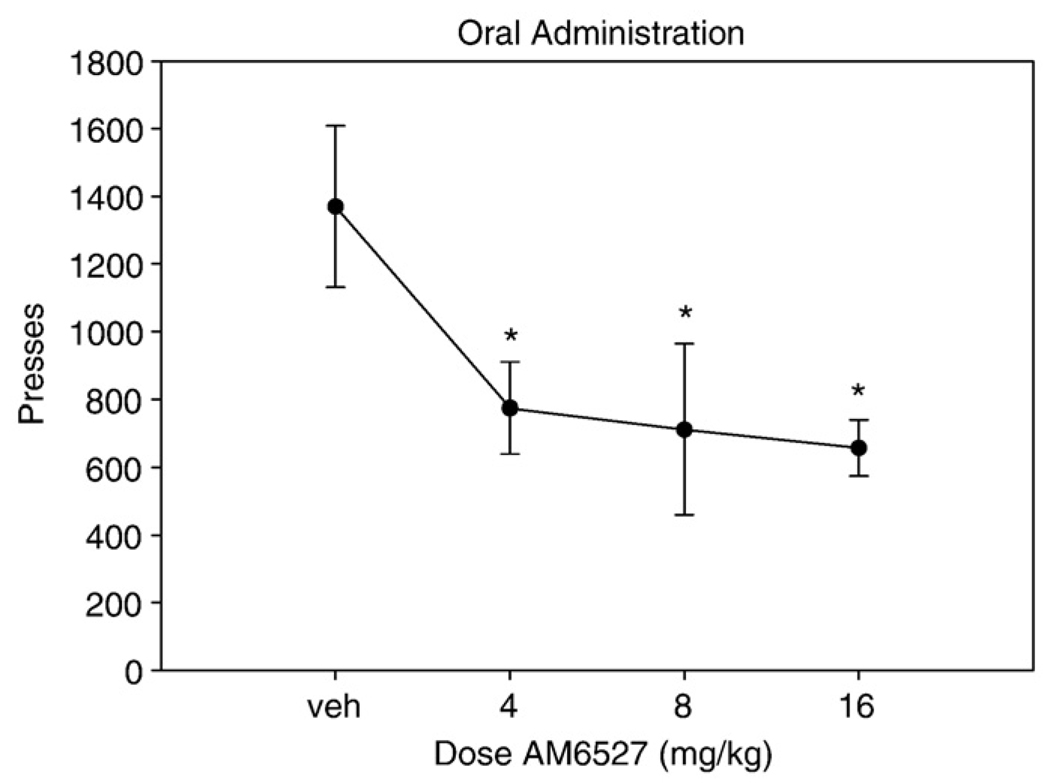
There was no significant change in lever pressing at any orally administered dose of AM4113 when compared to vehicle [F(3,21) = 0.522, n.s.; Fig. 2]. However, oral administration of AM6527 produced a dose-dependent decrease in responding on FR5 [F(3,21) = 4.901, p < 0.01; Fig. 3]. Planned comparisons demonstrated that every dose of AM6527 produced a significant suppression of responding compared to vehicle (p < 0.05). The ED50 of orally administered AM6527 for suppression of FR5 lever pressing was 1.49 mg/kg (R2 = 0.33).
Fig. 2.
The effect of orally administered (P.O.) AM4113 on FR5 lever pressing. Mean (±SEM) number of lever presses (FR5 schedule) during the 30-min session for rats that received treatment with vehicle or drug at 8.0, 16.0, or 32.0 mg/kg. There were no significant differences between treatments.
Fig. 3.
The effect of oral administration of AM6527 on FR5 lever pressing. Mean (±SEM) number of lever presses (FR5 schedule) during the 30-min session for rats that received treatment with vehicle or drug at 4.0, 8.0, or 16.0 mg/kg. Orally administered AM6527 significantly suppressed lever pressing on an FR5 operant schedule when compared to vehicle (*p < 0.05).
4. Discussion
Previously published results from our laboratory have demonstrated that AM4113, rimonabant (SR141716A), and AM251 are 100, 143, and 430 times more selective for CB1 than CB2 respectively (Lan et al., 1999; McLaughlin et al., 2006; Sink et al., 2008a). In the present study, AM6527 showed a similar selectivity for CB1 vs. CB2 receptors as AM4113 (i.e., approximately 100-fold), although the Ki values demonstrate greater affinity (0.89 nM and 92 nM for CB1 and CB2 respectively) for AM4113 compared to AM6527 (see Sink et al., 2008a; see also Table 1). The present behavioral results indicate that AM6527, like the cannabinoid CB1 receptor antagonist AM4113, and the inverse agonists rimonabant, AM251, and AM1387, can suppress food-reinforced operant lever pressing on a FR5 schedule of reinforcement (McLaughlin et al., 2003, 2006; Sink et al., 2008a,b). The potencies of AM4113 and AM6527 for suppression of lever pressing after IP administration were roughly comparable, with AM6527 having a slightly lower ED50, despite the fact that this drug appears to have a slightly lower affinity than AM4113 for CB1 receptors. Additional tests of feeding behavior, including other behavioral effects such as nausea (e.g. Sink et al., 2008a), should be conducted with AM6527 in order to provide a more detailed behavioral characterization of this compound. Nevertheless, AM6527, which is a very selective CB1 antagonist, does appear to be a potentially useful tool for studying the behavioral effects of CB1 receptor blockade.
The two CB1 antagonists differed markedly in terms of their effects after oral administration. Despite its relatively potent effects after IP administration, AM4113 failed to suppress food-reinforced operant responding after oral administration, even at doses up to 32.0 mg/kg. In contrast, AM6527 was effective at significantly reducing feeding after oral administration, with an ED50 of 1.49 mg/kg, suggesting that AM6527 has significantly higher oral bioavailability than AM4113. AM6527 was less potent after oral administration than it was after IP administration, which is a common finding for most drugs. Moreover, the shapes of the oral and IP dose response curves for AM6527 appeared to be somewhat different from each other. Nevertheless, it is clear that there is a striking difference between the oral potency of AM6527 and that of AM4113. These observations are consistent with unpublished mouse screening data indicating that AM6527 has approximately four times greater availability after oral administration than AM4113 (Wood and Makriyannis, unpublished observations). Several processes can influence the oral bioavailability of substances, including first-pass metabolism and other gastrointestinal and hepatic factors (Madden et al., 1995; Pastino and Conolloy, 2000; Choi et al., 2006; Gershkovich et al., 2007), and additional research will be necessary to determine which of these factors is important for cannabinoid CB1 antagonists.
Taken together, these results indicate that AM4113 may be very useful as a research tool because it is a neutral antagonist of CB1 receptors. However, its relative lack of potency with oral administration in rats suggests that this drug might not be very potent if orally administered in humans, which would make it less attractive as a candidate for drug development at this point. Additional research should determine if changes in vehicle composition (e.g. Carrier et al., 2007) could enhance the oral bioavailability of AM4113. Nevertheless, it does appear that AM6527, like rimonabant (Huestis et al., 2001; Costa et al., 2005; Davis and Nomikos, 2008), is an orally active drug that is capable of interfering with CB1 receptor transmission, and therefore AM6527 may be useful for clinical research on the appetite suppressant effects of interference with CB1 receptor transmission. Further studies should also assess the potential effects of AM6527 on processes other than appetite, such as forepaw usage during food handling and actions related to food aversions (McLaughlin et al., 2005; Sink et al., 2008a,b).
Acknowledgements
This research was supported by grants to JDS and AM from the United States NIH/NIDA. The authors would like to thank Ms. Shivangi Joci for performing the receptor binding assays published in this manuscript.
References
- Arnone M, Maruani J, Chaperon F, Thiébot MH, Poncelet M, Soubrié P, et al. Selective inhibition of sucrose and ethanol intake by SR 141716, an antagonist of central cannabinoid (CB1) receptors. Psychopharmacology. 1997;132:104–106. doi: 10.1007/s002130050326. [DOI] [PubMed] [Google Scholar]
- Carrier RL, Miller LA, Ahmed I. The utility of cyclodextrins for enhancing oral bioavailability. J Control Release. 2007;123(2):78–99. doi: 10.1016/j.jconrel.2007.07.018. [DOI] [PubMed] [Google Scholar]
- Chambers AP, Vemuri VK, Peng Y, Wood JT, Olszewska T, Pittman QJ, et al. A neutral CB1 receptor antagonist reduces weight gain in rat. Am J Physiol Regul Integr Comp Physiol. 2007;293:R2185–R2193. doi: 10.1152/ajpregu.00663.2007. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Prusoff WH. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 percent inhibition (I50) of an enzymatic reaction. Biochemical Pharmacology. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Choi YH, Kim SG, Lee MG. Dose-independent pharmacokinetics of metformin in rats: Hepatic and gastrointestinal first-pass effects. J Pharm Sci. 2006;95:2543–2552. doi: 10.1002/jps.20744. [DOI] [PubMed] [Google Scholar]
- Colombo G, Agabio R, Diaz G, Lobina C, Reali R, Gessa GL. Appetite suppression and weight loss after the cannabinoid antagonist SR 141716. Life Sci. 1998;63:PL113–PL117. doi: 10.1016/s0024-3205(98)00322-1. [DOI] [PubMed] [Google Scholar]
- Costa B, Trovato AE, Colleoni M, Giagnoni G, Zarini E, Croci T. Effect of the cannabinoid CB1 receptor antagonist, SR141716, on nociceptive response and nerve demyelination in rodents with chronic constriction injury of the sciatic nerve. Pain. 2005;116:52–61. doi: 10.1016/j.pain.2005.03.043. [DOI] [PubMed] [Google Scholar]
- Davis RJ, Nomikos GG. Oral administration of the antiobesity drugs, sibutramine and rimonabant, increases acetylcholine efflux selectively in the medial prefrontal cortex of the rat. Mol Psychiatry. 2008;13:240–241. doi: 10.1038/sj.mp.4002112. [DOI] [PubMed] [Google Scholar]
- Freedland CS, Poston JS, Porrino LJ. Effects of SR141716A, a central cannabinoid receptor antagonist, on food-maintained responding. Pharmacol Biochem Behav. 2000;67:265–270. doi: 10.1016/s0091-3057(00)00359-2. [DOI] [PubMed] [Google Scholar]
- Gardner A, Mallet PE. Suppression of feeding, drinking, and locomotion by a putative cannabinoid receptor ‘silent antagonist’. Eur J Pharmacol. 2006;530:103–106. doi: 10.1016/j.ejphar.2005.11.032. [DOI] [PubMed] [Google Scholar]
- Gershkovich P, Qadri B, Yacovan A, Amselem S, Hoffman A. Different impacts of intestinal lymphatic transport on the oral bioavailability of structurally similar synthetic lipophilic cannabinoids: dexanabinol and PRS-211,220. Eur J Pharm Sci. 2007;31:298–305. doi: 10.1016/j.ejps.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Huestis MA, Gorelick DA, Heishman SJ, Preston KL, Nelson RA, Moolchan E, et al. Blockade of effects of smoked marijuana by the CB1-selective cannabinoid receptor antagonist SR141716. Arch Gen Psychiatry. 2001;58:322–328. doi: 10.1001/archpsyc.58.4.322. [DOI] [PubMed] [Google Scholar]
- Jamshidi N, Taylor DA. Anandamide administration into the ventromedial hypothalamus stimulates appetite in rats. Br J Pharmacol. 2001;134:1151–1154. doi: 10.1038/sj.bjp.0704379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jogani VV, Shah PJ, Mishra P, Mishra AK, Misra AR. Nose-to-brain delivery of tacrine. J Pharm Pharmacol. 2007;59:1199–1205. doi: 10.1211/jpp.59.9.0003. [DOI] [PubMed] [Google Scholar]
- Keppel G. Design and Analysis: A Researchers Handbook. Englewood Cliffs, NJ: Prentice-Hall; 1982. [Google Scholar]
- Kirkham TC, Williams CM, Fezza F, Di Marzo V. Endocannabinoid levels in rat limbic forebrain and hypothalamus in relation to fasting, feeding and satiation: Stimulation of eating by 2-arachidonoyl glycerol. Br J Pharmacol. 2002;136:550–557. doi: 10.1038/sj.bjp.0704767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan R, Lui Q, Fan P, Lin S, Fernando SR, McCallion D, et al. Structure-activity relationships of pyrazole derivatives as cannabinoid receptor antagonists. J of Med Chem. 1999;42:769–776. doi: 10.1021/jm980363y. [DOI] [PubMed] [Google Scholar]
- Madden S, Spaldin V, Park BK. Clinical pharmacokinetics of tacrine. Clin Pharmacokinet. 1995;28:449–457. doi: 10.2165/00003088-199528060-00003. [DOI] [PubMed] [Google Scholar]
- Morse KL, Fournier DJ, Li X, Grzybowska J, Makriyannis A. A novel electrophilic high affinity irreversible probe for the cannabinoid receptor. Life Sci. 1995;56:1957–1962. doi: 10.1016/0024-3205(95)00176-7. [DOI] [PubMed] [Google Scholar]
- McLaughlin PJ, Winston K, Swezey L, Wisniecki A, Aberman J, Tardif DJ, et al. The cannabinoid CB1 antagonists SR 141716A and AM 251 suppress food intake and food-reinforced behavior in a variety of tasks in rats. Behav Pharmacol. 2003;14:583–588. doi: 10.1097/00008877-200312000-00002. [DOI] [PubMed] [Google Scholar]
- McLaughlin PJ, Winston KM, Limebeer CL, Parker LA, Makriyannis A, Salamone JD. The cannabinoid CB1 antagonist AM251 produces food avoidance and behaviors associated with nausea but does not impair feeding efficiency in rats. Psychopharmacology. 2005;180:286–293. doi: 10.1007/s00213-005-2171-0. [DOI] [PubMed] [Google Scholar]
- McLaughlin PJ, Qian L, Wood JT, Wisniecki A, Winston KM, Swezey LA, et al. Suppression of food intake and food-reinforced behavior produced by the novel CB1 receptor antagonist/inverse agonist AM1387. Pharmacol Biochem Behav. 2006;83:396–402. doi: 10.1016/j.pbb.2006.02.022. [DOI] [PubMed] [Google Scholar]
- Ohta H, Ishizaka T, Tatsuzuki M, Yoshinaga M, Iida I, Tomishima Y, et al. N-Alkylidenearylcarboxamides as new potent and selective CB(2) cannabinoid receptor agonists with good oral bioavailability. Bioorg Med Chem Lett. 2007;17:6299–6304. doi: 10.1016/j.bmcl.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Pastino GM, Conolly RB. Application of a physiologically based pharmacokinetic model to estimate the bioavailability of ethanol in male rats: distinction between gastric and hepatic pathways of metabolic clearance. Toxicol Sci. 2000;55:256–265. doi: 10.1093/toxsci/55.2.256. [DOI] [PubMed] [Google Scholar]
- Pi-Sunyer FX, Aronne LJ, Heshmati HM, Devin J, Rosenstock J RIO-North America Study Group. Effect of rimonabant, a cannabinoid-1 receptor blocker, on weight and cardiometabolic risk factors in overweight or obese patients: RIO-North America: a randomized controlled trial. JAMA. 2006;295:761–775. doi: 10.1001/jama.295.7.761. [DOI] [PubMed] [Google Scholar]
- Salamone JD, McLaughlin PJ, Sink K, Makriyannis A, Parker LA. Cannabinoid CB(1) receptor inverse agonists and neutral antagonists: effects on food intake, food-reinforced behavior and food aversions. Physiol Behav. 2007;91:383–388. doi: 10.1016/j.physbeh.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simiand J, Keane M, Keane PE, Soubrie P. SR141716, a CB1 cannabinoid receptor antagonist selectively reduces sweet food intake in marmoset. Behav Pharmacol. 1998;9:179–181. [PubMed] [Google Scholar]
- Sink KS, McLaughlin PJ, Wood JA, Brown C, Fan P, Vemuri VK, et al. The novel cannabinoid CB(1) receptor neutral antagonist AM4113 suppresses food intake and food-reinforced behavior but does not induce signs of nausea in rats. Neuropsychopharmacology. 2008a;43:946–955. doi: 10.1038/sj.npp.1301476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sink KS, Vemuri VK, Olszewska T, Makriyannis A, Salamone JD. Cannabinoid CB1 antagonists and dopamine antagonists produce different effects on a task involving response allocation and effort-related choice in food-seeking behavior. Psychopharmacology. 2008b;196:565–574. doi: 10.1007/s00213-007-0988-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Gaal LF, Rissanen AM, Scheen AJ, Ziegler O, Roessner S RIO-Europe Study Group. Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the RIO-Europe study. Lancet. 2005;365:1389–1397. doi: 10.1016/S0140-6736(05)66374-X. [DOI] [PubMed] [Google Scholar]
- Ward SJ, Dykstra LA. The role of CB1 receptors in sweet versus fat reinforcement: Effect of CB1 receptor deletion, CB1 receptor antagonism (SR141716A) and CB1 receptor agonism (CP-55940) Behav Pharmacol. 2005;16:381–388. doi: 10.1097/00008877-200509000-00010. [DOI] [PubMed] [Google Scholar]
- Williams CM, Kirkham TC. Anandamide induces overeating: mediation by central cannabinoid (CB1) receptors. Psychopharmacology. 1999;143:315–317. doi: 10.1007/s002130050953. [DOI] [PubMed] [Google Scholar]